Abstract
This study investigated the effects of dexmedetomidine on heart-type fatty acid binding protein (H-FABP), creatine kinase isoenzymes (CK-MB), and troponin I (cTnI) levels, neurological function and near-term prognosis in patients undergoing heart valve replacement. Patients undergoing heart valve replacement were randomly allocated to remifentanil anesthesia (control group, n=48) or dexmedetomidine anesthesia (observation group, n=48). Hemodynamic parameters were measured before anesthesia induction (T1), 1 min after intubation (T2), 10 min after start of surgery (T3), and on completion of surgery (T4). Levels of plasma H-FABP, CK-MB and cTnI were measured 10 min before anesthesia induction (C1), 10 min after start of surgery (C2), on completion of surgery (C3), 6 h after surgery (C4), and 24 h after surgery (C5). S100β protein and serum neuron-specific enolase (NSE) were detected 10 min before anesthesia induction (C1), and 24 h after surgery (C5). Neurological and cardiac function was evaluated 24 h after surgery. Incidence of cardiovascular adverse events was recorded for 1 year of follow-up. There were no significant differences in the average heart rate between the two groups during the perioperative period. The mean arterial pressure in the observation group was significantly lower than control group (P<0.05). Levels of H-FABP, CK-MB and cTnI at C2, C3, C4 and C5, were significantly higher than C1, but significantly lower in the observation versus control group (P<0.05). Twenty-four hours after surgery, levels of S100β and NSE in both groups were higher than those before induction (P<0.05), but significantly lower in the observation versus control group (P<0.05). Twenty-four hours after surgery, neurological function scores were better, and myocardial contractility and arrhythmia scores significantly lower in the observation versus control group (P<0.05 for all). After follow-up for 1 year, incidence of cardiovascular adverse events was significantly lower in the observation versus control group (P<0.05). Dexmedetomidine anesthesia can effectively maintain hemodynamic stability, reduce myocardial injury and the occurrence of cognitive dysfunction, and improve prognosis in patients undergoing heart valve replacement.
Keywords: dexmedetomidine, heart valve replacement, H-FABP, CK-MB, cTnI
Introduction
Heart valve disease is a common clinical heart disease, which involves the aortic valve, mitral valve, tricuspid valve and pulmonary valve; the main clinical manifestations are valve stenosis and insufficiency (1). Traditional cardiopulmonary bypass (CPB) is the main method of treating valvular heart disease. CPB represents a stress on the body, and intubation and sternotomy during surgery further enhances the stress response, resulting in hemodynamic fluctuations in patients, making them prone to myocardial ischemia and ischemia-reperfusion injury. This may lead to systemic inflammatory response syndrome, and multiple organ dysfunction, affecting the prognosis. Brain damage is one of the most serious potential complications after heart valve replacement surgery (2).
In the 1990s, non-CPB coronary artery bypass grafting, that is, heart off pump bypass surgery began to be applied clinically. This technique can help avoid damage caused by CPB, and is more conducive to the protection of heart and brain function and reducing postoperative complications (3). Heart-type fatty acid binding protein (H-FABP), creatine kinase myocardial isoenzyme (CK-MB), troponin I (cTnI) are markers that can reflect the degree of myocardial injury (4). S100β protein and serum neuron-specific enolase (NSE) are markers that can reflect the degree of brain injury and neurological impairment (5).
Anesthesia can also induce a stress response and inhibit immune function, while postoperative pain will prolong the hospital stay, increasing the economic burden. Dexmedetomidine (Dex) is a highly selective α2 adrenergic receptor agonist with analgesic, sedative and anti-sympathetic tonic effects (6). In this study, we examined the effects of performing Dex anesthesia on patients undergoing cardiac valve replacement on H-FABP, CK-MB, cTnI and neurological function of patients.
Materials and methods
Subjects
Ninety-six patients admitted to Shanxi Provincial People's Hospital from January 2015 to December 2015 for heart valve replacement surgery were selected. Inclusion criteria were New York Heart Society heart function grade II–III; non-CPB heart off-pump coronary artery bypass surgery opted for, with a certain degree of preoperative myocardial injury, and complete patient medical records; informed consent signed. Exclusion criteria were severe renal insufficiency, heart failure history and coagulation abnormalities; allergy to dextromethorphan. Patients were divided into observation group and control group according to random number table (both n=48). The studywas approved by the Ethics Committee of Shanxi Provincial People's Hospital and informed consents were signed by the patients and/or guardians.
