Abstract
In vitro fertilization-embryo transfer (IVF-ET) can be used by infertile couples to assist with reproduction; however, failure of the embryo to implant into the endometrial lining results in failure of the IVF treatment. The present study investigated the expression of chemokine receptor 7 (CCR7)(lo) programmed death-1(PD-1)(hi) chemokine receptor type 5 (CXCR5)+ cluster of differentiation 4 (CD4)+ T cells and associated factors in patients with repeated implantation failure (RIF). A total of 30 females with RIF and 30 healthy females were enrolled in the current study. Flow cytometry was used to detect the proportion of CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells in the peripheral blood. Cytokine bead arrays were performed to detect the levels of interleukin (IL)-6, −4 and −2 in the serum. ELISAs were used to detect the level of IL-21 in the serum. Quantitative real time polymerase chain reaction analysis and immunohistochemistry were used to investigate the expression of B-cell lymphoma 6 (Bcl-6), chemokine receptor type 5 (CXCR5) and IL-21 in the endometrium. The results revealed that the percentage of CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells was increased in the RIF group compared with the control group during the mid luteal phase. The mRNA and protein levels of Bcl-6, IL-21 and CXCR5 in the endometrium and the concentrations of IL-21 and IL-6 in the serum were significantly increased in the RIF group; however, no significant difference was observed between the two groups in regards to the expression of IL-4 and IL-2. Furthermore, a significant positive correlation was identified between the percentage of CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells and IL-21 and IL-6 levels. The expression of IL-21 also had a positive correlation with Bcl-6 and CXCR5 expression in the RIF group. These results suggest that increased levels of CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells and associated factors contribute to RIF and could therefore be a potential therapeutic target.
Keywords: repeated implantation failure, CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cell, follicular helper T cell, Bcl-6, CXCR5, interleukin 21
Introduction
The treatment options available to assist infertile couples in having children have progressed immensely during recent years to include a variety of assisted reproductive technologies (1). However, for in vitro fertilization-embryo transfer (IVF-ET), embryo implantation remains the rate-limiting step (2–4). Couples who fail to achieve a pregnancy following between two and six IVF cycles, in which >10 high-grade embryos were transferred to the uterus are defined by various clinicians as having repeated implantation failure (RIF) (5,6). With the tendency being that only one or two high-grade embryos are transferred in each cycle, certain clinicians have recommended that the definition of RIF be changed to the failure of implantation in at least three consecutive IVF attempts (6–8). In the present study, patients with failure of ≥3 consecutive IVF-ET cycles were defined as RIF.
Appropriate immune responses at the time of embryo introduction are key for successful implantation into the endometrial wall. Previous studies have reported that the prevalence of dominant T-helper (Th)1 and Th17 cells may cause multiple implantation failures in IVF cycles, while the prevalence of dominant Th2 and regulatory T cells (Tregs) is beneficial for a successful in vitro treatment outcome (3,8–12). Liang et al (13) identified that the Th1/Th2 ratio in circulating Th cells was significantly increased in women with RIF compared to those with a successful implantation. While Persson et al (9) demonstrated that unsuccessful IVF outcomes had an abnormality in the quantities of peripheral Th1 and Th17 cells, and that following embryo transfer pregnant women had higher numbers of Th2-associated cytokine-secreting cells. Another study revealed that pregnancy and live birth rates were significantly improved in women with >0.6% circling T cells as Tregs (11). However, these previous studies fail to clarify the enhancement of humoral immune responses in women with reproductive problems. T follicular helper (Tfh) cells are involved in humoral immunity (14,15) and the role they serve in RIF remain unclear.
Tfh cells are known as one subset of CD4+ T cells settled in the secondary lymph nodes, they have increased expression of chemokine receptor type 5 (CXCR5) and decreased expression of chemokine receptor 7 (CCR7), which guides Tfh cell migration towards B cell follicles (16,17). Currently, Tfh cells are defined by their expression of combinations of markers, including programmed death-1 (PD-1), interleukin (IL)-21 and IL-4, which are directly linked with the biology of Tfh cells. High expression of PD-1 in combination with CXCR5 is a reliable way to identify Tfh cells (18). IL-21 and IL-4 are the main cytokines secreted by Tfh cells, and their cooperation can regulate immunoglobulin G (IgG)-1 production and the production of multiple antibody classes (19,20). Transcription factor B-cell lymphoma 6 (Bcl-6) is the master regulator of Tfh cell differentiation (21–23). IL-6 is helpful in Tfh cell differentiation while IL-2 negatively regulates Tfh by inducing B lymphocyte-induced maturation protein 1 expression (24). These molecules are all considered therapeutic targets to prevent B cells from producing high affinity autoantibodies directed against self-antigens.
Circulating CD4+ T cells that express CXCR5 are considered counterparts to Tfh cells (25). They are thought to be primarily generated in germinal centers (GCs) (26). Very few circulating CD4+ T cells express high amounts of CXCR5 or PD-1, but a significant fraction express intermediate amounts of these molecules and are designated as circulating Tfh (cTfh) cells (18). Human cTfh cells are composed of distinct subsets with unique phenotypes and function. The CCR7(lo)PD-1(hi) subset has a partial Tfh effector phenotype that is indicative of active Tfh differentiation in lymphoid organs and correlated with clinical indices in autoimmune diseases. This provides a biomarker to monitor protective antibody responses during infection or vaccination and pathogenic antibody responses in autoimmune diseases (26–28). In certain diseases, including systemic lupus erythematosus (29–31), rheumatoid arthritis (25,32) and allograft rejection (33), the percentages of cTfh cells are increased and positively correlated with serum levels of autoantibodies; however, the underlying mechanisms of cTfh and associated factors in the development of RIF require further study.
In the present study, CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells and associated factors were investigated, and it was identified that their presence was significantly increased in patients with RIF, suggesting that they are involved in the development of RIF. These results provide a novel insight into the development of potential therapies to help prevent RIF.
Materials and methods
Patients and controls
A total of 60 females were enrolled in the present study. The patients were admitted to the First Affiliated Hospital of Xinjiang Medical University (Urumqi, China) between July 2013 and December 2015. They formed two separate groups: The RIF group and the control group (n=30/group). The inclusion criteria for the RIF group were females ≤43 years old with a history of ≥3 previous failed IVF-ET cycles, with a normal uterus, high quality embryos, menstrual regularity and normal sperm from the partner. The exclusion criteria were severe endometriosis, adhesiolysis, an abnormal uterus, having received immune therapy and the presence of possible causes for the failure of implantation, including autoimmune diseases, endocrine disorders, couples with chromosomal abnormalities and sexually transmitted diseases. A detailed history of the patients was taken, as well as a physical examination and investigations for infertility. The inclusion criteria for the control group were healthy females ≤43 years with infertile partners who got conceived and gave birth during their first cycle of IVF-ET, the exclusion criteria were the same as those for the RIF group. Baseline characteristics between the study groups were not significantly different, as shown in Table I.
Table I.
