Abstract
The present study aimed to investigate the effects of basic fibroblast growth factor (bFGF) and fibronectin (FN) on adhesion of osteoblasts seeded into bio-derived bone scaffolds. Rat calvarial osteoblasts were separated and their osteogenic phenotypes were determined by staining for alkaline phosphatase as well as alizarin red staining. The bio-derived bone scaffolds were prepared from the metaphysis of porcine femur and their physicochemical properties were assessed by scanning electron microscopy (SEM) and X-ray diffraction analysis. An MTT assay was used to detect the effects of bFGF and FN on osteoblast adhesion or proliferation on cell/scaffold constructs through blocking the extracellular matrix FN-integrin pathway by the Gly-Arg-Gly-Asp-Ser (GRGDS) peptide. Western blot analysis was used to measure the β1 integrin levels. Based on the adhesion of osteoblasts stimulated by various concentrations of bFGF (0.1, 1, 10 and 100 ng/ml) on bio-derived bone scaffolds modified by various concentrations of FN (0.1, 1, 10 and 100 µg/ml), the cell/scaffold constructs were divided into four groups: i) Control, non-stimulated and non-modified group; ii) 10 µg/ml FN-modified group; iii) 100 ng/ml bFGF-stimulated group; iv) 10 µg/ml FN + 100 ng/ml bFGF group. Cell proliferation curves acquired by MTT assay and micrographs obtained by SEM showed that the combination of bFGF and FN significantly improved cell adhesion, particularly in the 10 µg/ml FN + 100 ng/ml bFGF group vs. the other groups, and the effect on cell adhesion was inhibited by 1 mmol/l GRGDS peptide through the FN-integrin pathway. Western blot results showed that the combination of bFGF and FN significantly enhanced β1 integrin expression levels. These results suggested that osteoblasts stimulated by 100 ng/ml bFGF and bio-derived bone materials modified by 10 µg/ml FN should be combined to be applied in the bone tissue engineering.
Keywords: bio-derived bone material, fibronectin, basic fibroblast growth factor, osteoblast, bone tissue engineering
Introduction
The reconstruction of large bone defects remains a major orthopedic challenge (1). In bone tissue engineering, osteogenic cell types are seeded onto three-dimensional porous scaffolds to stimulate bone healing and remodel bone defects (2).
The bone material requires chemical and physical processing to minimize its antigenicity, maintain the physical structure and preserve its osteogenic induction properties (3). Besides the well-known limitations regarding the origin of materials and seed cells, cell adhesion to material is one of the problems that should be addressed in bone tissue engineering.
Fibronectin (FN), which interacts with integrin, is involved in the early stages of bone formation as a component of the extracellular matrix (ECM) (4,5). Its distribution in the areas of skeletogenesis mediates cell adhesion and supports osteoblast differentiation to form mineralized nodules. FN binds to osteoblast α5β1 integrin receptor through its tri-peptide amino acid sequence Arg-Gly-Asp (RGD) (5–7). FN is also required for the recruitment of growth factors and the assembly of multiple ECM proteins through the RGD sequence of its central-binding domain (8–10). The design of bioactive scaffolds involves mimicking naturally occurring ECM signaling via modifying the biomaterial with FN to enhance adhesion (11). Soluble Gly-Arg-Gly-Asp-Ser (GRGDS) peptide competes with FN for binding to osteoblast α5β1 integrin and inhibits integrin-mediated adhesion.
Basic fibroblast growth factor (bFGF) has been recognized as a therapeutic agent to enhance bone regeneration (12). bFGF may modulate bone formation through regulating the proliferation and differentiation of osteoblastic lineage cells, such as osteoblasts (12,13).
The objective of the present study was to investigate whether FN and bFGF have an effect on the β1 integrin-mediated adhesion of osteoblasts in bio-deprived bone materials through the ECM-integrin pathway.
