Significance
RNA editing is an enzymatic modification that leads to single-nucleotide changes in mRNA. Editing is particularly robust within cells of the immune lineage. Here, we focus on the macrophage and demonstrate that genetic inactivation of the RNA-editing enzyme Apobec1 affects protein levels of genes that underlie macrophage-specific behaviors including phagocytosis and transendothelial migration. We further show that loss of Apobec1 leads to an overabundance of proinflammatory monocytes, a hallmark of many chronic diseases. These data provide the first view of the consequences of editing for gene expression and cellular function. Overall, epitranscriptomic changes catalyzed by RNA editing might be important biomarkers of diseases associated with inflammation (e.g., neurodegenerative diseases), for which an association with DNA mutation has been lacking.
Keywords: epitranscriptomics, RNA, editing, APOBEC1, monocytes
Abstract
Epitranscriptomics refers to posttranscriptional alterations on an mRNA sequence that are dynamic and reproducible, and affect gene expression in a similar way to epigenetic modifications. However, the functional relevance of those modifications for the transcript, the cell, and the organism remain poorly understood. Here, we focus on RNA editing and show that Apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-1 (APOBEC1), together with its cofactor RBM47, mediates robust editing in different tissues. The majority of editing events alter the sequence of the 3′UTR of targeted transcripts, and we focus on one cell type (monocytes) and on a small set of highly edited transcripts within it to show that editing alters gene expression by modulating translation (but not RNA stability or localization). We further show that specific cellular processes (phagocytosis and transendothelial migration) are enriched for transcripts that are targets of editing and that editing alters their function. Finally, we survey bone marrow progenitors and demonstrate that common monocyte progenitor cells express high levels of APOBEC1 and are susceptible to loss of the editing enzyme. Overall, APOBEC1-mediated transcriptome diversification is required for the fine-tuning of protein expression in monocytes, suggesting an epitranscriptomic mechanism for the proper maintenance of homeostasis in innate immune cells.
The transfer of genomic information from DNA to mRNA is modulated epigenetically by chemical modifications to DNA or to the histones that package it. In mammals, DNA modifications include 5 mC methylation, hmU methylation, and, most recently, m6dA methylation, all of which are predicted to affect transcriptional output (reviewed in refs. 1–3). Similarly, histone modifications impact transcription by facilitating interactions and generally turning genes on or off (often together with DNA modifications). Recently, it has become clear that mRNA can also be modified (4, 5). In particular, the dynamic nature of modifications in mRNAs (as well as in many noncoding RNAs) has given rise to the idea of a heretofore invisible “code” that resides in nucleic acids but is divergent from their genetically encoded sequence. Detection of mRNA modifications has relied on increasingly sensitive methods for profiling common epitranscriptomic marks, and these methods are revealing an increasingly large number of modifications, suggesting that the regulation of RNA fate, and therefore of gene expression, is encoded, at least in part, within the epitranscriptome.
The most prominent (and easily verifiable) of these alterations are the deamination of adenosine to inosine (decoded as guanosine) and the deamination of cytosine (C) to uracil (U), collectively termed “RNA editing.” These are respectively mediated by the adenosine deaminases that act on RNA (ADARs) and the cytidine deaminase, Apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-1 [APOBEC1; as well as its homolog APOBEC3A in humans (6)]. Studies with animals deficient in ADARs have suggested that editing plays a key role in the brain as well as in the immune system. In both contexts, editing has been implicated in disease progression and manifestations [e.g., depression, epilepsy, schizophrenia, amyotrophic lateral sclerosis (7) in the brain; Aicardi and other syndromes in immunity (8, 9)]. As well, the frequency of ADAR editing has been shown to change dramatically between different tissues [e.g., fetal and adult brains (10)]. However, a major complication of work with ADAR-deficient animals, in terms of understanding function, is that loss of the enzyme leads to retrotransposon mobilization and eventual cell death in all organisms studied (11–14). In contrast, APOBEC1-deficient animals (which are null for C-to-U editing) are viable (15, 16), and thus amenable to functional studies.
Until recently, APOBEC1 has been thought to have a defined set of targets [e.g., the transcript of Apolipoprotein B (ApoB)], and thus a limited biological role, with constrained expression to the small intestine and liver. However, work on APOBEC1, together with its newly discovered cofactor RBM47 (17), has led to the identification and validation of hundreds of instances of C-to-U editing in many cell types, with APOBEC1 being widely expressed in the immune system (18, 19) (SI Appendix, Fig. S1). It is now apparent that the editing activity of APOBEC1 extends well beyond its canonical target, ApoB. However, the functional relevance of these abundant epitranscriptomic alterations, at either the molecular or cellular level, has yet to be described.
Here, we surveyed the effects of editing at the transcript level (to determine if and how it might affect transcript fate) and at the cellular level (to determine whether editing correlates with altered cellular phenotypes). We focused on a small number of transcripts that are highly edited (for which we would expect a larger measurable effect), which we have characterized at multiple levels. We have also pinpointed the developmental stages within bone marrow that are susceptible to loss of the editing enzyme and shown that editing is important for the proper maintenance of monocyte subsets. These experiments aim to broadly describe the effects of editing from the molecule to the organism, and should serve as a useful starting point for future work that delves deeper into mechanistic questions that remain outstanding.
