Significance
AMPA-type glutamate receptors are known to play critical roles in both basal synaptic transmission and acute forms of plasticity, such as long-term potentiation and long-term depression, but less is known about their role in neuronal homeostasis. A model for bidirectional synaptic scaling is emerging in which the GluA2 AMPA receptor subunit has a central role. Here we confirm that GluA2, but not GluA1, is necessary for scaling, and we show that GluA2 is also sufficient for mediating this phenomenon. In addition, we find that the membrane-proximal C-terminal domain of GluA2 is required for scaling following chronic pharmacologic silencing of network activity. How the GluA2 membrane-proximal C-terminal domain mediates synaptic insertion of AMPA receptors following chronic silencing remains to be elucidated.
Keywords: synaptic scaling, synaptic plasticity, AMPAR, GluA2, homeostatic plasticity
Abstract
Bidirectional scaling of synaptic transmission, expressed as a compensatory change in quantal size following chronic activity perturbation, is a critical effector mechanism underlying homeostatic plasticity in the brain. An emerging model posits that the GluA2 AMPA receptor (AMPAR) subunit may be important for the bidirectional scaling of excitatory transmission; however, whether this subunit plays an obligatory role in synaptic scaling, and the identity of the precise domain(s) involved, remain controversial. We set out to determine the specific AMPAR subunit required for scaling up in CA1 hippocampal pyramidal neurons, and found that the GluA2 subunit is both necessary and sufficient. In addition, our results point to a critical role for a single amino acid within the membrane-proximal region of the GluA2 cytoplasmic tail, and suggest a distinct model for the regulation of AMPAR trafficking in synaptic homeostasis.
In the vertebrate CNS, AMPA receptors (AMPARs) mediate the majority of fast excitatory synaptic transmission, and while these receptors are formed as tetrameric combinations of four subunits (GluA1–4), each subunit is differentially expressed over development and throughout the brain, and obeys distinct trafficking patterns during both basal activity and plasticity (1, 2). Numerous studies have explored the putative AMPAR subunit specificity in acute forms of plasticity, including both long-term potentiation (LTP) and long-term depression (LTD) (1–4). However, recent evidence suggests that there is no absolute requirement for a specific AMPAR subunit in supporting LTP (5) or LTD (6). In contrast, there has been comparatively little exploration of possible AMPAR subunit requirements in slower, homeostatic forms of plasticity.
First demonstrated in experiments characterizing the effects of chronic activity suppression in cultured neurons (7, 8), synaptic scaling is now an established phenomenon in excitatory neurons, in which chronic changes in neural activity induce counteracting changes in postsynaptic neurotransmitter receptor abundance, contributing to the restoration of baseline neuronal output (9). Previous studies have implicated the GluA2 AMPAR subunit in both cell-autonomous and network-wide synaptic scaling in pyramidal cells of the visual cortex and hippocampus (10–12; but see ref. 13). However, other lines of evidence suggest that GluA2-lacking, calcium-permeable, AMPARs are preferentially trafficked to the synapse during scaling (14–17; but see ref. 10).
Here we set out to find the AMPAR subunit and specific regions within it required to support scaling up (“scaling” hereinafter). To achieve this, we used a variety of molecular replacement techniques, replacing endogenous AMPARs with chimeric AMPAR subunits, or subunits containing point mutations within identified critical regions. We first explored the requirement for the individual AMPAR subunits GluA1 and GluA2 in synaptic scaling, as the vast majority of endogenous AMPARs in CA1 pyramidal neurons are GluA1/A2 heteromers (18, 19). We found a requirement for GluA2, but not for GluA1, in scaling of postsynaptic currents in rodent hippocampal pyramidal neurons in organotypic slice culture, consistent with previous observations from dissociated cultures of cortical neurons (10). In addition, we found that AMPAR subunits lacking the GluA2 cytoplasmic C-terminal domain (CTD) failed to support scaling, while both wild-type GluA2 and chimeric subunits containing the GluA2 CTD were sufficient to support scaling. In neurons expressing only GluA2(Q) homomers, scaling remained intact, indicating that GluA2 is sufficient to support scaling. Most surprisingly, we found no requirement for the distal GluA2 CTD, despite evidence from previous studies identifying the distal CTD as important (11). Instead, we identified a specific amino acid sequence within the membrane-proximal GluA2 CTD as necessary and sufficient, and demonstrated that point mutations within the region disrupt the ability of the GluA2 subunit to support scaling, suggesting a previously undescribed interaction.
Results
The GluA2 AMPAR Subunit, but Not GluA1, Is Necessary for Homeostatic Synaptic Scaling Following Chronic Activity Blockade.
Previous studies exploring the need for the GluA2 AMPAR subunit in synaptic scaling have relied primarily on recording miniature excitatory postsynaptic currents in dissociated cultures of cortical rodent neurons. Here we instead turned to organotypic hippocampal slice cultures, a system that is also pharmacologically accessible but allows the use of evoked stimulation of CA3 inputs onto CA1 pyramidal neurons, more closely modeling the input that these cells receive in vivo. Slices were prepared from P6–P8 animals and biolistically transfected the following day, a technique resulting in very sparse transfection, to investigate the effects of cell-autonomous genetic manipulations (Fig. 1A).
