Significance
The idea that early Australopithecus shaped stone tools to butcher large mammals before the emergence of Homo around 2 million years ago has excited both primatologists and archaeologists. Such claims depend on interpreting modifications found on the surfaces of fossil bones. Recent experiments involving the feeding of mammal carcasses to modern crocodiles have revealed that equifinality—the creation of similar products by different processes—is more important than previously appreciated by zooarchaeologists. Application of these findings to Ethiopian fossils casts doubt on claims for the earliest large mammal butchery and indicates the need for reassessment of all Oldowan-associated bone assemblages to determine the degree to which equifinality compromises earlier interpretations of hominid subsistence activities and their role in human evolution.
Keywords: zooarchaeology, Oldowan, taphonomy, cutmarks, equifinality
Abstract
Zooarchaeologists have long relied on linear traces and pits found on the surfaces of ancient bones to infer ancient hominid behaviors such as slicing, chopping, and percussive actions during butchery of mammal carcasses. However, such claims about Plio–Pleistocene hominids rely mostly on very small assemblages of bony remains. Furthermore, recent experiments on trampling animals and biting crocodiles have shown each to be capable of producing mimics of such marks. This equifinality—the creation of similar products by different processes—makes deciphering early archaeological bone assemblages difficult. Bone modifications among Ethiopian Plio–Pleistocene hominid and faunal remains at Asa Issie, Maka, Hadar, and Bouri were reassessed in light of these findings. The results show that crocodiles were important modifiers of these bone assemblages. The relative roles of hominids, mammalian carnivores, and crocodiles in the formation of Oldowan zooarchaeological assemblages will only be accurately revealed by better bounding equifinality. Critical analysis within a consilience-based approach is identified as the pathway forward. More experimental studies and increased archaeological fieldwork aimed at generating adequate samples are now required.
Traces on bone surfaces play an important role in documenting humanity’s ever-broadening subsistence base. Prominent recent claims of stone tool use at ∼3.4 million years (Ma) (1, 2), occupation of Eurasia by 2.6 Ma (3), and hominids in California at ∼130 Ky (4) employ such evidence.
Experimental and naturalistic investigations in the 1980s led to a claimed dichotomy between U-shaped marks left on bones by “carnivore” teeth, and V-shaped traces (cutmarks) inflicted by stone tools (5, 6). Assertions that internal striae and shoulder marks were uniquely associated with cutmarks (7) were augmented by similar claims for percussion pits and associated striae (8, 9). Today these features are treated as “signature criteria” (10) and widely thought to accurately diagnose stone tool butchery.
Oldowan bone and lithic assemblages from Olduvai (∼1.8 Ma) and other excavations have been intensively analyzed for decades and used in considerations of human evolution. Indeed, fundamentally different inferences about early hominid carcass procurement and butchery (e.g., hunting vs. scavenging) have been derived from such analyses of the very same assemblages (11, 12). Zooarchaeologists have largely followed the orthodoxy that stone tool modifications to bone surfaces are accurately diagnosable from mammalian carnivore damage, and routinely use carnivore to mean mammalian carnivore.
However, a few zooarchaeologists persistently doubted the proclaimed tool/carnivore dichotomy and cautioned against overreliance on microscopic techniques (13–15). Lyman consistently and prophetically cautioned that equifinality could cripple inferences about ancient behavior (16). Early experiments even revealed that animal trampling could mimic cutmarks (17). But with the tool/carnivore dichotomy meme embedded, paleoanthropological attention increasingly focused on subsistence.
It is now recognized that “linear marks” and other bone surface modifications can result from interacting agents that range from people to plants (18). On the basis of the mark(s) alone, it is often difficult to distinguish among the modifying objects (the tooth surface, stone tool edge, or sedimentary particle) and their effectors (the bone chewer, trampling animal, or hominid butcher). Decades of actualistic research have now demonstrated that equifinality cannot be reduced to insignificance with a few more technological advances or experimental studies (19). Indeed, rather than reducing equifinality, these studies have persistently shown the pervasiveness of equifinality.
This roadblock to knowledge is also a significant barrier to assessing claims about “archaeology” among living apes and monkeys (20). As the hominid fossil record pushed into the Late Miocene, living chimpanzees continued to be idolized as behavioral proxies for early hominids (21, 22). The discovery that chimpanzees break open nuts with wooden and stone hammers raised expectations that a technology even more rudimentary than the Oldowan would be found in >3.0-Ma deposits (23), inspiring an ongoing quest for mid-Pliocene artifacts and/or their bony traces.
Two recent Nature cover articles proclaimed just such evidence. Dubbed “The First Cut,” one cover featured a photo of linear marks on the surface of a small bone shaft fragment eroded from Pliocene Ethiopian sediments at Dikika (1). Controversy immediately arose because the marks were interpreted by the discovery team as “unambiguous stone-tool cut marks for flesh removal and percussion marks for marrow access.” (p. 857). Critics interpreted the traces as trampling damage (24, 25) rather than butchery marks. The second cover, “The Dawn of Technology,” shows two allegedly shaped stones from Kenya dating to ∼3.4 Ma (2). Were stone tools made and used by hominids to butcher large mammals much earlier than previously thought?
Crocodiles: Equifinality Expanded
In his seminal 1981 book on the meaning of Paleolithic assemblages, Binford (13) recognized four types of “tooth marking.” He noted some equifinality regarding carnivore tooth vs. stone tool marks on bones. He did not mention crocodiles as bone modifiers. Even the most comprehensive current atlas of bone modification agents (18) emphasizes the effects of crocodile digestion more than the marks that crocodile teeth leave on bones of their prey.
