ABSTRACT
Cryptococcus gattii and Cryptococcus neoformans are environmental fungi that cause cryptococcosis, which is usually treated with amphotericin B and fluconazole. However, therapeutic failure is increasing because of the emergence of resistant strains. Because these species are constantly isolated from vegetal materials and the usage of agrochemicals is growing, we postulate that pesticides could be responsible for the altered susceptibility of these fungi to clinical drugs. Therefore, we evaluated the influence of the pesticide tebuconazole on the susceptibility to clinical drugs, morphophysiology, and virulence of C. gattii and C. neoformans strains. The results showed that tebuconazole exposure caused in vitro cross-resistance (CR) between the agrochemical and clinical azoles (fluconazole, itraconazole, and ravuconazole) but not with amphotericin B. In some strains, CR was observed even after the exposure ceased. Further, tebuconazole exposure changed the morphology, including formation of pseudohyphae in C. neoformans H99, and the surface charge of the cells. Although the virulence of both species previously exposed to tebuconazole was decreased in mice, the tebuconazole-exposed colonies recovered from the lungs were more resistant to azole drugs than the nonexposed cells. This in vivo CR was confirmed when fluconazole was not able to reduce the fungal burden in the lungs of mice. The tolerance to azoles could be due to increased expression of the ERG11 gene in both species and of efflux pump genes (AFR1 and MDR1) in C. neoformans. Our study data support the idea that agrochemical usage can significantly affect human pathogens present in the environment by affecting their resistance to clinical drugs.
KEYWORDS: agrochemical, antifungal cross-resistance, fluconazole, pseudohyphae, tebuconazole
INTRODUCTION
Losses of crops due to pests represent a major problem that must be faced by agriculture to achieve increased food production (1). One of the most frequent strategies to avoid these losses is the use of pesticides, which has grown in recent years. The use of agrochemicals increased by an average of 93% worldwide in recent years, and in Brazil, pesticide use has increased by 190% (2).
Tebuconazole (TBZ), an agrochemical triazole, has a broad spectrum of action and is used to treat or prevent diseases in fruits, cereals, and vegetables. Tebuconazole inhibits fungal sterol-(lanosterol)-14-α demethylase, an enzyme that participates in ergosterol synthesis (3).
Evidence clearly shows that pesticides can cause problems for human health (2, 4, 5). It is unclear, however, (i) what the effects of agrochemicals are on human pathogens present in the environment and (ii) what the indirect effects are on human and animal health. Some studies have shown that exposure to environmental antifungals selects subpopulations of medically important pathogens that are less susceptible to clinical drugs (6–8). However, these studies did not describe the effects of pesticides on the virulence of these pathogens after exposure or indicate whether resistance is maintained in vivo.
Cryptococcus gattii and Cryptococcus neoformans, which are commonly isolated from vegetal materials, are medically important pathogens as the main etiological agents of cryptococcosis. These pathogens infect humans and other animals through inhalation of desiccated environmental yeasts and/or spores from the environment, causing pneumonia and severe meningoencephalitis (9). It is estimated that cryptococcosis affects 1,000,000 people annually, with 650,000 deaths (10).
Cryptococcosis is treated with amphotericin B combined with fluconazole (FLC) and/or 5-flucytosine. Other azole drugs, such as itraconazole (ITC), are also used in some cases (11). Although antibiotic therapy is frequently effective, there are important drawbacks associated with its use. Amphotericin B and 5-flucytosine are nephrotoxic and hepatotoxic, respectively, and they are not available in all countries (12). Regarding azole drugs, especially fluconazole, the isolation of Cryptococcus species strains with increased tolerance of these drugs is increasing (13, 14).
In recent years, it has been suggested that environmental pressures affect the virulence of Cryptococcus spp. and their susceptibility to clinical drugs (15, 16). However, no study has yet confirmed this hypothesis. Therefore, the main goal of our work was to study the effects of exposure to the agrochemical tebuconazole on the susceptibility to clinical drugs and virulence of C. gattii and C. neoformans.
RESULTS
Antifungal drug susceptibility testing and screening of subpopulations more tolerant of tebuconazole (tebuconazole adaptation).
As expected, all C. gattii and C. neoformans strains were inhibited by the drugs tested (fluconazole, amphotericin B, and tebuconazole) at temperatures of 30 and 35°C (data not shown). All strains were also sensitive to tebuconazole when the MIC was determined in solid medium (MICsolid) (Table 1).
TABLE 1.
Screening of C. gattii and C. neoformans subpopulations with increased tolerance of tebuconazolea
| Strain | MICsolid (μg/ml) (geometric mean) | MCA (μg/ml) (geometric mean) | MCA/sub-MICsolid (geometric mean) | |||
|---|---|---|---|---|---|---|
| C. gattii | 30°C | 35°C | 30°C | 35°C | 30°C | 35°C |
| R265 (C) | 2.0 | 1.0 | 7.5 | 2.0 | 7.5 | 4.0 |
| ATCC 24065 (R) | 1.0 | 2.0 | 5.0 | 2.0 | 10.0 | 2.0 |
| ATCC 320608 (R) | 2.0 | 2.0 | 10.0 | 2.0 | 10.0 | 2.0 |
| 547/OTTI/94-PI-10 (E) | 2.0 | 2.0 | 10.0 | 2.0 | 10.0 | 2.0 |
| ICB 181 (E) | 1.0 | 1.0 | 2.0 | 2.0 | 4.0 | 4.0 |
| L24/01 (C) | 2.0 | 2.0 | 10.0 | 2.0 | 10.0 | 2.0 |
| L27/01 (C) | 1.0 | 2.0 | 2.0 | 2.0 | 4.0 | 2.0 |
| L28/02 (C) | 2.0 | 1.0 | 10.0 | 1.0 | 10.0 | 2.0 |
| 1913/ER (C) | 1.0 | 0.5 | 1.5 | 0.75 | 3.0 | 3.0 |
| 196L/03 (C) | 2.0 | 4.0 | 4.0 | 16.0 | 4.0 | 8.0 |
| LMM 818 (C) | 1.0 | 2.0 | 3.5 | 3.0 | 7.0 | 3.0 |
| 23/10893 (C) | 1.0 | 0.5 | 0.75 | 0.5 | 1.5 | 2.0 |
| 29/10893 (C) | 1.0 | 0.5 | 1.5 | 1.0 | 3.0 | 4.0 |
| Range | 1.0–2.0 (1.37) | 0.5–4.0 (1.30) | 0.75–10.0 (3.80) | 0.5–16.0 (1.81) | 1.5–10.0 (5.53) | 0.5–8.0 (2.78) |
| C. neoformans | 30°C | 35°C | 30°C | 35°C | 30°C | 35°C |
| H99 (C) | 1.0 | 1.0 | 2.0 | 2.0 | 4.0 | 4.0 |
| ATCC 24067 (R) | 0.5 | 2.0 | 2.0 | 2.0 | 8.0 | 2.0 |
| ATCC 28957 (R) | 1.0 | 2.0 | 10.0 | 2.0 | 20.0 | 2.0 |
| ATCC 62066 (R) | 1.0 | 2.0 | 2.0 | 2.0 | 4.0 | 2.0 |
| Range | 0.5–1.0 (0.84) | 1.0–2.0 (1.68) | 2.0–10.0 (2.99) | 2.0 (2.0) | 4.0–20.0 (7.11) | 2.0–4.0 (2.38) |
MICsolid, MIC in solid medium for tebuconazole (TBZ) before the adaptation process; MCA, maximum concentration of tebuconazole achieved in the TBZ adaptation test; C, clinical strain; R, reference strain; E, environmental strain.
Further, we determined whether the strains were capable of growing in higher concentrations of tebuconazole in a stepwise manner, and we studied whether the temperature would affect this adaptation. Tables 1 and 2 show the maximum concentration achieved (MCA) of tebuconazole in the tebuconazole adaptation test and the MCA/sub-MICsolid ratios (sub-MIC, MIC/2) at 30 and 35°C (the higher the ratio, the more passages through tebuconazole-containing media occurred). When the adaptation was performed at 30°C, 38% (n = 5) of the C. gattii strains were able to grow in a concentration 10 times higher than before the adaptation (MCA/sub-MICsolid = 10.0) and the geometric mean of the ratio was 5.53 (Table 1). However, when the tests were carried out at 35°C, the strains grew in a lower concentration of tebuconazole (geometric mean = 2.78), demonstrating that temperature affected the adaptation process (Table 1). The same phenomenon was observed for C. neoformans strains, with a geometric mean of MCA/sub-MICsolid ratio at 30°C almost 3-fold higher than that seen when the test was performed at 35°C (Table 1).
