Significance
The relation between structural and functional connectivity has profound implications for our understanding of cerebral physiology and cognitive neuroscience. Yet, this relation remains incompletely understood. Cases in which the corpus callosum is sectioned for medical reasons provide a unique opportunity to study this question. We report functional connectivity assessed before and after surgical section of the corpus callosum, including multiyear follow-up in a limited subsample. Our results demonstrate a causal role for the corpus callosum in maintaining functional connectivity between the hemispheres. Additionally, comparison of results obtained in complete vs. partial callosotomy demonstrate that polysynaptic connections also play a role in maintaining interhemispheric functional connectivity.
Keywords: corpus callosum, resting state, functional connectivity, structural connectivity, callosotomy
Abstract
Resting state functional connectivity is defined in terms of temporal correlations between physiologic signals, most commonly studied using functional magnetic resonance imaging. Major features of functional connectivity correspond to structural (axonal) connectivity. However, this relation is not one-to-one. Interhemispheric functional connectivity in relation to the corpus callosum presents a case in point. Specifically, several reports have documented nearly intact interhemispheric functional connectivity in individuals in whom the corpus callosum (the major commissure between the hemispheres) never develops. To investigate this question, we assessed functional connectivity before and after surgical section of the corpus callosum in 22 patients with medically refractory epilepsy. Section of the corpus callosum markedly reduced interhemispheric functional connectivity. This effect was more profound in multimodal associative areas in the frontal and parietal lobe than primary regions of sensorimotor and visual function. Moreover, no evidence of recovery was observed in a limited sample in which multiyear, longitudinal follow-up was obtained. Comparison of partial vs. complete callosotomy revealed several effects implying the existence of polysynaptic functional connectivity between remote brain regions. Thus, our results demonstrate that callosal as well as extracallosal anatomical connections play a role in the maintenance of interhemispheric functional connectivity.
Infra-slow (<0.1 Hz) intrinsic brain activity is temporally correlated within functionally related systems currently known as resting state networks (RSNs) (1, 2). This phenomenon is widely known as functional connectivity (FC). RSNs are conveniently studied in humans using resting state functional magnetic resonance imaging (rs-fMRI). Although rs-fMRI is increasingly being used to map the representation of function in health and disease (3–6), the physiological principles underlying RSNs remain incompletely understood (7). In particular, the extent to which anatomical connectivity accounts for FC is unclear. On the one hand, the broad topographic features of RSNs correspond to major white matter tracts. For example, the cingulum bundle connects the anterior and posterior midline components (nodes) of the default mode network (DMN) (8). However, FC generally is more extensive than anatomical connectivity. For example, interhemispheric anatomical connections between the primary visual cortices (V1) in each hemisphere are sparse; yet, V1 homotopic FC is strong (9). Thus, the relation between anatomical and FC remains a topic of active investigation (for a recent review, see ref. 10).
One of the most striking features of resting state FC is symmetry about the midline. Thus, resting state correlations tend to be particularly strong between corresponding loci in each hemisphere (homotopic FC) (11, 12). The corpus callosum (CC) is the major commissure connecting the two hemispheres. Accordingly, it would be logical to suppose that the CC accounts for the prominent symmetry of FC. However, data pertaining to this question are mixed and partially contradictory. One set of pertinent observations derives from human studies of callosal agenesis, a condition in which the CC never develops. An early study reported that homotopic FC is decreased but not absent (13). More recent papers emphasize that homotopic FC in callosal agenesis may be nearly normal (14–17). Conflicting inferences might be drawn from observations made in patients with intractable epilepsy who have undergone therapeutic section of the CC (18–20). Specifically, Johnston et al. studied a 6-y-old boy before and after a complete callosotomy and reported that FC was normal before surgery but largely lost afterward (18). However, Uddin et al. found partially intact interhemispheric FC four decades after total callosotomy (20). A recent study in monkeys observed marked loss of homotopic FC following complete section of the CC, but only in cases in which the anterior commissure also was sectioned (21). Division of the anterior commissure along with corpus callosotomy varied among the three reports mentioned above. Specifically, the anterior commissure was spared in the case reported by Johnston et al., sectioned in the case reported by Uddin et al., and sectioned in two of the three monkeys studied by O’Reilly (21). Thus, the available data do not clearly define the role of the CC in the maintenance of FC.
We acquired rs-fMRI before and after corpus callosotomy in 22 epilepsy patients. The study cohort included partial as well as complete section of the CC, thereby enabling examination of graded effects of callosotomy. Importantly, we also studied select individuals 2–7 y following callosotomy. Our analysis reveals differential contributions of the CC to FC evaluated in different regions of the brain. Specifically, interhemispheric FC in primary sensorimotor and primary visual cortex is less dependent on the CC than multimodal cortex. Interhemispheric FC is decreased immediately after callosotomy and does not show signs of recovery on multiyear longitudinal follow-up.
Results
Structural Imaging.
