Significance
Maternal stress during gestation causes numerous effects on infant physiology that extend well into adulthood. We contribute to the ongoing debate on whether these effects are adaptive outcomes or merely the product of energetic constraints by presenting an integrated hypothesis that predicts the diversity of observed maternal effects on offspring growth, incorporating both theoretical explanations into one coherent framework. Empirical tests of this hypothesis across mammals suggest that the timing of the stressor during gestation and a simultaneous consideration of maternal investment and adaptive growth plasticity effects are crucial for a full comprehension of prenatal stress effects on offspring growth. The results support an adaptive life history perspective on maternal effects that is relevant for evolutionary biology, medicine, and psychology.
Keywords: phenotypic plasticity, developmental plasticity, growth plasticity, catch-up growth, developmental constraints
Abstract
Across mammals, prenatal maternal stress (PREMS) affects many aspects of offspring development, including offspring growth. However, how PREMS translates to offspring growth is inconsistent, even within species. To explain the full range of reported effects of prenatal adversity on offspring growth, we propose an integrative hypothesis: developmental constraints and a counteracting adaptive growth plasticity work in opposition to drive PREMS effects on growth. Mothers experiencing adversity reduce maternal investment leading to stunted growth (developmental constraints). Concomitantly, the pace of offspring life history is recalibrated to partly compensate for these developmental constraints (adaptive growth plasticity). Moreover, the relative importance of each process changes across ontogeny with increasing offspring independence. Thus, offspring exposed to PREMS may grow at the same rate as controls during gestation and lactation, but faster after weaning when direct maternal investment has ceased. We tested these predictions with a comparative analysis on the outcomes of 719 studies across 21 mammal species. First, the observed growth changes in response to PREMS varied across offspring developmental periods as predicted. We argue that the observed growth acceleration after weaning is not “catch-up growth,” because offspring that were small for age grew slower. Second, only PREMS exposure early during gestation produced adaptive growth plasticity. Our results suggest that PREMS effects benefit the mother’s future reproduction and at the same time accelerate offspring growth and possibly maturation and reproductive rate. In this sense, PREMS effects on offspring growth allow mother and offspring to make the best of a bad start.
In many mammals, including humans, prenatal maternal stress (PREMS) affects offspring development and adult health in terms of growth, immune function, metabolic syndrome, and life expectancy (1–7). Although these outcomes are harmful to offspring, it is currently debated whether such PREMS effects represent an unavoidable, nonadaptive constraint or an evolutionarily adaptive recalibration of an organism’s life history strategy (1, 8–18). At the heart of this debate is the pace of growth for the developing fetus/infant (19). Growth is a crucial aspect of early development and life history pace that is often associated with variation in infant and juvenile survival and offspring maturation and reproductive rate (6, 16, 19–24). Although growth plasticity and its effects have been well studied (1, 17, 20, 23–25), evidence for PREMS-triggered growth plasticity remains ambiguous. Indeed, PREMS effects on growth are known to range from positive to negative effects, even within the same species (18, 26–31). Furthermore, PREMS effects seem to be sensitive to the timing of PREMS during gestation and to vary across offspring life history stages (1, 4, 28–34). Consequently, there is no consensus about how PREMS affects offspring growth in mammals. We propose an integrated hypothesis that combines the two opposing processes of developmental constraints and adaptive developmental plasticity.
The developmental constraints hypothesis predicts that offspring show reduced pre- and postnatal growth in response to PREMS. Mothers reduce their energetic allocation to maternal investment, because they are experiencing a reduction in maternal energetic intake, an increase in expenditure, and/or a depletion of maternal capital (1, 8, 15, 31, 35–41). In support of the developmental constraints hypothesis, PREMS negatively affects placenta size, fetus weight, postnatal mammary gland size, and milk yield (1–3, 30, 31, 38–42). Additionally, artificial nursing and cross-fostering studies in rodents and ungulates indicate that reduced postnatal offspring growth rates result from PREMS of the nursing mother rather than the offspring’s prenatal environment (43–47). Critical to this hypothesis, reduced maternal investment constrains offspring development, while benefitting the mother’s ability to reproduce in the future (8, 16, 18, 19, 21, 48).
From the offspring’s perspective, reduced maternal investment during gestation and lactation results in nonadaptive growth plasticity in the form of inevitable developmental constraints (1, 11, 20). Apart from other developmental constraints, reduced growth rates result in later maturation and/or reduced adult body size, which in turn reduces lifetime reproductive success by shortening reproductive life span and reducing reproductive rate (6, 20, 21, 23, 24). Fitness may be further reduced, because low birth weight and postnatal body size are strong predictors of reduced early survival (6, 20, 22–24, 49, 50). Thus, minimizing the impact of developmental constraints on growth can benefit the offspring (18, 20, 23, 24). One process alleviating the negative impact of growth reduction is “catch-up growth,” i.e., a period of accelerated growth following a phase of stunted growth that allows offspring to return to their target growth canal (25, 47, 51–53). In the best case scenario, catch-up growth produces an individual matching the population mean in terms of body size for age and age at maturation (25, 51–53).
In contrast to the developmental constraints hypothesis, the adaptive developmental plasticity hypothesis predicts faster offspring growth and reproduction in response to PREMS (1, 11–14, 17, 54), which, in contrast to a catch-up process, lead to overshooting of growth trajectories and increased body size for age during development (“accelerated growth,” refs. 20, 26, 27, 29, 32, 53, and 55–57). From life history theory, the adaptive developmental plasticity hypothesis predicts that PREMS that is associated with reduced offspring longevity triggers a recalibration of offspring developmental trajectories toward a “faster” life history strategy altogether (11, 12, 14, 15, 20, 21, 54). In support of the hypothesis, prenatal adversity can be associated with accelerated growth (26, 27, 29, 32), earlier reproduction (27, 58–60), and a shorter life span (refs. 5–7, 49, 61, and 62; see also, refs. 15, 24, and 63). Accelerated development is thought to come at the costs of more quality-related functions like physical maintenance and immune defense (23, 24, 29, 47, 64). Despite these costs, adaptive growth plasticity can evolve, provided the fitness outcomes of an organism with an adjusted life history are higher than a nonadjusted one under similar constraints (11, 12, 15, 23, 24, 65). Under adaptive growth plasticity, offspring grow faster and achieve maturational milestones earlier and reproduce faster than they would otherwise.
Several hypotheses integrate these ideas, i.e., that PREMS causes deleterious effects in the offspring and may also lead to a recalibration of offspring phenotype (10, 11, 15, 17, 18). Nederhof and Schmidt (66) propose that developmental constraints result from the lack of (complete) recalibration of offspring phenotype, whereas adaptive developmental plasticity in the broader sense results from successful recalibration. Other hypotheses suggest that developmental constraints are inevitable consequences of PREMS, resulting from mothers with reduced capital investing less in their dependent offspring (11, 19, 67). In response to such unavoidable constraints, offspring may engage in later catch-up growth to avoid the fitness consequences of small body size and late maturation (17–19, 23, 47) at the costs of metabolic changes. Alternatively, offspring accelerate their life history strategy directly in response to their own disadvantaged prenatal somatic state or indirectly in response to a cue that reliably predicts this state (11, 15). One reliable cue may be maternal secretion of glucocorticoid (GC) hormones (like cortisol) during prenatal development (1–4, 8, 18). Maternal glucocorticoids are known to increase immediately in response to environmental challenges to the mother (1–3, 26, 27, 32). Offspring exposed to elevated maternal glucocorticoids (prenatally via the bloodstream or postnatally via the mother’s milk) experience a wide range of stress effects, including accelerated and decelerated offspring growth (1, 2, 4, 18, 26, 28, 29, 57, 68–72).
