Abstract
Background
Strigolactones (SLs) play important roles in controlling root growth, shoot branching, and plant-symbionts interaction. Despite the importance, the components of SL biosynthesis and signaling have not been unequivocally explored in soybean.
Results
Here we identified the putative components of SL synthetic enzymes and signaling proteins in soybean genome. Soybean genome contains conserved MORE AXILLARY BRANCHING (MAX) orthologs, GmMAX1s, GmMAX2s, GmMAX3s, and GmMAX4s. The tissue expression patterns are coincident with SL synthesis in roots and signaling in other tissues under normal conditions. GmMAX1a, GmMAX2a, GmMAX3b, and GmMAX4a expression in their Arabidopsis orthologs’ mutants not only restored most characteristic phenotypes, such as shoot branching and shoot height, leaf shape, primary root length, and root hair growth, but also restored the significantly changed hormone contents, such as reduced JA and ABA contents in all mutant leaves, but increased auxin levels in atmax1, atmax3 and atmax4 mutants. Overexpression of these GmMAXs also altered the hormone contents in wild-type Arabidopsis. GmMAX3b was further characterized in soybean nodulation with overexpression and knockdown transgenic hairy roots. GmMAX3b overexpression (GmMAX3b-OE) lines exhibited increased nodule number while GmMAX3b knockdown (GmMAX3b-KD) decreased the nodule number in transgenic hairy roots. The expression levels of several key nodulation genes were also altered in GmMAX3b transgenic hairy roots. GmMAX3b overexpression hairy roots had reduced ABA, but increased JA levels, with no significantly changed auxin content, while the contrast changes were observed in GmMAX3b-KD lines. Global gene expression in GmMAX3b-OE or GmMAX3b-KD hairy roots also revealed that altered expression of GmMAX3b in soybean hairy roots changed several subsets of genes involved in hormone biosynthesis and signaling and transcriptional regulation of nodulation processes.
Conclusions
This study not only revealed the conservation of SL biosynthesis and signaling in soybean, but also showed possible interactions between SL and other hormone synthesis and signaling during controlling plant development and soybean nodulation. GmMAX3b-mediated SL biosynthesis and signaling may be involved in soybean nodulation by affecting both root hair formation and its interaction with rhizobia.
Electronic supplementary material
The online version of this article (10.1186/s12870-017-1182-4) contains supplementary material, which is available to authorized users.
Keywords: Soybean, Strigolactones, Nodulation, Hormone interaction, GmMAX3, Genetic complementation
Background
The currently emerging hormones, the strigolactone (SLs), a novel group of terpeniod lactone derived from carotenoid, were first recognized as a constituent of root secretion for germination of parasitic witch weeds [71]. SLs then are found to be required for the establishment of symbiotic arbuscular mycorrhizal fungi in plant related to phosphor deficiency [3, 12, 63], and recently involved in legume-rhizobia interaction [22–24, 37]. The most prominent phenotypes controlled by SLs are the root growth, shoot branching, and overall plant architecture [25, 54, 55, 60].
Genetic and physiological studies on the carotenoid –derived long-distance signal molecules had revealed that SLs are mainly produced in the roots of plants and then transported upward to shoot regions [25, 60]. These studies have revealed the critical roles of SLs in controlling shoot branching in multi-branching mutants, including MORE AXILLARY GROWTH (MAX) in Arabidopsis, RAMOSUS (RMS) in pea (Pisum sativum), DECREASED APICAL DOMINANCE (DAD) in petunia (Petunia hybrida), and DWARF or HIGH-TILLERING DWARF (D/HTD) in rice (Oryza sativa) [5, 9, 11, 25, 60]. SLs also have roles in plant development and adaptive responses other than regulating shoot branching [16, 49]. It has been demonstrated that these MAX/D/RMS/DAD mutants are involved in either SL biosynthesis or SL signal perception and transductions [6, 7]. SL biosynthesis was derived from carotenoid pathway, firstly by D27 carotenoid isomerase catalyzed conversion of all-trans-β-carotene into produce 9-cis-β-carotene [4, 68]. 9-Cis-β-carotene is successively cleaved by the carotenoid cleavage dioxygenase 7 (CCD7), encoded by Arabidopsis AtMAX3, rice D17/HTD1, pea RMS5, or petunina DAD3 to produce 9-cis-β-apo-10-carotenal [4] and CCD8, encoded by AtMAX4, rice D10, pea RMS1 or petunia DAD1, to convert 9-cis-β-apo-10 carotenal into carlactone [4]. Carlactone can be further converted into 5-deoxylstrigol and other bioactive SLs by a P450 monooxygenase, encoded by Arabidopsis MAX1 and lotus LBO [1, 15, 48, 74]. These bioactive SLs are perceived by rice D14 or petunia DAD2, an α/β-fold hydrolase that can hydrolyze SLs, acting as a SL receptor. D14 interacts with the MAX2/D3 group of F-box proteins [18, 20, 26, 55, 72] to form a D14/ SKP1–CULLIN–F-BOX (SCF) E3 ubiquitin ligase complex D14-SCFD3/MAX2 in the presence of SLs [55]. The D14-SCFD3/MAX2 appears to play a vital role in mediating SLs-triggered its substrate protein degradation [18, 55, 68, 75]. D53 in rice or its orthologs, SMAX1-LIKE6 (SMXL6) and SMXL7 in Arabidopsis act as a substrate for SCFD3/AtMAX2 [29, 36, 65, 75]. As a repressor, D53/SMXL6/7 degradation by SCFD3/AtMAX2 ubiquitination complex and 26S proteosome then release the suppressed SL signaling pathways, even downstream targets of SL signaling remain to be disclosed [6, 13, 53]. Since SLs are found synthesized in roots and stems, then transported upwards to shoot through hypodermal passage cells instead of xylem in higher plant parts [32, 70, 73], an ATP-binding cassette (ABC) transporter PLEIOTROPIC DRUG RESISTANCE1 (PDR1) was identified as a SL exporter [33, 46]. Although great progress has been made in understanding SL biosynthesis and signaling, more essential details and underlying mechanisms underlying of many SLs-related phenomena, e.g. complex cross-talks or interactions between SLs and other hormones, remain to be determined.
Functions of SLs in plants are mostly through complex interactions with other hormones such as auxins, cytokinins, abscisic acid (ABA), jasmonate acid (JA) and oxylipins, and gibberellic acids (GAs). SLs and auxins together control shoot budding and branching [17, 50]. SLs and JA interaction in Arbuscular mycorrhizal colonization [40], SLs influencing root development through the cytokinin signaling network or interaction with ethylene and auxin [29, 31], gibberellin signaling regulating SL biosynthesis [27]. Among them, the interactions between SL and auxin biosynthesis, transport, and signaling are extensively studied [17, 19]. However, due to the complex and wide-effects of these interactions, mechanisms underlying the cross-talking hormones and relevant developmental or stress responsive phenotypes yet to be understood.
Despite of the important roles of SLs in controlling shoot and root architectures demonstrated in different model plants species such as Arabidopsis, rice, pea and petunia, and SLs are directly related to agronomic traits for many crops, the relevant parts in several important leguminous crops such as soybean and alfalfa remain to be explored. Current studies have revealed that diverse and parallel strigolactone biosynthesis pathways and signal transduction mechanisms could exist in different plants species [6]. It is of great interest and importance to investigate how strigolactones are synthesized, how their signals are transduced and function in soybean. Here we identified the putative orthologs of Arabidopsis MAX1, 2, 3, and 4 from soybean genome, based on homology to their counterparts in these model plants and the expression patterns. We analyzed their functions by genetic complementation of Arabidopsis corresponding mutants, with regarding to several developmental phenotypes. We further characterized GmMAX3b for its function in nodulation process, using transgenic hairy roots in combination of transcriptomic analysis. The study provided insights into our understanding of SL function in soybean-rhizobia interaction. We investigated the physiological functions of GmMAX3b by overexpression and knockdown in soybean transgenic hairy roots for their nodulation phenotype, hormone content, nodulation gene expression changes. GmMAX3b-OE chimerical transgenic plant hairy roots displayed more nodules while GmMAX3b-KD plants gave less nodules than the control hairy roots did. Expression of several key nodulation genes were changed correspondingly, and contents of hormones like IAA, ABA, and JA also altered in transgenic hairy roots. All these data suggest that GmMAX3b not only plays a conserved role in regulating shoot branching, and root developments, like its ortholog, but also functions in root hair formation and nodulation in soybean.
Methods
Plant materials and growth conditions
Arabidopsis thaliana wild-type (ecotype Columbia-0, Col-0) and atmax mutants, atmax1–1 (SAIL_25_A05, ABRC stock #: CS9564), atmax2–1 (SALK_028336, ABRC stock #: CS9566, #: CS9565), atmax3–9 (ABRC stock #: CS9567), atmax4–1(ABRC stock #: CS9568) were obtained from the Arabidopsis Biological Resources Center (ABRC, Columbus, Ohio, USA). Arabidopsis atmax2–1, atmax3 mutant has been described previously [35, 54]. All seeds were surface-sterilized and subsequently germinated on one-half strength Murashige and Skoog (MS) agar plates supplemented with 1% (w/v) sucrose in 12 h/12 h light/dark photoperiod at day/night temperatures of 23 °C/20 °C and 400 μmol m−2 s−1 with a 16-h photoperiod. 10 day-old Arabidopsis seedlings were transferred from MS medium to soil pots grown in growth chambers at 23 °C, approximately 125 μmol photons m−2 s−1 with 14 h/10 h (long-day conditions) or 10 h/14 h (short-day conditions) photoperiods, according to experiment requirements.
Gene cloning and vector construction
Homologues of Arabidopsis MAX genes and rice DWARF genes were identified through homology search against soybean genome on public database (Phytozome.org). For gene cloning, total RNA was extracted from 14-days old Glycine max leaves, stems, or roots of soybean (Glycine max) variety “Tianlong #1”. About 10 μg of total RNA was used to synthesize first-strand cDNA using the first-strand synthesis system (Invitrogen). The cDNA were used as a template for amplification of the open reading frames (ORFs) of GmMAX genes with pairs of gene-specific primers (Additional file 1: Table S1). After all GmMAX ORFs were cloned into T-easy vector and sequenced for verification, the ORFs for GmMAX1a (Glyma04g05510.3) GmMAX1b (Glyma06g05520.2), GmMAX2a (Glyma.12 g15360.1), GmMAX3b (Glyma11g16370.1), GmMAX4a (Glyma04g08910.1), GmMAX4b (Glyma06g09000.2), were amplified by using long primers for being subcloned into pDONR221 by using recombination enzyme BP clonase (Life Technologies). The resulted pDONR221 clones harboring various GmMAX ORFs were verified by sequencing and then recombined into different Gateway destination vectors, including pB2GW7 for overexpression and pB7GWIWGII for knockdown using LR clonase (Life technologies, CA, USA).
Arabidopsis transformation and mutant complementation
pB2GW7 binary vectors harboring 35S::GmMAX1a, 35S::GmMAX1b, 35S::GmMAX2a, 35S::GmMAX3b, 35S::GmMAX4a, or 35S::GmMAX4b, were transformed into Agrobacterium tumefaciens GV3101 by electroporation. Selected A. tumefaciens GV3101 clones were grown overnight for Arabidopsis transformation using flower dipping method. For overexpression of GmMAX genes in Arabidopsis thaliana, Col-0 plants were used for transformation. For max mutant complementation, at max1, atmax2, atmax3, and atmax4 mutant plants were transformed with A. tumefaciens GV3101 harboring each corresponding GmMAX homologue gene. All transformants were screened and selected by using BASTA spraying, and at least 10 independent homozygous T3 transgenic lines were selected for our phenotype observation. Three independent transgenic lines were used for further analysis.
