Significance
The acquisition of cytotoxic function by CD8+ T cells is critical for antiviral and antitumor responses. While cytotoxic differentiation is preprogrammed during CD8+ T-cell development in the thymus, the regulation of T-cell cytotoxic capacities by inflammatory cues is poorly understood, notably in cases of immune dysfunction observed in tumor-infiltrating lymphocytes or during chronic infections. Here, we demonstrate that the program underlying IL-17 production dampens cytotoxic function in both CD4+ and CD8+ T cells. Specifically, we show that two transcription factors involved in IL-17 production, STAT3 and RORγt, repress cytotoxic differentiation. These results highlight the role of the inflammatory environment on T-cell responses and have implications for the development of T cell-based immunotherapies.
Keywords: CD8 T cells, cytotoxicity, IL-17, STAT3 signaling
Abstract
CD8+ T cells are preprogrammed for cytotoxic differentiation in the thymus as they acquire expression of the transcription factor Runx3. However, a subset of effector CD8+ T cells (Tc17) produce IL-17 and fail to express cytotoxic genes. Here, we show that the transcription factors directing IL-17 production, STAT3 and RORγt, inhibit cytotoxicity despite persistent Runx3 expression. Cytotoxic gene repression did not require the transcription factor Thpok, which in CD4+ T cells restrains Runx3 functions and cytotoxicity; and STAT3 restrained cytotoxic gene expression in CD8+ T cells responding to viral infection in vivo. STAT3-induced RORγt represses cytotoxic genes by inhibiting the functions but not the expression of the “cytotoxic” transcription factors T-bet and Eomesodermin. Thus, the transcriptional circuitry directing IL-17 expression inhibits cytotoxic functions. However, by allowing expression of activators of the cytotoxic program, this inhibitory mechanism contributes to the instability of IL-17–producing T cells.
T cells are essential to fight intracellular pathogens, including viruses, bacteria, and protozoans. MHC I-restricted CD8+ T cells differentiate into cytotoxic (Tc1) effectors that produce the cytokine IFNγ and cytolytic molecules, including perforin and granzymes (1). Whereas acquisition of cytotoxic functions is not typical of MHC II-restricted CD4+ T cells, IFNγ secretion by Th1 CD4+ effector T cells is essential to combat intracellular pathogens (2). The differentiation of both Tc1 (CD8+) and Th1 (CD4+) T cells involves the transcription factor Runx3 and the T-box factors T-bet or Eomesodermin (Eomes). Runx3 is up-regulated during the differentiation of MHC I-restricted T cells in the thymus (3, 4) and remains expressed in postthymic resting and activated CD8+ T cells (5). Although not expressed in naïve CD4+ T cells, Runx3 is induced in differentiating CD4+ Th1 effectors (6, 7). While neither T-bet nor Eomes are expressed in resting T cells, they are up-regulated in differentiating Th1 and Tc1 effectors, in which they sustain production of IFNγ and cytotoxic molecules (2, 8, 9).
CD4+ T cells are also involved in the control of extracellular microbes, including bacteria, yeast, and fungi, through their production of IL-17 and related cytokines (10, 11). The differentiation of IL-17–producing CD4+ T cells (Th17) requires the transcription factors STAT3 and RORγt (12–15). There is evidence that the transcriptional circuitry directing IFNγ and cytotoxic gene expression in Th1 or Tc1 cells inhibits Th17-related gene expression (16). Mechanistically, T-bet and Eomes directly antagonize the expression of RORγt (17–19) and thereby restrain IL-17 production.
Because MHC I molecules typically present peptide antigens synthesized intracellularly, it had been considered that CD8+ T cells were not involved in IL-17–mediated control of extracellular pathogens. Nonetheless, CD8+ T cells producing IL-17 (Tc17) are found at effector sites both in humans and in experimental models, and there is evidence that such cells have potential pathogenic properties (20–23). Moreover, the differentiation of Tc17 cells involves STAT3 and RORγt, as does that of Th17 CD4+ effectors (20, 24). This indicates that a common transcriptional circuitry, called “Teff17” hereafter, directs IL-17 production in Th17 and Tc17 cells.
It was noted that Tc17 cells show reduced cytotoxic activity and cytotoxic gene expression relative to Tc1 cells (20, 24, 25). However, how this is achieved has not been investigated. Here, we demonstrate that repression of cytotoxic genes is an intrinsic property of the Teff17 circuitry, which we show acts in Tc17 CD8+ T cells by inhibiting the function but not the expression of Runx3. Such inhibition depends on the transcription factor STAT3, in part through its ability to promote RORγt expression. Accordingly, the Teff17 circuitry represses cytotoxic genes independently of Thpok in CD4+ T cells. Last, we show that RORγt itself restrains the activation of cytotoxic genes but fails to inhibit the expression of T-bet or Eomes. We propose that such persistent expression of key activators of cytotoxic differentiation contributes to the instability of IL-17–producing T cells.
Results
Teff17 Transcriptional Circuitry Represses Cytotoxic Functions Despite Persistent Runx3 Expression.
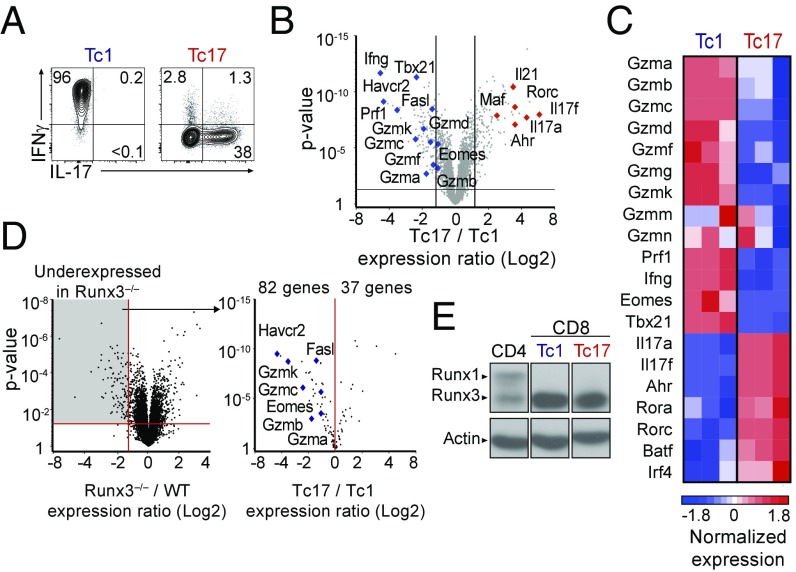
Upon antigen stimulation, naïve CD8+ T cells typically differentiate into Tc1 killer cells that express molecules essential for cytotoxicity, including perforin, granzymes A, B, and K, and the cytokine IFNγ. In contrast, CD8+ T cells signaled with TGF-β and IL-6 (Tc17 culture conditions) produce IL-17 and show little if any cytotoxic activity (Fig. 1A and Fig. S1A). Of note, CD8+ T cells activated in the presence of either TGF-β or IL-6 alone maintained cytotoxic activity (Fig. S1B), suggesting that repression of cytotoxic differentiation is characteristic of the Teff17 transcriptional circuitry, rather than resulting from signaling by either cytokine.
