Abstract
The purpose of the present study was to assess the protective effects of rhein lysinate (RHL) in a KK/HlJ mouse model of diabetic nephropathy (DN) and to explore its mechanism of action. A total of 4 groups were established: C57BL/J control, the KK/HlJ model and 25 and 50 mg/kg/day RHL-treated KK/HlJ groups. The KK/HlJ mouse model of DN was established by streptozotocin injection, followed by maintenance on a specific diet. The albumin-to-creatinine ratio (ACR) was determined at 5 weeks and at 16 weeks, the kidneys were harvested, and morphological examination and immunohistochemical analysis were performed. The levels of malondialdehyde (MDA), as well as superoxide dismutase (SOD) and glutathione peroxidase (GSH-px) activities in the kidneys were measured using appropriate assay kits. The expression of inflammatory factors and associated proteins was analyzed using western blot analysis. At 5 weeks, the levels of ACR in KK/HlJ mice were increased, which was inhibited by treatment with RHL. Treatment with RHL (50 mg/kg/day) decreased the body weight of KK/HlJ mice. Compared with the C57BL/J control, the KK/HlJ model mice had a significantly lower activity of SOD and GSH-px in the kidneys, but had significantly higher levels of MDA. Treatment of KK/HlJ mice with RHL significantly increased the activities SOD and GSH-px, and reduced the MAD level in the kidneys. Renal tubular epithelial cell edema was observed in KK/HlJ mice but not in C57BL/J mice. RHL decreased the incidence of renal tubular epithelial cell edema and significantly decreased the expression of TNF-α and IL-6 as well as the expression and phosphorylation of NF-κB in the kidneys. Therefore, DN is associated with the expression of inflammatory factors, renal tubular epithelial cell edema and renal dysfunction in KK/HlJ mice. RHL improves renal function by decreasing kidney inflammation.
Keywords: rhein lysinate, KK/HlJ mice, kidney, oxidative stress, inflammatory factors
Introduction
Diabetes mellitus is an increasing global health problem. Estimates for 2013 by the International Diabetes Federation (IDF) indicate that a total of 382 million have diabetes in 2013, and the number is expected to rise to 592 million by 2035 (1–3). Diabetic nephropathy (DN) is one of the most common complications of diabetes. An estimate of 30–50% of patients with diabetes develop renal manifestations (4–6). DN often leads to chronic kidney disease (CKD). Nearly 44% of end-stage renal disease (ESRD) patients that require hemodialysis are diabetic nephropathy patients (7). Inflammation and oxidative stress are associated with the pathogenesis of DN (8). Transcription factors such as nuclear factor κ of activated B cells (NF-κB) (9), pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin 1 (IL-1) (10) are associated with inflammatory pathways in DN. Thus, it is important to treat DN using anti-inflammatory drugs.
Research using experimental models of type 2 diabetes with nephropathy may provide an enhanced understanding of this complication in this multifactorial disease (11). Qi et al (12) reported that KK/HlJ mice are more prone to DN, whereas the most widely used C57BL/6J mice are relatively resistant to developing DN. Rhein lysinate (RHL) is the lysine salt of rhein, which is one of the active components of rhubarb root (Rheum palmatum Linn or Rheum tanguticum Maxim) (13). A previous study by our group found that RHL reduced the levels of TNF-α, IL-6 and NF-κB, decreased the incidence of glomerulonephritis and prolonged the median survival time of senescence-prone inbred strain 10 mice (14). However, to the best of our knowledge, the effect of RHL on DN has not been previously reported. The present study investigated the effect of RHL on DN in KK/HlJ mice.
Materials and methods
Chemicals and reagents
Rhein lysinate (RHL) was synthesized at the Oncology Department of the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China; patent no. 2008100890258). The structural formula of this compound is presented in our previous study (13). Streptozotocin (STZ) was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-px) kits were obtained from Nanjing Jiancheng Co. (Nanjing, China). Antibodies targeting TNF-α (3707s), IL-6 (12912s), NF-κB (8242s) and phosphorylated (p)-NF-κB (3033s) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibody targeting β-actin (sc-8432) was provided by Santa Cruz Biotechnology (Dallas, TX, USA). The appropriate anti-mouse (7076s) and anti-rabbit (7074s) horseradish peroxidase-conjugated secondary antibodies were obtained from Cell Signaling Technology, Inc. Prestained protein marker p7708V was provided by New England Biolabs, Ltd. (Ipswitch, MA, USA). Immobilon™ western kit and polyvinylidene difluoride (PVDF) membranes were obtained from Millipore (Billerica, MA, USA).
