Abstract
This prospective study aimed to estimate the efficacy of sorafenib therapy after transarterial chemoembolization (TACE) for unresectable hepatocellular carcinoma (HCC). Between July 2011 and March 2013, 17 patients were enrolled, 11 of whom received sorafenib therapy. Patients who previously received TACE for HCC and whose disease progressed within a six-month period were given 400–800 mg sorafenib orally, once or twice daily, within the 3 weeks after a second TACE (sorafenib after TACE group). The response to treatment, time to progression (TTP), overall survival (OS), and adverse events (AEs) were recorded. Of the 113 patients who underwent initial TACE for unresectable HCC between January 1995 and January 2013, 23 patients were selected who were treated with TACE alone, and for whom the interval between the second and third TACE treatments was <6 months (TACE alone group). The interval (TTP) was calculated between the third and fourth TACE treatments, then TTP was compared among the three groups: Sorafenib after TACE for > or <4 months; and TACE alone. During a median follow-up period of 34.4 months (range, 5.9–51.7 months) in both groups receiving sorafenib after TACE, sorafenib prolonged TTP (3.9 months) and OS (34.4 months). It was demonstrated that sorafenib use for >4 months prolonged TTP (5.7 months) significantly compared with use for <4 months (3.0 months) (P=0.002). The OS of patients given sorafenib for >4 months (35.9 months) was longer than that of patients who received the drug for <4 months (17.2 months), but this difference was not significant. In the TACE alone group, the median TTP between the third and fourth TACE treatments was 4.3 months. TTP decreased among the groups in the following order: Sorafenib for >4 months, TACE alone, and sorafenib for <4 months. There were three AEs of grade 3 in the present study. Two patients demonstrated a decrease in liver reserve function following sorafenib treatment, but improved immediately after sorafenib administration was stopped. Sorafenib induction early after TACE for unresectable HCC was generally well tolerated and significantly improved TTP. Further studies are required to confirm the safety and efficacy of this combination therapy.
Keywords: hepatocellular carcinoma, sorafenib, transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide in terms of number of cases (626,000, or 5.7% of new cancer cases) and it is the third most common cause of death from cancer (1). There are a variety of treatment guidelines for liver cancer, and their applicability in individual cases depends on the tumor stage (2–4).
Transarterial chemoembolization (TACE) is recommended for the treatment of unresectable HCC. TACE is a palliative rather than curative treatment. It can be carried out repeatedly for unresectable HCC, but if the effect is judged to be poor, or TACE-refractory, introduction of sorafenib is considered (5,6). However, the safety and efficacy of early sorafenib induction after TACE has yet to be established.
In the present study, we evaluated the safety and efficacy of sorafenib therapy within three weeks after a second TACE treatment was performed due to recurrence within six months of the first TACE procedure.
Patients and methods
Study design
This prospective study initially enrolled 17 patients whose tumors were treated with sorafenib post-TACE from July 2011 to March 2013 at Kagoshima University Hospital and Kagoshima Teishin Hospital. For the final analysis we selected the 11 patients who met the following inclusion criteria: (1) Classification by the Barcelona clinic liver cancer (BCLC) staging system (3) of stage B disease, which is generally not considered an indication for curative-intent treatment; (2) Eastern Cooperative Oncology Group performance status 0–1; (3) Child-Pugh grade A; and (4) progressive disease (PD), confirmed within six months after being treated with TACE. Exclusion criteria were as follows: (1) liver transplants at any time; (2) only nodal or distant metastases without viable lesions in the liver; (3) secondary malignancies; and (4) a history of concomitant use of some other targeting agent, chemotherapy, and immunotherapy.
All the patients were informed of the advantages and disadvantages of the treatment options, including treatment outcomes, treatment-related morbidities, and costs. The final treatment decision was made jointly by each patient and his/her physician, with full respect for the patient's option to decline participation. The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the ethics committees of Kagoshima University Medical and Dental Hospital and Kagoshima Teishin Hospital (approval no. 23-53). All the cases were judged to have PD within six months after TACE, and sorafenib administration was started within three weeks after the second TACE treatment.