Results
APOBEC1 RNA Editing Is Differentially Regulated in Different Tissues.
APOBEC1 is expressed in a wide array of tissues, most notably in cells from the immune system. Previous studies of Apobec1 have demonstrated that the frequency of editing is related to its expression (20–23). We tested this conjecture using data from intestinal enterocytes and macrophages. We found that in macrophages, the median editing frequency is 10% (Fig. 1A), which is lower than what is reported in intestinal enterocytes [36% (24) and 25% (25)], even though the expression of APOBEC1 is at least an order of magnitude greater in macrophages vis-à-vis enterocytes (Fig. 1B). This could be related to differences in cofactors: Neither Apobec1’s cofactor, required for the specificity with which Apobec1 edits ApoB in enterocytes (20, 26), nor ApoB is present in macrophages (Fig. 1B). Only a small subset of transcripts expressed and edited in enterocytes (combined data from refs. 24, 25) are also expressed and edited by bone marrow-derived macrophages (BMDMs) (Fig. 1C). We then compared editing in macrophages with that of bone marrow-derived dendritic cells (DCs) (data from ref. 19): Only one-third of the edited transcripts in DCs are shared with macrophages (Fig. 1C). This differential specificity between tissues (even those of shared origin) might be the result of differential “coating” of individual transcripts by RNA binding proteins, whose complement varies between cells (27).
Fig. 1.
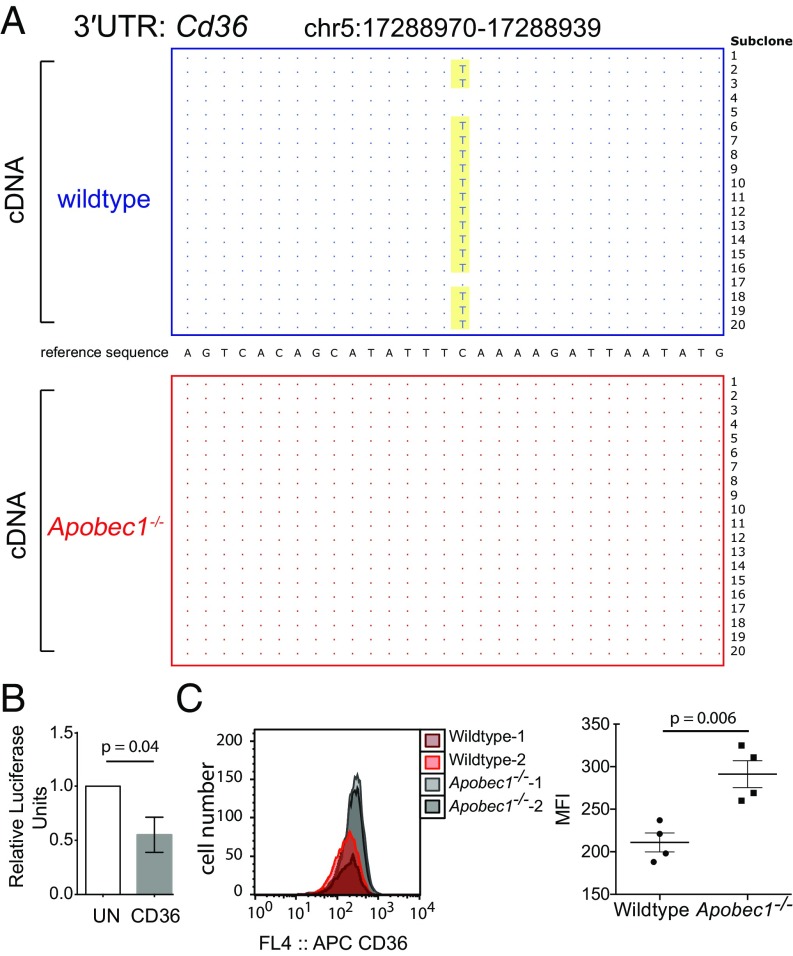
APOBEC1 editing differs even within related cell lineages. (A) Histogram of editing rates in BMDMs. (B) Semiquantitative PCR of APOBEC1, ApoB, Apobec1’s cofactor (A1cf), and GAPDH transcripts (Apobec1, Apob, A1cf, and Gapdh, respectively). (C) Venn diagram depicting the number of edited transcripts shared between macrophages (275 edited sites in 71 transcripts), DCs (16 edited sites in 15 transcripts), and enterocytes (61 edited sites in 59 transcripts).
RNA Editing in BMDMs Is Robust but Does Not Affect Transcript Stability or Localization.
To understand the role of editing for the targeted transcripts, we focused on BMDMs, which express high amounts of APOBEC1 and RBM47, a feature characteristic of most cells of the immune system (SI Appendix, Fig. S1). Within BMDMs, APOBEC1 expression results in robust RNA editing, which can be determined using a variety of bioinformatic approaches (e.g., ref. 19) and can be validated using Sanger sequencing of individual subclones from PCR products from wildtype and Apobec1−/− cells (Fig. 2A and SI Appendix, Fig. S2). As previously noted, the vast majority of APOBEC1 editing in BMDMs occurs within 3′UTRs, with editing frequencies ranging from 5 to 85%, a localization distribution common to both adenosine and cytosine deaminases (19, 28). Many of the transcripts edited in BMDMs are also edited in RNA-sequencing (RNA-seq) datasets from mouse intestinal enterocytes as well as in RNA-seq datasets derived from human small intestine (the only tissue where human APOBEC1 is robustly expressed at steady state), supporting the importance of coregulated editing across species (SI Appendix, Table S1).