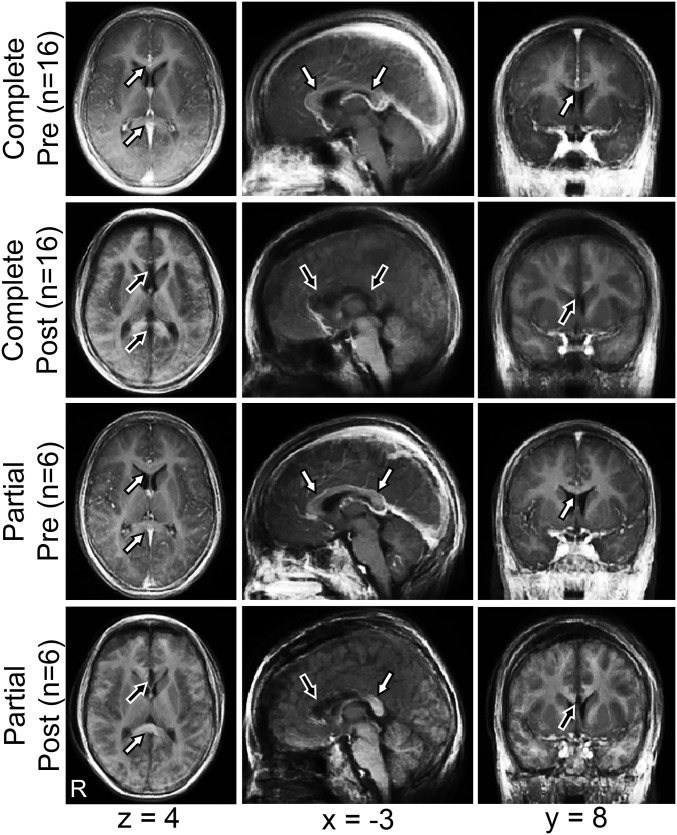
Atlas transformed anatomic images, averaged across subjects, precallosotomy and postcallosotomy, are shown in Fig. 1. White arrows identify normal CC. Black arrows indicate areas where the CC has been sectioned. Spared fibers in the splenium of the CC are evident in the postpartial callosotomy group (white arrows). A voxel-based analysis of the CC area spared by partial callosotomy is shown in Fig. S1.
Fig. 1.
Anatomic imaging precallosotomy and postcallosotomy. Mean T1-weighted images before (precallosotomy) and after (postcallosotomy) complete and partial callosotomy, represented in atlas space (right hemisphere on Left). MNI152 coordinates of axial, sagittal, and coronal planes are listed. White arrows indicate intact CC, and black arrows indicate areas of divided CC. Note residual splenium after partial callosotomy.
FC Maps.
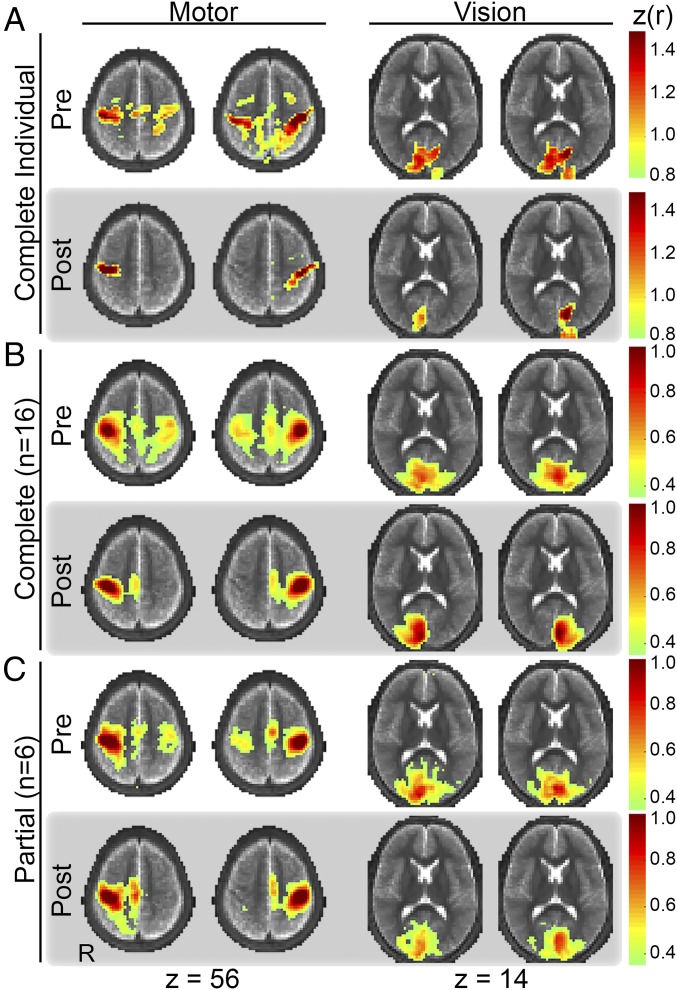
FC maps obtained with seeds in primary motor and visual areas, precallosotomy and postcallosotomy, are displayed in Fig. 2. The seed coordinates in MNI152 space were (−40, −23, 53) and (41, −22, 48) for left and right motor and (−8, −83, 0) and (7, −83, 0) for left and right vision, respectively. An exemplar individual is shown in Fig. 2A. Group-averaged results representing complete and partial callosotomy are shown in Fig. 2 B and C, respectively. Precallosotomy FC maps identify the expected sensorimotor (SMN) and visual (VIS) networks. The SMN includes primary motor cortex in the precentral gyrus, primary sensory cortex in the postcentral gyrus, and the supplementary motor area in the posterior aspect of the superior frontal gyrus. VIS areas include primary visual cortex in the calcarine sulcus and secondary visual areas in the lateral occipital lobe.