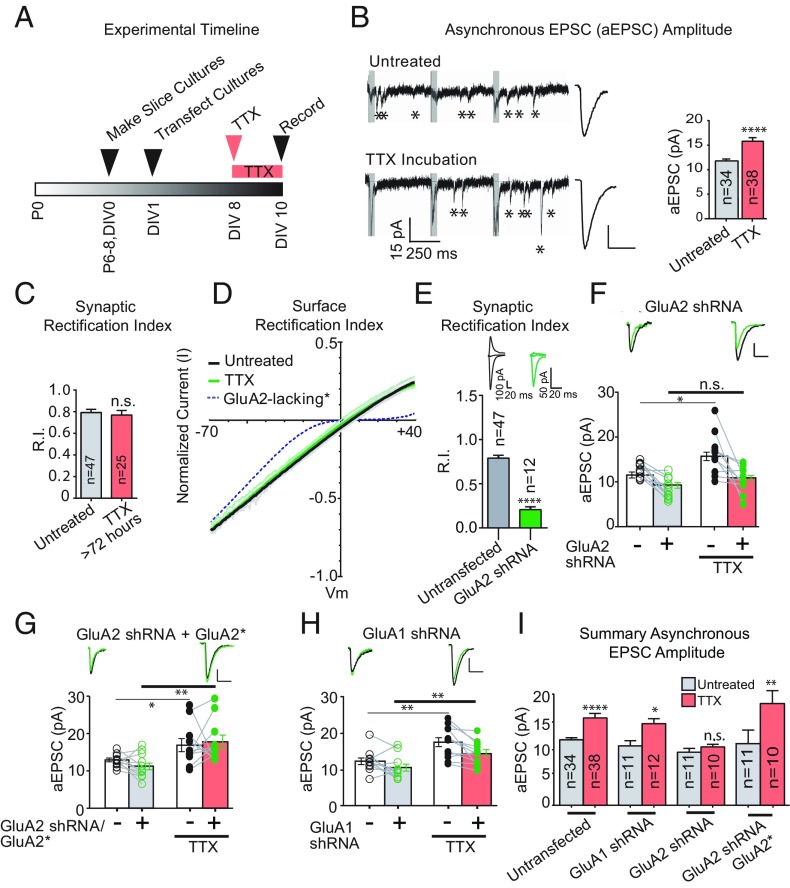
Fig. 1.
GluA2, not GluA1, is necessary for homeostatic synaptic scaling of quantal size. (A) Timeline of experiments. (B, Left) Sample traces of aEPSCs showing scaling of aEPSC amplitude with TTX treatment. The gray box shows the synchronous component of EPSC that is not analyzed. (B, Right) Averaged aEPSCs from TTX-treated and untreated control neurons. The bar graph shows averaged aEPSC amplitudes. (C) Synaptic rectification of AMPARs with or without TTX treatment (>72 h; Methods). (D) Surface rectification of AMPARs with and without TTX treatment. (E) Synaptic rectification of paired AMPA EPSCs in control neurons and neighboring neurons transfected with an shRNA against GluA2. (F) Paired asynchronous recordings without and with TTX treatment in control neurons and neighboring neurons transfected with GluA2 shRNA. Black, control; green, transfected. Untreated sample traces are at the top left, and TTX-treated sample traces are at the top right. (G) shRNA-insensitive GluA2 rescue under the same treatment conditions as in F. *Indicates shRNA resistance. (H) GluA1 shRNA under the same treatment conditions as in G. (I) Summary graph indicating unpaired scaling data, within the same transfection conditions. Significance was measured across treatment conditions. (Scale bars: 5 pA and 20 ms unless noted otherwise.) *P < 0.05; **P < 0.01; ****P < 0.0001; n.s., not significant.
We first set out to verify in slice culture that, as in dissociated culture, synaptic AMPAR content scales up following chronic silencing with saturating concentrations of tetrodotoxin (TTX; 1 μM). Using a bipolar electrode, we stimulated Schaffer collateral axons from CA3 and recorded both synchronous and asynchronous excitatory postsynaptic currents (EPSCs and aEPSCs, respectively) in CA1 pyramidal neurons. The aEPSC recordings were made in the presence of 4 mM extracellular strontium, which resulted in desynchronization of vesicle release from the presynaptic terminal, allowing for analysis of discrete aEPSCs (Fig. 1B, asterisks). For asynchronous recordings, a train of three stimuli spaced 500 ms apart was used to elicit aEPSCs, and following each stimulus artifact, a 50-ms period containing a synchronous component of the EPSC was not analyzed (Fig. 1B, Left, gray bars following stimulation artifacts).
As expected, aEPSCs scaled up by ∼40% in wild-type neurons following chronic activity blockade (Fig. 1B, Right). It has been reported that GluA2-lacking receptors, which generate strongly inwardly rectifying currents, are recruited to the synapse following scaling (14–17; but see ref. 10); however, the rectification index (RI) of evoked synaptic AMPAR currents was unaltered following protracted (>72 h) TTX exposure (Fig. 1C). We also examined the rectification of extrasynaptic AMPAR currents from somatic outside-out patches (Fig. 1D) by generating IV curves. Compared with the dramatic rectification seen in GluA2-lacking cells (broken line in Fig. 1D; data from ref. 18), the curves from control cells (black line) and cells exposed to TTX (green line) were very similar, although there was a slight, albeit significant, decrease in RI (P < 0.01; Fig. 1D).
We then sought to confirm the role for GluA2 in scaling. We first confirmed the efficacy of our short-hairpin RNA (shRNA) knockdown of the GluA2 subunit by comparing the RI of evoked EPSCs from shRNA-transfected cells and neighboring control cells (20). Expression of the shRNA for 7–9 d resulted in strongly rectifying EPSCs (Fig. 1E). In all subsequent experiments, we simultaneously recorded aEPSCs in a transfected neuron and a neighboring control neuron. By monitoring the control cells, we could verify the presence of TTX-induced scaling in any given set of experiments. We show statistical comparisons across treatment conditions to focus on the scaling phenomenon, because genetic manipulations in this study (such as GluA2 knockdown) produced baseline effects on aEPSC amplitude, thereby obscuring the utility of a direct paired comparison.