Fig. 2.
APOBEC1 editing of a highly edited 3′UTR can modulate protein production. (A) Representative subclone sequencing of the 3′UTR of Cd36 genomic DNA (gDNA) and cDNA, derived from wildtype and Apobec1−/− BMDMs. (B) Relative luciferase levels of the Cd36 3′UTR with single editing events pre-encoded into DNA, in the absence of the editing enzyme (Cd36; n = 5). UN, unedited construct. (C) Mean fluorescence intensity (MFI) of CD36 surface protein levels in wildtype and Apobec1−/− BMDMs (n = 4). Error bars represent the SEM; statistical significance was obtained using a t test.
The preferential localization of edited sites to the 3′UTR, a key regulatory region of transcripts, suggests that editing may play a role in the modulation of gene expression. To evaluate the possibility that APOBEC1-mediated editing might affect mRNA stability, as suggested for ADAR-mediated editing in the past (5), we first looked at transcript abundance differences at the steady state, between wildtype and Apobec1−/− cells. We found that transcript levels of individual genes were highly correlated between the two genotypes, suggesting that APOBEC1 does not broadly affect mRNA levels (SI Appendix, Fig. S3A). Because RNA editing imparts sequence heterogeneity among cells of a population (19, 28), any downstream analysis of the effects of editing on the rate of decay of individual transcripts using transcriptome-wide methods [e.g., 5′-bromo-uridine (BrU) immunoprecipitation chase-deep sequencing analysis (BRIC-seq) (29)] is problematic, as it could average out potentially substantial differences. We therefore turned to “spot-checking” the rates of decay of transcripts edited with very high frequency within populations of wildtype BMDMs. Focusing on such transcripts allows for a meaningful comparison with their unedited counterparts in Apobec1−/− BMDMs. We then used standard methods (e.g., actinomycin D to block mRNA synthesis, followed by quantitative RT-PCR at several time points after actinomycin-D treatment) to assess decay rates. Interestingly, we found no differences in stability between the largely edited and completely unedited mRNAs (SI Appendix, Fig. S3B). In fact, edited transcripts tend to have very long half-lives [e.g., B2m (30)]. We conclude that APOBEC1-mediated RNA editing is unlikely to broadly affect RNA stability.
The 3′UTR editing by ADARs has also been shown to modulate nuclear retention (6), and we wanted to determine whether APOBEC1-mediated editing could alter transcript localization. While methods exist to demarcate transcript localization in vivo and at the level of single cells [e.g., fluorescent in situ sequencing (31)], these are highly technically demanding and, as a result, are not widely used. We therefore turned to more traditional, low-throughput methods; using biochemical fractionation approaches (32), we determined that editing events could be identified in chromatin-associated nascent transcripts (SI Appendix, Fig. S3D), confirming that APOBEC1 acts soon after transcription, as previously demonstrated (31). However, in all cases we examined, the nuclear and cytoplasmic levels of transcripts were unaffected by editing (SI Appendix, Fig. S3C) and editing frequencies were similar between the nuclear and cytoplasmic compartments (SI Appendix, Fig. S3D), suggesting that C-to-U editing does not broadly modulate subcellular localization.
RNA Editing Affects the Translational Output of BMDMs.
APOBEC1-dependent editing imparts sequence diversity within transcript 3′UTRs even within single cells (19), and yet this diversity does not seem to alter RNA stability or localization (at least in bulk). We therefore asked whether it changed the final outcome of gene expression, which is protein abundance. To assess whether editing of individual transcripts changed the translational output vis-à-vis that of their unedited counterparts, we generated dual-luciferase constructs containing 3′UTRs of interest, which were directly amplified from wildtype BMDM cDNA. We then transfected those into APOBEC1-deficient BMDMs, which contain the physiologically relevant milieu of RNA binding factors, and assessed the effect of editing within a 3′UTR on one of the luciferase cassettes (Renilla) standardized against expression of the second luciferase cassette (firefly).
We first tested a “pre-edited” construct containing the C-to-thymine (T) change we had isolated from the Cd36 transcript (Fig. 2A), and found that this single-nucleotide variant led to significant modulation of luciferase levels (Fig. 2B). Cd36 encodes a well-studied surface scavenger receptor, for which good antibodies exist. Using these antibodies, we were able to confirm that wildtype macrophages (in which Cd36 is 80% edited) displayed significantly lower levels of CD36 at the cell surface (as measured by mean fluorescence intensity) than APOBEC1-deficient counterparts (Fig. 2C). In addition to demonstrating that editing of the Cd36 3′UTR leads to changes in protein abundance [confirming the findings of Mehta and Driscoll (26)], these data show that it is the editing function of APOBEC1 (i.e., the point mutations introduced within mRNA, phenocopied here in the luciferase assays in the absence of the editing enzyme) and not some other editing-independent activity, as previously documented for ADAR1 (34).
