ABSTRACT
The objective of this study was to analyze the relationship between the pharmacokinetic (PK)/pharmacodynamic (PD) parameters of a single 2-g dose of extended-release formulation of azithromycin (AZM-SR) and its microbiological efficacy against gonococcal urethritis. Fifty male patients with gonococcal urethritis were enrolled in this study. In 36 patients, the plasma AZM concentrations were measured using liquid chromatography-tandem mass spectrometry, the AZM MIC values for the Neisseria gonorrhoeae isolates were determined, and the microbiological outcomes were assessed. AZM-SR monotherapy eradicated N. gonorrhoeae in 30 (83%) of the 36 patients. AZM MICs ranged from 0.03 to 2 mg/liter. The mean value of the area under the concentration-time curve (AUC), estimated by population PK analysis using a two-compartment model, was 20.8 mg · h/liter. Logistic regression analysis showed that the PK/PD target value required to predict an N. gonorrhoeae eradication rate of ≥95% was a calculated AUC/MIC of ≥59.5. The AUC/MIC value was significantly higher in patients who achieved microbiological cure than in patients who achieved microbiological failure. Monte Carlo simulation using this MIC distribution revealed that the probability that AZM-SR monotherapy would produce an AUC/MIC exceeding the AUC/MIC target of 59.5 was 47%. Furthermore, the MIC distribution for strains isolated in this study was mostly consistent with that for strains currently circulating in Japan. In conclusion, in Japan, AZM-SR monotherapy may not be effective against gonococcal urethritis. Therefore, use of a single 2-g dose of AZM-SR either with or without other antibiotics could be an option to treat gonococcal urethritis if patients are allergic to ceftriaxone and spectinomycin or are diagnosed to be infected with an AZM-sensitive strain.
KEYWORDS: azithromycin, gonococcal urethritis, PK/PD, Neisseria gonorrhoeae, Monte Carlo simulation
INTRODUCTION
Gonorrhea is one of the most common sexually transmitted infections. Although several antimicrobial agents have been recommended for the treatment of gonorrhea, the introduction of new agents has repeatedly led to the development of resistance to these agents in clinical strains of Neisseria gonorrhoeae (1). Fluoroquinolones and oral third-generation cephalosporins were recommended as first-line agents for the treatment of gonorrhea after the emergence and spread of penicillin-resistant N. gonorrhoeae (2). However, these agents are no longer used to treat gonorrhea because of increases in the numbers of clinical strains exhibiting resistance to them (3). Although ceftriaxone monotherapy has been recommended as another first-line treatment (4), some clinical strains of N. gonorrhoeae exhibiting decreased susceptibility to ceftriaxone have already emerged (5, 6). Currently, we cannot expect the rapid development of promising new agents to treat gonorrhea caused by antimicrobial-resistant N. gonorrhoeae strains; therefore, it is necessary to manipulate existing agents to enhance their efficacy toward gonorrhea and prevent the emergence and spread of antimicrobial-resistant N. gonorrhoeae strains.
Azithromycin (AZM), a macrolide antibiotic containing a 15-member azalactone ring, exhibits antimicrobial activity against N. gonorrhoeae. Some studies report a good efficacy of a single 1-g dose of conventional AZM against gonorrhea (7, 8). However, since the early 1990s, clinical strains exhibiting decreased susceptibility to AZM have been observed in several countries worldwide (9–11). Although a single 2-g dose of an immediate-release formulation of AZM (conventional AZM) remains effective against uncomplicated gonorrhea (12), this monotherapy could cause an unacceptable rate of adverse events, particularly gastrointestinal upset. Recently, a single 2-g dose of a microsphere-based extended-release formulation of AZM (AZM-SR) was developed and was found to be effective against N. gonorrhoeae (13–15), and the frequency of adverse events also decreased compared with that achieved with a single 2-g dose of conventional AZM (12, 15–18). Therefore, a single 2-g dose of AZM-SR is considered useful for the treatment of gonorrhea. Generally, the efficacy of antimicrobial agents is known to be correlated with their pharmacokinetic (PK)/pharmacodynamic (PD) properties. With regard to AZM, previous studies have reported that the probability of success according to the clinical and bacteriological responses of respiratory tract infections is positively associated with the area under the concentration-time curve (AUC)/MIC ratio (19). However, the PK/PD properties of AZM-SR in Japanese male patients with gonococcal urethritis have been unclear. To find the target value of the PK/PD parameters positively associated with the efficacy of AZM-SR, we analyzed the relationship between the PK/PD properties and the efficacy of a single 2-g dose of AZM-SR for the treatment of gonococcal urethritis. Additionally, we explored the prospects of the regimen as an alternative treatment for gonorrhea caused by currently circulating clinical strains of N. gonorrhoeae using Monte Carlo simulation.
RESULTS
Efficacy of AZM-SR.
Overall, 13 patients who did not revisit the clinic for further examination and 1 additional patient were excluded because of a lack of laboratory data. Of the remaining 36 patients, 30 (83%) were judged to be microbiologically cured after treatment with AZM-SR. There was no significant difference in the background characteristics between the patients who achieved a microbiological cured and those who achieved a microbiological failure (Table 1).