TABLE 2.
MICs of fluconazole and tebuconazole for non-TBZ-adapted C. gattii colonies, C. gattii colonies subjected to TBZ adaptation at 30°C, and TBZ-adapted C. gattii colonies subcultured 10 times in agrochemical-free mediuma
| Strain | MIC (μg/ml) at indicated temp (geometric mean)b |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluconazole |
Tebuconazole |
|||||||||||
| 30°C |
35°C |
30°C |
35°C |
|||||||||
| NA | A | 10p | NA | A | 10p | NA | A | 10p | NA | A | 10p | |
| R265 | 8.0 | 64.0 [8×] | 16.0 | 8.0 | 64.0 [8×] | 16,0 | 0.5 | 4.0 [8×] | 1.0 | 1.0 | 4.0 [4×] | 2.0 |
| ATCC 24065 | 4.0 | 32.0 [8×] | 32.0 [8×] | 4.0 | 8.0 | ND | 0.5 | 4.0 [8×] | 4.0 [8×] | 0.5 | 1.0 | ND |
| ATCC 32608 | 16.0 | 64.0 [4×] | 32.0 | 8.0 | 16.0 | ND | 0.5 | 2.0 [4×] | 1.0 | 1.0 | 1.0 | ND |
| 547/OTTI/94-PI-10 | 16.0 | 32.0 | ND | 8.0 | 16.0 | ND | 2.0 | 2.0 | ND | 2.0 | 2.0 | ND |
| ICB 181 | 16.0 | 32.0 | ND | 8.0 | 16.0 | ND | 1.0 | 4.0 [4×] | 1.0 | 0.25 | 2.0 [8×] | 0.25 |
| L24/01 | 16.0 | 64.0 [4×] | 16.0 | 8.0 | 16.0 | ND | 4.0 | 4.0 | ND | 1.0 | 2.0 | ND |
| L27/01 | 16.0 | 64.0 [4×] | 16.0 | 32.0 | 32.0 | ND | 0.5 | 4.0 [8×] | 0.5 | 2.0 | 2.0 | ND |
| L28/02 | 32.0 | 64.0 | ND | 16.0 | 16.0 | ND | 1.0 | 1.0 | ND | 1.0 | 2.0 | ND |
| 1913R | 16.0 | 16.0 | ND | 16.0 | 16.0 | ND | 0.125 | 0.5 [4×] | 0.125 | 0.125 | 0.5 [4×] | 0.125 |
| 196L/03 | 16.0 | 128.0 [8×] | 16.0 | 16.0 | 128.0 [8×] | 16.0 | 1.0 | 8.0 [8×] | 1.0 | 1.0 | 8.0 [8×] | 1.0 |
| LMM 818 | 16.0 | 8.0 | ND | 16.0 | 8.0 | ND | 0.25 | 0.5 | ND | 0.25 | 0.5 | ND |
| 23/10893 | 8.0 | 16.0 | ND | 8.0 | 16.0 | ND | 0.125 | 1.0 [8×] | 0.25 | 0.125 | 1.0 [8×] | 0.25 |
| 29/10933 | 8.0 | 16.0 | ND | 4.0 | 4.0 | ND | 0.25 | 0.25 | ND | 0.25 | 0.25 | ND |
| Range | 4.0–32.0 (12.92) | 8.0–128.0 (35.60) | ND (ND) | 4.0–32.0 (9.90) | 4.0–128.0 (17.80) | ND (ND) | 0.125–4.0 (0.56) | 0.25–8.0 (1.79) | ND (ND) | 0.125–2.0 (0.56) | 0.25–8.0 (1.37) | ND (ND) |
Tests were performed at 30°C and 35°C.
MIC values represent endpoint values (MIC at 50% of growth inhibition). Numbers in square brackets indicate how many times (×) higher the drug MIC value for the TBZ-adapted colonies (A) or colonies subjected to 10 passages (10p) was (≥4×) than the drug MIC value for the non-TBZ-adapted (NA) colonies. Values highlighted in bold indicate MIC values that were at least 4× higher than those seen with the NA colonies. ND, not determined.
Tebuconazole-adapted colonies present cross-resistance (CR) with fluconazole and other azole drugs.
Tables 2 and 3 show the MIC in liquid medium (MICbroth) of fluconazole and tebuconazole for nonadapted (NA) and tebuconazole-adapted (A) colonies of C. gattii and C. neoformans, respectively, when the adaptation was performed at 30°C. Despite adaptation, not all C. gattii strains exhibited alterations of at least 2 dilutions of the MIC of tebuconazole compared to the MIC for NA colonies (Tables 2 and 3). Overall, 61.5% (n = 8) of C. gattii (Table 2) and 100% (n = 4) of C. neoformans (Table 3) tebuconazole-adapted cells became more resistant to the environmental antifungal than the NA colonies when the tests were performed at the same adaptation temperature. In contrast, when MIC assays were carried out at 35°C using cells adapted at 30°C, 38% (n = 5) of C. gattii (Table 2) and 50% (n = 2) of C. neoformans (Table 3) tebuconazole-adapted colonies became more resistant to tebuconazole.
TABLE 3.
MICs of fluconazole and tebuconazole for non-TBZ-adapted C. neoformans colonies, C. neoformans colonies subjected to TBZ adaptation at 30°C, and TBZ-adapted C. neoformans colonies subcultured 10 times in agrochemical-free mediuma
| Strain | MIC (μg/ml) at indicated temp (geometric mean)b |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluconazole |
Tebuconazole |
|||||||||||
| 30°C |
35°C |
30°C |
35°C |
|||||||||
| NA | A | 10p | NA | A | 10p | NA | A | 10p | NA | A | 10p | |
| H99 | 16.0 | 128.0 [8×] | 32.0 | 8.0 | 64.0 [8×] | 8.0 | 1.0 | 4.0 [4×] | 1.0 | 0.25 | 2.0 [8×] | 0.25 |
| ATCC 24067 | 16.0 | 64.0 [4×] | 16.0 | 4.0 | 32.0 [8×] | 4.0 | 0.5 | 2.0 [4×] | 0.5 | 0.25 | 1.0 [4×] | 0.25 |
| ATCC 28957 | 4.0 | 16.0 [4×] | 32.0 [8×] | 2.0 | 4.0 | ND | 0.5 | 4.0 [8×] | 2.0 [4×] | 0.5 | 0.5 | ND |
| ATCC 62066 | 4.0 | 16.0 [4×] | 4.0 | 4.0 | 4.0 | ND | 0.25 | 1.0 [4×] | 0.5 | 0.5 | 0.5 | ND |
| Range | 4.0–16.0 (8.0) | 16.0–128.0 (38.05) | ND (ND) | 2.0–8.0 (4.0) | 4.0–64.0 (13.45) | ND (ND) | 0.25–1.0 (0.5) | 1.0–4.0 (2.38) | ND (ND) | 0.25–0.5 (0.35) | 0.5–2.0 (0.84) | ND (ND) |
Tests were performed at 30°C and 35°C.
MIC values represent endpoint values (MIC at 50% of growth inhibition). Numbers in square brackets indicate how many times (×) higher the drug MIC value for the TBZ-adapted colonies (A) or colonies subjected to 10 passages (10p) was (≥4×) than the drug MIC value for the non-TBZ-adapted (NA) colonies. Values highlighted in bold indicate MIC values that were at least 4× higher than those seen with the NA colonies. ND, not determined.
We then tested whether tebuconazole adaptation can also decrease susceptibility to fluconazole. The geometric means of the drug MICs for colonies subjected to tebuconazole adaptation at 30°C and 35°C increased almost 3-fold and 2-fold for C. gattii and 5-fold and 3-fold for C. neoformans, respectively (Tables 2 and 3). Additionally, the adaptation resulted in selection of populations with cross-resistance (CR). A total of 38% (n = 5) of C. gattii tebuconazole-adapted colonies and a total of 100% (n = 4) of C. neoformans tebuconazole-adapted colonies presented higher MICs of fluconazole, as well as of tebuconazole (Tables 2 to 4). Four strains of C. gattii (R265, ATCC 32608, L27/01, and 196L/03) and three strains of C. neoformans (H99, ATCC 24067, and ATCC 62066) returned to the original phenotype when grown in medium without the agrochemical for 10 passages (10p) (Tables 2 and 3) (referred to here as “temporary CR”). This phenomenon occurred in a manner dependent on the temperature (ATCC 32608, L27/01, and ATCC 62066) or not dependent on the temperature (R265, 196L/03, H99, and ATCC 24067) for different strains (Tables 2 to 4). The other strain of C. gattii (ATCC 24065) and the other strain of C. neoformans (ATCC 28957), which showed CR with fluconazole, did not return to the original susceptibility phenotype even after growth in tebuconazole-free medium, demonstrating “permanent CR” (Tables 3 to 5). This profile also appears to be temperature dependent (Tables 2 to 4).
