Abstract
The aim of the present study was to investigate plausible explanations for the favorable outcome of female melanoma patients and determine the effect of biology on this outcome. Data from 1,169 cutaneous melanoma patients were retrospectively analyzed. Cox proportional hazards models were used and the confounding factors on the survival difference were analyzed by a forward step multivariate modification method. The majority of the factors contributing to poor prognosis were significantly more pronounced in male melanoma patients. After the survival advantage of female patients (P=0.0001 on univariate analysis) was confounded (P=0.708 on multivariate analysis) following adjustment for the prognostic factors, two factors (neurotropism and vertical growth phase) were identified as the confounders, and this effect was attributed to the small number of patients in the groups of these two variables. The already known female advantage in melanoma survival was not affected by other prognostic factors, and female sex remained an independent predictor of good survival in melanoma. This sex-related independent survival advantage was attributed to a biological characteristic that has not yet been fully elucidated, but may be more closely associated with host-related rather than melanoma-related factors.
Keywords: melanoma, sex, biology, survival
Introduction
The National Comprehensive Cancer Network guidelines, version 2.2016, stated that >76,000 new melanoma patients were diagnosed and >9,500 succumbed to this disease in 2014 in the United States (1). Since Clark et al found in 1969 that the disease was less malignant in women, numerous studies have been published, aiming to extensively investigate the cause of this difference (2).
Sex is considered to be an important independent prognostic factor for melanoma survival, and female patients have higher survival rates (3). In order to explain this difference, researchers have suggested two major hypotheses so far: The first hypothesis is based on behavioral characteristics, such as that men have more advanced disease at diagnosis due to certain lifestyle characteristics (i.e., men are exposed to the sun more often and for longer periods compared with women, and are less conscious of skin care and skin cancer, thus not taking sufficient preventive measures) that may result in delays in screening, detection and diagnosis of the disease (4). The second hypothesis is that there is a biological trait that has yet to be elucidated, which accounts for the sex-related survival difference in melanoma; this unclear biological trait is believed to be associated with either the tumor per se (namely, the disease in female patients is naturally less aggressive), or with factors within the host (namely, female sex prevents disease progression and spread) (5–9).
The first hypothesis was controverted and the second hypothesis was advocated by the fact that the female survival advantage persisted even following adjustment for factors such as Breslow's thickness, and that lymph node and visceral organ metastases did not affect the higher survival rate of female patients (4,5–11). Thus, it was concluded that the aggressiveness of the disease did not affect the survival benefit of female melanoma patients. Sex appeared to remain an independent prognostic factor. Thus, to assess this advantage, the survival benefit associated with sex difference in melanoma was investigated following adjustment for known prognostic and predictive variables in a large Turkish patient population.
Patients and methods
Patients
The data of 1,169 adult cutaneous melanoma patients who were treated and followed up at the Department of Medical Oncology, Institute of Oncology, Istanbul University (Istanbul, Turkey) from 1993 to 2015, were retrospectively examined. The American Joint Committee on Cancer (AJCC) staging system (12) was used to stage the disease. Sentinel lymph node biopsy (SLNB) or lymph node dissection were performed to assess lymph node status. If the SLNB revealed involvement, the patient then underwent elective complete lymphadenectomy. The BRAF V600E mutation DNA was detected by a real-time polymerase chain reaction-based assay using formalin-fixed parafin-embedded tissue samples. Standard international guideline recommendations and protocols were applied while treating and following up the patients. Only a small number of metastatic melanoma patients were treated with targeted therapies; instead, chemotherapy-based regimens (dacarbazine, temozolomide and cisplatin, as single agent or combined) were mainly used in these patients, as the Turkish Ministry of Health did not approve the use of both BRAF-targeting therapy and immunotherapy until the end of 2015. Only 4 and 2 patients received BRAF-targeting therapy (vemurafenib) and immunotherapy (ipilimumab), respectively. These small numbers were not considered to affect the statistical results. The present study was reviewed and approved by our local Ethics Committee and informed consent was obtained from all individual participants included in this study.
