ABSTRACT
Splenomegaly is a common feature of many infectious diseases, including schistosomiasis japonica. However, the immunopathogenesis and the treatment of splenomegaly due to schistosomiasis have been largely neglected. Praziquantel (PZQ), a classical schistosomicide, has been demonstrated by us and others to have antifibrotic and anti-inflammatory activities against schistosomiasis. In this study, we investigated the effect of PZQ on alleviating the splenomegaly caused by Schistosoma japonicum infection in mice. The results showed that the number of macrophages, especially the number of M1 macrophages, was significantly increased in the enlarged spleens of infected mice (P < 0.001). After PZQ treatment for 4 weeks, the number of splenic macrophages, especially the number of M1 macrophages, was significantly reduced (P < 0.001) by the way of apoptosis, and another schistosomicide, mefloquine, had no effect either on the splenomegaly or on reducing the number of macrophages. Furthermore, by using the murine macrophage line RAW 264.7, we found that PZQ could inhibit the formation of the NLRP3 inflammasome and attenuate phagocytic activity in M1 macrophages. Thus, our studies suggest that PZQ plays a powerful role in ameliorating the splenomegaly caused by S. japonicum infection, which presents a new strategy for the therapy of splenomegaly resulting from other pathological conditions.
KEYWORDS: splenomegaly, schistosomiasis, macrophage, praziquantel, NLRP3 inflammasome
INTRODUCTION
Schistosomiasis is a serious public health problem worldwide. To date, more than 200 million individuals are infected with schistosomes and 120 million people have associated clinical diseases (1). Schistosomiasis japonica is caused by the parasitic fluke Schistosoma japonicum. The most pathological characteristic of schistosomiasis japonica is the deposition of eggs by schistosomes in the liver, where they induce inflammatory granulomas and subsequent fibrosis formation (2). Meanwhile, schistosomiasis japonica is usually accompanied by splenomegaly and hypersplenism, especially in the chronic stage of infection (3). Splenomegaly is a common feature of many infectious diseases and leads to alterations of the splenic architecture as well as of the inherent immunological function of the spleen (4).
Macrophages are the main innate immune cell type in the spleen and play a pivotal role in hypersplenism (5, 6). The phagocytic capacity of splenic macrophages is upregulated in hypersplenism (7), and cytokine secretion by hypersplenic macrophages is abnormal (8). It is acknowledged that macrophages exhibit multiple functions during the immune response (9), and these important innate immune cells are usually classified into two different types: classically activated macrophages (called M1 macrophages), which exhibit proinflammatory and microbicidal functions, and alternatively activated macrophages (called M2 macrophages), which have immunoregulatory and tissue-repairing functions with a crucial role in the resolution of harmful inflammation through the production of anti-inflammatory mediators (10–13). It is reported that during schistosome infection, liver and peritoneal macrophages are of the M1 phenotype in the early stage and then transfer to the M2 phenotype in the chronic stage (13–16). However, the molecular basis of macrophage activation and polarization as well as the contribution of macrophages to the splenomegaly of mice with chronic schistosomiasis is still unclear.
The nucleotide-binding domain and leucine-rich repeat pyrin 3 domain (NLRP3) belongs to the NOD-like receptors (NLRs), which are an evolutionarily conserved family of intracellular receptors (17). NLRP3 can be activated by a wide variety of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), such as those of Escherichia coli (18), influenza A virus (19, 20), Candida albicans (21), extracellular ATP (22), β-amyloid plaques (23), and uric acid crystals (24). Once it is activated, NLRP3 recruits apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and procaspase-1 and then oligomerizes with them to form a functional inflammasome. Previous studies have demonstrated that the NLRP3 inflammasome is essential for the cleavage and secretion of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 in innate immune cells (25–27). Therefore, the NLRP3 inflammasome is a key component of the innate immune system and plays an important role not only in regulating the complex network of the cellular response but also in initiating local inflammatory reactions. Moreover, its dysregulation has been implicated in the development of many chronic and inflammatory diseases (28).
Praziquantel (PZQ), an antischistosomal drug, is well-known for its schistosomicidal effect (29). It is noted that after the pathogens are eliminated by PZQ treatment, the development of hepatic fibrosis and/or splenomegaly cannot be completely reversed and remains in progress in some patients (30, 31). Until now, the splenomegaly and hypersplenism induced by infectious factors have lacked effective therapeutic drugs in the clinic. Previous studies by us and other groups have indicated that PZQ treatment may ameliorate splenomegaly, portal hepertension, and hepatic fibrosis in schistosome-infected mice (32–35). However, the precise mechanisms by which PZQ administration regulates splenomegaly and splenic macrophages have not been fully elucidated.
In this study, we investigated the role of PZQ in ameliorating the splenic pathological injury caused by S. japonicum infection in mice. We found that PZQ could inhibit the formation of the NLRP3 inflammasome in M1 macrophages and attenuate its phagocytic activity. These results highlight additional directions of study to better understand the roles of PZQ in worm-induced splenic pathology.
RESULTS
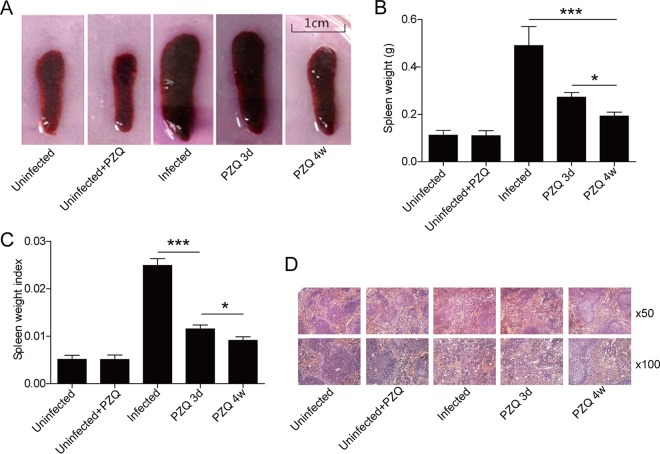
PZQ treatment ameliorated the spleen pathological conditions of mice infected with S. japonicum.
