Abstract
Activation of protein C is greatly enhanced by the presence of thrombomodulin (TM) and endothelial protein C receptor (EPCR) on the endothelial surface. Impairment of the anticoagulant protein C system occurs during endotoxemia and contributes to sepsis-associated hypercoagulability. Previous studies have demonstrated that unfractionated heparin (UFH) can attenuate coagulation in endotoxemic mice. However, whether UFH has an effect on the protein C system remains to be elucidated. The current study evaluated the therapeutic effect of UFH on the protein C system in a mouse model of lipopolysaccharide (LPS)-induced sepsis, and further investigated the effect of UFH on the expression of TM and EPCR in vitro using human umbilical vein endothelial cells (HUVECs). The in vivo data indicated that UFH preconditioning attenuated the decline in circulating activated protein C following LPS administration, and also reduced LPS-induced shedding of TM and EPCR. In HUVECs, LPS stimulation led to the downregulation of TM and EPCR expression, and UFH dose-dependently restored the mRNA and protein levels of TM and EPCR. In addition, UFH inhibited the LPS-induced activation of mitogen-activated protein kinase 14, proto-oncogene tyrosine-protein kinase Src and nuclear factor κB signaling in HUVECs. In summary, these results suggest that UFH has a protective effect on the protein C system during sepsis. Thus, UFH may be a candidate therapeutic agent for the treatment of patients with sepsis.
Keywords: unfractionated heparin, protein C, thrombomodulin, endothelial protein C receptor, lipopolysaccharide, sepsis
Introduction
Sepsis is a clinical condition that results from an overwhelming systemic host response to infection (1). Under certain circumstances, components of the innate immune system that are responsible for the host's defense against invading pathogens can cause damage to the host's tissues and organs, resulting in multiple organ failure, a hallmark of sepsis (2). Previously, studies have been conducted on the extensive crosstalk between inflammation and coagulation during sepsis (3). Systemic inflammation leads to activation of the coagulation system with concurrent inhibition of anticoagulant mechanisms; coagulation in turn exacerbates the inflammatory response (4). The protein C pathway is an important pathway in the crosstalk between coagulation and inflammation during sepsis (5). In normal situations, circulating protein C is cleaved and activated by thrombin to generate activated protein C (APC) that regulates blood coagulation. The endothelial cell layer provides an anticoagulant surface by expressing thrombomodulin (TM) and endothelial protein C receptor (EPCR), which support thrombin in generating APC (6,7). However, the protein C system is impaired during sepsis; APC is rapidly consumed during coagulation, while TM and EPCR are cleaved from the endothelial surface by proteases, including neutrophil elastase (8) and metalloproteases (9,10). Thus, a rapid drop in circulating APC, and concurrent rises in the serum levels of soluble TM (sTM) and soluble EPCR (sEPCR), are commonly observed in sepsis.
Previous studies have introduced human recombinant APC to replenish APC levels in order to reduce the effects of severe sepsis (11–13). However, the clinical trials performed discovered no evidence suggesting the effectiveness of recombinant APC for treating patients with severe sepsis or septic shock, and these trials have recently been discontinued (14). Comparatively, a number of in vitro and in vivo studies have suggested a potentially life-saving effect of heparin in the treatment of sepsis due to its beneficial anti-inflammatory actions (15–18). Additionally, treatment with unfractionated heparin (UFH) can also attenuate coagulation in endotoxemic mice (19). A previous study by our group reported that UFH inhibited inflammatory responses in endothelial cells and protected endothelial barrier integrity in in vitro models of lipopolysaccharide (LPS)-induced sepsis (20–22). In the present study, the effect of UFH on the protein C system in a mouse model of LPS-induced sepsis, as well as in human umbilical vein endothelial cells (HUVECs) with LPS challenge, was investigated.
Materials and methods
Mouse model of LPS-induced sepsis
Male C57BL/6J mice that were 10–12 weeks old and weighed 20–25 g were provided by the Animal Center of China Medical University (Shenyang, China). The mice were housed in the animal center under standard conditions (23±2°C, 65±5% humidity, 12 h light/dark cycle) with access to food and water ad libitum. The mice were randomly assigned into three groups as follows: Control, LPS and LPS + UFH, (12 mice/group). To induce sepsis, LPS derived from Escherichia coli 055:B5 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was administered via an intraperitoneal injection at a dose of 20 mg/kg body weight, while the control mice were administered an intraperitoneal injection of 100 µl saline. UFH with a mean molecular weight of 12,000 Da (Shanghai no. 1 Biochemical and Pharmaceutical Co., Ltd., Shanghai, China) was administered via the tail vein (5 U/20 g of body weight) 30 min prior to LPS administration, and the other two groups received an equal amount of saline.