Paleontologists working on Plio–Pleistocene African bone assemblages have long recognized crocodile presence, and indeed, diversity (26). However, the taphonomic impact of crocodiles was only recently recognized by zooarchaeologists, first with Njau (27) and Njau and Blumenschine (28)’s publications of experimental results from captive crocodile feeding. Their actualistic work followed the earlier traditions described above, focusing on establishing signature criteria by which crocodile activity could be uniquely identified, rather than emphasizing how their bite traces can mimic marks classically and exclusively attributed to defleshing, disarticulation, and percussion with stone tools.
Njau’s findings complement data from fields spanning alligator taphonomy (29), forensics (30), and Mesozoic paleontology (31), further raising this specter of equifinality. The revelation that traces left by crocodile teeth can match those previously thought to be diagnostic of stone tool butchery is a significant expansion of equifinality that threatens the binary orthodoxy employed by African zooarchaeologists to sort ancient mammalian carnivore traces from marks made by technological hominids.
Our observations on experimentally modified modern bones and fossils (below and SI Appendix, SI Text, Figs. S1–S12, and Table S1) confirm how pervasive this equifinality can be. We apply our findings to three time-successive occurrences of modified bones from the Plio–Pleistocene paleontological record of the Middle Awash study area of Ethiopia. Our team’s sustained research efforts there are summarized in refs. 32–34. We present below, in chronological order, modified mammalian bones from sediments dated to ∼4.2 Ma, ∼3.4 Ma, and ∼2.5 Ma. Our findings have broad implications for claims of hominid butchery of large mammals in waterside Plio–Pleistocene African settings.
This issue has so far been underappreciated by zooarchaeologists working on small assemblages from Plio–Pleistocene sediments deposited in proximity to crocodile-infested swamps, rivers, and lakes. We call for a more comprehensive, critical, assemblage-level zooarchaeological reassessment of the relatively small samples of modified fossil bones from Plio–Pleistocene African waterside localities.
Crocodile Modification of Middle Awash Fossils
Middle Awash Asa Issie locality 2 dates to 4.2 Ma (33). The remains of ∼50 Australopithecus anamensis specimens are among ∼650 generically identifiable vertebrate fossils collected. Specimen ASI-VP-2/420 is a distal hominid humeral shaft whose fracture morphology and adherent matrix indicate perimortem breakage (Fig. 1 and SI Appendix, Fig. S3).
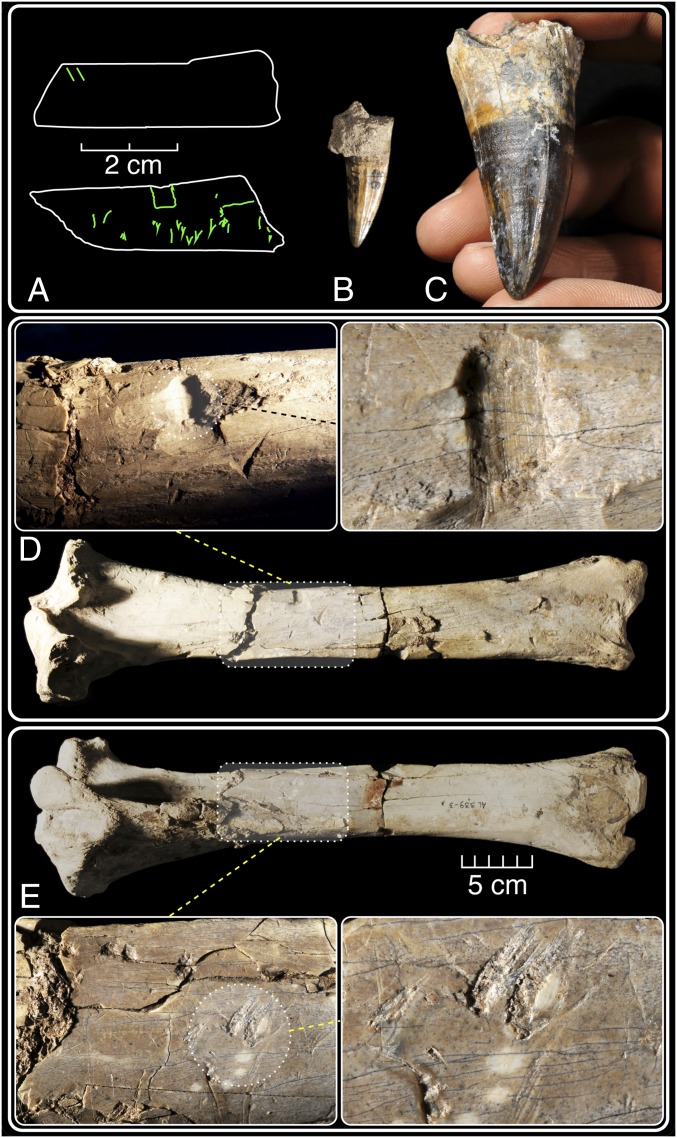
Fig. 1.
Bone surface modification marks on Pliocene hominid humeri from the Middle Awash study area, Ethiopia, visualized by photographs, SEM, and confocal microscopy. (A and B) The ∼4.2-Ma-old ASI-VP-2/420 distal humerus bears a jagged matrix-filled pit, shallow U-shaped grooves, and a long, straight score with snag pits at both ends. Straight, deep, V-shaped, intersecting linear marks with internal striae are illustrated. (C–I) The anterior surface of Maka humerus MAK-VP-1/3 exhibits linear bone modifications and bisected pits near its distal epiphysis. Note the presence of multiple internal striae and the deep, V-shaped profiles of most linear marks. Formerly thought diagnostic of cutmarks made by stone tools, such linear features are now known from crocodilian feeding experiments. Note that an interpretation of any one of these marks in isolation would lead to the impasse of equifinality described in the text. The anatomical, associational, geochronological, stratigraphic, and sedimentological contexts of these hominid fossils are interpreted as constituting a preponderance of evidence that the modifications are best attributed to crocodiles. F and G are casts. See text and SI Appendix, Figs. S4 and S5 for further details.