Surgical treatment
Patients were fasted for 8 h before surgery, with blood pressure, heart rate (HR), oxygen saturation (SpO2) and Bipolar Spectrum Index (BIS) monitoring. All patients underwent non-CPB off-pump coronary artery bypass surgery.
The control group was treated with remifentanil anesthesia, using propofol (3 mg/kg) (Sichuan Guorui Pharmaceutical Co., Ltd., approval no. H20040079), remifentanil (0.3 µg/kg; Yichang Humanwell Pharmaceutical Co., Ltd., approval no. H20054171) and vecuronium bromide (0.1 mg/kg) (Beijing Mengjin Medical Technology Development Co., Ltd., approval no. H20063122) to induce anesthesia; tracheal intubation was performed when BIS was less than 55, then the patient was connected to the ventilator (respiratory rate: 12–15 times/min, suction ratio: 1:2, tidal volume: 8–9 ml/kg), and remifentanil was intermittently added to maintain the BIS value at 40–45, with remifentanil dosage adjusted according to BIS value, the floating range was ±30%.
The observation group were treated with Dex anesthesia, and the anesthesia induction was the same as that of the control group. Dex (0.5 µg/kg/h) was pumped after tracheal intubation. Intravenous injection of remifentanil (0.3 µg/kg) was performed every 3–5 min during surgery to maintain anesthesia, the pumping of Dex was stopped 30 min before the end of surgery, and tracheal extubation was performed when the patient's spontaneous breathing tidal volume reached 5 ml/kg, heartrate 20 beats/min, oxygen SpO2 ≥95% and maintained for more than 5 min.
Detection of related indicators
Immediately before induction (C1), 10 min after the start of surgery (C2), at the end of surgery (C3), 6 h after the end of surgery (C4), and 24 h after surgery (C5), 4 ml of central venous blood was taken, centrifuged and the supernatant isolated and stored at −70°C. The levels of H-FABP, CK-MB and cTnI were detected by double antibody one step sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems Inc., Minneapolis, MN, USA). OD value was read at the wavelength of 450 nm with the microplate reader (Jiangsu Potebio Biotechnology Co., Ltd.), and the concentration of H-FABP, CK-MB and cTnI was calculated.
Venous blood samples (4 ml) were taken from the jugular bulb immediately before and 24 h after anesthesia induction. The sample was centrifuged at 4°C and the supernatant stored at −70°C. The concentration of S100β and NSE was detected by solid sandwich ELISA method according to the manufacturer's instructions (reagents supplied by RB systems, USA). The OD value was read at a wavelength of 450 nm using a microplate reader and the concentration of S100β and NSE were calculated.
The hemodynamics of the two groups before anesthesia induction (T1), 1 min after intubation (T2), after 10 min of surgery (T3) and at the end of surgery (T4) were compared, including HR, and mean arterial pressure (MAP).
Changes in the concentration of H-FABP, CK-MB and cTnI were measured by enzyme-linked immunosorbent assay (ELISA) before the induction of anesthesia (C1), after 10 min of surgery (C2), immediately at the end of surgery (C3), 6 h after surgery (C4), and 24 h after surgery (C5). The levels of S100β and NSE were measured by ELISA before and 24 h after anesthesia induction.
One day before and 24 h after surgery, the cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA) scale and the Simple Mental State Examination Scale (MMSE). The MoCA scale was evaluated from the eight areas including space and the ability of implementation, memory, attention, naming, language, delayed memories, abstract thinking and orientation, with a total score of 30 points. A total of <26 points was judged as impaired cognitive function. MMSE criteria scored the orientation force, language, memory, attention and computing power. Scores were judged as mild cognitive dysfunction: 21–24 points, moderate cognitive dysfunction 11–20 points, severe cognitive dysfunction 0–10 points.
