ABSTRACT
Meropenem-vaborbactam (Vabomere) is highly active against Gram-negative pathogens, especially Klebsiella pneumoniae carbapenemase (KPC)-producing, carbapenem-resistant Enterobacteriaceae. The objective of these studies was to evaluate the efficacy of meropenem alone and in combination with vaborbactam in mouse thigh and lung infection models. Thighs or lungs of neutropenic mice were infected with KPC-producing carbapenem-resistant Enterobacteriaceae, with meropenem MICs ranging from ≤0.06 to 8 mg/liter in the presence of 8 mg/liter vaborbactam. Mice were treated with meropenem alone or meropenem in combination with vaborbactam every 2 h for 24 h to provide exposures comparable to 2-g doses of each component in humans. Meropenem administered in combination with vaborbactam produced bacterial killing in all strains tested, while treatment with meropenem alone either produced less than 0.5 log CFU/tissue of bacterial killing or none at all. In the thigh model, 11 strains were treated with the combination of meropenem plus vaborbactam (300 plus 50 mg/kg of body weight). This combination produced from 0.8 to 2.89 logs of bacterial killing compared to untreated controls at the start of treatment. In the lung infection model, two strains were treated with the same dosage regimen of meropenem and vaborbactam. The combination produced more than 1.83 logs of bacterial killing against both strains tested compared to untreated controls at the start of treatment. Overall, these data suggest that meropenem-vaborbactam may have utility in the treatment of infections due to KPC-producing carbapenem-resistant Enterobacteriaceae.
KEYWORDS: meropenem, vaborbactam, KPC
INTRODUCTION
Carbapenem antibiotics are considered first-line agents for serious infections for Gram-negative bacteria featuring an extended spectrum of resistance to other agents. While carbapenems have an excellent profile of beta-lactamase stability, resistance can be mediated by class A serine carbapenemases, especially Klebsiella pneumoniae carbapenemase (KPC)-type carbapenemases (1, 2). The emergence of carbapenem-resistant Enterobacteriaceae in U.S. hospitals has prompted the U.S. Centers for Disease Control (CDC) to issue guidance for infection control procedures to prevent further spread (3). Widespread dissemination of carbapenemase-mediated resistance has had serious repercussions for clinical practice, leaving clinicians with few treatment options for serious Gram-negative infections (4–8). Therefore, the discovery and development of new treatment options for Gram-negative pathogens are pressing public health priorities.
Meropenem-vaborbactam (Vabomere) was recently approved by the FDA for the treatment of complicated urinary tract infections, including pyelonephritis, and a phase 3 clinical investigation of serious infections due to carbapenem-resistant Enterobacteriaceae (CRE), including hospital-acquired and ventilator-associated pneumonia (ClinicalTrials.gov identifier NCT02168946), is ongoing. Vaborbactam is a new cyclic boronic acid-based inhibitor of serine beta-lactamases (9). Vaborbactam inhibits multiple class A and C beta-lactamases but was specifically optimized to inhibit KPC carbapenemases and restore the activity of carbapenem antibiotics. The objective of these studies was to demonstrate the in vivo activity of meropenem in combination with vaborbactam in mouse thigh and lung infection models due to carbapenem-resistant KPC-producing Enterobacteriaceae.
(This work was presented in part at the 52nd and 54th Interscience Conferences on Antimicrobial Agents and Chemotherapy in 2012 and 2014, respectively).
RESULTS
Susceptibility studies.
The characteristics of the strains used in these studies are shown in Table 1. All strains produced a KPC beta-lactamase, and some had mixtures of other non-beta-lactamase-mediated resistance mechanisms known to affect the potency of carbapenems. Vaborbactam combined with meropenem markedly enhanced the in vitro potency of meropenem against these strains of Enterobacteriaceae by at least 8-fold, and all strains had a meropenem MIC of 8 mg/liter or less when tested in combination with 8 mg/liter vaborbactam.
TABLE 1.
