ABSTRACT
Leishmania donovani is the causing agent of visceral leishmaniasis, a common infection that affects millions of people from the most underdeveloped countries. Miltefosine is the only oral drug to treat infections caused by L. donovani. Nevertheless, its mechanism of action is not well understood. While miltefosine inhibits the synthesis of phosphatidylcholine and also affects the parasite mitochondrion, inhibiting the cytochrome c oxidase, it is to be expected that this potent drug also produces its effect through other targets. In this context, it has been reported that the disruption of the intracellular Ca2+ homeostasis represents an important object for the action of drugs in trypanosomatids. Recently, we have described a plasma membrane Ca2+ channel in Leishmania mexicana, which is similar to the L-type voltage-gated Ca2+ channel (VGCC) present in humans. Remarkably, the parasite Ca2+ channel is activated by sphingosine, while the L-type VGCC is not affected by this sphingolipid. In the present work we demonstrated that, similarly to sphingosine, miltefosine is able to activate the plasma membrane Ca2+ channel from L. donovani. Interestingly, nifedipine, the classical antagonist of the human channel, was not able to fully block the parasite plasma membrane Ca2+ channel, indicating that the mechanism of interaction is not identical to that of sphingosine. In this work we also show that miltefosine is able to strongly affect the acidocalcisomes from L. donovani, inducing the rapid alkalinization of these important organelles. In conclusion, we demonstrate two new mechanisms of action of miltefosine in L. donovani, both related to disruption of parasite Ca2+ homeostasis.
KEYWORDS: Leishmania donovani, Ca2+, miltefosine, sphingosine, visceral leishmaniasis, mechanism of action
INTRODUCTION
Leishmaniasis is a parasitic neglected tropical disease affecting millions of people all over the world. There are three main forms of this disease: visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucocutaneous leishmaniasis, all of which are caused by 20 different Leishmania species, which are transmitted by phlebotomine sand flies. Current estimates indicate that from 0.2 to 0.4 million people are affected by visceral leishmaniasis, which is the most severe clinical form of the disease and usually leads the patient to death if untreated. Its etiologic agents are the trypanosomatid parasites Leishmania infantum (in the Americas) and Leishmania donovani (Asia, Middle East and Africa) (1). The classical treatments against leishmaniasis include pentavalent antimonials (glucantime and pentostan), which present serious disadvantages, such as variable efficacy, parenteral, and marked side effects. More recently, amphotericin B administered in liposomal complex has been shown to be very efficient (2). Another class of compounds, alkyl phosphorylcholines and related derivatives, have shown efficacy against L. donovani (3). A similar compound derived from phosphocholine, miltefosine, was first used as an anti-neoplastic drug (4) and has shown large efficacy against L. donovani and other trypanosomatids like Trypanosoma cruzi and Trypanosoma brucei (5). Miltefosine also showed antiparasitic action in vivo on VL-infected patients in India (6). Accordingly, in the last few years miltefosine efficacy against different Leishmania species has been reported (7, 8). Furthermore, miltefosine has shown a synergistic effect with several drugs, among others, with nanotized curcumin against L. donovani (9), with amiodarone against L. mexicana (10), with allopurinol against canine VL produced by L. infantum (11), and with pentamidine against L. infantum-HIV coinfection (12). Despite its reported side effects as inducer of resistance and teratogenic action, evidence proving miltefosine antileishmanial action in vitro and in vivo led to its use as the first oral treatment for VL (13, 14).
In concerns to the mechanism of action of miltefosine, several compounds have been shown to act as inhibitors of lipid biosynthesis in kinetoplastid parasites. Among them, lysophospholipids produced a marked effect on the phospholipid composition of trypanosomatids, in which the biosynthesis of phosphatidylcholine (PC) is inhibited at the level of phosphatidylethanolamine N-methyltransferase (15). Miltefosine, as an alkyl-lysophospholipid, showed a reduction of the concentration of phosphatidylcholine in T. cruzi. Remarkably, it has been claimed that miltefosine inhibits the biosynthesis of PC in T. cruzi (16) with 10 to 20 times more potency compared to that of mammalian cells (17), thus explaining its high selectivity as antiparasitic drug. The same mechanism has also been reported in L. donovani, in which phosphatidylcholine concentration is decreased and phosphatidylethanolamine concentration is enhanced (18).
Previous reports demonstrate that miltefosine causes decreases in the oxygen consumption rate and ATP levels of L. donovani through inhibition of the mitochondrial cytochrome c oxidase (19). Furthermore, miltefosine also produces an apoptosis-like death in L. donovani promastigotes (20).
With regard to Ca2+ signaling, it is known that the mechanisms involved in Ca2+ regulation in trypanosomatids constitute a target for chemotherapeutic agents like amiodarone and dronedarone, which disrupt Ca2+ homeostasis in T. cruzi and L. mexicana (21–24) through their action on two organelles acting as Ca2+ compartments, the mitochondrion and the acidocalcisomes. Moreover, the antituberculosis compound SQ109, which also possesses a very potent trypanocidal effect, was recently found to act on T. cruzi (25) and L. mexicana (26) through the same mechanism of Ca2+ and mitochondrial disruption. Also in concerns to disruption of Ca2+ regulation, it has been reported that many Ca2+ channel antagonists produce a marked effect in several trypanosomatids (27), including L. donovani (28). In fact, a plasma membrane Ca2+ channel homolog to the human L-type voltage-gated Ca2+ channel (VGCC) has been described in L. mexicana (29). This channel shares many characteristics with its human homolog, such as antagonism by classical human channel blockers (nifedipine and verapamil). However, remarkably, the parasite channel is selectively stimulated by the sphingolipid sphingosine, while the VGCC is not (29). In the present work we show new mechanisms of action of miltefosine, demonstrating that this drug is able to activate a Ca2+ channel in the plasma membrane of L. donovani similar to the sphingosine-activated channel mentioned above for L. mexicana. Although miltefosine simulated the effect of sphingosine, the activation of the parasite channel by this drug was not completely blocked by dihydropyridines such as nifedipine, the classical human L-type VGCC antagonist. Furthermore, in the present work we also demonstrate that miltefosine has a direct effect on L. donovani acidocalcisomes.
