Abstract
Delphinidin, a flavonoid polyphenolic compound, is widely found in nature and is used as a food supplement due to its pharmacological activity. The aims of the present study were to examine the anti-inflammatory effect of delphinidin in alleviating spinal cord injury (SCI)-induced inflammation in a rat model and to determine the underlying mechanisms in SCI. The Basso, Beattie, Bresnahan (BBB) scores of rats were assessed to evaluate the effect of delphinidin on the recovery of motor function. ELISA kits were also used to analyze the activities of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2) and caspase-3. In addition, the protein expression levels of nuclear factor (NF)-κB, activator protein 1 (AP-1) and p38-MAPK protein expression were measured using western blot analysis. Treatment with delphinidin significantly increased the BBB scores, as well as inhibited the intramedullary spinal pressure in SCI rats. Delphinidin treatment also significantly suppressed the levels of inflammatory factors and NF-κB protein expression in SCI rats. Finally, treatment with delphinidin significantly inhibited NF-κB stimulation, COX-2 activity, PGE2 production, and AP-1 and p38-MAPK protein expression in SCI rats. These results suggest that the anti-inflammatory effect of delphinidin alleviated inflammation in the SCI rat model via alleviation of the intramedullary spinal pressure through the NF-κB and p38-MAPK signaling pathways.
Keywords: delphinidin, spinal cord injury, intramedullary spinal pressure, NF-κB, cyclooxygenase-2, p38-MAPK, inflammation
Introduction
Spinal cord injury (SCI) is a common trauma that threatens human health seriously and can occur at any age (1). It frequently results in failure in limb sensation, motor function and automatic nervous function. The high disability and mortality rates of SCI often have a huge physiological and psychological effect on individuals, their families and society (2). However, there is no effective treatment due to the complicated pathophysiological mechanism of SCI, although numerous clinical and animal studies have been conducted (3,4).
SCI involves a series of cytological and molecular biology cascade reactions (5). It can be divided into primary and secondary injury (1). Primary injury of the spinal cord may result in irreversible death of a large number of neurons in the damaged tissue (6), with no effective methods to regenerate the neurons available to date. Primary injury may then result in secondary injury after several min or hfrom SCI. Thus, it further results in neuronal injury and expansion of the SCI area (5,6). The early application of drugs prevents secondary injury; thus, the condition of spinal cord tissues can be improved to preserve the anatomical structure for functional recovery (6). Therefore, early drug administration is important for acute SCI treatment.
The inflammatory reaction is a major component of secondary SCI and is a key constituent of the pathophysiological mechanism of acute SCI. Inflammation may promote a series of molecular biological events, resulting in the activation of inflammatory cells from the spinal cord tissues and infiltration of the circulatory system, the release of various pro-inflammatory mediators and neurotoxins, and the generation of oxygen radicals and nitroso compounds, and consequently causing cell damage (7,8).
Delphinidin (Fig. 1) is a polyphenolic substance that is derived from catechinic acid and epicatechin (9). It is widely obtained from the pericarp, testa and bark of various plants. Delphinidin extracted from grape seeds has a high purity and oligomer content. As a representative of the flavonoid polyphenolic substances, delphinidin is widely found in plant-based food (10). It has powerful antibiosis, anti-inflammatory, anti-allergenic and anti-oxygenation activities (11).
Figure 1.
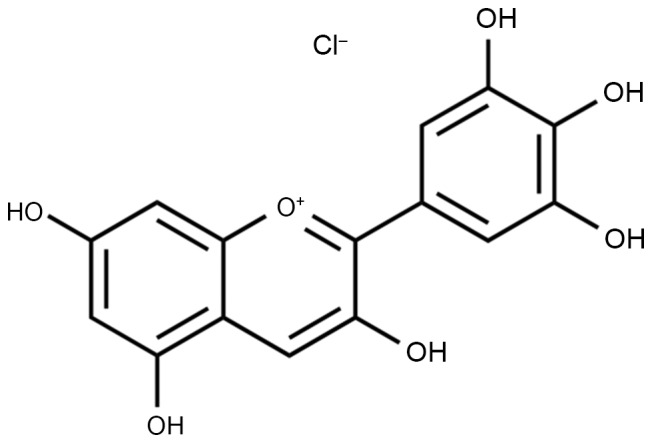
Chemical structure of delphinidin.
Delphinidin has been extensively used as a complementary anti-inflammation and trauma treatment in the form of a food supplement (12,13). The present study investigated whether the anti-inflammatory effect of delphinidin alleviates the SCI-induced inflammation in a rat model through intramedullary spinal pressure.