A deep, partially matrix-filled, steep-sided, bifurcate, V-shaped, pivoted pseudocut with internal striations (see ref. 35 for terminology) marks the distolateral diaphysis. More superficial, U-shaped marks lie a few millimeters proximally. A 20-mm-long, V-shaped groove with terminal snag pits at both ends lies 3 mm distal to the bifurcate mark. A large, matrix-filled, jagged-edged pit is nearby. Taken in isolation, several of these modifications could be interpreted as evidence of stone tool percussion and slicing. However, given the context of this fossil and the characteristics and distribution of ancient surface modifications on even this small fragment, the preponderance of evidence leads us to conclude that these modifications are likely crocodile induced, rather than made by stone tools (which are unknown at any occurrence of this antiquity).
The Pliocene Maka MAK-VP-1 locality is spatially larger and paleontologically richer than Asa Issie (34). A total of 753 generically identifiable surface and in situ vertebrate specimens is currently available from the Maka Sand Unit, a stratum also containing the embedded SHT tuff at ∼3.4 Ma. The assemblage from these fluviatile sands includes 27 Australopithecus afarensis specimens as well as crocodile remains and many other fossils exhibiting evidence of being bitten in the form of diamond-shaped (bisected) pits, hook scores, and pivoted drag-snags (ref. 35 and SI Appendix, Figs. S4 and S5; such modifications are also present on bones from penecontemporaneous paleontological localities in the nearby Hadar study area).
MAK-VP-1/3 is a left hominid humerus recovered in 1990 (ref. 34 and Fig. 1 and SI Appendix, Fig. S4). Surface preservation is imperfect, but ancient modifications include several deep linear marks with V-shaped cross-sections and multiple internal striae. Scanning electron microscope (SEM) images were immediately acquired and assessed by zooarchaeologists expert in mark identification. These experts diagnosed the Maka marks as having been made by stone tools. However, mark distribution did not correspond to an anatomically expected pattern of tissue removal during butchery. Other fossils with obvious crocodile modifications were also collected from this stratum (SI Appendix, Fig. S5), and no stone artifacts have ever been found in this or adjacent strata. Given this discordance, we withheld judgment for two decades, concerned that equifinality might prevent identification of the modifying agent. Njau’s initial results (27) and our subsequent analyses have now combined with contextual data to allow attribution of the surface modifications on the Maka hominid humeral shaft to perimortem crocodile biting. Fig. 2 illustrates our inferences regarding analogous crocodile modification on penecontemporaneous vertebrate fossils from Dikika (1) and Hadar.
Fig. 2.
Fossilized crocodile teeth and inferred crocodile biting damage to a fossil equid tibia from the Hadar A.L. 339 Pliocene locality illustrate the potential and probable effects of ancient crocodile feeding on a large mammal carcass. (A) Line drawings of two small bone shaft fragments from Dikika inferred to be evidence of the earliest butchery with stone tools (1). (B and C) Unworn large and small fossil crocodile teeth from Hadar that exhibit anatomy capable of modifying bone surfaces during biting. Even individual teeth can feature dozens of raised and serrated enamel edges that can each produce cutmark and percussion mimics upon contact with bone surfaces. Crocodile biting can therefore leave a variety of marks on bones of prey, depending on the age, size, and taxon of the individual crocodile (SI Appendix, Fig. S2). (D and E) A fossilized equid tibia from Hadar with extensive surface modifications. Some marks on this specimen are indistinguishable from traces that have been produced experimentally with stone tools used for slicing and percussion. Note the presence of striae fields, pits with internal striae, extensive ectocortical flake scars, and V-shaped, internally striated linear grooves. Numerous bisected and rounded pits and punctures, drag-snags, and hook marks are more diagnostic of crocodile biting. In the context of the damage pattern observable across the intact limb bone, the most likely bone modification agent was crocodile. Absent the context of the intact specimen with its diverse modifications, smaller bone shaft fragments such as the Dikika ones remain subject to equifinality.
The third Middle Awash case reassessed for the possibility of crocodile modification involves faunal elements from the ∼2.5 Ma Hatayae Member of the Bouri Formation (36). This small fossil collection was made within 500 m of remains attributed to Australopithecus garhi (37). Oldowan cores and flakes were found on the surface of nearby eroding sediments, but excavations failed to recover in situ stone tools. Abundant and in situ stone tools of even greater antiquity are present at Gona, <100 km to the north, associated with cutmarked bones (38).
A reassembled bovid tibial midshaft from the surface of Bouri locality 11 was first interpreted and illustrated as exhibiting stone tool percussion and chopping damage (Fig. 3 and SI Appendix, Figs. S6 and S7). An in situ equid femur was described as exhibiting “… stone-tool cut marks indicative of dismemberment and filleting.” (ref. 36, p. 627). Seven years before the perspective of Njau’s experimental work with crocodiles became available (27), these modified fossil bones were interpreted within the then-dichotomous zooarchaeological paradigm of mammalian carnivore chewing vs. butchery with stone tools. Our inferences were widely endorsed (24, 39), but here we reassess these specimens in light of the experimental work now available on crocodile bone modification (context and specimens detailed in Fig. 3 and SI Appendix, Figs. S6–S11).