Materials and methods
Preparation of rat calvarial osteoblasts
A total of 10 healthy Sprague Dawley rats weighing 250–300 g (5 male and 5 female) were provided by Animal Center of Nanjing Medical University (Nanjing, China). All animal protocols were approved by the Animal Ethics Committee of First People's Hospital of Jining City (Jining, China). They were anesthetized and sacrificed by decapitation. Osteoblasts were isolated according to a previously described procedure (9,14,15). Calvariae were dissected out and immersed in PBS. The calvariae were minced into 1-mm3 pieces and incubated with gentle shaking for 20 min at 37°C in PBS containing 0.1% collagenase II (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells dissociated by sequential enzymatic digestion were collected, centrifuged, re-suspended and seeded in T-75 cm2 flasks, maintained in Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12) plus 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.).
Staining of rat calvarial osteoblasts for alkaline phosphatase (ALP)
Cells of the 2nd passage, which had been grown on coverslips, were fixed with 95% ethanol for 10 min. ALP was stained with the bromochloroindolyl phosphate/nitro blue tetrazolium kit (Nanjing Jiancheng Biological Co., Nanjing, China) as previously described (16).
Alizarin Red S staining of rat calvarial osteoblasts
ECM mineralization was determined by Alizarin Red S staining (17). Cells of the 2nd passage growing to confluence on coverslips were further cultured for three weeks and then fixed with 95% ethanol for 10 min. Cells were stained with 0.1% Alizarin Red S-Tris HCl (pH 8.3) for 30 min at 37°C and rinsed with distilled water to quantify calcium deposition.
Preparation and identification of bio-derived bone materials
The metaphysis of fresh porcine femur purchased from Jining Slaughter Factory (Jining, China) was deprived of soft tissue, periosteum and arthrodial cartilage, and then sliced into elongated shapes of 0.5×0.5×1 cm3. After repeated washing, the bone fragments were deproteinized with 30% H2O2 for 48 h at 37°C and defatted with chloroform/methanol (1:1) for 4 h. The bio-derived bone pieces were cut into blocks sized 0.4×0.4×0.4 cm3. The material was finally lyophilized in a vacuum freeze-drier following storage for 2 h in a −80°C freezer.
The resultant material was assessed by scanning electronic microscopy (SEM) and X-ray diffraction analysis. The specimens of the bio-derived bones were fixed with 3% glutaraldehyde for 24 h, re-fixed with 1% osmium tetroxide for another 2 h and rinsed in PBS. After drying naturally, the specimens were mounted on aluminum stubs, sputter-coated with gold-palladium and observed by S-3000N SEM (Hitachi, Tokyo, Japan). X-ray diffraction analysis of the bio-derived bone materials was performed on an energy dispersive D/Max-III X-ray diffractometer (Rigaku Corp., Tokyo, Japan) in a Theta/Theta configuration and a Cu anode with a scanner speed of 1°/min. The exposure conditions were 40 kV and 40 mA (2,3,18–20).
Seeding of rat calvarial osteoblasts onto bio-derived bone materials
FN and bFGF (Merck KGaA; Darmstadt, Germany) were individually diluted in serum-free DMEM/F12 medium. After sterilization with ethylene oxide, the bio-derived bone materials were filled in 96-well plates (6 blocks/well), and optionally modified with poly-L-lysine (PLL; Sigma-Aldrich; Merck KGaA) or various concentrations of FN (0, 0.1, 1, 10 or 100 µg/ml) at 4°C overnight.
The rat calvarial osteoblasts were incubated with serum-free medium for 24 h and stimulated with various concentrations of bFGF (0, 0.1, 1, 10 or 100 ng/ml) for another 24 h. The osteoblasts were then digested, centrifuged, re-suspended and seeded into bio-derived bones at a density of 2×106 cells/ml with different concentrations of bFGF solution (0, 0.1, 1, 10 or 100 ng/ml). Thus, the study was designed to include 5 groups of cells and 6 groups of scaffolds, resulting in 30 groups.
MTT assay
Cell proliferation was assessed using an MTT assay. All procedures were performed according to the manufacturers' protocols of the MTT kit (Sigma-Aldrich; Merck KGaA) (21). The optical density at 490 nm was determined by microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Viable cell numbers on scaffolds were then determined based on the absorbance.