TABLE 4.
Percentage of cross-resistance between TBZ and FLC seen with C. gattii and C. neoformans strains after TBZ adaptation at 30 and 35°Ca
| CR category | % CR at indicated temp |
|||
|---|---|---|---|---|
|
C. gattii |
C. neoformans |
|||
| 30°C | 35°C | 30°C | 35°C | |
| Total | 39 | 23 | 100 | 25 |
| Temporary | 31 | 15 | 75 | 25 |
| Permanent | 8 | 8 | 25 | 0 |
CR, cross-resistance.
TABLE 5.
MICs of itraconazole and ravuconazole for non-TBZ-adapted C. gattii and C. neoformans colonies, C. gattii and C. neoformans colonies subjected to TBZ adaptation at 30°C, and TBZ-adapted C. gattii and C. neoformans colonies subcultured 10 times in agrochemical-free mediuma
| Strain | MIC at indicated tempb |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Itraconazole |
Ravuconazole |
|||||||||||
| 30°C |
35°C |
30°C |
35°C |
|||||||||
| NA | A | 10p | NA | A | 10p | NA | A | 10p | NA | A | 10p | |
| C. gattii | ||||||||||||
| R265 | 0.25 | 1.0 [4×] | ND | 0.25 | 1.0 [4×] | ND | 0.125 | 2.0 [16×] | ND | 0.03 | 0.5 [16×] | ND |
| ATCC 24065 | 0.5 | 1.0 | 1.0 | 0.25 | 0.5 | 1.0 | 0.06 | 2.0 [32×] | 2.0 [32×] | 0.03 | 0.125 [4×] | 0.125 [4×] |
| ATCC 32608 | 0.5 | 1.0 | ND | 0.5 | 0.5 | ND | 0.125 | 0.5 [4×] | ND | 0.125 | 0.125 | ND |
| L27/02 | 0.5 | 1.0 | ND | 0.25 | 0.5 | ND | 0.125 | 1.0 [8×] | ND | 0.03 | 0.125 [4×] | ND |
| 196L/03 | 0.5 | 1.0 | ND | 0.5 | 1.0 | ND | 0.25 | 2.0 [8×] | ND | 0.125 | 2.0 [16×] | ND |
| C. neoformans | ||||||||||||
| H99 | 0.125 | 1.0 [8×] | ND | 0.125 | 1.0 [8×] | ND | 0.06 | 1.0 [16×] | ND | 0.06 | 0.5 [8×] | ND |
| ATCC 24067 | 0.5 | 1.0 | ND | 0.25 | 0.5 | ND | 0.125 | 1.0 [8×] | ND | 0.015 | 0.25 [16×] | ND |
| ATCC 28957 | 0.25 | 1.0 [4×] | 0.25 | 0.125 | 0.5 [4×] | ND | 0.06 | 0.5 [8×] | 0.5 [8×] | 0.015 | 0.03 | ND |
| ATCC 62066 | 0.5 | 1.0 | ND | 0.25 | 0.25 | ND | 0.03 | 0.5 [16×] | ND | 0.03 | 0.03 | ND |
Tests were performed at 30°C and 35°C.
MIC values represent endpoint values (MIC at 50% of growth inhibition). Numbers in square brackets indicate how many times (×) higher the drug MIC value for the TBZ-adapted colonies (A) or colonies subjected to 10 passages (10p) was (≥4×) than the drug MIC value for the non-TBZ-adapted (NA) colonies. Values highlighted in bold indicate MIC values that were at least 4× higher than those seen with the NA colonies. ND, not determined.
We also evaluated whether CR with fluconazole in tebuconazole-adapted strains at 30°C would occur for other azoles, such as itraconazole and ravuconazole. As shown in Table 5, C. gattii and C. neoformans strains became less susceptible to ravuconazole after adaptation to tebuconazole, but only C. gattii R265 and C. neoformans H99 and ATCC 28957 showed CR with itraconazole. C. gattii ATCC 32608 and C. neoformans ATCC 28957 and ATCC 62066 exhibited altered susceptibility to ravuconazole only when the test was performed at 30°C (Table 5), confirming the importance of temperature in the resistance process.
We also performed tebuconazole adaptation at 35°C using the same procedure (Tables 6 and 7). Overall, 42% (n = 6) of C. gattii strains exhibited an increased tebuconazole MIC after tebuconazole adaptation (Table 6) and 23% (n = 3) presented CR with fluconazole. For two strains (R265 and 23/10893), the phenotype reverted after subcloning was performed several times on nonselective medium. For one strain (196L/03), the phenotype was stable even after several subcultures on agrochemical-free medium (Tables 4 and 6). However, only adapted cells from C. neoformans H99 exhibited an increased tebuconazole MIC and CR with fluconazole (Tables 4 and 7).
TABLE 6.
MICs of fluconazole and tebuconazole for non-TBZ-adapted C. gattii colonies, C. gattii colonies subjected to TBZ adaptation at 35°C, and TBZ-adapted C. gattii colonies subcultured 10 times in agrochemical-free mediuma
| Strain | MIC (μg/ml) (geometric mean)b |
|||||
|---|---|---|---|---|---|---|
| Fluconazole |
Tebuconazole |
|||||
| NA | A | 10p | NA | A | 10p | |
| R265 | 8.0 | 32.0 [4×] | 16.0 | 1.0 | 4.0 [4×] | 1.0 |
| ATCC 24065 | 4.0 | 8.0 | ND | 0.5 | 0.5 | ND |
| ATCC 32608 | 8.0 | 16.0 | ND | 1.0 | 2.0 | ND |
| 547/OTTI/94-PI-10 | 8.0 | 16.0 | ND | 2.0 | 4.0 | ND |
| ICB 181 | 8.0 | 16.0 | ND | 0.25 | 1.0 [4×] | 1.0 [4×] |
| L24/01 | 8.0 | 4.0 | ND | 1.0 | 2.0 | 1.0 |
| L27/01 | 32.0 | 16.0 | ND | 2.0 | 1.0 | ND |
| L28/02 | 16.0 | 16.0 | ND | 1.0 | 2.0 | ND |
| 1913R | 16.0 | 16.0 | ND | 0.125 | 1.0 [8×] | 0.25 |
| 196L/03 | 16.0 | 256.0 [16×] | 256.0 [16×] | 1.0 | 8.0 [8×] | 16.0 [16×] |
| LMM 818 | 16.0 | 16.0 | ND | 0.25 | 0.25 | ND |
| 23/10893 | 8.0 | 32.0 [4×] | 4.0 | 0.125 | 2.0 [16×] | 0.125 |
| 29/10933 | 4.0 | 8.0 | ND | 0.25 | 1.0 [4×] | 0.5 |
| Range | 4.0–32.0 (9.90) | 4.0–256.0 (17.80) | ND (ND) | 0.125–2.0 (0.56) | 0.25–8.0 (1.53) | ND (ND) |
Tests were performed at 35°C.
MIC values represent endpoint values (MIC at 50% of growth inhibition). Numbers in square brackets indicate how many times (×) higher the drug MIC value for the TBZ-adapted colonies (A) or colonies subjected to 10 passages (10p) was (≥4×) than the drug MIC value for the non-TBZ-adapted (NA) colonies. Values highlighted in bold indicate MIC values that were at least 4× higher than those seen with the NA colonies. ND, not determined.
TABLE 7.
MICs of fluconazole and tebuconazole for non-TBZ-adapted C. neoformans colonies, C. neoformans colonies subjected to TBZ adaptation at 35°C, and TBZ-adapted C. neoformans colonies subcultured 10 times in agrochemical-free mediuma
| Strain | MIC (μg/ml) (geometric mean)b |
|||||
|---|---|---|---|---|---|---|
| Fluconazole |
Tebuconazole |
|||||
| NA | A | 10p | NA | A | 10p | |
| H99 | 8.0 | 32.0 [4×] | 16.0 | 0.25 | 1.0 [4×] | 2.0 [8×] |
| ATCC 24067 | 8.0 | 8.0 | ND | 0.5 | 1.0 | ND |
| ATCC 28957 | 4.0 | 8.0 | ND | 0.5 | 1.0 | ND |
| ATCC 62066 | 4.0 | 32.0 [8×] | 4.0 | 1.0 | 2.0 | ND |
| Range | 4.0–8.0 (5.65) | 8.0–32.0 (16.00) | ND (ND) | 0.25–1.0 (0.50) | 1.0–2.0 (1.19) | ND (ND) |
Tests were performed at 35°C.
