ABSTRACT
Povidone-iodine (PI) and chlorhexidine (CHX) are widely used antiseptics active against conventional Staphylococcus aureus, Enterobacteriaceae, Candida species, and viruses, but their efficacy against Mycobacterium abscessus remains unproven. We determined the in vitro potency of alcoholic PI and CHX against M. abscessus subsp. abscessus (ATCC 19977), M. abscessus subsp. bolletii (BCRC 16915), and our outbreak strain of M. abscessus subsp. massiliense (TPE 101) in reference to Staphylococcus aureus (ATCC 29213) by standard quantitative suspension and carrier methods (EN 14563). By suspension, all mycobacterial strains compared to S. aureus were significantly more resistant to CHX, but not PI. By carrier, the mean logarithmic reductions (LR) achieved by PI under clean (dirty) conditions were 6.575 (2.482), 5.540 (2.298), 4.595 (1.967), and 1.173 (0.889), while those achieved by CHX under clean (dirty) conditions were 3.164 (5.445), 5.307 (2.564), 3.844 (2.232), and 0.863 (0.389) for S. aureus, M. abscessus subsp. bolletii, M. abscessus subsp. abscessus, and M. abscessus subsp. massiliense, respectively. M. abscessus subsp. massiliense (outbreak strain) was significantly more resistant than the other tested strains to PI and CHX. By both methods, the mean LR achieved by PI was higher than for CHX for all mycobacterial strains, but under dirty conditions, neither antiseptic was effectively mycobactericidal (LR < 5). These preliminary findings caution against the universal replacement of PI with CHX as the first-line skin antiseptic, since all M. abscessus isolates were resistant to CHX. More studies are needed to establish the best practice for skin antisepsis if mycobacterial infections are also to be prevented.
KEYWORDS: Mycobacterium abscessus, Mycobacterium abscessus subsp. massiliense, antiseptic resistance, chlorhexidine, disinfectants, in vitro, outbreak, povidone-iodine
INTRODUCTION
The expansion of body-modifying procedures and vulnerable populations has led to a global rise in outbreaks of nontuberculous mycobacterial (NTM) infections following cosmetic and medical procedures (1). The most common NTM causing such infections belongs to the Mycobacterium abscessus complex, whose members are notoriously difficult to treat, since they are inherently resistant to multiple antimicrobials (2–4). The M. abscessus complex comprises 3 subspecies of rapidly growing mycobacteria: M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii (5, 6).
Previously, in Taiwan, we found the emergence of a dominant clone of M. abscessus subsp. massiliense (TPE 101, MLST sequence type 48 [ST48], clonal complex 3) causing a prolonged outbreak of skin and soft tissue infections, with a peak attack rate in 2012 (7, 8). Outside Taiwan, an epidemic of surgical site infections in Brazil between 2004 and 2011 by a related clone (BRA100, ST23, clonal complex 3) coinciding with pulmonary infections among cystic fibrosis cohorts in the United Kingdom and United States has led to the identification of a globally successful clone of M. abscessus subsp. massiliense, perhaps with enhanced virulence and transmissibility (9–13).
Although the significance and specific virulence traits of these M. abscessus subsp. massiliense outbreak strains are only beginning to be unraveled, one untested hypothesis for their association with invasive procedures is their relative resistance to routine skin antisepsis (14). Povidone-iodine (PI) and chlorhexidine (CHX) are widely used antiseptics active against conventional Staphylococcus aureus, enteric bacteria, Candida species, and viruses, but their efficacy against individual strains of M. abscessus, particularly outbreak strains of M. abscessus subsp. massiliense, remains unproven (15, 16). Since various landmark studies in the last decade showing superiority of preoperative cleansing of the patient's skin with CHX over PI for preventing vascular catheter infections and surgical site infections after clean-contaminated surgery, there has been a shift toward recommending CHX over PI as the first-line skin antisepsis for a wide variety of procedures (17–19). We hypothesized that this secular trend has had an impact on the increasing frequency of rapidly growing NTM, particularly M. abscessus subsp. abscessus, isolated from catheter and surgical sites (20–24).
In this study, we determined the in vitro potencies of commonly applied formulations of alcoholic PI and CHX against our outbreak strain of M. abscessus subsp. massiliense (TPE 101) in reference to S. aureus (ATCC 29213), M. abscessus subsp. abscessus (ATCC 19977), and M. abscessus subsp. bolletii (BCRC 16915) by standard quantitative suspension and carrier (EN 14563-2008) methods.
RESULTS
Suspension method.
The mean log reductions (LRs) following suspension with PI and CHX under the various test conditions (exposure times and dilutions) for the three mycobacterial isolates and reference S. aureus strain (ATCC 29213) are shown in Table 1. There was a significant difference in the behaviors of the two antiseptics against the 4 tested strains (P < 0.05) (Table 1). PI consistently produced higher mean LRs than did CHX for all strains by the suspension method. However, there were significant interspecies and subspecies differences in the magnitude of the effect.
TABLE 1.
