ABSTRACT
Clostridium difficile causes diarrhea and colitis by releasing toxin A and toxin B. In the human colon, both toxins cause intestinal inflammation and stimulate tumor necrosis factor alpha (TNF-α) expression via the activation of NF-κB. It is well established that the macrolide antibiotic fidaxomicin is associated with reduced relapses of C. difficile infection. We showed that fidaxomicin and its primary metabolite OP-1118 significantly inhibited toxin A-mediated intestinal inflammation in mice in vivo and toxin A-induced cell rounding in vitro. We aim to determine whether fidaxomicin and OP-1118 possess anti-inflammatory effects against toxin A and toxin B in the human colon and examine the mechanism of this response. We used fresh human colonic explants, NCM460 human colonic epithelial cells, and RAW264.7 mouse macrophages to study the mechanism of the activity of fidaxomicin and OP-1118 against toxin A- and B-mediated cytokine expression and apoptosis. Fidaxomicin and OP-1118 dose-dependently inhibited toxin A- and B-induced TNF-α and interleukin-1β (IL-1β) mRNA expression and histological damage in human colonic explants. Fidaxomicin and OP-1118 inhibited toxin A-mediated NF-κB phosphorylation in human and mouse intestinal mucosae. Fidaxomicin and OP-1118 also inhibited toxin A-mediated NF-κB phosphorylation and TNF-α expression in macrophages, which was reversed by the NF-κB activator phorbol myristate acetate (PMA). Fidaxomicin and OP-1118 prevented toxin A- and B-mediated apoptosis in NCM460 cells, which was reversed by the addition of PMA. PMA reversed the cytoprotective effect of fidaxomicin and OP-1118 in toxin-exposed human colonic explants. Fidaxomicin and OP-1118 inhibit C. difficile toxin A- and B-mediated inflammatory responses, NF-κB phosphorylation, and tissue damage in the human colon.
KEYWORDS: Clostridium difficile infection, antibiotics, signaling
INTRODUCTION
Clostridium difficile infection (CDI) is a gastrointestinal disease associated primarily with the use of antibiotics in a hospital setting (1). With increasing incidence, morbidity, and mortality rates, CDI is a prominent constituent of antibiotic-associated diarrhea and intestinal inflammation (2, 3). C. difficile, an anaerobic bacterium, releases toxin A and toxin B, which mediate diarrhea and colitis in animals and humans. Toxin A and toxin B increase proinflammatory cytokine (tumor necrosis factor alpha [TNF-α] and interleukin-1β [IL-1β]) mRNA expression levels and cause histological damage in fresh human colonic explants (4). These toxins also cause epithelial cell apoptosis, as they inactivate GTPases and cause cytoskeleton disruption leading to cell rounding (3, 5). Our previous studies also showed that NF-κB activation plays a major role in the proinflammatory effect of C. difficile toxin A in vitro (6, 7).
Fidaxomicin is a poorly absorbed antimicrobial macrolide (8, 9), which emerged as a new therapeutic agent for CDI. Fidaxomicin has an antibacterial effect against various strains of C. difficile by inhibiting the RNA polymerase sigma subunit, which blocks the protein synthesis of bacteria (10). The MIC range of fidaxomicin against C. difficile is approximately 0.001 to 1 μg/ml (11). While CDI cure rates with vancomycin, metronidazole, and fidaxomicin are similar, fidaxomicin use is associated with substantially reduced rates of CDI relapse (12–14). Evidence suggests that fidaxomicin exerts its therapeutic effect primarily by preventing bacterial RNA transcription (15) and inhibiting toxin A and toxin B production in CDI (16). Fidaxomicin is hydrolyzed into a less active metabolite, OP-1118, in the intestine (17). The MIC range of OP-1118 against C. difficile bacteria is around 0.25 to 2 μg/ml (18).
We recently reported that fidaxomicin and its primary metabolite OP-1118 significantly inhibited C. difficile toxin A-mediated enteritis in mice and reduced toxin A-mediated cell rounding in fibroblasts, suggesting that in addition to its antibacterial effects against C. difficile, fidaxomicin may also exert potent anti-inflammatory effects against intestinal responses to C. difficile toxins (19). The potential anti-inflammatory effects of fidaxomicin and OP-1118 in human colonic tissue, which is the primary target tissue of C. difficile and its toxins, however, have never been determined. Also, the mechanism(s) by which fidaxomicin exerts its anti-inflammatory and cytoprotective effects against C. difficile toxin A and toxin B remains to be elucidated.
Based on these considerations, this study addressed the hypothesis that fidaxomicin and OP-1118 possess anti-inflammatory and cytoprotective effects against C. difficile toxins in the human colon by interfering with NF-κB signaling pathways commonly activated by these toxins.
RESULTS
Fidaxomicin and OP-1118 inhibit toxin A- and toxin B-induced TNF-α and IL-1β mRNA expression in human colonic explants.
