Significance
Microtubule dynamics is tightly regulated during fundamental biological processes such as mitosis, thereby representing a major target for anticancer therapies. To better understand the molecular mechanisms underlying the organization of the microtubule network, we systematically investigated proteins interacting with EB1, a major regulator of microtubules dynamics. We identified a specific isoform of myomegalin, which we termed “SMYLE,” that assembles a macromolecular complex associated with the centrosome, the major microtubule-organizing center in cells, and also connected to the microtubule nucleating complex. SMYLE promoted microtubule assembly from the centrosome and subsequent stabilization of microtubules at the cell periphery. This had consequences on cell motility, mitosis, and cell-cycle progression, suggesting that SMYLE might be an important player in tumor progression.
Keywords: microtubules, centrosome, mitotic spindle, cell motility
Abstract
Control of microtubule dynamics underlies several fundamental processes such as cell polarity, cell division, and cell motility. To gain insights into the mechanisms that control microtubule dynamics during cell motility, we investigated the interactome of the microtubule plus-end–binding protein end-binding 1 (EB1). Via molecular mapping and cross-linking mass spectrometry we identified and characterized a large complex associating a specific isoform of myomegalin termed “SMYLE” (for short myomegalin-like EB1 binding protein), the PKA scaffolding protein AKAP9, and the pericentrosomal protein CDK5RAP2. SMYLE was associated through an evolutionarily conserved N-terminal domain with AKAP9, which in turn was anchored at the centrosome via CDK5RAP2. SMYLE connected the pericentrosomal complex to the microtubule-nucleating complex (γ-TuRC) via Galectin-3–binding protein. SMYLE associated with nascent centrosomal microtubules to promote microtubule assembly and acetylation. Disruption of SMYLE interaction with EB1 or AKAP9 prevented microtubule nucleation and their stabilization at the leading edge of migrating cells. In addition, SMYLE depletion led to defective astral microtubules and abnormal orientation of the mitotic spindle and triggered G1 cell-cycle arrest, which might be due to defective centrosome integrity. As a consequence, SMYLE loss of function had a profound impact on tumor cell motility and proliferation, suggesting that SMYLE might be an important player in tumor progression.
The regulation of the microtubule (MT) network is central to many fundamental biological processes, including cell polarization, cell motility, and mitosis. MTs are polarized tubulin polymers that assemble from MT organizing centers (MTOCs) at centrosomal and noncentrosomal sites and grow radially until reaching stabilizing structures. Because MT nucleation is a kinetically unfavorable process, in cells it often occurs from templates such as the γ-tubulin ring complex (γ-TuRC), a multiprotein complex in which proteins of the γ-tubulin complex organize γ-tubulin into a ring (1). Nucleation is regulated by factors that attach the γ-TuRCs to the MTOCs (2). Dimers of α-β tubulin are then processively assembled into MTs through a highly dynamic process, with MTs constantly alternating between phases of growth and shortening while exploring the cytoplasmic space (3). MT stability is regulated by MT-associated proteins that bind to the MT lattice; stable MTs often carry posttranslational modifications, such as detyrosination and acetylation (4). Recent studies indicate that tubulin acetylation contributes to regulating microtubule architecture and mechanics (5–7). MT dynamics are controlled by MT plus-end tracking proteins (+TIPs), a family of unrelated proteins, including EB1/2/3, CLASP1/2, CLIP170, the tumor suppressor APC, and the spectraplakin ACF7, that form comet-shaped structures at MT distal ends (8). End-binding 1 (EB1) is considered a major binding hub for CAP-Gly domain or SxIP motif-containing +TIPs forming a complex protein-interaction network that was proposed to regulate MT dynamics and interactions with peripheral stabilizing structures (9). While investigating the mechanism through which the ErbB2 receptor tyrosine kinase controls breast tumor cell motility, we characterized a signaling pathway whereby the ErbB2 effector Memo controls the relocalization to the plasma membrane of a protein complex comprising APC and ACF7 that captures and stabilizes MT plus-ends at the cell periphery (10–12), thereby regulating directed cell motility (13, 14). To further characterize the mechanisms underlying MT capture, we aimed to define the MT plus-end–associated protein network by characterizing the EB1 interactome. We identified an EB1-interacting isoform of myomegalin that assembled a macromolecular complex at the centrosome and defined its function in microtubule assembly, directed cell motility, and orientation of cell division.
Results
The EB1-Associated Network Includes MTOC-Associated Proteins.
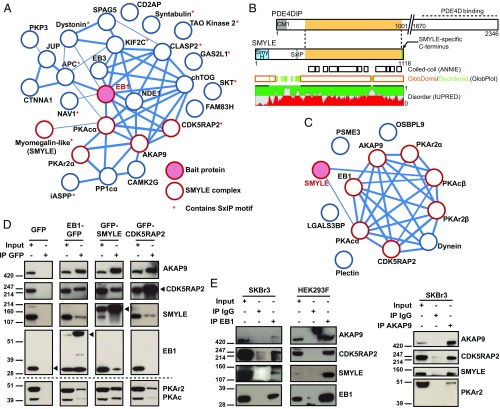
Upon addition of heregulin β (HRG), SKBr3 cells extend wide, flat protrusions populated by MTs that extend from the centrosomal area to the cell periphery. For further insights into MT plus-end complexes involved in MT extension and maintenance within cell protrusions, we investigated the EB1 protein-interaction network via affinity purification mass spectrometry. SKBr3 cells stably expressing moderate amounts of EB1-GFP displayed typical EB1 tip tracking (Fig. S1 A–C). GFP-trap pulldown from EB1-GFP cell lysates followed by label-free mass spectrometry analysis revealed a group of proteins that robustly associated with EB1 (Fig. 1A and Dataset S1). Of note, HRG treatment did not significantly modify the EB1 interactome (Dataset S1). Many proteins contained the typical SxIP motifs known to contribute to EB1 binding, suggesting that they are direct EB1 interactors. We identified EB3, known to dimerize with EB1, and several known MT plus-end–binding proteins, such as KIF2C/MCAK, dystonin, and NAV1. Unexpectedly, among the best interactors we identified a set of proteins previously known to be associated with MTOCs, in particular the cAMP-dependent PKA scaffold AKAP9 (also called “AKAP350” or “AKAP450”), regulatory and catalytic subunits of PKA, the pericentrosomal matrix protein CDK5RAP2, and a specific isoform of PDE4DIP/myomegalin. Sequence analysis of the peptides identified by tandem mass spectrometry (Fig. S1D) revealed that the 1,116-aa-long myomegalin-like protein identified here (UniProt accession no. E9PL24) is clearly distinct from the originally identified 2,346-aa myomegalin (UniProt accession no. Q5VU43; Human Genome Organization Gene Nomenclature Committee: phosphodiesterase 4D-interacting protein, PDE4DIP) (15). It has a specific 373-aa N-terminal sequence lacking the CM1 motif, aligns with PDE4DIP from residues 374–1,102, lacks the phosphodiesterase 4D-binding region, and has a unique 14-aa C-terminal sequence (Fig. 1B and Fig. S1D). The protein, which we termed “SMYLE” (for short myomegalin-like EB1-binding protein) to prevent confusion with the original long isoform, differs from the previously described Golgi-associated EB–MMG at its C terminus (16) but is identical to the Golgi-associated protein (MMG8) identified by Wang et al. (17). Extensive analysis of SMYLE sequences across distant species through homologous sequence alignments, secondary structure prediction, and computation of conservation scores revealed the presence of a well-conserved structured domain that we called the “SMYLE homology (SmyH) domain” in the N-terminal region (residues 1–101) (Fig. S2). The less conserved region of the SMYLE-specific N terminus was intrinsically disordered (Fig. 1B) and contained a short SxIP-like motif (residues 304–314), that was systematically associated with the SmyH domain.