MIC values represent endpoint values (MIC at 50% of growth inhibition). Numbers in square brackets indicate how many times (×) higher the drug MIC value for the TBZ-adapted colonies (A) or colonies subjected to 10 passages (10p) was (≥4×) than the drug MIC value for the non-TBZ-adapted (NA) colonies. Values highlighted in bold indicate MIC values that were at least 4× higher than those seen with the NA colonies. ND, not determined.
The Cryptococcus species strains adapted at 35°C behaved similarly to those adapted at the lower temperature regarding susceptibility to itraconazole and ravuconazole (Table 8). Adapted cells of C. gattii R265 and 196L/03 became more resistant to ravuconazole, whereas cells coming from C. neoformans H99 were more resistant to itraconazole and ravuconazole (Table 8). In contrast, cells adapted from C. gattii 23/10893 did not show altered susceptibility (Table 8).
TABLE 8.
MICs of itraconazole and ravuconazole for non-TBZ-adapted C. gattii and C. neoformans colonies, C. gattii and C. neoformans colonies subjected to TBZ adaptation at 35°C, and TBZ-adapted C. gattii and C. neoformans colonies subcultured 10 times in agrochemical-free mediuma
| Strain | MIC (μg/ml) (geometric mean)b |
|||||
|---|---|---|---|---|---|---|
| Itraconazole |
Ravuconazole |
|||||
| NA | A | 10p | NA | A | 10p | |
| C. gattii | ||||||
| R265 | 0.25 | 0.25 | 0.25 | 0.03 | 0.5 [16×] | ND |
| 196L/03 | 0.5 | 1.0 | 1.0 | 0.125 | 1.0 [8×] | 1.0 [8×] |
| 23/10893 | 0.5 | 0.5 | ND | 0.03 | 0.06 | ND |
| C. neoformans | ||||||
| H99 | 0.25 | 2.0 [8×] | ND | 0.03 | 0.5 [16×] | ND |
Tests were performed at 35°C.
MIC values represent endpoint values (MIC at 50% of growth inhibition). Numbers in square brackets indicate how many times (×) higher the drug MIC value for the TBZ-adapted colonies (A) or colonies subjected to 10 passages (10p) was (≥4×) than the drug MIC value for the non-TBZ-adapted (NA) colonies. Values highlighted in bold indicate MIC values that were at least 4× higher than those seen with the NA colonies. ND, not determined.
Two strains, one from each species (one isolated from C. gattii L24/01 adapted at 30°C and one isolated from C. neoformans ATCC 62066 adapted at 35°C), exhibited an increased MIC only of fluconazole (Tables 2 and 7). This phenomenon has also been observed when an environmental nonazole antifungal agent has been used (unpublished data).
Overall, our data demonstrated that exposing Cryptococcus spp. to tebuconazole can induce CR with other azole derivatives commonly used in the clinical setting. Interestingly, CR was not observed with amphotericin B (data not shown).
Tebuconazole adaptation induced morphophysiological changes.
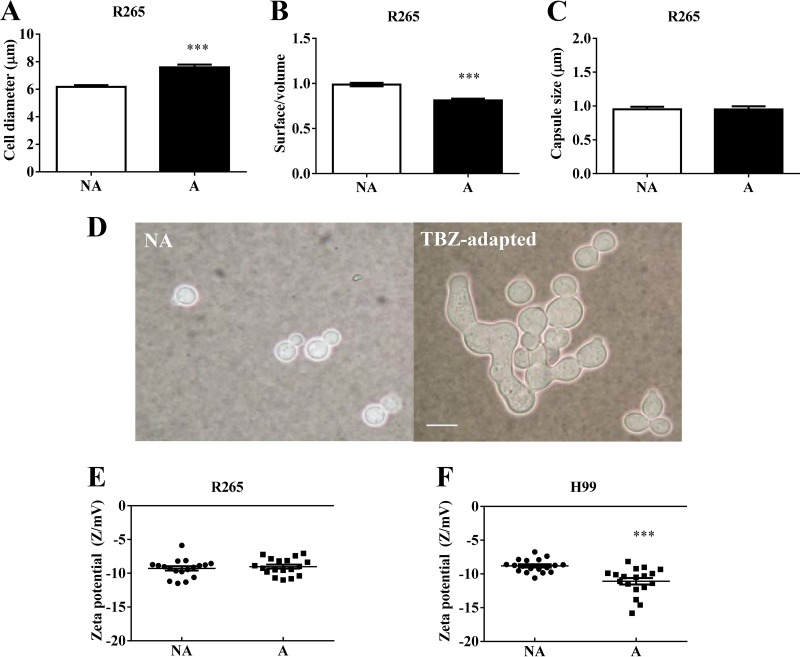
Tebuconazole adaptation caused morphological changes in Cryptococcus spp. The adapted cells of C. gattii R265 exhibited a significantly increased diameter (P < 0.05) (Fig. 1A) and a decreased surface/volume ratio (Fig. 1B) compared to NA cells (P < 0.05), but the capsule size was maintained (Fig. 1C). Strikingly, 70% to 90% tebuconazole-adapted cells of C. neoformans H99 presented elongated, irregular shapes, characterizing the formation of pseudohyphae, while the NA and 10p cells presented only yeast forms (Fig. 1D). These C. neoformans H99-derived elongated cells showed a significant (P < 0.05) increase in their surface electronegativity (Fig. 1F), but this was not observed for C. gattii R265 (Fig. 1E).
FIG 1.
TBZ exposure causes morphophysiological changes in Cryptococcus gattii R265 and C. neoformans H99. (A to C) TBZ-adapted cells of C. gattii R265 exhibited increased cell diameter (A) and decreased surface/volume ratio (B) but unaltered capsule thickness (C) compared to nonadapted (NA) cells. (D) C. neoformans H99 presented the pseudohyphal form after TBZ adaptation. An India ink suspension was used. Bar, 10 μm. (E and F) The electronegativity of the cellular surface was not altered in C. gattii R265 (E); however, it was increased in TBZ-adapted cells of C. neoformans H99 (F). NA, nonadapted; A, TBZ adapted; **, P < 0.01; ***, P < 0.001.
In contrast, adaptation and the morphological changes did not affect the growth rate in Sabouraud dextrose agar (SDA) (data not shown).
Tebuconazole adaptation decreased the virulence of C. gattii R265 and C. neoformans H99 in C57BL/6 mice.
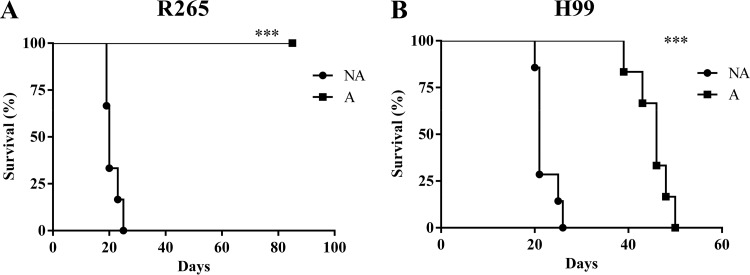
Mice infected with NA cells of C. gattii R265 and C. neoformans H99 succumbed significantly (P < 0.05) earlier than those infected with tebuconazole-adapted cells of the same strain (Fig. 2). Interestingly, R265-adapted cells were not able to kill the animals, even after 80 days (Fig. 2A).
FIG 2.
TBZ exposure decreases virulence in Cryptococcus gattii R265 and C. neoformans H99. C57BL/6 mice were infected by the intratracheal route with 1 × 105 CFU of nonadapted (NA) and TBZ-adapted (A) cells. The survival curve showed that animals infected with TBZ-adapted cells of C. gattii R265 (A) and C. neoformans H99 (B) survived longer than those infected with NA cells.