Of 113 patients who underwent initial TACE for HCC in Child-Pugh grade A and BCLC Stage B from January 1995 to January 2013 at Kagoshima University Hospital, 23 patients who were treated by TACE alone were selected, and the interval between the third TACE to the second was <6 months (TACE alone group). We calculated TTP in the fourth TACE with the third and compared each group.
Evaluation of outcomes
Time to progression (TTP) based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (7) was the primary endpoint of the analysis. The secondary endpoints were overall survival (OS), which was defined as the time from enrolment to death from any cause or to the last follow-up in censored patients, and treatment-related adverse events (AEs), which were assessed using the Common Terminology Criteria for AEs (CTCAE) version 4.0. (8).
Treatment protocol
TACE
We performed TACE using the Seldinger technique according to the following protocol. After a 3.5- or 4-Fr-sheath (Medikit Super Sheath; Medikit, Tokyo, Japan) was introduced into the femoral artery, a 3.5- or 4-Fr preshaped catheter (Selecon-PA Catheter; Terumo, Gifu, Japan) was inserted into a superior mesenteric artery and 30–40 ml of 50% iopamidol (Iomeron 350; Eisai, Osaka, Japan) was injected. Computed tomographic arterial portography was performed to determine whether there were one or more HCC lesions, and to assess the patency of the portal vein. Computed tomographic arteriography was then performed to detect HCC, and 15–20 ml of 50% iopamidol was injected via a common hepatic artery. Additionally, we selectively placed a 2-Fr microcatheter in the tumor-bearing artery of the HCC (nutrient artery), and injected an emulsified formulation of iodized oil (Lipiodol; Laboratoire Guerbet, AulnaySous-Bois, France) along with the following three anti-cancer agents: i) 20 mg epirubicin hydrochloride (Farmorubicin; Pfizer Japan, Tokyo, Japan) and 4 mg mitomycin C (Kyowa-Kirin, Tokyo, Japan); ii) miriplatin hydrate (Miripla®, Dainippon Sumitomo Pharma, Tokyo, Japan); and iii) cisplatin (Nihon-Kayaku, Tokyo, Japan). After injecting the emulsified formulation, we injected gelatin sponge particles (Gelpart®, Nippon Kayaku, Tokyo, Japan) as an embolus into the same location. We performed hepatic arteriography after the embolus injection to confirm the loss of blood flow to the tumor through the nutrient artery before performing the surgery. Sorafenib was administered within one to three weeks after TACE.
Re-TACE followed by sorafenib
All the patients were given detailed information regarding sorafenib after TACE treatment, including its efficacy and potential AEs. Patients received oral sorafenib (400 or 200 mg) twice daily after TACE, except for those with a contraindication to sorafenib treatment (e.g., insufficient liver function). For patients treated with sorafenib after TACE, the efficacy of the combined treatment was assessed using dynamic CT or MR imaging of the liver 6–8 weeks after treatment.
Clinical characteristics of patients and laboratory markers. The following patient clinical characteristics and laboratory markers were assessed: age; sex; tumor size; observation period; previous treatment; viral markers, including hepatitis B virus (HBV), hepatitis C virus (HCV), and NBNC [HBV (−) and HCV (−)]; hepatic function assessed using Child-Pugh grades based on both clinical (ascites and encephalopathy) and laboratory (serum albumin, total bilirubin and prothrombin time) parameters; body mass index (BMI); aspartate transaminase (AST); alanine aminotransferase (ALT); γ-glutamyl transpeptidase (γ-GTP); serum albumin; total bilirubin; prothrombin time; platelets; α-fetoprotein (AFP); and des-γ-carboxy prothrombin (DCP).
During follow-up, the levels of AST, AST, γ-GTP, serum albumin, total bilirubin, prothrombin time, platelets, AFP, and DCP were determined every 4–6 weeks to evaluate liver function. As mentioned above, dynamic liver CT or MR imaging was performed every 6–8 weeks after treatment to evaluate the response.
