Abstract
G protein-coupled bile acid receptor 1 (TGR5) serves a key function in regulating glycometabolism. TGR5 is highly expressed in the mitochondria of brown adipose tissue (BAT) and downregulates adenosine triphosphate synthesis via the bile acid-TGR5-cyclic adenosine monophosphate-2-iodothyronine deiodinase (D2)-triiodothyronine-uncoupling protein pathway, thus regulating energy homeostasis and reducing body weight. Chenodeoxycholic acid (CDCA), the primary bile acid, is a natural ligand of TGR5. The present study aimed to characterize the ability of CDCA to reduce high-fat diet-induced obesity and improve glucose tolerance. A mouse model of diet-induced obesity was constructed. The results demonstrated that a high-fat diet significantly increased the weight of mice after 10 weeks (P<0.05), but following the addition of CDCA and continued feeding for another 10 weeks, a decrease in weight was detected and no significant difference in final weight was observed between the high fat diet group treated with CDCA and the group fed a normal diet. Furthermore, CDCA treatment significantly increased glucose tolerance (P<0.001, P<0.01 and P<0.01 at 15, 40 and 60 min after glucose injection, respectively) and significantly decreased serum insulin levels compared with mice fed a high-fat diet alone. Staining of the liver with hematoxylin and eosin and oil red O revealed that the CDCA-treated group exhibited significantly lower fat accumulation in BAT and WAT compared with mice fed a high-fat diet alone (P<0.001). Reverse transcription-quantitative polymerase chain reaction analysis demonstrated that the expression of D2 activation system-related factors was significantly increased in BAT from mice treated with CDCA (P<0.001), confirming the role of TGR5 in modulating high-fat diet-induced obesity. In addition, CDCA inhibited adipocyte differentiation in 3T3-L1 cells and inhibited ligand-stimulated peroxisome proliferator-activated receptor γ (PPARγ) transcriptional activity. These results suggest that CDCA may prevent high-fat diet-induced obesity and hyperglycemia, and that these beneficial effects are mediated via the activation of TGR5 and inhibition of PPARγ transcriptional activity.
Keywords: G protein-coupled bile acid receptor 1 ligands, brown adipose tissue, high-fat diet, obesity, peroxisome proliferator-activated receptor γ
Introduction
The obesity epidemic has become a major public health crisis, contributing to an upsurge in cases of hypertension, coronary heart disease and diabetes (1). Obesity, defined as the excess accumulation of white adipose tissue (WAT), develops when energy intake exceeds expenditure (2). Weight reduction and increased physical activity are recommended as the most effective treatments for obesity; however, in many cases these lifestyle changes are not sustained in the long-term (3). Additionally, many anti-obesity drugs have been linked with adverse side effects and consequently withdrawn from the market, such as 2,4-dinitrophenol (2,4-DNP) (4).
Mammalian adipose tissue is comprised predominantly of WAT, with a proportionally smaller amount of brown adipose tissue (BAT) (5). WAT is the primary site of energy storage and contains a single large lipid droplet and a small number of mitochondriathat store excess energy in the form of triglycerides (5). WAT also secretes a variety of cytokines and hormones that regulate energy metabolism and insulin resistance (6). By contrast, BAT, which was determined to serve a role in adult human metabolism in 2009 (7–11), contains many small lipid droplets and a much higher number of mitochondria (6,12). BAT expresses uncoupling protein 1 (UCP1), which localizes on the inner mitochondrial membrane and uncouples the activity of the respiratory chain from adenosine triphosphate (ATP) synthesis, thereby releasing energy as heat via the process of thermogenesis (12,13). Recent findings have indicated that UCP2 also has an important role in mediated energy metabolism as UCP1 (14). This heat production process means that BAT effectively accelerates fat metabolism when stimulated. A study in humans by Rothwell and Stock (15) estimated that as little as 50 g maximally stimulated BAT could account for up to 20% of the daily energy expenditure of an individual. The exploitation of BAT has been proposed as a potential method of combatting obesity (7) and a growing body of evidence suggests that BAT recruitment (16–20) or BAT transplantation (19,20) may be effective at decreasing body mass and improving energy metabolism.