Clinicopathological characteristics of the RIF and control groups.
| Control (n=30) | RIF (n=30) | ||||
|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | P-value |
| Age (years) | 32.77 | 4.7 | 33.23 | 5.06 | 0.712 |
| BMI (kg/m2) | 21.69 | 2.5 | 22.96 | 2.74 | 0.066 |
| Length of infertility (years) | 4.78 | 3.76 | 4.71 | 3.5 | 0.936 |
| Endometrial thickness (mm) | 10.53 | 1.78 | 10.58 | 1.98 | 0.932 |
| No. of previous failed IVF-ET cycles | – | – | 3.4 | 0.56 | – |
SD, standard deviation; BMI, body mass index; RIF, repeated implantation failure; IVF-ET, in vitro fertilization-embryo transfer.
Ethical approval for the present study was obtained from the Human Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University prior to commencement of the study. Patients who fulfilled the inclusion criteria were recruited following the attainment of written informed consent.
Specimen collection
Endometrial tissues were collected from the RIF and control group 1 month prior to the first IVF-ET cycle (34). IVF-ET was performed in the mid luteal phase of the menstrual cycle when the uterus is most receptive to the embryo; the specific time period for this is known as the implantation window. One section of the tissue was fixed with 4% paraformaldehyde solution for 3 days at room temperature. Sections were embedded in paraffin and sliced into 4-µm-thick sections to be utilized for immunohistochemistry, while another section was used for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis. Peripheral blood was obtained from the RIF and control groups in the mid luteal phase of the menstrual cycle; this was then used to detect the proportion of Tfh cells and the concentration of cytokines.
Flow cytometric analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh heparinized peripheral blood samples (35), then suspended in PBS at 1×106 cells/ml. Next, 100 µl of PBMCs in suspension and anti-human monoclonal antibodies conjugated with different fluorescent dyes, including 2 µl peridinin chlorophyll-CD4 (1:60; cat no. 347324), 2 µl phycoerythrin (PE)-cyanin 7-CCR7 (1:60; cat no. 557648), 2.5 µl fluorescein isothiocyanate-CXCR5 (1:48; cat no. 558112), 2 µl allophycocyanin-CD45RA (1:60; cat no. 561210) and 2 µl PE-PD-1 (1:60; cat no. 12996941) were combined. All antibodies were purchased from BD Biosciences (San Jose, CA, USA), except anti-PD-1, which was from eBioscience (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The samples were incubated for 15 min at 4°C in dark conditions. The cells were washed with PBS and then centrifuged at 300 × g for 5 min at 4°C. After removing the supernatant, the last step was repeated. Then the cells were resuspended in 300 µl PBS. After gating for sideward scatter and CD4 cells positive for CD4 were further analyzed. CD45RA was used to identify the memory T cells (CD45RA) in the CD4+ T cell population. The CD45RA− CXCR5+ cells were then gated and the CCR7(lo)PD-1(hi) subset of CD45RA− CXCR5+ cells was analyzed. For each sample >20,000 cells were collected, which were detected using a BD LSR II flow cytometer (BD Biosciences), and analyzed using FlowJo software (version 7.6.1; Tree Star, Inc., Ashland, OR, USA).
RNA isolation and RT-qPCR analysis
Total RNA was isolated from endometrial tissue of each group with Lipofectamine (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol and reverse transcribed into complementary (c)DNA using the Roche Transcriptor cDNA Synthesis kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturers protocols. The cDNA was then used as the template for the qPCR reaction. SYBR-Green (Takara Biotechnology Co., Ltd., Dalian, China) incorporation-based qPCR was performed to quantify the mRNA levels of Bcl-6, IL-21 and CXCR5 with a CFX96™ Real-Time PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Reactions were carried out through 34 cycles of 30 sec at 94°C and 1 min at 55°C, followed by 30 sec at 72°C. The primers were designed by Sangon Biotech Co., Ltd. (Shanghai, China) and presented in Table II. The relative expression was calculated using the 2−∆∆Cq method (36). The results were normalized to the housekeeping gene GAPDH and represented as lg2−∆∆Cq. Each sample was conducted with two biological replicates.
Table II.
Primer sequences for reverse transcription-quantitative polymerase chain reaction analysis.
| Gene | Direction | Primer sequence (5′-3′) | Expected product size (bp) |
|---|---|---|---|
| Bcl-6 | F | ATGAGGAGTTTCGGGATGTC | 177 |
| R | CCTCTTCTGGGATTGTTTGC | ||
| CXCR5 | F | GCAAGAAAGAAACCCGACAG | 210 |
| R | TTATGGGAAGGGAGTGAGGA | ||
| IL-21 | F | CACAGACTAACATGCCCTTCAT | 224 |
| R | GAATCTTCACTTCCGTGTGTTCT | ||
| GAPDH | F | GAAGGTGAAGGTCGGAGT | 226 |
| R | GAAGATGGTGATGGGATTTC |
F, forward; R, reverse; Bcl-6, B-cell lymphoma 6; CXCR5, chemokine receptor type 5; IL-21, interleukin 21; bp, base pairs.
Pathological morphology and immunohistochemistry
Hematoxylin and eosin (H&E) staining was used to observe the endometrial tissue. Slides were deparaffinized with xylene and rehydrated in gradual dilutions of ethanol. The slides were then stained with hematoxylin for 3 min at room temperature. After rinsing under running water for 3 min, slides were differentiated with 1% HCl in ethanol for 1 sec at room temperature. Finally, sections were counterstained with eosin for 90 sec at room temperature. Slides were examined and images were captured using a light microscope at ×200 and ×400 magnification.
Immunohistochemical staining for Bcl-6, IL-21 and CXCR5 was performed on endometrial slides. Firstly, slides were deparaffinized with xylene; rehydrated in a graded series of ethanol, immersed in 3% H2O2 and then placed in buffered citric acid (pH 6.0) in a microwave oven (800 W) for 13 min. After being washed three times with PBS, the slides were incubated with 10% goat serum (Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd., Beijing, China) for 30 min at 37°C. Then, the slides were washed three times with PBS and incubated with rabbit anti-human polyclonal primary antibodies directed against Bcl-6 (1:100; cat no. bs2734R), IL-21 (1:250 cat no. bs2621R) and CXCR5 (1:100; cat no. bs3598R) (BIOSS, Beijing, China) overnight at 4°C. After three washes, slides were incubated with the corresponding peroxidase-conjugated Affinipure goat anti-rabbit IgG secondary antibodies (1:100; cat no. SPN9001; Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd.) for 25 min at 37°C. The antibody reaction was visualized using the chromogen diaminobenzidine (Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd.). Finally, all the slides were counterstained with hematoxylin for 2 min at room temperature. Immunoreactivity was assessed in five randomly selected high power fields (Leica DM3000; Leica Microsystems Inc., Wetzlar, Germany) and the average optical density was measured using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA) on the histogram-based mode.
