ABSTRACT
Carbapenem-resistant Enterobacteriaceae (CRE) are rapidly spreading and taking a staggering toll on all health care systems, largely due to the dissemination of genes coding for potent carbapenemases. An important family of carbapenemases are the Zn(II)-dependent β-lactamases, known as metallo-β-lactamases (MBLs). Among them, the New Delhi metallo-β-lactamase (NDM) has experienced the fastest and widest geographical spread. While other clinically important MBLs are soluble periplasmic enzymes, NDMs are lipoproteins anchored to the outer membrane in Gram-negative bacteria. This unique cellular localization endows NDMs with enhanced stability upon the Zn(II) starvation elicited by the immune system response at the sites of infection. Since the first report of NDM-1, new allelic variants (16 in total) have been identified in clinical isolates differing by a limited number of substitutions. Here, we show that these variants have evolved by accumulating mutations that enhance their stability or the Zn(II) binding affinity in vivo, overriding the most common evolutionary pressure acting on catalytic efficiency. We identified the ubiquitous substitution M154L as responsible for improving the Zn(II) binding capabilities of the NDM variants. These results also reveal that Zn(II) deprivation imposes a strict constraint on the evolution of this MBL, overriding the most common pressures acting on catalytic performance, and shed light on possible inhibitory strategies.
KEYWORDS: NDM, Zn(II) limitation, antibiotic resistance, carbapenemase, metallo-β-lactamase, nutritional immunity
INTRODUCTION
Carbapenem-resistant Enterobacteriaceae (CRE) are rapidly spreading and taking a staggering toll on all health care systems (1, 2), largely due to the dissemination of genes coding for potent carbapenemases (3). Metallo-β-lactamases (MBLs) are Zn(II)-dependent enzymes that represent one of the largest group of carbapenemases. MBLs are able to hydrolyze not only carbapenems but also penicillins and cephalosporins with comparable performance characteristics (4, 5). They include the families of plasmid-encoded IMP, VIM, and the New Delhi metallo-β-lactamase (NDM) enzymes, which have disseminated worldwide among opportunistic and pathogenic bacteria. These enzymes are not affected by the action of serine-β-lactamase inhibitors, including the newly developed avibactam, and there are no specific inhibitors for MBLs available in the clinic.
The NDM has experienced the fastest and widest geographical (6, 7) spread among MBLs in recent years. The clinical success of NDM has been attributed to the fact that it is a lipoprotein anchored to the outer membrane in Gram-negative bacteria (Fig. 1B) (8–10). This feature is exclusive to this enzyme, in contrast to all other MBLs, which are soluble periplasmic proteins (5). We have recently suggested (9) that this cellular localization can boost the fitness of NDM-1 under physiological conditions. At the sites of infection, pathogens must face the “nutritional immunity” response by the host immune system, which involves the release of large amounts of the metal-chelating protein calprotectin (CP). As a consequence, Zn(II) levels in the bacterial periplasm decrease, leading to accumulation of apo (nonmetallated) MBLs that are susceptible to proteolytic degradation (9). Membrane anchoring prevents degradation of apo-NDM-1 upon this Zn(II) starvation process (9).
FIG 1.
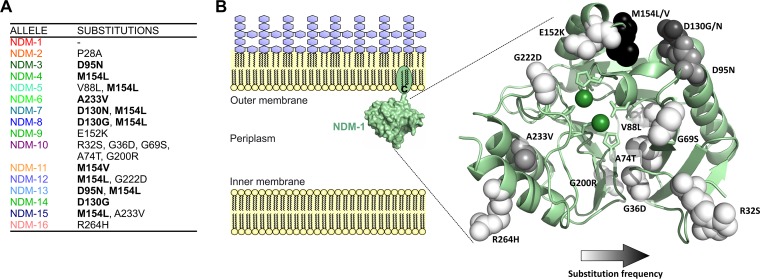
NDM alleles possess a limited number of substitutions located outside the active site. (A) NDM variants and their corresponding substitutions with respect to NDM-1. Substitutions occurring in two or more alleles are indicated in bold. (B) Diagram showing the cellular localization of NDM-1 within the inner leaflet of the outer membrane in Gram-negative bacteria and the crystal structure of NDM-1 (PDB accession number 4EYL), displaying the residues mutated in clinical alleles (spheres). Spheres are colored with a gradient from white to black according to increasing frequency of substitution at that position among alleles. Active-site Zn(II) ions are indicated as green spheres, and residues acting as metal ligands are displayed as sticks.