Mean LRs achieved by alcoholic PI and CHX for all tested M. abscessus strains and a reference S. aureus strain at each exposure time and dilution by quantitative suspension test
| Exposure time (s)a | Mean LRb at dilution: |
P1c,e | P2d,e | |||||
|---|---|---|---|---|---|---|---|---|
| 1:25 | 1:50 | 1:100 | 1:200 | 1:400 | 1:800 | |||
| S. aureus (ATCC 29213) | ||||||||
| PI | ||||||||
| 30 | 5.08 | 5.08 | 3.88 | 5.08 | 5.08 | 4.54 | 0.170 | Ref |
| 60 | 4.16 | 5.09 | 5.09 | 4.05 | 5.09 | 5.09 | 0.623 | |
| 90 | 4.32 | 4.71 | 5.15 | 4.68 | 5.15 | 5.15 | 0.667 | Ref |
| P3 | 0.665 | 0.536 | 0.102 | 0.572 | 0.592 | 0.414 | ||
| CHX | ||||||||
| 30 | 2.37 | 2.21 | 1.88 | 1.69 | 0.49 | 0.04 | 0.036 | Ref |
| 60 | 2.86 | 3.31 | 2.63 | 2.71 | 1.79 | 0.34 | 0.019 | |
| 90 | 3.93 | 2.90 | 3.35 | 4.22 | 2.25 | 1.06 | 0.141 | Ref |
| P4e | 0.382 | 0.443 | 0.277 | 0.208 | 0.028 | 0.023 | ||
| M. abscessus subsp. bolletii (BCRC 16915) | ||||||||
| PI | ||||||||
| 30 | 5.43 | 5.43 | 5.43 | 5.43 | 5.43 | 3.89 | 0.047 | 1.000 |
| 60 | 5.37 | 5.37 | 4.78 | 4.46 | 5.37 | 5.37 | 0.553 | |
| 90 | 5.35 | 5.35 | 5.35 | 5.35 | 4.82 | 5.35 | 0.480 | 1.000 |
| P3 | 0.277 | 0.277 | 0.423 | 0.388 | 0.377 | 0.129 | ||
| CHX | ||||||||
| 30 | −0.04 | −0.03 | −0.01 | −0.05 | 0.05 | 0.01 | 0.637 | 0.011 |
| 60 | −0.10 | −0.12 | −0.09 | −0.07 | −0.04 | −0.02 | 0.208 | |
| 90 | −0.13 | −0.18 | −0.10 | −0.07 | −0.02 | −0.07 | 0.085 | 0.007 |
| P4 | 0.099 | 0.066 | 0.204 | 0.860 | 0.184 | 0.459 | ||
| M. abscessus subsp. abscessus (ATCC 19977) | ||||||||
| PI | ||||||||
| 30 | 4.08 | 3.62 | 3.35 | 3.64 | 2.71 | 2.22 | 0.635 | 1.000 |
| 60 | 2.97 | 4.74 | 4.10 | 4.06 | 3.05 | 3.35 | 0.705 | |
| 90 | 2.95 | 4.15 | 3.35 | 4.11 | 4.12 | 3.34 | 0.904 | 0.495 |
| P3 | 0.451 | 0.616 | 0.825 | 0.934 | 0.563 | 0.666 | ||
| CHX | ||||||||
| 30 | −0.06 | −0.10 | −0.17 | −0.04 | −0.02 | −0.01 | 0.058 | 0.010 |
| 60 | −0.23 | −0.13 | −0.10 | −0.15 | −0.10 | −0.11 | 0.448 | |
| 90 | −0.14 | −0.07 | −0.12 | −0.12 | −0.05 | −0.07 | 0.846 | 0.008 |
| P4 | 0.075 | 0.670 | 0.497 | 0.458 | 0.674 | 0.151 | ||
| M. abscessus subsp. massiliense (TPE 101) | ||||||||
| PI | ||||||||
| 30 | 4.08 | 4.08 | 3.64 | 4.08 | 3.27 | 3.32 | 0.776 | 1.000 |
| 60 | 3.60 | 4.04 | 3.17 | 3.44 | 4.04 | 2.15 | 0.199 | |
| 90 | 4.07 | 4.07 | 4.07 | 3.15 | 2.78 | 2.74 | 0.300 | 0.177 |
| P3 | 0.385 | 0.911 | 0.351 | 0.617 | 0.446 | 0.641 | ||
| CHX | ||||||||
| 30 | −0.04 | −0.00 | −0.02 | −0.12 | −0.02 | −0.04 | 0.935 | 0.011 |
| 60 | −0.04 | −0.02 | −0.03 | 0.00 | −0.06 | −0.06 | 0.986 | |
| 90 | −0.04 | −0.06 | 0.01 | 0.02 | −0.03 | −0.03 | 0.821 | 0.010 |
| P4 | 0.999 | 0.792 | 0.893 | 0.138 | 0.795 | 0.981 | ||
P3, P values for whether the differences with increasing exposure times are statistically significant for povidone-iodine; P4, P values for whether the differences with increasing exposure times are statistically significant for chlorhexidine.
The experiments were conducted three times, and an average (mean) LR was obtained for each test condition. A mean LR of greater than 5 was considered biocidal.
P1, P values for whether the differences across the range of dilutions within species are statistically significant.
P2, P values for the interspecies comparison (whether the activities of PI or CHX is significantly different between mycobacterial strain and the reference S. aureus strain) at a dilution of 1:25 with exposure times of 30 s and at a dilution of 1:800 with exposure times of 90 s. Ref, reference.
P values of <0.05 are in boldface.
The effects of PI and CHX for S. aureus described below are consistent with previous studies (15). PI achieved an average LR of greater than 5 for most dilutions at 30 s for S. aureus. In contrast, CHX showed only a minimal killing effect, failing to attain an LR of greater than 5 under any of the test conditions for S. aureus. The effects of dilution and exposure time did not fit the decay model for PI, whereas for CHX, the predicted effect was obtained. This is well illustrated in Table 1, which shows a regular decay of activity of CHX throughout the dilution range (P < 0.036) and increased activity of CHX with increased contact time (P < 0.028), which is slowly bactericidal. In contrast, PI is rapidly lethal, acting mainly during the first 30 s and showing little enhanced killing when the time of exposure was increased.