Assessment of the effect of GRGDS peptide on cell/scaffold construction
To explore whether the combination of FN and bFGF influenced α5β1 integrin-mediated cell adhesion, 4 groups were established: i) The non-stimulated and non-modified group as a control; ii) 10 µg/ml FN-modified group; iii) 100 ng/ml bFGF-stimulated group; iv) 100 ng/ml bFGF-stimulated + 10 µg/ml FN-modified group. The osteoblasts were stimulated with bFGF and bio-derived bone scaffolds were modified with FN according to the methods mentioned above. The groups were then sub-divided into 2 groups, to one of which 2.1 mmol/l GRGDS was added. An MTT assay was used to detect osteoblast proliferation or adhesion on bio-derived bone scaffolds as described above.
Western blot analysis
The protein expression levels of osteoblasts/bio-derived bone scaffold constructs were analyzed by western blot analysis as previously described (22). Protein was extracted with radioimmunoprecipitation assay lysis buffer with phenylmethane sulfonylfluoride and quantified by using a bicinchoninic acid protein assay kit (Cell Signaling Technology, Inc., Danvers, MA, USA). A total of 30 µg protein was loaded per lane and separated by 10% SDS-PAGE before being transferred onto a polyvinylidene difluoride membrane. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing Tween-20 for 2 h at room temperature and incubated with the rabbit integrin β1 antibody (1:2,000 dilution; #4706, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. After washing, membranes were incubated with secondary anti-rabbit horseradish peroxidase-conjugated antibodies (1:2,000 dilution; #323-025-021; Zhongshan Goldenbridge Bio, Inc., Haimen, China) for 1 h at room temperature. Bands were visualized using a Phototope-HRP Western Blotting kit (Cell Signaling Technology, Inc.) and X ray diffractometer (Rigaku, Tokyo, Japan) and analyzed using a gel imaging system (UVP, LLC, Upland, CA, USA) (23).
Statistical analysis
Values are expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was used to detect multivariate differences. Student's t-test was used to analyze inter-group differences. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of the processed osteoblasts and bio-derived bone scaffolds
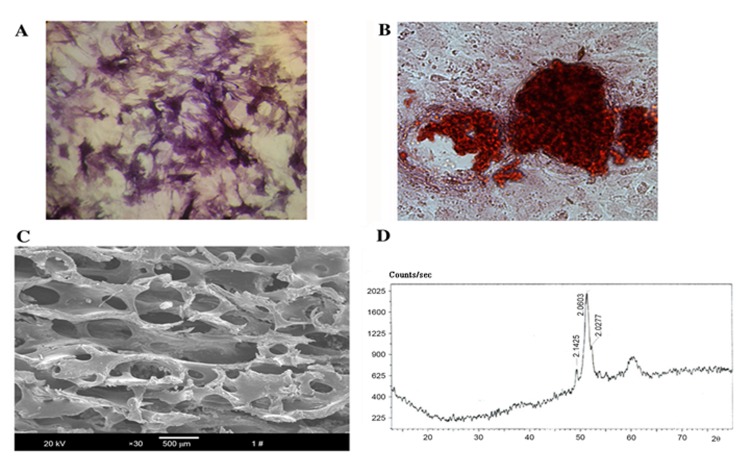
To identify the properties of the osteoblasts, ALP activity was confirmed by staining using a commercially available kit (Fig. 1A), while mineralized nodules and calcium deposition were assessed by Alizarin Red S staining (Fig. 1B). The microstructure revealed by SEM showed that the bio-derived bone scaffold materials maintained their natural interconnected pore framework with an average pore size of 623.67±12.31 µm and a porosity of 75±4.15% (Fig. 1C). The X-ray diffraction pattern showed that curvilinear type of the material was hydroxyapatite ceramic [Ca10(OH)2(PO4)6] (Fig. 1D).
Figure 1.
