ABSTRACT
Pseudomonas aeruginosa is a major cause of morbidity and mortality in chronically infected cystic fibrosis patients. Novel in vitro biofilm models which reliably predict the therapeutic success of antimicrobial therapies against biofilm bacteria should be implemented. The activity of fosfomycin, tobramycin, and the fosfomycin-tobramycin combination against 6 susceptible P. aeruginosa strains isolated from respiratory samples from cystic fibrosis patients was tested by using two in vitro biofilm models: a closed system (Calgary device) and an open model based on microfluidics (BioFlux). All but one of the isolates formed biofilms. The fosfomycin and tobramycin minimal biofilm inhibitory concentrations (MBIC) were 1,024 to >1,024 μg/ml and 8 to 32 μg/ml, respectively. According to fractional inhibitory concentration analysis, the combination behaved synergistically against all the isolates except the P. aeruginosa ATCC 27853 strain. The dynamic formation of the biofilm was also studied with the BioFlux system, and the MIC and MBIC of each antibiotic were tested. For the combination, the lowest tobramycin concentration that was synergistic with fosfomycin was used. The captured images were analyzed by measuring the intensity of the colored pixels, which was proportional to the biofilm biomass. A statistically significant difference was found when the intensity of the inoculum was compared with the intensity of the microchannel in which the MBIC of tobramycin, fosfomycin, or their combination was used (P < 0.01) but not when the MIC was applied (P > 0.01). Fosfomycin-tobramycin was demonstrated to be synergistic against cystic fibrosis P. aeruginosa strains in the biofilm models when both the Calgary and the microfluidic BioFlux systems were tested. These results support the clinical use of this combination.
KEYWORDS: BioFlux system, Calgary device, P. aeruginosa biofilms, cystic fibrosis, fosfomycin-tobramycin
INTRODUCTION
The biofilm mode of growth is directly involved in the pathogenesis of Pseudomonas aeruginosa, contributing to morbidity and mortality in chronically infected cystic fibrosis (CF) patients (1). The eradication of this biological structure is extremely difficult because of the increased tolerance to antimicrobials that microorganisms exhibit within its environment. Inhaled tobramycin (TOB) has been long used in CF treatments to control chronic colonization, but recently, the use of antibiotic combinations in CF patients has been suggested not only to reduce and delay antimicrobial resistance but also to enhance antibacterial activity, particularly against bacteria growing in biofilms (2). Previously, a combination of fosfomycin (FOF) and tobramycin (FT) in a 4:1 ratio was found to be synergistic in vitro against P. aeruginosa, especially in anaerobic environments, and its effectiveness has been proven in phase II clinical studies (3–5).
On the other hand, susceptibility testing results should predict therapeutic success, a situation hardly achieved when standard MIC values for planktonic bacteria are considered for the bacteria causing biofilm-related infections. Consequently, susceptibility testing of the bacteria in biofilms has been claimed to be a useful tool for this purpose (6). Currently, two types of assays are available to evaluate the in vitro activity of antibiotics against biofilms: open and closed systems. Closed, or static, systems analyze biofilm formation in the wells of microtiter plates and are suitable for high-throughput analysis, while open, or dynamic, systems produce conditions that better resemble those encountered in vivo (7).
The objectives of this work were to analyze the effects of FOF, TOB, and FT on CF P. aeruginosa strains growing in biofilms. With the Calgary closed system, pharmacodynamic (PD) parameters, that is, the minimal biofilm inhibitory concentration (MBIC) and the biofilm prevention concentration (BPC), were determined. Synergy was estimated by calculating the fractional inhibitory concentration index (∑FIC) adapted to the MBIC. To observe and describe the dynamics of CF P. aeruginosa biofilm formation, the BioFlux microfluidic open model (Fluxion Biosciences, South San Francisco, CA) was used. With this system, FOF, TOB, and FT activities were determined by measuring their effects on biofilm biomass through analysis of the image intensity of colored pixels.
RESULTS
Susceptibility testing results.
The FOF and TOB MIC values are shown in Table 1. All of them corresponded to the susceptible category.
TABLE 1.