To ensure that the APOBEC1-driven modulation of protein abundance is not limited to CD36, we then tested a small number of additional pre-edited constructs containing C-to-T changes isolated from the relevant transcripts isolated ex vivo from wild-type BMDM transcripts (Fig. 3A). Generally (although not always, as discussed below), an increase in the number of C-to-T changes led to a decrease in protein abundance, suggesting that APOBEC1-generated edits modulate the translational output of the transcripts we tested. To determine what could account for these differences, we considered several possibilities. The first one is that editing directly alters the interaction of a given transcript with the ribosome (loading, initiation, or translational efficiency). To directly assess this, standard “ribosomal profiling” experiments (e.g., Ribo-seq) have been used in the past (35). However, since ribosomes do not occupy 3′UTRs, these methods cannot be used to directly delineate how the single-nucleotide variants introduced by editing directly affect the process of translation in the edited subset in relation to the unedited subset within the same sample. Another possibility is that editing indirectly affects the interaction of a given transcript with the ribosome, for example, through “recoding” microRNA (miRNA) target sites within transcripts, a possibility raised in the past, based on the observation that APOBEC1-dependent 3′UTR edits were preferentially located in regions of substantial phylogenetic conservation (24) that had features of miRNA binding. To capture such sites together with miRNAs expressed in BMDMs, we performed high-throughput sequencing of mRNA and miRNA isolated by cross-linking and immunoprecipitation of Argonaute (Ago) proteins (36) in cells derived from wildtype and Apobec1−/− littermates (SI Appendix, Fig. S4). We focused on the subset of 3′UTRs where Ago occupancy overlapped with edited sites and assigned to these a set of likely miRNA targets, selected based on miRNA abundance (Fig. 3B) and recently defined rules for canonical as well as noncanonical binding (37, 38). We also accounted for sites potentially modified through editing, resulting in disruption or creation of miRNA/mRNA pairing (SI Appendix, Table S2). We then tested whether single-nucleotide changes through APOBEC1 editing at the miRNA target sites could disrupt miRNA regulation. We cloned edited and unedited 3′UTRs into dual-luciferase expression vectors and cotransfected them with their putative miRNA in HEK-293T cells (or an irrelevant control miRNA) in the absence of APOBEC1. Repression of miRNA was then determined by comparing the loss of luciferase in the presence of the miRNA between edited and unedited constructs. We identified a subset of 3′UTRs [e.g., Sptssa (also known as 1110002B05Rik), Rac1] where editing disrupts predicted miRNA–UTR interactions, resulting in derepression of luciferase levels in the edited construct compared with the unedited construct (Fig. 3C). Therefore, sequence changes consistent with APOBEC1-mediated editing can alter miRNA targeting and protein production. However, such changes will be hard to identify from global profiling data, because of cell-to-cell sequence heterogeneity (19). For example, the transcripts for which we have observed miRNA target site deletion show an ∼30% edit rate (suggesting potentially that 30% of the cells in the population edit). Thus, a miRNA-dependent twofold change in mRNA levels, when this twofold change is expected to impact only 30% of the mRNA transcripts in the population, would not lead to detectable changes, making their identification in a global screen problematic. In all, while APOBEC1-mediated editing can impact translation and protein output through miRNA-dependent mechanisms, editing can also affect protein output via mechanisms that do not directly implicate the recoding of a miRNA target site, including the loss or gain of binding of specific sets of RNA binding proteins (RBPs) that promote or disallow ribosomal loading (reviewed in refs. 38, 39).
Fig. 3.
APOBEC1-mediated editing modulates protein production by altering 3′UTR regulation. (A) Effect of editing in protein production, in the absence of the editing enzyme. The 3′UTRs of interest depicted at the top of each plot were cloned directly from cDNA derived from wildtype BMDMs. A schematic at the top of each graph depicts the range of edited 3′UTRs tested. All error bars represent the SEM; statistical significance was obtained using a t test. LUC, luciferase. (B) Putative miRNA targets in APOBEC1-edited regions that overlap with Ago footprints. “Edited” (with C-to-T mutations reflecting APOBEC1-dependent changes) and “Unedited” (reflecting the genomic reference) footprint sequences were scanned for miRNA target regions (match to position 2–7, 1–6, or 3–8 of mature miRNA sequence). The miRNA targets that would be created (green) or disrupted (red) by an APOBEC1-editing event are depicted. (C and D) APOBEC1-editing disruption of putative miRNA target regions in the Sptssa and Rac1 3′UTRs. UN, unedited construct with a sequence consistent with the reference genome. ED, edited construct, mutated to reflect the editing event in question. A schematic depicting miRNA-site deletion or creation by APOBEC1 editing is shown at the top of each graph. The miRNA repression was calculated using the ratio of relative luciferase values (Materials and Methods) between miRNA and unrelated miRNA for each edited and unedited pair. The star indicates values below 0 or no relative repression (n = 5).
APOBEC1-Mediated RNA Editing Alters Cellular Behavior by Targeting Transcripts That Belong to a Common Pathway.
