Abstract
Metastasis is one of the most aberrant behaviors of cancer cells. Patients with cancers, including colorectal cancer (CRC), have a higher risk of tumor recurrence and cancer-related mortality once metastasis is diagnosed. Existing treatment strategies fail to cure cancer mostly due to the onset of metastasis. Therefore, metastasis remains a challenge in cancer treatment. Some complementary and alternative medical therapies using traditional Chinese medicine have been demonstrated to be clinically effective in cancer treatment. Scutellaria barbata D. Don (SB) is a promising medicinal herb. It was previously reported that the ethanol extract of SB (EESB) is able to promote apoptosis, and inhibit cell proliferation and angiogenesis in human colon cancer cells. However, the anticancer effect of SB and the underlying mechanism require further investigation, particularly its role against metastasis. To further elucidate the antimetastatic effect of SB, MTT and Transwell assays were used in the present study to evaluate the effect of EESB on the proliferation, migration and invasion of the CRC cell line HCT-8. In addition, western blot analysis was performed to detect the expression of matrix metalloproteinases (MMPs), cadherins and other metastasis-associated proteins. EESB significantly reduced HCT-8 cell viability and attenuated the migration and invasion ability of HCT-8 cells in a dose-dependent manner. In addition, EESB decreased the expression of MMP-1, MMP-2, MMP-3/10, MMP-9 and MMP-13, and proteins in the phosphoinositide 3-kinase (PI3K)/AKT and transforming growth factor (TGF)-β/Smad pathways, but not the epithelial-mesenchymal transition (EMT)-related factors E-cadherin and N-cadherin. In conclusion, the results suggested that SB inhibits CRC cell metastasis via the suppression of PI3K/AKT and TGF-β/Smad signaling pathways, which may represent a mechanism by which SB exerts an anticancer effect.
Keywords: Scutellaria barbata D. Don, colorectal cancer, migration, invasion, PI3K/AKT pathway, TGF-β/Smad pathway
Introduction
Colorectal cancer (CRC) is one of the most common epithelial cancers (1). Epidemiologically, CRC is the third most prevalent cancer worldwide with a high mortality rate in males and females (2). Although CRC management and therapy are performed by screening, surgery, adjuvant irradiation and chemotherapy, CRC remains one of the most life-threatening malignancies, particularly when it reaches the advanced stages (3,4). Approximately 50% of patients with CRC develop metastasis, which is usually incurable and fatal (5). The majority of patients with CRC and distant metastasis are not suitable candidates for conventional intervention and exhibit a poor 5-year survival rate of <10% (6). From a therapeutic perspective, the identification of molecular mechanisms underlying the metastatic progression of CRC may contribute to the reduction of morbidity and mortality (7). In addition, the discovery of effective and safe compounds for the treatment of CRC is urgently required in order to reduce morbidity and mortality rates.
Tumor metastasis is a complex process, and is highly regulated by multiple mechanisms, including aberrant activation of the phosphoinositide 3-kinase (PI3K)/AKT and transforming growth factor (TGF)-β/Smad pathways (8–11). Furthermore, a large number of studies have shown that matrix metalloproteinase (MMP) overexpression is involved in numerous malignant tumors, including esophageal cancer, breast cancer, liver cancer and rectal cancer (12–16). MMPs serve a very important role in tumor invasion and metastasis (12). In addition, epithelial-mesenchymal transition (EMT) is closely associated with tumor occurrence and metastasis (12,17). Furthermore, the expression of MMP family-related factors or N-cadherin/E-cadherin is regulated by the aforementioned pathways in an interactive manner (18,19). As a result, targeting PI3K/AKT and TGF-β/Smad pathways may represent a novel therapeutic method to prevent metastasis without causing side effects.
Traditional Chinese medicine (TCM) is of interest to researchers as it induces relatively few side effects and has been clinically used for thousands of years as an important alternative remedy for a variety of diseases (20–23). TCMs are considered to be multi-component and multi-targeted agents that exert their therapeutic functions holistically (24). Scutellaria barbata D. Don (SB) is a medicinal herb widely distributed in northeast Asia. In TCM, SB is a well-known herb considered to be useful for heat-clearing, detoxification, promotion of blood circulation and removal of blood stasis (25). SB has long been used as an important component in several TCM formulas for the clinical treatment of various types of cancer. SB extracts have been shown to inhibit the growth of numerous cancer cell types (26–30). In a previous study, it was reported that SB promotes cancer cell apoptosis via activation of the mitochondrial-dependent pathway (31). However, studies in which the anticancer effect of SB and its mechanisms are elucidated, particularly studies relating to metastasis, are lacking. In the present study, the effects of SB on the migration and invasion abilities of HCT-8 human colorectal carcinoma cells and their regulation through PI3K/AKT and TGF-β/Smad signaling pathways were evaluated.
