Abstract
Context:
Multiple consensus statements decree that women with diabetes mellitus should have comparable birth outcomes to women without diabetes mellitus; however, there is a scarcity of contemporary population-based studies on this issue.
Objective:
To examine temporal trends in obstetric interventions and perinatal outcomes in a population-based cohort of women with type 1, type 2, or gestational diabetes mellitus compared with a control population.
Design:
Cross-sectional study.
Setting:
National hospitalization data (Canada except Quebec) from 2004 to 2015.
Patients:
Pregnant women with type 1 (n = 7362), type 2 (n = 11,028), and gestational diabetes mellitus (n = 149,780) and women without diabetes mellitus (n = 2,688,231).
Main Outcome Measures:
Rates of obstetric intervention, maternal morbidity, and neonatal morbidity/mortality.
Results:
A consistent relationship was generally observed between diabetes mellitus subtype and obstetric outcomes, with women with type 1 diabetes mellitus having the highest rate of intervention and the highest rates of adverse perinatal outcomes followed by women with type 2 diabetes mellitus and women with gestational diabetes mellitus. Rates of severe preeclampsia were 1.2% among women without diabetes mellitus, 2.1% among women with gestational diabetes mellitus, 4.2% among women with type 2 diabetes mellitus, and 7.5% among women with type 1 diabetes mellitus (P < 0.001). The rate of neonatal morbidity ranged from 8.7% in women without diabetes mellitus to 11.0%, 17.4%, and 24.1% in women with gestational, type 2, and type 1 diabetes mellitus, respectively (P < 0.001).
Conclusions:
In a contemporary obstetric population, women with diabetes mellitus remain at increased risk of adverse pregnancy outcomes compared with women without diabetes mellitus.
Keywords: diabetes mellitus, gestational diabetes mellitus, maternal morbidity, neonatal morbidity, pregnancy, temporal trends
This population-based study of Canadian women found that women with diabetes mellitus are increased risk of adverse pregnancy outcomes; however, results differed by subtype of diabetes mellitus.
Diabetes mellitus (encompassing type 1 diabetes mellitus, type 2 diabetes mellitus, and gestational diabetes mellitus) is the most common metabolic complication affecting pregnant women and, despite clinical advancements, remains associated with adverse pregnancy outcomes [1]. Although promising improvements have been documented in single-centered studies, there is a scarcity of recent information from large population-based studies examining the impact of diabetes mellitus on obstetrical interventions and outcomes [2].
A population-based study from Ontario, Canada, showed that deliveries to women with preexisting diabetes mellitus (type 1 or type 2 diabetes mellitus) increased between 1996 and 2001 and that preexisting diabetes mellitus was associated with almost twice the odds of labor induction or caesarean delivery and four times the odds of hypertension or preeclampsia [3]. Population-based data from northern England have also documented an increase in deliveries to women with preexisting diabetes mellitus between 1996 and 2004 and high rates of obstetric intervention and adverse pregnancy outcomes [2], and similar findings have been reported in Spain as well [4]. The increasing prevalence of preexisting diabetes mellitus among pregnant women in multiple jurisdictions highlights the need for ongoing surveillance of pregnancy outcomes in this population.
Studies of pregnancy outcomes in the population of diabetic women are difficult to interpret because women with type 1 or type 2 diabetes mellitus are typically categorized as a single group, whereas the conditions are heterogeneous in terms of the need for intervention and the frequency of adverse outcomes [5]. Examining outcomes separately in each group is critical for the identification of quality improvement initiatives [5]. Continued assessment in a more recent time period is also essential to monitor progress toward achieving the goals outlined in the St. Vincent’s Declaration and Istanbul Commitment, which call for continued work to ensure comparable birth outcomes among women with and without diabetes mellitus [6].
This study examined temporal trends in the use of obstetric interventions and adverse perinatal health outcomes in a contemporary population-based cohort of women with type 1, type 2, or gestational diabetes mellitus compared with a control population. Examining interventions and outcomes in these unique population groups may identify areas for continuous quality improvement initiatives.
1. Materials and Methods
A. Data Sources and Linkages
National hospitalization data from Canada (excluding Quebec) from 1 April 2004 to 31 March 2015 were obtained on pregnancies in women with type 1 diabetes mellitus, type 2 diabetes mellitus, and gestational diabetes mellitus and a comparison group of women without diabetes who delivered between 22 and 43 weeks of gestation. Deliveries were identified by the use of ICD-10-CA codes Z37.x (outcome of delivery) and Z38.x (liveborn infants according to place of birth). Morbidity among women and infants was identified in the Discharge Abstract Database using ICD-10-CA codes that were assigned by trained health records personnel based on physician notations made in the patient’s medical chart. Although the Discharge Abstract Database is valid for identifying easily visible congenital anomalies, it has a low sensitivity and positive predictive value for specific classes of anomalies [7], and therefore mother-infant dyads were excluded if the infant had a congenital or chromosomal anomaly (ICD-10-CA Q00 to Q99) (n = 170,577) or if gestational age data were missing (n = 5271).