G protein-coupled bile acid receptor 1 (TGR5), a G protein-coupled receptor highly expressed in the mitochondria of BAT, serves a key function in regulating glycometabolism (21). Bile acids, a family of steroid molecules generated in the liver by cholesterol oxidation, solubilize dietary lipids and promote their absorption in the digestive tract, as well as induce energy expenditure by promoting the activation of intracellular thyroid hormone (22). The primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA), and the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA), as well as oleanolic acid (OA), all act as signaling molecules and are natural ligands of TGR5 and farnesoid X receptor (FXR) (23–25). The bile acid-regulated receptor TGR5 downregulates ATP synthesis by activating cyclic adenosine monophosphate (cAMP). Increased levels of intracellular cAMP activate protein kinase A (PKA), which in turn phosphorylates cAMP response element binding protein (CREB), which transactivates target genes, including the Dio2 gene that encodes the enzyme 2-iodothyronine deiodinase (D2) (26). Bile acid-mediated induction of D2 has been detected in human skeletal muscle and murine BAT, the only tissues that co-express TGR5 and D2 (27). D2 promotes intracellular thyroid hormone activation by converting thyroxine to triiodothyronine (T3), which upregulates UCP expression, effectively decreasing ATP synthesis (energy expenditure) by dissipating the proton gradient generated by the electron transport chain (23). Thus, the bile acid-TGR5-cAMP-D2-T3-UCP pathway may serve a key function in regulating energy homeostasis and reducing body mass.
Another cellular receptor that serves an important role in regulating glycometabolism is the nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ). It has been demonstrated that the transcription factor PPARγ is essential for adipogenesis, coordinating the expression of hundreds of genes responsible for the development of mature adipocytes (28). Numerous growth factors that inhibit fat cell differentiation mediate the phosphorylation of PPARγ via mitogen-activated protein kinase and downregulate its transcriptional activity (29).
In the present study, a diet-induced obesity mouse model was used to assess the ability of bile acid ligands to reduce obesity induced by a high-fat diet and improve glucose tolerance. Immunohistochemical staining, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), western blotting and ELISA assays were performed to analyze the effects of these ligands on body weight, glucose tolerance, serum insulin levels, hepatic fat tissue and the expression of cAMP, UCP2 and D2 in mouse fat tissue. The results of the current study suggest that TGR5 serves a key function in modulating high-fat diet-induced obesity.
Materials and methods
Reagents
CDCA was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Hematoxylin and eosin (H&E) and the serum insulin detection ELISA kit (cat. no. CSB-E05071m) were purchased from Roche Diagnostics (Indianapolis, IN, USA). All other reagents were purchased from Sigma-Aldrich; Merck KGaA.
Alimentary obesity rodent model and drug treatment procedure
A total of 15C57BL/6 wild-type male mice (age, 6 weeks; weight, 15–20 g) were purchased from the Model Animal Research Center of Nanjing University (MARC, Nanjing, China) were maintained in the Animal Resource Facility of the Animal Experiment Center at Fujian Medical University (Fuzhou, China). The mice were housed in a temperature-, humidity and light-controlled environment (25°C; 5.6%; 12-h light/dark cycle). For alimentary-induced obesity rodent models, mice in the high-fat diet (HF) group (n=10) were gavaged with high-lipid food (carbohydrate, 40%; protein, 13%; fat, 40%; other, 7%) and mice in the normal food diet (NF) group (n=5), which acted as the control, were fed with standard rodent chow (carbohydrate, 60%; protein, 22%; fat, 10%; other, 8%). Food and water were supplied ad libitum. After 10 weeks, 5 mice from the HF group were gavaged with CDCA (5 g/kg) to form the HF+CDCA group (n=5). All mice were fed for a further 10 weeks and mice were weighed each week. All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals and all experiments were approved and performed according to the guidelines of the Ethics Committee of The Union Hospital of Fujian Medical University (Fuzhou, China).
Cell culture
3T3-L1 cells were obtained from Procell Life Science Co., Ltd. (Wuhan, China). 3T3-L1 preadipocytes were cultured in medium A (Dulbecco's modified Eagle's medium; Sigma-Aldrich; Merck KGaA; supplemented with 15% fetal bovine serum; Sigma-Aldrich; Merck KGaA) at 37°C in an atmosphere containing 5% CO2. A total of 2 days after confluence was reached, cells were differentiated into adipocytes following the addition of differentiation medium (medium A containing 0.5 mM 3-isobuthyl-1-methylxantine, 1 mM dexamethasone, 10 mg/ml insulin and 5 mM pioglitazone hydrochloride) in the presence or absence of 5 µg/ml CDCA (day 0). After 2 days, the 3T3-L1 cells were transferred to adipocyte-growing medium (medium A containing 5 mg/ml insulin and 5 mM pioglitazone hydrochloride) in the presence or absence of 5 µg/ml CDCA, which was replenished every 2 days. Dimethyl sulfoxide was used as the vehicle control for the test compounds. On day 8, the differentiated adipocytes were stained with oil red O at room temperature for 10 min and photographed at ×40 magnification using an SP350 digital camera (Olympus Corporation, Tokyo, Japan) and optical microscope (Olympus BX51; Olympus Corporation).