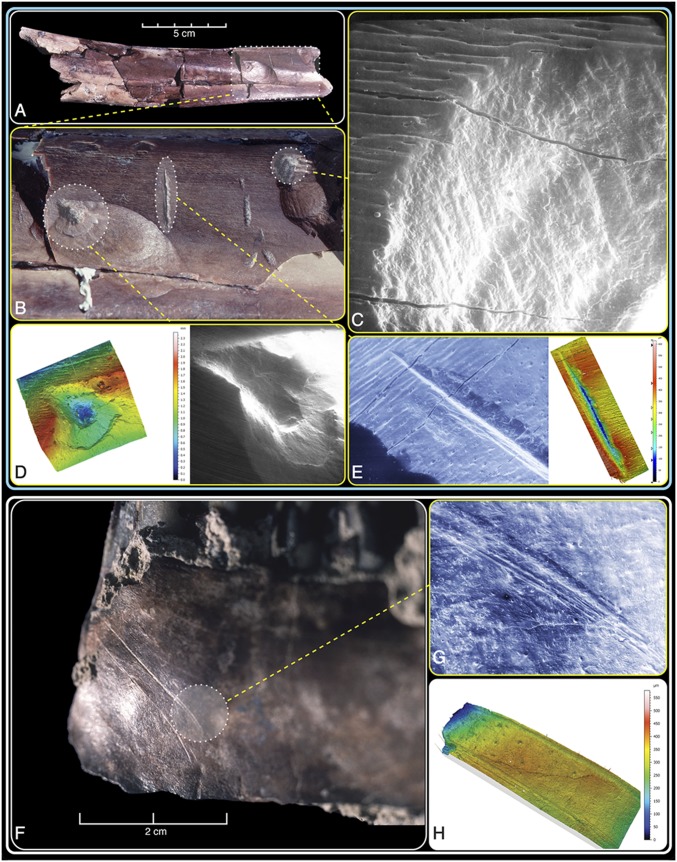
Fig. 3.
Bone surface modifications on ∼2.5-Ma-old fossilized ungulate bones from Bouri, visualized by photographs, SEM, and confocal microscopy. (A–E) The BOU-VP-11/14 bovid tibia showing numerous marks. (F–H) The BOU-VP-11/12 alcelaphine mandible exhibits long curvilinear marks across its posteromedial surface. Before experimental work on crocodile bite marks (refs. 27, 28, and 35 and SI Appendix, Figs. S1 and S2), such deep, V-shaped, internally striated marks and forceful production of jagged pits with internal striae and large external cortical flaking were considered diagnostic of stone tool use by early hominids and differentiated from marks made by mammalian carnivores. However, even the limited currently available experimental data on crocodilians indicate that assessment of individual marks on small fragments drawn from inadequate samples of fossil bones (1) can be problematic. See text for a detailed consideration of the equifinality plaguing behavioral interpretation of such specimens and SI Appendix, Figs. S7–S13 for a broader consideration of spatial, stratigraphic, and taphonomic contexts.
The unbroken but heavily modified Bouri fossilized equid femur BOU-VP-11/15 recovered from fine, water-lain sand shows marks similar to those made by crocodiles in actualistic and fossil contexts (27, 40). These marks include drag-snags (with and without striations), pseudocuts (with and without snags), and bisected perforations into thin cortex near the epiphyses. Slice marks with internal striae are deeply V shaped. Given its near-shore context, the overall patterning of marks, and the absence of in situ artifacts, we judge that crocodile biting created many—perhaps even all—of the marks on this equid femur.
The associated ungulate tibial midshaft specimen BOU-VP-11/14 is more difficult to interpret (Fig. 3 and SI Appendix, Fig. S8). We now appreciate that internally striated, irregular pits associated with deep negative flake scars on outer midshaft surfaces can result from crocodile biting and that these cannot always be distinguished from hammerstone percussion. Associated V-shaped linear pseudocutmarks made by crocodile teeth also frequently bear internal striae (27, 35). The BOU-VP-11/14 modified tibial shaft shows a plethora of damage types. More than 40 separate modifications of this 18-cm-long midshaft include curvilinear and straight pseudocuts as well as U-shaped grooves and V-shaped linear striations. Irregular pits with internal striations are present. Bisected pits expected of crocodile tooth perforation are absent, but thin cortex and underlying cancellous bone best suited to capture these are unavailable on this nonepiphyseal midshaft.
Many of the marks on the Bouri tibia fall squarely within the widened zone of equifinality between crocodile biting and stone tool modification. Differential diagnosis is therefore problematic, both at the level of the individual mark and across the entire element. Even if the dubious proposition that individual marks can each be unambiguously attributed to one agent or another, the relative timing of their formation would remain in doubt without mark overlap. Hence, we cannot attribute the marks on this BOU ungulate tibial shaft with certainty to either hominids, crocodiles, or to a potential contribution of both agents acting in sequence.
The BOU-A21 occurrence within BOU-VP-12 is located ∼250 m to the NW (SI Appendix, Fig. S9). It presents similar interpretive challenges. An excavated medium-sized alcelaphine bovid mandible (BOU-VP-12/11; Fig. 3 and SI Appendix, Fig. S10) found in situ with hominid remains was noted to bear “… three successive, curvilinear striae on its posteromedial surface; these striae are unambiguous cut marks made by a sharp stone flake, presumably during tongue removal…” (ref. 36, p. 627). Reassessment of this specimen must now also consider both crocodile biting and trampling as possible additional modifiers.
There is polishing accompanied by superficial random striae adjacent to the long, linear marks on this Bouri fossil mandible. Such modifications are indicative of abrasion before burial, and some of the other excavated specimens also show such superficial damage. For the mandible, mark superimposition indicates at least some surface abrasion, most likely from trampling. The induced random striae contrast strongly with the set of much longer, subparallel, curvilinear marks that contain multiple (up to eight) straight and continuous internal striae (Fig. 2 and SI Appendix, Fig. S10). These marks are ancient (still matrix obscured in places), shallow, wide, and U shaped in profile, but physical and hydraulic abrasion can lower the relief and change the cross-sections of stone tool cutmarks (41). Despite the lowered overall relief from abrasion, obvious parallel shoulder marks accompany the two longest marks.
