ABSTRACT
Although carbapenems are effective for treating serious multidrug-resistant Pseudomonas aeruginosa infections, carbapenem-resistant P. aeruginosa (CRPA) is now being reported worldwide. Ceftolozane-tazobactam (C/T) demonstrates activity against many multidrug-resistant isolates. We evaluated the activity of C/T and compared its activity to that of ceftazidime-avibactam (C/A) using a well-characterized collection of non-carbapenemase-producing CRPA isolates. Forty-two non-carbapenemase-producing CRPA isolates from a previous study (J. Y. Lee and K. S. Ko, Int J Antimicrob Agents 40:168–172, 2012, https://doi.org/10.1016/j.ijantimicag.2012.04.004) were included. All had been previously shown to be negative for blaIMP, blaVIM, blaSPM, blaGIM, blaSIM, and blaKPC by PCR. In the prior study, expression of oprD, ampC, and several efflux pump genes had been defined by quantitative reverse transcription-PCR. Here, antimicrobial susceptibility was determined by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) guidelines. Time-kill curve assays were performed using three C/T- and C/A-susceptible CRPA isolates. Among 42 non-carbapenemase-producing CRPA isolates, overall susceptibility to C/T was 95.2%, compared to 71.4%, 42.9%, 23.8%, 21.4%, and 2.4% for C/A, ceftazidime, piperacillin-tazobactam, cefepime, and meropenem, respectively. The C/T resistance rate was significantly lower than that of C/A among isolates showing decreased oprD and increased mexB expression (5.1% versus 25.6%, P = 0.025, and 4.3% versus 34.8%, P = 0.022, respectively). In time-kill curve studies, C/T was less bactericidal than C/A against an isolate with decreased oprD and increased ampC expression. C/T was active against 95.2% of non-carbapenemase-producing CRPA clinical isolates. No apparent correlation of C/T MIC values with specific mutation-driven resistance mechanisms was noted.
KEYWORDS: Pseudomonas aeruginosa, carbapenem resistant, ceftolozane-tazobactam
INTRODUCTION
Although carbapenems remain effective in treating serious multidrug-resistant (MDR) Pseudomonas aeruginosa infections, carbapenem-resistant P. aeruginosa (CRPA) has emerged and is being reported as a nosocomial pathogen worldwide and particularly in debilitated or immunocompromised patients (1, 2). Infections caused by CRPA are of concern in many hospitals since they have been shown to reduce the likelihood of appropriate initial antimicrobial therapy and are associated with significant mortality (3).
While the production of carbapenemases, mainly Ambler class B metallo-β-lactamases (MBLs), is noteworthy in P. aeruginosa as a mechanism of carbapenem resistance (4), the presence of non-carbapenemase-mediated carbapenem resistance is far more common (5–7). Loss of outer membrane porin D (OprD) function in conjunction with another mechanism, such as overexpression of ampC or overexpression of efflux pumps, is the major determinant of resistance to carbapenems (5–7). Chromosomally encoded AmpC β-lactamase together with the efflux pump MexAB-OprM operon contributes to resistance of P. aeruginosa to many β-lactam antibiotics. Nevertheless, isolates with reduced susceptibility to carbapenems because of inactivation of oprD in conjunction with other mechanism, such as overexpression of ampC or overexpression of efflux pumps, sometimes show susceptibility to other β-lactams besides carbapenems (8–10).
Ceftolozane-tazobactam (C/T) is a novel antibiotic with broad-spectrum activity against Gram-negative bacteria, including MDR P. aeruginosa. Ceftolozane is an oxyimino-aminothiazolyl cephalosporin that has stability against chromosomal AmpC β-lactamases, overexpressed MexAB-OprM efflux pumps, and deleted OprD porins (11). Ceftolozane's affinity for the penicillin-binding proteins of P. aeruginosa accounts for its activity against this organism (12). Although tazobactam does not play a critical role in enhancing the activity of ceftolozane against P. aeruginosa, it extends the activity of ceftolozane alone against extended-spectrum-β-lactamase-producing Enterobacteriaceae (12). However, C/T is not active against Klebsiella pneumoniae carbapenemase (KPC) or metallo-β-lactamases (13, 14). Therefore, C/T demonstrates activity against many MDR isolates of P. aeruginosa, including carbapenem-resistant strains that do not produce a carbapenemase (14).
Here, we evaluated the activity of C/T and compared its activity to that of ceftazidime-avibactam (C/A) in non-carbapenemase-producing CRPA clinical isolates (6). In addition, we assessed strains for underlying C/T and C/A resistance mechanisms.
RESULTS
Antimicrobial susceptibility of C/T and C/A against P. aeruginosa and its correlation with resistance mechanisms.
