Abstract
Background
Ocular syphilis is an inflammatory eye disease due to Treponema pallidum infection. In the United States, syphilis rates have increased since 2000; clusters of ocular syphilis were reported in 2015. We investigated ocular syphilis in North Carolina to describe the epidemiology and clinical course of disease.
Methods
We reviewed syphilis cases reported to North Carolina during 2014–2015 and abstracted information from health department interviews for cases with ocular symptoms and no other defined etiology. To assess duration and severity of ocular symptoms, we also reviewed medical records and conducted structured interviews. We compared the prevalence of ocular manifestations among reported syphilis cases by demographic and clinical characteristics.
Results
Among 4232 syphilis patients, 63 (1.5%) had ocular syphilis: 21 in 2014 and 42 in 2015, a 100% increase. Total syphilis cases increased 35% through 2015. No patient with ocular syphilis named another ocular syphilis patient as a sex partner. Patients presented in all syphilis stages; 24 (38%) were diagnosed in primary or secondary syphilis. Ocular manifestations were more prevalent among syphilis patients who were male, aged ≥40 years, white, and infected with human immunodeficiency virus. No risk behaviors were associated with ocular syphilis. Among 39 interviewed patients, 34 (87%) reported reduced vision during infection; 12 (31%) reported residual visual symptoms posttreatment.
Conclusions
In North Carolina, ocular syphilis increased from 2014 to 2015 and may be due to increased recognition of ocular manifestations, or a true increase in ocular syphilis. Many ocular syphilis patients experienced vision loss; however, most improved posttreatment.
Keywords: syphilis, epidemiology, Treponema pallidum, vision loss, surveillance
Syphilis is a genital ulcerative disease caused by the spirochete Treponema pallidum that can cause multisystem disease with a variety of clinical manifestations. In the United States, rates of syphilis have been increasing since 2001, with the number of total syphilis cases reported to the Centers for Disease Control and Prevention (CDC) increasing 17.7% in 2015 compared with 2014. These increases are largely attributable to an increase in syphilis among men who have sex with men (MSM) [1].
Previous studies describing clusters of ocular syphilis have identified involvement with most eye structures, in any stage of syphilis, and with acute or chronic manifestations [2–5]. Uveitis is the most common ophthalmologic diagnosis observed; optic neuritis, retinitis, and retinal detachment also have been diagnosed [4–7].
While syphilis is a nationally notifiable disease, CDC has not systematically collected surveillance data on ocular manifestations; therefore, national rates of ocular syphilis over time are unknown. Studies have found frequencies of ocular manifestations ranging from 0% to 7.9% of patients with secondary syphilis [8, 9], and up to 51% of patients with neurosyphilis [10]. A review of surveillance data during 2012–2013 from King County, Washington, revealed that 4.8% of patients with syphilis reported visual symptoms and 2.7% had objective findings consistent with ocular syphilis [11]. In England, national surveillance data showed that 0.6% of patients with early syphilis diagnoses had ocular manifestations [6].
In early 2015, CDC was notified about 2 clusters of ocular syphilis reported from Seattle and San Francisco [12] and issued a clinical advisory [13] in April 2015 to increase clinician awareness and public health reporting. Since then, many states have received reports regarding severe visual symptoms among patients diagnosed with syphilis. A review of ocular syphilis in 8 US jurisdictions found ocular manifestations from syphilis in 0.61% of total syphilis cases, increasing from 0.53% in 2014 to 0.65% in 2015 [14]. Molecular typing of Treponema pallidum strains was performed on samples from US patients with ocular syphilis; 5 strains were identified in 14 patients, suggesting there is not a single type responsible for this increase [15].
The North Carolina Division of Public Health (NC DPH) performed a preliminary review of syphilis surveillance data for ocular manifestations and found an increase from 2014 to 2015, potentially out of proportion to the rise in all reported cases of syphilis. Several accounts of severe outcomes related to ocular syphilis, including blindness, were reported as well. In response, the NC DPH invited CDC to assist with systematically reviewing North Carolina's ocular syphilis cases during 2014–2015. The aims of the investigation were to: estimate the prevalence of ocular syphilis; describe clinical symptoms, diagnosis, and treatment; and to identify possible risk factors for ocular manifestations.
