ABSTRACT
Penicillins are widely used to treat infections in children; however, the evidence is continuing to evolve in defining the optimal dosing. Modern pediatric pharmacokinetic study protocols frequently favor opportunistic, “scavenged” sampling. This study aimed to develop a small-volume single assay for five major penicillins and to assess the influence of sample degradation on inferences made using pharmacokinetic modeling, to investigate the suitability of scavenged sampling strategies. Using a rapid ultrahigh-performance liquid chromatographic-tandem mass spectrometric method, an assay for five penicillins (amoxicillin, ampicillin, benzylpenicillin, piperacillin, and flucloxacillin) in blood plasma was developed and validated. Penicillin stabilities were evaluated under different conditions. Using these data, the impact of drug degradation on inferences made during pharmacokinetic modeling was evaluated. All evaluated penicillins indicated good stability at room temperature (23 ± 2°C) over 1 h, remaining in the range of 98 to 103% of the original concentration. More-rapid analyte degradation had already occurred after 4 h, with stability ranging from 68% to 99%. Stability over longer periods declined: degradation of up to 60% was observed with delayed sample processing of up to 24 h. Modeling showed that analyte degradation can lead to a 30% and 28% bias in clearance and volume of distribution, respectively, and falsely show nonlinearity in clearance. Five common penicillins can now be measured in a single low-volume blood sample. Beta-lactam chemical instability in plasma can cause misleading pharmacokinetic modeling results, which could impact upon model-based dosing recommendations and the forthcoming era of beta-lactam therapeutic drug monitoring.
KEYWORDS: beta-lactams, drug stability, high-pressure liquid chromatography, mass spectrometry, penicillins, pharmacokinetics, scavenged sampling
INTRODUCTION
Penicillins have been widely used in both children (1) and adults for over 50 years and arguably remain the most important group of antibiotics. There is still considerable variation in the rate of antimicrobial prescribing in different countries (2) and in the doses used (3). Even for very common antibiotics, such as the penicillins, there remains a marked lack of information about optimal dosing (4). Pharmacokinetic (PK) variability in children can arise from the physiological changes related to growth and organ maturation, and also due to pathophysiology, especially in critical illness. Selecting the best antimicrobial dose to use in children is challenging because of this extensive variability in patients' pharmacokinetics (5). As a result, a drug's PK profile can be unpredictable (6), which in the case of antibiotics can lead to subtherapeutic concentrations (7), with associated treatment failure, and the possible emergence of antimicrobial resistance (AMR) (8). Identifying the optimum dosing regimens is thus key to improving therapeutic outcomes, reducing toxicity (9), and limiting the development of AMR. Given the wide use of beta-lactam antibiotics in clinical practice, the knowledge of PK variability has led researchers to question whether there is a potential role for beta-lactam therapeutic drug monitoring (TDM) to help individualize dosing strategies in an intensive care unit (ICU) setting (6).
Penicillins are unstable β-lactam antimicrobials, and spontaneous degradation of these drugs occurs. Diverse degradation pathways caused by the β-lactam group are common to all penicillins, yet they vary with storage conditions, resulting in different degradation products (10). One of the main challenges when determining the pharmacokinetics of these drugs is how to maintain their stability in biological samples under various storage conditions. The instability of penicillins, which is already well established, can result from the β-lactam ring opening under acidic and basic conditions, from enzymatic (hydrolysis and aminolysis) degradation, from degradation by the presence of metal ions, and from temperature changes (10). Therefore, detailed stability (ST%) studies during bioanalytical assay validation are crucial. The most important stability from the clinical study perspective is the short-term stability, taking into account possible delays and interferences during the sampling and sample handling in the hospital (both at the bedside and also when transporting the sample from the patient to the laboratory).
Importantly, opportunistic or so-called “scavenged” sampling techniques are being used increasingly in pediatric PK studies as a sparse sampling methodology (11, 12). These approaches mean that the blood samples from patients are initially processed in the same way as routine blood samples required for clinical care before being processed and stored specifically for antibiotic quantification, the results of which are then used for pharmacokinetic analysis. The use of scavenged sampling itself, which incorporates processing delays into the standard operating procedures, thus might influence the PK analysis results in the case of chemically unstable drugs. Understanding the impact of such delays on both laboratory and pharmacometric analysis is particularly important as opportunistic, scavenged sampling methods are now being further advocated specifically for neonatal pharmacokinetic studies (13).
Numerous simultaneous bioanalytical methods have been developed over the years for beta-lactam antibiotics (14–22), with evidence of increased interest recently (16, 18, 20). The number of simultaneously determined drugs varies (maximum, 21 [17]), and the required sample volumes range from as little as 20 μl (21) and 50 μl (14, 16, 18, 19, 22) to 1 ml (17).
