ABSTRACT
Evidence supports vancomycin therapeutic-drug monitoring by area under the concentration-time curve (AUC), but data to establish an AUC upper limit are limited and published nephrotoxicity thresholds range widely. The objective of this analysis was to examine the association between initial vancomycin AUC and nephrotoxicity. This was a multicenter, retrospective cohort study of adult patients receiving intravenous vancomycin from 2014 to 2015. Nephrotoxicity was defined as a serum creatinine increase of 0.5 mg/liter and 50% from baseline on consecutive measurements. Vancomycin exposure profile during the initial 48 h of therapy was estimated using maximum a posteriori probability Bayesian estimation. Vancomycin AUC and minimum-concentration (Cmin) thresholds most strongly associated with nephrotoxicity were identified via classification and regression tree (CART) analysis. Predictive performances of CART-derived and other candidate AUC thresholds was assessed through positive and negative predictive value and receiver operating characteristic curves. Poisson regression was used to quantify the association between exposure thresholds and nephrotoxicity while adjusting for confounders. Among 323 patients included, nephrotoxicity was significantly higher in patients with AUCs from 0 to 48 h (AUC0–48) of ≥1,218 mg · h/liter, AUC0–24 of ≥677 mg · h/liter, AUC24–48 of ≥683 mg · h/liter, and day 1 Cmin (Cmin24) of ≥18.8 mg/liter. Vancomycin exposure in excess of these thresholds was associated with a 3- to 4-fold-increased risk of nephrotoxicity in Poisson regression. The predictive performance of AUC for nephrotoxicity was maximized at daily AUC values between 600 and 800 mg · h/liter. Although these data support an AUC range for vancomycin-associated nephrotoxity rather than a single threshold, available evidence suggests that a daily AUC limit of 700 mg · h/liter is reasonable.
KEYWORDS: area under the concentration-time curve, AUC, trough concentration, Cmin, acute kidney injury, nephrotoxicity, therapeutic-drug monitoring
INTRODUCTION
Recent evidence regarding vancomycin pharmacokinetics/pharmacodynamics has initiated a paradigm shift in vancomycin therapeutic-drug monitoring (TDM) from targeting trough concentrations to area under the concentration-time curve (AUC) (1–8). The 2009 vancomycin TDM guidelines published by the Infectious Diseases Society of America in collaboration with the American Society of Health-System Pharmacists and the Society of Infectious Diseases Pharmacists recommended trough concentrations between 15 and 20 mg/liter to maximize the likelihood of achieving daily AUC/MIC ratios of ≥400 for organisms with MICs of ≤1 mg/liter (9). However, recent pharmacokinetic data suggest that the majority of patients can achieve AUC values of ≥400 mg · h/liter with trough concentrations of <15 mg/liter (1). Considering that vancomycin-associated nephrotoxicity has been linked to trough concentrations of >15 mg/liter, monitoring vancomycin by AUC would be expected to reduce unnecessarily high vancomycin exposure and thus reduce nephrotoxicity (10, 11). This notion is consistent with recent clinical data demonstrating reduced nephrotoxicity with AUC-guided dosing relative to targeting trough concentrations of 15 to 20 mg/liter (8). Coupled with clinical data confirming that AUC better predicts efficacy and preclinical data suggesting that AUC and maximum concentration (Cmax) are more strongly correlated with kidney injury than trough, it is apparent that AUC-guided vancomycin dosing is a more rational approach (2, 4, 7).
Although clinical data suggest that targeting daily vancomycin AUCs between 400 and 600 mg · h/liter will ensure efficacy, the AUC range associated with nephrotoxicity has not been clearly defined (2, 4, 12, 13). A number of published reports have examined the potential relationship between daily vancomycin AUC and nephrotoxicity. However, the AUC estimation methods have varied and the resulting AUC thresholds have ranged widely, from 563 to 1,300 mg · h/liter (6, 14–17). This leaves the therapeutic vancomycin AUC range without an upper limit, which further limits the widespread implementation of AUC monitoring. As vancomycin is one of the most commonly prescribed intravenous (i.v.) antibiotics in many countries and vancomycin-associated nephrotoxicity is reported to occur in 5 to 30% of patients, additional data to define an upper limit for AUC monitoring are crucial (10, 18–20). The objective of this analysis was to derive vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin, with a focus on AUC.
(Preliminary results of this analysis have been previously presented at the 2016 American Society for Microbiology Microbe in Boston, MA, and the 2017 European Congress of Clinical Microbiology and Infectious Disease in Vienna, Austria.)