The animal care and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (23), and the protocol was approved by the Institutional Animal Care and Use Committee of China Medical University.
Histopathology
A total of 6 h after LPS administration, 4 mice from each group were sacrificed. The lung, liver and kidneys were removed, fixed in 4% paraformaldehyde and embedded in paraffin. The paraffin blocks were cut into 5-µm-thick sections, and the sections were stained with hematoxylin for 5 min at room temperature and then with eosin for 3 min at room temperature. Tissue sections were observed under an optical microscope.
ELISA
Blood was sampled from the posterior orbital venous plexus of the mice at 3, 6 and 12 h post LPS administration. Serum levels of APC, sTM and sEPCR were measured using the respective commercial ELISA kits (APC, cat. no. SEA738Mu; sTM, cat. no. SEA529Mu; protein C receptor, cat. no. SEA022Mu; USCN Life Sciences, Inc., Wuhan, China) according to the manufacturer's protocol.
Cell culture
Primary HUVECs were obtained from American Type Culture Collection (Manassas, VA, USA) cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 µg/ml streptomycin, 100 U/ml penicillin and 2 mM L-glutamine. The cells were cultured in a humidified incubator at 37°C with 5% CO2. The cells were pretreated with 0.1, 1 or 10 U/ml UFH for 15 min, followed by treatment with 10 µg/ml LPS for 2, 6, 12, 24 or 48 h. Untreated cells were used as a control.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
HUVECs were harvested at 2, 6, 12 and 24 h after LPS induction, and total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. RT was performed at 40°C for 45 min in a reaction mix (10 µl total volume) consisting of 1 µl total RNA, 2.0 µl 5X PrimeScript™ Buffer, 0.5 µl recombinant RNase inhibitor, 0.5 µl dNTPs, 0.5 µl PrimeScript™ RTase (Takara Bio, Inc., Otsu, Japan), 0.5 µl random primers and 1 µl oligo (dT). qPCR for TM and EPCR was performed using SYBR-Green Master mix (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) with the following primers: TM forward, 5′-CTGCCGATGTCATTTCCTTGC-3′ and reverse, 5′-GCTGGTGTTGTTGTCTCCCGTA-3′; EPCR forward, 5′-GAGGCTGGCAAGGGAAAGT-3′ and reverse, 5′-GCAGATGTGGGAGAAGAAAG-3′; β-actin forward, 5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse, 5′-CTGTCACCTTCACCGTTCCAGTTT−3′. The thermocycling conditions for amplification were as follows: Initial denaturation at 95°C for 10 min, followed by 35 cycles of denaturation at 95°C for 10 sec and annealing/extension at 60°C for 30 sec. The housekeeping gene β-actin was used for normalization. Quantification of gene expression was performed using the 2−ΔΔCq method (24), and was expressed relative to the control group.
Western blot analysis
Proteins were extracted from HUVECs at 6, 12, 24 and 48 h post LPS induction to examine the expression of TM and EPCR, and the activation of signaling molecules was assessed 6 h after LPS induction. Whole cell lysates, nuclear extracts and cytosolic extracts were prepared as previously described (20). Protein concentration was determined using the Bradford assay. Equal amounts of protein (40 µg) were loaded into each lane, separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked at room temperature for 1 h with 5% non-fat milk solution in TBS, 50 mM Tris-HCl (pH 7.5) and 150 mM NaCl containing 0.1% Tween-20. The membranes were then incubated overnight at 4°C with primary antibodies directed against the following proteins: TM (cat. no. bs-20395R), EPCR (cat. no. bs-9506R), mitogen-activated protein kinase 14 (p38 MAPK; cat. no. bs-0637R), phosphorylated (p)-p38 MAPK (cat. no bs-5477R), proto-oncogene tyrosine-protein kinase Src (Src; cat. no. bs-10604R), p-Src (cat. no. bs-7619R), p-nuclear factor (NF)-κB inhibitor-α (IκBα; cat. no bs-1287R; all 1:500; all BIOSS, Beijing, China) or NF-κB p65 subunit (cat. no. BA0610; 1:400; Wuhan Boster Biological Technology, Wuhan, China). Following incubation with horseradish peroxidase-conjugated goat anti-rabbit (cat. no. A0208) or goat anti-mouse antibodies (cat. no. A0216) (both 1:5,000; both Beyotime Institute of Biotechnology, Haimen, China) for 45 min at room temperature, chemiluminescent detection was performed using an enhanced chemiluminescent reagent (7Sea-ECL; 7Sea Biotech, Shanghai, China) according the manufacturer's protocol. To verify equal loading and transfer, the membranes were stripped, and then re-blotted overnight at 4°C with anti-β-actin (1:1,000; cat. no. sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies for total and cytosolic proteins, or with anti-histone H3 (1:1,000; cat. no. bsm-33042M; BIOSS) antibodies for nuclear proteins. For quantification of the target proteins, the intensity of the bands was measured using ImageJ software (version 1.6.0; National Institutes of Health, Bethesda, MD, USA). The protein levels were presented as the ratio of the target protein to the respective internal reference protein (β-actin or H3), and then normalized to the control group.