Materials and methods
Materials and reagents
RPMI-1640 medium, fetal bovine serum (FBS), penicillin-streptomycin and trypsin-EDTA were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Rabbit polyclonal antibodies against AKT (cat. no. 10176-2-AP) and PI3K (cat. no. 13329-1-AP) were purchased from Proteintech Group (Wuhan, China). Rabbit polyclonal antibodies against phospho (p)-AKT (cat. no. sc-135650) and p-PI3K (cat. no. sc-12929), and goat polyclonal antibodies against phosphatase and tensin homolog (PTEN) (cat. no. sc-6818) were purchased from Santa Cruz Biotechnology (Shanghai) Co., Ltd. (Shanghai, China). Rabbit polyclonal antibodies against MMP1 (cat. no. D120093), 2 (cat. no. D161446), 9 (cat. no. D120097) and 13 (cat. no. D120098)] were purchased from Sangon Biotech Co., Ltd. (Shanghai, China), and MMP3/10 (cat. no. sc-30070) was purchased from Santa Cruz Biotechnology (Shanghai) Co., Ltd. Mouse monoclonal antibodies against E-cadherin (cat. no. ab76055) and N-cadherin (cat. no. ab98952) were purchased from Abcam (Hong Kong) Ltd. (Hong Kong, China). Rabbit polyclonal antibodies against TGF-β1 (cat. no. 3711S), Smad4 (cat. no. 38454S), Smad2/3 (cat. no. 8685S) and β-actin (cat. no. 4967), and horseradish peroxidase (HRP)-conjugated secondary antibodies (cat. no. 7074) were provided by Cell Signaling Technology, Inc. (Beverly, MA, USA). Transwell chambers were obtained from Corning Life Sciences (Tewksbury, MA, USA). BD BioCoat Matrigel Invasion Chamber was purchased from BD Biosciences (San Jose, CA, USA). All other chemicals were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) unless otherwise stated.
Ethanol extract of SB (EESB) preparation
Authentic plant material was purchased from Guo Yi Tang Chinese herbal medicine store (Fujian, China). The original herb was identified as SB by Dr Wei Xu at the Department of Pharmacology, Fujian University of Traditional Chinese Medicine (Fuzhou, China). The plants were dried and cut into small pieces, and EESB was obtained as previously described (32). EESB stock solutions were prepared by dissolving EESB powder in PBS at a concentration of 500 mg/ml, and stored at −20°C. EESB working concentrations were obtained by diluting the stock solution in the culture medium.
Cell culture
HCT-8 human colorectal carcinoma cells were obtained from Nanjing KeyGen Biotech. Co., Ltd. (Nanjing, China). Cells were grown in RPMI-1640 medium containing 10% (v/v) FBS, 100 U/ml penicillin and 100 µg/ml streptomycin in a 37°C humidified incubator with 5% CO2. Cells were digested at room temperature for 3 min using trypsin-EDTA and subcultured when 80–90% confluency was reached.
MTT assay
Cell viability was assessed using an MTT colorimetric assay. Cells were harvested, re-suspended at a final concentration of 1×105 cells/ml and then seeded into 96-well plates at a volume of 100 µl/well. After 12 h incubation at 37°C, cells were treated with EESB at different concentrations (0, 0.125, 0.25, 0.5, 1, 1.5 and 2 mg/ml) and incubated for 24 or 48 h. Subsequently, 100 µl MTT (0.5 mg/ml) was added to each well. The plates were incubated at 37°C for 4 h, and 100 µl DMSO was added to dissolve the purple formazan crystals. The absorbance was read at 570 nm using an ELISA reader (Model ELx800; BioTek Instruments, Inc., Winooski, VT, USA).
Microscopic observation of cell density
HCT-8 cells were seeded into 6-well plates at a density of 5×105 cells/well in 2 ml medium. Cells were treated with EESB at different concentrations (0, 0.125, 0.25 and 0.5 mg/ml) and incubated for 24 h. Cell density was observed using a phase-contrast microscope (Leica Microsystems GmbH, Wetzlar, Germany). Images were captured at a magnification of ×200.
Cell migration and invasion analysis using Transwell assays
Migration assays were performed using Transwell cell culture chambers with 8-µm pore filters (Corning Life Sciences). Following treatment with EESB at different concentrations (0, 0.125, 0.25 and 0.5 mg/ml) for 24 h, HCT-8 cells were trypsinized and resuspended in serum-free RPMI-1640. A total of 5×104 cells in 200 µl serum-free RPMI-1640 were plated in the upper chamber. RPMI-1640 media containing 10% (v/v) FBS was placed in the lower chamber as a chemoattractant. Cells were allowed to migrate for 12 h, and the non-migrated cells were removed from the upper surface of the Transwell membranes using a cotton swab. Membranes were fixed with ice-cold 4% paraformaldehyde for 10 min and stained using crystal violet at room temperature for 15 min. The average number of migrating cells per field was assessed by counting three random fields under a phase-contrast microscope (Leica) at a magnification of ×200. The procedure for the cell invasion assay was the same as that described for the migration assay, with the exception that the upper chamber was coated with Matrigel Matrix (BD Biosciences).
Western blot analysis
HCT-8 cells were seeded into 25-cm2 flasks at a density of 1.25×106 cells/flask in 5 ml medium. Following incubation for 12 h, cells were treated with EESB at different concentrations (0, 0.125, 0.25 and 0.5 mg/ml) and incubated for 24 h. The treated cells were lysed using Pierce radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Inc.) containing EASYpack protease and PhosSTOP phosphatase inhibitor cocktails (both Roche Diagnostics, Basel, Switzerland). The lysates were then centrifuged at 17,000 × g for 20 min at 4°C, and the total protein concentration was determined by BCA assay. Equal amounts of total proteins (50 mg) were resolved using 10% SDS-PAGE gels and then electroblotted onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk at room temperature for 2 h, and treated with primary antibodies against E-cadherin (1:1,000), N-cadherin (1:1,000), TGF-β1 (1:1,000), Smad2/3 (1:1,000), Smad4 (1:1,000), AKT (1:500), p-AKT (1:500), PTEN (1:500), PI3K (1:500), p-PI3K (1:500), MMP1 (1:1,000), MMP2 (1:1,000), MMP3/10 (1:1,000), MMP9 (1:1,000), MMP13 (1:1,000) and β-actin (1:1,000) overnight at 4°C. Subsequently, the membranes were incubated with HRP-conjugated secondary antibody at room temperature for 1 h and the protein bands were detected using an enhanced chemiluminescence detection reagent, SuperSignal West Pico Chemiluminescent substrate (Thermo Fisher Scientific, Inc.). β-actin was used as the internal control. Images were taken using a ChemiDoc XRS+ imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Image Lab™ software (version 3.0; Bio-Rad Laboratories, Inc.) was used for densitometric analysis and quantification of the western blots.