Since the first report of NDM-1 (11), new allelic variants (16 in total) have been identified in clinical isolates, differing by a limited number of substitutions (12). The 16 NDM variants are characterized by substitutions at a relatively small number of positions, all occurring outside the active site (Fig. 1). Residue M154 is the most frequently substituted, with M154L being the most common change (found in the single mutant NDM-4 and in six double mutants: NDM-5, NDM-7, NDM-8, NDM-12, NDM-13, and NDM-15) and one occurrence of M154V (in NDM-11). Residue D130 is replaced in three alleles, with substitutions D130G (NDM-8 and NDM-14) and D130N (NDM-7). Substitutions D95N and A233V are present in two alleles each, both as single variants (NDM-3 and NDM-6) and in combination with M154L (NDM-13 and NDM-15) (Fig. 1A). However, comparative studies of MBL proteins NDM-1 to NDM-8 have not revealed significant differences in their resistance profiles nor in the in vitro activities of the purified, soluble forms of these enzymes (with truncated lipidation sites) (13). These observations cannot account for the selection of these alleles in clinical environments.
We contend that the expression of NDM variants should be evaluated under conditions as close as possible to the physiological ones: (i) in the membrane-bound form and (ii) under environmental conditions of Zn(II) deprivation (9, 14, 15). Here, we examine the resistance profiles of the different NDM variants under these conditions, identify the molecular features responsible for the observed phenotypes, and demonstrate that NDM variants are evolving by enhancing their Zn(II) binding capability in vivo. These results also reveal that Zn(II) deprivation has imposed a strict constraint in the evolution of this MBL, overriding the most common pressures acting on catalytic performance, and shed light on possible inhibitory strategies.
RESULTS
To compare the performance of NDM variants within a common physiological background, we expressed the blaNDM genes coding for all 16 alleles in an isogenic Escherichia coli strain with their native signal peptide, targeting the variants to the outer membrane with expression levels similar to those observed in clinical strains (9, 16). To ensure homogeneous immunodetection and quantitation, all variants were expressed fused to a common C-terminal Strep-tag, which does not affect resistance (9). Expression of blaNDM alleles resulted in similar protein levels for all variants except NDM-10 (harboring five substitutions), which displayed a 5-fold reduction with respect to the NDM-1 level (see Fig. S1 in the supplemental material). In the case of NDM-2, carrying the substitution P28A, 20% of the total protein was found as a soluble, nonlipidated periplasmic lactamase, indicating that mutations proximal to the lipidation site (C26) can affect membrane anchoring.
In these constructs, MICs of piperacillin (PIP), cefepime (FEP), cefotaxime (CTX), and imipenem (IPM) for most variants did not reveal major differences among blaNDM alleles (Table S1), with the exception of blaNDM-10. This quintuple mutant displayed significantly lower MIC values that we attribute to the lower protein levels of this variant. Overall, these resistance profiles do not differ from those reported for alleles blaNDM-1 to blaNDM-8 (13). However, the experimental conditions employed to study the resistance profile may not always reflect the actual environment that is acting in selection (17). For instance, the standard conditions used for MIC determinations involve medium with a high Zn(II) content, which does not represent the environment at infection sites where potent metal-sequestering proteins such as calprotectin (CP) are released by the host immune system (10, 18–21). We therefore evaluated the impact of Zn(II) deprivation on MIC values by adding the chelating agent dipicolinic acid (DPA) to the medium. It has already been shown that DPA can mimic the effect elicited by the action of CP without being lethal for bacteria (9).
These experiments (summarized in Fig. 2) revealed (i) that MIC values were affected by addition of DPA and (ii) that the response was strongly allele dependent. In general, the impact of Zn(II) deprivation on MIC values was noticeable at DPA levels higher than 350 μM (Fig. 2 and Table S2). For most NDM variants, MIC values were higher than the MIC for NDM-1 at DPA levels beyond this value. NDM-10 was an exception since resistance levels for this variant were drastically reduced at low concentrations of DPA. These results clearly show that the amino acid substitutions have a defined and distinct impact on the ability to tolerate Zn(II) starvation.
FIG 2.
NDM alleles display an increased tolerance to Zn(II) starvation with respect to that of NDM-1. Cefotaxime MICs are given for E. coli DH5α cells expressing different NDM alleles in growth medium supplemented with the indicated concentrations of DPA relative to the MIC in 0 μM DPA. Data correspond to three independent experiments and are presented as means ± standard errors of the means.