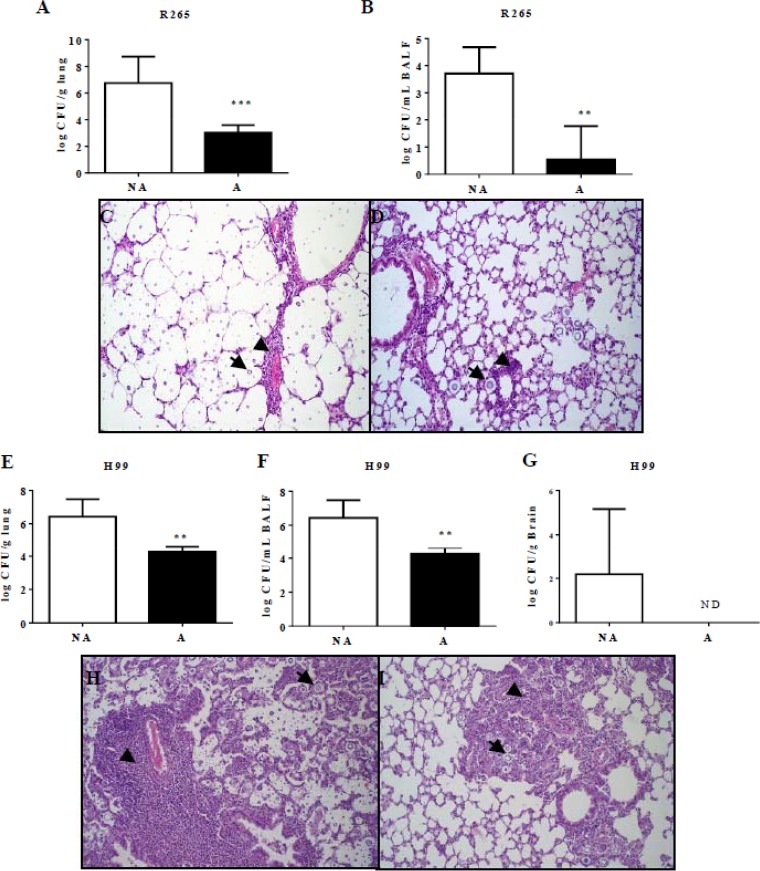
Additionally, animals were infected for a better characterization of the disease characteristics caused by the different cells. The fungal burden in the lungs (Fig. 3A and E) and bronchoalveolar lavage fluid (BALF) (Fig. 3B and F) was significantly higher (P < 0.05) in mice infected with NA cells in both strains. C. gattii R265 cells were not detected in the brain of any infected animal; however, NA cells of C. neoformans H99, unlike the tebuconazole-adapted cells, disseminated into the brain in 40% of infected animals (Fig. 3G).
FIG 3.
Animals infected with TBZ-adapted cells exhibit lower fungal load in the lungs, BALF, and brain and decreased lung inflammation. C57BL/6 mice were infected by the intratracheal route with 1 × 105 CFU of nonadapted (NA) and TBZ-adapted (A) cells for the determination of the CFU level per gram and for histopathology analysis. (A and B) After 15 days of infection, higher fungal burden was observed in the lungs (A) and bronchoalveolar lavage fluid (BALF) (B) of animals infected with NA cells of C. gattii R265. (C and D) Lung histopathology analysis showed that NA cells caused more inflammation (C) than A cells (D). (E to I) Animals infected with NA cells of C. neoformans H99 also exhibited a higher fungal load in the lungs (E), BALF (F), and brain (G) and more-intense inflammation in the lungs (H) than animals infected with A cells (I). **, P < 0.01; ***, P < 0.001; ND, not detected; NA, nonadapted; A, TBZ adapted. Arrows indicate yeast in the lungs, and arrowheads indicate the inflammatory infiltrate.
Histopathology analysis confirmed the decreased virulence of tebuconazole-adapted colonies. Mice infected with nonadapted cells of C. gattii R265 presented a moderate to accentuated amount of extracellular yeasts diffusely distributed in the alveolar and bronchial lumen (Fig. 3C). This was associated to discrete perivascular inflammatory infiltrate with a predominance of neutrophils and multifocal alveolar thickening due to discrete mononuclear inflammatory infiltrate (Fig. 3C). Mice infected with nonadapted C. neoformans H99 demonstrated an accentuated amount of yeasts diffusely distributed in the pulmonary parenchyma (Fig. 3H). We also observed accentuated perivascular inflammatory infiltrate with neutrophils, macrophages, and lymphocytes and an accentuated inflammatory infiltrate with predominance of macrophages and multinucleated giant cells in the alveolar space with multifocal to diffuse distribution (Fig. 3H). However, the animals infected with adapted cells exhibited a reduced amount of yeasts in lung parenchyma and, consequently, a significant reduction in associated inflammation (Fig. 3D and I). No change was observed in the lungs of control group mice (noninfected mice).
These results demonstrated that although C. neoformans was able to induce a greater inflammatory response in the lungs than C. gattii, the colonization and inflammatory response in mice infected with both species were reduced after tebuconazole adaptation.
Tebuconazole adaptation caused antifungal cross-resistance in vivo.
The MIC of all azole drugs in colonies recovered from the lungs of animals infected with tebuconazole-adapted cells was significantly (P < 0.05) higher than that of drugs recovered from animals infected with NA cells (Fig. 4A and B). Furthermore, fluconazole did not reduce the fungal burden in lungs (P > 0.05) from mice (Fig. 4C and D), as was also observed in histology analyses (data not shown).
FIG 4.
TBZ exposure causes antifungal resistance in vivo. The MICs of tebuconazole (TBZ), fluconazole (FLC), itraconazole (ITC), ravuconazole (RVC), and amphotericin B (AMB) were determined for colonies recovered from lungs of animals that had been infected with nonadapted (NA) and TBZ-adapted (A) cells of C. gattii R265 (A) or C. neoformans H99 (B) and treated (A + FCZ) or not treated (A) with FLC (10 mg/kg). After 15 days, the animals were euthanized and the lungs were collected for determination of the CFU level per gram. There were no statistical significant differences between the fungal loads in the lungs of animals that were infected with TBZ-adapted cells of C. gattii R265 (C) or C. neoformans H99 and (D) and treated or not treated with FLC. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Tebuconazole adaptation induced different mechanisms of resistance in C. gattii and C. neoformans.
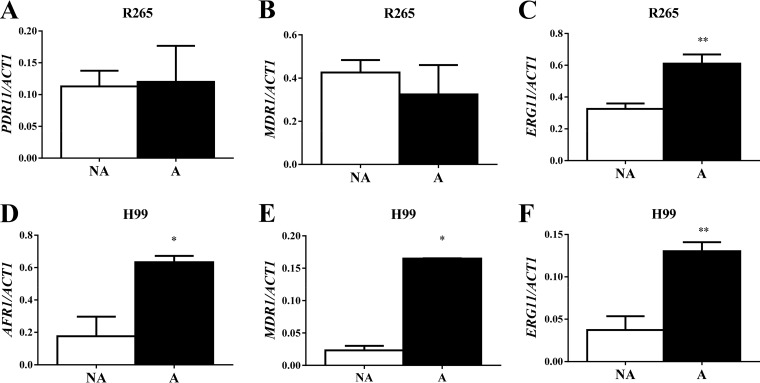
We investigated the mechanism involved in the increased MIC of azole drugs for the adapted versus nonadapted C. gattii R265 and C. neoformans H99 strains. Figure 5 shows that the expression levels of the ERG11 gene, but not those of the efflux pump PDR11 and MDR1 genes (Fig. 5A to C), were significantly (P < 0.05) higher in adapted C. gattii R265 cells than in the nonadapted cells. However, in C. neoformans H99, all genes (ERG11, AFR1, and MDR1) were expressed at a higher level (P < 0.05) in tebuconazole-exposed cells (Fig. 5D to F).
FIG 5.
TBZ exposure changes in the expression of efflux pumps and ERG11 genes. Expression of PDR1 (A) and MDR1 (B), but not that of ERG11 (C), was altered by tebuconazole exposure in C. gattii R265. In C. neoformans H99, the levels of expression of AFR1 (D), MDR1 (E), and ERG11 (F) were increased in adapted cells versus nonadapted cells. NA, nonadapted; A, TBZ adapted; *, P < 0.05; **, P < 0.01.
DISCUSSION
In this study, we showed that exposing C. gattii and C. neoformans to the triazole agrochemical tebuconazole resulted in greater tolerance, in vitro and in vivo, of clinical drugs (fluconazole and itraconazole) and less virulence in a murine model than were seen with cells not exposed to the agrochemical. We also tested an azole drug currently in clinical trials (ravuconazole) (17) to study whether there would be CR with drugs that are not yet commercially available, and such CR was confirmed.