To evaluate the effect of PZQ on splenomegaly, we established a mouse model of chronic schistosomiasis. The spleen size and the ratio of the spleen weight to the body weight for infected mice were significantly increased compared with the values for uninfected control mice (P < 0.001, n = 5). After treatment with PZQ for 3 days, the size and weight of the spleens were observed to decrease, but they were still higher than the normal levels (Fig. 1A). After treatment with PZQ for 4 weeks, the spleen size and spleen/body weight ratio for infected mice were much smaller than those for the infected group and the group treated with PZQ for 3 days (Fig. 1B and C). Hematoxylin and eosin staining showed that the histological structure of the spleens of mice infected with S. japonicum was disorganized, with a chaotic distribution of red and white pulp, congestion of the visible part of the splenic sinus, an irregular cell arrangement, and even tissue necrosis. After PZQ treatment for 3 days, the histology of the spleen improved, but the spleen still presented with a disorganized structure and an irregular cell arrangement. However, the splenic parenchymal structure was repaired, with the reformation of clear white pulp and red pulp and the regular cell distribution in the group treated with PZQ for 4 weeks (Fig. 1D). To test whether this effect could be related to its worm-killing effects, we treated a group of infected mice with another antischistosomiasis drug, mefloquine. As expected, no obvious improvements in the spleen size or the histological structure were observed, even though the reduction of the worm burden produced by mefloquine was indistinguishable from that produced by PZQ (see Fig. S1 and S2 in the supplemental material).
FIG 1.
Effects of PZQ treatment on the splenomegaly of mice with S. japonicum infection. (A) Gross examination of the spleens from S. japonicum-infected mice treated or not treated with PZQ (the scale is shown in the diagram). (B) Weights of spleens of S. japonicum-infected mice treated or not treated with PZQ. (C) The splenic weight index (spleen weight/body weight). (D) Representative images of paraffin-embedded splenic sections stained with hematoxylin and eosin. Uninfected, spleens from naive mice; Uninfected+PZQ, spleens from uninfected mice treated with PZQ for 4 weeks; Infected, spleens from mice that had been infected with S. japonicum for 16 weeks; PZQ 3d, spleens from mice that had been infected with S. japonicum for 12 weeks and then treated with PZQ (300 mg/kg) for 3 days; PZQ 4w, spleens from mice that had been infected with S. japonicum for 12 weeks and then treated with PZQ (300 mg/kg) for 4 weeks. *, P < 0.05; ***, P < 0.001.
PZQ treatment ameliorates the inflammation of spleens in mice infected with S. japonicum by downregulating the proportion of splenic macrophages.
Macrophages are the primary innate immune cells in the spleen. In order to determine the effect of PZQ on macrophages in the spleen, we used an anti-CD68 antibody to show the distribution of the entire macrophage population, as CD68 is the specific surface molecule of macrophages (Fig. 2A). The proportion of macrophages was significantly increased in mice infected with S. japonicum (P < 0.001, n = 5). After 4 weeks of PZQ treatment, the number of macrophages was significantly reduced compared with that in the infected groups (P < 0.001, n = 5). The number of macrophage in the group treated with PZQ for 4 weeks was much lower than that in the group treated with PZQ for 3 days (Fig. 2B). We also analyzed the macrophages with surface markers F4/80 and CD11b by flow cytometry. In line with the results of immunohistochemistry, the percentage of splenic macrophages was much higher in the infected group than in the control group. While both the 3-day and 4-week PZQ treatments reduced the proportion of macrophages, the rate of reduction with the latter treatment was much higher (Fig. 2C and D). However, the proportion of splenic macrophages in the mefloquine treatment group was identical to that in the infected group (Fig. S3A and B), indicating that PZQ might have special effects on macrophages compared with the effect of mefloquine.
FIG 2.
Effects of PZQ treatment on splenic macrophages and inflammatory factors in S. japonicum-infected mice. (A) Representative images of macrophages from immunohistochemistry analysis with anti-CD68 staining under a light microscope. The magnifications are indicated. (B) Quantification of the CD68+ macrophages in the different groups by detecting integral optical density (IOD) with image analysis software. (C) Flow cytometry analysis of the macrophage population using phenotypic surface markers F4/80 and CD11b. (D) Quantification of the proportion of splenic macrophages analyzed in the assay whose results are presented in panel C. (E and F) The levels of IL-4 (EF) and IL-6 (F) cytokines in the supernatants of splenic macrophages from healthy mice and S. japonicum-infected mice treated or not treated with PZQ for 4 weeks were determined by ELISA. *, P < 0.05; ***, P < 0.001.
To assess the secretory function of macrophages, we measured the levels of the IL-4 and IL-6 cytokines generated by macrophages. Interestingly, the level of IL-4 secreted by M2 macrophages was not affected by infection with S. japonicum with or without the 4-week PZQ treatment (Fig. 2E). Similarly, there were no significant changes in the levels of IL-6 secretion by M1 macrophages, though there was a trend toward a higher level of secretion in infected mice and a reduced level of secretion when those mice were treated with PZQ (Fig. 2F).
PZQ treatment reduced the number of splenic M1 macrophages.