When plants reached maturity, the number of primary rosette leaf branches was counted. A minimum of 10 individual plants per genotype were examined. Expression of the transgene in transformants was confirmed by qRT-PCR. T3 Arabidopsis transgenic plants were measured for branching inhibition and analysis of plants hormones.
Evaluation of branches number, leaf size, and leaf shape
Branches number and leaf development analyses were performed on soil-grown plants, according to the method described previously [50, 54]. The seeds of various genotypes were sown on soil pots, and Arabidopsis seedlings were separated each to one pot at 7 days after sowing. Plants were grown under continuous light (4 μmol/m2/s) at 22 °C. The number of primary rosette branches was counted and shoot height was measured at 56 days after sowing. For each genotype, 21-day-old plants were photographed for determination of leaf size. The leaf area was then measured using the Image-J software package. For evaluation of leaf shape, the leaves were removed from plants after 35-day-old plants and pictured for measurement of leaf length and width ration using the same software.
Analysis of hypocotyl length, primary root and root hair length
The hypocotyl length, primary root length, and root hair density and length in Arabidopsis roots and soybean hairy roots were assessed by using methods described previously [30] with little modifications. Briefly, plates containing Arabidopsis seeds were germinated and incubated vertically under continuous light. Observation and measurements were conducted at 8–10 days after sowing, by photographing and measurement using the Image-J software NIH ImageJ (https://imagej.nih.gov/ij/)(http://www.rsbweb.nihgov/ij/).
Soybean hairy root induction and nodulation assay with chimerical plants
To generate soybean hairy roots, A. rhizogenes strains K599 harboring pB2GW7-GmMAX3b for overexpressing, pB7GWIWGII-GmMAX3b for knockdown or GUS were grown on LB-agar medium at 28 °C, with spectinomycin and streptomycin as selection for transformation of soybean cultivar “Tianlong No.1”, according to methods as described previously [34]. Briefly, the young soybean seedlings were wounded at hypocotyls and incubated with A. rhizogenes for 24 h. The infected seedlings were grown in autoclaved soils at 25 °C for 1 week till hairy roots appeared on the wounding sites. About 1 week after hairy root emergence, the chimerical soybean plants were inoculated with rhizobia strain Bradyrhizobium japonicum strain USDA110, which were grown in the YMA on 28 °C and the OD600nm of rhizobia was adjusted at 0.8–1.0. The rhizobia bacteria were applied about 25 ml to each plant. After 4 weeks of rhizobium application, the hairy roots and nodules were examined and collected for RNA and hormone analyses. For each binary vector including GUS control, at least three independent in vitro transformation experiments with the identical treatments and growth conditions were carried out.
RNA isolation, cDNA library construction for Illumina deep sequencing
Total RNA was extracted with Trizol reagent (Invitrogen, CA, USA) or RNA kit (Biotech, Beijing) following the manufacturer’s instructions. RNA integrity was confirmed by using the 2100 Bioanalyzer. A total of 0.5–2 μg RNA per sample was used for cDNA library preparation using the TruSeq RNA sample preparation kit (Illumina, CA, USA). Each library was sequenced on an Illumina HiSeq2500 instrument and data analyses were carried out by the Biotech Company Novogene Corporation, as previously described. Approximately 70 million 100 bp pair-end reads were generated for each sample. The fragments per kilobase of transcript per million mapped reads (FPKM) and transcript level per million count values were calculated using eXpress. Differential gene expression was analyzed by using the DESeq (2012) R package. Hierarchical cluster analysis based on the differentially expressed genes (DEGs) were filtered with expression levels FPKM >5, false discovery rate < 0.01, log2 fold change >1 or <−1 in each pairwise comparison.
Analysis of gene expression
Quantitative and semi-quantitative reverse transcriptase-PCR (qRT-PCR) analysis of gene expression was conducted as described previously [34]. Total RNAs from various tissues (Seed, root, nodule, stem, leaf and flower) of soybean seedlings or leaf of Arabidopsis plants were isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) or RNA isolation kit (Biotech, Beijing) according to the manufacturer’s instructions. For each sample, 10 μg of total RNA were digested with RNase-free DNaseI (Promega, Madison, WI, USA) to remove any genomic DNA contamination. After DNaseI treatment, RNA concentration was determined again using a NanoDrop ND-2000 UV spectrophotometer (Thermo Scientific, USA). First-strand cDNA was synthesized from 2 μg total mRNA using the Superscript III first strand synthesis system (Invitrogen, CA, USA). For semi-quantitative RT-PCR, the specific primers spanning the full-length ORF of GmMAX genes were used. The amplification of AtACTIN8 (AtACT8) was used as internal control. For the examination of expression of GmMAX genes in Arabidopsis transgenic lines, GmMAX gene-specific primers were used for quantitative RT-PCR are listed in Additional file 1: Table S1. qRT-PCR reactions were performed in 96-well plates (iQ5 Real Time PCR System; Bio-Rad) for all tissues tested, and data were analyzed.
Hormone quantification analysis
Extraction of hormones from roots and shoots for LC-MS analysis were done as previously described [64]. In brief, 0.5 g of root or shoot samples was ground in a mortar with liquid nitrogen. The samples were extracted with 2 ml of cold ethyl acetate in a 10-ml glass vial. The vials were vortexed and sonicated for 20 min in cold water bath. Samples were centrifuged for 10 min at 2500 g at 4 °C after which the organic phase was carefully transferred to a 4-ml glass vial. The pellets were re-extracted with another 2 ml of ethyl acetate. The combined ethyl acetate fractions were dried under a flow of nitrogen gas and the residue dissolved in 250 μl of acetonitrile: water: formic acid (25: 75: 0.1, v: v: v). Before analysis, samples were filtered through Minisart SRP4 0.45 lm filters (Sartorius, Goettingen, Germany) and LC-MS ⁄MS was performed as described.
Subcellular localization of GmMAX3b
To monitor the transient expressions of fusion proteins, the constructs in A. tumefaciens strain EHA105 were transformed into N. benthamiana leaf epidermal cells by infiltration [34]. The ORF of GmMAX3b in pDONR221 was recombined into pK7WGF2 in fusion with GFP at N-terminal by using Gateway recombination LR Clonase (INVITROGEN). The sequencing-confirmed GFP-GmMAX3b vectors were transformed into A. tumefaciens strain GV3101 through electroporation. The positive colony was grown at 28 °C overnight and re-suspended in infiltration medium (10 mM MES, pH 5.6, 10 mM MgSO4, and 100 mM acetosyringone) for transformation of tobacco. The infiltrated tobacco leaves were checked for GFP signals of fusion proteins after 2 days of incubation. The Olympus BX53 microscope and Leica Sports cofocal microscope were used to imaging at an excitation wavelength of 488 nm and emissions collected at 500–530 nm filter to record GFP images and 650–700 nm to record chloroplast autofluorescence [34].
Bioinformatics analysis
GmMAX protein sequences obtained from sequencing of our clones were deposited onto GenBank with accession numbers: Glycine max GmD27a (KY486796), GmD14a (KY486797), GmMAX1a (KY486798), GmMAX1b (KY486799), GmMAX2a (KY486800), GmMAX3b (KY486801), GmMAX4a (KY486802), and GmMAX4b (KY486803). Amino acid multiple alignments were made with the ClustalW program (http://www.ebi.ac.uk/clustalw/) under default parameters. A phylogenetic tree was constructed using MEGA6. The significance level of the neighbor-joining analysis was examined by bootstrap testing with 1000 repeats.
Statistical analysis
Most data was recorded from at least three independent experiments and Student’s t-test was applied to analyze the difference in data comparisons. The confidence limit 95% represents the significant between two tailed data.
Results
Identification of AtMAX homologues from soybean genome
Arabidopsis AtMAX1, 2, 3, 4 or rice DWARF /HTD homologue protein sequences were used for blast against soybean genome (https://phytozome.jgi.doe.gov/pz/portal.html). This research resulted in the identification of at least two close homologues for each of AtMAX1, 2, 3, and 4. GmMAX1a and GmMAX1b were encoded by Glyma.04 g05510 and Glyma.06 g05520.2 loci, respectively (Additional file 2: Figure S1). Phytozome database show that GmMAX1a has three transcript variants and GmMAX1b has four transcript variants. Both GmMAX1a and GmMAX1b showed approximately 84–5% similarity and 69% identity with AtMAX1 at protein sequence. GmMAX1a and GmMAX1b proteins shared approximately 90% identity with each other. The two soybean homologues of AtMAX2 are encoded by loci Glyma12g15360.1 and Glyma06g43000.1, designated as GmMAX2a and GmMAX2b respectively (Additional file 3: Figure S2). GmMAX2a showed approximately 71% similarity and 59% identity with AtMAX2 at the amino acid level. GmMAX2a shows approximately 99% similarity and 59% identity with AtMAX2 protein. GmMAX2a and GmMAX2b shared 97% similarity and 92% identity with each other and GmMAX2a is expressed higher than these of GmMAX2b. Arabidopsis MAX3 (AtMAX3) has also two closest homologues in soybean genome encoded by loci Glyma01g14266.1 and Glyma11g16370.1, designated as GmMAX3a and GmMAX3b, respectively (Additional file 4: Figure S3). GmMAX3b showed approximately 77% similarity and 62% identity with AtMAX3 at the amino acid level. GmMAX3a is expressed at much lower level than GmMAX3b does; the expression patterns of GmMAX3b are also most similar to these of AtMAX3, we therefore only studied GmMAX3b. Arabidopsis MAX4 (AtMAX4) has two homologues in soybean genome encoded by loci Glyma04g08910.1 and Glyma06g09000.2, designated as GmMAX4a and GmMAX4b, respectively (Additional file 5: Figure S4). The primary variant of GmMAX4b showed approximately 78% similarities and 65% identity with AtMAX4. GmMAX4a and GmMAX4b shared approximately 97% similarity and 93% identity with each other at protein sequence level.
Tissue specific expression patterns of GmMAX genes
We examined the expression patterns of each GmMAX gene across various tissues and organs in soybean plants. GmMAX1a transcripts were at the highest level in root and nodules and the expression of GmMAX1a in the rest of other tissues was low (Fig. 1 ). GmMAX1b was also highly expressed in nodule and root, where GmMAX1a transcripts were more than 6 times greater than in stem and seeds (Fig. 1). GmMAX1a was expressed over 20-fold higher than GmMAX1b across most tissues and organs (Additional file 6: Figure S5). GmMAX2a were also expressed in most tissues, but with the highest expression levels in leaf and flower and seed. GmMAX3b was highly and more specifically expressed in soybean stem, nodule, and stem.Both GmMAX4a and GmMAX4b displayed highest expression levels in root, nodule, and stem. But GMMAX4a transcripts levels were much higher than these of GmMAX4b, suggesting that GmMAX4a should be the primary copy in the function (Fig. 1 ).
Fig. 1.