Among 42 non-carbapenemase-producing CRPA isolates, overall susceptibility to C/T was 95.2%, compared to 71.4%, 42.9%, 23.8%, 21.4%, and 2.4% for C/A, ceftazidime, piperacillin-tazobactam, cefepime, and meropenem, respectively (Table 1). Only two isolates showed resistance to C/T.
TABLE 1.
Antimicrobial activities tested against non-carbapenemase-producing CRPA isolatesa
| Antimicrobial agent | MIC (mg/liter) |
No. (%) of isolates with result (n = 42): |
||||
|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | S | I | R | |
| Imipenem | 16 | 32 | 8 to 32 | 0 (0) | 0 (0) | 42 (100) |
| Meropenem | 16 | 32 | 2 to >64 | 1 (2.4) | 9 (21.4) | 32 (76.2) |
| Piperacillin-tazobactam | 128/4 | >256/4 | 4/4 to >256/4 | 10 (23.8) | 3 (7.1) | 29 (69.0) |
| Ceftolozane-tazobactam | 2/4 | 4/4 | 1/4 to 32/4 | 40 (95.2) | 0 (0) | 2 (4.8) |
| Ceftazidime-avibactam | 8/4 | 16/4 | 2/4 to 32/4 | 30 (71.4) | NA | 12 (28.6) |
| Cefepime | 32 | >64 | 2 to >64 | 9 (21.4) | 8 (19.0) | 25 (59.5) |
| Ceftazidime | 16 | 64 | 2 to >64 | 18 (42.9) | 3 (7.1) | 21 (50.0) |
| Ciprofloxacin | 2 | 64 | ≤0.06 to >64 | 14 (33.3) | 8 (19.0) | 20 (47.6) |
| Amikacin | 16 | >128 | 2 to >128 | 25 (59.5) | 4 (9.5) | 13 (31.0) |
| Polymyxin B | 1 | 2 | 0.5 to 2 | 42 (100) | 0 (0) | 0 (0) |
| Colistin | 2 | 2 | 0.5 to 2 | 42 (100) | NA | 0 (0) |
Abbreviations: CRPA, carbapenem-resistant P. aeruginosa; S, susceptible; I, intermediate; R, resistant; NA, not applicable.
Of 42 non-carbapenemase-producing CRPA isolates, 39 (92.9%) showed decreased oprD expression (≤30%) compared with that of PAO1 (Table 2). Strains with decreased oprD expression displayed median C/T and C/A MIC values of 2 and 8 mg/liter, respectively. The resistance rate for C/T was significantly lower than that for C/A among isolates showing decreased oprD expression (5.1% versus 25.6%, P = 0.025). Among the isolates analyzed, 23 (54.8%) had elevated expression of mexB. Resistance to C/T was significantly lower than that to C/A among isolates showing elevated mexB expression (4.3% versus 34.8%, P = 0.022). Elevated expression of mexD was noted among 6 (14.3%) of the isolates. All isolates showing elevated expression of mexD were susceptible to both C/T and C/A. Overall, 17 (40.5%) isolates were considered to have a derepressed chromosomal ampC. The resistance rates for C/T and C/A among isolates showing derepressed chromosomal ampC were 5.9% and 23.5%, respectively, a statistically nonsignificant difference.
TABLE 2.
MIC range and resistance rates for ceftolozane-tazobactam and ceftazidime-avibactam according to results for expression of oprD, efflux pumps, and chromosomal ampC among non-carbapenemase-producing CRPA clinical isolates
| Resistance mechanism | No. of isolates | MIC range (median) (mg/liter) |
% resistance |
||
|---|---|---|---|---|---|
| Ceftolozane-tazobactam | Ceftazidime-avibactam | Ceftolozane-tazobactam | Ceftazidime-avibactam | ||
| Decreased oprD expression | |||||
| Positive (≤30% compared with PAO1)a | 39 | 1–32 (2) | 2–32 (8) | 5.1 | 25.6 |
| Negative (>30% compared with PAO1) | 3 | 2–4 (2) | 8–16 (16) | 0 | 66.7 |
| Overexpressed mexB | |||||
| Positive (≥3-fold compared with PAO1)a | 23 | 1–32 (2) | 2–32 (8) | 4.3 | 34.8 |
| Negative (<2-fold compared with PAO1) | 12 | 1–16 (2) | 2–16 (4) | 8.3 | 25.0 |
| Overexpressed mexD | |||||
| Positive (≥10-fold compared with PAO1) | 6 | 1–4 (2) | 2–8 (4) | 0 | 0 |
| Negative (<5-fold compared with PAO1) | 35 | 1–32 (2) | 2–32 (8) | 5.7 | 34.3 |
| Overexpressed mexF | |||||
| Positive (≥10-fold compared with PAO1) | 5 | 1–4 (2) | 2–16 (8) | 0 | 40 |
| Negative (<5-fold compared with PAO1) | 29 | 1–32 (2) | 2–32 (8) | 6.9 | 24.1 |
| Overexpressed ampC | |||||
| Positive (≥10-fold compared with PAO1) | 17 | 1–32 (4) | 2–16 (8) | 5.9 | 23.5 |
| Negative (<5-fold compared with PAO1) | 25 | 1–16 (2) | 2–32 (4) | 4.0 | 32.0 |
P < 0.05 in comparison of percent resistance to ceftolozane-tazobactam with that to ceftazidime-avibactam.