Methods
North Carolina providers are required by law to report all newly diagnosed cases of syphilis to local health departments. Once reported, health department staff verify treatment, collect syphilis-related signs and symptoms from the reporting provider, and attempt to interview each case to identify risk behaviors and sex partners at risk of transmitting syphilis. Health department staff enter all case report data into the North Carolina Electronic Disease Surveillance System (NC EDSS).
We reviewed cases in all stages of syphilis entered into NC EDSS; a patient meeting the syphilis surveillance case definition [16] with ocular symptoms not explained by another etiology was considered a case of ocular syphilis.
We identified 83 potential cases of ocular syphilis from 1 January 2014 to 31 December 2015 using 2 ascertainment methods (Figure 1). First, we identified cases where a checkbox indicated “eye/conjunctiva” was a site of infection. Second, we searched free-text fields from syphilis case investigation notes, using key words (eg, eye, vision, sight, or blind). The “eye/conjunctiva” checkbox was a standard component of the syphilis case report form before this investigation, but disease investigation specialists (DIS) were encouraged to ask all investigated syphilis cases about eye symptoms and utilize the checkbox in early 2015.
Figure 1.

Ocular syphilis case identification and follow-up interview participation—North Carolina, 2014–2015.
For all identified ocular syphilis cases, we reviewed data in NC EDSS and abstracted demographic information, syphilis testing, symptoms and treatment, sexual behavior, partner information, and other risk factor information. We also requested medical records from reporting providers and reviewed records for clinical visits associated with the ocular syphilis diagnosis to document additional symptoms, risk, treatment, and comorbidities. We characterized ocular syphilis cases and compared symptoms by human immunodeficiency virus (HIV) status. We estimated ocular syphilis prevalence among all syphilis cases reported to NC EDSS by demographic, behavioral, and clinical characteristics.
Follow-up Interviews
NC DIS from the Ocular Syphilis DIS Workgroup attempted to contact all ocular syphilis patients for a follow-up interview to assess symptoms and resolution, medical care, insurance status, and visual function. We assessed visual function using questions from the Visual Function Questionnaire (VFQ-25) [17]. We asked patients to rate overall visual function from blind to excellent. We also asked patients to rate the difficulty level of multiple vision-related tasks using a numeric scale from 1 (unable to perform activity because of eyesight) to 5 (no difficulty). Visual function questions were asked at 3 time points: before having eye/vision problems related to syphilis, when symptoms related to syphilis were at their worst, and at the time of the interview. At each time point, we calculated mean visual function score by activity.
We considered a severe eye diagnosis to be retinitis, optic neuritis, or retinal detachment. We defined recommended treatment as 10–14 days of intravenous (IV) aqueous penicillin G [18]. We defined vision loss as a follow-up questionnaire response indicating vision loss in either eye. We defined residual symptoms as reported visual function worse than baseline.
Statistical Analysis
We compared the distribution of demographic factors, syphilis characteristics, behavioral risk factors, clinical characteristics, and visual function using χ2 tests; Fisher exact P values were reported where cell sizes were <5. Associations between ocular syphilis and median rapid plasma reagin (RPR) titers were assessed using the Wilcoxon rank-sum test. We compared prevalence estimates using log binomial models to estimate prevalence ratios (PRs) and 95% confidence intervals (CIs). We considered an α value ≤.05 statistically significant. Data were analyzed using SAS version 9.3 software (Cary, North Carolina).
Results
In North Carolina, syphilis cases diagnosed in any stage increased 35%, from 1799 cases in 2014 to 2433 cases in 2015. A total of 63 ocular syphilis cases were identified: 21 in 2014 and 42 in 2015, a 100% increase. Ocular syphilis cases represented 1.5% of all reported syphilis cases in North Carolina during 2014–2015, and were distributed among 33 of 100 counties. No patients with ocular syphilis named another ocular syphilis patient as a sexual partner, and no other epidemiologic links were found between cases, such as travel or an institutionalized setting.