Glucose tolerance test and serum insulin detection assay
To test the effect of different ligands on blood glucose metabolism, mice from each group that had been gavage for 20 weeks were administered with an intraperitoneal injection of glucose (2 g/kg). A micro blood glucose instrument (Ningbo Kingkerry Medical Instrument Co., Ltd., Ningbo, China) was used to monitor the changes in blood sugar between 0 and 120 min. Serum insulin levels were measured using an ELISA kit. A total of 20 µl serum was obtained at 0 and 120 min after intraperitoneal injection of glucose and processed via centrifuging 200 µl whole mouse blood obtained from the tail vein at 2,000 × g at 4°C for 10 min as described previously (29).
Immunohistochemical staining
All mice were humanely euthanized with CO2 gas following 20 weeks treatment with the drug and gavage. Livers were harvested and a tissue sample from the right lobe of each liver was fixed in 4% PBS-buffered paraformalin at room temperature for 24 h. Livers were prepared as either paraffin sections or frozen sections and stained with H&E or oil red O, as described previously (30,31).
RT-qPCR analysis of factors involved in fatty acid synthesis and fat metabolism
Total RNA was prepared from the tissue samples using TRI reagent (Molecular Research Center, Inc., Cincinnati, OH, USA). First-strand cDNA was synthesized from the total RNA using Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Gene expression of cAMP, UCP2 and D2 was quantified by qPCR using an Applied Biosystems 7300 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The 20 ml reaction mixture contained 7.0 ml nuclease-free water, 1.0 ml cDNA (1 mg/ml), 1.0 ml (10 mM) each primer and 10.0 ml Maxima SYBR-Green/ROX qPCR Master Mix (2X) (Thermo Fisher Scientific, Inc.) and underwent the following thermocycling conditions: 95°C for 10 sec followed by 40 cycles of 94°C for 15 sec, annealing at 55°C for 30 sec, and a final extension at 70°C for 30 sec. The data was determined using default threshold settings and the mean Cq was calculated from the quintuplicate PCRs. The ratio of mRNA was calculated by using the equation 2−ΔCq, in which ΔCq=Cqtreatment-Cqcontrol (14). The sequences of specific forward and reverse primers are presented in Table I. The quantity of mRNA was normalized to an internal standard, mouse β-actin.
Table I.
Primers used in the present study.
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| cAMP | 5′-TATCACTGCTGCTGCTACTG-3′ | 5′-GCGGAGAAGTCCAGCCAGCC-3′ |
| Ucp2 | 5′-GTGGTGGTCGGAGATACCAGA-3′ | 5′-GGGCAACATTGGGAGAAGTCC-3′ |
| D2 | 5′-AGGACTGGAAGGGGTGATCC-3′ | 5′-CCGACCTGGACCTCAAAGC-3′ |
| β-actin | 5′-GGCTGTATTCCCCTCCATCG-3′ | 5′-CCAGTTGGTAACAATGCCATGT-3′ |
Western blot analysis
Samples from 3T3-L1 cells lysates were isolated using radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Protein concentration was determined using the bicinchoninic acid protein assay. Subsequently, 60 µg/ml protein were subjected to 10% SDS-PAGE and transferred to a polyvinylidenedifluoride membrane. Membranes were blocked with TBST containing 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20, and incubated at 4°C overnight. Subsequently membranes were incubated with goat monoclonal anti-mouse PPARγ (1:1,000; sc-22020 P; Santa Cruz Biotechnology, Inc.), β-actin (1:5,000; sc-58673; Santa Cruz Biotechnology, Inc.). β-actin was used as an internal control for protein loading. The membrane was further incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000; sc-34665; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Membranes visualized using the enhanced chemiluminescence system. Densitometric analysis was performed using Scion Image 3.0 software (Scion Corporation, Frederick, MD, USA).