TABLE 1.
Background characteristics of subjects treated for gonococcal urethritis with a single 2-g dose of AZM-SRc
| Characteristic | Value(s) for subjects who achieved microbiological: |
P valueb | |||
|---|---|---|---|---|---|
| Cure (n = 30) |
Failure (n = 6) |
||||
| Mean ± SD | Range | Mean ± SD | Range | ||
| Age (yr) | 31.5 ± 8.2 | 20–54 | 30.7 ± 9.4 | 24–45 | 0.83 |
| Wt (kg) | 68.5 ± 11.3 | 55–100 | 67.3 ± 13.8 | 47–80 | 0.82 |
| WBC count (no./μl) | 6,677 ± 2,457 | 3,800–16,500 | 7,083 ± 1,938 | 4,700–8,800 | 0.71 |
| AST concn (IU/liter) | 19.8 ± 10.7 | 12–72 | 20.3 ± 6.9 | 12–31 | 0.91 |
| ALT concn (IU/liter) | 24.0 ± 28.5 | 9–168 | 21.5 ± 10.7 | 8–39 | 0.84 |
| ALP concn (U/liter) | 234.0 ± 65.5 | 159–462 | 246.8 ± 58.8 | 139–315 | 0.66 |
| T-bil concn (mg/dl) | 0.63 ± 0.23 | 0.1–1.1 | 0.67 ± 0.25 | 0.3–0.9 | 0.70 |
| Cr concn (mg/dl) | 0.83 ± 0.09 | 0.65–0.98 | 0.86 ± 0.10 | 0.71–0.94 | 0.38 |
| CLCRa (ml/min) | 125.6 ± 22.7 | 95.1–187.1 | 117.8 ± 17.9 | 99.0–143.2 | 0.44 |
CLCR was calculated using the Cockcroft-Gault equation.
Determined by Student's t test.
The data are for 36 subjects. WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; T-bil, total bilirubin; Cr, creatinine; CLCR, creatinine clearance.
MIC.
The AZM MICs of the strains collected from the 36 patients ranged from 0.03 to 2 mg/liter. The distribution of the AZM MICs against the N. gonorrhoeae strains is shown in Table 2. All cases of microbiological failure were caused by strains with an AZM MIC of ≥0.5 mg/liter.
TABLE 2.
Microbiological efficacy of a single 2-g dose of AZM-SR for gonococcal urethritis treatment
| MIC (mg/liter) of AZM | No. of isolates from subjects who achieved microbiologicala: |
|
|---|---|---|
| Cure | Failure | |
| 0.03 | 1 | 0 |
| 0.06 | 1 | 0 |
| 0.125 | 0 | 0 |
| 0.25 | 15 | 0 |
| 0.5 | 11 | 3 |
| 1 | 2 | 1 |
| 2 | 0 | 2 |
| Total | 30 | 6 |
The judgments were performed microbiologically.
Determination of plasma AZM concentrations.
Plasma AZM concentrations were determined using our established method without interference from matrix products (Fig. 1A). The calibration curve was linear from 10 to 300 ng/ml, with correlation coefficients being >0.99. All validations, including intraday and interday accuracies and stabilities, met the criteria (within ±15%). The rates of AZM recovery ranged from 83.6% to 99.9%. The limits of quantification and detection were 2.3 and 0.7 ng/ml, respectively (signal-to-noise ratios, 10 and 3, respectively).
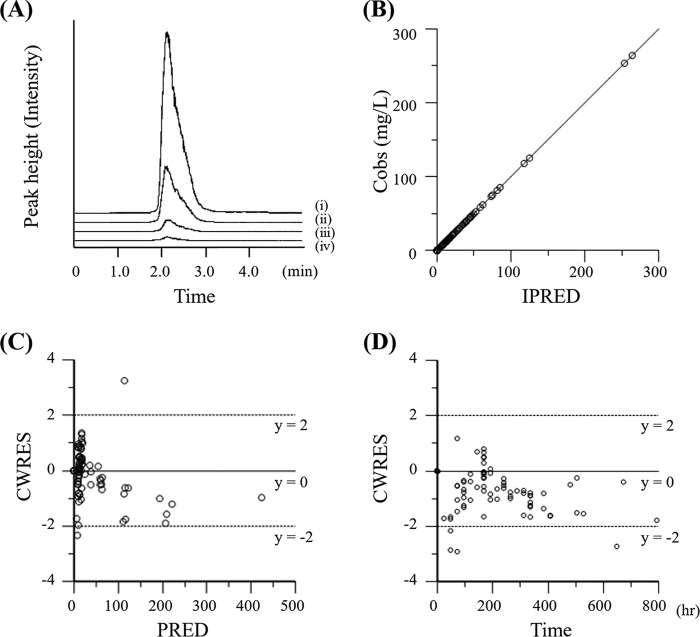
FIG 1.
Typical AZM LC-MS/MS chromatograms and goodness-of-fit plots for the final PK model. (A) Chromatograms showing the results for plasma AZM concentrations of 300 (i), 100 (ii), 30 (iii), and 10 (iv) ng/ml; (B) scatter plots of observed plasma AZM concentrations (Cobs) versus individual model predictions (IPRED); (C) conditional weighted residuals (CWRES) versus population predictions (PRED); (D) CWRES versus time after dose.