Materials and methods
Animal care and groups
All experimental procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by Liaocheng People's Hospital, Liaocheng Clinical School of Taishan Medical University (Liaocheng, China). The study was approved by the Ethics Committee of Liaocheng People's Hospital. Adult male Sprague-Dawley rats (n=40, weight, 220–250 g, 8–9 weeks-old) were purchased from the Laboratory Animal Center of the Taishan Medical University, and raised in the specific-pathogen-free Laboratory Animal Center at a constant environment with a temperature of 22±1°C, 50–60% humidity, an alternating 12 h light-dark cycle and free access to food/water. The rats were randomly divided into four groups (n=10 rats per group): Sham, SCI model, and two SCI + delphinidin-treated (40 or 200 mg/kg) groups. A total of 24 h after SCI, the delphinidin group rats were treated with 40 or 200 mg/kg delphinidin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 2 weeks.
SCI model
In order to establish an SCI model, the rats were anesthetized with 35 mg/kg pentobarbital sodium by intraperitoneal injection. The spinal cord was aseptically exposed following laminectomy at T9-10. Next, a 2-mm diameter hammer was implanted at 25 mm from the T9-10 spinal cord and removed after 1 min. Subsequently, manual bladder expression was performed three times daily.
Basso, Beattie, Bresnahan (BBB) open field locomotor assessment
Following delphinidin treatment, the BBB scores of rats were assessed to evaluate the recovery condition of the motor function. The BBB scores ranged between 0 and 21, with the lowest point (score of 0) indicating complete paralysis and the maximum point (score of 21) implying normal function.
ELISAs for determining the activities of various inflammatory factors
Following delphinidin treatment, rats was sacrificed using decapitation under anesthetization (35 mg/kg pentobarbital sodium) and spinal cord (2 mm cephalic and caudally around the injury epicenter) was removed. The tissues were dissolved in RIPA lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China), and a BCA kit (Beyotime Institute of Biotechnology) was used to quantify the protein concentration. In accordance with the specifications of the ELISA kits, protein samples (10 µg) were used to measure the activities of tumor necrosis factor-α (TNF-α; cat. no. H052), interleukin (IL)-6 (cat. no. H007), cyclooxygenase-2 (COX-2; cat. no. H200), prostaglandin E2 (PGE2; cat. no. H099) and caspase-3 (cat. no. G015; all Jiancheng Bioengineering Institute, Nanjing, China) using a luminometer (MicroLumat Plus LB 96V; Berthold Technologies, Bad Wildbach, Germany).
Western blot analysis
Following delphinidin treatment, protein samples were obtained from spinal cord tissues. The protein samples (40 µg) were separated by 8–10% SDS-PAGE and transferred into polyvinylidene difluoride membranes (EMD Millipore, Bedford, MA, USA). The membranes were blocked in 5% fat-free milk for 1 h and then incubated at 4°C overnight with primary antibodies, including antibodies against nuclear factor-κB (NF-κB)/p65, activator protein 1 (AP-1), phosphorylated-p38-MAPK and β-actin. Subsequently, the membranes were incubated with secondary antibodies for 2 h at room temperature, and images were captured using ChemiDoc-It TS2 Imager (UVP, LLC, Upland, CA, USA). The relative protein expression was determined using ImageJ2× software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation and the Student's t-test was used for single statistical comparisons using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) software package. P<0.05 was considered to indicate a statistically significant difference.
Results
Anti-inflammatory effect of delphinidin improves the BBB scores in the rat model of SCI
The present study investigated whether the anti-inflammatory effect of delphinidin was able to improve the BBB scores in a rat model of SCI. As shown in Fig. 2, BBB scores in the SCI model group were markedly reduced compared with those of the sham group (P=0.0023). However, treatment with 40 or 200 mg/kg delphinidin significantly increased the SCI-induced inhibition of BBB scores at 21 days after SCI (Fig. 2; P=0.0079 or 0.0056, respectively).
Figure 2.