Statistical analysis
Sex was compared with various clinicopathological variables using Chi-square tests. The recurrence-free survival (RFS) was defined from the time of diagnosis (pathological diagnosis) to the date of recurrence (clinical or radiological recurrence). Overall survival (OS) was measured from the date of diagnosis (pathological diagnosis) to either the date of death from any cause or the date of the last contact, whichever occurred first. Patient survival was analyzed with the Kaplan-Meier estimator and the differences in survival were assessed with log-rank statistics. Cox proportional hazards models were used to acquire univariate and multivariate analyses. The multivariate survival analysis was performed for all variables with a significant effect on OS and RFS in the univariate analysis, i.e., histology (histology 1: superficial or other types of spreading; and histology 2: nodular or other histological type); Clark's level (I–III or IV–V); Breslow's thickness (<2 or ≥2 mm); mitotic rate (≤3 or >3/mm2); extent of nodal involvement (1 or ≥2 nodes involved); and presence or absence of neurotropism, ulceration, lymphovascular invasion (LVI), nodal involvement, vertical growth phase, recurrence and metastasis (metastasis 1). Subsequently, another multivariate model, forward step multivariate modification, was produced, in that the variables were included in the analysis one by one in a forward step manner. A P-value of ≤0.05 was considered to indicate statistically significant differences. Statistical analysis was performed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Between 1993 and 2015, a total of 1,169 melanoma patients were assigned to the present study. The median patient age was 52 years (range, 16–104 years). The demographic and clinicopathological characteristics of the patients are summarized in Table I. The number of male patients was higher compared with that of female patients (634 vs. 535; 54.3 vs. 45.7%, respectively). Male melanoma patients were older at diagnosis compared with their female counterparts (P=0.034) and had more truncal lesions compared with extremity lesions (P=0.0001). Furthermore, male patients more often exhibited nodular histological subtype (P=0.005), higher Clark's level of invasion (IV–V) (P=0.004), greater Breslow's thickness (≥2 mm) (P=0.0001), ulceration (P=0.0001), absence of regression (P=0.032), lymph node involvement (P=0.047), disease recurrence (P=0.0001) and metastasis (P=0.003) (Table. I). However, sex was not found to be associated with the spreading pattern of the disease (superficial vs. others), mitotic rate, neurotropism, tumor-infiltrating lymphocytes (TIL), vertical growth phase, LVI, underlying precursor lesion, BRAF positivity/negativity, number of metastatic lymph nodes and type of distant metastasis (M1a-b vs. M1c).