Hospital delivery records are able to accurately identify women with preexisting and gestational diabetes mellitus. A systematic review found that sensitivity ranged from 71.0% to 81.3%, specificity ranged from 99.4% to 99.6%, and positive predictive values ranged from 50.0% to 88.8% for gestational diabetes mellitus, whereas sensitivity ranged from 78.0% to 95.3%, specificity ranged from 99.4% to 100.0%, and positive predictive value ranged from 94.0% to 97.6% for preexisting diabetes mellitus [8]. Canadian studies [9, 10] show that the diagnosis of diabetes mellitus in the Discharge Abstract Database is valid: a recent study from the British Columbia Perinatal Data Registry, which shares ICD-10 diagnostic codes with the Discharge Abstract Database, showed a sensitivity of 94.7%, a specificity of 99.6%, and a positive predictive value of 65.3% for diabetes mellitus [10]. The following hierarchical algorithm was used to classify women with diabetes mellitus: codes for type 1 diabetes mellitus (ICD-10-CA E10.x, O24.5) superseded all other diabetes mellitus codes, and women with these codes were classified as having type 1 diabetes mellitus. Codes for type 2 diabetes mellitus (ICD-10-CA E11.x, O24.6) superseded gestational diabetes mellitus codes, and women with these codes were classified as having type 2 diabetes mellitus. Women with the codes for gestational diabetes mellitus (ICD-10-CA O24.8; infant: ICD-10-CA P70.0) who did not have other codes for preexisting diabetes mellitus were classified as having gestational diabetes mellitus.
B. Outcomes
The Obstetric Comorbidity Index was used to identify various types of comorbidities and obstetric risk factors among women with different types of diabetes mellitus [11, 12]. The Obstetric Comorbidity Index is a weighted algorithm that assigns points for the presence of preexisting comorbidities, substance-related conditions, pregnancy-related conditions, and advanced maternal age (≥35 years) [11]. Originally developed in a US Medicaid population to predict maternal morbidity and mortality [11], this index has subsequently been validated in a Canadian population [12]. Obstetric intervention comprising labor induction (5.AC.30.^^) and caesarean section (5.MD.60.^^) was identified using relevant Canadian Classification of Interventions (CCI) codes. Maternal and newborn length of stay were obtained by subtracting the admission date from the discharge date. Length of stay refers to the entire time spent in the hospital inclusive of, but not restricted to, time spent in intensive care units. Prolonged maternal and neonatal length of stay was defined as length of stay >2 days following a vaginal birth and >4 days following a caesarean delivery based on contemporary population norms for length of stay in Canada [13]. Maternal morbidity/mortality was defined as the presence of at least one of the following: maternal death, obstetric embolism (ICD-10-CA O88), obstetric shock (ICD-10-CA O75.1, R57, T80.5, T88.6), postpartum hemorrhage with hysterectomy or other procedures to control bleeding (ICD-10-CA O72.0 to O72.3 and CCI 5MD60KE, 5MD60RC, 5MD60CB, 5MD60RD, 1RM87LAGX, 1RM89LA, 5PC91LA, 1KT51, or 1RM13 without 1PL74, 1RS80, 1RS74), sepsis (ICD-10-CA O75.3, O85), third- or fourth-degree perineal laceration (ICD-10-CA O70.2, O70.3), uterine rupture (ICD-10-CA O71.0, O71.1), or venous thromboembolism (ICD-10-CA G08, I26, I80.1, I80.2, I80.3, I80.8, 180.9, I82, K55.0, K55.9, K75.1, N28.0, O07.2, O07.7, O08.2, O22.3, O22.8, O22.9, O87.1, O87.9, O88.2). Neonatal morbidity comprised birth asphyxia (ICD-10-CA P21), fetal asphyxia (ICD-10-CA P20), grade 3 or 4 intraventricular hemorrhage (ICD-10-CA P52.2), neonatal convulsions (ICD-10-CA P90), other disturbances of cerebral status of the newborn (ICD-10-CA P91), respiratory distress syndrome (ICD-10-CA P22), birth injuries (ICD-10-CA P10, P11, P13, P14 or CCI 5.MD.45.QB, 5.MD.45.QC), or shoulder dystocia (ICD-10-CA O66.0). Preterm birth was defined as delivery prior to 34 and 37 weeks of gestation, and perinatal mortality was defined as stillbirth or neonatal death in the hospital. Analyses of perinatal mortality were restricted to pregnancies reaching at least 30 weeks of gestation to address the issue of immortal time bias [14]. As gestational diabetes is typically not diagnosed until 24 to 28 weeks of gestation, the fetus must survive until at least this time to permit a diagnosis of gestational diabetes [14]. No differences in perinatal mortality prior to 30 weeks of gestation were observed between women with type 1 and type 2 diabetes compared with the general population (P ≥ 0.05).
C. Ethics Statement
Ethics approval was obtained from the Conjoint Health Research Ethics Board at the University of Calgary.
D. Statistical Analysis
Nonparametric tests for trend were used to examine temporal patterns in the prevalence of comorbidities, obstetric interventions, and perinatal outcomes in women with diabetes mellitus [15]. χ2 Tests were used to examine the association between diabetes mellitus and severe maternal outcomes and between diabetes mellitus subtypes and neonatal morbidity, as well as perinatal mortality. Relative risk estimates of obstetrical interventions and maternal and neonatal complications between diabetes mellitus subtypes were obtained from multivariable log binominal models after adjusting for the Obstetric Comorbidity Index, which includes maternal comorbidities, obstetrical risk factors, and age. Incidence rate ratios for maternal and neonatal length of stay in days were obtained from multivariable Poisson regression models after adjusting for the Obstetric Comorbidity Index. For outcomes with increased risk among women with diabetes mellitus, population attributable fractions were calculated using the adjusted effect estimates obtained from the regression models to quantify the impact of diabetes mellitus subtype on overall risk in the population. All analyses were conducted in Stata SE version 14 (StataCorp LP, College Station, TX) and α <0.05 was used to determine statistical significance.