Fig. 2.
FC maps corresponding to seeds in primary motor (first and second columns) and primary visual (third and fourth columns) areas of the right (first and third columns) and left (second and fourth columns) hemispheres. (A) Results obtained in an exemplar individual. Note loss of interhemispheric FC after complete callosotomy with preserved intrahemispheric FC in both motor and visual networks. (B) Mean (n = 16) results before and after complete callosotomy. (C) Mean (n = 6) results before and after partial callosotomy. Note maintained visual but not motor interhemispheric FC after partial, but not complete, callosotomy. The FC maps are thresholded at z(r) > 0.80 in the exemplar individual and z(r) > 0.35 in the group results.
Complete callosotomy, on average (Fig. 2B), resulted in a marked loss of interhemispheric FC and, possibly, modest enhancement of intrahemispheric FC, both for motor and visual seeds. The effects of partial callosotomy were different in somatosensory vs. visual areas. Specifically, interhemispheric FC was lost in SMN areas, much as in complete callosotomy. In contrast, interhemispheric FC in VIS areas was largely unaffected by partial callosotomy (Fig. 2C). The contrast between partial vs. complete callosotomy most likely reflects sparing of occipital, but not more anterior, commissural fibers. Thus, spared splenial connections between posterior occipital areas continue to mediate interhemispheric FC.
FC Matrices.
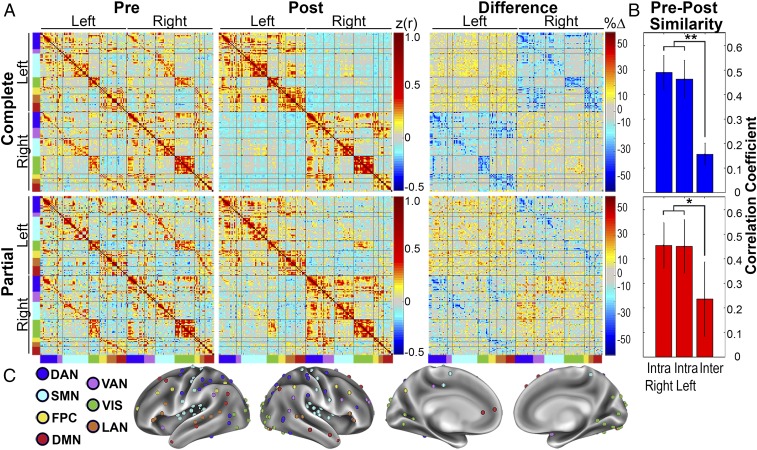
A previously defined seed set was used for further analysis (3). Seeds in this set were defined by systematic evaluation of previously published literature to best represent seven canonical networks commonly used in resting state fMRI studies. These networks include the dorsal attention network (DAN), ventral attention network (VAN), SMN, VIS, frontal-parietal control network (FPC), language network (LAN), and DMN. Seeds close to the midline (n = 29) were removed from the original set (n = 169). In Fig. 3C, the remaining seeds (n = 140) are color coded by RSN.
Fig. 3.
Contrast between interhemispheric vs. intrahemispheric FC. (A) FC matrices representing seven RSNs are organized according to hemisphere of seed. Diagonal and off-diagonal blocks represent intrahemispheric and interhemispheric FC, respectively. RSN color codes are defined in C. (B) Bar graphs representing similarity between precallosotomy and postcallosotomy for intrahemispheric and interhemispheric FC. The error bars represent 95% confidence intervals. **P < 0.001, *P < 0.05. (C) Seeds plotted on an inflated mean brain surface.
FC between all seed pairs, sorted by hemisphere and by RSN, is shown in matrix form in Fig. 3A. This seed ordering arranges left and right intrahemispheric FC in the top-left and bottom-right quadrants, respectively. The top-right quadrant shows interhemispheric FC. Percent difference was calculated in each individual as %Δz = 100 × (z(r)post − z(r)pre)/max(z(r)pre), where z(r) is the Fisher z-transformed Pearson correlation coefficient. The %Δz values shown in Fig. 3A are averaged over individuals. A striking decrease in interhemispheric FC is evident after complete callosotomy. Partial callosotomy decreased interhemispheric correlations to a lesser degree. The greatest residual interhemispheric FC was observed between seeds in the visual network. Of note, partial and complete callosotomy similarly decreased FC in the sensorimotor network. We also observed an increase in intrahemispheric FC after both complete and partial callosotomy, which is evident in the difference matrices of Fig. 3A. This finding may be related to similar functional imaging results in stroke studies where intrahemispheric FC is found to increase within the hemisphere contralateral to the lesion (22, 23).