Statistical analysis
All data are presented as the mean ± standard error of the mean. The two-tailed Student's t-test was used to evaluate differences between data groups using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of CDCA on mouse body weight
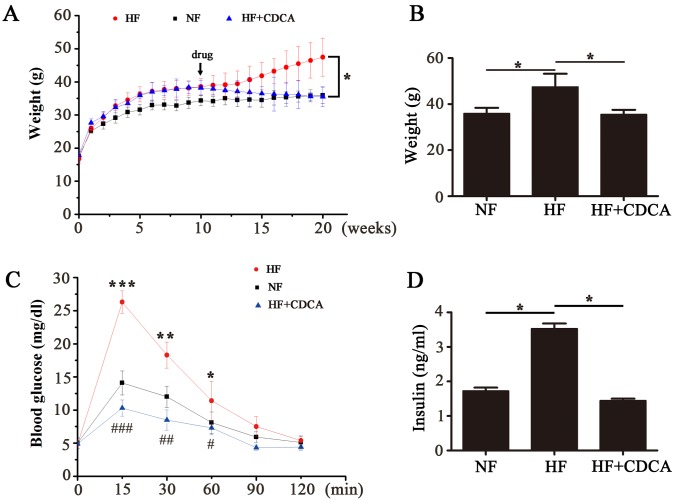
To analyze the effect of different ligands on fat accumulation in mice fed a high-fat diet, a 20-week feeding program was followed. Mice were fed an NF or HF diet for 10 weeks, then some of the HF mice were treated with CDCA (5 g/kg) and feeding was continued for all mice for another 10 weeks. The body weights of the mice were measured each week for 20 weeks (Fig. 1A). After the 20-week feeding period the body weights of the mice in each group were compared (Fig. 1B). The results indicated that there was a significant decrease in the body weight of mice in the HF+CDCA group compared with those fed an HF diet alone (P<0.05). These results suggest that treatment with CDCA, a natural TGR5 ligand, decreases fat accumulation.
Figure 1.
Effect of CDCA on mouse body weight, glucose tolerance and serum insulin levels in the drug-treated mice. Mice were fed with an NF or HF diet for 10 weeks before the addition of CDCA (5 g/kg) to the HF diet food. All mice were then fed for a further 10 weeks (n=5). (A) Body weight was measured each week from 0 to 20 weeks. (B) Body weights of each group at the end of the 20-week feeding period. *P<0.05. (C) After 20 weeks feeding, the mice were fasted for 16 h prior to intraperitoneal injection with glucose (2 g/kg), then blood glucose levels were measured over a 120-min time course by ELISA (n=5). *P<0.05, **P<0.01 and ***P<0.001 vs. NF. #P<0.05, ##P<0.01 and ###P<0.001 vs. HF. (D) Following the measurement of glucose tolerance, endpoint serum insulin levels were detected by ELISA. Data are presented as the mean ± standard error of the mean (n=5). *P<0.05. NF, normal food diet; HF, high-fat diet; CDCA, chenodeoxyclic acid.
Effect of TGR5 ligands on glucose tolerance and serum insulin levels
After 20 weeks feeding, the mice received an intraperitoneal injection with glucose (2 g/kg). Subsequently, blood glucose levels were monitored over a 120-min period to determine glucose tolerance (Fig. 1C). The results indicated that there was a significant decrease in blood glucose levels in mice fed an HF+CDCA diet compared with those fed an HF diet alone (P<0.001 at 15 min; P<0.01 at 30 min; P<0.05 at 60 min). This indicates that CDCA treatment improves glucose tolerance.
Serum insulin levels were determined using ELISA (Fig. 1D). Insulin levels in the serum of mice in the HF+CDCA group were significantly lower than those in the HF group (P<0.05). This indicates that CDCA protects against hyperinsulinemia induced by a high-fat diet.