The overall length and pattern of the curvilinear marks on the BOU-VP-12/11 bovid mandible’s posteromedial surface has not yet been matched in either trampling or crocodile studies. The anatomical placement of the marks is consistent with tongue removal. Other specimens from the surface adjacent to this excavation in silt-grained sediments also bear marks (SI Appendix, Fig. S11). Those eroded from nearby outcrops (SI Appendix, Fig. S12) provide additional evidence of butchery in the form of more typical stone tool cutmarks. Although these Hatayae Member specimens exhibit marks apparently diagnostic of stone tool butchery, associated in situ artifacts (the sine qua non of hominid presence) are still lacking (SI Appendix, Fig. S13). Accurate assessment of the relative roles of trampling, crocodile biting, and butchery by hominids in the Hatayae Member will obviously require larger fossil samples.
These Middle Awash examples demonstrate that the limitations of equifinality and small sample size must be recognized when assessing claims regarding early hominid butchery and subsistence. Claims of stone tool use at Dikika (1) were based on marks on two small midshaft fragments from a surface collection (Fig. 2). They have been questioned by authors who also note that: “… Gona and Bouri stand as the earliest, best evidence of the tool-assisted reduction of large animal carcasses by hominins …” (ref. 24, p. 20933). We concur with that conclusion, but also agree with those whom they criticize (25). Among the latter, James and Thompson aptly note that a mere 14 specimens predating 2.0 Ma have even been claimed to have unequivocal stone tool cut and percussion marks (42), and as shown above, equifinality makes it impossible to eliminate crocodiles as the agent responsible for some of these marks.
Beyond Equifinality.
Njau’s results (27) and ours indicate that stone tool cutmarks can be mimicked by crocodile biting as well as by trampling. Our work with fossils confirms that initial studies have not yet adequately explored the range of damage—the universe of equifinality—potentially created by crocodile biting. The equifinality already appears to extend beyond cutmarks to encompass even the irregular pits containing internal and external striae fields previously thought to be associated exclusively with hammerstone percussion. We predict an even greater expansion of equifinality when more crocodile experimentation with larger, hungrier animals and subsequent blind testing are conducted (43).
Meanwhile, it seems appropriate to abandon the quest to completely eliminate equifinality in many zooarchaeological contexts—particularly the ancient tropical and subtropical waterside locations in which crocodiles were potential bone modifiers. Simply dismissing equifinality by boldly asserting “high confidence” in mark diagnosis (1, 44) is a perilous pathway given the complex sedimentary and ecological envelopes containing the evidence of early hominids and their behaviors.
Logistically, the smaller the bone fragments themselves—and the smaller and more selective the fossil assemblage they comprise—the greater the risk that equifinality will lead to misinterpretation. The inferential potential of paleontological bone surfaces compared with their modern, relatively unaffected actualistic counterparts is often further compromised by processes such as pre- and postfossilization surface alterations (including bioturbation); hydraulic and aeolian erosion; matrix adhesion (often with attendant bone spalling); and damage through matrix removal, molding, photography, and study.
How should these lessons be applied more broadly across the earliest archaeological sites claimed to document large mammal butchery with stone tools? Most occurrences are from water-lain sediments and none of them have adequate zooarchaeological samples (42). Even larger Oldowan assemblages from well-known and intensively analyzed younger occurrences such as Olduvai FLK 22 (“Zinj”) are plagued by similar potential ambiguities. Each of these now requires more holistic reconsideration at several analytical levels, in part because published “cutmark” data from this universe of sites were compiled under the now dubious proposition that marks could be attributed in dialectic fashion either to hominid activity or to mammalian carnivores.
Methodologically, the zooarchaeological attraction to reductionist quantitative emphasis on individual marks persists. For example, a recent review of bone modification studies remains focused on the individual mark, proposing “standardization” as the key to progress and lauding a typological approach to mark identification based on “archetype” marks. The review mentions crocodiles only by way of citation (45). Another recent study concludes that three modern surface topography visualization methods yield equivalent results, but again fails to address equifinality by ignoring crocodiles (46). The quest for technological “solutions” to removing equifinality also obviously fails if actualistic and prehistoric sample sizes are inadequate. It is already evident that focus on the individual mark will always be a pathway to the long-recognized but often ignored roadblock of equifinality. There is probably not a technological fix to this problem.
The quest to eliminate equifinality through bottom-up approaches built on reductionist character quantification of individual mark attributes too often fails when published claims of diagnostic criteria (47) are falsified by new actualistic studies. Even top-down, assemblage-level assessments still rely on investigator decisions too often based on inadequate compensation for equifinality in small samples.
Each bone assemblage is different from the next, so searching for a formulaic analytical “menu” for universal application seems pointless. Even a linear investigative process in the assessment—stepping from marks, to specimens, to assemblage—is not the best way of building the broad knowledge that we seek. Rather, assessment and inference based on any fossil bone assemblage is best accomplished through a series of feedback loops. Rather than relying on inadequately established signature criteria, an iterative hierarchical assessment of the individual mark, the preserved bone, the assemblage, and the broader sedimentological/associational/distributional contexts and patterns will be the key to moving beyond equifinality.
Binford (13) recognized this and advocated an approach often termed “configurational” (although this term has deeper roots and different meanings in evolutionary biology) (48). Domínguez-Rodrigo et al. (24) described the configurational approach as “holistic assessment” while ascribing claimed butchery marks on two tiny Pliocene bone fragments to trampling damage. But Binford’s approach was not just holistic in terms of assessment of a bone within its provenance and assemblage contexts (see ref. 49 for expositions of these). Neither was it linear. It recognized “… the potential feedback of applying a methodology and then investigating the archaeological record in new ways…” (ref. 13, p. 282). As Lyman prophetically appreciated, if we fail to build “… multivariate interpretive models … at large inclusive scales, we may have a great deal of difficulty figuring out the behavioral meaning of cut-marked bones of Plio–Pleistocene age…” (ref. 50, p. 1,731). As in historical sciences from forensics to astrophysics, it is axiomatic that the more multistranded, adequately verified, and independent the evidence, the more solid the built inferences will be.