Most ocular syphilis patients were male (n = 59 [94%]); of those, 42 (71%) stated they had male sex partners (Table 1). The mean age was 45 years (range, 21–79 years). Primary or secondary syphilis was diagnosed in 24 (38%). Median RPR was 1:128 and varied slightly by stage of disease (primary or secondary syphilis = 1:256; all latent syphilis [early, late, and latent of unknown duration] = 1:128). A variety of syphilis symptoms were reported; a recent skin rash was most common. However, 25 (40%) patients reported no recent syphilis symptoms other than ocular symptoms. Forty-two patients (67%) received the recommended treatment for ocular syphilis, and 13 (21%) patients received intramuscular (IM) benzathine penicillin only. Among 38 ocular syphilis patients who had a lumbar puncture with cerebrospinal fluid (CSF) analysis, 24 (63%) had a reactive CSF Venereal Disease Research Laboratory test (VDRL).
Table 1. Demographic and Clinical Characteristics of Patients Diagnosed With Ocular Syphilis—North Carolina, 2014–2015 (n = 63).
| Characteristics | No. (%) |
|---|---|
| Male | 59 (94) |
| Known MSMa | 42 (71) |
| Race | |
| Black | 29 (46) |
| White | 32 (51) |
| Other | 2 (3) |
| Stage of syphilis | |
| Primary or secondary | 24 (38) |
| Early latent | 9 (14) |
| Late latent or latent of unknown durationb | 30 (48) |
| Syphilis symptoms | |
| Genital lesions | 3 (5) |
| Skin rash | 32 (51) |
| Alopecia | 4 (6) |
| Condyloma lata | 2 (3) |
| Ocular symptoms only | 25 (40) |
| HIV infected | 35 (56) |
| CSF analysis performed | 38 (60) |
| CSF VDRL reactivec | 24 (63) |
| Treatment | |
| Aqueous penicillin G IVd | 42 (67) |
| Benzathine penicillin IM only | 13 (21) |
| Other treatment | 5 (8) |
| No/unknown treatment | 3 (5) |
| Eye examination | |
| Consistent with ocular syphilis | 49 (78) |
| No eye examination documented | 14 (22) |
| Diagnosise | |
| Scleritis/keratitis | 1 (2) |
| Anterior uveitis | 13 (21) |
| Posterior uveitis | 8 (13) |
| Panuveitis | 17 (27) |
| Retinitis | 9 (14) |
| Optic neuritis | 5 (8) |
| Retinal detachment | 2 (3) |
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IM, intramuscular; I V, intravenous; MSM, men who have sex with men; VDRL, Venereal Disease Research Laboratory test.
Percentage calculated of males.
Includes late latent, late with clinical manifestations, and latent of unknown duration.
Percentage calculated of those with CSF analysis performed.
Patient could have received benzathine penicillin IM in addition to aqueous penicillin G IV.
Patient can be included in >1 category.
Overall, 49 patients (78%) had a documented eye examination consistent with ocular syphilis. Uveitis (anterior uveitis, posterior uveitis, or panuveitis) was the most common diagnosis documented in the eye examination. Fifteen patients received a severe eye diagnosis; 1 patient was diagnosed with retinitis and retinal detachment.
A total of 35 (56%) patients were HIV infected; 11 of whom (31%) were diagnosed with HIV at the same time as ocular syphilis. Similar proportions of HIV-infected and HIVuninfected ocular syphilis patients had a severe eye diagnosis, CSF analysis performed, or positive CSF VDRL. However, a larger proportion of HIV-infected patients with a CD4 count <350 cells/μL reported only ocular symptoms of syphilis (55.6%), compared with HIV-infected patients with a CD4 count ≥350 cells/μL (10%).
Factors Associated With Ocular Syphilis
Ocular syphilis was more prevalent in syphilis patients who were male (PR, 2.82; 95% CI, 1.03–7.73), white (PR, 2.63; 95% CI, 1.60–4.32), >40 years of age (PR, 3.27; 95% CI, 1.98–5.39), and HIV infected (PR, 1.78; 95% CI, 1.09–2.92) (Table 2). Patients diagnosed with ocular syphilis had a higher median RPR titer than patients with nonocular syphilis (1:128 vs 1:32, P < .0001). No behaviors were associated with ocular syphilis (eg, drug use, sex parties, meeting partners via internet sites or apps).