We confirmed the absence of scaling in neurons expressing the shRNA (Fig. 1 F and I), as the size of the aEPSCs in untransfected cells (clear bars) was increased following TTX treatment, while the size of aEPSCs recorded from the shRNA-expressing cells treated with TTX (salmon bar) were no different from those not treated with TTX (gray bar). The scaling of aEPSC amplitudes was fully restored with cotransfection of an RNAi-resistant GluA2 (Fig. 1 G and I, with shRNA resistance denoted by an asterisk appended to the subunit name). In addition, the relative amplitude of synchronous EPSCs in GluA2-lacking cells compared with neighboring controls was not maintained after chronic network silencing (Fig. S1), further verifying the requirement for GluA2 in scaling.
Is the GluA1 AMPAR subunit required for scaling? To test this possibility, we biolistically transfected an shRNA targeting GluA1 (Fig. S2) and examined the effect of chronic activity blockade with TTX. The scaling of aEPSCs in GluA1-lacking cells was the same as that observed in untransfected cells (Fig. 1 H and I). These data confirm the need for GluA2, but not for GluA1, in scaling of EPSCs. The finding that scaling is intact in the absence of GluA1 raises the possibility that GluA2 may be sufficient for this phenomenon.
The GluA2 AMPAR Subunit Is Sufficient for Homeostatic Synaptic Scaling Following Chronic Activity Blockade.
We next asked whether GluA2 alone is sufficient for synaptic scaling in the absence of all endogenous AMPARs. To address this question, we used a molecular replacement strategy with conditional knockout mice homozygous for floxed GluA1, GluA2, and GluA3 (GRIA1–3fl/fl), from which we generated slice cultures between postnatal days 6 and 9. We then sparsely transfected Cre recombinase along with the unedited rectifying GluA2 subunit GluA2(Q) using biolistic transfection on the day after preparation of cultures (Fig. 2A). We used GluA2(Q) rather than the GluA2(R), because neurons expressing only GluA2(R) generate little AMPAR current (18). This strategy allows for a complete replacement of endogenous heteromeric AMPARs with GluA2 homomers in transfected neurons lacking GRIA1–3, confirmed by measuring the RI (Fig. 2B).
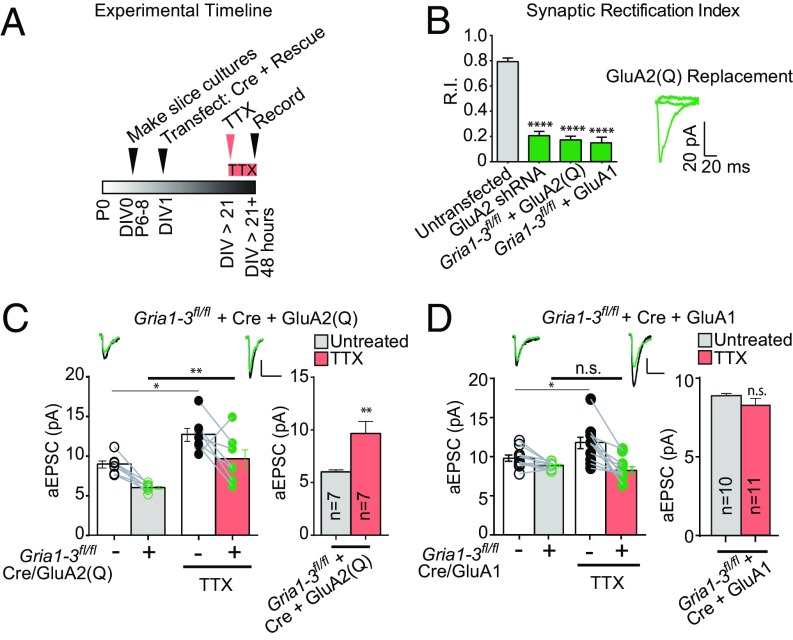
Fig. 2.
GluA2 is sufficient to support synaptic scaling in the absence of other AMPAR subunits. (A) Timeline of organotypic slice culture preparation, TTX incubation, and recording for GluA2(Q) replacement experiments. (B) Synaptic rectification of paired AMPA EPSCs in GRIA1–3fl/fl control neurons and neighboring neurons transfected with Cre and GluA2(Q) or Cre and GluA1. GluA2 shRNA synaptic rectification is shown for comparison. (C) Paired asynchronous recordings without and with preceding chronic TTX treatment in GRIA1–3fl/fl control neurons and neighboring GRIA1–3fl/fl neurons transfected with Cre + GluA2(Q). (Right) Within-transfection condition summary bar graph. (D) Paired asynchronous recordings without and with preceding chronic TTX treatment in GRIA1–3fl/fl control neurons and neighboring GRIA1–3fl/fl neurons transfected in utero with Cre + GluA1. *P < 0.05; **P < 0.01; ****P < 0.0001; n.s., not significant.
On this null background, GluA2(Q) rescued ∼70% of synchronous EPSC amplitude at −70 mV, relative to neighboring untransfected cells (Fig. S3 A and B), and cells expressing only GluA2(Q) exhibited normal scaling (Fig. 2C). These aEPSC data were supported by synchronous EPSC experiments (Fig. S3 A and B). In addition, GluA1 replacement on the GRIA1–3fl/fl + Cre background was unable to support scaling (Fig. 2D). These results support a model in which GluA2 is not only necessary, but also sufficient, for the synaptic expression of scaling.
The GluA2 C Tail Is Critical for Homeostatic Synaptic Scaling.