2. Results
Overall, 2,856,401 births met all eligibility criteria, including 7362 births to women with type 1 diabetes mellitus, 11,028 births to women with type 2 diabetes mellitus, and 149,780 births to women with gestational diabetes mellitus. The prevalence of diabetes mellitus in pregnancy increased over time for all subtypes (P < 0.001). The prevalence of type 1 diabetes mellitus in pregnancy increased from 0.3% [95% confidence interval (CI), 0.2% to 0.3%] in 2004 to 0.3% (95% CI, 0.3% to 0.3%) in 2014, the prevalence of type 2 diabetes mellitus in pregnancy increased from 0.2% (95% CI, 0.2% to 0.3%) in 2004 to 0.5% (95% CI, 0.4% to 0.5%) in 2014, and the prevalence of gestational diabetes mellitus increased from 4.0% (95% CI, 4.0% to 4.1%) in 2004 to 7.0% (95% CI, 6.9% to 7.1%) in 2014.
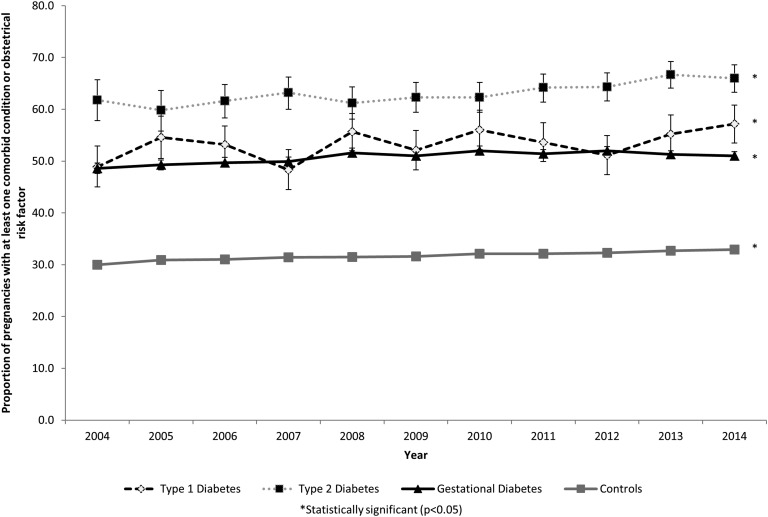
Comorbidities and obstetric risk factors were common among women with diabetes mellitus, with 53.3% of women with type 1 diabetes mellitus, 63.4% of women with type 2 diabetes mellitus, and 50.9% of women with gestational diabetes mellitus having at least one comorbid condition (Table 1). A statistically significant higher prevalence of comorbidities and obstetrical risk factors was observed in women with type 1 diabetes mellitus (P = 0.02), type 2 diabetes mellitus (P < 0.001), and gestational diabetes mellitus (P < 0.001) pregnancies over time (Fig. 1). The types of comorbidities present differed between groups (Table 1). For example, the prevalence of severe preeclampsia was almost twofold higher among women with type 1 diabetes mellitus compared with women with type 2 diabetes mellitus, and chronic hypertension was fivefold more common in women with type 2 diabetes mellitus compared with women with gestational diabetes mellitus.
Table 1.
Types of Preexisting Comorbidities and Obstetrical Risk Factors in the Current Pregnancy as Defined by the Obstetrical Comorbidity Index
| Comorbidity |
Prevalence |
P Value | |||
|---|---|---|---|---|---|
| Women Without Diabetes Mellitus (n = 2,688,231), n (%, 95% CI) | Type 1 Diabetes Mellitus (n = 7362), n (%, 95% CI) | Type 2 Diabetes Mellitus (n = 11,028), n (%, 95% CI) | Gestational Diabetes Mellitus (n = 149,780), n (%, 95% CI) | ||
| Alcohol abuse | 2248 (0.08, 0.08–0.09) | 6 (0.08, 0.04–0.18) | 21 (0.19, 0.12–0.29) | 76 (0.05, 0.04–0.06) | <0.001 |
| Asthma | 10,325 (0.38, 0.38–0.39) | 53 (0.72, 0.55–0.94) | 116 (1.05, 0.88–1.26) | 849 (0.57, 0.53–0.61) | <0.001 |
| Cardiac valvular disease | 2106 (0.08, 0.08–0.08) | 8 (0.11, 0.05–0.22) | 11 (0.10, 0.06–0.18) | 109 (0.07, 0.06–0.09) | 0.55 |
| Chronic congestive heart failure | 269 (0.01, 0.01–0.01) | 10 (0.14, 0.07–0.25) | 9 (0.08, 0.04–0.16) | 31 (0.02, 0.01–0.03) | <0.001 |
| Chronic ischemic heart disease | 108 (0.00, 0.00–0.00) | <5 | 13 (0.12, 0.07–0.20) | 17 (0.01, 0.01–0.02) | <0.001 |
| Chronic renal disease | 626 (0.02, 0.02–0.03) | 96 (1.30, 1.07–1.59) | 70 (0.63, 0.50–0.80) | 68 (0.05, 0.04–0.06) | <0.001 |
| Congenital heart disease | 9580 (0.36, 0.35–0.36) | 51 (0.69, 0.53–0.91) | 90 (0.82, 0.66–10.02) | 599 (0.40, 0.37–0.43) | <0.001 |
| Drug abuse | 13,304 (0.49, 0.49–0.50) | 25 (0.34, 0.23–0.50) | 84 (0.76, 0.62–0.94) | 342 (0.23, 0.21–0.25) | <0.001 |