To confirm the effects of the 4-week PZQ treatment on splenic M1 macrophages in schistosome-infected mice, we marked macrophages with the M1 macrophage marker F4/80+ CD11b+ CD16/32+ and the M2 macrophage marker F4/80+ CD11b+ CD206+ and determined the proportion of M1 macrophages/M2 macrophages by flow cytometry. The results showed that M1 macrophages dominated among the splenic macrophages of infected mice. PZQ treatment, especially the 4-week treatment, obviously decreased the number of M1 macrophages in the spleens from infected mice (Fig. 3A and B). However, M2 macrophages showed no obvious changes in number (Fig. 3C). Meanwhile, macrophages labeled with CD16/32+ CD206+ were also analyzed, and no significant changes were seen (Fig. 3D). These results suggest that the polarization from M1 to M2 macrophages was not influenced by the PZQ treatment.
FIG 3.
PZQ treatment for 4 weeks reduced the percentage of M1 macrophages from mice with splenomegaly by way of apoptosis. (A) Flow cytometry analysis of M1-type and M2-type macrophage populations using phenotypic surface markers CD16/32 and CD206. (B to D) Quantification of the proportion of splenic M1-type macrophages (B), M2-type macrophages (C), or both M1-type and M2-type macrophages (D). (E) The apoptosis of M1-type macrophages was analyzed by flow cytometry. *, P < 0.05; ***, P < 0.001; ns, not significant.
To further elucidate the effect of PZQ on splenic M1 macrophages, we analyzed the apoptosis of M1 macrophages. It was shown that a higher proportion of M1 macrophages in the PZQ treatment groups than in the control groups experienced apoptosis (Fig. 3E).
PZQ inhibited the expression of the NLRP3 inflammasome in M1 macrophages.
Many studies have identified the NLRP3 inflammasome to be closely related to the immune and inflammatory responses (36–39). Analysis of splenic mRNA showed that the group with S. japonicum infection exhibited higher levels of NLRP3 expression than the control group, and 4 weeks of treatment with PZQ significantly inhibited the expression of NLRP3 (Fig. 4A). To further study the effect of PZQ on M1 macrophages, lipopolysaccharide (LPS)/gamma interferon (IFN-γ) or IL-4 was used to induce the murine macrophage line RAW 264.7 to the M1 or M2 phenotype, respectively. Real-time reverse transcriptase (RT) PCR was employed to measure the markers of the M1 phenotype (tumor necrosis factor alpha [TNF-α] and inducible nitric oxide synthase [iNOS]) and the M2 phenotype (Arg-1). As shown in Fig. 4B, we succeed in establishing M1/M2 polarization in vitro. Expression of the gene for NLRP3 RNA was upregulated 15-fold in M1 macrophages and showed nearly no change in M2 macrophages (Fig. 4C). However, the expression of the IL-1β and NLRP3 genes in M1 macrophages was conspicuously downregulated by PZQ pretreatment for 24 h (Fig. 4D). Moreover, Western blotting showed that PZQ inhibited the expression of NLRP3 at the protein level (Fig. 4E). In line with the change in the level of mRNA expression, the protein level of IL-1β in the supernatants of cultured M1 macrophages was also downregulated after PZQ treatment (Fig. 4F). Thus, PZQ likely suppresses M1 macrophages through downregulation of the expression of NLRP3.
FIG 4.
PZQ inhibited the expression of the NLRP3 inflammasome in M1-type macrophages. (A) The levels of NLRP3 mRNA in splenic macrophages from the different groups were evaluated by quantitative PCR. (B and C) Fold change in the levels of expression of mRNA for classical M1 (TNF-α, iNOS) and M2 (Arg-1) genes (B) and the NLRP3 gene (C) during RAW 264.7 macrophage polarization. (D) Effects of PZQ on the levels of NLRP3 (right) and IL-1β (left) mRNA in M1-type macrophages determined by quantitative PCR. (E) The effect of PZQ on the expression of NLRP3 in M1-type macrophages was evaluated by Western blotting. (F) Effect of PZQ on IL-1β levels in the supernatants of cultured M1 macrophages. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
PZQ attenuated the phagocytic activity of M1 macrophages.
Phagocytosis is one of the most important functions of macrophages in the innate immune response. To assess whether PZQ has an effect on the phagocytic activity of M1 macrophages, RAW 264.7 cells were stimulated with LPS/IFN-γ with or without pretreatment with PZQ. Then, the cells were incubated in the presence of fluorescently labeled E. coli BioParticles. The number of E. coli bioparticles engulfed by M1 macrophages was remarkably reduced after PZQ treatment (Fig. 5A). This finding was further confirmed by flow cytometry analysis (Fig. 5B).
FIG 5.
PZQ decreases the phagocytosis capacity of M1-type macrophages. (A) RAW 264.7 macrophages were induced into the M1 type with or without pretreatment with PZQ for 24 h. The number of engulfed E. coli bioparticles in the PZQ-treated group compared to the number in the healthy untreated group (Normal) could clearly be observed under a fluorescence microscope. The magnifications are indicated on the right. (B) Cells were prepared and were then analyzed by flow cytometry. Fluorescence data were collected for 10,000 cells by flow cytometry and analyzed using BD CELLQUEST Pro software. In each experiment, samples were taken from each group in triplicate, and the experiment was conducted three times. The results are presented as the mean ± SD from three independent experiments. ***, P < 0.001.
DISCUSSION
During the chronic stage of schistosomiasis japonica, granulomas develop around the eggs trapped in the liver, and these induce the excessive repair of tissue, leading to hepatic fibrosis (40). In line with pathogenic hepatic fibrosis, the spleen enlarges abnormally due to schistosome infections. Here we found that 4 weeks of treatment with PZQ ameliorates the splenomegaly in mice infected with S. japonicum by inhibiting M1 macrophages.