Fig. 1.
The Tc17 transcriptional program represses cytotoxic functions. (A) Contour plots of IL-17 vs. IFNγ expression on CD8+ T cells cultured under Tc1 and Tc17 conditions. (B) Volcano plot displays Tc17/Tc1 expression ratios (log2 values, full gene set) vs. P values; each symbol represents a distinct gene. Relevant genes are indicated. Data are from three replicates. Lines represent 1.5-fold change, P value 0.05. (C) Heatmap displays normalized expression on selected genes in Tc1 and Tc17 cells (Z score, color scale at Bottom). Data are from three replicates. (D) Volcano plot (Left) displays expression ratios (log2 values, full gene set) vs. P values of differential expression in Runx3−/− over wild-type CD8+ T cells; original data are from ref. 26. The Right volcano plot displays Tc17/Tc1 expression ratio vs. P values of differential expression for genes significantly underexpressed in Runx3−/− cells (1.5-fold change, P < 0.05, gray shading on Left plot). Each symbol represents a gene; relevant genes are indicated. (E) Immunoblot analyses of Runx protein expression in effector CD4+ (ThN) or CD8+ T cells cultured under Tc1 or Tc17 conditions. Data are representative of five (A) or two (B–D) mice analyzed in four (A) or two (B–D) independent experiments.
To determine the impact of the Teff17 transcriptional circuitry on the cytotoxic program, we compared gene expression in Tc1 vs. Tc17 CD8+ T cells by microarray analyses. We identified 269 genes differentially expressed (1.5-fold change, P < 0.05) between these two subsets (Fig. 1B). Consistent with previous reports (21, 24), expression of genes associated with IL-17 production, such as Il17a, Il17f, Rorc (encoding RORγt), and Ahr, was higher in Tc17 than Tc1 cells (Fig. 1C). Strikingly, we found that Tc17 differentiation was associated with a broad repression of the cytotoxic program, including genes encoding T-bet (Tbx21, called T-bet here), Eomes (Eomes), and cytotoxic molecules (Fig. 1C). Quantitative RT-PCR (qPCR) experiments confirmed lower expression in Tc17 than in Tc1 cells of genes encoding granzymes A and B, and perforin (Gzma, Gzmb, and Prf1, respectively) (Fig. S1C). These observations suggest that the transcriptional circuitry involved in Tc17 differentiation broadly inhibits cytotoxic gene expression.
The transcription factor Runx3 promotes cytotoxic gene expression and IFNγ production in CD8+ effector T cells (5); in addition, both Runx3 and the related protein Runx1 promote the production of IFNγ by “pathogenic” Th17 CD4+ T cells (17, 18). Given that Tc17 effectors expressed neither IFNγ nor cytotoxic genes, we predicted that they would express little or no Runx3. Consistent with this idea, many previously identified Runx3-dependent genes (Fig. 1D, Left) (26) were underexpressed in Tc17 compared with Tc1 cells (Fig. 1D, Right), including canonical cytotoxic genes Gzma, Gzmb, Gzmc, Fasl, or Havcr2 (encoding Tim-3). However, and contrary to the prediction, immunoblot analyses showed equivalent amounts of Runx3 protein in Tc1 and Tc17 cells (Fig. 1E); importantly, Runx1 was not detected in either subset. These findings indicate that the Teff17 transcriptional circuitry inhibits Runx3-dependent expression of cytotoxic genes without affecting the expression of Runx3 itself.
The transcription factor Thpok antagonizes Runx-mediated expression of cytotoxic genes in CD4+ T cells and is expressed, although at modest levels, in activated CD8+ T cells (27–30). Thus, we considered the possibility that Thpok may contribute to cytotoxic gene repression in Tc17 cells. To address this, we assessed wild-type (WT) and Thpok-deficient Tc17 effector cells for the expression of granzyme B, a sensitive marker of Thpok repression in both CD4+ and CD8+ T cells (28, 31). To ensure that Tc17 effectors were MHC I restricted, they were derived from naïve CD8+ T cells obtained from Cd4-cre+ Thpokfl/fl mice expressing the MHC I-restricted P14 transgenic TCR. Thpok disruption did not increase granzyme B expression (Fig. S1D), supporting the conclusion that the transcriptional circuitry of Tc17 cells overcomes Runx3-mediated activation of the cytotoxic program independently of Thpok.
Stat3 Represses Cytotoxic Gene Expression.
