Abstract
The aim of this study was to determine the mechanisms driving the protective effects of squid ink polysaccharide (SIP) against cyclophosphamide (CP)-induced testicular damage, focusing on germ cells. In the testes of mice exposed to CP and/or SIP, the present study examined the levels of reactive oxygen species (ROS) and malondialdehyde, activity of superoxide dismutase levels, protein expression levels of B-cell lymphoma 2 (Bcl2), Bcl2-associated X protein (Bax), and total Caspase 3, activation of p-p38 and p-Akt proteins, and tissue morphology. The findings indicated that CP induced ROS production and oxidative stress, resulting in testicular damage. However, under administration of SIP, oxidative stress was impaired and the testicular toxicity induced by CP was weakened, which implied that SIP may have an important role in preventing chemotherapeutic damage to the male reproductive system via promoting antioxidant ability. Furthermore, the altered expression levels, including the upregulation of Bax and Caspase 3, downregulation of Bcl-2 and the increased Bax/Bcl-2 ratio, indicated that apoptosis occurred in CP exposed testes of mice; however, the alterations were reversed in mice treated with SIP. Moreover, in CP-exposed testes, p38 and Akt proteins were significantly phosphorylated (P<0.05), whereas in the testes of mice co-treated with SIP and CP, phosphorylation of the two proteins was inhibited, demonstrating that the two signalling pathways participated in the regulative processes of the deleterious effects caused by CP, and the preventive effects SIP mediated.
Keywords: squid ink polysaccharide, cyclophosphamide, testicular germ cells, apoptosis
Introduction
Cyclophosphamide (CP) exhibits positive therapeutic effects in clinical practice; however, it also causes undesirable harm to normal organs and tissues, such as the testes (1). Previous studies (2–4) have indicated that a large number of free radicals and oxidation products are produced in mice after intraperitoneal injection of CP, which may cause testicular oxidative stress resulting in DNA double-strand breaks and germ cell apoptosis. Intracellular accumulation of excess reactive oxygen species (ROS) can induce apoptosis, resulting in programmed cell death (5). Apoptosis, a mode of programmed cell death, has important roles in cell growth, differentiation and development, and acts as an effective way to induce tumor cell death. Typically, apoptosis is targeted in cancer treatment as an anti-tumor mechanism (6,7). The specific mechanisms involved in apoptosis are yet to be fully understood. The majority of studies suggest that multiple mediators of cell damage, which are triggered by adverse exogenous factors, are closely related to apoptotic cells, including oxygen free radicals, radiation and chemotherapy (8–10).
Squid ink polysaccharide (SIP), a natural marine product, is considered to be a potentially effective, non-toxic, broad-spectrumcy to protective agent due to its anti-oxidant (1), anti-tumor (11) and anti-chemotherapy (1,12–16) functions. SIP has been identified as a type of glycosaminoglycan, with a unique structure: [3GlcAβ1-4(GalNAc α1-3)-Fucα1]n (17). In previous studies, SIP alleviated toxicity of the chemotherapeutic drug, CP, on the heart, liver, spleen, lung, kidney and intestines (12–16,18). There is also evidence of the protective effect of SIP on the male reproductive system. In a previous study, SIP effectively reduced testicular pathological damage caused by CP in mice, thus increasing testicular weight and sperm quantity, reducing sperm deformity rates, improving testis iconic enzyme activity and redox homeostasis, and maintaining natural hormone levels in serum and testes (1). Although the preventative effects of SIP towards CP-induced damage of testes and apoptosis in murine germ cells have been identified (10), its regulative processes remain unclear. To elucidate the preventive mechanisms of SIP on the testes of mice exposed to CP, apoptosis of testicular germ cells in mice treated with CP and SIP was investigated in the present study.
Materials and methods
Preparation of SIP
Fresh squid (Sepia esculenta) were caught from the East coast of Beibu Gulf. Their ink sacs were removed by the fishermen and stored at −70°C in an ultra-low temperature freezer. SIP was prepared with a slightly modified method as described by Chen et al (17). Briefly, the frozen squid ink was thawed at 4°C, diluted with an equal volume of PBS (0.01 mol/l; pH 7.4) and treated by sonication in an ice bath. Following storage at 4°C for >8 h, the mixture was centrifuged (7,155 × g) at 4°C for 50 min. The supernatant was collected and hydrolyzed with papain (1.5%) at 50°C for 90 min and heated in boiling water to denature the protease. Proteins in the treated supernatant were removed by the Sevag method (19). The aqueous phase was mixed with four volumes of ethanol to precipitate the polysaccharides. Crude polysaccharides were obtained from the precipitate and subsequently separated into three fractions by DEAE-52 cellulose column chromatography (GE Healthcare Life Sciences, Chalfont, UK). The first fraction, in which the peak area was far larger than the others, was collected, dialyzed, concentrated, and further purified in a Sephacryl S-300HR column (GE Healthcare Life Sciences). One elution peak was obtained from a S-300HR column and the fraction from that peak was named SIP. The collected SIP was dialyzed, concentrated, freeze-dried and stored at −20°C.