Statistical analysis
All data were obtained as the mean of three experiments. Statistical analysis was performed using SPSS software (version 17.0) for Windows (SPSS, Inc., Chicago, IL, USA) using one-way analysis of variance, followed by Fisher's least significant difference and Dunnett's tests. Data are expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of EESB on HCT-8 cell viability
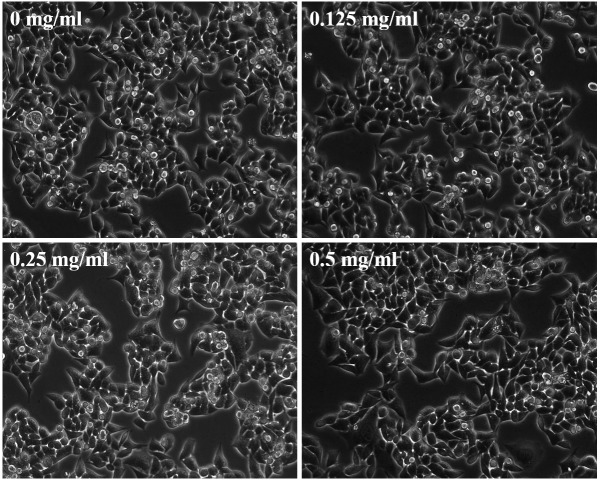
As shown in Fig. 1, EESB at low concentrations (0.125, 0.25 and 0.5 mg/ml) did not exhibit a significant effect on HCT-8 cell proliferation, while EESB at high concentrations (1, 1.5 and 2 mg/ml) significantly inhibited HCT-8 cell growth compared with that of the untreated cells. On the basis of these results, EESB concentrations of 0.125, 0.25 and 0.5 mg/ml were selected for the subsequent experiments. To further verify that the selected concentrations of EESB were not cytotoxic, the effect of EESB on HCT-8 cell density was analyzed under a microscope. As shown in Fig. 2, as the drug concentration increased from 0 to 0.5 mg/ml, there was no clear change in cell density, which indicated that these low doses of EESB had no marked effect on cell growth.
Figure 1.
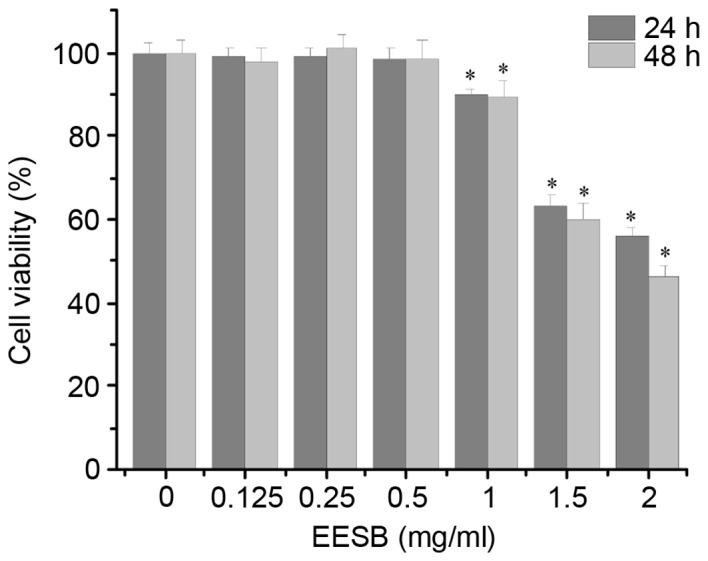
Effect of EESB on HCT-8 cell viability. Cells were treated with EESB at different concentrations for 24 and 48 h. Cell viability was measured via MTT assay. Data were normalized to the viability of control cells (100%). Data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 vs. the control cells. EESB, ethanol extract of Scutellaria barbata D. Don.
Figure 2.
Effect of EESB on HCT-8 cell density. HCT-8 cells were treated with EESB at various concentrations for 24 h. Density changes were observed using phase-contrast microscopy. Photographic images were captured at a magnification of ×200. Images are representative of three independent experiments. EESB, ethanol extract of Scutellaria barbata D. Don.