Previous studies have suggested that the GluA2 CTD may be integral for the scaling pathway. Dialysis of a GluA2 CTD peptide into the cell blocked scaling (10), presumably by acting in a dominant negative fashion. Further evidence pointed to a homeostatic mechanism by which GRIP interacts with the distal GluA2 CTD (11). To clarify further the role of the GluA2 CTD in scaling, we performed a series of experiments replacing endogenous GluA2 with GluA1/A2 chimeric subunits or with truncated GluA2 CTDs.
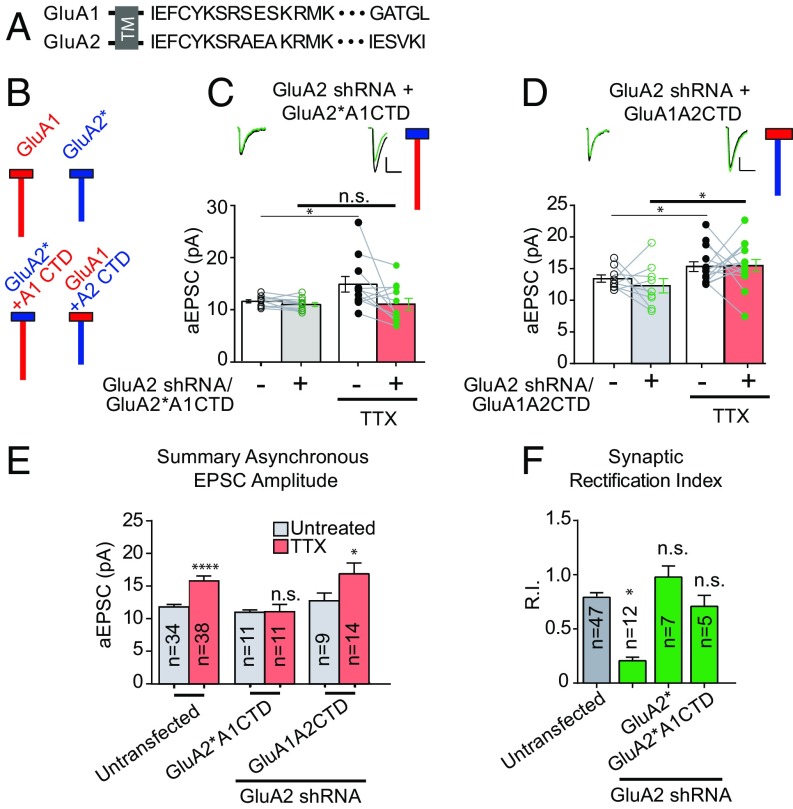
In neurons expressing GluA2 shRNA, we rescued synaptic currents with chimeric, truncated, or mutated RNAi-resistant AMPAR subunits to assess the role of the GluA2 CTD in scaling. We first rescued with chimeric AMPAR subunits in which the CTDs of the GluA1 and GluA2 were swapped (GluA1A2CTD and GluA2*A1CTD) (Fig. 3 A and B). We confirmed the synaptic incorporation of the GluA2*A1CTD by the loss of rectification (Fig. 3F). Despite its targeting to the synapse, this chimera was unable to rescue scaling, indicating a requirement for the GluA2 CTD (Fig. 3 C and E). The possibility remained that the GluA2 CTD was not sufficient to restore scaling in the absence of the rest of the GluA2 subunit. However, when we rescued with GluA2 CTD appended to GluA1, scaling was rescued (Fig. 3 D and E), confirming the key role of the GluA2 CTD in scaling.
Fig. 3.
The C-tail of the GluA2 subunit is critical for homeostatic synaptic scaling. (A) Endogenous GluA1 and GluA2 C-tail amino acid sequences. TM, transmembrane. (B) Schematic diagram of endogenous GluA1 and GluA2 AMPAR subunits next to schemata of chimeric AMPARs with swapped C-tails. Red indicates GluA1 subunit origin; blue, GluA2 subunit origin. Boxes indicate amino terminal domains and transmembrane regions of AMPARs, and vertical lines indicate intracellular C-tails. (C) Paired asynchronous recordings without and with preceding chronic TTX treatment in control neurons and neighboring neurons transfected with GluA2 shRNA and shRNA-insensitive AMPAR chimeric subunit GluA2*A1CTD. (D) GluA2 shRNA + shRNA-insensitive AMPAR chimeric subunit GluA1A2CTD. Treatment conditions are the same as in C. (E) Summary bar graph indicating unpaired scaling data under the same transfection conditions. Significance was measured across treatment conditions. (F) Comparison of synaptic rectification of chimeric AMPAR GluA2*A1CTD (with pore residue conferring calcium and intracellular polyamine block present) with cells transfected with GluA2 shRNA for comparison and cells transfected with GluA2 shRNA + full-length shRNA-insensitive GluA2. (Scale bars for aEPSC sample traces: 5 pA and 20 ms unless indicated otherwise.) *P < 0.05; ****P < 0.0001; n.s., not significant.
The Membrane Proximal Cytoplasmic Tail of the GluA2 Subunit Is Critical for Homeostatic Synaptic Scaling.
What domain of the CTD of GluA2 is required for scaling? To address this question, we designed a series of truncations (Fig. 4 A and B). In neurons expressing a construct in which the entire CTD of GluA2 (GluA2*∆838, referred to as GluA2*∆CTD) is deleted, scaling was absent (Fig. 4 C and F and Fig. S4 A and C). However, this negative result could be due to a failure of GluA2∆*CTD to form functional receptors, or from its inability to traffic to the synapse. To address this, we quantified the synaptic RI of evoked EPSCs (Fig. 4G). The fact that rectification was the same as in control cells indicates that this receptor is functional and traffics to the synapse. These findings establish the need for the GuA2 CTD in scaling.