MICs and enzymatic production data for the Enterobacteriaceae strains used in the studies
| Strain | β-Lactamase | Porin mutation(s)a |
Meropenem MIC (mg/liter) |
||
|---|---|---|---|---|---|
| OmpK35 | OmpK36 | Alone | With 8 mg/liter vaborbactam | ||
| E. coli EC1007 | KPC-3 | ND | ND | 8 | ≤0.06 |
| E. cloacae ECL1004 | NMC-A | ND | ND | 16 | ≤0.06 |
| E. cloacae ECL1026 | KPC-2, TEM-1 | ND | ND | 8 | ≤0.06 |
| E. cloacae ECL1055 | KPC-3, TEM | FS aa 287 | FL | 8 | ≤0.06 |
| K. pneumoniae KP1004 | KPC-2, TEM-1, SHV-11 | FS aa 42 | FL | 16 | ≤0.06 |
| K. pneumoniae KP1061 | KPC-3, TEM-1, SHV-11 | FS aa 42 | FL | 16 | ≤0.06 |
| K. pneumoniae KP1074 | KPC-3, SHV-11, TEM | FS aa 42 | GD | >64 | 0.5 |
| K. pneumoniae KP1093 | KPC-3, SHV-11, TEM | FS aa 42 | GD | 128 | 0.5 |
| K. pneumoniae KP1094 | KPC-2, TEM-1, LEN-17 | Stop aa 230 | Stop aa 92 | 512 | 4 |
| K. pneumoniae KP1099 | KPC-2, SHV-11, SHV12, CTX-M-14 | FS aa 29 | GD | 128 | 1 |
| K. pneumoniae KP1100 | KPC-3, TEM, SHV | FS aa 42 | GD | >256 | 4 |
| K. pneumoniae KP1223 | KPC-2, SHV, TEM | FS aa 29 | GD | >64 | 8 |
FL, full length (functional); stop aa, nonsense mutations resulting in a truncated nonfunctional protein; FS aa, frameshift mutation resulting in a nonfunctional protein; GD, insertion of two amino acids, Gly134-Asp135, resulting in a narrow semifunctional channel; ND, not determined.
Protein binding.
Vaborbactam protein binding in mouse and human serum is shown in Table 2. The average values for protein binding across the range of concentrations studied were 6% in mice and 33% in humans. Meropenem protein binding has been reported to be 10% in mice (10) and 2% in humans (11).
TABLE 2.
Vaborbactam protein binding in mice and humans
| Concn (μg/ml) | Serum protein binding (%) |
|
|---|---|---|
| Human | Mouse | |
| 1 | 37 | 7 |
| 5 | 30 | 8 |
| 15 | 29 | 4 |
| 50 | 33 | 4 |
| Avg | 33 | 6 |
Pharmacokinetics.
The decline in plasma concentrations for both meropenem and vaborbactam were best described by a one-compartment model with first-order elimination. The plasma pharmacokinetic parameters for meropenem alone, vaborbactam alone, or both drugs in combination in uninfected neutropenic mice are shown in Table 3. The pharmacokinetic parameters of meropenem and vaborbactam were also similar when administered alone and in combination in uninfected immunocompetent mice (data not shown). Based on these data, a dose of 300 mg/kg of body weight meropenem every 2 h for 24 h produces a free drug time above 8 mg/liter similar to that with 2 g administered every 8 h by a 3-h infusion in humans (12). A dose of 50 mg/kg vaborbactam every 2 h for 24 h produces a free drug 24-h vaborbactam are under the concentration-time curve (AUC) similar to that with 2 g administered every 8 h by a 3-h infusion in humans (12) (Table 4).
TABLE 3.
Pharmacokinetic parameters following a single dose of meropenem and vaborbactam alone and in combination administered by the intraperitoneal route in neutropenic mice
| Compounds | Dose (mg/kg) | AUC (h · mg/liter) | CL (liters/h/kg)a | Cmax (mg/liter)b |
|---|---|---|---|---|
| Meropenem alone | 100 | 45.15 | 2.21 | 106.00 |
| Meropenem (with vaborbactam) | 100 (+50) | 49.19 | 2.03 | 138.00 |
| Meropenem alone | 300 | 153.03 | 1.96 | 244.51 |
| Meropenem (with vaborbactam) | 300 (+50) | 130.85 | 2.29 | 260.44 |
| Vaborbactam alone | 50 | 29.24 | 1.71 | 62.45 |
| Vaborbactam (with meropenem) | 50 (+100) | 27.74 | 1.80 | 53.16 |
| Vaborbactam (with meropenem) | 50 (+300) | 30.15 | 1.66 | 44.62 |
CL, clearance.
Cmax, maximum concentration of drug in serum.
TABLE 4.
Comparison of the pharmacokinetics of meropenem and vaborbactam in mice and in humans
| Compound | Species | Dosage regimena | 24-h free AUC (mg · h/liters) | %T>8 mg/literb |
|---|---|---|---|---|
| Meropenem | Human | 2 g q8h by 3-h infusion | 402 | 56 |
| Mouse | 300 mg/kg q2h | 1,572 | 51 | |
| Human | 1.5 g q8h by 3-h infusion | 282 | 47 | |
| Mouse | 200 mg/kg q2h | 1,080 | 47 | |
| Human | 1 g q8h by 3-h infusion | 162 | 38 | |
| Mouse | 100 mg/kg q2h | 588 | 39 | |
| Vaborbactam | Human | 4 g q8h by 3-h infusion | 686 | 100 |
| Mouse | 100 mg/kg q2h | 720 | 70 | |
| Human | 2 g q8h by 3-h infusion | 343 | 72 | |
| Mouse | 50 mg/kg q2h | 360 | 53 | |
| Human | 1 g q8h by 3-h infusion | 172 | 44 | |
| Mouse | 25 mg/kg q2h | 180 | 30 | |
| Human | 500 mg q8h by 3-h infusion | 86 | 24 | |
| Mouse | 12.5 mg/kg q2h | 90 | 18 | |
| Human | 250 mg q8h by 3-h infusion | 43 | 0 | |
| Mouse | 6.25 mg/kg q2h | 45 | 0 |
q8h, every 8 h; q2h, every 2 h.