Effect of TGR5 ligands on hepatic fat deposition
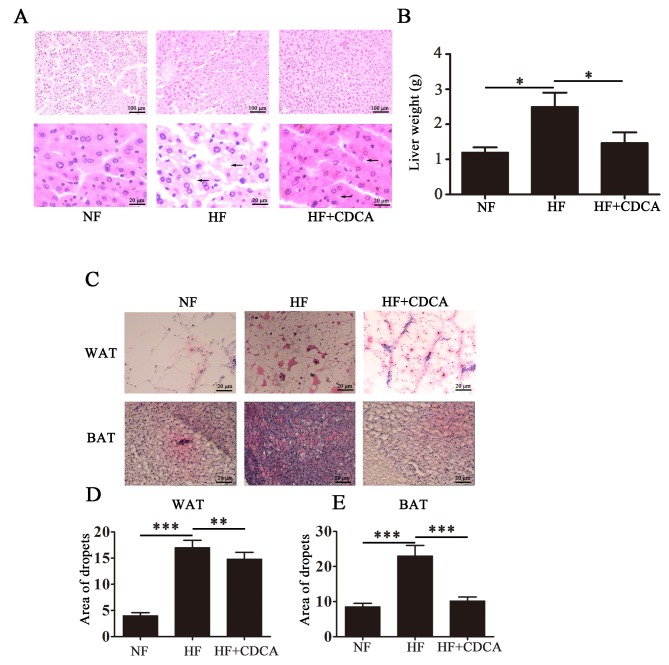
To analyze fat deposition in the livers of the mice, liver sections taken from mice euthanized after 20 weeks feeding were stained with H&E and representative images are presented in Fig. 2A. As expected, the HF diet promoted the accumulation of fat particles, as indicated by black arrows on the images. This phenomenon was reversed following treatment with CDCA. Liver weight analysis indicated that treatment with CDCA significantly reversed the HF diet-induced hepatic fat deposition observed in mice fed a HF diet alone (P<0.05; Fig. 2B). Treatment with CDCA resulted in liver weight similar to that of mice fed a NF diet. These findings indicate that CDCA is able to downregulate the hepatic fat deposition that occurs in response to a HF diet.
Figure 2.
Lipid deposition in the liver and adipose tissue. (A) Representative hematoxylin and eosin staining of liver sections from the mice following 20 weeks of feeding and drug treatment. Magnification, ×100 (top row); ×400 (bottom row). Black arrows indicate accumulated fat particles. (B) Liver weight analysis of the mice from different treatment groups. The results are presented as the mean ± standard error of the mean (n=5). (C) WAT and BAT in the livers of mice were separated and lipid droplets were detected by oil red O staining (magnification, ×400). (D and E) Relative lipid content in the liver for each of the treatment groups. Results are presented as mean ± standard error of the mean (n=10). *P<0.05, **P<0.01, ***P<0.001. NF, normal food diet; HF, high-fat diet; CDCA, chenodeoxyclic acid; WAT, white adipose tissue; BAT, brown adipose tissue.
Effect of TGR5 ligands on fat accumulation
To determine the effect of TGR5 ligand treatmenton fat accumulation, WAT and BAT from liver sections taken from mice after 20 weeks of feeding were separated and lipid droplets were detected by oil red O staining. Representative images of liver sections from the different treatment groups (n=10) are presented in Fig. 2C. The relative lipid content was quantified (Fig. 2D and E) and the results indicated that for WAT, CDCA treatment significantly reduced lipid deposition compared with untreated mice on the HF diet (P<0.01). Furthermore, for BAT, treatment with CDCA significantly reduced lipid deposition compared with untreated mice on the HF diet (P<0.001). These findings indicate that CDCA may inhibit fat accumulation induced by a HF diet.
Effect of CDCA on fatty acid synthesis and fat metabolism
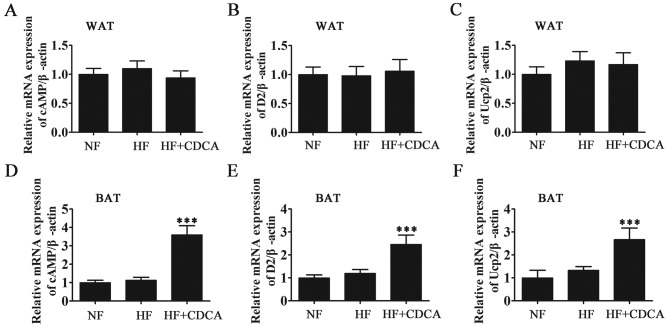
To determine the effect of the TGR5 ligand CDCA at the molecular level, the mRNA levels of the oxidation-related factors UCP2, D2 and cAMP in the WAT and BAT of mice were measured by RT-qPCR (Fig. 3). The results demonstrated that CDCA treatment significantly promoted the activation of fatty acid oxidation-related factors in BAT (P<0.001). In WAT, drug treatment did not promote the activation of fatty acid oxidation-related factors. Thus, it was demonstrated that the activity of the bile acid-TGR5-cAMP-D2-T3-UCP signal in BAT (but not in WAT) contributes to a reduction in adiposity. These findings suggest that the genes involved in the regulation of energy expenditure are upregulated following ligand binding by bile acids in BAT.