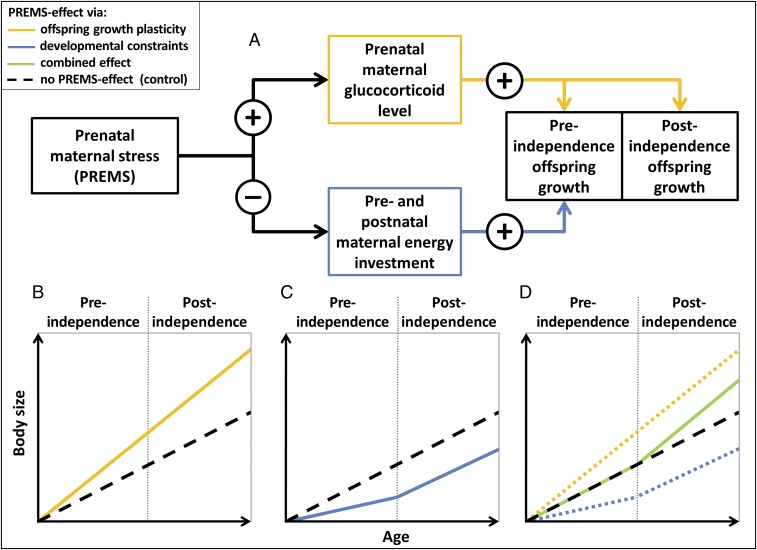
We borrow the notions that maternal phenotype is the only relevant information about the environment available to the unborn offspring (9, 10, 17, 67) and that information about expected levels of maternal investment and other aspects of maternal capital may be used to prenatally recalibrate offspring life history pace (11, 15). Building on these hypotheses, we propose an integrated hypothesis of PREMS effects on offspring growth, combining the effects of reduced maternal investment and a counteracting adaptive growth plasticity (Fig. 1; see also refs. 8, 10, and 18). We suggest that PREMS negatively affects maternal capital and investment and is associated with a rise in maternal glucocorticoid level that triggers an adaptive recalibration of offspring life history strategy toward accelerated maturation. The reduced maternal investment and the accelerated life history pace then both act on offspring growth, and the observed variation in PREMS effects on offspring growth captures the cumulative impact of these two opposing processes, which changes with the developmental period (Fig. 1). We predict that the effects originally caused by developmental constraints due to reduced maternal investment cease with increasing levels of offspring independence (Fig. 1 A and C; see also refs. 17, 19, 35, and 73), whereas the effects of adaptive growth plasticity (and its corresponding prenatal life history recalibration) are maintained throughout development (Fig. 1 A and B). As such, during gestation, both processes coincide and potentially cancel each other out, while after weaning, only the growth-accelerating life history recalibration is evident (Fig. 1D). The lactation period, as a period of gradually increasing nutritional independence in mammals (35, 73), is intermediate between gestation (full dependence) and postindependence (full independence; Fig. 1).
Fig. 1.
Schematic illustration of the integrated hypothesis predicting PREMS effects on offspring growth resulting from the opposing effects of reduced maternal investment and adaptive growth plasticity. (A) PREMS negatively impacts maternal physical condition resulting in reduced maternal investment and increased glucocorticoid levels. (B) Elevated prenatal glucocorticoid levels increase offspring growth rate throughout development, whereas (C) reduced maternal investment results in developmental constraints on preindependence offspring growth. (D) If both processes coincide, effects may cancel each other out during preindependence, resulting in a realized growth rate that is not different from an unaltered growth rate. Importantly, postindependence growth rate is not affected by maternal investment and therefore reflects adaptive growth plasticity only with increased growth rates compared with controls.
We tested these predictions in comparative analyses using published data on nonhuman mammals. As the timing of PREMS can differentially affect outcomes, we modeled the effects of early vs. late PREMS (4, 28, 30, 33, 59, 74). We assessed whether the effects are elicited by glucocorticoid manipulation without applying external stressors. Because PREMS effects may be particularly strong if caused by food restriction resulting in a direct reduction in maternal capital and investment (29, 75), we controlled for the type of stressor. PREMS effects may be further confounded by the length of PREMS exposure, the length of the potential recovery period following PREMS exposure until parturition, offspring sex, and species-level precociality; hence, we controlled for these variables in all analyses (1, 18, 30, 73). We tested whether growth acceleration follows periods of reduced growth and leads to canalization to potentially rule out conventional catch-up growth processes as alternative explanations of results.
Methods
We searched the literature for studies reporting PREMS effects on offspring growth during gestation, lactation, and/or after weaning. We conducted a full text search in Google Scholar (February 5, 2017) with the following search terms: (intitle:stress OR intitle:cortisol OR intitle:glucocorticoid OR intitle:hca OR intitle:acth OR intitle:dexamethasone OR intitle:“food restriction” OR intitle:“food deprivation” OR intitle:undernutrition) AND (intitle:prenatal OR intitle:gestation OR intitle:gestational OR intitle:pregnant OR intitle:intrauterine) AND (intext:growth OR intext:size OR intext:length OR intext:weight). To minimize potential limitations caused by Google Scholar, we parceled this search up into several subsearches searching for three to four of the stress-related terms, respectively.
We identified 3,035 papers that were surveyed for mammal studies that met the following criteria: the study (i) provides a clearly identifiable control group and PREMS-treatment group, which only differ in PREMS treatment; (ii) provides one clearly defined period of PREMS exposure that was restricted to the gestation period, with no additional differences in treatment before conception and after parturition, and no direct treatment or surgery of the fetus or placenta; (iii) was conducted on intact individuals (e.g., excluding studies involving gene knockouts, adrenalectomy, and poison administration); (iv) was without reported significant differences in offspring mortality between treatment groups (which is usually size dependent); (v) reports data and/or statistics on offspring body size or growth rate, allowing the comparison of offspring growth rates between the treatment and the control group explicitly for gestation, lactation, and/or the postweaning period, respectively; (vi) cannot explain growth rate differences between the control and the treatment group by reported significant group differences in gestation length or litter size; and (vii) presents data on postnatal traits in offspring being either nursed by mother (main dataset) or cross-fostered (including unconstrained artificial nursing). We checked for study redundancy, and in the case of multiple publications reporting on the same study population, results were combined into one dataset. We further excluded all values based on unknown sample size (see below). Altogether, we found 719 studies from 388 publications that met our criteria, comprising the main dataset (658 studies from 385 publications providing 1,125 values across developmental periods, Dataset S1, Table S1a) and the cross-foster dataset (61 studies from 28 publications, Dataset S1, Table S1b). The studies were conducted on 21 different mammal species ranging from rodents to ungulates and primates (Datasets S1 and S2).