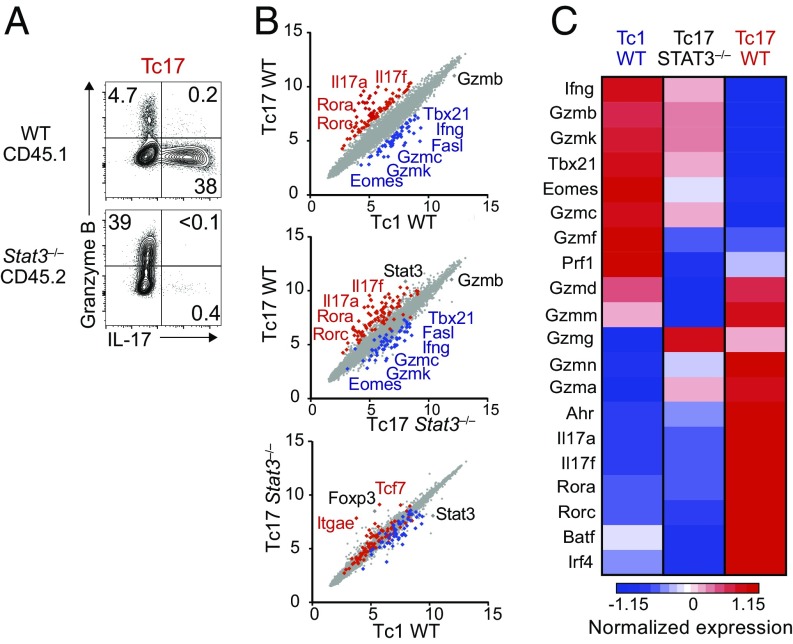
The preceding findings suggested that repression of the cytotoxic program was integral to the Teff17 transcriptional circuitry. Because the transcription factor STAT3, activated by IL-6, is required for the differentiation of both Th17 and Tc17 cells (20, 32), we examined whether it represses cytotoxic gene expression. We differentiated CD8+ T cells from Cd4-cre+ Stat3fl/fl mice (called here Stat3−/−) under Tc17 conditions. To avoid noncell-intrinsic effects, we compared Stat3−/− and wild-type CD8+ T cells cocultured in the same environment (Fig. S2A). Unlike control cells in the same coculture, Stat3-deficient CD8+ T cells failed to produce IL-17, and they displayed increased granzyme B expression (Fig. 2A), suggesting that STAT3 represses cytotoxic genes. To further evaluate this possibility, we performed microarrays on RNAs prepared from Stat3−/− and wild-type CD8+ T cells purified after coculture in Tc17 conditions. In parallel, we analyzed RNAs from wild-type and Stat3−/− CD8+ T cells cocultured in Tc1 conditions. Gene expression in Stat3−/− Tc17 cells was highly similar to that in wild-type Tc1 cells (Fig. 2B). Specifically, Stat3−/− Tc17 cells were skewed toward expression of cytotoxic genes, including those encoding granzymes B, C, and K, T-bet, and Eomes, in addition to their impaired expression of canonical Tc17 genes, including Rorc, Il17a, or Il17f (Fig. 2C). In contrast STAT3 disruption had no detectable effect on the transcriptome of in vitro Tc1 effectors, which display high-level expression of cytotoxic genes (Fig. S2B).
Fig. 2.
STAT3 represses cytotoxic gene expression in CD8+ T cells. (A) Contour plots of IL-17 vs. granzyme B intracellular expression in CD8+ T cells cocultured under Tc17 conditions as shown in Fig. S2A. Data are gated on WT CD45.1+ or Stat3−/− CD45.2+ cells and are representative of three mice per genotype analyzed in three independent experiments. (B) Scatterplots show microarray gene expression (log2 values, full gene set) in indicated cell populations after sorting from mixed cultures set as in Fig. S2A. Genes with 1.5-fold or greater expression change in wild-type Tc17 vs. Tc1 cells (P < 0.05) are defined in the Top plot and shown in red and blue in all three plots. Relevant genes are indicated. Data are from three replicates. (C) Heatmap displays normalized expression on selected genes in Tc1 WT, Tc17 WT, and Tc17 Stat3−/− cells (Z score, color scale at Bottom). Data are from three replicates.
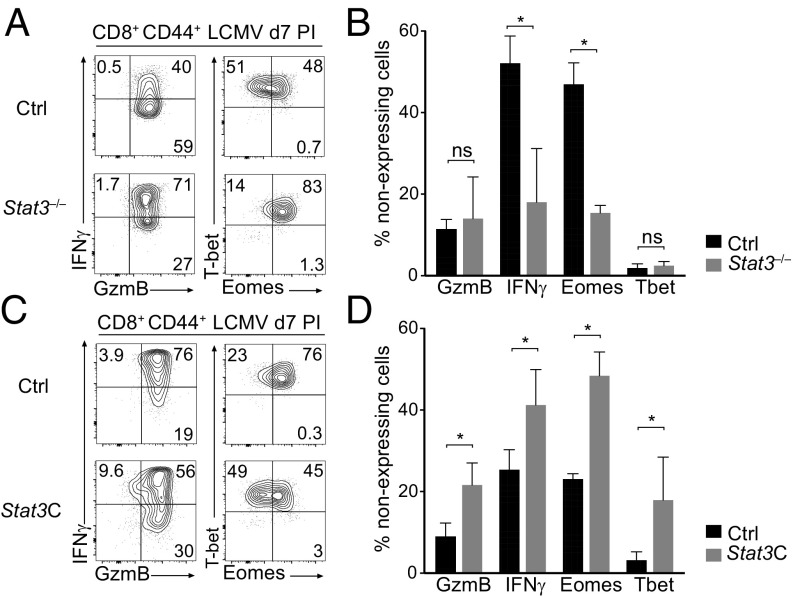
To examine whether STAT3 restrains cytotoxic gene expression in vivo, we evaluated the response of STAT3-deficient CD8+ T cells during infection by the Armstrong strain of lymphocytic choriomeningitis virus (LCMV). While LCMV Armstrong is cleared by a strong cytotoxic CD8+ T-cell response (16, 33), it causes acute IL-6 production (34), allowing us to assess the potential impact of STAT3 activation on cytotoxic genes. Consistent with our hypothesis, disruption of Stat3 increased Eomes expression and IFNγ production in effector CD8+ T cells at the peak of the LCMV response (Fig. 3 A and B); analyses in mixed bone-marrow chimeras (Stat3 deficient: wild type; 1:1) showed that this effect is cell intrinsic (Fig. S2 C and D).
Fig. 3.
