Significance
The studies described here are relevant to the cure of diabetic retinopathy, a leading cause of blindness with currently limited therapeutic options. Here we provided evidence showing that agonists of growth hormone-releasing hormone (GHRH) can significantly diminish retinal neurovascular injury characterizing the early stages of diabetic retinopathy through antioxidant and anti-inflammatory effects. The results of the presented studies provide information on the potential therapeutic effects of GHRH agonists and shed light on the role of hypothalamic hormones in retinal physiology and their effect on visual disorders. In addition, our findings suggest protective effects of GHRH analogs in other disease conditions affecting retinal neuronal cells and, possibly, other nonretinal neurons.
Keywords: GHRH, GHRH-R, GH, diabetic retinopathy, type 1 diabetes
Abstract
The potential therapeutic effects of agonistic analogs of growth hormone-releasing hormone (GHRH) and their mechanism of action were investigated in diabetic retinopathy (DR). Streptozotocin-induced diabetic rats (STZ-rats) were treated with 15 μg/kg GHRH agonist, MR-409, or GHRH antagonist, MIA-602. At the end of treatment, morphological and biochemical analyses assessed the effects of these compounds on retinal neurovascular injury induced by hyperglycemia. The expression levels of GHRH and its receptor (GHRH-R) measured by qPCR and Western blotting were significantly down-regulated in retinas of STZ-rats and in human diabetic retinas (postmortem) compared with their respective controls. Treatment of STZ-rats with the GHRH agonist, MR-409, prevented retinal morphological alteration induced by hyperglycemia, particularly preserving survival of retinal ganglion cells. The reverse, using the GHRH antagonist, MIA-602, resulted in worsening of retinal morphology and a significant alteration of the outer retinal layer. Explaining these results, we have found that MR-409 exerted antioxidant and anti-inflammatory effects in retinas of the treated rats, as shown by up-regulation of NRF-2-dependent gene expression and down-regulation of proinflammatory cytokines and adhesion molecules. MR-409 also significantly down-regulated the expression of vascular endothelial growth factor while increasing that of pigment epithelium-derived factor in diabetic retinas. These effects correlated with decreased vascular permeability. In summary, our findings suggest a neurovascular protective effect of GHRH analogs during the early stage of diabetic retinopathy through their antioxidant and anti-inflammatory properties.
Diabetic retinopathy (DR) is a common complication of diabetes mellitus and the leading cause of blindness in adults in the Western world (1). There is a paucity of effective therapeutic strategies for this potentially blinding condition (2, 3) because of the complex nature of the disease and limited knowledge of its pathogenic mechanisms.
DR is a progressive disease that develops in defined clinical stages (4). The early stages of DR are characterized by dysfunction of retinal vessels, capillary loss, activation of Muller glia cells, and progressive damage to ganglion cells (4). Although the exact sequence and interdependence of hyperglycemia-induced neural and vascular alterations in retina remains to be established, there is no doubt that the development of therapies that can halt both these pathogenic events would be of great relevance.
Growth hormone-releasing hormone (GHRH) is a neurosecretory peptide, synthesized in neurons of the hypothalamus, which regulates the secretion of growth hormone (GH) from the pituitary gland (5–7). GHRH also possesses extrapituitary activities, and its function has been identified and characterized in multiple organs, including brain, heart, kidney, and retina (8). Numerous agonistic and antagonistic analogs of GHRH have been synthesized (6, 7, 9) and evaluated biologically (10). In the last few years, these investigations have been extended to diabetes and ophthalmology (10–12).
The presence of a GH-related axis within the retina has been postulated for decades (5) and has been linked to ganglion cell development and survival (13). In addition, different components of this GH axis have been investigated for their activity in retinal tissue (14–16). The contribution of systemic GH and insulin like growth factor-1 (IGF1) to retinal diseases is somewhat complex, and although evidence is provided supporting neuroprotective effects (17), experimental and clinical studies also suggest proangiogenic activities leading to retinal neovascularization in proliferative diabetic retinopathy (18).
Much less is known on the biological significance of retinal GHRH, however. In agreement with the notion of retinal production of GH from ganglion cells (19), GHRH receptors have been identified and immunolocalized in these retinal cells (20, 21). Evidence is provided suggesting protective effects of GHRH on retinal neurons (20); other studies have shown that GHRH antagonism is beneficial in experimental uveitis (12). Ultimately, the role of GHRH in the development of retinal diseases still remains controversial and understudied.
Here we investigated the effects of diabetes on retinal expression and function of GHRH and examined the effects of modulating its activity, by means of the specific GHRH agonist, MR-409, and the GHRH antagonist, MIA-602, in preventing hyperglycemia-induced retinal neurovascular injury.
Results
Effects of Diabetes on Expression of GHRH and GHRH-R in Rat and Human Retinas.
We assessed the expression of GHRH receptors (GHRH-Rs) in normal and diabetic rat retinas (SI Appendix, Fig. S1 A–C). Western blotting analysis showed a 2.5-fold reduction of GHRH-R-specific immunoreactivity in retinas of STZ-rats after 8 wk of hyperglycemia in comparison with age-matched normoglycemic control rats (*P < 0.01; n = 8) (SI Appendix, Fig. S1A). Immunofluorescence, in normal tissue, showed GHRH-R immunolocalization predominantly in ganglion cell (GCL) and outer plexiform layers (SI Appendix, Fig. S1B, white arrows). This analysis confirmed a marked reduction of GHRH-R-specific immunoreactivity in the diabetic retina, particularly in the GCL (SI Appendix, Fig. S1C).
Assessment of GHRH expression by qPCR showed a significant reduction of specific mRNA levels in the diabetic rat retina at 8 wk of hyperglycemia in comparison with normoglycemic control rats (SI Appendix, Fig. S1D). Finally, qPCR analysis measuring retinal expression of GH showed a fivefold down-regulation (*P < 0.001; n = 8) of GH-specific mRNA in the STZ-rat retinas versus control (SI Appendix, Fig. S1E).
The expression patterns of GHRH-R, GHRH, and GH were also assessed in retinas of diabetic and nondiabetic human donors (postmortem). Levels of GHRH-R protein, measured by Western blotting, were significantly down-regulated (*P < 0.01; n = 8) in the human diabetic postmortem retina compared with nondiabetic control donors (SI Appendix, Fig. S2A). Significant loss of expression of mRNA for GHRH (*P < 0.01; n = 8) and for GH (*P < 0.01; n = 8), measured by qPCR, was also detected in the human postmortem diabetic retinas compared with nondiabetic donors (SI Appendix, Fig. S2 B and C, respectively).