Characteristics of the processed osteoblasts and bio-derived bone scaffolds. (A) Alkaline phosphatase staining of osteoblasts after purification and culturing for 3 days (magnification, ×200); (B) mineralized nodule staining of osteoblasts (magnification, ×200); (C) scanning electron microstructure of the prepared bio-derived bones (magnification, ×30; scale bar, 500 µm); (D) X-ray diffraction pattern of bio-derived bones.
bFGF and FN enhance adhesion of viable cells in cell/scaffold constructs
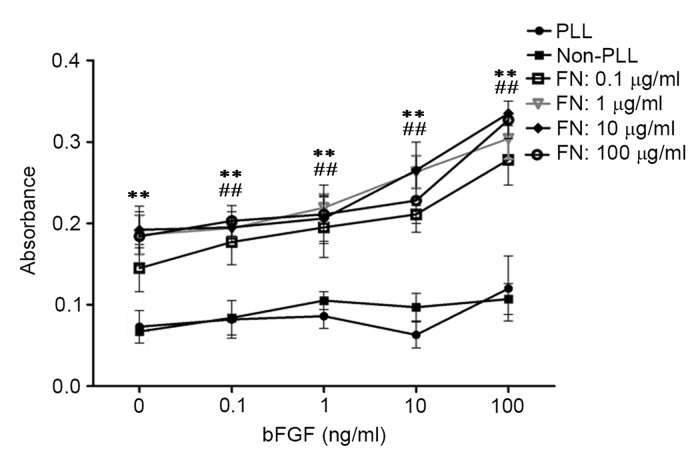
To explore whether the combination of FN and bFGF influenced cell adhesion to the material, osteoblasts stimulated with bFGF were seeded on bio-derived bone scaffolds modified with PLL or FN. The MTT results showed that modification with FN significantly improved adhesion of osteoblasts seeded into bio-derived bone scaffolds in comparison with that in the unmodified group (P<0.01) and the PLL only group (P<0.01). In addition, stimulation with bFGF slightly improved cell adhesion. Of note, the combination of bFGF and FN significantly improved cell adhesion compared with that in the unstimulated group and unmodified group (P<0.01) The statistical and observed results also revealed that cell adhesion was highest for osteoblasts with 100 ng/ml bFGF stimulation seeded into scaffolds modified with 10 µg/ml FN (Fig. 2).
Figure 2.
Effects of bFGF and FN on osteoblasts seeded into bio-derived bone scaffolds after culturing for 25 days assessed by MTT assay. bFGF, basic fibroblast growth factor; FN, fibronectin; PLL, poly-L-lysine. **P<0.01, FN-modified groups at different concentrations of bFGN (0, 0.1, 1, 10, 100 ng/ml) vs. the unmodified groups (Non-PLL and PLL-groups); ##P<0.01, bFGF-stimulated groups (the groups whose concentration of bFGF were above 0 ng/ml) at different concentrations of FN (0.1, 1, 10, 100 µg/ml) vs. the unstimulated groups (the groups whose concentration of bFGF were 0 ng/ml).
SEM results of cell/scaffold constructs
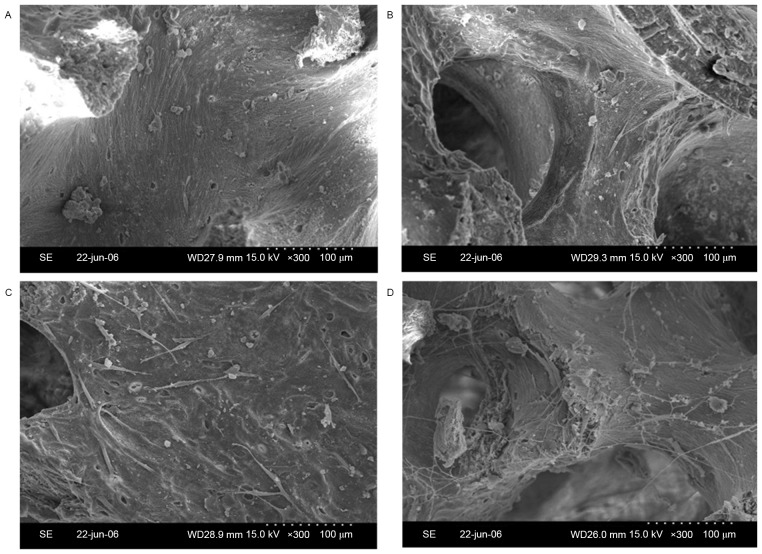
To observe the adhesion of osteoblasts to the bio-derived bone scaffolds, specimens of cell/scaffold constructs were visually observed by SEM. The number of spindle-shaped osteoblasts in the 100 ng/ml bFGF-stimulated and 10 µg/ml FN-modified group (Fig. 3A) was higher than that in the 100 ng/ml bFGF-stimulated group (Fig. 3B), the 10 µg/ml FN-modified group (Fig. 3C) and the non-stimulated and non-modified group (Fig. 3D).