Characteristics of the P. aeruginosa strains used in the biofilm assays
| Strain | Morphotype | MIC (μg/ml)a |
Infection | Patient age (yr) | |
|---|---|---|---|---|---|
| FOF | TOB | ||||
| Pab1 | Mucoid | 64 | 1 | Initial infection | 21 |
| Pab2 | Smooth | 64 | 1 | Initial infection | 15 |
| Pab3 | Smooth | 64 | 2 | Chronic infection | 45 |
| Pab4 | Mucoid | 64 | 1 | Chronic infection | 22 |
| Pab5 | Smooth | 64 | 4 | Chronic infection | 22 |
| Pab6 | Small colony | 64 | 4 | Chronic infection | 26 |
| ATCC 27853 | 4 | 0.5 | |||
FOF, fosfomycin; TOB, tobramycin.
Biofilm assays using the Calgary device.
All the isolates except the Pab6 strain, which corresponded to a small-colony variant, were able to form a biofilm. The difference in the optical density at 450 nm (OD450) between 0 and 6 h (ΔOD) after the start of incubation was ≥0.05 for strains Pab1 to Pab5 and the P. aeruginosa control strain ATCC 27853. For strain Pab6, the ΔOD was 0.01 (Fig. 1).
FIG 1.
Biofilm formation by each isolate in the Calgary device, represented by the difference in the OD450 values (ΔOD) between 0 and 6 h.
The MBICs of FOF, TOB, and FT are shown in Table 2. The FOF MBICs ranged from 1,024 to >1,024 μg/ml, and the range of TOB MBICs was 8 to 32 μg/ml. For the ATCC 27853 P. aeruginosa control strain, the FOF MBIC and TOB MBIC were >1,024 μg/ml and 2 μg/ml, respectively. For strains Pab1 to Pab5, ∑FIC was ≤0.5 for at least one of the concentrations tested, indicating synergy between FOF and TOB. However, for ATCC 27853, FT was not synergistic at any of the concentrations tested, probably due to the low TOB MBIC values (Table 3).
TABLE 2.
Fosfomycin, tobramycin, and fosfomycin-tobramycin MBICs obtained with the Calgary device
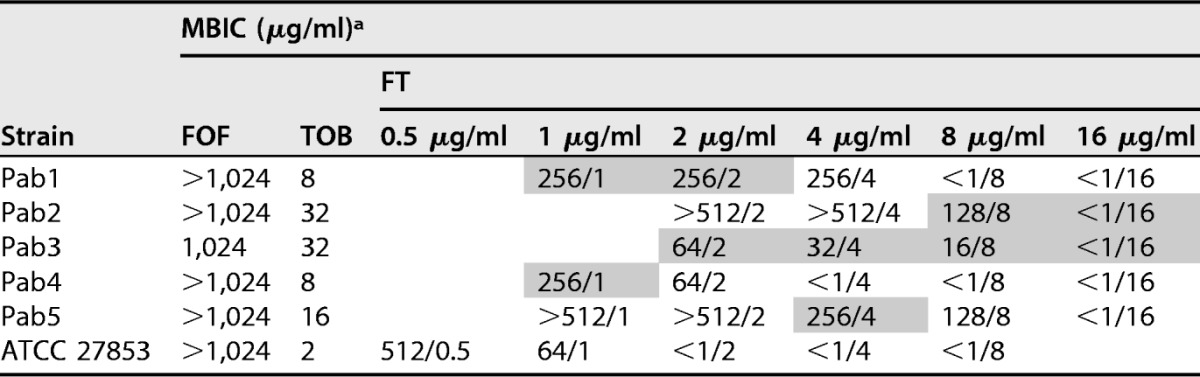
a Fosfomycin-tobramycin concentrations that were synergistic for each strain are shaded. The concentrations in the column heads under FT represent the TOB concentration used in the combination. FOF, fosfomycin; TOB, tobramycin; FT, fosfomycin-tobramycin combination.
TABLE 3.
∑FIC results for the fosfomycin-tobramycin combination concentrations tested in the Calgary device
| TOBa concn (μg/ml) | ∑FICb |
|||||
|---|---|---|---|---|---|---|
| Pab1 | Pab2 | Pab3 | Pab4 | Pab5 | ATCC 27853 | |
| 16 | 1 | 0.5 | 0.5 | |||
| 8 | 1 | 0.375 (128/8) | 0.265 | 0.625 | ||
| 4 | 0.75 | 1.125 | 0.156 | 0.5 (256/4) | ||
| 2 | 0.5 | 1.06 | 0.125 (64/2) | 0.562 | ||
| 1 | 0.375 (256/1) | 0.5 (256/1) | 0.562 | |||
| 0.5 | 0.75 | |||||
TOB, tobramycin.