PK modeling and model development.
The population PK model was based on that described in a previous report (19), and a two-compartment model with age and body weight as covariates was chosen as the final model. Goodness-of-fit plots for observed versus population predicted concentrations (PRED), observed versus individual predicted concentrations (IPRED), and conditional weighted residuals (CWRES) versus population predicted concentrations are presented in Fig. 1B to D. The plots of the observed versus the predicted concentrations suggested a slight overestimation at higher concentrations and indicated that the model described the data well with no systematic bias.
PK/PD analysis.
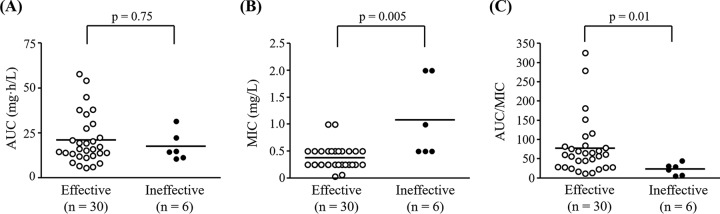
AUC values were calculated using population PK parameters and ranged from 5.7 to 58.1 mg · h/liter, with the mean value being 20.8 mg · h/liter. AUC values did not significantly differ between the patients with microbiological cure and those with microbiological failure (Fig. 2A). The mean MIC was 0.5 mg/liter (range, 0.03 to 2.0 mg/liter). In the treatment failure group, all MICs were >0.5 mg/liter. As shown in Fig. 2B, the AZM MICs in the microbiologically cured patients were significantly lower than those in patients exhibiting treatment failure. The mean AUC/MIC ratio was 68.9 (range, 5.9 to 324.9), and the AUC/MIC ratios for patients who failed treatment were significantly higher than those for patients who were cured (Fig. 2C).
FIG 2.
Comparison of AZM PK parameters (AUC, MIC, and AUC/MIC) in 36 patients with gonococcal urethritis. (A) AUC; (B) MIC; (C) AUC/MIC. Each line represents the median. P values were calculated by the Mann-Whitney U test.
The probability of the correct classification as treatment cures or failures was calculated to be 83% for AUC and 86% for AUC/MIC. The probability of microbiological cure (POC) from the AUC/MIC was computed using a logistic function, where POC = {1/[1 + e(0.798 − 0.063 × AUC/MIC)]}, and then the target value of the AUC/MIC required to predict a POC of ≥95% was determined to be 59.5 (Fig. 3).
FIG 3.
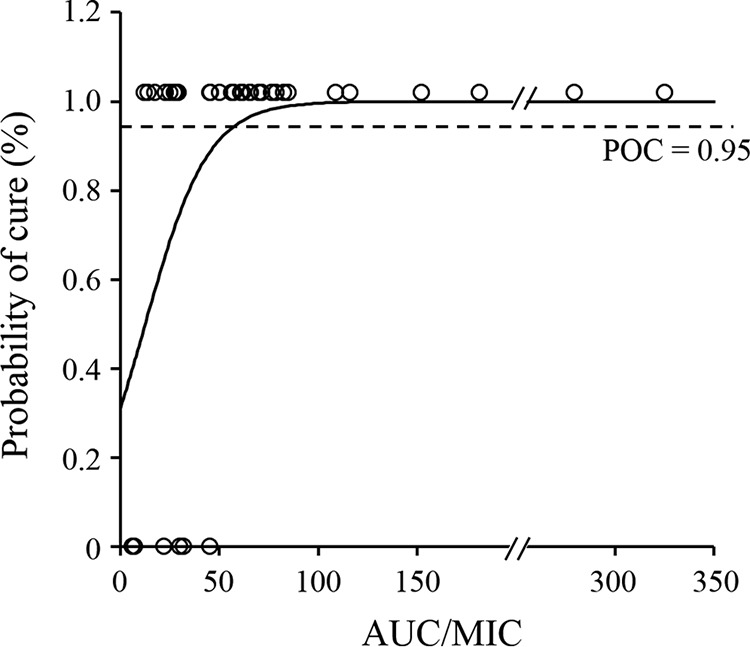
Probability of cure following AZM-SR administration. The solid line shows the logistic regression curve, and open circles represent clinical data.