From these studies, we recorded the species, type of stressor, beginning and end of PREMS exposure, gestation length, and offspring sex (male, female, or mixed). For each of the three developmental periods, we recorded how the offspring growth rate and final body size of the treatment group differed from the control group (relative growth rate, relative body size). Appropriate growth rate data were reported, however, in only 6% of the studies.
Although 84% of studies reported body size data that enabled assessment of differences in general growth rate, none of the studies reported individual values, data on temporal individual correlations, or any other statistics that would allow assessing the inevitable dependency of the data due to repeated measurements. Thus, although numerous studies reported on offspring growth, we were unable to access error terms of effect sizes in all but a few studies. We therefore ran a metaanalysis on ordinal instead of continuous data, that is, on the direction instead of the size of the PREMS effects. We extracted information on the existence and direction of PREMS effects scoring whether the offspring of PREMS mothers showed higher (1), equal (0), or lower (−1) growth rates and body sizes than the offspring of control (i.e., untreated) mothers. These effect scores were used in all statistical analyses. Effect scores were based on the statistical significance of the results or, if the respective significance levels were not reported, estimated from group-specific means ± SE (and their age trajectories). Postindependence (juvenile) values were derived within the periods of linear growth, that is, before growth trajectories asymptotically approach zero (25).
Considering the nature of the response variable, we applied cumulative link mixed models (CLMMs) [with Logit link function, R package “ordinal” (76); and Kendall’s tau B correlations (function cor.test) and one-sample Wilcoxon tests [R package “exactRankTests” (77)], which are both corrected for the number of ties. All analyses were run with R 3.3.2 (78), all tests were two-tailed with alpha level set to 0.05, and all P values were adjusted for multiple testing using p.adjust with Holm correction. All CLMMs included species and study population as random effects to control for species-specific attributes like gestation length, life expectancy, and number of studies, as well as study population-specific repeated measures. All models further included five control variables: length of PREMS exposure and length of the subsequent recovery period until parturition in percent of gestation length (continuous measures), species precociality (binomial measure, with primates labeled as precocial), offspring sex (male, female, or both), and sample size. If sample size for a certain developmental period was not reported, we estimated the sample size based on reported degrees of freedom or calculated it from the number of mothers per treatment, litter size, survival rates, and/or sex ratios. Sample size, however, was undetectable for ∼4% of the values. Since the CLMM algorithm handles missing values by casewise deletion only, these cases were excluded from all analyses to maintain consistency. All potential two- and three-way interaction terms involving sample size were nonsignificant and excluded from the final models.
We additionally ran all models using more conservative, reduced datasets (reduced main dataset: 499 studies from 303 publications providing 877 values across developmental periods, Dataset S1, Table S1a; reduced cross-foster dataset: 45 studies from 22 publications, Dataset S1, Table S1b). In these reduced datasets we excluded all studies that reported neither a PREMS effect on prenatal maternal glucocorticoid levels nor an effect on offspring growth, since in these cases it remains unclear whether the stressor was sufficient to provoke the PREMS effect of interest. This is of particular importance as nondetectable PREMS effects on offspring growth due to a balancing of the two proposed processes during gestation/lactation as well as the postindependence absence of developmental constraints are central to our predictions (Fig. 1).
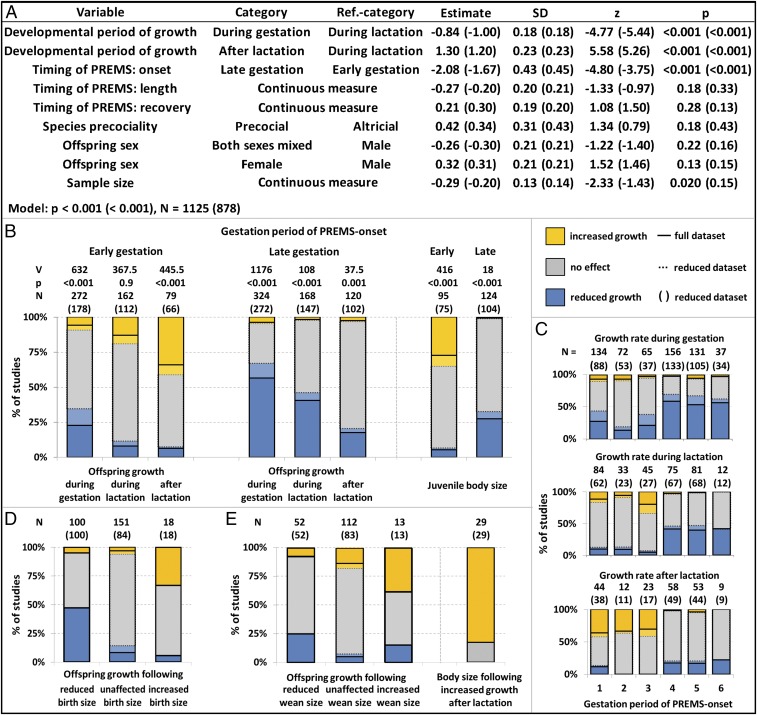
To test our predictions (Fig. 1), we first ran a CLMM on the main dataset (Fig. 2). We analyzed how PREMS effects on offspring growth depend on the level of offspring independence (as reflected by the three consecutive developmental periods) and the timing of PREMS onset (first half vs. second half of gestation).
Fig. 2.
Effects of early vs. late gestation maternal stress on offspring growth rates during gestation, during lactation and after lactation across mammals. Early/late gestation means first/second half of gestation. Percentage of studies reporting a higher (orange), equal (gray), or lower (blue) growth rate in offspring from PREMS mothers compared with control mothers (relative growth rate). (A–C) For each developmental period, relative growth rates following PREMS during the first half of gestation were higher than those following PREMS during the second half of gestation. Within this pattern, the relative growth rate increased with increasing levels of offspring independence (developmental period). (A) In a cumulative Logit link mixed model (CLMM) (random factors: species, within study repeated measures), relative growth rates were independently predicted by developmental period of growth measurement and gestation period of PREMS onset. Length/recovery means period between onset and end of PREMS/between end of PREMS and parturition (both in percent of gestation period). (B) PREMS effects on offspring growth following PREMS during the first half of gestation largely conformed to predictions if both developmental constraints and an adaptive growth plasticity coincide, whereas PREMS effects following PREMS during the second half of gestation largely conformed to predictions of mere developmental constraints in the absence of an adaptive growth plasticity. (C) Presenting PREMS effects on offspring growth depending on finer-scaled periods of PREMS onset (half gestational trimester) illustrates the difference between early and late gestation PREMS onset. (D and E) PREMS effects on offspring growth were not due to catch-up growth in reaction to a preceding growth reduction because accelerated offspring growth was preceded by increased rather than decreased body size at both birth and weaning, and accelerated postindependence growth led to increased rather than unaffected juvenile body sizes.
To test whether the effects of food restriction were different from the effects elicited by other types of stressors, we ran two additional models: one model on data only from studies using nonfood stressors (Fig. S2C) and one model on the full dataset, including the interaction term between stressor type and our test variables developmental period and PREMS onset (Fig. S2D).