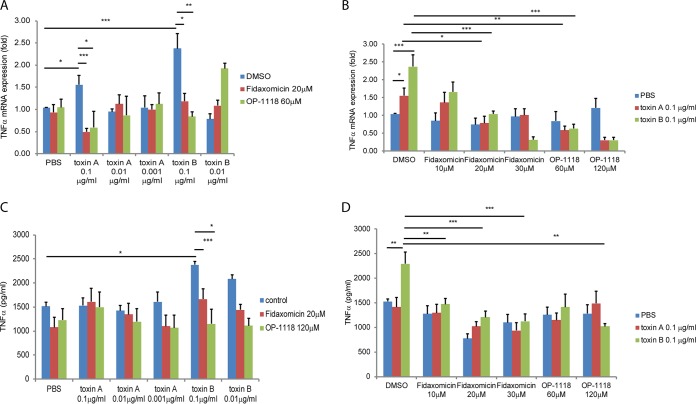
We previously reported that the administration of fidaxomicin or OP-1118 in mouse ileal loops significantly reduced ileal IL-1β expression in response to toxin A injection (19). To assess the potential anti-inflammatory role of fidaxomicin and OP-1118 in the human colon, we treated fresh human colonic explants with various concentrations of fidaxomicin or OP-1118, followed by exposure to toxin A or toxin B. The levels of mRNA expression in explant tissues and protein secretion into conditioned medium were then determined. As shown in Fig. 1, toxin A and toxin B stimulated TNF-α mRNA expression (Fig. 1A). Fidaxomicin and OP-1118 dose-dependently reduced the TNF-α mRNA expression level induced by toxin A and toxin B (Fig. 1B). However, only toxin B significantly induced TNF-α protein secretion into conditioned medium (Fig. 1C and D). Fidaxomicin and OP-1118 dose-dependently reduced TNF-α protein secretion induced by toxin B.
FIG 1.
Fidaxomicin and OP-1118 inhibit toxin A- and B-induced TNF-α mRNA expression in human colonic explants. (A) Dose response of toxin A- and B-induced TNF-α mRNA expression. DMSO was used as a solvent for fidaxomicin and OP-1118. (B) Dose response of fidaxomicin and OP-1118 in toxin A- and B-induced TNF-α mRNA expression. (C) Dose response of toxin A- and B-induced TNF-α protein expression. (D) Dose response of fidaxomicin and OP-1118 in toxin A- and B-induced TNF-α protein expression. Each group consisted of 6 fresh human colonic explants. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We also observed that toxin A and toxin B stimulated IL-1β mRNA expression (Fig. 2A). Fidaxomicin and OP-1118 dose-dependently reduced IL-1β mRNA expression induced by both toxins (Fig. 2B). Only toxin B significantly induced IL-1β protein secretion into conditioned medium, but both fidaxomicin and OP-1118 dose-dependently reduced IL-1β protein secretion induced by toxin B (Fig. 2C and D).
FIG 2.
Fidaxomicin and OP-1118 inhibit toxin A- and B-induced IL-1β mRNA expression in human colonic explants. (A) Dose response of toxin A- and B-induced IL-1β mRNA expression. (B) Dose response of fidaxomicin and OP-1118 in toxin A-and B-induced IL-1β mRNA expression. (C) Dose response of toxin A-and B-induced IL-1β protein expression. (D) Dose response of fidaxomicin and OP-1118 in toxin A- and B-induced IL-1β protein expression. Each group consisted of 6 fresh human colonic explants. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Fidaxomicin and OP-1118 inhibit toxin A-induced TNF-α expression via NF-κB phosphorylation.
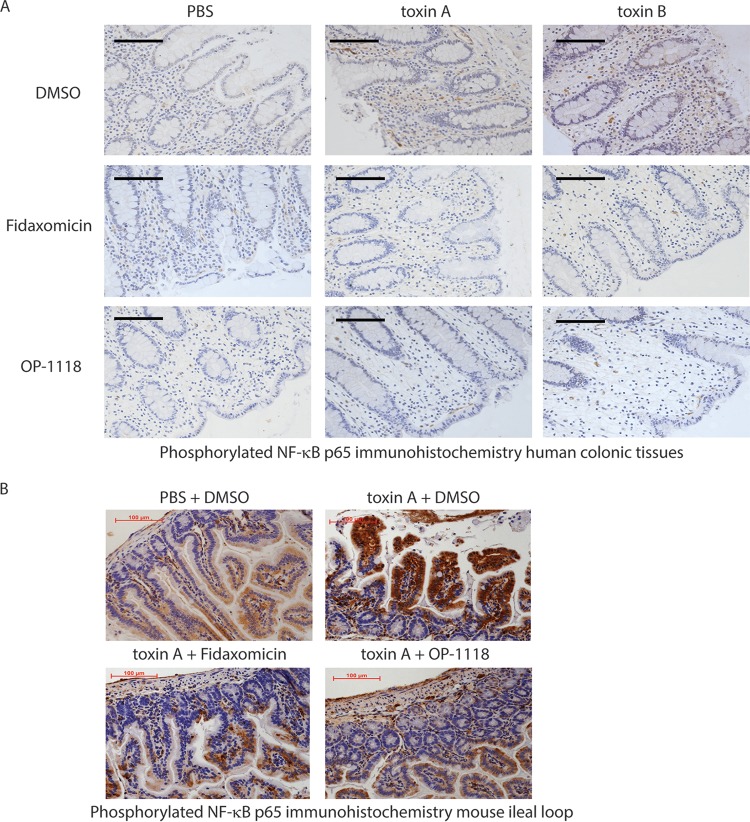
Toxin A and toxin B induced mucosal NF-κB phosphorylation in human colonic tissues (Fig. 3A; see also Fig. S1A in the supplemental material), which was reduced by the pretreatment of human colonic explants with fidaxomicin or OP-1118. Similarly, fidaxomicin or OP-1118 also substantially reduced NF-κB phosphorylation in toxin A-treated mouse ileal loops (Fig. 3B and Fig. S1B). Toxin B did not induce NF-κB phosphorylation in mouse ileal loops (data not shown). Based on this evidence, we next determined whether the anti-inflammatory mechanism of fidaxomicin and OP-1118 against toxin A and toxin B involved NF-κB activation.
FIG 3.