Effect of EESB on HCT-8 cell migration and invasion
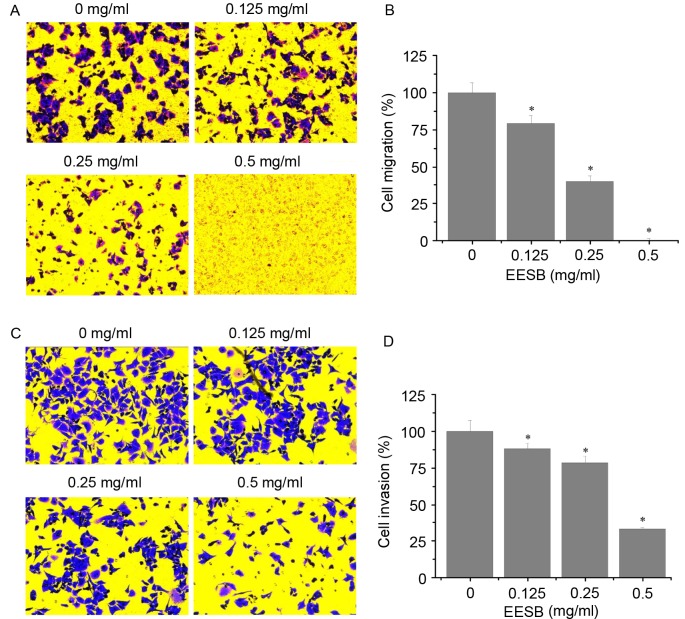
As shown in Fig. 3A and B, EESB significantly reduced the number of migrated cells compared with the untreated control. Similarly, EESB treatment significantly reduced cell invasion through the Matrigel membrane compared with the untreated control (Fig. 3C and D). The inhibitory effects on migration and invasion were concentration-dependent. The number of migrated cells was reduced by 20.89% using 0.125 mg/ml EESB and by 99.89% using 0.5 mg/ml EESB, as compared with the untreated control cells (Fig. 3B). The invasion assay results showed that EESB treatment for 24 h reduced the HCT-8 cell invasion rate by 11.86% when 0.125 mg/ml EESB was used and by 66.90% when 0.5 mg/ml EESB was used, as compared with the untreated control cells (Fig. 3D).
Figure 3.
Effect of EESB on HCT-8 cell migration and invasion. HCT-8 cells were treated with EESB at the indicated concentrations for 24 h. The (A and B) migration and (C and D) invasion of HCT-8 cells were determined using Transwell cell culture chambers and Transwell cell culture chambers with Matrigel matrix-coated membranes, respectively. (A) Migrated and (C) invaded cells were stained using crystal violet, and images were captured at a magnification of ×200. The average number of (B) migrated cells and (D) invaded cells were counted in three randomly selected fields. Data were normalized to the migration and invasion of control cells (100%). Data are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 vs. the control cells. EESB, ethanol extract of Scutellaria barbata D. Don.
Effect of EESB on MMP and E-/N-cadherin expression
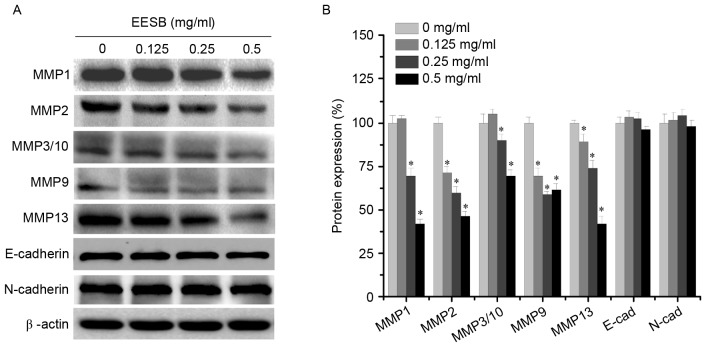
To elucidate the antimetastatic mechanisms of EESB, the expression levels of MMPs (MMP1, MMP2, MMP3/10, MMP9 and MMP13) and the EMT-regulated factors (E-cadherin and N-cadherin) were analyzed using western blotting. As shown in Fig. 4, EESB significantly inhibited the expression of MMP1, MMP2, MMP3/10, MMP9 and MMP13 to different extents, but exerted only a slight effect on the expression of the mesenchymal marker N-cadherin and the epithelial marker E-cadherin. These results indicated that EESB inhibited HCT-8 metastasis via the suppression of MMP expression but not via EMT.
Figure 4.
Effect of EESB on MMP and E-/N-cadherin expression. Cells were treated with EESB at different concentrations for 24 h. (A) MMP, E-cadherin and N-cadherin protein expression levels in HCT-8 cells were determined using western blotting. β-actin was used as the internal control. Images are representatives of three independent experiments. (B) Densitometric analysis. Data are expressed as the mean ± standard deviation and were normalized to the mean protein expression of untreated control (100%). *P<0.05. vs. the control cells. EESB, ethanol extract of Scutellaria barbata D. Don; MMP, matrix metalloproteinase; E-cad, E-cadherin; N-cad, N-cadherin.
Effect of EESB on PI3K/AKT and TGF-β/Smad signaling pathways
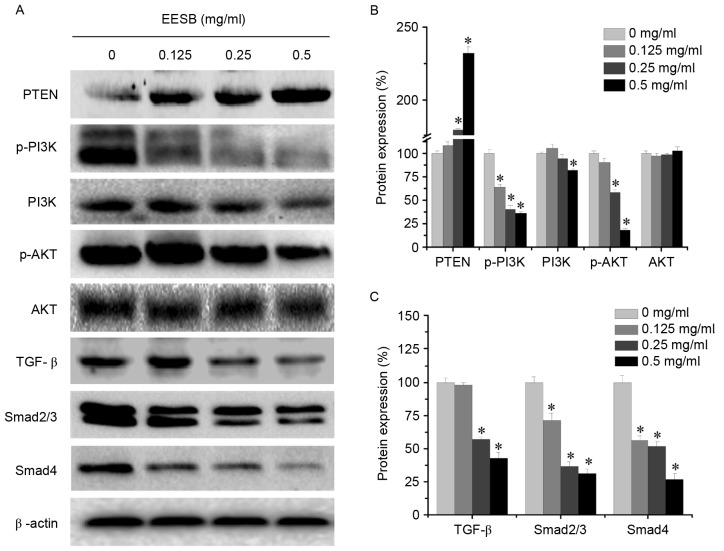
EESB markedly suppressed the activation of the PI3K/AKT pathway by significantly downregulating p-PI3K, PI3K and p-AKT protein levels (Fig. 5). In addition, PTEN, which is a tumor suppressor and PI3K/AKT upstream factor (33), was significantly upregulated following EESB treatment. Furthermore, EESB treatment significantly inhibited the expression of TGF-β, Smad2/3 and Smad4. These results suggested that the antimetastatic effect of EESB on CRC may be partly mediated by suppression of the PI3K/AKT and TGF-β/Smad signaling pathways.