STAT3 opposes CD8+ T cell cytotoxic differentiation in vivo. (A and B) CD8+ CD44+ T cells were sorted from the spleen of Stat3−/− or control mice 7 d after LCMV infection. (A) Contour plots show intracellular expression of granzyme B vs. IFNγ (Left) and Eomes vs. T-bet (Right) in CD44hi CD8+ T cells. (B) Percentage of cells with no detectable expression of the indicated protein among CD8+ T cells. (C and D) GFP+ or YFP+ CD8+ CD44hi T cells were sorted from the spleen of Ox40-cre+ Rosa26Stat3C-GFP (Stat3C) or Ox40-cre+ Rosa26YFP (Ctrl) animals 7 d after LCMV infection. (C) Contour plots show intracellular expression of granzyme B vs. IFNγ (Left) or Eomes vs. T-bet (Right). (D) Percentage of cells with no detectable expression of the indicated protein among CD8+ T cells. Note that, in control animals, a greater fraction of YFP+ CD8+ cells express granzyme B and IFNγ, relative to YFP– CD8+ cells in the same mouse (which represent the vast majority of CD8+ responders) (Fig. S2E). This is consistent with the preferential expression of Ox40 on highly activated CD8+ T cells (73). (A–D) Data are representative of two independent experiments, each with two mice of each genotype. *P < 0.05; ns, not significant.
This suggested that STAT3 represses cytotoxic genes in vivo. Accordingly, we speculated that ectopic activation of STAT3 in Tc1 cells should counteract their cytotoxic differentiation. To test this, we used a Cre-inducible allele (Rosa26Stat3C-GFP) in which the Rosa26 locus contains a floxed transcription termination site followed by a bicistronic insert encoding both a constitutively active version of STAT3 (STAT3C) and GFP as a reporter for Cre expression (35). To avoid constitutive STAT3 activity in developing thymocytes and resting T cells, we generated Rosa26Stat3C-GFP/+ mice carrying Ox40-cre, which is expressed in 10–15% of effector CD8+ T cells after LCMV infection (Fig. S2E) but not in naïve CD8+ T cells. As controls, we used Rosa26YFP/+Ox40-cre mice, in which YFP identifies cells with a history of Cre expression (Fig. S2E). In LCMV-infected Rosa26Stat3C-GFP/+ Ox40-cre+ mice, expression of STAT3C resulted in a significant inhibition of canonical Tc1 markers, as shown by the reduced frequency of cells expressing granzyme B, IFNγ, T-bet, and Eomes (Fig. 3 C and D). Thus, both loss- and gain-of-function experiments support the conclusion that STAT3 inhibits cytotoxic gene expression in CD8+ T in vivo.
STAT3 Target RORγt Represses Cytotoxic Effector Genes.
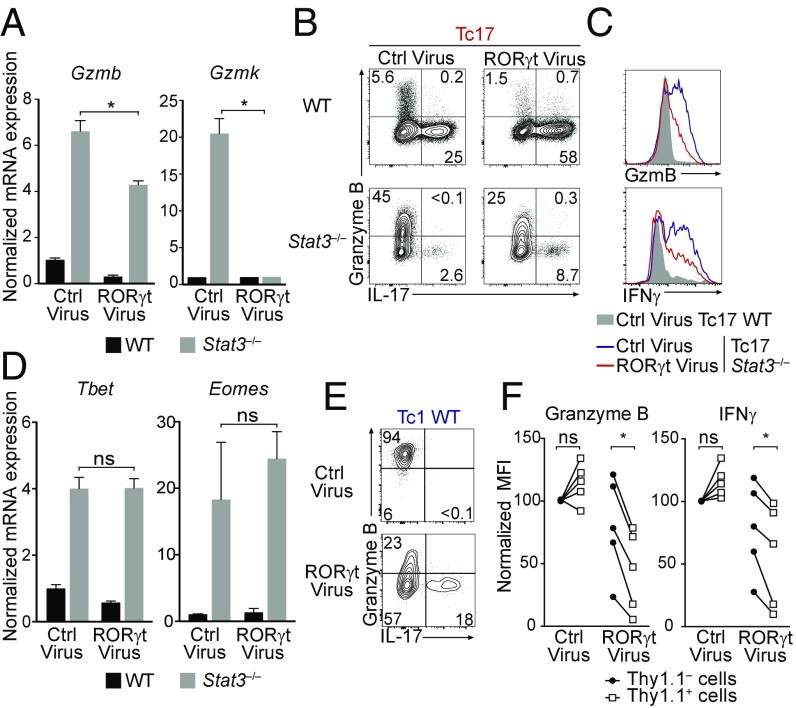
In addition to STAT3, expression of Teff17 genes involves the transcription factors Irf4, Batf, and RORγt (13, 36–40). Both Irf4 and Batf are expressed in Tc1 cells and promote IFNγ and cytotoxic gene expression in vivo during viral infection (41–44). In contrast, RORγt is specific to the Teff17 program. Because STAT3 promotes RORγt expression, we considered the possibility that RORγt would repress cytotoxic genes. To evaluate this, we expressed RORγt in Stat3−/− CD8+ T cells cocultured with WT CD8+ T cells under Tc17 conditions. Enforced RORγt expression failed to restore IL-17 production to wild-type levels, but strongly repressed Gzmk and to a lesser extent Gzmb (Fig. 4 A and B). However, even though it inhibited expression of IFNγ, a prototypical T-bet target (Fig. 4C), RORγt failed to affect expression of T-bet or Eomes, the “master regulators” of cytotoxic genes (Fig. 4D). Consistent with these results, reanalysis of previously published ChIP-seq data from Th17 CD4+ T cells detected STAT3 binding at T-bet, but little or no binding at Ifng, Gzmb, and Gzmk, which were bound by RORγt (Fig. S3). Of note, RORγt binding sites also recruited T-bet in Th1 cells (45) (Fig. S3).
Fig. 4.