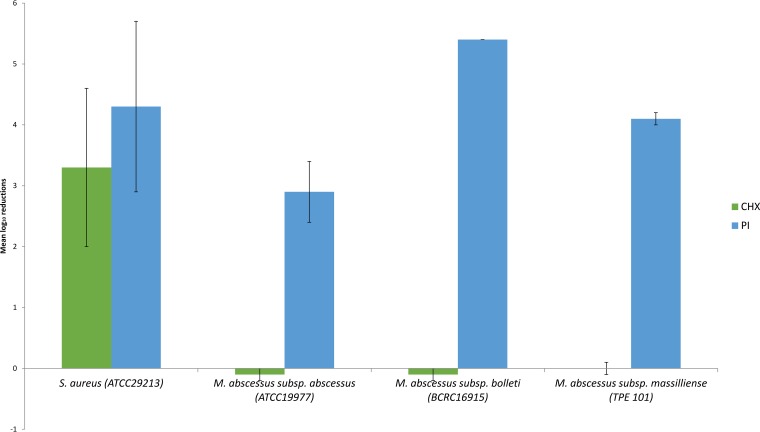
For the clinically prevalent M. abscessus subsp. abscessus and M. abscessus subsp. massiliense outbreak strains, the LRs for CHX were less than 1, while those for PI were less than 5 for all test conditions. No enhanced activity for CHX was observed with increased contact time. The potency of PI against the less frequently clinically implicated M. abscessus subsp. bolletii approached that of S. aureus, with LRs of greater than 5 for most dilutions and maximal killing at 30 s. However, no mycobactericidal effect of CHX could be demonstrated against M. abscessus subsp. bolletii, with LRs of less than 1 for all test conditions. Taken together, M. abscessus subsp. abscessus and M. abscessus subsp. massiliense demonstrated moderate in vitro resistance against PI and high resistance against CHX in comparison to S. aureus, while M. abscessus subsp. bolletii appeared to be as susceptible to PI as S. aureus and as resistant to CHX as the other two mycobacterial strains. The comparative activities of PI (4,000 mg/liter) and CHX (800 mg/liter) at the maximal concentration (1:25 dilution) and contact time (90 s) against all strains tested are shown in Fig. 1.
FIG 1.
Potency of alcoholic PI and CHX by quantitative suspension testing against different strains of M. abscessus expressed as the mean logarithmic reductions of CFU at the maximal concentrations of 4,000 mg/liter (PI) and 800 mg/liter (CHX) and at the maximal exposure time of 90 s. The error bars indicate SD.
Quantitative carrier method.
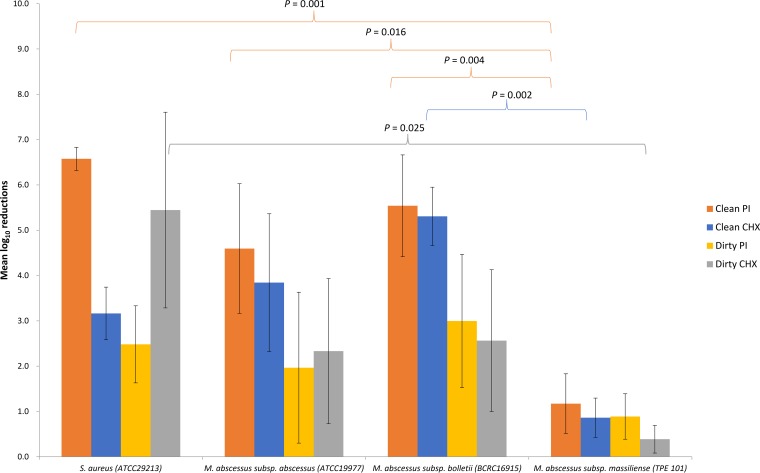
The mean (standard deviation [SD]) LRs achieved by PI under clean versus dirty conditions were 6.575 (0.255) versus 2.482 (0.851), 5.540 (1.123) versus 2.298 (1.471), 4.595 (1.431) versus 1.967 (1.665), and 1.173 (0.658) versus 0.889 (0.501), while those achieved by CHX under clean versus dirty conditions were 3.164 (0.581) versus 5.445 (2.159), 5.307 (0.643) versus 2.564 (1.556), 3.844 (1.519) versus 2.232 (1.602), and 0.863 (0.433) versus 0.389 (0.306) for S. aureus, M. abscessus subsp. bolletii, M. abscessus subsp. abscessus, and M. abscessus subsp. massiliense, respectively (Table 2). M. abscessus subsp. massiliense (outbreak strain) was significantly more resistant than the other tested strains to PI and CHX (P < 0.05) (Fig. 2). While the mean LR achieved by PI was higher than for CHX for all mycobacterial strains, this was not statistically significant. For S. aureus, the efficacy of PI was significantly (P = 0.019) reduced under dirty conditions but that of CHX activity was not affected (P = 0.293) (16). Neither antiseptic was effectively mycobactericidal under dirty conditions (LR < 5). The least killing activity (LR < 2) was observed for the outbreak M. abscessus subsp. massiliense strain regardless of antiseptic or the presence or absence of organic debris.
TABLE 2.
Mean LRs achieved by alcoholic PI and CHX for all tested M. abscessus strains and a reference S. aureus strain by quantitative carrier test (EN 14563-2008)
| Conditiona | Mean LRb (SD) |
P1c,e | P2d,e | |||
|---|---|---|---|---|---|---|
| S. aureus (ATCC 29213) | M. abscessus subsp. bolletii (BCRC 16915) | M. abscessus subsp. abscessus (ATCC 19977) | M. abscessus subsp. massiliense (TPE 101) | |||
| PI | ||||||
| Clean | 6.58 (0.25) | 5.54 (1.12) | 4.60 (1.43) | 1.17 (0.66) | 1.000 (1.000) | |
| Dirty | 2.48 (0.85) | 3.00 (1.47) | 1.97 (1.66) | 0.89 (0.50) | 0.226 (1.000) | |
| P3e | 0.019 | 0.228 | 0.435 | 1.000 | 0.001 (0.883) | |
| CHX | ||||||
| Clean | 3.16 (0.58) | 5.31 (0.64) | 3.84 (1.52) | 0.86 (0.43) | 0.117 (0.324) | |
| Dirty | 5.45 (2.16) | 2.56 (1.57) | 2.33 (1.60) | 0.39 (0.31) | 1.000 (0.224) | |
| P4 | 0.293 | 0.168 | 1.000 | 1.000 | 0.084 (0.025) | |
| P5e | 0.050 (0.100) | 1.000 (1.000) | 1.000 (1.000) | 1.000 (1.000) | ||
P3 is the P value for comparisons of clean versus dirty conditions for PI within species; P4 is the P value for comparisons of clean versus dirty conditions for CHX within species; P5 is the P value for comparisons of PI versus CHX under clean (dirty) conditions within species.