Fidaxomicin and OP-1118 inhibit toxin A- and B-induced NF-κB phosphorylation in human colonic explants and mouse ileal loops. The phosphorylated NF-κB signal in human colonic tissues (A) and mouse ileal loops (B) is shown in brown. (A) Each group consisted of 6 fresh human colonic explants. (B) Each group consisted of 6 mice. Magnification, ×200. Black bars, 100 μm.
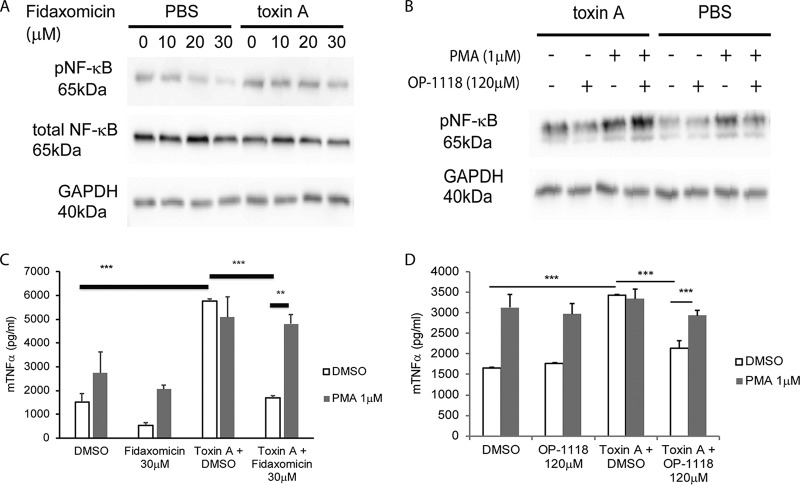
To address this, we used the well-established RAW264.7 mouse macrophage cell line, which is known to respond to toxin A (6). Our results show that, similar to our findings for mouse ileum and human colon (Fig. 3 and Fig. S1), exposure of macrophages to toxin A induced NF-κB phosphorylation (Fig. 4A and B). Preincubation of cells with fidaxomicin dose-dependently reduced toxin A-induced NF-κB phosphorylation (Fig. 4A and Fig. S1C). Similarly, OP-1118 inhibited toxin A-induced NF-κB phosphorylation in RAW264.7 cells. This inhibition was reversed by the NF-κB activator phorbol myristate acetate (PMA) (Fig. 4B and Fig. S1D). Together, these results suggest that fidaxomicin and its active metabolite OP-1118 inhibit NF-κB activation.
FIG 4.
Fidaxomicin inhibits toxin A-mediated TNF-α expression via NF-κB inhibition. (A and B) Western blot showing the protein signals of phosphorylated NF-κB (pNF-κB), total NF-κB, and GAPDH in RAW264.7 macrophages. (C and D) TNF-α levels in conditioned medium of RAW264.7 macrophages. The results are pooled from three separate experiments. **, P < 0.01; ***, P < 0.001.
NF-κB activation is involved in the transcription of several proinflammatory cytokines, including TNF-α (20). To examine the functional importance of the anti-NF-κΒ response exerted by fidaxomicin and OP-1118 in the proinflammatory effects of toxin A, we tested the effects of fidaxomicin and OP-1118 on toxin A-induced TNF-α secretion in RAW264.7 cells. Consistent with previously reported observations (21), toxin A induced TNF-α protein expression in RAW264.7 macrophages, which was significantly reduced by the pretreatment of cells with fidaxomicin or OP-1118 (Fig. 4C and D). Toxin B did not induce TNF-α protein expression in RAW264.7 cells (data not shown). Furthermore, pretreatment of the cells with PMA reversed the inhibitory effects of fidaxomicin and OP-1118 on TNF-α expression in the presence of toxin A (Fig. 4C and D). These findings suggest that fidaxomicin and OP-1118 inhibit toxin A-induced TNF-α expression via NF-κB inhibition.
Fidaxomicin and OP-1118 inhibit toxin A- and B-mediated tissue damage in human colonic explants via NF-κB inhibition.
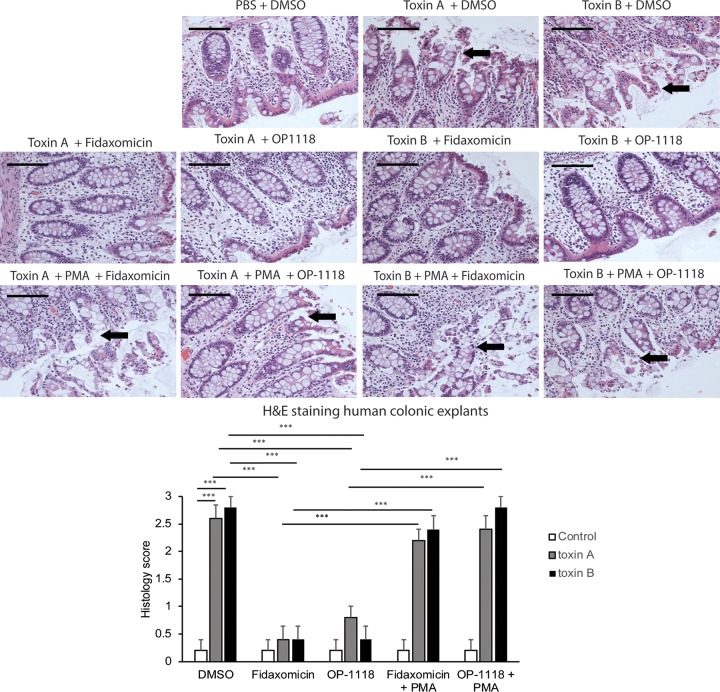
We next studied this protective response in normal human colonic explants. Toxin A and toxin B caused epithelial layer disruption of fresh human colonic explants (Fig. 5), in line with data from our previous reports (4, 22, 23). Incubation of human colonic explants with fidaxomicin or OP-1118 reversed toxin A- and B-mediated tissue damage (Fig. 5B). Exposure to fidaxomicin, OP-1118, or PMA in the absence of toxin A or toxin B did not cause tissue damage (data not shown). However, the addition of PMA reversed all cytoprotective effects of fidaxomicin and OP-1118 against toxin A and toxin B in human colonic explants (Fig. 5 and data not shown).