%T>8 mg/liter, cumulative percentage of a 24-h period that the drug concentration exceeds 8 mg/liter.
Thigh infection model.
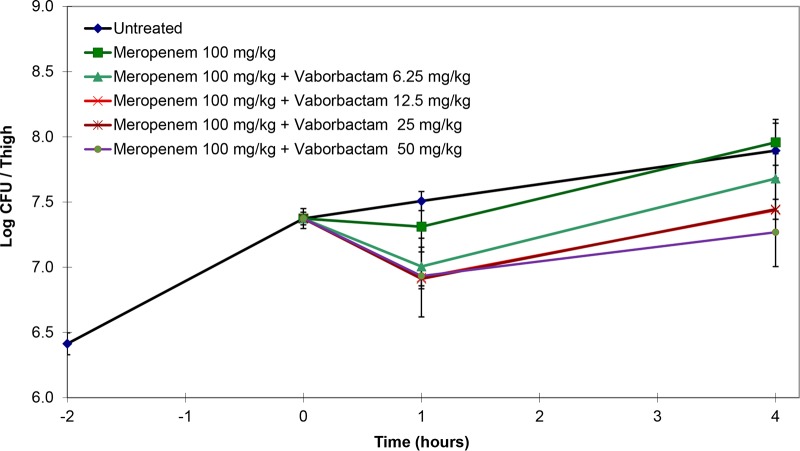
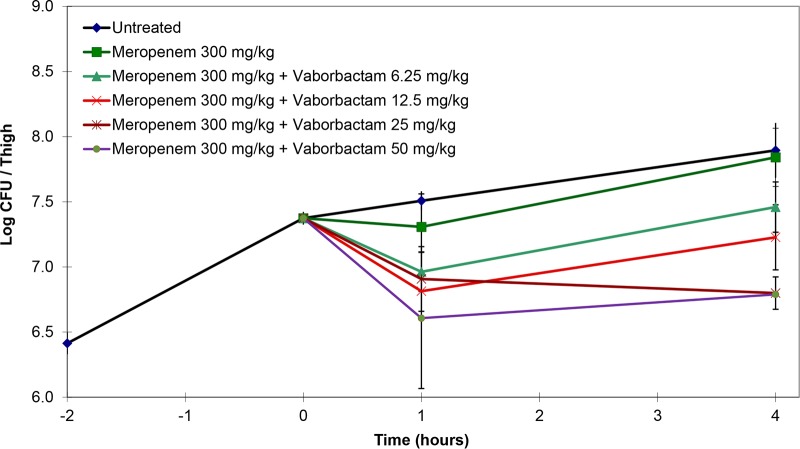
In pilot single-dose studies, mice infected with K. pneumoniae KP1074 were treated with meropenem at 100 or 300 mg/kg alone or in combination with various doses of vaborbactam. Mice treated with either 100 or 300 mg/kg meropenem alone had bacterial counts similar to those of the untreated controls. Bacterial killing with both doses of meropenem increased with the addition of vaborbactam in a dose-dependent fashion (Fig. 1 and 2). However, 300 mg/kg meropenem combined with 50 mg/kg vaborbactam produced the greatest reduction in bacterial counts compared to the untreated controls.
FIG 1.
Activity of 100 mg/kg meropenem alone and in combination with various doses of vaborbactam against K. pneumoniae KP1074 (meropenem MIC of ≥64 mg/liter; with 8 mg/liter vaborbactam, 0.5 mg/liter) in a neutropenic mouse thigh infection model. Treatment was administered as a single intraperitoneal dose at 2 h postinfection.
FIG 2.
Activity of 300 mg/kg meropenem alone and in combination with various doses of vaborbactam against K. pneumoniae KP1074 (meropenem MIC ≥64 mg/liter; with 8 mg/liter vaborbactam, 0.5 mg/liter) in a neutropenic mouse thigh infection model. Treatment was given as a single intraperitoneal dose at 2 h postinfection.