RORγt antagonizes cytotoxic functions in CD8+ T cells. (A and D) RT-qPCR experiments assess expression of Gzmb and Gzmk (A) or T-bet and Eomes (D) from WT (black bars) or Stat3−/− (gray bars) cells cocultured in Tc17 conditions as described in Fig. S2A, transduced with RORγt or control (empty) retroviruses, and sorted for CD45 allele expression before RNA preparation. Data are expressed relative to expression in WT Tc17 cells transduced with control virus (set to 1) and is representative of two mice per genotype analyzed in two independent experiments. (B) Contour plots show intracellular expression of IL-17 vs. granzyme B on WT or Stat3−/− cells cocultured in Tc17 conditions as described in Fig. S2A and retrovirally transduced as indicated. Data are gated on transduced cells and representative of three mice per genotype in three independent experiments. (C) Overlaid histograms show intracellular granzyme B and IFNγ expression in WT (gray shaded) or Stat3−/− (transduced as indicated) CD8+ T cells assessed as in B. Data are representative of three mice per genotype in three independent experiments. (E and F) WT CD8+ T cells were transduced with RORγt or control Thy1.1-expressing retrovirus and cultured in Tc1 conditions. (E) Contour plots show intracellular expression of IL-17 vs. granzyme B, gated on retrovirus-expressing (Thy1.1+) cells. (F) Before–after plots compare intracellular granzyme B and IFNγ expression in Thy1.1+ (empty squares) and Thy1.1– (filled circles) cells within the same culture. Data [mean fluorescent intensity (MFI)] is expressed relative to that in Thy1.1– cells in control virus-transduced cultures, set to 100 within each mouse. Each pair of symbols represents a separate culture; data are from five mice analyzed in three independent experiments *P < 0.05; ns, not significant.
This suggested that RORγt inhibits the function of T-bet or Eomes rather than their expression and prompted us to examine whether ectopic expression of RORγt in WT Tc1 cells, which express T-bet and Eomes, would dampen the cytotoxic program. Indeed, retroviral RORγt transduction impaired both granzyme B and IFNγ expression in wild-type Tc1 CD8+ effectors (Fig. 4 E and F). We conclude from these experiments that RORγt inhibits cytotoxic differentiation at least in part independently of STAT3, and that it acts by restraining the function but not the expression of T-bet and Eomes.
Teff17 Effector Program Represses Cytotoxic Differentiation in CD4+ T Cells.
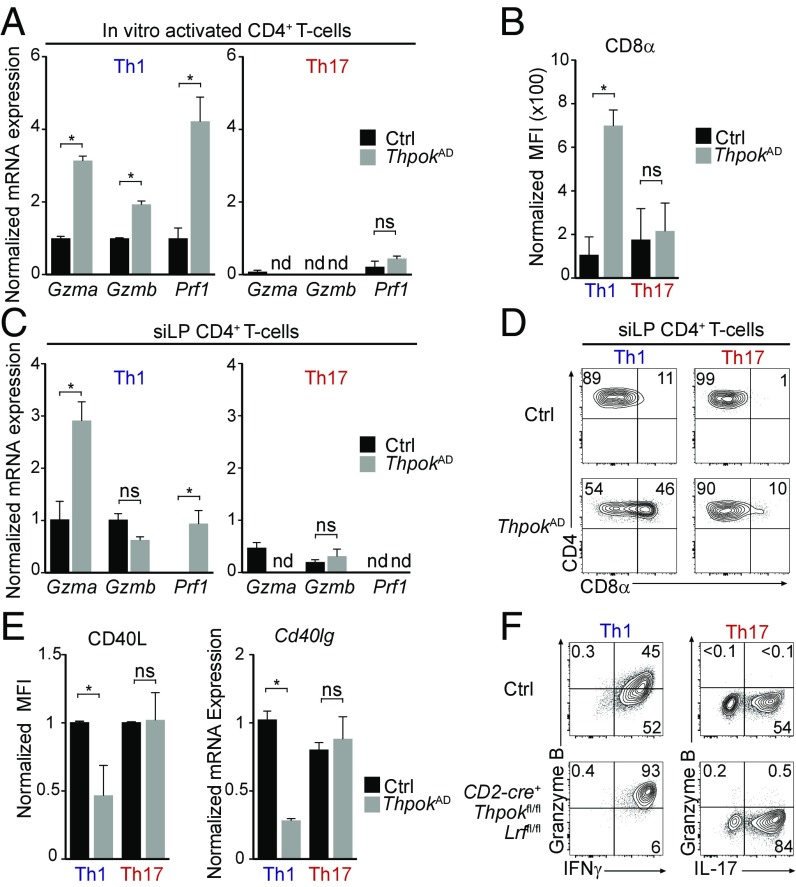
While CD8+ T cells are preprogrammed for cytotoxic differentiation, CD4+ T cells are preprogrammed to repress cytotoxic genes by their expression of Thpok, which inhibits Runx3 expression and functions (28, 29, 46–48). Accordingly, we previously showed that postthymic Thpok deletion diverts Th1 and Th2 CD4+ effectors toward cytotoxic differentiation (29). However, the preceding findings raised the possibility that Th17 CD4+ T cells, which also express STAT3 and RORγt (12–14, 49), would restrain cytotoxic gene expression independently of Thpok. We addressed this question by activating naïve CD4+ T cells from Ox40-cre+ Thpokfl/fl mice (called ThpokAD for “activation deleted”) in which Thpok disruption occurs during CD4+ T cell activation (50, 51). In line with previous results (29, 52), Thpok disruption did not impair IL-17 production (Fig. S4A). Importantly, Thpok was dispensable for the repression of Prf1, Gzma, and Gzmb in Th17- but not in Th1-activated cells (Fig. 5A). We previously reported that Thpok prevents CD8α reexpression in naïve and Th1 effector CD4+ T cells (29). In contrast, Th17 effector CD4+ T cells restrained CD8α expression despite Thpok disruption (Fig. 5B). To determine whether repression of cytotoxic genes requires Thpok in Th17 effectors in vivo, we examined the small intestine lamina propria (siLP), a site highly enriched in effector T cells in unmanipulated mice. Using cytokine capture assays, we isolated T cells producing IFNγ (Th1) or IL-17 (Th17) (Fig. S4B). Similar to in vitro analyses, repression of Gzma and Prf1 in Th17 cells was independent of Thpok, unlike in Th1 cells in which both genes were up-regulated after Thpok disruption (Fig. 5C); the same was true of repression of CD8α (Fig. 5D).
Fig. 5.