Analog MR-409 Enhances the Expression of GHRH and GHRH-R in the Diabetic Retina.
Next we determined the effects of GHRH agonist, MR-409, and the antagonist, MIA-602, in STZ-rats. These peptides were administered subcutaneously at a dose of 15 μg/kg every other day. The treatment started 2 wk after the onset of diabetes and was prolonged for a further 6 wk. Neither MR-409 nor MIA-602 changed blood glucose and glycated hemoglobin (HbA1c) levels, and they did not prevent diabetes-induced body weight loss (SI Appendix, Table S2) or corrected diabetes-induced alterations of other metabolic parameters [i.e., alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine, serum albumin and total protein, cholesterol, and triglycerides] (SI Appendix, Table S3).
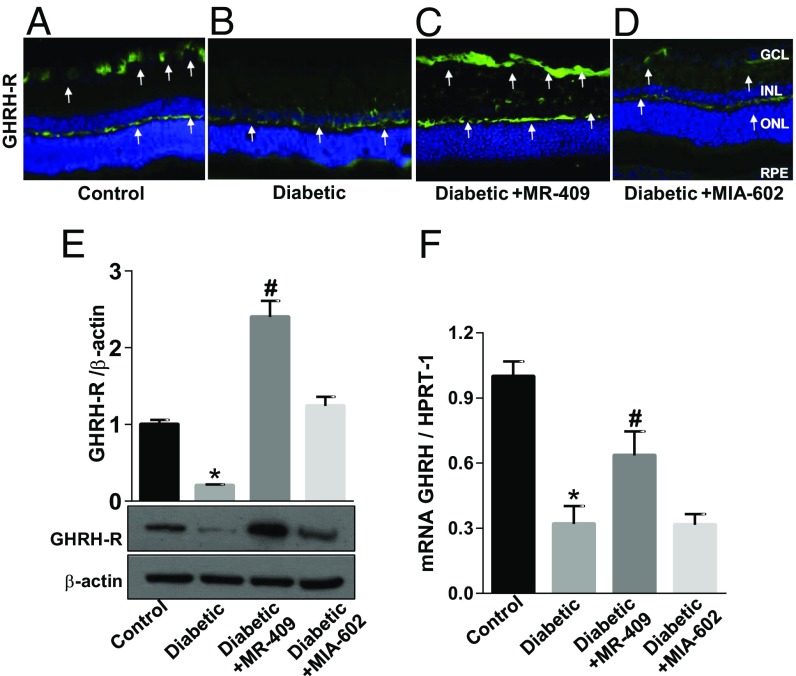
Immunofluorescence analysis of GHRH-R (Fig. 1 A–D) showed a marked increase in specific immunoreactivity of GHRH-R, particularly in the neurofibrillar retinal layer of STZ-rats treated with the GHRH agonist, MR-409 (Fig. 1C), whereas GHRH-R immunoreactivity in retinas of STZ-rats treated with the GHRH antagonist, MIA-602, was unchanged (Fig. 1D).
Fig. 1.
Effects of GHRH agonist MR-409 or antagonist MIA-602 on GHRH-R and GHRH expression levels. (A–D) Microimages illustrating GHRH-R-specific immunoreactivity (green fluorescence and white arrows) in retinal cryosections of STZ-rats (Diabetic) (B–D) compared with age-matched normal rats (Control, A). In C and D, the STZ-rats received, every other day, s.c. injections of 15 μg/kg MR-409 or MIA-602 (respectively). Treatment was started 2 wk after the onset of diabetes and prolonged for 6 wk (6 wk treatment in a total of 8 wk hyperglycemia). These images show that GHRH-R is down-regulated in the diabetic retina, particularly in the GCL (Diabetic) (B), but treatment with MR-409 (Diabetic+MR-409) drastically increased GHRH-R-specific immunoreactivity in the GCL and neurofibrillary layer (C, green fluorescence, white arrows). (E) Representative immunoblotting showing GHRH-R-specific immunoreactivity measured in retinal extracts of rats subjected to different treatment conditions. Bar histograms represent the optical density values normalized to the loading control, β-actin. Diabetes suppressed GHRH-R protein levels in comparison with normal age-matched controls (*P < 0.001 vs. Control; n = 8). MR-409 stimulated a significant increase in levels of GHRH-R protein in STZ-rats at 8 wk of hyperglycemia (Diabetic+MR-409; #P < 0.001 vs. Diabetic; n = 8). (F) qPCR analysis measuring GHRH mRNA expression levels in retinas of rats from the different experimental groups. These data show that GHRH-specific mRNA levels are down-regulated in the diabetic retina (*P < 0.001 vs. Control; n = 8), and treatment with MR-409 partially rescues GHRH mRNA (#P < 0.05 vs. Diabetic), whereas the antagonist, MIA-602, has no effect.
Western blotting (Fig. 1E) analysis showed that treatment of STZ-rats with the agonist, MR-409, boosted the expression of GHRH-R in the diabetic retina (#P < 0.001; n = 8) whereas the antagonist, MIA-602, normalized it to the control level (Fig. 1E). MRNA for GHRH was significantly increased in retinas of STZ-rats treated with MR-409 (Fig. 1F). Conversely, GHRH expression levels in STZ-rats treated with the antagonist, MIA-602, remained unchanged (Fig. 1F). Changes in GHRH and GHRH-R may affect the production of GH and of genes belonging to the GH axis. We, therefore, assessed by qPCR the retinal expression levels of these related factors. Levels of mRNAs specific for GH (SI Appendix, Fig. S3A), IGF1 (SI Appendix, Fig. S3B), insulin-like growth factor binding protein 3 (IGFBP-3) (SI Appendix, Fig. S3C), and somatostatin (SST) (SI Appendix, Fig. S3D) were significantly down-regulated in retinas of STZ-rats versus control age-matched normoglycemic rats (*P < 0.05; n = 8). Treatment with the GHRH agonist, MR-409, enhanced the expression levels of all of the mRNAs tested (SI Appendix, Fig. S3) and particularly boosted the expression of SST (SI Appendix, Fig. S3D). Treatment of the STZ-rats with the GHRH antagonist, MIA-602, also promoted a slight increase of mRNAs for GH, IGF1, and IGFBP-3; however, these differences did not reach statistical significance (SI Appendix, Fig. S3 A–C). Interestingly, MIA-602 further decreased the level of SST, although the obtained values failed statistical significance. Retinal expression of glucagon-like peptide-1 (GLP-1), another GH- and diabetes-related hormone, was down-regulated in the diabetic retina, and systemic treatment with the GHRH agonist increased its expression even above control levels (SI Appendix, Fig. S3E). Treatment of the STZ-rats with the GHRH antagonist, MIA-602, had no effect.