RESULTS
A total of 323 patients were included. The cohort was predominantly African American (74.6%), approximately half male (51.7%), with a mean (standard deviation [SD]) age of 61.7 (16.8) years. Hypertension (75.9%), heart failure (37.5%), diabetes mellitus (35.9%), and renal disease (18.6%) were common comorbidities. The median (interquartile range [IQR]) Elixhauser comorbidity index and acute physiology and chronic health evaluation II (APACHE II) score were 5 (4 to 7) and 13 (9 to 20), respectively. The majority of patients received at least one concomitant nephrotoxic medication (61.6%), and the most common were furosemide (41.5%), lisinopril (26.0%), and i.v. contrast (18.0%). Vancomycin indication per initial physician order was bacteremia in 57.0% and pneumonia in 43.0% of patients. Therapeutic-drug monitoring was performed using AUC in 52.3% of patients. The median (IQR) duration of therapy was 6 (5 to 8) days, and the median (IQR) total daily doses on days 1 and 2 were 3,000 (2,250 to 4,000) mg and 2,000 (1,250 to 3,000) mg, respectively. The median (IQR) Bayesian estimated AUC from 0 to 24 h (AUC0–24), AUC24–48, day 1 minimum concentration (Cmin24), and day 2 Cmin (Cmin48) were 572 (416 to 738) mg · h/liter, 586 (467 to 743) mg · h/liter, 11.1 (7.9 to 14.7) mg/liter, and 13.6 (9.4 to 17.3) mg/liter, respectively. A total of 670 vancomycin serum concentrations were used in the Bayesian analysis. The median (IQR) number of serum concentrations per patient and time to concentration were 2 (2, 3) concentrations and 47.4 (29.8 to 68.9) h, respectively. Linear regression of observed versus Bayesian predicted vancomycin serum concentrations indicate that the observed concentrations were explained well (R2 = 0.990 [data not shown]).
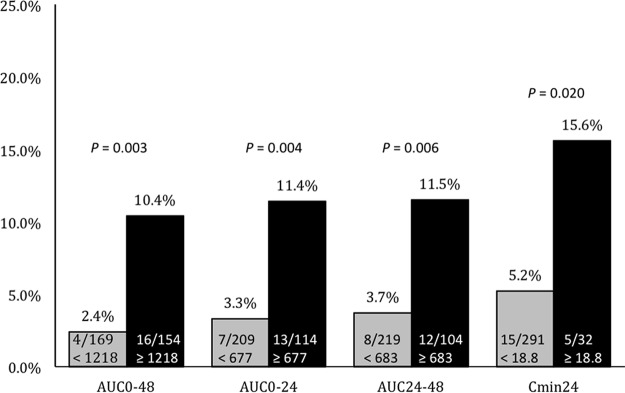
Bivariate comparisons between patients who experienced and did not experience nephrotoxicity are listed in Table 1. Patients who experienced nephrotoxicity had significantly higher Elixhauser comorbidity index and APACHE II scores, were more likely to have renal disease, liver disease, or heart failure, and were more likely to have received concomitant furosemide or concomitant i.v. contrast dye. Those experiencing nephrotoxicity had significantly higher vancomycin exposure quantified by AUC0–48 and AUC24–48 and numerically higher AUC0–24, Cmin24, and Cmin48. In the classification and regression tree (CART) analysis, nephrotoxicity was significantly higher among patients with AUC0–48s of ≥1,218 mg · h/liter, AUC0–24s of ≥677 mg · h/liter, AUC24–48s of ≥683 mg · h/liter, and Cmin24s of ≥18.8 mg/liter, while no threshold was discovered for Cmin48 (Table 1 and Fig. 1).
TABLE 1.
Bivariate comparisons of demographic and clinical characteristics between nephrotoxic and non-nephrotoxic patientsa
| Characteristic | Value for patients |
P value | |
|---|---|---|---|
| Non-nephrotoxic (n = 303) | Nephrotoxic (n = 20) | ||
| Demographics | |||
| Age (yrs), mean (SD) | 61.5 (16.5) | 64.2 (21.3) | 0.593 |
| Male, no. (%) | 152 (50.2) | 15 (75.0) | 0.031 |
| Race, no. (%) | 0.636 | ||
| African American | 228 (75.2) | 13 (65.0) | |
| Caucasian | 56 (18.5) | 6 (30.0) | |
| Asian | 2 (0.7) | 0 | |
| Other/unknown | 17 (5.6) | 1 (5.0) | |
| Selected comorbidities | |||
| Renal disease, no. (%) | 50 (16.5) | 10 (50.0) | <0.001 |
| Liver disease, no. (%) | 26 (8.6) | 5 (25.0) | 0.016 |
| Heart failure, no. (%) | 107 (35.3) | 14 (70.0) | 0.002 |
| Diabetes, no. (%) | 107 (35.3) | 9 (45.0) | 0.382 |
| Peripheral vascular disease, no. (%) | 38 (12.5) | 4 (20.0) | 0.337 |