Macrophages are key mediators of the immune response during infection and play a pivotal role in enlargement of the spleen due to its responsibility for the removal of old and damaged platelets, old erythrocytes, and apoptotic cells (41, 42). In our study, the number of macrophages in the spleens of mice infected with S. japonicum obviously increased. Three days of PZQ treatment slightly decreased the number of macrophages compared with the number in the control group. Nevertheless, 4 weeks of PZQ treatment could efficiently reduce the number of macrophages and improve the splenic physiological function. This revealed that long-term PZQ treatment but not the routine dosage of PZQ used for the treatment of schistosomiasis could effectively ameliorate splenic complications, like splenomegaly and hypersplenism. In comparison, mefloquine treatment showed no obvious effect on splenomegaly and the number of macrophages, even though it could effectively kill the worms (43–45). Meanwhile, according to the findings of a previous study, the efficacy of mefloquine is independent of the host immune response (46). Given this, we speculated that PZQ improved the splenomegaly through regulating the immune microenvironment of the spleen via its direct effect on splenic macrophages.
Plasticity and functional polarization are characteristics of macrophages (47). Two specific subsets of macrophages have been identified to play different roles in the pathogenic process. M1 macrophages play a role in the proinflammatory response during persistent tissue damage, which mediates resistance to pathogens and causes acute tissue injury, whereas M2 macrophages secrete anti-inflammatory factors, helping to diminish the inflammatory reaction (48). In different disease models, including models of infection and neurodegenerative disorders, the polarization of macrophages results in macrophages that express mixed or unique phenotypes. For example, the unrestrained activation of proinflammatory M1 macrophages is considered to be a critical event in the pathogenesis of chronic venous leg ulcers (49). Improved cognition in patients with mild cognitive impairment is closely related to increased intermediate M1-M2 macrophage polarization (50). The prolonged accumulation of M1 macrophages leads to reactive oxygen species-mediated DNA damage, fibroblast cellular senescence, and defective tissue repair. These changes strongly support the view that the development of chronic inflammation is due to the fact that macrophages fail to switch from the M1 to the M2 phenotype (49). In schistosomiasis, the liver and peritoneal macrophages undergo the transition from the M1 to the M2 phenotype (51, 52). The M2 macrophages in the liver account for the repair but not the inflammation that occurs during the hepatic fibrosis induced by S. japonicum infection (51). Our previous study revealed that the 4-week PZQ treatment has an anti-inflammatory effect (53). While we have found that the 4-week PZQ treatment reduced the overall number of macrophages in the spleen, the exact effect of PZQ on the macrophage subtype is still unclear. In this study, M1 macrophages with the F4/80+ CD11b+ CD16/32+ marker in the spleen accounted for a large proportion of the macrophages in mice with chronic S. japonicum infection. This phenotype of macrophages in the spleen is different from that of macrophages in the liver and peritoneum, whose pattern changes from the M1 phenotype in the acute stage to the M2 phenotype in the chronic stage of schistosome infection. Our results suggest that M1 macrophages are mainly responsible for the splenomegaly caused by S. japonicum infection.
So far, the NLRP3 inflammasome has been well studied. The NLRP3 inflammasome is constitutively expressed in many immune cells, especially in macrophages, where it plays a central role in the response to infection and the pathogenesis of inflammation (54). It has been reported that the NLRP3 inflammasome is vital in M1 macrophages but not in M2 macrophages (55). Once it is activated, NLRP3 triggers the transformation of procaspase-1 to caspase-1, as well as the secretion of mature IL-1β and IL-18 (56). As a result, large amounts of IL-1β are present in M1-polarized macrophages, while no IL-1β is found in M2-polarized macrophages (57). Our finding also demonstrated that NLRP3 expression was highly induced in M1 macrophages but not in M2 macrophages. In addition, the NLRP3 protein is involved in many regulatory mechanisms contributing to a number of biological functions. For example, the NLRP3 protein regulates myofibroblast differentiation independently of the inflammasome, indicating that NLRP3 not only is intrinsically linked to the inflammasome but also exerts a distinct role in response to signals of injury to regulate tissue homeostasis (58). NLRP3, as a transcriptional factor, plays an important role in regulating Th2 cell differentiation (59). Besides, an inflammasome-independent effect of NLRP3 has also been observed in the kidney epithelium (60). Our present study showed that the level of expression of NLRP3 increased significantly in the spleens of mice, while PZQ inhibited the function of M1 macrophages through downregulating the gene and protein expression of NLRP3. These results indicate that PZQ attenuates the splenomegaly induced by S. japonicum infection probably through inhibiting NLRP3 expression in M1 macrophages.
In addition to resulting in the processing of IL-1β and IL-18, the activation of NLRP3/caspase-1 triggers a form of cell death called pyroptosis (61). Normally, just as apoptosis needs caspase-3 and caspase-7 activation, pyroptosis requires the proteolytic activation of a specific caspase, caspase-1. In contrast to the anti-inflammatory effect of apoptosis, however, pyroptosis is predicted to be proinflammatory because of the rapid loss of cell membrane integrity and the release of cytosolic contents (62). Some evidence also suggests that multiple pathways may be activated in single dying cells and cross talk between cell death programs may allow fine control over the ultimate outcome (63, 64). What is more, inhibition of the dominant molecular route of cell death may not result in survival but, rather, may allow the occurrence of alternative programs, leading to different types of cell death (65). Our work indicates that the 4-week PZQ treatment inhibited the increase in the number of M1 macrophages in the spleen by increasing the apoptotic cell death of M1 macrophages. Whether the increase in apoptosis is due to the inhibition of NLRP3 inflammasome-dependent pyroptosis still needs to be further studied.