The lowest tobramycin concentration of the combination that resulted in synergistic activity is shaded. Values in parentheses are the fosfomycin/tobramycin concentrations (in micrograms per milliliter).
The TOB BPC was ≤1 μg/ml for all the isolates, including those with higher TOB MICs (Pab3, TOB MIC = 2 μg/ml, Pab5 TOB MIC = 4 μg/ml). Therefore, all the TOB BPC/MIC indexes were ≤1. However, although all the isolates presented the same FOF MIC (64 μg/ml), the FOF BPC/MIC indexes ranged from 1 to 32. This means that for strains Pab1, Pab4, and Pab5 and the ATCC 27853 strain, the BPC was close to the MIC, while the Pab2 and Pab3 strains presented a BPC 3 to 5 2-fold dilutions higher than the MIC (Table 4). The biofilm prevention TOB concentrations within the combination were ≤1 μg/ml; thus, TOB alone was able to prevent the development of the biofilm at this concentration, and the addition of fosfomycin did not increase the activity.
TABLE 4.
Fosfomycin and tobramycin BPC and BPC/MIC results obtained for each isolate by the Calgary devicea
| Strain | TOB |
FOF |
||
|---|---|---|---|---|
| BPC (μg/ml) | BPC/MIC | BPC (μg/ml) | BPC/MIC | |
| Pab1 | ≤1 | 1 | 128 | 2 |
| Pab2 | ≤1 | 1 | >1,024 | 32 |
| Pab3 | ≤1 | 0.5 | 512 | 8 |
| Pab4 | ≤1 | 1 | 64 | 1 |
| Pab5 | ≤1 | 0.25 | 64 | 1 |
| ATCC 27853 | ≤0.5 | 1 | 8 | 2 |
TOB, tobramycin; FOF, fosfomycin.
Biofilm assays using the BioFlux device.
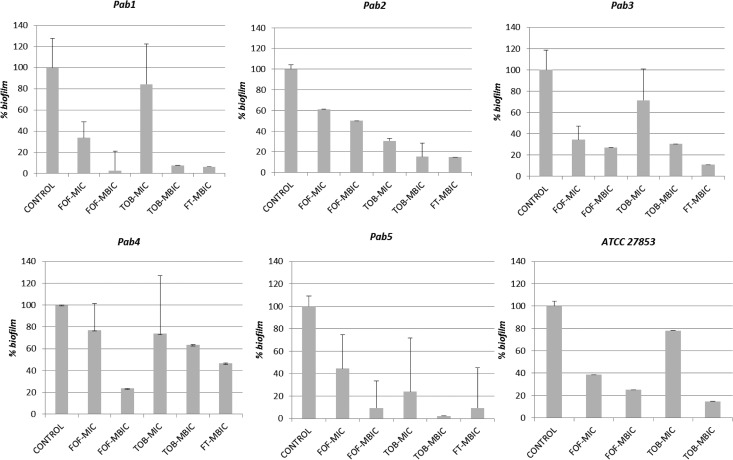
When strains Pab1 to Pab5 were grown in the positive-control microchannels of the Bioflux device, they exhibited a biofilm mode of growth, while strain Pab6 did not. This result is in agreement with that observed using the Calgary device. At 8 h of incubation, the percentage of formed biofilms for strains Pab1 to Pab4 and ATCC 27853 ranged from 37% to 59.7% of the total. However, for the Pab5 strain, it was only 7.1%. The incubation for this strain was therefore prolonged to 24 h, a point at which the percentage of the formed biofilm was 40.1%. At this moment, the antibiotics were added, and the incubation was subsequently prolonged to 48 h. Images of each microchannel were captured and can be observed in Fig. S1 in the supplemental material. The intensity of the colored pixels and the transformed percentage of the remaining biofilm values after the antimicrobial challenge are shown in Fig. 2. FT was not tested with the ATCC 27853 strain, as this combination did not exhibit synergy against that strain with the Calgary device.
FIG 2.
Percentage of the biofilm remaining in the microchannel after 24 h of incubation (48 h in the case of Pab5). The standard deviation was calculated by considering the areas of maximum intensity. The fosfomycin-tobramycin combination was not tested with the ATCC 27853 strain as it did not exhibit synergy with the Calgary device. P values were as follows: 0.0374 for the FOF MIC, 0.0039 for the FOF MBIC, 0.0547 for the TOB MIC, and 0.0062 for the FT MBIC.