Table 2. Demographic and Other Characteristics Associated With Ocular Syphilis, North Carolina, 2014–2015.
| Characteristic | Total Syphilis Casesa (N = 4232) | Ocular Syphilis Cases (n = 63) | Prevalence of Ocular Syphilis, % | Prevalence Ratio (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Female | 677 | 4 | 0.6 | Ref |
| Male | 3542 | 59 | 1.7 | 2.82 (1.03–7.73) |
|
| ||||
| Race | ||||
| Black | 2674 | 29 | 1.1 | Ref |
| White | 1124 | 32 | 2.9 | 2.63 (1.60–4.32) |
| Other | 429 | 2 | 0.5 | 0.45 (.11–1.86) |
|
| ||||
| Age, y | ||||
| <40 | 2889 | 25 | 0.9 | Ref |
| ≥40 | 1343 | 38 | 2.8 | 3.27 (1.98–5.39) |
|
| ||||
| Year diagnosed | ||||
| 2014 | 1799 | 21 | 1.2 | Ref |
| 2015 | 2435 | 42 | 1.7 | 1.48 (.88–2.49) |
|
| ||||
| Reported MSM (among men only) | ||||
| No reported MSM behavior | 1170 | 22 | 1.9 | Ref |
| MSM | 2372 | 37 | 1.6 | 0.83 (.49–1.40) |
|
| ||||
| Previous history of syphilis | ||||
| No | 3549 | 56 | 1.6 | Ref |
| Yes | 683 | 7 | 1.0 | 0.65 (.30–1.42) |
|
| ||||
| Stage of syphilis | ||||
| Primary/secondary | 1757 | 24 | 1.4 | Ref |
| Early latent | 1083 | 9 | 0.8 | 0.61 (.28–1.30) |
| Late latent or latent of unknown durationb | 1392 | 30 | 2.2 | 1.58 (.93–2.69) |
|
| ||||
| HIV status | ||||
| HIV uninfected/unknown | 2488 | 28 | 1.1 | Ref |
| HIV infected | 1744 | 35 | 2.0 | 1.78 (1.09–2.92) |
Bolded prevalence ratios are considered statistically significant, where the confidence intervals do not include 1.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; MSM, men who have sex with men.
Numbers may not add to 4232 due to missing values.
Includes late latent, late with clinical manifestations, and latent of unknown duration.
Follow-up Interviews
Among the 63 ocular syphilis patients identified, 39 (62%) were interviewed (Figure 1). Median time from ocular syphilis diagnosis to interview was 10 months (range, 3–25 months). Among 39 patients who completed interviews, 27 (69%) had health insurance at the time of ocular syphilis diagnosis: 18 (67%) patients had private insurance, and 9 (33%) had government insurance. A total of 26 (67%) patients received care from an eye doctor, 7 of whom also received care elsewhere (ie, emergency room, primary care physician, sexually transmitted disease, HIV, or infectious disease clinic). An additional 12 (32%) patients did not receive care from an eye doctor, but did receive care at another location. One patient had no documented treatment, 8 (21%) received IM penicillin only, 28 (72%) received recommended IV penicillin, and 2 (5%) received other treatment (doxycycline or ceftriaxone).
The median number of days from onset of symptoms to initial healthcare visit was 14 days; median number of days from initial visit to initiation of syphilis treatment was 10 days. We did not detect any associations between age, race, HIV status, insurance, presenting symptoms, and location of care with number of days between symptom onset and initial healthcare visit. However, patients presenting only with ocular symptoms were more likely to be seen by an eye doctor (86.7% vs 54.2%; P = .04) and to have >10 days between seeking care and treatment initiation (55.6% vs 23.8%; P = .04), compared to those presenting with additional syphilis symptoms. The median time from care to initiation of treatment for patients who saw an eye doctor (including patients who also sought care at another location) was 13 days (range, 1–210 days); median time for patients who did not see an eye doctor was 7 days (range, 0–210 days).
Thirty-three (85%) patients reported good or excellent eyesight (with glasses or contact lenses, if needed) prior to ocular syphilis. When ocular symptoms were at their worst, only 5 (13%) patients reported good or excellent eyesight, and 4 (10%) reported blindness. At the time of the interview, 31 (80%) reported good or excellent eyesight; 1 (3%) patient reported blindness. A similar pattern was reported when patients were asked about visual impairment during daily activities such as driving, reading, and working, at the same 3 time points (Figure 2).
Figure 2.