RESULTS
Effect of miltefosine on the intracellular Ca2+ concentration of L. donovani promastigotes.
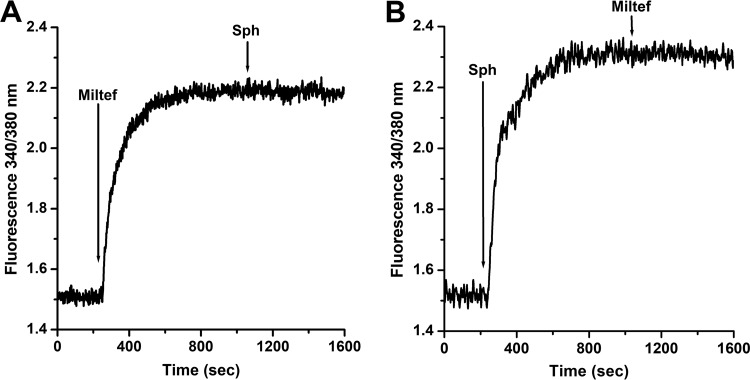
Several mechanisms have been proposed for the mode of action of miltefosine on Leishmania spp. These include disturbances of the lipid-dependent signaling pathways (16), inhibition of cytochrome C oxidase (19), and an apoptosis-like cell death (30). However, there is increasing evidence that Ca2+ homeostasis could be a target for the action of drugs against trypanosomatids (21–24), and the role of Ca2+ on different cellular processes, including cell death by apoptosis and necrosis, is well known. In order to determine the effect of miltefosine on the [Ca2+]i (intracellular Ca2+ concentration) in L. donovani promastigotes, the parasites were loaded with the fluorescent Ca2+ indicator Fura-2. It can be observed (Fig. 1) that the addition of miltefosine (4 μM) induced a large increase in the [Ca2+]i. We used this concentration because it has been previously shown, based on a dose-response curve, that at 4 μM miltefosine exerts its maximal effect on the magnitude of the [Ca2+]i increase in L. mexicana (10). Addition of sphingosine (10 μM), which at this concentration is known to optimally activate the plasma membrane Ca2+ channel in L. mexicana (29), showed no further effect. Accordingly, when miltefosine was added after the rapid increase in the [Ca2+]i induced by sphingosine, the drug did not produce any further increase in fluorescence. These results suggest that miltefosine and sphingosine share the same mechanism of action, namely, the opening of a Ca2+ channel at the plasma membrane.
FIG 1.
Effect of miltefosine and sphingosine on the intracellular Ca2+ concentration of L. donovani promastigotes. Promastigotes were loaded with Fura-2 and the indicated compounds were added directly to the cuvette, as described in Materials and Methods. (A) Miltefosine (4 μM) was added (arrow) in the presence of 2 mM extracellular Ca2+, followed by the addition of sphingosine (10 μM). (B) Sphingosine (10 μM) was added as indicated (arrow), followed by miltefosine (4 μM). Traces are representative of at least three independent experiments.
We then sought to verify if the observed Ca2+ channel activated by miltefosine corresponds to the same entity of the sphingosine-sensitive plasma membrane Ca2+ channel already described in L. mexicana (29). For this, we used Bay K 8644, a very specific agonist of the human L-type VGCC, widely used for its functional characterization, and which has been demonstrated to indeed also activate the Ca2+ channel reported in L. mexicana (29). At 4 μM the agonist is known to induce the maximal opening of the human L-type VGCC and also the similar channel in L. mexicana (29). It was observed that upon addition of Bay K 8644 (Fig. 2) this agonist totally substituted the effect of miltefosine. That is, Bay K 8644 (4 μM) did not produce any further effect after addition of miltefosine (Fig. 2A) and, accordingly, addition of miltefosine after Bay K 8644 did not induce any further Ca2+ release (Fig. 2B). These results support the notion that both miltefosine and sphingosine act on the same channel.
FIG 2.
Effect of miltefosine and the Ca2+ channel agonist Bay K 8644 on the intracellular Ca2+ concentration of L. donovani promastigotes. (A) Miltefosine (4 μM) and then Bay K 8644 (4 μM) were added (arrows) directly to the cuvette in the presence of 2 mM extracellular Ca2+. (B) Bay K 8644 (4 μM) was added (arrow), followed by miltefosine (4 μM) when indicated, in the presence of 2 mM extracellular Ca2+. Traces are representative of at least three independent experiments. (See Materials and Methods section for details).