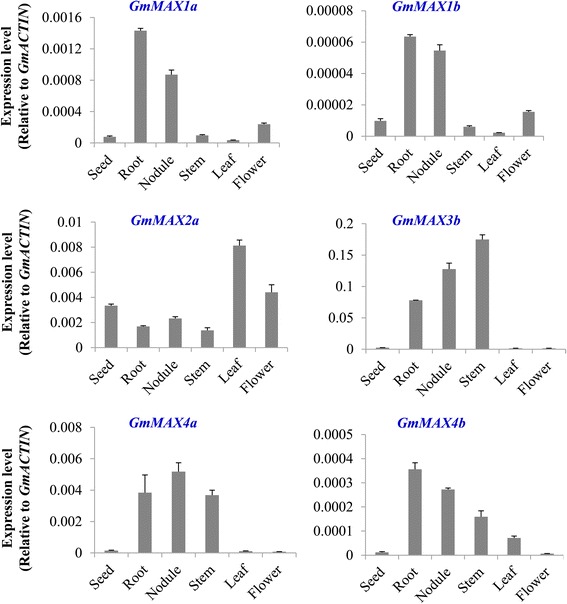
Tissue-specific expression patterns of GmMAX1a, 1b, 2a, 3b, and 4a. Relative expression of these GmMAXs to GmACTIN was measured with samples from soybean cultivar “Tianlong 1” at different development stages by using qRT-PCR. Data are expressed as means ± s.d from 3 independent experiments with biological replicates
GmMAX4a was expressed to an extremely high level in the stems, and then in root and nodules. GmMAX4b was highly expressed in nodules, then in stem and root. Both GmMAX4a and b had low expression levels in the rest of other tissues (Fig. 1). GmMAX4a was expressed over 10-fold higher than GmMAX4b across most tissues and organs, which is consistent with transcriptomic data from public database (Additional file 6: Figure S5).
Functions of GmMAX1a when expressed in Arabidopsis
In order to test whether GmMAX genes function similarly as corresponding AtMAX ortholog genes, we conducted genetic complementation by expressing GmMAX genes, driven by the constitutive 35S promoter, in corresponding Arabidopsis max mutants. We focused on a number of primary phenotypes that were observed with these mutants, to determine whether heterogonous expression of soybean GmMAX gene could rescue the corresponding Arabidopsis max mutants.
AtMAX1 is essentially involved in SL biosynthesis and the loss-of-function of AtMAX1 caused smaller leaf blade size, shorter primary root, reduced height and more branches, than wild-type Col-0 (Fig. 2a, b) [11], whereas 35S::GmMAX1a transgenic atmax1 plants restored the leaf blade size to Col-0. We verified all transgenic Arabidopsis lines; the Col-0 and atmax1 mutants showed no GmMAX1a transcripts while the complement 35S::GmMAX1a transgenic lines showed strong expression (Additional file 7: Figure S6A). GmMAX1a could rescue atmax1 defective phenotype in leaf shape development; leaves of atmax1 mutant were rounder than those of Col-0, whereas 35S::GmMAX1a plants showed very similar leaf shape with Col-0 plants (Fig. 2c). The most typical phenotype of atmax1 mutant plants is more auxiliary branches as compared with Col-0,a significant reduction in the branching number, about 3 to 7 in average, was observed when GmMAX1a was introduced into atmax1 mutant or overexpression of GmMAX1a in the Col-0 background (Fig. 2b). Under normal growth conditions, 45-day old atmax1 mutants were shorter than the wild-type Col-0, and GmMAX1a restored the mutant’s shoot height to the wild-type level (Fig. 2b). The siliques of atmax1 mutant plants were shorter with less seed than these of Col-0, whereas overexpression of GmMAX1a in atmax1 completely recovered this phenotypes (Fig. 2d). Therefore, GmMAX1a expression completely rescued the more auxiliary branches and lower shoot height phenotypes that are solely due to loss-of-function of AtMAX1. These results demonstrated that GmMAX1a and AtMAX1 possess conserved function in plant development and growth.
Fig. 2.
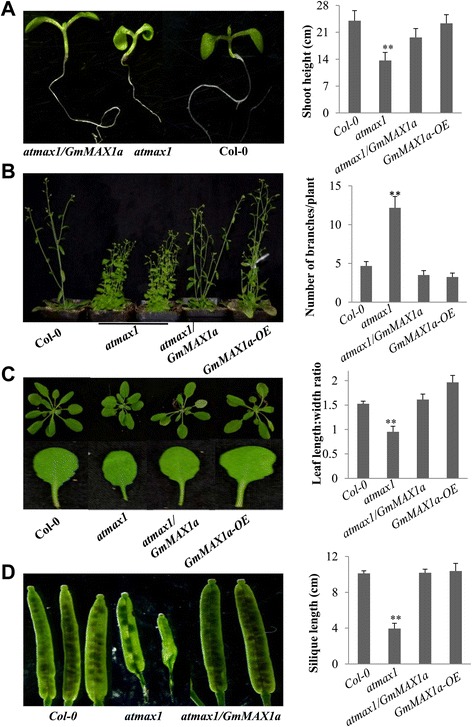
Function analysis of GmMAX1a expressed in Arabidopsis. Complementation of GmMAX1a in atmax1–1 mutant and overexpression in the wild-type Col-0 were done to compare the functions of GmMAX1a with AtMAX1. a The seedlings of atmax1–1, Col-0, and atmax1–1 complementation plant with GmMAX1a (atmax1/GmMAX1a) with different primary root lengths (left panel). Quantification of shoot height was conducted and calculated on at least three independent lines of more than 20 plants (right panel). b Mature plants of Col-0, atmax1–1 mutant, complementation (atmax1/GmMAX1a), and GmMAX1a overexpression lines (left panel). The numbers of branches were determined and calculated on at least three independent lines of more than 20 plants (right panel). c The differences between seedlings (top panel) and leaf shapes (bottom panel) of Col-0, atmax1–1, complementation of (atmax1–1/GmMAX1a) and GmMAX1a-OE plants (left panel). Ratios of leaf length to leaf width were collected on 2-week-old seedlings (right panel). Data represent means ± s.d (n = 20 plants for each genotyping). d Silique lengths of Col-0, atmax1–1, and complementation of (atmax1/GmMAX1a) plants (left panel). Quantification of silique length (cm) of Col-0, atmax1–1, complementation of (atmax1/GmMAX1a) and GmMAX1a-OE plants (right panel). Data represent means ± s.d (n = 20 plants for each genotyping). Data are expressed as means ± s.d. from 3 independent experiments with biological replicates. Asterisks indicate significant difference according to a Student’s t-test (**P < 0.001, * P < 0.05)
Functional analysis of GmMAX2a in Arabidopsis
F-box protein AtMAX2/OsD3/PtRMS4 is essentially involved in SL signal perception and transduction. Arabidopsis atmax2 mutant displays an auxiliary branch phenotype as other atmax mutants [54], reduce root hairs, delayed seed germination [43, 50], and an increased hypocotyls length in light-grown seedlings [59]. We confirmed these phenotypes of atmax2 in our growth conditions as compared with the wild-type control (Fig. 3). We generated GmMAX2a transgenic lines in Col-0 and atmax2 mutant backgrounds to texted its function. The Col-0 and atmax2 mutants showed no GmMAX2a transcript while the 35S::GmMAX2a transgenic lines showed much more GmMAX2a transcripts (Additional file 7: Figure S6B). Analysis of most independent homozygous T3 transgenic lines showed that GmMAX2a overexpression can inhibit the auxiliary branching numbers of atmax2 mutant (Fig. 3c). Under the identical conditions, wild-type Col-0 plants had an average of 5 branches, while the atmax2 mutant had ~16 braches. The atmax2 mutant expressing GmMAX2a had clearly reduced branch number, similar to that of the wild-type (Fig. 3d). While the 35S::GmMAX2a overexpression in wild-type caused no difference in branch numbers with the wild-type Col-0 (Fig. 3d). The GmMAX2a overexpression plants have shoot heights similar to these of wild-type Col-0, and were significantly higher than the atmax2 mutant plants (Fig. 3c, d). The hypocotyls of 35S::GmMAX2 transgenic atmax2 plants became shorter than those in atmax2 and approximately resembled to Col-0, suggesting that the hypocotyls of atmax2 mutants were restored by GmMAX2a (P < 0.01) (Fig. 3b). The hypocotyl lengths of GmMAX2a overexpression in wild-type were also significantly shorter (P < 0.01) than the wild-type. The leaf shape phenotypes of atmax2 mutant were also rescued by overexpression of GmMAX2a in the atmax2 mutant background (Fig. 3a).
Fig. 3.
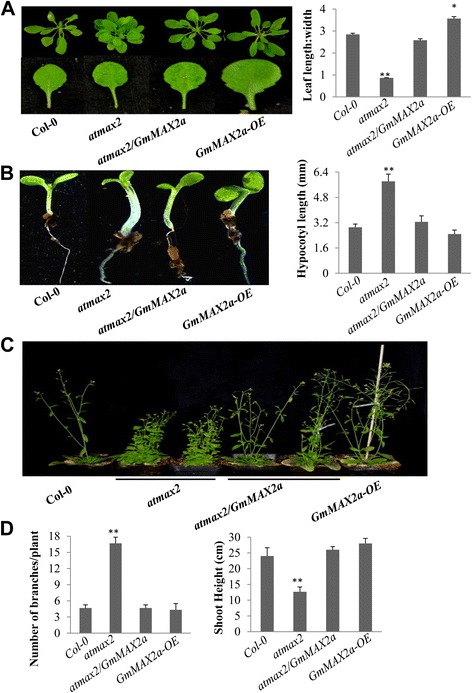
Functions of GmMAX2a expressed in Arabidopsis plants. a The differences between seedlings (top panel) and leaf shapes (bottom panel) of Col-0, atmax2, complementation of (atmax2/GmMAX2a) and GmMAX2a-OE plants (left panel). Ratios of leaf length to leaf width were collected on 2-week-old seedlings (right panel). Data represent means ± s.d (n = 20 plants for each genotyping). b Hypocotyl lengths of wild type (Col-0), atmax2, mutant complementation (atmax2/GmMAX2a) and GmMAX2a-OE plants. Data represent means ± s.d (n = 30 seedlings for each genotyping). c Mature plants of Col-0, atmax2, mutant complementation (atmax2/GmMAX2a), and GmMAX2a overexpression lines. d Quantification of the number of branches/plant (left panel) and shoot height (cm) (right panel) was conducted and calculated on at least three independent lines of more than 20 plants. Data are expressed as means ± s.d. from 3 independent experiments with biological replicates. Asterisks indicate significant difference according to a Student’s t-test (**P < 0.001, * P < 0.05)
Functional analysis of GmMAX3b in Arabidopsis
GmMAX3, a putative carotenoid cleavage dioxygenase 7, is ortholog to Arabidopsis AtMAX3, rice HTD1, petunia DAD3 or pea RMS5 involved in SL biosynthesis [10, 76]. GmMAX3b was ectopically expressed in atmax3–9 mutant and wild-type Col-0 backgrounds for examination of its function. The Col-0 and atmax3–9 mutants showed no GmMAX3b transcript while the 35S::GmMAX3b complement transgenic lines showed stronger expression (Additional file 7: Figure S6C). Compared with atmax3–9 mutant plants, the number of primary rosette-leaf branches in GmMAX3b/atmax3 transgenic lines was significantly reduced, suggesting that GmMAX3b was able to complement the shoot branching phenotype of atmax3–9 mutant plants. While the GmMAX3b overexpression lines of Arabidopsis did not show any difference in the rosette branch number from the wild-type (Fig. 4a). GmMAX3b also complemented the reduced shoot height of atmax3–9 as compared to the wild-type, as well as the short primary roots of atmax3–9 seedlings to those of the wild-type (Fig. 4c).The overexpression of GmMAX3b slightly increased the shoot height as compared to the wild-type, but restored the long primary roots of GmMAX3b/atmax3 seedlings (Fig. 4c). The leaf shape phenotypes of atmax3–9 mutant were also rescued by overexpression of GmMAX3b in the mutant background (Fig. 4b, d).
Fig. 4.