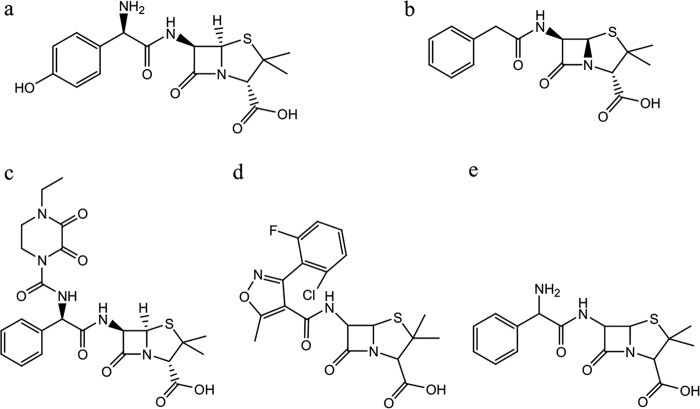
The aim of this laboratory study was to develop and validate a bioanalytical method for measuring penicillins in small-volume plasma samples from pediatric and neonatal patients, in order to use the data for population PK modeling and dose optimization studies (2, 23). The influence of sample degradation on measured concentrations was then studied using pharmacokinetic modeling in order to evaluate the suitability of scavenged sampling strategies for the penicillins as chemically unstable drugs. The penicillins studied included amoxicillin, ampicillin, benzylpenicillin (penicillin G), piperacillin, and flucloxacillin (Fig. 1). The bioanalytical methods were developed for the Neonatal and Pediatric Pharmacokinetics of Antimicrobials study (NAPPA, EudraCT 2013-002366-40, NCT01975493) (24).
FIG 1.
Chemical structures of amoxicillin (a), penicillin G (b), piperacillin (c), flucloxacillin (d), and ampicillin (e).
RESULTS
LC-MS/MS method development.
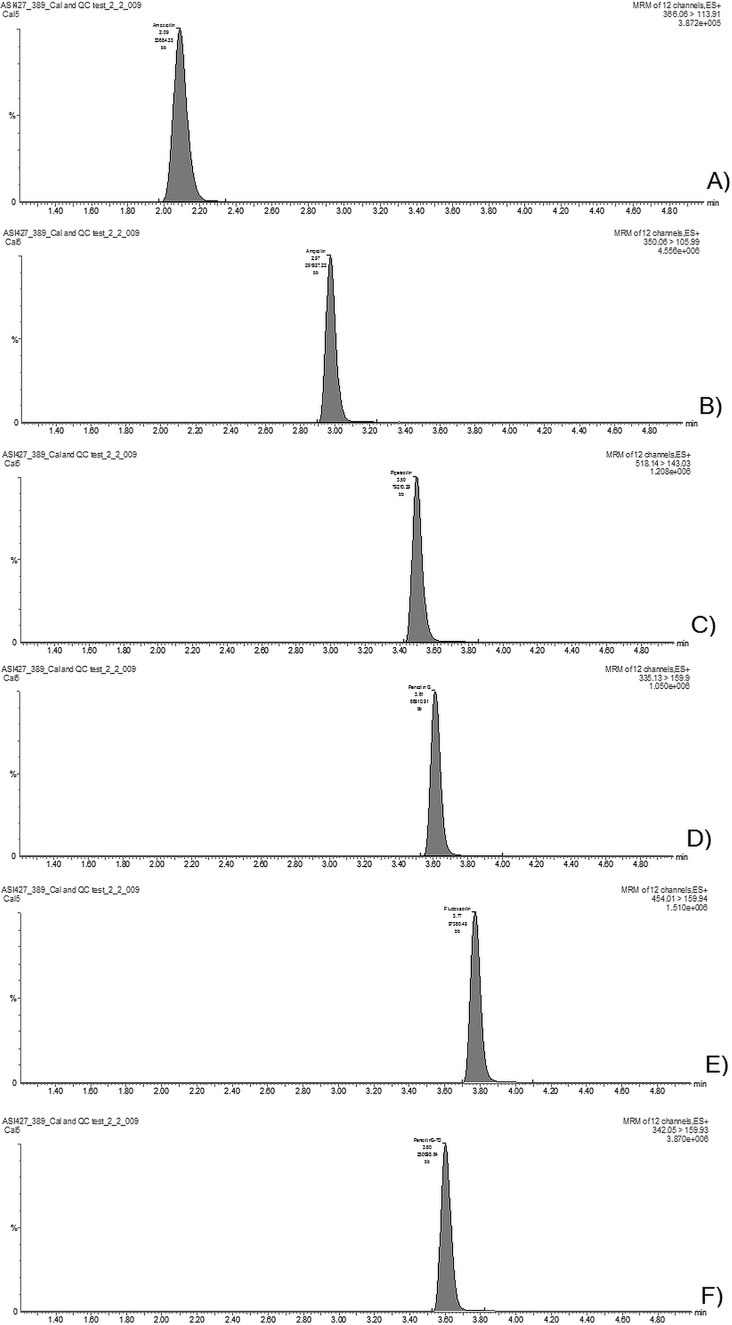
The liquid chromatography-tandem mass spectrometric (LC-MS/MS) assay was developed to simultaneously quantify the concentrations of 5 major penicillins in blood plasma: amoxicillin, ampicillin, benzylpenicillin, piperacillin, and flucloxacillin. Chromatographic separation was achieved within 4 min for all analytes (a representative chromatogram from a sample with a concentration of 25 mg/liter is shown in Fig. 2).
FIG 2.
Chromatograms in the plasma sample with a concentration of 25 mg/liter for amoxicillin (A), ampicillin (B), piperacillin (C), penicillin G (D), flucloxacillin (E), and penicillin G-D7 (IS) (F).
Method validation and selectivity.
The method was fully validated according the 2011 edition of the European Medicines Agency's Guideline on Bioanalytical Method Validation (25). Selectivity was evaluated by analyzing blood plasma samples from six different sources.
LLOQ.