First, we showed that previous exposure to tebuconazole selects cells with permanent or temporary CR with fluconazole. The major mechanism of resistance to azole drugs that has been demonstrated for Cryptococcus spp. is the overexpression of efflux pump genes (AFR1, AFR2, PDR11, and MDR1) (18, 19) and, in some cases, overexpression of the target of these drugs, ERG11p (20). Here, we showed that the mechanisms of azole tolerance in C. gattii and C. neoformans can be different in strains that presented temporary CR, such as H99 and R265. We observed increased expression of the efflux pump and ERG11 genes in C. neoformans H99 but observed increased expression only of ERG11 in C. gattii R265. These results demonstrated that although the adapted cells of both species exhibited increased MICs of all the same azole drugs, the mechanisms were different for each species, and possibly for each strain. Another study confirmed that resistance mechanisms can be strain dependent, demonstrating that one azole-heteroresistant strain of C. gattii expressed more PDR11 and ERG11 than the original cells (20). Rocha and colleagues reported that in Candida parapsilosis, exposure to the agrochemical tetraconazole selected cells more resistant to azole drugs because of overexpression of efflux pumps, but they did not observe altered ERG11 expression (7).
Tebuconazole exposure also caused changes in azole susceptibility that were observed even after 10 passages on agrochemical-free medium. We called this phenomenon “permanent CR,” and it can indicate that a mutation could have occurred to confer resistance to the cells. Moreover, a study with Aspergillus fumigatus exposed to environmental antifungals, including tebuconazole, showed that ERG11 mutation causes resistance to fluconazole and other azoles, except for itraconazole (8). Other studies also showed that CR between fluconazole and itraconazole is not common (21, 22). These phenomena may occur because itraconazole, in addition to inhibiting ERG11p, also inhibits NADH-dependent 3-ketosteroid reductase (an enzyme that catalyzes one of the last reactions of ergosterol synthesis) (21, 23), and the mechanism of resistance can be different from that seen with fluconazole (24). More studies should be performed to determine the mechanisms involved in the permanent CR caused by agrochemical exposure.
Temperature is a critical factor that the human-pathogenic fungus must overcome to cause illness (25). To test whether temperature can also affect the process of acquiring resistance, the strains were exposed to tebuconazole and incubated at 30 or 35°C. When adaptation was carried out at the lower temperature, the strains supported higher concentrations of the drug and more strains became tolerant of the pesticide than at 35°C. This indicates that the temperature of 30°C is more favorable for the fungus to develop resistance, probably because the optimal temperature of growth of Cryptococcus is around 25°C (26). In addition to the importance of temperature during the adaptation process, we showed that it is relevant for determining MIC (incubation temperature). The tebuconazole-adapted colonies grown at 30°C were more tolerant to the drugs than colonies grown at 35°C, indicating that resistance in the environment may not occur in vivo because of the body temperature of endothermic animals. These data may also explain, at least in part, why Cryptococcus spp. are not considered to be a major problem in antimicrobial resistance in clinical practice (12).
The morphological characteristics of Cryptococcus cells influence their virulence (24). Usually, cells with a larger diameter and a smaller capsule are less virulent (27–29). Here, we observed that tebuconazole-adapted cells, which exhibited larger diameters than the NA cells, were less virulent in both species. Morphological analysis also showed that tebuconazole-adapted cells of C. neoformans H99 exhibited formation of pseudohyphae. Pseudofilamentation happens when budding cells do not fully separate, resulting in formation of a chain of bound cells (30). This process is common in Candida (30) but is rare and little studied in Cryptococcus. Pseudofilamentation seems to be a response to overcome environmental stresses (31), but during this process, the cells become less virulent (32), as our results demonstrated. This decreased virulence may occur because cell surface molecules become differentially presented and because they cannot be phagocytosed by macrophages (33) and do not reach the central nervous system (30, 32). The zeta potential data confirmed that formation of pseudohyphae can cause changes in cell surface molecules that influence the electronegativity of the cell surface.
To better characterize the influence of tebuconazole exposure on virulence, we analyzed the microscopic changes and the fungal quantity in the lungs. The higher fungal burden in the lungs and BALF of animals infected with NA cells of both species agreed with the survival curve data. Further, we detected fungus in the brain only of the animals infected with NA cells of C. neoformans H99. These results support those of previous studies indicating that pseudohyphae do not reach the central nervous system (30, 32), which explains why these cells are less virulent than NA cells.
Other researchers have reported that strains with secondary resistance to fluconazole are less virulent than susceptible strains (28, 34) and that strains that are heteroresistant to itraconazole and fluconazole are more virulent (27, 35). In most of these cases, morphological changes are crucial for altered virulence (27, 28, 34). In itraconazole-heteroresistant cells, increased virulence attributable to decreased cell size was observed (27), the inverse of what was observed in the cross-resistant cells in this study (cells were bigger and less virulent). Our study reinforced the idea of the importance of morphology for the virulence in Cryptococcus spp.
Although cells exposed to tebuconazole were less virulent than NA cells, they presented antifungal tolerance in vivo, as confirmed by the higher MIC values of azoles for the colonies recovered from mice and by the inability of fluconazole to reduce the fungal burden in mouse lungs.
In conclusion, exposure to tebuconazole selected cells with cross-resistance with clinical azole drugs in vivo and in vitro but not with amphotericin B. Tebuconazole exposure also altered fungal morphology and decreased the virulence of C. gattii and C. neoformans. To the best of our knowledge, this work is the first to demonstrate the implications of exposure to agrochemicals for the virulence and in vivo resistance of Cryptococcus spp.
MATERIALS AND METHODS
Microorganisms and study design.
We used 13 strains of C. gattii (9 clinical and 2 environmental isolates, all from the culture collection of the Laboratório de Micologia da Universidade Federal de Minas Gerais, Minas Gerais, Brazil, and 2 reference strains from the culture collection of the University of Georgia, Atlanta, GA) (Table 1) (36). We also used four strains of C. neoformans (one clinical strain and three reference strains) (Table 1) (37). All isolates were maintained on Sabouraud dextrose broth at −80°C.
Antifungal susceptibility, tebuconazole adaptation, and cross-resistance tests were performed for all strains. The C. gattii R265 and C. neoformans H99 strains were chosen for further tests (i.e., morphophysiological, virulence change, in vivo antifungal resistance, and RT-PCR analyses).
Antifungal drug susceptibility testing.
The MICs of fluconazole (FLC) (Sigma-Aldrich, St. Louis, MO), amphotericin B (AMB) (Sigma-Aldrich), and the agricultural fungicide tebuconazole (TBZ) (Alterne) were determined using the microdilution method proposed by the Clinical and Laboratory Standards Institute (CLSI) (M27-A3 method) (MICbroth) (38). The MIC of tebuconazole was also determined by spot tests on Sabouraud dextrose agar (SDA) supplemented with different concentrations of the pesticide (MICsolid) (27). For the spot tests, cell suspensions containing 1 to 5 × 104 cells were plated onto SDA plates containing different concentrations of tebuconazole (from 0.125 to 256.0 μg/ml). The growth pattern was determined after 72 h of incubation. The MICbroth and MICsolid tests were performed at two different incubation temperatures: 30 and 35°C. All tests were performed in duplicate for each strain, and the tests were repeated at least twice to confirm the results.
Tebuconazole adaptation (screening for subpopulations more tolerant of tebuconazole).
After susceptibility testing on solid medium (MICsolid) was performed, the strains were grown on SDA with increasing concentrations of the pesticide. Initially, all strains were grown on medium supplemented with tebuconazole at the MIC/2 (sub-MIC). After 1 week, an inoculum using at least five colonies was prepared in sterile saline solution, and the transmittance (530 nm) of the suspensions was adjusted to a range of 75% to 77% (1 × 106 to 5 × 106 fungal cells). Subsequently, 10 μl of this suspension was inoculated on a medium containing tebuconazole at the MIC. After 1 week, the process was repeated and the strains were grown, in a stepwise manner, at increasing concentrations of tebuconazole until the concentration where the growth ceased was reached. These tests were performed at both 30°C and 35°C. The colonies that were exposed to tebuconazole were named tebuconazole-adapted (A) colonies, and the original colonies (no exposure) were named nonadapted (NA) colonies.