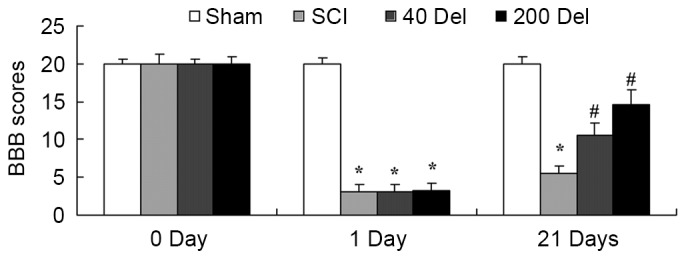
Anti-inflammatory effect of Del alleviates the BBB scores in a rat model of SCI, following treatment with 40 or 200 mg/kg Del. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; BBB, Basso, Beattie, Bresnahan; SCI, spinal cord injury.
Anti-inflammatory effect of delphinidin alleviates the intramedullary spinal pressure in the SCI rat model
The effect of delphinidin treatment on the intramedullary spinal pressure in SCI rats was investigated. There was a significant increase in the intramedullary spinal pressure in SCI rats when compared with the sham group (Fig. 3; P=0.0037). However, 40 or 200 mg/kg delphinidin treatment significantly reduced the induction of intramedullary spinal pressure when compared with the untreated SCI rats (Fig. 3; P=0.0072 or 0.0044, respectively).
Figure 3.
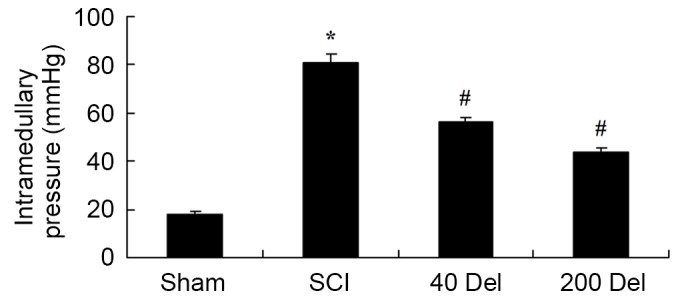
Anti-inflammatory effect of Del treatment (40 or 200 mg/kg) alleviates the intramedullary spinal pressure in a rat model of SCI. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; SCI, spinal cord injury.
Delphinidin treatment reduces the activities of inflammatory factors in the rat model of SCI
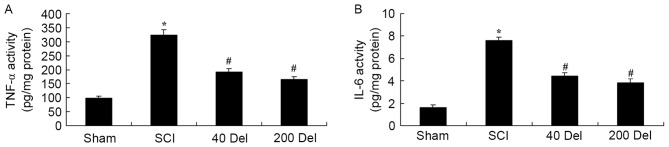
To examine the anti-inflammatory effect of delphinidin in SCI rats, the activities of anti-inflammatory factors TNF-α and IL-6 were analyzed by ELISA. The TNF-α and IL-6 activities were found to be markedly increased in SCI rats, compared with the sham group (Fig. 4; P=0.0021 or 0.0014, respectively). However, 40 or 200 mg/kg delphinidin treatment significantly suppressed the promotion of TNF-α and IL-6 activities in the SCI rats (Fig. 4; P=0.0055 or 0.0038 and P=0.0051 or 0.0024, respectively). Taken together, these results indicated delphinidin has an anti-inflammatory effect in SCI.
Figure 4.
Effect of Del treatment (40 or 200 mg/kg) on inflammatory factors in a rat model of SCI. The treatment increased the (A) TNF-α and (B) IL-6 activities in the spinal cord tissues of SCI rats. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; SCI, spinal cord injury; TNF, tumor necrosis factor; IL, interleukin.
Delphinidin inhibits the COX-2 activity and PGE2 production in the rat model of SCI
In order to investigate the anti-inflammatory effect of delphinidin in SCI rats, the COX-2 and PGE2 levels were investigated by ELISA. The induction of SCI in the rats resulted in enhanced COX-2 activity (Fig. 5; P=0.0025) and PGE2 production (Fig. 6; P=0.0012) when compared with the levels in the sham group. By contrast, upon treatment with different concentrations of delphinidin for 2 weeks, the activity of COX-2 in the SCI rats was significantly inhibited when compared with the untreated SCI model rats (Fig. 5). In addition, 40 or 200 mg/kg delphinidin treatment markedly inhibited the activation of PGE2 production in SCI rats (Fig. 6; P=0.0031 or 0.0026, respectively).
Figure 5.