ELISA
Serum levels of IL-21 from patients with RIF and healthy females were determined using a human IL-21 ELISA kit (cat no. bsK00366; BIOSS) according to the manufacturer's protocol. Firstly, 100 µl/well of diluted serum (1:2) was added to a microwell plate coated with monoclonal antibodies directed against human IL-21, this was performed at 37°C for 30 min. The plate was then washed three times with PBS with 1% Tween (PBST), and then streptavidin-HRP was added to the plate at 37°C for 40 min. After washing with PBST four times, 50 µl/well of TMB substrate was added to react with the enzyme in the dark. Then concentrated H2SO4 was used to terminate the reaction. Finally, the optical density of the plates at 450 nm was detected using a VersaMax™ microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Cytometric bead array (CBA)
Serum levels of IL-6, IL-4 and IL-2 were detected using a CBA kit (cat no. 560484; BD Biosciences) according to the manufacturer's protocol. In summary, 50 µl of mixed capture beads, serum sample and PE detection reagents were added to the tubes, which were then incubated for 3 h in the dark at room temperature. After washing and centrifuging at 300 × g for 5 min at 4°C, the supernatant was discarded and 300 µl of wash buffer was added to each tube. Then, the levels of IL-6, IL-4 and IL-2 were detected using a BD LSR II flow cytometer, and the data was analyzed using FlowJo software (version 7.6.1; FlowJo LLC, Ashland, OR, USA).
Statistical analysis
All group data analysis was performed using SPSS statistical software (version 17.0; SPSS, Inc., Chicago, IL, USA). Results are expressed as the mean ± standard deviation. An independent-samples t-test was used to assess differences between two groups. Pearson's correlation coefficient was used to analyze the correlation between two variables. P<0.05 (two-tailed) was considered to indicate a statistically significant difference.
Results
An increased proportion of CCR7(lo)PD-1(hi) T cells are found in the peripheral blood of women with RIF
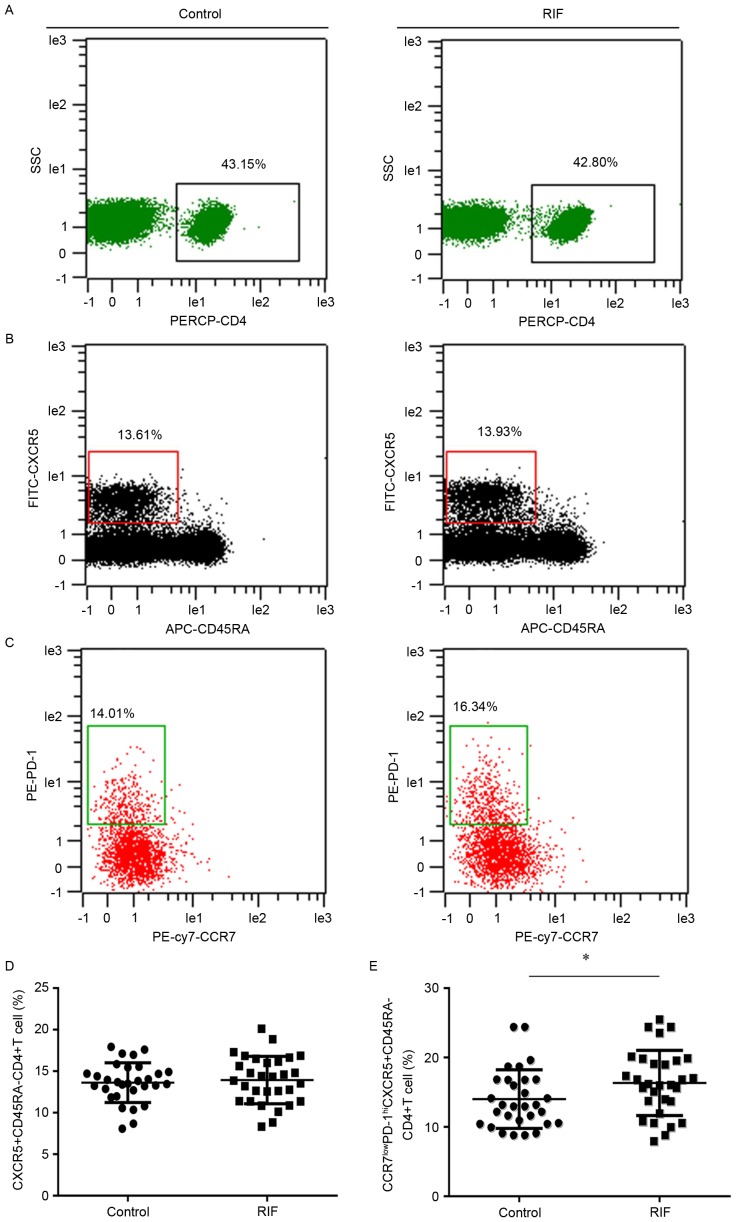
The activated Tfh cell subset was defined as the CCR7(lo)PD-1(hi) subset. It was hypothesized that the quantities of CCR7(lo)PD-1(hi) Tfh cells may be different in patients with RIF compared to the control group. Flow cytometry analysis was performed to detect the percentage of this subset within the total CXCR5+ CD4+ T cell population in the peripheral blood. This could then reflect the systemic contribution of Tfh cells to implantation failure in IVF. CD4+ T cells were discriminated from total cells (Fig. 1A) and memory Tfh cells (CD45RA− CXCR5+ CD4+ T) were identified in the CD4+ T cell population (Fig. 1B); the CXCR5+ CD45RA− CD4+ cells expressing CCR7(lo)PD-1(hi) were analyzed (Fig. 1C). The percentage of CXCR5+ CD45RA− CD4+ T cells in the total number of CD4+ T cells was not significantly different between the two groups (Fig. 1D). Whereas, the percentage of subset CCR7(lo)PD-1(hi) within the CXCR5+ CD4+ T cells in the RIF (16.34±4.69) group was significantly increased (14.01±4.22) compared with the control group (P<0.05; Fig. 1E).
Figure 1.
Expression of CCR7(lo)PD-1(hi) T follicle helper cells in the peripheral blood. Cytometric plots indicating how (A) PERCP-CD4 was used to identify CD4+ T cells. These cells were further analyzed to identify cells expressing (B) CXCR5 and CD45RA. (C) CXCR5+ CD45RA− cells were analyzed to reveal the presence of CCR7 and PD-1. The presence of (D) CXCR5+ CD45RA− CD4+ T cells and (E) CCR7(lo)PD-1(hi) CXCR5+ CD45RA− CD4+ T cells in the RIF group and control group were compared. *P<0.05 vs. the control group. CCR7, chemokine receptor 7; PD-1, programmed death 1; Tfh, T follicle helper; CXCR5, chemokine receptor type 5; SSC, side scatter; RIF, repeated implantation failure; CD, cluster of differentiation; CD45RA, CD45 RA isotope; PE, phycoerythrin; FITC, fluorescein isothiocyanate; APC, allophycocyanin; cy7, cyanin 7.