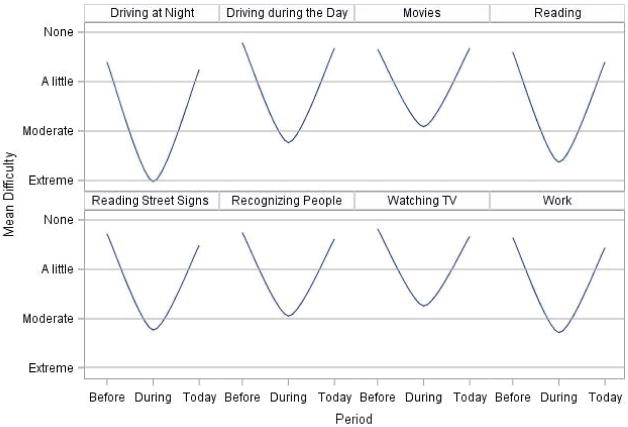
Mean visual function score by activity and time period—North Carolina, 2014–2015. “Before” was defined as prior to having eye or vision problems related to syphilis; “During” was defined as when syphilis-related eye or vision symptoms were at their worst; “Today” was defined as the time of the follow-up interview.
Twelve patients reported worse visual function at the time of the interview, compared with before ocular syphilis diagnosis. Patients with residual deficits had been diagnosed with panuveitis, anterior uveitis, chorioretinitis, and retinal detachment; none diagnosed with scleritis, keratitis, optic neuritis, or posterior uveitis alone had residual deficits. Age, race, HIV status, timing of care and treatment, and location of treatment were not associated with residual visual function deficits. However, a lack of more common syphilis-related symptoms was associated with residual deficits; of 15 patients presenting only with ocular symptoms, 6 (40%) developed residual deficits, compared to 6 of 24 (25%) who presented with additional syphilis-related symptoms. Five (42%) patients with residual deficits had health insurance at the time of ocular syphilis diagnosis; in comparison, 22 of 27 (81%) patients without residual disease were insured at the time of diagnosis (P = .01).
Discussion
In this in-depth analysis of ocular syphilis, we found an increase in ocular syphilis cases in North Carolina from 2014 to 2015, in the setting of increased awareness of ocular manifestations of syphilis. Ocular syphilis was diagnosed in all stages of syphilis and in both HIV-infected and HIV-uninfected patients. Although a subset of patients experienced severe symptoms, including vision loss, most patients recovered visual function following treatment.
The epidemiology of ocular syphilis in North Carolina is similar to that reported in previous investigations. The predominance of ocular syphilis in men, particularly men aged >40 years, is consistent with what has been reported recently in the United States [14], and elsewhere [6, 7]. The high proportion of cases in men and MSM is also consistent with the epidemiology of syphilis in the United States [1]. However, studies from the United Kingdom [6] and the Netherlands [7] found a lower proportion of ocular syphilis patients known to be HIV infected (31.7% and 35.9%, respectively) than our investigation.
The extent to which HIV infection influences the development or severity of ocular symptoms in ocular syphilis is unclear. This investigation found a higher prevalence of ocular syphilis among HIV-infected syphilis cases, compared to syphilis cases without HIV. However, in this investigation and another US-based investigation, only half of patients diagnosed with ocular syphilis were HIV infected [14]. In previous studies of ocular syphilis, HIV-infected patients were more likely to be diagnosed with posterior uveitis [4, 19], and have findings in follow-up suggesting possible treatment failure [20, 21]. More recent studies, however, have not found a relationship between HIV status and anatomical location of uveitis, CSF abnormalities, bilateral eye disease, presenting or final visual acuity, or reactive CSF VDRL [6, 7, 14]. While the presence of HIV infection alone might not determine ocular involvement, the degree of immunosuppression from HIV could modify ocular symptoms. CDC's 8-jurisdiction evaluation found that patients with a CD4 count <500 cells/μL were more likely to have a reactive CSF VDRL and bilateral ocular disease [14]. Although not statistically significant, this investigation found that, among HIV-infected ocular syphilis patients, those with a low CD4 count were more likely to present without other more common syphilis symptoms, compared to patients with a higher CD4 count.