NDM variants can be classified into four groups according to the dependence of resistance on DPA concentration. Tier 1 alleles are those displaying the highest tolerance to Zn(II) scarcity: NDM-15 (M154L A233V), NDM-13 (D95N M154L), NDM-12 (M154L G222D), NDM-8 (D130G, M154L), NDM-7 (D130N M154L), and NDM-5 (V88L M154L). Tier 2 alleles possess greater tolerance to Zn(II) starvation than NDM-1, with MICs at least one 2-fold dilution below the lowest MIC of the tier 1 variants. Tier 2 includes NDM-14 (D130G), NDM-9 (E152K), NDM-6 (A233V), NDM-4 (M154L), and NDM-3 (D95N). Tier 3 MBLs behave similarly to NDM-1 and include NDM-16 (R264H), NDM-11 (M154V), NDM-2 (P28A), and NDM-1. Finally, tier 4 contains NDM-10, the only allele that performs worse than NDM-1.
Tier 1 consists of double mutants while tier 2 contains only single mutants, indicating that variants with two mutations possess a higher ability to confer resistance under low-Zn(II) conditions. This observation suggests that tolerance to Zn(II) starvation is selected as mutations accumulate during the evolution of NDM. Furthermore, all tier 1 variants possess the substitution M154L, which seems to be crucial for adaptation to low Zn(II) availability. This enhancement can in principle be attributed to the specific presence of a leucine residue in position 154 since NDM-11 (M154V) displays behavior similar to that of NDM-1 upon addition of DPA (Fig. 2).
We interrogated the role of residue 154 by performing site saturation mutagenesis on NDM-1 at this position. MIC values of cefotaxime were within one dilution of the value for NDM-1 for 7 of the 19 mutants, revealing that position 154 is highly tolerant to substitutions (Table S3), in agreement with its location near the protein surface (Fig. 1B). In the presence of DPA, however, only variant M154L (NDM-4) provided higher levels of resistance than NDM-1 upon Zn(II) starvation, while all other variants were more susceptible (Fig. S2). The selection of replacement M154L in a position highly tolerant of substitutions strongly suggests that Zn(II) deprivation has exerted a significant evolutionary pressure on the natural selection of NDM alleles.
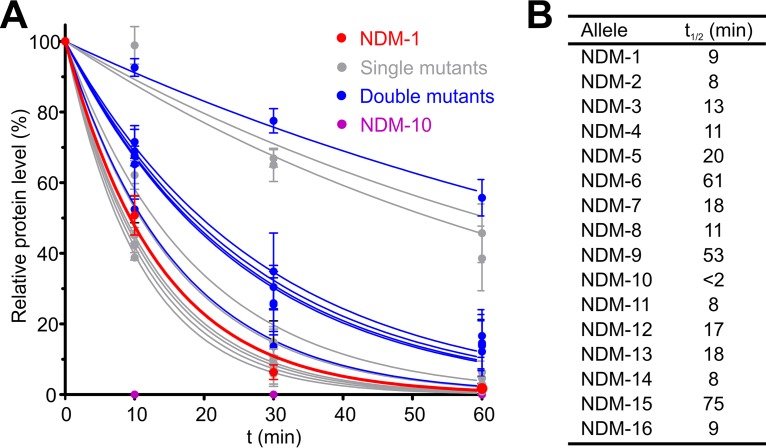
We next aimed to characterize the molecular features giving rise to these phenotypes. Zn(II) starvation elicits degradation of MBLs within the periplasmic space since the apoenzymes [Zn(II)-free forms] are susceptible to proteolysis while the metal-bound proteins are stable (9). We interrogated the in vivo stability of NDM alleles upon Zn(II) depletion by monitoring the variation over time of the NDM protein levels in E. coli cells after addition of DPA to the growth medium (Fig. S3). Under these conditions, NDM-1 experienced >90% degradation within 60 min, with a half-life (t1/2) of ca. 9 min. Degradation rates were highly variable among NDM alleles, with half-lives spanning from 2 to 75 min (Fig. 3). Most variants performed better than or similarly to NDM-1.
FIG 3.
Clinical NDM variants possess enhanced in vivo stability upon Zn(II) starvation. (A) MBL protein levels for NDM alleles in whole E. coli cells quantified from Western blots (see Fig. S3 in the supplemental material) as a function of time after addition of 500 μM DPA and normalized to the level in control samples not treated with DPA. Data are presented as percentages of initial protein levels remaining after treatment with DPA and correspond to means ± standard errors of the means of three independent experiments. Data points for each allele were fitted to a first-order exponential decay, indicated as a continuous curve. (B) Half-lives for NDM alleles obtained from exponential decay fit of the data in panel A.