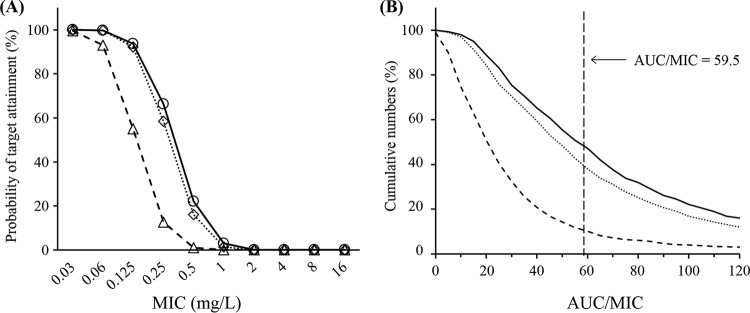
Although AZM-SR provided high a probability of target attainment (PTA; >90%) at an MIC of ≤0.125 mg/liter, the PTA for MICs of 0.25 and 0.5 mg/liter decreased to 66% and 22%, respectively (Fig. 4A). In our patient population, the PTA obtained with 2 g AZM was almost similar to that obtained with AZM-SR; however, a satisfactory PTA was not obtained with 1 g AZM, even at an MIC of >0.125 mg/liter (PTA < 55%). Furthermore, as a result of the Monte Carlo simulation according to the MIC distribution in the subjects in the present study, the cumulative fractions of responses (CFRs) of three AZM monotherapies at the target AUC/MIC value of 59.5 were calculated to be 47% for AZM-SR, 41% for 2 g AZM, and 10% for 1 g AZM (Fig. 4B).
FIG 4.
Probability of target attainment achieved with 2 g AZM-SR, 2 g AZM, and 1 g AZM at each MIC and the cumulative probability of AUC/MIC distributions calculated by Monte Carlo simulation. (A) The plots show data for 2 g AZM-SR (open circles, solid lines), 1 g AZM (open triangles, dashed lines), and 2 g AZM (open diamonds, dotted lines). (B) Each line represents 2 g AZM-SR (solid lines), 1 g AZM (dashed lines), and 2 g AZM (dotted lines).
DISCUSSION
In the present study with Japanese male patients with gonococcal urethritis, we established the way to measure plasma AZM concentrations, with good validation. Moreover, we calculated the PK parameters by the use of population PK and found that AUC/MIC ratios were related to microbiological efficacy. To our knowledge, this is the first study showing the PK parameters associated with microbiological efficacy and the target values of the PK parameters associated with microbiological efficacy.
A simple liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the measurement of AZM was established. In this method, solid-phase extraction (SPE) instead of liquid-liquid extraction was used, unlike in previous studies (20, 21). Plasma AZM concentrations were measured within 5 min, with good validation. Moreover, the measurement range was sufficient to measure the trough levels; therefore, this method was considered applicable to the PK analysis of AZM.
Estimation of the values of PK parameters optimally requires large numbers of blood samples; however, it is difficult and clinically invasive to collect multiple samples with various concentrations from patients. Moreover, iterative analysis and the development of new population models with every addition of patient data are discouraged. Therefore, the present analyses of the PK parameters were performed using the two-compartment model developed by Muto et al. (19). This model includes age and body weight as covariates, and our data showed a sufficient goodness of fit, indicating its wide applicability to the analysis of PK parameters for AZM, regardless of the disorder. Our estimated AUC value was slightly higher than that reported from a previous study of patients with respiratory tract infections (19), potentially reflecting the relatively small number of patients, with only two plasma samples from each patient being tested. These limitations warrant future studies with larger numbers of samples and patients.
Here, we attempted to elucidate the relationship between PK parameters and microbiological efficacy in Japanese male patients with gonococcal urethritis treated with a single 2-g dose of AZM-SR. As a result of determination of the PK parameters reflecting the effectiveness of AZM, an AUC/MIC of 59.5 was calculated to be the target value for predicting the rate of N. gonorrhoeae eradication (≥95%) in males with gonococcal urethritis. However, a Monte Carlo simulation using the distribution of MICs for the N. gonorrhoeae strains recovered in the present study indicated that the CFR of AZM-SR monotherapy required to exceed an AUC/MIC of 59.5 is 47%. The recent distributions of MICs for N. gonorrhoeae strains in Japan were reported to have median values of 0.125 and 0.25 mg/liter in 2002 and 2014, respectively (22), whereas in this study (conducted with isolates collected from 2014 to 2016), the median MIC was 0.5 mg/liter. The distribution of MICs for the strains isolated in our study was mostly consistent with that previously reported (22), suggesting that the strains tested are typical of those circulating in Japan. Furthermore, the proportion of strains with MICs of ≥1 mg/liter (the breakpoint of the AZM MIC) significantly increased from 6.4% in 2014 to 24.1% in 2015 (22). In this study, the breakpoint AZM MIC associated with microbiological failure was 0.5 mg/liter. These findings indicate that the susceptibility of N. gonorrhoeae isolates to AZM is decreasing; therefore, there is concern that treatment failures will increase. Taken together with the findings described in previous reports (22, 23), our results suggest that adequate therapeutic effects cannot be obtained, even upon AZM-SR administration. Although descriptions of the effectiveness of AZM for the treatment of gonococcal urethritis have been added to the guidelines revised in 2016 (4), the decreasing susceptibility of N. gonorrhoeae to AZM has been reported in Japan (22, 24). In our current study, the microbiological efficacy of a single 2-g dose of AZM-SR was 83.3%, which was lower than that previously reported (93.8%) (14). Additionally, gonococcal strains exhibiting high levels of AZM resistance (MICs ≥ 256 mg/liter) have been reported in an increasing number of countries (10, 11, 25–27). The failure of treatment of gonorrhea with a single 2-g dose of AZM-SR (28) and conventional AZM (23) has also been reported; therefore, there is concern that microbiological failures will increase. These reports suggest that the use of AZM probably could induce AZM resistance. Therefore, AZM monotherapy cannot be recommended as a first-line treatment for gonorrhea; this is consistent with the results obtained by Yasuda et al. (22). Therefore, a single use of ASM-SR should be restricted to limited cases (e.g., patients allergic to ceftriaxone).