The Teff17 effector program represses cytotoxic gene expression in CD4+ T cells. (A and C) RT-qPCR expression of Gzma, Gzmb, and Prf1 on Th1 or Th17 cells from ThpokAD (Ox40-cre+ Thpokfl/fl, gray bars) or control (Ox40-cre+ Thpok+/+, black bars) mice. Data are shown for effectors derived in vitro from naïve CD4+ T cells (A) or for CD4+ T cells isolated from the siLP (C), and is shown relative to gene expression values in control Th1 cells, set to 1 (except in C for Prf1, set to 1 on ThpokAD Th1 cells). (B) Bar graphs show the MFI of surface CD8α expression on effectors CD4+ T cells derived as in A. (D) Contour plot show CD8α vs. CD4 expression on TCRβ+ CD4+ CD44+ IFNγ+ (Th1) and IL-17+ (Th17) siLP cells from control or ThpokAD animals; data are gated on YFP+ cells (as an indicator of Cre activity; mice carried a Rosa26YFP allele). (E) Bar graphs show the MFI of surface CD40L expression or RT-qPCR expression of Cd40lg (encoding CD40L) on effector CD4+ T cells derived in vitro as in A. Data are expressed relative to values in control Th1 cells, set to 1. (F) Contour plots show intracellular expression of IFNγ (Left) or IL-17 (Right) vs. granzyme B in CD4+ effectors derived in Th1 or Th17 culture conditions from CD2-cre+ Thpokfl/fl Lrffl/fl or control (CD2-cre– Thpokfl/fl Lrffl/fl). Data are representative of two (A, E, and F) or three (B–D) mice per genotype analyzed in two (A, B, E, and F) or three (C and D) independent experiments. *P < 0.05; nd, not detected; ns, not significant.
The preceding findings demonstrate that Th17 effectors repress cytotoxic genes independently of Thpok, both in vitro and in vivo. To examine the potential role of STAT3 in such repression, we compared expression of IFNγ and granzyme B in Stat3−/− and control CD4+ T cells cultured under Th17 conditions. STAT3 disruption increased expression of both molecules (Fig. S4C), a result consistent with previous transcriptome analyses (36, 53). However, the up-regulation of granzyme B and IFNγ expression in Stat3−/− CD4+ T cells was lower than in Stat3−/− CD8+ T cells cultured in the same conditions (Fig. S4C), consistent with a STAT3-independent inhibition by Thpok.
In addition to repressing cytotoxic genes, Thpok promotes expression of genes characteristic of the helper program, including Cd40lg, encoding a surface protein essential for helper activity. In Th1 cells, Thpok activation of Cd40lg is mediated in part through antagonism of Runx functions (29). In contrast to Th1 cells, Thpok was dispensable for CD40L expression in Th17 effectors (Fig. 5E), supporting the conclusion that the Th17 effector program of CD4+ T cells antagonizes Runx functions independently of Thpok.
In CD4+ T cells, Thpok serves in part redundantly with the related transcription factor LRF (encoded by Zbtb7a, called Lrf here) (29, 54). Thus, we considered that LRF could repress cytotoxic genes in Thpok-deficient Th17 effectors. To address this question, we cultured CD4+ T cells that postthymically delete both Thpok and LRF [from CD2-cre Thpokfl/fl Lrffl/fl mice (29)] under Th1 and Th17 conditions. Double-deficient Th17 cells fully repressed granzyme B expression (Fig. 5F) and, as previously reported (29), produced IL-17. In contrast, double-deficient Th1 cells failed to repress the expression of cytotoxic molecules compared with controls. Thus, repression of cytotoxic gene expression requires neither Thpok nor LRF in Th17 cells, unlike in other helper effector subtypes (29).
Discussion
The present report demonstrates that the transcriptional circuitry involved in IL-17 production in T cells broadly represses cytotoxic functions. Such repression is dependent on the transcription factor STAT3, in part via the induction of RORγt. Importantly, RORγt represses expression of cytotoxic effector genes despite persistent expression of canonical transcription factors Runx3, T-bet, and Eomes, implying that persistent inhibition of cytotoxic functions in Tc17 cells is highly dependent on cytokine-activated STAT3.
While T-bet and Eomes had been shown to restrain RORγt expression and thereby Th17 or Tc17 differentiation (16–18), whether STAT3 or RORγt reciprocally inhibit cytotoxic gene expression had not been elucidated. Although Th1-related and cytotoxic genes are not expressed in Th17 CD4+ T cells (36, 55), this observation did not imply repression by the Teff17 circuitry because Th17 CD4+ T cells express Thpok, which itself inhibits expression of cytotoxic genes (28, 29). In fact, Batf and Irf4, key components of the Teff17 circuitry, are also needed for proper Tc1 responses to viral infection (41–44). Here, we demonstrate that a STAT3–RORγt-based Teff17 transcriptional circuitry represses cytotoxic gene expression and the development of cytotoxic functions in Tc17 CD8+ T cells.
While RORγt represses effector genes (including those encoding granzymes), it does not inhibit T-bet or Eomes expression, in contrast to T-bet inhibition of RORγt gene expression. Such an asymmetric control has important functional implications. Whereas T-bet repression of RORγt stabilizes Tc1 differentiation, the inability of RORγt to repress T-bet, Eomes, and Runx3 compromises the stability of IL-17–producing T cells. In circumstances where STAT3 activation is not sustained (e.g., by IL-6 signaling), or is counteracted through signaling by other cytokines (e.g., IL-12), the persistent expression of T-bet, Eomes, and Runx3 would favor the reemergence of cytotoxic gene expression.
Consistent with this asymmetric antagonism, IFNγ and IL-17 double-producing CD8+ T cells are found in experimental colitis (22). Similar dual producers contribute to graft versus host disease (GVHD) after allogeneic stem cell transplantation (21), and therefore are presumably equivalent to pathogenic Th17 cells described in experimental models of colitis and multiple sclerosis (18, 56, 57). While these IFNγ- and IL-17–producing CD8+ T cells expressed T-bet, they showed reduced expression of Eomes and cytotoxic genes, including Gzmb. Consistent with the idea that Tc17 cells are unstable, they were shown by fate-mapping analyses to revert to a cytotoxic fate (21).