FIG 5.
Fidaxomicin inhibits toxin A- and B-mediated epithelial cell damage in fresh human colonic explants via NF-κB inhibition. (Top) The histological structure of the treated human colonic tissues was evaluated by H&E staining at a ×200 magnification. Luminal epithelial cell linings were damaged in human colonic explants exposed to toxin A or toxin B. Arrows indicate the locations of epithelial damage. Black bars, 100 μm. (Bottom) Histology scores for human colonic explants. C. difficile toxins caused damage to the colonic mucosal epithelial layer. The data are pooled from 6 patients per group. ***, P < 0.001.
Fidaxomicin and OP-1118 inhibit toxin A- and B-mediated apoptosis in colonic epithelial cells via NF-κB inhibition.
We also determined whether fidaxomicin and OP-1118 protect cells from apoptosis in response to C. difficile toxins. As expected (22, 24), both toxin A and toxin B caused apoptosis of human colonic NCM460 epithelial cells as detected by a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (Fig. S2A and S2B). Coincubation of NCM460 cells with fidaxomicin or OP-1118 significantly reduced toxin A- and B-mediated apoptosis (Fig. S2A and S2B). The activation of NF-κB by PMA reversed the antiapoptotic effect of fidaxomicin and OP-1118 on toxin A- and B-exposed cells (Fig. S2A and S2B). In contrast, we did not observe TUNEL-positive cells in fidaxomicin-, OP-1118-, or PMA-treated cells in the absence of toxin exposure (data not shown). This finding suggests that fidaxomicin and OP-1118 inhibit toxin A- and B-induced apoptosis, likely through modulation of the NF-κB pathway.
DISCUSSION
Our recent results using mouse ileal loops indicated that fidaxomicin and its primary metabolite OP-1118 inhibit C. difficile toxin A-associated inflammatory responses (19). We showed that both toxins increased TNF-α and IL-1β mRNA expression levels in human colonic explants, as previously described (4), and increased the phosphorylation of the p65 subunit of NF-κB (6). All of these responses were reduced by fidaxomicin and OP-1118 (Fig. 1 to 3). In addition, fidaxomicin and OP-1118 significantly inhibited toxin A- and B-mediated histologic damage in human colonic tissues and apoptosis in human colonic epithelial cells, respectively (Fig. 5; see also Fig. S2 in the supplemental material). Together, these results strongly indicate that these anti-inflammatory and antiapoptotic mechanisms may contribute to the therapeutic effects of fidaxomicin on C. difficile infection by preserving the integrity of the human colonic mucosa following toxin exposure. These results are also consistent with anti-inflammatory effects of other macrolides on nonintestinal cells and tissues (25–27).
Compared to toxin A, toxin B had a potent effect in inducing increased TNF-α and IL-1β mRNA levels after 4 h of incubation (Fig. 1 to 2). Previous studies indicated that toxin B is more potent than toxin A in inducing epithelial cell damage in the human colon at 5 h (22) and that both toxins are proinflammatory toxins in human intestinal mucosa (28). Data from previous studies by Lyras et al. using toxin B mutants in the C. difficile hamster model also underline the importance of toxin B in CDI pathophysiology (29, 30). Overall, our results demonstrate that fidaxomicin exerts anti-inflammatory and cytoprotective effects in the human colon against not only toxin A but also toxin B, which may be relevant for the potential effectiveness of fidaxomicin in CDI caused by hypervirulent toxin A-negative, toxin B-positive C. difficile strains such as those of ribotype 017 (31).
Although both toxins induced TNF-α and IL-1β mRNA expression, only toxin B sufficiently induced the protein secretion of TNF-α and IL-1β in human colonic tissues after 4 h of exposure (Fig. 1 and 2). This discrepancy between the levels of mRNA and protein secretion of these cytokines in response to toxin A may be due to the sensitivities of the methods used for their detection (more sensitive reverse transcription-PCR [RT-PCR] for mRNA and a less sensitive protein enzyme-linked immunosorbent assay [ELISA] for protein).
We previously demonstrated that toxin A induces TNF-α expression via an NF-κB-dependent pathway in mouse macrophages (6). Previous results also indicate that both toxin A and toxin B can activate NF-κB-driven cytokine genes in human colonocytes and monocytes (7, 32). Furthermore, toxin A can activate NF-κB in human colonic biopsy specimens via the activation of reactive oxygen species (7). Here we found that fidaxomicin and OP-1118 inhibited NF-κB activation and reduced the expression level of the NF-κB-driven cytokine TNF-α in response to both toxins in human colonic explants as well as in response to toxin A in mouse ileal loops and macrophages. The NF-κB activator PMA was able to reverse the inhibition of toxin A-induced TNF-α expression caused by fidaxomicin and OP-1118, strongly suggesting that the anti-inflammatory effects of this drug and its active metabolite involve the inhibition of the NF-κB system.