To quantify the change in FC after callosotomy, we computed the similarity (element-wise Pearson correlation) between pre- and post-FC matrices. This analysis was partitioned by interhemispheric and intrahemispheric FC. The precallosotomy FC matrices were similar between complete and partial groups with a correlation of r = 0.79. We expected intrahemispheric FC to be affected less by callosotomy and, therefore, served as a control for the changes in interhemispheric FC. Accordingly, the similarity of intrahemispheric FC matrices was not significantly different between right and left hemispheres for either complete {t (30) = 0.505, confidence interval (CI) of difference = [−0.081, 0.134], P = 0.618} or partial {t (30) = 0.054, CI = [−0.158, 0.166], P = 0.958} callosotomy (“Intra” bars in Fig. 3B). In contrast, interhemispheric FC was markedly reduced following callosotomy after both complete {t (30) = −7.863, CI = [−0.418, −0.246], P < 0.001 for inter- vs. intraright; and t (30) = −6.797, CI = [−0.397, −0.214], P = <0.001 for inter- vs. intraleft} and partial {t (10) = −2.427, CI = [−0.420, −0.018], P = 0.036 for inter- vs. intraright; and t (10) = −2.267, CI = [−0.426, −0.007], P = 0.047 for inter- vs. intraleft} callosotomy (“Inter” bars in Fig. 3B).
Voxel Mirrored Homotopic FC.
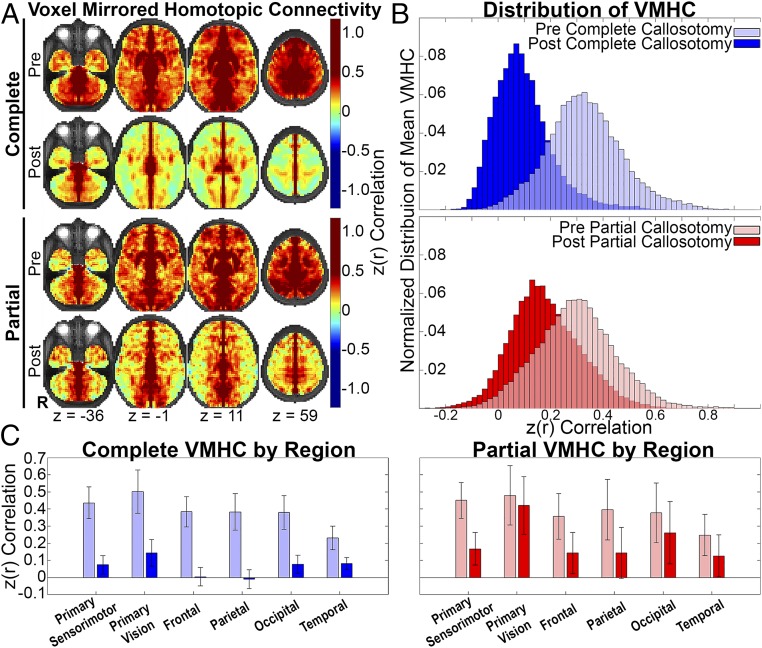
The present data inform the question of how much FC is or is not attributable to anatomic connectivity. Thus, if homotopic FC were entirely mediated by the CC, then this measure should be eliminated by complete callosotomy. Similarly, partial callosotomy should demonstrate a topographic distinction between preserved vs. eliminated homotopic FC in close relation to the extent of callosotomy. As illustrated in Fig. 4, these predictions are only partially supported by the data. Specifically, as predicted, homotopic FC is markedly reduced in many parts of the brain following complete callosotomy. Similarly, homotopic FC is almost intact in visual areas following partial callosotomy, which spares the splenium, that is, the interhemispheric connection between the occipital lobes. However, homotopic FC is partially preserved in primary sensorimotor and visual areas following complete callosotomy. Similarly, following partial callosotomy, homotopic FC is reduced but not eliminated in many parts of the cerebral hemispheres that, theoretically, have been disconnected. Complete maps for each group before and after are presented in Fig. S3.
Fig. 4.
Topography of CC-mediated FC and distribution of VMHC. (A) VMHC computed as the Fisher z-transformed Pearson correlation between voxels mirrored about the midline. By definition, these displays are bilaterally symmetric. Spatial blurring during preprocessing generates artifactually high homotopic FC along the midline. The underlay is the T2-weighted atlas representative image. (B) Distributions of mean VMHC across all voxels after vs. before callosotomy. Note larger shift toward zero after complete relative to partial callosotomy. (C) Bar graphs (mean ± 95% confidence interval) representing homotopic FC organized according to anatomical region. Note partial preservation of FC in primary sensorimotor and visual cortices after complete callosotomy but nearly complete loss of VMHC in multimodal associative areas. Note also more retained VMHC after partial callosotomy.