Statistical analysis
Statistical analyses were performed using the χ2-test or the Mann-Whitney U test, as appropriate. The Kaplan-Meier method was used to estimate cumulative survival and time to progression of local and other tumors. To ensure patient safety, the dose of sorafenib was reduced or treatment was delayed or temporarily discontinued when we observed clinically significant toxicity (≥grade 3) based on the National Cancer Institute's CTCAE version 4.0 (8) or at the physician's discretion. P<0.05 was regarded as statistically significant. All the statistical analyses were conducted using IBM SPSS Statistics v. 20 (IBM SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics and rates of TTP and OS in the 11 patients
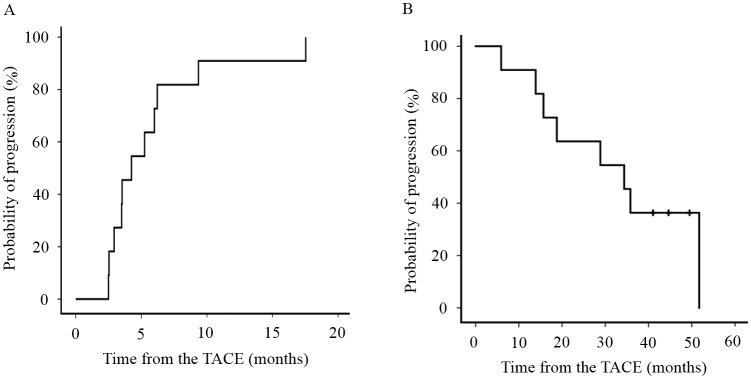
Eleven patients met the aforementioned inclusion criteria. Table I summarizes the baseline clinical characteristics of the 11 patients treated with sorafenib after TACE for HCC. The median TTP of all the patients during the follow-up period was 3.9 months (median range, 2.3–16.1) (Fig. 1A) and the median OS of all patients was 34.4 months (median range, 5.9–51.7) (Fig. 1B). The median underlying cause of HCC was HBV in two patients, HCV in six patients, and NBNC in three patients. The ECOG performance score was 0 in nine patients and 1 in one patient. Nine patients had chronic hepatitis and three had liver cirrhosis. All the patients were Child-Pugh grade A. LCSGJ stage was II in two patients and III in nine patients. AFP was 7.3 ng/ml (median range, 3.5–188 ng/ml) and DCP was 24.0 mAU/ml (median range, 8–3589 mAU/ml). All the patients had pretreatment for HCC. The initial sorafenib dose was 800 mg in nine patients and 400 mg in two patients.
Table I.
Baseline sorafenib after TACE patient's characteristics.
| Patient's characteristics | All patients (n=11) |
|---|---|
| Age (years), median (range) | 70.6±7.5 |
| Gender | |
| Male | 10 (90.9) |
| Female | 1 (9.1) |
| TTP, months | 3.9 (2.3–16.1) |
| OS, months | 34.4 (5.9–51.7) |
| Underlying cause | |
| HBV | 2 (18.2) |
| HCV | 6 (54.5) |
| NBNC | 3 (27.3) |
| ECOG performance score | |
| 0 | 10 (90.9) |
| 1 | 1 (9.1) |
| Child-Pugh score | |
| A | 11 (100.0) |
| B | 0 (0.0) |
| C | 0 (0.0) |
| LCSGJ staging | |
| II | 2 (18.2) |
| III | 9 (91.8) |
| AFP, ng/ml | 7.3 (3.5–188.0) |
| DCP, mAU/ml | 24.0 (8.0–3589.0) |
| Pretreatment HCC | |
| Yes | 11 (100.0) |
| No | 0 (0.0) |
| Initial sorafenib dose, mg | |
| 800 | 9 (91.8) |
| 400 | 2 (18.2) |
Categorical data are expressed as n (%). Continuous data are expressed as mean ± standard deviation or median (range) by group. HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, HBV (−) and HCV (−); ECOG, Eastern Cooperative Oncology Group; LCSGJ, Liver Cancer Study Group of Japan; AFP, α-fetoprotein; DCP, des-γ-carboxy prothrombin; HCC, hepatocellular carcinoma. Child-Pugh score based on both clinical (ascites and encephalopathy) and laboratory (serum albumin, total bilirubin and prothrombin time) parameters.