The NDM variants can be classified into four groups according to their stability profiles. In group 1 are proteins with an enhanced stability against degradation (t1/2 between 75 and 53 min): NDM-15 (M154L A233V), NDM-6 (A233V), and NDM-9 (E152K). In group 2 are proteins that are more stable than NDM-1 (t1/2 between 20 and 17 min): NDM-5 (V88L M154L), NDM-7 (D130N M154L), NDM-12 (M154L G222D), and NDM-13 (D95N M154L). In group 3 are proteins with stability similar to that of NDM-1 (t1/2 between 13 and 8 min): NDM-2 (P28A), NDM-3 (D130G), NDM-4 (M154L), NDM-11 (M154V), and NDM-16 (R264H). Group 4 consists of a single protein, NDM-10, that has markedly lower stability than NDM-1 (t1/2 of <2 min). Analysis of these data reveals that most double variants exhibited greater stability than NDM-1, with the exception of NDM-8 (M154L D130G), which had a stability profile comparable to that of NDM-1. Among point mutations present in NDM alleles, substitutions A233V and E152K were shown to be stabilizing, giving rise to the only single variants displaying high stability: NDM-6 and NDM-9. The most stable NDM mutants (groups 1 and 2) outperform even SPM-1, which was previously shown to be the most stable MBL upon metal depletion under conditions similar to those reported here (Fig. S4 and S5) (9, 15).
We next analyzed possible epistatic interactions between mutations. NDM-15 (M154L A233V) shows a degradation profile similar to that of the single mutant NDM-6, suggesting that its high stability is mostly due to the substitution A233V while the stabilizing role of M154L is minor. A comparable stabilizing effect by the M154L substitution is observed when NDM-4 with NDM-1 are compared. Variant NDM-13 (D95N M154L) is more stable than the corresponding single mutants NDM-3 (D95N) and NDM-4 (M154L), and both substitutions may contribute to increased stability. NDM-8 (D130G M154L) is as stable as NDM-4, and thus D130G does not appear to significantly contribute to stability. We conclude that the effects of the different substitutions on stability are additive, with no evident epistatic interactions.
The degradation profiles upon Zn(II) starvation do not account for the resistance provided by the alleles under these conditions: NDM-4 (M154L) and NDM-11 (M154V) are degraded at a rate similar to that of NDM-1 (Fig. 3), but NDM-4 provides higher levels of resistance under Zn(II) deprivation (Fig. 2). Conversely, NDM-6 (A233V) and NDM-15 (M154L A233V) show similarly high stabilities, but NDM-15 outperforms NDM-6 under Zn(II) starvation (Fig. 2). These observations, together with the presence of the M154L substitution in all tier 1 mutants, reveal that M154L enhances resistance under Zn(II)-limiting conditions without imparting protein stabilization.
Resistance at low Zn(II) levels can be optimized by two main mechanisms: (i) improved stability of the apoproteins or (ii) optimization of the Zn(II) binding affinity in the different variants so that the accumulation of apoproteins is minimized. Thus, we evaluated the impact of positions 154 and 233 in the metal binding affinity of variants NDM-1, NDM-4, NDM-6, NDM-11, and NDM-15, which present substitutions at these positions. To assess this property, we measured the β-lactamase activity of these enzymes in spheroplasts containing the membrane-bound forms challenged with DPA. Studies on spheroplasts allow direct assessment of enzymatic activity since they are devoid of periplasmic proteases that elicit protein degradation. In all cases, we observed a decrease in activity (Fig. 4) with increasing concentrations of DPA. Immunoblotting experiments revealed similar protein levels under these conditions (Fig. S6), showing that the decrease in activity is not due to protein degradation and therefore can be attributed to accumulation of the inactive apoproteins generated by the chelating agent. Thus, the different behaviors of the alleles (Fig. 4) provide an estimate of the Zn(II) affinity of each variant. NDM-4 (M154L) and NDM-15 (M154L A233V) displayed the lowest susceptibility to inactivation by DPA, revealing that the M154L substitution indeed increases the metal binding ability while the stabilizing substitution A233V does not. Substitution M154V did not affect Zn(II) binding, in agreement with the resistance profile observed for NDM-11 (Fig. 2). The apparent Zn(II) affinity of NDM-6 was similar to that of NDM-1, confirming that the A233V substitution impacts only protein stability. This experiment allows us to propose that the most frequent mutation (M154L) plays a crucial role in NDM fitness by increasing the Zn(II) binding affinity.
FIG 4.
Substitution M154L leads to increased Zn(II) binding affinity. Relative β-lactamase activity of spheroplasts from E. coli cells expressing NDM alleles after incubation for 10 min at 30°C with different concentrations of DPA. Values are presented as relative to the activity at 0 μM DPA. Data correspond to three independent experiments and are plotted as means ± standard errors of the means.