The experiments were conducted three times, and an average (mean) LR was obtained for each test condition. A mean LR of greater than 5 was considered biocidal.
P1 is the P value for interspecies comparisons under clean (dirty) conditions for each mycobacterial species in reference to S. aureus in the following order: M. abscessus subsp. bolletii, M. abscessus subsp. abscessus, and M. abscessus subsp. massiliense for PI.
P2 is the P value for interspecies comparisons under clean (dirty) conditions for each mycobacterial species in reference to S. aureus in the following order: M. abscessus subsp. bolletii, M. abscessus subsp. abscessus, and M. abscessus subsp. massiliense for CHX.
P values of <0.05 are in boldface.
FIG 2.
Potencies of alcoholic PI and CHX by quantitative carrier testing (EN 14563) against different strains of M. abscessus expressed as the mean logarithmic reductions of CFU. The error bars indicate SD.
DISCUSSION
This in vitro study using two established methodologies to assess the susceptibility of M. abscessus subsp. massiliense (TPE101; outbreak strain), M. abscessus subsp. abscessus (ATCC 19977; nonoutbreak strain), M. abscessus subsp. bolletii (nonoutbreak strain), and S. aureus (reference strain) to commercial formulations of chlorhexidine and povidone-iodine suggests that clinically prevalent M. abscessus strains are highly resistant to the most commonly used commercial formulation of 2% chlorhexidine-alcohol and only partially susceptible to 10% povidone-iodine-alcohol. Since the skin is the major source of pathogens following an invasive procedure and a potential reservoir for mycobacteria contaminating hospital water or products applied to the skin prior to the invasive procedure, it is conceivable that inadequate mycobactericidal activities of the currently used antiseptics may lead to an increase in postprocedural mycobacterial infections (1, 4, 7, 21).
The inactivity of CHX in suspension against these M. abscessus strains is particularly worrisome, as there is a potential for the antiseptic itself, commonly stored in multiuse bottles, to be contaminated by M. abscessus in a manner similar to that in which an outbreak of postinjection M. abscessus infection was linked to the contamination of the benzalkonium chloride used to disinfect the skin prior to articular steroid injection (25). In contrast, based on the suspension results, multiuse bottles of PI are unlikely to permit the growth of M. abscessus, although its activity did appear to be attenuated by organic material and on a two-dimensional surface.
Chlorhexidine gluconate is a nonvolatile, slowly bactericidal agent (26, 27). In susceptible bacteria, it collapses the membrane potential, and membrane disruption is followed by leakage of intracellular constituents. Its mechanism of action is concentration dependent. Higher concentrations of CHX cause coagulation of cytoplasmic proteins and nucleic acids, which is also slowly lethal (27). One of its main attributes is residual antimicrobial activity, which is beneficial in skin antiseptics used for catheter care but not necessarily in hand hygiene or wound care (18, 27). Another benefit of CHX over PI is the absence of skin staining, which is an important consideration in cosmetic surgery. The combination of CHX and an alcohol, with the alcohol providing rapid bactericidal effect, has gradually replaced PI as the first-line skin antisepsis in the last 2 decades (18, 19), at a time coinciding with increasing reports of outbreaks of nontuberculous mycobacterial skin and soft tissue infections after surgery, particularly cosmetic surgery (1).
CHX's general lack of activity against mycobacteria, particularly Mycobacterium tuberculosis, has been exploited in sputum decontamination to improve isolation rates (28, 29). However, CHX cannot be assumed to spare all mycobacteria, since it demonstrated highly mycobactericidal effects (>6 LRs) even in the presence of sputum in both suspension and carrier tests against M. smegmatis, and the MICs for some mycobacterial strains are on the order of those for CHX-sensitive Gram-positive cocci (27, 30, 31). The marginally better activity of CHX in the surface carrier tests for standard strains of M. abscessus subsp. bolletii and M. abscessus subsp. abscessus compared to the suspension test in our study may be due to the increased length of exposure before neutralization (120 s instead of 30 to 90 s in the suspension test), the lack of dilution of 2% chlorhexidine-75% alcohol in the surface carrier test (4,000 mg/liter versus 800 mg/liter in the suspension test), and the effect of the coformulated alcohol, which was allowed to evaporate in the surface carrier test but not in the suspension test. However, there was really no difference in the activity of CHX assayed (LRs all <1) by suspension or carrier tests against M. abscessus subsp. massiliense (TPE 101).
This dominant clone of M. abscessus subsp. massiliense (TPE 101) emerged in 2010, causing skin and soft tissue infections in northern and central Taiwan after invasive procedures, with a peak attack rate in 2012, whose common source was finally traced to a brand of contaminated ultrasonography transmission gel (8). At our institution, this coincided with implementation of the Joint Commission International-endorsed use of chlorhexidine in central vascular catheter infection prevention bundles in 2010. In fact, the pivotal case that led to identification of the ultrasonography gel as the point source of the outbreak was a patient diagnosed with metastatic pancreatic adenocarcinoma, who had received an ultrasound-guided tunneled central vascular catheter in the operating room in anticipation of chemotherapy and developed TPE 101 bacteremia within 24 h. The investigation revealed that both used and unopened sonography gels were also contaminated by nonfermentative Gram-negative bacilli (NFGNB) and Candida species (8). However, neither the above-mentioned patient nor the other cases receiving ultrasound-guided procedures who developed M. abscessus subsp. massiliense procedural site infections were concomitantly infected by these NFGNB or yeasts. Hence, we hypothesized that inadequate mycobactericidal decontamination of the skin after ultrasonography may have led to the emergence of M. abscessus subsp. massiliense TPE 101.
