Abstract
Gastric mucosal cells, particularly parietal and chief cells, are usually affected by exogenous, and endogenous stimuli-induced gastritis. The integrity of these cells and their alterations are involved in the pathogenesis of numerous gastric disorders. Omeprazole, a gastric acid secretion blocker, is commonly used for gastrointestinal diseases due to its antioxidative stress and anti-inflammatory properties. Little is known regarding how omeprazole modulates the re-epithelialized effect on gastric mucosal cells associated with gastrointestinal disorders. The present study aimed to determine whether omeprazole attenuates parietal and chief cell damage in association with its antioxidative property. An in vivo ethanol-induced gastritis rat model was used. Histopathological, scanning and transmission electron microscopic, and immunohistochemical studies were performed. The results revealed that omeprazole improved the gastric mucosal surface, and reduced the severity of mucosal inflammation and hemorrhaging. Notably, ethanol-induced gastritis caused dysmorphic rough endoplasmic reticulum (RER) in chief cells, which was accompanied by mitochondrial swelling. This alteration was modulated by omeprazole due to its antioxidant effect characterized by upregulation of superoxide dismutase in gastric mucosal cells. In addition, expression of aquaporin-4 was increased in the omeprazole treatment group, which may be due to the expansion of regenerative parietal cells and acid suppression. The results of the present study suggest that omeprazole preserves the RER in chief cells and enhances parietal cell regeneration through its antioxidative property by exerting anti-inflammatory effects.
Keywords: aquaporin (AQP)-4, chief cells, gastritis, omeprazole, parietal cells, super-oxide dismutase (SOD)
Introduction
Gastrointestinal homeostasis is one of several factors for longevity and human health. Although the common causes of gastritis are related to non-steroidal anti-inflammatory drugs, burns, brain injury, autoimmunity, and Helicobacter pylori infection (1), modern lifestyles and dietary habits, such as non-exercise related obesity, cigarette smoking, heavy alcohol intake, and fast food consumption, are also major driving forces of gastric disorders (2). There are several mechanisms involved in the pathogenesis of gastritis, depending on its cause. However, the pathological outcomes are similar, including inflammation, gastric mucosal cell alterations (degeneration, apoptosis, and necrosis), barrier damage, and hemorrhage. Interestingly, endoplasmic reticulum (ER) stress has been associated with the pathogenicity of gastritis through apoptotic pathways (3). Moreover, oxidative-induced gastric disorders play a crucial role in gastric mucosal cell injury, particularly from exogenous stimuli such as diet and drugs (4). Parietal cell, acid secreting cell, and chief cell, pepsinogen secreting cell, are affected during ethanol-induced gastritis (5) that is characterized by narrowing of the mucosal membrane and atrophy of secretory cells in the glands. Morphological identification of these cells is important for new therapeutic modalities (6). Unfortunately, there are no reports describing the ultrastructural integrity of rough ER (RER) in parietal and chief cells in association with ethanol-induced gastritis.
Recently, several natural products have been introduced to alleviate gastrointestinal disorders with their anti-oxidative property (7,8). Nevertheless, omeprazole, a basic medication on the World Health Organization's list of essential medicine, is now globally available for treatment of some gastro-intestinal diseases. Omeprazole has the ability to inhibit a proton pump to block acid secretion in the stomach. Importantly, it also modulates endogenous oxidative stress and prevents proinflammatory cytokines release (9). However, it is doubtful that whether omeprazole can preserve the RER integrity in gastric mucosal cells related to gastrointestinal disorders.
The present study aimed to demonstrate the anti-oxidative property and epithelial protective effect of omeprazole in an ethanol-induced gastritis rat model. Microscopic and fine morphological structures of the cells in gastric-mucosal, -submucosal, and -muscular layers were compared in rats with or without omeprazole-treatment. Anti-oxidative stress marker, super-oxide dismutase (SOD), and aquaporin (AQP)-4, acid suppression (10) and re-epithelialized marker (11,12), were also examined using immunohistochemical study. The results of the present study may provide a better understanding of the mechanical action of omeprazole correlates to the pathogenesis of gastritis, together with for the benefit of a new therapeutic approach.
Materials and methods
Ethic statement
All activities related to the animal studies were performed in accordance with the Ethical Principles and Guidelines for the Use of Animals, National Research Council of Thailand, and approved by National Laboratory Animal Center-Animal Care and Use Committee (ACUC), Mahidol University. Sprague-Dawley rats were provided by National Laboratory Animal Center, Mahidol University and housed in the barrier system with temperature, ventilation, and humidity control. Animal were provided with standard diet (Perfect Companion Co., Ltd., Bangkok, Thailand) and chlorinated water ad libitum and a 12/12 h light/dark cycle.
Omeprazole preparation
Omeprazole was purchased from the Government Pharmaceutical Organization, Thailand. Omeprazole granules were removed from the capsule and ground into a powder. To dissolve the powder, 8.4% (w/v) of sodium bicarbonate (8.4 g in 100 ml deionized water) was made as a diluent. An omeprazole suspension was prepared by dissolving the powder in a diluent with strong mixing. The suspension was kept at room temperature for no more than 15 days.
Induction gastritis rats
Thirty rats were included to the study and equally divided into two groups with or without omeprazole treatment. In each group, five rats were randomly allocated into each of three subgroups according to induction period: 4, 7, and 14 days. To induce gastritis, all rats were fasted overnight with free access to water. Fasted rats were orally administrated a single dose of 5 ml/kg absolute ethanol. Therefore, 20 mg/kg of omeprazole and water were administered daily to treated and untreated groups, respectively (13). Routine clinical observations were performed under attending veterinarian control.
Specimen collection
All rats in each subgroup were humanely euthanized with overdose of carbon dioxide (CO2) inhalation on 4, 7, and 14 days post-induction. Grandular stomach was removed and cut into three parts, one was fixed in 10% neutral buffer formalin for 48 h and subjected to the histopathological examination, the second part was separated for PGE-2 activity study, and the remaining part was fixed in 2.5% glutaraldehyde in 0.1 M sucrose phosphate buffer (SPB), pH 7.4 for 1 h and attended to the electron microscopic studies.