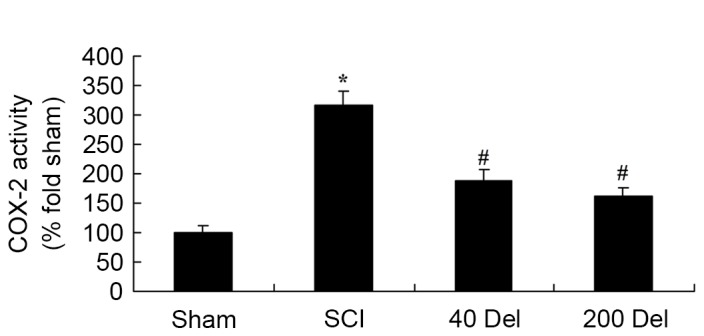
Del treatment (40 or 200 mg/kg) in a rat model of SCI reduced the COX-2 activity, as determined by ELISA. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; SCI, spinal cord injury; COX-2, cyclooxygenase-2.
Figure 6.
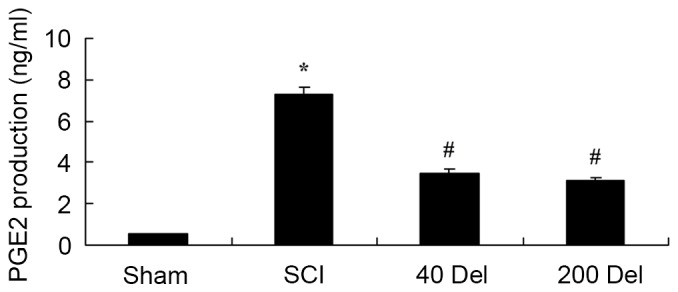
Del treatment (40 or 200 mg/kg) in a rat model of SCI reduced the PGE2 production, as determined by ELISA. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; SCI, spinal cord injury; PGE2, prostaglandin E2.
Delphinidin treatment reduces caspase-3 activity in the rat model of SCI
To further determine the effect of delphinidin treatment on apoptosis in the SCI rats, caspase-3 activity was detected in the present study using ELISA. As shown in Fig. 7, the caspase-3 activity was evidently activated in SCI rats compared with the sham group (P=0.0049). However, 40 or 200 mg/kg delphinidin treatment significantly inhibited the caspase-3 activity induced by SCI in the rat model of SCI (Fig. 7; P=0.0058 or 0.0045, respectively).
Figure 7.
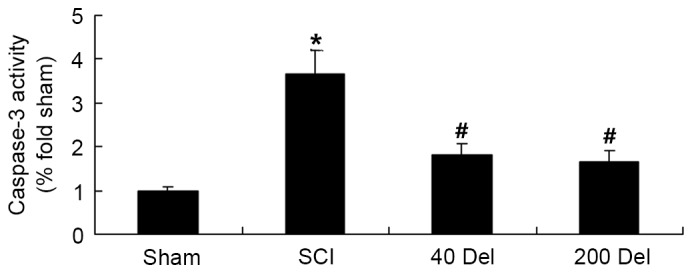
Del treatment (40 or 200 mg/kg) in a rat model of SCI reduced the activity of caspase-3, which is an indicator of apoptosis, as determined by ELISA. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; SCI, spinal cord injury.
Delphinidin reduces NF-κB and Ap-1 protein levels in a rat model of SCI
To further investigate the anti-inflammatory effect of delphinidin in the rat model of SCI, NF-κB/p65 and Ap-1 protein expression levels were detected using western blot analysis. As shown in Figs. 8 and 9, the induction of SCI evidently increased the NF-κB/p65 (P=0.0012 or 0.0009, respectively) and Ap-1 protein expression levels when compared with those in the sham group rats. However, 40 or 200 mg/kg delphinidin treatment significantly inhibited the protein expression levels of NF-κB/p65 (Fig. 8; P=0.0044 or 0.0032, respectively) and Ap-1 (Fig. 9; P=0.0052 or 0.0041) in SCI rats (Fig. 8).
Figure 8.
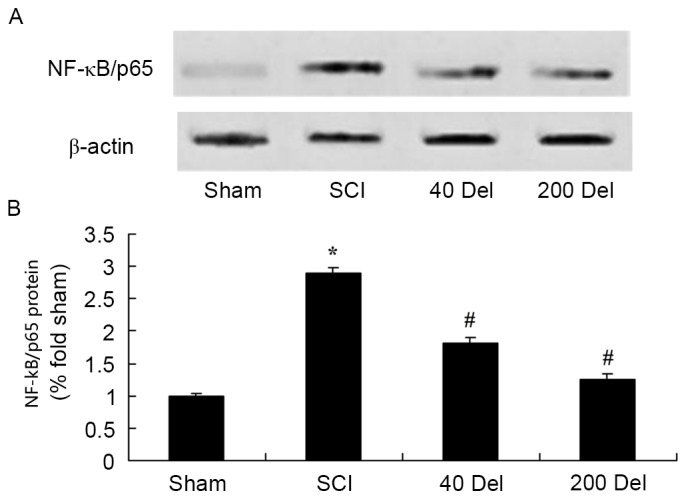
Effect of Del treatment (40 or 200 mg/kg Del) on NF-κB expression in a rat model of SCI, as determined by western blot analysis. The anti-inflammatory effect of Del increased the NF-κB/p65 protein expression, shown in the (A) western blots and (B) quantified levels. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; SCI, spinal cord injury; NF, nuclear factor.