Animals and induction of DN in inbred mice
Induction of DN was performed according to a previous protocol (12). The inbred male C57BL/J mice (n=12) and KK/HlJ mice (n=36) (weight, 18–24 g; age, 8 weeks) and mouse food used in the present study were purchased from Beijing HFK Bioscience Co. (Beijing, China). The diet contained water (≤8%), crude protein (≥18%), crude fat (≥6%), crude fiber (≥5%), crude ash (≤7%), as well as minerals and trace elements. All protocols were approved by the institutional animal care and use committee of Beijing Hospital (Beijing, China). Mice were housed in an environmentally-controlled facility maintained on an automatic 12-h light/dark cycle. Food and water were provided ad libitum throughout the study. The study used the following 4 groups: C57BL/J control mice (n=12), the KK/HlJ model mice (n=12), the 25 mg/kg/day RHL-treated KK/HlJ mice (n=12) and the 50 mg/kg/day RHL-treated KK/HlJ mice (n=12). At 10 weeks of age, STZ was administered to KK/HlJ mice by intraperitoneal injection (50 mg/kg/day, made freshly in 0.1 mol/l citrate buffer, pH 4.5) for 5 consecutive days with normal diet and mice received the diabetic diet for the remaining treatment time. After all of STZ injections, 25 or 50 mg/kg/day RHL was respectively administered to the animals by gavage in the 25 or 50 mg/kg/day RHL-treated KK/HlJ group for 15 weeks.
Analysis of urinary albumin
Urinary albumin was assessed by determining the albumin-creatinine ratio (ACR) in morning urine. Urine was collected monthly by a home-made mouse urine collection device which used a 96-well plate as the floor. Mice were able to move freely on this 96-well plate until they naturally urinated. Urine was collected by a pipette without contamination by feces. Urinary albumin and creatinine concentrations were determined using a microalbumin/creatinine reagent kit (SIEM-6011A) supplied by Siemens medical solutions diagnostics (Tarrytown, NY, USA).
Measurement of laboratory parameters in serum
Serum was collected at the end of the experiment. Serum creatinine, urea nitrogen and blood glucose were measured in the clinical laboratory using standard protocols.
Analysis of antioxidant activity
At the end of the experiment, mice were anesthetized by intraperitoneal injection of 10% chloral hydrate and sacrificed; the kidneys were quickly removed and placed in cold PBS. Renal tissue (50 mg) was dissected and homogenized in a glass Teflon homogenizer containing 450 ml precooled PBS and the homogenate was then centrifuged at 3,000 × g for 15 min at 4°C. The activities of SOD and GSH-px and the MDA content in the obtained supernatant were measured using test kits, referring to the supplier's manual.
Histological and immunohistochemical analysis
For histological examination, kidney tissues fixed with 4% buffered paraformaldehyde were dehydrated in ethanol and embedded in paraffin wax. The embedded kidney tissues were serially sectioned into 3-µm slices and stained with hematoxylin (staining 5 min)-eosin (staining 2 min) (Beijing Solarbio Science & Technology Co., Ltd., Beijing China) at room temperature. To better characterize the inflammation of kidney tissues, immunohistochemistry detection of TNF-α and IL-6 was performed. After dewaxing with xylene, samples were incubated with 3% H2O2 to block endogenous peroxidase. All slices were incubated with 5% bovine serum albumin (Sigma Aldrich; Meck KGaA, Darmstadt, Germany) at 37°C for 1 h to block non-specific binding. Subsequently, the slices were incubated with anti-TNF-α (1:1,000) or anti-IL-6 (1:1,000) antibodies at 4°C overnight according to the manufacturer's protocol. As a negative control, the primary antibody was replaced with 5% bovine serum albumin at 4°C overnight. Positive staining was identified by visual observation of a yellow/brown pigmentation under the light microscope. Images were captured with an Olympus IX81 inverted microscope (Olympus, Tokyo, Japan).
Western blot analysis
The expression of TNF-α, IL-6 and NF-κB, as well as the phosphorylation of NF-κB in the kidneys were determined by western blot analysis using a procedure identical to that used in a previous study by our group (14).
Statistical analysis
Values are expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS 11.0 for Windows (SPSS, Inc., Chicago, IL, USA). Differences between groups of values were compared using a one-way analysis of variance and the Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
RHL improves the kidney function of mice with DN
The ACR in mice in each group was determined at 5 weeks. Compared with that in the C57BL/6J control group, the ACR was significantly increased in the KK/HlJ model group (P<0.05; Table I). Compared with those in the KK/HlJ model group, RHL (25 and 50 mg/kg/day) significantly decreased the ACR (P<0.05; Table I). The ACR was 40.8±8.6, 486.5±82.9, 424.7±78.6, 385.1±52.4 µg/mg in the C57BL/6J control group, KK/HlJ model group, 25 and 50 mg/kg/day RHL treatment groups, respectively at 5 weeks (Table I).