PGE-2 activity assessment
Collected grandular stomachs were homogenized in 1.15% potassium chloride with 1:5 ratio (w/v) and then centrifuged at 10,000 g, 4°C for 10 min. The supernatant was collected for PGE-2 activity using commercial enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chemical, Ann Arbor, MI, USA). The level of PGE-2 in grandular stomach was measured based on the conjugate reaction of PGE-2 and PGE-2 acetylcholinesterase (AChE) which was obviously detected at 412 nm absorbance by ELISA reader.
Electron microscopic studies
Scanning electron microscopy (SEM)
Fixed stomachs were washed three times with 0.1 M SPB for 10 min each and secondarily fixed with 1% osmium tetroxide in 0.1 M SPB for 1 h. After three consecutive wash, the stomachs were dehydrated with graded ethyl-alcohol and dried in liquid CO2 using critical point dryer (HCP-2; HITACHI, Ltd., Tokyo, Japan). Then, the stomachs were mounted on an aluminum stub using double-side carbon tape and coated with gold film to 20 nm thickness using a sputter coater (K550; EMITECH, UK). All specimens were examined under a scanning electron microscope (JSM-6610LV; JEOL, Tokyo, Japan) with a 15 kV acceleration voltage.
Transmission electron microscopy (TEM)
Fixed stomachs were washed and secondary fixed as mentioned above. The specimens were then dehydrated in graded ethanol, infiltrated with LR White resin (EMS, Hatfield, PA, USA), embedded in capsule beams, and finally polymerized at 65°C for 48 h. Then the specimens were cut in 90–100 nm thickness and stained with uranyl acetate and lead citrate. The ultra-thin sections were investigated under a transmission electron microscope (HT7700; HITACHI, Ltd.).
Ultrastructural changes in the chief and parietal cells were evaluated by focusing on the RER and mitochondrial alterations, and then semi-quantified by the H-score, a multiplication between percentage of the cells with an RER or mitochondrial alterations (0–100%) and severity score (four grades; 0=no changing, 1=low severity, 2=moderate severity, and 3=severe changing). At least 50 chief and parietal cells each were counted per animal.
Histopathological studies
Fixed stomachs were dehydrated in graded ethanol, infiltrated and embedded in paraffin, sectioned to 4-µm thickness, and then stained with hematoxylin and eosin (H&E). All histopathological changes in gastric-mucosa, -submucosa, and -muscular layer were examined under light microscope and scored by H-score, as a multiplication between the severity score (0–3) and the histological changing area (0–100%). Briefly, the severity was scored in four grades (0=no changing, 1=mild severity, 2=moderate severity or progressive stage, and 3=severe or end stage). In association with the distribution of each histological change, percentage of affected area per section of each animal was simultaneously estimated.
The gross hemorrhagic area was quantified by an imaging analysis program (ImageJ® v.1.36; National Institutes of Health; Bethesda, MD, USA). Briefly, stomach images were acquired in color. The non-hemorrhagic area was adjusted to white by the replace mode. The adjusted images were transformed to gray scale and then the hemorrhagic area was localized by the threshold mode. A line was drawn over the area of glandular portion and finally the hemorrhagic area was measured as the percentage hemorrhagic area/grandular portion.
Immunohistochemical studies
SOD and AQP-4 expression on gastric mucosal layer were used to demonstrate anti-oxidative property and acid suppression/re-epithelialized effect of omeprazole by immunohistochemical technique. Polyclonal rabbit anti-AQP-4 (D2408; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and polyclonal rabbit anti-Mn-SOD (2683863; Millipore, Billerica, MA, USA) were used as primary antibodies in cooperation with rabbit anti-IgG (Santa Cruz Biotechnology, Inc.) as a negative control for the staining system validation. In addition, rat brain and liver were used as a positive control for AQP-4 and SOD labeling, respectively. Sections were deparaffinized in xylene, rehydrated in graded ethanol, unmasked antigens in heated citrate buffer pH 6.0, and peroxidase/non-specific binding blocked with EnVision FLEX/HRP blocking reagent (K8002; DAKO; Agilent Technologies, Inc., Santa Clara, CA, USA). The sections were incubated with primary antibody for 1 h and labeled polymer HRP anti-mouse/rabbit (K8002; DAKO) for 20 min, then visualization with diaminobenzidine; DAB (K8002; DAKO). Finally, the sections were counterstained in hematoxylin and mounted with Permount®.
In each animal (belong to any subgroup; 4 and 7 days post induction), ten color images of mucosal layer were randomly acquired by a light microscope (BX51; Olympus Corp., Tokyo, Japan) and digital camera (DP70; Olympus Corp.) at ×400 magnifications. Therefore fifty images were included to the analysis per subgroup. SOD and AQP-4 expression were evaluated using the H-score. Percentage area of expression/field (0–100%) and intensity score (three grades; 0=negative staining, 1=low intensity staining, 2=moderate intensity staining, and 3=strong intensity staining) were computed. The area of expression was measured by an imaging analysis program as described elsewhere (14). Briefly, color images were converted to gray scale. The expression areas were then located by threshold adjustment and measured as percentage area of the expression/field.
Immunogold electron microscopic studies
For aldehyde blocking, gastric sections were incubated in 50 mM glycine in phosphate buffer (PBS), pH 7.4. A section's non-specific binding was consequently blocked in 5% bovine serum albumin (BSA, 25557; EMS) in PBS, pH 7.4. The sections were washed with 0.1% BSA in PBS (incubated buffer). The sections were incubated in polyclonal rabbit anti-AQP-4 (D2408; Santa Cruz Biotechnology, Inc.) or polyclonal rabbit anti-Mn-SOD (2683863; Millipore) for 1 h and then incubated in goat anti-rabbit IgG conjugated with 10 nm gold (G7402-.4ML; Sigma Chemical Co., St. Louis, MO, USA) for 1 h. After that the sections were rigorously washed with incubated buffer and distilled water. To enhance gold signalling, silver enhancement kit (Aurion R-Gent SE-EM kit, 25521; EMS) was used followed by the manufacture guideline. Finally, the sections were then stained by uranyl acetate and lead citrate. To quantify the AQP-4 and SOD expression, gold labelled particle in gastric cell (n=10 cells/animal) was counted under transmission electron microscope (HT7700, HITACHI, Ltd.).