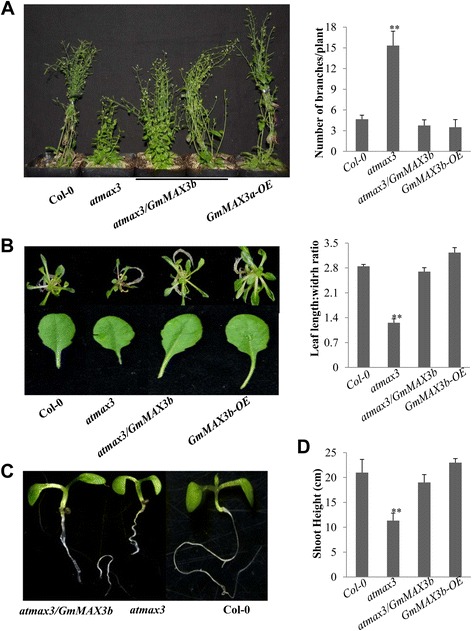
Functions of GmMAX3b when expressed in Arabidopsis plants. a Mature plants of Col-0, atmax3–9, mutant complementation (atmax3/GmMAX3b), and GmMAX3b overexpression lines. Plants were photographed at 35 days after sowing (left panel). Quantification of the number of branches of each plant (right panel) was conducted and calculated on at least three independent lines of more than 20 plants. b The differences between seedlings (top panel) and leaf shapes (bottom panel) of Col-0, atmax3, mutant complementation plant (atmax3/GmMAX3b), and GmMAX3b-OE plants (left panel). Ratios of leaf length to leaf width (right panel) were collected on 2-week-old seedlings. Data represent means ± s.d (n = 20 plants for each genotyping). c The different primary root lengths seedlings of atmax3/GmMAX3b complementation, atmax3, and Col-0 plant with GmMAX3b (atmax3/GmMAX3b). d Quantification of shoot height was conducted and calculated on at least three independent lines of more than 20 plants. Data represent means ± s.d from three independent experiments. Asterisks indicate significant difference according to a Student’s t-test (**P < 0.001, * P < 0.05)
Genetic complementation of Arabidopsis atmax4–1 by GmMAX4a
AtMAX4, ortholog to rice DWARF10, pea RMS1, or petunia DAD1, encoding a carotenoid cleavage dioxygenase 8 (CCD8) involved in SL biosynthesis [51, 52]. The atmax4–1 mutant showed more auxiliary branches and reduced shoot height, as compared to the wild-type Col-0, due to the deficiency of SL biosynthesis [51, 52]. We generated 35S:GmMAX4a transgenic lines in both atmax4 mutant and wild-type backgrounds (Fig. 5). The Col-0 and atmax4 mutants showed no GmMAX4a transcript while the complement 35S::GmMAX4a transgenic lines showed more GmMAX4a transcripts (Additional file 7: Figure S6D). Compared with atmax4 mutants, the numbers of primary rosette-leaf branches were significantly reduced in these GmMAX4a-complementation lines, and almost completely restored to the wild-type’s branch numbers (Fig. 5a). However, GmMAX4a overexpression lines had not obviously further inhibited the branch number of the wild-type. GmMAX4a-complementation plants also appeared to be taller than atmax4 mutant plants, but the overexpression lines of GmMAX4a were not significantly higher than the wild type (Fig. 5a). The primary root lengths of GmMAX4a-complementation seedlings were much longer than that of atmax4 mutant, and similar to the wild-type seedlings (Fig. 5b). In terms of leaf development and leaf shape, GmMAX4a-overexpression also restored the short petiole and round shape of atmax4 mutant to the wild-type (Fig. 5c).
Fig. 5.
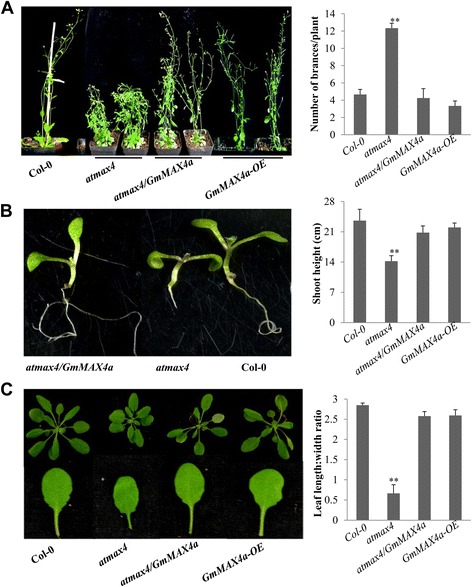
Functions of GmMAX4a when expressed in Arabidopsis. a Mature plants of Col-0, atmax4 mutant, complementation (atmax4/GmMAX4a), and GmMAX4a-OE lines. Plants were photographed at 25 days after sowing (left panel). Quantification of the number of branches of each plant (right panel) was conducted and calculated on at least three independent lines of more than 20 plants. b The different primary root lengths seedlings (left panel) of atmax4 complementation plant with GmMAX4a (atmax4/GmMAX4a), atmax4 and Col-0 plants. Quantification of the shoot height (right panel) were conducted and calculated on at least three independent lines of more than 20 plants. c Leaf shapes of Col-0, atmax4, complementation (atmax4/GmMAX4a), and GmMAX4a-OE plants (left panel). Ratios of leaf length to leaf width were collected on 2-week-old seedlings (right panel). Data represent means ± s.d (n = 20 plants for each genotyping). Data represent means ± s.d from three independent experiments. Asterisks indicate significant difference according to a Student’s t-test (**P < 0.001, * P < 0.05)
GmMAX3b complemented the atmax3 mutants’ defective root hair phenotype
As for the root growth, the knockout mutation in max3 resulted in shorter primary root and root hair lengths, but longer lateral root length [28, 30]. We also tested root phenotypes of the overexpression lines for GmMAX3b in atmax3 mutants, respectively. 35S: GmMAX3b transgenic plants showed the increased primary root and root hair length (Fig. 6a, b), suggesting that GmMAX3b can complement the primary root and root hair defects in these mutants.
Fig. 6.
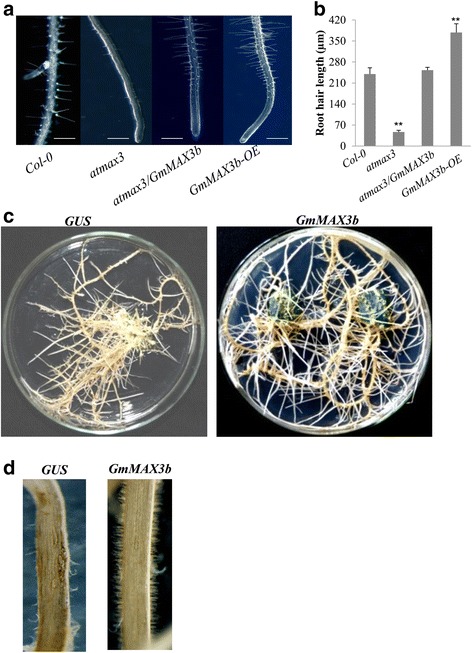
Effects of GmMAX3b-overexpression on morphology of hairy roots in mutant Arabidopsis and Soybean. a Root hairs of Col-0, atmax3–9, and complementation (atmax3/GmMAX3b) plants. Bar = 1 mm. b Quantification of root hairy length of Col-0, atmax3–9, and complementation (atmax3/GmMAX3b) plants. c Soybean hairy roots expressing GmMAX3b and GUS (control). d Root hair patterns in the soybean hairy roots overexpressing GmMAX3b or GUS. Data represent means ± s.d (n = 20 roots for each genotyping). 20 root hairs of each root were used for determination of root hair length
The GmMAX3b-OE also increases the root hairs in the soybean transgenic hairy roots in vitro (Fig. 6c). The observation of transgenic hairy roots under microscope suggested that GmMAX3b-OE had developed more and better root hairs than the GUS control, as suggested by the higher root hair density and longer root hairs than the GUS control (Fig. 6d).
GmMAX expression in Arabidopsis affected hormone levels
One important role of MAX genes as SL biosynthesis and signaling components is that they affect various other hormones, through which SLs exerted their physiological functions on different physiological processes [31, 49]. We measured hormones in the fresh leaves of Arabidopsis plant in various genetic backgrounds. It was found that the atmax1, atmax2, atmax3, and atmax4 mutant plants had significantly reduced ABA and JA contents as compared with the wild-type (Fig. 7). Moreover, except for atmax2, other mutants such as atmax1, atmax3, and atmax4 had significantly increased IAA levels as compared with the wild-type (Fig. 7). While each complementation line had significantly rescued ABA and JA contents as, compared with the atmax1, atmax2, atmax3, and atmax4 mutant plants and wild-type Col-0 plants. The IAA levels in atmax1, atmax3, and atmax4 complementation plants, atmax1/GmMAX1a, atmax3/GmMAX3b, and atmax4/GmMAX4a plants, respectively, had also reduced almost to the wild-type levels (Fig. 7). Meanwhile, the overexpression lines of GmMAX1a, GmMAX2a, GmMAX3b and GmMAX4a had increased ABA and JA contents, but reduced IAA levels, as compared to the wild type. The overexpression of GmMAX1a, GmMAX2a, GmMAX3b, and GmMAX4a in their corresponding orthologous mutants and the wild-type Col-0 had promoted the biosynthesis of ABA and JA (Fig. 7). These data demonstrated that overexpression of GmMAXs affected the hormone levels in these transgenic Arabidopsis plants, and this may be the major mechanisms, by which these GmMAXs rescued atmax mutant phenotypes. With an exception, atmax2 and its complementation line atmax2/GmMAX2a, and GmMAX2a overexpression lines did not shown significantly increased IAA contents as compared with the control wild-type (Fig. 7).
Fig. 7.
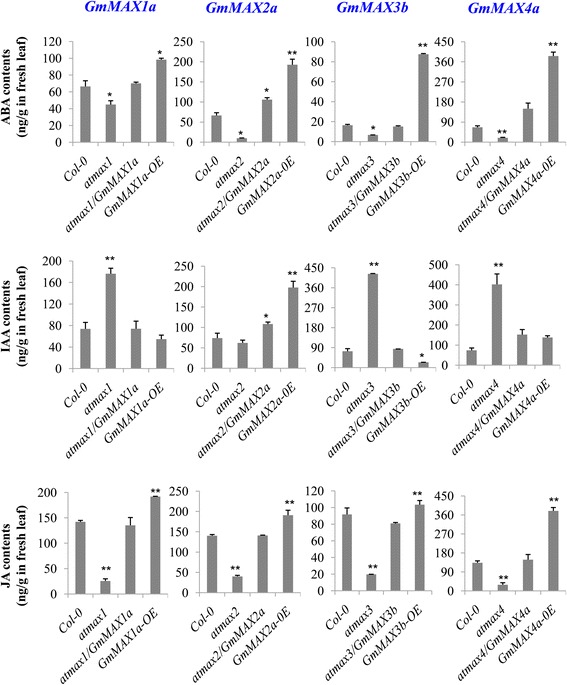
Hormone analyses on GmMAXs-transgenic Arabidopsis plants. Quantification of hormones IAA, ABA, JA (both free plus JA-Ile) in leaves of wild-type Col-0, atmax1, atmax2 atmax3, and atmax4 mutants, and their complementation (atmax1/GmMAX1a, atmax2/GmMAX2a, atmax3/GmMAX3b, and atmax4/GmMAX4a), and overexpression (GmMAX1a-OE, GmMAX2a-OE, GmMAX3b-OE, and GmMAX4a-OE) plants, respectively. Data are expressed as means ± s.d (n > 2). Asterisks indicate significant difference between wild-type control, max mutants, complementation, and overexpression lines, according to a Student’s t-test (**P < 0.001, * P < 0.05)
Overexpression and knockdown of GmMAX3b in soybean hairy roots altered nodulation
We then examined the functions of GmMAX3b in soybean nodulation. By transformation of wild-type soybean hypocotyls to generate hairy roots, we generated chimerical transgenic soybean plants with wild-type shoots and transgenic hairy roots overexpression and knockdown of GmMAX3b . In comparison with similar chimerical soybean transgenic plant with hairy roots overexpressing GUS as a control, GmMAX3b-OE plants gave more nodules (>30%) while GmMAX3b-KD plants developed less nodules than control (Fig. 8a, c).