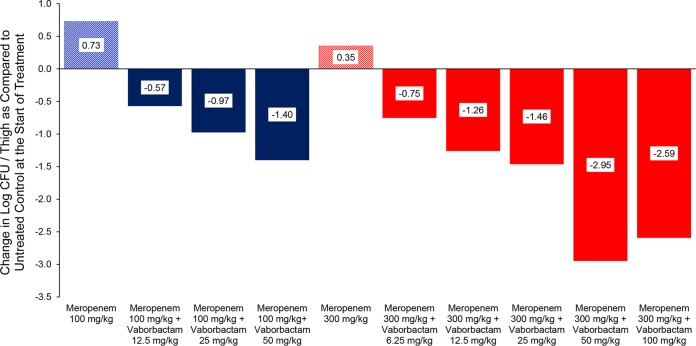
The single-dose studies were followed with a 24-h thigh infection model using K. pneumoniae KP1094. Following infection, mice were treated with either 100 or 300 mg/kg meropenem alone every 2 h for 24 h or in combination with vaborbactam ranging from 6.25 to 100 mg/kg every 2 h for 24 h. In these studies, meropenem alone did not reduce bacterial counts compared to the untreated controls at the start of treatment. The addition of vaborbactam to both meropenem regimens produced bacterial killing in a dose-dependent fashion, with a maximum of bacterial killing of 2.50 log CFU/thigh (Fig. 3).
FIG 3.
Activity of meropenem alone and in combination with vaborbactam against K. pneumoniae KP1094 (meropenem MIC, 512 mg/liter; with 8 mg/liter vaborbactam, 4 mg/liter) in a neutropenic mouse thigh infection model. Treatments were started at 2 h postinfection and continued every 2 h for 24 h by intraperitoneal route.
Finally, the activity of 300 mg/kg meropenem alone and in combination with 50 mg/kg vaborbactam administered every 2 h over 24 h was assessed against seven carbapenem-resistant KPC-containing K. pneumoniae, one Escherichia coli, and three Enterobacter cloacae strains (Tables 5 and 6). Treatment with meropenem alone did not significantly reduce bacterial counts compared to the untreated controls at the start of treatment. However, the combination of meropenem plus vaborbactam produced bacterial killing against all strains tested ranging from 0.82- to 2.37-log CFU/thigh reductions in bacterial counts.
TABLE 5.
Activity of meropenem alone and in combination with vaborbactam against carbapenem-resistant KPC-containing K. pneumoniae in a neutropenic mouse thigh infection modela
| K. pneumoniae strain | Compound | Dose (mg/kg) | Log CFU/thigh ± SDb | P valuec |
|---|---|---|---|---|
| KP1004 | Untreated control at start of treatment | 0 | 7.24 ± 0.10 | |
| Untreated control at 24 h | 0 | Not done | ||
| Meropenem alone | 300.00 | 7.18 ± 0.14 | 0.298 vs NT; <0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 5.55 ± 0.18 | <0.0001 vs NT; <0.0001 vs alone | |
| KP1074 | Untreated control at start of treatment | 0 | 7.06 ± 0.25 | |
| Untreated control at 24 h | 0 | Not done | ||
| Meropenem alone | 300 | 8.07 ± 0.43 | 0.0002 vs NT; 0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 5.85 ± 0.25 | 0.0005 vs NT; 0.0001 vs alone | |
| KP1099 | Untreated control at start of treatment | 0 | 7.03 ± 0.02 | |
| Untreated control at 24 h | 0 | 9.22 ± 0.6 | ||
| Meropenem alone | 300 | 8.78 ± 0.29 | <0.0001 vs NT; <0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 5.78 ± 0.53 | 0.00132vs NT; <0.0001 vs alone | |
| KP1100 | Untreated control at start of treatment | 0 | 6.75 ± 0.59 | |
| Untreated control at 24 h | 0 | 9.13 ± 0.13 | ||
| Meropenem alone | 300 | 6.36 ± 0.17 | 0.0002 vs NT; <0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 5.93 ± 0.28 | 0.043 vs NT; <0.0001 vs alone | |
| KP1093 | Untreated control at start of treatment | 0 | 6.76 ± 0.10 | |
| Untreated control at 24 h | 0 | 8.69 ± 0.29 | ||
| Meropenem alone | 300 | 7.34 ± 0.19 | 0.0017vs NT; <0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 4.90 ± 0.15 | <0.0001vs NT; <0.0001 vs alone | |
| KP1094 | Untreated control at start of treatment | 0 | 6.70 ± 0.17 | |
| Untreated control at 24 h | 0 | 8.76 ± 0.31 | ||
| Meropenem alone | 300 | 6.80 ± 0.56 | 0.733 vs NT; 0.0033 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 4.33 ± 0.89 | 0.0019 vs NT; 0.0033 vs alone | |
| KP1223 | Untreated control at start of treatment | 0 | 6.92 ± 0.16 | |
| Untreated control at 24 h | 0 | 9.34 ± 0.18 | ||
| Meropenem alone | 300 | 10.12 ± 0.11 | <0.0001vs NT; <0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 5.88 ± 0.70 | <0.0001 vs NT; <0.0001 vs alone |
Treatment started at 2 h postinfection and continued every 2 h for 24 h. Untreated controls were euthanized at the start of treatment. Treated groups were euthanized 2 h after the last treatment.
Those labeled “not done” were due to high mortality rate.
NT, untreated at the start of treatment.
TABLE 6.