GHRH Agonist MR-409 Preserves the Structural Morphology of the Diabetic Retina.
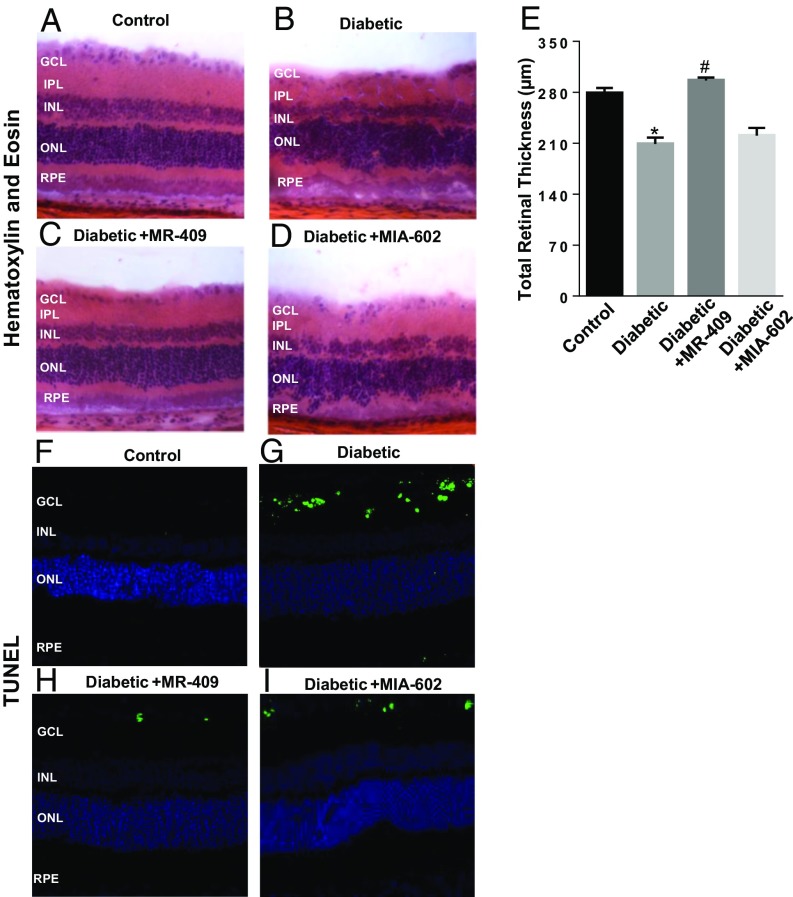
Morphological and morphometric analyses were conducted in retinal cryosections stained with hematoxylin and eosin (Fig. 2 A–D) and showed that diabetes promotes a reduction of total retinal thickness (Fig. 2B) compared with control age-matched normoglycemic rats (Fig. 2A). Administration of the GHRH agonist, MR-409, normalized the morphology of retinal layers (Fig. 2C), whereas the GHRH antagonist, MIA-602, further altered retinal morphology by promoting distortion of the retinal layers, particularly the outer retina (Fig. 2D). Morphometric analysis showed a significant preservation of total retinal thickness in STZ-rats treated with the GHRH agonist, MR-409, whereas MIA-602 had no effects (Fig. 2E).
Fig. 2.
Effects of MR-409 and MIA-602 on retinal morphological changes and apoptosis in diabetic retinas. Histopathological analysis assessing retinal morphology in response to the different treatment conditions was conducted by using H&E staining of retinal cryosections (A–D). In D, a pronounced distortion of the outer nuclear layer (ONL) is evidenced. Morphometric analysis assessing total retinal thickness (E) values measured in H&E retinal cryosections obtained from the different treatment groups. The sum of the results obtained from these analyses demonstrates a significant preservation of the retinal inner nuclear layer (INL) in the STZ-rats. (F–I) TUNEL staining to measure apoptosis in retinal cryosections of the different groups. Higher number of TUNEL-positive cells evident in diabetic retina (G) compared with control rats (F); whereas treatment with MR-409 (H) reduced the number of apoptotic cells, MIA-602 (I) treatment did not alter the rate of apoptosis in STZ-rats.
GHRH Agonist MR-409 Prevents Loss of Ganglion Cells in the Diabetic Rat Retina.
Next we assessed the extent of retinal cell death in the different treatment conditions. Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling (TUNEL) assay showed (Fig. 2 F–I) more TUNEL-positive cells in the diabetic retina in comparison with normoglycemic control rats (Fig. 2 G and F, respectively). However, treatment of STZ rats with MR-409 (Fig. 2H) markedly decreased the number of TUNEL-positive nuclei, whereas the GHRH antagonist, MIA-602, had no effects (Fig. 2I).
We also analyzed the expression pattern and retinal immunolocalization of the 17–19-kDa active form of caspase-3, an early marker of cellular apoptosis. Western blotting showed an up-regulation of cleaved caspase-3 in retinas of diabetic rats, which was significantly down-regulated in STZ-rats treated with the GHRH agonist, MR-409, but not with the antagonist, MIA-602 (SI Appendix, Fig. S4A). Immunohistochemistry showed increased immunoreactivity for the cleaved (active) form of caspase-3 in the inner retina of STZ-rats (diabetic; SI Appendix, Fig. S4C, white arrows) in comparison with control rats (SI Appendix, Fig. S4B). Treatment of STZ rats with the GHRH agonist, MR-409, markedly decreased immunoreactivity to cleaved caspase-3, particularly in the GCL (SI Appendix, Fig. S4D), whereas MIA-602 had no effect (SI Appendix, Fig. S4E).
Furthermore, immunostaining of retinal cryosections with the neuronal marker β-III tubulin (SI Appendix, Fig. S5 A–D) showed a significant reduction of retinal immunoreactivity to this neuronal marker in STZ-rats (SI Appendix, Fig. S5B) compared with control rats (SI Appendix, Fig. S5A). MR-409, however, increased retinal immunoreactivity to β-III tubulin (SI Appendix, Fig. S5C) in diabetic rats, whereas the antagonist, MIA-602, had no effect (SI Appendix, Fig. S5D).