Table I.
Patient characteristics and correlations between patient sex and various clinicopathological variables.
| Variables | n (%) | Male, n (%) | Female, n (%) | P-value |
|---|---|---|---|---|
| No. of patients | 1,169 (100.0) | 634 (54.3) | 535 (45.7) | |
| Age, years | 0.034 | |||
| <50 | 531 (45.4) | 270 (42.6) | 261 (48.8) | |
| ≥50 | 638 (54.6) | 364 (57.4) | 274 (51.2) | |
| Site of lesion | 0.0001 | |||
| Trunk | 659 (58.4) | 402 (66.7) | 257 (49.0) | |
| Extremities | 469 (41.6) | 201 (33.3) | 268 (51.0) | |
| Histology 1 | 0.698 | |||
| Superficial spreading | 405 (48.7) | 216 (49.3) | 189 (48.0) | |
| Others | 427 (51.3) | 222 (50.7) | 205 (52.0) | |
| Histology 2 | 0.005 | |||
| Nodular | 254 (30.5) | 152 (34.7) | 102 (25.8) | |
| Others | 580 (69.5) | 286 (65.3) | 294 (74.2) | |
| Clark's level | 0.004 | |||
| I–III | 290 (32.1) | 137 (28.0) | 153 (36.9) | |
| IV–V | 614 (67.9) | 352 (72.0) | 262 (63.1) | |
| Breslow's thickness, mm | 0.0001 | |||
| <2 | 311 (35.5) | 134 (28.2) | 177 (44.1) | |
| ≥2 | 565 (64.5) | 341 (71.8) | 224 (55.9) | |
| Neurotropism | 0.410 | |||
| Present | 23 (4.4) | 14 (5.1) | 9 (3.6) | |
| Absent | 499 (95.6) | 260 (94.9) | 239 (96.4) | |
| Mitotic rate, n/mm2 | 0.241 | |||
| ≤3 | 436 (57.9) | 226 (55.9) | 210 (60.2) | |
| >3 | 317 (42.1) | 178 (44.1) | 139 (39.8) | |
| Ulceration | 0.0001 | |||
| Present | 429 (54.3) | 257 (60.2) | 172 (47.4) | |
| Absent | 361 (45.7) | 170 (39.8) | 191 (52.6) | |
| Vertical growth phase | 0.065 | |||
| Present | 422 (89.4) | 227 (91.9) | 195 (86.7) | |
| Absent | 50 (10.6) | 20 (8.1) | 30 (13.3) | |
| LVI | 0.302 | |||
| Present | 80 (11.3) | 47 (12.5) | 33 (10.0) | |
| Absent | 627 (88.7) | 330 (87.5) | 297 (90.0) | |
| TIL | 0.519 | |||
| Absent | 264 (35.2) | 138 (34.2) | 126 (36.4) | |
| Present | 486 (64.8) | 266 (65.8) | 220 (63.6) | |
| Regression | 0.032 | |||
| Present | 169 (25.3) | 104 (28.7) | 65 (21.4) | |
| Absent | 498 (74.7) | 259 (71.3) | 239 (78.6) | |
| Underlying precursor lesion | 0.924 | |||
| Present | 176 (31.5) | 90 (31.4) | 86 (31.7) | |
| Absent | 382 (68.5) | 197 (68.6) | 185 (68.3) | |
| BRAF | 0.493 | |||
| Negative | 39 (56.5) | 27 (54.0) | 12 (63.2) | |
| Positive | 30 (43.5) | 23 (46.0) | 7 (36.8) | |
| Lymph node involvement (node 1) | 0.047 | |||
| Yes | 374 (50.1) | 223 (53.3) | 151 (46.0) | |
| No | 372 (49.9) | 195 (46.7) | 177 (54.0) | |
| No. of involved lymph nodes (node 2) | 0.732 | |||
| 1 | 211 (57.0) | 125 (56.3) | 86 (58.1) | |
| ≥2 | 159 (43.0) | 97 (43.7) | 62 (41.9) | |
| Metastasis (metastasis 1) | 0.003 | |||
| Present | 131 (11.2) | 87 (13.7) | 44 (8.2) | |
| Absent | 1,037 (88.8) | 546 (86.3) | 491 (91.8) | |
| Type of metastasis (metastasis 2) | 0.198 | |||
| M1a-b | 41 (31.3) | 24 (27.6) | 17 (38.6) | |
| M1c | 90 (68.7) | 63 (72.4) | 27 (61.4) | |