Figure 9.
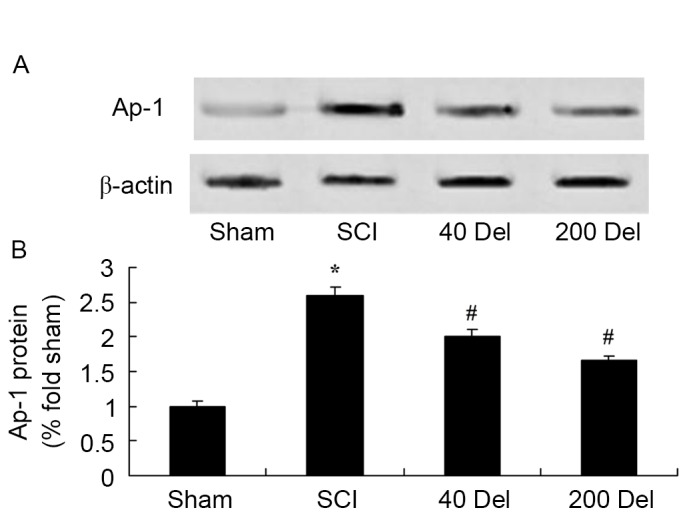
Effect of Del treatment (40 or 200 mg/kg) on Ap-1 in a rat model of SCI as determined by western blot analysis. The anti-inflammatory effect of Del inhibited Ap-1 protein expression, shown in the (A) western blots and (B) quantified levels. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; SCI, spinal cord injury; AP-1, activator protein 1.
Anti-inflammatory effect of delphinidin blocks the activation of the p38-MAPK signaling pathway in the rat model of SCI
To further study the molecular and cellular effects of delphinidin on SCI, the expression of phosphorylated p38-MAPK in the rat model of SCI was detected. At 2 weeks after SCI, the p-p38-MAPK protein expression in the SCI model group was markedly higher in comparison with that of the sham group (Fig. 10; P=0.0029). However, treatment with 40 or 200 mg/kg delphinidin significantly suppressed the SCI-induced activation of p-p38-MAPK protein expression in SCI rats (Fig. 10; P=0.0047 or 0.0035, respectively).
Figure 10.
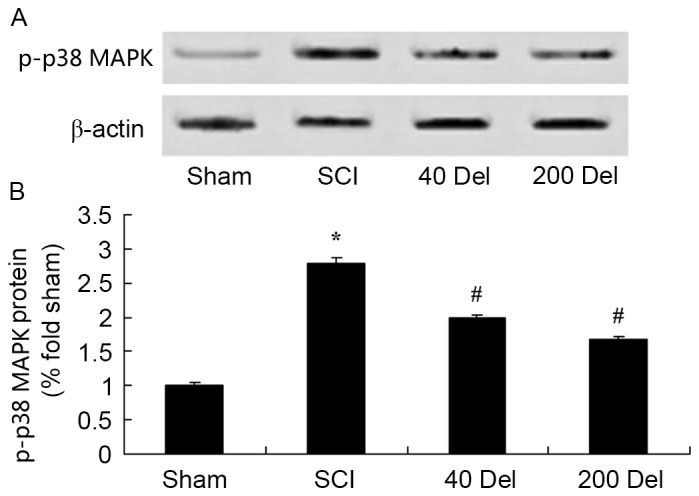
Effect of Del treatment (40 or 200 mg/kg) on p38-MAPK in a rat model of SCI as determined by western blot analysis. The anti-inflammatory effect of Del inhibited p38-MAPK protein expression, shown in the (A) western blots and (B) quantified levels. *P<0.01 vs. SCI model group; #P<0.01 vs. sham group. Del, delphinidin; SCI, spinal cord injury.