The resistance mechanisms described above were observed alone (15 isolates) or in combinations of two to four mechanisms in the isolates tested (Table 3). Overall, 10 resistance mechanisms or combinations thereof were observed, with decreased oprD expression alone being most prevalent (13 strains), followed by a combination of decreased expression of oprD and overexpression of mexB (9 isolates). Resistance to C/T was significantly lower than that to C/A among isolates showing decreased oprD and increased mexB expression (0% versus 55.6%, P = 0.005).
TABLE 3.
MIC range and resistance rates for ceftolozane-tazobactam and ceftazidime-avibactam according to results for resistance mechanisms among non-carbapenemase-producing CRPA clinical isolates
| Resistance mechanism | No. of isolates | MIC range (median) (mg/liter) |
% resistance |
||
|---|---|---|---|---|---|
| Ceftolozane-tazobactam | Ceftazidime-avibactam | Ceftolozane-tazobactam | Ceftazidime-avibactam | ||
| Decreased oprD expression | 13 | 1–16 (2) | 2–16 (4) | 7.7 | 15.4 |
| Decreased oprD and increased mexB expressiona | 9 | 1–4 (2) | 2–32 (16) | 0 | 55.6 |
| Decreased oprD and increased mexY expression | 1 | 1 | 2 | 0 | 0 |
| Decreased oprD and increased ampC expression | 3 | 1–4 (2) | 4–8 (4) | 0 | 0 |
| Decreased oprD and increased mexB and ampC expression | 4 | 2–32 (4) | 8–16 (16) | 25.0 | 50.0 |
| Decreased oprD and increased mexY and ampC expression | 1 | 2 | 16 | 0 | 100 |
| Decreased oprD and increased mexB, mexD, and ampC expression | 6 | 1–4 (2) | 2–8 (4) | 0 | 0 |
| Decreased oprD and increased mexB, mexY, and ampC expression | 2 | 2 | 8 | 0 | 0 |
| Increased mexB expression | 2 | 2 | 8–16 | 0 | 50 |
| Increased mexY and ampC expression | 1 | 4 | 16 | 0 | 100 |
P < 0.05 in comparison of percent resistance to ceftolozane-tazobactam with that to ceftazidime-avibactam.
Killing effects of C/T and C/A on CRPA clinical isolates.
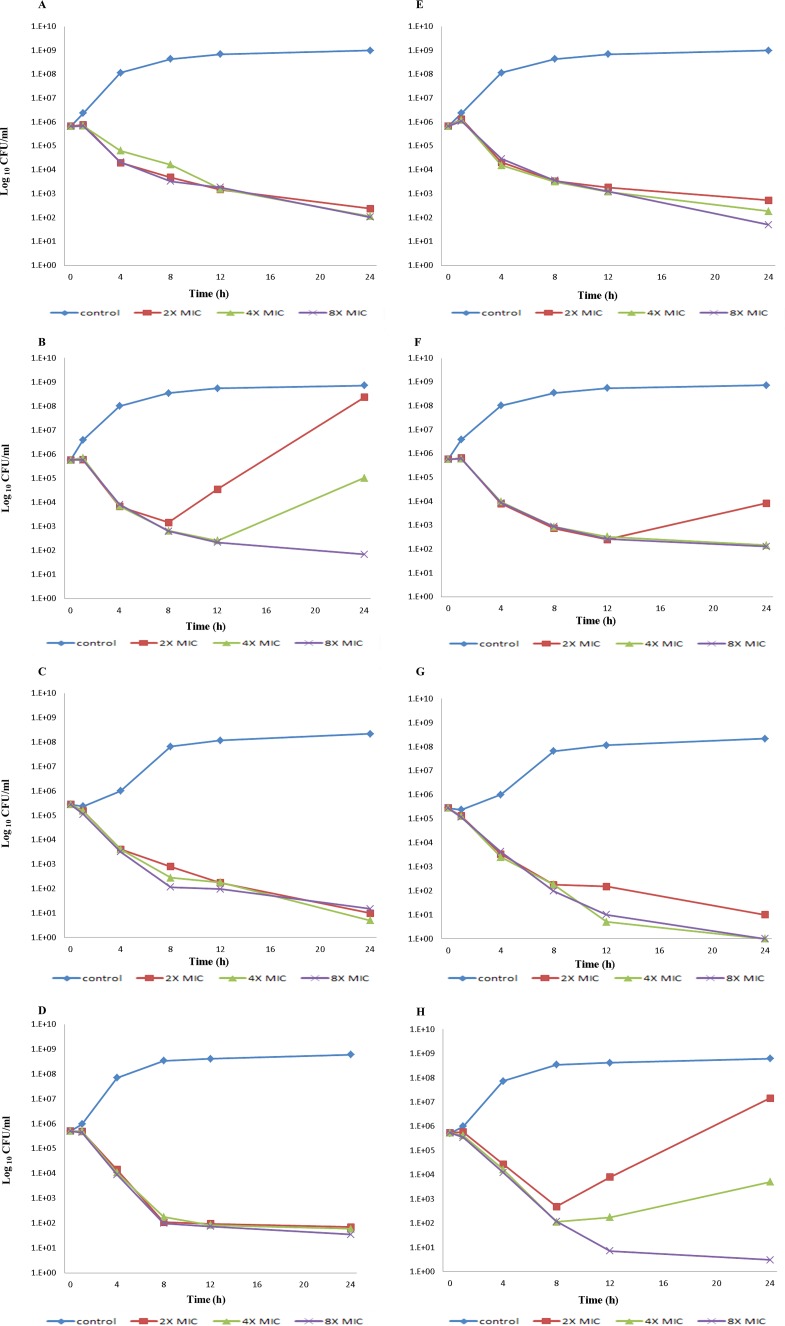