Figure 1.
Rates of TTP and OS in all 11 patients. (A) The median TTP of all the patients during the follow-up period was 3.9 (range, 2.3–16.1) months. (B) The median OS of all patients was 34.4 months (range, 5.9–51.7). TTP, time to progression; OS, overall survival.
Rates of TTP and OS in HCC patients treated with sorafenib after TACE for more or less than 4 months, and with TACE alone
Table II summarizes the baseline clinical characteristics of the HCC patients treated with sorafenib after TACE for more than 4 months, less than 4 months, and TACE alone.
Table II.
Baseline clinical characteristics of HCC patients treated with sorafenib after TACE for more than 4 months, less than 4 months, and with TACE alone.
| Patient characteristic | Sorafenib after TACE group (n=11) | ||
|---|---|---|---|
| Period of sorafenib | <4 months (n=6) | 4 months ≦ (n=5) | TACE alone group (n=23) |
| Age, years | 72.8±6.4 | 68.0±8.6 | 67.4 ± 8.5 |
| Sex | |||
| Male | 6 (100.0) | 4 (80.0) | 16 (69.6) |
| Female | 0 (0.0) | 1 (20.0) | 7 (30.4) |
| TTP, months | 3.0 (2.3–3.9) | 5.7 (4.8–16.1) | 4.3 (0.7- 24.0) |
| OS, months | 17.2 (5.9–38.9) | 35.9 (28.9–49.5) | – |
| Underlying cause | |||
| HBV | 1 (16.7) | 1 (20.0) | 1 (4.3) |
| HCV | 4 (66.6) | 2 (40.0) | 21 (83.3) |
| NBNC | 1 (16.7) | 2 (40.0) | 1 (4.3) |
| Child-Pugh score | |||
| A | 6 (100.0) | 5 (100.0) | 23 (100.0) |
| B | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| LCSGJ staging | |||
| II | 1 (16.7) | 1 (20.0) | 10 (43.5) |
| III | 5 (83.3) | 4 (80.0) | 13 (56.5) |
| AFP, ng/ml | 12.1 (3.5–137.0) | 7.3 (4.9–188.0) | 110.0 (1.7- 67996.0) |
| DCP, mAU/ml | 41.0 (15.0–3589.0) | 20.0 (8.0–67.0) | 29.0 (11.0- 7200.0) |
| Pretreatment HCC | |||
| Yes | 6 (100.0) | 5 (100.0) | 30 (100.0) |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sorafenib, mg | |||
| 800 | 3 (50.0) | 5 (100.0) | – |
| 400 | 3 (50.0) | 0 (0.0) | – |
Categorical data are expressed as n (%). Continuous data are expressed as mean ± standard deviation or median (range) by group. TTP, Time to progression; OS, overall survival; HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, HBV (−) and HCV (−); LCSGJ, Liver Cancer Study Group of Japan; AFP, α-fetoprotein; DCP, des-γ-carboxy prothrombin; HCC, hepatocellular carcinoma. Child-Pugh score based on both clinical (ascites and encephalopathy) and laboratory (serum albumin, total bilirubin and prothrombin time) parameters.