| Hypertension, no. (%) | 229 (75.6) | 16 (80.0) | 0.792 |
| Obesity, no. (%) | 54 (17.8) | 2 (10.0) | 0.546 |
| Elixhauser comorbidity index, median (IQR) | 5 (3–7) | 7.5 (5.25–8.75) | <0.001 |
| Clinical data | |||
| Baseline SCrb (mg/dl), median (IQR) | 0.88 (0.69–1.18) | 0.95 (0.87–1.22) | 0.164 |
| APACHE II score,b median (IQR) | 13 (9–18) | 20.5 (12.75–31.25) | 0.001 |
| Concomitant nephrotoxin,d no. (%) | 183 (60.4) | 16 (80.0) | 0.098 |
| Intravenous contrast dye, no. (%) | 49 (16.2) | 9 (45.0) | 0.001 |
| Furosemide, no. (%) | 119 (39.3) | 15 (75.0) | 0.002 |
| Lisinopril, no. (%) | 78 (25.7) | 6 (30.0) | 0.674 |
| Aminoglycoside, no. (%) | 23 (7.6) | 2 (10.0) | 0.660 |
| Vancomycin treatment data | |||
| Vancomycin indication,c no. (%) | 0.777 | ||
| Bacteremia | 172 (56.8) | 12 (60.0) | |
| Pneumonia | 131 (43.2) | 8 (40.0) | |
| AUC monitoring, no. (%) | 160 (52.8) | 9 (45.0) | 0.498 |
| Total daily dose from 0 to 48 h (mg), median (IQR) | 5,000 (4,000–6,500) | 4,625 (3,062–7,375) | 0.593 |
| Total daily dose from 0 to 24 h (mg), median (IQR) | 3,000 (1,250–4,000) | 2,625 (2,312–4,875) | 0.741 |
| Total daily dose from 24 to 48 h (mg), median (IQR) | 2,000 (1,250–3,000) | 1,625 (1,000–2,500) | 0.257 |
| Vancomycin duration (days), median (IQR) | 6 (5–8) | 6.5 (5–9.75) | 0.163 |
| AUC0–48 (mg · h/liter), median (IQR) | 1,162 (913–1,462) | 1,413 (1,220–1,600) | 0.031 |
| AUC0–24 (mg · h/liter), median (IQR) | 569 (413–735) | 700 (547–821) | 0.058 |
| AUC24–48 (mg · h/liter), median (IQR) | 577 (459–731) | 719 (568–853) | 0.029 |
| Cmin24 (mg/liter), median (IQR) | 11 (7.7–14.4) | 13 (9.5–18.4) | 0.089 |
| Cmin48 (mg/liter), median (IQR) | 13.5 (9.3–17.0) | 16.1 (11.2–21.5) | 0.055 |
| AUC0–48 of ≥1,218 mg · h/liter, no. (%) | 138 (45.5) | 16 (80.0) | 0.003 |
| AUC0–24 of ≥677 mg · h/liter, no. (%) | 101 (33.3) | 13 (65.0) | 0.004 |
| AUC24–48 of ≥683 mg · h/liter, no. (%) | 92 (30.4) | 12 (60.0) | 0.006 |
| Cmin24 of ≥18.8 mg/liter, no. (%) | 27 (8.9) | 5 (25.0) | 0.020 |
Abbreviations: APACHE, acute physiology and chronic health evaluation; SCr, serum creatinine.
At time of 1st vancomycin dose.
Indication per physician order for vancomycin.
Seventy-two hours before initial vancomycin dose to 72 h after last vancomycin dose.
FIG 1.
Incidence of nephrotoxicity by CART-derived exposure thresholds.
The predictive performance of the CART-derived vancomycin AUC thresholds and other candidate AUC thresholds are listed in Table 2. Despite generally poor sensitivity, negative predictive value (NPV) was high and remained consistent across all candidate nephrotoxicity thresholds for AUC0–48, AUC0–24, and AUC24–48 between 93.5 and 97.6%. In contrast, positive predictive value (PPV) was low but varied across the candidate nephrotoxicity thresholds from 3.4 to 11.5% and thus was more informative than NPV in selecting a nephrotoxicity threshold. Positive predictive value for all three AUC exposure variables was maximized at the CART-derived thresholds and was highest for AUC0–48s between 1,200 and 1,400 mg · h/liter, for AUC0–24s between 677 and 800 mg · h/liter, and for AUC24–48s between 683 and 800 mg · h/liter. Consistent with maximization of PPV, the CART-derived thresholds for AUC0–48, AUC0–24, and AUC24–48 were most predictive in receiver operating characteristic (ROC) curve analysis and were the only thresholds with a statistically significant predictive value. The AUC0–48 threshold of ≥1,218 mg · h/liter had the highest predictive value, with an area under the ROC curve (95% confidence interval [CI]) of 0.672 (0.561 to 0.784).
TABLE 2.