Treatment of ocular syphilis is important for visual function improvement. In a report from the preantibiotic era, 9%–12% of patients with syphilitic iritis developed blindness [22]. In a retrospective review, worse posttreatment visual acuity was noted with a treatment delay of >12 weeks after symptom onset, while improved posttreatment visual acuity was observed in those treated with IV antibiotics [7]. A recent report of 6 cases described 2 patients with blindness after delayed treatment for ocular syphilis [5]. In our investigation, lack of other syphilis manifestations was associated with delayed treatment. In addition, patients who were assessed by an eye doctor had almost a week's delay in initiation of treatment. While we did not find this to be associated with vision loss or residual visual defects, other studies have found clinical implications associated with treatment delays [5, 7]. Provider education, especially for eye doctors who might not be accustomed to screening for syphilis, could potentially shorten delays in treatment.
We were unable to assess differences in outcome after treatment between patients who received IV penicillin and those who received IM penicillin only, because of small numbers and substantial differences between these populations; none of the patients who received only IM penicillin had a severe eye diagnosis or presented with vision loss.
Most patients reported health insurance at the time of diagnosis. Lack of health insurance coverage was associated with residual visual deficits. Although no difference was found in time to care or treatment by insurance status, it is possible that differences in healthcare access could have impacted outcomes. Furthermore, lack of health insurance could have resulted in a subset of persons with ocular syphilis remaining undiagnosed, and therefore not identified by our investigation.
This investigation had several limitations. First, awareness of ocular syphilis in North Carolina increased over the course of our investigation; DIS were encouraged to ask about ocular symptoms and utilize the “eye/conjunctiva” checkbox in 2015, potentially leading to an increase in case ascertainment in 2015, compared to 2014. If ocular symptoms were not uniformly elicited and recorded during routine syphilis investigations, our numbers could be an underestimate of the true ocular syphilis burden. This could also have led to misclassification of true ocular cases as nonocular syphilis cases, potentially biasing results of our comparisons. Additionally, patients presenting to a clinician with ocular symptoms who never received a syphilis diagnosis are missing from our analysis. With respect to patient interviews, follow-up time varied by patient and could have impacted symptom recall. Additionally, eyesight in follow-up interviews was based on self-reported visual function, not standardized examinations. Finally, we did not interview syphilis patients without ocular manifestations, limiting our ability to compare some characteristics (eg, healthcare access).
The increase in reports of ocular syphilis, in North Carolina and across the United States, may be due to increased recognition of ocular manifestations in the setting of increased syphilis cases, or a true increase in the proportion of syphilis cases with ocular disease. We could not assess trends over time, because systematically collected information on suspected ocular syphilis is not available before 2014 in North Carolina. Preliminary reports from North Carolina in 2016 show a stable or slight increase in the proportion of syphilis patients with ocular manifestations (data not shown). While this was one of the largest ocular syphilis investigations published to date, further investigation is needed to better understand the increase in reports of ocular syphilis, and to identify potential risk factors influencing development of ocular disease.
The number of ocular syphilis cases increased in North Carolina during 2014–2015, in the context of an increase in all syphilis diagnoses. Ocular manifestations of syphilis can have substantial impact on visual function, even causing vision loss. Patients with syphilis should be asked about symptoms of syphilis complications, including vision or neurologic changes. All patients diagnosed with syphilis who have ocular manifestations should immediately be treated for neurosyphilis with IV penicillin and referred for ophthalmologic examination. In addition, patients with inflammatory eye disease without a known cause should have an evaluation of syphilis, regardless of risk factors. Prompt identification of ocular syphilis, urgent ophthalmologic evaluation, and appropriate treatment are critical for management of ocular syphilis.
Acknowledgments
The authors thank the Ocular Syphilis DIS Workgroup: Jason Hall, Victor Hough, Andre Ivey, Stephanie Hawks, Samantha Greene, Dishonda Taylor, Mike Mercurio, and Miraida Gipson. We also thank Evelyn Foust, Vicki Mobley, Megan Davies, and the North Carolina Public Health epidemiologists, as well as Elissa Meites at the CDC.