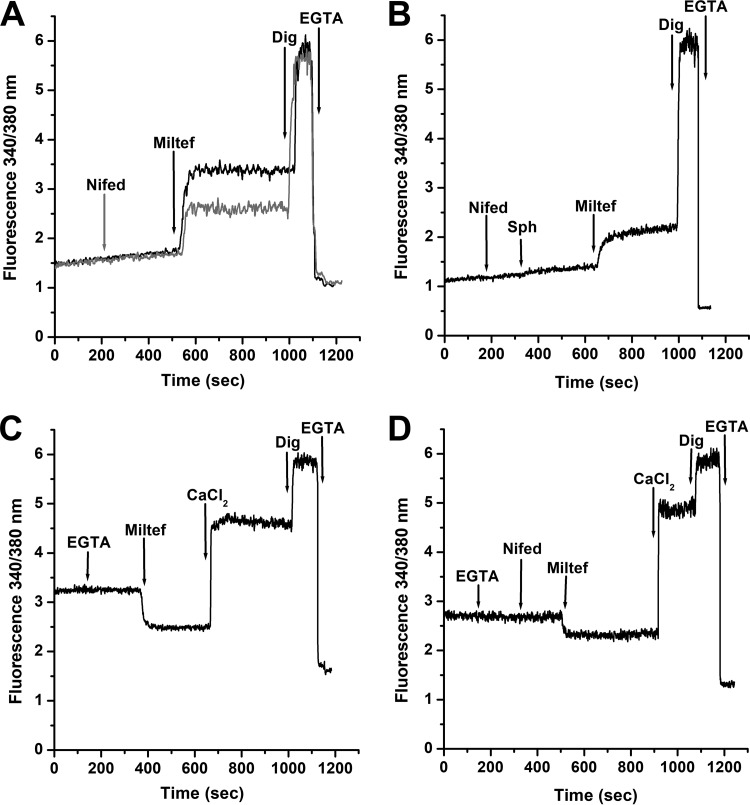
We performed experiments to determine whether the effect of a dihydropyridine (nifedipine), a classical human L-type VGCC inhibitor, was able to also block the effect of miltefosine. It was observed (Fig. 3A) that nifedipine (4 μM) partially blocks the effect generated by miltefosine, while this channel blocker produces total blockade of the sphingosine action, as previously reported in L. mexicana (29), and shown here in Fig. 3B. In these experiments, we used nifedipine at 4 μM, since this concentration is twice the amount of this antagonist known to totally block the sphingosine-activating effect on the L. mexicana channel (29). Addition of the mild detergent digitonin (40 μM), known to disrupt the permeability barrier of the plasma membrane without affecting intracellular organelles, induced a further increase in the Ca2+ signal reaching the maximal fluorescence level, as expected. Further addition of EGTA (10 mM) to chelate all extracellular Ca2+ caused the fluorescence signal to reach the lowest level. These results suggest that the mechanism of action of miltefosine is similar but not identical to that of sphingosine.
FIG 3.
Effect of the L-type VGCC channel blocker nifedipine on the action of miltefosine on the intracellular Ca2+ concentration of L. donovani promastigotes. (A) Black line indicates effects of miltefosine (4 μM) in the presence of extracellular CaCl2 (2 mM), followed by digitonin (40 μM) and EGTA (arrows), respectively. Gray line indicates effects of nifedipine (4 μM), followed by miltefosine (4 μM) in the presence of 2 mM extracellular Ca2+, followed by digitonin (40 μM) and EGTA (arrows), respectively. (B) Effect of nifedipine (4 μM) followed by sphingosine (10 μM), miltefosine (4 μM), digitonin (40 μM) and EGTA, respectively (arrows) in the presence of 2 mM extracellular Ca2+. (C) Effect of miltefosine in the absence of extracellular Ca2+. EGTA was added to chelate any contaminating extracellular Ca2+ (arrow), followed by miltefosine (4 μM), CaCl2 (2 mM), digitonin (40 μM), and EGTA, respectively. (D) Effect of miltefosine after addition of nifedipine in the absence of extracellular Ca2+. EGTA was added to chelate any contaminating extracellular Ca2+ (arrow), followed by nifedipine (4 μM), miltefosine (4 μM), CaCl2 (2 mM), digitonin (40 μM), and EGTA, respectively. Traces are representative of at least three independent experiments.
We then determined the effect of miltefosine in the absence of extracellular Ca2+. Figure 3C shows that addition of the drug, instead of inducing an increase in the [Ca2+]i, reduced it to well below the basal level. This is due to the presence of EGTA, which chelates all extracellular Ca2+ and forces the intracellular basal Ca2+ to leave the cytoplasm toward the outside medium. When Ca2+ is restored at the extracellular milieu, a large increase was now observed, indicating that the channel had indeed been opened by miltefosine.
The effect of miltefosine after the addition of nifedipine and in the absence of extracellular Ca2+ was then tested (Fig. 3D). According with the results obtained in Fig. 3A and B, the release of intracellular Ca2+ obtained after miltefosine addition in the presence of the blocker was less than that with miltefosine alone (Fig. 3C), indicating a partial blockage of the channel and confirming that nifedipine does not completely block the activating effect exerted by miltefosine on this Ca2+ channel.
Effect of miltefosine on intracellular organelles of L. donovani.