We further tested whether the observed patterns in the data hold if mothers’ glucocorticoid levels were directly manipulated or whether the observed effects depend on external stress treatment. We first ran a model on a subset of the data that were generated with glucocorticoid manipulation only (Fig. S3C), and then a model on our entire dataset with interaction terms of stress manipulation type (direct glucocorticoid administration/manipulation vs. external stressor) with our test variables (Fig. S3D).
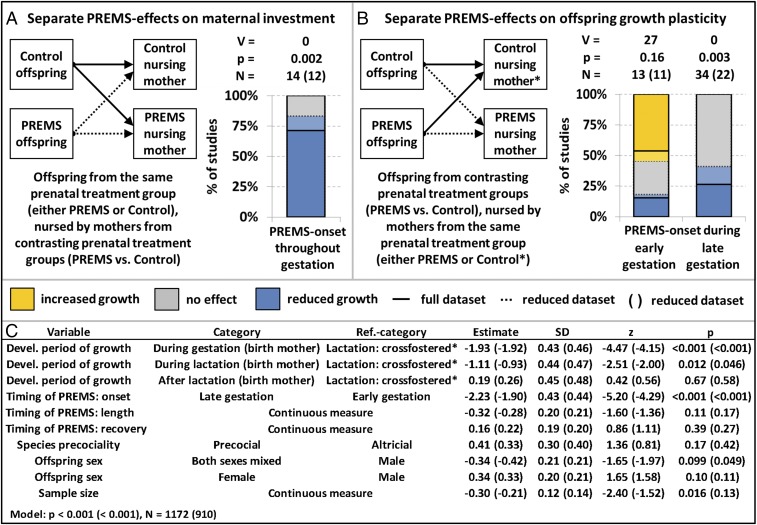
Then, we examined cross-fostering and unconstrained artificial nursing studies. These studies allowed us to test whether it is indeed the change in maternal investment that causes developmental constraints in the offspring. We first tested whether offspring from the same treatment group (either PREMS or control) differed in growth, depending on whether they were nursed by PREMS mothers or controls. We expected PREMS mothers to invest less during lactation and therefore lower growth rates in offspring nursed by PREMS mothers. Then we compared only offspring that were nursed by the same class of mothers. Since offspring classes receive the same energy supply during lactation, we predicted that the growth rate differences between PREMS-exposed offspring and controls match our expectation for the postindependence rather than for the lactation period (Fig. 1).
To test whether catch-up growth could explain our results, we expected that (i) the relative growth rates during lactation and after lactation would be negatively correlated to offspring body sizes at birth and at weaning, respectively (canalization), and (ii) accelerated postindependence growth would not result in overshooting body size. To test these predictions, we extracted and used all data points from the main dataset that were matched as outlined above (see Fig. 2 D and E for sample sizes) and ran Kendall correlations.
We further controlled our results for potential publication bias. Due to the nature of our dataset, we were unable to run effect size analyses (such as funnel plots or conventional sensitivity analyses). We therefore reran our main analysis with the subset of the data from studies reporting offspring body size and/or growth as a methodological byproduct rather than a part of a tested hypothesis (∼68% of all studies, Fig. S1 and Dataset S1, Table S1a). For this subset, a publication bias for significant PREMS effects on offspring growth should be negligible, because the reported PREMS effects or lack thereof would be irrelevant for publication probability.
Results
Across studies, PREMS effects on offspring growth were largely due to two independent effects. First, the negative effects of PREMS on offspring growth ceased with increasing independence and thus decreasing impact of maternal investment (effect of developmental period, Figs. 1C and 2A). As offspring progressed from gestation (most dependence on the mother) to postindependence (least dependence on the mother), the proportion of studies reporting negative effects of PREMS on offspring growth decreased, consistent with the developmental constraints hypothesis (blue bars in Fig. 2B). Second, relative growth rates were higher when the onset of PREMS occurred during early compared with late gestation (Fig. 2 A–C), independent of the length of PREMS exposure or the length of the recovery period. This effect of PREMS timing might reflect a general difference in maternal investment, in which case the effect would decrease with increasing offspring independence (Fig. 1C). Alternatively, higher growth rates in response to earlier compared with later PREMS exposure may result from an early acceleration of offspring growth that would act consistently throughout development (Fig. 1B). To test between these alternatives, we assessed the statistical interaction term between the timing of PREMS onset and developmental period and found that this interaction term did not significantly affect the response (P > 0.2 in all models). Thus, the effect of PREMS timing did not change across developmental periods with varying degrees of offspring dependence (Fig. 2B), i.e., early PREMS exposure led to increased growth rates across all developmental stages compared with controls.
Additional models were built to assess whether the type of manipulation used to cause PREMS affected the results. The patterning of early and late PREMS effects remained unchanged when limiting the analysis to nonfood stressors such as social, heat, restraint, or predation stress; the main effects of developmental period and PREMS onset were still significant (Fig. S2 A and C). The developmental constraints effects were exacerbated in studies using food restriction to elicit PREMS (Fig. S2D interaction effects of stressor type with developmental period, Fig. S2B).
The overall patterns were also similar across studies that only manipulated maternal glucocorticoid levels without exposure to external stressors; the main effects of PREMS onset and of developmental period remained significant when limiting the analysis to GC manipulation only (Fig. S3 A and C). External stressors did not have an effect over and above the effect of maternal glucocorticoids. Instead, with external stressors the timing effects of PREMS on growth patterns were less pronounced. (Fig. S3D interaction terms of stressor type and PREMS onset on the one hand and developmental period on the other, Fig. S3B).
PREMS effects on offspring growth did not appear to be caused by catch-up growth. Relative growth rates were positively, instead of negatively, correlated with the preceding body size both at birth [Fig. 2D; full (reduced) dataset: Kendall’s tau B = 0.415 (0.370), z = 7.13 (5.58), P < 0.001 (0.009), n = 269 (202)] and at weaning [Fig. 2E; Kendall’s tau B = 0.243 (0.249), z = 3.42 (3.23), P < 0.001 (0.016), n = 177 (148)]. It was the large newborns and weanlings growing faster, not the small ones. For the few studies that provided information on both postindependence growth and later size, accelerated growth after PREMS did not result in similar body size in treated and control juveniles, but instead, treated juveniles were larger than controls (Fig. 2E; Wilcoxon signed-rank test: V = 300, P < 0.001, n = 29).
Lastly, we focused on cross-fostering and unconstrained artificial nursing studies. When offspring from the same prenatal treatment group were nursed by mothers from different prenatal treatment groups, those nursed by PREMS mothers grew slower than those nursed by control mothers (Fig. 3A). Thus, growth restriction during lactation was due to PREMS exposure of the nursing mother, rather than the offspring’s prenatal environment. Furthermore, if offspring from different prenatal treatment groups were equally nursed during lactation (Fig. 3B), then differences in maternal investment should be eliminated and offspring should exhibit the growth patterns found in postindependence PREMS offspring in our main analyses (Fig. 2). To test this, we used data from cross-fostered offspring that were similarly nursed as reference category for developmental phases in our main model (Fig. 3C). Indeed, the growth patterns in this reference category were different from growth patterns during lactation in offspring nursed by their birth mothers, but not different from growth patterns after lactation in these offspring (Fig. 3C; see also comparison between Figs. 2B and 3B). Finally, separate plots for early and late onset PREMS indicate that PREMS offspring grew faster than controls if both were nursed by the same control mothers or were hand raised (Fig. 3B).