Statistical analysis
The data are expressed as the mean ± standard deviation among the individuals in each group for the in vivo assays or of three independent experiments for the in vitro assays. Differences between multiple groups were assessed by one-way analysis of variance with a post hoc Bonferroni correction test. The statistical analyses were performed using GraphPad Prism 5.0 (GraphPad software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
UFH preconditioning attenuates LPS-induced damage of the APC system in a mouse model
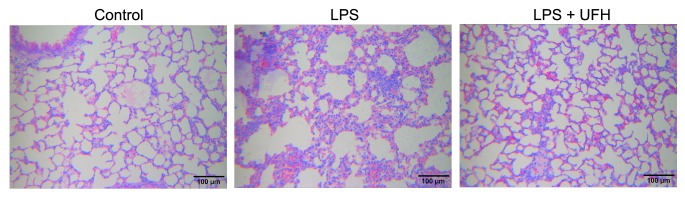
Previous studies have demonstrated that UFH could rescue sepsis-associated acute lung injury and lethality in LPS-induced rodents (17,25). In the present study, LPS was administered to the mice with or without UFH preconditioning, and histopathological examinations of the lung, liver and kidneys were conducted 6 h after LPS induction. As shown in Fig. 1, administration of LPS led to disruption of alveolar structure and integrity, as well as the infiltration of leukocytes into the pulmonary tissues. In the mice pretreated with UFH, the alveolar structure was preserved with markedly reduced leukocyte infiltration compared to the LPS-induced mice. No notable pathological changes were observed in the liver or kidney 6 h post LPS induction (data not shown). These results were consistent with those of previous studies, which demonstrated that UFH attenuated LPS-induced acute lung injury in mice, thus indicating the successful establishment of the animal model.
Figure 1.
UFH alleviates LPS-induced acute lung injury in mice. Mice received an intraperitoneal injection of LPS with or without pretreatment with UFH. The mice were sacrificed 6 h post LPS administration, and the lung sections were subjected to hematoxylin and eosin staining for pathological examination. Representative images of each group (n=4/group) are shown. Magnification, ×200. UFH, unfractionated heparin; LPS, lipopolysaccharide.
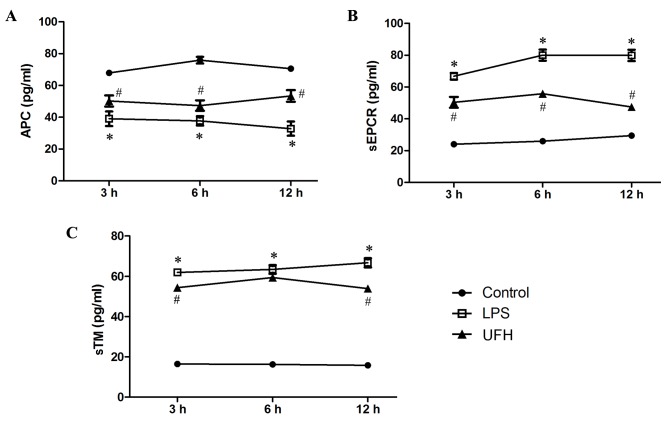
Levels of APC, sEPCR and sTM in the serum of LPS-induced mice were measured during early sepsis. Serum APC dropped significantly in LPS-treated mice (39.06±4.58 pg/ml) compared with the control mice (67.88±0.51 pg/ml) at 3 h post LPS induction, and the level slowly declined with time (37.72±2.95 pg/ml at 6 h; 32.81±4.45 pg/ml at 12 h) (Fig. 2A). Serum APC was significantly increased at all time points in the mice that received UFH preconditioning compared with LPS-treated mice (50.22±3.47 pg/ml at 3 h; 47.32±3.31 pg/ml at 6 h; 53.35±3.67 pg/ml at 12 h). Furthermore, serum levels of sEPCR and sTM experienced >3-fold increases following LPS induction (Fig. 2B and C). UFH preconditioning reduced LPS-induced elevation of serum sEPCR by 38.41–62.48% during the early response (3, 6 and 12 h), while it attenuated LPS-induced elevation of serum sTM to a lesser extent (8.53–25.15%). These results demonstrate that UFH preconditioning prevented the exhaustion of circulating APC, and inhibited the shedding of EPCR and TM, following LPS induction.
Figure 2.