Figure 3.
Scanning electron micrographs of osteoblasts seeded into bio-derived bone scaffolds. (A) Non-stimulated and non-modified group; (B) 100 ng/ml bFGF-stimulated and non-modified group; (C) non-stimulated and 10 µg/ml FN-modified group; (D) 100 ng/ml bFGF-stimulated and 10 µg/ml FN-modified group (magnification, ×300; scale bar, 100 µm). bFGF, basic fibroblast growth factor; FN, fibronectin.
GRGDS peptide affects cell adhesion in cell/scaffold constructs
To explore whether bFGF and FN have an effect on cell adhesion through the integrin-mediated pathway, an MTT assay was used to detect osteoblast proliferation, while the 1 mmol/l GRGDS peptide was used to block RGD in the FN-integrin β1 interaction. Cell adhesion was significantly better in the groups that were not treated with GRGDS in comparison with that in the GRGDS-treated groups (P<0.01). Among the GRGDS-treated groups, cell adhesion in the 10 µg/ml FN + 100 ng/ml bFGF group was significantly higher compared with that in the 10 µg/ml FN-modified group, the 100 ng/ml bFGF-stimulated group and the control group, respectively (P<0.001). In addition, cell adhesion in the 10 µg/ml FN-modified group was better than that in the control group (P<0.01; Fig. 4A).
Figure 4.
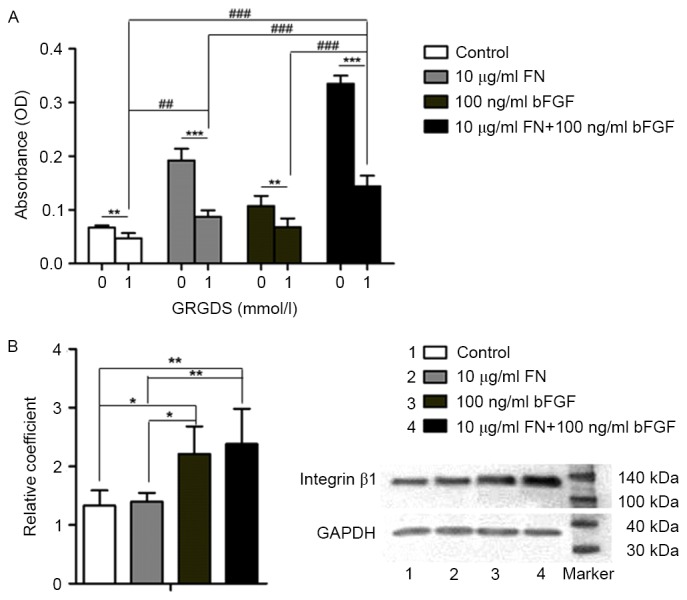
Involvement of the integrin-β1 pathway in the cell/scaffold constructs. (A) MTT results of osteoblasts adhering to bio-derived bone scaffolds. Groups: 0, not treated with GRGDS; 1, treated with 1 mmol/l GRGDS. (B) Western blot results of integrin β1 in osteoblasts seeded into bio-derived bone scaffolds. Lanes: 1, Control; 2, 10 µg/ml FN-modified group; 3, 100 ng/ml bFGF-stimulated group; 4, 100 ng/ml bFGF-stimulated and 10 µg/ml FN-modified group. ***P<0.001, **P<0.01, *P<0.05, 0 vs. 1; ###P<0.001, ##P<0.01 among the respective GRGDS-treated groups. bFGF, basic fibroblast growth factor; FN, fibronectin; OD, optical density.