Discussion
Acute SCI is a trauma that may result in disability (2). One of the most important targets of early treatment for SCI is to prevent the secondary injury. The pathological development process of secondary injury in the spinal cord involves capillary changes, edema, altered energy metabolism, various biochemical changes and apoptosis (14). Inhibiting the inflammatory reaction and lipid peroxidation is also regarded as one of the most important targets of early treatment for SCI (15). Following SCI, the early inflammatory reaction serves a role in protecting the spinal cord nerves and promoting functional recovery (16). For these reasons, strategies for relieving the inflammatory reaction after SCI, and promoting structural reconstruction and functional recovery of the damaged areas is the focus of research in the fields of neuroscience, orthopedics and sports medicine (17,18). In the present study, delphinidin treatment significantly increased the SCI-inhibited BBB scores and reduced the induction of intramedullary spinal pressure in SCI rats. These findings suggest that delphinidin may be used to treat SCI and to reduce intramedullary spinal pressure.
The inflammatory reaction is mainly regulated by the expression of several genes, while the NF-κB family is the major regulatory factor of inflammatory gene expression (7,19). NF-κB can regulate expression of many cellular factors and regulate inflammatory reactions. Abnormally activated NF-κB may launch the apoptosis of neurons in trauma of central nervous system, excitotoxic injury, ischemic damage and neurodegenerative disease (12). NF-κB is abnormally activated following SCI, activating inducible nitric oxide synthase in neurons (7). The expression of various genes in SCI is regulated by NF-κB, including the expression of pro-inflammatory cell factors, such as TNF-α, IL-1β and IL-6, as well as proteases, such as matrix metalloproteinase (7). Pal et al (12) reported that delphinidin reduces psoriasiform lesions by inducing epidermal differentiation and inhibiting inflammation in flaky skin mice. Cho et al (9) suggested that delphinidin inhibited extracellular matrix production in nasal polyp-derived fibroblasts through the MAPK/NF-κB signaling pathway induced by TGF-β1 stimulation. In addition, the molecular mechanism of delphinidin regulating SCI-induced inflammation through the suppression of NF-κB after SCI was initially observed in the present study.
At present, TGF-β1 is increasingly investigated due to its association with the majority of fiber hyperplastic diseases (20). In addition, TGF-β1 is recognized as one of the most powerful cell factors that cause fibrosis (21). The present study results revealed that delphinidin significantly inhibited the activation of PGE2 production, and suppressed the protein expression levels of AP-1 and p38-MAPK in SCI rats. This data suggests that delphinidin prevents SCI through regulation of PGE2 production, AP-1 and p38-MAPK expression.
COX-2 is typically considered to be harmful under pathological conditions, such as inflammation, pain, cellular damage and tumor. However, recent studies have proven that COX-2 also has an effect on the normal physiological function of humans (22). In the central nervous system, COX-2 is expressed in the glutamate neurons of the hippocampus and cerebral cortex, and is critical to neurovascular coupling during the period of synaptic activity, long-term synaptic plasticity and functional congestion (23). Previous data indicated that COX-2 expression is associated with hypoxia, peroxidation and neuronal cell death induced by excitatory amino acids after SCI (24). The application of COX-2 inhibitors subsequent to SCI serves a certain neuroprotective effect, indicating that COX-2 is harmful after SCI (25). Hwang et al (26) suggested that delphinidin inhibited COX-2 expression via TNF-α in JB6 P+ mouse epidermal cells. In the present study, delphinidin treatment significantly reduced COX-2 activity in SCI rats.
It is known that p-p38-MAPK participates in signal transduction in the process of nerve cell apoptosis. Thus, strong or weak expression of p-p38 predicts whether nerve cells are alive (27). As a result of the apoptosis process of neurons and colloid cells subsequent to SCI, p-p38 expression increases, indicating that the p-p38 signal transduction pathway is altered after SCI (28). SCI results in the activation of p-p38, which then increases NOS activity and promotes apoptosis (29). The results of the present study demonstrated that the anti-inflammatory effect of delphinidin reduced the p-p38-MAPK protein expression and caspase-3 activity in a rat model of SCI. Furthermore, Oak et al (30) reported that delphinidin prevents the activation of p38-MAPK and JNK in vascular smooth muscle cells. Overall, these data suggest that administration of delphinidin alleviates the SCI-induced inflammation through suppression of the p38-MAPK signaling pathway.
In conclusion, the anti-inflammatory effect of delphinidin was able to alleviate the SCI-induced inflammation and intramedullary spinal pressure in a rat model of SCI. The present study confirms that the anti-inflammatory effect of delphinidin was achieved in SCI rats through theinhibition of COX-2 and PGE2 production, and the suppression of NF-κB and p38 MAPK signaling pathways.