To obtain a quantitative view of the contrasting effects of complete vs. partial callosotomy, the distribution of voxel mirrored homotopic FC (VMHC) between all voxels was averaged across individuals. These results are displayed in histogram format in Fig. 4B. At baseline (precallosotomy), the distribution of VMHC is similar between complete and partial groups (Cohen’s d = 0.23). The postcallosotomy distributions clearly are shifted toward zero (i.e., no homotopic FC). However, this effect is much more marked in the complete as opposed to partial callosotomy results (Cohen’s d between precallosotomy and postcallosotomy is 0.80 for partial and 1.80 for complete). Positive skew, evident in the postcomplete callosotomy histogram, most likely reflects focal areas of preserved homotopic FC.
To quantify the regional specificity in CC-mediated FC, we evaluated the mean VMHC in primary and multimodal regions before and after callosotomy (Fig. 4C). These regions were defined by Brodmann areas corresponding to primary sensorimotor cortex, primary visual cortex, and multimodal areas of frontal, parietal, occipital, and temporal lobes (Fig. S4). The results of this anatomic region of interest analysis are consistent with a more significant decrease in all regions after complete compared with partial callosotomy (SI Results). More specifically, after complete callosotomy, multimodal areas of the frontal and parietal lobes are reduced to near zero VMHC, while primary sensorimotor and vision areas are reduced but not lost. In contrast, after partial callosotomy, the primary visual cortex remains near precallosotomy levels, likely owing to the spared splenium fibers.
Follow-Up Imaging at Delayed Time Interval.
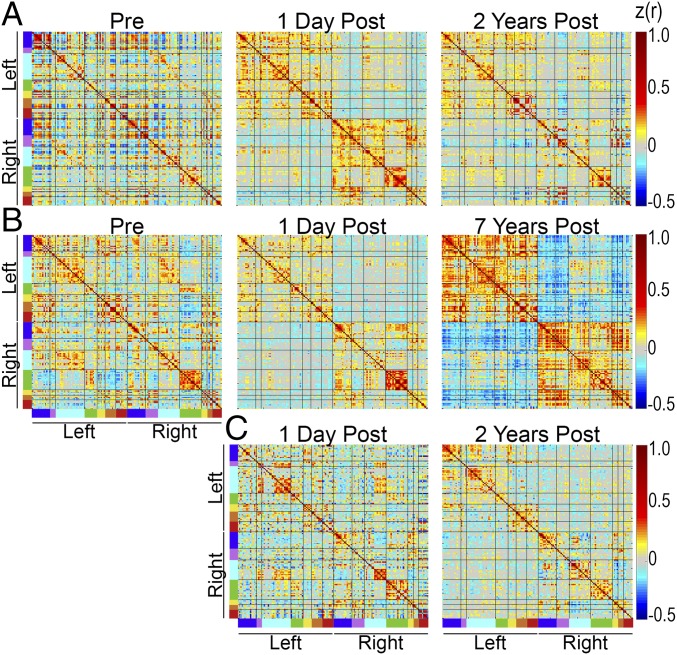
The postcallosotomy results shown so far demonstrate a marked loss of interhemispheric FC following callosotomy. However, these data were obtained 1 d after surgery, whereas previously reported, albeit limited, evidence raises the possibility that interhemispheric FC may recover after a prolonged interval following complete callosotomy (20). We were able to examine this question in three individuals at intervals between 2 and 7 y after callosotomy (Fig. 5) (SI Results). All show no evidence of recovered interhemispheric FC.
Fig. 5.
FC matrices obtained in three individuals including longitudinal imaging at follow-up intervals of 2–7 y. (A) Partial callosotomy at age 2 y; follow-up (sedated) at age 4 y. (B) Complete callosotomy at age 13 y; follow-up (nonsedated) at age 20 y. (C) Complete callosotomy at age 15 y; follow-up (sedated) at age 17 y. The precallosotomy study in this case was excluded as this patient initially presented with epileptic encephalopathy. Follow-up imaging 2 y after complete callosotomy was obtained under sedation for clinical indications. Note no sign of recovery of interhemispheric FC at follow-up in any of these individuals.
Discussion
The extent to which interhemispheric FC depends on the CC is uncertain owing to conflicting evidence. To address this issue, we report a series of human subjects studied before and after surgical section of an intact CC. Our data reveal a causal role of the CC in maintaining interhemispheric FC throughout the brain. Complete section of the CC dramatically reduced interhemispheric FC assessed in the immediate postoperative period, as previously reported in one case study (18). The effects of partial callosotomy were less dramatic and not entirely consistent with a simple relation between structural and FC. We also obtained longitudinal rs-fMRI in a restricted sample of individuals studied between 2 and 7 y following callosotomy. This data speaks to the question of FC plasticity. In the following discussion, we address the relation between structural and FC. We also touch on the question of interhemispheric FC recovery following a prolonged postoperative interval.
Structural Versus FC.