Integrin β1 has a role in osteoblast attachment to bio-derived bone scaffolds
Western blot analysis was used to detect integrin β1 protein expression in the absence of GRGDS. The level of integrin β1 protein in the 10 µg/ml FN + 100 ng/ml bFGF group was higher than that in the 10 µg/ml FN-modified group (P<0.001) and that in the control group (P<0.001). Furthermore, the level of integrin β1 in the 100 ng/ml bFGF group was higher than that in the 10 µg/ml FN-modified group (P<0.05) and that in the control group (P<0.05; Fig. 4B).
Discussion
Bone regeneration to restore the original bone's form and function is limited by the size of the bone defect. The general consensus is that a highly porous microstructure and large surface area are conducive to tissue ingrowth (24,25). The selection of the most promising scaffold material and seed cell type to be used in bone regeneration remains critically important for bone tissue engineering (26).
In the present study, osteoblasts we separated from Sprague Dawley rat calvariae and bio-derived bone scaffolds were manufactured from the metaphysis of fresh porcine femur. Osteoblasts were identified via staining for ALP and Alizarin Red S staining. ALP activity is used as an early phenotypic marker for mature osteoblasts, while the formation of mineralized nodules is a phenotypic marker for a later stage of osteogenic differentiation (17). The deproteinized and defatted bone materials were processed into small 0.4×0.4×0.4 cm3 blocks with an average pore size of 623.67±12.31 µm and met the clinical requirements as indicated in the SEM images. The X-ray diffraction pattern showed that the main component of bio-derived bone materials was non-crystal phase hydroxyapatite [Ca10(OH)2(PO4)6], which was consistent with normal bone structure.
FN is an adhesive protein that contributes to the structural stability of ECM. It has an important role in cell attachment to surfaces of various materials through binding to integrin (27). Thus, in the present study, the bio-deprived bone scaffolds were modified with FN at various concentrations. In addition, the effects of the FGF family of proteins, particularly bFGF (also known as FGF-2) have been well investigated in osteogenesis and bFGF is essential for bone formation (24). Thus, in the present study, rat calvarial osteoblasts were stimulated with various concentrations of bFGF prior to seeding into the bone scaffolds. To explore the most appropriate FN and bFGF concentrations for cell-scaffold constructs, osteoblasts stimulated with various concentrations of bFGF (0.1, 1, 10 or 100 ng/ml) were seeded into bio-derived bone scaffolds modified with various concentrations of FN (0.1, 1, 10 or 100 µg/ml) to quantify the number of viable adherent cells by an MTT assay. The MTT assay evaluates the number of living cells and the metabolic activity of cells present. The results indicated that scaffold modification with FN only or cell stimulation with bFGF only significantly improved the adhesion of osteoblasts seeded into bio-derived bone scaffolds. Cell adhesion was highest when a combination of osteoblast stimulation with 100 ng/ml bFGF and scaffold modification with 10 µg/ml FN was used.
Integrins, which trigger cell adhesion, are the major class of receptors implicated in cell-ECM interactions, which are mediated via the FN-integrin signaling pathway (6). The RGD peptide sequence derived from ECM proteins such as FN has been used to modulate cell attachment, proliferation and migration (11). The RGD peptide of FN, to which cell adhesion ligands present in the ECM attach, is blocked by the GRGDS peptide. The results of the present study demonstrated that after GRGDS treatment, cell adhesion in the 10 µg/ml FN-modified group and in the 10 µg/ml FN + 100 ng/ml bFGF group was significantly declined to a greater extent than that in the control and 100 ng/ml bFGF-stimulated groups. This result indicated that osteoblast adhesion to bio-derived bone scaffolds may be enhanced through the ECM-FN-integrin pathway. Western blot analysis further confirmed that the level of integrin β1 was affected by bFGF, particularly by the combination of bFGF and FN. As bFGF had a more significant effect than that of FN, this may indicate that the mechanism is more complex and further investigation is required to fully elucidate the mechanism involved.