Three non-carbapenemase-producing CRPA clinical isolates susceptible to both C/T and C/A (genotypes ST277, ST641, and ST233) were selected for a time-kill assay using C/T and C/A (Table 4). Figure 1 shows the killing curves at C/T or C/A concentrations of 2 times, 4 times, and 8 times the MIC. Both C/T and C/A caused decreases in the number of CFU per milliliter over the 1- to 8-h time period for all strains at all multiples of MICs tested. The onset of bacterial killing showed a lag time of approximately 1 h in all strains. PAO1 had at least a 3-log10 decrease in the number of CFU per milliliter with both C/T and C/A at all MICs at 24 h (Fig. 1A and E). Both C/T and C/A were bactericidal after 8 h when tested at 2 times, 4 times, and 8 times the MIC against an isolate with downregulated oprD (Fig. 1C and G). C/T was less bactericidal against an isolate with downregulated oprD and overexpressed ampC (Fig. 1B and F). At the 8-h time point, all concentrations of C/T caused a <3-log10 decrease in CFU per milliliter. In addition, regrowth was observed at 12 h when tested at 2 times the MIC and at 24 h when tested at 4 times the MIC against this isolate (Fig. 1B and F). On the other hand, C/A was bactericidal against an isolate with downregulated oprD and overexpressed mexB at 8 h at all MICs tested (Fig. 1D and H). However, regrowth was observed at the 12-h time point when tested at 2 times and 4 times the MIC (Fig. 1D and H).
TABLE 4.
Antimicrobial resistance and genotype of three non-carbapenemase-producing CRPA isolates tested in a time-kill assayb
| Isolate no. | ST | MIC (mg/liter) |
Expression of genea |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IMP | MEM | C/T | C/A | FEP | CAZ | P/T | oprD | mexB | ampC | ||
| 42 | 277 | 32 | 8 | 1/4 | 4/4 | 8 | 8 | 8/4 | 0.0618 | 0.7769 | 61.3534 |
| 91 | 641 | 16 | 4 | 4/4 | 4/4 | 32 | 4 | 128/4 | 0.0001 | 1.2536 | 0.2649 |
| 186 | 233 | 16 | 16 | 2/4 | 8/4 | 8 | 8 | 16/4 | 0.0015 | 19.6462 | 0.4798 |
Values in bold indicate a significant overexpression (or underexpression for oprD) of the corresponding gene according to the defined thresholds relative to PAO1 (see Table 2).
Abbreviations: ST, sequence type; IMP, imipenem; MEM, meropenem; C/T, ceftolozane-tazobactam; C/A, ceftazidime-avibactam; FEP, cefepime; CAZ, ceftazidime; P/T, piperacillin-tazobactam.
FIG 1.