For all analytes, the lower limit of quantification (LLOQ) was 0.1 mg/liter. The within-day accuracy for amoxicillin ranged from 98 to 106%, for ampicillin from 105 to 110%, for penicillin G from 105 to 108%, for piperacillin from 97 to 106%, and for flucloxacillin from 96 to 105% at the LLOQ level. Within-day precision for amoxicillin ranged from 2.5 to 5.4%, for ampicillin from 2 to 3.5%, for penicillin G from 4.6 to 10.6%, for piperacillin from 2.9 to 4.2%, and for flucloxacillin from 4.3 to 11.7% at the LLOQ level.
The signal-to-noise ratio remained higher than 5 for all analytes, ranging from 89 to 251.
Calibration, carry-over, matrix effects, accuracy, and precision.
Nine calibration concentration levels (0.1, 0.5, 1, 5, 25, 60, 100, 150, and 200 mg/liter) were used to compose the matrix-matched calibration lines; in addition, the calibrators of the blank sample (processed using an internal standard [IS]) and the double-blank sample (processed without internal standard) were analyzed. All samples were analyzed in duplicates. Back-calculated concentrations using linear regression fitting with 1/x2 weighting (Table 1) ranged from 96 to 110% for all analytes in all concentration levels from 0.1 mg/liter to 200 mg/liter.
TABLE 1.
Calibration line parameters for all analytesa
| Analyte | Slope | Intercept | r2 |
|---|---|---|---|
| Amoxicillin | 0.1120 | 0.0087 | 0.9997 |
| Ampicillin | 0.5005 | 0.0008 | 0.9998 |
| Penicillin G | 0.0241 | 0.0042 | 0.9996 |
| Piperacillin | 0.0447 | 0.0018 | 0.9999 |
| Flucloxacillin | 0.0192 | 0.0012 | 0.9992 |
Weighting was 1/x2 for all analytes. r2, coefficient of determination.
Carry-over (see the Supplemental text in the supplemental material) was considered acceptable for all analytes and the IS. Matrix effects (Supplemental text) and the calibration (Table 1) were evaluated for all analytes. Matrix effects ranged from 96 to 107.6% for all penicillins.
Accuracy and precision were tested in four different concentrations (in 5 replicates): 0.1 mg/liter (as the LLOQ), 0.5 mg/liter as the low concentration, 50 mg/liter as the medium concentration, and 150 mg/liter as the high concentration.
Within-run and between-run assay accuracies for all analytes ranged from 1.4% to 10.5% at the LLOQ concentration and from 0.3% to 8.8% for the low, medium, and high concentrations, respectively (Tables 2 and 3). Within-run assay precisions for all analytes ranged from 2.0 to 6.1% at the LLOQ concentration and from 2.0% to 5.4% for low, medium, and high concentrations (Table 2). Between-run assay precision for all analytes ranged from 1.6 to7.3% at the LLOQ concentration and from 1.3% to 7.4% for low, medium, and high concentrations (Table 3).
TABLE 2.
Within-run assay precision and accuracy (% mean deviation from the nominal concentration) (n = 5)
| Analyte | Accuracy (%) |
Precision (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| LLOQ (0.1 mg/liter) | Low (0.5 mg/liter) | Medium (50 mg/liter) | High (150 mg/liter) | LLOQ (0.1 mg/liter) | Low (0.5 mg/liter) | Medium (50 mg/liter) | High (150 mg/liter) | |
| Amoxicillin | −6.6 | 6.8 | −3.3 | −4.7 | 6.1 | 5.4 | 3.1 | 3.8 |
| Ampicillin | 5.1 | 5.6 | 0.3 | −3.9 | 3.4 | 2.0 | 2.5 | 3.8 |
| Penicillin G | 4.7 | 8.8 | 1.5 | 6.6 | 2.0 | 5.0 | 3.7 | 3.3 |
| Piperacillin | 1.4 | −2.2 | 2.4 | −3.5 | 2.7 | 3.0 | 4.4 | 3.5 |
| Flucloxacillin | 2.1 | 5.2 | 1.2 | −5.1 | 4.6 | 4.4 | 2.4 | 3.1 |
TABLE 3.
Between-run assay precision and accuracy (n = 3)
| Analyte | Accuracy (%) |
Precision (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| LLOQ (0.1 mg/liter) | Low (0.5 mg/liter) | Medium (50 mg/liter) | High (150 mg/liter) | LLOQ (0.1 mg/liter) | Low (0.5 mg/liter) | Medium (50 mg/liter) | High (150 mg/liter) | |
| Amoxicillin | −10.5 | −0.7 | 1.8 | 1.2 | 7.3 | 5.1 | 5.6 | 2.5 |
| Ampicillin | 7.6 | 2.8 | 1.8 | −1.4 | 2.3 | 5.3 | 5.6 | 1.3 |
| Penicillin G | 7.5 | 4.8 | −2.6 | −3.2 | 1.6 | 5.7 | 5.7 | 2.6 |
| Piperacillin | 1.8 | 5.7 | 1.5 | −3.7 | 4.1 | 4.7 | 7.4 | 3.2 |
| Flucloxacillin | 2.2 | 5.7 | 1.5 | −1.3 | 4.7 | 5.0 | 7.0 | 2.2 |
Stability. (i) Short-term stability of analytes in blood plasma at room temperature.