Statistical analysis
Statistical analysis was performed using GraphPad Prism® v.5. The non-parametric t-test was used to compare the difference in histopathological/ultrastructural changes between subgroups. P<0.05 was considered to indicate a statistically significant difference.
Results
Histopathology and electron microscopy
Gross appearance and SEM of the gastric mucosa
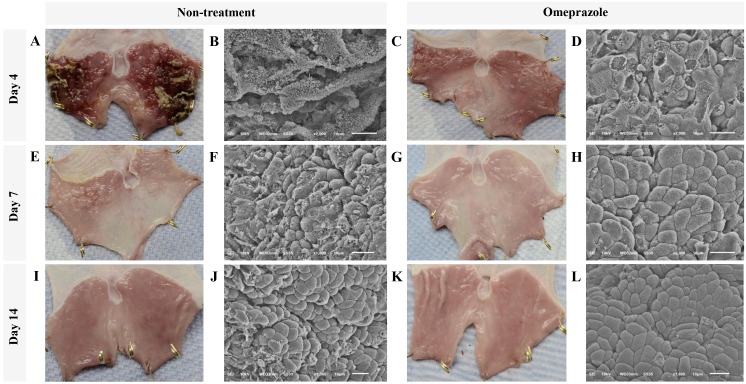
At gross level by 4 days post induction, a single dose of 5 ml/kg absolute ethanol (Fig. 1) induced severe generalized hemorrhages and necrotizing membrane deposit on the mucosal surface of glandular stomach in all rats without treatment whereas only pinpoint hemorrhages were observed in omeprazole treated rats (Fig. 1A and C). In addition, ultrastructure of gastric mucosal areas in untreated rats was highly affected as shown by extensive epithelial sloughing and a completely lost, stripped and folded membrane, loss of gastric pits, and cellular debris accumulation (Fig. 1B). In contrast to the treated rats, epithelial cells were better preserved. Although many epitheliums were flattened and ruptured in treated rats, they were not transformed as presented in the untreated rats. However, the cellular arrangement was not well formed, especially in gastric pit and cellular interface (Fig. 1D).
Figure 1.
Gross appearance and scanning electron micrographs of the glandular stomach of rats treated with or without omeprazole after ethanol-induced gastritis. (A-D) 4 days post induction. (E-H) 7 days post induction. (I-L) 14 days post induction. Gross appearance and ultrastructure of glandular stomach from non-treated (A, E and I and B, F and J, respectively) and treated rats (C, G and K and D, H and L, respectively). Bars in gross legion images: 1 cm.
The improvement of gross lesions on gastric surface was rapidly resolved in all rats with or without treatment (Fig. 1A, E & I and C, G & K) at 7 and 14 days post induction. Nevertheless, scarce hemorrhagic areas and irregular membranes remained in all rats without treatment (Fig. 1E). At 4 days post induction, the gross hemorrhagic area in untreated rats was significantly higher than that in omeprazole treated rats (Table I). However, the severity of bleeding was intensely decreased in both groups at 4 days after induction. Corresponding to the ultrastructural appearance, the cellular arrangement was almost completely regenerated by 7 days post induction in omeprazole treated rats. Moreover, cell to cell interfaces and gastric pits were clearly localized and almost reformed (Fig. 1F-J). Meanwhile, the cellular arrangement of untreated rats was closed to intact at 14 days post induction (Fig. 1L).
Table I.
The comparison of histopathological, PGE-2 activity, and ultrastructural changes in glandular stomach of omeprazole-treated and untreated rats at 4, 7, and 14 days post gastritis induction.
| 4 Day PI | 7 Day PI | 14 Day PI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathology/parameter | Omeprazole (n=5 rats) | Non-treatment (n=5 rats) | P-value | Omeprazole (n=5 rats) | Non-treatment (n=5 rats) | P-value | Omeprazole (n=5 rats) | Non-treatment (n=5 rats) | P-value |
| Mucosa | |||||||||
| Glandular atrophy/dilatation | 52.5±35.4 | 97.5±35.4 | 0.404 | 0.0±0.0 | 30.0±30.0 | 0.374 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Necrosis | 45.5±35.7 | 202.5±70.7 | 0.094 | 0.0±0.0 | 30.0±30.0 | 0.374 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Tissue debris | 22.5±14.3 | 150.0±71.4 | 0.131 | 0.0±0.0 | 0.0±0.0 | 1.000 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Inflammation | 15.0±15.0 | 262.5±22.5 | 0.000 | 0.0±0.0 | 50.0±50.0 | 0.374 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Hemorrhage | 30.0±17.3 | 210.7±71.4 | 0.049 | 0.0±0.0 | 0.0±0.0 | 1.000 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Congestion | 77.5±31.1 | 37.5±10.3 | 0.269 | 5.0±2.8 | 30.0±0.0 | 0.001 | 5.0±2.8 | 3.3±1.6 | 1.000 |