Finally, we sought to directly compare the fitness of NDM alleles under various conditions of Zn(II) availability. To this end, we performed competition experiments between E. coli cells expressing NDM-1, NDM-4, NDM-6, or NDM-15 in the presence of different concentrations of cefotaxime and with or without the addition of metal chelators to the growth medium (Fig. 5). NDM-1 and the single mutants NDM-4 and NDM-6 displayed similar fitness levels under Zn(II)-rich conditions, i.e., growth medium not supplemented with DPA, and no allele seemed to be favored over the other within the range of antibiotic concentrations tested (Fig. 5A and B). In contrast, NDM-1 and NDM-4 were selected over the double mutant NDM-15, particularly at higher antibiotic concentrations (Fig. 5C and D), in accordance with their slightly higher CTX MICs (128 versus 64 to 128 μg/ml). Competition experiments carried out in the presence of 250 μM DPA presented a radically different scenario. Single mutants NDM-4 and NDM-6 were selected over NDM-1, and NDM-4 was outcompeted by NDM-15. The double mutant was able to outperform NDM-1 more effectively than single-mutant variants, highlighting the gain in fitness due to accumulation of beneficial mutations. Finally, we performed competition experiments between NDM-1 and NDM-15 in the presence of calprotectin (Fig. 5D) and obtained results comparable to those with DPA. Thus, a similar differential fitness is observed when metal depletion by the host's nutritional immune response upon pathogenesis is reproduced. The concentration of CP used for the experiment (250 μg/ml) is within the physiological range since levels of up to 1,000 μg/ml have been reported at infection sites (20). These competition experiments clearly reveal that alleles with better Zn(II) binding capabilities can outcompete NDM-1 under these conditions, even at antibiotic concentrations well below the MICs.
FIG 5.
NDM alleles outcompete NDM-1 under Zn(II)-limiting conditions. Competition experiments were performed between E. coli W3110 cells expressing different NDM alleles in the presence of increasing concentrations of cefotaxime in growth medium, with and without supplementation with metal chelators, as indicated (e.g., NDM-1 versus 4 indicates NDM-1 versus NDM-4). Data are presented as means ± standard errors of the means of two independent determinations reversing the Lac+/Lac− background of the strain carrying each allele.
DISCUSSION
Assessment of the molecular features involved during the evolution of resistance requires recreating as closely as possible the environmental conditions that may have driven adaptation. For example, study of the evolution of the serine-β-lactamase TEM at sublethal antibiotic concentrations, such as those present in the environment, allowed an exhaustive exploration of the adaptive landscapes of this protein, leading to increased diversity of this enzyme (17). Here, we consider that, during an infection, the host's immune system releases metal-chelating proteins to inhibit the growth of bacteria, which in turn compete for these essential nutrients by producing high-affinity metal importers. In this context, MBLs should possess optimized Zn(II) binding capabilities to effectively mediate β-lactam resistance. In particular, clinically relevant MBLs require binding of two Zn(II) ions in the periplasm to confer resistance (22). The requirements for Zn(II) binding are stringent since metal depletion leads to protein degradation, and loss of resistance is more dramatic. Here, we show that the resistance profiles of the known NDM variants are similar when measured in Zn(II)-rich medium. In contrast, significant differences appear under Zn(II) deprivation conditions. We further show that most variants are better suited than NDM-1 to resist these conditions.
Our results suggest that the pathways along which NDM alleles are currently evolving in clinical settings enhance the performance of these enzymes under low Zn(II) availability. We identified two mechanisms behind this adaptation: (i) stabilization of otherwise unstable apoenzymes and (ii) enhancement of the Zn(II) affinity to maintain high levels of the active species. Substitutions A233V and E152K dramatically increase protein stability in the periplasm, while the highly frequent M154L substitution enhances metal affinity. Both mechanisms can act independently or combine without epistatic interactions to render enzymes with a higher fitness under Zn(II) deprivation conditions (NDM-15). A small group of NDM alleles (NDM-2, NDM-11, and NDM-16) did not show any advantages over NDM-1 under our conditions. The substitutions present in these variants may be neutral or the result of a host-specific adaptation, as previously shown for SPM-1 (15), that may not be evident in E. coli (e.g., NDM-2 has been detected only in Acinetobacter baumannii) (12).