Predictive performance of CART-derived and other candidate AUC toxicity thresholdsa
| AUC (mg · h/liter) | Sensitivity (%) | NPV (%) | Specificity (%) | PPV (%) | Area under ROC curve (95% CI) |
|---|---|---|---|---|---|
| AUC0–48 | |||||
| ≥1,000 | 85.0 | 97.2 | 34.7 | 7.9 | 0.598 (0.482–0.714) |
| ≥1,200 | 80.0 | 97.6 | 53.1 | 10.1 | 0.666 (0.554–0.778) |
| ≥1,218 | 80.0 | 97.6 | 54.5 | 10.4 | 0.672 (0.561–0.784) |
| ≥1,400 | 50.0 | 95.5 | 70.3 | 10.0 | 0.601 (0.469–0.734) |
| ≥1,600 | 25.0 | 94.3 | 82.5 | 8.6 | 0.538 (0.402–0.673) |
| ≥1,800 | 5.0 | 93.5 | 90.8 | 3.4 | 0.479 (0.352–0.605) |
| AUC0–24 | |||||
| ≥500 | 80.0 | 96.7 | 38.3 | 7.9 | 0.591 (0.472–0.711) |
| ≥600 | 65.0 | 96.0 | 55.8 | 8.8 | 0.604 (0.478–0.730) |
| ≥677 | 65.0 | 96.7 | 66.7 | 11.4 | 0.658 (0.534–0.783) |
| ≥700 | 50.0 | 95.5 | 70.0 | 9.9 | 0.600 (0.467–0.733) |
| ≥800 | 30.0 | 94.7 | 82.5 | 10.2 | 0.563 (0.425–0.700) |
| ≥900 | 10.0 | 93.8 | 89.4 | 5.9 | 0.497 (0.367–0.628) |
| AUC24–48 | |||||
| ≥500 | 80.0 | 96.2 | 33.0 | 7.3 | 0.565 (0.443–0.687) |
| ≥600 | 65.0 | 95.9 | 53.5 | 8.4 | 0.592 (0.466–0.718) |
| ≥683 | 60.0 | 96.3 | 69.6 | 11.5 | 0.648 (0.520–0.777) |
| ≥700 | 50.0 | 95.6 | 71.6 | 10.4 | 0.608 (0.475–0.741) |
| ≥800 | 30.0 | 94.7 | 81.8 | 9.8 | 0.559 (0.422–0.696) |
| ≥900 | 10.0 | 93.8 | 89.1 | 5.7 | 0.496 (0.366–0.626) |
Abbreviations: AUC, area under the concentration-time curve; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic; CI, confidence interval.
The final Poisson regression models for the independent impact of each CART-derived vancomycin exposure thresholds on nephrotoxicity are listed in Table 3. After adjustment for Elixhauser comorbidity index and receipt of concomitant i.v. contrast dye, an AUC0–48 of ≥1,218 mg · h/liter (risk ratio [RR], 4.366; 95% CI, 1.559 to 12.226) and an AUC24–48 of ≥683 mg · h/liter (RR, 2.982; 95% CI, 1.293 to 6.878) were associated with 4- and 3-fold-increase risks of nephrotoxicity, respectively. Adjusting for heart failure and APACHE II score, an AUC0–24 of ≥677 mg · h/liter was associated with a nearly 4-fold-increased risk of nephrotoxicity (RR, 3.734; 95% CI, 1.646 to 8.470). Adjusting for renal disease and heart failure, a Cmin24 of ≥18.8 mg/liter was associated with a 3-fold-increased risk of nephrotoxicity (RR, 3.733; 95% CI, 1.710 to 8.150). The results of the Cox proportional hazards regression for time to nephrotoxicity were consistent with those of the Poisson regression with (Table 4). Vancomycin exposure in excess of each CART-derived exposure threshold was independently associated with time to nephrotoxicity while adjusting for Elixhauser comorbidity index and concomitant i.v. contrast dye.
TABLE 3.
Final Poisson regression models for vancomycin exposure thresholds and nephrotoxicity
| Exposure threshold | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| AUC0–48 of ≥1,218 mg · h/liter | 4.390 (1.500–12.845) | 0.003 | 4.366a (1.559–12.226) | 0.005 |
| AUC0–24 of ≥677 mg · h/liter | 3.405 (1.398–8.291) | 0.004 | 3.734b (1.646–8.470) | 0.002 |
| AUC24–48 of ≥683 mg · h/liter | 3.159 (1.332–7.491) | 0.006 | 2.982a (1.293–6.878) | 0.010 |
| Cmin24 of ≥18.8 mg/liter | 3.031 (1.179–7.791) | 0.020 | 3.104c (1.243–7.755) | 0.015 |
Adjusted for Elixhauser comorbidity index and concomitant intravenous contrast dye.
Adjusted for heart failure and APACHE II score.
Adjusted for renal disease and heart failure.
TABLE 4.