Previous investigations of the functional consequences of RNA editing have focused on instances where transcripts are edited with very high frequency and with clear biological implications (e.g., refs. 40–43). However, the collective functional consequence of targeting a large number of transcripts has never been examined. Subsets of APOBEC1-edited transcripts in BMDMs encode proteins important for discernible macrophage functions like lysosome maturation/phagocytosis (e.g., Lamp1, Lamp2, Atp6ap1), as well as proteins important for cytokine signaling/migration (e.g., Rac1, Kras, Pak2, Brb2; SI Appendix, Table S3). Given that editing can affect protein abundance, we asked whether small alterations in the levels of such proteins could collectively alter cell physiology, by assessing the relative performance of wildtype and Apobec1−/− BMDMs in both phagocytosis and migration.
To test phagocytosis, we added pHrodo-labeled Staphylococcus aureus particles to BMDM cultures at two different concentrations, equivalent to two “multiplicities of infection.” The pHrodo-labeled S. aureus particles are taken up by the macrophages and become fluorescent within the acidic environment of the lysosome, enabling quantification by flow cytometry. Consistent with reports that increased abundance of LAMP1 leads to an increase in phagosome maturation (44), Apobec1−/− macrophages (which show decreased Lamp1 expression after editing; Fig. 3A) showed an increase in particle uptake, compared with their wildtype counterparts (Fig. 4A). To test migration, we employed a chemotaxis assay where BMDMs are incubated in a two-chamber cell culture dish that separates cells from the chemotactic agent (CXCL12, selected because its receptor CXCR4 was well expressed in wildtype and Apobec1−/− BMDMs). Over a range of chemokine concentrations, we observed a diminished ability of Apobec1−/− cells to migrate in comparison to their wildtype counterparts (Fig. 4B). This diminished capacity is not due to changes in receptor levels, which are comparable between genotypes (not shown), suggesting that the functional difference we observe is due to changes in downstream signaling pathways, which include a number of proteins encoded by transcripts that are targets of editing (e.g., RAC1, KRAS, PAK2, BRB2).
Fig. 4.
APOBEC1 is required for the proper phagocytosis and migration of BMDMs. (A) Phagocytosis of S. aureus pHrodo particles. (Left) Schematic of the phagocytosis setup: Phrodo-labeled particles are nonfluorescent in cell culture media; however, upon phagocytosis, they are transported to the lysosome inside the cell, whose acidic environment allows the particle to become fluorescent. (Right) Phagocytosis assay (n = 5). Error bars represent the SEM; statistical significance was obtained using a t test. MOI, multiplicity of infection. (B) Transendothelial migration assay. (Left) Schematic of the migration setup: Cells are plated in a two-chamber well (Top blue), which separates the cells from the chemokine (purple) via a porous membrane (green). Cells then transverse the membrane and can be quantified. (Right) Quantification of migration toward CXCL12. Error bars represent the SEM; statistical analysis was performed using the multiple measured one-way ANOVA, followed by a t test with Bonferroni’s correction (n = 3). *P < 0.05; **P < 0.01; ***P < 0.0001.
Loss of APOBEC1-Mediated RNA Editing Results in Subtle Fluctuations in the Number of Proinflammatory Monocytes.
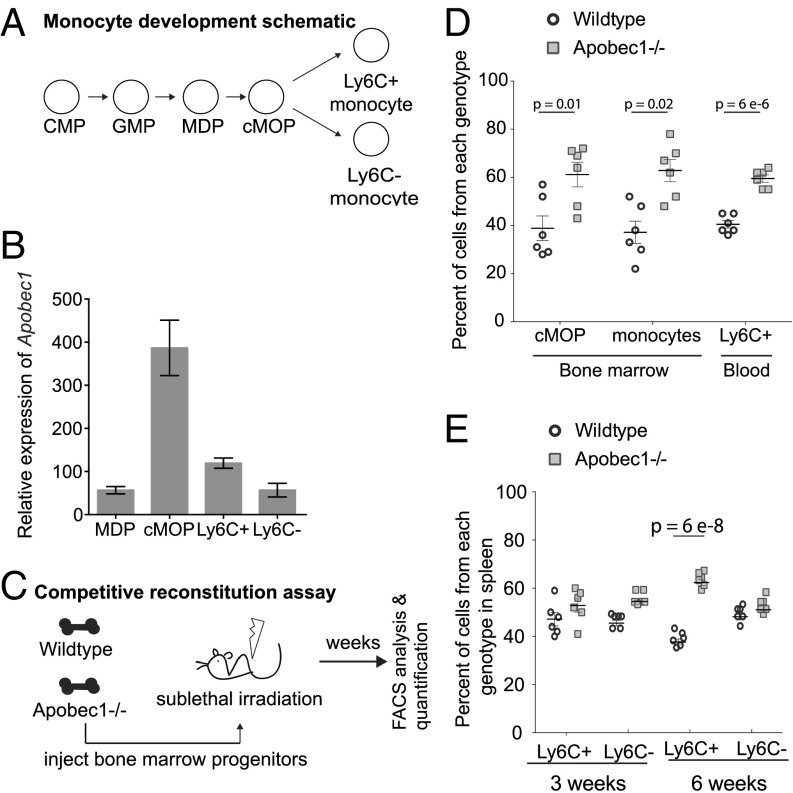
To address whether the in vitro differences we observe have in vivo consequences, we first surveyed the population of bone marrow-derived immune cell progenitors (Fig. 5A) that would be sensitive to loss of APOBEC1. We used fluorescence-activated cell sorting of specific subsets of cells followed by quantitative RT-PCR to estimate APOBEC1 expression levels. We found increased transcript levels for the editing enzyme within the common monocyte progenitor (cMOP) population (Fig. 5B). To then isolate APOBEC1-dependent effects on the monocyte lineage in the absence of other known monocyte-extrinsic APOBEC-1–related phenotypes (e.g., lipid metabolism), we turned to a competitive reconstitution experiment (Fig. 5C). We found that Apobec1−/− cMOP cells significantly outcompeted their wildtype counterparts at 6 wk after transplantation (Fig. 5D), suggesting that APOBEC1 mRNA editing has a role in generating this population. Looking further downstream in the developmental process, within the secondary lymphoid organs, we found a significant increase in numbers of M1-like (Ly6C+) monocytes of the Apobec1−/− genotype vis-à-vis their wildtype counterparts in the spleen at 6 wk (Fig. 5E), a trend that initiated at 3 wk (Fig. 5E). Meanwhile, the frequency of the other major type of monocytes, the M2-like (Ly6C−) cells, was not altered (Fig. 5E). The increase in Ly6C+ monocytes is not due to an increase in their proliferative capacity [we did not observe expression of the cell proliferation marker Ki-67 in monocytes (not shown)]. We hypothesize that the increase is due, in part, to the reduced capacity to signal through CXCR4 (Fig. 4B), whose deficiency in vivo results in loss of retention and an increased capacity to egress from bone marrow (45).