Fig. 1.
Characterization of the SMYLE protein interaction network. (A) EB1-GFP interaction partners in SKBr3 cells were identified by GFP-Trap pulldown and mass spectrometry analysis. Proteins identified in at least two of three independent experiments were integrated in a protein–protein interaction network based on physical and functional interactions per the STRING database. Edge thickness reflects confidence. Proteins that our study identified as part of the SMYLE complex are indicated. (B) Myomegalin sequence analysis. Note that SMYLE harbors neither the centrosome-targeting CM1 motif nor the C terminus known to interact with PDE4D but contains a SxIP-like motif within a disordered region, a SmyH domain as shown in Fig. S2, and a specific C terminus. (C) The SMYLE interactome was characterized as in A. Proteins identified in at least two of four independent experiments were integrated in a protein–protein interaction network per the STRING database. (D and E) Protein–protein interactions were confirmed by GFP pulldown (D) or immunoprecipitation (IP) of endogenous EB1 or AKAP9 (E), followed by Western blotting with the specified antibodies. The arrowhead points to the GFP-tagged bait proteins.
We generated cell lines stably expressing moderate levels of GFP-CDK5RAP2 or GFP-SMYLE and analyzed associated proteins by mass spectrometry (Fig. S1 B and C). CDK5RAP2 pulled down AKAP9, SMYLE, and a regulatory subunit of PKA (Fig. S1E and Dataset S1). SMYLE robustly bound to a limited number of proteins including AKAP9, PKA regulatory and catalytic subunits, EB1, and CDK5RAP2 (Fig. 1C and Dataset S1).
Interaction of EB1 with its partners was confirmed by Western blotting analysis of the EB1-GFP pulldown (Fig. 1D) and also by immunoprecipitation of endogenous EB1 in both SKBr3 and HEK293F cells (Fig. 1E). Pulldown of GFP-CDK5RAP2 and GFP-SMYLE and Western blotting confirmed the existence of the reciprocal interactions (Fig. 1D). Since we could not express GFP-AKAP9 in SKBr3 cells, we performed immunoprecipitation of endogenous AKAP9, which confirmed the interaction with the identified partners of the SMYLE complex (Fig. 1E). Together these data show that the plus-end tracking protein EB1 is part of a megadalton protein complex including the large scaffolding proteins AKAP9 and CDK5RAP2 usually found in association with MTOCs and SMYLE, an isoform of PDE4DIP with specific structural features.
A Centrosome-Associated SMYLE Complex.
To perform further molecular and functional studies of the complex, we designed and validated siRNAs to knock down each of the candidate partners (Fig. S3A). SMYLE is the major isoform of myomegalin in the cell lines used in this study (Fig. S3B). We designed an siRNA (SMYLE siRNA no. 2) that targets a sequence unique to SMYLE and is not present in the originally described PDE4DIP or in EB1-MMG. Of note, lipid-mediated siRNA targeting of SMYLE RNA led to the down-regulation of both SMYLE and AKAP9, and, reciprocally, targeting AKAP9 inhibited SMYLE expression (Fig. S3A), as documented previously (17).
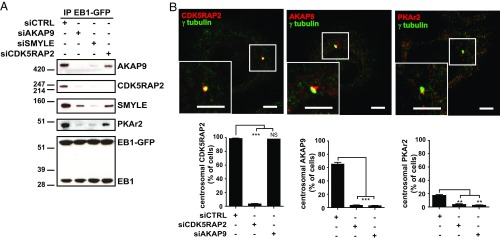
These siRNAs allowed us to further explore the organization of the SMYLE complex. Both SMYLE and CDK5RAP2 possess a potential SxIP EB1-binding motif. To understand which proteins interact with EB1 directly and for insight into the hierarchical organization of the complex, we knocked down SMYLE, AKAP9, or CDK5RAP2 before EB1 pulldown. We observed that depleting SMYLE or AKAP9 prevented EB1 binding to CDK5RAP2, while depletion of CDK5RAP2 did not disturb EB1 association with SMYLE, AKAP9, and PKA, indicating that SMYLE/AKAP9 mediates the binding of EB1 to CDK5RAP2 (Fig. 2A).
Fig. 2.
Hierarchical organization of the SMYLE complex at the centrosome. (A) AKAP9 and SMYLE mediate CDK5RAP2 binding to EB1. To define the hierarchy of interaction with EB1, AKAP9, SMYLE, and CDK5RAP2 protein expression was knocked down before EB1-GFP pulldown and Western blotting analysis. (B, Upper) CDK5RAP2, AKAP9, and PKA colocalize with γ-tubulin in SKBr3 cells. (Scale bars: 5 μm.) (Lower) AKAP9 and PKA centrosomal localization in SKBr3 cells depends on CDK5RAP2. Graphs show the percentage of cells displaying CDK5RAP2, AKAP9, and PKAr2 association with the centrosome after CDK5RAP2 or AKAP9 siRNA-mediated depletion. See typical immunofluorescence images in Fig. S4. Data presented are the mean ± SEM from three independent experiments, 150 cells per data point; **P < 0.01, ***P < 0.001, NS (not significant) P > 0.05.
We then investigated the hierarchical organization of the complex within the cell. Previous studies have observed CDK5RAP2 and AKAP9 at the Golgi apparatus (18, 19). However, we found that in SKBr3 cells endogenous CDK5RAP2, AKAP9, and PKA colocalized with γ-tubulin at the centrosomes in 98, 65, and 17% of the cells, respectively (Fig. 2B). CDK5RAP2 also colocalized with AKAP9 at the centrosome in HEK293F cells (Fig. S4A). We observed occasional AKAP9 colocalization with the Golgi marker GM130 in SKBr3 cells. However, in contrast to centrosome-associated AKAP9 labeling, Golgi-associated staining was never decreased upon AKAP9 siRNA and thus had to be considered nonspecific (Fig. S4B). Thus, AKAP9 and CDK5RAP2 were associated with the centrosomal area, but not with the Golgi apparatus, in SKBr3 and HEK293F cells. Importantly, CDK5RAP2 knockdown (Fig. S3C) abolished the centrosomal localization of AKAP9 and PKA, whereas AKAP9 depletion (Fig. S3B) had no effect on CDK5RAP2 localization (Fig. 2B and Fig. S5). As expected, PKA recruitment to the centrosomes was dependent on AKAP9 and thus on CDK5RAP2 (Fig. 2B and Fig. S5).