Activity of meropenem alone and in combination with vaborbactam against carbapenem-resistant KPC-containing E. coli and E. cloacae in the neutropenic mouse thigh infection modela
| Strain | Compound | Dose (mg/kg) | Log CFU/thigh ± SDb | P valuec |
|---|---|---|---|---|
| E. coli EC1007 | Untreated control at start of treatment | 0 | 6.66 ± 0.08 | |
| Untreated control at 24 h | 0 | Not done | ||
| Meropenem alone | 300.00 | 6.62 ± 0.22 | 0.733 vs NT; <0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 5.42 ± 0.12 | <0.0001 vs NT; <0.0001 vs alone | |
| E. cloacae ECL1004 | Untreated control at start of treatment | 0 | 7.21 ± 0.12 | |
| Untreated control at 24 h | 0 | Not done | ||
| Meropenem alone | 300.00 | 6.89 ± 0.32 | 0.114 vs NT; 0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 5.39 ± 0.13 | <0.0001 vs NT; 0.0001 vs alone | |
| E. cloacae ECL1026 | Untreated control at start of treatment | 0 | 6.58 ± 0.22 | |
| Untreated control at 24 h | 0 | Not done | ||
| Meropenem alone | 300.00 | 6.84 ± 0.37 | 0.261 vs NT; 0.0001 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 4.52 ± 0.38 | <0.0001 vs NT; 0.0001 vs alone | |
| E. cloacae ECL1055 | Untreated control at start of treatment | 0 | 6.49 ± 0.10 | |
| Untreated control at 24 h | 0 | Not done | ||
| Meropenem alone | 300.00 | 6.38 ± 0.25 | 0.465 vs NT; 0.0039 vs combo | |
| Meropenem (with vaborbactam) | 300 (+50) | 5.53 ± 0.27 | 0.006 vs NT; 0.0039vs alone |
Treatments started 2 h postinfection and continued every 2 h for 24 h. Untreated controls were euthanized at the start of treatment. Treated groups were euthanized 2 h after the last treatment.
Those labeled “not done” were due to high mortality rate.
NT, untreated at the start of treatment.
Lung infection model.
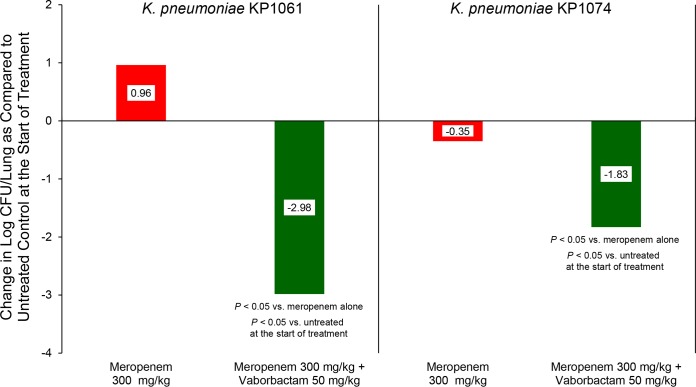
Similar to the studies in the neutropenic mouse thigh infection model, treatment with meropenem alone produced 0.35 logs of bacterial killing against K. pneumoniae KP1074 and allowed for 0.96 logs of bacterial growth against K. pneumoniae KP1061. In contrast, treatment with the combination of meropenem plus vaborbactam produced over 1.5 logs of bacterial killing against both strains tested (Fig. 4).
FIG 4.
Activity of meropenem alone and in combination with vaborbactam against carbapenem-resistant, KPC-containing K. pneumoniae in strains in a neutropenic mouse lung infection model. Treatments were administered every 2 h for 24 h by intraperitoneal route.
DISCUSSION
Meropenem is a carbapenem antibiotic that can be administered at high doses (up to 2 g every 8 h) and by prolonged infusion (up to 3 h), thus improving the pharmacokinetic and pharmacodynamic target attainment compared to lower doses administered by shorter infusions (13–15).
Meropenem is stable to hydrolysis by many class A and class C beta-lactamases that mediate resistance to extended-spectrum penicillins and cephalosporins (16, 17). However, the dissemination of carbapenemases, particularly the KPC carbapenemases, has resulted in a loss of activity of carbapenems and of other beta-lactam antibiotics. Vaborbactam, the first member of a new class of cyclic boronic acid beta-lactamase inhibitors, has potent inhibition of the KPC carbapenemase and restores the activity of meropenem against carbapenemase-producing strains (9).
All strains evaluated in these studies produced the KPC beta-lactamase and were resistant to meropenem alone (i.e., MICs were 8 mg/liter or higher) in vitro. These strains were selected for study, as they also produced other serine beta-lactamases and had changes in outer membrane porins that are associated with reduced permeability to meropenem (12). Thus, these strains represented a subset of clinical isolates with multiple resistance mechanisms that reduce sensitivity to carbapenems. The addition of vaborbactam at 8 mg/liter reduced the MICs 8- to 64-fold, reducing the meropenem MIC to 8 mg/liter or less for all strains.