In contrast to Tc17 CD8+ T cells, in which inhibition of cytotoxic gene expression relies on the STAT3-driven Teff17 circuitry, both that circuitry and the CD4+ lineage-specific transcription factor Thpok contribute to restrain cytotoxic genes in Th17 CD4+ T cells. Of note, Thpok-mediated repression of IFNγ can be overcome by Th1-inducing environmental cues, despite persistent Thpok expression (28, 29, 58). Accordingly, Th17 effectors, which harbor epigenetic marks of activity at Th1 loci, can acquire IFNγ production and contribute to immunopathology during inflammation (59, 60).
STAT3 and RORγt may inhibit cytotoxic genes hierarchically, as suggested by ChIP binding results: in this scenario, STAT3 acts on transcriptional regulators T-bet, Eomes, and RORγt, which themselves control cytotoxic effector genes. Mechanistically, STAT3 may serve by opposing the positive effect of STAT5 on cytotoxic genes, including T-bet and Eomes (61). As STAT3 competes with STAT5 for DNA binding genome-wide (62), sustained STAT3 activation may displace STAT5 and thereby inhibit expression of cytotoxic genes. Additionally, because STAT5 and Runx3 molecules directly interact (63), the competition between STAT3 and STAT5 may affect Runx3-dependent genes, including Eomes (5).
In cells that coexpress RORγt and T-bet or Eomes, the present study indicates that RORγt can counteract T-bet and Eomes and restrain cytotoxic gene expression. The binding of RORγt to Ifng, Gzmb, and Gzmk cis-regulatory regions suggests that such an effect could be direct, through RORγt recruitment to these genes. Because RORγt binds cis-regulatory elements that can also recruit T-bet, it is possible that competition between these factors for DNA binding controls cytotoxic gene expression. Challenging this idea, RORγt and T-bet recognize distinct DNA sequences (36, 64). Alternatively, RORγt could inhibit T-bet or Eomes without affecting their DNA binding, e.g., by affecting their recruitment of transcriptional coactivators.
Cytotoxic gene repression by STAT3 and RORγt is expected to reduce the antitumor potential of CD8+ T cells in inflammatory tumor microenvironments. Indeed, Stat3 disruption promotes responses against experimental tumors (65). Even though the exact mechanisms by which STAT3 inhibits antitumoral activity remain to be elucidated, a growing number of reports suggest a critical role of STAT3 and IL-6 signaling in T cells and natural killer cells, consistent with an effect on cytotoxic gene expression (66–69). Thus, the ability to manipulate and target this pathway might be a valuable approach to enhance antitumor responses in cancer immunotherapy strategies.
Materials and Methods
Mice.
Mice carrying floxed alleles for Thpok (28), Stat3 (70), Rosa26Stat3C-GFP (35), or Lrf (71) were from our own colony or obtained from J. O'Shea (National Institutes of Health, Bethesda), S. Koralov (New York University, New York), and P. P. Pandolfi (Harvard University, Boston), respectively. Additional strains are described in SI Materials and Methods. Animal procedures were approved by the National Cancer Institute Animal Care and Use Committee.
In Vitro Cell Procedures.
Sorted naïve (CD44lo) T cells were activated with anti-CD3 and anti-CD28, in the presence of T cell-depleted irradiated WT splenocytes and cytokines and anti-cytokines antibodies as described in SI Materials and Methods. Retroviral transductions were performed as previously described (31), using either MIGR-RORγt-Thy1.1 or PMRX-Thy1.1 retroviruses (72). In vitro cytotoxicity was determined using pan-T-depleted WT splenocytes coated with relevant GP33 (KAVYNFATM) or irrelevant (SIIFNEKL) peptides, labeled with distinct CFSE concentrations, and cocultured with in vitro derived CD8+ effector T cells for 24 h.
Microarrays and ChIP-Seq Data.
Affymetrix Mouse Exon 2.0 ST arrays were processed as described in SI Materials and Methods and analyzed with Partek Genomic Suite; data are deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database under accession nos. GSE104143 and GSE104144. The Runx3 dataset (26) was obtained from the GEO (accession no. GSE50131). The STAT3 and RORγt (36) and T-bet ChIP-seq datasets (45) were obtained from the GEO (GSE40918 and GSE40623, respectively), aligned to the mouse genome (mm10 release) using the Bowtie package and analyzed with Partek Flow on the National Institutes of Health high-performance computing Biowulf cluster.
Statistical Analyses.
All statistical analyses were performed using Prism software. Bars in graphs indicate average ± SEM. Comparisons were performed by two-tailed unpaired t test. *P values <0.05.
Additional information is available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank E. Castro, H. Kwak, and T.-A. Lewis for expert animal care and genotyping; Q. Xiao for technical assistance and genotyping; N. Killeen, S. Koralov, A. Laurence, D. McGavern, J. O’Shea, and J. Zhu for mice and reagents; X. Wu for microarray analyses; and G. Abou Ezzi, Y. Belkaid, J. Brenchley, C. Harly, and V. Lazarevic for reading the manuscript. Supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE104143 and GSE104144).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711160114/-/DCSupplemental.