Fig. 3.
Separating the effects of maternal investment and offspring growth plasticity: How cross-fostering and artificial nursing influences PREMS effects on offspring growth during lactation. Percentage of studies reporting a higher (orange), equal (gray), or lower (blue) growth rate in offspring (A) nursed by or (B) born to PREMS mothers (compared with control mothers). Early/late gestation means first/second half of gestation. (A) Pure maternal investment effect (Fig. 1A blue path, Fig. 1C). Offspring of the same prenatal treatment group showed a significantly reduced growth rate during lactation if nursed by a PREMS compared with a control mother. (B and C) Pure effect of offspring growth plasticity (Fig. 1A orange path, Fig. 1B). Offspring from different prenatal treatment groups were equally nursed by mothers that did not differ from each other in their prenatal treatments. The resulting PREMS effects on offspring growth during lactation largely conform to predictions for adaptive growth plasticity in the absence of maternal investment effects and are similar to PREMS effects on postindependence growth (see Fig. 2 for comparison). A cumulative Logit link mixed model (CLMM) (random factors: species, within study repeated measures) revealed that compared with PREMS effects in offspring naturally nursed by their birth mothers, the exclusion of differences in maternal investment during lactation (reference category “lactation: cross-fostered”) resulted in increased growth rates, i.e., a significant shift from PREMS effects predicted for lactation to effects predicted for postindependence. Length/recovery means period between onset and end of PREMS/between end of PREMS and parturition (both in percent of gestation period). *Control nursing includes both nursing by control females and artificial nursing.
Discussion
In this metaanalysis including 719 studies from 388 publications across 21 mammal species ranging across diverse taxa, we provide a comprehensive description of PREMS effects on offspring growth. Growth differences between PREMS offspring and controls varied with the timing of the stressor during gestation and the developmental period assessed. Growth patterns in response to PREMS did not differ between nutritional and other stressors and were elicited by glucocorticoid manipulation alone. The main limitation of our study is that all analyses had to use the proportion of study outcomes as a response variable, because none of the studies reported quantitative differences in growth rates with error terms. Our approach may have masked additional variation that could be due to nonlinear effects or differences between species, sexes, and stressors, and nonsignificant results should therefore be treated with caution. As continuous effects become available, analyses can also be controlled for the phylogenetic relationships between the species studied.
Our results are consistent with two opposing processes acting on offspring growth in response to PREMS. The first process results from developmental constraints and reduces offspring growth, with effects diminishing as offspring becomes increasingly independent. The second process is a single recalibration of an offspring’s growth trajectory that initially buffers the effects of reduced maternal investment during gestation, then fully compensates for it during lactation, and, eventually, leads to accelerated growth, overshooting an offspring’s original target body size during juvenility. These outcomes were observed only after PREMS exposure early but not late during gestation, suggesting that a sensitive window has closed by then. Since we found that larger, not smaller, neonates grew faster, the resulting growth pattern is not consistent with classic catch-up growth (51–53) as the underlying process. Instead, our results suggest developmental plasticity in response to early PREMS with lasting effects. These growth patterns were observed in response to a variety of stressors and not limited to food-restriction studies. Our results also suggest that both reduced maternal investment and the counteracting growth acceleration can be induced by elevated maternal glucocorticoid levels alone, even in the absence of an external stressor. This highlights the potential role of glucocorticoids in shaping and linking maternal investment strategies and offspring adaptive developmental plasticity (1–4, 8, 18).
In response to (expected) developmental constraints imposed by the mother, offspring shift to a faster life history trajectory, accelerating their own somatic growth. By accelerating growth, offspring are better able to mature quickly and to produce offspring early and fast (15, 20, 65). Mothers gain by reserving residual reproductive value (8, 19), and offspring gain by increasing the chance that they reproduce at all after a bad start (20, 23, 65). This life history pace recalibration probably comes at a cost, because energy is allocated more to growth, maturation, and reproduction and away from other functions such as immune function, neurodevelopment, and cognitive function, all of which are known to be affected by PREMS (1, 2, 4, 9, 29, 31, 63, 79). Thus, these latter functions are jeopardized by energetic constraints caused by reduced maternal investment and additionally by energy allocation toward somatic growth and maturation (23, 24). Although the combined effects of early gestation PREMS may lead to reduced fitness overall for both mother and offspring compared with control conditions, these effects may maximize fitness for both under suboptimal early conditions that cause PREMS (8, 9, 11, 16, 18, 19, 65).
A comprehensive test of our integrative hypothesis will require lifetime fitness data from sophisticated experimental settings that, to our knowledge, are not yet available (18, 80–82). It has been established, however, that PREMS often is associated with reduced offspring life span (5–7, 49, 61, 62), and that, across vertebrates, such reduced life span correlates with accelerated growth, maturation, reproduction, and earlier reproductive senescence, suggesting an overall faster life history pace (24, 63). As an indicator for the relevance of timing effects in mammals, PREMS during the first half of gestation tends to result in an earlier age at maturation without affecting body size at maturation compared with controls (refs. 27, 59, and 60, but see refs. 83–85). By contrast, PREMS during the second half of gestation generally leads to a later age at maturation (refs. 86–90, but see refs. 91–93). Evidence for adaptive developmental plasticity in humans comes from the Dutch hunger winter cohort. Without considering PREMS timing, prenatal exposure to the famine caused accelerated reproduction (ref. 58, but see ref. 94) and cognitive aging (95). Consistent with our hypothesis, mothers’ exposure to the famine during late but not early gestation led to reduced offspring birth weight (ref. 33, see also refs. 34 and 96, but see ref. 97), whereas early but not late exposure altered offspring DNA methylation of regions associated with growth and metabolism (74). PREMS generally reduced offspring health and life span (ref. 49, see also refs. 61 and 62), and these effects were strongest after PREMS during the first half of gestation (98), potentially reflecting the somatic costs of accelerated growth (23, 24, 47, 64).
Accelerated growth has often been interpreted as a catch-up process that is part of canalized growth (17, 19, 20, 23, 25, 47, 51–53). Catch-up growth occurs in small-for-age offspring and can cause constraints on other aspects of development, because more resources are allocated to growth (17, 19, 20, 23–25, 47, 51–53). Catch-up growth has offered a promising explanation for PREMS effects, because PREMS often leads to reduced birth size and reduced birth size is often associated with disadvantaged adult phenotypes (1–3, 16, 42, 47, 99). Neither of these correlations is ubiquitous, however. PREMS effects on offspring growth are highly variable, and the long-term costs observed in adulthood may or may not be related to reduced birth size (1, 13, 16, 17, 19, 42). Our results indicate that PREMS effects on offspring growth do not generally involve classic catch-up growth in a large sample across mammals.
To recalibrate offspring phenotype to accelerated growth and faster life history, it seems necessary that PREMS occurs during a critical period that is limited to early gestation. Late gestation PREMS not only fails to initiate a life history recalibration, but even seems to prevent the offspring from enlisting catch-up growth. Such timing effects can be explained by a general decrease of fetal plasticity with increasing age (1, 9, 19, 42), changing effects of PREMS on the placenta (42), changes in the interplay between maternal and fetal glucocorticoid metabolism (3), or changes in fetal glucocorticoid sensitivity (3). Thus, more work on the mechanisms underlying timing effects, together with research on the consequences of PREMS for different aspects of life history, including maturation, reproductive rate, and longevity, as well as studies spanning different sensitive periods in ontogeny where PREMS effects may be modified, and work on the interplay of PREMS and maternal investment will advance our understanding of the interplay of developmental constraints and adaptive developmental plasticity.