Final Cox proportional hazards regression models for time to nephrotoxicity
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| AUC0–48 of ≥1,218 mg · h/liter | 4.963 (1.634–15.072) | 0.005 |
| Concomitant i.v. contrast dye | 4.141 (1.696–10.109) | 0.002 |
| Elixhauser comorbidity index | 1.224 (1.057–1.418) | 0.007 |
| AUC0–24 of ≥677 mg · h/liter | 4.167 (1.639–10.594 | 0.003 |
| Concomitant i.v. contrast dye | 3.798 (1.560–9.248) | 0.003 |
| Elixhauser comorbidity index | 1.283 (1.095–1.502) | 0.002 |
| AUC24–48 of ≥683 mg · h/liter | 3.513 (1.393–8.859) | 0.008 |
| Concomitant i.v. contrast dye | 4.270 (1.723–10.585) | 0.002 |
| Elixhauser comorbidity index | 1.224 (1.059–1.414) | 0.006 |
| Cmin24 of ≥18.8 mg/liter | 3.707 (1.336–10.287) | 0.012 |
| Concomitant i.v. contrast dye | 3.454 (1.422–8.390) | 0.006 |
| Elixhauser comorbidity index | 1.267 (1.080–1.486) | 0.004 |
DISCUSSION
Given the anticipated paradigm shift in vancomycin TDM from trough concentrations to AUC, this study sought to derive vancomycin exposure-toxicity thresholds with a focus on AUC to establish an upper therapeutic limit. Using a validated Bayesian approach to estimate AUC from limited vancomycin serum concentration sampling and CART analysis, thresholds in AUC over the first 48 h of therapy that impacted nephrotoxicity risk were derived. Adjusting for comorbidity, severity of illness, and receipt of concomitant nephrotoxins, vancomycin AUC0–48 of ≥1,218 mg · h/liter, AUC0–24 of ≥677 mg · h/liter, and AUC24–48 of ≥683 mg · h/liter were associated with approximately 3- to 4-fold-increased nephrotoxicity risk. In this cohort, nephrotoxicity was low (<4%) among patients with vancomycin exposures below these thresholds.
Although these CART-derived thresholds are inherently able to provide vancomycin AUC thresholds where the incidence of nephrotoxicity is most disproportionate, nephrotoxicity risk theoretically increases as a function of vancomycin AUC. Because of this, it is important to recognize that a range of AUC values can provide value in predicting likelihood of nephrotoxicity rather than a single threshold. This is evident in the fact that the AUC0–48 CART threshold of 1,218 mg · h/liter was slightly lower than the sum of the daily AUC0–24 and AUC24–48 CART thresholds (677 + 683 = 1,360 mg · h/liter) and likely reflects AUC fluctuation from day 1 to day 2 of therapy. To address this, we assessed the predictive performance of the CART-derived and other possible nephrotoxicity thresholds across the first 48 h of therapy (21). Negative predictive value was high for all thresholds, but this finding is a result of low overall nephrotoxicity incidence rather than high sensitivity. However, it was notable that NPV remained relatively constant across candidate toxicity thresholds. This means that the rate of “false negatives” (patients below a given threshold who experienced toxicity anyway) did not improve much with lower toxicity thresholds. Positive predictive value, despite being low, was more informative of the varying predictive value of the candidate thresholds. Positive predictive value was maximized at daily AUC values between approximately 600 and 800 mg · h/liter over the first 48 h of therapy. Maximization of PPV allows for identification of patients with vancomycin exposures most likely to result in nephrotoxicity. This is desirable when establishing an upper range of recommended vancomycin exposure for invasive Staphylococcus aureus infections where morbidity and mortality concern may outweigh toxicity concern. The collective positive predictive value data suggest that limiting daily vancomycin AUC values to <700 mg · h/liter can maximize PPV without compromising NPV, limiting the likelihood of unnecessary efficacy reductions without a proportionate toxicity reduction. However, it is important to recognize that this and other thresholds have limited ability to predict which patients will or will not experience toxicity and can only serve as a guide toward limiting toxicity.
Despite the wealth of published data relating vancomycin AUC values with efficacy for invasive Staphylococcus aureus infections, there is a relative dearth of evidence regarding vancomycin AUC and nephrotoxicity (2–4, 12, 13, 22). Among the few published reports, resulting AUC nephrotoxicity thresholds vary widely (6, 14–17). Using approaches similar to the present study, Lodise et al. and Chavada et al. identified steady-state vancomycin AUC nephrotoxicity thresholds of 1,300 and 563 mg · h/liter, respectively, in adult patients (14, 17). An AUC threshold of 700 mg · h/liter has also been proposed based on a small study of adult patients by Suzuki et al. and nephrotoxicity thresholds from vancomycin administered by continuous infusion (1, 6, 15). In pediatric patients, Le et al. identified ≥800 mg · h/liter as predictive of nephrotoxicity (16). A major difference between this and prior studies is the focus on the initial 48 h of therapy rather than steady-state or highest observed value. Although no data compare the importance of initial versus steady-state exposure in determining nephrotoxicity risk, initial therapy may be more reliable for capturing vancomycin exposure that preceded nephrotoxicity rather than exposure during or after nephrotoxicity in patients with early-onset nephrotoxicity. To limit this, we also excluded patients meeting the definition for nephrotoxicity within the first 48 h of therapy.