Short-term stability data of the penicillin-containing plasma samples when stored at room temperature (23 ± 2°C) for 24 h indicated degradation of flucloxacillin, piperacillin, and penicillin G in the plasma samples (at low, medium, and high concentrations), since only 40 to 63%, 52 to 64%, and 66 to 70%, respectively, of the drug was detectable after applying room temperature as a stress condition. Ampicillin and amoxicillin, however, had slightly better stability on the bench-top, with 89 to 96% and 71 to 89%, respectively, of the drugs detectable after 24 h at room temperature.
In addition, short-term stability was tested over a 4-h period at room temperature, which also indicated compound degradation: flucloxacillin, piperacillin, and penicillin G maintained 68 to 80%, 83 to 89%, and 89 to 95% of their original concentration. Both ampicillin and amoxicillin remained in the range of 95 to 98% and 96 to 99% after 4 h at the room temperature.
All penicillins indicated good stability at room temperature over 1 h, remaining in the range of 98 to 103% from the original concentration.
(ii) Autosampler stability.
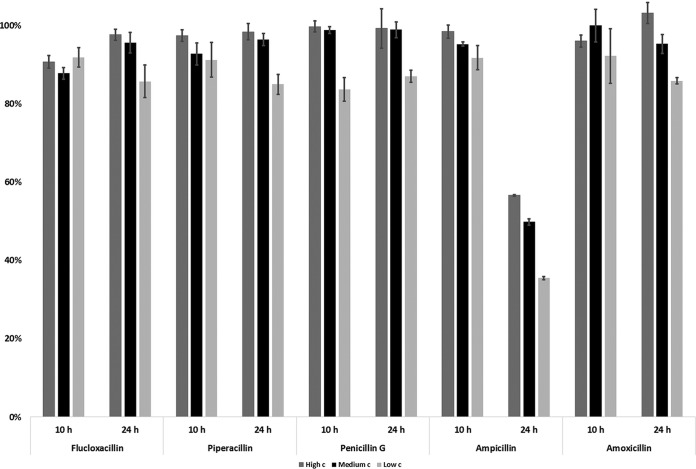
Rapid degradation of ampicillin occurred in the samples kept in the cooled (+10°C) autosampler for 24 h: only 35 to 57% of the original drug concentration remained in the samples (Fig. 3). However, all other penicillins maintained 85 to 99% of their original content. In order to improve the autosampler stability, rapid analysis of beta-lactams is required. Therefore, ampicillin stability was tested over shorter time periods. Within a 10-hour period, approximately 92 to 96% of the original concentration of ampicillin remained in the samples stored in the autosampler in five replicates.
FIG 3.
Average autosampler stability of penicillins over 10 and 24 h (n = 5); error bars represent ±1 standard deviation.
(iii) Freeze-thaw stability.
The freeze-thaw stability of ampicillin also indicated some degradation of the compound in plasma samples, since 82 to 99% of the original content remained in the plasma samples after 3 freeze-thaw cycles, while the other penicillins maintained approximately 98 to 100% of their original content in the plasma samples.
(iv) Long-term stability.
Long-term stability of analytes in the plasma samples was evaluated over a 6-month time period at −80°C. All penicillins remained within the range of 95 to 104% of their original content at low, medium, and high concentrations (tested in five aliquots of the same sample, i.e., n = 5).
PK modeling and simulations results.
Table 4 gives the estimated piperacillin clearance (CL) at different dose levels assuming various levels of degradation using the doses reported by Landersdorfer et al. (26) (3,000 mg and 1,500 mg), the current usual dose (4,000 mg), and 400 mg for illustration of a 10-fold range.
TABLE 4.
Effect of sample degradation on potential conclusion of nonlinear piperacillin CLa
| Dose (mg) | CL (liters/h) after delay in sample processing of: |
|||||
|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 4 h | 8 h | 24 h | |
| 4,000 | 10.9 | 11.1 | 11.2 | 11.6 | 12.3 | 15.5 |
| 3,000 | 10.9 | 11.1 | 11.3 | 11.7 | 12.6 | 16.4 |
| 1,500 | 10.9 | 11.2 | 11.4 | 11.9 | 12.9 | 18.1 |
| 400 | 10.9 | 11.2 | 11.5 | 12.1 | 13.5 | 20.4 |
A simulated CL of 11.0 liters/h was used for all doses (assumed linear pharmacokinetics).
DISCUSSION
A simultaneous method for measuring amoxicillin, ampicillin, benzylpenicillin, piperacillin, and flucloxacillin was developed. To the best of our knowledge, the combination of these five penicillins using sample volumes as low as 50 μl in a single LC-MS assay has not previously been reported, although other simultaneous beta-lactam LC-MS assays have been described (15, 17, 21, 22). As the associated clinical study (NAPPA) was investigating penicillin pharmacokinetics in neonatal and pediatric patients, the main challenge of the method development was the restricted sample volume in this patient population. Knowing the stability issues of beta-lactam antibiotics, sample preparation was done rapidly using protein precipitation and dilution afterwards to avoid unnecessary contamination of the LC-MS system from the high content of sample matrix.
Using data from the stability studies performed during method development, the impact of analyte degradation was assessed, and we have shown, using piperacillin as an example drug, that inferences made during PK modeling may be biased if analytes have degraded within clinical samples. As scavenged sampling methods are increasingly recommended for neonatal pharmacokinetic studies, these findings are of clear significance and serve as an important reminder of the importance of tailoring scavenged sampling protocols in future pediatric pharmacokinetic studies according to each specific analyte's stability. Furthermore, given the growing interest in the use of TDM for beta-lactams (6) to optimize pharmacotherapy in clinical practice, these findings have further implications for TDM sampling protocols.