α5β1 integrin, a specific Fn receptor, mediates critical interactions between osteoblasts and Fn required for bone morphogenesis (28). The present study showed that direct osteoblast interactions with the extracellular matrix are mediated by a selective group of integrin receptors, including α5β1, α3β1, αvβ3, and α4β1 (29). A study performed by Tang et al (30) demonstrated that bFGF increased Fn fibrillogenesis and the cell surface expression of α5 and β1 integrins via a PKC-dependent pathway. All the above studies are consistent with our findings and further emphasize the significance of our study.
In conclusion, the combined use of bFGF and FN has a significant enhancing effect on osteoblast adhesion on bio-derived bones. When 100 ng/ml bFGF-stimulated osteoblasts were seeded on the 10 µg/ml FN-modified bio-derived bones, the greatest enhancement of cell adhesion could be generated. The present findings suggest the mechanism of the enhancing effect is likely mediated by RGD-integrin α5β1 pathway.
References
- 1.Liu F, Zhang X, Yu X, Xu Y, Feng T, Ren D. In vitro study in stimulating the secretion of angiogenic growth factors of strontium-doped calcium polyphosphate for bone tissue engineering. J Mater Sci Mater Med. 2011;22:683–692. doi: 10.1007/s10856-011-4247-1. [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZY, Teoh SH, Chong WS, Foo TT, Chng YC, Choolani M, Chan J. A biaxial rotating bioreactor for the culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials. 2009;30:2694–2704. doi: 10.1016/j.biomaterials.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Xie H, Yang F, Deng L, Luo J, Qin T, Li X, Zhou GQ, Yang Z. The performance of a bone-derived scaffold material in the repair of critical bone defects in a rhesus monkey model. Biomaterials. 2007;28:3314–3324. doi: 10.1016/j.biomaterials.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Lee DS, Choi I, Le Pham BH, Jang JH. Design of an osteoinductive extracellular fibronectin matrix protein for bone tissue engineering. Int J Mol Sci. 2015;16:7672–7681. doi: 10.3390/ijms16047672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pegueroles M, Aparicio C, Bosio M, Engel E, Gil FJ, Planell JA, Altankov G. Spatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energy. Acta Biomater. 2010;6:291–301. doi: 10.1016/j.actbio.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: Reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 7.Brunner M, Millon-Frémillon A, Chevalier G, Nakchbandi IA, Mosher D, Block MR, Albigès-Rizo C, Bouvard D. Osteoblast mineralization requires beta1 integrin/ICAP-1-dependent fibronectin deposition. J Cell Biol. 2011;194:307–322. doi: 10.1083/jcb.201007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentmann A, Kawelke N, Moss D, Zentgraf H, Bala Y, Berger I, Gasser JA, Nakchbandi IA. Circulating fibronectin affects bone matrix, whereas osteoblast fibronectin modulates osteoblast function. J Bone Miner Res. 2010;25:706–715. doi: 10.1359/jbmr.091011. [DOI] [PubMed] [Google Scholar]
- 9.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–713. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Houseman BT, Mrksich M. The microenvironment of immobilized Arg-Gly-Asp peptides is an important determinant of cell adhesion. Biomaterials. 2001;22:943–955. doi: 10.1016/S0142-9612(00)00259-3. [DOI] [PubMed] [Google Scholar]
- 11.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials. 2004;25:895–906. doi: 10.1016/S0142-9612(03)00602-1. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Q, Kubo T, Doi K, Morita K, Takeshita R, Katoh S, Shiba T, Gong P, Akagawa Y. Effect of combined application of bFGF and inorganic polyphosphate on bioactivities of osteoblasts and initial bone regeneration. Acta Biomater. 2009;5:1716–1724. doi: 10.1016/j.actbio.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Bai Y, Yin G, Pu X, Huang Z, Liao X, Chen X, Yao Y. Synergistic and sequential effects of BMP-2, bFGF and VEGF on osteogenic differentiation of rat osteoblasts. J Bone Miner Metab. 2014;32:627–635. doi: 10.1007/s00774-013-0538-6. [DOI] [PubMed] [Google Scholar]