For all biofilm-producing isolates, statistically significant differences (P < 0.01) were observed between the biofilm formed in the inoculum control and the biofilms formed in the microchannels with FOF, TOB, or FT when they were tested at concentrations equal to the MBIC, indicating antibiofilm and antimicrobial activity at those concentrations. However, no significant differences were found for these bacteria when they were incubated with FOF or TOB at concentrations identical to the MICs (P = 0.0374 and P = 0.0547, respectively).
The ∑FIC results obtained with the Calgary device, which indicated the synergy of FOF and TOB, were confirmed with the BioFlux device, as the TOB concentrations within the FT combination required to achieve an antibiofilm effect were 2 to 4 2-fold dilutions lower than those required to achieve the same effect when TOB was tested alone.
DISCUSSION
Biofilms are involved in more than 80% of all microbial infections (8). The penetration and activity of antibiotics are usually reduced in this type of growth, compromising their antimicrobial effect within these environments. Biofilms of P. aeruginosa are particularly relevant in chronic pulmonary infections in CF patients, where eradication is very difficult. About 54% of CF patients under the age of 18 years are colonized by this microorganism, while the percentage rises to 80% in adults (9).
Two types of in vitro biofilm models are currently being used to predict antimicrobial therapeutic success against biofilm bacteria: closed and open systems. In closed systems, nutrients are limited and metabolic waste accumulates, which can create a bias in biofilm quantification. This technique, however, can easily be performed for high-throughput analysis. Moreover, PD parameters, which establish the activities of antibiotics against biofilms, can be also determined. On the other hand, open systems better reproduce the conditions encountered in vivo, as there is a permanent control of nutrient delivery, flow, and temperature, and the antibiofilm pharmacokinetics (PK)/PD of antibiotics can be determined. However, these systems are more expensive, and assays with these systems are labor intensive. The BioFlux system is a microfluidic system in which multiple biofilms can be run in parallel, covering all the advantages of methods with open systems. In this work, the activities of FOF, TOB, and FT against P. aeruginosa biofilms were tested in a complementary way using both open and closed systems. Through the use of a mathematical formula, the image intensity results from the BioFlux system were translated to a remaining biofilm percentage that enabled a graphic representation.
The use of both the Calgary and the BioFlux devices to study the dynamics of biofilm formation showed that all isolates except Pab6 were able to form a biofilm. The Pab6 strain was isolated from a CF patient with a prolonged chronic infection, indicating, as previously stated, that biofilm development is not essential for the ultimate survival of P. aeruginosa in chronic lung infection (10).
When the closed system was used to analyze the activities of antibiotics against biofilms, the closed system showed high MBIC values for FOF (1,024 to >1,024 μg/ml, which were 4 2-fold dilutions higher than the MIC) and TOB (MBICs were 2 to 5 2-fold dilutions higher than the MICs). According to the FIC index determined on the basis of the MBICs, FT showed synergy against all biofilm-producing CF strains tested.
BPC is a parameter that could be useful for the evaluation of treatment in the early stage of colonization in CF patients. Our BPC results showed that TOB effectively prevents biofilm development, while FOF has an erratic behavior that depends on the strain tested. These results match those previously described, where fluoroquinolones, tobramycin, and colistin presented the lowest BPC values (11).
With the BioFlux device, the FOF, TOB, and FT MBICs exhibited a statistically significant difference in biofilm intensity compared to that for the inoculum control. However, tobramycin and fosfomycin concentrations in the FT were lower than those used when each compound was tested alone.
These results reinforce the fact that antibiotic concentrations that inhibit planktonic cells are not able to inhibit the same microorganism when they are growing in biofilms. In fact, for most antibiotics, the MBICs are at least 1 2-fold dilution higher than the MICs (12). So, high antibiotic concentrations must penetrate into the biofilm structure for the antibiotics to exert their action. In CF patients, these concentrations can be achieved through inhaled therapy. To evaluate the activities of antibiotics in these biofilm infection models, clinical laboratories perform classical antibiotic susceptibility tests with planktonic cells, as there is neither a feasible technique for routine testing of biofilm bacteria nor a standardized procedure. Moreover, when an antibiotic is administered by inhaled therapy, susceptibility breakpoints should be based on the PK/PD parameters adapted for this route of administration; however, CLSI and EUCAST have not yet defined them. Therefore, in vitro conventional MIC testing is not adequate to predict the possible in vivo therapeutic effect of antibiotics in biofilm-mediated infections.