Myocardial contractility score formula 24 h after operation was (dopamine + dobutamine) × 1 + milrinone × 15 + (adrenaline + norepinephrine + isoproterenol) × 100 µg/kg/min; ventricular arrhythmia criteria was judged as 0 points: no arrhythmia occurred, 1 point: atrial arrhythmia or <10 pre-ventricular contractions, 2 points: ≥10 times ventricular contraction, 3 points: ventricular tachycardia attack 1–2 times, 4 points: ventricular tachycardia or ventricular tachycardia attack ≥3 times. Patients were followed up for 1 year, and the incidence of adverse cardiovascular events was observed, including cardiac arrest, arrhythmia, and heart functional failure.
Statistical analysis
Data were processed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA), measurement data were expressed as mean ± standard deviation, using t-test; enumeration data were expressed as a percentage, using χ2 test, and P<0.05 indicated statistical significance.
Results
Comparison of hemodynamics
There was no significant difference between the two groups in terms of baseline characteristics (P>0.05) (Table I). There was no significant difference in perioperative HR between the two groups (P>0.05). The MAP of the observation group was lower than that of the control group after anesthesia (P<0.05) (Table II).
Table I.
Baseline characteristics.
| Item | Control group n=48 | Observation group n=48 | t-value/χ2 | P-value |
|---|---|---|---|---|
| Sex (M/F) | 26/22 | 25/23 | 0.042 | 0.838 |
| Age (years) | 45–76 | 45–75 | ||
| Mean age (years) | 56.38±6.47 | 56.43±6.57 | 0.038 | 0.970 |
| BMI (Kg/m2) | 22.23±3.15 | 22.56±3.18 | 0.511 | 0.611 |
| NYHA heart function (n, %) | ||||
| Grade II | 18 (37.50) | 20 (41.67) | 0.174 | 0.676 |
| Grade III | 30 (62.50) | 28 (58.33) | ||
| Anesthesia (min) | 243.56±81.24 | 243.73±80.34 | 0.010 | 0.992 |
| Operation time (min) | 212.54±63.17 | 210.43±63.36 | 0.163 | 0.871 |
Table II.
Comparison of hemodynamic indicators at different time points.
| Indicator | Group | Case no. | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| HR (times) | Observation | 48 | 84.23±7.18 | 74.37±6.38 | 68.43±5.25 | 64.73±4.48 |
| Control | 48 | 85.06±7.15 | 75.02±6.57 | 67.94±5.17 | 65.06±4.72 | |
| MAP (mmHg) | Observation | 48 | 56.19±3.57 | 67.15±4.13a | 65.56±3.18a | 56.35±3.17a |
| Control | 48 | 57.02±3.68 | 79.32±4.24 | 75.18±3.24 | 59.68±3.45 |
P<0.05, compared with the control group.
Concentration changes of H-FABP, CK-MB, cTnI
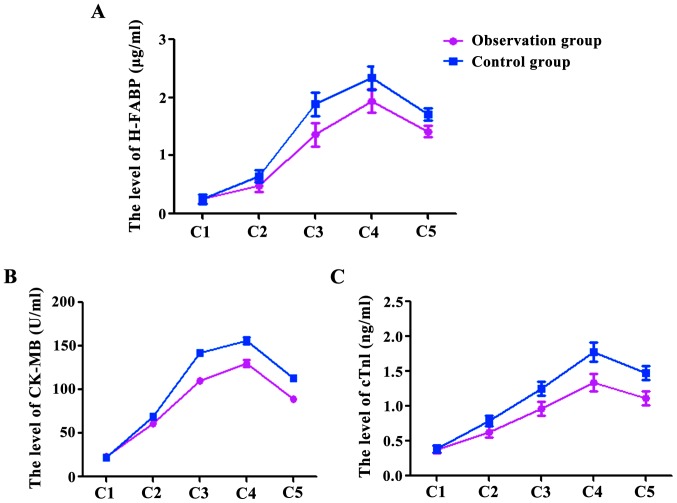
At the time points C1, C2, C3, C4, and C5, the levels of H-FABP were 0.26±0.06, 0.48±0.13, 1.36±0.27, 1.94±0.23, 1.42±0.37 µg/ml in the observation group, and were 0.25±0.04, 0.65±0.14, 1.89±0.26, 2.34±0.25, 1.71±0.45 µg/ml in the control group (Fig. 1A). The levels of CK-MB in the observation group were 22.78±1.56, 60.68±2.53, 110.36±2.47, 130.64±2.13, 89.52±2.37 U/ml, and in the control group were 22.44±1.34, 69.63±2.74, 141.75±3.26, 156.64±3.25, 112.73±3.45 U/ml (Fig. 1B). The levels of cTnI in the observation group were 0.38±0.06, 0.63±0.13, 0.96±0.17, 1.34±0.13, 1.12±0.27 ng/ml, and in the control group 0.39±0.08, 0.79±0.14, 1.25±0.26, 1.78±0.25, 1.48±0.25 ng/ml (Fig. 1C). The levels of H-FABP, CK-MB and cTnI at C2, C3, C4 and C5 were significantly higher than at C1 in both groups (P<0.05), however, the levels in the observation group at C2, C3, C4 and C5 were significantly lower than those in the control group (P<0.05).