The highest concentration of tebuconazole that the fungus was capable of growing in after the tebuconazole adaptation tests was called the maximum concentration achieved (MCA). We also quantified the ability of the microorganisms to grow in the presence of the agrochemical by determining the MCA-to-sub-MIC ratio (MCA/sub-MIC).
Cross-resistance tests (CR).
The MICbroth of fluconazole, amphotericin B, and tebuconazole was determined for nonadapted and tebuconazole-adapted colonies. The test was performed at 30 and 35°C for the colonies adapted at 30°C and was performed at 35°C for the colonies adapted at 35°C. A strain was considered cross-resistant when it presented decreased susceptibility to both tebuconazole and clinical drugs.
To test the stability of the cross-resistance to fluconazole and tebuconazole, at least five colonies of each adapted strain that showed an increased drug MICbroth (increase of at least four times) were mixed and then subcultured every 48 h on SDA plates without tebuconazole for 10 passages (10p colonies) (27). Next, we determined the MICbroth for 10p colonies.
We also tested the CR between tebuconazole and itraconazole (ITC) (Sigma-Aldrich) and ravuconazole (RVC) (Sigma-Aldrich) (an azole in phase II trials) (17) for the tebuconazole-adapted and 10p colonies that showed CR with fluconazole.
Morphometric and zeta potential analysis.
Nonadapted and tebuconazole-adapted colonies were grown on SDA and on SDA supplemented with tebuconazole at the MCA, respectively, for 72 h at 30°C. Subsequently, the cells were visualized in a suspension in India ink with an optical microscope (Axioplan; Carl Zeiss) and the slides were photographed using a Coolpix 4500 (Nikon) digital camera. The capsule and diameter of at least 50 cells with regular form were measured using ImageJ 1.40 g software (http://rsb.info.nih.gov/ij/; National Institutes of Health, NIH, Bethesda, MD). In addition, the surface-to-volume ratio (S/V) was calculated using the formula 3/r, where r is the radius (27). For cells with irregular form, qualitative analyses were performed. The zeta potentials of the NA and A yeast cells were calculated using a zeta potential analyzer (Zetasizer NanoZS90; Malvern, United Kingdom) as described previously (39).
Ethics statement, virulence, and cross-resistance in vivo.
C57BL/6 male mice, 6 to 8 weeks of age, were used for animal experiments. All experimental procedures were carried out according to the standards of the Brazilian Society of Laboratory Animal Science/Brazilian College for Animal Experimentation (available at http://www.sbcal.org.br). The study was approved by the Ethics Committee in Animal Experimentation of the Universidade Federal de Minas Gerais (CEUA/UFMG; protocol 306/2015).
The animals (six per group) were anesthetized by intraperitoneal (i.p.) injection with ketamine hydrochloride (60 mg/kg of body weight) and xylazine (10 mg/kg) in sterile saline solution. Next, each animal received 30 μl of 1 × 105 cells of C. gattii R265 or C. neoformans H99 by the intratracheal route. The mice were monitored daily for survival (27).
Other groups of animals were infected and euthanized under anesthesia 15 days postinoculation to obtain lungs, bronchoalveolar lavage fluid (BALF), and brain tissue. The organ homogenates and BALF were plated onto SDA to determine the fungal burden, expressed as CFU per gram or per milliliter (28). We also determined the MICbroth of tebuconazole, fluconazole, itraconazole, ravuconazole, and amphotericin B for the colonies recovered from the lungs. Moreover, lungs were collected, fixed in formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin (HE) for histopathological analysis. Histopathology was evaluated in two aspects: presence of yeast and inflammation in the lung parenchyma. The amount (discrete, moderate, or accentuated) and distribution (multifocal or diffuse) of yeasts and the type of inflammatory cells, as well as the location (perivascular or parenchyma), intensity (discrete, moderate, or accentuated), and distribution (multifocal or diffuse) of inflammation, were evaluated. Change described as “multifocal to diffuse” means that the lesion distribution varied in this way in the mice of this group.
To test the antifungal cross-resistance in vivo, mice infected with tebuconazole-adapted C. gattii R265 and C. neoformans H99 received 10 mg/kg of fluconazole daily by the intraperitoneal route. At 15 days postinoculation, the animals were euthanized and the lungs collected for determination of the CFU levels per gram.
RNA extraction and RT-PCR analysis.
Nonadapted and adapted cells of C. gattii R265 and C. neoformans H99 were grown on SDA and SDA plus tebuconazole plates, respectively, at 30°C. After 72 h, the colonies were collected and the RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer's instructions. Total RNA (5 μg) was subjected to DNase I treatment (Roche), and then 1 μg of the DNase I-treated RNA was used for reverse transcription (RT) using a QuantiTect reverse transcription (Qiagen) kit. Subsequently, the cDNAs were subjected to PCR amplification in the presence of dCTP (α33P) (PerkinElmer) with the primers for the following genes: ACT1, AFR1, and MDR1 for C. neoformans and ACT1, MDR1 (18), and PDR11 (20) for C. gattii. PCR products were resolved on a 7.5% polyacrylamide gel and quantified using a Typhoon 9200 imager and ImageQuant 5.2 software (Molecular Dynamics) (40).
Statistical analyses.
All statistical analyses were performed using GraphPad Prism, version 6.00, for Windows (GraphPad Software, San Diego, CA, USA), with P values of <0.05 considered significant. The results of antifungal experiments (performed in vitro and in vivo), morphometric analysis, zeta potential determinations, quantification of CFU levels per gram of organs, BALF analysis, and RT-PCR were analyzed by the use of Student's t test. Survival curves were plotted by the use of Kaplan-Meier analysis, and results were analyzed using the log rank test. All tests, including animal experiments, were repeated at least twice.
ACKNOWLEDGMENTS
This study was supported by Fundação de Amparo a Pesquisa do Estado de Minas Gerais—FAPEMIG (grant APQ-00727-16) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (grant 403006/2016-3). R.W.B. received fellowships from CNPq and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PDSE-CAPES-88881.131683/2016-01). D.A.S. is a research fellow of the CNPq (grant 305154/2014-1).
We declare that we have no conflicts of interest.