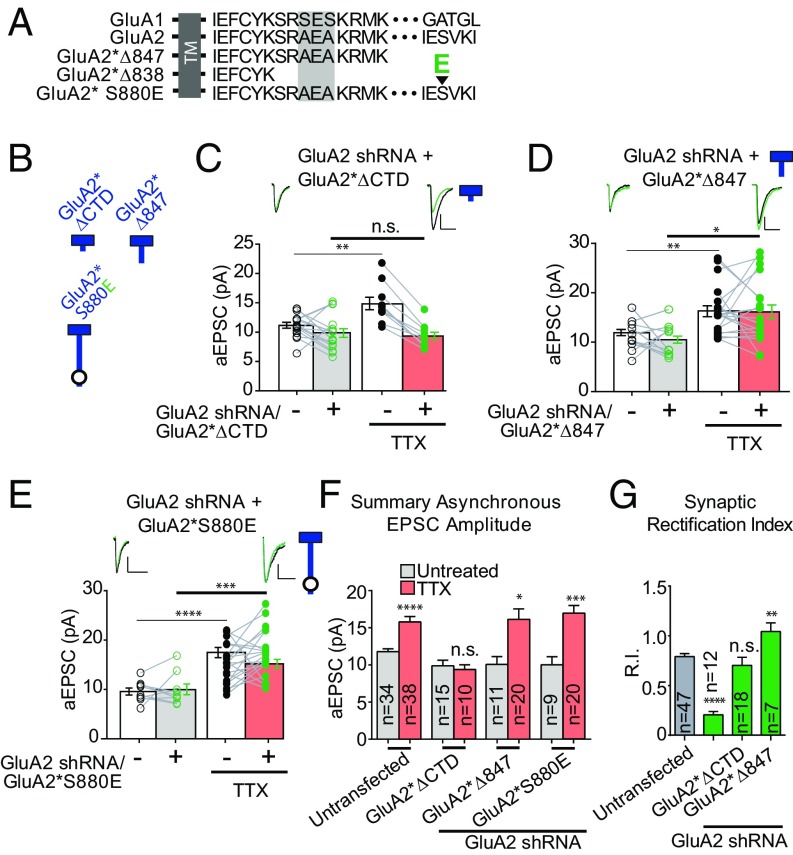
Fig. 4.
The membrane-proximal cytoplasmic tail of GluA2 is critical for synaptic scaling. (A) Endogenous GluA1 and GluA2 C-tail amino acid sequences, and GluA2 C-tail sequences with truncations (ΔCTD and Δ847) or point mutation (S880E; large arrowhead indicates location of phosphomimetic point mutation). The shaded-gray box in the membrane-proximal sequence illustrates the region with divergent sequences in the first 14 amino acids of AMPAR C-tails. TM, transmembrane. (B) Schematic diagram of truncated or mutated GluA2 subunits. Blue indicates the origin of the GluA2 subunit, and the circle indicates the location of the specific S880E point mutation. (C) Paired asynchronous recordings without and with preceding chronic TTX treatment in control neurons and neighboring neurons transfected with GluA2 shRNA and the shRNA-insensitive AMPAR chimeric subunit GluA2*ΔCTD. (D) GluA2 shRNA + shRNA-insensitive AMPAR chimeric subunit GluA2*Δ847. Treatment conditions are the same as in C. (E) GluA2 shRNA + shRNA-insensitive AMPAR chimeric subunit GluA2*S880E. Treatment conditions are the same as in C. (F) Summary bar graph indicating unpaired scaling data under the same transfection conditions. Significance was measured across treatment conditions (color-coded from C–F). (G) Comparison of synaptic rectification of truncated or mutated GluA2 subunits with cells transfected with GluA2 shRNA. (Scale bars for aEPSC sample traces: 5 pA and 20 ms unless indicated otherwise.) *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n.s., not significant.
We next reintroduced sections of the GluA2 CTD to the GluA2*∆CTD construct, adding back nine amino acids to the membrane-proximal region (MPR) of the CTD (Fig. 4 A and B). Surprisingly, this replacement subunit, GluA2*∆847 (CTD truncated following K847) was able to fully rescue scaling of aEPSCs (Fig. 4 D and F and Fig. S4 B and C). This result, in which scaling is rescued with a GluA2 subunit lacking the majority of the CTD, appears to be at odds with previous studies in which phosphorylation of distal residues, Y876 or S880, reduced the GRIP1/GluA2 interaction and blocked scaling (11, 21, 22). Therefore, we carried out phosphomimetic experiments in which we mutated the distal serine to glutamate (GluA2*S880E). In our hands, robust scaling occurred with this mutation (Fig. 4 E and F).
Specific Membrane Proximal Cytoplasmic Residues in the GluA2 AMPAR Subunit Are Necessary for Scaling.