Fig. 8.
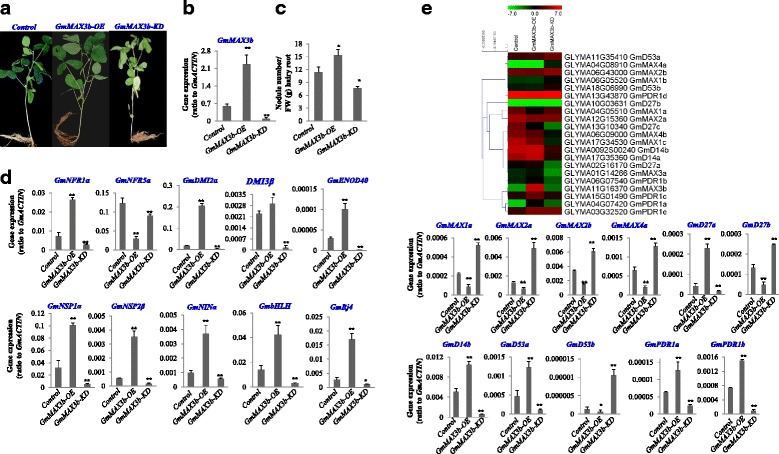
Effect of GmMAX3b overexpression and GmMAX3b knockdown in hairy roots on soybean nodulation. Chimerical soybean plants were generated by transformation with K599 harboring, GmMAX3b-overexpression (GmMAX3b-OE), GmMAX3b-knockdown (GmMAX3b-KD) or GUS vector. Plants with wild-type shoots and transgenic hairy roots were inoculated with Bradyrhizobium japonicum strain USDA110. Nodule numbers from the hairy roots were counted and the roots were sampled for gene expression analysis at 28 days post inoculation. a Chimerical soybean plants with wild-type shoots but transgenic hairy roots. GmMAX3b-OE plants developed more nodules as compared with GUS control. b qRT-PCR confirmation of GmMAX3b-OE and GmMAX3b-KD in transgenic hairy roots as compared to GUS control. c Hairy root fresh weight (g) and nodule numbers ratio in GmMAX3b-OE, GmMAX3b-KD and GUS control lines. d Expression levels of nodulation genes in GmMAX3b-OE, GmMAX3b-KD and GUS transgenic hairy roots as control. e Validation of SL biosynthesis and signaling genes in GmMAX3b-OE, GmMAX3b-KD and GUS as control hairy root lines. Gene expression was determined by qRT-PCR with GmACTIN as an internal control. Data are expressed as means ± s.d. from at least 3 independent experiments with biological replicates. Differences were analyzed, *p < 0.05; **p < 0.01 in student’s t-test
In order to address how GmMAX3b-OE and GmMAX3b-KD chimerical plants produced more nodules than the GUS control did, we analyzed nodulation genes in these transgenic hairy roots (Fig. 8d). Some of the key genes involved in legume nodulation were expressed much higher in GmMAX3b-OE while expression was lower in GmMAX3b-KD transgenic hairy roots than in GUS control (Additional file 8: Data S1, Additional file 9: Figure S7), as confirmed by qRT-PCR, which also showed the up-regulation of GmNFR1α, GmDMI2α, GmNSP2β, GmNINα, GmENOD40, GmbHLH, and GmRj4 and vice versa in GmMAX3b-KD (Fig. 8d). Meanwhile, the expression of GmNFR5α were lower in both GmMAX3b-OE and GmMAX3b-KD than in GUS hairy root control. GmDMI3β showed no substantial change in GmMAX3b-OE but significantly decreased expression in GmMAX3b-KD and GUS hairy roots (p < 0.05, Fig. 8d). The differences on expression of these key nodulation genes may explain why GmMAX3b-OE and GmMAX3b-KD transgenic hairy roots had altered the nodules than the GUS control.
Global gene expression changed in GmMAX3b-OE and GmMAX3b-KD soybean hairy roots
To learn how and what GmMAX3b overexpression and knockdown had changed the hairy roots in transgenic chimerical soybean plants, we did RNA-Seq in comparison to GUS control. The transcriptomic analysis showed that expression of more than 2000 genes was changed in GmMAX3b-OE hairy roots compared with GUS control. The expression of nodulation genes, SL biosynthesis and signaling genes was changed (Fig. 8e,Additional file 8: Data S1). Besides the significantly overexpression and knockdown of GmMAX3b, expression of SL biosynthesis and signaling genes, such as GmD27a, GmD14b, GmD53a, GmPDR1a and GmPDR1a was significantly up-regulated (P < 0.05) while GmMAX1a, 2a, 2b, and 4a significantly down-regulated (P < 0.05) and vice versa in GmMAX3b-KD hairy roots (Fig. 8e).
Some of the key genes involved in auxin biosynthesis pathway were expressed much higher in GmMAX3b-OE and down-regulated in GmMAX3b-KD than in GUS control (Additional file 10: Figure S8). The expression of auxin biosynthesis genes, such as YUC8a, YUC8b, YUC5a, and YUC9a was higher while the expression levels of YUC12a and YUC5b was lower in GmMAX3b-OE transgenic hairy roots than in the GUS control, and the opposite expression patterns for these genes were observed in GmMAX3b-KD hairy root lines (Additional file 8: Data S1).
Complex interactions between SL signaling with other hormone signaling were revealed by transcriptome of GmMAX3b overexpression and knockdown hairy roots (Additional file 8: Data S1). JA biosynthetic genes, such as several allene oxide synthases GmAOSa (GLYMA17G36530 and GmAOSb (GLYMA07G21100) and 12-oxophytodienoate reductase GmOPDR (GLYMA01G44600), jasmonate O-methyltransferase GmJAOMa (GLYMA18G47370), as well as JA-specific GH3 genes GmGH3s (GLYMA01G39780), were up-regulated in GmMAX3b-OE and down-regulated in GmMAX3b-KD hairy roots as compared with GUS control. Meanwhile, more than 10 ethylene-responsive transcription factor (ERF) genes were up-regulated in GmMAX3b-OE and down-regulated in GmMAX3b-KD hairy roots. Carotenoid pathway genes showed differential expression patterns in GmMAX3b-OE and GUS control hairy root lines. Beta-carotene 3-hydroxylase, zeta-carotene desaturase, carotene epsilon-monooxygenase; phytoene dehydrogenase, phytoene desaturase genes did not show difference. Other genes encoding carotene epsilon-monooxygene GmCEMa (GLYMA13G21110), prolycopene isomerase GmPCI (GLYMA07G40340); xanthoxin dehydrogenase GmXDH (GLYMA11G21180); lycopene epsilon cyclase; violaxanthin de-epoxidase GmVEO1 (GLYMA03G41420), GmVEO2 (GLYMA19G44010); zeaxanthin epoxidase GmZEO (GLYMA17G20020) were down-regulated in GmMAX3b-OE and up-regulated in GmMAX3b-KD hairy roots compared to the GUS control. ABA catabolic genes encoding abscisic acid 8′-hydroxylase (GLYMA09G35250) genes were up-regulated in GmMAX3b-OE hairy root. ABA synthesis genes, such as abscisic-aldehyde oxidase genes (GLYMA02G44000) were down-regulated in GmMAX3b-OE line, compared with the GUS control. ABSCISIC ACID-INSENSITIVE 5-like GmABI5 (GLYMA09G35250) was up-regulated by GmMAX3b-OE and down-regulated in GmMAX3b-KD hairy roots. For gibberellic acid (GA) biosynthesis and catabolism, gibberellin 2-beta-dioxygenase genes GLYMA15G10070, GLYMA13G33300, and GLYMA17G04150, and gibberellin 3-beta-dioxygenase genes GLYMA03G01190 and GLYMA13G0725, were up-regulated. Whereas gibberellin 20 oxidase genes GLYMA02G15390, GLYMA02G15380, and GLYMA02G15370 were down-regulated in GmMAX3b-OE hairy roots. Many genes encoding different types of transcription factors were up- or down-regulated in GmMAX3b-OE hairy roots, suggesting that they might be involved in mediation of SL-regulated downstream effects. TCP transcription factors BRCs were once been shown to act as down-stream effectors of SL signaling [2, 13]. In GmMAX3b-OE hairy roots, TCP genes such as GLYMA03G02090 and GLYMA09G42120 were markedly up-regulated as compared with that in GUS control. The C2H2 zinc-finger transcription factor STOP homolog genes, such as GLYMA18G02010 and GLYMA12G30285 were down-regulated in GmMAX3b-OE, suggesting that GmMAX3b or SL signaling also negatively regulates GmSTOPs and affects plant response to acidic soils and Al3+ stresses. In consistence, several aluminum-activated malate transporter (ALMT) family genes were also changed in GmMAX3b-OE hairy roots.
GmMAX3b-OE and GmMAX3b-KD hairy roots altered the endogenous hormone levels
Given various hormones had significant effects on legume nodulation processes, and GmMAX3b can affect the hormone levels in Arabidopsis atmax3 mutant and wild-type plants, we thus examined hormone levels in the hairy roots of GmMAX3b-OE, GmMAX3b-KD and GUS chimerical soybean plants. It was found that GmMAX3b-OE hairy roots had a significantly increased while GmMAX3b-KD hairy roots had significantly decreased IAA level compared with GUS control (Fig. 9a). TRYPTOPHAN--PYRUVATE AMINOTRANSFERASE (TAA1) and flavone containing- proteins YUCCAs (YUC) have been shown to be critical for auxin biosynthesis [39, 69]. The expression of three major TAA1s, the major YUC genes in soybean roots, GmYUC12a (Glyma.03G208900.1) and GmYUC12b (Glyma.19G206200.1), as well as the putative GmPIN genes, GmPIN1a (Glyma.07G102500.1) and GmPIN1b (Glyma.08G054700.1), were examined in GmMAX3b-OE, GmMAX3b-KD and GUS hairy roots. TAA1b (Glyma17g09401), GmYUC12a, GmPIN1a and GmPIN1b were all expressed to higher levels in GmMAX3b-OE and lower in GmMAX3b-KD than in GUS control (Fig. 9b). More interestingly, the ABA level in GmMAX3b-OE hairy roots was significantly lower and higher in GmMAX3b-KD transgenic lines compared to the GUS control (Fig. 9a). JA contents were increased in GmMAX3b-OE and decreased in GmMAX3b-KD hairy roots compared to the GUS control (Fig. 9a). Examination of JA and ABA biosynthetic genes in GmMAX3b-OE, GmMAX3b-KD and GUS hairy roots lines confirmed that the altered expression of these genes revealed by RNA-seq data analyses. JA biosynthetic genes, GmAOSa, GmOPDR, GmJAOMa, as well as JA-specific GH3 gene GmGH3a, were up-regulated in GmMAX3b-OE and down-regulated in GmMAX3b-KD compared with GUS control hairy roots. Meanwhile, carotenoid pathway genes toward ABA biosynthesis, GmCEMa, GmPCI, GmXDH, GmVEO1 GmVEO2, and GmZEO were down-regulated in GmMAX3b-OE and up-regulated in GmMAX3b-KD hairy roots compared to the GUS control (Fig. 9c), supporting that ABA levels were decreased in GmMAX3b-OE and elevated in GmMAX3b-KD hairy roots.