Time-kill curves of carbapenem-resistant ceftolozane-tazobactam-susceptible and ceftazidime-avibactam-susceptible P. aeruginosa clinical isolates exposed to ceftolozane-tazobactam (A, B, C, and D) and ceftazidime-avibactam (E, F, G, and H). (A and E) PAO1. (B and F) An isolate with downregulated oprD and upregulated ampC (isolate 42). (C and G) An isolate with downregulated oprD (isolate 91). (D and H) An isolate with downregulated oprD and upregulated mexB (isolate 186). The lower limit of detection for this assay was 1 CFU/ml.
DISCUSSION
Our C/T susceptibility data demonstrate that it is an active agent against non-carbapenemase-producing CRPA. In comparison to C/A MICs, C/T MICs were lower. Our study also demonstrates that decreased oprD transcription and increased transcription of efflux pump genes or ampC do not fully explain the correlation of C/T MIC values with specific mutation-driven resistance mechanisms. However, C/T was less bactericidal against an isolate having decreased oprD and increased ampC expression than against isolates having other resistance mechanisms, and C/A showed regrowth at the 12-h time point when tested at 2 times and 4 times the MIC against an isolate with decreased oprD and increased mexB expression in time-kill studies.
The increase in carbapenem resistance among P. aeruginosa clinical isolates is worrisome, because there has been little progress in the development of new antimicrobial agents targeting this organism (4, 15). Delaying the initiation of appropriate antimicrobial therapy is well established as being associated with increased morbidity and mortality in patients with severe P. aeruginosa infections (16, 17). In this setting, colistin has been recently deployed as a last-resort treatment option (18). However, concerns about nephrotoxicity, a well-known adverse effect of colistin, and challenging pharmacokinetics have led to limited use of this drug (19). C/T has shown potent in vitro activity against Pseudomonas species (20, 21). Previous studies showed C/T activity against 86% to 95% of clinical P. aeruginosa isolates, and when specifically evaluating more-resistant strains, 60 to 80% of ceftazidime-resistant and meropenem-resistant pseudomonal isolates displayed MICs to C/T of ≤8 mg/liter (22, 23). Consistent with this, our study showed 40 isolates (95.2%) to be susceptible to C/T among 42 non-carbapenemase-producing CRPA clinical isolates collected before the clinical availability of ceftolozane. All isolates were colistin susceptible, 79.4% were C/A susceptible, 59.5% were amikacin susceptible, and <50% were susceptible to the remaining antibiotics tested.
Previous studies investigated the possible molecular mechanisms of P. aeruginosa resistance to C/T and C/A (12, 24–27). High-level resistance to C/T occurred only in a strain with multiple mutations leading to overexpression and structural modifications of AmpC (12). AmpC overexpression was suggested to contribute to resistance of P. aeruginosa to C/T, but underlying resistance mechanisms remain poorly defined. In the current study, two isolates showed resistance to C/T. The first showed decreased oprD expression, and the second showed decreased oprD expression combined with increased ampC and mexB expression. However, due to the apparent limited correlation of C/T MIC values with specific mutation-driven resistance mechanisms, we are unable to explain resistance mechanisms for C/T with decreased oprD transcription and increased transcription of efflux genes or of the ampC gene. Parenthetically, there was a correlation between MICs and resistance mechanism in 15 isolates with a metallo-β-lactamase (13 with blaIMP-6 and 2 with blaVIM-2), which showed MICs of ≥128/4 mg/liter for both C/T and C/A (data not shown). Intriguingly, nine isolates with decreased oprD expression combined with increased mexB expression did not display resistance to C/T, in contrast to the situation with C/A. Ceftolozane is known to not be affected by overexpressed MexAB-OprM because it is not a substrate of this pump, nor is it affected by deletion of OprD porins, because it does not enter bacterial cells through OprD (13). Further study will be needed to understand the drivers of resistance to C/T to support efforts for preserving the potency of this last-resort antibiotic.
Our study also showed antimicrobial effects of C/T and C/A against non-carbapenemase-producing CRPA clinical isolates using time-kill assays. C/T produced a decrease in the number of CFU per milliliter at 8 h for an isolate having decreased oprD and increased ampC expression; however, this combination was not bactericidal against this isolate, in contrast to C/A, which showed a bactericidal effect without regrowth. An important potential use of C/A is in the treatment of P. aeruginosa infections, as this drug has been shown to have potent inhibitory activity against the class C β-lactamase of P. aeruginosa (28). In addition, avibactam binds covalently and reversibly to β-lactamases (29). This reversibility is a unique feature that allows avibactam to undergo recyclization to inactivate another β-lactamase. We are uncertain of the clinical significance of regrowth after the 8-h time point, because both C/T and C/A clinical dosing regimens are every 8 h. Similar observations of regrowth in time-kill studies have been made with other commercially available β-lactam–β-lactamase-inhibitor combinations, such as piperacillin-tazobactam (30, 31). Despite this phenomenon, piperacillin-tazobactam has been in successful clinical use for many years, suggesting that regrowth in time-kill studies for β-lactam–β-lactamase-inhibitor agents might be an in vitro phenomenon that does not necessarily translate to clinical activity. With regard to lag time of bacterial killing, both C/T and C/A showed a lag in bacterial killing for all three isolates studied. β-Lactams bind to penicillin-binding proteins, stimulating an autolysin effect; turnover of the autolysin effect may result in a lag in killing (32, 33).