There are multiple considerations to bear in mind when interpreting these findings. Most importantly, these findings were derived from hospitalized adult patients with confirmed or suspected bacteremia and/or pneumonia from a single health system, potentially limiting external generalizability. We focused on patients with bacteremia or pneumonia indication for vancomycin to generate a relatively homogenous patient population at high risk of nephrotoxicity receiving aggressive vancomycin therapy. Despite this, nephrotoxicity incidence remained low relative to that in some other studies (10). A possible explanation is the exclusion of patients receiving concomitant piperacillin-tazobactam and those with baseline renal insufficiency. Exclusion of patients receiving piperacillin-tazobactam was essential due to a drastic reduction in piperacillin-tazobactam use at the Detroit Medical Center (DMC) coinciding with implementation of AUC monitoring. Had these patients been included, piperacillin-tazobactam exposure would have been more common among those monitored by trough concentration relative to those monitored by AUC. Considering that patients monitored by trough concentration would also have higher AUC values, the disproportionate exposure to a medication known to increase the risk of vancomycin-associated nephrotoxicity would falsely amplify nephrotoxicity differences observed between high- and low-AUC patients (23, 24). However, it is important to recognize that the vancomycin nephrotoxicity threshold may be different among patients receiving piperacillin-tazobactam, so these findings may not apply to that population. It is also important to note that although we accounted for bacteremia indication, this is not synonymous with confirmed bacteremia, which could influence nephrotoxicity risk. Because confirmation of bacteremia usually does not occur until after initial vancomycin dosing, we expect that this distinction would not influence vancomycin exposure, and thus, this should not substantially alter these findings. In addition, these data were derived from vancomycin administered as intermittent infusion. Continuous infusion is another potential approach to maximize AUC while limiting exposure and nephrotoxicity (5). Although our findings are similar to those derived from continuous-infusion vancomycin, these results should be applied to continuous infusion with caution (6, 25). Finally, although serum creatinine (SCr) is the current standard for clinical diagnosis of acute kidney injury, SCr increases can lag behind the onset of kidney damage and glomerular filtration rate (GFR) decline by several hours (26). Future studies involving novel urinary biomarkers or urine output may enhance precision in deriving vancomycin exposure-toxicity relationships (7).
In conclusion, daily vancomycin AUC values between 600 and 800 mg · h/liter during the first 48 h of therapy were associated with a 3- to 4-fold-increased nephrotoxicity risk. Nephrotoxicity among patients with vancomycin exposure below this range was uncommon. Although these data support an AUC range for vancomycin-associated nephrotoxity rather than a single threshold, available evidence suggests that a daily AUC limit of 700 mg · h/liter is reasonable. However, additional data addressing the limitations of this and other published studies are needed to establish a consensus vancomycin AUC therapeutic window.
MATERIALS AND METHODS
Study design and population.
This was a retrospective, observational cohort study of hospitalized adult patients receiving intravenous vancomycin for confirmed or suspected bacteremia or pneumonia from January 2014 to December 2015 at the Detroit Medical Center (DMC). Patients age ≥18 years receiving ≥72 h of intravenous vancomycin therapy with an indication of bacteremia or pneumonia entered by the prescriber at the time of initial vancomycin order were eligible for inclusion. Patients with a baseline serum creatinine (SCr) of ≥2 mg/liter, receiving renal replacement therapy during initial 96 h of vancomycin therapy, without a vancomycin serum concentration measured during the initial 96 h of vancomycin therapy, and those meeting the definition for vancomycin-associated nephrotoxicity during the initial 48 h of vancomycin therapy were excluded. During the study period, the DMC switched from trough concentration to AUC monitoring and also significantly reduced piperacillin-tazobactam use due to a nationwide shortage and emerging evidence of its association with increased vancomycin-associated nephrotoxicity (23, 24). To reduce the potential confounding effect of these nearly simultaneous clinical practice changes, patients receiving concomitant piperacillin-tazobactam were also excluded from this analysis. This study was approved by the institutional review board at Wayne State University, and waiver of informed consent was granted.
Patient data elements and collection.