Figure 1.
The levels of H-FABP (A), CK-MB (B) and cTnI (C) at C2, C3, C4 and C5 were significantly higher than at C1, P<0.05, and there was no significant difference between the two groups at C1, P>0.05; at C2, C3, C4 and C5, the observation group was significantly lower than the control group, P<0.05.
The levels of S100β and NSE in the two groups
The levels of S100β and NSE after surgery were significantly higher than those before surgery (P<0.05). The levels of S100β and NSE in the observation group were significantly lower than those in the control group (P<0.05) (Table III).
Table III.
Comparison of S100β and NSE concentrations in both groups.
| S100β (pg/ml) | NSE (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | 1 day before surgery | 24 h after surgery | t-value | P-value | 1 day before surgery | 24 h after surgery | t-value | P-value |
| Observation | 103.24±5.13 | 326.45±8.29 | 156.632 | <0.001 | 12.42±1.32 | 18.12±1.73 | 18.148 | <0.001 |
| Control | 104.73±6.24 | 375.27±12.38 | 135.199 | <0.001 | 12.78±1.23 | 23.36±1.84 | 33.119 | <0.001 |
| t-value | 1.280 | 22.701 | 1.382 | 14.374 | ||||
| P-value | 0.204 | <0.001 | 0.170 | <0.001 | ||||
The MoCA and MMSE scores of the two groups
The MoCA and MMSE scored in the two groups after surgery were lower than those before surgery, and the decrease in scores in the observation group was lower than that of the control group, P<0.05 (Table IV).
Table IV.
Comparison of MoCA and MMSE scores in both groups.
| MoCA | MMSE | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | 1 day before surgery | 24 h after surgery | t-value | P-value | 1 day before surgery | 24 h after surgery | t-value | P-value |
| Observation | 28.24±1.33 | 25.15±1.39 | 11.128 | <0.001 | 30.62±3.12 | 23.12±2.43 | 13.139 | <0.001 |
| Control | 28.53±1.64 | 26.37±1.38 | 6.982 | <0.001 | 29.98±2.13 | 26.26±3.23 | 6.661 | <0.001 |
| t-value | 0.952 | 4.315 | 1.174 | 5.382 | ||||
| P-value | 0.344 | <0.001 | 0.243 | <0.001 | ||||
Cardiac parameters
The myocardial contractility and cardiac arrhythmias scores in the observation group were significantly lower than those of the control group 24 h after surgery (P<0.05); after 1-year follow-up, the incidence of adverse events observed in the observation group was significantly lower than in the control group (P<0.05) (Table V).
Table V.