Animals and experimental design
A total of 40 sexually mature male Kunming mice (weighing 25±2 g), aged 6 weeks, were purchased from the Experimental Animal Centre of Guangxi Medical University (Nanning, China; SCXK(Gui) 2009-002). Mice were adaptively domesticated for one week under the following constant experimental conditions: Relative humidity, 55±5%; temperature, 22±2°C; quasi-diurnal cycle of 12 h light and 12 h darkness; and ad libitum access to food and water.
A total of 40 mice were randomly divided into four equal experimental groups: Control group (CON; orally administered and abdominally injected with normal saline); CP-treated group (CP; orally administered normal saline and abdominally injected with CP in normal saline); SIP-treated group (SIP; orally administered SIP and abdominally injected with normal saline); and a co-treated group (SIP+CP; orally administered SIP and abdominally injected with CP in normal saline). SIP was administered once a day for two weeks with 80 mg/kg body weight chosen as an optimal concentration of SIP from our previous studies (1,20). CP was intraperitoneally injected once on the 7th day of SIP or saline administration; 120 mg/kg body weight was chosen as the dose to obtain optimal effects in this model, since a large dose may result in testicular damage due to increased cellular sensitivity when exposed to high doses of CP (21,22). Following treatment, all mice were sacrificed by dislocation of cervical vertebra at 24 h after the last administration. Bilateral testes were collected, rapidly cleared of surrounding fat and connective tissue and stored at −70°C.
Detection of testicular oxidative stress level
Samples were homogenised quickly in ice-cold PBS at 4°C. The homogenate was centrifuged 2,000 × g for 10 min at 4°C. The supernatant was determined by measuring the activity of superoxide dismutase (SOD), contents of malondialdehyde (MDA) and reactive oxygen species (ROS) with detection kits according to manufacturers' protocols. SOD assay kit (Hydroxylamine method, cat. no. A001-1), ROS assay kit (Dichlorofluorescein method, cat. no. E004) and MDA assay kit (TBA method, cat. no. A003-1) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Transmission electron microscopy detection
Testes were fixed with 5% glutaraldehyde in PBS followed by post-fixation with 1% osmic acid formulated with PBS. Following washing three times with PBS, the tissue was dehydrated with ethanol and acetone, embedded in Epon812 and sectioned into 60–70 nm with an ultra-microtome. Sections were stained with 2% uranyl acetate and lead citrate and the ultra-structure of testicular germ cells was observed by transmission electron microscopy.
Terminal-deoxynucleoitidyl transferase-mediated nick-end labelling (TUNEL) assay
TUNEL assay was performed according to the manufacturer's instructions, Situ TUNEL apoptosis detection kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Procedure was as follows: De-waxed and hydrated testes paraffin sections were washed three times with PBS and incubated in DNase-free Proteinase K for 30 min at 37°C. Following washing, sections were immersed in methanol solution at room temperature for 5 min, the excess liquid was removed with filter paper and the sample was incubated in TdT enzyme reaction solution for 60 min at 37°C prior to washing with PBS. Excess liquid was removed with filter paper and the sections were incubated in Streptavidin-HRP solution at 37°C for 30 min in the dark. Following washing, each section was stained with 3,3′-diaminobenzidinesolution at room temperature for 10 min and washed with PBS. The sections were observed under an optical microscope (magnification, ×200), and brown particles were deemed to be indicative of apoptosis-positive cells. The number of positive cells per 100 germ cells was calculated as the positive rate of apoptosis, which indicated the percentage of apoptosis-positive cells in the total cell count.