UFH reduces APC depletion and the shedding of EPCR and TM following LPS induction. Serum levels of (A) APC, (B) sEPCR and (C) sTM were measured by ELISA at 3, 6 and 12 h post LPS administration (n=4/group). *P<0.05 vs. the control group; #P<0.05 vs. the LPS group. UFH, unfractionated heparin; APC, activated protein c; sEPCR, soluble endothelial protein C receptor; sTM, soluble thrombomodulin; LPS, lipopolysaccharide.
LPS-induced downregulation of EPCR and TM expression is inhibited by UFH in HUVECs
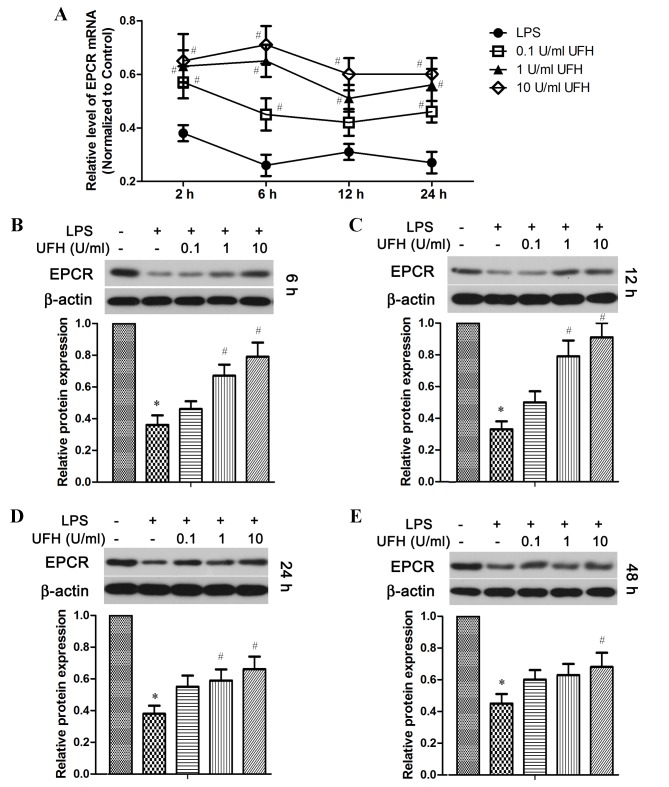
The activation of protein C is enhanced by the presence of EPCR and TM on the endothelial surface (6,7). As considerable shedding of EPCR and TM was observed following LPS induction in the mouse model, it was then investigated whether endothelial cells could regenerate and replenish these two molecules during sepsis, and whether UFH had an effect on the expression of these two molecules in endothelial cells (Figs. 3 and 4). RT-qPCR analysis revealed that the level of EPCR mRNA was reduced by ~70% in LPS-treated HUVECs compared with the control cells, whereas pretreatment with UFH significantly reduced LPS-induced downregulation of EPCR mRNA expression in a dose-dependent manner compared with LPS-treated cells (Fig. 3A). At the protein level, EPCR was significantly decreased in LPS-treated HUVECs compared with the control group shortly following LPS induction (6 h), as well as in the long term (48 h) (Fig. 3B and E). A prominent dose-dependent increase in EPCR protein levels with UFH pretreatment was observed in HUVECs at 6 h and 12 h following LPS induction (Fig. 3B and C); however, the dose-dependency was not as strong after 24 and 48 h (Fig. 3D and E). Notably, UFH of a high dose (10 U/ml) abated LPS-repressed EPCR transcription at 24 h as effectively as it did at 6 h (Fig. 3A). However, high-dose UFH-mediated protection of EPCR protein was weakened at a later stage of LPS treatment (48 h) compared with that at an earlier stage (6 and 12 h; Fig. 3 B-E).
Figure 3.
UFH inhibits LPS-induced downregulation of EPCR expression in HUVECs. HUVECs were treated with various concentrations of UFH for 15 min prior to exposure to 10 µg/ml LPS. (A) Levels of EPCR mRNA following LPS induction were determined by reverse transcription-quantitative polymerase chain reaction analysis, and the values were normalized to that of the control group. The levels of EPCR protein were detected by western blot analysis at (B) 6, (C) 12, (D) 24 and (E) 48 h post LPS exposure. *P<0.05 vs. the control group; #P<0.05 vs. the LPS group. UFH, unfractionated heparin; LPS, lipopolysaccharide; EPCR, endothelial protein C receptor; HUVECs, human umbilical vein endothelial cells.