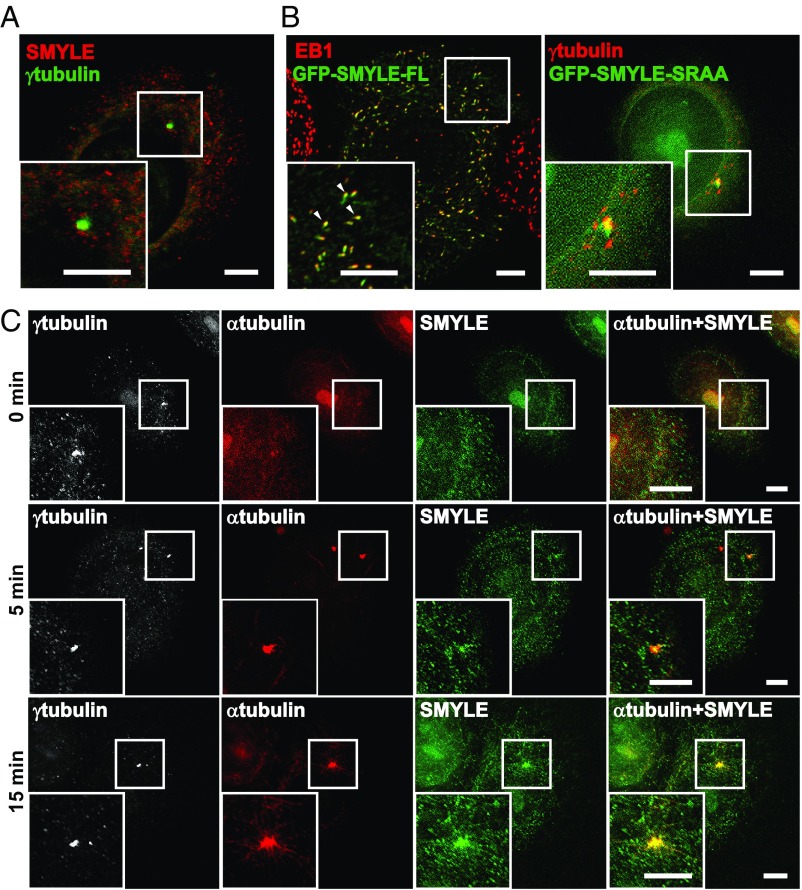
We have produced a monoclonal antibody directed against the C-terminal peptide specific for the SMYLE isoform, and validated the antibody for Western blotting and immunofluorescence (Fig. S6 A and B). However, using this antibody, we could not detect SMYLE labeling at the centrosome (Fig. 3A). Exploration of previously published proteomic data (20) indicates the presence of PDE4DIP isoforms in centrosome-enriched fractions, suggesting that endogenous SMYLE might be present below immunofluorescence detection levels. We investigated ectopically expressed SMYLE. GFP-SMYLE did not localize at the Golgi apparatus (Fig. S4C) but associated with EB1 comets at MT ends (Fig. 3B). Interestingly, mutation of the SMYLE SxIP-like motif to SRAA prevented GFP-SMYLE plus-end binding and revealed GFP-SMYLE’s association with the centrosomal area (Fig. 3B). To confirm that endogenous SMYLE interacted with EB1 in the cellular context (and not only after cell lysis), we produced and selected SKBr3 clones stably expressing EB1 fused to the promiscuous biotin ligase BirA (R118G mutant) (Fig. S7A) (21). The addition of biotin to the cultured cells triggered the biotinylation of EB1 neighbor proteins before cell disruption. Mass spectrometry analysis of the isolated biotinylated proteins identified SMYLE, together with known partners of EB1, as a genuine partner of EB1 (Fig. S7B).
Fig. 3.
SMYLE associates with newly assembled centrosomal MTs. (A) SMYLE was immunolabeled with an antibody directed against the SMYLE-specific C terminus. No centrosomal labeling was detectable at steady state. (B) SKBr3 cells expressing full-length (FL) GFP-SMYLE or the GFP-SMYLE SRAA mutant were immunostained for GFP, EB1, or γ-tubulin. Ectopically expressed SMYLE colocalized with EB1 at MT plus-ends (arrowheads), while the SMYLE SRAA mutant relocalized to the centrosomal area. (C) MT disassembly was induced by cold and nocodazole treatment. Cells were fixed 0, 5, and 15 min after washout before coimmunolabeling with γ-tubulin, α-tubulin, and SMYLE. SMYLE decorates MTs at and extending from the centrosome. (Scale bars: 5 μm.)
We assumed that we might facilitate the detection of endogenous SMYLE in the centrosomal area by disturbing the dense MT network. Thus, we induced MT depolymerization via nocodazole and cold treatment. Immunofluorescence analysis using the specific anti-SMYLE antibody revealed that, after nocodazole washout, SMYLE was associated with newly nucleated MTs at the centrosome (Fig. 3C and Fig. S6C).
Architecture of the SMYLE Complex.
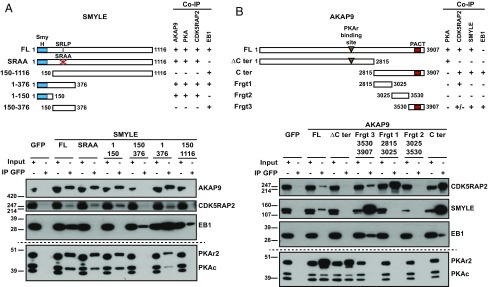
We explored the interaction of SMYLE with its partners through a biochemical approach (Fig. 4A). The SMYLE region residues 1–376, unique to this isoform, was able to recruit all partners of SMYLE: AKAP9, PKA, CDK5RAP2, and EB1. The SRLP-to-SRAA mutation prevented the interaction of SMYLE with EB1, as expected, but preserved AKAP9 and CDK5RAP2 binding. The very N-terminal region of SMYLE (residues 1–150), which included the conserved SmyH domain, was required and sufficient for AKAP9 and CDK5RAP2 binding.
Fig. 4.
Molecular mapping of protein–protein interactions within the SMYLE complex. GFP-labeled full-length (FL) and deletion-mutant SMYLE (A) or AKAP9 (B) cDNA were expressed in HEK293F cells before pulldown and Western blotting with the indicated antibodies. (A) SMYLE interacts with AKAP9, PKA, and CDK5RAP2 via the SmyH domain-containing N terminus and with EB1 via the SxIP-like SRLP sequence. (B) AKAP9 interacts with SMYLE via the PACT-containing C terminus. Interaction with CDK5RAP2 involves both the residues 2815–3025 and the PACT-containing regions. Co-IP, coimmunoprecipitation.
Furthermore, the C-terminal fragment of AKAP9, residues 2815–3907, was required and sufficient to recruit CDK5RAP2, SMYLE, and EB1 (Fig. 4B). CDK5RAP2 bound mostly with the 2815–3025 region, while SMYLE (and EB1, via SMYLE) interacted more specifically with the 3530–3907 sequence that includes the PACT domain.