This hypothesis of antiseptic failure effectively leading to selective pressure for TPE 101 was supported by our findings (Fig. 2). Of the tested M. abscessus strains, including the less frequently isolated M. abscessus subsp. bolletii and the M. abscessus subsp. abscessus ATCC 19977 strain, both not implicated in skin and soft tissue infections at our institution, CHX achieved LRs of >3 under clean and >2 under dirty conditions by carrier testing, possibly qualifying it as mycobacteriostatic. However, CHX LRs were uniformly <1 against TPE 101 regardless of the presence or absence of an organic load. This intraspecies differential resistance to CHX was statistically significant (Fig. 2). Intraspecies differential resistance to CHX has been demonstrated before by a transmission electron microscopy (EM) study on glutaraldehyde-resistant Mycobacterium chelonae strains compared to a reference strain of M. chelonae, NCTC 946 (32). Fraud et al. were able to demonstrate significant cell wall alterations (by EM analysis and the dramatic loss of lipids) observed in M. chelonae NCTC 946 spheroplasts at low CHX concentrations of 25 to 100 mg/liter, which resulted in cytosolic protein precipitation and cell death of the NCTC 946 strains, but not in their two glutaraldehyde-resistant M. chelonae strains, which required much higher concentrations of CHX (500 mg/liter) to induce the same effects, ostensibly because the permeability barrier had been altered to a lesser extent (32).
By the surface carrier test, TPE 101 also demonstrated high resistance to PI. The possible mechanisms for the enhanced CHX and PI resistance phenotype of this outbreak strain, such as decreased cell wall permeability or “rough colony” morphology, deserve further characterization by EM studies, as described above (32–34). Furthermore, TPE 101 was typed as ST48, differing by only 1 of 7 multilocus sequence type (MLST) loci (murC gene) from the globally successful clone ST23, and belonging to the same clonal complex 3 as ST23 (8). ST23 has been responsible for an epidemic of postsurgical infections involving at least 2,032 cases across 63 hospitals in Brazil (BRA100) and two respiratory outbreaks among cystic fibrosis cohorts in the United States and the United Kingdom (9, 11, 12). BRA100 has been shown to tolerate high concentrations of glutaraldehyde (up to 7%), which is a common disinfectant for endoscopic or heat-intolerant surgical equipment, and other clinical strains of M. abscessus subsp. massiliense have demonstrated in vitro resistance to quaternary ammonium compounds (35, 36). The resistance of these epidemic strains to skin antiseptics such as CHX and PI has not been previously determined, yet it is highly likely that outbreak strains of M. abscessus subsp. massiliense, including ST23 and ST48, share multiple disinfectant resistance mechanisms that facilitate their adaptation to health care settings.
The results of S. aureus bactericidal suspension and carrier tests performed in this study are in agreement with published studies (15, 16). Although there have been several studies on the activities of PI and CHX against other mycobacterial species, we found only one comparable study on the mycobactericidal activities of PI and CHX against M. abscessus (30, 37, 38). In that study, one M. abscessus strain was tested using the suspension method. Nevertheless, similar to our suspension results, PI was shown to be rapidly mycobactericidal even at dilutions to 0.05% for 30 s for their M. abscessus clinical isolate, as well as for standard strains of Mycobacterium avium, Mycobacterium kansasii, and M. tuberculosis. Although CHX activity was not tested against their M. abscessus clinical isolate, they also showed a lack of CHX activity against M. tuberculosis H37Rv, M. kansasii ATCC 12478, and M. avium ATCC 15769 (37). The conclusion from this and other published studies by this group on other mycobacterial strains was that PI remains a useful antiseptic against mycobacteria, albeit with an expected reduction of activity with an organic load and when tested on surrogate or skin surfaces (39, 40).
The limitations of this study include the interreplicate variability, which could have been reduced by increasing the replicates (currently tested in triplicate), and the testing of only two well-established skin antiseptics and not of newer, potentially more mycobactericidal antiseptics. CHX's activity may be improved at higher concentrations, so it would be worth testing commercial formulations of 4% and 20% CHX. The strengths of this study include the use of two methods to assess antiseptic activity (since antiseptics act differently on planktonic and surface-adherent microorganisms, yielding discrepancies between suspension and carrier tests), realistic contact times, and the use of an organic load to mimic the skin and biofilms (30). In addition, both antiseptics studied were alcohol based to avoid the previous criticism that the differences between CHX and PI may be solvent related (41).
Nevertheless, these in vitro findings will need to be corroborated by skin surface (ex vivo) and clinical data to determine their clinical impact. Of note, the various randomized clinical trials showing superiority of CHX over PI documented a decrease of infections by mostly bacteria (and Candida species) only; mycobacteria were absent from all reports, due to either rarity or omission (17, 18, 42). It may thus be prudent for future clinical trials of CHX and PI to consider obtaining cultures specifically for recovering mycobacteria, since they may be overlooked by routine culture methods and there is poor evidence to assume that the broad-spectrum antimicrobial activity of these two common antiseptic preparations encompass pathogenic mycobacteria.
The principal conclusion from this work is that alcohol-based 2% CHX is insufficient to prevent, and may actually facilitate, health care-associated infections with epidemic M. abscessus strains. The observed growing preference for using CHX as the first-line antisepsis in central vascular catheter placement and surgery might feasibly contribute to the increasing frequency of M. abscessus isolated from the bloodstream and surgical sites (24). More studies are needed to establish the best practice for skin antisepsis if mycobacterial infections are also to be prevented and to establish the virulence traits conferring the epidemic potential of M. abscessus subsp. massiliense ST48 and ST23 strains.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The organisms studied were M. abscessus subsp. abscessus (ATCC 19977), M. abscessus subsp. bolletii (BCRC 16915), and M. abscessus subsp. massiliense (TPE 101, ST48, clonal complex 3) and S. aureus (ATCC 29213). Subculture and manipulation of the test organisms were kept to a minimum. Suspensions of all the test organisms were prepared and frozen in 1-ml aliquots at −80°C until required.
Skin antiseptics tested.