Previous studies report a correlation between cortical areas with strong structural and FC, but this relationship is incomplete in other areas with strong FC but weak structural connectivity (8, 24, 25). These findings imply that the relation between structural and FC is not one-to-one. Nevertheless, the most salient characteristics of resting state is strong homotopic FC (11), and the largest white matter structure in the brain is the CC. It is therefore reasonable to assume that the CC plays a major role in the maintenance of homotopic FC. Acknowledging expected differences at baseline from typically developing individuals, we focus our study on precallosotomy vs. postcallosotomy FC differences within subject.
Our results show partially preserved homotopic FC following complete callosotomy in primary sensorimotor and visual areas (Fig. 4 A and C). Hence, structures other than the CC must be contributory. Two observations inform this question. First, invasive tracer studies show relatively sparse axonal connectivity via the CC in the hand area of primary motor cortex (“callosal holes”) (26–28). Also, it is known that callosal connections between primary visual areas are very sparse (29–31). Thus, it may be inferred that homotopic FC in primary cortical areas is less dependent on the CC. Second, prior studies have established that subcortical structures participate in resting-state cortical RSNs (32, 33). Importantly, the most robust thalamocortical structural connectivity, as assessed by DTI tractography, is found in primary sensorimotor and visual cortices, whereas the weakest connections are found in multimodal areas (34). This anatomy is consistent with the residual FC evident in Fig. 4A.
Further evidence of polysynaptic FC is apparent after partial callosotomy (Fig. 4A). Homotopic FC in multimodal areas of the frontal lobes is reduced less after partial relative to complete callosotomy, despite callosal fibers connecting these areas sectioned in both procedures. This is in contrast to residual interhemispheric FC in posterior parietal and occipital areas, which is expected from known structural connections in the splenium (35). This finding suggests that posterior parietal-occipital areas, the callosal fibers of which are spared by partial callosotomy, are able to support frontal homotopic FC via intrahemispheric anatomic connections, e.g., via the superior longitudinal fasciculus. Thus, the posterior areas with maintained callosal structural connectivity act as hubs between widely separated regions in posterior and anterior parts of the brain. These findings help to explain the absence of disconnection syndrome after partial callosotomy where interhemispheric information transfer remains when the splenium is spared (36).
Homotopic FC data has been reported in prior studies (11) and summarized by metaanalysis (37). Homotopy is a consistent characteristic of resting-state fMRI (12) with electrophysiological correlates (38). Notable exceptions are language and attention functionality, which are asymmetrically represented in the human brain (39, 40). Stark et al. (11) show greatest homotopic FC in primary sensorimotor areas, followed by unimodal and heteromodal association areas. We observe similar results of greatest residual interhemispheric FC after callosotomy in the sensorimotor and vision networks, as well as near zero VMHC after complete callosotomy in frontal and parietal regions.
Longitudinal Follow-Up.
Previously reported postcallosotomy imaging has been obtained at intervals ranging from 1 d (18) to 6 mo (21) to 4 decades (20). The strongest evidence of FC recovery following a prolonged interval was reported in ref. 20. In our cohort, only one individual was able to tolerate nonsedated follow-up imaging. In two other individuals, sedated imaging was obtained for clinical indications. Our follow-up data reveal no convincing evidence of recovery of interhemispheric FC several years after callosotomy. These results tend to validate our postcallosotomy data obtained at an interval of 1 d.
The observation of relatively intact interhemispheric FC in callosal agenesis (e.g., ref. 16) raises the question whether compensation for the absence of a CC is possible very early in development within a critical period. Indeed, diffusion tensor MRI results indicate that compensatory tracts in the anterior and posterior commissures develop in these cases (16). The present follow-up data suggest that such compensation does not occur postnatally even in a case as young as 2 y. Thus, if a critical period does exist, it would seem to be over by age 2 y. Accordingly, it is not surprising that we saw no evidence of recovery in the other two longitudinally studied individuals. Our data, however, do not exclude the possibility of recovery decades following callosotomy (20).
Conclusion
We expand the available data that heretofore has been derived from a very limited number of case studies of corpus callosotomy. In particular, this is the only study to date reporting longitudinal human FC data acquired at an interval of years. We find no evidence of interhemispheric FC recovery. We provide strong evidence supporting a causal role of the CC in maintaining interhemispheric FC. We also provide evidence that extracallosal pathways are important, specifically in mediating residual homotopic FC in primary sensorimotor and visual areas following complete callosotomy. More generally, our results reinforce the principle that polysynaptic pathways account for a substantial fraction of FC (41, 42).
Methods
Corpus Callosotomy Subjects.
Twenty-two individuals with medically refractory epilepsy underwent complete (n = 16) or partial (n = 6) corpus callosotomy according to standard practice (43). All aspects of the study were approved by the Human Research and Protection Office Institutional Review Board (IRB) at Washington University School of Medicine in St. Louis. All subjects were pediatric patients with cognitive disabilities, therefore informed consent was initially obtained from the parent or legal guardian with assent from the subject where appropriate. The IRB subsequently approved waiver of written consent for imaging sequences obtained alongside clinical studies. The subjects who returned for delayed follow-up imaging provided an additional informed consent from the parent or legal guardian with assent when appropriate. Surgical candidacy was determined by clinical criteria alone. See SI Methods for further details.