It is well accepted that both C. difficile toxins trigger apoptosis and apoptosis-related cell death in several target cells, including intestinal epithelial cells (33–35). Our results indicate that in NCM460 human colonic epithelial cells, both toxins increased the number of TUNEL-positive cells, and coincubation of cells with fidaxomicin and OP-1118 can inhibit this effect (Fig. S2). Interestingly, the activation of NF-κB signaling by the addition of PMA reversed the antiapoptotic effect of fidaxomicin and OP-1118 on toxin A- and B-exposed cells (Fig. S2). This finding suggests that fidaxomicin and OP-1118 inhibit toxin A- and B-induced apoptosis in human colonocytes by modulating the NF-κB pathway. Previous results indicated that toxin A-induced apoptosis in vitro and in vivo (evidenced by increased Fas ligand expression) was linked to NF-κB activation (34). Moreover, NF-κB has been shown to regulate inflammasome expression (36), as the activation of the inflammasome mediates apoptosis (37). Both toxin A and toxin B also mediate cell apoptosis and damage via the inflammasome-dependent release of IL-1β (38). Gerhard et al., however, presented evidence suggesting that toxin A-induced apoptosis (evidenced by caspase activation) depends on the glucosylation of Rho GTPases (39), consistent with previous observations that perturbations of the actin cytoskeleton can mediate apoptosis (40, 41). Although the details of these complex associations need further investigation, the inhibitory effects of fidaxomicin and OP-1118 on the NF-κB pathway and on cytoskeletal disruption may be associated with the suppression of the inflammasome and subsequent apoptosis.
Certain patients with CDI develop systemic infections, which require concomitant antibiotic treatment. However, these concomitant antibiotics often compromise the cure rate for vancomycin against CDI. Fidaxomicin has a higher cure rate than does vancomycin in patients with concomitant antibiotic treatment (42). Fidaxomicin-treated patients also have a lower relapse rate than do vancomycin-treated patients in this concomitant antibiotic-exposed patient cohort (42). We speculate that the anti-inflammatory effect of fidaxomicin may be important for CDI because certain anti-inflammatory drugs (such as aspirin and statin) may reduce the risk of CDI (43, 44). The pleiotropic mechanisms of fidaxomicin may confer advantages over vancomycin for the treatment of CDI.
In summary, fidaxomicin and its active metabolite OP-1118 significantly reduce C. difficile toxin A- and B-mediated proinflammatory cytokine expression and histologic damage in human colonic tissues. These protective effects provide novel insights into the therapeutic mechanism of this antibiotic for CDI.
MATERIALS AND METHODS
C. difficile culture and toxin purification.
C. difficile strain VPI 10463 (ATCC 43255) was cultured in Difco cooked meat medium (catalog number 226730; BD, Fisher Scientific) at 37°C under anaerobic conditions, and toxin A and toxin B were purified as previously reported (45). The purity of the purified toxin A and toxin B preparations was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by Coomassie blue staining, as described previously (45). The cytotoxicity of toxin A and toxin B was determined by cell rounding in 3T3 fibroblasts, as described previously (19).
Human colonic explants.
Fresh human colonic explants were obtained from the UCLA surgical pathology department. Institutional Review Board (IRB) approval was waived since no patient-identifiable information was obtained. We used fresh human colonic explants collected from noncancerous regions of colon cancer patients, as previously described (4). The fresh colonic explants were cut into 3- by 3-mm pieces. The explants were placed into RPMI 1640 medium and treated with dimethyl sulfoxide (DMSO) (0.8%), fidaxomicin (10 to 30 μM), or OP-1118 (60 to 120 μM) for 30 min, followed by phosphate-buffered saline (PBS) (1 μl/ml), toxin A (0.001 to 0.1 μg/ml), or toxin B (0.01 to 0.1 μg/ml) for 1 or 4 h as indicated. Some groups were pretreated with DMSO (1 μl/ml) or PMA (1 μM) for 30 min before exposure to fidaxomicin or OP-1118.
Histology scoring.
Fresh human colonic explants were treated with DMSO (1 μl/ml) or PMA (1 μM) for 30 min, followed by fidaxomicin (30 μM), OP-1118 (120 μM), or DMSO (0.8%) for 30 min. Tissues were then incubated with PBS (1 μl/ml), toxin A (1 μg/ml), or toxin B (1 μg/ml) for 4 h, followed by formalin fixation overnight. The fixed tissues were sectioned and paraffin embedded at the UCLA tissue processing core laboratory (TPCL).
Some of the tissue sections were stained with hematoxylin and eosin (H&E). The stained slides were analyzed by two independent observers in a blind manner. Epithelial damage was quantified by observing at least 20 different fields of H&E-stained intestinal sections from each group. A histology score of 0 to 3 was assigned, as previously described (46).
Mouse ileal loops.
Paraffin-embedded sections of mouse ileal loop tissues were obtained from a previous study (19). Ileal loops of mice were pretreated with fidaxomicin (20 μM), OP-1118 (120 μM), or DMSO (0.8%) for 30 min, followed by exposure to C. difficile toxin A (10 μg per ileal loop) or PBS alone (200 μl per ileal loop) for 4 h. The animal study was approved by the UCLA Institutional Animal Research Committee (approval number 2007-116).
Phosphorylated NF-κB immunohistochemistry.
Phosphorylated NF-κB immunohistochemistry of human colonic and mouse ileal tissues was performed by the UCLA TPCL. Paraffin-embedded sections were cut at a 4-μm thickness, and paraffin was removed with xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min. Heat-induced antigen retrieval (HIER) was carried out for all sections with 0.01 M citrate buffer (pH 6) by using a Biocare decloaker at 95°C for 25 min. After treatment with blocking buffer (2% bovine serum albumin [BSA]) for 1 h, the slides were then incubated overnight at 4°C with rabbit polyclonal antibody to phosphorylated NF-κB p65 in 2% BSA at a 1:100 dilution (catalog number ab86299; Abcam). The signal was detected by using the rabbit horseradish peroxidase EnVision kit (catalog number K4003; DakoCytomation). This secondary antibody kit was directly applied to the slides, without dilution. All sections were visualized with the diaminobenzidine reaction and counterstained with hematoxylin.