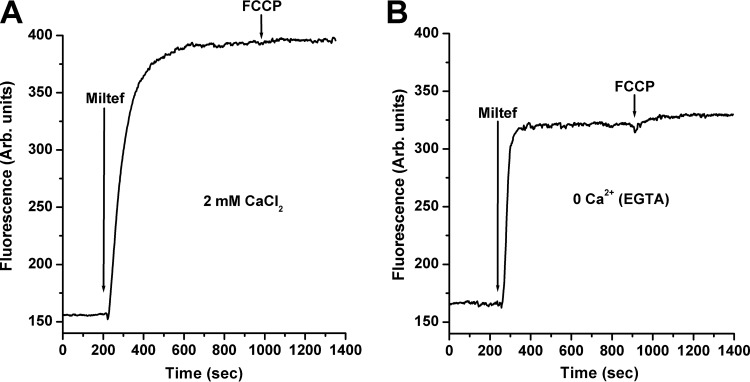
We also studied the possible effect of miltefosine on the intracellular organelles known to be involved in Ca2+ homeostasis, such as the acidocalcisomes and the unique giant mitochondrion present in these parasites. Concerning this last organelle, it was previously shown that miltefosine has a mitochondrial depolarizing effect, reported as an impairment of the ability of the parasites to accumulate rhodamine 123 after 14 h of treatment with miltefosine in L. donovani promastigotes (19). This effect was predictable since miltefosine inhibits the cytochrome c oxidase, which in turn would affect the mitochondrial membrane potential. We now show that miltefosine produces a very short-term collapse of the mitochondrial electrochemical membrane potential, since a fast, large increase in rhodamine 123 fluorescence was observed upon addition of miltefosine (Fig. 4A). In principle, this effect was also predictable, since miltefosine induces the entrance of Ca2+ and therefore its accumulation in the mitochondrion. This is known to occur via a mitochondrial Ca2+ uniporter (31) also present in the Leishmania parasite (32), whose driving force is the mitochondrial electrochemical membrane potential. Thus, any Ca2+ entry would dissipate this potential, which will be translated into the release of rhodamine 123. However, the depolarizing effect of miltefosine on the parasite's mitochondrion was also observed, albeit at a lesser extent, in the absence of extracellular Ca2+ (Fig. 4B), indicating that this effect is partially independent of the entrance of the cation to the cell, but is a direct effect of miltefosine on this organelle. In both extracellular Ca2+ condition experiments we added the mitochondrial electron chain uncoupler fluorocarbonyl cyanide-p-(trifluoromethoxy)phenylhydrazone (FCCP) (2 μM), which is expected to completely deenergize the mitochondria at this concentration (32, 33). Only a small response was obtained for this effector after miltefosine was added in the absence of extracellular Ca2+ (Fig. 4B), confirming again the large effect of miltefosine on this organelle and its partial dependence on the entrance of extracellular Ca2+.
FIG 4.
Effect of miltefosine on the mitochondrial electrochemical potential of L. donovani promastigotes. Parasites were incubated in the presence of rhodamine123 (10 mg/ml) for 30 min at room temperature, as indicated in Materials and Methods. (A) In the presence of 2 mM extracellular Ca2+, miltefosine (4 μM) was added (arrow), followed by FCCP (2 μM). (B) Miltefosine (4 μM) was added (arrow), followed by addition of FCCP (2 μM). This was performed in the absence of extracellular Ca2+. Traces are representative of at least three independent experiments.
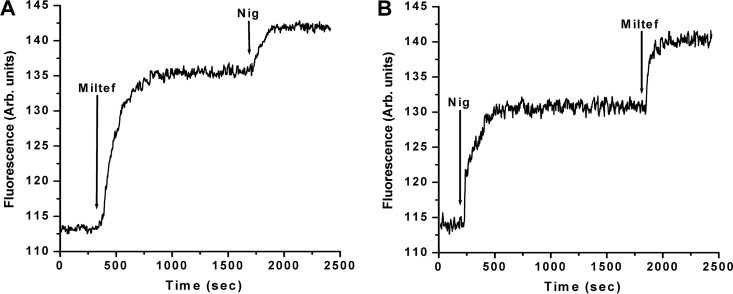
We then studied the effect of miltefosine in another very relevant compartment, associated as well with intracellular Ca2+ regulation and also involved in the L. donovani parasite's bioenergetics, the acidocalcisomes (34). We determined whether miltefosine had an effect on this organelle by the use of acridine orange, which is known to be accumulated in acidic reservoirs. These experiments were performed in the absence of extracellular Ca2+, to exclude the possible effect associated with the entrance of Ca2+ through the plasma membrane channel to the cytoplasm that could interfere with the basal Ca2+ content in the acidocalcisomes, and therefore with the degree of acidity. Figure 5A shows that the addition of miltefosine (4 μM) to promastigotes loaded with the fluorescent indicator produced a large increase in fluorescence due to the release of the fluorophore from the acidocalcisomes after its alkalinization by action of the drug. The consecutive addition of nigericin (2 μM), a known K+/H+ exchanger and therefore an inducer of the alkalinization of these organelles, produced a further increase in fluorescence. This might mean that miltefosine was not able to completely alkalinize the acidocalcisomes, or alternatively it would imply the existence in the parasite of other acidic compartments affected by this drug. In Fig. 5B we performed the same experiment but inverted the order of addition of the effectors. It can be noticed that nigericin induced alkalinization of the acidocalcisomes. Addition of miltefosine after nigericin induced a further effect, which could be attributed to the action of this compound on different acidic organelles in which acridine orange accumulates.
FIG 5.