| Submucosa | |||||||||
| Edema | 215.0±31.22 | 187.5±71.8 | 0.737 | 0.0±0.0 | 30.0±30.0 | 0.394 | 0.0±0.0 | 0.0±0.0 | 0.589 |
| Inflammation | 250.0±50.0 | 225.0±75.0 | 0.791 | 0.0±0.0 | 163.3±74.4 | 0.093 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Hemorrhage | 97.5±59.2 | 127.5±43.0 | 0.696 | 0.0±0.0 | 0.0±0.0 | 1.000 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Congestion | 22.5±16.0 | 22.5±7.5 | 1.000 | 3.3±1.6 | 40.0±10.0 | 0.022 | 1.6±1.6 | 1.6±1.6 | 1.000 |
| Fatty degeneration | 82.5±51.0 | 67.5±25.6 | 0.802 | 0.0±0.0 | 16.6±8.8 | 0.132 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Muscularis | |||||||||
| Vacuolated degeneration | 90.0±37.6 | 87.5±55.5 | 0.972 | 0.0±0.0 | 90.0±56.8 | 0.189 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Inflammation | 0.0±0.0 | 22.5±13.1 | 0.138 | 0.0±0.0 | 0.0±0.0 | 1.000 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Hemorrhage | 0.0±0.0 | 22.5±14.3 | 0.168 | 0.0±0.0 | 0.0±0.0 | 1.000 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Congestion | 0.0±0.0 | 7.5±2.5 | 0.024 | 0.0±0.0 | 6.6±1.6 | 0.016 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Grandular PGE-2 (pg/ml) | 2,277.1±291.7 | 1,643.1±89.89 | 0.047 | 3,580.4±714.7 | 1,263.0±20.44 | 0.017 | 1,408.7±13.41 | 986.5±19.21 | 0.000 |
| Gross hemorrhagic area | 14.0±7.2 | 28.2±10.2 | 0.040 | 0.1±0.1 | 1.2±0.5 | 0.985 | 0.0±0.0 | 0.0±0.0 | 1.000 |
| Defected RER in chief cells | 5.2±0.6 | 174.0±8.7 | 0.000 | 5.6±0.8 | 250.0±15.1 | 0.000 | 6.8±0.8 | 6.4±1.0 | 0.899 |
| Defected RER in parietal cells | 5.7±0.8 | 4.7±1.0 | 0.483 | 6.2.0±1.1 | 5.5±0.6 | 0.580 | 6.2±1.2 | 6.5±1.9 | 0.890 |
| Swelling Mito in chief cells | 24.0±6.1 | 26.0±7.0 | 0.796 | 87.3±2.8 | 84.6±9.2 | 0.654 | 15.0±1.1 | 11.0±1.1 | 0.07 |
| Swelling Mito in parietal cells | 36.3±2.9 | 24.0±6.4 | 0.156 | 47.3±2.7 | 28.3±21.6 | 0.840 | 44.0±4.7 | 32.6±6.9 | 0.248 |
Data are presented as the mean ± SEM. RER, rough endoplasmic reticulum; mito, mitochondria.
Pathological changes in the glandular stomach
Pathological changes of ethanol-induced gastritis in each gastric layer are shown in Table I, and Figs. 2 and 3. The mucosal layer exhibited a shortening in thickness (Fig. 2), severe hemorrhage (Fig. 3F), acute inflammation (Fig. 3B), cellular-swelling, -degeneration, and -necrosis (Fig. 3B), and glandular atrophy/dilattion (Fig. 3C). Predominant changes in submucosal layer composed of edema, inflammation, hemorrhage (Figs. 2 and 3H), and fatty degeneration (Fig. 3I). Interestingly, vacuolated degeneration was found in the muscular layer accompanied by inflammation (Fig. 3K and L). Similar to the other studies (7,13), at 4 days post induction, mucosal inflammation and hemorrhage in untreated rats were significantly higher than those in treated rats. Although glandular atrophy/dilatation and necrosis in mucosal layer were similar in both groups, untreated rats showed a higher trended than omeprazole treated rats. Subsequently, at 7 and 14 days post induction, the main histopathological changes in each gastric layer were similar in both groups.
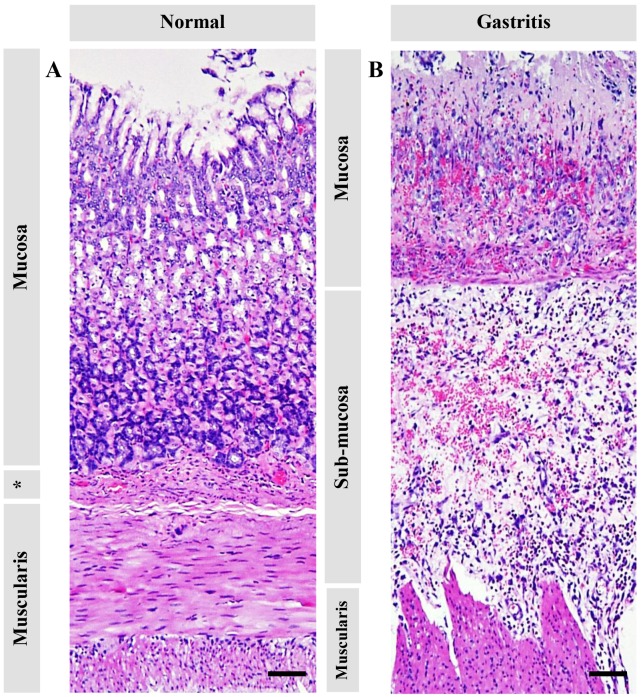
Figure 2.
Histopathological changes of the glandular stomach in normal rats compared with ethanol-induced gastritis rats. (A) Intact stomach. (B) Gastritis induced severe mucosal hemorrhages and degeneration with submucosal edema and muscularis degeneration. *Submucosal area. Bars, 50 µm.
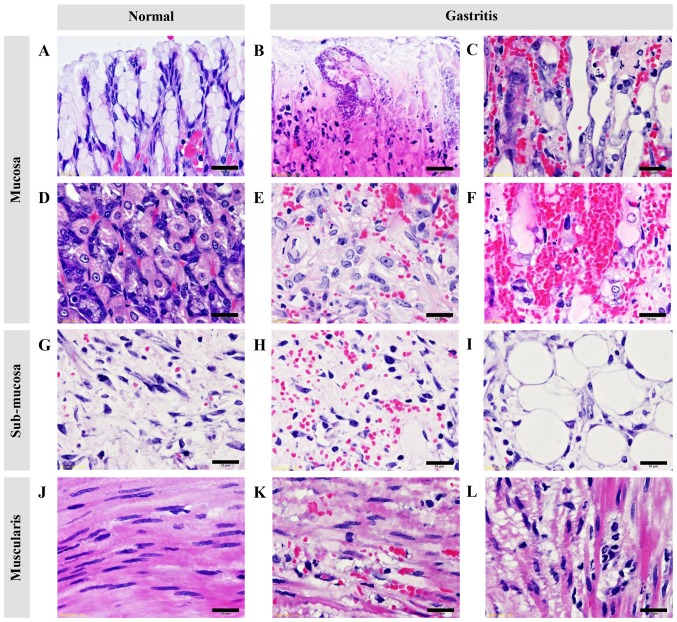
Figure 3.