Data and Materials Availability.
The raw data from the study are available as Dataset S1.
Supplementary Material
Acknowledgments
We thank Jacinta Beehner, Thore Bergman, Marco Del Giudice, Martin N. Muller, Melissa Emery Thompson, the members of the Anthropology Department at the University of New Mexico, and the members of the research unit “Sociality and Health in Primates” (DFG FOR 2136) for stimulating discussions. Financial support was granted by the Leibniz Association (Leibniz Graduate School for the Foundation of Primate Social Behavior) and the German Initiative of Excellence to the University of Göttingen and the National Institutes of Health (Grant R01AG049395).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707152114/-/DCSupplemental.
References
- 1.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol Rev. 2014;94:1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: Outcomes. Nat Rev Endocrinol. 2014;10:391–402. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- 3.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 2: Mechanisms. Nat Rev Endocrinol. 2014;10:403–411. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- 4.Zijlmans MA, Riksen-Walraven JM, de Weerth C. Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neurosci Biobehav Rev. 2015;53:1–24. doi: 10.1016/j.neubiorev.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Ozanne SE, Hales CN. Lifespan: Catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- 6.Lummaa V, Clutton-Brock T. Early development, survival and reproduction in humans. Trends Ecol Evol. 2002;17:141–147. [Google Scholar]
- 7.Vaiserman AM. Early-life nutritional programming of longevity. J Dev Orig Health Dis. 2014;5:325–338. doi: 10.1017/S2040174414000294. [DOI] [PubMed] [Google Scholar]
- 8.Sheriff MJ, Love OP. Determining the adaptive potential of maternal stress. Ecol Lett. 2013;16:271–280. doi: 10.1111/ele.12042. [DOI] [PubMed] [Google Scholar]
- 9.Wells JC. The thrifty phenotype as an adaptive maternal effect. Biol Rev Camb Philos Soc. 2007;82:143–172. doi: 10.1111/j.1469-185X.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- 10.Wells JCK. Maternal capital and the metabolic ghetto: An evolutionary perspective on the transgenerational basis of health inequalities. Am J Hum Biol. 2010;22:1–17. doi: 10.1002/ajhb.20994. [DOI] [PubMed] [Google Scholar]
- 11.Nettle D, Bateson M. Adaptive developmental plasticity: What is it, how can we recognize it and when can it evolve? Proc Biol Sci. 2015;282:20151005. doi: 10.1098/rspb.2015.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nettle D, Frankenhuis WE, Rickard IJ. The evolution of predictive adaptive responses in human life history. Proc Biol Sci. 2013;280:20131343. doi: 10.1098/rspb.2013.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the predictive adaptive response hypothesis. J Physiol. 2014;592:2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Giudice M. Early stress and human behavioral development: Emerging evolutionary perspectives. J Dev Orig Health Dis. 2014;5:270–280. doi: 10.1017/S2040174414000257. [DOI] [PubMed] [Google Scholar]
- 15.Wells JC, Yao P, Williams JE, Gayner R. Maternal investment, life-history strategy of the offspring and adult chronic disease risk in South Asian women in the UK. Evol Med Public Health. 2016;2016:133–145. doi: 10.1093/emph/eow011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coall DA, Callan AC, Dickins TE, Chisholm JS. Evolution and prenatal development: An evolutionary perspective. In: Lerner RM, Lamb ME, editors. Handbook of Child Psychology and Developmental Science. Vol 3:3. Wiley; New York: 2015. pp. 57–105. [Google Scholar]
- 17.Wells JC. Worldwide variability in growth and its association with health: Incorporating body composition, developmental plasticity, and intergenerational effects. Am J Hum Biol. 2017;29 doi: 10.1002/ajhb.22954. [DOI] [PubMed] [Google Scholar]
- 18.Sheriff MJ, et al. Integrating ecological and evolutionary context in the study of maternal stress. Integr Comp Biol. 2017;57:437–449. doi: 10.1093/icb/icx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells JC. The thrifty phenotype hypothesis: Thrifty offspring or thrifty mother? J Theor Biol. 2003;221:143–161. doi: 10.1006/jtbi.2003.3183. [DOI] [PubMed] [Google Scholar]
- 20.Dmitriew CM. The evolution of growth trajectories: What limits growth rate? Biol Rev Camb Philos Soc. 2011;86:97–116. doi: 10.1111/j.1469-185X.2010.00136.x. [DOI] [PubMed] [Google Scholar]
- 21.Stearns SC. The Evolution of Life Histories. Oxford Univ Press; Oxford: 1992. [Google Scholar]
- 22.Plard F, et al. Disentangling direct and growth-mediated influences on early survival: A mechanistic approach. J Anim Ecol. 2015;84:1363–1372. doi: 10.1111/1365-2656.12378. [DOI] [PubMed] [Google Scholar]
- 23.Metcalfe NB, Monaghan P. Compensation for a bad start: Grow now, pay later? Trends Ecol Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- 24.Metcalfe NB, Monaghan P. Growth versus lifespan: Perspectives from evolutionary ecology. Exp Gerontol. 2003;38:935–940. doi: 10.1016/s0531-5565(03)00159-1. [DOI] [PubMed] [Google Scholar]
- 25.Hector KL, Nakagawa S. Quantitative analysis of compensatory and catch-up growth in diverse taxa. J Anim Ecol. 2012;81:583–593. doi: 10.1111/j.1365-2656.2011.01942.x. [DOI] [PubMed] [Google Scholar]
- 26.Dantzer B, et al. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science. 2013;340:1215–1217. doi: 10.1126/science.1235765. [DOI] [PubMed] [Google Scholar]
- 27.Schöpper H, Klaus T, Palme R, Ruf T, Huber S. Sex-specific impact of prenatal stress on growth and reproductive parameters of guinea pigs. J Comp Physiol B. 2012;182:1117–1127. doi: 10.1007/s00360-012-0680-9. [DOI] [PubMed] [Google Scholar]
- 28.Hauser J, et al. Effects of prenatal dexamethasone treatment on postnatal physical, endocrine, and social development in the common marmoset monkey. Endocrinology. 2007;148:1813–1822. doi: 10.1210/en.2006-1306. [DOI] [PubMed] [Google Scholar]
- 29.Berghänel A, Heistermann M, Schülke O, Ostner J. Prenatal stress effects in a wild, long-lived primate: Predictive adaptive responses in an unpredictable environment. Proc Biol Sci. 2016;283:20161304. doi: 10.1098/rspb.2016.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooke JA, Arnott G, Dwyer CM, Rutherford KMD. The importance of the gestation period for welfare of lambs: Maternal stressors and lamb vigour and wellbeing. J Agric Sci. 2015;153:497–519. [Google Scholar]
- 31.Merlot E, Quesnel H, Prunier A. Prenatal stress, immunity and neonatal health in farm animal species. Animal. 2013;7:2016–2025. doi: 10.1017/S175173111300147X. [DOI] [PubMed] [Google Scholar]
- 32.Rendina DN, Lubach GR, Coe CL. Gestational timing of prenatal disturbance and fetal sex determine the developmental outcomes. Neonatology. 2016;109:314–320. doi: 10.1159/000443717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roseboom TJ, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donnell MH, Behie AM. Effects of bushfire stress on birth outcomes: A cohort study of the 2009 Victorian Black Saturday bushfires. Int J Disaster Risk Reduct. 2013;5:98–106. [Google Scholar]