Comparison of myocardial contractility and arrhythmia scores and cardiovascular adverse event incidence.
| Group | N | Myocardial contractility score | Arrhythmias score | Cardiovascular adverse event incidence (n, %) |
|---|---|---|---|---|
| Observation | 48 | 2.53±0.78 | 4.24±1.42 | 6 (12.50) |
| Control | 48 | 3.74±1.43 | 6.37±2.68 | 20 (41.67) |
| t-value/χ2 | 5.147 | 4.866 | 10.338 | |
| P-value | <0.001 | <0.001 | 0.001 |
Discussion
The incidence of neurological complications of heart valve replacement surgery is higher than other surgeries, and the degree of postoperative neurological impairment of patients is more severe than other heart surgeries. Possible reasons for this are the formation of brain micro-thrombi during surgery, the strong stimulation of the operation, hypoxia due to low perfusion, ischemia and systemic inflammatory responses (7). Related studies have shown that the incidence of postoperative cognitive dysfunction (POCD) after heart valve replacement can be as high as 69% (8). Heart valve replacement surgery can cause damage to the myocardium, and different methods of anesthesia can lead to certain stress responses during the perioperative period, which may have some influence on the surgical outcomes. It has recently become a clinical focus to define strategies to reduce the neurological impairment and myocardial damage after heart valve replacement, and how to preserve heart and brain function and improve the success rates of surgery and prognosis (9).
Both tracheal intubation and the surgical procedure in the perioperative period of heart valve replacement can result in stress responses that cause HR acceleration and elevated blood pressure (10). Studies have shown that Dex activates the α2 receptor in the medullary dorsal motor neuron complex, thereby reducing blood pressure (11). The results of this study showed that there was no significant difference in perioperative HR between the two groups (P>0.05). MAP in the observation group was lower than that in the control group (P<0.05) after anesthesia, which was consistent with the results of related studies. Various anesthetic methods can bring varying degrees of stress response, resulting in the increased release of glucagon, catecholamines and norepinephrine and other secretions, therefore exciting the sympathetic nervous system, causing neuroendocrine system changes and hemodynamic instability (12). Dex can inhibit a variety of stress responses, maintaining hemodynamic stability. Dex has both analgesic and sedative effects, inhibits the release of norepinephrine, reduces the tension of the sympathetic nervous system, reduces the increased blood pressure response during surgery, and regulates blood pressure in the recovery period (13).
H-FABP is a low molecular weight cytosolic protein present in cardiomyocytes, which is commonly used as an early indicator of myocardial injury. Usually in early myocardial injury H-FABP quickly leaks from the cardiomyocytes, and is released into the peripheral blood, entering into the energy metabolism system by binding with long-chain fatty acid, and providing energy for the myocardium; its release is positively correlated with the level of myocardial injury (14). CK-MB is composed of the four isomers of creatine kinase, which is related to muscle contraction and intracellular energy transport. It is clinically used as an indicator of myocardial injury, and its concentration level is positively correlated with the degree of myocardial injury (15).
CTnI is an inhibitory protein in the troponin-protoelin-modulating complex that regulates the interaction between myofibrin and myosin and can inhibit muscle contractions, and is rapidly released into the peripheral blood during myocardial ischemia and reaches a peak in a few hours (16). The results of this study showed that H-FABP, CK-MB and cTnI were significantly increased in the two groups at the beginning of surgery for 10 min, at the end of surgery, 6 and 24 h after surgery, however, the increases occurring in the observation group were significantly lower than those in the control group (P<0.05). This may be due to the fact that Dex can directly act on ischemic myocardium, reducing the level of coronary norepinephrine, and reducing coronary blood flow by activating the α2 receptor, triggering ischemic preconditioning signals at all levels, and thereby playing a cardio-protective role (17). In addition, Dex can inhibit the release of catecholamines, thereby changing the balance of myocardial oxygen supply and demand, resulting in protection of the myocardium (17).
S100β is an acidic calcium binding protein that exists in glial cells and is a glial cell marker (18). Under normal conditions S100β is metabolized by the kidney without passing through the blood-cerebrospinal fluid barrier, but when brain injury occurs, the blood-brain barrier is compromised, and permeability increases. Therefore, when S100β enters the blood circulation through the blood-brain barrier, S100β concentration level can be used as a diagnostic indicator of brain injury, and is directly related to the recovery of neurological function in patients after surgery (18). NSE is a dimeric enzyme often present in the neuronal cytoplasm, and when neuronal damage occurs, it is released into the extracellular fluid (19). The results of this study showed that levels of S100β and NSE were higher after surgery before, but the levels of S100β and NSE in the observation group were significantly lower than in the control group (P<0.05), indicating that different degrees of brain injury occurred in both groups. The lower levels of S100β and NSE in the observation group is likely due to the use of Dex anesthesia, which reduces cerebral oxygen uptake rate, promoting cerebral oxygen supply and demand balance, and thereby reducing oxidative stress injury (20); Dex anesthesia may also inhibit neuronal discharge, resulting in anti-sympathetic nerve effect and reduced neurotoxicity, reducing the damage to neurons, and thereby playing a neuro-protective role (20).