A high level of penetration of FOF into biofilms has been reported (13), but monotherapy against P. aeruginosa, even FOF-susceptible strains, is not recommended due to the high MICs for the wild-type population (epidemiological cutoff value [ECOFF] ≤ 128 μg/ml) and the possibility of the rapid emergence of resistant mutants (14). The FOF MIC (4 μg/ml) for the ATCC 27853 strain was much lower than the modal MIC (64 μg/ml) of the FOF MIC distribution for P. aeruginosa (15). The FOF hypersusceptibility of this strain could be due to inactivation of the peptidoglycan recycling process (16); however, even for this strain, a high FOF MBIC (1,024 μg/ml) was recorded. This fact reflects the frequent emergence of high-level fosfomycin-resistant mutants within the high bacterial inoculum present in the biofilm that is due to the mutation of the glycerol-3-phosphate permease (GlpT). Furthermore, although after administration of 120 mg of aerosolized fosfomycin a concentration of 2,500 μg/ml has been found in tracheal aspirates (17), the high mutant prevention concentration values reported (>2,048 μg/ml) (14) again prevent its use in monotherapy.
On the other hand, TOB is less active against bacteria growing in biofilms than against bacteria growing planktonically, as the anaerobic environments reduce its penetration into bacterial cells (3). The peak concentrations of tobramycin measured in sputum after aerosolized administration are approximately 1,000 μg/ml (18). This peak concentration of tobramycin exceeds the MBIC; however, after exposure to 1,000 μg/ml of tobramycin, areas of living cells remain within the inner part of biofilms (19). In this case, the association with fosfomycin could be advantageous, as FT has increased activity under anaerobic conditions because the expression of nitrate reductase genes, which are essential for the growth of P. aeruginosa, is downregulated (3).
Thus, within the FT combination, FOF could behave as a TOB enhancer, inducing its active uptake (20). Use of the combination guarantees concentrations of both antibiotics above the MBIC, so the TOB levels reached inside the biofilm structure should be adequate, thus ameliorating the negative side effects of tobramycin during treatment (4). In a previous study, prevention of the generation of resistant mutants and synergy between FOF and TOB were observed in isolates which were susceptible to both antibiotics, while FOF and TOB showed very weak or no synergy with high mutant prevention concentration values against high-level tobramycin-resistant isolates harboring aminoglycoside-modifying enzymes. So, the possible use of this combination is restricted to patients infected with susceptible isolates. In CF isolates with an altered MexXY-OprM efflux system that are susceptible to TOB but for which the MIC is close to the breakpoint (4 μg/ml), the synergy of FOF and TOB has been explained by their rapid accumulation inside the cell through the induction of the active uptake of TOB (14).
Also, the FOF and TOB combination has been proven to have disrupting activity on CF biofilms grown on cultured airway cells derived CF patients (4). FT was used as an inhaled treatment option in a multicenter study in CF patients and showed promising results (5). In addition, the amikacin-FOF combination has undergone a clinical trial in patients with mechanical ventilation-associated pneumonia, obtaining a significant reduction in bacterial burden in tracheal aspirates compared to the placebo group (17).
In conclusion, P. aeruginosa biofilms are implicated in numerous infections. In CF patients, the biofilm mode of growth makes treatment a real challenge; therefore, novel therapeutic interventions are needed. In vitro biofilm models should be implemented in clinical microbiology laboratories for routine susceptibility testing to predict therapeutic success when this mode of growth is present. The combination of FOF and TOB has been demonstrated to be synergistic against CF P. aeruginosa isolates when using both the Calgary device and the BioFlux microfluidic open system. The latter system is a new tool that permits the study of biofilm formation under conditions resembling those encountered in vivo.
MATERIALS AND METHODS
Bacterial strains and susceptibility testing.
Six P. aeruginosa clinical strains (strains Pab1 to Pab6) were collected from respiratory samples from 6 CF patients (2 initial infections and 4 chronic infections). These strains represented different morphotypes (the mucoid, small-colony, and smooth morphotypes) and were nonhypermutable. P. aeruginosa ATCC 27853 was used as the control strain. The MICs of FOF (Laboratorios Ern, S.A., Barcelona, Spain) and TOB (Sigma-Aldrich Chemical Co., St. Louis, MO) were determined by the agar dilution method (on BBL Mueller-Hinton II cation-adjusted broth and agar; BD, Sparks, MD), as recommended for CF P. aeruginosa isolates (21). As previously stated, fosfomycin enters P. aeruginosa cells only through the GlpT transporter because this microorganism lacks the UhpT permease (15, 22), so glucose-6-phosphate (a UhpT inducer) was not added to the medium when fosfomycin was tested.