- 14.Tang DZ, Hou W, Zhou Q, Zhang M, Holz J, Sheu TJ, Li TF, Cheng SD, Shi Q, Harris SE, et al. Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-BMP signaling. J Bone Miner Res. 2010;25:1234–1245. doi: 10.1002/jbmr.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi R, Kumar S, Kumar A, Siddiqui JA, Swarnkar G, Gupta V, Kendurker A, Dwivedi AK, Romero JR, Chattopadhyay N. Kaempferol has osteogenic effect in ovariectomized adult sprague-dawley rats. Mol Cell Endocrinol. 2008;289:85–93. doi: 10.1016/j.mce.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Huo K, Zhang X, Wang H, Zhao L, Liu X, Chu PK. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials. 2013;34:3467–3478. doi: 10.1016/j.biomaterials.2013.01.071. [DOI] [PubMed] [Google Scholar]
- 17.Yao D, Xie XH, Wang XL, Wan C, Lee YW, Chen SH, Pei DQ, Wang YX, Li G, Qin L. Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis-an in vitro efficacy study. PLoS One. 2012;7:e41264. doi: 10.1371/journal.pone.0041264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang ZY, Teoh SH, Chong MS, Schantz JT, Fisk NM, Choolani MA, Chan J. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem cells. 2009;27:126–137. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 19.Lin YC, Brayfield CA, Gerlach JC, Rubin JP, Marra KG. Peptide modification of polyethersulfone surfaces to improve adipose-derived stem cell adhesion. Acta Biomater. 2009;5:1416–1424. doi: 10.1016/j.actbio.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Mariscal-Muñoz E, Costa CA, Tavares HS, Bianchi J, Hebling J, Machado JP, Lerner UH, Souza PP. Osteoblast differentiation is enhanced by a nano-to-micro hybrid titanium surface created by Yb: YAG laser irradiation. Clin Oral Investig. 2016;20:503–511. doi: 10.1007/s00784-015-1533-1. [DOI] [PubMed] [Google Scholar]
- 21.Wang YW, Wu Q, Chen GQ. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials. 2004;25:669–675. doi: 10.1016/S0142-9612(03)00561-1. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Li H, Li T, Fan J, Zhao RC, Weng X. MicroRNA expression profile of dexamethasone-induced human bone marrow-derived mesenchymal stem cells during osteogenic differentiation. J Cell Biochem. 2014;115:1683–1691. doi: 10.1002/jcb.24831. [DOI] [PubMed] [Google Scholar]
- 23.Hendesi H, Barbe MF, Safadi FF, Monroy MA, Popoff SN. Integrin mediated adhesion of osteoblasts to connective tissue growth factor (CTGF/CCN2) induces cytoskeleton reorganization and cell differentiation. PLoS One. 2015;10:e0115325. doi: 10.1371/journal.pone.0115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida K, Haudenschild DR. Interactions between FGF21 and BMP-2 in osteogenesis. Biochem Biophys Res Commun. 2013;432:677–682. doi: 10.1016/j.bbrc.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/S0142-9612(02)00635-X. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28:3338–3348. doi: 10.1016/j.biomaterials.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro N, Sousa SR, Monteiro FJ. Influence of crystallite size of nanophased hydroxyapatite on fibronectin and osteonectin adsorption and on MC3T3-E1 osteoblast adhesion and morphology. J Colloid Interface Sci. 2010;351:398–406. doi: 10.1016/j.jcis.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J Cell Sci. 1997;110:2187–2196. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 29.Lim JY, Taylor AF, Li Z, Vogler EA, Donahue HJ. Integrin expression and osteopontin regulation in human fetal osteoblastic cells mediated by substratum surface characteristics. Tissue Eng. 2005;11:19–29. doi: 10.1089/ten.2005.11.19. [DOI] [PubMed] [Google Scholar]
- 30.Tang CH, Yang RS, Huang TH, Liu SH, Fu WM. Enhancement of fibronectin fibrillogenesis and bone formation by basic fibroblast growth factor via protein kinase c-dependent pathway in rat osteoblasts. Mol Pharmacol. 2004;66:440–449. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]