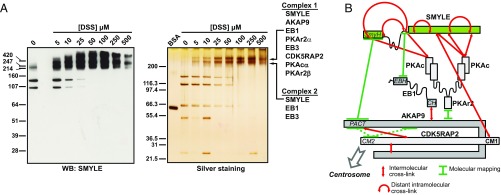
We gained further insights into the organization of the complex via cross-linking of proximal lysine residues and mass spectrometry analysis (22). We first purified the native SMYLE complex via Strep-Flag tandem-affinity purification (Fig. S7C). Upon the addition of the short-range cross-linker disuccinimidyl suberate (DSS), SMYLE was completely retained within two major high-molecular-weight complexes that could be distinguished on SDS/PAGE (Fig. 5A). Mass spectrometry analysis indicated that the high-mobility complex (complex 2) included essentially SMYLE associated with EB1 and EB3, whereas the low-mobility complex (complex 1) also included AKAP9, PKA regulatory and catalytic subunits, and, to a lesser extent, CDK5RAP2 (Dataset S2). Initial mass spectrometry-based identification of cross-linked peptides revealed only SMYLE–SMYLE dipeptides (Dataset S2). Cross-linking of distant Lys residues suggested possible folding of the molecule, in particular across the intrinsically disordered region that includes the EB1-binding site (Fig. S7D). We also identified unambiguous intermolecular self-links within the SMYLE C-terminal region indicative of SMYLE oligomerization (Fig. S7D). To detect intermolecular cross-links, we scaled up the experiment 10-fold. Dipeptide search with pLINK detected around 80 potential cross-links between complex components (Fig. S7E and Dataset S2). Manual curation of cross-linked peptides confirmed a small set of cross-links (Fig. S7F and Dataset S2) and underscored an unexpected proximity between SMYLE and a PKA catalytic subunit. Together, molecular mapping and cross-linking mass spectrometry provided complementary insights into the convoluted organization of the SMYLE complex (Fig. 5B).
Fig. 5.
Identification of SMYLE supramolecular complexes via cross-linking mass spectrometry. (A) Increasing concentrations of the DSS cross-linker were added to TAP-tag–purified native SMYLE complex before SDS/PAGE separation of the cross-linked complexes. (Left) Western blotting (WB) analysis showed that the addition of the cross-linker induced a complete shift of SMYLE toward high-molecular-weight complexes. (Right) Silver staining revealed two major SMYLE complexes. Composition of the two complexes, as determined by mass spectrometry (Dataset S2), is indicated. (B) Schematic organization of the SMYLE complex, as determined by molecular mapping (Fig. 4) and cross-linking mass spectrometry (Dataset S2 and Fig. S7 D–F) analyses. Previous data indicate that CDK5RAP2 interacts with AKAP9 via the CM2 domain (dashed lines).
Interaction of SMYLE with both AKAP9 and EB1 Is Required for MT Extension to the Cell Periphery.
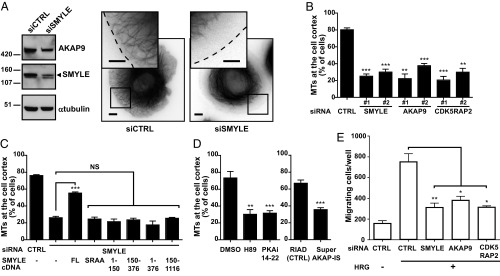
We then evaluated the impact of SMYLE silencing on the MT network. We used nucleofection-mediated instead of lipid-mediated SMYLE siRNA transfection, since we observed that it had a more limited impact on AKAP9, allowing us to distinguish the effects directly due to SMYLE (Fig. 6A). Using the siRNA that targets myomegalin short isoforms EB-MMG and SMYLE (siRNA no. 1) or uniquely SMYLE (siRNA no. 2), we found that SMYLE depletion (Fig. S3D) led to defective peripheral MTs (Fig. 6 A and B) in live GFP-α-tubulin–expressing SKBr3 cells. The knockdown of AKAP9 or CDK5RAP2 (Fig. S3D) led to the same defect in cortical MT distribution at the cell periphery (Fig. 6B).
Fig. 6.
The SMYLE complex is required for MTs extension to the cell cortex and directed migration. (A–D) MT extension to the cell cortex was examined in GFP-α-tubulin–expressing SKBr3 cells upon the addition of HRG. (A, Right) Snapshots of control (CTRL) and SMYLE siRNA-treated SKBr3 cells with a zoom on the cortical area. (Scale bars: 5 μm.) (Left) Representative Western blots. (B) Impact of the depletion of SMYLE, AKAP9, or CDK5RAP2, using two distinct siRNAs for each targeted mRNA, on the percentage of cells having extended MTs. (C) Quantification of MT extension to the cell cortex upon expression of full-length (FL) or mutant SMYLE constructs in endogenous SMYLE-knockdown cells. The ability to bind EB1 and AKAP9 is required but not sufficient for SMYLE function. (D) Inhibition of PKA activity via the H89 small molecule or the PKAi 14-22 inhibitory peptide or prevention of PKAr2 anchoring to AKAP via the SuperAKAP-IS peptide precludes stabilization of cortical MTs. Data in B–D show the mean ± SEM from three independent experiments, 150 cells per data point. (E) SKBr3 cell migration in response to HRG was evaluated in control cells and SMYLE-, AKAP9-, or CDK5RAP2-depleted cells in Boyden-like chambers; Data presented are the mean ± SEM of cell numbers from triplicate wells in three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001; NS (not significant) P > 0.05.
Reexpression of wild-type SMYLE in SMYLE knockdown cells restored MTs at the cell periphery, confirming that the effect was due to SMYLE loss of function (Fig. 6C and Fig. S3E). In contrast, EB1 binding-defective SMYLE (SMYLESRAA) or AKAP9 binding-defective SMYLE (SMYLE150–1116) failed to restore peripheral MTs (Fig. 6C and Fig. S3E), demonstrating that SMYLE functions only in the presence of the intact complex. Of note, the N-terminal fragment (residues 1–376) bound EB1, AKAP9, PKA, and CDK5RAP2 (Fig. 4A) but was unable to restore normal MTs (Fig. 6C), showing that SMYLE function also involves the coiled-coil regions.
A similar functional analysis showed that the AKAP92815–3907 C-terminal region, which efficiently bound CDK5RAP2, SMYLE, and EB1 but not PKA (Fig. 4B), was not functional (Fig. S3F), suggesting that other AKAP9 regions that include the PKA-binding motifs are also required for function. Pharmacological inhibition of PKA activity led to defective cortical MTs (Fig. 6D). Similarly, disrupting AKAP anchoring of PKAr2 via the SuperAKAP-IS competitive peptide (23) led to microtubule defects, showing that AKAP-associated PKA activity is required for stabilization of cortical MTs (Fig. 8A).
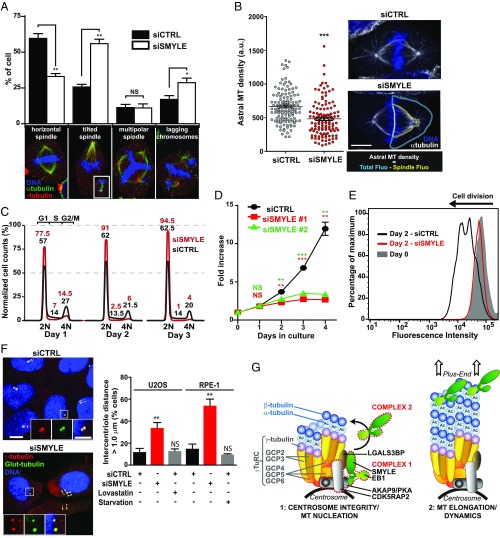
Fig. 8.