Western blot analysis
Western blot was used to assess the levels of B-cell lymphoma 2 (Bcl2), Bcl2-associated X (Bax), Caspase 3, LC3B, Beclin-1, phospho-p38, phospho-Akt and β-actin. Samples were homogenized in a 1 ml/0.1 g tissue RIPA lysis buffer with 1 mM phenylmethyylsulfonyl fluoride (Beyotime Institute of Biotechnology, Shanghai, China) in a homogenizer. Following lysis for 20 min on ice and centrifugation at 10,000 × g for 3 min at 4°C, the protein content was determined using the Enhanced BCA Protein Assay kit (Beyotime Institute of Biotechnology). All the resulting extracts were adjusted to the same concentration and electrophoresed on a 10% SDS-PAGE. The loading quantity was 40 µg. The separated proteins were then transferred onto polyvinylidene difluoride membranes. The membranes were blocked by incubation with a phosphate-buffered saline and Tween-20 (PBST) buffer (0.05% Tween-20, 100 mM NaCl, 10 mM Tris-HCl pH 7.4), containing 10% nonfat dry milk for 2 h, then incubated with one of the following antibodies: Caspase 3 rabbit polyclonal antibody (cat. no. 19677-1-AP, Proteintech Group, Inc., Chicago, IL, USA), Bax rabbit polyclonal antibody (cat. no. 50599-2-Ig; Proteintech Group, Inc.), Bcl-2 rabbit polyclonal antibody (cat. no. 12789-1-AP; Proteintech Group, Inc.), Phospho-p38 MAPK rabbit monoclonal antibody (cat. no. 4092S; Cell Signaling Technology, Inc., Danvers, MA, USA), Akt-phospho-S473 mouse monoclonal antibody (cat. no. 66444-1-Ig; Proteintech Group, Inc.), beta actin rabbit polyclonal antibody (cat. no. 20536-1-AP; Proteintech Group, Inc.), beta actin mouse monoclonal antibody (cat. no. 60008-1-Ig; Proteintech Group, Inc.), at concentrations of 1:2,000. They were then washed three times with 0.05% Tween-20 in PBST for 5 min by slowly shaking and finally incubated for 2 h with anti-rabbit IgG (H+L) secondary antibody (KPL, Inc., Gathersburg, MD, USA) at concentrations of 1:2,000. The membranes were washed with PBST and the proteins were detected with a substrate super ECL Plus solution (Applygen Technologies Inc., Beijing, China). All these steps were carried out at room temperature. Densitometric analysis of digitalized images was done with the Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All experimental data were presented as mean ± standard deviation. Data were analysed using JMP 7.0.2 (SAS Institute Inc., Cary, NC, USA). One-way analysis of variance and the post-hoc Tukey's honest significant difference test were used to evaluate differences between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
SIP inhibits CP-induced oxidative stress in mice testes
ROS levels, SOD activity and MDA content in testes are shown in Table I. Significant increases in ROS level and MDA content, and a reduction in SOD activity were observed in mice exposed to CP (P<0.05). After SIP treatment, SOD activity of CP-treated mice was obviously higher (P<0.05), ROS levels and MDA content were also greatly reduced (P<0.05). To sum up, its preventive effects on the testes of CP-treated mice were found by evaluating the three indicators, which implied marked inhibition of oxidative stress in CP-exposed testes following treatment with SIP.
Table I.
ROS level, MDA content and SOD activity in testes.
| Group | ROS (a.u./mg) | SOD (U/mg) | MDA (nmol/m) |
|---|---|---|---|
| CON | 32.39±5.76 | 101.17±5.34a | 11.99±3.80 |
| CP | 46.74±5.37a,b | 76.48±5.70 | 21.90±2.36a,b |
| SIP+CP | 31.42±5.35 | 99.62±4.51b | 12.10±3.89 |
| SIP | 29.87±4.84 | 96.95±6.49 | 12.21±4.41 |
Mice were orally administered 80 mg/kg SIP once a day for two weeks (SIP+CP and SIP, respectively). After 2 h on the 7th day of SIP treatment, mice were injected with 120 mg/kg cyclophosphamide (CP and SIP+CP, respectively). Control mice were orally administered and/or injected with saline.
P<0.05 vs. CON.
P<0.05 vs. CP. ROS, reactive oxygen species; SOD, superoxide dismutase; MDA, malondialdehyde; CON, control mice; CP, CP-treated mice; SIP+CP, mice treated with squid ink polysaccharide and CP; SIP, squid ink polysaccharide-treated mice.