On the basis of the existence of a GHRH-related axis in ganglion cells, we directly assessed survival of retinal ganglion cells in the different treatment groups. As shown in SI Appendix, Fig. S5 E and F, double-labeling of retinal neurons with the neuronal marker NeuN (red) and the retinal ganglionic cell-specific marker Brn3a (green) showed loss of double-labeled (yellow) nuclei in the GCL in the diabetic retina (SI Appendix, Fig. S5F) compared with control rat retinas (SI Appendix, Fig. S5E). Treatment of the STZ-rats with MR-409 increased the number of double-labeled nuclei (SI Appendix, Fig. S5G), whereas MIA-602 had no effect (SI Appendix, Fig. S5H). Finally, counting of double-labeled nuclei within the retinal GCL further confirmed the previous data (SI Appendix, Fig. S5I).
GHRH Agonist Halts Oxidative/Nitrative Stress Markers in the Diabetic Retina and Promotes Up-Regulation of Endogenous Antioxidant Activities.
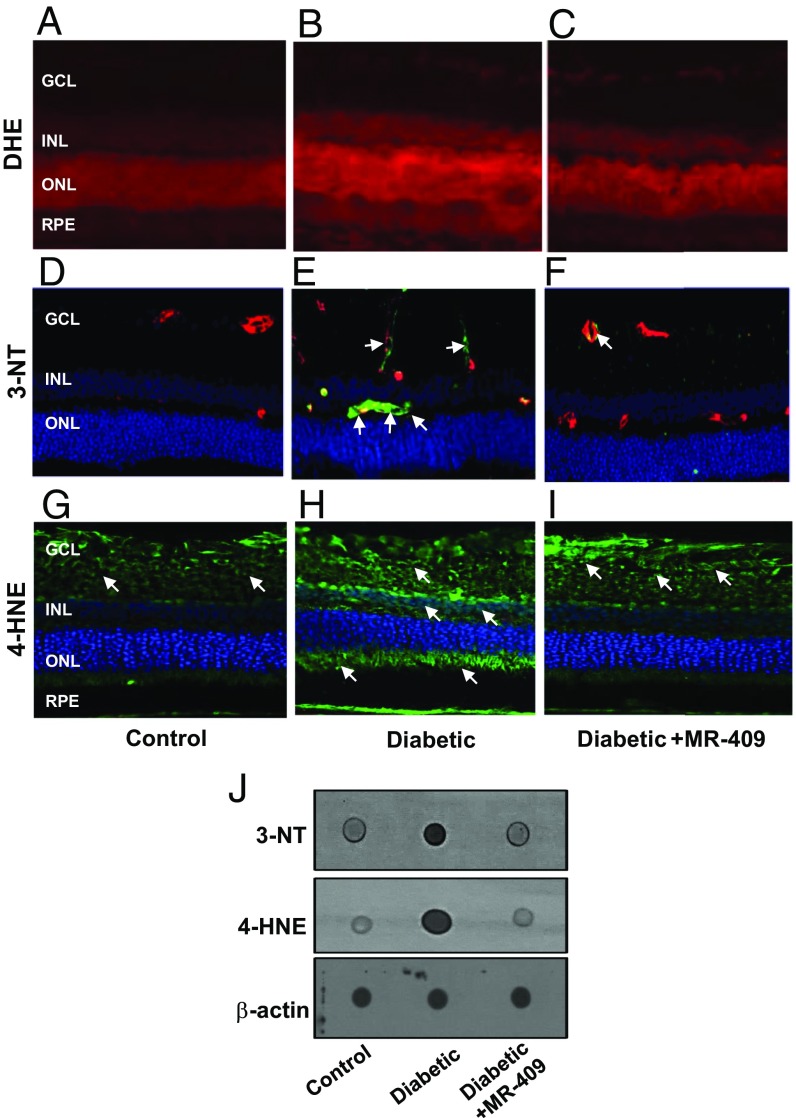
We further characterized the protective effects of the GHRH agonist, MR-409, by investigating the potential molecular mechanisms involved in this process. Retinal cell injury in diabetes results from metabolic and redox stress (22). As shown in Fig. 3 A–C, probing retinal tissues with the superoxide indicator dihydroethidium showed increased staining in the diabetic rat retinas (Fig. 3B) compared with control (Fig. 3A). This effect was partially blocked by treatment of the STZ-rats with MR-409 (Fig. 3C).
Fig. 3.
Oxidative/nitrative markers. (A–C) Dihydroethidium (DHE) staining to evaluate superoxide production. Retinal sections from diabetic rats (B) show higher intensity of DHE staining indicating increased superoxide levels compared with control rats (A). Treatment with MR-409 (C) reduced diabetes-associated superoxide production. (D–F) Immunostaining for 3-NT and double staining with isolectin B4 for double labeling with retinal vessels. Immunoreactivity for 3-NT is evidenced around blood vessels (green, 3-NT; red, isolectin B4; yellow, merging; white arrows), particularly in the diabetic retina (E, Diabetic). Treatment with GHRH agonist (F, Diabetic+MR-409) prevents this effect. (G–I) 4-HNE immunostaining (green fluorescence) in retinal cryosections shows increased immunoreactivity in the diabetic retina (H, Diabetic) versus normoglycemic age-matched control (G, Control). Treatment of STZ-rats with MR-409 (I) decreased retinal immunoreactivity of 4-HNE (green fluorescence, white arrows, at 20× magnification). (J) Dot blot analysis assessing 3-NT and 4-HNE and showing increased immunoreactivity for both these markers in diabetic retina (Diabetic), which is prevented by treatment of STZ-rats with MR-409 (Diabetic+MR-409). Beta-actin was used as loading control.
Overproduction of reactive oxygen species in the diabetic retina rapidly results in oxidative modifications including formation of 3-nitrotyrosine (3-NT) and 4-hydroxynonenal (4-HNE). Specific immunoreactivity to 3-NT and 4-HNE were found to be augmented in the STZ-rat retinas (Fig. 3 E and H, respectively) compared with control retinas (Fig. 3 D and G). Treatment of STZ-rats with MR-409 considerably decreased both 3-NT and 4-HNE immunoreactivity (Fig. 3 F and I, respectively). Dot blot analysis of 3-NT and 4-HNE confirmed the immunohistochemistry data and showed a marked reduction in 3-NT and 4-HNE in retinas of diabetic rats treated with MR-409 (Fig. 3J).