Single-dose studies with meropenem alone or in combination with various doses of vaborbactam against K. pneumoniae KP1074 showed that vaborbactam increased the activity of meropenem in a dose-dependent fashion, with maximum bacterial killing achieved after a single 300-mg/kg dose of meropenem in combination with 50 mg/kg vaborbactam. Due to the rapid clearance of both compounds in mice, maximum bacterial killing was observed at 1 h, and all dosage regimens showed regrowth.
In the 24-h neutropenic mouse thigh infection model against K. pneumoniae KP1094, as was observed in the single-dose study, the addition of vaborbactam increased the activity of meropenem in a dose-dependent fashion up to 50 mg/kg. In this study, maximum bacterial killing was achieved with a 300-mg/kg dose of meropenem administered in combination with 50 mg/kg vaborbactam every 2 h for 24 h. This dosage regimen produces meropenem and vaborbactam exposures in uninfected mice that are similar to those with 2 g of meropenem and 2 g of vaborbactam given every 8 h by a 3-h infusion in humans. The pharmacokinetics of meropenem and vaborbactam in infected mice were not determined, which may be a limitation to the direct comparison of exposures between mice and humans.
The activity of meropenem alone and in combination with vaborbactam against KPC-producing carbapenem-resistant strains of K. pneumoniae, E. coli, and E. cloacae was assessed in a 24-h neutropenic mouse thigh infection model. Notably, many of the strains tested in vivo had mixtures of carbapenem resistance mechanisms, including multiple beta-lactamases and mutations involving the OmpK35 and OmpK36 porins that are important for meropenem and vaborbactam entry into K. pneumoniae (18). As predicted based on the MICs, meropenem alone did not produce bacterial killing, but when combined with vaborbactam, it showed bacterial killing for strains with meropenem-vaborbactam MICs of up to 8 mg/liter. The activity of meropenem alone and in combination with vaborbactam was confirmed in a neutropenic mouse lung infection model against two KPC-producing strains. As observed in the thigh infection model, the combination was highly active in this model as well.
In conclusion, these data demonstrate that meropenem-vaborbactam has excellent activity against carbapenem-resistant KPC-containing strains of Enterobacteriaceae. These data also show that vaborbactam has low serum protein binding and a pharmacokinetic profile that is well matched for use in combination with meropenem. Therefore, further development of meropenem-vaborbactam for the treatment of infections due to carbapenem-resistant KPC-containing Enterobacteriaceae is warranted.
MATERIALS AND METHODS
All studies using animals were performed under protocols approved by an Institutional Animal Care and Use Committee (IACUC).
Antimicrobial agents.
Meropenem for injection (Sandoz) was purchased from commercial sources. Vaborbactam was synthesized at The Medicines Company, San Diego, CA.
Bacterial strains and MIC testing.
Eight clinical isolates of K. pneumoniae, three clinical isolates of E. cloacae, and one E. coli isolate were used in these studies. Meropenem MICs were determined using a broth microdilution assay according to CLSI reference methods (19). The MICs of meropenem were determined alone and in combination with a fixed concentration of 8 mg/liter vaborbactam. Assays were performed using a final volume of 100 μl. The inocula were adjusted to yield a final cell density of ca. 5 × 105 CFU/ml. Meropenem was diluted directly into 96-well microtiter plates by serial 2-fold dilution, and then vaborbactam was added at a fixed concentration. Microtiter plates were read using a plate reader (Molecular Devices, Sunnyvale, CA) at 600 nm (optical density [OD] value of less than 0.065 = no growth), as well as by visual observation using a reading mirror. The MIC was defined as the lowest concentration of antibiotic at which the visible growth of the organism was completely inhibited.
Protein binding.
Vaborbactam protein binding in pooled mouse and human serum (BioreclamationIVT, Baltimore, MD) was determined using ultrafiltration at concentrations of 1, 5, 15, and 50 μg/ml. Protein binding was measured in duplicate at each concentration. Briefly, serum was spiked with vaborbactam and incubated at 37°C for 15 min. Five-hundred-microliter aliquots of spiked serum or spiked prefiltered serum was added to the upper reservoir of a Centrifree cartridge (YM-30; Millipore, Bedford, MA) and centrifuged at 2,000 × g for 15 min at room temperature. The filtrates were analyzed by liquid chromatography-mass spectrometry (LC-MS). The peak areas were used to calculate serum protein binding as follows: % serum bound = 100 − (ASF/ASWF × 100), where ASF is the peak area of vaborbactam from spiked serum after ultrafiltration, and ASWF is the peak area of vaborbactam from spiked prefiltered serum after ultrafiltration.
Pharmacokinetics.