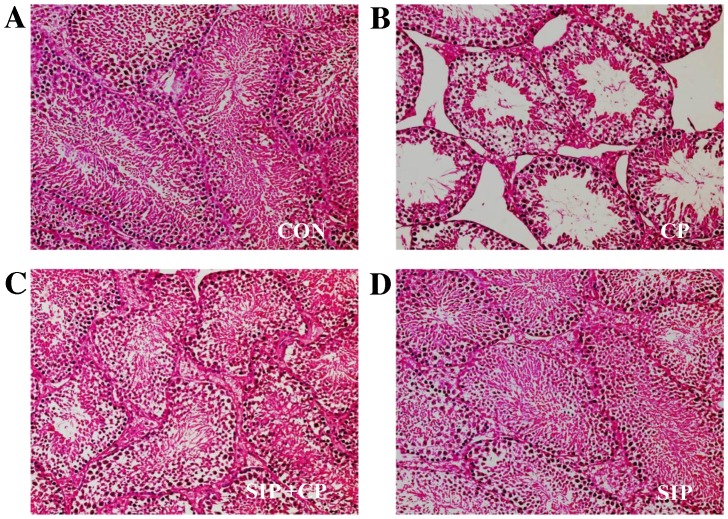
SIP prevents CP-induced damage of histological structures in mice testes
As demonstrated in Fig. 1, testicular tissues of vehicle- and SIP-treated mice exhibited normal histological structures, including the correct epithelial thickness of the seminiferous tubule, plentiful layers of cells, compactness and regularity of spermatogenic cells, as well as rich capillaries inthe periphery of the seminiferous tubule. However, the histological structure of the CP-treated group showed reduced epithelial thickness of the seminiferous tubule and fewer layers of cells. Furthermore, spermatogenic cell counts decreased and their structures were disarranged; in addition, few capillaries were observed in the enlarged space between seminiferous tubules. In the group co-treated with SIP and CP, histological structure was visibly better than that of CP-treated mice, as indicated by comprehensive assessment of the aforementioned parameters.
Figure 1.
Histopathological observation of testicular seminiferous tubules in (A) control, (B) cp-treated, (C) SIP+CP treated and (D) SIP-treated mice.. Testicular seminiferous tubules were observed by optical microscopy and hematoxylin and eosin staining techniques. Images were captured at a magnification of ×200. CON, control mice; CP, CP-treated mice; SIP+CP, mice treated with squid ink polysaccharide and CP; SIP, squid ink polysaccharide-treated mice. Scale bar 0.05 mm.
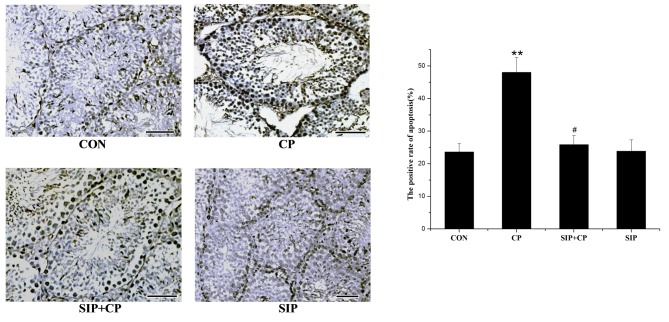
SIP prevents CP-induced apoptosis of testicular germ cells
TUNEL was used to detect DNA strand breaks generated during early-stage apoptosis and to observe the direct location of apoptosis (Fig. 2). In the control group, few apoptotic cells were present in the cell layers of spermatogonia. CP-treated mice exhibited a large number of apoptotic cells distributed predominantly in the spermatogonial layer, and the positive rate of apoptosis was significantly increased compared with the other groups (P<0.05). Similar to the control mice, minimal evidence of apoptosis was observed in the cell layers of the SIP-treated mice. Compared with the CP-treated mice, mice co-treated with SIP and CP exhibited lower levels of apoptosis in each cell layer. These results demonstrated that the positive rate of apoptosis was significantly decreased by SIP (P<0.05).
Figure 2.
SIP prevents CP-induced apoptosis of testicular germ cells. Testicular seminiferous tubules was observed by optical microscopy and TUNEL techniques, images were captured at a magnification of ×400. **P<0.01 vs. CON. #P<0.05 vs. CP. Scale bar 0.05 mm. CON, control mice CP, CP-treated mice; SIP+CP, mice treated with squid ink polysaccharide and CP; SIP, squid ink polysaccharide-treated mice.