Table I.
Effect of RHL on ACR, blood glucose, creatinine and urea.
| KK/HlJ | ||||
|---|---|---|---|---|
| Groups | C57BL/6J control | Model | RHL 25 mg/kg/day | RHL 50 mg/kg/day |
| ACR (µg/mg) 5 weeks | 40.8±8.6 | 486.5±82.9a | 424.7±78.6a,b | 385.1±52.4a,b |
| ACR (µg/mg) 15 weeks | 45.12±10.5 | 553.6±82.1a | 442.3±61.5a,b | 310.5±49.7a,b |
| Blood glucose (mmol/l) | 6.53±0.51 | 12.36±3.0a | 9.53±2.81a,b | 8.50±1.90a,b |
| Creatinine (mmol/l) | 65.5±24.8 | 130.0±28.8a | 92.5±26.7a,b | 70.0±25.1b |
| Urea (mmol/l) | 7.0±2.1 | 12.5±3.2a | 11.7±1.8a | 9.6±2.2a,b |
P<0.05, compared with C57BL/J control group.
P<0.05, compared with KK/HlJ control group. Values are expressed as the mean ± standard error (n=12). RHL, rhein lysinate; ACR, albumin-to-creatinine ratio.
RHL protects kidney damage in diabetic KK/HlJ mice
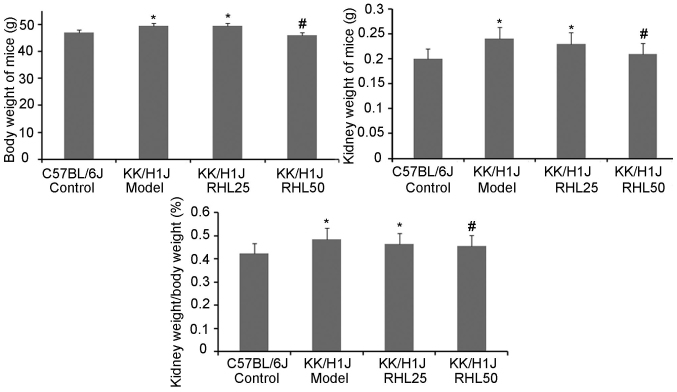
To assess the effects of RHL on DN in KK/HlJ mice, the effect of RHL on body and kidney weight was investigated (Fig. 1). Compared with those in the C57BL/6J control group, the body and kidney weights in the KK/HlJ model group and the 25 mg/kg/day RHL-treated group were increased. Compared with those in the KK/HlJ model group, the body and kidney weights in the 50 mg/kg/day RHL-treated group were decreased. The kidney weight-to-body weight ratio in KK/HlJ and C57BL/6J mice was also determined. As presented in Fig. 1, a significant increase in the kidney weight-to-body weight ratio was observed in all KK/HlJ mice, compared with that in C57BL/6J mice. The kidney weight-to-body weight ratio in the RHL 50 mg/kg/day group was decreased compared with that in the KK/HlJ model group. In addition, renal hypertrophy and hydronephrosis were observed in the KK/HlJ groups, including the RHL treatment groups.
Figure 1.
Kidney and body weights of the mice and the kidney weight to body weight ratio. The diabetic nephropathy model group mice were intraperitoneally injected with streptozotocin for 5 consecutive days and received a specific diet for 16 weeks. Values are expressed as the mean ± standard deviation. *P<0.05 vs. C57BL/J control group; #P<0.05 vs. KK/HlJ model group.
RHL decreases the ACR, as well as blood glucose, creatinine and urea in mice with DN
To assess the effect of RHL on the kidney function of KK/HlJ mice with DN, the ACR, as well as blood glucose, creatinine and urea were detected at 15 weeks (Table I). Compared with those in the C57BL/6J control group, the ACR, as well as blood glucose, creatinine and urea were increased in the KK/HlJ model group, and certain parameters were also increased in the 25 and 50 mg/kg/day RHL-treated groups. Compared with those in the KK/HlJ model group, RHL (25 and 50 mg/kg/day) decreased the ACR, as well as blood glucose and creatinine, and RHL at 50 mg/kg/day also decreased the levels of urea (Table I).