Oxidative/nitrative stress in the diabetic retina may result from the combination of increased production of free radicals and the down-regulation of endogenous antioxidant activities (22). The nuclear factor, erythroid-related factor 2 (NRF2), is a master regulator of genes expressing endogenous antioxidants (23, 24). We analyzed the expression pattern of NRF2-dependent genes including hemeoxygenase-1, NAD(P)H quinone dehydrogenase, glutathione peroxidase-1, glutamate-cysteine ligase catalytic subunit, and glutamate-cysteine ligase modifier subunit (SI Appendix, Fig. S6). Retinal expression at the mRNA level of these NRF2-dependent genes (SI Appendix, Fig. S6 A–E) was significantly down-regulated in diabetic rats, and treatment with MR-409 restored the expression of all the analyzed NRF2-dependent genes (SI Appendix, Fig. S6 A–E). We also analyzed the expression levels of another key endogenous antioxidant, thioredoxin-1 (Trx-1) (SI Appendix, Fig. S6F). Although the Trx-1 gene is not transcriptionally regulated by NRF2, its expression is augmented in conditions of oxidative stress and can be used as a marker of cellular/tissue redox imbalance (22). Trx-1 gene was significantly up-regulated in the diabetic retina (SI Appendix, Fig. S6F) compared with controls, and treatment with MR-409 down-regulated it, thus confirming the antioxidant effects of MR-409.
GHRH Agonist MR-409 Normalizes the Expression of Markers of Gliosis and Inflammation in the Diabetic Rat Retina.
Reactive gliosis and up-regulation of intercellular adhesion molecule-1 (ICAM-1) are key features of hyperglycemia-induced retinal injury (4). In the diabetic retina, we found that (SI Appendix, Fig. S7B) there was an up-regulation in glial fibrillary acidic protein (GFAP), a marker of gliosis, in comparison with controls (SI Appendix, Fig. S7A). GFAP staining displayed a typical localization in Muller glial cells (white arrows), thus suggesting reactive gliosis. Treatment of STZ-rats with MR-409 down-regulated GFAP immunoreactivity (SI Appendix, Fig. S7C). Direct assessment of GFAP protein levels by immunoblotting (SI Appendix, Fig. S7G) confirmed these data (*P < 0.001 vs. control and #P < 0.01 vs. diabetic).
The same trend was observed by analyzing the expression pattern of ICAM-1. As shown in SI Appendix, Fig. S7 D, E, and H, ICAM-1 expression was increased in the STZ-rat retina compared with controls (SI Appendix, Fig. S7 E and D, respectively). Treatment with MR-409 significantly down-regulated ICAM-1 to control level (SI Appendix, Fig. S7F). Western blotting to assess ICAM-1 protein levels (SI Appendix, Fig. S7H) confirmed the immunohistochemistry data (SI Appendix, Fig. S7H).
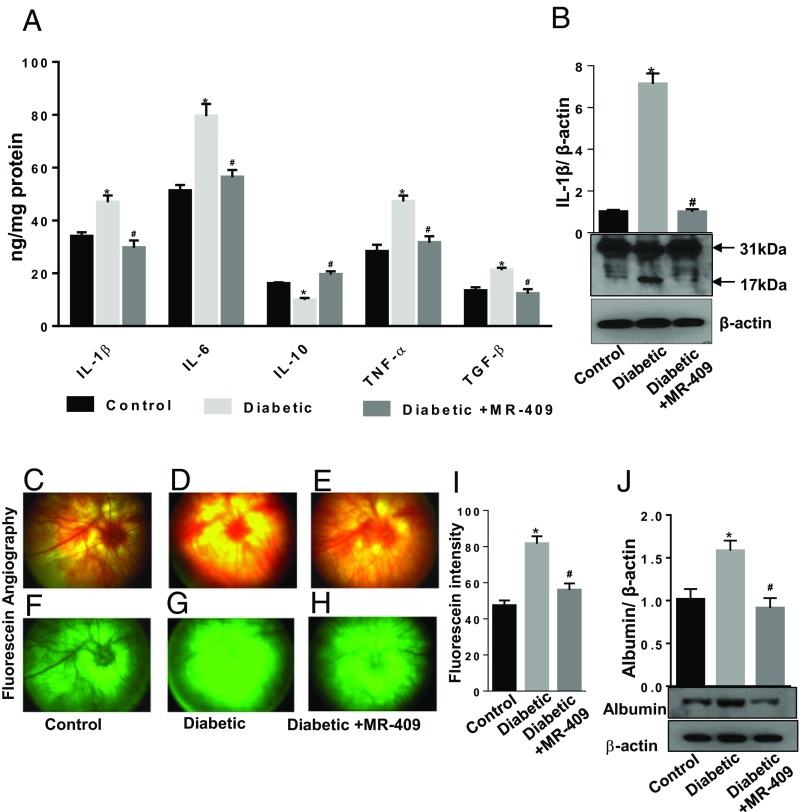
We further investigated the expression pattern of inflammatory cytokines implicated in DR pathogenesis, such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), and the anti-inflammatory IL-10. We used a custom cytokine ELISA plate array and found that protein levels of IL-1β, IL-6, TNF-α, and TGF-β were significantly up-regulated in the diabetic retina compared with controls (Fig. 4A). This effect was blocked by treatment of the STZ-rats with MR-409 (*P < 0.05; n = 8; Fig. 4A). On the contrary, IL-10 protein levels were significantly down-regulated in the untreated diabetic retina versus controls (*P < 0.05; n = 8), and treatment with MR-409 increased IL-10 levels even above the control (Fig. 4A). Western blotting analysis further showed increased levels of a cleaved 17-kDa form of IL-1β in the diabetic retina, but MR-409 blocked this effect (Fig. 4B).
Fig. 4.