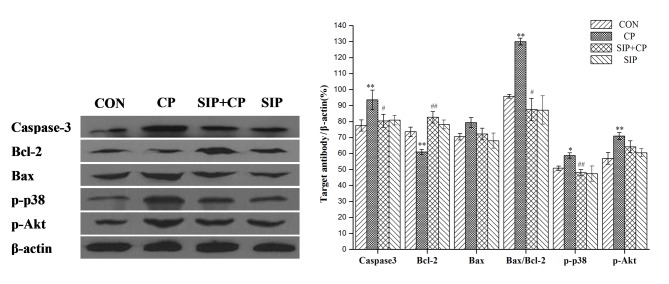
Effect of SIP on the CP-mediated expression of caspase-3, Bcl-2 and Bax genes, and phosphorylation of p38 and Akt proteins
As shown in Fig. 3, CP upregulated Caspase-3 protein expression (P<0.01) and improved photophosphorylation of p38 (P<0.05) and Akt proteins (P<0.01) in mice testes. Bcl-2 protein expression was significantly decreased by CP (P<0.01) and the Bax/Bcl-2 ratio was significantly increased (P<0.01). Following administration of SIP, testes of CP-exposed mice showed a significant reduction of photophosphorylation of p38 protein (P<0.01) and a significant decrease in the protein expression of Caspase-3 (P<0.05), while the expression level of the Bcl-2 gene was significantly increased (P<0.01). A significant decrease in Bax/Bcl-2 ratio was also observed in the SIP + CP group compared with the CP group (P<0.05). Compared with the CP-treated group, the protein expression of p-Akt in mice co-treated with SIP and CP decreased, but no significant difference was observed (P>0.05).
Figure 3.
Effects of SIP on CP-mediated expression of Caspase-3, Bcl-2 and Bax genes, as well as phosphorylation of p38 and Akt proteins. *P<0.05, **P<0.01 vs. CON. #P<0.05, ##P<0.01 vs. CP. CON, control mice; CP, CP-treated mice; SIP+CP, mice treated with squid ink polysaccharide and CP; SIP, squid ink polysaccharide-treated mice; p-, phosphorylated; Akt, AKT serine/threonine kinase; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
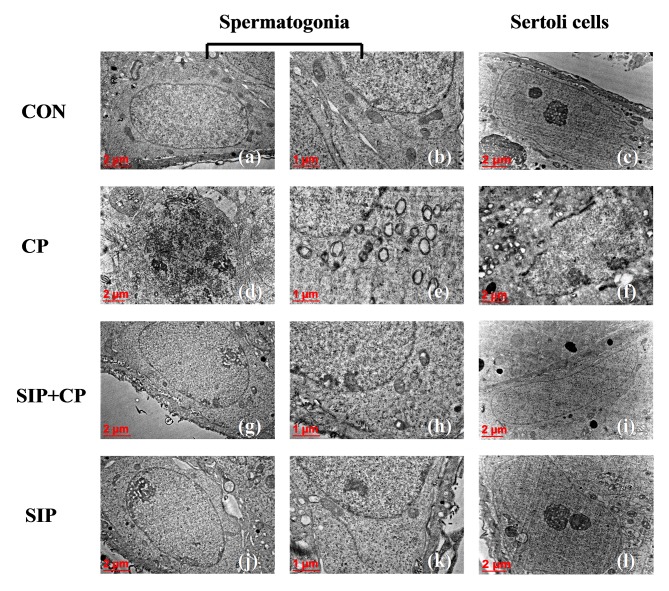
SIP attenuates the destruction of the ultra-structure of testicular germ cells caused by CP
As shown in Fig. 4, spermatogonia and Sertoli cells in control mice were healthy, as indicated by their large nuclei, homogeneous electron density in the nucleoplasm and prominent nucleoli. Additionally, the cytoplasm of spermatogonia and Sertoli cells contained rod-shaped mitochondria with substantial cristae. However, following treatment with CP alone, spermatogonia nuclei exhibited chromatorrhexis and chromatin pyknosis, and Sertoli cells appeared to undergo chromatin margination. Moreover, mitochondria in the two types of cells were swollen and displayed a lack of cristae. In some cases, spherical cellular monolayers were evident. Under administration of SIP and CP, spermatogonia and Sertoli cells demonstrated improved ultrastructure, indicated by clear and evident nucleoli, the homogenous electron density of the nucleoplasm and clear cristae present in numerous mitochondria. In SIP-treated mice, nucleoli in spermatogonia and Sertoli cells were clear and obvious, the karyoplasm was light in colour and of uniform density, and the cytoplasm had many mitochondria with rich cristae.
Figure 4.
Ultrastructure of testicular spermatogonia and Sertoli cells in four groups of mice as assessed by transmission electron microscopy. Images were obtained at magnifications of ×12,000 (c, f, i, l), ×15,000 (a, d, g, j), ×30,000 (b, e, h, k). CON, control mice; CP, CP-treated mice; SIP+CP, mice treated with squid ink polysaccharide and CP; SIP, squid ink polysaccharide-treated mice.