Effect of miltefosine on acidocalcisomes in L. donovani promastigotes. Parasites were loaded with acridine orange (2 mM) as described in Materials and Methods. The excitation wavelength was 488 nm, and emission was at 530 nm. (A) Miltefosine (4 μM) was added (arrow) directly to the stirring cuvette with promastigotes loaded with acridine orange, followed by the addition of nigericin (2 μM). This was performed in the absence of extracellular Ca2+. (B) In the absence of extracellular Ca2+, nigericin was added at 2 μM (arrow), followed by miltefosine (4 μM). Traces are representative of at least three independent experiments.
DISCUSSION
Miltefosine is the first oral drug prescribed against leishmaniasis and its effects against L. donovani are well known, the Old World visceral-leishmaniasis-causing agent. Nevertheless, until the last decade little was known about the mechanism of action of this drug. One of its most remarkable effects is the inhibition of phosphatidylcholine synthesis, being 10 to 20 times more selective for the phosphatidylethanolamine N-methyl-transferase from the parasite compared to its human counterpart, thus explaining its large selectivity against trypanosomatids (15). Other relevant well-described effects of miltefosine are associated with its action on the cytochrome c oxidase, explaining the disruption of overall mitochondrial function (19). In this work we report new mechanisms of action for miltefosine. First, we demonstrated that this compound activates a plasma membrane Ca2+ channel in L. donovani, similar to the human L-type VGCC homolog previously described in L. mexicana. Thus, similar to its human counterpart, the channel is activated by the specific L-type VGCC agonist Bay K 8644 and is blocked by dihydropyridines (like nifedipine), which are classical L-type VGCC antagonists. Also similar to the Ca2+channel from L. mexicana, this channel is opened by the sphingolipid sphingosine, which is a distinctive feature of the trypanosomatid channel (29). In this context, disruption of intracellular Ca2+ homeostasis has been recognized as a putative target for drug action on trypanosomatids (35). The action of many other drugs used against these parasites, such as pentamidine (36), amiodarone (10, 21, 22, 22), dronedarone (23, 24, 37), and SQ109 (25, 26) is mainly through disruption of Ca2+ regulation. Accordingly, a large Ca2+ entrance to the cell induced by miltefosine should produce a massive impairment of Ca2+ function, causing the death of the parasite.
We also demonstrated in this work that miltefosine produced a dramatic, fast, and direct effect on the acidocalcisomes of L. donovani. This would also cause an increase in cytoplasmic Ca2+, since alkalinization of these organelles would lead to the release of this cation, thus adding its effect to the action produced by Ca2+ entrance through the plasma membrane Ca2+ channel. Besides, acidocalcisome impairment would have consequences on the bioenergetics of the parasite, since this organelle is involved in the production and accumulation of pyrophosphates (38), which are considered an alternative energetic coin in trypanosomatids. In turn, this effect should reinforce the well-recognized action of miltefosine on the mitochondrion, since, as mentioned, this drug inhibits the cytochrome c oxidase, which produces impairment of the membrane electrochemical membrane potential, the driving force for Ca2+ accumulation inside this organelle (32). Related to this point, we cannot discard a possible direct effect of miltefosine on the mitochondrial function, beyond its action on the cytochrome c oxidase. Thus, the experiments performed in this work showing the total collapse of the mitochondrial electrochemical membrane potential in seconds, very different from the previously reported long-lasting effect of miltefosine on the membrane potential observed after several hours (19), would support a third effect of this compound in these parasites. This is reinforced by the fact that the results obtained when the experiments were performed in the absence of extracellular Ca2+ were very similar, thus discarding an effect that could be attributed to Ca2+ entry to the cell through the just-opened plasma membrane Ca2+ channel, which would induce the entry of the cation to the mitochondria, causing the collapse of its membrane potential. Although these experiments are not conclusive, this possibility remains open. The results suggest that the effect discussed on the mitochondrial membrane potential induced by miltefosine would reinforce its global effect on the increase in the intracellular Ca2+ concentration, with the expected overall consequences on the parasite biology. This large increase in the intracellular concentration of this cation could be also the basis for the apoptotic effects on these parasites attributed to miltefosine (20), since it have been demonstrated that an increase in cytoplasmic Ca2+ concentration is a condition for cells to take the decision to start the apoptotic fate (39).
In concerns to the presence of a sphingosine-activated homolog of the human L-type VGCC in L. donovani, Fig. 6 depicts the sequence alignment of the α1C subunit of the human channel with L. mexicana and L. donovani homologs on the relevant domains, including the binding sites for the specific channel blocker nifedipine. This sequence alignment shows that, although there is a 26% homology between the human and the L. donovani sequences, there is a 100% homology sequences of the two Leishmania species. Furthermore, the complete sequence of the gene for the L. mexicana channel is about 94% similar to the L. donovani sequence (40). This high homology between this two species explains the similarities observed during this work concerning the opening of the channel by sphingosine, as well as the Bay K 8644 activation and nifedipine antagonism of this parasite channel.
FIG 6.
Sequence alignments of the IIIS6 and IVS6 domains of human L-Type VGCC (NCBI accession number NP_955630.3) with L. mexicana (NCBI accession number XP_003878633.1, gene ID according to TriTrypDB LmxM.33.0480) and L. donovani (NCBI accession number CBZ37533.1, gene ID according to TriTrypDB LdBPK_340500.1) homologs. The amino acid sequences next to the selectivity filter are highlighted in gray and the amino acids associated with dihydropyridines (nifedipine) responsiveness are highlighted in gray and underlined (29).