It has been reported that PZQ can reduce the hepatic damage induced by schistosoma eggs, which is attributed not only to its effect on decreasing the number of eggs but also to its possible anti-inflammatory properties (66). PZQ can also affect the host immune reaction during Schistosoma mansoni infection (67). However, there is little evidence of the effects of PZQ during the whole process of splenomegaly. Although we have provided evidence that PZQ is able to inhibit the NLRP3 inflammasome, what activates the NLRP3 inflammasome, how the activated NLRP3 inflammasome induces splenomegaly, and how PZQ is involved in inhibiting the NLRP3 inflammasome remain unclear. Our results suggest that the improvement of splenomegaly produced by PZQ results not only from its ability to reduce the proportion of M1 macrophages but also from its inhibitory effect on the function of M1 macrophages, probably due to a comprehensive effect. Therefore, it is necessary to perform further studies in vivo to demonstrate its mechanism in detail.
The spleen is one of the major organs affected during schistosomiasis japonica, and schistosome infection causes not only splenomegaly but also complicated changes in the local immune environment, yet its pathology has long been overlooked (68). However, almost no targeted routine chemotherapy for splenomegaly has been identified. Our present study shows that the persistence of unrestrained proinflammatory M1 macrophages may be the main reason for the splenomegaly due to murine schistosomiasis japonica and allows us to gain deeper insights into the role of PZQ in the alleviation of splenomegaly through the induction of apoptosis of M1 macrophages and the inhibitions of NLRP3 expression and phagocytic function in M1 macrophages. Our findings provide new insight into the role of PZQ in ameliorating the splenomegaly caused by S. japonicum infection and may present evidence for the use of PZQ as clinical therapy for splenomegaly resulting from other pathogenic factors.
MATERIALS AND METHODS
Ethical statement.
Animal care and all animal experiment protocols were approved by the Animal Ethics Committee of Nanjing Medical University. All experiments were performed in accordance with the approved guidelines (IACUC no. 1601130).
Animals and drug.
Six- to 8-week old female BABL/c mice (Comparative Medicine Center of Yangzhou University, Yangzhou, China) were used in the study and maintained according to guidelines approved by the Animal Ethics Committee of Nanjing Medical University. S. japonicum cercariae of the Chinese mainland strain were obtained from infected Oncomelania hupensis tropical freshwater snails (Jiangsu Institute for Schistosomiasis Control, Wuxi, China). PZQ and mefloquine were purchased from Sigma and resuspended in a 1% carboxymethyl cellulose carrier for use in vivo. Sterile dimethyl sulfoxide (DMSO) was used as the solvent for PZQ, and in the in vitro experiments, the concentration of DMSO in the culture medium was less than 0.1%.
Establishment of model of chronic infection and drug treatment strategy.
The BABL/c mice were infected cutaneously with S. japonicum cercariae (14 ± 2) and fed to 12 weeks postinfection to establish the model of chronic schistosomiasis (69). Intragastric administrations of PZQ (250 mg/kg of body weight/24 h) for 3 days were used to eliminate the worms. Continuous administrations of PZQ (300 mg/kg/12 h) or mefloquine (100 mg/kg/12 h) were carried out for 4 weeks. Uninfected and infected animals with continuous administrations of 1% carboxymethyl cellulose for 4 weeks were used as controls. Each group contained 5 mice.
Cell isolation and culture.
The mice were anesthetized with 2% pentobarbital sodium, and the spleens were harvested. Splenocytes were isolated by pressing the spleens through a nylon mesh. Erythrocytes were lysed by use of an ammonium chloride solution for 2 min, and splenic cells were washed twice. After 2 h of incubation in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (37°C, 95% humidity, 5% CO2), nonadherent cells were removed, and adherent splenic macrophages were recovered. The procedure of adherence for macrophage isolation has been described previously (70). The purity of the macrophages was >90%, as estimated by cytometry after staining with anti-CD11b and anti-CD68.
The method of macrophage polarization described previously was used (71). Briefly, RAW 264.7 macrophages plated on 12-well plates were primed with fresh medium, and then M1-polarized macrophages were achieved by a 4-h stimulation with LPS (1 μg/ml) and IFN-γ (20 ng/ml); IL-4 (20 ng/ml) was used for 4 h to differentiate the M2 phenotype. The cells were pretreated with PZQ (20 ng/ml) for 24 h before induction of the M1 or M2 phenotype. iNOS and TNF-α genes were examined as M1 macrophage-related factors, while the Arg-1 gene was examined as the M2 macrophage-related factor.
Histology and immunohistochemistry.
The spleens were fixed in 10% neutral buffered formalin and were embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin and eosin and were examined using light microscopy. Immunoperoxidase staining for macrophages in spleen tissue was done with the specific cell surface marker CD68 (primary antibody, rat anti-mouse F4/80; Abcam, Cambridge, MA, USA) and horseradish peroxidase-conjugated secondary antibodies (Merck). Slides were scanned by use of an Aperio slide scanner (Aperio Technologies, Vista, CA, USA). Positive staining for F4/80 was measured by using Aperio's Spectrum Plus software positive-pixel-count algorithm (version 8.2; Aperio Technologies, Vista, CA, USA).
Flow cytometry.
Single-cell suspensions were washed in staining buffer and adjusted to 1 × 106/100 μl. For analysis of the purity of the macrophages, cells were incubated with phycoerythrin-conjugated antibody against mouse F4/80 and peridinin chlorophyll protein-Cy5.5-conjugated antibody against mouse CD11b (eBioscience). For M1 and M2 macrophage surface marker analysis, cells were incubated with fluorescein isothiocyanate-conjugated antibodies against mouse CD16/32 or allophycocyanin-conjugated antibodies against mouse CD206 (eBioscience). All antibodies were used at 1.25 μg/ml. Cells were incubated with the antibodies for 30 min at 4°C and washed with phosphate-buffered saline (PBS). Samples were fixed with 1% paraformaldehyde-PBS and analyzed by using a BD FACSCalibur flow cytometer. The results were analyzed by using FlowJo software (TreeStar Inc., Ashland, OR).