RHL improves kidney function in mice with DN
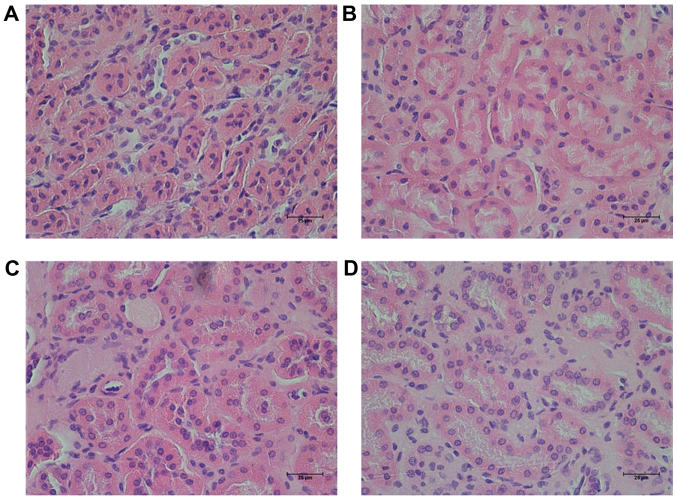
In the present study, it was observed that the size and weight (0.05 g) of one of the kidneys from one mouse was decreased in the KK/HlJ model group. However, this was not observed in the other groups. Hematoxylin-eosin staining revealed that the characteristic changes of the kidney in the KK/HlJ mouse models of DN were renal tubular epithelial cell edema (Fig. 2), which demonstrated that renal tubular epithelial cell edema was shallow. No other structural changes were observed in the DN model group. In comparison, administration of RHL (25 and 50 mg/kg/day) improved renal tubular epithelial cell edema (Fig. 2).
Figure 2.
Effect of RHL on changed of the histopathological profile of the kidneys of mice, as determined by hematoxylin and eosin staining (magnification, ×200). Renal sections from (A) the C57BL/6J control group, (B) the KK/HlJ model group, (C) the 25 mg/kg/day RHL-treated KK/HlJ group and (D) the 50 mg/kg/day RHL-treated KK/HlJ group. Renal tubular epithelial cell edema was present in the KK/HlJ control mice. RHL, rhein lysinate.
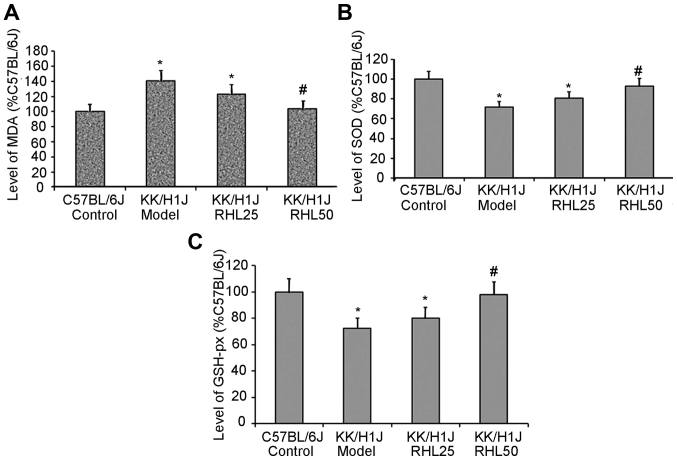
RHL increases the activity of SOD and GSH-px and decreases the levels of MDA in kidney tissue
In the KK/HlJ model group, the activities of SOD and GSH-px were 28 and 27% lower, respectively, than those in the C57BL/6J control group; however, the MDA levels were 40% higher. In the KK/HlJ group treated with 25 mg/kg/day RHL, the activities of SOD and GSH-px were 12 and 10% higher, respectively, than those in the KK/HlJ model group; however, the MDA levels were 13% lower. In the KK/HlJ group treated with 50 mg/kg/day RHL, the activities of SOD and GSH-px were 30 and 35% higher, respectively, than those in the KK/HlJ model group; however, the MDA levels were 26% lower (Fig. 3).
Figure 3.
Effects of RHL on the MDA content and on the activity of SOD and GSH-Px in the kidney tissues of mice in each group. (A) MDA content and the activities of (B) SOD and (C) GSH-px were determined using test kits. *P<0.05 vs. C57BL/6J control group; #P<0.05 vs. KK/HlJ model group. RHL, rhein lysinate; SOD, superoxide dismutase; MDA, malondialdehyde; GSH-Px, glutathione peroxidase.