Effects of MR-409 on retinal expression of cytokines and blood retinal barrier integrity. (A) Expression of IL-1β, IL-6, IL-10, TNF-α, and TGF-β were evaluated in retinal tissue using a customized ELISA kit. Bar histograms showing a significant up-regulation of retinal levels of IL-1β, IL-6, TNF-α, and TGF-β and decreased levels of IL-10 in diabetic rats (light gray bars) compared with control (black bars). Treatment with MR-409 normalized the levels of measured cytokines (dark gray bars). (B) Western blot analysis of IL-1β in the different treatment conditions showing the mature (active) 17-kDa form of IL-1β and the pro-IL-1β isoform (31 kDa). Representative images of fundus photographs (C–E) and fluorescein angiography (F–H) of rat eyes of control, diabetic, and diabetic treated with MR-409. Pictures were taken at constant interval for every rat studied in each experimental group. The fluorescence intensity per rat retina was calculated by Image J software and expressed as arbitrary units of fluorescence intensity (I). This analysis shows increased fluorescence intensity (extravasation) in diabetic rat retinas (D and G) compared with control normoglycemic rats (C and F) (Diabetic, *P < 0.01 vs. Control; n = 8). Treatment of STZ-rats with the GHRH agonist, MR-409, significantly decreases fluorescein extravasation (Diabetic+MR-409; #P < 0.01 vs. Diabetic; n = 8) (E and H). (J) Western blot analysis assessing albumin protein levels in retinal extracts of perfused rats from the different experimental groups. This analysis shows increased albumin immunoreactivity in the retinal lysates of STZ-rats after perfusion (Diabetic, *P < 0.05 vs. Control; n = 8). Albumin-specific immunoreactivity is significantly reduced in retinal extracts of (perfused) STZ-rats treated with MR-409 (Diabetic+MR-409, #P < 0.01 vs. Diabetic; n = 8). The data are expressed as arbitrary units of optical density and normalized for the loading control β-actin.
The GHRH Agonist MR-409 Normalizes Expression of Growth Factors in the Diabetic Retina.
Hyperglycemia alters the expression pattern of growth factors such as vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF) (4, 25), which may result in breakdown of the blood–retinal barrier. Protein levels and immunolocalization of VEGF and PEDF in the different experimental groups were assessed by Western blotting and immunohistochemistry (SI Appendix, Fig. S8). As shown in SI Appendix, Fig. S8 A–C, specific immunoreactivity for PEDF demonstrated lower intensity in retinas of STZ-rats (SI Appendix, Fig. S8B) compared with control rats (SI Appendix, Fig. S8A) and MR-409–treated STZ-rats showed an increase in PEDF immunoreactivity (SI Appendix, Fig. S8C). Conversely, retinas of STZ-rats showed higher intensity of VEGF immunoreactivity compared with control (SI Appendix, Fig. S8 D and E), while treatment with the agonist, MR-409, decreased the intensity (SI Appendix, Fig. S8F). Immunoblotting demonstrating protein levels of PEDF (SI Appendix, Fig. S8G) and VEGF (SI Appendix, Fig. S8H) further confirmed the immunohistochemistry data.
The GHRH Agonist MR-409 Prevents Hyperglycemia-Induced Vascular Leakage in the Diabetic Retina.
Dysfunction of retinal blood vessels was assessed by monitoring the integrity of the blood retinal barrier (4). Fundoscopy (Fig. 4 C–E) and fluorescein angiography (Fig. 4 F–H) were performed in rats after different treatments and showed that the agonist, MR-409 (Fig. 4 E and H), significantly down-regulated the fluorescein extravasation induced by hyperglycemia (Fig. 4 D and G), an effect that was evident despite the high endogenous autofluorescence of the (albino) rat fundus. Morphometric analysis measuring fluorescence intensity (Fig. 4I) demonstrated statistical significance of the detected differences (*P < 0.01 vs. control; #P < 0.01 vs. diabetic; n = 8). Finally, albumin extravasation was measured by Western blot in retinal extracts obtained from rats after perfusion. As shown in Fig. 4J, albumin levels were up-regulated in retinas of STZ-rats versus controls (*P < 0.05 vs. control; n = 8), and MR-409 significantly decreased them (#P < 0.01 vs. diabetic; n = 8; Fig. 4J).
Discussion
We have investigated the effects of drugs altering GHRH signaling in the diabetic retina during the early stages of the disease. The presence of a GH- and GHRH-related axis on retinal neurons (5, 13) and studies demonstrating that its inhibition may halt retinal inflammation and neovascularization (11, 12, 18, 26) prompted the present study assessing the effect of GHRH in the diabetic retina during the early stages of DR, where loss of capillaries and retinal neurons have an important pathogenic role (4).
Our findings confirmed the presence of GHRH-R and of a GHRH-dependent axis on retinal neurons and demonstrated that these are significantly down-regulated in experimental DR and human postmortem retinas of diabetic donors. Despite the documented effects of GH in promoting retinal neovascularization and contributing to proliferative diabetic retinopathy (11, 18, 26), our findings show that loss of GHRH and its receptors during the early stages of experimental DR may play a potential pathogenic role, as treatment of STZ-rats with the agonist, MR-409, significantly preserves retinal ganglion cell survival and normalizes vascular barrier function. On the contrary, the GHRH antagonist, MIA-602, had no effects on survival of retinal neurons and worsened tissue morphology by altering the integrity of ONL (outer nuclear layer), a highly undesirable effect.
Full-length (pituitary) and spliced isoforms (SV1) of GHRH receptors have been identified and shown to have tissue-specific functions (27, 28). Both forms of these receptors are expressed in retina (12). The agonistic analog, MR-409, boosted GHRH-R expression particularly in the retinal neurofibrillar layer (Fig. 1C). Interestingly, the antagonist, MIA-602, also partially stimulated GHRH-R expression to levels higher than the diabetic but not different from control (Fig. 1E). This effect could result from a compensatory mechanism triggered by complete loss of GHRH-dependent retinal signaling (as a result of the combined action of diabetes and the antagonistic analog, MIA-602), thus further underscoring the importance of the maintenance of GHRH function on retinal homeostasis.
Neuroprotective effects of GHRH in the diabetic retina were remarkably accompanied by preservation of the blood–retinal barrier, of which dysfunction is another important pathological feature of DR (4). Interestingly, previous studies have shown that a related GHRH agonist, JI-34, displayed antipermeability effects (29). Macular edema and increased retinal vascular permeability have been associated with up-regulation of VEGF, ICAM-1, and inflammatory cytokines (4). Our findings show that MR-409 prevented retinal expression of VEGF and ICAM-1 and suppressed the effects of diabetes in promoting retinal levels of IL-1β, IL-6, TNF-α, and TGF-β while up-regulating protein levels of the anti-inflammatory cytokine, IL-10.