In conclusion, the results shown demonstrate a double effect of miltefosine on L. donovani, namely, the opening of the sphingosine-activated plasma membrane Ca2+ channel and a direct effect on the acidocalcisomes, which in combination should produce a large intracellular Ca2+ accumulation. Interestingly, both mechanisms of actions are parasite-specific. Both effects are correlated with the abrupt increase in the intracellular Ca2+ concentration observed in L. donovani upon addition of miltefosine. Since the disruption of the parasite Ca2+ homeostasis has been claimed as a target for the action of several drugs against trypanosomatids, the results presented here, added to the well-recognized action of miltefosine on phospholipid synthesis and on cytochrome c oxidase inhibition, would contribute to the dramatic parasite death induced by this drug and could explain the large benefits attributed to miltefosine.
MATERIALS AND METHODS
Chemicals.
Miltefosine (hexadecyl phosphocholine), sphingosine, Bay K 8644, verapamil, EGTA, digitonin, fluorocarbonyl cyanide-p-(trifluoromethoxy)phenylhydrazone (FCCP), and nigericin were from Sigma (St. Louis, MO). Fura-2-acetoxymethyl ester (Fura-2-AM), acridine orange, and rhodamine 123 were from Molecular Probes (Eugene, OR).
Culture of L. donovani promastigotes.
L. donovani (DD8 strain) promastigotes were cultured in liver infusion tryptose (LIT) medium supplemented with 10% of fetal bovine serum at 26°C as reported previously (7).
Intracellular Ca2+ measurements.
L. donovani promastigotes were loaded with the Ca2+ radiometric indicator Fura-2 as reported previously (24). The fluorophore Fura-2 is excited by two different wavelengths, 340 nm when it is Ca2+-bound and 380 nm when it is free of Ca2+, and emission is recorded at a unique wavelength of 510 nm. Briefly, 1 × 107 parasites were collected by centrifugation at 600 × g for 2 min and washed twice in a loading buffer (137 mM NaCl, 4 mM KCl, 1.5 mM KH2PO4, 8.5 mM Na2HPO4, 11 mM glucose, 1.8 mM CaCl2, 0.8 mM MgSO4, 20 mM HEPES-NaOH [pH 7.4]). The pellet was resuspended in 1 μM Fura-2-AM (the acetoxymethyl ester derivative of Fura-2), and probenecid (2.4 mM) and pluronic acid (0.05%) were added to the loading buffer. The parasites were incubated at 29°C in the dark with continuous agitation for 2 h. Fura-2-AM-loaded parasites were washed twice in the same buffer, in either the presence or absence of Ca2+. The CaCl2 concentration used in all the experiments where the cation was present was done in the presence of 2 mM Ca2+, mimicking the extracellular concentration present in the growth medium. Additionally, EGTA (500 μM) was added when measurements were made in the absence of extracellular Ca2+. This concentration of EGTA is high enough to chelate all possible contaminant Ca2+ and to lower its concentration to a level which favorably competes with the fluorescent Ca2+ indicator, making it possible to obtain the minimal fluorescence value. Digitonin (40 μM) is known to permeabilize the parasite cell membrane, allowing Ca2+ entrance from the extracellular medium (22). 10 mM EGTA was added at the end of the experiments in order to obtain the maximal and minimal fluorescence values, respectively (29). Fluorescence measurements were carried out on a stirred cuvette at 29°C, using a Perkin-Elmer LS-55 fluorescence spectrometer with a double wavelength excitation beam (340 nm and 380 nm), recording the emission at 510 nm.
Determination of the mitochondrial membrane potential.
The effect of miltefosine on the mitochondrial membrane potential of L. donovani promastigotes was evaluated using the fluorescent dye rhodamine 123 as reported previously (21), taking advantage of the internationalization of the fluorophore, according to the mitochondrial electrochemical membrane potential (Δφ). Briefly, 8 × 106 parasites were collected by centrifugation at 600 × g for 2.5 min and washed in phosphate-buffered saline (PBS) plus 1% glucose. The pellet was resuspended in the same buffer in the presence of rhodamine 123 (20 μM) and incubated for 45 min at 29°C in the dark with continuous stirring. Subsequently, parasites were washed twice and resuspended in the same buffer, and then transferred to a stirred cuvette. Measurements (excitation wavelength [λext], 488 nm; emission wavelength [λem], 530 nm) were made in a Hitachi 7000 spectrofluorimeter at 29°C. The protonophore FCCP (2 μM) was used as a positive control.
Determination of acidocalcisome alkalinization.
The effect of miltefosine on acidocalcisomes was evaluated using acridine orange, which is accumulated in acidic compartments (22). Promastigotes (8 × 106 cells/ml) were collected, washed, and incubated in a loading buffer (the same used in mitochondrial membrane potential measurements) with acridine orange at 2 μM for 5 min at 29°C in the dark and with constant stirring. Measurements were performed with λext at 488 nm and λem at 530 nm at 29°C in a Hitachi 7000 spectrofluorimeter under magnetic stirring. Nigericin, a K+/H+ exchanger that is known to alkalinize the acidocalcisomes, was used at 2 μM as a positive control. This concentration of nigericin exceeds the amount required for complete release of acridine orange from acidocalcisomes (41).
ACKNOWLEDGMENTS
We thank Lourdes Plaza from Loyola University and Cecilia Castillo from Instituto de Estudios Avanzados (IDEA) for critically revising the manuscript.