Bcl-6, IL-21 and CXCR5 mRNA expression is increased within the endometrium of the RIF group in the mid luteal phase
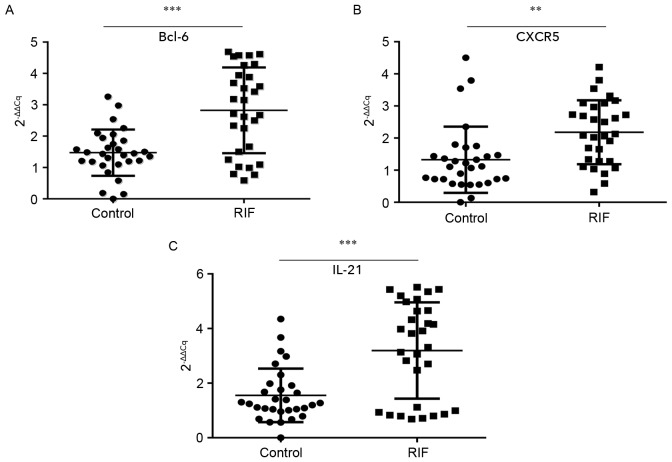
A successful pregnancy is dependent upon synchronized, coordinated cross talk between the local and systemic immune systems. The endometrium is the beginning of the maternal interface and embryo attachment requires active local endometrial reactivity on the maternal side. Therefore, the endometrial local immune microenvironment is important to investigate. It was proposed that the endometrial immune microenvironment may change as the proportion of circulating Tfh cells increased in patients with RIF. The mRNA expression of Bcl-6, IL-21 and CXCR5 in endometrial tissues during the luteal phase was tested by RT-qPCR. The mean expression of Bcl-6 (2.82±1.37; Fig. 2A), CXCR5 (2.18±1.00; Fig. 2B) and IL-21 (3.20±1.76; Fig. 2C) were all significantly higher in the RIF group than in the control group (1.47±0.74, P<0.001; 1.33±1.03, P<0.01; and 1.56±0.98, P<0.001 respectively). These results suggest that the overexpression of the Tfh-associated markers Blc-6, CXCR5 and IL-21 in the endometrium during the mid luteal phase is associated with embryo implantation failure.
Figure 2.
Bcl-6, CXCR5 and IL-21 mRNA expression is increased in patients with RIF. The mRNA expression of (A) Bcl-6, (B) CXCR5 and (C) IL-21 in the control and RIF groups in the endometrium during the mid-luteal phase. **P<0.01 and ***P<0.001 vs. the control group. Bcl-6, B-cell lymphoma 6; CXCR5, chemokine receptor type 5; IL, interleukin; RIF, repeated implantation failure.
Protein expression of Bcl-6, IL-21 and CXCR5 is increased within the endometrium during the mid luteal phase
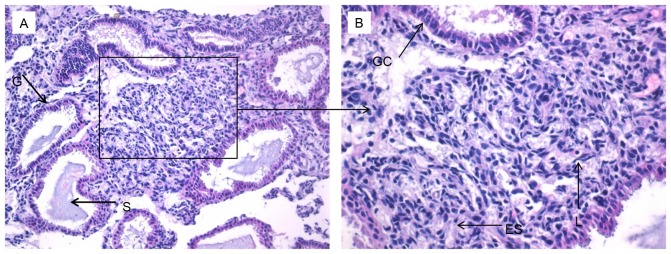
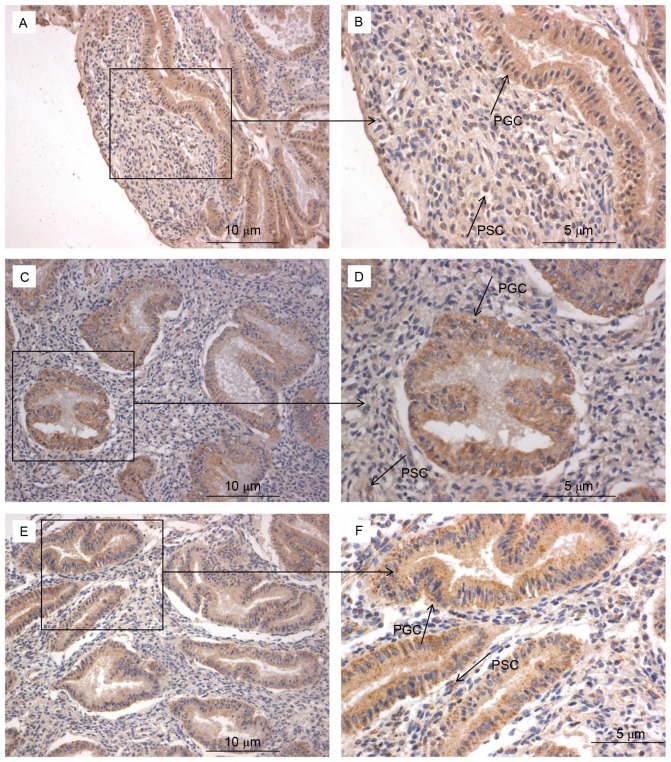
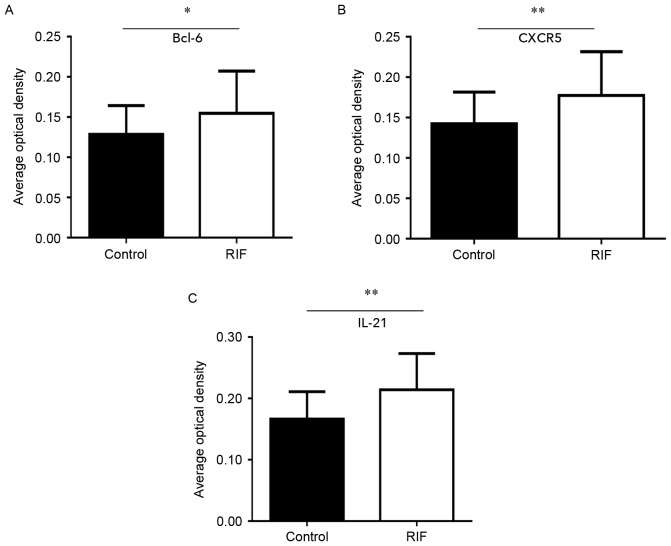
An improved understanding of the modulation of Bcl-6, IL-21 and CXCR5 molecules in the endometrium may reveal the underlying mechanisms of preparation by the endometrium for the arrival of an embryo. This in turn could provide novel insights into RIF by exposing deviations from normal activity. Firstly, H&E staining was performed to observe the pathological morphology of endometrial tissue (Fig. 3). Nuclear palisading, ferning of the glandular epithelium and several lymphocytes were observed. Secondly, immunohistochemistry was used to examine the expression and localization of Bcl-6 (Fig. 4A and B), IL-21 (Fig. 4C and D) and CXCR5 (Fig. 4E and F) in the endometrium. These factors were expressed in glandular cells, endometrial stroma and lymphocytes. Finally, quantification of Bcl-6, IL-21 and CXCR5 immunostaining was performed. The expression of Bcl-6 (0.15±0.05; Fig. 5A), IL-21 (0.21±0.06; Fig. 5B) and CXCR5 (0.18±0.06; Fig. 5C) in RIF endometrium were significantly increased compared with the control group Bcl-6 (0.13±0.04), IL-21 (0.17±0.04) and CXCR5 (0.14±0.04) levels (all P<0.05). These results suggest that high expression levels of Bcl-6, CXCR5 and IL-21 proteins in the endometrium during the middle luteal phase of menstruation.