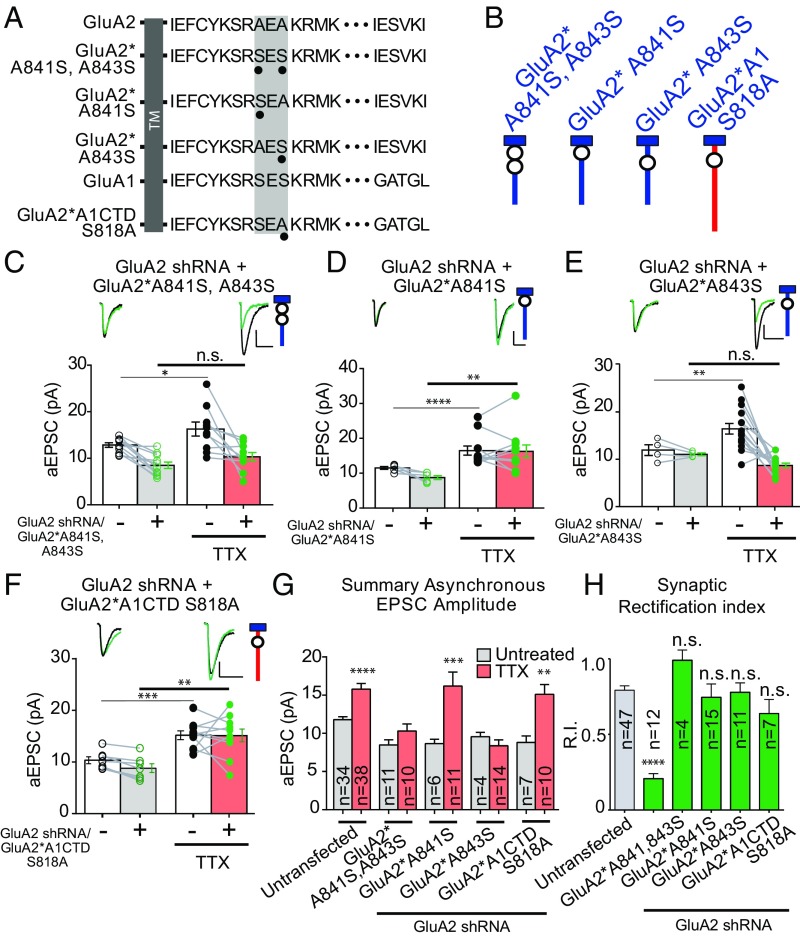
After establishing a requirement for the MPR of the GluA2 CTD in scaling, we set out to determine the role of specific residues. Examination of this region (Fig. 5 A and B) revealed that the only difference between GluA2 and GluA1 is the presence of two alanines in GluA2 (A841 and A843) instead of two serines in GluA1 (S816 and S818). We focused on these residues. In all subsequent experiments, we quantified the RI of the evoked EPSCs to ensure that all constructs successfully trafficked to the synapse (Fig. 5H). Mutating the two GluA2 alanines to “GluA1-like” serines (GluA2*A841S and A843S) abolished scaling (Fig. 5 C and G). In addition, scaling was absent when the same mutations were made in the truncated GluA2 (GluA2*∆847) (Fig. S5). These results establish the requirement for either one or both membrane-proximal alanines for scaling.
Fig. 5.
A specific residue in the membrane-proximal CTD of the GluA2 AMPAR subunit is necessary for scaling. (A) Endogenous GluA1 and GluA2 C-tail amino acid sequences, and GluA2 or chimeric AMPAR C-tail sequences with point mutations. The shaded-gray box in the membrane-proximal sequence shows the region with divergent sequences in the first 14 amino acids of AMPAR C-tails, as well as the location of mutations. TM, transmembrane. (B) Schematic diagram of truncated or mutated GluA subunits. Blue and red indicate GluA2 and GluA1 subunit origin, respectively. (C) Paired asynchronous recordings without and with previous chronic TTX treatment in control neurons and neighboring neurons transfected with GluA2 shRNA and shRNA-insensitive AMPAR chimeric subunit GluA2*A841S, A843S. The schematic of the GluA2 replacement receptor repeated in the top right. (D) GluA2 shRNA + shRNA-insensitive AMPAR chimeric subunit GluA2*A841S. Treatment conditions were the same as in C. (E) GluA2 shRNA + shRNA-insensitive AMPAR chimeric subunit GluA2*A843S. Treatment conditions were the same as in C. (F) GluA2 shRNA + shRNA-insensitive AMPAR chimeric subunit GluA2*A1CTD S818A. Treatment conditions are the same as in C. (G) Summary bar graph indicating unpaired scaling data, under the same transfection conditions. Significance was measured across treatment conditions. (H) Comparison of synaptic rectification of mutated GluA2 or chimeric subunits to cells transfected with GluA2 shRNA alone. (Scale bars for aEPSC sample traces: 5 pA and 20 ms unless indicated otherwise.) *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n.s., not significant.
We next tested the requirement for these two residues individually. Surprisingly, scaling was restored when endogenous GluA2 was replaced with GluA2*A841S (Fig. 5 D and G), but not when replaced with GluA2*A843S (Fig. 5 E and G), indicating a specific role for A843 of GluA2. If this alanine is critical for scaling, then we might predict that mutating the equivalent serine in GluA1 (S818) to an alanine would rescue scaling. Indeed, we found that this construct (GluA2*A1CTD S818A) rescued scaling (Fig. 5 F and G).
Discussion
Homeostatic synaptic scaling, operating at a much slower time scale than more acute forms of plasticity, such as LTP and LTD, is a critical mechanism by which the cell tunes the strength of its synaptic inputs up or down to counteract normal or pathological activity perturbations, contributing to the restoration of baseline neuronal output. Even subtle deficits in a neuron’s ability to maintain a setpoint of activity in response to chronic perturbations would result in catastrophic degradation of salient information. Thus, accurately characterizing the molecular interactions of downstream effectors—including the postsynaptic receptors themselves—that drive synaptic scaling is of critical importance in understanding the mechanism by which a cell is able to maintain this setpoint of activity.
Using molecular replacement strategies to dissect the role of specific AMPAR subunits and associated domains, we established that the GluA2 subunit is both necessary and sufficient for scaling. In addition, we identify a specific and novel role for the GluA2 CTD. While previous reports have implicated the GluA2 CTD in homeostasis (10, 11), our results define a critical residue in the MPR. In contrast to previous studies (11, 21), we could find no requirement for more distal sequences or specific residues therein. Perhaps the difference in results is due to the preparation; we used hippocampal slice culture, while previous studies used dissociated visual cortical or hippocampal neurons. Finally, we identified a specific uncharged residue, S843, within the membrane-proximal CTD of the GluA2 subunit that, when mutated, renders the GluA2 CTD unable to support scaling. Taken together, these results reinforce the crucial role of the GluA2 subunit in bidirectional synaptic scaling, and suggest a novel molecular interaction mediating the phenomenon.