| Gestational hypertension | 107,612 (4.00, 3.98–4.03) | 924 (12.55, 11.81–13.33) | 1326 (12.02, 11.43–12.64) | 11,874 (7.93, 7.79–8.07) | <0.001 |
| Human immunodeficiency virus | 1494 (0.06, 0.05–0.06) | 8 (0.11, 0.05–0.22) | 20 (0.18, 0.12–0.28) | 99 (0.07, 0.05–0.08) | <0.001 |
| Mild/unspecified preeclampsia | 2707 (0.10, 0.10–0.10) | 105 (1.43, 1.18–1.72) | 223 (2.02, 1.78–2.30) | 473 (0.32, 0.29–0.35) | <0.001 |
| Multiple gestation | 44,854 (1.67, 1.65–1.68) | 133 (1.81, 1.53–2.14) | 234 (2.12, 1.87–2.41) | 3502 (2.34, 2.26–2.42) | <0.001 |
| Placenta previa | 15,602 (0.58, 0.57–0.59) | 37 (0.50, 0.36–0.69) | 91 (0.83, 0.67–1.01) | 1355 (0.90, 0.86–0.94) | <0.001 |
| Preexisting hypertension | 12,944 (0.48, 0.47–0.49) | 307 (4.17, 3.74–4.65) | 1055 (9.57, 9.03–10.13) | 2686 (1.79, 1.73–1.86) | <0.001 |
| Previous caesarean delivery | 278,459 (10.36, 10.32–10.39) | 1689 (22.94, 22.00–23.92) | 2489 (22.57, 21.80–23.36) | 24,152 (16.12, 15.94–16.31) | <0.001 |
| Pulmonary hypertension | 160 (0.01, 0.00–0.01) | 5 (0.07, 0.03–0.16) | <5 | 15 (0.01, 0.01–0.02) | <0.001 |
| Severe preeclampsia | 31,844 (1.18, 1.17–1.20) | 554 (7.53, 6.94–8.15) | 464 (4.21, 3.85–4.60) | 3071 (2.05, 1.98–2.12) | <0.001 |
| Sickle cell disease | 2877 (0.11, 0.10–0.11) | 8 (0.11, 0.05–0.22) | 30 (0.27, 0.19–0.39) | 299 (0.20, 0.18–0.22) | <0.001 |
| Systemic lupus erythematosus | 1279 (0.05, 0.05–0.05) | <5 | 8 (0.07, 0.04–0.14) | 62 (0.04, 0.03–0.05) | 0.44 |
| Maternal age at delivery (y) | |||||
| >44 | 3766 (0.14, 0.14–0.14) | 7 (0.10, 0.05–0.20) | 79 (0.72, 0.57–0.89) | 784 (0.52, 0.49–0.56) | |
| 40–44 | 76,363 (2.84, 2.82–2.86) | 196 (2.66, 2.32–3.06) | 1071 (9.71, 9.17–10.28) | 11,108 (7.42, 7.28–7.55) | <0.001 |
| 35–39 | 407,407 (15.16, 15.11–15.20) | 1161 (15.77, 14.96–16.62) | 3115 (28.25, 27.41–29.09) | 38,923 (25.99, 25.77–26.21) | |
| Any of the above | 851,956 (31.69, 31.63–31.75) | 3925 (53.31, 52.17–54.45) | 6994 (63.42, 62.52–64.31) | 76,231 (50.90, 50.64–51.15) | <0.001 |
Figure 1.
Temporal trends in the proportion of pregnancies in women with type 1, type 2, and gestational diabetes mellitus with at least one comorbid condition (excluding diabetes mellitus) compared with women without diabetes mellitus (Canada, excluding Quebec, 2004 to 2015).
A consistent relationship was generally observed between diabetes mellitus subtype and obstetric intervention; women with type 1 diabetes mellitus had the highest rate of intervention and the longest length of stay, followed by women with type 2 diabetes mellitus and women with gestational diabetes mellitus (Table 2). These relationships were attenuated after controlling for comorbidities, obstetrical risk factors, and maternal age but remained higher than observed in the control group. Despite the increased risk of obstetric interventions and adverse perinatal outcomes for women with diabetes mellitus, population attributable fractions were small (Table 2). Population attributable fractions were typically highest for gestational diabetes mellitus, reflecting the higher prevalence of this condition compared with type 1 and type 2 diabetes mellitus.
Table 2.