MoCA and MMSE scores in the both groups 1 day after surgery were significantly decreased, with the scores of the control group significantly lower than those of the observation group. Twenty-four hours after surgery, the myocardial contractility and arrhythmia scores of the observation group were significantly lower than those of the control group (P<0.05). After one year of follow-up, the incidence of cardiovascular adverse event in the observation group was significantly lower than in the control group (P<0.05), and this may be due to patient discomfort after surgery, excessive tension of the sympathetic nervous system, and inflammatory reactions leading to POCD in the control group. Dex has anti-inflammatory effects, which can inhibit the production of inflammatory factors, thereby reducing the damage to the nervous system, improving the level of acetylcholine, reducing cognitive impairment and protecting the brain (20). Dex can also reduce the surgical stress response, inhibit apoptosis, reduce myocardial ischemia and reperfusion injury, therefore reducing intraoperative and postoperative ventricular arrhythmia (21).
In conclusion, Dex can maintain perioperative hemodynamic stability of patients undergoing heart valve replacement surgery and reduce myocardial and brain damage, playing a protective role on the heart and the brain, which is conducive to a better prognosis.
References
- 1.Cavero I, Guillon JM. Safety Pharmacology assessment of drugs with biased 5-HT(2B) receptor agonism mediating cardiac valvulopathy. J Pharmacol Toxicol Methods. 2014;69:150–161. doi: 10.1016/j.vascn.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Nombela-Franco L, Eltchaninoff H, Zahn R, Testa L, Leon MB, Trillo-Nouche R, D'Onofrio A, Smith CR, Webb J, Bleiziffer S, et al. Clinical impact and evolution of mitral regurgitation following transcatheter aortic valve replacement: A meta-analysis. Heart. 2015;101:1395–1405. doi: 10.1136/heartjnl-2014-307120. [DOI] [PubMed] [Google Scholar]
- 3.Gurbuz O, Kumtepe G, Yolgosteren A, Ozkan H, Karal IH, Ercan A, Ener S. A comparison of off- and on-pump beating-heart coronary artery bypass surgery on long-term cardiovascular events. Cardiovasc J Afr. 2016;27:1. doi: 10.5830/CVJA-2016-049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willemsen RT, Buntinx F, Winkens B, Glatz JF, Dinant GJ. ‘RAPIDA’-study team: The value of signs, symptoms and plasma heart-type fatty acid-binding protein (H-FABP) in evaluating patients presenting with symptoms possibly matching acute coronary syndrome: Background and methods of a diagnostic study in primary care. BMC Fam Pract. 2014;15:203. doi: 10.1186/s12875-014-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patro N, Naik A, Patro IK. Differential temporal expression of S100β in developing rat brain. Front Cell Neurosci. 2015;9:87–99. doi: 10.3389/fncel.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Tomasi R, Dossow-Hanfstingl VV. Analgesie-, sedierungs- und delir- management auf der intensivstation. Intensiv- und Notfallbehandlung. 2015;39:167–172. doi: 10.5414/IBX00412. (In German) [DOI] [Google Scholar]
- 7.Whitlock RP, Devereaux PJ, Teoh KH, Lamy A, Vincent J, Pogue J, Paparella D, Sessler DI, Karthikeyan G, Villar JC, et al. SIRS Investigators: Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): A randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:1243–1253. doi: 10.1016/S0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 8.Sigaut S, Tremey B, Ouattara A, Couturier R, Taberlet C, Grassin-Delyle S, Dreyfus JF, Schlumberger S, Fischler M. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2014;120:590–600. doi: 10.1097/ALN.0b013e3182a443e8. [DOI] [PubMed] [Google Scholar]