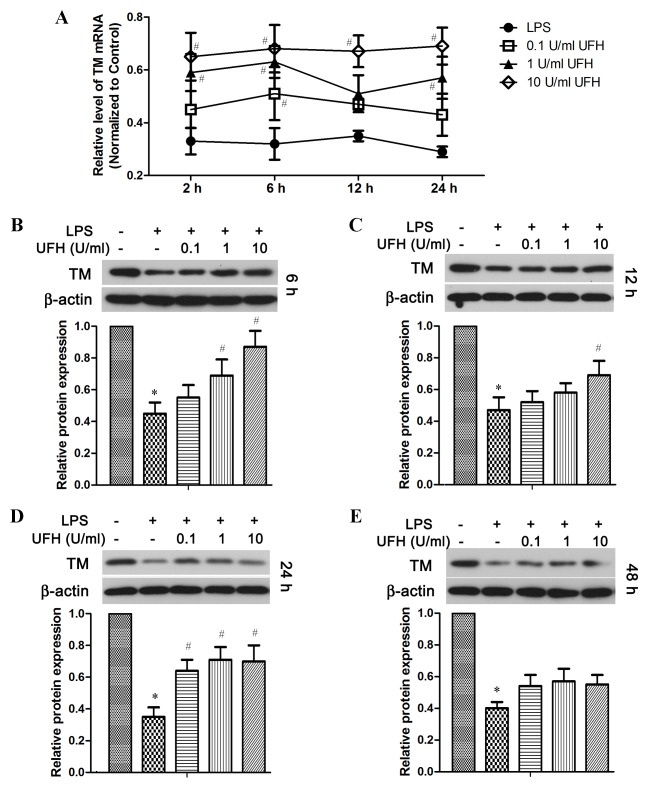
Figure 4.
LPS-induced downregulation of TM expression is decreased by UFH in HUVECs. HUVECs were treated with UFH prior to LPS stimulation. (A) The levels of TM mRNA following LPS induction were determined by reverse transcriptase-quantitative polymerase chain reaction analysis, and the values were normalized to that of the control group. The levels of TM protein at (B) 6, (C) 12, (D) 24 and (E) 48 h after LPS exposure were detected by western blot analysis using β-actin as the internal control. *P<0.05 vs. the control group; #P<0.05 vs. the LPS group. LPS, lipopolysaccharide; TM, thrombomodulin; UFH, unfractionated heparin; HUVECs, human umbilical vein endothelial cells.
TM mRNA expression was also significantly reduced in HUVECs following LPS stimulation, and UFH dose-dependently increased TM mRNA expression in LPS-treated HUVECs (Fig. 4A). In addition, compared with LPS-treated cells, UFH treatment led to dose-dependent elevation of the TM protein at 6 h post LPS induction (Fig. 4B). UFH of all doses significantly attenuated LPS-induced loss of TM protein at 24 h, whereas UFH did not show significant rescue of TM protein after 48 h LPS treatment (Fig. 4C-E).
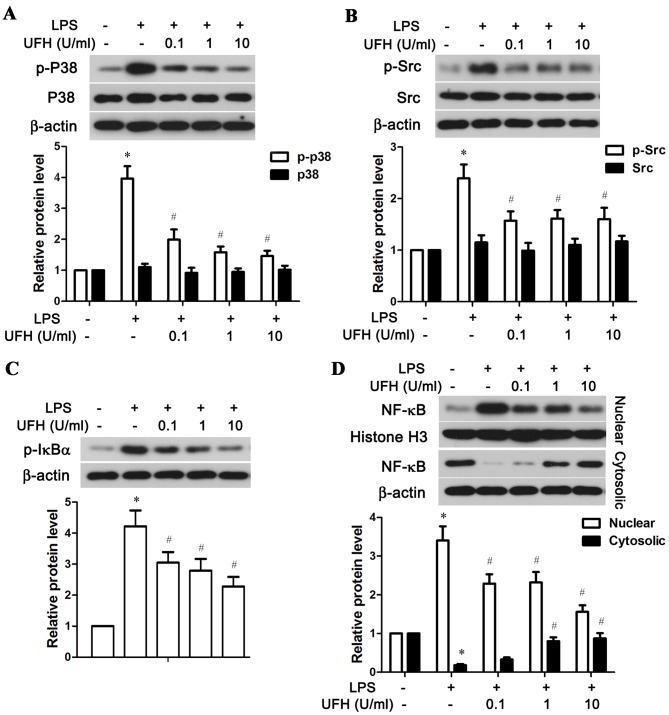
UFH suppresses LPS-induced activation of the p38 MAPK, Src and NF-κB signaling pathways
The possible mechanisms underlying UFH-mediated rescue of EPCR and TM in LPS-stimulated HUVECs were explored by examining the activation status of several associated signaling molecules, including p38 MAPK, Src and NF-κB. As shown in Fig. 5A, LPS significantly stimulated the phosphorylation of p38 MAPK in HUVECs compared with the control group, while a low dose of UFH (0.1 U/ml) was able to significantly reduce LPS-induced p38 MAPK phosphorylation. A low dose of UFH also significantly lowered LPS-induced activation of Src in HUVECs (Fig. 5B). In addition, UFH dose-dependently inhibited phosphorylation of IκBα, an inhibitor of NF-κB, and blocked the nuclear translocation of NF-κB following LPS stimulation (Fig. 5C and D). Taken together, these resulted demonstrated that UFH at a low dose was sufficient to block LPS-induced activation of p38 MAPK and Src in HUVECs, while it inhibited activation of the NF-κB pathway in a dose-dependent manner.