Rapid and simultaneous multidrug assays are the key for successful TDM services that need to be delivered in a timely manner (22). This novel assay, which has both good accuracy and good precision, also benefits from rapid sample preparation and decreased matrix effects compared to previous assays (15), even when more-complex and cleaner sample preparation was used. The lower matrix effects obtained were most likely due to the appropriate dilution during sample preparation.
One of the key validation parameters for beta-lactams is stability, for both short and long terms. Despite previous recognition of both the instability of beta-lactams at room temperature and the importance of preanalytical stability (27), there is a persisting lack of stability data in published assay validations (17, 22). Our results indicated more-rapid degradation in plasma samples with EDTA for piperacillin and amoxicillin than reported in the literature previously for lithium-heparinized tubes and tubes without the gel separator (27). Another study (15) indicated slightly better stability over 4 h at room temperature for penicillin G, piperacillin, and flucloxacillin without mentioning the anticoagulant used for plasma. Importantly, beyond assay validation, we then sought to interrogate the chemical stability data to understand in a quantifiable way its potential impact on the output from pharmacokinetic modeling, which when used for model-based dosing recommendations could have direct clinical implications in future.
From the results of the pharmacokinetic modeling, it can be seen that in the absence of sample degradation, the estimated CL was close to the simulated value, and while CL did increase with increasing processing time, it was not until samples were left for 24 h that degradation started to falsely show nonlinearity. It should be noted, however, that the nonlinear CL reported by Landersdorfer et al. (26) included data on urinary piperacillin. Since we did not test piperacillin stability in urine, it is still possible that more-rapid urinary piperacillin degradation coupled to longer times between sample collection inherent in urinary pharmacokinetic studies may enhance an apparent nonlinear pharmacokinetic effect.
In the assessment of scavenged sampling, the estimates for CL and volume of distribution (V) were 11.3 liters/h and 12.3 liters, when sample degradation was assumed not to have occurred. While for a more-complex 2-compartment pharmacokinetic model, scavenged sampling has been shown to bias parameter estimates and should therefore generally not be preferred over optimally timed samples (28), this result indicates that for this simple 1-compartment model the scavenged design could potentially work if samples were processed immediately. It should be noted that truly immediate processing is rarely possible in a clinical environment, however, when it comes to so-called “scavenged” samples. Further, when degradation was allowed by having samples processed between 4 and 24 h postcollection (representing lengths of delays that can occur when samples are scavenged from the laboratory), the CL and V were 14.3 liters/h and 15.4 liters, respectively. This represents a 30% and 28% bias in CL and V, which could potentially cause unnecessarily high doses to be recommended on the basis of pharmacometric (PK model-based) analyses. Indeed, the authors of the scavenged sampling study (11) acknowledge this potential problem, and further penicillin studies using scavenged samples require accurate recording of sample processing times so that degradation can be accounted for during pharmacokinetic modeling. Nonetheless, for future beta-lactam TDM methods, we have demonstrated the acceptability of storage for up to 1 h at room temperature for plasma samples collected from patients, which is very promising, and this should make the TDM standard operating procedures both realistic and acceptable in the context of a busy clinical setting.
To our knowledge, this is the first study to use data obtained during bioanalytical method validation to demonstrate how the use of scavenged sampling methods could impact significantly upon the results of pharmacokinetic modeling. This is relevant when dealing with unstable analytes present in patient samples destined for drug quantification assays, whether these be samples for pediatric pharmacokinetic research using opportunistic sampling strategies or samples from ICU patients intended for TDM. These findings underscore the importance of a detailed evaluation of the stability of beta-lactam antibiotics in specific matrices during the bioanalytical method validation. This not only is important for planning the laboratory workflow but also should be considered all the way from bedside to bench, with consideration of sample collection methods, transportation, and storage. For researchers developing dosing guidance using pharmacokinetic analyses of such data, the instability of these analytes can cause significant bias in the prediction of pharmacokinetic parameters. With the implementation of novel TDM initiatives based on individualized pharmacokinetic profiles and forecasting, this could lead to suboptimal dosing recommendations, which could adversely affect clinical outcomes. However, when carefully evaluated, the instability can be accounted for when creating pharmacokinetic models. This work highlights the importance of continued close collaboration between bioanalytical chemists, pharmacometricians, and clinicians when developing novel TDM protocols. For future work, we would recommend that the importance of strict sampling handling procedures be incorporated into all standard operating procedures for beta-lactam TDM methods, to ensure that our knowledge of beta-lactam stability is fully embedded in forthcoming innovative dose individualization strategies. A potentially simple way to overcome these challenges is through the use of point-of-care methods for beta-lactam quantification at the bedside, which if economically viable, would present an ideal solution.
MATERIALS AND METHODS
Instrumentation.
Chromatographic separation and mass-spectrometric detection of five analytes were carried out using a Waters Acquity ultrahigh-performance liquid chromatography (UPLC) system equipped with a Waters TQ detector (Waters, Milford, MA, USA). The UPLC system consists of a binary solvent manager, a sample manager, and a column thermostat. Electrospray ionization (ESI)-MS detection was carried out in the positive-ion detection mode. The UPLC-MS/MS instrument was controlled by Waters MassLynx software version 4.1 (Waters, Milford, MA, USA). Data analysis was carried out using TargetLynx software version 4.1 (Waters, Milford, MA, USA).