A recent work has revealed that evolution of the serine-β-lactamase TEM has been shaped by optimization of the enzymatic efficiency (23) and not by stabilization of the protein itself. Our results show that the adaptive landscape of the metallo-β-lactamase NDM has been shaped by Zn(II) deprivation conditions, leading to optimization of cofactor binding. In this regard, the identification of the ubiquitous substitution M154L as responsible for increasing the Zn(II) binding affinity in different alleles also provides a unique example of optimization of cofactor assembly during evolution. This finding is in line with the reconstruction of the evolutionary trajectory of an in vitro-evolved MBL that disclosed that the Zn(II) binding affinity may be an essential feature in defining its fitness landscape (13).
Membrane localization is conserved among all NDM alleles, in contrast to serine-β-lactamases, which have in most cases foregone their membrane anchoring during evolution from penicillin binding proteins (PBPs) (24). This contrast may be caused by the different fitness effects of membrane anchoring in each class of enzyme, which ultimately impacts its evolutionary fixation. It has been shown that TEM is functional when, like its PBP predecessors, it is anchored to the outer leaflet of the inner membrane, conferring the same resistance levels as the native soluble protein without an apparent fitness advantage (24). Meanwhile, we have previously shown that membrane anchoring of NDM-1 allows it to better tolerate Zn(II) deprivation conditions (9) as soluble variants of the enzyme are less stable and confer lower levels of resistance in low-Zn(II) environments. The role of membrane anchoring in stabilization would guarantee the conservation of this characteristic. It should be noted that anchoring is but one mechanism accessible to MBLs to improve stability under these conditions as other MBLs, such as SPM-1 or NDM variants carrying the A233V or E152K substitution, also possess enhanced stability against proteolytic degradation.
Despite the lack of clinically useful inhibitors, recent research has shown that MBLs may also be challenged by compounds such as aspergillomarasmine A (AMA) (25), a Zn(II) chelator able to reverse resistance mediated by MBLs in animal models. While NDM-1 was susceptible to inactivation by this compound, SPM-1 proved to be refractory (25). Our results show that NDMs are evolving beyond the AMA-resistant SPM-1 MBL, indicating that tolerance to low Zn(II) availability will undoubtedly allow MBLs to circumvent this type of inhibition. This stresses the need to develop specifically tailored inhibitors not dependent on metal chelation to combat the growing threat posed by these enzymes.
MATERIALS AND METHODS
Bacterial strains and reagents.
Escherichia coli DH5α was used for expression of plasmid pMBLe and microbiological and biochemical studies. Unless otherwise noted, all strains were grown aerobically at 37°C in lysogeny broth (LB) medium supplemented with 20 μg/ml gentamicin when necessary. Chemical reagents were purchased from Sigma-Aldrich, molecular biology enzymes were from Promega, and primers were from Invitrogen.
Construction of NDM alleles.
blaNDM variant genes were generated from pMBLe-blaNDM-1 (9), which contains the full-length blaNDM-1 gene fused to a C-terminal Strep-tag II sequence under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible pTac promoter. Variants were constructed by site-directed mutagenesis as previously described (14) using the primers detailed in Table S4 in the supplemental material. All constructs were verified by DNA sequencing (University of Maine, USA).
Periplasm and spheroplast preparations.
Extraction of periplasmic proteins was performed as previously described (9). Briefly, 2 to 3 ml of E. coli pMBLe-blaNDM cultures was pelleted, and cells were washed once with 20 mM Tris–150 mM NaCl, pH 8.0. The washed cells were resuspended in 20 mM Tris, 0.1 mM EDTA, 20% (wt/vol) sucrose, 1 mg/ml lysozyme (from chicken egg white, ≥90% protein; Sigma-Aldrich), and 0.5 mM phenylmethylsulfonyl fluoride (PMSF), pH 8 (the resuspension volume [V] was normalized according to the formula V = 100 μl × OD600 × Vc, where Vc is the starting volume of the culture sample and OD600 is the optical density at 600 nm). Cells were then incubated with gentle agitation at 4°C for 30 min and finally pelleted with the periplasmic extract in the supernatant. The pellet consisting of spheroplasts was washed in 20 mM Tris plus 20% (wt/vol) sucrose, pH 8, and resuspended in the same volume of this buffer.
MBL detection.
MBL protein levels were determined by SDS-PAGE followed by Western blotting with Strep-tag II monoclonal antibodies (at a 1:1,000 dilution from 200 μg/ml solution) (Novagen) and immunoglobulin G-alkaline phosphatase conjugates (at a 1:3,000 dilution). Protein band intensities were quantified from polyvinylidene difluoride (PVDF) membranes with ImageJ software (26) and converted to relative protein amounts through a calibration curve constructed under the same experimental conditions. In all cases, Western blotting with antibodies detecting GroEL was performed as a loading control.
MIC determinations.