Patient data, including demographics, comorbidities, medication therapy, laboratory values, physiological parameters, and indication for vancomycin therapy, were obtained from the electronic medical record by querying the organization's business intelligence software. Comorbidities were defined using International Classification of Disease, 9th revision, clinical modification (ICD-9-CM) and 10th revision, clinical modification (ICD-10-CM), codes (34, 35). The degree of patient comorbidity was quantified using the Elixhauser comorbidity index (27). Severity of illness was quantified using the acute physiology and chronic health evaluation II (APACHE II) score using the worst physiological parameters within 24 h of vancomycin initiation (28). Indication for vancomycin therapy was obtained from the antibiotic indication field completed by the prescriber at the time of initial electronic order entry. Administration dates and times of vancomycin and other potentially nephrotoxic medications were identified from electronic barcoded medication administration records. The following medications or medication classes were considered potential nephrotoxins: aminoglycosides, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, amphotericin B, calcineurin inhibitors, i.v. contrast dye, loop diuretics, polymyxins, and vasopressors. Renal function was assessed by SCr and creatinine clearance (CLCR) estimated by Cockcroft-Gault formula (29).
Outcome.
The primary outcome was vancomycin-associated nephrotoxicity, defined as an SCr increase of 0.5 mg/liter and 50% from baseline on two consecutive measurements from initial vancomycin dose to 72 h after the last dose (9, 14).
Data analysis.
In the primary analysis, the relationship between initial vancomycin concentration-time profile and vancomycin-associated nephrotoxicity was examined. The vancomycin concentration-time profile during the initial 48 h of therapy was estimated via the maximum a posteriori probability (MAP) Bayesian function of ADAPT V using a previously published 2-compartment population pharmacokinetic model as the Bayesian prior (30, 31). This approach has been validated for vancomycin AUC estimation using trough-only sampling (1). For patients experiencing nephrotoxicity, vancomycin serum concentrations drawn 48 h before the definition of nephrotoxicity was met and later were excluded from Bayesian analysis to limit bias in initial exposure profile estimation. The following vancomycin exposure variables were then compared between patients who experienced nephrotoxicity and those who did not: day 1 AUC (AUC0–24), day 2 AUC (AUC24–48), cumulative day 1 and 2 AUC (AUC0–48), day 1 Cmin (Cmin24); day 2 Cmin (Cmin48). Thresholds in the distribution of the vancomycin exposure variables where the incidence of nephrotoxicity was most disproportionate were derived using classification and regression tree (CART) analysis (32). The predictive performance of the vancomycin exposure variables, including the CART-derived and other a priori defined exposure thresholds, for predicting nephrotoxicity was evaluated using receiver operating characteristic (ROC) curves along with negative and positive predictive values (NPV and PPV, respectively). The impact of the CART-derived exposure thresholds on nephrotoxicity risk while accounting for confounding variables was quantified using Poisson regression with robust variance estimation (33). Candidate confounding variables were entered into regression models with each individual exposure threshold using a stepwise approach and retained in the model if the point estimate of the effect of the exposure variable on nephrotoxicity was altered by >10% (2). Associations between the CART-derived exposure thresholds and days to nephrotoxicity were also examined using Cox proportional hazards regression.
All statistical tests were two-sided; P values of ≤0.05 were considered statistically significant. Analyses were performed using SPSS Statistics, IBM SPSS software, version 24.0 (IBM Corp., Armonk, NY).
ACKNOWLEDGMENTS
We thank Shravya Kidambi and Sahil Bhatia for their assistance with data collection and management.
We investigators received no external funding for execution of this study.
M.J.R. is a grant recipient of, consultant for, advisory board member for, and is on the speaker's bureau for Allergan, Bayer, Cempra Inc., Merck & Co., The Medicines Company, Sunovian, and Theravance and is supported in part by NIH grants R21 AI109266-01 and R01 AI121400-01. All other authors have no conflicts of interest to declare.