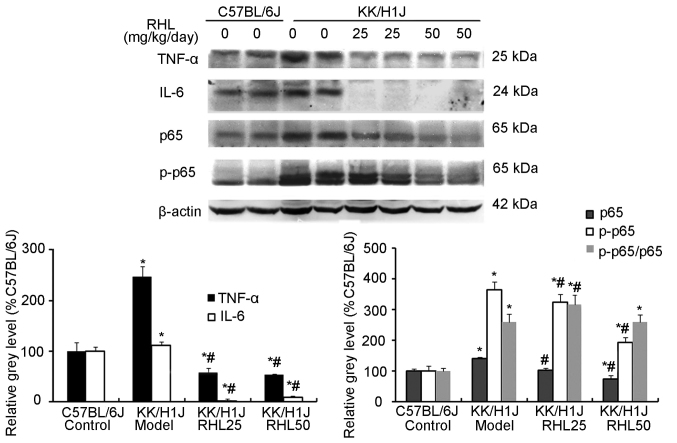
RHL suppresses the expression of inflammatory factors and associated proteins in the kidney of mice with DN
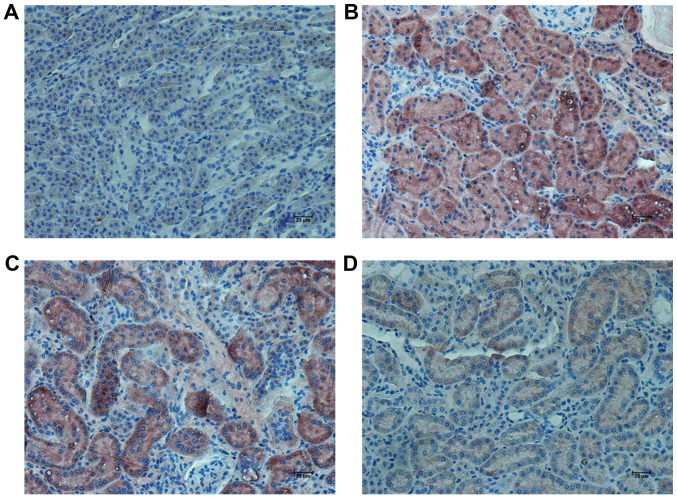
In the KK/HlJ model group, the TNF-α expression levels were higher than those in the C57BL/6J control group. RHL treatment (25 and 50 mg/kg/day) decreased the expression of TNF-α and IL-6, as indicated by immunohistochemical and western blot analysis (Figs. 4–6). In addition, compared with the C57BL/6J control group, the KK/HlJ model group exhibited increased expression and phosphorylation of NF-κB. Compared with the KK/HlJ model group, the RHL groups (25 and 50 mg/kg/day) exhibited decreased phosphorylation and expression of NF-κB. Compared with the C57BL/6J control group, the KK/HlJ model and RHL groups (25 and 50 mg/kg/day) exhibited increased p-NF-κB/NF-κB ratios; however, compared with the KK/HlJ model group, 25 mg/kg/day RHL treatment exhibited increased p-NF-κB/NF-κB ratios and 50 mg/kg/day RHL treatment exhibited no significant effect on the p-NF-κB/NF-κB ratios (Fig. 6).
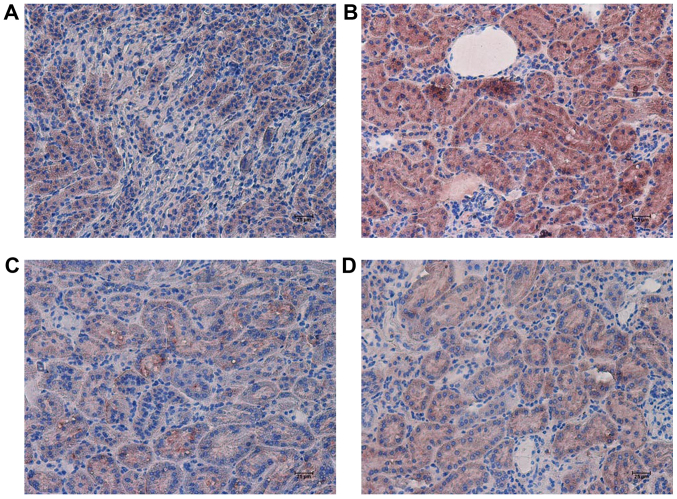
Figure 4.
Effects of RHL on the expression of TNF-α in mouse kidney tissue. Representative immunohistochemically stained renal tissue sections from (A) the C57BL/6J control group, (B) the KK/HlJ model group, (C) the 25 mg/kg/day RHL-treated KK/HlJ group and (D) the 50 mg/kg/day RHL-treated KK/HlJ group (magnification, ×200). TNF-α expression was indicated by brown staining. TNF, tumor necrosis factor; RHL, rhein lysinate.