Impact of Diabetes Mellitus Subtypes on Obstetrical Interventions and Maternal and Neonatal Health Outcomes
| Characteristic | Women Without Diabetes Mellitus | Type 1 Diabetes Mellitus | Type 2 Diabetes Mellitus | Gestational Diabetes Mellitus |
|---|---|---|---|---|
| Labor induction | ||||
| Rate per 100 deliveries (%, 95% CI) | 22.04 (21.99–22.09) | 41.04 (39.92–42.16) | 43.61 (42.68–44.53) | 35.33 (35.09–35.57) |
| Crude RR (95% CI) | 1.86 (1.81–1.91) | 1.98 (1.94–2.02) | 1.60 (1.59–1.61) | |
| Adjusted RRa (95% CI) | 1.81 (1.76–1.86) | 1.91 (1.87–1.95) | 1.58 (1.56–1.59) | |
| Population attributable fraction (95% CI) | 0.22 (0.21–0.24) | 0.38 (0.36–0.39) | 2.99 (2.93–3.05) | |
| Caesarean section | ||||
| Rate per 100 deliveries (%, 95% CI) | 26.59 (26.53–26.64) | 60.55 (59.43–61.67) | 51.72 (50.79–52.65) | 38.25 (38.00–38.49) |
| Crude RR (95% CI) | 2.28 (2.24–2.32) | 1.95 (1.91–1.98) | 1.44 (1.43–1.45) | |
| Adjusted RRa (95% CI) | 1.70 (1.64–1.78) | 1.42 (1.39–1.45) | 1.25 (1.24–1.26) | |
| Population attributable fraction (95% CI) | 0.25 (0.22–0.28) | 0.23 (0.21–0.24) | 1.47 (1.42–1.53) | |
| Prolonged maternal length of stay | ||||
| Rate per 100 deliveries (%, 95% CI) | 17.88 (17.83–17.93) | 43.06 (41.93–44.19) | 38.18 (37.27–39.09) | 22.08 (21.89–22.29) |
| Crude RR (95% CI) | 2.41 (2.35–2.47) | 2.14 (2.08–2.19) | 1.23 (1.22–1.25) | |
| Adjusted RRa (95% CI) | 2.16 (2.10–2.22) | 1.87 (1.82–1.91) | 1.16 (1.15–1.17) | |
| Population attributable fraction (95% CI) | 0.35 (0.33–0.37) | 0.40 (0.38–0.42) | 0.89 (0.83–0.95) | |
| Prolonged neonatal length of stay | ||||
| Rate per 100 deliveries (%, 95% CI) | 15.45 (15.40–15.49) | 40.56 (39.44–41.69) | 34.77 (33.88–35.66) | 18.18 (17.98–18.37) |
| Crude RR (95% CI) | 2.63 (2.55–2.70) | 2.25 (2.19–2.31) | 1.18 (1.16–1.19) | |
| Adjusted RRa (95% CI) | 2.29 (2.22–2.35) | 1.90 (1.85–1.95) | 1.09 (1.08–1.10) | |
| Population attributable fraction (95% CI) | 0.40 (0.38–0.42) | 0.43 (0.41–0.46) | 0.50 (0.44–0.57) | |
| Maternal morbidity/mortalityb | ||||
| Rate per 100 deliveries (%, 95% CI) | 3.34 (3.32–3.36) | 3.80 (3.39–4.27) | 2.86 (2.56–3.18) | 3.40 (3.31–3.49) |
| Crude RR (95% CI) | 1.14 (1.01–1.28) | 0.85 (0.77–0.95) | 1.02 (0.99–1.04) | |
| Adjusted RRa (95% CI) | 1.21 (1.08–1.36) | 0.93 (0.84–1.04) | 1.06 (1.03–1.09) | |
| Population attributable fraction (95% CI) | 0.05 (0.02–0.09) | — | 0.31 (0.16–0.46) | |
| Preterm birth (<37 weeks) | ||||
| Rate per 100 deliveries (%, 95% CI) | 6.73 (6.70–6.76) | 32.42 (31.36–33.50) | 23.70 (22.92–24.51) | 10.51 (10.35–10.66) |
| Crude RR (95% CI) | 4.82 (4.66–4.98) | 3.52 (3.41–3.64) | 1.56 (1.54–1.59) | |
| Adjusted RRa (95% CI) | 3.32 (3.10–3.56) | 2.40 (2.31–2.49) | 1.32 (1.30–1.34) | |
| Population attributable fraction (95% CI) | 0.90 (0.80–1.00) | 0.82 (0.77–0.87) | 1.91 (1.78–2.03) | |
| Preterm birth (<34 weeks) | ||||
| Rate per 100 deliveries (%, 95% CI) | 1.76 (1.75–1.78) | 7.56 (6.97–8.18) | 6.15 (5.71–6.61) | 2.34 (2.27–2.42) |
| Crude RR (95% CI) | 4.27 (3.94–4.63) | 3.48 (3.23–3.74) | 1.32 (1.28–1.37) | |
| Adjusted RRa (95% CI) | 2.60 (2.24–3.01) | 2.17 (2.01–2.35) | 1.08 (1.04–1.12) | |
| Population attributable fraction (95% CI) | 0.71 (0.52–0.88) | 0.75 (0.64–0.86) | 0.47 (0.23–0.72) | |
| Neonatal morbidityc | ||||
| Rate per 100 deliveries (%, 95% CI) | 8.66 (8.63–8.70) | 24.11 (23.15–25.10) | 17.42 (16.72–18.14) | 11.02 (10.86–11.18) |
| Crude RR (95% CI) | 2.78 (2.67–2.90) | 2.01 (1.93–2.09) | 1.27 (1.25–1.29) | |
| Adjusted RRa (95% CI) | 2.54 (2.44–2.64) | 1.79 (1.72–1.87) | 1.21 (1.19–1.23) | |
| Population attributable fraction (95% CI) | 0.46 (0.43–0.49) | 0.36 (0.33–0.39) | 1.13 (1.03–1.23) | |
| Perinatal mortalityd | ||||
| Rate per 100 deliveries (%, 95% CI) | 0.32 (0.31–0.32) | 1.30 (1.06–1.59) | 1.74 (1.51–2.01) | 0.44 (0.41–0.47) |
| Crude RR (95% CI) | 4.12 (3.37–5.04) | 5.53 (4.79–6.38) | 1.39 (1.29–1.51) | |
| Adjusted RRa (95% CI) | 3.54 (2.89–4.34) | 4.61 (3.98–5.33) | 1.28 (1.18–1.39) | |
| Population attributable fraction (95% CI) | 0.79 (0.57–1.01) | 1.71 (1.40–2.02) | 1.55 (0.98–2.11) |
Abbreviation: RR, relative risk.