SMYLE contributes to mitotic spindle orientation and centrosome cohesion. (A) Metaphase figures were evaluated in control or SMYLE-depleted metaphase HeLa cells, as exemplified below the graph, in three independent experiments (n = 380 cells for SMYLE siRNA and 348 cells for control siRNA). (Magnification, 735×.) The Inset (image reduced by a factor of 2) shows the opposite pole of the same tilted spindle in a different focal plan. SMYLE depletion disturbs mitotic spindle orientation and chromosome alignment. (B) Effect of SMYLE on HeLa cell astral MT density. The fluorescence intensity of astral MTs was determined as depicted on the right; n = 120 half spindles per experiment in two independent experiments. (Scale bar: 10 μm.) (C) Typical cell-cycle profile (representative of two experiments) of SMYLE-depleted HeLa cells 1–3 d after knockdown shows drastic G1 phase accumulation relative to control. (D) SMYLE impacts cell proliferation. SMYLE expression was knocked down using two different siRNAs. HeLa cell numbers were quantified in three independent experiments and expressed relative to cell numbers at day 0. (E) SMYLE-knockdown cells failed to exit G1. Starved RPE-1 cells accumulated in G0/G1, were loaded with CellTrace Violet to track population doubling, and then were treated with SMYLE siRNA for 48 h to deplete SMYLE in G1-arrested cells. Flow cytometry was performed before (day 0) and 2 d after the addition of serum. The result is representative of two experiments. (F) SMYLE silencing affects centriole cohesion. Intercentriole distance (double arrowheads) was measured in control cells, SMYLE-depleted cells, and G1-arrested RPE-1 and U2OS cells. The percentage of cells showing intercentriole distance >1 μm was determined in two experiments (120 cells). (Scale bar: 10 μm; scale bar in Inset: 5 μm.) Graphs in A, B, D, and F show mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, NS (not significant) P > 0.05. (G) A model illustrating how SMYLE might form distinct complexes that control centrosome function and the early steps of MT assembly on the one hand and MT dynamics on the other hand.
SMYLE Promotes MT Nucleation and Is Connected to the γ-TuRC via LGALS3BP.
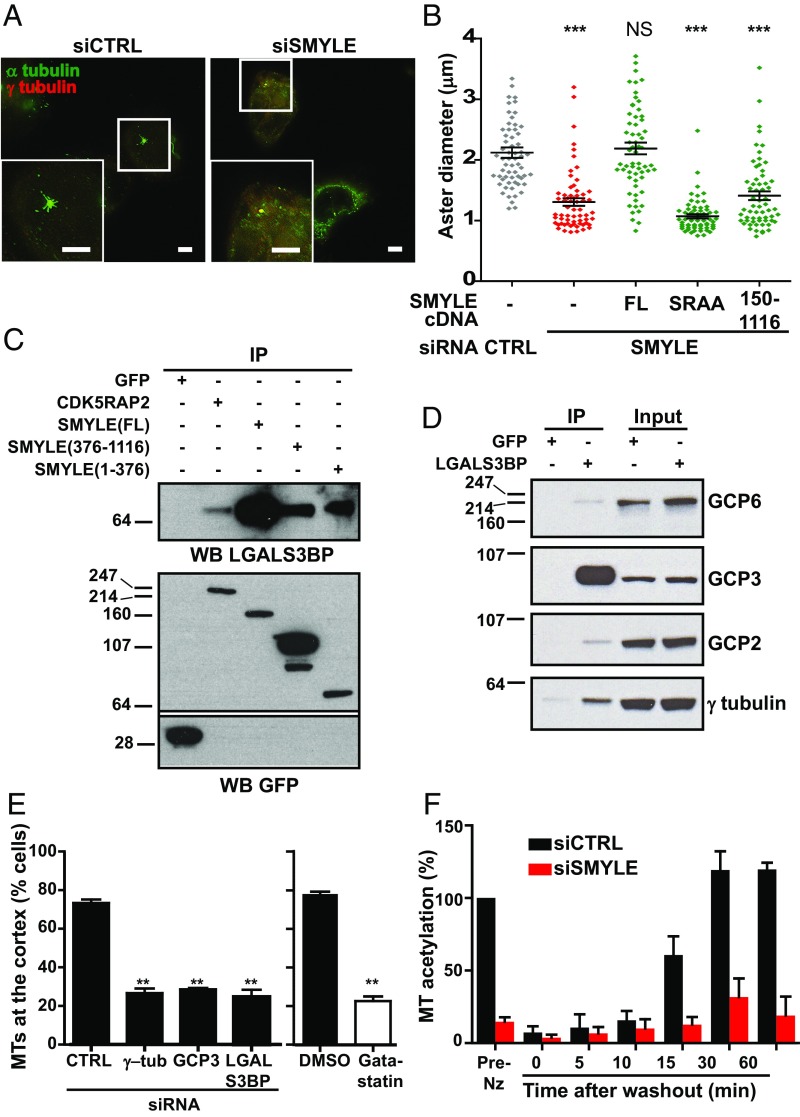
In other cellular models, myomegalin-like proteins associate with the Golgi apparatus where they contribute to the regulation of MT nucleation (17, 24). Our cellular model provided the opportunity to explore the potential contribution of the SMYLE complex to MT assembly from the centrosome. We induced the depolymerization of MTs in SKBr3 cells by nocodazole and cold treatment and followed the early steps of MT regrowth from the centrosome after nocodazole washout. We observed that, in the absence of SMYLE, MT aster formation was significantly slowed (Fig. 7 A and B), indicating that SMYLE contributes to MT nucleation. Reexpression of SMYLE restored nucleation, but SMYLESRAA and SMYLE150–1116 did not (Fig. 7B), indicating that nucleation-promoting activity required both EB1 and AKAP9 binding. Previous studies investigating Golgi-associated myomegalin suggested that it interacted with γ-tubulin and γ-tubulin–associated proteins. SMYLE interactome in SKBr3 or HEK293F cells did not reveal an interaction with γ-tubulin. However, proteomic analysis (Fig. 1C and Fig. S1E) showed that SMYLE and CDK5RAP2 bound LGALS3BP, a protein that was associated with the centrosome (25). We thus evaluated whether LGALS3BP might mediate the interaction of the SMYLE complex with the γ-TuRC and found that SMYLE (and to a lesser extend CDK5RAP2) interacted with a 65-kDa form of LGALS3BP (Fig. 7C), corresponding to the nonglycosylated protein (26). This interaction required both the SMYLE N-terminal and coiled-coiled regions. In turn, LGALS3BP coprecipitated γ-TuRC proteins, most robustly GCP3 but also GCP2, GCP6, and γ-tubulin (Fig. 7D), indicating that LGALS3BP is a connecting point between the centrosome-associated SMYLE complex and the γ-TuRC MT-nucleating complex.
Fig. 7.