For susceptibility categorization, EUCAST criteria were followed. As there are no clinical breakpoints for fosfomycin, the EUCAST ECOFF (128 μg/ml) was used. All the strains were susceptible to FOF and TOB (Table 1).
Biofilm assays using the Calgary static device.
Biofilm assays using the Calgary static device were performed as previously described with minimal variations (see Fig. S2 in the supplemental material) (23). Briefly, a culture with a turbidity equivalent to that of a 0.5 McFarland standard was transferred to a flat-bottom 96-well microtiter plate (Nunc International, Rochester, NY). The bacterial biofilm formed around the pegs of a modified polystyrene microtiter plate lid. This lid with pegs was immersed into a growth plate and incubated for 20 h at 37°C. After the pegs were rinsed 3 times in sterile water, the lid was placed into antimicrobial-containing Mueller-Hinton broth and incubated for 20 h at 37°C. Twofold increasing FOF (2 to 1,024 μg/ml) and TOB (0.5 to 64 μg/ml) concentrations and a variable FOF concentration (1 to 512 μg/ml) with different fixed TOB concentrations (0.5 to 32 μg/ml) for the combination were used. After this incubation, the biofilm was recovered by centrifuging (800 rpm, 10 min) the lid with pegs in an antibiotic-free Muller Hinton microtiter plate. The MBIC was calculated after measuring the optical density at 450 nm (OD450) before and after a 6-h incubation (ΔOD). Biofilm growth was defined as a mean ΔOD of ≥0.05. The MBIC was defined as the lowest antibiotic concentration that resulted in an OD difference at or below 10% of the OD for the positive control.
To determine synergy between FOF and TOB, the FIC index (∑FIC), which is commonly used in checkerboard assays (24), was adapted to the MBIC and was calculated as follows (25): ∑FIC = (MBIC of TOBc/MBIC of TOB) + (MBIC of FOFc/MBIC of FOF), where MBIC of TOBc and MBIC of FOFc refer to the MBICs of TOB and FOF within the FT combination, respectively. Synergy was defined when ∑FIC was ≤0.5.
Using the static method with the Calgary device, the PD parameter BPC was also estimated for FOF, TOB, and FT following the protocol described by Fernández-Olmos et al. (11). In this protocol, the inoculum and the antimicrobials are simultaneously incubated in the microtiter plate with the pegs at the same time (Fig. S2).
Biofilm formation and susceptibility determination were performed in duplicate for all the isolates.
Biofilm assays using the BioFlux microfluidic open system.
Using the BioFlux device, 24 biofilms were simultaneously developed in 48 wells following a protocol adapted from that of Benoit et al. (Fig. S2) (26). Microchannels were filled with 100 μl of prewarmed Luria broth medium (LB; Oxoid, Ltd., Basingstoke, Hampshire, UK) through the input wells (5 min, 1 dyne/cm2). For cell attachment, 20 μl of a 108- to 109-CFU/ml bacterial suspension was inoculated into the output wells for 5 s at 2 dyne/cm2 and the plate was incubated for 2 h at 37°C. For the positive-control wells, fresh medium was added to the input wells and the biofilms were incubated for 24 h at 37°C (0.15 dyne/cm2). In the first step, registration of the positive-control microchannel of each isolate was made after 8 and 24 h to see the dynamics of biofilm formation. Negative controls, for which medium without the bacterial suspension was injected, were included in all assays. Antibiotic addition was performed after 8 h of incubation, only if the percentage of the formed biofilm was equal to or greater than approximately 40% of the total. The antibiotic concentrations tested corresponded the FOF and TOB MICs and MBICs obtained with the Calgary assays. For FT, the lowest TOB concentration that resulted in synergistic activity using the Calgary device was then applied in the BioFlux system (Table 3). The results were analyzed by quantifying the image intensity of colored pixels in an 8-bit gray size, which was registered by the BioFlux software after microscopic observation of the selected area in the microchannel.
The percentage of the biofilm remaining after the 24-h incubation (or the 48-h incubation, in the case of Pab5) was estimated through the following equation and subsequently graphically represented (Fig. 2): {[(Imax − X)/(Imin − Imax)] × 100} + 100, where the image of the positive control was considered to have the maximum intensity (Imax), the image of the negative control was considered to have the minimum intensity (Imin), and X was the intensity of the evaluated sample. In order to reflect areas of congregation within the biofilm, the standard deviations presented in Fig. 2 correspond to the maximum intensity values recorded along the microchannel. In all cases, the results from at least two independent experiments were considered.