Figure 3.
Hematoxylin and eosin staining of endometrial tissue. Images at a magnification of (A) ×200 or (B) ×400. G, gland; S, secreted material; GC, glandular cells; ES, endometrial stroma; L, lymphocytes.
Figure 4.
Immunohistochemistry of Bcl-6, CXCR5 and IL-21 in the endometrium. Bcl-6 staining at a magnification of (A) ×200 and (B) ×400. CXCR5 staining at a magnification of (C) ×200 and (D) ×400. IL-21 staining at a magnification of (E) ×200 and (F) ×400. Bcl-6, B-cell lymphoma 6; CXCR5, chemokine receptor type 5; IL, interleukin; PSC, positive stroma cell; PGC, positive glandular cell.
Figure 5.
Bcl-6, CXCR5 and IL-21 protein expression is increased in patients with RIF. Analysis of (A) Bcl-6, (B) CXCR5 and (C) IL-21 immunohistostaining quantification. *P<0.05, **P<0.01 vs. the control group. Bcl-6, B-cell lymphoma 6; CXCR5, chemokine receptor type 5; IL, interleukin; RIF, repeated implantation failure.
Serum IL-21 and IL-6 concentrations are increased in females with RIF
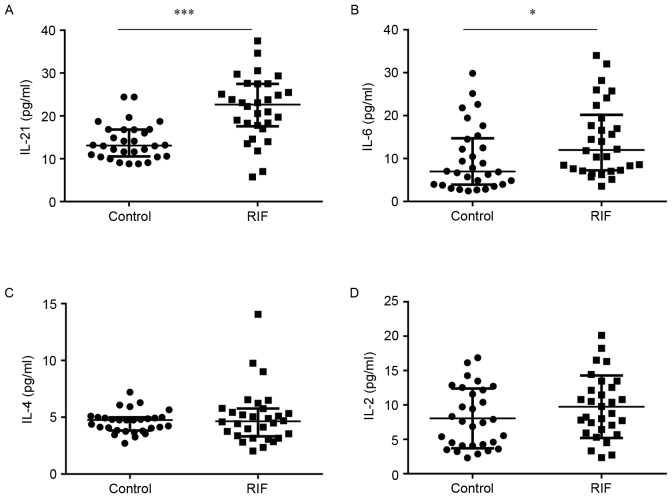
Systemic immunity is affected by cytokines; certain variations in their expression confer an advantage for embryo implantation, whereas others do not (37,38). In the current study, ELISAs or CBAs were used to estimate the concentrations of IL-21, IL-6, IL-4 and IL-2 in the serum. The concentration of IL-21 was significantly higher in the RIF group (21.80±7.40) compared to the control group (14.01±4.22) (P<0.05; Fig. 6A). Similarly, IL-6 levels were significantly increased in the RIF group (14.45±8.54) compared with the control group (9.87±7.58) (P<0.05; Fig. 6B). However, the levels of IL-4 (5.03±2.44) and IL-2 (9.74±4.55) in the RIF group did not exhibit a significant difference compared with the control group (4.60±0.97; Fig. 6C and 8.05±4.35; Fig. 6D). These results suggest that increased concentrations of IL-21 and IL-6 in the serum. Whether overexpression of IL-21 and IL-6 correlates to cTfh levels requires further research.
Figure 6.
Concentration of IL-21, −6, −4 and −2 in the RIF and control groups. Comparison of (A) IL-21, (B) IL-6, (C) IL-4 and (D) IL-2 between the RIF and control groups. *P<0.05, ***P<0.001 vs. the control group. IL, interleukin; RIF, repeated implantation failure.
Correlation analysis
To find correlations between cTfh cells and associated factors in patients with RIF, Pearson correlation analyses were performed. It was revealed that the proportion of cTfh cells was positively correlated with IL-21 and IL-6 (P<0.01; Table III). No significant correlations were identified between CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells and IL-4 and IL-2. As shown in Table IV, IL-21 mRNA levels were significantly positively correlated with the levels of Bcl-6 and CXCR5 in the endometrium (P<0.05). These results suggest that CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells and cytokines synergistically regulate local immunity in women with RIF, and that the occurrence of RIF is not caused by a single factor.
Table III.
Correlation between CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells and cytokines in the peripheral blood of patients with repeated implantation failure.
| Cytokine (pg/ml) | |||||
|---|---|---|---|---|---|
| Cell type | Correlation | IL-21 | IL-6 | IL-4 | IL-2 |
| CCR7(lo)PD-1(hi) | Correlation coefficient | 0.649 | 0.497 | 0.279 | 0.358 |
| CXCR5+ CD4+ T | P-value | <0.001b | 0.005a | 0.135 | 0.052 |
P<0.01
P<0.001 vs. the control group. CCR7, chemokine receptor 7; PD-1, programmed death-1; CXCR5, chemokine receptor type 5; CD, cluster of differentiation; IL, interleukin.
Table IV.
Correlation between IL-21 and CXCR5 and Bcl-6 mRNA in the endometrium of patients with repeated implantation failure.
| mRNA | |||||
|---|---|---|---|---|---|
| Factors | Correlation | CXCR5 | Bcl-6 | ||
| IL-21 | Correlation coefficient | 0.544 | 0.569 | ||
| P-value | 0.002a | 0.001a | |||
P<0.01 vs. the control group. IL, interleukin; CXCR5, chemokine receptor type 5; Bcl-6, B-cell lymphoma 6.
Discussion
In the present study, CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells and associated factors were detected in increased concentrations in patients with RIF compared to the control group during the mid-luteal phase in the endometrium and the peripheral blood. This suggests that CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells are involved in the pathogenesis and pathophysiology of RIF. Increased PD-1 on the surface of Tfh cells represents an activated status. By co-culturing with autologous memory B cells, a previous study identified that CCR7(lo)PD-1(hi) CXCR5+ but not naive CD4+ T cells potently induced CD27 (hi) CD38 (hi) plasmablast or plasma cell differentiation and total IgG production (26). Similar results were identified in the studies of Zhang et al (27) and Akiyama et al (39). This indicates that CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells can induce RIF by promoting antibody production.
Autoantibodies and alloantibodies have been identified to be associated with infertility, miscarriage and embryo implantation failure (40,41). The embryo, as a semi-allograft, can trigger antigenic responses and induce the production of maternal antibodies (41,42). These alloantibodies have no pathogenic effect on the embryo; on the contrary, a lack of these antibodies can cause recurrent miscarriage (42). Women with recurrent miscarriage and RIF also have higher levels of autoantibodies, including antinuclear autoantibodies, antiphospholipid antibodies, anti-thyroperoxidase and anti-thyroglobulin antibodies (43–45). These autoantibodies can damage the endometrium and hinder the implantation of the embryo, resulting in infertility and recurrent spontaneous abortion (43,46). A lack of alloantibodies and overexpression of autoantibodies can lead to abnormalities in humoral immunity (42,44). Tfh cells prime B cells to initiate antibody responses and maintain humoral immunity (22,23). Realizing the role of Tfh cells in embryo implantation is crucial for the development of potential novel therapies against RIF.