Histopathological changes of the glandular stomach in normal rats compared with ethanol-induced gastritis rats. (A) Intact mucus surface cells. (B) Mucosal necrosis. (C) Mucosal gland atrophy, dilatation, and degeneration. (D) Intact parietal and chief cells. (E) Degenerative and necrotic parietal and chief cells. (F) Severe hemorrhage in the mucosal surface. (G) Intact sub-mucosa. (H) Submucosal hemorrhages and edema. (I) Fatty degeneration. (J) Intact smooth muscle cells. (K-L) Muscular degeneration with hemorrhage and congestion. Bars, 10 µm.
Ultrastructural changes (TEM) in chief and parietal cells
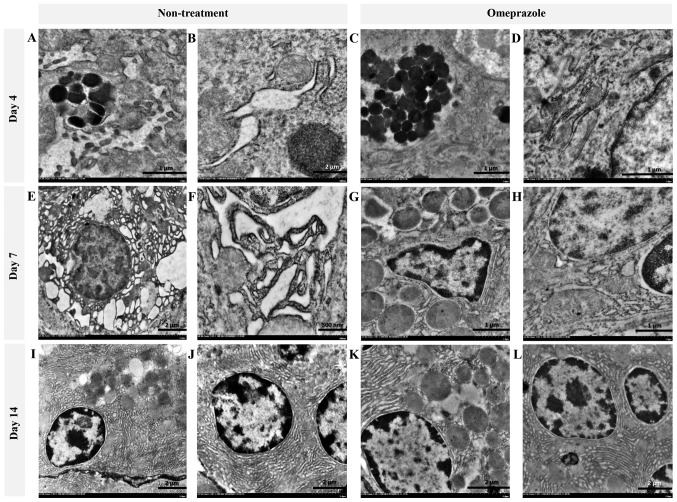
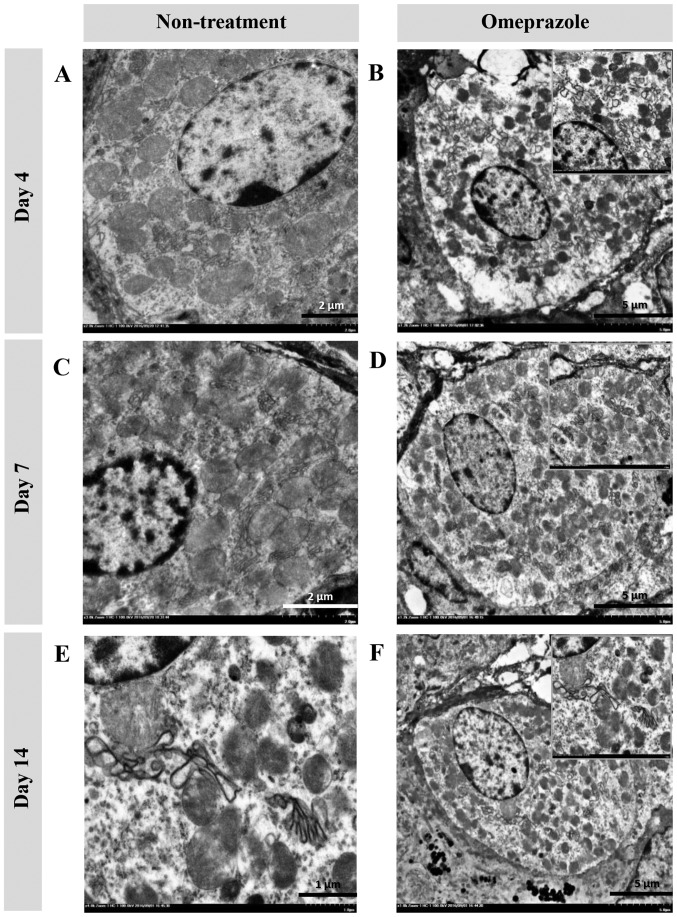
In the present study, the ultrastructural changes of cells were examined using TEM (Fig. 4). RER changes in chief cells were mostly found in untreated rats at 4 and 7 days post induction (Fig. 4A, B, E and F), which were characterized by a dark electron-dense crinkled-like structure with dilatation and enlargement. The highest severity of RER changes was presented at 7 days post induction, which was accompanied by mitochondrial swelling (Fig. 4E and F). At 14 days post induction, the RER was completely regenerated as shown by the sheath-like structure in all groups of rats (Fig. 4I-L). In addition, H-score demonstrated that untreated rats had a number of defected RERs in chief cells at 4 and 7 days post induction with increasing severity (Table I). Unlike chief cells, defected RERs in parietal cells were similarly low in both groups of rats (Table I). It appeared that parietal cells were more preserved than chief cells in ethanol-induced gastritis as shown in the Fig. 5.
Figure 4.
Ultrastructure changes of chief cells in omeprazole-treated rats compared with untreated rats. RER alterations were found in untreated rats as indicated by the presence of cytoplasmic vacuolation and moderate RER dilatation (A and B) while the RER architecture in omeprazole-treated rats was preserved (C, D, G, H, K and L). A severe grade of peculiar dark, dense and dilated RERs accompanied by degenerative mitochondria were observed in non-treated rats at 7 days post-induction (E and F). RER integrity was preserved in rats with or without omeprazole treatment at 14 days post-induction (I-L). B, E and I-L scale bar, 2 µm; F scale bar, 500 nm; A, C, D, G and H, scale bar, 1 µm.
Figure 5.