Finally, MR-409 prevented the up-regulation of stress factors (i.e., GFAP) and of oxidative/nitrative stress markers induced by hyperglycemia, which are also important pathological features of DR (4). This suggests that the protective effects of MR-409 are associated with decreased reactive gliosis and possibly linked to its antioxidant properties. This was confirmed by our results showing that MR-409 restored NRF-2-dependent gene expression and normalized stress-induced Trx-1 overexpression. The protective effects of the GHRH agonist, MR-409, could be direct and/or indirect. GHRH-mediated rescue of IGF1, IGFBP-3, GLP-1, and GH could indirectly contribute to the observed neuroprotective effects during the early stages of DR (30–37). Of particular interest are our results on the expression pattern of SST, which was boosted above control levels by the GHRH agonist, MR-409, and was further down-regulated below control levels in response to the antagonist, MIA-602 (SI Appendix, Fig. S3D). Along with previous evidence demonstrating that SST can promote GHRH expression at the hypothalamic level (38), these data support the existence of positive feedback between the GHRH and SST pathways, further emphasizing the stringency of the system and its homeostatic importance at retinal level. On the basis of the documented beneficial effects of SST analogs in DR (39, 40), it is possible that this boost in SST expression could be contributing to the protective effects of MR-409 in the diabetic retina. GHRH protective effects in DR may also directly involve antioxidant activities. This hypothesis is supported by our data indicating that the agonistic analog, MR-409, stimulates NRF2-dependent gene expression. Previous studies have demonstrated that impairment of the NFR2 pathway plays a key contributing role in the pathogenesis of diabetic retinopathy (as reviewed in refs. 41 and 42). Further studies should be conducted to analyze GHRH-R-mediated activation of the NRF2 pathway.
Our results are in agreement with previous evidence suggesting the existence of a supportive GH-related axis in retinal neurons (20), but partially differ from other studies showing that the GHRH antagonist (MIA-602) prevents lipopolysaccharide-induced experimental uveitis (12). Acute versus chronic retinal inflammatory responses may involve alternate pathways and explain the different effect of GHRH modulation on diverse retinal diseases. Moreover, some of us have previously shown that in STZ-rats, higher doses (25 mg/kg/d) of GHRH antagonist, MIA-602, prevent hypertriglyceridemia and exert general vasculoprotective effects (43). We did not observe the same results, and although different dosage and treatment conditions can partially explain the observed differences, there is little doubt that differential expression of GHRH receptor isoforms could influence tissue-specific responses, further increasing complexity of the biology of this hormone.
In conclusion, our results suggest the merit of further studies to investigate the mechanisms involved in GHRH protective effects in the diabetic retina. These investigations may lead to therapeutic tools to halt retinal neurovascular injury in the early stages of DR, thus potentially preventing visual loss in diabetes.
Materials and Methods
Human Samples.
Deidentified, postmortem human retina samples were obtained from the Georgia Eye Bank through their approved research program and used in the present study per protocol approved by the institutional biosafety committee at Augusta University. All tissue samples were deidentified prior to receipt; therefore, IRB approval was not required.
Animals and Treatments.
All the animal procedures were performed in compliance with the Association for Research in Vision and Ophthalmology Statement for the humane use of laboratory animals. All experiments involving animals adhered to the Public Health Service Policy on Humane Care and Use of Laboratory Animals (revised July 2017) and were approved by the Augusta University institutional animal care and use committee. Adult male Sprague-Dawley rats (250–300 g) obtained from Evigo Laboratories were made diabetic by a single intravenous injection of STZ (Sigma-Aldrich). In some experiments, STZ-rats received, on alternate days, s.c. injections of 15 µg/kg of GHRH agonist, MR-409, or the antagonist, MIA-602. Control rats received vehicle injection.
For a detailed description of materials and methods used, please see SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Sean Shaw and Dr. Jianghe Yuan for their excellent technical assistance. The work on GHRH agonists and antagonists in the laboratory of A.V.S. was supported by the Medical Research Service of the Department of Veterans Affairs and the University of Miami School of Medicine. We acknowledge the financial support of the National Eye Institute [EY022416 (to M.B.) and EY022704 (to P.M.M.)] and of the Jon Simowitz philanthropic gift, a gift to the Department of Ophthalmology and the Culver-Vision Discovery Institute at Augusta University.
Footnotes
Conflict of interest statement: N.L.B. owns equity in Biscayne Pharmaceuticals. A.V.S. is a coinventor on the patent for GHRH agonist, assigned to the University of Miami and the Veterans Affairs Medical Center, Miami, FL. However, the investigation of the effects of GHRH agonist MR-409 was an academic endeavor without any commercial interests. The other authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718592114/-/DCSupplemental.
References
- 1.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: A worldwide perspective. Surv Ophthalmol. 2012;57:347–370. doi: 10.1016/j.survophthal.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Tan GS, Cheung N, Simó R, Cheung GC, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5:143–155. doi: 10.1016/S2213-8587(16)30052-3. [DOI] [PubMed] [Google Scholar]
- 3.Tolentino MS, Tolentino AJ, Tolentino MJ. Current and investigational drugs for the treatment of diabetic retinopathy. Expert Opin Investig Drugs. 2016;25:1011–1022. doi: 10.1080/13543784.2016.1201062. [DOI] [PubMed] [Google Scholar]
- 4.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 5.Murray PG, Higham CE, Clayton PE. 60 years of neuroendocrinology: The hypothalamo-GH axis: The past 60 years. J Endocrinol. 2015;226:T123–T140. doi: 10.1530/JOE-15-0120. [DOI] [PubMed] [Google Scholar]
- 6.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 7.Cai R, et al. Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities. Peptides. 2014;52:104–112. doi: 10.1016/j.peptides.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granata R. Peripheral activities of growth hormone-releasing hormone. J Endocrinol Invest. 2016;39:721–727. doi: 10.1007/s40618-016-0440-x. [DOI] [PubMed] [Google Scholar]
- 9.Zarandi M, et al. Synthesis and structure-activity studies on novel analogs of human growth hormone releasing hormone (GHRH) with enhanced inhibitory activities on tumor growth. Peptides. 2017;89:60–70. doi: 10.1016/j.peptides.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol Metab. 2011;22:311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Growth Hormone Antagonist for Proliferative Diabetic Retinopathy Study Group The effect of a growth hormone receptor antagonist drug on proliferative diabetic retinopathy. Ophthalmology. 2001;108:2266–2272. doi: 10.1016/s0161-6420(01)00853-3. [DOI] [PubMed] [Google Scholar]