This study has some limitations. First, although we investigated the main resistance mechanisms of P. aeruginosa causing carbapenem resistance, we did not interrogate all resistance mechanisms. Although uncommon, class A extended-spectrum β-lactamases, such as TEM, SHV, CTX-M, PER, VEB, GES, and IBC families, have been detected in P. aeruginosa. Extended-spectrum β-lactamases from the class D OXA-type enzymes have also been encountered in P. aeruginosa (34). Second, even though these isolates were collected from eight South Korean hospitals, the sample size is too small to determine statistical significance. Therefore, further study will be needed to investigate the correlation of C/T MIC values with specific resistance mechanisms using a large number of non-carbapenemase-producing CRPA isolates.
In conclusion, C/T showed excellent activity against non-carbapenemase-producing CRPA clinical isolates. We were unable to fully correlate C/T MIC values with specific mutation-driven resistance mechanisms.
MATERIALS AND METHODS
Bacterial isolates.
A total of 213 P. aeruginosa isolates (bacteremia, n = 101; urinary tract infection, n = 112) from eight South Korean hospitals assessed in a previous study (6) were considered for inclusion. Among 213 P. aeruginosa isolates, a total of 57 isolates (26.8%) resistant to imipenem and/or meropenem were determined to be resistant to carbapenems. Among the 57 CRPA isolates, 15 isolates were metallo-β-lactamase producers (13 blaIMP-6 and 2 blaVIM-2). Pathogens harboring carbapenemases, such as KPCs and metallo-β-lactamases, are known to be resistant to C/T (14), and these 15 isolates were not further studied in detail. Therefore, 42 non-carbapenemase-producing CRPA isolates, 14 of which were isolated from blood, 28 of which were isolated from urine, and all of which were negative for blaIMP, blaVIM, blaSPM, blaGIM, blaSIM, and blaKPC, were studied. These 42 isolates were evaluated for the presence of cryptic carbapenemases using the Carba NP test, as previously described (35, 36); they were all phenotypically negative for carbapenemase activity. Multilocus sequence typing (MLST) revealed 26 sequence types (STs). The expression of oprD, ampC, and several efflux pump genes had been previously defined by quantitative reverse transcription-PCR to define mechanisms conferring carbapenem resistance. Reduced oprD expression was considered relevant when it was ≤30% compared with that of P. aeruginosa PAO1. Strains were considered positive for ampC, mexD, mexF, or mexY overexpression when the corresponding mRNA level was at least 10-fold higher than that of PAO1, negative if it was lower than 5-fold, and borderline if it was between 5- and 10-fold. Strains were considered positive for mexB overexpression when the corresponding mRNA level was at least 3-fold higher than that of PAO1, negative if it was lower than 2-fold, and borderline if it was between 2- and 3-fold, according to previously defined criteria (37). All but three exhibited a relevant decrease in oprD expression compared to the reference strain. Twenty-nine overexpressed efflux pumps (primarily mexB but also mexD, mexY, and mexF) or ampC.
Antimicrobial susceptibility testing.
In our previous work (6), susceptibility to 10 antimicrobial agents including imipenem, meropenem, piperacillin-tazobactam, cefepime, ceftazidime, tetracycline, ciprofloxacin, amikacin, polymyxin B, and colistin had been determined. In the current study, antimicrobial susceptibility to C/T and C/A was tested by broth microdilution according to the CLSI guidelines (38). Interpretation of susceptibility for all antimicrobial agents except C/A was done according to CLSI breakpoints (36). For C/A, the FDA susceptibility breakpoint (≤8/4 mg/liter) was applied. Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 served as quality control strains. All quality control results were within CLSI-specified ranges.
Correlation of C/T or C/A activity with mechanisms of resistance.
The relationship between C/T or C/A activity and overexpression of efflux pumps or ampC and decreased oprD expression was also investigated.
In vitro time-kill studies.