Equifinality will not be entirely eliminated at the level of the individual mark on a bone. We therefore propose, at least for occurrences in which crocodiles are potential agents of bone modification, an approach that is configurational, broadly contextual (49), and comprehensive (51). Our goal should be what E. O. Wilson has championed as “consilience” for each occurrence (52). In this regard, “context” thought of in the singular is too simplistic and misleading to express the biophysical milieu in which individual marks formed. A formal evaluation undertaken via an iterative feedback process will usually be necessary in circumstances where equifinality is a potential problem. For any assemblage, such an iterative, cyclical, feedback approach considers each bone modification in the context of the bone fragment, element, assemblage, geology, and ecology it occupies. This approach will more accurately determine whether and how hominids might have participated in the formation of any prehistoric assemblage.
For tropical and subtropical waterside bone assemblages, the consilience approach situates the question of butcher, cannibal, or crocodile into a deeper and more comprehensive evidentiary frame. In the legal forum, this is how forensic scientists help prosecutors make their cases, whether the requirement is a preponderance of evidence or establishment beyond doubt.
Finally, even assemblage-level efforts to establish consilience as outlined above will not allow inferential robusticity without adequate samples of fossil and contextual evidence. What can currently be inferred soundly about hominid butchery before 2.0 Ma? With the primary evidence still inadequate (42) narrowly mark-focused approaches (53, 54), including recently proposed “non-expert machine learning-based methods” (ref. 54, p. 79) promise little illumination. Indeed, larger questions about procurement, processing frequency across space and through time, and myriad other important behavioral aspects remain in the realm of speculation (55). Even for the iconic Pleistocene FLK-22 (Zinj) occurrence excavated 57 y ago, Domínguez-Rodrigo et al. ask: “Is there any other Plio–Pleistocene site where the faunal assemblage could be identified as completely (or mostly) accumulated and modified by hominins?” (ref. 56, p. 260).
Nearly 40 y after serious taphonomic work began on Oldowan-associated African Plio–Pleistocene bone assemblages, the biggest zooarchaeological challenge remains the notoriously small sizes of Plio–Pleistocene occurrences with sufficiently high “integrity” and “resolution” (13). This barrier to knowledge production is owed to several factors, ranging from the limited availability of appropriate deposits to the inadequacy of field exploration and from the increasing financial and career costs of sustained field research (including excavation) to an academic reward system increasingly linked to clickbait entertainment. Worse, paleoanthropological funding is lavished on digital laboratory technologies and quantitative methods even as vital actualistic studies remain unconducted.
Only by redoubling archaeological fieldwork to generate adequate samples, by conducting parallel experimental studies to fully bound equifinality, and by critical analysis aimed at consilience can zooarchaeological studies generate more reliable light on ancient events to allow a fuller assessment of the dynamics of interacting hominids, crocodiles, and other bone modifiers whose combined actions produce the zooarchaeological record of hominid evolution.
Supplementary Material
Acknowledgments
We thank the Afar people and numerous other field and laboratory workers for their contributions; W. Kimbel and K. Reed for access to fossils from Hadar; D. DeGusta, G. Richards, and B. Plowman for all SEM images; M. Brasil for NextEngine laser scanning; J. Carlson for figure preparation; several students for assistance during the butchery experiment; and A. Blanco-Lapaz for maceration of bones. T.D.W. thanks Z. Alemseged for showing him the two original Dikika surface-modified fossils, and T. Pickering for discussion over same. Thanks to the Human Evolution Research Center's donors, particularly for Glynn Isaac postdoctoral fellowship support for Y.S., who also acknowledges support from the German Research Foundation (DFG FOR 2237). This work benefitted from discussions and support from W. H. Gilbert, J. Carlson, B. Asfaw, J. Njau, Y. Beyene, and G. WoldeGabriel. We thank the Authority for Research and Conservation of the Cultural Heritage, the Cultural Heritage Collection and Laboratory Service Directorate at the National Museum of Ethiopia (at which all antiquities used in this study are permanently curated), and the Afar Regional Bureau for permission and facilitation. Sensofar confocal profilometry microscopy was acquired via award from the Ministerium für Wissenschaft, Forschung und Kunst Baden-Württemberg to K. Harvati.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 13066.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716317114/-/DCSupplemental.