Apoptotic assay.
Apoptosis was examined with an annexin V kit (eBioscience). Cells were detected using a BD FACSCalibur flow cytometer. The results were analyzed with FlowJo software (TreeStar Inc., Ashland, OR).
Real-time RT-PCR.
Tissues or cells were homogenized in the TRIzol reagent (Invitrogen, Carlsbad, CA), and their RNAs were extracted according to the manufacturer's protocol. Reverse transcriptase (RT) reactions for cDNA synthesis were carried out with a RevertAid first-strand cDNA synthesis kit with oligo(dT) primer (Fermentas, EU). The relative expression of mRNA was determined by real-time RT-PCR with Fast-Start Universal SYBR green PCR master mix (Roche Diagnostics, Indianapolis, IN, USA) on an Applied Biosystems 7300 real-time PCR system (Foster City, CA). The reaction conditions were as follows: stage 1, 50°C for 2 min; stage 2, 95°C for 10 min; stage 3, 40 cycles of 95°C for 15 s and 60°C for 1 min. The PCR procedures concluded with melting curve analysis. The fold changes in the levels of gene expression were calculated by using the 2−ΔΔCT threshold cycle (CT) method. Each experiment was conducted three times. The sequences of the primer pairs used in this study are shown in Table S1 in the supplemental material.
ELISA.
Splenic macrophages from different groups of mice or LPS/IFN-γ-induced M1 macrophages were harvested and cultured, and the supernatants were collected for cytokine assay using an IL-4, IL-6, and IL-1β enzyme-linked immunosorbent assay (ELISA) kit (eBioscience). The assays were performed according to the manufacturer's protocol.
Western blot analysis.
An equal amount of total protein from each sample was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% bovine serum albumin in Tris-buffered saline–Tween 20, probed with a primary antibody to NLRP3 (1:1,000; Santa Cruz), and then incubated with peroxidase-conjugated secondary antibody (1:5,000; Santa Cruz). Protein bands were detected by enhanced chemiluminescence (Merck Millipore) and visualized by use of a gel imaging system (Bio-Rad). β-Actin (1:1,000; Cell Signaling Technology) was used as a control.
RAW 264.7 cell phagocytosis assay.
RAW 264.7 cells were seeded into a 12-well plate (1 × 107cells/well) and were stimulated with LPS and IFN-γ for 4 h to induce M1 macrophages with or without PZQ pretreatment for 24 h. Then, Alexa Fluor 488-conjugated Escherichia coli (K-12 strain) BioParticles (Invitrogen, USA) were added, and the mixture was incubated in a dark environment for 1 h in serum-free DMEM. The plate was placed in a dark container on ice, with the BioParticle suspension being removed at the end of the incubation. To exclude the surface-bound fluorescence as well as the fluorescence of the cell, trypan blue (1.25 mg/ml) was added for 1 min to quench all of the fluorescence outside the cell. Then, the cells were washed with PBS and the plates were analyzed immediately by using a flow cytometer and a fluorescence microscope.
Statistics.
The data were analyzed by two-tailed Student's t test to compare the results for two groups. One-way analysis of variance was used to analyze the results for more than two groups. All statistical analyses were conducted with GraphPad Prism (version 5.0) software.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 81273234, no. 81471573), the Six Talent Peaks Project in Jiangsu Province (China), and the Natural Science Foundation of Higher Education of Jiangsu Province (China) (no. 11KJA330003).
We are grateful to the Department of Biochemistry and Molecular Biology of Nanjing Medical University for helping to do the fluorescence microscopy analysis. We thank Stadecker Miguel (Department of Integrative Physiology and Pathobiology, Sackler School of Biomedical Sciences, Tufts University School of Medicine, USA) for his suggestions and revision of the manuscript.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00005-17.
REFERENCES
- 1.Wu W, Feng A, Huang Y. 2015. Research and control of advanced schistosomiasis japonica in China. Parasitol Res 114:17–27. doi: 10.1007/s00436-014-4225-x. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. 2007. Immunopathology of schistosomiasis. Immunol Cell Biol 85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. 2009. Immunopathogenesis of human schistosomiasis. Parasite Immunol 31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 4.Burke ML, McManus DP, Ramm GA, Duke M, Li Y, Jones MK, Gobert GN. 2010. Co-ordinated gene expression in the liver and spleen during Schistosoma japonicum infection regulates cell migration. PLoS Negl Trop Dis 4:e686. doi: 10.1371/journal.pntd.0000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lifshitz L, Tabak G, Gassmann M, Mittelman M, Neumann D. 2010. Macrophages as novel target cells for erythropoietin. Haematologica 95:1823–1831. doi: 10.3324/haematol.2010.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosche KL, Aljasham AT, Kipfer JN, Piatkowski BT, Konjufca V. 2015. Infection with Salmonella enterica serovar Typhimurium leads to increased proportions of F4/80+ red pulp macrophages and decreased proportions of B and T lymphocytes in the spleen. PLoS One 10:e0130092. doi: 10.1371/journal.pone.0130092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yongxiang W, Zongfang L, Guowei L, Zongzheng J, Xi C, Tao W. 2002. Effects of splenomegaly and splenic macrophage activity in hypersplenism due to cirrhosis. Am J Med 113:428–431. doi: 10.1016/S0002-9343(02)01210-X. [DOI] [PubMed] [Google Scholar]
- 8.Li A, Li Z, Ma S, Su Q, Zhang S, Sun X, Li G. 2008. Dysfunction of splenic macrophages in cirrhotic patients with hypersplenism and HBV infection. Am J Med Sci 336:32–38. doi: 10.1097/MAJ.0b013e31815b69e7. [DOI] [PubMed] [Google Scholar]