References
- 1.de Rivero Vaccari JP, Marcillo A, Nonner D, Dietrich WD, Keane RW. Neuroprotective effects of bone morphogenetic protein 7 (BMP7) treatment after spinal cord injury. Neurosci Lett. 2009;465:226–229. doi: 10.1016/j.neulet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Lim JH, Muguet-Chanoit AC, Smith DT, Laber E, Olby NJ. Potassium channel antagonists 4-aminopyridine and the T-butyl carbamate derivative of 4-aminopyridine improve hind limb function in chronically non-ambulatory dogs; a blinded, placebo-controlled trial. PLoS One. 2014;9:e116139. doi: 10.1371/journal.pone.0116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DePaul MA, Palmer M, Lang BT, Cutrone R, Tran AP, Madalena KM, Bogaerts A, Hamilton JA, Deans RJ, Mays RW, et al. Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Sci Rep. 2015;5:16795. doi: 10.1038/srep16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi F, De Rensis F, Saleri R, Spattini G. Effect of urethral infusion of atracurium besylate on manual bladder expression in dogs and cats with spinal cord injuries: A randomised trial. Vet Rec. 2015;176:545. doi: 10.1136/vr.102825. [DOI] [PubMed] [Google Scholar]
- 5.Wilson JR, Grossman RG, Frankowski RF, Kiss A, Davis AM, Kulkarni AV, Harrop JS, Aarabi B, Vaccaro A, Tator CH, et al. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J Neurotrauma. 2012;29:2263–2271. doi: 10.1089/neu.2012.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosety-Rodriguez M, Camacho A, Rosety I, Fornieles G, Rosety MA, Diaz AJ, Bernardi M, Rosety M, Ordonez FJ. Low-grade systemic inflammation and leptin levels were improved by arm cranking exercise in adults with chronic spinal cord injury. Arch Phys Med Rehabil. 2014;95:297–302. doi: 10.1016/j.apmr.2013.08.246. [DOI] [PubMed] [Google Scholar]
- 7.Oudega M. Inflammatory response after spinal cord injury. Exp Neurol. 2013;250:151–155. doi: 10.1016/j.expneurol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Fan QQ, Li L, Wang WT, Yang X, Suo ZW, Hu XD. Activation of α2 adrenoceptors inhibited NMDA receptor-mediated nociceptive transmission in spinal dorsal horn of mice with inflammatory pain. Neuropharmacology. 2014;77:185–192. doi: 10.1016/j.neuropharm.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Cho JS, Kang JH, Shin JM, Park IH, Lee HM. Inhibitory effect of delphinidin on extracellular matrix production via the MAPK/NF-κB pathway in nasal polyp-derived fibroblasts. Allergy Asthma Immunol Res. 2015;7:276–282. doi: 10.4168/aair.2015.7.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayoub O, Andriantsitohaina R, Clere N. Pleiotropic beneficial effects of epigallocatechin gallate, quercetin and delphinidin on cardiovascular diseases associated with endothelial dysfunction. Cardiovasc Hematol Agents Med Chem. 2013;11:249–264. doi: 10.2174/1871525712666140309233048. [DOI] [PubMed] [Google Scholar]
- 11.Yuan B, Okusumi S, Yoshino Y, Moriyama C, Tanaka S, Hirano T, Takagi N, Toyoda H. Delphinidin induces cytotoxicity and potentiates cytocidal effect in combination with arsenite in an acute promyelocytic leukemia NB4 cell line. Oncol Rep. 2015;34:431–438. doi: 10.3892/or.2015.3963. [DOI] [PubMed] [Google Scholar]
- 12.Pal HC, Chamcheu JC, Adhami VM, Wood GS, Elmets CA, Mukhtar H, Afaq F. Topical application of delphinidin reduces psoriasiform lesions in the flaky skin mouse model by inducing epidermal differentiation and inhibiting inflammation. Br J Dermatol. 2015;172:354–364. doi: 10.1111/bjd.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM, Saleem M, Din M, Mukhtar H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: Involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564–8572. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutbeyaz ST, Koseoglu BF, Gokkaya NK. The combined effects of controlled breathing techniques and ventilatory and upper extremity muscle exercise on cardiopulmonary responses in patients with spinal cord injury. Int J Rehabil Res. 2005;28:273–276. doi: 10.1097/00004356-200509000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Schulz R, Czaja SJ, Lustig A, Zdaniuk B, Martire LM, Perdomo D. Improving the quality of life of caregivers of persons with spinal cord injury: A randomized controlled trial. Rehabil Psychol. 