Callosotomy Procedure.
Corpus callosotomy was performed following a standard clinical protocol via open craniotomy and microsurgical technique, as previously described (43) (SI Methods). Complete callosotomy divides the entire length of the CC including the splenium. In partial callosotomy, the posterior third to fourth of the CC (always including the splenium) is spared. Postoperative imaging is routinely obtained 1 d after surgery to confirm planned extent of callosotomy and rule out any surgical complications.
Image Acquisition and Preprocessing.
All imaging was performed with a 3T Siemens Trio scanner. Structural imaging included one T1w MP-RAGE [repetition time (TR) = 2,000 ms, echo time (TE) = 2.5 ms, flip angle = 12°, voxel size 1.0 × 1.0 × 1.0 mm] and one T2-weighted (T2w) turbo-spin echo sequence [TR = 9,000 ms, TE = 115 ms, flip angle = 120°, voxel size 1.0 × 1.0 × 2.5 mm]. For clinical reasons, the preoperative (but not postoperative) MP-RAGE was acquired with i.v. gadolinium contrast (at the end of the session). The remaining sequences were identical across sessions. Resting-state fMRI was acquired using an echo-planar imaging (EPI) sequence sensitive to blood oxygen level-dependent (BOLD) contrast (TR = 2,070 ms, TE = 25 ms, flip angle = 90°, voxel size 4.0 × 4.0 × 4.0 mm). Two runs of 200 frames each (∼14 min total) were acquired in each subject. Preprocessing followed previously published methods (44) (SI Methods).
FC.
FC was computed using seed-based correlation analysis with a previously defined seed set (3) (Fig. 3). Each 6-mm spherical seed was assigned to one of seven canonical RSNs. Of the original 169 seeds, 29 near the midline were excluded to reduce overlap from common source location and spatial blurring. FC, defined as the Pearson correlation coefficient (r), was computed between the seed and every other voxel in the brain. Pearson r values were Fisher z-transformed in all subsequent analysis.
Supplementary Material
Acknowledgments
We thank Nicholas Szrama, Mrinal Pawa, Ravi Chacko, Joseph Humphries, Donna Dierker, Nicholas Metcalf, and Mario Ortega for useful contributions, discussions, and guidance during data analysis and manuscript preparation, and we thank the patients and their families for contributing to this research. This study was supported by NIH Grant R25 NS090978, the Knight-Davidson family, The McDonnell Center for Systems Neuroscience, Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University in St. Louis, and Neuroimaging Informatics and Analysis Center Grant 1P30NS098577.
Footnotes
Conflict of interest statement: E.C.L. discloses financial relationships with the following companies: Intellectual Ventures, Monteris Medical, Acera Medical, Pear Therapeutics, General Sensing, Immunovalent, Face to Face Biometrics, Neurolutions, and Osteovantage.
This article is a PNAS Direct Submission. M.T.d.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707050114/-/DCSupplemental.
References
- 1.Shen HH. Core concept: Resting-state connectivity. Proc Natl Acad Sci USA. 2015;112:14115–14116. doi: 10.1073/pnas.1518785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder AZ. Intrinsic brain activity and resting state networks. In: Pfaff DW, Volkow ND, editors. Neuroscience in the 21st Century. Springer; New York: 2016. pp. 1–52. [Google Scholar]
- 3.Hacker CD, et al. Resting state network estimation in individual subjects. Neuroimage. 2013;82:616–633. doi: 10.1016/j.neuroimage.2013.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leuthardt EC, et al. Resting-state blood oxygen level-dependent functional MRI: A paradigm shift in preoperative brain mapping. Stereotact Funct Neurosurg. 2015;93:427–439. doi: 10.1159/000442424. [DOI] [PubMed] [Google Scholar]
- 5.Brier MR, Day GS, Snyder AZ, Tanenbaum AB, Ances BM. N-methyl-D-aspartate receptor encephalitis mediates loss of intrinsic activity measured by functional MRI. J Neurol. 2016;263:1083–1091. doi: 10.1007/s00415-016-8083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyser CD, et al. Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb Cortex. 2016;26:322–333. doi: 10.1093/cercor/bhu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schölvinck ML, Leopold DA, Brookes MJ, Khader PH. The contribution of electrophysiology to functional connectivity mapping. Neuroimage. 2013;80:297–306. doi: 10.1016/j.neuroimage.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 10.Behrens TE, Sporns O. Human connectomics. Curr Opin Neurobiol. 2012;22:144–153. doi: 10.1016/j.conb.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark DE, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvador R, et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- 13.Quigley M, et al. Role of the corpus callosum in functional connectivity. AJNR Am J Neuroradiol. 2003;24:208–212. [PMC free article] [PubMed] [Google Scholar]
- 14.Tyszka JM, Kennedy DP, Adolphs R, Paul LK. Intact bilateral resting-state networks in the absence of the corpus callosum. J Neurosci. 2011;31:15154–15162. doi: 10.1523/JNEUROSCI.1453-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna PC, et al. Preserved interhemispheric functional connectivity in a case of corpus callosum agenesis. Neuroradiology. 2012;54:177–179. doi: 10.1007/s00234-011-0883-x. [DOI] [PubMed] [Google Scholar]