Successful implantation of an embryo requires synchronous development of the embryo and the endometrium (47,48). Endometrial receptivity is generally at its highest during the mid-luteal phase of the menstrual cycle, which makes it the best time for implantation. Reduced receptivity can result in failed or inadequate implantation (49). This means that the microenvironment provided by uterine fluid, particularly glandular secretions, is essential for implantation. Analysis of endometrial fluid has identified cytokines, chemokines, proteases, antiproteases and other factors that modulate blastocyst functions relevant to implantation (50). The present study discovered that Bcl-6, CXCR5 and IL-21 were highly expressed in endometrial glands and the stroma of the RIF group. These results suggest that Tfh-associated factors may be involved in regulating the implantation microenvironment.
During early pregnancy, immune cells are recruited extensively to the endometrium. Chemokines and their receptors mediate immune cell recruitment and subsequent chronic activation of the immune system. They serve an important role in embryonic attachment and placental development (51). Franasiak et al (52) revealed that the chemokine CXCL13 reached optimal levels in the endometrium during the mid-luteal phase. Notably, the present study identified that the level of its receptor CXCR5 was increased in the endometrium during the same phase. CXCR5 is critical for the migration of Tfh and B cells into B cell follicles (26). Therefore, it has been suggested that the increased CXCR5 was involved in Tfh cells. In vivo, CXCR5 is closely associated with Bcl-6 expression (53). Bcl-6 is involved in migration control, differentiation and products of Tfh cells, and is also required for the formation of GCs. Overexpression of Bcl-6 results in the repression of CCR7 and the upregulation of PD-1 and CXCR5 (54). Bcl-6 is usually upregulated in GC Tfh cells and B cells, but hardly expressed in non-Tfh and cTfh cells (16,17). Normally, Bcl-6 acts mainly as a transcriptional repressor, interfering with the differentiation of non-Tfh cells; for instance, by antagonizing the expression of transcription factors T-bet, trans-acting T-cell-specific transcription factor GATA-3 and nuclear receptor ROR γ inhibit polarization of Th1, Th2 and Th17 cells (53,55,56). Previous studies have demonstrated that Bcl-6 is the master regulator of Tfh cell differentiation (16,54). Bcl-6 is upregulated during Tfh cell differentiation and Bcl6− T cells fail to differentiate into Tfh cells. In the present study, the expression of Bcl-6 was clearly increased in the RIF endometrium, and there was a significant positive correlation between Bcl-6 and IL-21 levels.
IL-21 is the main cytokine secreted by Tfh cells, it can enhance the differentiation of Tfh cells, regulate B cell differentiation and proliferation, stimulate plasma cell differentiation and immunoglobulin production, and induce the expression of Bcl-6 (57). IL-21 is the most potent inducer of plasma-cell differentiation in vitro (58,59). Altered IL-21 production induces miscarriage by promoting an inflammatory state. In the present study, the expression of IL-21 was similar to that of Bcl-6 and CXCR5 in the RIF endometrium. Wang et al (60) observed that interferon regulatory factor 4 mediated Th17 cell activation and upregulated the expression of IL-17A and IL-21, which resulted in pregnancy loss. Messaoudi et al (61) revealed that polymorphisms of IL-21 were associated with recurrent spontaneous miscarriage through pro-inflammatory pathways. The results of the present study demonstrated that the mRNA and protein levels of Blc-6, CXCR5 and IL-21 were increased in the RIF endometrium at the mid luteal phase. Furthermore, Blc-6 and CXCR5 levels had a positive correlation with IL-21 levels. From the results of the current study and the previous studies discussed, it was proposed that increased Bcl-6, CXCR5 and IL-21 collaborate to cause an imbalance of the endometrial immune microenvironment in women with RIF. The activation of these factors may be involved in Tfh and B cell differentiation and antibody secretion.
Cytokines are critical for embryo implantation and pregnancy maintenance (12,13). Several cytokines are involved in the regulation of Tfh cell differentiation. IL-21, IL-6 and IL-4 are considered to support Tfh differentiation, while IL-2 is a potent inhibitor of this. IL-6 is a pro-inflammatory cytokine secreted by numerous cell types. IL-6 derived from follicular dendritic cells is essential for Tfh cell maintenance (62). Plasmablast-derived IL-6 can also increase the number of human cTfh cells (63). IL-6 induces an early wave of Bcl-6 expression and is important for the initiation of Tfh cell production. A lack of IL-6 results in a severe reduction in CXCR5+ Bcl6+ early Tfh cells in vivo (64). Previously, a significantly higher expression of IL-6 in women with unexplained recurrent spontaneous abortion has been reported (12). IL-21 and IL-4 are two cytokines expressed by Tfh cells (19,65,66). Production of IL-4 and IL-21 is coupled in Tfh cells and required for optimal antibody responses (19,20). IL-21 is a novel susceptibility gene for recurrent spontaneous miscarriage (61). Upregulating the expression of IL-21 may result in pregnancy loss (60) and infertility (31). In the current study, IL-21 and IL-6 were increased in the serum of the RIF group, but IL-4 and IL-2 levels did not demonstrate a statistically significant difference compared to the control group. There is a significant positive correlation between the number of cTfh cells and IL-21 and IL-6 levels. These results suggest that IL-21 may co-operate with IL-6, resulting in RIF by promoting Tfh cell differentiation.
In conclusion, the results of the present study indicate that CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells, CXCR5, IL-21, Bcl-6 and IL-6 are involved in the pathogenesis of RIF. Whether CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells and associated factors contribute to abnormal humoral immunity in patients with RIF requires additional research. CCR7(lo)D-1(hi) CXCR5+ CD4+ T cells and associated factors could be a potential therapeutic target to help reduce the risk of implantation failure.
Acknowledgements
The present study was supported by the Natural Science Foundation of Xinjiang Autonomous Region (grant no. 2013211A087) and the National Natural Science Foundation of China (grant nos. 81660343, 8160202 and 81460307).