Images were taken with a Zeiss AX10 microscope in a blind manner. The stained slides were analyzed by two independent observers in a blind manner. Four different locations per tissue section were observed and scored. The quantitative intensity of the phosphorylated NF-κB signal was observed and scored as 0 for normal, 1 for mild, 2 for moderate, or 3 for strong intensity, as described previously (47).
Cell culture.
Mouse RAW264.7 macrophages were purchased from the ATCC and cultured in T75 flasks containing Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen), as described previously (6). Human colonic NCM460 epithelial cells were cultured in M3D medium (Incell) containing 10% fetal bovine serum (Invitrogen) and 1% penicillin-streptomycin (Invitrogen), as described previously (48).
TNF-α and IL-1β ELISAs.
The levels of proinflammatory mediators, including human TNF-α (catalog number DY210; R&D Systems), human IL-1β (catalog number DY201; R&D Systems), and mouse TNF-α (catalog number DY410; R&D Systems), were measured according to the manufacturer's instructions. RAW264.7 macrophages in 12-well plates (1 × 105 cells/well; 1 ml/well) were pretreated with DMSO (1 μl/ml) and PMA (1 μM) for 30 min, followed by the addition of DMSO (0.8%), fidaxomicin (30 μM), or OP-1118 (120 μM) for 30 min, before exposure to toxin A (0.1 μg/ml; 1 μl/well) for 4 h. Conditioned media were then collected for mouse TNF-α ELISA measurements on 96-well plates (100 μl/well in triplicate).
Quantitative real-time RT-PCR.
Total RNA was isolated by using an RNeasy kit (catalog number 74106; Qiagen, CA) and reverse transcribed into cDNA by using a Superscript III kit (catalog number 11752; Invitrogen, Carlsbad, CA). Quantitative PCRs were run in a Bio-Rad CFX384 PCR detector. The mRNA expression level was determined by using cataloged primers (Invitrogen) for human TNF-α (Hs00174128_m1), IL-1β (Hs01555410_m1), and 18S (Hs99999901_s1). Results were expressed as relative fold differences normalized to the 18S signal.
Western blot analyses.
Mouse RAW264.7 macrophages in 12-well plates (1 × 105 cells/well; 1 ml/well) were treated with DMSO (1 μl/ml) or PMA (1 μM) for 30 min, followed by various concentrations of fidaxomicin (10 to 30 μM), OP-1118 (120 μM), or DMSO (0.8%) for 30 min. Cells were then incubated with PBS (1 μl/ml), toxin A (1 μg/ml), or toxin B (1 μg/ml) for 1 h.
Treated cells were lysed in radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors (catalog number sc-24948; Santa Cruz Biotechnology). The protein concentrations were measured by using a bicinchoninic acid assay (BCA) method (catalog number 23225; ThermoFisher Scientific). The lysates were normalized to 20 μg protein per lane and added with blue loading buffer (catalog number 7722; Cell Signaling) to form a 1× loading mixture.
Equal amounts of cell extracts were fractioned by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were transferred onto 0.45-μm nitrocellulose membranes (400 mA for 1.5 h; Bio-Rad). Membranes were blocked in 5% nonfat milk in Tris-buffered saline–Tween 20 (TBST) (50 mM Tris [pH 7.5], 0.15 M NaCl, 0.05% Tween 20) and then incubated with antibodies (phosphorylated NF-κB [catalog number ab86299; Abcam], total NF-κB [catalog number sc-372; Santa Cruz Biotechnology], and glyceraldehyde-3-phosphate dehydrogenase [GAPDH] [catalog number sc-25778; Santa Cruz Biotechnology]) at 1:500 ratios for 16 h at 4°C. The horseradish peroxidase-labeled antibody signal was detected by chemiluminescence using the LAS4000 luminescent-image analyzer (Fujifilm, Tokyo, Japan). The protein signal was quantified by using Multi Gauge software (Fujifilm).
TUNEL apoptosis detection.
Human NCM460 colonic epithelial cells (105 cells/well; 1 ml/well) were cultured in chambered cell culture slides (catalog number 354104; Corning). Cells were treated with DMSO (1 μl/ml) or PMA (1 μM) for 30 min, followed by fidaxomicin (30 μM), OP-1118 (120 μM), or DMSO (0.8%) for 30 min. Cells were then incubated with PBS (1 μl/ml), toxin A (1 μg/ml), or toxin B (1 μg/ml) for 8 h.
The TPCL assisted with TUNEL staining. The relative severity of apoptosis was observed and scored based on TUNEL-positive signal as follows: 0 for normal, 1 for mild, 2 for moderate, and 3 for strong. Four different locations per well were observed and scored.
Statistical analyses.
Quantitative results were expressed as means ± standard errors of the means. Results were analyzed by using the Prism 5 statistics software program (GraphPad). Ordinary two-way analysis of variance (ANOVA) (for examining effects of drug treatment and toxin treatment), followed by Bonferroni posttests, was used for intergroup comparisons unless indicated otherwise. Statistical significance is indicated in the figure legends. Error bars represent standard errors of the means.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Merck, Inc. (C.P.). H.W.K. was also supported by NIH grant R03 DK103964. Support to C.P. was also provided by NIH/NIAID grant UO-1 AI124290, the Blinder Research Foundation for Crohn's Disease, and the Eli and Edythe Broad Chair. C.P.K. was supported by NIH grant R01 AI095256, and X.C. was supported by a career development award from the Crohn's and Colitis Foundation of America, Inc.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01513-17.