Ultrastructural changes of parietal cells in omeprazole-treated rats compared with untreated rats. Fine structure of parietal cell in non-treated and treated rats during 4 (A and B, respectively), 7 (C and D, respectively) and 14 (E and F, respectively) days spost induction. E scale bar, 1 µm; A and C scale bar, 2 µm; B, D and F, scale bar, 5 µm.
PGE-2 activity
PGE-2 activity is described in Table I. The results revealed that omeprazole treatment induced a significantly higher level of gastric PGE-2 compared with untreated rats at all time points. PGE-2 activity was increased over time until 7 days post induction and then declined at 14 days post induction. Unfortunately, in the present study, there was no correlation of the level of PGE-2 activity with histopathological or ultrastructural changes.
Immunohistochemistry
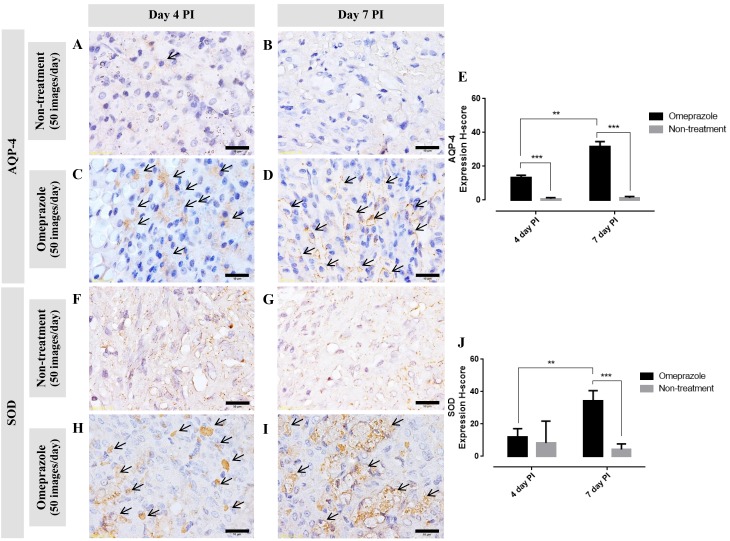
AQP-4 expression in gastric mucosa
Generally, AQP-4 is expressed on the baso-lateral area of the parietal cells. In this study, at 4 and 7 days post induction, the AQP-4 expression score in omeprazole treated rats was significantly higher than that in untreated rats (Fig. 6A-E). Furthermore, the expression increased over time. Concerning to the localized area, at 4 days post induction, AQP-4 was labeled in the cytoplasm which located on either side of the cell (Fig. 6C arrow). In contrast to 7 days post induction, AQP-4 was labeled on the baso-lateral cell as presented by ‘fried egg appearance’ (Fig. 6D arrow). These labeling patterns are associated with the regeneration processes of the parietal cells.
Figure 6.
Immunohistochemical staining of (A-E) AQP-4 and (F-J) SOD in the glandular stomach of omeprazole-treated rats compared with untreated rats. (arrow; expression area) **P<0.01, ***P<0.001. A-D, H and I scale bar, 10 µm; F and G scale bar, 50 µm.
SOD expression in gastric mucosa
Similar to the AQP-4 expression score, at 7 days post induction, SOD expression in omeprazole treated rats was significantly higher than that in untreated rats (Fig. 6F-J). Up-regulation of SOD was depended on the post induction time. The positive immuno-localization of SOD was presented on the cytoplasm of gastric mucosal cells.
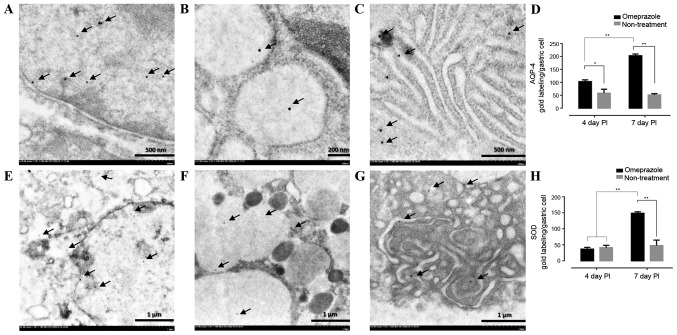
AQP-4 and SOD gold labeling in gastric mucosa
To confirm the levels of AQP-4 and SOD expression in the gastric cells, immunoelectron microscopic study was performed. The results revealed that both AQP-4 and SOD were labeled in the nucleus, cytoplasm and rough endoplasmic reticulum (Fig. 7A-C and E-G). Like immunohistochemical staining, the treated rats had significant level of AQP-4 when compared to untreated rats in all observation periods while a level of SOD in treated rats was up-regulated on day 7 post induction (Fig. 7D and H).
Figure 7.
AQP-4 and SOD gold labeled in the gastric mucosal cell of omeprazole-treated rats compared with untreated rats. The positive gold particles were observed in the nucleus (A and E), cytoplasm (B and F) and rough endoplasmic reticulum (C and G). The number of their labelling was indicated by bar graphs (D and H). (*P<0.05, **P<0.01). A and C scale bar, 500 nm; B scale bar, 200 nm; E-G scale bar, 1 µm.
Discussion
The balance of protective and aggressive factors in the gastric mucosal barrier is an important consideration in gastrointestinal diseases (4). Gastric acid, pepsin, and external stimuli are known aggravating factors of gastric mucosal cells, while microcirculatory system, HCO3−, prostaglandins, epidermal growth factor synthesis, and epithelial cell reconstitution are gastro-protective factors that maintain integrity of the gastric mucosal layer. Obviously, there are several viewpoints in association with the effect of ethanol on gastric mucosal cells. However, the main pathogenesis of ethanol-induced gastritis is vascular damage followed by mucosal cell-hypoxia, -degeneration, and -necrosis, respectively. The decline of anti-oxidant level and over production of oxygen free radicals especially super-oxide and hydrogen peroxide, are also link to ethanol-induced gastric injury and play a crucial role to further induce gastric inflammatory response. In addition, ethanol itself destroys gastric mucosal cells and causes them to slough. Then, the remaining cells and submucosal layer are directly exposed to luminal gastric acid. Submucosal edema is a consequence of the increase in vascular permeability, while vascular congestion is exacerbated by alcohol penetration. According to the histopathological and immunohistochemical results, omeprazole attenuated the severity of hemorrhage and inflammation on gastric mucosa (Table I) with upregulation of anti-oxidative stress marker, SOD (Figs. 6 and 7), which contributed to the mucosal protective effect of omeprazole in ethanol-induced gastritis.