Figure 6.
Effects of RHL on the expression of proteins associated with inflammation-associated genes in the kidney were assessed by western blot analysis. β-actin was used as the internal control. Values are expressed as the mean ± standard deviation. *P<0.05 vs. C57BL/6J control group; #P<0.05 vs. KK/HlJ model group. RHL, rhein lysinate.
Discussion
The role of rhein and its analogues in the management of chronic kidney disease (CKD) has been addressed in several previous studies. Rhein improved the symptoms of nephropathy through decreasing the production of proinflammatory cytokines, including IL-1β, prostaglandin E2 and TNF-α and inhibiting the expression of transforming growth factor-β1 (15). A previous study by our group also observed that RHL protects the kidney from impairment in a senescence-prone inbred strain 10 mice (14). However, the effect of RHL, the lysin salt of rhein, on DN has remained elusive. In the present study, a KK/HlJ mouse model of DN was induced by STZ injection and a specific diet. The ACR was detected at 5 weeks; compared with that in the C57BL/6J control group, the ACR was increased in the KK/HlJ model group and in the KK/HlJ RHL treatment groups. It was demonstrated that the kidney function in the KK/HlJ model group and the KK/HlJ RHL treatment groups was impaired. The levels of ACR were also detected at 15 weeks; overall, it was demonstrated that RHL improved kidney function impairment of KK/HlJ mice at 5 and 15 weeks. After 16 weeks of treatment, the mice were sacrificed, and kidney weights and the kidney weight-to-body weight ratio in the KK/HlJ model group were revealed to be higher than those in the C57BL/6J control group, and to be reduced by treatment with RHL at 50 mg/kg/day. A previous study by our group reported that RHL decreased the body weight and blood glucose in high-fat diet and STZ-induced diabetic mice (16). In the present study, it was also observed that RHL treatment at 25 and 50 mg/kg/day decreased blood glucose, and RHL at 50 mg/kg/day also decreased the body weight, compared with that in the KK/HlJ model group. Blood biochemistry analysis indicated that creatinine and urea, the biomarkers of kidney function, were increased in the KK/HlJ model group, compared with those in the C57BL/6J control group, which was decreased by RHL treatment.
The pathophysiological characteristics of DN include renal hypertrophy, decrease of renal function, glomerular and tubular basement membranes thickening, mesangial matrix expansion, ultimately causing glomerulosclerosis and interstitial fibrosis (17–19). In the present study, a renal function decrease, renal hypertrophy and renal tubular edema were observed, while glomerulosclerosis and interstitial fibrosis were not seen. It may be speculated that DN in KK/HlJ mice induced in the present study was at its early stage. Thus, early DN may be cured by RHL; however, if glomerulosclerosis and interstitial fibrosis had been present, DN would have hardly been cured. Oxidative stress has been reported to be responsible for the development of peripheral diabetic neuropathy (20,21). MDA, SOD and GSH-px are indicators of the oxidative stress status (22,23). In the present study, the activities of SOD and GSH-px in the KK/HlJ model group were lower than those in the C57BL/6J control group; however, the levels of MDA in the KK/HlJ model group were higher than those in the C57BL/6J control group. These results indicated that RHL increased the levels of SOD and GSH-px and decreased the levels of MDA in kidney tissues of KK/HlJ mice. Thus, it may be deduced that RHL protects the kidney by reducing the levels of reactive oxygen species. These results were similar to those of a previous study by our group (14).
Inflammatory factors reportedly have a role in diabetes and in the progression of DN (24–26). Furthermore, diabetes is associated with increased levels of inflammatory biomarkers, including IL-6 and TNF-α (27,28). The TNF-α-308G/A polymorphism was reported to be associated with the expression levels of TNF-α and may be as a genetic susceptibility factor for DN (29). However, the effect of RHL on inflammatory factors associated with DN has remained elusive. The present results demonstrated that the levels of TNF-α and IL-6 in the KK/HlJ model group were higher than those in the C57BL/6J control group, and the expression levels of TNF-α and IL-6 were markedly suppressed in RHL-treated KK/HlJ mice. Therefore, it was speculated that inflammatory factors (TNF-α and IL-6) take part in the progression of DN in KK/HlJ mice and that RHL treatment decreases the production of inflammatory factors to thereby protect renal function.