Glossary
Abbreviations
- RIF
repeated implantation failure
- Bcl-6
B-cell lymphoma 6
- CXCR5
chemokine receptor type 5
- Tfh
follicular helper T
- cTfh
circulating Tfh
- IVF-ET
in vitro fertilization-embryo transfer
- CCR7
chemokine receptor 7
- PD-1
programmed death-1
References
- 1.Valenzuela OA, Couturier-Tarrade A, Choi YH, Aubrière MC, Ritthaler J, Chavatte-Palmer P, Hinrichs K. Impact of equine assisted reproductive technologies (standard embryo transfer or intracytoplasmic sperm injection (ICSI) with in vitro culture and embryo transfer) on placenta and foal morphometry and placental gene expression. Reprod Fertil Dev. 2017 doi: 10.1071/RD16536. [DOI] [PubMed] [Google Scholar]
- 2.Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. Am J Reprod Immunol. 2014;72:141–147. doi: 10.1111/aji.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledee N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, Dubanchet S, Gahéry H, Bensussan A, Chaouat G. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol. 2016;75:388–401. doi: 10.1111/aji.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford G, Ray A, Gudi A, Shah A, Homburg R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: Review of the literature and meta-analysis. Hum Reprod Update. 2015;21:275–284. doi: 10.1093/humupd/dmu052. [DOI] [PubMed] [Google Scholar]
- 5.Tan BK, Vandekerckhove P, Kennedy R, Keay SD. Investigation and current management of recurrent IVF treatment failure in the UK. BJOG. 2005;112:773–780. doi: 10.1111/j.1471-0528.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- 6.Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–3043. doi: 10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- 7.Simon A, Laufer N. Repeated implantation failure: Clinical approach. Fertil Steril. 2012;97:1039–1043. doi: 10.1016/j.fertnstert.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa K, Kwak-Kim J, Ota K, Kuroda K, Hisano M, Sugiyama R, Yamaguchi K. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am J Reprod Immunol. 2015;73:353–361. doi: 10.1111/aji.12338. [DOI] [PubMed] [Google Scholar]
- 9.Persson M, Ekerfelt C, Jablonowska B, Jonsson Y, Ernerudh J, Jenmalm MC, Berg G. Immunological status in patients undergoing in vitro fertilisation: Responses to hormone treatment and relationship to outcome. J Reprod Immunol. 2012;96:58–67. doi: 10.1016/j.jri.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Schlossberger V, Schober L, Rehnitz J, Schaier M, Zeier M, Meuer S, Schmitt E, Toth B, Strowitzki T, Steinborn A. The success of assisted reproduction technologies in relation to composition of the total regulatory T cell (Treg) pool and different Treg subsets. Hum Reprod. 2013;28:3062–3073. doi: 10.1093/humrep/det316. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Wang Z, Zhao X, Wang J, Sun H, Hu Y. An increase of Treg cells in the peripheral blood is associated with a better in vitro fertilization treatment outcome. Am J Reprod Immunol. 2012;68:100–106. doi: 10.1111/j.1600-0897.2012.01153.x. [DOI] [PubMed] [Google Scholar]
- 12.Saifi B, Rezaee SA, Tajik N, Ahmadpour ME, Ashrafi M, Vakili R, SoleimaniAsl S, Aflatoonian R, Mehdizadeh M. Th17 cells and related cytokines in unexplained recurrent spontaneous miscarriage at the implantation window. Reprod Biomed Online. 2014;29:481–489. doi: 10.1016/j.rbmo.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Liang PY, Diao LH, Huang CY, Lian RC, Chen X, Li GG, Zhao J, Li YY, He XB, Zeng Y. The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod Biomed Online. 2015;31:823–826. doi: 10.1016/j.rbmo.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Figueiredo MM, Costa PAC, Diniz SQ, Henriques PM, Kano FS, Tada MS, Pereira DB, Soares IS, Martins-Filho OA, Jankovic D, et al. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog. 2017;13:e1006484. doi: 10.1371/journal.ppat.1006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteiro C, Kasahara TM, Castro JR, Sacramento PM, Hygino J, Centurião N, Cassano T, Lopes LMF, Leite S, Silva VG, et al. Pregnancy favors the expansion of circulating functional follicular helper T Cells. J Reprod Immunol. 2017;121:1–10. doi: 10.1016/j.jri.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly-TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 17.Ueno H. Human circulating T follicular helper cell subsets in health and disease. J Clin Immunol. 2016;36(Suppl 1):S34–S39. doi: 10.1007/s10875-016-0268-3. [DOI] [PubMed] [Google Scholar]
- 18.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T Cells. Annu Rev Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein JS, Herman EI, Lainez B, Licona-Limón P, Esplugues E, Flavell R, Craft J. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17:1197–1205. doi: 10.1038/ni.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuire HM, Vogelzang A, Warren J, Loetsch C, Natividad KD, Chan TD, Brink R, Batten M, King C. IL-21 and IL-4 collaborate to shape T-dependent antibody responses. J Immunol. 2015;195:5123–5135. doi: 10.4049/jimmunol.1501463. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crotty S. T follicular helper cell differentiation, function and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badell IR, Ford ML. T follicular helper cells in the generation of alloantibody and graft rejection. Curr Opin Organ Transplant. 2016;21:1–6. doi: 10.1097/MOT.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, Huang H, Wen R, Wang J, Li HS, et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arroyo-Villa I, Bautista-Caro MB, Balsa A, Aguado-Acín P, Bonilla-Hernán MG, Plasencia C, Villalba A, Nuño L, Puig-Kröger A, Martín-Mola E, Miranda-Carús ME. Constitutively altered frequencies of circulating follicullar helper T cell counterparts and their subsets in rheumatoid arthritis. Arthritis Res Ther. 2014;16:500. doi: 10.1186/s13075-014-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Pang N, Zhu Y, Zhou D, Zhao H, Hu J, Ma X, Li J, Wen H, Samten B, et al. CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells are positively correlated with levels of IL-21 in active and transitional cystic echinococcosis patients. BMC Infect Dis. 2015;15:457. doi: 10.1186/s12879-015-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, Li X, Zhang Z, Zhou M, Sun Y, Su D, Feng X, Gao X, Shi S, Chen W, Sun L. Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Sci Rep. 2015;5:12777. doi: 10.1038/srep12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco P, Ueno H, Schmitt N. T follicular helper (Tfh) cells in lupus: Activation and involvement in SLE pathogenesis. Eur J Immunol. 2016;46:281–290. doi: 10.1002/eji.201545760. [DOI] [PubMed] [Google Scholar]