Discussion
As a clinical chemotherapeutic agent that is widely used, CP inevitably has some negative effects on the normal tissues and organs of patients with cancer, such as infertility or genetic toxicity (23,24). Two main physiological functions of testes is the generation of sperm and endocrine regulation, the latter restricts the former through they interact with each other (25,26). Apoptosis within seminiferous tubules is necessary to maintain a dynamic equilibrium in the testes and the generation of healthy sperm. However, chemotherapeutic drugs, such as CP, may generate excess free radicals and oxidation products and weaken testicular antioxidant functions, which may destroy the oxidation-antioxidation balance system in testes and result in testicular oxidative stress (27,28). Oxidative stress-induced accumulation of ROS may result in serious damage to mitochondria, including DNA mutation, lipid peroxidation and mitochondrial membrane channel opening, which leads to reduced mitochondrial function and male reproductive disorder (29). In the present study, CP induced injury to testis in male mice, including excessive ROS and MDA, decreased SOD activity and number of germ cells, as well as hollowed seminiferous tubules. Moreover, nuclear fragmentation and condensation, enlarged mitochondria and reduced cristae were observed in spermatogonial cells and Sertoli cells, the number of apoptosis-positive cells was elevated and these apoptotic cells were predominantly distributed in spermatogonial cell layers. These data support the CP-induced theory of oxidative stress (1,20). Additionally, mitochondria are organelles that are highly sensitive to stressful stimulations, oxidative stress alters mitochondrial membrane permeability and potential, which leads to mitochondrial swelling (29). In the present study, mitochondria were swollen and displayed a lack of cristae in spermatogonial cells and Sertoli cells. Therefore, cristae are important structures involved in mitochondrial energy metabolism, and their destruction inevitably causes mitochondrial energy metabolism disorder (30). Thus, based on the results of the current study, there was an association between oxidative stress and the apoptosis in cells, with low activities of antioxidative enzymes, nuclear pyknosis, mitochondrial swelling and vesiculation.
The marine polysaccharide, SIP, has demonstrated strong antioxidant capacity and it is able to effectively weaken the toxicity of CP on the male reproductive system (1). The present findings, demonstrated that SIP inhibited apoptosis of germ cells via improving antioxidant ability by hindering oxidative damage in mitochondria and suppressing activation of Caspase-3 protein in CP-exposed mice. Furthermore, SIP depleted ROS and therefore reduced the expression levels of pro-apoptotic Bax genes in spermatogonia and Sertoli cells, allowing these cells to maintain their morphological structural integrity. As a result, the blood-testis barrier was protected and normal physiological functions in the testes were conserved.
Multiple factors of signaling pathways are involved in the inhibition of apoptosis. Anti-apoptotic protein, Bcl-2, combines with Bax to form a homodimeror heterodimer that inhibits apoptosis (31). The present study indicated that SIP increased the content of Bcl-2 and decreased the Bax/Bcl-2 ratio, which suggested that SIP inhibited CP-induced apoptosis by regulating expression levels of Bcl-2 and Bax genes in the testes of CP-treated mice. The p38 mitogen-activated protein kinase (MAPK) signal transduction pathway relies on p38, which is an important member of the MAPK familythat can be activated by anti-tumor drugs or ROS; p38 has been used to inhibit tumors by inducing tumor cells apoptosis (32). The present results suggested that CP-activated p38 protein was dephosphorylated by SIP, or that SIP suppressed the phosphorylation of p38 protein induced by CP, which indicated that SIP may impair p38 activation by scavenging CP-mediated ROS to depress apoptosis. Akt is an important target kinase in the downstream actions of phosphatidylinositol-2-kinase, its phosphorylation can activate or inhibit downstream target proteins such as Bad, Caspase-9, nuclear factor kappa B and prostate apoptosis response-4 to block apoptosis (33–36). Akt signaling is considered to be an important mechanism that contributes toward sapoptosis inhibition (37,38). In the present study, it was identified that CP upregulated the content of phospho-Akt and the level of Akt phosphorylation was not significantly reduced after inhibition of apoptosisby SIP. We speculate that SIP may improve the cellular environmental impact of CP and contribute to Akt-mediated inhibition of apoptosis.