The Monte Carlo simulation performed in this study showed that the CFRs of single 1-g and 2-g doses of conventional AZM and a single 2-g dose of AZM-SR were 10%, 41%, and 47%, respectively. These results suggest that a single use of AZM would be ineffective for the treatment of gonorrhea. The guidelines of other countries (29–32) recommend the use of a combination of AZM with other antibiotics for gonorrhea treatment. Most countries, with the exception of Canada, recommend only intramuscular ceftriaxone (250 or 500 mg as a single dose) plus oral AZM (1 to 2 g as a single dose) as a first-line treatment (29–33). In Canada, oral cefixime (800 mg as a single dose) plus oral AZM (1 g as a single dose) is also recommended as a first-line therapy (33). A novel dual antimicrobial therapy has also been evaluated: intramuscular gentamicin (240 mg as a single dose) plus oral AZM (2 g as a single dose) and oral gemifloxacin (320 mg as a single dose) plus oral AZM (2 g as a single dose) (34). This treatment with agents with different mechanisms of action may hinder resistance development (35).
With regard to adverse events, particularly gastrointestinal symptoms, a single use of 1-g and 2-g doses of conventional AZM and a 2-g dose of AZM-SR caused adverse events in <10% (8, 36), 24.4% to 35.3% (12, 18), and 17.9% to 21.5% (4, 15) of Caucasian patients, respectively. These results show that a higher dose of AZM would more frequently induce adverse events. In cases in which a single 2-g dose of AZM-SR was used, gastrointestinal symptoms were more likely to occur in Japanese patients (approximately 30%) (13, 14). However, the symptoms were mostly temporary and were resolved within a day when the patients took AZM-SR (13, 14). Taken together, when a single 2-g dose of AZM-SR is administered with or without other antibiotics to Japanese patients, the patients should be carefully monitored for gastrointestinal adverse events, particularly within 1 day after AZM-SR administration.
In conclusion, assessment of the microbiological responses to AZM treatment of gonococcal urethritis can be performed using the AUC/MIC ratio. Our present results suggest that a single 2-g dose of AZM-SR may not be effective for the treatment of gonococcal urethritis in Japan. Therefore, AZM-SR monotherapy should not be used for the treatment of gonococcal urethritis. Currently, in Japan, the guideline recommends monotherapy with intramuscular ceftriaxone (1 g as a single dose) or intramuscular spectinomycin (2 g as a single dose) as a first-line treatment of gonococcal urethritis (4). So far, no reports have indicated the failure of treatment with these two drugs. Thus, if patients are allergic to ceftriaxone and spectinomycin and/or are diagnosed to be infected with AZM-sensitive N. gonorrhoeae isolates, either the combined use of a single 2-g dose of AZM-SR and other antibiotics or monotherapy using a higher dose of AZM-SR could be an option instead of monotherapy using a single 2-g dose of AZM-SR to treat gonococcal urethritis.
MATERIALS AND METHODS
Subjects.
We enrolled 50 Japanese male patients who visited iClinic between May 2014 and January 2016 and were diagnosed with gonococcal urethritis. All patients provided written informed consent, which was approved by the ethics committees of Gifu University (reference number 26-10). N. gonorrhoeae was isolated from these patients by culture of a swab specimen from the urethra and stored at −70°C. Patients were administered a single 2-g dose of AZM-SR. Clinical data, including age, body weight, and laboratory values, such as white blood cell count, the levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, and creatinine, and creatinine clearance, were also recorded.
Microbiological outcomes.
The microbiological efficacy of AZM-SR against gonococcal urethritis was evaluated by use of the Aptima Combo 2 test for N. gonorrhoeae (Hologic, San Diego, CA, USA) and culture of a specimen collected from each patient by a clinician on every visit day between 5 and 41 days after AZM-SR administration, except for one patient who revisited 2 days after the first visit. If a positive judgment at the initial examination did not become a negative judgment by 14 days after administration, the treatment was defined to be ineffective.
Antimicrobial susceptibility testing.
The AZM MICs for the pretreatment strains of N. gonorrhoeae which were isolated from the urethra of the patients and survived storage were determined by an agar dilution method authorized by the Clinical and Laboratory Standards Institute (37), as previously described (22).
Determination of plasma AZM concentrations.
Plasma samples were collected twice, once between days 2 and 8 and then between days 7 and 33, from every patient and stored at −30°C until use in AUC/MIC testing. The plasma AZM concentrations were measured using LC-MS/MS, as previously reported (38), with slight modifications. Briefly, an LC-MS/MS system (a Waters 2695 separations module and a Waters Quattro micro-mass spectrometer; Waters Corporation, Milford, MA, USA) with a Cadenza CD-C18 column (2 mm by 100 mm; particle size, 3 μm; Imtakt Corporation, Kyoto, Japan) was set at 40°C. The mobile phase comprised 50 mM aqueous ammonium acetate and acetonitrile. The mass spectrometer was operated in the positive ionization mode to monitor transition ions m/z 749.5 → 157.9 for AZM and m/z 752.6 → 594.4 for the internal standard (IS; AZM-d3; Cosmo Bio Co., Ltd., Tokyo, Japan). The optimal parameters for MS were as follows: capillary voltage, 1.2 kV; cone voltage, 35 kV; source temperature, 110°C; and desolvation temperature, 350°C. Nitrogen gas was used for desolvation and as a cone gas at flow rates of 600 liters/h and 50 liters/h, respectively. All data were processed using a MassLynx system (Waters Corporation).