Figure 5.
Effect of EESB on the activation of PI3K/AKT and TGF-β/Smad signaling pathways. Cells were treated with EESB at different concentrations for 24 h. (A) PI3K/AKT and TGF-β/Smad protein expression levels were determined by western blotting. β-actin was used as the internal control. Images are representatives of three independent experiments. Densitometric analysis for (B) PTEN, p-PI3K, PI3K, p-AKT and AKT and (C) TGF-β, Smad2/3 and Smad4. Data are expressed as the mean ± standard deviation and were normalized to the mean protein expression of the untreated control (100%). *P<0.05. vs. the control cells. EESB, ethanol extract of Scutellaria barbata D. Don; PI3K, phosphoinositide 3-kinase; TGF-β, transforming growth factor-β; PTEN, phosphatase and tensin homolog; p, phospho.
Discussion
CRC remains a potentially lethal disease with a poor prognosis, mostly due to metastasis in the majority of patients. Multi-drug combination therapies have been developed leading to significantly improved patient response and overall survival. However, resistance to these drugs is inevitable and continues to be a notable problem (34,35). Therefore, novel agents, including natural products, are currently being considered for more efficient cancer treatment. TCM, with its relatively high safety and long history of pharmacological applications, has attracted attention in the field of cancer treatment (20,23). SB is a herb used in TCM formulations, where it is considered to have ‘heat-clearing and detoxifying’ actions, and has many reported applications in cancer treatment (36–41). Similar to other medicinal herbs, SB is a multi-targeted agent that is considered to exert its therapeutic function holistically (27,31,32,42); thus, it may be a good candidate as an anticancer drug. However, the specific mechanism of its anticancer effect, particularly its antimetastatic ability, is not yet clear.
The process of tumor metastasis is complex. One of the most studied mechanisms relates to MMP involvement. MMPs are a group of important proteases that degrade extracellular matrix (ECM). Numerous studies have shown that MMPs are associated with tumor growth, metastasis and invasion (43–45). The main members of the family may be divided into five groups according to their domain, enzyme and substrate specificity as follows: Collagenase (MMP1, MMP8 and MMP13), gelatinase (MMP2 and MMP9), matrix soluble elements (MMP3, MMP7, MMP10 and MMP11) and matrix dissolution factor and membrane type (MT) metalloproteinase (MT1-MMP, MT2-MMP and MT3-MMP) (46). It has been reported that MMP1 expression is increased significantly in gastric cancer, which destroys the basement membrane, and promotes tumor lymphangiogenesis, tumor invasion and metastasis (47). MMP2 has been demonstrated to be closely associated with migration and invasion in several types of tumors, including breast cancer, ovarian cancer and lung cancer (48,49). MMP9 expression was identified to be increased in osteosarcoma, lung cancer, pancreatic cancer and CRC tissues to different extents, which was positively correlated with tumor metastasis (48–50). Similarly, it has been observed that MMP3 and MMP10 expression levels in lung cancer, esophageal cancer, liver cancer and endometrial adenocarcinoma tissues are higher than those in normal tissues, and that their expression has an association with tumor invasion, metastasis and proliferation (51–55). In addition, a review of a number of studies has demonstrated that MMP13 expression in malignant tumors, such as colon cancer, breast cancer, non-small cell lung cancer and oral squamous cell carcinoma, is closely associated with tumor occurrence, development, invasion and metastasis (56). On the basis of this evidence, MMPs have a close association with tumor invasion and metastasis, serving a very important role. In the present study, EESB downregulated the expression of the MMPs, to different extents, suggesting that EESB may inhibit CRC metastasis by inhibiting the expression of these specific MMPs to balance the ECM environment.
EMT is an important phenomenon in the occurrence and development of tumors and is also an important mechanism allowing tumor invasion and metastasis (57,58). E-cadherin and N-cadherin are two important factors in the maintenance of the EMT balance; EMT regulation is influenced by these two cadherins (17). However, in the present study, EESB showed only a weak effect on the expression of the mesenchymal marker N-cadherin and epithelial marker E-cadherin, suggesting that EESB inhibits CRC metastasis thorough mechanisms other than EMT.
In addition to the aforementioned factors, numerous pathways also contribute to tumor metastasis. For example, activation of the PI3K/AKT signaling pathway accelerates angiogenesis, tumor invasion and metastasis through the disturbance of tumor-suppressor PTEN or other causal factors, which is important in the occurrence and development of malignant tumors (59). Furthermore, the PI3K/AKT signaling pathway is involved in regulating the expression of MMP-2 and MMP-9 in a variety of tumor tissues and cells, to regulate multidrug resistance, as well as tumor invasion and metastasis (60). Furthermore, TGF-β promotes tumor metastasis by increasing tumor angiogenesis, immune suppression, and the production and deposition of ECM (61). Previous studies have shown that TGF-β may cause tumor metastasis through activation of the Smad pathway, and Smad2 and Smad4 are important proteins that regulate the transcription and the antiproliferative response of TGF-β, and regulate the expression of genes downstream of TGF-β that are involved in tumor metastasis (62,63). Furthermore, TGF-β may mediate tumor cell invasion by regulating ECM-degrading proteinases (64). Among the increasing number of ECM-degrading proteinases implicated in tumor cell invasion, the majority of the attention has been focused on the MMP family and the plasminogen activator system (65,66). TGF-β1 has been suggested to activate the Smad signaling pathway, and significantly promote the expression of MMPs and other invasion and metastasis-related genes in highly invasive breast cancer cells, thereby enhancing the ability of the cells to invade and metastasize (67,68). In the present study, EESB decreased the expression of proteins in the PI3K/AKT and TGF-β/Smad2/3 pathways, and upregulated the expression of the tumor-suppressor PTEN, thus indicating that the inhibitory effect of EESB on metastatic CRC cells may be mediated by the suppression of certain members of the MMP family, and PI3K/AKT and TGF-β/Smad signaling pathways.