Figure 3.
RT-qPCR analysis of oxidation-related factors (D2 activation system) from WAT and BAT. The mRNA levels of fatty acid synthesis and oxidation-related factors cAMP, D2 and UCP2 from (A-C) WAT and (D-F) BAT were measured by RT-qPCR. Expression was measured relative to β-actin. Results are presented as mean ± standard error of the mean (n=5). ***P<0.001 vs. NF. NF, normal food diet; HF, high-fat diet; CDCA, chenodeoxyclic acid; WAT, white adipose tissue; BAT, brown adipose tissue; cAMP, cyclic adenosine monophosphate; D2, 2-iodothyronine deiodinase; UCP2, uncoupled protein 2; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
CDCA antagonizes PPARγ and inhibits lipid accumulation in differentiating adipocytes
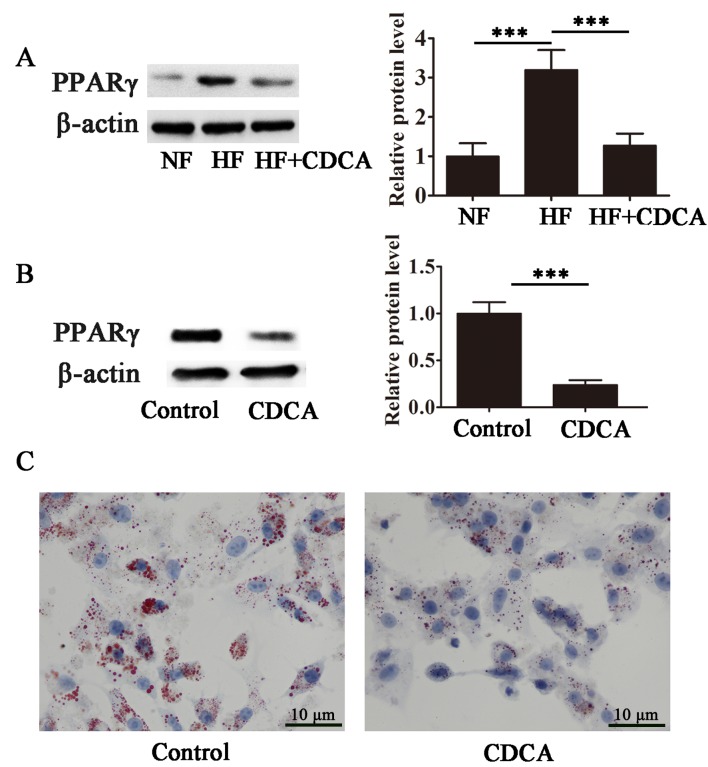
The effect of CDCA on the transcriptional activity of PPARγ, a master regulator of adipogenesis, was evaluated in differentiating 3T3-L1 cells. As presented in Fig. 4A, high-fat diet-stimulated PPARγ transcriptional activity was significantly suppressed in the presence of CDCA (P<0.001), indicating that CDCA antagonized ligand-stimulated PPARγ activity. Furthermore, CDCA significantly inhibited lipid accumulation during adipocyte differentiation in 3T3-L1 cells (P<0.001; Fig. 4B and C). These findings suggest that CDCA inhibits PPARγ activity and that the antiobesity effects of CDCA may, at least in part, be due to the antagonism of PPARγ.
Figure 4.
CDCA inhibits ligand-activated PPARγ transcription and lipid accumulation in differentiating adipocytes. (A) Results of western blotting indicating the expression of PPARγ in BAT. Results are presented as the mean ± standard error of the mean (n=5). (B) Western blot analysis indicating the effect of CDCA on PPARγ expression during adipogenesis in 3T3-L1 cells. The results are presented as the mean ± standard error of the mean (n=5). (C) Representative images of the differentiated adipocytes on day 8. Cells were stained with oil red O. Magnification, ×40. ***P<0.001. NF, normal food diet; HF, high-fat diet; CDCA, chenodeoxyclic acid; Control, without CDCA treatment; PPARγ, peroxisome proliferator-activated receptor γ.