An SPE cleanup procedure was used to extract AZM from plasma (39). Briefly, 50-μl aliquots of distilled water were added to 200-μl patient plasma samples or blank plasma (Cosmo Bio Co., Ltd.) that was spiked with an AZM reference standard (Sigma-Aldrich, St. Louis, MO, USA) at 10, 30, 100, and 300 ng/ml. Subsequently, 50-μl aliquots of the IS (1,000 ng/ml) were vigorously mixed and loaded onto Oasis HLB columns (Waters Corporation) equilibrated with 1 ml of methanol and distilled water. After washing three times with 2% formic acid, the analytes were eluted with 1 ml of methanol and evaporated to dryness using a gentle stream of nitrogen. The residues were then dissolved in 100 μl of the mobile phase and sonicated at an ultrasonic frequency of 42 kHz for 10 min. Subsequently, the diluents were passed through Millex-LG filters (pore size, 0.2 μm; 4 mm; Merck, Darmstadt, Germany), and 15-μl aliquots were injected into the LC-MS/MS system.
The selectivity, calibration curve, accuracy, intraday and interday precisions, recovery, and stability were validated according to Food and Drug Administration guidelines (40).
Population PK analysis.
Population PK analysis of AZM-SR was performed using the Phoenix NLME program (v1.3; Certara, Princeton, NJ, USA). On the basis of the findings of a previous study (19), the population PK model was generated and evaluated using the nonparametric bootstrap and visual predictive check options.
PK/PD analysis and simulation.
AUC was calculated using the PK parameters obtained from the population PK analysis. To explore the relationship between the PK/PD parameters and antimicrobial effects, the predictive values of the microbiological efficacies were calculated for the PK parameters, such as AUC, the maximum concentration (Cmax), the AUC/MIC ratio, and Cmax/MIC. The POC was computed using logistic regression, and the PK/PD target values were defined as the threshold for a 95% POC (41).
Simulation of the AUC for 1,000 cases was performed using the PK data from this study for AZM-SR and from previous studies for 1-g and 2-g doses of AZM (19, 42, 43) and Phoenix NLME software (Certara). AUC and MIC data were combined by Monte Carlo simulation (Crystal Ball; Oracle Corporation, Redwood Shores, CA, USA) on the basis of the MIC distribution from this study. The PTA was examined using the target AUC/MIC value. The cumulative fractions of responses (CFRs) of three monotherapies, 2 g AZM-SR, 2 g AZM, and 1 g AZM, were calculated on the basis of the AUC/MIC values.
Statistical analysis.
Descriptive statistics were performed for all continuous data, and data are presented as the means ± standard deviations, unless specified otherwise. Comparisons of continuous data between groups were performed using Student's t test after confirming the normal distribution of the data. PK parameters between groups were compared using the Mann-Whitney U test, and a P value of <0.05 was considered statistically significant. Logistic regression analyses were performed to identify univariate predictors of treatment success (i.e., microbiological cure). All statistical analyses were performed using SPSS software (IBM Corp., Armonk, NY, USA).
ACKNOWLEDGMENTS
This study was supported by internal funding.
Although Global Regulatory Science, Gifu Pharmaceutical University, is financially supported by donations from Otsuka Pharmaceutical Co, Ltd., we report no conflicts of interest regarding the content of this article.