SMYLE contributes to MT nucleation and acetylation and is linked to the γ-TuRC via LGALS3BP. (A and B) MT disassembly was induced by cold and nocodazole treatment of SKBr3 cells. (A) MT assembly was examined 5 min after nocodazole washout by immunolabeling α-tubulin and γ-tubulin. (Scale bars: 5 μm.) (B) MT aster regrowth was quantified in control cells, SMYLE-depleted cells and cells reexpressing GFP-SMYLEFL, -SMYLESRAA, or -SMYLE150–1116. Both EB1- and AKAP9-binding– defective SMYLE failed to rescue MT nucleation. Aster diameter was measured in 60 asters per data point in two independent experiments. Black lines indicate mean ± SEM. ***P < 0.001; NS, not significant (P > 0.05). (C) Coprecipitation of LGALS3BP with GFP-tagged CDK5RAP2, SMYLEFL, SMYLE1–376, and SMYLE376–116 constructs expressed in SKBr3 cells. (D) GFP-LGALS3BP expressed in SKBr3 cells pulled down γ-TuRC components. (E) Interfering with pericentrosomal/γ-TuRC proteins affects properties of MTs at the cell periphery (evaluated as in Fig. 6). **P < 0.01. (F) SMYLE depletion leads to decreased levels of acetylation in newly assembled MTs. MT acetylation levels after nocodazole washout were determined by Western blotting and quantified. Data shown are the mean ± SEM from three independent experiments. Data were normalized to α-tubulin amounts and pre-nocodazole treatment (Pre-Nz) levels. Representative Western blot and immunofluorescence are shown in Fig. S8.
As our data showed that the SMYLE complex regulated centrosomal MT nucleation on the one hand and peripheral MT distribution on the other hand, we wondered whether the two events might be related. Targeting the MT nucleation complex with siRNA (against γ-tubulin or GCP3) or with the γ-tubulin inhibitor gatastatin (27), we observed that both molecular and pharmacological inhibition of γ-TuRC activity prevented the extension of MTs within SKBr3 cell protrusions (Fig. 7E and Fig. S3I), in accordance with a model connecting early steps of MT assembly with the formation of peripheral MTs. Interestingly, similar results were obtained upon silencing LGALS3BP (Fig. 7E and Fig. S3I), supporting its tight connection to the γ-TuRC function.
It is not yet clear how SMYLE might affect MT properties, leading to defective peripheral MTs. However, we have observed that newly assembled MTs were heavily acetylated and, importantly, that SMYLE silencing led to a drastic reduction in MT acetylation from the earliest time point observed, as indicated by semiquantitative Western blotting (Fig. 7F and Fig. S8A) and immunofluorescence (Fig. S8B). As recent studies indicate that acetylation modifies MT structure, enhancing mechanical resilience (7), the reduced acetylation associated with SMYLE silencing might also contribute to the defect in peripheral MTs.
SMYLE Silencing Disturbs Cancer Cell Migration, Mitosis, and Cell-Cycle Progression.
We have repeatedly observed that a normal distribution of peripheral MTs is required for directed migration (13, 14, 28). We thus evaluated the contribution of SMYLE and SMYLE-associated proteins to HRG-induced directed cell migration and observed that depletion of SMYLE, AKAP9, or CDK5RAP2 led to a strong inhibition of SKBr3 cell motility (Fig. 6E).
Centrosome-nucleated MTs are critically involved in cell mitosis. Thus, we investigated whether SMYLE also contributed to cell division. SMYLE knockdown in HeLa cells led to a drastic reduction in the number of mitotic figures but increased mitotic abnormalities, including imperfect chromosome alignments (Fig. 8A). Interestingly, upon SMYLE silencing, we observed a significant reduction of astral MTs (Fig. 8B). HeLa cells cultured on collagen or fibronectin orient their mitotic spindle parallel to the substrate before dividing, a phenomenon which is under the control of astral MTs (29). Accordingly, in control cells, 60% of HeLa cells had a horizontal bipolar spindle, and 33% showed a tilted bipolar spindle (the remainder of the cells had multipolar spindles). SMYLE knockdown strikingly increased the number of misoriented spindles (Fig. 8A).
We evaluated the consequences of SMYLE knockdown (Fig. S3G) on HeLa cell-cycle progression (Fig. 8C). Upon SMYLE silencing, there was a rapid and persistent accumulation of HeLa cells in the G1 phase of the cycle, with a concomitant reduction in both S and G2/M cell counts. SMYLE knockdown induced a similar G1 arrest in RPE-1 cells (98% of the cells in G1 vs. 63% in control cells). In accordance with the observed cell arrest in the G1 phase, siRNA-mediated down-regulation of SMYLE led to a striking decrease in HeLa cell proliferation relative to control cells (Fig. 8D).
G1 arrest might be the consequence of defects in late mitosis, as we have seen that SMYLE-depleted cells showed imperfect chromosome alignment (Fig. 8A); alternatively, it might be induced within G1. To address this issue, we evaluated the impact of SMYLE silencing in postmitotic cells. RPE-1 cells were accumulated in G0–G1 by serum starvation, before treatment with siRNA to deplete SMYLE. Upon the addition of serum, a majority of control cells underwent one or two divisions, whereas most SMYLE-depleted cells failed to divide (Fig. 8E), showing that G1 arrest can be triggered from within G1 and that abnormal mitotic events, such as spindle or cytokinesis dysfunctions, are not required to induce the arrest. We then examined whether G1 arrest of SMYLE knockdown cells might be the consequence of defective centrosome function, as observed previously upon silencing of other centrosome-associated proteins (30, 31). We observed that SMYLE silencing led to defective centrosome cohesion in both U2OS and RPE-1 cells, with a strong increase in the population of cells showing separated centrioles (Fig. 8F and Fig. S3H). This was not a mere consequence of cell-cycle arrest, as lovastatin- or starvation-arrested G1 cells did not show increased intercentriolar distance (Fig. 8F). Together, these results suggest that SMYLE knockdown leads to defective centrosome function which might contribute to G1 arrest.
Discussion
Searching for proteins that associate with EB1, a major regulator of MT dynamics, we characterized a protein complex that contributes to centrosomal MT functions. Indeed, we found that the EB1-binding isoform of myomegalin, SMYLE, organizes a large protein complex that supports de novo MT assembly at the centrosome and has an impact on MT distribution at the cell periphery. This has critical consequences for directed migration, mitotic spindle orientation, and cell-cycle progression.
In contrast to previous reports (19, 26), we observed that CDK5RAP2 and AKAP9 localized to the centrosomal area and not to the Golgi apparatus. Centrosome vs. Golgi localization is, in fact, cell line-dependent, as we found that AKAP9 was exclusively observed at the centrosome of SKBr3 and HEK293F cells but was present at the Golgi and the centrosome of RPE-1 and MDA-MB231 cells. In accordance, we detected SMYLE only in the centrosomal area and never at the Golgi apparatus of SKBr3 cells. Moreover, the SMYLE interactome in SKBr3 and HEK293F cells did not reveal proteins involved in MTOC anchoring to the Golgi apparatus, such as GM130, CLASP2, or CAMPSAP (19, 26, 32). Thus, while previous studies have underlined the function of myomegalin proteins at the Golgi apparatus, our cellular models allow us to specifically analyze the impact of the SMYLE isoform associated with the centrosome.
Previous data suggest that the Golgi-associated myomegalin regulates nucleation via its interaction with γ-tubulin (17, 18). While γ-tubulin was not present in SMYLE pulldowns, we found that SMYLE coprecipitated LGALS3BP. LGALS3BP (also known as “90K” or “Mac-2 BP”) is a glycosylated, secreted molecule that has been mostly investigated for its role in cell adhesion in tumor and immune functions and as a circulating marker for tumor progression (33). Of note, SMYLE interacted with a 65-kDa form of LGALS3BP, which corresponds to the protein without N-linked glycans, suggesting that unglycosylated LGALS3BP might have specific intracellular functions associated with the centrosome. A recent study suggested that LGALS3BP regulated centriole biogenesis without distinguishing between glycosylated and unglycosylated forms (27). Interestingly, examination of large-scale proteomic analyses (34, 35) reveals that LGALS3BP is one of the few proteins to systematically copurify with γ-TuRC components. We found that, in SKBr3 cells LGALS3BP associates with γTuRC proteins and especially with GCP3, which plays a key role in allosteric γ-TuRC activation (36). We propose that unglycosylated LGALS3BP functions as a linker between the centrosomal SMYLE complex and the γ-TuRC. It will clearly be important to investigate LGALS3BP’s precise mode of action and determine whether it functions as a tether, as an adapter that brings components such as EB1 or PKA closer to the γ-TuRC, or as a switch that transmits an activating signal to the γ-TuRC.