Figure 5.
UFH inhibits activation of the p38 MAPK, Src and NF-κB signaling pathways in LPS-stimulated human umbilical vein endothelial cells. A total of 6 h after LPS treatment, the phosphorylation status of (A) p38 MAPK, (B) Src, (C) IκBα, and (D) nuclear and cytosolic NF-κB were detected by western blot analysis. β-actin was used as the internal control for total protein and cytosolic protein, and histone H3 was used as the internal control for nuclear protein. *P<0.05 vs. the control group; #P<0.05 vs. the LPS group. UFH, unfractionated heparin; p38, p38 mitogen-activated protein kinase; NF-κB, nuclear factor-κB; LPS, lipopolysaccharide; IκBα, NF-κB inhibitor α; p, phosphorylated.
Discussion
The impairment of the protein C anticoagulant pathway is an important contributor to sepsis-associated hypercoagulability. LPS and cytokine-induced alterations in various components of the protein C pathway lead to an increased risk of thrombosis and uncontrolled inflammation during sepsis (26). Consistent with previous studies, an immediate decline in APC, and elevated levels of sEPCR and sTM were observed in mice serum following LPS administration in the present study. By contrast, preconditioning with UFH prevented APC depletion and reduced the shedding of EPCR and TM from the endothelial surface in LPS-treated mice. The protection of endothelial TM and EPCR was possibly achieved by UFH-exerted direct inhibition of neutrophil elastase (27) and metalloproteinases (28), the key sheddases for TM and EPCR. The intact membrane-bound TM and EPCR guaranteed regular production of APC, and thus maintained a physiological level of circulating APC. The in vivo findings of the present study demonstrated that UFH conveyed immediate protection upon protein C pathway components against LPS-induced disruption, and thus disruption of the anticoagulant system.
It has been reported that TM expression begins to diminish in a dose- and time-dependent manner in the endothelium of lung, liver and kidneys of rats within 2–4 h following LPS administration (29). In addition, decreased expression of TM and EPCR mRNA has been observed in HUVECs following LPS stimulation (30). The present study demonstrated that LPS-induced downregulation of TM and EPCR transcription in HUVECs was reversed by UFH in a dose-dependent manner. UFH has been demonstrated to induce TM expression in HUVECs in the presence or absence of LPS (31), and it could also prevent tumor necrosis factor α-induced downregulation of EPCR mRNA in trophoblast cells (32). These findings support the protective role of UFH on TM and EPCR expression under inflammatory conditions. In addition, UHF can suppress inflammation-mediated expression of procoagulant tissue factors and increase the release of tissue factor pathway inhibitors in endothelial cells, thereby restoring the anticoagulant activity and modulating the hemostatic properties of the endothelium (31).
Although a high dose of UFH maintained mRNA expression of TM and EPCR in HUVECs at a comparative efficiency during early and late phase of LPS stimulation in the present study, it failed to preserve the protein levels of TM and EPCR at a later stage of LPS treatment. Ishii et al (33) previously reported that an increased release of sTM in LPS-stimulated HUVECs was correlated with the degree of cell damage in a time-dependent manner. Thus, the decrease of TM observed in the present study may be attributed to TM shedding and release in damaged HUVECs due to LPS. In addition, sEPCR can be generated in vitro through proteolytic cleavage by metalloproteases, and this process is inducible by several inflammatory mediators (10). Therefore, UFH is able to protect TM and EPCR expression in endothelial cells during early endotoxemia, while anti-inflammatory agents and inhibitors of metalloproteases are also recommended for optimal protection of these protein C pathway components.
A previous study demonstrated that inhibition of endothelial NF-κB activation prevented LPS-induced downregulation of EPCR and TM expression, reduced EPCR shedding and restored plasma APC levels (34). In addition, activation of the MAPK signaling pathway is implicated in basal and induced EPCR shedding (35), and Src activation is involved in the initiation of TM expression (36). The present study demonstrated that UFH interfered with the activation of the p38 MAPK, Src and NF-κB signaling pathways in LPS-stimulated HUVECs, suggesting that it may exert a protective effect on protein C system molecules by inhibiting these signaling pathways.