Female Swiss Webster mice (5 to 6 weeks of age) were obtained from Envigo Laboratories (Livermore, CA). The pharmacokinetics of meropenem alone, vaborbactam alone, and both drugs in combination were determined in both immunocompetent and neutropenic mice. For neutropenic mice, neutropenia was achieved by the administration of 150 mg/kg cyclophosphamide (Baxter, IL), by the intraperitoneal route, 4 days and 1 day prior to the start of the study. Mice were administered meropenem (100 and 300 mg/kg) and vaborbactam (50 mg/kg) alone or in combination by the intraperitoneal route. At the designated time points, mice were euthanized, and their blood was collected by cardiac puncture and transferred to EDTA-containing tubes. Blood samples were centrifuged within 5 min of collection at 12,000 × g for 5 min to obtain plasma. An equal volume of 3-(N-morpholino) propanesulfonic acid (MOPS) buffer (pH 7) was added to plasma samples that contained meropenem, which were then stored at −80°C until analyzed.
Bioanalytical assay.
Vaborbactam and meropenem standard curves were prepared in plasma at concentrations of 0.04 to 50.0 μg/ml. Twenty-five-microliter aliquots of sample were placed in 1.5-ml microcentrifuge tubes containing 200 μl of 4.0 μg/ml doripenem (internal standard for meropenem) and 4.0 μg/ml of RPX7015 (internal standard for vaborbactam) in 10%/45%/45% (vol/vol/vol) water-methanol-acetonitrile. The samples were mixed using a vortex mixer and then centrifuged for 10 min at 15,000 × g using a tabletop centrifuge. The supernatant (∼150 μl) was removed and added to 400 μl of water in a 96-well plate. The samples were mixed again using a vortex mixer. Twenty microliters of each sample was injected onto a high-performance LC-mass spectrometer (HPLC-MS) for quantification. The lower limit of quantitation for both meropenem and vaborbactam was 0.04 μg/ml. Plasma concentrations were fitted using a one-compartment first-order model (Phoenix WinNonlin version 6.4; Certara USA, Inc., Princeton, NJ).
Neutropenic mouse thigh and lung infection models.
Female Swiss Webster mice (n = 2 to 5/group) were rendered neutropenic, as described above. Test strains were grown in Mueller-Hinton broth (MHB) at 37°C under constant aeration (300 rpm) for 20 h. The infecting inoculum was prepared by removal of an aliquot and subculturing into fresh MHB; this was allowed to regrow at 37°C, under constant aeration, for 3 h to reach an absorbance at 600 nm of 0.30 to 0.35. The bacterial suspensions were diluted in fresh MHB to yield ∼106 to 107 CFU/ml by correlation of absorbance at 600 nm with predetermined plate counts. For the thigh infection model, mice were infected by intramuscular injection of 0.1 ml of inoculum (107 CFU/ml) into both thigh muscles while under isoflurane anesthesia (5% isoflurane in oxygen running at 4 liters/min) (20). For the lung infection model, isoflurane-anesthetized mice were infected by intratracheal instillation of 0.05 ml of inoculum (106 CFU/ml) using a curved oral gavage tip attached to a 1-ml syringe (21).
Treatment regimens.
For the initial experiments, meropenem was administered intraperitoneally at 100 and 300 mg/kg alone or in combination with 6.25, 12.5, 25, or 50 mg/kg of vaborbactam either as single doses or every 2 h for 24 h for multiple-dose studies.
Treatment regimens were chosen in order to simulate exposures in humans. Briefly, meropenem administered at 100 mg/kg or 300 mg/kg every 2 h over a 24-h period in mice produces an exposure equivalent to 1 g or 2 g of meropenem administered every 8 h by a 3-h infusion in humans, respectively (12, 22). Vaborbactam administered at 6.25 mg/kg, 12.5 mg/kg, 25 mg/kg, 50 mg/kg, and 100 mg/kg every 2 h over a 24-h period in neutropenic mice produces an exposure equivalent to 0.25 g, 0.5 g, 1 g, 2 g, or 4 g of vaborbactam administered every 8 h by a 3-h infusion in humans, respectively (12, 22).
Following the initial experiments, the meropenem treatment regimen was limited to 300 mg/kg every 2 h for 24 h, and the meropenem-vaborbactam treatment regimen was limited to 300 mg/kg meropenem and 50 mg/kg vaborbactam every 2 h for 24 h in order to simulate an exposure of 2 g of meropenem and 2 g of vaborbactam administered every 8 h by a 3-h infusion in humans. All treatments were administered by the intraperitoneal route.
Bacterial load in tissues.
For each strain, 2 to 5 untreated mice were euthanized prior to the start of treatment to determine baseline bacterial counts. All treatment and control groups were euthanized 2 h following the last dose by carbon dioxide asphyxiation. The thighs (n = 2) or lungs (n = 5 to 6) were removed aseptically and homogenized (Pro200 homogenizer; Pro Scientific, Monroe, CT) in ice-cold sterile saline. Serial 10-fold dilutions of the homogenized thighs and lungs were plated on Mueller-Hinton agar, and the colonies were counted.
Statistical analysis.