- 30.Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One. 2013;8:e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Liu J, Cui X, Zuo Y, Zhang Z, Li Y, Tao R, Li Y, Pang J. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol. 2015;295:46–51. doi: 10.1016/j.cellimm.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Park HJ, Kim DH, Lim SH, Kim WJ, Youn J, Choi YS, Choi JM. Insights into the role of follicular helper T cells in autoimmunity. Immune Netw. 2014;14:21–29. doi: 10.4110/in.2014.14.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi J, Luo F, Shi Q, Xu X, He X, Xia Y. Increased circulating follicular helper T cells with decreased programmed death-1 in chronic renal allograft rejection. BMC Nephrol. 2015;16:182. doi: 10.1186/s12882-015-0172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu KH, Loose DS, Yates MS, Nogueras-Gonzalez GM, Munsell MF, Chen LM, Lynch H, Cornelison T, Boyd-Rogers S, Rubin M, et al. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res (Phila) 2013;6:774–781. doi: 10.1158/1940-6207.CAPR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia L, Wang Y, Li J, Li S, Zhang Y, Shen J, Tan W, Wu C. Detection of IL-9 producing T cells in the PBMCs of allergic asthmatic patients. BMC immunology. 2017;18:38. doi: 10.1186/s12865-017-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Franasiak JM, Scott RT. Contribution of immunology to implantation failure of euploid embryos. Fertil Steril. 2017;107:1279–1283. doi: 10.1016/j.fertnstert.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Wang WJ, Liu FJ, Zhang X, Liu XM, Qu QL, Li FH, Zhuang LL, Li XX, Hao CF. Periodic elevation of regulatory T cells on the day of embryo transfer is associated with better in vitro fertilization outcome. J Reprod Immunol. 2017;119:49–53. doi: 10.1016/j.jri.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama M, Yasuoka H, Yamaoka K, Suzuki K, Kaneko Y, Kondo H, Kassai Y, Koga K, Miyazaki T, Morita R, et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther. 2016;18:167. doi: 10.1186/s13075-016-1064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An LF, Zhang XH, Sun XT, Zhao LH, Li S, Wang WH. Unexplained infertility patients have increased serum IL-2, IL-4, IL-6, IL-8, IL-21, TNFα, IFNgamma and increased Tfh/CD4 T cell ratio: Increased Tfh and IL-21 strongly correlate with presence of autoantibodies. Immunol Invest. 2015;44:164–173. doi: 10.3109/08820139.2014.932377. [DOI] [PubMed] [Google Scholar]
- 41.Perricone C, de Carolis C, Perricone R. Pregnancy and autoimmunity: A common problem. Best Pract Res Clin Rheumatol. 2012;26:47–60. doi: 10.1016/j.berh.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Chen JL, Yang JM, Huang YZ, Li Y. Clinical observation of lymphocyte active immunotherapy in 380 patients with unexplained recurrent spontaneous abortion. Int Immunopharmacol. 2016;40:347–350. doi: 10.1016/j.intimp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Ticconi C, Pietropolli A, Borelli B, Bruno V, Piccione E, Bernardini S, Di Simone N. Antinuclear autoantibodies and pregnancy outcome in women with unexplained recurrent miscarriage. Am J Reprod Immunol. 2016;76:396–399. doi: 10.1111/aji.12560. [DOI] [PubMed] [Google Scholar]
- 44.Breen KA, Sanchez K, Kirkman N, Seed PT, Parmar K, Moore GW, Hunt BJ. Endothelial and platelet microparticles in patients with antiphospholipid antibodies. Thromb Res. 2015;135:368–374. doi: 10.1016/j.thromres.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 45.Kwak-Kim J, Skariah A, Wu L, Salazar D, Sung N, Ota K. Humoral and cellular autoimmunity in women with recurrent pregnancy losses and repeated implantation failures: A possible role of vitamin D. Autoimmun Rev. 2016;15:943–947. doi: 10.1016/j.autrev.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Unuane D, Velkeniers B, Deridder S, Bravenboer B, Tournaye H, De Brucker M. Impact of thyroid autoimmunity on cumulative delivery rates in in vitro fertilization/intracytoplasmic sperm injection patients. Fertil Steril. 2016;106:144–150. doi: 10.1016/j.fertnstert.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: It is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil Steril. 2017;108:15–18. doi: 10.1016/j.fertnstert.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 48.Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet. 2016;33:1419–1430. doi: 10.1007/s10815-016-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar V, Soni UK, Maurya VK, Singh K, Jha RK. Integrin beta8 (ITGB8) activates VAV-RAC1 signaling via FAK in the acquisition of endometrial epithelial cell receptivity for blastocyst implantation. Sci Rep. 2017;7:1885. doi: 10.1038/s41598-017-01764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salamonsen LA, Evans J, Nguyen HP, Edgell TA. The microenvironment of human implantation: Determinant of reproductive success. Am J Reprod Immunol. 2016;75:218–225. doi: 10.1111/aji.12450. [DOI] [PubMed] [Google Scholar]
- 51.Bidarimath M, Khalaj K, Kridli RT, Wessels JM, Koti M, Tayade C. Altered expression of chemokines and their receptors at porcine maternal-fetal interface during early and mid-gestational fetal loss. Cell Tissue Res. 2016;366:747–761. doi: 10.1007/s00441-016-2470-2. [DOI] [PubMed] [Google Scholar]
- 52.Franasiak JM, Burns KA, Slayden O, Yuan L, Fritz MA, Korach KS, Lessey BA, Young SL. Endometrial CXCL13 expression is cycle regulated in humans and aberrantly expressed in humans and Rhesus macaques with endometriosis. Reprod Sci. 2015;22:442–451. doi: 10.1177/1933719114542011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, Crotty S. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190:4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaeth M, Eckstein M, Shaw PJ, Kozhaya L, Yang J, Berberich-Siebelt F, Clancy R, Unutmaz D, Feske S. Store-operated Ca(2+) entry in follicular T cells controls humoral immune responses and autoimmunity. Immunity. 2016;44:1350–1364. doi: 10.1016/j.immuni.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belanger S, Crotty S. Dances with cytokines, featuring TFH cells, IL-21, IL-4 and B cells. Nat Immunol. 2016;17:1135–1136. doi: 10.1038/ni.3561. [DOI] [PubMed] [Google Scholar]
- 58.Spolski R, Leonard WJ. Interleukin-21: A double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13:379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 59.Tangye SG. Advances in IL-21 biology-enhancing our understanding of human disease. Curr Opin Immunol. 2015;34:107–115. doi: 10.1016/j.coi.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Yin T, Wen Y, Tian F, He X, Zhou D, Lin Y, Yang J. Potential effects of interferon regulatory factor 4 in a murine model of polyinosinic-polycytidylic acid-induced embryo resorption. Reprod Fertil Dev. 2015 doi: 10.1071/RD14499. [DOI] [PubMed] [Google Scholar]
- 61.Messaoudi S, Al-Khateeb GM, Dendana M, Sater MS, Jazia KB, Nouira M, Almawi WY, Mahjoub T. Genetic variations in the interleukin-21 gene and the risk of recurrent idiopathic spontaneous miscarriage. Eur Cytokine Netw. 2011;22:123–126. doi: 10.1684/ecn.2011.0287. [DOI] [PubMed] [Google Scholar]
- 62.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chavele KM, Merry E, Ehrenstein MR. Cutting edge: Circulating plasmablasts induce the differentiation of human T follicular helper cells via IL-6 production. J Immunol. 2015;194:2482–2485. doi: 10.4049/jimmunol.1401190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol. 2013;190:3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahoo A, Wali S, Nurieva R. T helper 2 and T follicular helper cells: Regulation and function of interleukin-4. Cytokine Growth Factor Rev. 2016;30:29–37. doi: 10.1016/j.cytogfr.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fairfax KC, Everts B, Amiel E, Smith AM, Schramm G, Haas H, Randolph GJ, Taylor JJ, Pearce EJ. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J Immunol. 2015;194:2999–3010. doi: 10.4049/jimmunol.1401225. [DOI] [PMC free article] [PubMed] [Google Scholar]