Discussion
TGR5 is a membrane receptor that mediates bile acid signaling. In a previous study by the current authors, it was reported that TGR5 regulates glucose and energy metabolism and is therefore a candidate to combat obesity (32). Ligand binding to TGR5 induces adenylate cyclase, leading to an increase in intracellular cAMP (23). Elevated levels of cAMP activate PKA, which phosphorylates the CREB transcription factor, transactivating its target genes by binding to cAMP response elements within their promoter sequences (23). In BAT, D2 expression is promoted, boosting the local production of thyroid hormone and consequently increasing energy expenditure (23). Several other genes involved in the regulation of energy expenditure are upregulated following ligand binding by bile acids in WAT and BAT, as demonstrated in the current study and a previous report (23). In the current study, the mRNA levels of fatty acid synthesis and oxidation-related factors were increased in BAT but not in WAT. This increase in energy expenditure and acceleration of metabolism in response to TGR5 activation leads to decreased body weight via the suppression of fat deposition. This was demonstrated in the current study using a mouse model of diet-induced obesity. A significant decrease in body weight was detected in mice fed an HF diet and treated with bile acid (CDCA) compared with those fed an HF diet alone. Furthermore, H&E and oil red O staining determined that CDCA significantly inhibits the hepatic fat deposition and accumulation induced by an HF diet. The results of the current study also suggested that CDCA inhibits adipocyte differentiation in 3T3-L1 cells. This may be explained by the fact that while CDCA stimulates the transcriptional activity of TGR5, it also antagonizes ligand-dependent PPARγ transactivation. PPARγ is a master regulator of adipogenesis (28) and the antagonistic effect of CDCA on PPARγ may potentially downregulate the expression of a variety of genes essential for adipocyte differentiation.
In a previous study by the current authors, it was hypothesized that different TGR5 ligands may trigger different downstream pathways and distinct gene expression profiles, leading to specific functional outcomes (30). It was previously demonstrated that activation of TGR5 by its ligand in the absence of other pro-inflammatory stimuli, such as lipopolysaccharide, leads to the upregulation of interleukin-1β and tumor necrosis factor-α expression without PKA activation. In this case, cytokine production is mediated by the activation of c-Jun N-terminal kinase, which in turn upregulates microRNA-26a expression (30). Therefore, TGR5 mediates its biological functions via at least two distinct downstream signaling pathways.
Another effect of TGR5 activation is the stimulation of glucagon-like peptide 1 (GLP1) secretion (24,32–34). GLP1 is a member of the incretin family of insulinotropic hormones secreted from intestinal enteroendocrine cells in response to a meal and its primary role is regulating insulin secretion (35). In the present study, significantly lower insulin levels were detected in the serum of mice on a HF diet treated with CDCA compared with those on a HF diet alone, indicating that these ligands protect against hyperinsulinemia induced by a HF diet. As well as regulating insulin levels, TGR5 activation also reduces blood glucose levels in mice fed an HF diet. In the present study, a significant decrease in blood glucose levels was observed in mice fed a HF diet and subsequently with treated with CDCA, compared with those fed an HF diet alone. This indicates that CDCA decreases blood sugar levels and suggests that TGR5 enhances glucose tolerance, further confirming its potential in the treatment of metabolic disorders.
It is imperative that careful consideration is given to selecting the most suitable TGR5 ligand for drug development to ensure safety and efficacy. In the present study, the effects of CDCA was considered for weight reduction. It was determined that CDCA is effective at reducing weight, however optimizing the safety of CDCA is essential before it can be considered an anti obesity drug candidate.
A previous study has investigated the physiological role of FXR, another bile acid-activated receptor, and FXR agonists have been investigated for their potential in treating metabolic disorders (36). However, intrinsic toxicity has been detected in preclinical trials and variable gene expression profiles have been reported. This demonstrates the need for further research to fully elucidate bile acid-activated signaling networks, in order to develop safe and effective drug therapies from bile acid ligands. In conclusion, the present findings indicated that CDCA treatment may effectively suppress HG-induced obesity.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81372092), Fujian Municipal Natural Science Foundation (grant no. 2011Y0029), Fujian Health Department Foundation (grant no. 2013-ZQN-ZD-15), Fujian Finance Department Foundation (grant no. 010110002), Research Foundation of Fujian Provincial Department of Science & Technology (grant no. 2016Y4003), Research Foundation of Fujian Development and Reform Commission (grant no. 201603), Key Program of National Clinical Specialty Discipline Construction of China and Key Clinical Specialty Discipline Construction Program of Fujian, China.