In conclusion, SIP weakened CP-induced oxidative stress to improve the antioxidant ability, protected functions of mitochondria, increased expression levels of Bcl-2 and reduced expression levels of Caspase-3 and Bax. Furthermore, SIP inhibited the p38 MAPK signal pathway, thus suppressing CP-induced apoptosis in testicular germ cells.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 31171667), the Special Fund for Distinguished Experts in Guangxi and the Guangxi Talent Highland of Preservation and Deep Processing Research in Fruit and Vegetables.
References
- 1.Le XY, Luo P, Gu YP, Tao YX, Liu HZ. Interventional effects of squid ink polysaccharides on cyclophosphamide-associated testicular damage in mice. Bratisl Lek Listy. 2015;116:334–339. doi: 10.4149/bll_2015_063. [DOI] [PubMed] [Google Scholar]
- 2.Mythili Y, Sudharsan PT, Selvakumar E, Varalakshmi P. Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem Biol Interact. 2004;151:13–19. doi: 10.1016/j.cbi.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar-Mahecha A, Hales B, Robaire B. Effects of acute and chronic cyclophosphamide treatment on meiotic progression and the induction of DNA double-strand breaks in rat spermatocytes. Biol Reprod. 2005;72:1297–1304. doi: 10.1095/biolreprod.104.038620. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Ma K, Wang H. Cyclophosphamide suppresses thioredoxin reductase in bladder tissue and its adaptive response via inductions of thioredoxin reductase and gluththione peroxidase. Chem Biol Interact. 2006;162:24–30. doi: 10.1016/j.cbi.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Faleiro L, Lazebnik Y. Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol. 2000;151:951–959. doi: 10.1083/jcb.151.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, Wu L, Li L, Tashiro S, Onodera S, Ikejima T. p53-mediated cell cycle arrest and apoptosis induced by shikonin via a caspase-9-dependent mechanism in human malignant melanoma A375-S2 cells. J Pharmacol Sci. 2004;94:166–176. doi: 10.1254/jphs.94.166. [DOI] [PubMed] [Google Scholar]
- 7.Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 8.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhang X, Rabbani ZN, Jackson IL, Vujaskovic Z. Oxidative stress mediates radiation lung injury by inducing apoptosis. Int J Radiat Oncol Biol Phys. 2012;83:740–748. doi: 10.1016/j.ijrobp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997;56:1490–1497. doi: 10.1095/biolreprod56.6.1490. [DOI] [PubMed] [Google Scholar]
- 11.Zong A, Zhao T, Zhang Y, Song X, Shi Y, Cao H, Liu C, Cheng Y, Qu X, Cao J, Wang F. Anti-metastatic and anti-angiogenic activities of sulfated polysaccharide of Sepiella maindroni ink. Carbohyd Polym. 2013;91:403–409. doi: 10.1016/j.carbpol.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Zuo T, He X, Cao L, Xue C, Tang QJ. The dietary polysaccharide from Ommastrephes bartrami prevents chemotherapeutic mucositis by promoting the gene expression of antimicrobial peptides in Paneth cells. J Funct Foods. 2015;12:530–539. doi: 10.1016/j.jff.2014.12.015. [DOI] [Google Scholar]
- 13.Zuo T, Cao L, Xue C, Tang QJ. Dietary squid ink polysaccharide induced goblet cells to protect small intestine from chemotherapy induced injury. Food Funct. 2015;6:981–986. doi: 10.1039/C4FO01191K. [DOI] [PubMed] [Google Scholar]
- 14.Tang Q, Zuo T, Lu S, Wu J, Wang J, Zheng R, Chen S, Xue C. Dietary squid ink polysaccharides ameliorated the intestinal microflora dysfunction in mice undergoing chemotherapy. Food Funct. 2014;5:2529–2535. doi: 10.1039/C4FO00408F. [DOI] [PubMed] [Google Scholar]
- 15.Zuo T, Cao L, Li X, Zhang Q, Xue C, Tang QJ. The squid ink polysaccharides protect tight junctions and adherens junctions from chemotherapeutic injury in the small intestinal epithelium of mice. Nutr Cancer. 2015;67:364–371. doi: 10.1080/01635581.2015.989369. [DOI] [PubMed] [Google Scholar]