In conclusion, the current study demonstrated that UFH has protective effects on the protein C system in a mouse sepsis model and in LPS-stimulated HUVECs. The mechanisms of action of these protective effects may involve direct inhibition of sheddases and the maintenance of endothelial EPCR and TM expression. The preliminary clinical data suggest that heparin is associated with reduced mortality in patients with sepsis (37,38). The results of the present study support the potential therapeutic value of UFH as a treatment for sepsis; however, its efficacy and safety should be evaluated in future clinical trials.
Acknowledgements
The present study was supported by the Natural Science Foundation of Liaoning Province (grant no. 2013021073).
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 3.Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 4.Opal SM, Esmon CT. Bench-to-bedside review: Functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 2003;7:23–38. doi: 10.1186/cc2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 6.Esmon CT. The protein C pathway. Chest. 2003;124(3 Suppl):26S–32S. doi: 10.1378/chest.124.3_suppl.26S. [DOI] [PubMed] [Google Scholar]
- 7.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24:1374–1383. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki Y, Inoue T, Kyi M, Sawada M, Miyake S, Yoshizawa Y. Effects of a neutrophil elastase inhibitor (ONO-5046) on acute pulmonary injury induced by tumor necrosis factor alpha (TNFalpha) and activated neutrophils in isolated perfused rabbit lungs. Am J Respir Crit Care Med. 1998;157:89–94. doi: 10.1164/ajrccm.157.1.9612021. [DOI] [PubMed] [Google Scholar]
- 9.Qu D, Wang Y, Esmon NL, Esmon CT. Regulated endothelial protein C receptor shedding is mediated by tumor necrosis factor-alpha converting enzyme/ADAM17. J Thromb Haemost. 2007;5:395–402. doi: 10.1111/j.1538-7836.2007.02347.x. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Qu D, Esmon NL, Esmon CT. Metalloproteolytic release of endothelial cell protein C receptor. J Biol Chem. 2000;275:6038–6044. doi: 10.1074/jbc.275.8.6038. [DOI] [PubMed] [Google Scholar]
- 11.Bemard GR, Ely EW, Wright TJ, Fraiz J, Stasek JE, Jr, Russell JA, Mayers I, Rosenfeld BA, Morris PE, Yan SB, Helterbrand JD. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med. 2001;29:2051–2059. doi: 10.1097/00003246-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Bemard GR. Drotrecogin alfa (activated) (recombinant human activated protein C) for the treatment of severe sepsis. Crit Care Med. 2003;31(1 Suppl):S85–S93. doi: 10.1097/00003246-200301001-00012. [DOI] [PubMed] [Google Scholar]
- 13.Fowler RA, Hill-Popper M, Stasinos J, Petrou C, Sanders GD, Garber AM. Cost-effective of recombinant human activated protein C and the influence of severity of illness in the treatment of patients with severe sepsis. J Crit Care. 2003;18:181–194. doi: 10.1016/j.jcrc.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Martí-Carvajal AJ, Solà I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12:CD004388. doi: 10.1002/14651858.CD004388.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornet AD, Smit EG, Beishuizen A, Groeneveld AB. The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost. 2007;98:579–586. [PubMed] [Google Scholar]
- 16.Li Y, Sun JF, Cui X, Mani H, Danner RL, Li X, Su JW, Fitz Y, Eichacker PQ. The effect of heparin administration in animal models of sepsis: A prospective study in Escherichia coli-challenged mice and a systematic review and metaregression analysis of published studies. Crit Care Med. 2011;39:1104–1112. doi: 10.1097/CCM.0b013e31820eb718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao D, Ding R, Mao Y, Wang L, Zhang Z, Ma X. Heparin rescues sepsis-associated acute lung injury and lethality through the suppression of inflammatory responses. Inflammation. 2012;35:1825–1832. doi: 10.1007/s10753-012-9503-0. [DOI] [PubMed] [Google Scholar]
- 18.Wildhagen KC, de Frutos García P, Reutelingsperger CP, Schrijver R, Aresté C, Ortega-Gómez A, Deckers NM, Hemker HC, Soehnlein O, Nicolaes GA. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123:1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- 19.Ding R, Zhao D, Guo R, Zhang Z, Ma X. Treatment with unfractionated heparin attenuates coagulation and inflammation in endotoxemic mice. Thromb Res. 2011;128:e160–e165. doi: 10.1016/j.thromres.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Zheng Z, Li X, Ma X. Unfractionated heparin inhibits lipopolysaccharide-induced inflammatory response through blocking P38 MAPK and NF-κB activation on endothelial cell. Cytokine. 2012;60:114–121. doi: 10.1016/j.cyto.