Models are adjusted for the Obstetric Comorbidity Index.
Maternal morbidity/mortality includes maternal death, obstetric embolism, obstetric shock, postpartum hemorrhage with hysterectomy or other procedures to control bleeding, sepsis, third- or fourth-degree perineal laceration, uterine rupture, and venous thromboembolism.
Neonatal morbidity includes birth asphyxia, fetal asphyxia, grade 3 or 4 intraventricular hemorrhage, neonatal convulsions, other disturbances of cerebral status of the newborn, respiratory distress syndrome, birth injuries, and shoulder dystocia.
Perinatal mortality includes stillbirth and neonatal death in the hospital, and this analysis was restricted to pregnancies with a gestational age ≥30 weeks.
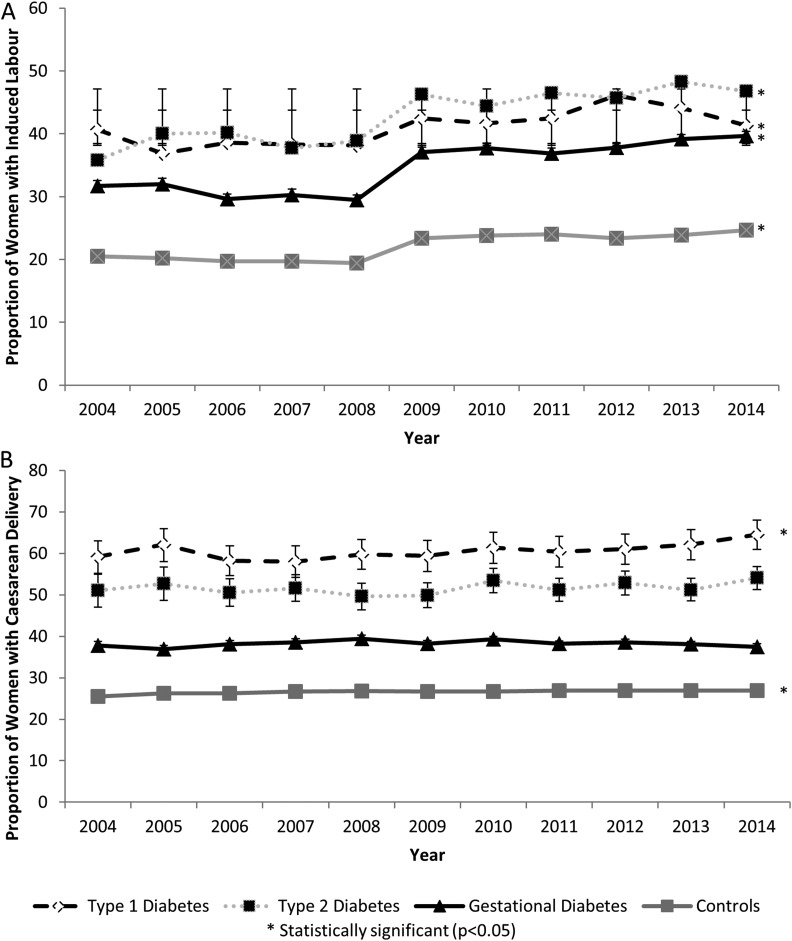
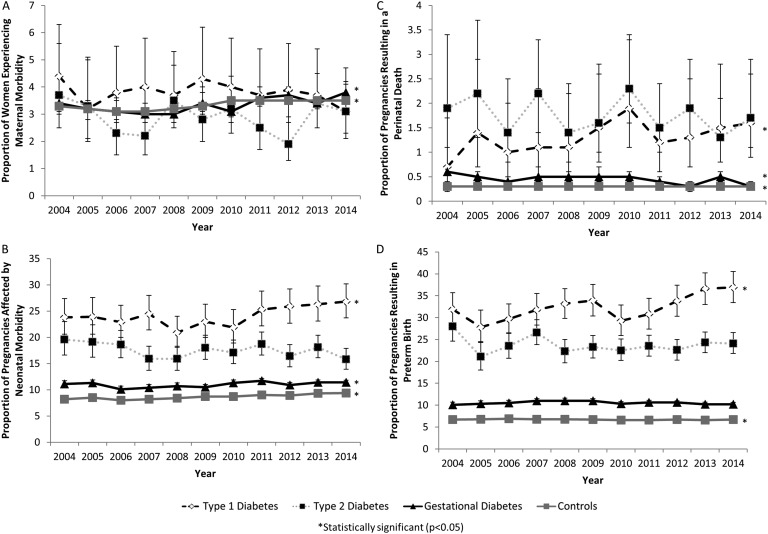
Although the use of labor induction increased significantly over time for women with type 1 (P = 0.001), type 2 (P < 0.001), and gestational (P < 0.001) diabetes, the rate of caesarean delivery remained constant among women with type 2 diabetes (P = 0.18) and gestational diabetes (P = 0.94) but increased from 51.2% (95% CI, 55.2% to 63.0%) to 64.5% (95% CI, 60.9% to 68.0%) in women with type 1 diabetes (P = 0.02) (Fig. 2). Overall, women with diabetes were more likely to experience maternal morbidity/mortality and neonatal morbidity (Table 2). No significant changes were observed in the rate of maternal morbidity/mortality over time for women with type 1 (P = 0.54) or type 2 diabetes (P = 0.80). However, the rate of maternal morbidity/mortality increased significantly in women with gestational diabetes from 3.4% (95% CI, 3.1% to 3.8%) in 2004 to 3.8% (95% CI, 3.6% to 4.1%) in 2014 (P < 0.001) (Fig. 3). The rate of neonatal morbidity increased among women with type 1 diabetes from 23.8% (95% CI, 20.6% to 27.4%) to 26.8% (95% CI, 23.7% to 30.2%) (P = 0.03) and among women with gestational diabetes from 11.1% (95% CI, 10.5% to 11.7%) to 11.4% (95% CI, 10.9% to 11.8%) (P = 0.003). No temporal changes in patterns were observed for women with type 2 diabetes (P = 0.19) (Fig. 3). The increased rate of neonatal morbidity among women with type 1 diabetes was partly due to the increased rate of preterm birth in this population, which increased from 31.9% (95% CI, 28.4% to 35.7%) in 2004% to 36.9% (95% CI, 33.4% to 40.5%) in 2014 (P = 0.001). No temporal changes in the preterm birth rate were observed for women with type 2 (P = 0.45) or gestational diabetes (P = 0.30) (Fig. 3). The rate of perinatal mortality decreased from 0.6% (95% CI, 0.4% to 0.7%) in 2004 to 0.3% (95% CI, 0.3% to 0.4%) in 2014 among women with gestational diabetes (P = 0.01). No temporal changes in perinatal mortality rates were observed for women with type 1 diabetes (P = 0.13) or women with type 2 diabetes (P = 0.43) (Fig. 3).
Figure 2.
Temporal trends in obstetric intervention (A, labor induction; B, caesarean section) among pregnancies in women with type 1, type 2, and gestational diabetes mellitus compared with women without diabetes mellitus (Canada, excluding Quebec, 2004 to 2015).
Figure 3.
Temporal trends in obstetric outcomes (A, maternal morbidity; B, neonatal morbidity; C, perinatal death; D, preterm birth) among pregnancies in women with type 1, type 2, and gestational diabetes mellitus compared with women without diabetes mellitus (Canada, excluding Quebec, 2004 to 2015).
3. Discussion