In conclusion, EESB exerted significant antimetastatic effects on CRC cells by inhibition of their migration and invasion ability, and via the regulation of PI3K/AKT and TGF-β/Smad signaling pathways. These mechanisms are potentially those by which EESB exhibits its effectiveness in cancer treatment.
Acknowledgements
The present study was sponsored by the Research Fund for the Doctoral Program of Higher Education of China (grant no. 20133519110003), the Project Funding for the Training of Young and Middle-aged Backbone Personnel of Fujian Provincial Health and Family Planning Commission (grant no. 2016-ZQN-67), and the Developmental Fund of Chen Keji Integrative Medicine (Fujian, China; grant nos. CKJ2014013 and CKJ2015007).
Glossary
Abbreviations
- CRC
colorectal cancer
- EESB
ethanol extract of Scutellaria barbata D. Don
- TCM
traditional Chinese medicine
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- EMT
epithelial-mesenchymal transition
References
- 1.Tenesa A, Dunlop MG. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet. 2009;10:353–358. doi: 10.1038/nrg2574. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Grávalos C, Cassinello J, Fernández-Rañada I, Holgado E. Role of tyrosine kinase inhibitors in the treatment of advanced colorectal cancer. Clin Colorectal Cancer. 2007;6:691–699. doi: 10.3816/CCC.2007.n.038. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Oliveira J. ESMO Guidelines Working Group: Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):S61–S63. doi: 10.1093/annonc/mdp130. [DOI] [PubMed] [Google Scholar]
- 6.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talmadge JE, Fidler IJ. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen A, Lin W, Chen Y, Liu L, Chen H, Zhuang Q, Lin J, Sferra TJ, Peng J. Pien Tze Huang inhibits metastasis of human colorectal carcinoma cells via modulation of TGF-β1/ZEB/miR-200 signaling network. Int J Oncol. 2015;46:685–690. doi: 10.3892/ijo.2014.2772. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu T, Jing C, Shi Y, Miao R, Peng L, Kong S, Ma Y, Li L. microRNA-20a enhances the epithelial-to-mesenchymal transition of colorectal cancer cells by modulating matrix metalloproteinases. Exp Ther Med. 2015;10:683–688. doi: 10.3892/etm.2015.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam SK, Yadav VK, Bajaj S, Datta A, Dutta SK, Bhattacharyya M, Bhattacharya S, Debnath S, Roy S, Boardman LA, et al. DNA damage-induced ephrin-B2 reverse signaling promotes chemoresistance and drives EMT in colorectal carcinoma harboring mutant p53. Cell Death Differ. 2016;23:707–722. doi: 10.1038/cdd.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo S. Research progress in the mechanism of colorectal cancer metastasis. J Mudanjiang Med College. 2008;29:65–67. [Google Scholar]
- 13.Juchniewicz A, Kowalczuk O, Milewski R, Laudański W, Dzięgielewski P, Kozłowski M, Nikliński J. MMP-10, MMP-7, TIMP-1 and TIMP-2 mRNA expression in esophageal cancer. Acta Biochim Pol. 2017;64:295–299. doi: 10.18388/abp.2016_1408. [DOI] [PubMed] [Google Scholar]
- 14.Yun EJ, Song KS, Shin S, Kim S, Heo JY, Kweon GR, Wu T, Park JI, Lim K. Docosahexaenoic acid suppresses breast cancer cell metastasis by targeting matrix-metalloproteinases. Oncotarget. 2016;7:49961–49971. doi: 10.18632/oncotarget.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dou CY, Cao CJ, Wang Z, Zhang RH, Huang LL, Lian JY, Xie WL, Wang LT. EFEMP1 inhibits migration of hepatocellular carcinoma by regulating MMP2 and MMP9 via ERK1/2 activity. Oncol Rep. 2016;35:3489–3495. doi: 10.3892/or.2016.4733. [DOI] [PubMed] [Google Scholar]
- 16.Fuksiewicz M, Kotowicz B, Rutkowski A, Kowalska M. The matrix metalloproteinase-7 and pro-enzyme of metalloproteinase-1 as a potential marker for patients with rectal cancer without distant metastasis. Tumour Biol. 2015;36:3629–3635. doi: 10.1007/s13277-014-3000-z. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Zhuo N, Guo Z. Molecular mechanism of epithelial-mesenchymal transition and its role in tumor metastasis. Chin Med Herald. 2014;11:163–165. [Google Scholar]
- 18.Wei F, Shen Q, Liu C. Phenethyl isothiocyanate inhibits PI3K/NF-kB to down-regulate MMP-9 expression in human colon cancer cells. Med Sci J Central South China. 2014;42:351–354. [Google Scholar]
- 19.Liang S, Lv Y, Wang X. Study of the correlation and expression of focal adhesion kinase and matrix metalloproteinase-9 in colorectal carcinoma. J Colorectal Anal Surger. 2008;14:157–160. [Google Scholar]
- 20.Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Jiang P, Zhang W. Molecular networks for the study of TCM pharmacology. Brief Bioinform. 2010;11:417–430. doi: 10.1093/bib/bbp063. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Chen Y, Wei L, Chen X, Xu W, Hong Z, Sferra TJ, Peng J. Hedyotis Diffusa Willd extract induces apoptosis via activation of the mitochondrion-dependent pathway in human colon carcinoma cells. Int J Oncol. 2010;37:1331–1338. doi: 10.3892/ijo_00000785. [DOI] [PubMed] [Google Scholar]