- 9.Howard BT, Iles TL, Coles JA, Sigg DC, Iaizzo PA. Handbook of Cardiac Anatomy, Physiology, and Devices. 3rd edition. Springer International Publishing; 2015. Reversible and irreversible damage of the myocardium: Ischemia/reperfusion injury and cardioprotection; pp. 279–293. [DOI] [Google Scholar]
- 10.Green JS, Tsui BC. Impact of anesthesia for cancer surgery: Continuing Professional Development. Can J Anaesth. 2013;60:1248–1269. doi: 10.1007/s12630-013-0037-1. [DOI] [PubMed] [Google Scholar]
- 11.Kiliç K, Hanci V, Selek S, Sözmen M, Kiliç N, Citil M, Yurtlu DA, Yurtlu BS. The effects of dexmedetomidine on mesenteric arterial occlusion-associated gut ischemia and reperfusion-induced gut and kidney injury in rabbits. J Surg Res. 2012;178:223–232. doi: 10.1016/j.jss.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 12.Jung SM, Cho CK. The effects of deep and light propofol anesthesia on stress response in patients undergoing open lung surgery: A randomized controlled trial. Korean J Anesthesiol. 2015;68:224–231. doi: 10.4097/kjae.2015.68.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell MT, Agoston VA, Freeman KA, Puskas F, Herson PS, Mares J, Fullerton DA, Reece TB. Interruption of spinal cord microglial signaling by alpha-2 agonist dexmedetomidine in a murine model of delayed paraplegia. J Vasc Surg. 2014;59:1090–1097. doi: 10.1016/j.jvs.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 14.Glatz JF, Renneberg R. Added value of H-FABP as plasma biomarker for the early evaluation of suspected acute coronary syndrome. Clin Lipidol. 2014;9:205–220. doi: 10.2217/clp.13.87. [DOI] [Google Scholar]
- 15.Banning A, Musumeci F, Penny W, Tovey JA. Reference intervals for cardiac troponin T, creatine kinase and creatine kinase-MB isoenzyme following coronary bypass graft surgery. Ann Clin Biochem. 1996;33:561–562. doi: 10.1177/000456329603300613. [DOI] [PubMed] [Google Scholar]
- 16.Sandoval Y, Smith SW, Schulz KM, Murakami MM, Love SA, Nicholson J, Apple FS. Diagnosis of type 1 and type 2 myocardial infarction using a high-sensitivity cardiac troponin I assay with sex-specific 99th percentiles based on the third universal definition of myocardial infarction classification system. Clin Chem. 2015;61:657–663. doi: 10.1373/clinchem.2014.236638. [DOI] [PubMed] [Google Scholar]
- 17.Funai Y, Pickering AE, Uta D, Nishikawa K, Mori T, Asada A, Imoto K, Furue H. Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: An in vivo patch-clamp analysis of analgesic mechanisms. Pain. 2014;155:617–628. doi: 10.1016/j.pain.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeifer R, Franz M, Figulla HR. Hypothermia after cardiac arrest does not affect serum levels of neuron-specific enolase and protein S-100b. Acta Anaesthesiol Scand. 2014;58:1093–1100. doi: 10.1111/aas.12386. [DOI] [PubMed] [Google Scholar]
- 19.Benedict C, Cedernaes J, Giedraitis V, Nilsson EK, Hogenkamp PS, Vågesjö E, Massena S, Pettersson U, Christoffersson G, Phillipson M, et al. Acute sleep deprivation increases serum levels of neuron-specific enolase (NSE) and S100 calcium binding protein B (S-100B) in healthy young men. Sleep. 2014;37:195–198. doi: 10.5665/sleep.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun L, Guo R, Sun L. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: A meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand. 2014;58:642–650. doi: 10.1111/aas.12292. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Hua F, Lu J, Jiang Y, Tang Y, Tao L, Zou B, Wu Q. Effect of dexmedetomidine on myocardial ischemia-reperfusion injury. Int J Clin Exp Med. 2015;8:21166–21172. [PMC free article] [PubMed] [Google Scholar]