TNF-α is one of the key proinflammatory cytokines and is involved in a number of inflammatory processes. TNF-α activates the NF-κB transcription factor. NF-κB has two functions: First, in malignant cells, it promotes cell survival and proliferation. Furthermore, NF-κB activates the immune response, particularly the production of proinflammatory cytokines (30), and inflammation mediated by NF-κB has a critical role in the pathogenesis of DN (31). The present study revealed that TNF-α was involved in renal tubular edema via the TNF-α/NF-κB biochemical pathway. RHL decreased the expression of TNF-α and NF-κB, and inhibited the phosphorylation of NF-κB downstream of TNF-α. Therefore, RHL had the ability to inhibit the immune response by directly or indirectly blocking the TNF-α/NF-κB biochemical pathway.
In conclusion, oxygen free radicals and inflammatory factors took part in the progression of DN in KK/HlJ mice. RHL (25 and 50 mg/kg/day) significantly decreased kidney inflammation by reducing the levels of oxygen free radicals, blocking the TNF-α/NF-κB biochemical pathway and reducing renal function impairment. This finding is expected to inspire future investigations on the efficacy of RHL in DN.
Figure 5.
Effects of RHL on the expression of IL-6 in the mouse kidney tissue. Representative immunohistochemically stained renal tissue sections from (A) the C57BL/6J control group, (B) the KK/HlJ model group, (C) the 25 mg/kg/day RHL-treated KK/HlJ group and (D) the 50 mg/kg/day RHL-treated KK/HlJ group (magnification, ×200). IL-6 expression was indicated by brown staining. IL, interleukin; RHL, rhein lysinate.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81671391), Beijing Hospital Nova Project (grant no. BJ-2016-033) and the General Program of Natural Science Foundation of Hebei Province of China (grant no. H2012401030).
References
- 1.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon) 2014;42:698–702. doi: 10.1016/j.mpmed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Groop PH. Epidemiology and risk factors for diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:260–266. doi: 10.1053/j.ackd.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Noubiap JJ, Naidoo J, Kengne AP. Diabetic nephropathy in Africa: A systematic review. World J Diabetes. 2015;6:759–773. doi: 10.4239/wjd.v6.i5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assogba GF, Couchoud C, Roudier C, Pornet C, Fosse S, Romon I, Druet C, Stengel B, Fagot-Campagna A. Prevalence, screening and treatment of chronic kidney disease in people with type 2 diabetes in France: The ENTRED surveysnd (2001 and 2007) Diabetes Metab. 2012;38:558–566. doi: 10.1016/j.diabet.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Bakris GL. Recognition, pathogenesis, and treatment of different stages of nephropathy in patients with type 2 diabetes mellitus. Mayo Clin Proc. 2011;86:444–456. doi: 10.4065/mcp.2010.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME, Mathew TH. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study) Med J Aust. 2006;185:140–144. doi: 10.5694/j.1326-5377.2006.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 7.Chokhandre MK, Mahmoud MI, Hakami T, Jafer M, Inamdar AS. Vitamin D & its analogues in type 2 diabetic nephropathy: A systematic review. J Diabetes Metab Disord. 2015;14:58. doi: 10.1186/s40200-015-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Wang C, Liu F, Lu Y, Cheng J. Metabonomics revealed xanthine oxidase-induced oxidative stress and inflammation in the pathogenesis of diabetic nephropathy. Anal Bioanal Chem. 2015;407:2569–2579. doi: 10.1007/s00216-015-8481-0. [DOI] [PubMed] [Google Scholar]
- 9.Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, Schneider H, Ruiz-Ortega M, Egido J. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19:2505–2512. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- 10.Ayepola OR, Cerf ME, Brooks NL, Oguntibeju OO. Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes-induced nephrotoxic rats. Phytomedicine. 2014;21:1785–1793. doi: 10.1016/j.phymed.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Soler MJ, Riera M, Batlle D. New experimental models of diabetic nephropathy in mice models of type 2 diabetes: Efforts to replicate human nephropathy. Exp Diabetes Res. 2012;2012:616313. doi: 10.1155/2012/616313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 2005;54:2628–2637. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- 13.Lin YJ, Zhen YS. Rhein lysinate suppresses the growth of breast cancer cells and potentiates the inhibitory effect of taxol in athymic mice. Anticancer Drugs. 