- 12.Qin YJ, et al. Antagonist of GH-releasing hormone receptors alleviates experimental ocular inflammation. Proc Natl Acad Sci USA. 2014;111:18303–18308. doi: 10.1073/pnas.1421815112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders EJ, Lin WY, Parker E, Harvey S. Growth hormone promotes the survival of retinal cells in vivo. Gen Comp Endocrinol. 2011;172:140–150. doi: 10.1016/j.ygcen.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Otteson DC, Cirenza PF, Hitchcock PF. Persistent neurogenesis in the teleost retina: Evidence for regulation by the growth-hormone/insulin-like growth factor-I axis. Mech Dev. 2002;117:137–149. doi: 10.1016/s0925-4773(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 15.Hellström A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87:3413–3416. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 16.Lambooij AC, et al. Somatostatin receptor 2A expression in choroidal neovascularization secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:2329–2335. [PubMed] [Google Scholar]
- 17.Harvey S, Martinez-Moreno CG. Growth hormone and ocular dysfunction: Endocrine, paracrine or autocrine etiologies? Growth Horm IGF Res. 2016;29:28–32. doi: 10.1016/j.ghir.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson-Berka JL, Wraight C, Werther G. The role of growth hormone, insulin-like growth factor and somatostatin in diabetic retinopathy. Curr Med Chem. 2006;13:3307–3317. doi: 10.2174/092986706778773086. [DOI] [PubMed] [Google Scholar]
- 19.Harvey S, et al. Release of retinal growth hormone in the chick embryo: Local regulation? Gen Comp Endocrinol. 2012;176:361–366. doi: 10.1016/j.ygcen.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Moreno CG, Giterman D, Henderson D, Harvey S. Secretagogue induction of GH release in QNR/D cells: Prevention of cell death. Gen Comp Endocrinol. 2014;203:274–280. doi: 10.1016/j.ygcen.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Moreno CG, Trudeau VL, Harvey S. Co-storage and secretion of growth hormone and secretoneurin in retinal ganglion cells. Gen Comp Endocrinol. 2015;220:124–132. doi: 10.1016/j.ygcen.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta. 2015;1852:2474–2483. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Gupta RK, et al. Oxidative stress and antioxidants in disease and cancer: A review. Asian Pac J Cancer Prev. 2014;15:4405–4409. doi: 10.7314/apjcp.2014.15.11.4405. [DOI] [PubMed] [Google Scholar]
- 24.Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125:1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caldwell RB, et al. Vascular endothelial growth factor and diabetic retinopathy: Role of oxidative stress. Curr Drug Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 26.Hellström A, Svensson E, Carlsson B, Niklasson A, Albertsson-Wikland K. Reduced retinal vascularization in children with growth hormone deficiency. J Clin Endocrinol Metab. 1999;84:795–798. doi: 10.1210/jcem.84.2.5484. [DOI] [PubMed] [Google Scholar]
- 27.Mullis PE. Genetics of GHRH, GHRH-receptor, GH and GH-receptor: Its impact on pharmacogenetics. Best Pract Res Clin Endocrinol Metab. 2011;25:25–41. doi: 10.1016/j.beem.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Barabutis N, Schally AV. Growth hormone-releasing hormone: Extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9:4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- 29.Lucas R, et al. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc Natl Acad Sci USA. 2012;109:2084–2089. doi: 10.1073/pnas.1121075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong D, et al. Insulin-like growth factor 1 rescues R28 retinal neurons from apoptotic death through ERK-mediated BimEL phosphorylation independent of Akt. Exp Eye Res. 2016;151:82–95. doi: 10.1016/j.exer.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, et al. IGF-1 signaling via the PI3K/Akt pathway confers neuroprotection in human retinal pigment epithelial cells exposed to sodium nitroprusside insult. J Mol Neurosci. 2015;55:931–940. doi: 10.1007/s12031-014-0448-7. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, et al. Liraglutide alleviates H2O2-induced retinal ganglion cells injury by inhibiting autophagy through mitochondrial pathways. Peptides. 2017;92:1–8. doi: 10.1016/j.peptides.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich N, et al. The DPP4 inhibitor linagliptin protects from experimental diabetic retinopathy. PLoS One. 2016;11:e0167853. doi: 10.1371/journal.pone.0167853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarajapu YP, et al. Protection of blood retinal barrier and systemic vasculature by insulin-like growth factor binding protein-3. PLoS One. 2012;7:e39398. doi: 10.1371/journal.pone.0039398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Steinle JJ. IGFBP-3 inhibits TNF-α production and TNFR-2 signaling to protect against retinal endothelial cell apoptosis. Microvasc Res. 2014;95:76–81. doi: 10.1016/j.mvr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders EJ, Parker E, Harvey S. Growth hormone-mediated survival of embryonic retinal ganglion cells: Signaling mechanisms. Gen Comp Endocrinol. 2008;156:613–621. doi: 10.1016/j.ygcen.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-Moreno CG, et al. Neuroprotection by GH against excitotoxic-induced cell death in retinal ganglion cells. Gen Comp Endocrinol. 2016;234:68–80. doi: 10.1016/j.ygcen.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Proudan N, Peroski M, Grignol G, Merchenthaler I, Dudas B. Juxtapositions between the somatostatinergic and growth hormone-releasing hormone (GHRH) neurons in the human hypothalamus. Neuroscience. 2015;297:205–210. doi: 10.1016/j.neuroscience.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez JM, 2nd, Yorek MA, Grant MB. Combination therapies prevent the neuropathic, proinflammatory characteristics of bone marrow in streptozotocin-induced diabetic rats. Diabetes. 2015;64:643–653. doi: 10.2337/db14-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rai U, Thrimawithana TR, Valery C, Young SA. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol Ther. 2015;152:98–110. doi: 10.1016/j.pharmthera.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Batliwala S, Xavier C, Liu Y, Wu H, Pang IH. Involvement of Nrf2 in ocular diseases. Oxid Med Cell Longev. 2017;2017:1703810. doi: 10.1155/2017/1703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowluru RA, Mishra M. Epigenetic regulation of redox signaling in diabetic retinopathy: Role of Nrf2. Free Radic Biol Med. 2017;103:155–164. doi: 10.1016/j.freeradbiomed.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero MJ, et al. Role of growth hormone-releasing hormone in dyslipidemia associated with experimental type 1 diabetes. Proc Natl Acad Sci USA. 2016;113:1895–1900. doi: 10.1073/pnas.1525520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.