Fig. 9.
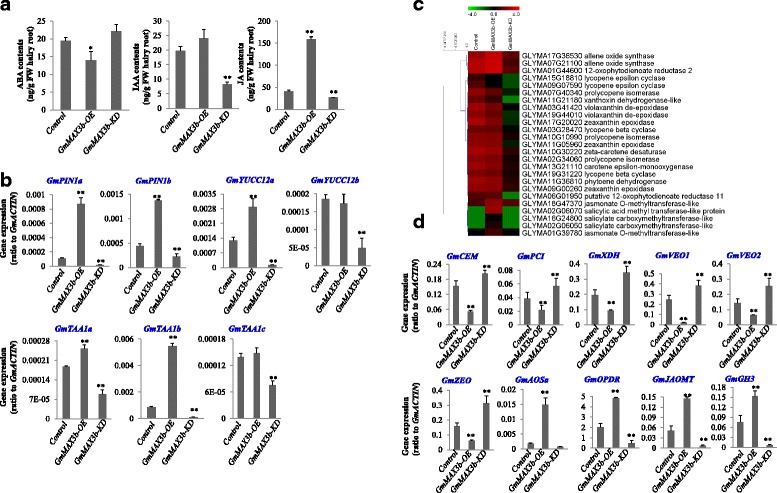
Effects of GmMAX3b-overexpression and GmMAX3b knockdown on hormone levels in hairy roots. Chimerical soybean plants with wild-type shoots and transgenic hairy roots overexpressing GmMAX3b (GmMAX3b-OE), GmMAX3b-knockdown (GmMAX3b-KD) and GUS vector control were inoculated with Bradirhizobium japonicum strain USDA110. Hairy roots were sampled for hormones and gene expression analysis at 28 days post inoculation. a Hormone contents in GmMAX3b-OE, GmMAX3b-KD and GUS hairy roots. b Validation of auxin biosynthetic genes TAAs and YUCs in GmMAX3b-OE, GmMAX3b-KD and GUS hairy root lines. c Heat map analysis of hormone biosynthesis and signaling genes in GmMAX3b-OE, GmMAX3b-KD and GUS hairy root lines. d Validation of SL biosynthesis and signaling genes in GmMAX3b-OE, GmMAX3b-KD and GUS hairy root lines. Data are expressed as means ± s.d from at least three independent experiments with duplicates. Differences are analyzed with student’s t test, *p < 0.05, **p < 0.01
Subcellular localization of GmMAX3b
When GFP-GmMAX3b fusion was transiently expressed in the leaf epidermal cells of Nicotiana benthamiana, GFP-GmMAX3b signals was largely localized to chloroplasts and remained some in the cytosol (Fig. 10). This is consistent with the prediction that GmMAX3b protein has the first 30-aa chloroplast transit peptide for targeting to the chloroplasts by iPSORT (http://ipsort.hgc.jp/index.html). The signal of GFP-GmMAX3 in the cytosol may be due to the overexpression, which caused the many proteins not processed into the chloroplast (Fig. 10). This is in agreement with AtMAX3, which was also localized to the chloroplast in Arabidopsis.
Fig. 10.
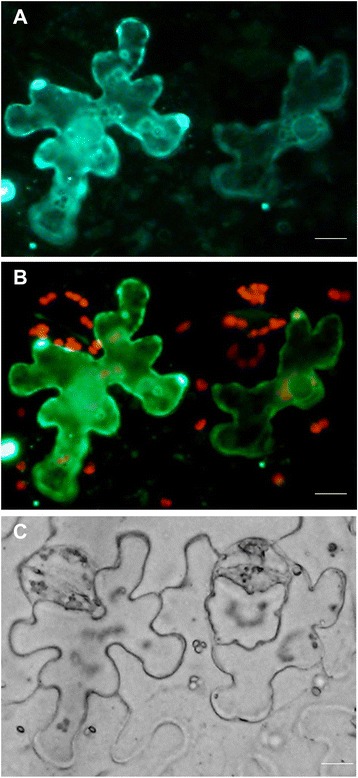
Subcellular localization of GFP-GmMAX3b. Transient expression of GFP- GmMAX3b fusion driven by a 35S promoter in tobacco leaf epidermal cells was observed under Olympus BX53 microscopy after 2 days of infiltration. a GFP-GmMAX3b image; b the merge of both GFP-GmMAX3b and chloroplast autofluorescence; c Bright field image. Bar = 30 μm
Discussion
As one of the most important economic crops, soybean becomes increasingly demanded for animal feeds, food industry, and sustainable agriculture for its nitrogen-fixation capability. As SLs play important roles in regulating plant architecture, shoot branching, root growth, plant-mycorrhizal and legume-rhizobium interaction, understanding of SLs in soybean are essential for soybean breading and agricultural practices. Unfortunately, so far SLs in soybean have not been systematically explored. The study tries to explore the unverified area to present a fresh scenario for the functions of SL biosynthesis and signaling genes in soybean in determining plant development and architecture and soybean nodulation.
GmMAXs have conserved functions similar to their Arabidopsis counterparts
Unlike diploid model legumes Medicago or Lotus that usually contains only one copy of SLs biosynthesis gene homologues, tetraploid soybean genome owns multiple copies of each MAX or D gene. Many of them share high identity or similarity with pea, Arabidopsis and rice orthologs. As revealed in other plants, SLs usually are synthesized in roots and stem and transported upward to shoots and leaves [32, 73], SL biosynthesis genes show higher expression in the roots and stems, but SL signaling genes can be expressed everywhere. Indeed, the conserved SL biosynthesis and signaling components in soybean are also confirmed by expression of each GmMAX gene. Furthermore, ectopic expression of GmMAXs, GmMAX1a, GmMAX2a, GmMAX3b, and GmMAX4a, in Arabidopsis corresponding orthologous knockout mutants for genetic complementation and in wild-type Col-0 for overexpression, we observed the rescue of several typical phenotypes in the Arabidopsis mutants, or enhanced phenotypes by overexpression of corresponding GmMAX orthologs in the wild-type. GmMAX1a, GmMAX2a, GmMAX3b, and GmMAX4a could restore phenotypes of the atmax mutants in various tested aspects, including shoot height, shoot branching, leaf morphological development, root hair, and primary or lateral roots, to the wild-type (Figs. 2, 3, 4 and 5). All atmax mutants share some common phenotypes attributable to the lack of the SL biosynthesis and signaling, such as increased shoot branching, reduced height, decreased petiole length, leaf shape, and delayed leaf senescence (Figs. 2, 3, 4 and 5). Because AtMAX1, 3, and 4 are the biosynthesis enzymes, whereas AtMAX2 is a SL signaling component, different from atmax1, atmax3, and atmax4 mutants, the atmax2 mutants do not completely phenocopy atmax1, atmax3, and atmax4 mutants [6, 16]. Although many atmax1, 3, and 4 mutant adult shoot phenotype are evident in the atmax2–1 plant, the normal leaf blade length but wider leaf blade are observed in atmax2. AtMAX2 also has an additional role in both KAI2 signaling pathway, besides SL signaling pathway, thus atmax2 has loss-of-functions in both SL- and KAI2 receptors-mediated signaling pathway [47, 67]. Additional phenotypes of atmax2 mutants include the increased sensitivity to drought tolerance because of defects in cuticle development [16].
GmMAX3b regulated hormone biosynthesis when expressed in Arabidopsis
Besides the phenotype complementation, GmMAXs also exerted effects on the biosynthesis of other hormones. GmMAXs rescued the abnormal hormone levels in these mutants, such as reduced auxin levels and increased JA and ABA contents, by overexpression of these GmMAXs. Overexpression of GmMAX2a in either atmax2 mutant or in the wild-type could increase auxin levels. Overexpression of other GmMAXs, such as GMAX1a, GmMAX3b, and GmMAX4a repressed auxin biosynthesis, and rescued the increased IAA levels their orthologous mutants of Arabidopsis to the normal low levels (Fig. 7). However, all four GmMAXs could enhance JA and ABA contents in their Arabidopsis corresponding mutants, and the wild-type backgrounds.
It has been reported in a previously study that the SL synthesis mutants atmax1–1, atmax3–9, and atmax4–1 have increased auxin transport in the primary inflorescence stem [8], and that atmax1–1 and atmax3–9 have increased levels of the PIN1 auxin efflux carrier at the basal plasma membrane of cambial and xylem parenchyma cells in the stem [6, 8]. Our results are consistent with these observations except for atmax2 mutant, atmax mutant such as atmax1, atmax3, and atmax4 plants all had significantly increased IAA levels. These increased levels of IAA, and enhanced transport of IAA caused more branches in these mutants. Expression of these GmMAXs in their corresponding mutants clearly restored the IAA levels to the normal (Fig. 7). All tested Arabidopsis atmax mutants had reduced levels of ABA and JA, which may be responsible for the delayed leaf senescence. Expression of these GmMAXs in their corresponding mutant backgrounds also enhanced the ABA and JA contents in these mutant backgrounds to the normal levels.
GmMAX3b regulated soybean hairy root nodulation
Besides multiple physiological effects of SLs in root growth, shoot branching, and mycorrhizal branching, SLs have been implicated in legume nodulation [22–24]. The pea SLs-deficient mutant rms1/CCD8 produces fewer nodules ~ 40% of the wild type, but application of synthetic SLs analog GR24 partially rescued the phenotype [22]. Lotus japonicus LjCCD7-silenced plants, showing 80% reduction in SLs, also reduced nodules by 20% compared with control plants [37, 38]. We also observed that overexpression and knockdown of GmMAX3b altered soybean nodulation. More than 26% increase in nodule number was detected in GmMAX3b-OE and 33% reduction was observed in GmMAX3b-KD hairy roots compared with GUS control, suggesting that GmMAX3b affected nodulation in chimerical transgenic hairy roots compared with GUS control. Nodulation gene expression confirmed that GmMAX3b overexpression and knockdown affected many key nodulation genes, like NFR1α genes involved in Nod factor perception, genes involved in Nod factor signal transduction such as DMI2α, and DMI3β, NINα, and NSP2β, to downstream factors, such as nodulin genes, ENOD40, Rj4, and bHLH [44, 45]. Transcriptome analysis also showed that overexpression of GmMAX3b down-regulated and GmMAX3b knockdown up-regulated several SL biosynthetic genes, such as GmD27b, GmMAX1a, GmMAX4s, and GmMAX2s, but slightly increased or decreased expression levels of GmD14s and GmD53. Thus overexpression and knockdown of GmMAX3b seems slightly down- or up-regulated SL biosynthesis and signaling. These data suggest that GmMAX3b affected SL biosynthesis and signaling, through which it may affect nodulation.