- 35.Hinde K, Milligan LA. Primate milk: Proximate mechanisms and ultimate perspectives. Evol Anthropol. 2011;20:9–23. doi: 10.1002/evan.20289. [DOI] [PubMed] [Google Scholar]
- 36.Hinde K, Power ML, Oftedal OT. Rhesus macaque milk: Magnitude, sources, and consequences of individual variation over lactation. Am J Phys Anthropol. 2009;138:148–157. doi: 10.1002/ajpa.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowen WD. Maternal effects on offspring size and development in pinnipeds. In: Maestripieri D, Mateo JM, editors. Maternal Effects in Mammals. University of Chicago Press; Chicago: 2009. pp. 104–132. [Google Scholar]
- 38.Swanson TJ, et al. Effects of gestational plane of nutrition and selenium supplementation on mammary development and colostrum quality in pregnant ewe lambs. J Anim Sci. 2008;86:2415–2423. doi: 10.2527/jas.2008-0996. [DOI] [PubMed] [Google Scholar]
- 39.Klaus T, Schöpper H, Huber S. Effects of chronic stress during pregnancy on maternal performance in the guinea pig (Cavia aperea f. porcellus) Behav Processes. 2013;94:83–88. doi: 10.1016/j.beproc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Tygesen MP, Tauson AH, Blache D, Husted SM, Nielsen MO. Late foetal life nutrient restriction and sire genotype affect postnatal performance of lambs. Animal. 2008;2:574–581. doi: 10.1017/S1751731107001516. [DOI] [PubMed] [Google Scholar]
- 41.Tao S, Dahl GE. Invited review: Heat stress effects during late gestation on dry cows and their calves. J Dairy Sci. 2013;96:4079–4093. doi: 10.3168/jds.2012-6278. [DOI] [PubMed] [Google Scholar]
- 42.Rutherford JN. The primate placenta as an agent of developmental and health trajectories across the life course. In: Clancy KBH, Hinde K, Rutherford JN, editors. Building Babies. Vol 37. Springer; New York: 2013. pp. 27–53. [Google Scholar]
- 43.Hauser J, Feldon J, Pryce CR. Prenatal dexamethasone exposure, postnatal development, and adulthood prepulse inhibition and latent inhibition in Wistar rats. Behav Brain Res. 2006;175:51–61. doi: 10.1016/j.bbr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 44.Cabrera RJ, Rodríguez-Echandía EL, Jatuff AS, Fóscolo M. Effects of prenatal exposure to a mild chronic variable stress on body weight, preweaning mortality and rat behavior. Braz J Med Biol Res. 1999;32:1229–1237. doi: 10.1590/s0100-879x1999001000009. [DOI] [PubMed] [Google Scholar]
- 45.Tao S, Monteiro APA, Thompson IM, Hayen MJ, Dahl GE. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J Dairy Sci. 2012;95:7128–7136. doi: 10.3168/jds.2012-5697. [DOI] [PubMed] [Google Scholar]
- 46.Roussel S, Boissy A, Montigny D, Hemsworth PH, Duvaux-Ponter C. Gender-specific effects of prenatal stress on emotional reactivity and stress physiology of goat kids. Horm Behav. 2005;47:256–266. doi: 10.1016/j.yhbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 47.Hales CN, Ozanne SE. The dangerous road of catch-up growth. J Physiol. 2003;547:5–10. doi: 10.1113/jphysiol.2002.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emery Thompson M, et al. Faster reproductive rates trade off against offspring growth in wild chimpanzees. Proc Natl Acad Sci USA. 2016;113:7780–7785. doi: 10.1073/pnas.1522168113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roseboom TJ, et al. Adult survival after prenatal exposure to the Dutch famine 1944–45. Paediatr Perinat Epidemiol. 2001;15:220–225. doi: 10.1046/j.1365-3016.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- 50.Théoret-Gosselin R, Hamel S, Côté SD. The role of maternal behavior and offspring development in the survival of mountain goat kids. Oecologia. 2015;178:175–186. doi: 10.1007/s00442-014-3198-x. [DOI] [PubMed] [Google Scholar]
- 51.Boersma B, Wit JM. Catch-up growth. Endocr Rev. 1997;18:646–661. doi: 10.1210/edrv.18.5.0313. [DOI] [PubMed] [Google Scholar]
- 52.Prader A, Tanner JM, von Harnack G. Catch-up growth following illness or starvation. An example of developmental canalization in man. J Pediatr. 1963;62:646–659. doi: 10.1016/s0022-3476(63)80035-9. [DOI] [PubMed] [Google Scholar]
- 53.Cameron N. Growth patterns in adverse environments. Am J Hum Biol. 2007;19:615–621. doi: 10.1002/ajhb.20661. [DOI] [PubMed] [Google Scholar]
- 54.Del Giudice M, Gangestad SW, Kaplan HS. The Handbook of Evolutionary Psychology. Wiley; New York: 2015. Life history theory and evolutionary psychology. [Google Scholar]
- 55.Demerath EW, et al. Genetic and environmental influences on infant weight and weight change: The Fels longitudinal study. Am J Hum Biol. 2007;19:692–702. doi: 10.1002/ajhb.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science. 1982;215:1518–1519. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- 57.Swolin-Eide D, et al. Affected skeletal growth but normal bone mineralization in rat offspring after prenatal dexamethasone exposure. J Endocrinol. 2002;174:411–418. doi: 10.1677/joe.0.1740411. [DOI] [PubMed] [Google Scholar]
- 58.Painter RC, et al. Increased reproductive success of women after prenatal undernutrition. Hum Reprod. 2008;23:2591–2595. doi: 10.1093/humrep/den274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zehr JL, Van Meter PE, Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): Role of prenatal androgens, social rank, and adolescent body weight. Biol Reprod. 2005;72:1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]
- 60.Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS One. 2009;4:e6744. doi: 10.1371/journal.pone.0006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanson HA, Smith KR. Early origins of longevity: Prenatal exposures to food shortage among early Utah pioneers. J Dev Orig Health Dis. 2013;4:170–181. doi: 10.1017/S2040174412000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Todd N, Valleron A-J, Bougnères P. Prenatal loss of father during world war one is predictive of a reduced lifespan in adulthood. Proc Natl Acad Sci USA. 2017;114:4201–4206. doi: 10.1073/pnas.1617911114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lemaître J-F, et al. Early-late life trade-offs and the evolution of ageing in the wild. Proc Biol Sci. 2015;282:20150209. doi: 10.1098/rspb.2015.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douhard F, Gaillard J-M, Pellerin M, Jacob L, Lemaître J-F. The cost of growing large: Costs of post-weaning growth on body mass senescence in a wild mammal. Oikos. 2017;126:1329–1338. [Google Scholar]
- 65.Jones JH. Fetal programming: Adaptive life-history tactics or making the best of a bad start? Am J Hum Biol. 2005;17:22–33. doi: 10.1002/ajhb.20099. [DOI] [PubMed] [Google Scholar]