Subcellular distribution and cross-linking experiments revealed that SMYLE belongs to at least two distinct complexes, one containing the whole SMYLE complex (including AKAP9, EB1/EB3, PKA subunits, and CDK5RAP2) and the other including only SMYLE and EB1/EB3. Our data also suggest interplay between SMYLE centrosomal and plus-end localizations: Preventing SMYLE–EB1 interaction had a direct consequence on SMYLE relocalization from the MT plus-end to the centrosomal area. It is tempting to speculate that the two observed complexes might be associated with distinct functions in the centrosomal area and at MT plus-ends (Fig. 8G).
The nature of the connection between SMYLE function at the centrosome and its role in stabilizing MTs at the cell periphery is an intriguing question. We have clearly shown that in SKBr3 cells impacting the early steps of MT assembly (via disturbance of the γ-TuRC) has consequences on cortical MTs. The mechanism, however, remains elusive. The defect of MTs at the periphery might be the consequence of abnormal MT dynamics due to altered EB1 function. Indeed, we have previously demonstrated that even a small decrease in the speed of MT growth induced by low concentrations of a MT-targeting drug was sufficient to lead to defective MT distribution at the periphery of migrating cells (28). Another possibility is that the SMYLE complex at the minus end enforces a structural change of the MT lattice that is propagated along the length of the MT wall. In this regard, it is remarkable that SMYLE has a strong influence on MT acetylation. Given recent data showing that MT acetylation modifies MT protofilaments cohesion, thus promoting resilience to mechanical stress (6, 7), SMYLE knockdown might end up undermining the extending MTs.
Abnormal centrosome MT nucleating function had an impact not only on directed cell migration but also on mitosis. In animal cells, oriented cell division involves the transmission of localized pulling forces located at the cell cortex to astral MTs, resulting in spindle positioning. SMYLE knockdown strongly compromises mitotic spindle planar orientation, which most likely results from the observed reduction in astral MT density. Our data also show that SMYLE knockdown leads to HeLa and RPE-1 cells’ accumulation in the G1 phase of the cycle. While this could be the consequence of defects in late mitosis, our data indicate that the G1 accumulation most probably results from the activation of a centrosome damage checkpoint (30, 31). Precisely how SMYLE controls centrosome function remains to be determined.
While deciphering the molecular basis of the cross-talk between the SMYLE complex and the γ-TuRC represents a challenge because of the large size of the complexes, it appears a necessary task if one wants to understand SMYLE’s mode of action and potential contribution to oncogenic progression.
Materials and Methods
Protein Pulldowns and Analysis by Mass Spectrometry.
Cells expressing the GFP-tagged constructs were lysed, and GFP pulldowns were performed using GFP-Trap agarose beads (ChromoTek). The samples were either immunoblotted or analyzed by mass spectrometry as described previously (15). For purification of the native complex, HEK293F cells stably expressing SF-SMYLE were lysed in 0.5% Nonidet P-40 lysis buffer. Cell lysates were incubated with Strep-Tactin Superflow resin (IBA) before elution with 2 mM biotin. The eluates then were incubated with anti-Flag M2 agarose (Sigma-Aldrich) and eluted with 200 μg/mL Flag peptide. The isolated complexes samples were cross-linked by adding 50 μM DSS (Creative Molecules), prepared from an equimolar mixture of isotopically light [d0]- and heavy [d12]-labeled DSS, for 30 min at 37 °C. Cross-linking was quenched by the addition of 50 mM ammonium bicarbonate. Cross-linked samples were run in SDS/PAGE. Coomassie-stained bands were cut into small pieces before cysteine reduction, alkylation, and trypsin digestion. Resulting peptides were analyzed by nano-LC coupled to MS/MS (Q-Exactive Plus; Thermo Fisher). Raw data were converted into .mgf files using msconvert and were submitted to a combined target-decoy database search via the Mascot (version 2.5.1) search engine. Then, .mgf files were submitted to a search for cross-linked peptides with pLINK (37). Only peptides cross-linked with both [d0]- and [d12]-DSS were considered positive. A manual validation was performed to confirm the identification.
Analysis of Cortical MTs by Time-Lapse Microscopy.
Detailed procedures have been described previously (12). Briefly, SKBr3 cells cotransfected with EGFP-α-tubulin and the indicated siRNA or cDNA were grown on collagen-coated glass coverslips for 48 h and observed upon the addition of 5 nM HRGβ1 (R&D Systems) using the 63× objective (plan Apochromat NA 1.4) of a fluorescence microscope (Zeiss Axiovert 200) driven by MetaMorph 6.3 software. When indicated, cells were pretreated with 10 μM H89, 10 μM PKA inhibitor 14-22 myristoylated peptide (Merck Millipore), or 30 μM gatastatin (a kind gift of T. Usui, University of Tsukuba, Tsukuba, Japan). Images were acquired using a CoolSNAP HQ digital camera (Roper).
MT Regrowth Assay.
MTs were completely depolymerized by treating cells with 10 µM nocodazole (Sigma-Aldrich) for 1 h on ice. To initiate microtubule regrowth, nocodazole-treated cells were washed twice with ice-cold PBS and then were incubated in prewarmed medium containing 5 nM HRG. Cells were permeabilized for 15 s in PHEM (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, 4 mM MgSO4, pH 7.0) buffer containing 0.25% Triton X-100 and 320 mM sucrose and were fixed with −20 °C methanol for 5 min before immunolabeling of α- and γ-tubulin. MT aster diameter was measured with ZEN software (Zeiss).
Analysis of Mitotic Cells and Centrosome Defects.
HeLa cells seeded on collagen-coated coverslips were fixed with 4% formaldehyde and permeabilized with 0.2% Triton X-100. Cells were stained with DAPI, and antibodies against α- and γ-tubulin. Mitotic cells were detected on the basis of metaphase condensed DNA. Mitotic figures were categorized as (i) horizontal spindles with both γ-tubulin–labeled poles in the same plane; (ii) tilted spindles with only one of the two poles visible in a given plane; (iii) multipolar spindles; and (iv) spindles with lagging chromosomes. Astral MT density was determined by measuring α-tubulin total fluorescence minus spindle fluorescence, as indicated in Fig. 8, after correcting for background fluorescence. To identify centrioles, cells were incubated on ice for 30 min before fixation and immunolabeling glutamylated- and γ-tubulin. G1-arrested RPE-1 cells (starved) and U2OS cells (treated with 40 μM lovastatin; Sigma-Aldrich) were also analyzed to exclude a causative effect of G1 arrest. Distance between centrioles was measured with Zen.