Within a past decade, although several products have been considered as candidates to alleviate gastrointestinal diseases according to the mucosal lesion index, mucous production, level of PGE-2, and specific cytokines (15), no studies have described improvement of RER integrity in ethanol-induced gastritis because RER alterations are also crucially involved in the pathogenesis of several gastrointestinal disturbances. SEM usually employed to evaluate mucosal surface cells affected by ethanol, which reveals a flattened or swollen mucosal epithelium and irregularly gastric pits in animal models (16). The gastric mucosal epithelium in human with chronic gastritis presents the swelling, vacuolar degeneration, ribosome dissociation, dilatation of the RER and Golgi's apparatus together with mitochondrial swelling in chief cells (17,18). In the present study, we demonstrated additional histopathological changes in ethanol-induced gastritis other than those in previous reports, including fatty degeneration in submucosal layer and vacuolated degeneration in muscular layer (Table I and Fig. 3I and L). The results also presented that omeprazole improved cellular architecture in the stomach after 4 days post induction. In treatment group, the gastric cells were almost recovered and complete healed on 7 and 14 days post induction, respectively. However, at ultrastructural level, some defects on RER and mitochondria were still observed. It has been appeared that ethanol and luminal gastric acid penetrated deeply to damage all of the epitheliums from mucosal to muscularis layer. We also examined alterations in chief and parietal cells induced by ethanol. Interestingly, we firstly confirmed that ethanol-induced gastritis caused RER alterations in chief cells indicated by a number of large, dilated, and fragmented RERs as shown in Fig. 4A, B, E and F, and omeprazole preserves their integrity in relation to its anti-oxidative and anti-inflammatory effects.
Although, currently it is well described that omeprazole, which is prescribed to suppression acid secretion in the stomach, preserves the gastric mucosal layer, there is no clear evidence that omeprazole reduces ER stress in ethanol-induced gastritis. A recent study suggested that ethanol exacerbates ER stress responses and promotes hepatic, cardiac, and pancreatic diseases through apoptotic pathways (19) however, ethanol-induced ER stress in the stomach is still controversial because of the lack of reports (16). The ER is a protein factory for protein synthesis and folding, after which synthesized proteins are transported to the Golgi. ER stress is caused by retention of proteins in the ER because of unfolding (20) or the so called ‘unfolded protein response’, leading to protein degradation and initiation of cellular apoptosis (21). Moreover, ER stress and impaired protein folding cause up-regulation of reactive oxygen species production (22). A Collapsed RER is closely connected to misfolded protein as shown by our results. It has been postulated that omeprazole may reduce ER stress. Another proposed mechanism for ethanol-induced gastritis is energetic mitochondrial disturbance by oxidative stress (23). In the present study, ultrastructural finding demonstrated that mitochondrial swelling was accompanied by RER alterations (Table I and Fig. 4E and F). This result supports that ER stress similarly triggers mitochondrial alterations and dysfunction, and especially apoptosis (24) with an increase in cytosolic calcium (25). However this mechanistic detail needs to be further studies.
There are several markers used to study parietal cells morphology and functions depending on their roles, AQP-4, water channel membrane protein in gastric acid physiology, is relates to the degree of regenerating parietal cells (10). Early immature parietal cells weakly express AQP-4, which gradually increases upon maturation. Furthermore, because of its function, AQP-4 is typically used as an improvement criterion in studies of gastric mucosal injury (26,27). In the present study, omeprazole maintained the integrity and induced re-epithelialization of the parietal cell as indicated by a number of cell positive for AQP-4 at 4 and 7 days post gastritis induction with increasing trends (Fig. 6A, D and E) as confirmed by immunogold electron microscopic study (Fig. 7). The results indicated that the expression was located throughout of cell especially in the nucleus and rough endoplasmic reticulum. Apart from regenerative aspect, the present of AQP-4 is associated with acid suppression by proton pump inhibitors (11,12). Moreover, inflammatory condition induced by histamine results gastric AQP-4 rearrangement or down-regulation (28). Therefore, other than the re-epithelializing effect, omeprazole exerts anti-acidic and anti-inflammatory effects in the gastric environment. Unfortunately, the correlation between PGE-2 activity to histopathological and ultrastructural changes was not observed in the present study. This limitation may relate to the selection of sampling date post induction due to the difference recovering rat e of gastric mucosal between treated and non-treated rats. The earlier detection, such as on 3, 5 and 7 day, may give better correlation.
In conclusion, deformation of the RER in chief cells is caused by ethanol-induced gastritis. Omeprazole modulates mucosal cells injury affected by ethanol as shown by the improvement of the RER architecture in chief cells and the enhancement of parietal cell regeneration together with exerting gastric anti-inflammatory and anti-oxidative effects.
Acknowledgements
The present study was supported by the National Laboratory Animal Center, Faculty of Tropical Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, and National Research Council of Thailand (NRCT) under the project ‘The Added Value of Herbs Used for Cooking in Households’.