GmMAX3b overexpression and knockdown changed expression of subsets of gene in soybean hairy roots
Our RNA-Seq data showed that more than 1077 genes were up-regulated and 1521 genes were down-regulated in GmMAX3b-OE hairy roots as compared with GUS control (Additional file 8: Data S1). Among these genes, some nodulation genes were up-regulated in GmMAX3b-OE and down-regulated in GmMAX3b-KD hairy roots, including several key nodulation genes. These regulation of genes were also confirmed by qRT-PCR. Therefore, the data supported that GmMAX3b-OE did promote soybean nodulation, by up-regulation of nodulation genes and GmMAX3b-KD decrease the soybean nodulation, by down-regulation of nodulation genes. In the further analysis of transcriptomic data, we did observe significantly increased expression levels of major auxin biosynthesis genes such as TAA1s, YUCCA1, 2, 4 in GmMAX3b-OE and vice versa in GmMAX3b-KD hairy roots. In Arabidopsis, PIN genes were regarded as one of major targets of SL downstream genes [6, 8]. Although it was shown that SLs regulate PIN1 through post-translational mechanism [6, 8], we had observed that several PIN genes were up-regulated in GmMAX3b-OE and down-regulated in GmMAX3b-KD hairy roots as compared with GUS lines. The up- or down-regulation of auxin signaling genes was consistent with slightly enhanced auxin levels in GmMAX3b-OE or decreased level in GmMAX3b-KD hairy roots than in GUS control lines. Obviously increased or decreased expression levels of jasmonate biosynthesis and metabolism genes, as well as JA downstream signaling genes in GmMAX3b-OE and GmMAX3b-KD, as supported by qRT-PCR analysis (Fig. 9), indicated that GmMAX3b-OE hairy roots had enhanced JA biosynthesis and signaling genes. This is exactly in line with our hormone analysis results, which showing that JA levels in GmMAX3b-OE are higher and lower in GmMAX3b-KD lines than these in GUS control. On the contrary, we see significantly decreased expression levels of many carotenoid genes, as well as ABA biosynthesis and signaling genes. These were consistent with qRT-PCR data, as well as the hormone analysis results, showing ABA contents were decreased in GmMAX3b-OE, but increased in GmMAX3b-KD hairy roots compared with GUS lines.
GmMAX3b may modulate SL, auxin and other hormone signaling in soybean hairy roots to affect soybean nodulation
Auxins, JA, and ABA all impact mixed effects on SLs biosynthesis [15, 23]. SLs in turn affect these hormone biosynthesis and transport, and thereby regulate physiological processes [6, 8, 14, 61, 66]. We showed that GmMAX3b-OE hairy roots had slightly increased IAA levels, but drastically decreased ABA level, and significantly increased JA levels while GmMAX3b-KD hairy roots had significantly decreased IAA and JA levels, but slightly increased ABA level (Fig. 9a). MtD27 expression in nodulation not only depends on NSP1 and NSP2, but also is dependent of other symbiosis signaling pathway, including MtDMI1, MtDMI2, MtDMI3/MtCCaMK [62]. Pea SLs-deficient mutant rms1/CCD8 showed defects in nodulation is dependent of SL signaling [22]. Lotus japonicus LjCCD7-silenced plants also showed defects on nodulation compared with control plants [37, 38]. Here we showed that GmMAX3b-OE caused alteration in SL biosynthesis and signaling genes, and most likely the SL effects. GmMAX3b-OE also caused slightly increased IAA levels and significantly increased JA levels, but decreased ABA levels, but GmMAX3b-KD had contrast hormone levels.
SL can either act as a suppressor or activator of JA and ABA biosynthesis, as complex hormone cross-talking networks. Jasmonic acid (JA) and ethylene could either negatively and positively regulate Nod factor signaling and nodulation process, depending on the different circumstances [41, 45, 56, 57]. ABA also can either suppress or support Nod factor signal transduction and nodule formation, depending on the ABA concentrations [21, 42, 58]. GmMAX3b-OE may promote the nodulation by decreasing ABA accumulation, increasing JA and IAA contents, both of them positively affect nodulation in soybean, whereas GmMAX3b-KD displayed opposite changes in these hormones (Fig. 8). Further investigations are needed to reveal how these hormones affect nodulation in soybean.
Conclusion
In this study, an attempt was made to understand whether and how soybean SL biosynthesis and signaling are also suited for regulation of the relevant aspects of soybean plants. We identified the most closed homologues of AtMAX1/, AtMAX2/, AtMAX3/ and AtMAX4/ from soybean genome and further verified their capabilities on genetic complementation of Arabidopsis corresponding orthologs’ mutants. Not only morphological and developmental phenotypes were complemented by GmMAXs, the altered endogenous hormone levels were also complemented by overexpression of these GmMAX orhologs in these mutants. Further studies using GmMAX3b as an example, showed that GmMAX3b is involved in soybean root hair formation and nodulation, most likely through regulating various hormone levels in soybean hairy roots, as indicated by both transcriptomic profiling and hormone analyses. This study showed that SL modulates the level of other hormones and induces changes in plant development, including in soybean root hair developemnt and nodulation. GmMAX3b-medicated SL biosynthesis and signaling may affect soybean-rhizobia interaction, as indicated by altered early nodulation gene expression in GmMAX3b overexpression and knockdown mutant hairy roots.
Additional files
List of primers used in this study. (DOC 84 kb)
Amino acid sequence alignment and phylogenetic analyses of GmMAX1a. (PDF 778 kb)
Amino acid sequence alignment and phylogenetic analyses of GmMAX2a. (PDF 772 kb)
Amino acid sequence alignment and phylogenetic analyses of GmMAX3b. (PDF 981 kb)
Amino acid sequence alignment and phylogenetic analyses of GmMAX4a. (PDF 719 kb)
Expression patterns of SL biosynthesis and signaling genes in soybean. (PDF 809 kb)
Semi qRT-PCR of GmMAX1a, 2a, 3b and 4a in Col-0, max mutants, complementation and overexpression Arabidopsis lines. (PDF 320 kb)
Transcriptomic expression of different genes in GmMAX3b-OE and GmMAX3b-KD hairy roots. (XLS 89 kb)
Heat map analysis for the effects of GmMAX3b overexpression and knockdown on nodulation genes. (PDF 315 kb)
Heat map analysis for the effects of GmMAX3b overexpression and knockdown on auxin biosynthesis and transport genes. (PDF 497 kb)
Acknowledgements
The authors thank lab members in Prof. ZHao’s lab for all assistances in experiments and data analyses. Dr. Xinan Zhou for providing the soybean seeds of “Tianlong #1” for experiments. The first authors would like to thank the China Scholarship Council (CSC) for the scholarship.
Funding
This work was supported by the National Natural Science Foundation of China (31670294).
Availability of data and materials
All data supporting my findings can be available and found in the supplementary data, materials can be available for distribution upon request.
Abbreviations
- ABA
Abscisic acid
- ABC
ATP-binding cassette
- CCD
Carotenoid cleavage dioxygenase
- D/HTD
Dwarf or high-tillering dwarf
- D27
Dwarf27
- DAD
Decreased apical dominance
- DEGs
Differentially expressed genes
- FPKM
Fragments per kilobase of transcript per million mapped reads
- GA
Gibberellic acids
- JA
Jasmonate acid
- MAX
More axillary branching
- MS
Murashige and Skoog
- PDR1
Pleiotropic drug resistance1
- qRT-PCR
Quantitative reverse transcriptase-PCR
- RMS
Ramosus
- SCF
SKP1–Cullin–F-Box
- SLs
Strigolactones
- SMXL6
SMAX1-Like6
Authors’ contributions
JZ planned and designed the research. BU, MZ, NR, JW, PL and DL performed experiments and analyzed data. BU, MZ. and JW conducted bioinformatics analyses. JZ, BU, and MZ wrote the manuscript. All authors have read and approved the manuscript.
Ethics approval and consent to participate
No investigations were undertaken using humans/human samples in this study. No experimental animals were used to conduct any of the experiments reported in this manuscript. Our study did not involve endangered or protected species. No specific permits were required from the studies. and Professor Jian Zhao should be contacted for future permissions.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12870-017-1182-4) contains supplementary material, which is available to authorized users.
Contributor Information
Basir UI Haq, Email: basirulhaq3star@yahoo.com.
Muhammad Zulfiqar Ahmad, Email: zulfiqar_nuas@yahoo.com.
Naveed ur Rehman, Email: naveed.urrehman@yahoo.com.
Junjie Wang, Email: junjiewang88@sina.com.
Penghui Li, Email: lphui2012@126.com.
Dongqin Li, Email: ldq@mail.hzau.edu.cn.
Jian Zhao, Phone: +86 27 87385199, Email: jzhao2@qq.com.
References
- 1.Abe S, Sado A, Tanaka K, et al. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Natl Acad Sci USA. 2014;111(50):18084–18089. doi: 10.1073/pnas.1410801111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar-Martinez JA, Poza-Carrion C, Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007;19(2):458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama K, Matsuzaki K, Hayashi H, et al. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Natl. 2005;435(7043):824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 4.Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S. The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335(6074):1348–1351. doi: 10.1126/science.1218094. [DOI] [PubMed] [Google Scholar]
- 5.Arite T, Iwata H, Ohshima K, et al. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007;51:1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x. [DOI] [PubMed] [Google Scholar]
- 6.Bennett T, Liang Y, Seale M, et al. Strigolactone regulates shoot development through a core signalling pathway. Biol Open. 2016;5:1806–1820. doi: 10.1242/bio.021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett T, Leyser O. Strigolactone signaling standing on the shoulders of DWARFs. Curr Opin Plant Biol. 2014;22:7–13. doi: 10.1016/j.pbi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol. 2006;16(6):553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Beverage CA, Symons GM, Turnbull CG, et al. Auxin inhibition of decapitation-induced branching is dependent on graft transmissible signals regulated by genes RMS1 and RMS2. Plant Physiol. 2000;123:689–698. doi: 10.1104/pp.123.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol. 2004;14(14):1232–1238. doi: 10.1016/j.cub.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 11.Booker J, Sieberer T, Wright W, et al. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell. 2005;8:443–449. doi: 10.1016/j.devcel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. Secondary metabolite signaling in host–parasitic plant interactions. Curr Opin Plant Biol. 2003;6:358–364. doi: 10.1016/S1369-5266(03)00065-7. [DOI] [PubMed] [Google Scholar]
- 13.Braun N, de Saint GA, Pillot JP, et al. The pea TCP transcription factor PsBRC1 acts downstream of strigolactone to control shoot branching. Plant Physiol. 2012;158:225–238. doi: 10.1104/pp.111.182725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breakspear A, Liu CW, Roy S, et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell. 2014;26:4680–4701. doi: 10.1105/tpc.114.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer PB, Yoneyama K, Filardo F, et al. LATERAL BRANCHING OXIDOREDUCTASE acts in the final stages of strigolactone biosynthesis in Arabidopsis. Proc Natl Acad Sci U S A. 2016;113:6301–6306. doi: 10.1073/pnas.1601729113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bu Q, Lv T, Shen H, et al. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014;164:424–439. doi: 10.1104/pp.113.226837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatfield SP, Stirnberg P, Forde BG, et al. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 2000;24:159–169. doi: 10.1046/j.1365-313x.2000.00862.x. [DOI] [PubMed] [Google Scholar]
- 18.Chevalier F, Nieminen K, Sanchez-Ferrero JC, et al. Strigolactone promotes degradation of DWARF14, an alpha/beta hydrolase essential for strigolactone signaling in Arabidopsis. Plant Cell. 2014;26:1134–1150. doi: 10.1105/tpc.114.122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford S, Shinohara N, Sieberer T, et al. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010;137:2905–2913. doi: 10.1242/dev.051987. [DOI] [PubMed] [Google Scholar]
- 20.de Saint GA, Clavé G, Badet-Denisot MA, et al. An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat Chem Biol. 2016;12:787–794. doi: 10.1038/nchembio.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, Kalo P, Yendrek C, et al. Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago Truncatula. Plant Cell. 2008;20(10):2681–2695. doi: 10.1105/tpc.108.061739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foo E. Auxin influences strigolactones in pea mycorrhizal symbiosis. Plant Physiol. 2013;170:523–528. doi: 10.1016/j.jplph.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant. 2013;6:76–87. doi: 10.1093/mp/sss115. [DOI] [PubMed] [Google Scholar]