- 16.Tovar-Moll F, et al. Structural and functional brain rewiring clarifies preserved interhemispheric transfer in humans born without the corpus callosum. Proc Natl Acad Sci USA. 2014;111:7843–7848. doi: 10.1073/pnas.1400806111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen JP, et al. Resting-state networks and the functional connectome of the human brain in agenesis of the corpus callosum. Brain Connect. 2013;3:547–562. doi: 10.1089/brain.2013.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston JM, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizoli CE, et al. Resting-state activity in development and maintenance of normal brain function. Proc Natl Acad Sci USA. 2011;108:11638–11643. doi: 10.1073/pnas.1109144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uddin LQ, et al. Residual functional connectivity in the split-brain revealed with resting-state functional MRI. Neuroreport. 2008;19:703–709. doi: 10.1097/WNR.0b013e3282fb8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Reilly JX, et al. Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proc Natl Acad Sci USA. 2013;110:13982–13987. doi: 10.1073/pnas.1305062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Meer MP, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel JS, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci USA. 2016;113:E4367–E4376. doi: 10.1073/pnas.1521083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch MA, Norris DG, Hund-Georgiadis M. An investigation of functional and anatomical connectivity using magnetic resonance imaging. Neuroimage. 2002;16:241–250. doi: 10.1006/nimg.2001.1052. [DOI] [PubMed] [Google Scholar]
- 25.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killackey HP, Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of new and old world monkeys. J Comp Neurol. 1983;219:384–419. doi: 10.1002/cne.902190403. [DOI] [PubMed] [Google Scholar]
- 27.Pappas CL, Strick PL. Anatomical demonstration of multiple representation in the forelimb region of the cat motor cortex. J Comp Neurol. 1981;200:491–500. doi: 10.1002/cne.902000404. [DOI] [PubMed] [Google Scholar]
- 28.Iwamura Y, Taoka M, Iriki A. Bilateral activity and callosal connections in the somatosensory cortex. Neuroscientist. 2001;7:419–429. doi: 10.1177/107385840100700511. [DOI] [PubMed] [Google Scholar]
- 29.Van Essen DC, Newsome WT, Bixby JL. The pattern of interhemispheric connections and its relationship to extrastriate visual areas in the macaque monkey. J Neurosci. 1982;2:265–283. doi: 10.1523/JNEUROSCI.02-03-00265.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berlucchi G. Visual interhemispheric communication and callosal connections of the occipital lobes. Cortex. 2014;56:1–13. doi: 10.1016/j.cortex.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Saenz M, Fine I. Topographic organization of V1 projections through the corpus callosum in humans. Neuroimage. 2010;52:1224–1229. doi: 10.1016/j.neuroimage.2010.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell PT, Shine JM. Subcortical contributions to large-scale network communication. Neurosci Biobehav Rev. 2016;71:313–322. doi: 10.1016/j.neubiorev.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 34.Toulmin H, et al. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc Natl Acad Sci USA. 2015;112:6485–6490. doi: 10.1073/pnas.1422638112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao YP, et al. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009;30:3172–3187. doi: 10.1002/hbm.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon HW, Bogen JE, Sperry RW. Absence of deconnexion syndrome in two patients with partial section of the neocommissures. Brain. 1971;94:327–336. doi: 10.1093/brain/94.2.327. [DOI] [PubMed] [Google Scholar]
- 37.Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew PJ, Duyn JH, Golanov E, Kleinfeld D. Finding coherence in spontaneous oscillations. Nat Neurosci. 2008;11:991–993. doi: 10.1038/nn0908-991. [DOI] [PubMed] [Google Scholar]
- 39.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAvoy M, et al. Unmasking language lateralization in human brain intrinsic activity. Cereb Cortex. 2016;26:1733–1746. doi: 10.1093/cercor/bhv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adachi Y, et al. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex. 2012;22:1586–1592. doi: 10.1093/cercor/bhr234. [DOI] [PubMed] [Google Scholar]
- 42.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: A review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 43.Jalilian L, et al. Complete versus anterior two-thirds corpus callosotomy in children: Analysis of outcome. J Neurosurg Pediatr. 2010;6:257–266. doi: 10.3171/2010.5.PEDS1029. [DOI] [PubMed] [Google Scholar]
- 44.Shulman GL, et al. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010;30:3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maccotta L, et al. Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin. 2013;2:862–872. doi: 10.1016/j.nicl.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reyes A, et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57:1475–1484. doi: 10.1111/epi.13456. [DOI] [PubMed] [Google Scholar]
- 47.Luo C, et al. Altered functional connectivity in default mode network in absence epilepsy: A resting-state fMRI study. Hum Brain Mapp. 2011;32:438–449. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mhuircheartaigh RN, et al. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: A functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckner RL, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.