REFERENCES
- 1.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. 2014. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lodise TP, Drusano GL, Zasowski E, Dihmess A, Lazariu V, Cosler L, McNutt LA. 2014. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis 59:666–675. doi: 10.1093/cid/ciu398. [DOI] [PubMed] [Google Scholar]
- 3.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Warren SJ, Gao W, Howden BP, Johnson PD. 2013. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 57:1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casapao AM, Lodise TP, Davis SL, Claeys KC, Kullar R, Levine DP, Rybak MJ. 2015. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother 59:2978–2985. doi: 10.1128/AAC.03970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Maarseveen EM, Gipmans S, Vasbinder E, Petjak M, van Zanten AR. 2016. Switching from intermittent to continuous infusion of vancomycin in critically ill patients: toward a more robust exposure. Ther Drug Monit 38:398–401. doi: 10.1097/FTD.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 6.Ingram PR, Lye DC, Tambyah PA, Goh WP, Tam VH, Fisher DA. 2008. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother 62:168–171. doi: 10.1093/jac/dkn080. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O'Donnell JN, Pais G, Cluff C, Lamar PC, Neely MN, Gulati A, Scheetz MH. 2016. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 60:5742–5751. doi: 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finch NA, Zasowski EJ, Murray KP, Mynatt RP, Zhao JJ, Yost R, Pogue JM, Rybak MJ. 18 September 2017. The impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity: a quasi-experiment. Antimicrob Agents Chemother doi: 10.1128/aac.01293-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 10.van Hal SJ, Paterson DL, Lodise TP. 2013. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 57:734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanrahan TP, Kotapati C, Roberts MJ, Rowland J, Lipman J, Roberts JA, Udy A. 2015. Factors associated with vancomycin nephrotoxicity in the critically ill. Anaesth Intensive Care 43:594–599. [DOI] [PubMed] [Google Scholar]
- 12.Zelenitsky S, Rubinstein E, Ariano R, Iacovides H, Dodek P, Mirzanejad Y, Kumar A, Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. 2013. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents 41:255–260. doi: 10.1016/j.ijantimicag.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Song KH, Kim HB, Kim HS, Lee MJ, Jung Y, Kim G, Hwang JH, Kim NH, Kim M, Kim CJ, Choe PG, Chung JY, Park WB, Kim ES, Park KU, Kim NJ, Kim EC, Oh MD. 2015. Impact of area under the concentration-time curve to minimum inhibitory concentration ratio on vancomycin treatment outcomes in methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents 46:689–695. doi: 10.1016/j.ijantimicag.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Kawasaki K, Sato Y, Tokimatsu I, Itoh H, Hiramatsu K, Takeyama M, Kadota J. 2012. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant staphylococcus aureus pneumonia. Chemotherapy 58:308–312. [DOI] [PubMed] [Google Scholar]
- 16.Le J, Ny P, Capparelli E, Lane J, Ngu B, Muus R, Romanowski G, Vo T, Bradley J. 2015. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc 4:e109–e116. doi: 10.1093/jpids/piu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ.. 2017. Establishment of an AUC0–24 threshold for nephrotoxicity is a step towards individualized vancomycin dosing for methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 61:e02535-16. doi: 10.1128/AAC.02535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. 2007. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 44:664–670. doi: 10.1086/511640. [DOI] [PubMed] [Google Scholar]
- 19.Taylor G, Gravel D, Saxinger L, Bush K, Simmonds K, Matlow A, Embree J, Le Saux N, Johnston L, Suh KN, Embil J, Henderson E, John M, Roth V, Wong A. 2015. Prevalence of antimicrobial use in a network of Canadian hospitals in 2002 and 2009. Can J Infect Dis Med Microbiol 26:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Australian Commission on Safety and Quality in Health Care. 2015. Antimicrobial use in Australian hospitals: 2014 annual report of the National Antimicrobial Utilisation Surveillance Program. Australian Commission on Safety and Quality in Health Care, Sydney, Australia: https://www.safetyandquality.gov.au/wp-content/uploads/2016/03/2014-NAUSP-Report-AU-Australian-Hospitals.pdf Accessed 26 June 2017. [Google Scholar]
- 21.Rhodes NJ, O'Donnell JN, Lizza BD, McLaughlin MM, Esterly JS, Scheetz MH. 2016. Tree-based models for predicting mortality in Gram-negative bacteremia: avoid putting the CART before the horse. Antimicrob Agents Chemother 60:838–844. doi: 10.1128/AAC.01564-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown J, Brown K, Forrest A. 2012. Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob Agents Chemother 56:634–638. doi: 10.1128/AAC.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navalkele B, Pogue JM, Karino S, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Koons J, Hussain T, Perry W, Evans R, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Kaye KS. 2017. Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis 64:116–123. doi: 10.1093/cid/ciw709. [DOI] [PubMed] [Google Scholar]
- 24.Giuliano CA, Patel CR, Kale-Pradhan PB. 2016. Is the combination of piperacillin-tazobactam and vancomycin associated with development of acute kidney injury? A meta-analysis. Pharmacotherapy 36:1217–1228. [DOI] [PubMed] [Google Scholar]
- 25.Hanrahan TP, Harlow G, Hutchinson J, Dulhunty JM, Lipman J, Whitehouse T, Roberts JA. 2014. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med 42:2527–2536. doi: 10.1097/CCM.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 26.Waikar SS, Bonventre JV. 2009. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. 2005. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 28.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 30.D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA. [Google Scholar]
- 31.Rodvold KA, Pryka RD, Garrison M, Rotschafer JC. 1989. Evaluation of a two-compartment Bayesian forecasting program for predicting vancomycin concentrations. Ther Drug Monit 11:269–275. doi: 10.1097/00007691-198905000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HSB. 1999. Recursive partitioning in the health sciences. Springer, New York, NY. [Google Scholar]
- 33.McNutt LA, Wu C, Xue X, Hafner JP. 2003. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 34.U.S. National Center for Health Statistics International classification of diseases, ninth revision, clinical modification (ICD-9-CM). www.cdc.gov/nchs/icd/icd9cm.htm.
- 35.U.S. National Center for Health Statistics International classification of diseases, tenth revision, clinical modification (ICD-10-CM). www.cdc.gov/nchs/icd/icd10cm.htm. [PubMed]