REFERENCES
- 1.Dupont HL. 2013. Diagnosis and management of Clostridium difficile infection. Clin Gastroenterol Hepatol 11:1216–1223; quiz e73. doi: 10.1016/j.cgh.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. 2008. Clostridium difficile—more difficult than ever. N Engl J Med 359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 4.Koon HW, Shih DQ, Hing TC, Yoo JH, Ho S, Chen X, Kelly CP, Targan SR, Pothoulakis C. 2013. Human monoclonal antibodies against Clostridium difficile toxins A and B inhibit inflammatory and histologic responses to the toxins in human colon and peripheral blood monocytes. Antimicrob Agents Chemother 57:3214–3223. doi: 10.1128/AAC.02633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leffler DA, Lamont JT. 2015. Clostridium difficile infection. N Engl J Med 372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 6.Hing TC, Ho S, Shih DQ, Ichikawa R, Cheng M, Chen J, Chen X, Law I, Najarian R, Kelly CP, Gallo RL, Targan SR, Pothoulakis C, Koon HW. 2013. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut 62:1295–1305. doi: 10.1136/gutjnl-2012-302180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He D, Sougioultzis S, Hagen S, Liu J, Keates S, Keates AC, Pothoulakis C, Lamont JT. 2002. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 122:1048–1057. doi: 10.1053/gast.2002.32386. [DOI] [PubMed] [Google Scholar]
- 8.Duggan ST. 2011. Fidaxomicin: in Clostridium difficile infection. Drugs 71:2445–2456. doi: 10.2165/11208220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Sears P, Crook DW, Louie TJ, Miller MA, Weiss K. 2012. Fidaxomicin attains high fecal concentrations with minimal plasma concentrations following oral administration in patients with Clostridium difficile infection. Clin Infect Dis 55(Suppl 2):S116–S120. doi: 10.1093/cid/cis337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tupin A, Gualtieri M, Leonetti JP, Brodolin K. 2010. The transcription inhibitor lipiarmycin blocks DNA fitting into the RNA polymerase catalytic site. EMBO J 29:2527–2537. doi: 10.1038/emboj.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein EJ, Babakhani F, Citron DM. 2012. Antimicrobial activities of fidaxomicin. Clin Infect Dis 55(Suppl 2):S143–S148. doi: 10.1093/cid/cis339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, Sears P, Gorbach S, OPT-80-004 Clinical Study Group. 2012. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 12:281–289. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 13.Cornely OA, Nathwani D, Ivanescu C, Odufowora-Sita O, Retsa P, Odeyemi IA. 2014. Clinical efficacy of fidaxomicin compared with vancomycin and metronidazole in Clostridium difficile infections: a meta-analysis and indirect treatment comparison. J Antimicrob Chemother 69:2892–2900. doi: 10.1093/jac/dku261. [DOI] [PubMed] [Google Scholar]
- 14.Crook DW, Walker AS, Kean Y, Weiss K, Cornely OA, Miller MA, Esposito R, Louie TJ, Stoesser NE, Young BC, Angus BJ, Gorbach SL, Peto TE, Study 003/004 Teams. 2012. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis 55(Suppl 2):S93–S103. doi: 10.1093/cid/cis499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artsimovitch I, Seddon J, Sears P. 2012. Fidaxomicin is an inhibitor of the initiation of bacterial RNA synthesis. Clin Infect Dis 55(Suppl 2):S127–S131. doi: 10.1093/cid/cis358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babakhani F, Bouillaut L, Sears P, Sims C, Gomez A, Sonenshein AL. 2013. Fidaxomicin inhibits toxin production in Clostridium difficile. J Antimicrob Chemother 68:515–522. doi: 10.1093/jac/dks450. [DOI] [PubMed] [Google Scholar]
- 17.Babakhani F, Gomez A, Robert N, Sears P. 2011. Killing kinetics of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. J Med Microbiol 60:1213–1217. doi: 10.1099/jmm.0.029470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babakhani F, Gomez A, Robert N, Sears P. 2011. Postantibiotic effect of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. Antimicrob Agents Chemother 55:4427–4429. doi: 10.1128/AAC.00104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koon HW, Ho S, Hing TC, Cheng M, Chen X, Ichikawa Y, Kelly CP, Pothoulakis C. 2014. Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum. Antimicrob Agents Chemother 58:4642–4650. doi: 10.1128/AAC.02783-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JM, Lee JY, Yoon YM, Oh YK, Youn J, Kim YJ. 2006. NF-kappa B activation pathway is essential for the chemokine expression in intestinal epithelial cells stimulated with Clostridium difficile toxin A. Scand J Immunol 63:453–460. doi: 10.1111/j.1365-3083.2006.001756.x. [DOI] [PubMed] [Google Scholar]
- 21.Castagliuolo I, Kelly CP, Qiu BS, Nikulasson ST, LaMont JT, Pothoulakis C. 1997. IL-11 inhibits Clostridium difficile toxin A enterotoxicity in rat ileum. Am J Physiol 273:G333–G341. [DOI] [PubMed] [Google Scholar]
- 22.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont JT. 1995. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest 95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. 1999. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun 67:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H, Kokkotou E, Na X, Rhee SH, Moyer MP, Pothoulakis C, Lamont JT. 2005. Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology 129:1875–1888. doi: 10.1053/j.gastro.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Hiwatashi Y, Maeda M, Fukushima H, Onda K, Tanaka S, Utsumi H, Hirano T. 2011. Azithromycin suppresses proliferation, interleukin production and mitogen-activated protein kinases in human peripheral-blood mononuclear cells stimulated with bacterial superantigen. J Pharm Pharmacol 63:1320–1326. doi: 10.1111/j.2042-7158.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- 26.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, Iuvone T, D'Acquisto F, Di Rosa M. 2000. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther 292:156–163. [PubMed] [Google Scholar]