Our study showed that in a contemporary obstetric population, infants of women with gestational diabetes mellitus, type 2 diabetes mellitus, and type 1 diabetes mellitus had higher rates of neonatal morbidity and neonatal length of stay compared with infants of women without diabetes mellitus. Maternal morbidity/mortality rates were significantly higher among women with gestational diabetes mellitus and type 1 diabetes mellitus, and maternal length of stay was increased among women with all diabetes mellitus subtypes. Rates of labor induction and caesarean delivery were also higher among women with diabetes mellitus. Although perinatal mortality rates remained increased in all diabetes subtypes, rates of perinatal mortality decreased in women with gestational diabetes.
These findings are concordant with other published findings [2–4], and confirm that progress on achieving the St. Vincent’s Declaration and Istanbul Commitment to having comparable birth outcomes in women with and without diabetes mellitus have been slow [6]. Of particular concern is that some adverse outcomes may be becoming more common among certain diabetes mellitus subtypes.
This study highlights key differences in obstetric management and pregnancy outcomes by diabetes mellitus subtype, indicating the need to study women with type 1, type 2, and gestational diabetes mellitus separately. Although outcomes in women with type 1 and type 2 diabetes mellitus tend to be worse than in women with gestational diabetes mellitus, the increased rate of adverse outcomes in women with gestational diabetes mellitus compared with controls is still noteworthy due to the large and increasing size of this population. The larger impact of gestational diabetes mellitus at the population level is reflected by the increased population attributable fractions in this group. Furthermore, women with gestational diabetes mellitus are at increased risk of developing type 2 diabetes mellitus and may enter subsequent pregnancies with type 2 diabetes mellitus [1]. The difference in preconception health status between these three groups also highlights that different management strategies may be needed to modify the risk of adverse perinatal outcomes.
This study has both strengths and limitations. The use of a large population-based database permitted the examination of rare outcomes stratified by type of diabetes mellitus. This stratification of outcomes by diabetes mellitus subtype is critical for clinicians to be able to provide accurate patient counseling and may provide important baseline data for quality improvement initiatives [5]. Unfortunately, important clinical data on diabetes mellitus control, medication use, obesity status, and indications for obstetric interventions were not available in the Discharge Abstract Database. Previous Canadian studies suggest that diabetes mellitus control prior to pregnancy is still suboptimal for women with both type 1 and type 2 diabetes mellitus, and only 43.1% and 18.4% of women with type 1 and 2 diabetes mellitus, respectively, receive preconception care [16]. The lack of adequate preconception care in these populations likely contributes to poor outcomes in both groups. Data were also not available on timing of screening for gestational diabetes and on the diagnostic criteria used. The use of different diagnostic criteria over time may explain the observed temporal increase in gestational diabetes as well as the temporal reduction in perinatal mortality rates among such women, as more women are classified as having gestational diabetes according to the International Association of Diabetes in Pregnancy Study Group criteria [17]. Despite using a validated case definition to identify women with diabetes, misclassification is still possible whereby women with insulin-dependent type 2 diabetes could have been classified as having type 1 diabetes, or overweight women with gestational diabetes could have been classified as having type 2 diabetes. As this case definition is more specific than sensitive, this misclassification would typically result in women being mistakenly assigned to a more severe class of diabetes.