Financial support. This work was supported jointly by the CDC and the North Carolina Division of Public Health.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveil-lance 2015. Atlanta, GA: US Department of Health and Human Services; 2016. [Google Scholar]
- 2.Kiss S, Damico FM, Young LH. Ocular manifestations and treatment of syphilis. Semin Ophthalmol. 2005;20:161–7. doi: 10.1080/08820530500232092. [DOI] [PubMed] [Google Scholar]
- 3.Spoor TC, Wynn P, Hartel WC, Bryan CS. Ocular syphilis. Acute and chronic. J Clin Neuroophthalmol. 1983;3:197–203. [PubMed] [Google Scholar]
- 4.Ormerod LD, Puklin JE, Sobel JD. Syphilitic posterior uveitis: correlative findings and significance. Clin Infect Dis. 2001;32:1661–73. doi: 10.1086/320766. [DOI] [PubMed] [Google Scholar]
- 5.Marx GE, Dhanireddy S, Marrazzo JM, et al. Variations in clinical presentation of ocular syphilis: case series reported from a growing epidemic in the United States. Sex Transm Dis. 2016;43:519–23. doi: 10.1097/OLQ.0000000000000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew RG, Goh BT, Westcott MC. British Ocular Syphilis Study (BOSS): 2-year national surveillance study of intraocular inflammation secondary to ocular syphilis. Invest Ophthalmol Vis Sci. 2014;55:5394–400. doi: 10.1167/iovs.14-14559. [DOI] [PubMed] [Google Scholar]
- 7.Bollemeijer JG, Wieringa WG, Missotten TO, et al. Clinical manifestations and outcome of syphilitic uveitis. Invest Ophthalmol Vis Sci. 2016;57:404–11. doi: 10.1167/iovs.15-17906. [DOI] [PubMed] [Google Scholar]
- 8.Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161–4. doi: 10.1097/00007435-198010000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hira SK, Patel JS, Bhat SG, Chilikima K, Mooney N. Clinical manifestations of secondary syphilis. Int J Dermatol. 1987;26:103–7. doi: 10.1111/j.1365-4362.1987.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Symptomatic early neurosyphilis among HIV-positive men who have sex with men—four cities, United States, January 2002–June 2004. MMWR Morb Mortal Wkly Rep. 2007;56:625–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Dombrowski JC, Pedersen R, Marra CM, Kerani RP, Golden MR. Prevalence estimates of complicated syphilis. Sex Transm Dis. 2015;42:702–4. doi: 10.1097/OLQ.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 12.Woolston S, Cohen SE, Fanfair RN, Lewis SC, Marra CM, Golden MR. A cluster of ocular syphilis cases—Seattle, Washington, and San Francisco, California, 2014-2015. MMWR Morb Mortal Wkly Rep. 2015;64:1150–1. doi: 10.15585/mmwr.mm6440a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. [Accessed February 15, 2016];Clinical advisory: ocular syphilis in the United States. Available at: http://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm.
- 14.Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65:1185–8. doi: 10.15585/mmwr.mm6543a2. [DOI] [PubMed] [Google Scholar]
- 15.Oliver S, Sahi SK, Tantalo LC, et al. Molecular typing of Treponema pallidum in ocular syphilis. Sex Transm Dis. 2016;43:524–7. doi: 10.1097/OLQ.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. [Accessed April 6, 2016];STD surveillance case definitions. 2011 Available at: http://www.cdc.gov/std/stats10/app-casedef.htm.
- 17.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute visual function questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 18.Workowski KA, Bolan GA Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 19.Villanueva AV, Sahouri MJ, Ormerod LD, Puklin JE, Reyes MP. Posterior uveitis in patients with positive serology for syphilis. Clin Infect Dis. 2000;30:479–85. doi: 10.1086/313689. [DOI] [PubMed] [Google Scholar]
- 20.Browning DJ. Posterior segment manifestations of active ocular syphilis, their response to a neurosyphilis regimen of penicillin therapy, and the influence of human immunodeficiency virus status on response. Ophthalmology. 2000;107:2015–23. doi: 10.1016/s0161-6420(00)00457-7. [DOI] [PubMed] [Google Scholar]
- 21.Li JZ, Tucker JD, Lobo AM, et al. Ocular syphilis among HIV-infected individuals. Clin Infect Dis. 2010;51:468–71. doi: 10.1086/654797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore JE. The modern treatment of syphilis. 2nd. Baltimore, MD: Charles C Thomas Books; 1943. [Google Scholar]