Cefotaxime, cefepime, piperacillin, and imipenem MIC determinations were performed in LB medium using the agar macrodilution method according to CLSI guidelines (27). In order to measure the effect of Zn(II) availability on antibiotic resistance, the growth medium was supplemented with various concentrations of the metal chelator dipicolinic acid (DPA) (>98%; Merck). In all cases, blaNDM expression was induced with 100 μM IPTG.
Relative MICs as plotted in Fig. 2 and S2 were calculated as follows: (MICNDM − MICcontrol)/(MICNDM + 0 μM DPA − MICcontrol + 0 μM DPA), where MICNDM and MICcontrol refer to values measured for E. coli DH5α pMBLe-blaNDM and pMBLe, respectively, under each set of conditions, and MICNDM + 0 μM DPA and MICcontrol + 0 μM DPA refer to the corresponding values in medium without addition of DPA.
Effect of external Zn(II) depletion on MBL protein levels.
E. coli pMBLe-blaNDM cells were grown at 37°C to an OD600 of 0.4. MBL expression was induced by the addition of 100 μM IPTG, and growth was continued at 37°C for 2 h. At this time, cultures were divided into two equal parts. One portion was treated with 500 μM DPA, and the other was kept as an untreated control; both cultures were grown at 37°C. Aliquots of DPA-treated and untreated cultures were taken at different time intervals (0, 10, 30, and 60 min) after DPA addition and processed for immunodetection. Protein values in DPA-treated samples were reported relative to the corresponding values in untreated samples.
Competition experiments.
Isogenic E. coli W3110 (28) and E. coli W3110 ΔlacZ (29) strains were transformed with pMBLe-blaNDM-15, pMBLe-blaNDM-6, pMBLe-blaNDM-4, and pMBLe-blaNDM-1. Pairs of strains expressing different NDM alleles in opposite Lac phenotype backgrounds (e.g., E. coli W3110 pMBLe-blaNDM-1 and E. coli W3110 ΔlacZ pMBLe-blaNDM-4) were grown at 37°C.
Cultures were then diluted 1/100 in fresh LB broth and grown at 37°C to an OD600 of 0.6. Equal amounts (according to the OD600 values) of Lac− cells expressing one allele and Lac+ cells producing the competing allele were mixed and diluted 1/500 in fresh LB broth supplemented with 20 μg/ml gentamicin, 100 μM IPTG, and different concentrations of cefotaxime and either with or without addition of 250 μM DPA or 250 μg/ml CP. The greatest concentration of cefotaxime used was the maximum allowing visible growth under each condition. The competition was then carried out by growing the cells overnight at 37°C, after which ca. 100 to 300 cells were plated in LB agar plates supplemented with 60 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and 100 μM IPTG and grown overnight at 37°C. Blue and white colonies were counted to determine the proportion of Lac+ and Lac− cells, and thus of each variant, in the population after the competition. In order to ensure that there was no effect on fitness due to the Lac+/Lac− background, the experiments were repeated reversing the Lac backgrounds of the strains carrying each allele.
Determination of Zn(II) affinity in spheroplasts.
Spheroplasts from E. coli cells expressing NDM variants were centrifuged and resuspended in Chelex-100-treated HEPES (10 mM) and 200 mM NaCl, pH 7.5. Spheroplasts were diluted 1/50 in Chelex-100-treated HEPES (10 mM) and 200 mM NaCl, pH 7.5, with variable amounts of DPA (0 to 50 μM). After incubation at 30°C for 10 min, 550 μM imipenem was added, and β-lactamase activity measured in a Jasco V-670 spectrophotometer at 30°C. Imipenem hydrolysis was monitored at 300 nm (Δε300 nm = −9,000 M−1 cm−1).
Supplementary Material
ACKNOWLEDGMENTS
G.B. is the recipient of a doctoral fellowship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). A.J.V. and L.J.G. are staff members from CONICET. This work was also supported by funds from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI100560 to A.J.V. and R.A.B. and by the Agencia Nacional de Promoción Científica y Tecnológica to A.J.V. R.A.B. is also supported by the Cleveland Department of Veterans Affairs, Veterans Affairs Merit Review Program award number 1I01BX001974, and the Geriatric Research Education and Clinical Center VISN 10. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
We thank Elizabeth Nolan (MIT) for providing calprotectin.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01849-17.