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (FONACIT) grant no. 2017000274, Venezuela, and the Consejo de Desarrollo Científico y Humanístico from the Universidad Central de Venezuela (CDCH—UCV) grant PG 03-8728-2013/2 to G.B.
We declare that we have no conflicts of interest.
REFERENCES
- 1.Ready PD. 2014. Epidemiology of visceral leishmaniasis. Clin Epidemiol 6:147–154. doi: 10.2147/CLEP.S44267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freitas-Junior LH, Chatelain E, Kim HA, Siqueira-Neto JL. 2012. Visceral leishmaniasis treatment: what do we have, what do we need and how to deliver it? Int J Parasitol Drugs Drug Resist 2:11–19. doi: 10.1016/j.ijpddr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft SL, Neal RA, Pendergast W, Chan JH. 1987. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem Pharmacol 36:2633–2636. doi: 10.1016/0006-2952(87)90543-0. [DOI] [PubMed] [Google Scholar]
- 4.Eibl H, Unger C. 1990. Hexadecylphosphocholine: a new and selective antitumor drug. Cancer Treat Rev 17:233–242. doi: 10.1016/0305-7372(90)90053-I. [DOI] [PubMed] [Google Scholar]
- 5.Croft SL, Snowdon D, Yardley V. 1996. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J Antimicrob Chemother 38:1041–1047. doi: 10.1093/jac/38.6.1041. [DOI] [PubMed] [Google Scholar]
- 6.Jha TK, Sundar S, Thakur CP, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med 341:1795–1800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 7.Morais-Teixeira E de, Damasceno QS, Galuppo MK, Romanha AJ, Rabello A. 2011. The in vitro leishmanicidal activity of hexadecylphosphocholine (miltefosine) against four medically relevant Leishmania species of Brazil. Mem Inst Oswaldo Cruz 106:475–478. doi: 10.1590/S0074-02762011000400015. [DOI] [PubMed] [Google Scholar]
- 8.Escobar P, Matu S, Marques C, Croft SL. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Trop 81:151–157. doi: 10.1016/S0001-706X(01)00197-8. [DOI] [PubMed] [Google Scholar]
- 9.Tiwari B, Pahuja R, Kumar P, Rath SK, Gupta KC, Goyal N. 2017. Nanotized curcumin and miltefosine, a potential combination for treatment of experimental visceral leishmaniasis. Antimicrob Agents Chemother 61:e01169-16. doi: 10.1128/AAC.01169-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrano-Martín X, Payares G, De Lucca M, Martinez JC, Mendoza-León A, Benaim G. 2009. Amiodarone and miltefosine act synergistically against Leishmania mexicana and can induce parasitological cure in a murine model of cutaneous leishmaniasis. Antimicrob Agents Chemother 53:5108–5113. doi: 10.1128/AAC.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farca AM, Miniscalco B, Badino P, Odore R, Monticelli P, Trisciuoglio A, Ferroglio E. 2012. Canine leishmaniosis: in vitro efficacy of miltefosine and marbofloxacin alone or in combination with allopurinol against clinical strains of Leishmania infantum. Parasitol Res 110:2509–2513. doi: 10.1007/s00436-011-2792-7. [DOI] [PubMed] [Google Scholar]
- 12.Faucher J-F, Morquin D, Reynes J, Chirouze C, Hoen B, Le Moing V. 2016. Serial use of pentamidine and miltefosine for treating Leishmania infantum-HIV coinfection. Parasitol Int 65:444–446. doi: 10.1016/j.parint.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Sundar S, Gupta LB, Makharia MK, Singh MK, Voss A, Rosenkaimer F, Engel J, Murray HW. 1999. Oral treatment of visceral leishmaniasis with miltefosine. Ann Trop Med Parasitol 93:589–597. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal VK, Singh Z. 2006. Miltefosine: First oral drug for treatment of visceral leishmaniasis. Med J Armed Forces India 62:66–67. doi: 10.1016/S0377-1237(06)80162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbina JA. 2006. Mechanisms of action of lysophospholipid analogues against trypanosomatid parasites. Trans R Soc Trop Med Hyg 100(Suppl 1):S9–S16. doi: 10.1016/j.trstmh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Lira R, Contreras LM, Rita RMS, Urbina JA. 2001. Mechanism of action of anti-proliferative lysophospholipid analogues against the protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. J Antimicrob Chemother 47:537–546. doi: 10.1093/jac/47.5.537. [DOI] [PubMed] [Google Scholar]
- 17.Wieder T, Orfanos CE, Geilen CC. 1998. Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine. J Biol Chem 273:11025–11031. doi: 10.1074/jbc.273.18.11025. [DOI] [PubMed] [Google Scholar]
- 18.Rakotomanga M, Blanc S, Gaudin K, Chaminade P, Loiseau PM. 2007. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob Agents Chemother 51:1425–1430. doi: 10.1128/AAC.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luque-Ortega JR, Rivas L. 2007. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob Agents Chemother 51:1327–1332. doi: 10.1128/AAC.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paris C, Loiseau PM, Bories C, Bréard J. 2004. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother 48:852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benaim G, Sanders JM, Garcia-Marchán Y, Colina C, Lira R, Caldera AR, Payares G, Sanoja C, Burgos JM, Leon-Rossell A, Concepcion JL, Schijman AG, Levin M, Oldfield E, Urbina JA. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem 49:892–899. doi: 10.1021/jm050691f. [DOI] [PubMed] [Google Scholar]