Fig. 5.
APOBEC1 is expressed within specific monocyte progenitors, and is required for the proper maintenance of monocyte populations in the periphery. (A) Schematic depicting the differentiation of monocytes from the cMOP. MDP, monocyte DC progenitor. (B) Apobec1 expression in sorted monocyte progenitors, relative to Gapdh expression, determined via quantitative PCR. (C) Schematic of the competitive reconstitution assay. Briefly, progenitor cells from both lineages were obtained from bone marrow and introduced into a recipient, whose progenitors were depleted via sublethal irradiation. The recipient mouse reconstitutes all blood lineages from the mixed wildtype and Apobec1−/− progenitors. (D) Analysis of bone marrow monocyte progenitor populations in radiation chimeras reconstituted with equal numbers of wildtype and Apobec1−/− progenitor cells 6 wk after transplantation (n = 6). (E) Analysis of spleens from mice reconstituted as in D. Error bars in D and E represent the SEM with statistical significance calculated using a t test.
Discussion
RNA editing is an active process that introduces base changes within hundreds of transcripts, while leaving the genome intact. The vast majority of effort in the field of editing has been spent on cataloguing editing instances in transcripts within different tissues, and attempting to understand how some of those altered transcripts correlate with disease progression and manifestations [AZIN1 and others (46, 47)]. In contrast to DNA mutation, where the mutational landscape represents a sum total of events that could have been introduced at different times, some of which have functional consequences (“driver” mutations) yet others do not (“passenger” mutations), the functional consequences of RNA editing are likely the result of the alteration of the sequence of tens, even hundreds, of transcripts at the same time [although a few single events (e.g., ApoB editing) can also be consequential].
To date, very little has been done to functionally characterize the molecular consequences of editing, in aggregate, and how these might affect cellular activity. Here, we have attempted to survey the global effects of editing at the level of the molecule (e.g., transcript stability, localization, translational output) and at the level of the cell (e.g., impacts on specific cellular behaviors as defined by sets of transcripts). Our survey suggests that, mechanistically, editing affects translation (but not RNA stability or localization). This effect on translation is, in part, indirect: We show that editing can erase miRNA target sites, thus relieving miRNA-mediated translational repression [thought to occur at early steps of the translation process (48, 49)]. While the majority of editing events do not alter miRNA target site sequences, leaving the precise mechanism of how editing interferes with translation unclear, it is tempting to hypothesize that edited transcripts are differentially loaded onto the ribosome. The adaptation of methods that assess translational efficiency, while also scanning the 3′UTR (untranslated) sequence, will help address such mechanistic questions in the future.
In terms of how editing alters cellular behavior, we have demonstrated that editing of transcripts within cellular pathways of relevance to the life of a monocyte can be correlated with changes in cellular performance: Apobec1−/− BMDMs tend to migrate less, but phagocytose more (Fig. 4A). However, it is important to note that these experiments are done with populations of cells, which are heterogeneous with regard to editing (a limitation that has hampered our ability to globally check how editing affects transcript processing). As well, while we suggest that editing of specific sets of transcripts alters, for example, transendothelial migration profiles, it is important to remember that these transcripts are coordinately altered together with nearly a hundred others. In future work, we aim to utilize targeted approaches within Apobec1−/− cell lines to conclusively demonstrate that it is editing alone, within specific transcripts, that alters cellular activity.