REFERENCES
- 1.Patel G, Bonomo RA. 2013. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol 4:48. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med 18:263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 4.Palzkill T. 2013. Metallo-beta-lactamase structure and function. Ann N Y Acad Sci 1277:91–104. doi: 10.1111/j.1749-6632.2012.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowder MW, Spencer J, Vila AJ. 2006. Metallo-beta-lactamases: novel weaponry for antibiotic resistance in bacteria. Acc Chem Res 39:721–728. doi: 10.1021/ar0400241. [DOI] [PubMed] [Google Scholar]
- 6.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 7.dortet L, poirel L, nordmann P. 2014. Worldwide dissemination of the NDM-type carbapenemases in gram-negative bacteria. Biomed Res Int 2014:249856. doi: 10.1155/2014/249856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King D, Strynadka N. 2011. Crystal structure of New Delhi metallo-beta-lactamase reveals molecular basis for antibiotic resistance. Protein Sci 20:1484–1491. doi: 10.1002/pro.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez LJ, Bahr G, Nakashige TG, Nolan EM, Bonomo RA, Vila AJ. 2016. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-beta-lactamase. Nat Chem Biol 12:516–522. doi: 10.1038/nchembio.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez LJ, Bahr G, Vila AJ. 2016. Lipidated beta-lactamases: from bench to bedside. Future Microbiol 11:1495–1498. doi: 10.2217/fmb-2016-0176. [DOI] [PubMed] [Google Scholar]
- 11.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan AU, Maryam L, Zarrilli R. 2017. Structure, genetics and worldwide spread of New Delhi metallo-beta-lactamase (NDM): a threat to public health. BMC Microbiol 17:101. doi: 10.1186/s12866-017-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makena A, Brem J, Pfeffer I, Geffen RE, Wilkins SE, Tarhonskaya H, Flashman E, Phee LM, Wareham DW, Schofield CJ. 2015. Biochemical characterization of New Delhi metallo-beta-lactamase variants reveals differences in protein stability. J Antimicrob Chemother 70:463–469. doi: 10.1093/jac/dku403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meini MR, Tomatis PE, Weinreich DM, Vila AJ. 2015. Quantitative description of a protein fitness landscape based on molecular features. Mol Biol Evol 32:1774–1787. doi: 10.1093/molbev/msv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez LJ, Moreno DM, Bonomo RA, Vila AJ. 2014. Host-specific enzyme-substrate interactions in SPM-1 metallo-beta-lactamase are modulated by second sphere residues. PLoS Pathog 10:e1003817. doi: 10.1371/journal.ppat.1003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasteran F, Gonzalez LJ, Albornoz E, Bahr G, Vila AJ, Corso A. 2016. Triton Hodge test: improved protocol for modified Hodge test for enhanced detection of NDM and other carbapenemase producers. J Clin Microbiol 54:640–649. doi: 10.1128/JCM.01298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mira PM, Meza JC, Nandipati A, Barlow M. 2015. Adaptive landscapes of resistance genes change as antibiotic concentrations change. Mol Biol Evol 32:2707–2715. doi: 10.1093/molbev/msv146. [DOI] [PubMed] [Google Scholar]
- 18.Brophy MB, Hayden JA, Nolan EM. 2012. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc 134:18089–18100. doi: 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerasi M, Ammendola S, Battistoni A. 2013. Competition for zinc binding in the host-pathogen interaction. Front Cell Infect Microbiol 3:108. doi: 10.3389/fcimb.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 21.Capdevila DA, Wang J, Giedroc DP. 2016. Bacterial strategies to maintain zinc metallostasis at the host-pathogen interface. J Biol Chem 291:20858–20868. doi: 10.1074/jbc.R116.742023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez JM, Meini MR, Tomatis PE, Medrano Martin FJ, Cricco JA, Vila AJ. 2012. Metallo-beta-lactamases withstand low Zn(II) conditions by tuning metal-ligand interactions. Nat Chem Biol 8:698–700. doi: 10.1038/nchembio.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knies JL, Cai F, Weinreich DM. 2017. Enzyme efficiency but not thermostability drives cefotaxime resistance evolution in TEM-1 beta-lactamase. Mol Biol Evol 34:1040–1054. doi: 10.1093/molbev/msx053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suvorov M, Vakulenko SB, Mobashery S. 2007. Cytoplasmic-membrane anchoring of a class A β-lactamase and its capacity in manifesting antibiotic resistance. Antimicrob Agents Chemother 51:2937–2942. doi: 10.1128/AAC.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC, Walsh TR, Coombes BK, Wright GD. 2014. Aspergillomarasmine A overcomes metallo-beta-lactamase antibiotic resistance. Nature 510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Bachmann BJ. 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev 36:525–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humbert MV, Rasia RM, Checa SK, Soncini FC. 2013. Protein signatures that promote operator selectivity among paralog MerR monovalent metal ion regulators. J Biol Chem 288:20510–20519. doi: 10.1074/jbc.M113.452797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.