- 66.Nederhof E, Schmidt MV. Mismatch or cumulative stress: Toward an integrated hypothesis of programming effects. Physiol Behav. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Wells JCK, Johnstone RA. Modeling developmental plasticity in human growth: Buffering the past or predicting the future? In: Jasienska G, Sherry DS, Holmes DJ, editors. The Arc of Life: Evolution and Health Across the Life Course. Springer; New York: 2017. pp. 21–39. [Google Scholar]
- 68.Hinde K, et al. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav Ecol. 2015;26:269–281. doi: 10.1093/beheco/aru186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belsky J, Ruttle PL, Boyce WT, Armstrong JM, Essex MJ. Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Dev Psychol. 2015;51:816–822. doi: 10.1037/dev0000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glynn LM, et al. Postnatal maternal cortisol levels predict temperament in healthy breastfed infants. Early Hum Dev. 2007;83:675–681. doi: 10.1016/j.earlhumdev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 71.Dettmer AM, et al. Cortisol in neonatal mother’s milk predicts later infant social and cognitive functioning in rhesus monkeys. Child Dev. 2017 doi: 10.1111/cdev.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sullivan EC, Hinde K, Mendoza SP, Capitanio JP. Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Dev Psychobiol. 2011;53:96–104. doi: 10.1002/dev.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langer P. The phases of maternal investment in eutherian mammals. Zoology (Jena) 2008;111:148–162. doi: 10.1016/j.zool.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 74.Tobi EW, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun. 2014;5:5592. doi: 10.1038/ncomms6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson JS, et al. The impact of in utero heat stress and nutrient restriction on progeny body composition. J Therm Biol. 2015;53:143–150. doi: 10.1016/j.jtherbio.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Christensen RHB. 2015 Ordinal–Regression Models for Ordinal Data. R Package Version 2015.6-28. Available at www.cran.r-project.org/package=ordinal/. Accessed November 20, 2016.
- 77.Hothorn T, Hornik K. 2015 exactRankTests: Exact Distributions for Rank and Permutation Tests. R Package Version 0.8-28. Available at https://CRAN.R-project.org/package=exactRankTests. Accessed November 20, 2016.
- 78.R Development Core Team 2011. A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna), Version 3.3.2.
- 79.Veru F, Laplante DP, Luheshi G, King S. Prenatal maternal stress exposure and immune function in the offspring. Stress. 2014;17:133–148. doi: 10.3109/10253890.2013.876404. [DOI] [PubMed] [Google Scholar]
- 80.Beehner JC, Bergman TJ. The next step for stress research in primates: To identify relationships between glucocorticoid secretion and fitness. Horm Behav. 2017;91:68–83. doi: 10.1016/j.yhbeh.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Taborsky B. Developmental plasticity: Preparing for life in a complex world. Adv Stud Behav. 2017;49:49–99. [Google Scholar]
- 82.Groothuis TGG, Taborsky B. Introducing biological realism into the study of developmental plasticity in behaviour. Front Zool. 2015;12(Suppl 1):S6. doi: 10.1186/1742-9994-12-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Torrente M, Colomina MT, Domingo JL. Effects of prenatal exposure to manganese on postnatal development and behavior in mice: Influence of maternal restraint. Neurotoxicol Teratol. 2002;24:219–225. doi: 10.1016/s0892-0362(02)00188-5. [DOI] [PubMed] [Google Scholar]
- 84.Rojo M, Marin B, Menendez-Patterson A. Effects of low stress during pregnancy on certain parameters of the offspring. Physiol Behav. 1985;34:895–899. doi: 10.1016/0031-9384(85)90010-1. [DOI] [PubMed] [Google Scholar]
- 85.Colomina MT, Roig JL, Torrente M, Vicens P, Domingo JL. Concurrent exposure to aluminum and stress during pregnancy in rats: Effects on postnatal development and behavior of the offspring. Neurotoxicol Teratol. 2005;27:565–574. doi: 10.1016/j.ntt.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 86.Smith JT, Waddell BJ. Increased fetal glucocorticoid exposure delays puberty onset in postnatal life. Endocrinology. 2000;141:2422–2428. doi: 10.1210/endo.141.7.7541. [DOI] [PubMed] [Google Scholar]
- 87.Marchlewska-Koj A, Kruczek M, Kapusta J, Pochroń E. Prenatal stress affects the rate of sexual maturation and attractiveness in bank voles. Physiol Behav. 2003;79:305–310. doi: 10.1016/s0031-9384(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 88.Iwasa T, et al. Effects of intrauterine undernutrition on hypothalamic Kiss1 expression and the timing of puberty in female rats. J Physiol. 2010;588:821–829. doi: 10.1113/jphysiol.2009.183558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borges CS, et al. Reproductive disorders in female rats after prenatal exposure to betamethasone. J Appl Toxicol. 2017;37:1065–1072. doi: 10.1002/jat.3457. [DOI] [PubMed] [Google Scholar]
- 90.Politch JA, Herrenkohl LR. Effects of prenatal stress on reproduction in male and female mice. Physiol Behav. 1984;32:95–99. doi: 10.1016/0031-9384(84)90077-5. [DOI] [PubMed] [Google Scholar]
- 91.Herrenkohl LR, Scott S. Prenatal stress and postnatal androgen: Effects on reproduction in female rats. Experientia. 1984;40:101–103. doi: 10.1007/BF01959126. [DOI] [PubMed] [Google Scholar]
- 92.Llorente E, Brito ML, Machado P, González MC. Effect of prenatal stress on the hormonal response to acute and chronic stress and on immune parameters in the offspring. J Physiol Biochem. 2002;58:143–149. doi: 10.1007/BF03179851. [DOI] [PubMed] [Google Scholar]
- 93.Dunn E, Kapoor A, Leen J, Matthews SG. Prenatal synthetic glucocorticoid exposure alters hypothalamic-pituitary-adrenal regulation and pregnancy outcomes in mature female guinea pigs. J Physiol. 2010;588:887–899. doi: 10.1113/jphysiol.2009.182139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lumey LH, Stein AD. In utero exposure to famine and subsequent fertility: The Dutch Famine Birth Cohort Study. Am J Public Health. 1997;87:1962–1966. doi: 10.2105/ajph.87.12.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci USA. 2010;107:16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Class QA, Lichtenstein P, Långström N, D’Onofrio BM. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: A population study of 2.6 million pregnancies. Psychosom Med. 2011;73:234–241. doi: 10.1097/PSY.0b013e31820a62ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dancause KN, et al. Disaster-related prenatal maternal stress influences birth outcomes: Project ice storm. Early Hum Dev. 2011;87:813–820. doi: 10.1016/j.earlhumdev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 98.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Grove BJ, Lim SJ, Gale CR, Shenkin SD. Birth weight and cognitive ability in adulthood: A systematic review and meta-analysis. Intelligence. 2017;61:146–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.