Our experiments demonstrate that RNA editing of clusters of transcripts that aggregate in common pathways is correlated with the difference in output of those pathways and, by extension, with differences in cell physiology. They also predict that slight differences in the maintenance of monocyte subsets might, over time, have critical consequences for the health of the organism (50). Finally, although human monocytes express a different C-to-U mRNA-editing enzyme (APOBEC3A), we hypothesize that the process of RNA editing (the generation of single-nucleotide variants at the RNA level) will be broadly relevant as a novel regulatory mechanism in both mice and humans.
Materials and Methods
All experimental procedures were approved by the Rockefeller University Animal Care and Use Committee, and adhere to NIH guidelines for the care and use of experimental animals. Details regarding RNA-seq and downstream bioinformatic analyses, and all other molecular and cellular methods reported herein, are available in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Fotini Gounari (University of Chicago) for advice on bone marrow reconstitution experiments and Robert Darnell (The Rockefeller University) for help with high-throughput sequencing of mRNA and miRNA isolated by cross-linking and immunoprecipitation (HITS-CLIP). We thank Sydney Sieh-Takata for help with validation of editing, Connie Zhao and the Rockefeller genomics core for help with sequencing applications, and Svetlana Mazel and the Rockefeller flow cytometry core for help with cell sorting (via the New York State Department of Health Contract C023046). The work herein was supported by European Research Council Grant 649019 (to F.N.P.), by a grant from the Peter Deane Trust (to K.B.), and by NCI/F32 Award F32CA183318 (to D.H.). All RNA-seq and HITS-CLIP raw sequence data reported here have been deposited in the National Center for Biotechnology Information Sequence Read Archive (accession no. GSE58798), along with the appropriate methods used to derive all relevant figures.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the National Center for Biotechnology Information Sequence Read Archive (accession no. GSE58798).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714227114/-/DCSupplemental.
References
- 1.Jones PA. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 2.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 3.Wagner JR, et al. The relationship between DNA methylation, genetic and expression inter-individual variation in untransformed human fibroblasts. Genome Biol. 2014;15:R37. doi: 10.1186/gb-2014-15-2-r37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, et al. APOBEC3A cytidine deaminase induces RNA editing in monocytes and macrophages. Nat Commun. 2015;6:6881. doi: 10.1038/ncomms7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maas S, Kawahara Y, Tamburro KM, Nishikura K. A-to-I RNA editing and human disease. RNA Biol. 2006;3:1–9. doi: 10.4161/rna.3.1.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice GI, et al. Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature. Nat Genet. 2012;44:1243–1248. doi: 10.1038/ng.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crow YJ, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A. 2015;167A:296–312. doi: 10.1002/ajmg.a.36887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang T, et al. Dynamic regulation of RNA editing in human brain development and disease. Nat Neurosci. 2016;19:1093–1099. doi: 10.1038/nn.4337. [DOI] [PubMed] [Google Scholar]
- 11.Savva YA, et al. RNA editing regulates transposon-mediated heterochromatic gene silencing. Nat Commun. 2013;4:2745. doi: 10.1038/ncomms3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orecchini E, et al. ADAR1 restricts LINE-1 retrotransposition. Nucleic Acids Res. 2017;45:155–168. doi: 10.1093/nar/gkw834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2004;2:e391. doi: 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DDY, et al. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res. 2004;14:1719–1725. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamuta M, et al. Complete phenotypic characterization of apobec-1 knockout mice with a wild-type genetic background and a human apolipoprotein B transgenic background, and restoration of apolipoprotein B mRNA editing by somatic gene transfer of Apobec-1. J Biol Chem. 1996;271:25981–25988. doi: 10.1074/jbc.271.42.25981. [DOI] [PubMed] [Google Scholar]
- 16.Morrison JR, et al. Apolipoprotein B RNA editing enzyme-deficient mice are viable despite alterations in lipoprotein metabolism. Proc Natl Acad Sci USA. 1996;93:7154–7159. doi: 10.1073/pnas.93.14.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fossat N, et al. C to U RNA editing mediated by APOBEC1 requires RNA-binding protein RBM47. EMBO Rep. 2014;15:903–910. doi: 10.15252/embr.201438450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heng TSP, Painter MW. Immunological Genome Project Consortium The Immunological Genome Project: Networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 19.Harjanto D, et al. RNA editing generates cellular subsets with diverse sequence within populations. Nat Commun. 2016;7:12145. doi: 10.1038/ncomms12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamanaka S, Poksay KS, Driscoll DM, Innerarity TL. Hyperediting of multiple cytidines of apolipoprotein B mRNA by APOBEC-1 requires auxiliary protein(s) but not a mooring sequence motif. J Biol Chem. 1996;271:11506–11510. doi: 10.1074/jbc.271.19.11506. [DOI] [PubMed] [Google Scholar]
- 21.Dance GSC, et al. Two proteins essential for apolipoprotein B mRNA editing are expressed from a single gene through alternative splicing. J Biol Chem. 2002;277:12703–12709. doi: 10.1074/jbc.M111337200. [DOI] [PubMed] [Google Scholar]