- 23.Demain AL, Zhang L. Natural Products and Drug Discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009;10:194–200. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen AL, Hong F, Liu LY, Lin JM, Zhuang QC, Hong ZF, Peng J. Effects of Pien Tze Huang on angiogenesis in vivo and in vitro. Chin J Integr Med. 2012;18:431–436. doi: 10.1007/s11655-012-1121-z. [DOI] [PubMed] [Google Scholar]
- 25.Read BE. The Chinese Pharmacopoeia. Can Med Assoc J. 1930;23:568–570. [PMC free article] [PubMed] [Google Scholar]
- 26.Cha YY, Lee EO, Lee HJ, Park YD, Ko SG, Kim DH, Kim HM, Kang IC, Kim SH. Methylene chloride fraction of Scutellaria barbata induces apoptosis in human U937 leukemia cells via the mitochondrial signaling pathway. Clin Chim Acta. 2004;348:41–48. doi: 10.1016/j.cccn.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Lin J, Chen Y, Cai Q, Wei L, Zhan Y, Shen A, Sferra TJ, Peng J. Scutellaria barbata D Don inhibits colorectal cancer growth via suppression of multiple signaling pathways. Integr Cancer Ther. 2014;13:240–248. doi: 10.1177/1534735413508811. [DOI] [PubMed] [Google Scholar]
- 28.Suh SJ, Yoon JW, Lee TK, Jin UH, Kim SL, Kim MS, Kwon DY, Lee YC, Kim CH. Chemoprevention of Scutellaria bardata on human cancer cells and tumorigenesis in skin cancer. Phytother Res. 2007;21:135–141. doi: 10.1002/ptr.2010. [DOI] [PubMed] [Google Scholar]
- 29.Yin X, Zhou J, Jie C, Xing D, Zhang Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004;75:2233–2244. doi: 10.1016/j.lfs.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Lee TK, Lee DK, Kim DI, Lee YC, Chang YC, Kim CH. Inhibitory effects of Scutellaria barbata D. Don on human uterine leiomyomal smooth muscle cell proliferation through cell cycle analysis. Int Immunopharmacol. 2004;4:447–454. doi: 10.1016/j.intimp.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Wei L, Chen Y, Lin J, Zhao J, Chen X, Xu W, Liu X, Sferra T, Peng J. Scutellaria barbata D. Don induces apoptosis of human colon carcinoma cell through activation of the mitochondrion-dependent pathway. J Med Plants Res. 2011;5:1962–1970. [Google Scholar]
- 32.Wei L, Lin J, Wu G, Xu W, Li H, Hong Z, Peng J. Scutellaria barbata D. Don induces G1/S arrest via modulation of p53 and Akt pathways in human colon carcinoma cells. Oncol Rep. 2013;29:1623–1628. doi: 10.3892/or.2013.2250. [DOI] [PubMed] [Google Scholar]
- 33.Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1:1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodel C, Hofheinz R, Liersch T. Rectal cancer: State of the art in 2012. Curr Opin Oncol. 2012;24:441–447. doi: 10.1097/CCO.0b013e328352ea02. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 36.Tan P, Lu B, Bao W. Analysis on the clinical application of Scutellaria barbata D. Don in the anti-cancer therapy. Jiangxi Tradit China Med. 2006;37:57–58. [Google Scholar]
- 37.Dai ZJ, Liu XX, Tang W, Xue Q, Wang XJ, Ji ZZ, Kang HF, Diao Y. Antitumor and immune-modulating effects of Scutellaria barbata extract in mice bearing hepatocarcinoma H22 cells-derived tumor. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1835–1837. (In Chinese) [PubMed] [Google Scholar]
- 38.Goh D, Lee YH, Ong ES. Inhibitory effects of a chemically standardized extract from Scutellaria barbata in human colon cancer cell lines, LoVo. J Agric Food Chem. 2005;53:8197–8204. doi: 10.1021/jf051506+. [DOI] [PubMed] [Google Scholar]
- 39.Marconett CN, Morgenstern TJ, San Roman AK, Sundar SN, Singhal AK, Firestone GL. BZL101, a phytochemical extract from the Scutellaria barbata plant, disrupts proliferation of human breast and prostate cancer cells through distinct mechanisms dependent on the cancer cell phenotype. Cancer Biol Ther. 2010;10:397–405. doi: 10.4161/cbt.10.4.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong BY, Nguyen DL, Lin T, Wong HH, Cavalcante A, Greenberg NM, Hausted RP, Zheng J. Chinese medicinal herb Scutellaria barbata modulates apoptosis and cell survival in murine and human prostate cancer cells and tumor development in TRAMP mice. Eur J Cancer Prev. 2009;18:331–341. doi: 10.1097/CEJ.0b013e32832c3859. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Z, Holle L, Song W, Wei Y, Wagner TE, Yu X. Antitumor and anti-angiogenic activities of Scutellaria barbata extracts in vitro are partially mediated by inhibition of Akt/protein kinase B. Mol Med Rep. 2012;5:788–792. doi: 10.3892/mmr.2011.694. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Cai Q, Lin J, Fang Y, Zhan Y, Shen A, Wei L, Wang L, Peng J. Chloroform fraction of Scutellaria barbata D. Don promotes apoptosis and suppresses proliferation in human colon cancer cells. Mol Med Rep. 2014;9:701–706. doi: 10.3892/mmr.2013.1864. [DOI] [PubMed] [Google Scholar]