Statistical analysis.
Results from analysis of the image intensity of the inoculum control, the image intensity corresponding to the antimicrobials, as well as the image intensity corresponding to the combination were analyzed using a Mann-Whitney nonparametric test. To maintain the overall boundary for statistical significance at 0.05, the threshold P value was divided by our 5 independent hypotheses (comparison of the intensity obtained with the inoculum control with the intensity obtained with the FOF MIC, FOF MBIC, TOB MIC, TOB MBIC, and FT MBIC), so a P value of <0.01 was considered statistically significant. Stata statistical software was used (Data Analysis and Statistical Software, version 11.0).
Supplementary Material
ACKNOWLEDGMENTS
María Díez-Aguilar was supported by the Innovative Medicines Initiative (IMI), a European Commission-funded project (iABC grant 115721-2), and the Fundación Francisco Soria Melguizo (Madrid, Spain). The content and scientific background of this work were also supported by Plan Nacional de I+D+i 2013-2016 and the Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015/0004 and RD12/0015/0006, REIPI RD16/0016/0004 and RD16/0016/0011, and grants PI15/00088 and PI12/00734), which is cofinanced by the European Development Regional Fund (EDRF; A way to achieve Europe), Operative Program Intelligent Growth 2014-2020.
We thank Alfonso Muriel (Unidad de Bioestadística Clínica, Hospital Universitario Ramón y Cajal) for advice on statistical analysis during manuscript preparation.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01650-17.
REFERENCES
- 1.Gibson RL, Burns JL, Ramsey BW. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Döring G, Flume P, Heijerman H, Elborn JS. 2012. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros 11:461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.McCaughey G, Gilpin DF, Schneiders T, Hoffman LR, McKevitt M, Elborn JS, Tunney MM. 2013. Fosfomycin and tobramycin in combination downregulate nitrate reductase genes narG and narH, resulting in increased activity against Pseudomonas aeruginosa under anaerobic conditions. Antimicrob Agents Chemother 57:5406–5414. doi: 10.1128/AAC.00750-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson GG, Kenney TF, MacLeod DL, Henig NR, O'Toole GA. 2013. Eradication of Pseudomonas aeruginosa biofilms on cultured airway cells by a fosfomycin/tobramycin antibiotic combination. Pathog Dis 67:39–45. doi: 10.1111/2049-632X.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trapnell BC, McColley SA, Kissner DG, Rolfe MW, Rosen JM, McKevitt M, Moorehead L, Montgomery AB, Geller DE. 2012. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with Pseudomonas airway infection. Am J Respir Crit Care Med 185:171–178. doi: 10.1164/rccm.201105-0924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macià MD, Rojo-Molinero E, Oliver A. 2014. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect 20:981–990. doi: 10.1111/1469-0691.12651. [DOI] [PubMed] [Google Scholar]
- 7.McBain AJ. 2009. In vitro biofilm models: an overview. In Advances in applied microbiology, 1st ed Elsevier Inc, New York, NY. [DOI] [PubMed] [Google Scholar]
- 8.Tolker-Nielsen T. 2014. Pseudomonas aeruginosa biofilm infections: from molecular biofilm biology to new treatment possibilities. APMIS Suppl 138:1–51. doi: 10.1111/apm.12335. [DOI] [PubMed] [Google Scholar]
- 9.de Dios Caballero J, del Campo R, Royuela A, Solé A, Máiz L, Olveira C, Quintana-Gallego E, de Gracia J, Cobo M, de la Pedrosa EGG, Oliver A, Cantón R GEIFQ (Grupo Español para el Estudio de la Colonización/Infección Broncopulmonar en Fibrosis Quística). 2016. Bronchopulmonary infection-colonization patterns in Spanish cystic fibrosis patients: results from a national multicenter study. J Cyst Fibros 15:357–365. doi: 10.1016/j.jcf.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Lee B, Haagensen JAJ, Ciofu O, Bo J, Høiby N, Molin S, Andersen JB. 2005. Heterogeneity of biofilms formed by nonmucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J Clin Microbiol 43:5247–5255. doi: 10.1128/JCM.43.10.5247-5255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Olmos A, García-Castillo M, Maiz L, Lamas A, Baquero F, Cantón R. 2012. In vitro prevention of Pseudomonas aeruginosa early biofilm formation with antibiotics used in cystic fibrosis patients. Int J Antimicrob Agents 40:173–176. doi: 10.1016/j.ijantimicag.