Chemicals.
Pharmaceuticals, i.e., ampicillin, amoxicillin, flucloxacillin, penicillin G, and piperacillin, and an internal standard (IS), i.e., penicillin G-D7, N-ethylpiperidinium salt, and LC-MS grade methanol and formic acid were purchased from Sigma (St. Louis, MO, USA). Acetonitrile was obtained from Rathburn Chemicals Ltd. (Walkerburn, Scotland). Water was purified (18.2 MΩ · cm at 25°C and a total organic carbon [TOC] value of <3 ppb) in-house using a Millipore Advantage A10 system from Millipore (Bedford, MA, USA). Blood plasma with EDTA was obtained from the Biological Specialties Corporation (Colmar, PA, USA).
Sample preparation.
Plasma samples were kept at −80°C for storage. For analysis, they were removed from the freezer and kept at room temperature for thawing. Once at room temperature, matrix-matched calibrators and quality control samples were mixed on an IKA Vibrax VXR mixer (Esslab, Essex, England). For 50 μl of each calibrator and quality control sample, 200 μl of internal standard (penicillin G-D7) in acetonitrile (stored at −20°C, prepared once every 2 weeks) was added. Samples were mixed on the IKA Vibrax VXR for 5 min and centrifuged at 13,500 × g for 5 min. A 1,000-μl volume of deionized water was added to 100 μl of supernatant, and then the samples were vortexed for 10 s. A 10-μl volume of the sample was injected into the LC-MS/MS system.
Liquid chromatography-mass spectrometry.
For the chromatographic separation of penicillins, 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in methanol (mobile phase B) with gradient elution and a reversed-phase analytical column (50 mm by 2.1 mm by 1.7 μm; Acquity UPLC BEH C18) were used. Separation was obtained using the gradient program starting from 5% of mobile phase B for the first 1.2 min (directed to the waste), after which mobile phase B content was raised to 100% over 2.3 min and kept at 100% for 1 min and thereafter lowered again to 5% over 0.2 min and then kept at 5% of mobile phase B for 1.8 min. Eluent flow rate was 0.25 ml/min. ESI interface was in use for the mass-spectrometric detection in the positive multiple-reaction monitoring (MRM) mode for detection of penicillins. Triple quadrupole detector transitions were used for quantification and qualification, as follows: m/z 335 [M + 1]; → m/z 160; 176 (for penicillin G); m/z 350 [M + 1] → m/z 160; 106 (for ampicillin); m/z 366 [M + 1] → m/z 114; 208 (for amoxicillin); m/z 518 [M + 1] → m/z 143; 160 (for piperacillin); m/z 454 [M + 1] → m/z 160; 295 (for flucloxacillin), and m/z 342 [M + 1] → m/z 160 (for penicillin G-D7, IS).
Optimized parameters for ESI and MS were used with capillary voltage of 0.8 kV, cone voltage of 31 V, source temperature of 120°C, desolvation gas temperature of 350°C, desolvation gas flow rate of 800 liters/h, and cone gas flow rate of 30 liters/h.
Stability experiments.
Short-term stability data of the penicillin-containing plasma samples were obtained by storing the quality control samples (at low, medium, and high concentrations) at room temperature (23 ± 2°C) for 4 h and 24 h and comparing the results obtained after these storage periods with the analysis performed at the starting time (time 0 h) of the analysis. The freeze-thaw stability of penicillins was evaluated in low- and high-concentration plasma samples (each in 5 replicates) after 3 freeze-thaw cycles. Plasma samples were kept frozen at −80°C and thawed, keeping them at room temperature (23 ± 2°C) for 1 h. Thereafter, samples were refrozen and thawed again after 24 h. Long-term stability of analytes in the plasma samples was evaluated for storage over a 6-month time period at −80°C (each tested in 5 aliquots of the sampled plasma sample, i.e., n = 5). Autosampler stability at the temperature of +10°C was evaluated over 24 h.
Each time, freshly prepared calibration solutions were measured and the concentration in quality control samples was calculated. All penicillins were present in the same sample over the time of storage. Stability of the analyte was evaluated by the following formula: ST% = c0/ct, where c0 is the initial concentration, determined without introducing any extra pauses in the analysis process, and ct is the concentration obtained when analysis is carried out with a pause of duration t in the analysis.
Pharmacokinetic modeling and simulations.
To assess the impact of drug degradation on inferences made during pharmacokinetic modeling, data from the stability tests were used. The slope of the log concentration with time was estimated for each initial concentration of each drug using linear regression in the statistical software R version 3.1.0 (29). Piperacillin was chosen as the model drug to assess the impact of sample degradation on two aspects of pharmacokinetic modeling results: reported nonlinearity in clearance (CL) and the utility of laboratory-scavenged samples.
To investigate whether more-rapid sample degradation at lower concentrations could account for observed nonlinear pharmacokinetics, concentration-time data were simulated using NONMEM version 7.3. The 1,500-mg and 3,000-mg doses described by Landersdorfer et al. (26) were used. This study estimated a 3-compartment model using NONMEM version 7.3 (30) and found nonlinear clearance with 3,000 mg, yielding a value of 11.0 liters/h, whereas 1,500 mg yielded a CL of 13.5 liters/h. In this study, the renal component of CL, estimated by urinary piperacillin excretion, decreased 24% with a doubling of dose. Simulated data were adjusted according to the degradation rate constants estimated above (with linear extrapolations made to account for changing rate constant with concentration) to assume that samples were left for 1, 2, 4, 8, and 24 h postcollection before freezing, in keeping with known scavenged sampling protocols. Using this adjusted data, CL was recalculated using a noncompartmental pharmacokinetic estimation of the area under the plasma drug concentration-time curve from 0 h to infinity [AUC(0–∞)]. Under the hypothesis of linear CL, the PK profiles for 4,000, 1,500, and 400 mg were also simulated and the degradation adjustment discussed above was made to the simulated concentrations in order to assess whether more-rapid degradation of lower concentrations could yield apparent nonlinear CL upon reestimation.