- 22.Blanc V, Xie Y, Luo J, Kennedy S, Davidson NO. Intestine-specific expression of Apobec-1 rescues apolipoprotein B RNA editing and alters chylomicron production in Apobec1 -/- mice. J Lipid Res. 2012;53:2643–2655. doi: 10.1194/jlr.M030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Severi F, Conticello SG. Flow-cytometric visualization of C>U mRNA editing reveals the dynamics of the process in live cells. RNA Biol. 2015;12:389–397. doi: 10.1080/15476286.2015.1026033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg BR, Hamilton CE, Mwangi MM, Dewell S, Papavasiliou FN. Transcriptome-wide sequencing reveals numerous APOBEC1 mRNA-editing targets in transcript 3′ UTRs. Nat Struct Mol Biol. 2011;18:230–236. doi: 10.1038/nsmb.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanc V, et al. Genome-wide identification and functional analysis of Apobec-1-mediated C-to-U RNA editing in mouse small intestine and liver. Genome Biol. 2014;15:R79. doi: 10.1186/gb-2014-15-6-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta A, Driscoll DM. Identification of domains in apobec-1 complementation factor required for RNA binding and apolipoprotein-B mRNA editing. RNA. 2002;8:69–82. doi: 10.1017/s1355838202015649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picardi E, Horner DS, Pesole G. Single-cell transcriptomics reveals specific RNA editing signatures in the human brain. RNA. 2017;23:860–865. doi: 10.1261/rna.058271.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imamachi N, et al. BRIC-seq: A genome-wide approach for determining RNA stability in mammalian cells. Methods. 2014;67:55–63. doi: 10.1016/j.ymeth.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Ogretmen B, McCauley MD, Safa AR. Molecular mechanisms of loss of beta 2-microglobulin expression in drug-resistant breast cancer sublines and its involvement in drug resistance. Biochemistry. 1998;37:11679–11691. doi: 10.1021/bi980573c. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH. Quantitative approaches for investigating the spatial context of gene expression. Wiley Interdiscip Rev Syst Biol Med. 2017;9:1–13. doi: 10.1002/wsbm.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt DM, et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell. 2012;150:279–290. doi: 10.1016/j.cell.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prohaska KM, Bennett RP, Salter JD, Smith HC. The multifaceted roles of RNA binding in APOBEC cytidine deaminase functions. Wiley Interdiscip Rev RNA. 2014;5:493–508. doi: 10.1002/wrna.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ota H, et al. ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing. Cell. 2013;153:575–589. doi: 10.1016/j.cell.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor PBF, Andreev DE, Baranov PV. Comparative survey of the relative impact of mRNA features on local ribosome profiling read density. Nat Commun. 2016;7:12915. doi: 10.1038/ncomms12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimson A, et al. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helwak A, Tollervey D. Mapping the miRNA interactome by cross-linking ligation and sequencing of hybrids (CLASH) Nat Protoc. 2014;9:711–728. doi: 10.1038/nprot.2014.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abaza I, Gebauer F. Trading translation with RNA-binding proteins. RNA. 2008;14:404–409. doi: 10.1261/rna.848208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpenter S, Ricci EP, Mercier BC, Moore MJ, Fitzgerald KA. Post-transcriptional regulation of gene expression in innate immunity. Nat Rev Immunol. 2014;14:361–376. doi: 10.1038/nri3682. [DOI] [PubMed] [Google Scholar]
- 41.Teng B-B, et al. Mutational analysis of apolipoprotein B mRNA editing enzyme (APOBEC1). Structure-function relationships of RNA editing and dimerization. J Lipid Res. 1999;40:623–635. [PubMed] [Google Scholar]
- 42.Chen L, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat Med. 2013;19:209–216. doi: 10.1038/nm.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lomeli H, et al. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- 44.Huynh KK, et al. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007;26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q, et al. CXCR4 antagonist AMD3100 redistributes leukocytes from primary immune organs to secondary immune organs, lung, and blood in mice. Eur J Immunol. 2015;45:1855–1867. doi: 10.1002/eji.201445245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi L, Chan THM, Tenen DG, Chen L. RNA editome imbalance in hepatocellular carcinoma. Cancer Res. 2014;74:1301–1306. doi: 10.1158/0008-5472.CAN-13-3485. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Chen L, Chan THM, Guan X-Y. Hepatocellular carcinoma: Transcriptome diversity regulated by RNA editing. Int J Biochem Cell Biol. 2013;45:1843–1848. doi: 10.1016/j.biocel.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 48.Meister G. miRNAs get an early start on translational silencing. Cell. 2007;131:25–28. doi: 10.1016/j.cell.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Zinovyev A, Morozova N, Gorban AN, Harel-Belan A. 2013. Mathematical modeling of microRNA–mediated mechanisms of translation repression. MicroRNA Cancer Regulation, Advances in Experimental Medicine and Biology, eds Schmitz U, Wolkenhauer O, Vera J (Springer, Dordrecht), Vol 774, pp 189–224. [DOI] [PubMed]
- 50.Cole DC, et al. Loss of Apobec1 RNA-editing function in microglia exacerbates age-related CNS pathophysiology. Proc Natl Acad Sci USA. 2017;114:13272–13277. doi: 10.1073/pnas.1710493114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.