Thigh and lung bacterial counts were analyzed by unpaired t test (GraphPad Prism version 6.03), respectively. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work, including the efforts of M. Sabet, Z. Tarazi, T. Nolan, J. Parkinson, D. Rubio-Aparicio, O. Lomovskaya, M. N. Dudley, and D. C. Griffith, was funded in part by the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under contract HHSO100201400002C.
M. Sabet, Z. Tarazi, T. Nolan, J. Parkinson, D. Rubio-Aparicio, O. Lomovskaya, M. N. Dudley, and D. C. Griffith are employees of The Medicines Company.
REFERENCES
- 1.Lynch JP III, Clark NM, Zhanel GG. 2013. Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum β-lactamases and carbapenemases). Expert Opin Pharmacother 14:199–210. doi: 10.1517/14656566.2013.763030. [DOI] [PubMed] [Google Scholar]
- 2.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE, Antibacterial Resistance Leadership Group. 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clin Infect Dis 60:1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 4.Bassetti M, Ginocchio F, Mikulska M. 2011. New treatment options against Gram-negative organisms. Crit Care 15:215. doi: 10.1186/cc9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassetti M, Ginocchio F, Mikulska M, Taramasso L, Giacobbe DR. 2011. Will new antimicrobials overcome resistance among Gram-negatives? Expert Rev Anti Infect Ther 9:909–922. doi: 10.1586/eri.11.107. [DOI] [PubMed] [Google Scholar]
- 6.Nicasio AM, Kuti JL, Nicolau DP. 2008. The current state of multidrug-resistant Gram-negative bacilli in North America. Pharmacotherapy 28:235–249. doi: 10.1592/phco.28.2.235. [DOI] [PubMed] [Google Scholar]
- 7.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 8.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DC, King P, Tsivkovski R, Sun D, Sabet M, Tarazi Z, Clifton MC, Atkins K, Raymond A, Potts KT, Abendroth J, Boyer SH, Loutit JS, Morgan EE, Durso S, Dudley MN. 2015. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility versus class A serine carbapenemases. J Med Chem 58:3682–3692. [DOI] [PubMed] [Google Scholar]
- 10.Mattie H, Zhang LC, van Strijen E, Sekh BR, Douwes-Idema AE. 1997. Pharmacokinetic and pharmacodynamic models of the antistaphylococcal effects of meropenem and cloxacillin in vitro and in experimental infection. Antimicrob Agents Chemother 41:2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AstraZeneca Pharmaceuticals. 2006. MERREM I.V. (meropenem for injection) package insert. AstraZeneca Pharmaceuticals, Wilmington, DE: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050706s022lbl.pdf. [Google Scholar]
- 12.Griffith DC, Sabet M, Tarazi Z, Lomovskaya O, Dudley MN. 2017. Pharmacodynamics of vaborbactam when administered in combination with meropenem, abstr Sunday 194. ASM Microbe 2017, 1 to 5 June 2017, New Orleans, LA. [Google Scholar]
- 13.Dandekar PK, Maglio D, Sutherland CA, Nightingale CH, Nicolau DP. 2003. Pharmacokinetics of meropenem 0.5 and 2 g every 8 hours as a 3-hour infusion. Pharmacotherapy 23:988–991. doi: 10.1592/phco.23.8.988.32878. [DOI] [PubMed] [Google Scholar]
- 14.Nicolau DP. 2008. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis 47(Suppl 1):S32–S40. doi: 10.1086/590064. [DOI] [PubMed] [Google Scholar]
- 15.Nicolau DP. 2008. Carbapenems: a potent class of antibiotics. Expert Opin Pharmacother 9:23–37. doi: 10.1517/14656566.9.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev 23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun D, Rubio-Aparicio D, Dudley MN, Lomovskaya O. 2014. Characterization of mutants selected in vitro using sub-optimal exposure of meropenem alone and with RPX7009, abstr C-103. Abstr 54th Intersci Conf Antimicrob Agents Chemother, 1 October 2014, Washington, DC. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (CLSI). 2015. Performance standards for antimicrobial susceptibility testing, 25th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Griffith DC, Harford L, Williams R, Lee VJ, Dudley MN. 2003. In vivo antibacterial activity of RWJ-54428, a new cephalosporin with activity against Gram-positive bacteria. Antimicrob Agents Chemother 47:43–47. doi: 10.1128/AAC.47.1.43-47.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabet M, Miller CE, Nolan TG, Senekeo-Effenberger K, Dudley MN, Griffith DC. 2009. Efficacy of aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:3923–3928. doi: 10.1128/AAC.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffith DC, Loutit JS, Morgan EE, Durso S, Dudley MN. 2016. Phase 1 study of the safety, tolerability, and pharmacokinetics of the beta-lactamase inhibitor vaborbactam (RPX7009) in healthy adult subjects. Antimicrob Agents Chemother 60:6326–6332. doi: 10.1128/AAC.00568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]