- 9.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. 2014. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benoit M, Desnues B, Mege JL. 2008. Macrophage polarization in bacterial infections. J Immunol 181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 11.Lugo-Villarino G, Verollet C, Maridonneau-Parini I, Neyrolles O. 2011. Macrophage polarization: convergence point targeted by Mycobacterium tuberculosis and HIV. Front Immunol 2:43. doi: 10.3389/fimmu.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sica A, Mantovani A. 2012. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. 2011. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem 286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreider T, Anthony RM, Urban JF Jr, Gause WC. 2007. Alternatively activated macrophages in helminth infections. Curr Opin Immunol 19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. 2011. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. 2008. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab 7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinon F, Burns K, Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 18.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas PG, Dash P, Aldridge JR Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 22.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 23.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 24.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamkanfi M, Dixit VM. 2014. Mechanisms and functions of inflammasomes. Cell 157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Lamkanfi M, Kanneganti TD. 2010. Nlrp3: an immune sensor of cellular stress and infection. Int J Biochem Cell Biol 42:792–795. doi: 10.1016/j.biocel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroder K, Tschopp J. 2010. The inflammasomes. Cell 140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Tschopp J. 2011. Mitochondria: sovereign of inflammation? Eur J Immunol 41:1196–1202. doi: 10.1002/eji.201141436. [DOI] [PubMed] [Google Scholar]
- 29.Gonnert R, Andrews P. 1977. Praziquantel, a new board-spectrum antischistosomal agent. Z Parasitenkd 52:129–150. doi: 10.1007/BF00389899. [DOI] [PubMed] [Google Scholar]
- 30.Chapadeiro E, Pitanga LC. 1997. On the reversal of schistosomiasis hepatic fibrosis after specific therapy. Histopathologic study. Rev Soc Bras Med Trop 30:53–56. doi: 10.1590/S0037-86821997000100010. [DOI] [PubMed] [Google Scholar]
- 31.Spear RC, Seto EY, Carlton EJ, Liang S, Remais JV, Zhong B, Qiu D. 2011. The challenge of effective surveillance in moving from low transmission to elimination of schistosomiasis in China. Int J Parasitol 41:1243–1247. doi: 10.1016/j.ijpara.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel-Hafeez EH, Ahmad AK, Abdulla AM, Aabdel-Wahab S, Mosalem FA. 2012. Therapeutic effect of alpha lipoic acid combined with praziquantel on liver fibrosis induced by Schistosoma mansoni challenged mice. Parasitol Res 111:577–586. doi: 10.1007/s00436-012-2871-4. [DOI] [PubMed] [Google Scholar]
- 33.El-Lakkany NM, Hammam OA, El-Maadawy WH, Badawy AA, Ain-Shoka AA, Ebeid FA. 2012. Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against Schistosoma mansoni-induced liver fibrosis. Parasit Vectors 5:9. doi: 10.1186/1756-3305-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang YX, Xu YL, Yu CX, Li HJ, Yin XR, Wang TS, Wang W, Liang YS. 2011. Effect of praziquantel prolonged administration on granuloma formation around Schistosoma japonicum eggs in lung of sensitized mice. Parasitol Res 109:1453–1459. doi: 10.1007/s00436-011-2485-2. [DOI] [PubMed] [Google Scholar]
- 35.Zhu D, Song K, Chen J, Wang J, Sun X, Qian H, Gu X, Zhang L, Qin Y, Duan Y. 2015. Expression of Septin4 in Schistosoma japonicum-infected mouse livers after praziquantel treatment. Parasit Vectors 8:19. doi: 10.1186/s13071-015-0640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandanger O, Gao E, Ranheim T, Bliksoen M, Kaasboll OJ, Alfsnes K, Nymo SH, Rashidi A, Ohm IK, Attramadal H, Aukrust P, Vinge LE, Yndestad A. 2016. NLRP3 inflammasome activation during myocardial ischemia reperfusion is cardioprotective. Biochem Biophys Res Commun 469:1012–1020. doi: 10.1016/j.bbrc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 37.Ren JD, Wu XB, Jiang R, Hao DP, Liu Y. 2016. Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochim Biophys Acta 1863:50–55. doi: 10.1016/j.bbamcr.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Pan CW, Pan ZZ, Hu JJ, Chen WL, Zhou GY, Lin W, Jin LX, Xu CL. 2016. Mangiferin alleviates lipopolysaccharide and d-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur J Pharmacol 770:85–91. doi: 10.1016/j.ejphar.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 39.McRae S, Iqbal J, Sarkar-Dutta M, Lane S, Nagaraj A, Ali N, Waris G. 2016. The hepatitis C virus-induced NLRP3 inflammasome activates the sterol regulatory element-binding protein (SREBP) and regulates lipid metabolism. J Biol Chem 291:3254–3267. doi: 10.1074/jbc.M115.694059. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Shaker Y, Samy N, Ashour E. 2014. Hepatobiliary schistosomiasis. J Clin Transl Hepatol 2:212–216. doi: 10.14218/JCTH.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mebius RE, Kraal G. 2005. Structure and function of the spleen. Nat Rev Immunol 5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 42.Brendolan A, Rosado MM, Carsetti R, Selleri L, Dear TN. 2007. Development and function of the mammalian spleen. Bioessays 29:166–177. doi: 10.1002/bies.20528. [DOI] [PubMed] [Google Scholar]