References
- 1.Schneider AS, Szanto PA, editors. Pathology. Fifth Edition. Lippincott William & Wilkins; Philadelphia, PA: 2013. Board Review Series. [Google Scholar]
- 2.Al-Humayed SM, Mohamed-Elbagir AK, Al-Wabel AA, Argobi YA. The changing pattern of upper gastro-intestinal lesions in southern Saudi Arabia: An endoscopic study. Saudi J Gastroenterol. 2010;16:35–37. doi: 10.4103/1319-3767.58766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou LX, Geng B, Yu F, Zhang J, Pan CS, Chen L, Qi YF, Ke Y, Wang X, Tang CS. Endoplasmic reticulum stress response is involved in the pathogenesis of stress induced gastric lesions in rats. Life Sci. 2006;79:1856–1864. doi: 10.1016/j.lfs.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2012;50:35–39. doi: 10.3164/jcbn.11-115SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarebska A, Sekita-Krzak J, Hernik D, Matusiewicz J, Czerny K. Histological examinations of chief and parietal cells of the rat gastric glands after experimental administration of cephalexin and ethanol. Ann Univ Mariae Curie Sklodowska Med. 2004;59:99–104. [PubMed] [Google Scholar]
- 6.Karam SM. A focus on parietal cells as a renewing cell population. World J Gastroenterol. 2010;16:538–546. doi: 10.3748/wjg.v16.i5.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teschke R, Wolff A, Frenzel C, Eickhoff A, Schulze J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World J Gastroenterol. 2015;21:4466–4490. doi: 10.3748/wjg.v21.i15.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidi SF, Muhammad JS, Usmanghani K, Sugiyama T. Review: Pharmacological ins and outs of medicinal plants against Helicobacter pylori: A review. Pak J Pharm Sci. 2015;28(3 Suppl):S1171–S1176. [PubMed] [Google Scholar]
- 9.Chanchal SK, Mahajan UB, Siddharth S, Reddy N, Goyal SN, Patil PH, Bommanahalli BP, Kundu CN, Patil CR, Ojha S. In vivo and in vitro protective effects of omeprazole against neuropathic pain. Sci Rep. 2016;6:30007. doi: 10.1038/srep30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka T, Kobayashi M, Sugimoto T, Araki K. An immunocytochemical study of regeneration of gastric epithelia in rat experimental ulcers. Med Mol Morphol. 2005;38:233–242. doi: 10.1007/s00795-005-0297-0. [DOI] [PubMed] [Google Scholar]
- 11.Fukuhara S, Matsuzaki J, Tsugawa H, Masaoka T, Miyoshi S, Mori H, Fukushima Y, Yasui M, Kanai T, Suzuki H. Mucosal expression of aquaporin-4 in the stomach of histamine type 2 receptor knockout mice and Helicobacter pylori-infected mice. J Gastroenterol Hepatol. 2014;4(29 Suppl):S53–S59. doi: 10.1111/jgh.12771. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki J, Suzuki H, Minegishi Y, Sugai E, Tsugawa H, Yasui M, Hibi T. Acid suppression by proton pump inhibitors enhances aquaporin-4 and KCNQ1 expression in gastric fundic parietal cells in mouse. Dig Dis Sci. 2010;55:3339–3348. doi: 10.1007/s10620-010-1167-8. [DOI] [PubMed] [Google Scholar]
- 13.Rahim NA, Hassandarvish P, Golbabapour S, Ismail S, Tayyab S, Abdulla MA. Gastroprotective effect of ethanolic extract of Curcuma xanthorrhiza leaf against ethanol-induced gastric mucosal lesions in Sprague-Dawley rats. Biomed Res Int. 2014;2014:416409. doi: 10.1155/2014/416409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ampawong S, Aramwit P. Tolerogenic responses of CD206+, CD83+, FOXP3+, and CTLA-4 to sericin/polyvinyl alcohol/glycerin scaffolds relevant to IL-33 and HSP60 activity. Histol Histopathol. 2016;31:1011–1027. doi: 10.14670/HH-11-733. [DOI] [PubMed] [Google Scholar]
- 15.Wang QS, Zhu XN, Jiang HL, Wang GF, Cui YL. Protective effects of alginate-chitosan microspheres loaded with alkaloids from Coptis chinensis Franch. and Evodia rutaecarpa (Juss.) Benth. (Zuojin Pill) against ethanol-induced acute gastric mucosal injury in rats. Drug Des Devel Ther. 2015;9:6151–6165. doi: 10.2147/DDDT.S96056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho KR, Kwon KY, Chang ES. Ultrastructural study of alcohol-induced gastric mucosal change of rat. Korean J Pathol. 1993;27:362–370. [Google Scholar]
- 17.Zhang B, Jin R. Ultrastructural characteristics of gastric mucosal epithelial cells of AIDS patients complicated with chronic gastritis. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2007;21:17–19. (In Chinese) [PubMed] [Google Scholar]
- 18.Zhang ZL, Bu JK, Zhao JX. Ultrastructural observation of the gastric mucosa in chronic gastritis patients treated by traditional Chinese medicine. World J Gastroenterol. 1997;3:185–188. doi: 10.3748/wjg.v3.i3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji C. New insights into the pathogenesis of alcohol-induced ER stress and liver diseases. Int J Hepatol. 2014;2014:513787. doi: 10.1155/2014/513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan JS, He SZ, Xu HZ, Zhan XJ, Yang XN, Xiao HM, Shi HX, Ren JL. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J Gastroenterol. 2008;14:5857–5867. doi: 10.3748/wjg.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apostolova N, Gomez-Sucerquia LJ, Alegre F, Funes HA, Victor VM, Barrachina MD, Blas-Garcia A, Esplugues JV. ER stress in human hepatic cells treated with Efavirenz: Mitochondria again. J Hepatol. 2013;59:780–789. doi: 10.1016/j.jhep.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Chen GX, Lao SX, Huang ZX. Effect of Chinese herbs on expression of aquaporin 3,4 gene in gastric mucosa of patients with Pi-Wei Damp-Heat syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:199–202. (In Chinese) [PubMed] [Google Scholar]
- 27.Feng G, Xu X, Wang Q, Liu Z, Li Z, Liu G. The protective effects of calcitonin gene-related peptide on gastric mucosa injury after cerebral ischemia reperfusion in rats. Regul Pept. 2010;160:121–128. doi: 10.1016/j.regpep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Carmosino M, Procino G, Nicchia GP, Mannucci R, Verbavatz JM, Gobin R, Svelto M, Valenti G. Histamine treatment induces rearrangements of orthogonal arrays of particles (OAPs) in human AQP4-expressing gastric cells. J Cell Biol. 2001;154:1235–1243. doi: 10.1083/jcb.200103010. [DOI] [PMC free article] [PubMed] [Google Scholar]