References
- 1.McPherron SP, et al. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature. 2010;466:857–860. doi: 10.1038/nature09248. [DOI] [PubMed] [Google Scholar]
- 2.Harmand S, et al. 3.3-million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature. 2015;521:310–315. doi: 10.1038/nature14464. [DOI] [PubMed] [Google Scholar]
- 3.Malassé AD, et al. Intentional cut marks on bovid from the Quranwala zone, 2.6 Ma, Siwalik Frontal Range, northwestern India. C R Palevol. 2016;15:317–339. [Google Scholar]
- 4.Holen SR, et al. A 130,000-year-old archaeological site in southern California, USA. Nature. 2017;544:479–483. doi: 10.1038/nature22065. [DOI] [PubMed] [Google Scholar]
- 5.Shipman P. Applications of scanning electron microscopy to taphonomic problems. Ann N Y Acad Sci. 1981;376:357–385. doi: 10.1111/j.1749-6632.1981.tb28179.x. [DOI] [PubMed] [Google Scholar]
- 6.Bunn HT. Archaeological evidence for meat-eating by Plio-Pleistocene hominids from Koobi Fora and Olduvai Gorge. Nature. 1981;291:574–577. [Google Scholar]
- 7.Potts R, Shipman P. Cutmarks made by stone tools on bones from Olduvai Gorge, Tanzania. Nature. 1981;291:577–580. [Google Scholar]
- 8.Turner CG. Taphonomic reconstruction of human violence and cannibalism based on mass burials in the American Southwest. In: LeMoine GM, MacEachern AS, editors. Carnivores, Human Scavengers and Predators: A Question of Bone Technology. Univ of Calgary Archaeol Ass; Calgary, Canada: 1983. pp. 219–240. [Google Scholar]
- 9.Blumenschine RJ, Selvaggio MM. Percussion marks on bone surfaces as a new diagnostic of hominid behaviour. Nature. 1988;333:763–765. [Google Scholar]
- 10.Lyman RL. Archaeofaunas and butchery studies: A taphonomic perspective. Adv Archaeol Method Theory. 1987;10:249–337. [Google Scholar]
- 11.Blumenschine RJ, Prassack KA, Kreger CD, Pante MC. Carnivore tooth-marks, microbial bioerosion, and the invalidation of Domínguez-Rodrigo and Barba’s (2006) test of Oldowan hominin scavenging behavior. J Hum Evol. 2007;53:420–426. doi: 10.1016/j.jhevol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Domínguez-Rodrigo M, Barba R. Five more arguments to invalidate the passive scavenging version of the carnivore-hominid-carnivore model: A reply to Blumenschine et al. (2007a) J Hum Evol. 2007;53:427–433. doi: 10.1016/j.jhevol.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Binford LR. Bones: Ancient Men and Modern Myths. Academic; New York: 1981. [Google Scholar]
- 14.Binford LR, Stone NM. Zhoukoudian: A closer look (comments and reply) Curr Anthropol. 1986;27:453–475. [Google Scholar]
- 15.Haynes G. Spiral fractures and cut mark-mimics in noncultural elephant bone assemblages. Curr Res Pleistocene. 1986;3:45–46. [Google Scholar]
- 16.Lyman RL. The concept of equifinality in taphonomy. J Taphon. 2004;2:15–26. [Google Scholar]
- 17.Behrensmeyer AK, Gordon KD, Yanagi GT. Trampling as a cause of bone surface damage and pseudo-cutmarks. Nature. 1986;319:768–771. [Google Scholar]
- 18.Fernández-Jalvo Y, Andrews P. Atlas of Taphonomic Identifications. Springer; Dordrecht, The Netherlands: 2016. [Google Scholar]
- 19.Gifford-Gonzalez D. Bones are not enough: Analogues, knowledge, and interpretive strategies in zooarchaeology. J Anthropol Archaeol. 1991;10:215–254. [Google Scholar]
- 20.McGrew WC. 2015. The cultured chimpanzee: Nonsense or breakthrough? Human Ethology Bulletin-Proceeding XXII ISHE Conference, pp 41–52.
- 21.Wrangham R, Pilbeam D. African apes as time machines. In: Galdikas B, Briggs E, Shapiro S, Goodall J, editors. All Apes Great and Small: Volume One: African Apes. Kluwer Academic; Plenum, NY: 2001. pp. 5–17. [Google Scholar]
- 22.Carvalho S, McGrew WC. The origins of the Oldowan: Why chimpanzees (Pan troglodytes) still are good models for technological evolution in Africa. In: Domínguez-Rodrigo M, editor. Stone Tools and Fossil Bones: Debates in the Archaeology of Human Origins. Cambridge Univ Press; Cambridge, UK: 2012. pp. 201–221. [Google Scholar]
- 23.Haslam M. On the tool use behavior of the bonobo-chimpanzee last common ancestor, and the origins of hominine stone tool use. Am J Primatol. 2014;76:910–918. doi: 10.1002/ajp.22284. [DOI] [PubMed] [Google Scholar]
- 24.Domínguez-Rodrigo M, Pickering TR, Bunn HT. Configurational approach to identifying the earliest hominin butchers. Proc Natl Acad Sci USA. 2010;107:20929–20934. doi: 10.1073/pnas.1013711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domínguez-Rodrigo M, Alcalá L. 3.3-million-year-old stone tools and butchery traces? More evidence needed. PaleoAnthropol. 2016;2016:46–53. [Google Scholar]
- 26.Tchernov E. Evolution of the Crocodiles in East and North Africa. Cahiers de Paléontologie, Éditions du Centre National de la Recherche Scientifique; Paris: 1986. [Google Scholar]
- 27.Njau JK. 2006. The relevance of crocodiles to Oldowan hominin paleoecology at Olduvai Gorge, Tanzania. PhD dissertation (Rutgers, The State Univ of New Jersey, New Brunswick, NJ), p 325.