Previous attempts to establish specific AMPAR subunit contributions to synaptic scaling are confusing. Several studies have reported evidence of an increase in the relative abundance of GluA1 homomers after long-term incubation in TTX (14–17; but see ref. 10), while other reports have found little evidence that any AMPAR subunit is, alone, necessary for scaling (13). We attempted to address these discrepancies by protracted incubation (>72 h) of organotypic hippocampal slice cultures with TTX to assay both synaptic and surface rectification, finding no change in synaptic rectification and a very small, albeit significant, decrease in the rectification of extrasynaptic AMPARs. These findings make it highly unlikely that GluA2-lacking receptors play an appreciable role in scaling.
In contrast to previous results suggesting that GluA2-lacking receptors are critical for scaling, we found that GluA2 is essential for scaling, reinforcing the GluA2-centric model of synaptic homeostasis supported by evidence from multiple studies. For example, GluA2 is required for scaling down following chronic, cell-autonomous optogenetic excitation (12). In addition, GluA2 is necessary for distance-dependent scaling of AMPARs along the dendrite (20). Finally, we previously identified a requirement for the GluA2 subunit in AMPAR consolidation following loss of synaptic scaffolding proteins (23). Taken together, these findings lay the groundwork for a model of bidirectional homeostatic control of postsynaptic strength through the GluA2 AMPAR subunit.
An intriguing question arises that we were unable to resolve within the scope of this study: given the otherwise striking similarity between the MPR of the GluA1 and GluA2 subunits, how might the presence of a single nonpolar amino acid (A843)—or the absence of a polar amino acid—confer such distinct synaptic trafficking behavior? We speculate that there exists some unique interaction between an effector protein and the proximal GluA2 CTD that is blocked by a polar serine residue, through either phosphorylation or steric hindrance.
Intriguingly, few protein interactions have been described within the MPR of the GluA2 CTD. The MPR of the GluA1 subunit has been found to interact with 4.1N/Band 4.1, a neuronally enriched FERM domain cytoskeletal-associated protein from the 4.1 family of membrane organizers that coordinates synaptic receptors with the actin cytoskeleton (24, 25). Germ line knockout of both 4.1N and 4.1G, two closely related members of the 4.1 family, does not grossly perturb glutamatergic synapses, pointing to a possible functional redundancy within the family of proteins (26). However, there is little evidence for any interaction between 4.1N and GluA2. Several groups have investigated interactions between iGluR subunits and 4.1N, with initial evidence pointing toward the GluA1 CTD (27). Subsequent studies identified interactions with GluA4 as well as the kainate receptor subunits, GluK1 and GluK2 (28, 29), but an interaction with GluA2 has not been ruled out. Intriguingly, it was recently found that posttranslational modifications to the MPR in the CTD of GluK2—a region with some sequence similarity to the AMPAR membrane-proximal CTDs—can dramatically impact association between these receptor subunits and 4.1N (29). The palmitoylation of GluK2 within this membrane-proximal sequence promotes association with 4.1N, while activation of PKC and the subsequent phosphorylation of the GluK2 MPR at a serine residue proximal to a series of positively charged amino acids serves to decrease the interaction with 4.1N. These posttranslational modifications of iGluRs (to govern the differential association with 4.1N) control activity-dependent receptor endocytosis, and thus could provide a mechanism by which AMPAR identity and abundance are regulated in forms of synaptic homeostasis.
Other possible “scaling effector” proteins include the AP2 adapter complex and NSF, which are known to interact with a specific stretch of amino acids in the GluA2 CTD (30, 31), but these proteins are unlikely to play a role in scaling, as a partial truncation of the GluA2 CTD (Δ847) that eliminates these binding sites either in part or in their entirety does not block scaling. These results could point toward a mechanism by which GluA2 is stabilized at synapses following chronic silencing through some modification or interaction of residues in the proximal CTD, thus ensuring that GluA2-containing, calcium-impermeable AMPARs are preferentially targeted to synapses following global scaling.
Other explanations, while less parsimonious, are nevertheless possible. For example, phosphorylation of S818 in the membrane-proximal GluA1 CTD may serve as a weak synaptic exclusion or ER retention signal in the absence of a GluA2 subunit. The occupation of synapses by AMPARs is a tightly-regulated process, and under basal conditions, preventing excess AMPARs from entering synapses is likely critical for preserving cellular patterns of information storage. Further experiments are needed to identify the protein or proteins upstream of synaptic AMPAR insertion that interact with the membrane-proximal CTD of GluA2.
Methods
Mouse Genetics.
Animals were housed according to the guidelines of the Institutional Animal Care and Use Committee the University of California, San Francisco. Mice with the GRIA1fl/fl, GRIA2fl/fl, and GRIA3fl/fl (GRIA1–3fl/fl) were generated and genotyped as described previously (18).
Neuronal Transfection.
Sparse biolistic transfections of organotypic slice cultures were performed as described previously (32). In utero electroporations were performed as described previously (33). Additional details and experimental constructs are provided in SI Methods.
Electrophysiology in Slice Cultures.