2009;20:65–72. doi: 10.1097/CAD.0b013e3283182913. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Liu J, Zhen YZ, Xu R, Qiao Y, Wei J, Tu P, Lin YJ. Rhein lysinate increases the median survival time of SAMP10 mice: Protective role in the kidney. Acta Pharmacol Sin. 2013;34:515–521. doi: 10.1038/aps.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng Z, Yan Y, Tang Z, Guo C, Li N, Huang W, Ding G, Wang Z, Xiao W, Yang Z. Anti-hyperuricemic and nephroprotective effects of rhein in hyperuricemic mice. Planta Med. 2015;81:279–285. doi: 10.1055/s-0034-1396241. [DOI] [PubMed] [Google Scholar]
- 16.Lin YJ, Hu G, Li KJ, Zhao YF, Wei J, Zhen YZ. The protection of Rhein lysinate to liver in diabetic mice induced by high-fat diet and streptozotocin. Arch Pharm Res. 2015;38:885–892. doi: 10.1007/s12272-014-0423-4. [DOI] [PubMed] [Google Scholar]
- 17.Cao Z, Cooper ME. Pathogenesis of diabetic nephropathy. J Diabetes Investig. 2011;2:243–247. doi: 10.1111/j.2040-1124.2011.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa M, Gohda T, Tanimoto M, Tomino Y. Pathogenesis and novel treatment from the mouse model of type 2 diabetic nephropathy. ScientificWorldJournal. 2013;2013:928197. doi: 10.1155/2013/928197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobretsov M, Romanovsky D, Stimers JR. Early diabetic neuropathy: Triggers and mechanisms. World J Gastroenterol. 2007;13:175–191. doi: 10.3748/wjg.v13.i2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wattanathorn J, Thiraphatthanavong P, Muchimapura S, Thukhammee W, Lertrat K, Suriharn B. The combined extract of Zingiber officinale and Zea mays (purple color) improves neuropathy, oxidative stress, and axon density in streptozotocin induced diabetic rats. Evid Based Complement Alternat Med. 2015;2015:301029. doi: 10.1155/2015/301029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng H, Zhang D, Yang H. Effects of amyloid precursor protein 17 peptide on the protection of diabetic encephalopathy and improvement of glycol metabolism in the diabetic rat. J Diabetes Res. 2013;2013:689841. doi: 10.1155/2013/689841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XW, Liu FQ, Guo JJ, Yao WJ, Li QQ, Liu TH, Xu LP. Antioxidation and anti-inflammatory activity of Tang Bi Kang in rats with diabetic peripheral neuropathy. BMC Complement Altern Med. 2015;15:66. doi: 10.1186/s12906-015-0600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Rejaie SS, Aleisa AM, Abuohashish HM, Parmar MY, Ola MS, Al-Hosaini AA, Ahmed MM. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol Res. 2015;37:924–933. doi: 10.1179/1743132815Y.0000000079. [DOI] [PubMed] [Google Scholar]
- 25.Semeraro F, Cancarini A, Dell'Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic retinopathy: Vascular and inflammatory disease. J Diabetes Res. 2015;2015:582060. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu JS, Liu Y, Shi R, Lu X, Ma YM, Cheng NN. Effects of combinations of Xiexin decoction constituents on diabetic nephropathy in rats. J Ethnopharmacol. 2014;157:126–133. doi: 10.1016/j.jep.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Akram KA, Tofighiyan T, Rakhshani MH. Effects of synbiotics on inflammatory markers in patients with type 2 diabetes mellitus. Glob J Health Sci. 2015;7:1–5. doi: 10.5539/gjhs.v7n7p1. (7 Spec No) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolasco EL, Zanoni FL, Nunes FP, Ferreira SS, Freitas LA, Silva MC, Martins JO. Insulin modulates liver function in a type I diabetes rat model. Cell Physiol Biochem. 2015;36:1467–1479. doi: 10.1159/000430311. [DOI] [PubMed] [Google Scholar]
- 29.Peng Y, Li LJ. TNF-α-308G/A polymorphism associated with TNF-α protein expression in patients with diabetic nephropathy. Int J Clin Exp Pathol. 2015;8:3127–3131. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang SS, Purdue MP, Cerhan JR, Zheng T, Menashe I, Armstrong BK, Lan Q, Hartge P, Kricker A, Zhang Y, et al. Common gene variants in the tumor necrosis factor (TNF) and TNF receptor superfamilies and NF-kB transcription factors and non-Hodgkin lymphoma risk. PLoS One. 2009;4:e5360. doi: 10.1371/journal.pone.0005360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie X, Chang X, Chen L, Huang K, Huang J, Wang S, Shen X, Liu P, Huang H. Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling. Mol Cell Endocrinol. 2013;381:56–65. doi: 10.1016/j.mce.2013.07.019. [DOI] [PubMed] [Google Scholar]