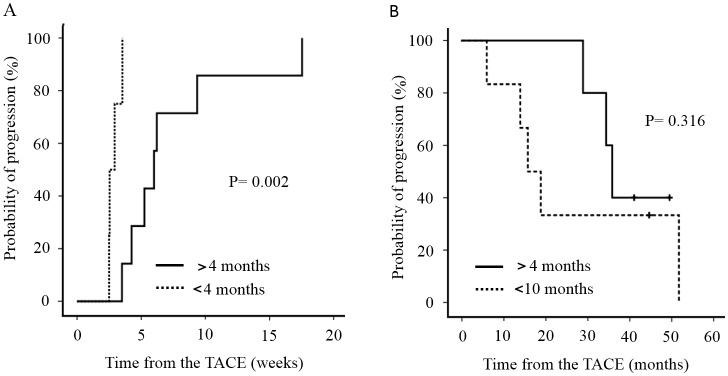
We divided the patients into two groups based on whether sorafenib was administered for more or less than 4 months. TTP in patients treated with sorafenib for >4 months was 5.7 months (median range, 4.8–16.1), while that in patients receiving sorafenib for <4 months was 3.0 months (median range, 2.3–3.9), indicating a significant difference (P=0.002) (Fig. 2A). OS in patients treated with sorafenib for >4 months was 35.9 (median range, 28.9–49.5) months, while that in patients receiving sorafenib for <4 months was 17.2 months (median range, 5.9–38.9). There was no significant difference in OS between the two groups (Fig. 2B). In the TACE alone group, the interval (TTP) between the third and fourth TACE treatments was 4.3 months (range, 0.7–24.0). Thus, TTP decreased among the groups in the following order: Sorafenib for more than 4 months > TACE alone > sorafenib for <4 months.
Figure 2.
Two groups based on whether sorafenib was administered for more or less than 4 months. (A) TTP in patients treated with sorafenib for >4 months was significantly higher than that in patients treated with sorafenib for <4 months (P=0.002). (B) There was no significant difference in OS between the two groups (P=0.316). TTP, time to progression; OS, overall survival.
Treatment-related AEs
Treatment-related AEs, which were assessed using the CTCAE version 4.0, (8) are shown in Table III. The most common AEs were hand-foot skin reactions (27%); these occurred at grade 1 (G1) in seven patients and G2 in three patients. The second most common AE was hypertension (10.8%), which occurred at G1 in two patients and G2 in two patients. One patient each had G3 increased serum amylase, decreased platelet count and white blood cells. In Table IV, AEs are divided into two groups based on whether they initially developed within 2 weeks of the start of sorafenib treatment or >2 weeks later. Hand-foot skin reactions occurred in each group, whereas hypertension was only observed within 2 weeks. The G3 increased serum amylase and decreased G3 white blood cells occurred within 2 weeks, whereas the G3 decreased platelet count occurred after >2 weeks. Total Child-Pugh scores decreased in only two patients by the end of sorafenib treatment (Fig. 3), but these improved immediately after sorafenib administration was stopped.
Table III.
Drug-related, treatment-emergent adverse events.
| AE | Grade 1 n=23 | Grade 2 n=11 | Grade 3 n=3 | Total n (%) |
|---|---|---|---|---|
| Hand-foot skin reaction | 7 (30.4) | 3 (27.2) | 0 (0.0) | 10 (27.0) |
| Diarrhea | 3 (13.0) | 0 (0.0) | 0 (0.0) | 3 (8.1) |
| Alopecia | 3 (13.0) | 0 (0.0) | 0 (0.0) | 3 (8.1) |
| Pruritus | 2 (8.7) | 0 (0.0) | 0 (0.0) | 2 (5.4) |
| Hypertension | 2 (8.7) | 2 (18.2) | 0 (0.0) | 4 (10.8) |
| Hoarseness | 2 (8.7) | 0 (0.0) | 0 (0.0) | 2 (5.4) |
| Serum amylase increased | 1 (4.3) | 1 (9.0) | 1 (33.3) | 3 (8.1) |
| Platelet count decreased | 1 (4.3) | 1 (9.0) | 1 (33.3) | 3 (8.1) |
| Hepatobiliary disorders | 1 (4.3) | 1 (9.0) | 0 (0.0) | 2 (5.4) |
| Anorexia | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (5.4) |
| White blood cell decreased | 0 (0.0) | 1 (9.0) | 1 (33.3) | 2 (5.4) |
| Blood bilirubin increased | 0 (0.0) | 1 (9.0) | 0 (0.0) | 1 (2.7) |
| Acute coronary syndrome | 0 (0.0) | 1 (9.0) | 0 (0.0) | 1 (2.7) |
Categorical data are expressed as n (%). AE, adverse event.