The mycobactericidal activities of the two most common skin disinfectants, Sindine antiseptic solution (alcoholic) containing 10% PI with 0.7 ml/1 ml 95% alcohol (Sinphar Pharmaceutical Co. Ltd., Taiwan) and Easy Antiseptic Liquid 2% (alcoholic) containing 2% CHX gluconate and 75% alcohol (2% CHX; Panion & BF Biotech Inc., Taiwan), were assessed over concentrations of 125 to 4,000 mg/liter (PI) and 25 to 800 mg/liter (CHX) (corresponding to dilutions of 1:25 to 1:800), respectively.
Suspension test.
The methods for preparing the test suspension and performing the disinfectant tests have been previously described in detail (43, 44). Briefly, bacteria were harvested from blood/Mueller-Hinton agar, added to moistened glass beads, and shaken for 5 min. Ten milliliters of double-distilled water was added, agitated, and left to settle for 30 min. The supernatant was removed to a second sterile bottle and left to settle for a further 2 h. Following sonication at 50 to 60 Hz for 10 min, the bacterial suspension in distilled water was adjusted turbidimetrically to 0.5 McFarland standard; 100 μl of the supernatant was added to 900 μl of the disinfectant at room temperature for contact times of 30, 60, and 90 s, thus spanning realistic in-use contact times. After the required contact time, 10 μl was removed to 990 μl of the neutralization/recovery system and serially diluted to 10−2. A combination of 3% Asolectin from soybean, 10% Tween 80, and 0.3% sodium thiosulfate in double-distilled water was used to inactivate or neutralize the antiseptic solution according to published results and our validation tests (45). Ten microliters of the neat solution and subsequent dilutions were plated on Mueller-Hinton agar, and surviving colonies were enumerated following appropriate incubation for 3 to 5 days at 35°C. The results of the test were expressed as the LR, which is the log10 value of the counts after exposure to the test antiseptic (Na) subtracted from the log10 value of the counts after exposure to the control (Nc). The experiments were performed in triplicate. An average (mean) LR of greater than 5 was considered biocidal.
Quantitative carrier test.
The European standard EN 14563, dedicated to testing products used in the medical area for mycobactericidal activity, was established in 2008 (46, 47). The potencies of alcoholic PI and CHX against the three mycobacterial and the reference S. aureus isolates was evaluated under “dirty” and “clean” conditions in accordance with EN 14563 with the following minor modifications.
Briefly, a bacterial suspension yielding 1.5 × 109 to 5 × 109 CFU/ml was freshly prepared, homogenized, and used within 2 h (46). One milliliter of interfering substances (0.3 g/liter bovine albumin under clean conditions or a mixture of 3 ml/liter sheep erythrocytes and 3.0 g/liter bovine albumin under dirty conditions) was mixed with 9 ml of the bacterial suspension, and 0.05 ml of this mixture was pipetted and evenly spread on the inoculation square of a frosted glass carrier. The carrier was maintained at 35°C for 60 min ± 10 s. After drying, the test antiseptic solution (CHX or PI) or distilled water (control) was applied to cover the entire surface of the inoculation square using a sterile cotton swab in the manner that preoperative skin is disinfected. At the end of 2 min of contact time to simulate recommended practice with the test antiseptic or control, the carrier was transferred into a neutralizing solution containing glass beads. The bacteria were dislodged from the surface by shaking. The number of surviving bacteria in each sample was determined, and the reduction was calculated and expressed as the LR as described above. The experiments were conducted three times, and an average (mean) LR was obtained for each test condition.
Statistical methods.
Statistical analysis was performed using SPSS Statistics software, version 21 (IBM Corp.). The statistical method was based upon analysis of variance of the mean LRs partitioned into components attributable to differences in concentration (dilutions), exposure time, bacterial strain, and choice of treatment (PI or CHX). Pairwise differences among the different experimental and control groups were detected by the Bonferroni method. Two-sided P values of less than or equal to 0.05 were considered significant.
ACKNOWLEDGMENTS
We thank the Third Core Facility at the National Taiwan University Hospital for technical assistance and facility support.
This study was funded by Taiwan's Ministry of Science and Technology (grant no.105-2628-B-002-019-MY3) awarded to Aristine Cheng.
We declare no conflicts of interest.
REFERENCES
- 1.Atkins BL, Gottlieb T. 2014. Skin and soft tissue infections caused by nontuberculous mycobacteria. Curr Opin Infect Dis 27:137–145. doi: 10.1097/QCO.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 2.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 3.Chen HY, Chen CY, Huang CT, Ruan SY, Chou CH, Lai CC, Liao CH, Tan CK, Huang YT, Yu CJ, Hsueh PR. 2011. Skin and soft-tissue infection caused by non-tuberculous mycobacteria in Taiwan, 1997–2008. Epidemiol Infect 139:121–129. doi: 10.1017/S0950268810001603. [DOI] [PubMed] [Google Scholar]
- 4.Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, Yang PC, Luh KT, Hsueh PR. 2010. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis 16:294–296. doi: 10.3201/eid1602.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tortoli E, Kohl TA, Brown-Elliott BA, Trovato A, Leao SC, Garcia MJ, Vasireddy S, Turenne CY, Griffith DE, Philley JV, Baldan R, Campana S, Cariani L, Colombo C, Taccetti G, Teri A, Niemann S, Wallace RJ Jr, Cirillo DM. 2016. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb nov. Int J Syst Evol Microbiol 66:4471–4479. doi: 10.1099/ijsem.0.001376. [DOI] [PubMed] [Google Scholar]