- 24.Foo E, Davies NW. Strigolactones promote nodulation in pea. Planta. 2011;234:1073–1081. doi: 10.1007/s00425-011-1516-7. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Roldan V, Fermas S, Brewer PB, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 26.Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC. DAD2 is an alpha/beta hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol. 2012;22:2032–2036. doi: 10.1016/j.cub.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Ito S, Yamagami D, Umehara M, et al. Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol. 2017;174(2):301–2017. doi: 10.1104/pp.17.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang L, Matthys C, Marquez-Garcia B, De Cuyper C, Smet L, De Keyser A, Boyer FD, Beeckman T, Depuydt S, Goormachtig S. Strigolactones spatially influence lateral root development through the cytokinin signaling network. J Exp Bot. 2016;67:379–389. doi: 10.1093/jxb/erv478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, Liu X, Xiong G, et al. DWARF 53 acts as a repressor of strigolactone signaling in rice. Nature. 2013;504:401–405. doi: 10.1038/nature12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapulnik Y, Delaux PM, Resnick N, et al. Strigolactone affect lateral root formation and root-hair elongation in Arabidopsis. Planta. 2011;233:209–216. doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- 31.Kapulnik Y, Resnick N, Mayzlish-Gati E, Kaplan Y, Wininger S, Hershenhorn J, Koltai H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J Exp Bot. 2011;62:2915–2924. doi: 10.1093/jxb/erq464. [DOI] [PubMed] [Google Scholar]
- 32.Kohlen W, Charnikhova T, Liu Q, et al. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in non arbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011;155:974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretzschmar T, Kohlen W, Sasse J, et al. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature. 2012;483:341–344. doi: 10.1038/nature10873. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Dong Q, Ge S, He X, Verdier J, Li D, Zhao J. Metabolic engineering of proanthocyanidin production by repressing the isoflavone pathways and redirecting anthocyanidin precursor flux in legume. Plant Biotech J. 2016;14:1604–1618. doi: 10.1111/pbi.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Czarnecki O, Chourey K, et al. Strigolactone regulated proteins revealed by iTRAQ-based quantitative proteomics in Arabidopsis. J Prot Rh. 2014;13:1359–1372. doi: 10.1021/pr400925t. [DOI] [PubMed] [Google Scholar]
- 36.Liang Y, Ward S, Li P, Bennett T, Leyser O. SMAX1-LIKE7 signals from the nucleus to regulate shoot development in Arabidopsis via partially EAR motif-independent mechanisms. Plant Cell. 2016;28:1581–1601. doi: 10.1105/tpc.16.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Novero M, Charnikhova T, et al. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus Japonicus. J Exp Bot. 2013;64:967–981. doi: 10.1093/jxb/ert056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, Kohlen W, Lillo A, et al. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell. 2011;23:3853–3865. doi: 10.1105/tpc.111.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mashiguchi K, Tanaka K, Sakai T, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108(45):18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagata M, Yamamoto N, Shigeyama T, et al. Red/far red light controls Arbuscular Mycorrhizal colonization via Jasmonic acid and Strigolactone signaling. Plant Cell Physiol. 2015;56:2100–2109. doi: 10.1093/pcp/pcv135. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa T, Kawaguchi M. Shoot-applied MeJA suppresses root nodulation in Lotus Japonicus. Plant Cell Physiol. 2006;47:176–180. doi: 10.1093/pcp/pci222. [DOI] [PubMed] [Google Scholar]
- 42.Nakatsukasa-Akune M, Yamashita K, Shimoda Y, et al. Suppression of root nodule formation by artificial expression of the TrEnodDR1 (coat protein of white clover cryptic virus 1) gene in Lotus japonicus. Mol Plant-Microbe Interact. 2005;18:1069–1080. doi: 10.1094/MPMI-18-1069. [DOI] [PubMed] [Google Scholar]
- 43.Nelson DC, Scaffidi A, Dun EA, Waters MT, Flematti GR, Dixon KW, Beveridge CA, Ghisalberti EL, Smith SM. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis Thaliana. Proc Natl Acad Sci U S A. 2011;108:8897–8902. doi: 10.1073/pnas.1100987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oldroyd GE, Engstrom EM, Long SR. Ethylene inhibits the nod factor signal transduction pathway of Medicago Truncatula. Plant Cell. 2011;13:1835–1849. doi: 10.1105/tpc.13.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oldroyd GE. Speak, friend, enter signaling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol. 2013;11:252–263. doi: 10.1038/nrmicro2990. [DOI] [PubMed] [Google Scholar]
- 46.Sasse J, Simon S, Gübeli C, Liu GW, Cheng X, Friml J, Bouwmeester H, Martinoia E, Borghi L. Asymmetric localizations of the ABC transporter PaPDR1 trace paths of directional strigolactone transport. Curr Biol. 2015;25:647–655. doi: 10.1016/j.cub.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Scaffidi A, Waters MT, Sun YK, Skelton BW, Dixon KW, Ghisalberti EL, Flematti GR, Smith SM. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiol. 2014;165:1221–1232. doi: 10.1104/pp.114.240036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seto Y, Sado A, Asami K, Hanada A, Umehara M, Akiyama K, Yamaguchi S. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc Natl Acad Sci U S A. 2014;111:1640–1645. doi: 10.1073/pnas.1314805111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen H, Luong P, Huq E. The F-box protein MAX2 functions as a positive regulator of photo morphogenesis in Arabidopsis. Plant Physiol. 2007;145:1471–1483. doi: 10.1104/pp.107.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen H, Zhu L, Bu QY, Huq E. MAX2 affects multiple hormones to promote photo morphogenesis. Mol Plant. 2012;5:750–762. doi: 10.1093/mp/sss029. [DOI] [PubMed] [Google Scholar]
- 51.Snowden KC, Simkin AJ, Janssen BJ, et al. The decreased apical dominance1/Petunia Hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell. 2005;17:746–759. doi: 10.1105/tpc.104.027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorefan K, Booker J, Haurogne K, et al. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003;17(12):1469–1474. doi: 10.1101/gad.256603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soundappan I, Bennett T, Morffy N, Liang Y, Stanga JP, Abbas A, Leyser O, Nelson DC. SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell. 2015;27:3143–3159. doi: 10.1105/tpc.15.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stirnberg P, van De Sande KP, Leyser HM. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development. 2002;129:1131–1141. doi: 10.1242/dev.129.5.1131. [DOI] [PubMed] [Google Scholar]
- 55.Stirnberg P, Furner IJ, Leyser HMO. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2007;50:80–94. doi: 10.1111/j.1365-313X.2007.03032.x. [DOI] [PubMed] [Google Scholar]
- 56.Sun J, Cardoza V, Mitchell DM, Bright L, Oldroyd G, Harris JM. Crosstalk between jasmonic acid, ethylene and nod factor signaling allow integration of diverse inputs for regulation of nodulation. Plant J. 2006;46:961–970. doi: 10.1111/j.1365-313X.2006.02751.x. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki A, Akune M, Kogiso M, et al. Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol. 2004;45:914–922. doi: 10.1093/pcp/pch107. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki A, Suriyagoda L, Shigeyama T, et al. Lotus Japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc Natl Acad Sci U S A. 2011;108(40):16837–16842. doi: 10.1073/pnas.1105892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, McCourt P. A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol. 2010;6:741–749. doi: 10.1038/nchembio.435. [DOI] [PubMed] [Google Scholar]
- 60.Umehara M, Hanada A, Yoshida S, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 61.Van Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U. Defective long-distance auxin transport regulation in the Medicago Truncatula super numeric nodules mutant. Plant Physiol. 2006;140:1494–1506. doi: 10.1104/pp.105.075879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Zeijl A, Liu W, Xiao TT, Kohlen W, Yang WC, Bisseling T, Geurts R. The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biol. 2015;15:260. doi: 10.1186/s12870-015-0651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waldie T, McCulloch H, Leyser O. Strigolactones and the control of plant development.Lessons from shoot branching. Plant J. 2014;79:607–622. doi: 10.1111/tpj.12488. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Hou Q, Li P, Yang L, Chen B, Sun X, Benedito V, Wen J, Mysore K, Zhao J. Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis in Medicago truncatula. Plant J. 2017;10:1111–13471. doi: 10.1111/tpj.13471. [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Wang B, Jiang L, Liu X, Li X, Lu Z, Meng X, Wang Y, Smith SM, Li J. Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation. Plant Cell. 2015;27:3128–3142. doi: 10.1105/tpc.15.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, Kohlen W, Rossmann S, Vernoux T, Theres K. Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. Plant Cell. 2014;26:2068–2079. doi: 10.1105/tpc.114.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol. 2012;159:1073–1085. doi: 10.1104/pp.112.196253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waters MT, Nelson DC, Scaffidi A, Flematti GR, Sun YK, Dixon KW, Smith SM. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development. 2012;139:1285–1295. doi: 10.1242/dev.074567. [DOI] [PubMed] [Google Scholar]
- 69.Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y. Conversion of tryptophan to indole-3-acetic acid by tryptophan aminotransferases of arabidopsis and YUCCAs in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108(45):18518–18523. doi: 10.1073/pnas.1108436108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie X, Yoneyama K, Kisugi T, Nomura T, Akiyama K, Asami T, Yoneyama K. Strigolactones are transported from roots to shoots, although not through the xylem. J Pesticide Sci. 2015;40:214–216. doi: 10.1584/jpestics.D15-045. [DOI] [Google Scholar]
- 71.Xie X, Yoneyama K, Yoneyama K. The strigolactone story. Annu Rev Phytopathol. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- 72.Yao R, Ming Z, Yan L, et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature. 2016;536:469–473. doi: 10.1038/nature19073. [DOI] [PubMed] [Google Scholar]
- 73.Yoneyama K, Xie X, Sekimoto H, Takeuchi Y, Ogasawara S, Akiyama K, Hayashi H, Yoneyama K. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008;179:484–494. doi: 10.1111/j.1469-8137.2008.02462.x. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, van Dijk AD, Scaffidi A, et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat Chem Biol. 2014;10:1028–1033. doi: 10.1038/nchembio.1660. [DOI] [PubMed] [Google Scholar]
- 75.Zhou F, Lin Q, Zhu L, et al. D14-SCF (D3)-dependent degradation of D53 regulates strigolactone signaling. Nature. 2013;504:406–410. doi: 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006;48(5):687–698. doi: 10.1111/j.1365-313X.2006.02916.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of primers used in this study. (DOC 84 kb)
Amino acid sequence alignment and phylogenetic analyses of GmMAX1a. (PDF 778 kb)
Amino acid sequence alignment and phylogenetic analyses of GmMAX2a. (PDF 772 kb)
Amino acid sequence alignment and phylogenetic analyses of GmMAX3b. (PDF 981 kb)
Amino acid sequence alignment and phylogenetic analyses of GmMAX4a. (PDF 719 kb)
Expression patterns of SL biosynthesis and signaling genes in soybean. (PDF 809 kb)
Semi qRT-PCR of GmMAX1a, 2a, 3b and 4a in Col-0, max mutants, complementation and overexpression Arabidopsis lines. (PDF 320 kb)
Transcriptomic expression of different genes in GmMAX3b-OE and GmMAX3b-KD hairy roots. (XLS 89 kb)
Heat map analysis for the effects of GmMAX3b overexpression and knockdown on nodulation genes. (PDF 315 kb)
Heat map analysis for the effects of GmMAX3b overexpression and knockdown on auxin biosynthesis and transport genes. (PDF 497 kb)
Data Availability Statement
All data supporting my findings can be available and found in the supplementary data, materials can be available for distribution upon request.