Table IV.
Drug-related adverse events within 2 weeks or more than 2 weeks after initiating sorafenib treatment.
| Within 2 weeks | More than 2 weeks | ||
|---|---|---|---|
| AE | Any Grade (n=21) | AE | Any Grade (n=15) |
| Hand-foot skin reaction | 5 (23.8) | Hand-foot skin reaction | 5 (33.3) |
| Hypertension | 4 (19.0) | Diarrhea | 3 (20.0) |
| Serum amylase increased | 3 (14.3) | Alopecia | 3 (20.0) |
| Hoarseness | 2 (9.5) | Platelet count decreased | 1 (6.7) |
| White blood cell decreased | 2 (9.5) | Blood bilirubin increased | 1 (6.7) |
| Acute coronary syndrome | 1 (4.8) | Hepatobiliary disorders | 1 (6.7) |
| Anorexia | 1 (4.8) | Pruritus | 1 (6.7) |
| Hepatobiliary disorders | 1 (4.8) | ||
| Platelet count decreased | 1 (4.8) | ||
| Pruritus | 1 (4.8) | ||
Categorical data are expressed as n (%). AE, adverse event.
Figure 3.
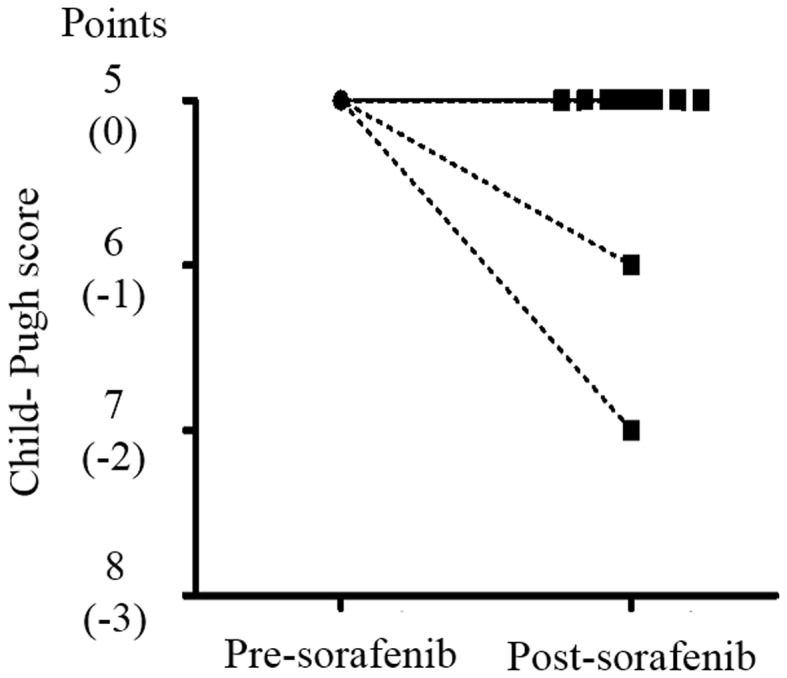
Child-Pugh scores of pre-sorafenib treatment and post-sorafenib treatment. Child-Pugh scores decreased in only two patients by the end of sorafenib treatment, but these improved immediately after sorafenib administration was stopped.