- 22.Serrano-Martín X, García-Marchan Y, Fernandez A, Rodriguez N, Rojas H, Visbal G, Benaim G. 2009. Amiodarone destabilizes intracellular Ca2+ homeostasis and biosynthesis of sterols in Leishmania mexicana. Antimicrob Agents Chemother 53:1403–1410. doi: 10.1128/AAC.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benaim G, Hernandez-Rodriguez V, Mujica-Gonzalez S, Plaza-Rojas L, Silva ML, Parra-Gimenez N, Garcia-Marchan Y, Paniz-Mondolfi A, Uzcanga G. 2012. In vitro anti-Trypanosoma cruzi activity of dronedarone, a novel amiodarone derivative with an improved safety profile. Antimicrob Agents Chemother 56:3720–3725. doi: 10.1128/AAC.00207-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benaim G, Casanova P, Hernandez-Rodriguez V, Mujica-Gonzalez S, Parra-Gimenez N, Plaza-Rojas L, Concepcion JL, Liu Y-L, Oldfield E, Paniz-Mondolfi A, Suarez AI. 2014. Dronedarone, an amiodarone analog with improved anti-Leishmania mexicana efficacy. Antimicrob Agents Chemother 58:2295–2303. doi: 10.1128/AAC.01240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veiga-Santos P, Li K, Lameira L, Carvalho TMU de, Huang G, Galizzi M, Shang N, Li Q, Gonzalez-Pacanowska D, Hernandez-Rodriguez V, Benaim G, Guo R-T, Urbina JA, Docampo R, Souza W de, Oldfield E. 2015. SQ109, a new drug lead for chagas disease. Antimicrob Agents Chemother 59:1950–1961. doi: 10.1128/AAC.03972-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-García V, Oldfield E, Benaim G. 2016. Inhibition of Leishmania mexicana growth by the tuberculosis drug SQ109. Antimicrob Agents Chemother 60:6386–6389. doi: 10.1128/AAC.00945-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Rycker M, Thomas J, Riley J, Brough SJ, Miles TJ, Gray DW. 2016. Identification of trypanocidal activity for known clinical compounds using a new Trypanosoma cruzi hit-discovery screening cascade. PLoS Negl Trop Dis 10:e0004584. doi: 10.1371/journal.pntd.0004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashif M, Manna PP, Akhter Y, Alaidarous M, Rub A. 2017. Screening of novel inhibitors against Leishmania donovani calcium ion channel to fight leishmaniasis. Infect Disord Drug Targets 17:120–129. doi: 10.2174/1871526516666161230124513. [DOI] [PubMed] [Google Scholar]
- 29.Benaim G, García-Marchán Y, Reyes C, Uzcanga G, Figarella K. 2013. Identification of a sphingosine-sensitive Ca2+ channel in the plasma membrane of Leishmania mexicana. Biochem Biophys Res Commun 430:1091–1096. doi: 10.1016/j.bbrc.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Verma NK, Dey CS. 2004. possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob Agents Chemother 48:3010–3015. doi: 10.1128/AAC.48.8.3010-3015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benaim G, Bermudez R, Urbina JA. 1990. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol Biochem Parasitol 39:61–68. doi: 10.1016/0166-6851(90)90008-A. [DOI] [PubMed] [Google Scholar]
- 33.Vercesi AE, Bernardes CF, Hoffmann ME, Gadelha FR, Docampo R. 1991. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J Biol Chem 266:14431–14434. [PubMed] [Google Scholar]
- 34.Vercesi AE, Rodrigues CO, Catisti R, Docampo R. 2000. Presence of a Na+/H+ exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett 473:203–206. doi: 10.1016/S0014-5793(00)01531-3. [DOI] [PubMed] [Google Scholar]
- 35.Benaim G, Garcia CR. 2011. Targeting calcium homeostasis as the therapy of Chagas' disease and leishmaniasis—a review. Trop Biomed 28:471–481. [PubMed] [Google Scholar]
- 36.Benaim G, Lopez-Estraño C, Docampo R, Moreno SN. 1993. A calmodulin-stimulated Ca2+ pump in plasma-membrane vesicles from Trypanosoma brucei; selective inhibition by pentamidine. Biochem J 296(Part 3):759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benaim G, Paniz Mondolfi AE. 2012. The emerging role of amiodarone and dronedarone in Chagas disease. Nat Rev Cardiol 9:605–609. doi: 10.1038/nrcardio.2012.108. [DOI] [PubMed] [Google Scholar]
- 38.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SNJ. 2005. Acidocalcisomes—conserved from bacteria to man. Nat Rev Microbiol 3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 39.Pimentel AA, Benaim G. 2012. [Ca2+ and sphingolipids as modulators for apoptosis and cancer]. Invest Clin 53:84–110. (In Spanish.) [PubMed] [Google Scholar]
- 40.Downing T, Imamura H, Decuypere S, Clark TG, Coombs GH, Cotton JA, Hilley JD, de Doncker S, Maes I, Mottram JC, Quail MA, Rijal S, Sanders M, Schönian G, Stark O, Sundar S, Vanaerschot M, Hertz-Fowler C, Dujardin J-C, Berriman M. 2011. Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Res 21:2143–2156. doi: 10.1101/gr.123430.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Docampo R, Scott DA, Vercesi AE, Moreno SN. 1995. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem J 310(Part 3):1005–1012. doi: 10.1042/bj3101005. [DOI] [PMC free article] [PubMed] [Google Scholar]