References
- 1.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 2.Lazarevic V, Glimcher LH, Lord GM. T-bet: A bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taniuchi I, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 4.Woolf E, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci USA. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Guilloty F, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djuretic IM, et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 7.Naoe Y, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 9.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 10.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 15.Harris TJ, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 16.Intlekofer AM, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarevic V, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, et al. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity. 2014;40:355–366. doi: 10.1016/j.immuni.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichiyama K, et al. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of eomesodermin. Immunity. 2011;34:741–754. doi: 10.1016/j.immuni.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Yen HR, et al. Tc17 CD8 T cells: Functional plasticity and subset diversity. J Immunol. 2009;183:7161–7168. doi: 10.4049/jimmunol.0900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gartlan KH, et al. Tc17 cells are a proinflammatory, plastic lineage of pathogenic CD8+ T cells that induce GVHD without antileukemic effects. Blood. 2015;126:1609–1620. doi: 10.1182/blood-2015-01-622662. [DOI] [PubMed] [Google Scholar]
- 22.Tajima M, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med. 2008;205:1019–1027. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naik S, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber M, et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 25.Liu SJ, et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol. 2007;82:354–360. doi: 10.1189/jlb.0207111. [DOI] [PubMed] [Google Scholar]
- 26.Lotem J, et al. Runx3-mediated transcriptional program in cytotoxic lymphocytes. PLoS One. 2013;8:e80467. doi: 10.1371/journal.pone.0080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y, et al. Thpok-independent repression of Runx3 by Gata3 during CD4+ T-cell differentiation in the thymus. Eur J Immunol. 2013;43:918–928. doi: 10.1002/eji.201242944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, et al. The zinc finger transcription factor Zbtb7b represses CD8-lineage gene expression in peripheral CD4+ T cells. Immunity. 2008;29:876–887. doi: 10.1016/j.immuni.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vacchio MS, et al. A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells. Nat Immunol. 2014;15:947–956. doi: 10.1038/ni.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setoguchi R, Taniuchi I, Bevan MJ. ThPOK derepression is required for robust CD8 T cell responses to viral infection. J Immunol. 2009;183:4467–4474. doi: 10.4049/jimmunol.0901428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkinson SR, et al. Expression of the transcription factor cKrox in peripheral CD8 T cells reveals substantial postthymic plasticity in CD4-CD8 lineage differentiation. J Exp Med. 2007;204:267–272. doi: 10.1084/jem.20061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Shea J. A first look at TH cell transcriptomes. Nat Rev Immunol. 2015;15:668. doi: 10.1038/nri3913. [DOI] [PubMed] [Google Scholar]
- 33.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogli LK, et al. 2013. T cell-derived IL-17 mediates epithelial changes in the airway and drives pulmonary neutrophilia. J Immunol 191:3100–3111, erratum (2013) 191:5318.
- 36.Ciofani M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber M, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci USA. 2008;105:20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P, et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012;490:543–546. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schraml BU, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber M, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grusdat M, et al. IRF4 and BATF are critical for CD8+ T-cell function following infection with LCMV. Cell Death Differ. 2014;21:1050–1060. doi: 10.1038/cdd.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin G, et al. A critical role of IL-21-induced BATF in sustaining CD8-T-cell-mediated chronic viral control. Cell Rep. 2015;13:1118–1124. doi: 10.1016/j.celrep.2015.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Man K, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol. 2013;14:1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- 44.Kurachi M, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol. 2014;15:373–383. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gökmen MR, et al. Genome-wide regulatory analysis reveals that T-bet controls Th17 lineage differentiation through direct suppression of IRF4. J Immunol. 2013;191:5925–5932. doi: 10.4049/jimmunol.1202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muroi S, et al. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nat Immunol. 2008;9:1113–1121. doi: 10.1038/ni.1650. [DOI] [PubMed] [Google Scholar]
- 47.Wildt KF, et al. The transcription factor Zbtb7b promotes CD4 expression by antagonizing Runx-mediated activation of the CD4 silencer. J Immunol. 2007;179:4405–4414. doi: 10.4049/jimmunol.179.7.4405. [DOI] [PubMed] [Google Scholar]
- 48.Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol. 2008;9:1131–1139. doi: 10.1038/ni.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 50.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 51.Klinger M, et al. Thymic OX40 expression discriminates cells undergoing strong responses to selection ligands. J Immunol. 2009;182:4581–4589. doi: 10.4049/jimmunol.0900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat Immunol. 2013;14:271–280. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durant L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carpenter AC, et al. The transcription factors Thpok and LRF are necessary and partly redundant for T helper cell differentiation. Immunity. 2012;37:622–633. doi: 10.1016/j.immuni.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray JP, et al. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mucida D, Salek-Ardakani S. Regulation of TH17 cells in the mucosal surfaces. J Allergy Clin Immunol. 2009;123:997–1003. doi: 10.1016/j.jaci.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee Y, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vacchio MS, Bosselut R. What happens in the thymus does not stay in the thymus: How T cells recycle the CD4+-CD8+ lineage commitment transcriptional circuitry to control their function. J Immunol. 2016;196:4848–4856. doi: 10.4049/jimmunol.1600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirota K, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grange M, et al. Active STAT5 regulates T-bet and eomesodermin expression in CD8 T cells and imprints a T-bet-dependent Tc1 program with repressed IL-6/TGF-β1 signaling. J Immunol. 2013;191:3712–3724. doi: 10.4049/jimmunol.1300319. [DOI] [PubMed] [Google Scholar]
- 62.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogawa S, Satake M, Ikuta K. Physical and functional interactions between STAT5 and Runx transcription factors. J Biochem. 2008;143:695–709. doi: 10.1093/jb/mvn022. [DOI] [PubMed] [Google Scholar]
- 64.Kanhere A, et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268. doi: 10.1038/ncomms2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kortylewski M, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 66.Gotthardt D, et al. Loss of STAT3 in murine NK cells enhances NK cell-dependent tumor surveillance. Blood. 2014;124:2370–2379. doi: 10.1182/blood-2014-03-564450. [DOI] [PubMed] [Google Scholar]
- 67.Yue C, et al. STAT3 in CD8+ T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 axis. Cancer Immunol Res. 2015;3:864–870. doi: 10.1158/2326-6066.CIR-15-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsukamoto H, Senju S, Matsumura K, Swain SL, Nishimura Y. IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age. Nat Commun. 2015;6:6702. doi: 10.1038/ncomms7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kujawski M, et al. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 2010;70:9599–9610. doi: 10.1158/0008-5472.CAN-10-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee CK, et al. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 71.Maeda T, et al. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature. 2005;433:278–285. doi: 10.1038/nature03203. [DOI] [PubMed] [Google Scholar]
- 72.Villarino AV, Gallo E, Abbas AK. STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and -independent mechanisms. J Immunol. 2010;185:6461–6471. doi: 10.4049/jimmunol.1001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taraban VY, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32:3617–3627. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 74.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pircher H, Bürki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 76.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 77.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciucci T, et al. Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut. 2015;64:1072–1081. doi: 10.1136/gutjnl-2014-306947. [DOI] [PubMed] [Google Scholar]
- 79.Manna S, et al. Histone H3 Lysine 27 demethylases Jmjd3 and Utx are required for T-cell differentiation. Nat Commun. 2015;6:8152. doi: 10.1038/ncomms9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.