REFERENCES
- 1.Workowski KA, Berman SM, Douglas JM Jr. 2008. Emerging antimicrobial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Ann Intern Med 148:606–613. doi: 10.7326/0003-4819-148-8-200804150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1994. Sexually transmitted diseases treatment guidelines. J Sch Health 64:156–159. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2007. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 56:332–336. [PubMed] [Google Scholar]
- 4.Japanese Society for Sexually Transmitted Infections. 2016. Sexually transmitted infections treatment guideline 2016. Jpn J Sex Transm Infect 27(Suppl 1):51–56. (In Japanese.) [Google Scholar]
- 5.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deguchi T, Yasuda M, Hatazaki K, Kameyama K, Horie K, Kato T, Mizutani K, Seike K, Tsuchiya T, Yokoi S, Nakano M, Yoh M. 2016. New clinical strain of Neisseria gonorrhoeae with decreased susceptibility to ceftriaxone, Japan. Emerg Infect Dis 22:142–144. doi: 10.3201/eid2201.150868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters LJ, Boag FC, Betournay R. 2005. Efficacy of azithromycin 1 g single dose in the management of uncomplicated gonorrhoea. Int J STD AIDS 16:84. [DOI] [PubMed] [Google Scholar]
- 8.Waugh MA. 1993. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women. J Antimicrob Chemother 31(Suppl E):193–198. [DOI] [PubMed] [Google Scholar]
- 9.Dillon JA, Trecker MA, Thakur SD, Gonococcal Antimicrobial Surveillance Program Network in Latin America and Caribbean 1990-2011 . 2013. Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities. Sex Transm Infect 89(Suppl 4):iv36–iv41. doi: 10.1136/sextrans-2012-050905. [DOI] [PubMed] [Google Scholar]
- 10.Palmer HM, Young H, Winter A, Dave J. 2008. Emergence and spread of azithromycin-resistant Neisseria gonorrhoeae in Scotland. J Antimicrob Chemother 62:490–494. doi: 10.1093/jac/dkn235. [DOI] [PubMed] [Google Scholar]
- 11.Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA, Ison CA. 2009. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother 64:353–358. doi: 10.1093/jac/dkp188. [DOI] [PubMed] [Google Scholar]
- 12.Handsfield HH, Dalu ZA, Martin DH, Douglas JM Jr, McCarty JM, Schlossberg D. 1994. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Azithromycin Gonorrhea Study Group. Sex Transm Dis 21:107–111. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S, Kiyota H, Ito S, Iwasawa A, Hiyama Y, Uehara T, Ichihara K, Hashimoto J, Masumori N, Sunaoshi K, Takeda K, Suzuki N, Hosobe T, Goto H, Suzuki H, Onodera S. 2014. Clinical efficacy of a single two gram dose of azithromycin extended release for male patients with urethritis. Antibiotics (Basel) 3:109–120. doi: 10.3390/antibiotics3020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasuda M, Ito S, Kido A, Hamano K, Uchijima Y, Uwatoko N, Kusuyama H, Watanabe A, Miyamura R, Miyata K, Deguchi T. 2014. A single 2 g oral dose of extended-release azithromycin for treatment of gonococcal urethritis. J Antimicrob Chemother 69:3116–3118. doi: 10.1093/jac/dku221. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen DM. 2009. Single-dose extended-release oral azithromycin vs. 3-day azithromycin for the treatment of group A beta-haemolytic streptococcal pharyngitis/tonsillitis in adults and adolescents: a double-blind, double-dummy study. Clin Microbiol Infect 15:1103–1110. doi: 10.1111/j.1469-0691.2009.02718.x. [DOI] [PubMed] [Google Scholar]
- 16.Chandra R, Liu P, Breen JD, Fisher J, Xie C, LaBadie R, Benner RJ, Benincosa LJ, Sharma A. 2007. Clinical pharmacokinetics and gastrointestinal tolerability of a novel extended-release microsphere formulation of azithromycin. Clin Pharmacokinet 46:247–259. doi: 10.2165/00003088-200746030-00005. [DOI] [PubMed] [Google Scholar]
- 17.Riedner G, Rusizoka M, Todd J, Maboko L, Hoelscher M, Mmbando D, Samky E, Lyamuya E, Mabey D, Grosskurth H, Hayes R. 2005. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med 353:1236–1244. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 18.Hook EW III, Behets F, Van Damme K, Ravelomanana N, Leone P, Sena AC, Martin D, Langley C, McNeil L, Wolff M. 2010. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. J Infect Dis 201:1729–1735. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 19.Muto C, Liu P, Chiba K, Suwa T. 2011. Pharmacokinetic-pharmacodynamic analysis of azithromycin extended release in Japanese patients with common respiratory tract infectious disease. J Antimicrob Chemother 66:165–174. doi: 10.1093/jac/dkq398. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Eltriki M, Somayaji V, Padwal RS, Brocks DR. 2013. A liquid chromatography-mass spectrometric method for the quantification of azithromycin in human plasma. Biomed Chromatogr 27:1012–1017. doi: 10.1002/bmc.2896. [DOI] [PubMed] [Google Scholar]