2009;54:1–15. doi: 10.1037/a0014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteneck GG, Gassaway J, Dijkers MP, Lammertse DP, Hammond F, Heinemann AW, Backus D, Charlifue S, Ballard PH, Zanca JM. Inpatient and postdischarge rehabilitation services provided in the first year after spinal cord injury: Findings from the SCIRehab study. Arch Phys Med Rehabil. 2011;92:361–368. doi: 10.1016/j.apmr.2010.07.241. [DOI] [PubMed] [Google Scholar]
- 17.Ellenbroek D, Kressler J, Cowan RE, Burns PA, Mendez AJ, Nash MS. Effects of prandial challenge on triglyceridemia, glycemia, and pro-inflammatory activity in persons with chronic paraplegia. J Spinal Cord Med. 2015;38:468–475. doi: 10.1179/2045772314Y.0000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen V, Elfving B, Opheim A. Assessment of unsupported sitting in patients with spinal cord injury. Spinal Cord. 2011;49:838–843. doi: 10.1038/sc.2011.9. [DOI] [PubMed] [Google Scholar]
- 19.Tyagi P, Kadekawa K, Kashyap M, Pore S, Yoshimura N. Spontaneous recovery of reflex voiding following spinal cord injury mediated by anti-inflammatory and neuroprotective factors. Urology. 2016;88:57–65. doi: 10.1016/j.urology.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji H, Tang H, Lin H, Mao J, Gao L, Liu J, Wu T. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep. 2014;2:787–792. doi: 10.3892/br.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rathore KI, Redensek A, David S. Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-α and TGF-β1. Glia. 2012;60:738–750. doi: 10.1002/glia.22303. [DOI] [PubMed] [Google Scholar]
- 22.Banovac K, Williams JM, Patrick LD, Levi A. Prevention of heterotopic ossification after spinal cord injury with COX-2 selective inhibitor (rofecoxib) Spinal Cord. 2004;42:707–710. doi: 10.1038/sj.sc.3101628. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Huang C, Wang Y, Guo Q, Jiang H, Wen J. Changes in expression of cyclooxygenase-2 in the spinal dorsal horn after intrathecal p38MAPK inhibitor SB203580 on neuropathic pain in rats. Ann Palliat Med. 2013;2:124–129. doi: 10.3978/j.issn.2224-5820.2013.07.02. [DOI] [PubMed] [Google Scholar]
- 24.Quan HH, Kang KS, Sohn YK, Li M. Tempol reduces injury area in rat model of spinal cord contusion injury through suppression of iNOS and COX-2 expression. Neurol Sci. 2013;34:1621–1628. doi: 10.1007/s10072-013-1295-y. [DOI] [PubMed] [Google Scholar]
- 25.Coronel MF, Labombarda F, De Nicola AF, González SL. Progesterone reduces the expression of spinal cyclooxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur J Pain. 2014;18:348–359. doi: 10.1002/j.1532-2149.2013.00376.x. [DOI] [PubMed] [Google Scholar]
- 26.Hwang MK, Kang NJ, Heo YS, Lee KW, Lee HJ. Fyn kinase is a direct molecular target of delphinidin for the inhibition of cyclooxygenase-2 expression induced by tumor necrosis factor-alpha. Biochem Pharmacol. 2009;77:1213–1222. doi: 10.1016/j.bcp.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 27.He BR, Xie ST, Wu MM, Hao DJ, Yang H. Phagocytic removal of neuronal debris by olfactory ensheathing cells enhances neuronal survival and neurite outgrowth via p38MAPK activity. Mol Neurobiol. 2014;49:1501–1512. doi: 10.1007/s12035-013-8588-2. [DOI] [PubMed] [Google Scholar]
- 28.Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji RR. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2016;55:70–81. doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwak YS, Unabia GC, Hulsebosch CE. Activation of P-38alpha MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Exp Neurol. 2009;220:154–161. doi: 10.1016/j.expneurol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oak MH, Bedoui JE, Madeira SV, Chalupsky K, Schini-Kerth VB. Delphinidin and cyanidin inhibit PDGF(AB)-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br J Pharmacol. 2006;149:283–290. doi: 10.1038/sj.bjp.0706843. [DOI] [PMC free article] [PubMed] [Google Scholar]