Dual whole-cell recordings in area CA1 were done by simultaneously recording responses from a fluorescent transfected neuron and neighboring untransfected control neuron. Dual whole-cell recordings measuring strontium-evoked aEPSCs used an extracellular solution bubbled with 95% O2/5% CO2 consisting of 119 NaCl mM, 2.5 mM KCl, 4 mM SrCl2 (substituted with 4 mM CaCl2 in synchronous EPSC recordings), 4 mM MgSO4, 1 mM NaH2PO4, 26.2 mM NaHCO3, and 11 mM glucose. One hundred micromolar picrotoxin was added to block inhibitory currents, and in synchronous EPSC experiments, 2 µM 2-chloroadenosine was used to control epileptiform activity. Intracellular solution contained 135 mM CsMeSO4, 8 mM NaCl, 10 mM Hepes, 0.3 mM EGTA, 5 mM QX314-Cl, 4 mM MgATP, 0.3 mM Na3GTP, and 0.1 mM spermine. The TTX treatment protocol and aEPSC recording protocol and analysis are described in SI Methods. Synaptic and surface rectification were measured as described in SI Methods.
Statistical Analysis.
The Mann–Whitney U test was used for all experiments involving unpaired data, including all outside-out patch data, and the Kruskal–Wallis test with Dunn correction for multiple comparisons was used for rectification experiments. For all experiments using paired data, a two-tailed Wilcoxon signed-rank test was used. Data analysis was conducted in Igor Pro (Wavemetrics), Excel (Microsoft), and GraphPad Prism.
Supplementary Material
Acknowledgments
We thank M. Horn, K. Lovero, and others in the R.A.N. laboratory for critical discussions and reading of the manuscript, M. Cerpas and D. Qin for technical help with organotypic slice cultures, and P. Seeburg and R. Sprengel for the GRIA1–3fl/fl mice. R.A.N. is supported by the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716022114/-/DCSupplemental.
References
- 1.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 3.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 4.Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- 5.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493:495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granger AJ, Nicoll RA. LTD expression is independent of glutamate receptor subtype. Front Synaptic Neurosci. 2014;6:15. doi: 10.3389/fnsyn.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien RJ, et al. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 8.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 9.Turrigiano GG. The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gainey MA, Tatavarty V, Nahmani M, Lin H, Turrigiano GG. Activity-dependent synaptic GRIP1 accumulation drives synaptic scaling up in response to action potential blockade. Proc Natl Acad Sci USA. 2015;112:E3590–E3599. doi: 10.1073/pnas.1510754112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altimimi HF, Stellwagen D. Persistent synaptic scaling independent of AMPA receptor subunit composition. J Neurosci. 2013;33:11763–11767. doi: 10.1523/JNEUROSCI.1102-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 15.Groth RD, Lindskog M, Thiagarajan TC, Li L, Tsien RW. Beta Ca2+/CaM-dependent kinase type II triggers up-regulation of GluA1 to coordinate adaptation to synaptic inactivity in hippocampal neurons. Proc Natl Acad Sci USA. 2011;108:828–833. doi: 10.1073/pnas.1018022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soares C, Lee KF, Nassrallah W, Béïque JC. Differential subcellular targeting of glutamate receptor subtypes during homeostatic synaptic plasticity. J Neurosci. 2013;33:13547–13559. doi: 10.1523/JNEUROSCI.1873-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu W, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shipman SL, Herring BE, Suh YH, Roche KW, Nicoll RA. Distance-dependent scaling of AMPARs is cell-autonomous and GluA2-dependent. J Neurosci. 2013;33:13312–13319. doi: 10.1523/JNEUROSCI.0678-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang SS, et al. Regulation of STEP61 and tyrosine-phosphorylation of NMDA and AMPA receptors during homeostatic synaptic plasticity. Mol Brain. 2015;8:55. doi: 10.1186/s13041-015-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy JM, Chen X, Reese TS, Nicoll RA. Synaptic consolidation normalizes AMPAR quantal size following MAGUK loss. Neuron. 2015;87:534–548. doi: 10.1016/j.neuron.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoover KB, Bryant PJ. The genetics of the protein 4.1 family: Organizers of the membrane and cytoskeleton. Curr Opin Cell Biol. 2000;12:229–234. doi: 10.1016/s0955-0674(99)00080-0. [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Merino C, Sigrist SJ, Featherstone DE. The 4.1 protein coracle mediates subunit-selective anchoring of Drosophila glutamate receptors to the postsynaptic actin cytoskeleton. J Neurosci. 2005;25:6667–6675. doi: 10.1523/JNEUROSCI.1527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wozny C, et al. The function of glutamatergic synapses is not perturbed by severe knockdown of 4.1N and 4.1G expression. J Cell Sci. 2009;122:735–744. doi: 10.1242/jcs.037382. [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman SK, Cai C, Mottershead DG, Haapalahti JP, Keinänen K. Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J Neurosci. 2003;23:798–806. doi: 10.1523/JNEUROSCI.23-03-00798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copits BA, Swanson GT. Kainate receptor post-translational modifications differentially regulate association with 4.1N to control activity-dependent receptor endocytosis. J Biol Chem. 2013;288:8952–8965. doi: 10.1074/jbc.M112.440719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- 31.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnell E, et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro-Quiroga I, Chittajallu R, Gallo V, Haydar TF. Long-term, selective gene expression in developing and adult hippocampal pyramidal neurons using focal in utero electroporation. J Neurosci. 2007;27:5007–5011. doi: 10.1523/JNEUROSCI.0867-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliet SH, Malenka RC, Nicoll RA. Bidirectional control of quantal size by synaptic activity in the hippocampus. Science. 1996;271:1294–1297. doi: 10.1126/science.271.5253.1294. [DOI] [PubMed] [Google Scholar]
- 35.Bemben MA, et al. CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nat Neurosci. 2014;17:56–64. doi: 10.1038/nn.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.