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism software. The unpaired one-tailed t test, with Welch correction, was used to determine significant differences between data groups. Graphs were plotted using Prism, to show the mean and SEM. P values are indicated on the graphs as *P < 0.05, **P < 0.01, ***P < 0.001, or NS (not significant) P > 0.05.
Detailed descriptions of antibodies, siRNA, cDNA, and standard methods are provided in Supporting Information.
Supplementary Material
Acknowledgments
We thank D. Isnardon [Centre de Recherche en Cancérologie de Marseille (CRCM) Microscopy Platform] and M. Richaud (CRCM Cytometry Platform) for support and N. Galjart, C. J. Gloeckner, O. Rosnet, K. J. Roux, J. Scott, M. Takahashi, K. Tasken, and T. Usui for sharing reagents. This work was supported by INSERM, Site de Recherche Intégrée sur le Cancer (SIRIC) Grant INCa-DGOS-Inserm 6038, Agence Nationale de la Recherche Grant ANR-16-CE11-0008, and Groupement des Entreprises Françaises dans la Lutte contre le Cancer (GEFLUC) Marseille-Provence. H.B. was supported by a Ministère de l’Enseignement Supérieur et de la Recherche Fellowship. The Marseille Proteomics core facility was supported by Infrastructures en Biologie Santé et Agronomie, Région Provence-Alpes-Côte d’Azur (PACA) and Cancerôpole PACA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.J.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705682114/-/DCSupplemental.
References
- 1.Kollman JM, Merdes A, Mourey L, Agard DA. Microtubule nucleation by γ-tubulin complexes. Nat Rev Mol Cell Biol. 2011;12:709–721. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin TC, Neuner A, Schiebel E. Targeting of γ-tubulin complexes to microtubule organizing centers: Conservation and divergence. Trends Cell Biol. 2015;25:296–307. doi: 10.1016/j.tcb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 4.Janke C. The tubulin code: Molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–472. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cueva JG, Hsin J, Huang KC, Goodman MB. Posttranslational acetylation of α-tubulin constrains protofilament number in native microtubules. Curr Biol. 2012;22:1066–1074. doi: 10.1016/j.cub.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portran D, Schaedel L, Xu Z, Théry M, Nachury MV. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol. 2017;19:391–398. doi: 10.1038/ncb3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, et al. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science. 2017;356:328–332. doi: 10.1126/science.aai8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galjart N. Plus-end-tracking proteins and their interactions at microtubule ends. Curr Biol. 2010;20:R528–R537. doi: 10.1016/j.cub.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Akhmanova A, Steinmetz MO. Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 10.Marone R, et al. Memo mediates ErbB2-driven cell motility. Nat Cell Biol. 2004;6:515–522. doi: 10.1038/ncb1134. [DOI] [PubMed] [Google Scholar]
- 11.Zaoui K, Honoré S, Isnardon D, Braguer D, Badache A. Memo-RhoA-mDia1 signaling controls microtubules, the actin network, and adhesion site formation in migrating cells. J Cell Biol. 2008;183:401–408. doi: 10.1083/jcb.200805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaoui K, Benseddik K, Daou P, Salaün D, Badache A. ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells. Proc Natl Acad Sci USA. 2010;107:18517–18522. doi: 10.1073/pnas.1000975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benseddik K, Sen Nkwe N, Daou P, Verdier-Pinard P, Badache A. ErbB2-dependent chemotaxis requires microtubule capture and stabilization coordinated by distinct signaling pathways. PLoS One. 2013;8:e55211. doi: 10.1371/journal.pone.0055211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daou P, et al. Essential and nonredundant roles for Diaphanous formins in cortical microtubule capture and directed cell migration. Mol Biol Cell. 2014;25:658–668. doi: 10.1091/mbc.E13-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verde I, et al. Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem. 2001;276:11189–11198. doi: 10.1074/jbc.M006546200. [DOI] [PubMed] [Google Scholar]
- 16.Roubin R, et al. Myomegalin is necessary for the formation of centrosomal and Golgi-derived microtubules. Biol Open. 2013;2:238–250. doi: 10.1242/bio.20123392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Zhang C, Qi RZ. A newly identified myomegalin isoform functions in Golgi microtubule organization and ER-Golgi transport. J Cell Sci. 2014;127:4904–4917. doi: 10.1242/jcs.155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivero S, Cardenas J, Bornens M, Rios RM. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 2009;28:1016–1028. doi: 10.1038/emboj.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, et al. Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J Biol Chem. 2010;285:22658–22665. doi: 10.1074/jbc.M110.105965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakobsen L, et al. Novel asymmetrically localizing components of human centrosomes identified by complementary proteomics methods. EMBO J. 2011;30:1520–1535. doi: 10.1038/emboj.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzog F, et al. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science. 2012;337:1348–1352. doi: 10.1126/science.1221483. [DOI] [PubMed] [Google Scholar]
- 23.Gold MG, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–395. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, et al. Molecular pathway of microtubule organization at the Golgi apparatus. Dev Cell. 2016;39:44–60. doi: 10.1016/j.devcel.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Fogeron M-L, et al. LGALS3BP regulates centriole biogenesis and centrosome hypertrophy in cancer cells. Nat Commun. 2013;4:1531. doi: 10.1038/ncomms2517. [DOI] [PubMed] [Google Scholar]
- 26.Laferté S, Loh LC, Keeler V. Monoclonal antibodies specific for human tumor-associated antigen 90K/Mac-2 binding protein: Tools to examine protein conformation and function. J Cell Biochem. 2000;77:540–559. doi: 10.1002/(sici)1097-4644(20000615)77:4<540::aid-jcb3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.Chinen T, et al. The γ-tubulin-specific inhibitor gatastatin reveals temporal requirements of microtubule nucleation during the cell cycle. Nat Commun. 2015;6:8722. doi: 10.1038/ncomms9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chanez B, Gonçalves A, Badache A, Verdier-Pinard P. Eribulin targets a ch-TOG-dependent directed migration of cancer cells. Oncotarget. 2015;6:41667–41678. doi: 10.18632/oncotarget.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.di Pietro F, Echard A, Morin X. Regulation of mitotic spindle orientation: An integrated view. EMBO Rep. 2016;17:1106–1130. doi: 10.15252/embr.201642292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tillement V, Haren L, Roullet N, Etievant C, Merdes A. The centrosome protein NEDD1 as a potential pharmacological target to induce cell cycle arrest. Mol Cancer. 2009;8:10. doi: 10.1186/1476-4598-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikule K, et al. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol. 2007;9:160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- 32.Efimov A, et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007;12:917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassadonia A, et al. 90K (Mac-2 BP) and galectins in tumor progression and metastasis. Glycoconj J. 2002;19:551–556. doi: 10.1023/B:GLYC.0000014085.00706.d4. [DOI] [PubMed] [Google Scholar]
- 34.Hein MY, et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell. 2015;163:712–723. doi: 10.1016/j.cell.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 35.Hutchins JRA, et al. Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science. 2010;328:593–599. doi: 10.1126/science.1181348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kollman JM, et al. Ring closure activates yeast γTuRC for species-specific microtubule nucleation. Nat Struct Mol Biol. 2015;22:132–137. doi: 10.1038/nsmb.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang B, et al. Identification of cross-linked peptides from complex samples. Nat Methods. 2012;9:904–906. doi: 10.1038/nmeth.2099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.