To investigate the effects of laboratory-scavenged samples (any excess from routine clinical samples assayed for piperacillin when reaching the lab), as reported by Cohen-Wolkowiez et al. (11), the above-described model was simplified to a one-compartment structure with CL of 11 liters/h and volume of distribution (V) of 12 liters. Fifty simulated subjects received 3,000 mg every 8 hours over a 32-hour interval (i.e., 4 dose intervals), and 4 random sample times within this interval were simulated from a uniform distribution. The simplified model was used to simulate concentration-time profiles, assuming interindividual variability on CL and V to be 30% and proportional residual variability to be 10%. The simulated concentrations were then adjusted according to the procedure described above to assume degradation. The time of processing for each sample was randomly generated from a uniform distribution with an interval of 4 to 24 h. Pharmacokinetic parameters were then estimated from the adjusted and unadjusted data sets to assess potential bias caused by degradation.
Supplementary Material
ACKNOWLEDGMENTS
The technical assistance of Hua Xu and support from the Analytical Services International Ltd. were much appreciated throughout this study.
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under REA grant agreement number 608765. This work was also supported by PUTJD 22 from the Estonian Research Council. C.I.S.B. was funded as a Clinical Research Fellow by the Global Research in Pediatrics (GRiP) Network of Excellence, part of the European Union's Seventh Framework Programme for research, technological development, and demonstration (FP7/2007–2013, grant agreement number 261060), which also funded the NAPPA study (EudraCT 2013-002366-40, NCT01975493) (24). M.S. chairs the UK Department of Health Expert Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection, is an independent scientific advisor to NICE (National Institute for Health and Care Excellence), and also receives institutional academic research grants from the NIHR (National Institute for Health Research) and the European Union. J.F.S. has received funding from United Kingdom Medical Research Council Fellowships (grants G1002305 and M008665). C.I.S.B. and J.F.S. have been supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01540-17.
REFERENCES
- 1.Barker CI, Germovsek E, Sharland M. 2016. What do I need to know about penicillin antibiotics? Arch Dis Child Educ Pract Ed 102:44–50. doi: 10.1136/archdischild-2015-309068. [DOI] [PubMed] [Google Scholar]
- 2.Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, ARPEC project group. 2016. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother 71:1106–1117. doi: 10.1093/jac/dkv418. [DOI] [PubMed] [Google Scholar]
- 3.Metsvaht T, Nellis G, Varendi H, Nunn AJ, Graham S, Rieutord A, Storme T, McElnay J, Mulla H, Turner MA, Lutsar I. 2015. High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: implications for research and dissemination. BMC Pediatr 15:41. doi: 10.1186/s12887-015-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker CI, Standing JF, Turner MA, McElnay JC, Sharland M. 2012. Antibiotic dosing in children in Europe: can we grade the evidence from pharmacokinetic/pharmacodynamic studies—and when is enough data enough? Curr Opin Infect Dis 25:235–242. doi: 10.1097/QCO.0b013e328353105c. [DOI] [PubMed] [Google Scholar]
- 5.Zuppa A, Barrett J. 2008. Pharmacokinetics and pharmacodynamics in the critically ill child. Pediatr Clin North Am 55:735–755. doi: 10.1016/j.pcl.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Huttner A, Harbarth S, Hope WW, Lipman J, Roberts JA. 2015. Therapeutic drug monitoring of the beta-lactam antibiotics: what is the evidence and which patients should we be using it for? J Antimicrob Chemother 70:3178–3183. doi: 10.1093/jac/dkv201. [DOI] [PubMed] [Google Scholar]
- 7.De Cock PA, Standing JF, Barker CI, de Jaeger A, Dhont E, Carlier M, Verstraete AG, Delanghe JR, Robays H, De Paepe P. 2015. Augmented renal clearance implies a need for increased amoxicillin-clavulanic acid dosing in critically ill children. Antimicrob Agents Chemother 59:7027–7035. doi: 10.1128/AAC.01368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rees VE, Bulitta JB, Nation RL, Tsuji BT, Sorgel F, Landersdorfer CB. 2015. Shape does matter: short high-concentration exposure minimizes resistance emergence for fluoroquinolones in Pseudomonas aeruginosa. J Antimicrob Chemother 70:818–826. doi: 10.1093/jac/dku437. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande AD. 2004. Degradation of β-lactam antibiotics. Curr Sci 87:1684–1695. [Google Scholar]
- 11.Cohen-Wolkowiez M, Ouellet D, Smith PB, James LP, Ross A, Sullivan JE, Walsh MC, Zadell A, Newman N, White NR, Kashuba AD, Benjamin DKJ. 2012. Population pharmacokinetics of metronidazole evaluated using scavenged samples from preterm infants. Antimicrob Agents Chemother 56:1828–1837. doi: 10.1128/AAC.06071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laughon MM, Benjamin DKJ, Capparelli EV, Kearns GL, Berezny K, Paul IM, Wade K, Barrett J, Smith PB, Cohen-Wolkowiez M. 2011. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol 4:643–652. doi: 10.1586/ecp.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leroux S, Turner MA, Guellec C, Hill H, van den Anker JN, Kearns GL, Jacqz-Aigrain E, Zhao W. 2015. Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet 54:1273–1285. doi: 10.1007/s40262-015-0291-1. [DOI] [PubMed] [Google Scholar]