- 43.Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012;13:13240–13263. doi: 10.3390/ijms131013240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Littlepage LE, Sternlicht MD, Rougier N, Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI, Werb Z. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010;70:2224–2234. doi: 10.1158/0008-5472.CAN-09-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laurie AS, Sandra JT, William GS. Matrix metalloproteinases: Changing roles in tumor progression and metastasis. Am J Pathol. 2012;181:1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Y, Wang S. Relationship between matrix metalloproteinases and their inhibitors and their relationship with invasion and metastasis of malignant tumors. J New Med. 2011;42:341–343. [Google Scholar]
- 47.Liu T, Ma Y, Zhang R. Advance research on relationship between matrix metalloproteinases and invasion and metastasis of malignant tumors. J Jilin Univ. 2004;30:662–664. [Google Scholar]
- 48.Meng F, Liu X, Qi S. Correlation of matrix metalloproteinases-2 and-9 in ovarian cancer. Chin J Gerontology. 2013;33:3505–3506. [Google Scholar]
- 49.Ming S, Sun T, Xiao W. Role of matrix metalloproteinases −2, −9 and its inhibitor 1 in the invasion and metastasis of lung cancer. Chin J Respirator Crit Care. 2005;4:198–202. [Google Scholar]
- 50.Zheng H, Shen B, Nie Y, Du Y. The expression of MMP-9, MMP-13 and TIMP-3 in hepatocellular carcinoma. Guangdong Med J. 2013;34:1995–1998. [Google Scholar]
- 51.Yan Z, Xu X, Yang G. Abnormal expression and clinical significance of matrix metalloprteinase 10 in esophagus carcinoma. Ningxia Med J. 2005;27:14–15. [Google Scholar]
- 52.He J, Ding C, He G, Huang Q. Relationship between expression of ESM-1 and MMP-3 and invasion and metastasis of human hepatocellular carcinoma. Med Sci J Central South China. 2012;40:368–372. [Google Scholar]
- 53.Yue X, Zhang Q, Xu A, Xing Y, Zhang F. Expression and clinical significance of MMP10 and CD105 in patients with non-small-cell lung carcinoma. Shandong Med J. 2009;49:13–15. [Google Scholar]
- 54.Feng J, Gou W, Liu D, Li X. Expressions of matrix metalloproteinase-3 and matrix metalloproteinase-10 in endometrial carcinoma. J Xian Jiaotong Univ (Med Sci) 2010;31:97–101. [Google Scholar]
- 55.Tian J, Xu M, Jing H. Matrix metalloproteinases 3 and its inhibitory factor 2 expression in lung squamous carcinoma. West China Med J. 2013;28:369–372. [Google Scholar]
- 56.Ma R, Zhang C. Review of matrix metalloproteinases-13 and its relationship with invasion and metastasis of malignant tumor. J Community Med. 2012;10:47–49. [Google Scholar]
- 57.Da C, Liu Y, Zhan Y, Liu K, Wang R. Nobiletin inhibits epithelial-mesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3 signaling pathway. Oncol Rep. 2016;35:2767–2774. doi: 10.3892/or.2016.4661. [DOI] [PubMed] [Google Scholar]
- 58.Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15:97. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L, Wang Q. Correlation of PI3K/AKT/mTOR signal pathway to infiltration and metastasis of malignant tumor. Modern Oncol. 2009;17:1585–1589. [Google Scholar]
- 60.Guo C, Ke W, Song K, Wang J, Zhou L, Li K. PI3K/AKT signalling pathway involves in the modulation of multidrug resistance and metastasis in breast cancer. Prog Mod Biomed. 2012;12:4809–4812. [Google Scholar]
- 61.Jia BY, Wang YJ. Metastasis associated signaling pathway in colorectal cancer. Int J Dig Dis. 2015;3:183–185. [Google Scholar]
- 62.Ding P, Song B, Zhu L. Effect of TGF-β/Smad 4 on the tumor metastasis of colorectal cancer cells. Chin J Cancer Prev Treat. 2011;18:1518–1520. [Google Scholar]
- 63.Qi Y, Li H. Research of Qilian Fuzheng capsule's function on anti-lung cancer metastasis by regulating TGF-β pathway. Chin Archives Tradit Chin Med. 2014;32:2567–2569. [Google Scholar]
- 64.Liotta LA. Tumor invasion and metastases-role of the extracellular matrix. Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 65.Stetler-Stevenson WG, Hewitt R, Corcoran M. Matrix metalloproteinases and tumor invasion: From correlation and causality to the clinic. Semin Cancer Biol. 1996;7:147–154. doi: 10.1006/scbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- 66.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 67.Perera M, Tsang CS, Distel RJ, Lacy JN, Ohno-Machado L, Ricchiuti V, Samaranayake LP, Smejkal GB, Smith MG, Trachtenberg AJ, Kuo WP. TGF-beta1 interactome: Metastasis and beyond. Cancer Genomics Proteomics. 2010;7:217–229. [PubMed] [Google Scholar]
- 68.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci USA. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]