- 27.Shinkai M, Foster GH, Rubin BK. 2006. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 290:L75–L85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- 28.Savidge TC, Pan WH, Newman P, O'Brien M, Anton PM, Pothoulakis C. 2003. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125:413–420. doi: 10.1016/S0016-5085(03)00902-8. [DOI] [PubMed] [Google Scholar]
- 29.Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter GP, Chakravorty A, Pham Nguyen TA, Mileto S, Schreiber F, Li L, Howarth P, Clare S, Cunningham B, Sambol SP, Cheknis A, Figueroa I, Johnson S, Gerding D, Rood JI, Dougan G, Lawley TD, Lyras D. 2015. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio 6:e00551-15. doi: 10.1128/mBio.00551-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jafari NV, Songane M, Stabler RA, Elawad M, Wren BW, Allan E, Bajaj-Elliott M. 2014. Host immunity to Clostridium difficile PCR ribotype 017 strains. Infect Immun 82:4989–4996. doi: 10.1128/IAI.02605-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flegel WA, Muller F, Daubener W, Fischer HG, Hadding U, Northoff H. 1991. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun 59:3659–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matarrese P, Falzano L, Fabbri A, Gambardella L, Frank C, Geny B, Popoff MR, Malorni W, Fiorentini C. 2007. Clostridium difficile toxin B causes apoptosis in epithelial cells by thrilling mitochondria. Involvement of ATP-sensitive mitochondrial potassium channels. J Biol Chem 282:9029–9041. doi: 10.1074/jbc.M607614200. [DOI] [PubMed] [Google Scholar]
- 34.Kim H, Rhee SH, Pothoulakis C, Lamont JT. 2007. Inflammation and apoptosis in Clostridium difficile enteritis is mediated by PGE2 up-regulation of Fas ligand. Gastroenterology 133:875–886. doi: 10.1053/j.gastro.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 35.Chumbler NM, Farrow MA, Lapierre LA, Franklin JL, Lacy DB. 2016. Clostridium difficile toxins TcdA and TcdB cause colonic tissue damage by distinct mechanisms. Infect Immun 84:2871–2877. doi: 10.1128/IAI.00583-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boaru SG, Borkham-Kamphorst E, Van de Leur E, Lehnen E, Liedtke C, Weiskirchen R. 2015. NLRP3 inflammasome expression is driven by NF-kappaB in cultured hepatocytes. Biochem Biophys Res Commun 458:700–706. doi: 10.1016/j.bbrc.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 37.Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, Stacey KJ. 2013. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, Armstrong GD, Tschopp J, Macdonald JA, Muruve DA, Beck PL. 2010. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 139:542–552, 552.e1–552.e3. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Gerhard R, Nottrott S, Schoentaube J, Tatge H, Olling A, Just I. 2008. Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J Med Microbiol 57:765–770. doi: 10.1099/jmm.0.47769-0. [DOI] [PubMed] [Google Scholar]
- 40.Desouza M, Gunning PW, Stehn JR. 2012. The actin cytoskeleton as a sensor and mediator of apoptosis. Bioarchitecture 2:75–87. doi: 10.4161/bioa.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gourlay CW, Ayscough KR. 2005. The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol Cell Biol 6:583–589. doi: 10.1038/nrm1682. [DOI] [PubMed] [Google Scholar]
- 42.Mullane KM, Miller MA, Weiss K, Lentnek A, Golan Y, Sears PS, Shue YK, Louie TJ, Gorbach SL. 2011. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis 53:440–447. doi: 10.1093/cid/cir404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SW, Choi AR, Lee HJ, Chung H, Park JC, Shin SK, Lee SK, Lee YC, Kim JE, Lee H. 2013. The effects of statins on the clinical outcomes of Clostridium difficile infection in hospitalised patients. Aliment Pharmacol Ther 38:619–627. doi: 10.1111/apt.12439. [DOI] [PubMed] [Google Scholar]
- 44.Saliba W, Barnett-Griness O, Elias M, Rennert G. 2014. Statins use and risk of mortality in patient with Clostridium difficile infection. Clin Microbiol Infect 20:1061–1066. doi: 10.1111/1469-0691.12672. [DOI] [PubMed] [Google Scholar]
- 45.Pothoulakis C, LaMont JT, Eglow R, Gao N, Rubins JB, Theoharides TC, Dickey BF. 1991. Characterization of rabbit ileal receptors for Clostridium difficile toxin A. Evidence for a receptor-coupled G protein. J Clin Invest 88:119–125. doi: 10.1172/JCI115267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O'Keane JC, Snider RM, Leeman SE. 1994. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci U S A 91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koon HW, Su B, Xu C, Mussatto CC, Tran DH, Lee EC, Ortiz C, Wang J, Lee JE, Ho S, Chen X, Kelly CP, Pothoulakis C. 2016. Probiotic Saccharomyces boulardii CNCM I-745 prevents outbreak-associated Clostridium difficile-associated cecal inflammation in hamsters. Am J Physiol Gastrointest Liver Physiol 311:G610–G623. doi: 10.1152/ajpgi.00150.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koon HW, Shih DQ, Hing TC, Chen J, Ho S, Zhao D, Targan SR, Pothoulakis C. 2011. Substance P induces CCN1 expression via histone deacetylase activity in human colonic epithelial cells. Am J Pathol 179:2315–2326. doi: 10.1016/j.ajpath.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.