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Ciofu O, Rojo-Molinero E, Macià MD, Oliver A. 2017. Antibiotic treatment of biofilm infections. APMIS 125:304–319. doi: 10.1111/apm.12673. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Martínez J, Ballesta S, Pascual A. 2007. Activity and penetration of fosfomycin, ciprofloxacin, amoxicillin/clavulanic acid and co-trimoxazole in Escherichia coli and Pseudomonas aeruginosa biofilm. Int J Antimicrob Agents 30:366–368. doi: 10.1016/j.ijantimicag.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Díez-Aguilar M, Morosini MI, Tedim AP, Rodríguez I, Aktaş Z, Cantón R. 2015. Antimicrobial activity of fosfomycin and tobramycin combination against Pseudomonas aeruginosa isolates assessed by time-kill assays and mutant prevention concentrations. Antimicrob Agents Chemother 59:6039–6045. doi: 10.1128/AAC.00822-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Díez-Aguilar M, Morosini MI, Del Campo R, García-Castillo M, Zamora J, Cantón R. 2013. In vitro activity of fosfomycin against a collection of clinical Pseudomonas aeruginosa isolates from 16 Spanish hospitals: establishing the validity of standard broth microdilution as susceptibility testing method. Antimicrob Agents Chemother 57:5701–5703. doi: 10.1128/AAC.00589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamou-Segarra M, Zamorano L, Vadlamani G, Chu M, Sanchez-Diener I, Juan C, Blazquez J, Hattie M, Stubbs KA, Mark BL, Oliver A. 2017. Synergistic activity of fosfomycin, β-lactams and peptidoglycan recycling inhibition against Pseudomonas aeruginosa. J Antimicrob Chemother 72:448–454. doi: 10.1093/jac/dkw456. [DOI] [PubMed] [Google Scholar]
- 17.Kollef MH, Ricard JD, Roux D, Francois B, Ischaki E, Rozgonyi Z, Boulain T, Ivanyi Z, János G, Garot D, Koura F, Zakynthinos E, Dimopoulos G, Torres A, Danker W, Montgomery AB. 2017. A randomized trial of the amikacin fosfomycin inhalation system for the adjunctive therapy of gram-negative ventilator-associated pneumonia: IASIS Trial. Chest 151:1239–1246. doi: 10.1016/j.chest.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Geller DE, Pitlick WH, Nardella PA, Tracewell WG, Ramsey BW. 2002. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest 122:219–226. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- 19.Rojo-Molinero E, Macià MD, Rubio R, Moyà B, Cabot G, López-Causapé C, Pérez JL, Cantón R, Oliver A. 2016. Sequential treatment of biofilms with aztreonam and tobramycin: a novel strategy for combating Pseudomonas aeruginosa chronic respiratory infections. Antimicrob Agents Chemother 60:2912–2922. doi: 10.1128/AAC.00196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLeod DL, Velayudhan J, Kenney TF, Therrien JH, Sutherland JL, Barker LM, Baker WR. 2012. Fosfomycin enhances the active transport of tobramycin in Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:1529–1538. doi: 10.1128/AAC.05958-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns JL, Saiman L, Whittier S, Larone D, Krzewinski J, Liu Z, Marshall SA, Jones RN. 2000. Comparison of agar diffusion methodologies for antimicrobial susceptibility testing of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J Clin Microbiol 38:1818–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castañeda-García A, Rodríguez-Rojas A, Guelfo JR, Blázquez J. 2009. The glycerol-3-phosphate permease GlpT is the only fosfomycin transporter in Pseudomonas aeruginosa. J Bacteriol 191:6968–6974. doi: 10.1128/JB.00748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moskowitz SM, Foster JM, Emerson J, Burns JL. 2004. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J Clin Microbiol 42:1915–1922. doi: 10.1128/JCM.42.5.1915-1922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 25.Reffuveille F, De La Fuente-Núnez C, Mansour S, Hancock REW. 2014. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob Agents Chemother 58:5363–5371. doi: 10.1128/AAC.03163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benoit MR, Conant CG, Ionescu-zanetti C, Schwartz M, Matin A. 2010. New device for high-throughput viability screening of flow biofilms. Appl Environ Microbiol 76:4136–4142. doi: 10.1128/AEM.03065-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.