Three CRPA isolates having different resistance mechanisms and PAO1 were selected for time-kill assays. Time-kill studies were performed according to a previously published study with some modifications (15). Briefly, freshly prepared colonies collected from the surface of an overnight agar culture were suspended in cation-adjusted Mueller-Hinton broth (CAMHB) and incubated for 1 to 2 h. Cultures were then diluted to a 0.5 McFarland standard (approximately 1.5 × 108 CFU/ml). An appropriate amount of bacteria was diluted in CAMHB to achieve a concentration of 5 × 105 CFU/ml in a final volume of 10 ml of CAMHB. Then, ceftolozane and ceftazidime were added to the prepared bacterial suspensions, so that the final drug concentration was 2 times, 4 times, or 8 times the MIC of ceftolozane and ceftazidime; tazobactam and avibactam were added to a final concentration of 4 mg/liter. A growth control with no antibiotic was also included. Tubes were incubated in a 37°C room air incubator with shaking (180 rpm); viability counts were performed at 0 h, 1 h, 4 h, 8 h, 12 h, and 24 h by removing 100 μl. A ≥3-log10 decrease in the number of CFU per milliliter was considered evidence of bactericidal activity.
Statistical analysis.
Categorical variables were compared using Fisher's exact test. All tests were 2 sided, and P values less than 0.05 were considered statistically significant. Statistical analysis was performed using PASW Statistics for Windows v.18.0 (SPSS Inc., Chicago, IL).
ACKNOWLEDGMENTS
This study was generously supported by Merck & Co., Inc.
All P. aeruginosa isolates used in this study were obtained from the Asian Bacterial Bank (ABB) of the Asia-Pacific Foundation for Infectious Diseases (APFID) (Seoul, Republic of Korea). We thank Angela P. Strasburg for performing the Carba NP test.
REFERENCES
- 1.Bodey GP, Jadeja L, Elting L. 1985. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med 145:1621–1629. doi: 10.1001/archinte.1985.00360090089015. [DOI] [PubMed] [Google Scholar]
- 2.Poole K. 2011. Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giamarellou H. 2002. Prescribing guidelines for severe Pseudomonas infections. J Antimicrob Chemother 49:229–233. doi: 10.1093/jac/49.2.229. [DOI] [PubMed] [Google Scholar]
- 4.Wi YM, Choi JY, Lee JY, Kang CI, Chung DR, Peck KR, Song JH, Ko KS. 2017. Emergence of colistin resistance in Pseudomonas aeruginosa ST235 clone in South Korea. Int J Antimicrob Agents 49:767–769. doi: 10.1016/j.ijantimicag.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Ochs MM, McCusker MP, Bains M, Hancock RE. 1999. Negative regulation of the Pseudomonas aeruginosa outer membrane porin OprD selective for imipenem and basic amino acids. Antimicrob Agents Chemother 43:1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JY, Ko KS. 2012. OprD mutations and inactivation, expression of efflux pumps and AmpC, and metallo-beta-lactamases in carbapenem-resistant Pseudomonas aeruginosa isolates from South Korea. Int J Antimicrob Agents 40:168–172. doi: 10.1016/j.ijantimicag.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Livermore DM. 1992. Interplay of impermeability and chromosomal beta-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 36:2046–2048. doi: 10.1128/AAC.36.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng ZR, Wang WP, Huang M, Shi LN, Wang Y, Shao HF. 2014. Mechanisms of carbapenem resistance in cephalosporin-susceptible Pseudomonas aeruginosa in China. Diagn Microbiol Infect Dis 78:268–270. doi: 10.1016/j.diagmicrobio.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Ortega C, Wiegand I, Olivares J, Hancock RE, Martinez JL. 2010. Genetic determinants involved in the susceptibility of Pseudomonas aeruginosa to beta-lactam antibiotics. Antimicrob Agents Chemother 54:4159–4167. doi: 10.1128/AAC.00257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubonja-Sonje M, Matovina M, Skrobonja I, Bedenic B, Abram M. 2015. Mechanisms of carbapenem resistance in multidrug-resistant clinical isolates of Pseudomonas aeruginosa from a Croatian hospital. Microb Drug Resist 21:261–269. doi: 10.1089/mdr.2014.0172. [DOI] [PubMed] [Google Scholar]
- 11.Livermore DM, Mushtaq S, Ge Y, Warner M. 2009. Activity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolates. Int J Antimicrob Agents 34:402–406. doi: 10.1016/j.ijantimicag.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagace-Wiens PR, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP III, Karlowsky JA. 2014. Ceftolozane/tazobactam: a novel cephalosporin/beta-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 74:31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 13.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation beta-lactam/beta-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright H, Bonomo RA, Paterson DL. 2017. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 23:704–712. doi: 10.1016/j.cmi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Wi YM, Choi JY, Lee JY, Kang CI, Chung DR, Peck KR, Song JH, Ko KS. 2017. Antimicrobial effects of beta-lactams on imipenem-resistant ceftazidime-susceptible Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e00054-17. doi: 10.1128/AAC.00054-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morata L, Cobos-Trigueros N, Martinez JA, Soriano A, Almela M, Marco F, Sterzik H, Nunez R, Hernandez C, Mensa J. 2012. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 56:4833–4837. doi: 10.1128/AAC.00750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 49:1306–1311. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafailidis PI, Falagas ME. 2014. Options for treating carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis 27:479–483. doi: 10.1097/QCO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 19.Choi IS, Lee YJ, Wi YM, Kwan BS, Jung KH, Hong WP, Kim JM. 2016. Predictors of mortality in patients with extensively drug-resistant Acinetobacter baumannii pneumonia receiving colistin therapy. Int J Antimicrob Agents 48:175–180. doi: 10.1016/j.ijantimicag.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Walkty A, Karlowsky JA, Adam H, Baxter M, Lagace-Wiens P, Hoban DJ, Zhanel GG. 2013. In vitro activity of ceftolozane-tazobactam against Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals in the CANWARD study, 2007 to 2012. Antimicrob Agents Chemother 57:5707–5709. doi: 10.1128/AAC.01404-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell DJ, Flamm RK, Sader HS, Jones RN. 2013. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2011–2012). Antimicrob Agents Chemother 57:6305–6310. doi: 10.1128/AAC.01802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sader HS, Farrell DJ, Flamm RK, Jones RN. 2014. Ceftolozane/tazobactam activity tested against aerobic Gram-negative organisms isolated from intra-abdominal and urinary tract infections in European and United States hospitals (2012). J Infect 69:266–277. doi: 10.1016/j.jinf.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Farrell DJ, Sader HS, Flamm RK, Jones RN. 2014. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 43:533–539. doi: 10.1016/j.ijantimicag.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Winkler ML, Papp-Wallace KM, Hujer AM, Domitrovic TN, Hujer KM, Hurless KN, Tuohy M, Hall G, Bonomo RA. 2015. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 59:1020–1029. doi: 10.1128/AAC.04238-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabot G, Bruchmann S, Mulet X, Zamorano L, Moya B, Juan C, Haussler S, Oliver A. 2014. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob Agents Chemother 58:3091–3099. doi: 10.1128/AAC.02462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berrazeg M, Jeannot K, Ntsogo Enguene VY, Broutin I, Loeffert S, Fournier D, Plesiat P. 2015. Mutations in beta-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob Agents Chemother 59:6248–6255. doi: 10.1128/AAC.00825-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castanheira M, Mills JC, Farrell DJ, Jones RN. 2014. Mutation-driven beta-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob Agents Chemother 58:6844–6850. doi: 10.1128/AAC.03681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Reville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D beta-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proc Natl Acad Sci U S A 109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess DS, Hall RG II. 2004. In vitro killing of parenteral beta-lactams against standard and high inocula of extended-spectrum beta-lactamase and non-ESBL producing Klebsiella pneumoniae. Diagn Microbiol Infect Dis 49:41–46. doi: 10.1016/j.diagmicrobio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Robin F, Krebs M, Delmas J, Gibold L, Mirande C, Bonnet R. 2011. In vitro efficiency of the piperacillin/tazobactam combination against inhibitor-resistant TEM- and complex mutant TEM-producing clinical strains of Escherichia coli. J Antimicrob Chemother 66:1052–1056. doi: 10.1093/jac/dkr045. [DOI] [PubMed] [Google Scholar]
- 32.Livermore DM. 1987. Radiolabelling of penicillin-binding proteins (PBPs) in intact Pseudomonas aeruginosa cells: consequences of beta-lactamase activity by PBP-5. J Antimicrob Chemother 19:733–742. doi: 10.1093/jac/19.6.733. [DOI] [PubMed] [Google Scholar]
- 33.Hayes MV, Orr DC. 1983. Mode of action of ceftazidime: affinity for the penicillin-binding proteins of Escherichia coli K12, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother 12:119–126. doi: 10.1093/jac/12.2.119. [DOI] [PubMed] [Google Scholar]
- 34.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasoo S, Cunningham SA, Kohner PC, Simner PJ, Mandrekar JN, Lolans K, Hayden MK, Patel R. 2013. Comparison of a novel, rapid chromogenic biochemical assay, the Carba NP test, with the modified Hodge test for detection of carbapenemase-producing Gram-negative bacilli. J Clin Microbiol 51:3097–3101. doi: 10.1128/JCM.00965-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing: 27th informational supplement. CLSI document M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 37.Cabot G, Ocampo-Sosa AA, Tubau F, Macia MD, Rodriguez C, Moya B, Zamorano L, Suarez C, Pena C, Martinez-Martinez L, Oliver A. 2011. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Antimicrob Agents Chemother 55:1906–1911. doi: 10.1128/AAC.01645-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]