- 14.Ohmori T, Suzuki A, Niwa T, Ushikoshi H, Shirai K, Yoshida S, Ogura S, Itoh Y. 2011. Simultaneous determination of eight β-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B 879:1038–1042. doi: 10.1016/j.jchromb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Sime F, Roberts M, Roberts J, Robertson T. 2014. Simultaneous determination of seven beta-lactam antibiotics in human plasma for therapeutic drug monitoring and pharmacokinetic studies. J Chromatogr B 960:134–144. doi: 10.1016/j.jchromb.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Abdulla A, Bahmany S, Wijma R, van der Nagel B, Koch B. 2017. Simultaneous determination of nine β-lactam antibiotics in human plasma by an ultrafast hydrophilic-interaction chromatography-tandem mass spectrometry. J Chromatogr B 1060:138–143. doi: 10.1016/j.jchromb.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Cazorla-Reyes R, Romero-González R, Frenich AG, Maresca MAR, Vidal JLM. 2014. Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 89:203–212. doi: 10.1016/j.jpba.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Hu Z, Laizure S, Hudson J. 2017. Simultaneous assay of multiple antibiotics in human plasma by LC-MS/MS: importance of optimizing formic acid concentration. Bioanalysis 5:469–483. doi: 10.4155/bio-2016-0157. [DOI] [PubMed] [Google Scholar]
- 19.Barco S, Bandettini R, Maffia A, Tripodi G, Castagnola E, Cangemi G. 2015. Quantification of piperacillin, tazobactam, meropenem, ceftazidime, and linezolid in human plasma by liquid chromatography/tandem mass spectrometry. J Chemother 27:343–347. doi: 10.1179/1973947814Y.0000000209. [DOI] [PubMed] [Google Scholar]
- 20.Rigo-Bonnin R, Ribera A, Arbiol-Roca A, Cobo-Sacristan S, Padulles A, Murillo O, Shaw E, Granada R, Perez-Fernandez X, Tubau F, Alia P. 2017. Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of β-lactam antibiotic concentration in human plasma. Clin Chim Acta 468:215–224. doi: 10.1016/j.cca.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Carlier M, Stove V, Roberts JA, Van de Velde E, De Waele JJ, Verstraete AG. 2012. Quantification of seven beta-lactam antibiotics and two beta-lactamase inhibitors in human plasma using a validated UPLC-MS/MS method. Int J Antimicrob Agents 40:416–422. doi: 10.1016/j.ijantimicag.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Colin P, De Bock L, T'jollyn H, Boussery K, Van Bocxlaer J. 2013. Development and validation of a fast and uniform approach to quantify β-lactam antibiotics in human plasma by solid phase extraction-liquid chromatography–electrospray-tandem mass spectrometry. Talanta 103:285–293. doi: 10.1016/j.talanta.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 23.Barker CI, Germovsek E, Hoare RL, Lestner JM, Lewis J, Standing JF. 2014. Pharmacokinetic/pharmacodynamic modelling approaches in paediatric infectious diseases and immunology. Adv Drug Deliv Rev 73:127–139. doi: 10.1016/j.addr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US National Institutes of Health. 2016. Neonatal and Paediatric Pharmacokinetics of Antimicrobials Study (NAPPA). A service of the U.S. National Institutes of Health. https://clinicaltrials.gov/ct2/show/NCT01975493 Accessed 6 December 2016.
- 25.EMA. 2011. European Medicines Agency: guideline on bioanalytical method validation. http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500109686&murl=menus/document_library/document_library.jsp&mid=WC0b01ac058009a3dc Accessed 15 August 2016.
- 26.Landersdorfer CB, Bulitta JB, Kirkpatrick CM, Kinzig M, Holzgrabe U, Drusano GL, Stephan U, Sorgel F. 2012. Population pharmacokinetics of piperacillin at two dose levels: influence of nonlinear pharmacokinetics on the pharmacodynamic profile. Antimicrob Agents Chemother 56:5715–5723. doi: 10.1128/AAC.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlier M, De Waele JJ, Verstraete AG, Stove V. 2015. Exploration of the pre-analytical stability of beta-lactam antibiotics in plasma and blood—implications for therapeutic drug monitoring and pharmacokinetic studies. Clin Chem Lab Med 53:e227–230. doi: 10.1515/cclm-2014-0833. [DOI] [PubMed] [Google Scholar]
- 28.Standing JF, Anderson BJ, Holford NH, Lutsar I, Metsvaht T. 2015. Comment on pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet 54:1287–1288. doi: 10.1007/s40262-015-0344-5. [DOI] [PubMed] [Google Scholar]
- 29.The R Project for Statistical Computing. 2014. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Accessed 15 August 2016 http://www.R-project.org/. [Google Scholar]
- 30.Boeckmann AJ, Beal SL, Sheiner LB. 1999. NONMEM users guide. University of California at San Francisco, San Francisco, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.