REFERENCES
- 1.Wu W, Ma B. 2015. Integrated nutrient management (INM) for sustaining crop productivity and reducing environmental impact: a review. Sci Total Environ 512–513:415–427. [DOI] [PubMed] [Google Scholar]
- 2.Rigotto RM, Vasconcelos DP, Rocha MM. 2014. Pesticide use in Brazil and problems for public health. Cad Saude Publica 30:1360–1362. doi: 10.1590/0102-311XPE020714. [DOI] [PubMed] [Google Scholar]
- 3.Kwok IM-Y, Loeffler RT. 1993. The biochemical mode of action of some newer azole fungicides. Pest Manag Sci 39:1–11. doi: 10.1002/ps.2780390102. [DOI] [Google Scholar]
- 4.Council On Environmental Health. 2012. Pesticide exposure in children. Pediatrics 130:e1757–e1763. doi: 10.1542/peds.2012-2757. [DOI] [PubMed] [Google Scholar]
- 5.Crinnion WJ. 2009. Chlorinated pesticides: threats to health and importance of detection. Altern Med Rev 14:347–359. [PubMed] [Google Scholar]
- 6.Serfling A, Wohlrab J, Deising HB. 2007. Treatment of a clinically relevant plant-pathogenic fungus with an agricultural azole causes cross-resistance to medical azoles and potentiates caspofungin efficacy. Antimicrob Agents Chemother 51:3672–3676. doi: 10.1128/AAC.00654-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocha MF, Alencar LP, Paiva MA, Melo LM, Bandeira SP, Ponte YB, Sales JA, Guedes GM, Castelo-Branco DS, Bandeira TJ, Cordeiro RA, Pereira-Neto WA, Brandine GS, Moreira JL, Sidrim JJ, Brilhante RS. 2016. Cross-resistance to fluconazole induced by exposure to the agricultural azole tetraconazole: an environmental resistance school? Mycoses 59:281–290. doi: 10.1111/myc.12457. [DOI] [PubMed] [Google Scholar]
- 8.Ren J, Jin X, Zhang Q, Zheng Y, Lin D, Yu Y. 2017. Fungicides induced triazole-resistance in Aspergillus fumigatus associated with mutations of TR46/Y121F/T289A and its appearance in agricultural fields. J Hazard Mater 326:54–60. doi: 10.1016/j.jhazmat.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Kronstad JW, Attarian R, Cadieux B, Choi J, D'Souza CA, Griffiths EJ, Geddes JM, Hu G, Jung WH, Kretschmer M, Saikia S, Wang J. 2011. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol 9:193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 11.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–222. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perfect JR, Bicanic T. 2015. Cryptococcosis diagnosis and treatment: what do we know now? Fungal Genet Biol 78:49–54. doi: 10.1016/j.fgb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith KD, Achan B, Hullsiek KH, McDonald TR, Okagaki LH, Alhadab AA, Akampurira A, Rhein JR, Meya DB, Boulware DR, Nielsen K; ASTRO-CM/COAT Team. 2015. Increased antifungal drug resistance in clinical isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother 59:7197–7204. doi: 10.1128/AAC.01299-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YC, Chang TY, Liu JW, Chen FJ, Chien CC, Lee CH, Lu CH. 2015. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: a 12-year longitudinal study. BMC Infect Dis 15:277. doi: 10.1186/s12879-015-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Springer DJ, Ren P, Raina R, Dong Y, Behr MJ, McEwen BF, Bowser SS, Samsonoff WA, Chaturvedi S, Chaturvedi V. 2010. Extracellular fibrils of pathogenic yeast Cryptococcus gattii are important for ecological niche, murine virulence and human neutrophil interactions. PLoS One 5:e10978. doi: 10.1371/journal.pone.0010978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Poeta M, Casadevall A. 2012. Ten challenges on Cryptococcus and cryptococcosis. Mycopathologia 173:303–310. doi: 10.1007/s11046-011-9473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen D, Wilson D, Drew R, Perfect J. 2015. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev Anti Infect Ther 13:787–798. doi: 10.1586/14787210.2015.1032939. [DOI] [PubMed] [Google Scholar]
- 18.Basso LR, Gast CE, Bruzual I, Wong B. 2015. Identification and properties of plasma membrane azole efflux pumps from the pathogenic fungi Cryptococcus gattii and Cryptococcus neoformans. J Antimicrob Chemother 70:1396–1407. doi: 10.1093/jac/dku554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang ML, Uhrig J, Vu K, Singapuri A, Dennis M, Gelli A, Thompson GR. 2015. Fluconazole susceptibility in Cryptococcus gattii is dependent on the ABC transporter Pdr11. Antimicrob Agents Chemother 60:1202–1207. doi: 10.1128/AAC.01777-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sykes JE, Hodge G, Singapuri A, Yang ML, Gelli A, Thompson GR 3rd. 2017. In vivo development of fluconazole resistance in serial Cryptococcus gattii isolates from a cat. Med Mycol 55:396–401. [DOI] [PubMed] [Google Scholar]
- 21.Espinel-Ingroff A, Aller AL, Canton E, Castañón-Olivares LR, Chowdhary A, Cordoba S, Cuenca-Estrella M, Fothergill A, Fuller J, Govender N, Hagen F, Illnait-Zaragozi MT, Johnson E, Kidd S, Lass-Flörl C, Lockhart SR, Martins MA, Meis JF, Melhem MS, Ostrosky-Zeichner L, Pelaez L, Pfaller MA, Schell WA, St-Germain G, Trilles L, Turnidge J. 2012. Cryptococcus neoformans-Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 56:5898–5906. doi: 10.1128/AAC.01115-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trpković A, Pekmezović M, Barać A, Crnčević Radović L, Arsić Arsenijević V. 2012. In vitro antifungal activities of amphotericin B, 5-fluorocytosine, fluconazole and itraconazole against Cryptococcus neoformans isolated from cerebrospinal fluid and blood from patients in Serbia. J Mycol Med 22:243–248. doi: 10.1016/j.mycmed.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Vanden Bossche H, Marichal P, Le Jeune L, Coene MC, Gorrens J, Cools W. 1993. Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethylation and reduction of 3-ketosteroids in Cryptococcus neoformans. Antimicrob Agents Chemother 37:2101–2105. doi: 10.1128/AAC.37.10.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sionov E, Chang YC, Garraffo HM, Dolan MA, Ghannoum MA, Kwon-Chung KJ. 2012. Identification of a Cryptococcus neoformans cytochrome P450 lanosterol 14α-demethylase (Erg11) residue critical for differential susceptibility between fluconazole/voriconazole and itraconazole/posaconazole. Antimicrob Agents Chemother 56:1162–1169. doi: 10.1128/AAC.05502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielska E, May RC. 2016. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res 16:fov106. doi: 10.1093/femsyr/fov106. [DOI] [PubMed] [Google Scholar]
- 26.Howard DH. 1961. Some factors which affect the initiation of growth of Cryptococcus neoformans. J Bacteriol 82:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira GF, Santos JR, Costa MC, Holanda RA, Denadai AM, Freitas GJ, Santos AR, Tavares PB, Paixão TA, Santos DA. 2015. Heteroresistance to itraconazole alters the morphology and increases the virulence of Cryptococcus gattii. Antimicrob Agents Chemother 59:4600–4609. doi: 10.1128/AAC.00466-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos JR, Holanda RA, Frases S, Bravim M, Araujo GeS Santos PC, Costa MC, Ribeiro MJ, Ferreira GF, Baltazar LM, Miranda AS, Oliveira DB, Santos CM, Fontes AC, Gouveia LF, Resende-Stoianoff MA, Abrahão JS, Teixeira AL, Paixão TA, Souza DG, Santos DA. 2014. Fluconazole alters the polysaccharide capsule of Cryptococcus gattii and leads to distinct behaviors in murine cryptococcosis. PLoS One 9:e112669. doi: 10.1371/journal.pone.0112669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NC, Chrétien F, Heitman J, Dromer F, Nielsen K. 2010. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 6:e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trevijano-Contador N, Rueda C, Zaragoza O. 2016. Fungal morphogenetic changes inside the mammalian host. Semin Cell Dev Biol 57:100–109. doi: 10.1016/j.semcdb.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Lee SC, Phadke S, Sun S, Heitman J. 2012. Pseudohyphal growth of Cryptococcus neoformans is a reversible dimorphic transition in response to ammonium that requires Amt1 and Amt2 ammonium permeases. Eukaryot Cell 11:1391–1398. doi: 10.1128/EC.00242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhai B, Zhu P, Foyle D, Upadhyay S, Idnurm A, Lin X. 2013. Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infect Immun 81:2626–2637. doi: 10.1128/IAI.00259-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J, Idnurm A, Lin X. 2015. Morphology and its underlying genetic regulation impact the interaction between Cryptococcus neoformans and its hosts. Med Mycol 53:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi SA, Trevijano-Contador N, Scorzoni L, Mesa-Arango AC, de Oliveira HC, Werther K, de Freitas Raso T, Mendes-Giannini MJ, Zaragoza O, Fusco-Almeida AM. 2016. Impact of resistance to fluconazole on virulence and morphological aspects of Cryptococcus neoformans and Cryptococcus gattii isolates. Front Microbiol 7:153. doi: 10.3389/fmicb.2016.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sionov E, Chang YC, Garraffo HM, Kwon-Chung KJ. 2009. Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob Agents Chemother 53:2804–2815. doi: 10.1128/AAC.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos JR, Gouveia LF, Taylor EL, Resende-Stoianoff MA, Pianetti GA, César IC, Santos DA. 2012. Dynamic interaction between fluconazole and amphotericin B against Cryptococcus gattii. Antimicrob Agents Chemother 56:2553–2558. doi: 10.1128/AAC.06098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magalhães TF, da Silva CM, de Fátima A, da Silva DL, Modolo LV, Martins CV, Alves RB, Ruiz AL, Longato GB, de Carvalho JE, de Resende-Stoianoff MA. 2013. Hydroxyaldimines as potent in vitro anticryptococcal agents. Lett Appl Microbiol 57:137–143. doi: 10.1111/lam.12086. [DOI] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts: fourth informational supplement, CLSI document M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.Nosanchuk JD, Cleare W, Franzot SP, Casadevall A. 1999. Amphotericin B and fluconazole affect cellular charge, macrophage phagocytosis, and cellular morphology of Cryptococcus neoformans at subinhibitory concentrations. Antimicrob Agents Chemother 43:233–239. doi: 10.1093/jac/43.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Hilarion S, Paulet D, Lee KT, Hon CC, Lechat P, Mogensen E, Moyrand F, Proux C, Barboux R, Bussotti G, Hwang J, Coppée JY, Bahn YS, Janbon G. 2016. Intron retention-dependent gene regulation in Cryptococcus neoformans. Sci Rep 6:32252. doi: 10.1038/srep32252. [DOI] [PMC free article] [PubMed] [Google Scholar]