Discussion
The present study investigated a loco-regional control of early induction of sorafenib after transarterial chemoembolization for unresectable hepatocellular carcinoma in relapsed patients within six months after the TACE procedure. We evaluated how early induction of sorafenib after TACE contributes to prolonging the interval between TACE treatments (TTP). TTP in patients treated with sorafenib for >4 months was 5.7 months and <4 months was 3.0 months. TTP in patients treated with sorafenib for >4 months was significantly higher than that. OS in patients treated with sorafenib for >4 months was 35.9 months and for <4 months was 17.2 months. There was no significant difference in OS between the two groups. In the TACE alone group, the interval (TTP) between the third and the fourth TACE treatments was 4.2 months. Sorafenib administered for >4 months resulted in longer TTP than TACE alone. In addition, early induction of sorafenib after TACE within three weeks was identified; however, only two patients experienced mild reduction in liver reserve function during the course of sorafenib treatment, but we did not observe any other severe AEs, only G1 and G2 hand-foot skin reactions (27.0%) and hypertension (10.8%) were observed. We considered sorafenib after TACE was generally well tolerated.
TACE is recommended for unresectable HCC by the Japan Society of Hepatology Consensus-Based Clinical Practice Guidelines for the management of HCC (2) and intermediate BCLC stages (3). Ikeda et al reported that the median OS of patients with unresectable HCC was 3.1 years, the one-year OS was 89.9%, and the two-year OS was 75.0% (9). There were no significant differences between Japanese and Korean patients. The use of TACE is beneficial in patients with intermediate stage HCC, but TACE is a palliative treatment. When TACE is performed repeatedly, its effect can gradually wane as patients become refractory (10). The treatment of TACE-refractory patients is controversial and there is no globally established approach to the problem. Several studies have reported the beneficial effects of sorafenib for TACE-refractory patients, and the relatively early administration of sorafenib extended overall survival (5,6,11). The development of sorafenib as a systemic chemotherapy is essential for patients who have no choice but to repeat TACE. However, a phase III clinical trial in the Asia-Pacific region revealed that the median TTP was 2.8 months and the median OS was only 6.5 months in HCC patients with sufficient liver function who were treated with sorafenib after TACE (12).
Currently, local treatments, i.e., TACE and radiofrequency ablation, have been shown to induce the production of VEGF (13–16), which may facilitate disease progression and metastasis. Additionally, VEGF is elevated after TACE, but to a lesser extent in patients who respond more poorly to TACE therapy (17). Sorafenib monotherapy appears to be inferior to TACE in terms of local control of HCC; however, the antiangiogenic and antiproliferative effects of sorafenib may inhibit VEGF production and multikinase signaling and prevent angiogenesis when administered after TACE (17–19). We planned this study with this background in mind, and expected prolongation of TTP and OS. There have been several trials based on the same concept, namely the antiangiogenic and antiproliferative effects of sorafenib after TACE. A double-blind and placebo-controlled phase III trial from Japan and Korea, the so-called post-TACE trial (20), found that the addition of sorafenib provided no additional benefit to TTP in HCC patients who responded to TACE. One of the most significant problems with this trial was that the median duration from TACE to the onset of sorafenib therapy was 9.3 weeks. Previous findings have shown that the transient elevation of VEGF occurs within seven days after TACE therapy (14,15). Therefore, it is unlikely that sorafenib contributed to TTP and OS as sorafenib was introduced after the seventh day. We took this into consideration and sorafenib was initially administered within three weeks of TACE in this study. We used a single-arm prospective design, and used historical data as controls (in other words, patients with intermediate BCLC Stage and less than six months between the second and third TACE) for comparison to TTP between the third and fourth TACE treatments. Patients who took sorafenib for more than 4 months demonstrated significantly longer TTP than those who received TACE alone.