- 43.Van Nassauw L, Toovey S, Van Op den Bosch J, Timmermans JP, Vercruysse J. 2008. Schistosomicidal activity of the antimalarial drug, mefloquine, in Schistosoma mansoni-infected mice. Travel Med Infect Dis 6:253–258. doi: 10.1016/j.tmaid.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis 3:e350. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao SH, Mei JY, Jiao PY. 2009. Further study on mefloquine concerning several aspects in experimental treatment of mice and hamsters infected with Schistosoma japonicum. Parasitol Res 106:131–138. doi: 10.1007/s00436-009-1640-5. [DOI] [PubMed] [Google Scholar]
- 46.Keiser J, N′Guessan NA, Adoubryn KD, Silue KD, Vounatsou P, Hatz C, Utzinger J, N′Goran EK. 2010. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis 50:1205–1213. doi: 10.1086/651682. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton JA. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 48.Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. 2011. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Famenini S, Rigali EA, Olivera-Perez HM, Dang J, Chang MT, Halder R, Rao RV, Pellegrini M, Porter V, Bredesen D, Fiala M. 2016. Increased intermediate M1-M2 macrophage polarization and improved cognition in mild cognitive impairment patients on ω-3 supplementation. FASEB J 31:148–160. doi: 10.1096/fj.201600677RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barron L, Wynn TA. 2011. Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur J Immunol 41:2509–2514. doi: 10.1002/eji.201141869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. 2010. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev 16:105–118. [PubMed] [Google Scholar]
- 53.Liang YJ, Luo J, Yuan Q, Zheng D, Liu YP, Shi L, Zhou Y, Chen AL, Ren YY, Sun KY, Sun Y, Wang Y, Zhang ZS. 2011. New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum. PLoS One 6:e20247. doi: 10.1371/journal.pone.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gross O, Thomas CJ, Guarda G, Tschopp J. 2011. The inflammasome: an integrated view. Immunol Rev 243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 55.Liu W, Zhang X, Zhao M, Zhang X, Chi J, Liu Y, Lin F, Fu Y, Ma D, Yin X. 2015. Activation in M1 but not M2 macrophages contributes to cardiac remodeling after myocardial infarction in rats: a critical role of the calcium sensing receptor/NRLP3 inflammasome. Cell Physiol Biochem 35:2483–2500. doi: 10.1159/000374048. [DOI] [PubMed] [Google Scholar]
- 56.Rabeony H, Pohin M, Vasseur P, Petit-Paris I, Jegou JF, Favot L, Frouin E, Boutet MA, Blanchard F, Togbe D, Ryffel B, Bernard FX, Lecron JC, Morel F. 2015. IMQ-induced skin inflammation in mice is dependent on IL-1R1 and MyD88 signaling but independent of the NLRP3 inflammasome. Eur J Immunol 45:2847–2857. doi: 10.1002/eji.201445215. [DOI] [PubMed] [Google Scholar]
- 57.Scotton CJ, Martinez FO, Smelt MJ, Sironi M, Locati M, Mantovani A, Sozzani S. 2005. Transcriptional profiling reveals complex regulation of the monocyte IL-1 beta system by IL-13. J Immunol 174:834–845. doi: 10.4049/jimmunol.174.2.834. [DOI] [PubMed] [Google Scholar]
- 58.Bracey NA, Gershkovich B, Chun J, Vilaysane A, Meijndert HC, Wright JR Jr, Fedak PW, Beck PL, Muruve DA, Duff HJ. 2014. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome. J Biol Chem 289:19571–19584. doi: 10.1074/jbc.M114.550624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruchard M, Rebe C, Derangere V, Togbe D, Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, Chevriaux A, Limagne E, Apetoh L, Vegran F, Ghiringhelli F. 2015. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol 16:859–870. doi: 10.1038/ni.3202. [DOI] [PubMed] [Google Scholar]
- 60.Wang W, Wang X, Chun J, Vilaysane A, Clark S, French G, Bracey NA, Trpkov K, Bonni S, Duff HJ, Beck PL, Muruve DA. 2013. Inflammasome-independent NLRP3 augments TGF-beta signaling in kidney epithelium. J Immunol 190:1239–1249. doi: 10.4049/jimmunol.1201959. [DOI] [PubMed] [Google Scholar]
- 61.Bergsbaken T, Fink SL, Cookson BT. 2009. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. 2010. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leist M, Jaattela M. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2:589–598. [DOI] [PubMed] [Google Scholar]
- 64.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. 1997. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 185:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fink SL, Cookson BT. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen BL, Zhang GY, Wang SP, Li Q, Xu MH, Shen YM, Yan L, Gu H, Li J, Huang YL, Mu YB. 2012. The combined treatment of praziquantel with osteopontin immunoneutralization reduces liver damage in Schistosoma japonicum-infected mice. Parasitology 139:522–529. doi: 10.1017/S0031182011002241. [DOI] [PubMed] [Google Scholar]
- 67.Mahmoud TY, Rizk SM, Maghraby AS, Shaheen AA. 2014. Propolis enhances the effectiveness of praziquantel in experimental schistosomiasis: biochemical and histopathological study. Parasitol Res 113:4513–4523. doi: 10.1007/s00436-014-4141-0. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Zhang J, Yin J, Shen Y, Wang Y, Xu Y, Cao J. 2015. The formation of egg granulomas in the spleens of mice with late Schistosoma japonicum infection alters splenic morphology. Parasit Vectors 8:375. doi: 10.1186/s13071-015-0988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tao FF, Yang YF, Wang H, Sun XJ, Luo J, Zhu X, Liu F, Wang Y, Su C, Wu HW, Zhang ZS. 2009. Th1-type epitopes-based cocktail PDDV attenuates hepatic fibrosis in C57BL/6 mice with chronic Schistosoma japonicum infection. Vaccine 27:4110–4117. doi: 10.1016/j.vaccine.2009.04.073. [DOI] [PubMed] [Google Scholar]
- 70.Lolait SJ, Lim AT, Toh BH, Funder JW. 1984. Immunoreactive beta-endorphin in a subpopulation of mouse spleen macrophages. J Clin Invest 73:277–280. doi: 10.1172/JCI111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pelegrin P, Surprenant A. 2009. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 28:2114–2127. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.