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Zheng Z, Mao Y, Ma X. Unfractionated heparin promotes LPS-induced endothelial barrier dysfunction: A preliminary study on the roles of angiopoietin/Tie2 axis. Thromb Res. 2012;129:e223–e228. doi: 10.1016/j.thromres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Li X, Zheng Z, Liu Y, Ma X. Unfractionated heparin suppresses lipopolysaccharide-induced monocyte chemoattractant protein-1 expression in human microvascular endothelial cells by blocking Krüppel-like factor 5 and nuclear factor-κB pathway. Immunobiology. 2014;219:778–785. doi: 10.1016/j.imbio.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council, corp-author. Guide for the care and use of laboratory animals (8th edition) The National Academies Press; Washington DC: 2011. [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Mu E, Ding R, An X, Li X, Chen S, Ma X. Heparin attenuates lipopolysaccharide-induced acute lung injury by inhibiting nitric oxide synthase and TGF-β/Smad signaling pathway. Thromb Res. 2012;129:479–485. doi: 10.1016/j.thromres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi T, Suzuki K. Changes of expression of the protein C pathway components in LPS-induced endotoxemia-implication for sepsis. Cardiovasc Hematol Disord Drug Targets. 2015;15:2–9. doi: 10.2174/1871529X15666150108110821. [DOI] [PubMed] [Google Scholar]
- 27.Redini F, Tixier JM, Petitou M, Choay J, Robert L, Hornebeck W. Inhibition of leucocyte elastase by heparin and its derivatives. Biochem J. 1988;252:515–519. doi: 10.1042/bj2520515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenagy RD, Nikkari ST, Welgus HG, Clowes AW. Heparin inhibits the induction of three matrix metalloproteinases (stromelysin, 92-kD gelatinase and collagenase) in primate arterial smooth muscle cells. J Clin Invest. 1994;93:1987–1993. doi: 10.1172/JCI117191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terada Y, Eguchi Y, Nosaka S, Toba T, Nakamura T, Shimizu Y. Capillary endothelial thrombomodulin expression and fibrin deposition in rats with continuous and bolus lipopolysaccharide administration. Lab Invest. 2003;83:1165–1173. doi: 10.1097/01.LAB.0000080606.96797.A5. [DOI] [PubMed] [Google Scholar]
- 30.Gao XH, Xu XX, Pan R, Li Y, Luo YB, Xia YF, Murata K, Matsuda H, Dai Y. Saponin fraction from Astragalus membranaceus roots protects mice against polymicrobial sepsis induced by cecal ligation and puncture by inhibiting inflammation and upregulating protein C pathway. J Nat Med. 2009;63:421–429. doi: 10.1007/s11418-009-0348-2. [DOI] [PubMed] [Google Scholar]
- 31.Vignoli A, Marchetti M, Balducci D, Barbui T, Falanga A. Differential effect of the low-molecular-weight heparin, dalteparin, and unfractionated heparin on microvascular endothelial cell hemostatic properties. Haematologica. 2006;91:207–214. [PubMed] [Google Scholar]
- 32.Faioni EM, Fontana G, Razzari C, Avagliano L, Bulfamante G, Calvi E, Doi P, Marconi AM. Activation of protein C in human trophoblasts in culture and downregulation of trophoblast endothelial protein C receptor by TNF-α. Reprod Sci. 2015;22:1042–1048. doi: 10.1177/1933719115570904. [DOI] [PubMed] [Google Scholar]
- 33.Ishii H, Uchiyama H, Kazama M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemost. 1991;65:618–623. [PubMed] [Google Scholar]
- 34.Song D, Ye X, Xu H, Liu SF. Activation of endothelial intrinsic NF-{kappa}B pathway impairs protein C anticoagulation mechanism and promotes coagulation in endotoxemic mice. Blood. 2009;114:2521–2529. doi: 10.1182/blood-2009-02-205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menschikowski M, Hagelgans A, Eisenhofer G, Siegert G. Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways. Exp Cell Res. 2009;315:2673–2682. doi: 10.1016/j.yexcr.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Lo IC, Lin TM, Chou LH, Liu SL, Wu LW, Shi GY, Wu HL, Jiang MJ. Ets-1 mediates platelet-derived growth factor-BB-induced thrombomodulin expression in human vascular smooth muscle cells. Cardiovasc Res. 2009;81:771–779. doi: 10.1093/cvr/cvn351. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Chi C, Guo L, Wang X, Guo L, Sun J, Sun B, Liu S, Chang X, Li E. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: A systematic review and meta-analysis. Crit Care. 2014;18:563. doi: 10.1186/s13054-014-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarychanski R, About-Setta AM, Kanji S, Turgeon AF, Kumar A, Houston DS, Rimmer E, Houston BL, McIntyre L, Fox-Robichaud AE, et al. The efficacy and safety of heparin in patients with sepsis: A systemati review and metaanalysis. Crit Care Med. 2015;43:511–518. doi: 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]