- 28.Njau JK, Blumenschine RJ. A diagnosis of crocodile feeding traces on larger mammal bone, with fossil examples from the Plio-Pleistocene Olduvai Basin, Tanzania. J Hum Evol. 2006;50:142–162. doi: 10.1016/j.jhevol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Drumheller-Horton S, Brochu C. A diagnosis of Alligator mississippiensis bite marks with comparisons to existing crocodylian datasets. Ichnos. 2014;21:131–146. [Google Scholar]
- 30.Sinton TJ, Byard RW. Pathological features of fatal crocodile attacks in northern Australia, 2005–2014. J Forensic Sci. 2016;61:1553–1555. doi: 10.1111/1556-4029.13171. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen AR. Feeding behaviour of carnivorous dinosaurs as determined by tooth marks on dinosaur bones. Hist Biol. 1998;13:17–26. [Google Scholar]
- 32.Gilbert WH, Asfaw B, editors. Homo Erectus: Pleistocene Evidence from the Middle Awash, Ethiopia. Univ of California Press; Berkeley, CA: 2008. [Google Scholar]
- 33.White TD, et al. Asa Issie, Aramis and the origin of Australopithecus. Nature. 2006;440:883–889. doi: 10.1038/nature04629. [DOI] [PubMed] [Google Scholar]
- 34.White TD, et al. New discoveries of Australopithecus at Maka in Ethiopia. Nature. 1993;366:261–265. doi: 10.1038/366261a0. [DOI] [PubMed] [Google Scholar]
- 35.Njau JK, Gilbert HG. A taxonomy for crocodile-induced bone modifications and their relevance to paleoanthropology. FOROST Occas Publ. 2016;3:1–13. [Google Scholar]
- 36.de Heinzelin J, et al. Environment and behavior of 2.5-million-year-old Bouri hominids. Science. 1999;284:625–629. doi: 10.1126/science.284.5414.625. [DOI] [PubMed] [Google Scholar]
- 37.Asfaw B, et al. Australopithecus garhi: A new species of early hominid from Ethiopia. Science. 1999;284:629–635. doi: 10.1126/science.284.5414.629. [DOI] [PubMed] [Google Scholar]
- 38.Domínguez-Rodrigo M, Pickering TR, Semaw S, Rogers MJ. Cutmarked bones from Pliocene archaeological sites at Gona, Afar, Ethiopia: Implications for the function of the world’s oldest stone tools. J Hum Evol. 2005;48:109–121. doi: 10.1016/j.jhevol.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Lupo KD. On early hominin meat eating and carcass acquisition strategies: Still relevant after all these years? In: Domínguez-Rodrigo M, editor. Stone Tools and Fossil Bones: Debates in the Archaeology of Human Origins. Cambridge Univ Press; Cambridge, UK: 2012. pp. 115–151. [Google Scholar]
- 40.Baquedano E, Domínguez-Rodrigo M, Musiba C. An experimental study of large mammal bone modification by crocodiles and its bearing on the interpretation of crocodile predation at FLK Zinj and FLK NN3. J Archaeol Sci. 2012;39:1728–1737. [Google Scholar]
- 41.Yravedra J, et al. FLK west (Lower Bed II, Olduvai Gorge, Tanzania): A new early Acheulean site with evidence for human exploitation of fauna. Boreas. 2017;46:816–830. [Google Scholar]
- 42.James EC, Thompson JC. On bad terms: Problems and solutions within zooarchaeological bone surface modification studies. Environ Archaeol. 2015;20:89–103. [Google Scholar]
- 43.Njau J. Paleontology. Reading Pliocene bones. Science. 2012;336:46–47. doi: 10.1126/science.1216221. [DOI] [PubMed] [Google Scholar]
- 44.Thompson JC, et al. Taphonomy of fossils from the hominin-bearing deposits at Dikika, Ethiopia. J Hum Evol. 2015;86:112–135. doi: 10.1016/j.jhevol.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Egeland CP. The use of bone surface modifications to model hominid lifeways during the Oldowan. In: Domínguez-Rodrigo M, editor. Stone Tools and Fossil Bones: Debates in the Archaeology of Human Origins. Cambridge Univ Press; Cambridge, UK: 2012. pp. 80–114. [Google Scholar]
- 46.Maté-González MA, et al. Assessment of statistical agreement of three techniques for the study of cut marks: 3D digital microscope, laser scanning confocal microscopy and micro-photogrammetry. J Microsc. 2017;267:356–370. doi: 10.1111/jmi.12575. [DOI] [PubMed] [Google Scholar]
- 47.Domínguez-Rodrigo M, Barba R, Egeland CP. Deconstructing Olduvai: A Taphonomic Study of the Bed I Sites. Springer; New York: 2007. pp. 23–32. [Google Scholar]
- 48.Wolverton S, Lyman RL. Immanence and configuration in analogical reasoning. North Am Archaeol. 2000;21:233–247. [Google Scholar]
- 49.Lyman RL. A historical sketch on the concepts of archaeological association, context, and provenience. J Archaeol Method Theory. 2012;19:207–240. [Google Scholar]
- 50.Lyman RL. Analyzing cut marks: Lessons from artiodactyl remains in the northwestern United States. J Archaeol Sci. 2005;32:1722–1732. [Google Scholar]
- 51.White TD. Prehistoric Cannibalism at Mancos 5MTUMR-2346. Princeton Univ Press; Princeton: 1992. [Google Scholar]
- 52.Wilson EO. Consilience: The Unity of Knowledge. Knopf; New York: 1998. [Google Scholar]
- 53.Pickering TR, Bunn HT. Meat foraging by Pleistocene African hominins: Tracking behavioral evolution beyond baseline inferences of early access to carcasses. In: Domínguez-Rodrigo M, editor. Stone Tools and Fossil Bones: Debates in the Archaeology of Human Origins. Cambridge Univ Press; Cambridge, UK: 2012. pp. 152–173. [Google Scholar]
- 54.Harris JA, Marean CW, Ogle K, Thompson J. The trajectory of bone surface modification studies in paleoanthropology and a new Bayesian solution to the identification controversy. J Hum Evol. 2017;110:69–81. doi: 10.1016/j.jhevol.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Domínguez-Rodrigo M, et al. Use and abuse of cut mark analyses: The Rorschach effect. J Archaeol Sci. 2017;86:14–23. [Google Scholar]
- 56.Domínguez-Rodrigo M, et al. Unraveling hominin behavior at another anthropogenic site from Olduvai Gorge (Tanzania): new archaeological and taphonomic research at BK, Upper Bed II. J Archaeol Sci. 2009;57:260–283. doi: 10.1016/j.jhevol.2009.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.