- 6.Sassi M, Drancourt M. 2014. Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics 15:359. doi: 10.1186/1471-2164-15-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng A, Liu YC, Chen ML, Hung CC, Tsai YT, Sheng WH, Liao CH, Hsueh PR, Chen YC, Chang SC. 2013. Extrapulmonary infections caused by a dominant strain of Mycobacterium massiliense (Mycobacterium abscessus subspecies bolletii). Clin Microbiol Infect 19:E473–E482. doi: 10.1111/1469-0691.12261. [DOI] [PubMed] [Google Scholar]
- 8.Cheng A, Sheng WH, Huang YC, Sun HY, Tsai YT, Chen ML, Liu YC, Chuang YC, Huang SC, Chang CI, Chang LY, Huang WC, Hsueh PR, Hung CC, Chen YC, Chang SC. 2016. Prolonged postprocedural outbreak of Mycobacterium massiliense infections associated with ultrasound transmission gel. Clin Microbiol Infect 22:382 e1–382 e11. doi: 10.1016/j.cmi.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Tettelin H, Davidson RM, Agrawal S, Aitken ML, Shallom S, Hasan NA, Strong M, de Moura VC, De Groote MA, Duarte RS, Hine E, Parankush S, Su Q, Daugherty SC, Fraser CM, Brown-Elliott BA, Wallace RJ Jr, Holland SM, Sampaio EP, Olivier KN, Jackson M, Zelazny AM. 2014. High-level relatedness among Mycobacterium abscessus subsp. massiliense strains from widely separated outbreaks. Emerg Infect Dis 20:364–371. doi: 10.3201/eid2003.131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shallom SJ, Gardina PJ, Myers TG, Sebastian Y, Conville P, Calhoun LB, Tettelin H, Olivier KN, Uzel G, Sampaio EP, Holland SM, Zelazny AM. 2013. New rapid scheme for distinguishing the subspecies of the Mycobacterium abscessus group and identifying Mycobacterium massiliense isolates with inducible clarithromycin resistance. J Clin Microbiol 51:2943–2949. doi: 10.1128/JCM.01132-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aitken ML, Limaye A, Pottinger P, Whimbey E, Goss CH, Tonelli MR, Cangelosi GA, Dirac MA, Olivier KN, Brown-Elliott BA, McNulty S, Wallace RJ Jr. 2012. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 185:231–232. doi: 10.1164/ajrccm.185.2.231. [DOI] [PubMed] [Google Scholar]
- 12.Duarte RS, Lourenco MC, Fonseca LDS, Leao SC, Amorim EDL, Rocha IL, Coelho FS, Viana-Niero C, Gomes KM, da Silva MG, Lorena NS, Pitombo MB, Ferreira RM, Garcia MH, de Oliveira GP, Lupi O, Vilaca BR, Serradas LR, Chebabo A, Marques EA, Teixeira LM, Dalcolmo M, Senna SG, Sampaio JL. 2009. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J Clin Microbiol 47:2149–2155. doi: 10.1128/JCM.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 15.McLure AR, Gordon J. 1992. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect 21:291–299. doi: 10.1016/0195-6701(92)90139-D. [DOI] [PubMed] [Google Scholar]
- 16.Block C, Robenshtok E, Simhon A, Shapiro M. 2000. Evaluation of chlorhexidine and povidone iodine activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis using a surface test. J Hosp Infect 46:147–152. doi: 10.1053/jhin.2000.0805. [DOI] [PubMed] [Google Scholar]
- 17.Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, Miller HJ, Awad SS, Crosby CT, Mosier MC, Alsharif A, Berger DH. 2010. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 362:18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 18.Humar A, Ostromecki A, Direnfeld J, Marshall JC, Lazar N, Houston PC, Boiteau P, Conly JM. 2000. Prospective randomized trial of 10% povidone-iodine versus 0.5% tincture of chlorhexidine as cutaneous antisepsis for prevention of central venous catheter infection. Clin Infect Dis 31:1001–1007. doi: 10.1086/318145. [DOI] [PubMed] [Google Scholar]
- 19.Privitera GP, Costa AL, Brusaferro S, Chirletti P, Crosasso P, Massimetti G, Nespoli A, Petrosillo N, Pittiruti M, Scoppettuolo G, Tumietto F, Viale P. 2017. Skin antisepsis with chlorhexidine versus iodine for the prevention of surgical site infection: a systematic review and meta-analysis. Am J Infect Control 45:180–189. doi: 10.1016/j.ajic.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Liao CH, Lai CC, Ding LW, Hou SM, Chiu HC, Chang SC, Hsueh PR. 2007. Skin and soft tissue infection caused by non-tuberculous mycobacteria. Int J Tuberc Lung Dis 11:96–102. [PubMed] [Google Scholar]
- 21.Wu TS, Yang CH, Brown-Elliott BA, Chao AS, Leu HS, Wu TL, Lin CS, Griffith DE, Chiu CH. 2016. Postcesarean section wound infection caused by Mycobacterium massiliense. J Microbiol Immunol Infect 49:955–961. doi: 10.1016/j.jmii.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Galmes-Truyols A, Gimenez-Duran J, Bosch-Isabel C, Nicolau-Riutort A, Vanrell-Berga J, Portell-Arbona M, Segui-Prat B, Guma-Tora M, Marti-Alomar I, Rojo-Arias MA, Ruiz-Veramendi M. 2011. An outbreak of cutaneous infection due to Mycobacterium abscessus associated to mesotherapy. Enferm Infecc Microbiol Clin 29:510–514. doi: 10.1016/j.eimc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Lee MR, Cheng A, Lee YC, Yang CY, Lai CC, Huang YT, Ho CC, Wang HC, Yu CJ, Hsueh PR. 2012. CNS infections caused by Mycobacterium abscessus complex: clinical features and antimicrobial susceptibilities of isolates. J Antimicrob Chemother 67:222–225. doi: 10.1093/jac/dkr420. [DOI] [PubMed] [Google Scholar]
- 24.Lee MR, Ko JC, Liang SK, Lee SW, Yen DH, Hsueh PR. 2014. Bacteraemia caused by Mycobacterium abscessus subsp. abscessus and M. abscessus subsp. bolletii: clinical features and susceptibilities of the isolates. Int J Antimicrob Agents 43:438–441. doi: 10.1016/j.ijantimicag.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Tiwari TS, Ray B, Jost KC Jr, Rathod MK, Zhang Y, Brown-Elliott BA, Hendricks K, Wallace RJ Jr. 2003. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis 36:954–962. doi: 10.1086/368192. [DOI] [PubMed] [Google Scholar]