There was concern about AEs given the short duration between TACE and the introduction of sorafenib, but the combination of these treatments was well tolerated and did not appear to lead to worse AEs than were observed with either TACE or sorafenib therapy alone. While a previous combination trial reported a higher incidence of AEs when TACE was administered before sorafenib (20), and a European study evaluating the combination of TACE and sorafenib was stopped prematurely because of safety concerns (21), the AE profile and incidence observed in this study were similar to those in several trials of TACE plus sorafenib combination therapy (22,23), and did not differ from those observed in the SHARP (24) and Asia-Pacific (12) sorafenib-only trials. In this study, the most common AE was hand-foot skin reaction, occurring in 27% of patients, and the second most common was hypertension, experienced by 10.8% of patients. Moreover, the AE profile differed slightly depending on whether sorafenib was administered within two weeks or after two weeks, but severe reactions were not observed in either case. Sorafenib eventually became ineffective in all the patients in this study; however, only two cases demonstrated mild declines in liver reserve, and the remainder maintained their baseline Child-Pugh scores after they stopped taking sorafenib. All the patients were able to undergo further TACE after sorafenib discontinuation because sufficient liver reserve function was maintained.
This clinical study was useful because of its prospective nature, as many previous reports have used a retrospective design. Nevertheless, there were some limitations to our study. First, our sample size was relatively small. Since the efficacy, side effects, and cost-benefit of sorafenib after TACE were unknown because of the prospective nature of the study, we could not obtain informed consent from many patients. Second, this was not a placebo-controlled randomized clinical trial. Ideally, a phase III clinical trial is needed with appropriate controls to avoid selection bias. However, it is relatively difficult to recruit patients to a placebo-controlled randomized clinical trial within a reasonable time period.
In conclusion, the use of sorafenib in combination with TACE significantly improved TTP and OS in patients with intermediate HCC. Further studies are needed to confirm the safety and efficacy of this combination therapy.
Acknowledgements
The English language service Zenis™ aided in the preparation of the current study manuscript for publication.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver1, corp-author. European Organisation For Research And Treatment Of Cancer: EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, Kanai F, Yoshikawa M, Yokosuka O. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87:330–341. doi: 10.1159/000365993. [DOI] [PubMed] [Google Scholar]
- 6.Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4:253–262. doi: 10.1159/000367743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 8.http://evs.nci.nih.gov/ftp1/CTCAE/About.html. [May 14;2015 ];NCI common terminology criteria for adverse events (CTCAE) v.4 data files. [Google Scholar]
- 9.Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK, Inaba Y, Aramaki T, Kwon SH, Yamamoto S, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: An Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013;24:490–500. doi: 10.1016/j.jvir.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):S22–S31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda M, Mitsunaga S, Shimizu S, Ohno I, Takahashi H, Okuyama H, Kuwahara A, Kondo S, Morizane C, Ueno H, et al. Efficacy of sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. J Gastroenterol. 2014;49:932–940. doi: 10.1007/s00535-013-0853-7. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 13.Mise M, Arii S, Higashituji H, Furutani M, Niwano M, Harada T, Ishigami S, Toda Y, Nakayama H, Fukumoto M, et al. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996;23:455–464. doi: 10.1002/hep.510230309. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Mori M, Kawaguchi C, Adachi M, Miura S, Ishii H. Serum vascular endothelial growth factor in the course of transcatheter arterial embolization of hepatocellular carcinoma. Int J Oncol. 1999;14:1087–1090. doi: 10.3892/ijo.14.6.1087. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878–2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poon RT, Lau C, Pang R, Ng KK, Yuen J, Fan ST. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: Importance of tumor biomarker in ablative therapies. Ann Surg Oncol. 2007;14:1835–1845. doi: 10.1245/s10434-007-9366-z. [DOI] [PubMed] [Google Scholar]
- 17.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 18.Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Sieghart W, Pinter M, Reisegger M, Müller C, Ba-Ssalamah A, Lammer J, Peck-Radosavljevic M. Conventional transarterial chemoembolisation in combination with sorafenib for patients with hepatocellular carcinoma: A pilot study. Eur Radiol. 2012;22:1214–1223. doi: 10.1007/s00330-011-2368-z. [DOI] [PubMed] [Google Scholar]
- 22.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960–3967. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabrera R, Pannu DS, Caridi J, Firpi RJ, Soldevila-Pico C, Morelli G, Clark V, Suman A, George TJ, Jr, Nelson DR. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;34:205–213. doi: 10.1111/j.1365-2036.2011.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]