Adverse perinatal outcomes are a consequence of numerous factors, including the individual’s underlying health status and receipt of health services—both factors likely contribute to the higher rate of adverse outcomes in women with diabetes mellitus [18]. Importantly, this study demonstrates the differences in the prevalence of comorbidities, obstetric risk factors, and the rate of adverse obstetric outcomes among women with different subtypes of diabetes mellitus, which may help identify targets for interventions. Furthermore, a greater recognition of the obstetric risks associated with different types of diabetes mellitus in pregnancy may result in a better alignment of health care resources with patient needs.
Acknowledgments
Financial Support: This study was funded by a grant from the Canadian Institutes of Health Research (TOG-341535). A.M. is supported by a New Investigator award (NIA-360943) and K.S.J. is supported by a Chair award (APR-126338) from the Canadian Institutes of Health Research.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CCI
- Canadian Classification of Interventions
- CI
- confidence interval
- ICD-10-CA
- International Classification of Disease Version 10 Canadian Modification.
References and Notes
- 1.Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, Posner SF, Callaghan WM. Diabetes trends among delivery hospitalizations in the U.S., 1994–2004. Diabetes Care. 2010;33(4):768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell R, Bailey K, Cresswell T, Hawthorne G, Critchley J, Lewis-Barned N; Northern Diabetic Pregnancy Survey Steering Group . Trends in prevalence and outcomes of pregnancy in women with pre-existing type I and type II diabetes. BJOG. 2008;115(4):445–452. [DOI] [PubMed] [Google Scholar]
- 3.Feig DS, Razzaq A, Sykora K, Hux JE, Anderson GM. Trends in deliveries, prenatal care, and obstetrical complications in women with pregestational diabetes: a population-based study in Ontario, Canada, 1996–2001. Diabetes Care. 2006;29(2):232–235. [DOI] [PubMed] [Google Scholar]
- 4.de Andrés AL, Jiménez-García R, Carrasco-Garrido P. Trends in pregestational diabetes among women delivering in Spain, 2001–2008. Int J Gynaecol Obstet. 2012;117(2):182–183. [DOI] [PubMed] [Google Scholar]
- 5.Owens LA, Sedar J, Carmody L, Dunne F. Comparing type 1 and type 2 diabetes in pregnancy—similar conditions or is a separate approach required? BMC Pregnancy Childbirth. 2015;15(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall M, Felton A. The St Vincent declaration 20 years on—defeating diabetes in the 21st century. Diabetes Voice. 2009;54:42–44. [Google Scholar]
- 7.Metcalfe A, Sibbald B, Lowry RB, Tough S, Bernier FP. Validation of congenital anomaly coding in Canada’s administrative databases compared with a congenital anomaly registry. Birth Defects Res A Clin Mol Teratol. 2013;100(2):59–66. [DOI] [PubMed] [Google Scholar]
- 8.Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Matern Child Health J. 2008;13(5):660–666. [DOI] [PubMed] [Google Scholar]
- 9.Frosst G, Hutcheon J, Joseph KS, Kinniburgh B, Johnson C, Lee L. Validating the British Columbia Perinatal Data Registry: a chart re-abstraction study. BMC Pregnancy Childbirth. 2015;15(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen VM, Dodds L, Spencer A, Cummings EA, MacDonald N, Kephart G. Application of a national administrative case definition for the identification of pre-existing diabetes mellitus in pregnancy. Chronic Dis Inj Can. 2012;32(3):113–120. [PubMed] [Google Scholar]
- 11.Bateman BT, Mhyre JM, Hernandez-Diaz S, Huybrechts KF, Fischer MA, Creanga AA, Callaghan WM, Gagne JJ. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122(5):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalfe A, Lix LM, Johnson JA, Currie G, Lyon AW, Bernier F, Tough SC. Validation of an obstetric comorbidity index in an external population. BJOG. 2015;122(13):1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalfe A, Mathai M, Liu S, Leon JA, Joseph KS. Proportion of neonatal readmission attributed to length of stay for childbirth: a population-based cohort study. BMJ Open. 2016;6(9):e012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutcheon JA, Kuret V, Joseph KS, Sabr Y, Lim K. Immortal time bias in the study of stillbirth risk factors: the example of gestational diabetes. Epidemiology. 2013;24(6):787–790. [DOI] [PubMed] [Google Scholar]
- 15.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. [DOI] [PubMed] [Google Scholar]
- 16.Kallas-Koeman M, Khandwala F, Donovan LE. Rate of preconception care in women with type 2 diabetes still lags behind that of women with type 1 diabetes. Can J Diabetes. 2012;36(4):170–174. [Google Scholar]
- 17.Kong JM, Lim K, Thompson DM. Evaluation of the International Association of the Diabetes in Pregnancy Study Group new criteria: gestational diabetes project. Can J Diabetes. 2015;39(2):128–132. [DOI] [PubMed] [Google Scholar]
- 18.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet Gynecol. 2009;113(5):1075–1081. [DOI] [PubMed] [Google Scholar]