- 26.Russell AD. 1986. Chlorhexidine: antibacterial action and bacterial resistance. Infection 14:212–215. doi: 10.1007/BF01644264. [DOI] [PubMed] [Google Scholar]
- 27.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferroni A, Vu-Thien H, Lanotte P, Le Bourgeois M, Sermet-Gaudelus I, Fauroux B, Marchand S, Varaigne F, Berche P, Gaillard JL, Offredo C. 2006. Value of the chlorhexidine decontamination method for recovery of nontuberculous mycobacteria from sputum samples of patients with cystic fibrosis. J Clin Microbiol 44:2237–2239. doi: 10.1128/JCM.00285-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asmar S, Drancourt M. 2015. Chlorhexidine decontamination of sputum for culturing Mycobacterium tuberculosis. BMC Microbiol 15:155. doi: 10.1186/s12866-015-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best M, Sattar SA, Springthorpe VS, Kennedy ME. 1988. Comparative mycobactericidal efficacy of chemical disinfectants in suspension and carrier tests. Appl Environ Microbiol 54:2856–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell AD. 1996. Activity of biocides against mycobacteria. Soc Appl Bacteriol Symp Ser 25:87S–101S. [PubMed] [Google Scholar]
- 32.Fraud S, Hann AC, Maillard JY, Russell AD. 2003. Effects of ortho-phthalaldehyde, glutaraldehyde and chlorhexidine diacetate on Mycobacterium chelonae and Mycobacterium abscessus strains with modified permeability. J Antimicrob Chemother 51:575–584. doi: 10.1093/jac/dkg099. [DOI] [PubMed] [Google Scholar]
- 33.Ruger K, Hampel A, Billig S, Rucker N, Suerbaum S, Bange FC. 2014. Characterization of rough and smooth morphotypes of Mycobacterium abscessus isolates from clinical specimens. J Clin Microbiol 52:244–250. doi: 10.1128/JCM.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernut A, Herrmann JL, Ordway D, Kremer L. 2017. The diverse cellular and animal models to decipher the physiopathological traits of Mycobacterium abscessus infection. Front Cell Infect Microbiol 7:100. doi: 10.3389/fcimb.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorena NS, Pitombo MB, Cortes PB, Maya MC, Silva MG, Carvalho AC, Coelho FS, Miyazaki NH, Marques EA, Chebabo A, Freitas AD, Lupi O, Duarte RS. 2010. Mycobacterium massiliense BRA100 strain recovered from postsurgical infections: resistance to high concentrations of glutaraldehyde and alternative solutions for high level disinfection. Acta Cir Bras 25:455–459. doi: 10.1590/S0102-86502010000500013. [DOI] [PubMed] [Google Scholar]
- 36.Cortesia C, Lopez GJ, de Waard JH, Takiff HE. 2010. The use of quaternary ammonium disinfectants selects for persisters at high frequency from some species of non-tuberculous mycobacteria and may be associated with outbreaks of soft tissue infections. J Antimicrob Chemother 65:2574–2581. doi: 10.1093/jac/dkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rikimaru T, Kondo M, Kondo S, Oizumi K. 2000. Efficacy of common antiseptics against mycobacteria. Int J Tuberc Lung Dis 4:570–576. [PubMed] [Google Scholar]
- 38.Best M, Sattar SA, Springthorpe VS, Kennedy ME. 1990. Efficacies of selected disinfectants against Mycobacterium tuberculosis. J Clin Microbiol 28:2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rikimaru T, Kondo M, Kondo S, Oizumi K. 1997. Bactericidal activities of povidone-iodine against Mycobacterium. Dermatology 195(Suppl 2):S104–S106. doi: 10.1159/000246041. [DOI] [PubMed] [Google Scholar]
- 40.Rikimaru T, Kondo M, Kajimura K, Hashimoto K, Oyamada K, Miyazaki S, Sagawa K, Aizawa H, Oizumi K. 2002. Efficacy of common antiseptics against multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 6:763–770. [PubMed] [Google Scholar]
- 41.Maiwald M, Chan ES. 2012. The forgotten role of alcohol: a systematic review and meta-analysis of the clinical efficacy and perceived role of chlorhexidine in skin antisepsis. PLoS One 7:e44277. doi: 10.1371/journal.pone.0044277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V, Mercat A, Bouadma L, Lasocki S, Alfandari S, Friggeri A, Wallet F, Allou N, Ruckly S, Balayn D, Lepape A, Timsit JF, CLEAN Trial Investigators . 2015. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 386:2069–2077. doi: 10.1016/S0140-6736(15)00244-5. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths PA, Babb JR, Fraise AP. 1998. Mycobacterium terrae: a potential surrogate for Mycobacterium tuberculosis in a standard disinfectant test. J Hosp Infect 38:183–192. doi: 10.1016/S0195-6701(98)90273-0. [DOI] [PubMed] [Google Scholar]
- 44.Griffiths PA, Babb JR, Fraise AP. 1999. Mycobactericidal activity of selected disinfectants using a quantitative suspension test. J Hosp Infect 41:111–121. doi: 10.1016/S0195-6701(99)90048-8. [DOI] [PubMed] [Google Scholar]
- 45.Sheikh W. 1981. Development and validation of a neutralizer system for in vitro evaluation of some antiseptics. Antimicrob Agents Chemother 19:429–434. doi: 10.1128/AAC.19.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Standardisation ECf. 2008. European standard EN 14563: chemical disinfectants—quantitative carrier test for evaluation of mycobactericidal activity of chemical disinfectants for instruments used in medical area. Test method and requirements (phase2/step2). European Committee for Standardization, Brussels, Belgium. [Google Scholar]
- 47.Bocian E, Grzybowska W, Tyski S. 2014. Evaluation of mycobactericidal activity of selected chemical disinfectants and antiseptics according to European standards. Med Sci Monit 20:666–673. doi: 10.12659/MSM.890175. [DOI] [PMC free article] [PubMed] [Google Scholar]