- 16.Zuo T, Cao L, Sun X, Li X, Wu J, Lu S, Xue C, Tang Q. Dietary squid ink polysaccharide could enhance SIgA secretion in chemotherapeutic mice. Food Funct. 2014;5:3189–3196. doi: 10.1039/C4FO00569D. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Xu J, Xue C, Dong P, Sheng W, Yu G, Chai W. Sequence determination of a non-sulfated glycosaminoglycan-like polysaccharide from melanin-free ink of the squid Ommastrephes bartrami by negative-ion electrospray tandem mass spectrometry and NMR spectroscopy. Glycoconj J. 2008;25:481–492. doi: 10.1007/s10719-007-9096-2. [DOI] [PubMed] [Google Scholar]
- 18.Liu HZ, Wang G, Wu JL, Shi LS, Zhong JP, Pan JQ. Amelioratory effects of squid ink polysaccharides on partial internal organs injuried by cyclophosphamide. Chinese J Mod Appl Pharm. 2012;2:89–93. [Google Scholar]
- 19.Staub AM. Removal of protein-Sevag method. Methods Carbohydrate Chem. 1965;5:5–6. [Google Scholar]
- 20.Le X, Luo P, Gu Y, Tao Y, Liu H. Squid ink polysaccharides reduces cyclophosphamide-induced testicular damage via Nrf2/ARE activation pathway in mice. Iran J Basic Med Sci. 2015;18:827–831. [PMC free article] [PubMed] [Google Scholar]
- 21.Elangovan N, Chiou TJ, Tzeng WF, Chu ST. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology. 2006;222:60–70. doi: 10.1016/j.tox.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Brodsky RA. High-dose cyclophosphamide for autoimmunity and alloimmunity. Immunol Res. 2010;47:179–184. doi: 10.1007/s12026-009-8149-y. [DOI] [PubMed] [Google Scholar]
- 23.Motoyoshi Y, Kaminoda K, Saitoh O, Hamasaki K, Nakao K, Ishii N, Nagayama Y, Eguchi K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep. 2006;16:141–146. [PubMed] [Google Scholar]
- 24.Guittin P, Labbe V, Cavalier JC, Delongeas JL, Hodge T. Effects of cyclophosphamide administration on different fertility evaluation tests in male rats. Teratology. 1996;53:30A. [Google Scholar]
- 25.Luboshitzky R, Shen-Orr Z, Herer P. Seminal plasma melatonin and gonadal steroids concentrations in normal men. Arch Androl. 2002;48:225–232. doi: 10.1080/01485010252869324. [DOI] [PubMed] [Google Scholar]
- 26.Martin CW, Riley SC, Everington D, Groome NP, Riemersma RA, Baird DT, Anderson RA. Dose-finding study of oral desogestrel with testosterone pellets for suppression of the pituitary-testicular axis in normal men. Hum Reprod. 2000;15:1515–1524. doi: 10.1093/humrep/15.7.1515. [DOI] [PubMed] [Google Scholar]
- 27.Iuchi Y, Kaneko T, Matsuki S, Ishii T, Ikeda Y, Uchida K, Fujii J. Carbonyl stress and detoxification ability in the male genital tract and testis of rats. Histochem Cell Biol. 2004;121:123–130. doi: 10.1007/s00418-003-0607-3. [DOI] [PubMed] [Google Scholar]
- 28.Selvakumar E, Prahalathan C, Sudharsan PT, Varalakshmi P. Protective effect of lipoic acid on cyclophosphamide-induced testicular toxicity. Clin Chim Acta. 2006;367:114–119. doi: 10.1016/j.cca.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Motawi TM, Sadik NA, Refaat A. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: An experimental study on rat myocardium, testicles and urinary bladder. Food Chem Toxicol. 2010;48:2326–2336. doi: 10.1016/j.fct.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 30.Tinari A, Garofalo T, Sorice M, Esposti MD, Malorni W. Mitoptosis: Different pathways for mitochondrial execution. Autophagy. 2007;3:282–284. doi: 10.4161/auto.3924. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria-specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Xie N, Li H, Wei D, LeSaga G, Chen L, Wang S, Zhang Y, Chi L, Ferslew K, He L, et al. Glycogen synthase kinase-3 and p38 MAPK are required for opioid-induced microglia apoptosis. Neuropharmacology. 2010;59:444–451. doi: 10.1016/j.neuropharm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 34.Niquet J, Wasterlain CG. Bim, Bad, and Bax: A deadly combination in epileptic seizures. J Clin Invest. 2004;113:960–962. doi: 10.1172/JCI21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005;24:6719–6728. doi: 10.1038/sj.onc.1208825. [DOI] [PubMed] [Google Scholar]
- 36.Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T, Rangnekar VM. Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell. 2005;20:33–44. doi: 10.1016/j.molcel.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2016;291:22846. doi: 10.1074/jbc.A116.312044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang F, Lee JT, Navolanic PN, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]