- 21.Filist M, Bus-Kwasnik K, Ksycinska H, Rudzki PJ. 2014. Simplified LC-MS/MS method enabling the determination of azithromycin in human plasma after a low 100mg dose administration. J Pharm Biomed Anal 100:184–189. doi: 10.1016/j.jpba.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda M, Ito S, Hatazaki K, Deguchi T. 2016. Remarkable increase of Neisseria gonorrhoeae with decreased susceptibility of azithromycin and increase in the failure of azithromycin therapy in male gonococcal urethritis in Sendai in 2015. J Infect Chemother 22:841–843. doi: 10.1016/j.jiac.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Gose SO, Soge OO, Beebe JL, Nguyen D, Stoltey JE, Bauer HM. 2015. Failure of azithromycin 2.0 g in the treatment of gonococcal urethritis caused by high-level resistance in California. Sex Transm Dis 42:279–280. doi: 10.1097/OLQ.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka M, Furuya R, Irie S, Kanayama A, Kobayashi I. 2015. High prevalence of azithromycin-resistant Neisseria gonorrhoeae isolates with a multidrug resistance phenotype in Fukuoka, Japan. Sex Transm Dis 42:337–341. doi: 10.1097/OLQ.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 25.Katz AR, Komeya AY, Soge OO, Kiaha MI, Lee MV, Wasserman GM, Maningas EV, Whelen AC, Kirkcaldy RD, Shapiro SJ, Bolan GA, Holmes KK. 2012. Neisseria gonorrhoeae with high-level resistance to azithromycin: case report of the first isolate identified in the United States. Clin Infect Dis 54:841–843. doi: 10.1093/cid/cir929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starnino S, Stefanelli P, Neisseria gonorrhoeae Italian Study Group . 2009. Azithromycin-resistant Neisseria gonorrhoeae strains recently isolated in Italy. J Antimicrob Chemother 63:1200–1204. doi: 10.1093/jac/dkp118. [DOI] [PubMed] [Google Scholar]
- 27.Unemo M, Golparian D, Skogen V, Olsen AO, Moi H, Syversen G, Hjelmevoll SO. 2013. Neisseria gonorrhoeae strain with high-level resistance to spectinomycin due to a novel resistance mechanism (mutated ribosomal protein S5) verified in Norway. Antimicrob Agents Chemother 57:1057–1061. doi: 10.1128/AAC.01775-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita-Ishihara T, Unemo M, Furubayashi K, Kawahata T, Shimuta K, Nakayama S, Ohnishi M. 2014. Treatment failure with 2 g of azithromycin (extended-release formulation) in gonorrhoea in Japan caused by the international multidrug-resistant ST1407 strain of Neisseria gonorrhoeae. J Antimicrob Chemother 69:2086–2090. doi: 10.1093/jac/dku118. [DOI] [PubMed] [Google Scholar]
- 29.Workowski KA, Bolan GA, Centers for Disease Control and Prevention . 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommend Rep 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 30.Bignell C, Unemo M, European STI Guidelines Editorial Board . 2013. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 24:85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 31.Bignell C, Fitzgerald M, Guideline Development Group, British Association for Sexual Health and HIV UK . 2011. UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS 22:541–547. doi: 10.1258/ijsa.2011.011267. [DOI] [PubMed] [Google Scholar]
- 32.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Public Health Agency of Canada. 2013. Gonococcal infections chapter. In Canadian guidelines on sexually transmitted infections. Public Health Agency of Canada, Ottawa, Ontario, Canada: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/std-mts/sti-its/cgsti-ldcits/assets/pdf/section-5-6-eng.pdf Accessed 12 September 2017. [Google Scholar]
- 34.Kirkcaldy RD, Weinstock HS, Moore PC, Philip SS, Wiesenfeld HC, Papp JR, Kerndt PR, Johnson S, Ghanem KG, Hook EW III. 2014. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 59:1083–1091. doi: 10.1093/cid/ciu521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidd S, Workowski KA. 2015. Management of gonorrhea in adolescents and adults in the United States. Clin Infect Dis 61(Suppl 8):S785–S801. doi: 10.1093/cid/civ731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanston WH, Prabhakar P, Barrow L, Mahabir BS, Furlonge C. 2001. Single dose (direct observed) azithromycin therapy for Neisseria gonorrhoeae and Chlamydia trachomatis in STD clinic attenders with genital discharge in Trinidad and Tobago. West Indian Med J 50:198–202. [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing: twenty-second informational supplement. M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 38.Chen L, Qin F, Ma Y, Li F. 2007. Quantitative determination of azithromycin in human plasma by ultra performance liquid chromatography-electrospray ionization mass spectrometry and its application in a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 855:255–261. doi: 10.1016/j.jchromb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Nirogi RV, Kandikere V, Shukla M, Mudigonda K, Maurya S, Boosi R, Yerramilli A. 2005. Sensitive and selective liquid chromatography-tandem mass spectrometry method for the quantification of azithromycin in human plasma. Anal Chim Acta 553:1–8. doi: 10.1016/j.aca.2005.08.007. [DOI] [Google Scholar]
- 40.Gao L, Li J, Kasserra C, Song Q, Arjomand A, Hesk D, Chowdhury SK. 2011. Precision and accuracy in the quantitative analysis of biological samples by accelerator mass spectrometry: application in microdose absolute bioavailability studies. Anal Chem 83:5607–5616. doi: 10.1021/ac2006284. [DOI] [PubMed] [Google Scholar]
- 41.Handsfield HH, McCutchan JA, Corey L, Ronald AR. 1992. Evaluation of new anti-infective drugs for the treatment of uncomplicated gonorrhea in adults and adolescents. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis 15(Suppl 1):S123–S130. doi: 10.1093/clind/15.Supplement_1.S123. [DOI] [PubMed] [Google Scholar]
- 42.Liu P, Allaudeen H, Chandra R, Phillips K, Jungnik A, Breen JD, Sharma A. 2007. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother 51:103–109. doi: 10.1128/AAC.00852-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beringer P, Huynh KM, Kriengkauykiat J, Bi L, Hoem N, Louie S, Han E, Nguyen T, Hsu D, Rao PA, Shapiro B, Gill M. 2005. Absolute bioavailability and intracellular pharmacokinetics of azithromycin in patients with cystic fibrosis. Antimicrob Agents Chemother 49:5013–5017. doi: 10.1128/AAC.49.12.5013-5017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]