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world-a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Alrushud AS, Rushton AB, Kanavaki AM, Greig CA. Effect of physical activity and dietary restriction interventions on weight loss and the musculoskeletal function of overweight and obese older adults with knee osteoarthritis: A systematic review and mixed method data synthesis. BMJ Open. 2017;7:e014537. doi: 10.1136/bmjopen-2016-014537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: A systematic review. BMC Med. 2016;14:191. doi: 10.1186/s12916-016-0553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B, Shin AC, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546:107–112. doi: 10.1038/nature22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend K, Tseng YH. Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte. 2012;1:13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:143–149. doi: 10.1097/MED.0b013e328337a81f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 11.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 12.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 13.Beranger GE, Karbiener M, Barquissau V, Pisani DF, Scheideler M, Langin D, Amri EZ. In vitro brown and ‘brite’/‘beige’ adipogenesis: Human cellular models and molecular aspects. Biochim Biophys Acta. 2013;1831:905–914. doi: 10.1016/j.bbalip.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Rubattu S, Stanzione R, Bianchi F, Cotugno M, Forte M, Della Ragione F, Fioriniello S, D'Esposito M, Marchitti S, Madonna M, et al. Reduced brain UCP2 expression mediated by microRNA-503 contributes to increased stroke susceptibility in the high-salt fed stroke-prone spontaneously hypertensive rat. Cell Death Dis. 2017;8:e2891. doi: 10.1038/cddis.2017.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: The case in favour. Clin Sci (Lond) 1983;64:19–23. doi: 10.1042/cs0640019. [DOI] [PubMed] [Google Scholar]
- 16.Arch JR. beta(3)-Adrenoceptor agonists: Potential, pitfalls and progress. Eur J Pharmacol. 2002;440:99–107. doi: 10.1016/S0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- 17.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316, 243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Pennisi PA, Gavrilova O, Pack S, Jou W, Setser-Portas J, East-Palmer J, Tang Y, Manganiello VC, Leroith D. Effect of adipocyte beta3-adrenergic receptor activation on the type 2 diabetic MKR mice. Am J Physiol Endocrinol Metab. 2006;290:E1227–E1236. doi: 10.1152/ajpendo.00344.2005. [DOI] [PubMed] [Google Scholar]
- 19.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagase I, Yoshida T, Kumamoto K, Umekawa T, Sakane N, Nikami H, Kawada T, Saito M. Expression of uncoupling protein in skeletal muscle and white fat of obese mice treated with thermogenic beta 3-adrenergic agonist. J Clin Invest. 1996;97:2898–2904. doi: 10.1172/JCI118748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen Y, Chi Q, Wang D, Zhang Z, Li C, et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013;23:851–854. doi: 10.1038/cr.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/S0006-291X(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 25.Moore DD, Kato S, Xie W, Mangelsdorf DJ, Schmidt DR, Xiao R, Kliewer SA. International union of pharmacology. LXII. The NR1H and NR1I receptors: Constitutive androstane receptor, pregnene X receptor, farnesoid X receptor alpha, farnesoid X receptor beta, liver X receptor alpha, liver X receptor beta, and vitamin D receptor. Pharmacol Rev. 2006;58:742–759. doi: 10.1124/pr.58.4.6. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen A, Bouscarel B. Bile acids and signal transduction: Role in glucose homeostasis. Cell Signal. 2008;20:2180–2197. doi: 10.1016/j.cellsig.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A, Schaart G, Kouach M, Charton J, Deprez B, et al. The Bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 2015;22:418–426. doi: 10.1016/j.cmet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/S1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 29.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Xu H, Ding L, Lou G, Liu Y, Yao Y, Chen L, Huang W, Fu X. Identification of miR-26a as a target gene of bile acid receptor GPBAR-1/TGR5. PLoS One. 2015;10:e0131294. doi: 10.1371/journal.pone.0131294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–68. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- 32.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Lou G, Meng Z, Huang W. TGR5: A novel target for weight maintenance and glucose metabolism. Exp Diabetes Res. 2011;2011:853501. doi: 10.1155/2011/853501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 35.Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun. 2007;362:793–798. doi: 10.1016/j.bbrc.2007.06.130. [DOI] [PubMed] [Google Scholar]
- 36.Ye L, Jiang Y, Zuo X. Farnesoid-X-receptor expression in monocrotaline-induced pulmonary arterial hypertension and right heart failure. Biochem Biophys Res Commun. 2015;467:164–170. doi: 10.1016/j.bbrc.2015.09.067. [DOI] [PubMed] [Google Scholar]