| Recurrence | 0.0001 | |||
| Yes | 360 (30.8) | 225 (35.5) | 135 (25.3) | |
| No | 808 (69.2) | 409 (64.5) | 399 (74.7) |
Bold print indicates statistical significance. LVI, lymphovascular invasion; TIL, tumor-infiltrating lymphocytes.
Univariate analysis
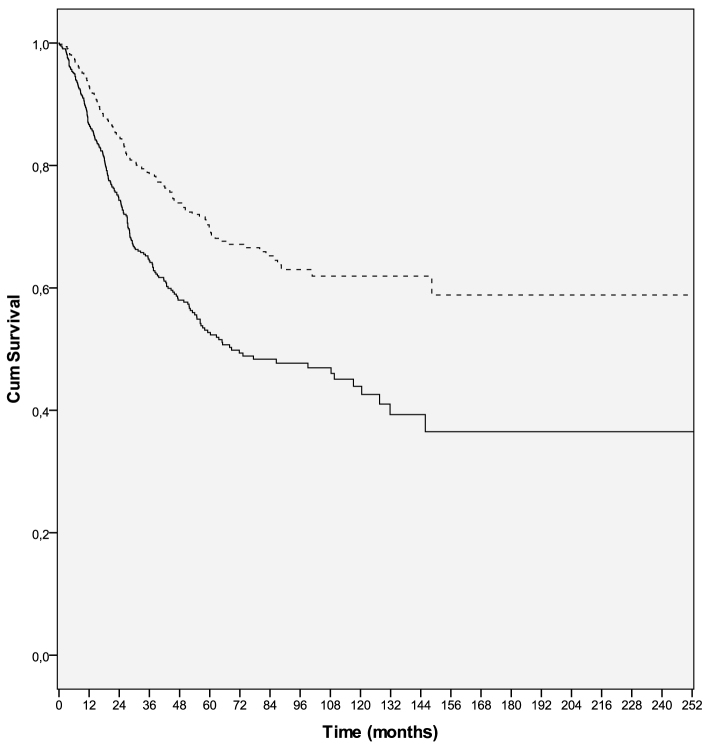
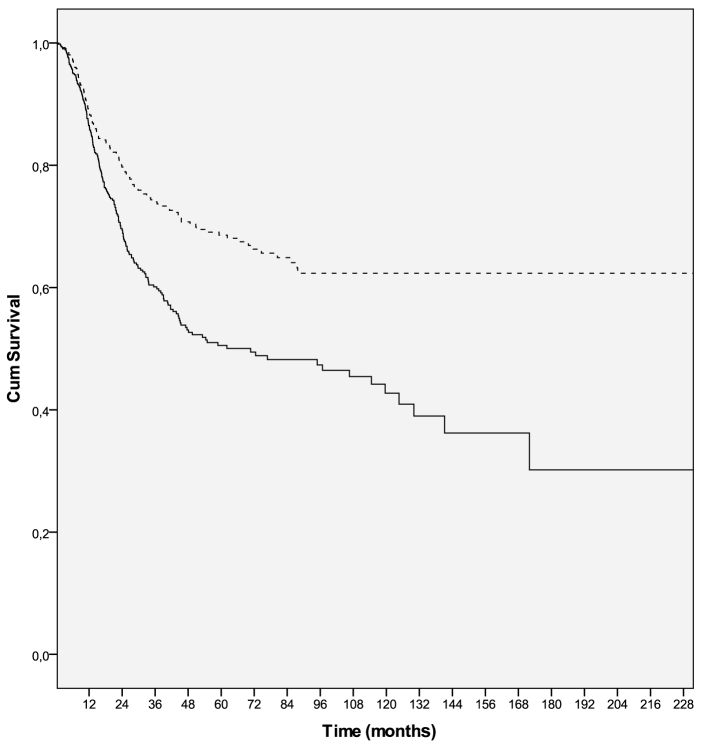
Univariate analyses of clinical variables demonstrated that male sex, non-superficial spreading pattern, nodular histological subtype, higher Clark's level, greater Breslow's thickness, higher mitotic rate, presence of ulceration, neurotropism, vertical growth phase, LVI, lymph node involvement, multiple lymph node involvement and metastasis, were all associated with poorer RFS and OS. However, no association was observed between age, tumor localization, BRAF status, type of metastasis (M1a-b vs. M1c), presence of regression, TIL, and underlying precursor lesion, and either RFS or OS. Male sex was significantly associated with poorer RFS (HR=0.594; 95% CI: 0.479–0.735; P=0.0001) and OS (HR=0.564; 95% CI: 0.456–0.697; P=0.0001) (Tables II and III, Figs. 1 and 2).
Table II.
Univariate and multivariate analyses of factors associated with overall survival.
| Univariate analyses | Multivariate analyses | |||
|---|---|---|---|---|
| Variables | Risk ratio (95% CI) | P-value | Risk ratio (95% CI) | P-value |
| Age (<50 vs. ≥50 years) | 1.216 (0.989–1.493) | 0.063 | ||
| Site of lesion (trunk vs. extremities) | 0.925 (0.748–1.144) | 0.471 | ||
| Histology 1 (superficial spreading vs. others) | 1.921 (1.454–2.539) | 0.0001 | ||
| Histology 2 (nodular vs. others) | 0.408 (0.312–0.534) | 0.0001 | ||
| Clark's level (I–III vs. IV–V) | 2.679 (1.921–3.738) | 0.0001 | ||
| Breslow's thickness (<2 vs. ≥2 mm) | 3.016 (2.156–4.220) | 0.0001 | ||
| Neurotropism (present vs. absent) | 3.673 (2.061–6.546) | 0.0001 | ||
| Mitotic rate (≤3 vs. >3/mm2) | 2.293 (1.705–3.083) | 0.0001 | ||
| Ulceration (present vs. absent) | 2.789 (2.035–3.823) | 0.0001 | ||
| Vertical growth phase (present vs. absent) | 12.46 (1.737–89.34) | 0.012 | ||
| LVI (present vs. absent) | 2.471 (1.678–3.638) | 0.0001 | 3.001 (1.207–7.459) | 0.018 |
| TIL (present vs. absent) | 0.890 (0.659–1.201) | 0.447 | ||
| Regression (present vs. absent) | 0.730 (0.495–1.077) | 0.113 | ||
| Precursor lesion (present vs. absent) | 0.796 (0.536–1.182) | 0.258 | ||
| BRAF (positive vs. negative) | 0.927 (0.473–1.815) | 0.825 | ||
| Node 1 (lymph node involvement, yes vs. no) | 3.511 (2.630–4.687) | 0.0001 | ||
| Node 2 (no. of involved lymph nodes, 1 vs. ≥2) | 1.412 (1.029–1.938) | 0.033 | ||
| Metastasis 1 (presence vs. absence) | 9.478 (7.490–11.99) | 0.0001 | ||
| Metastasis 2 (type M1a-b vs. M1c) | 1.480 (0.975–2.247) | 0.066 | ||
| Recurrence (yes vs. no) | 4.116 (3.324–5.097) | 0.0001 | 10.90 (4.446–26.71) | 0.0001 |
| Sex (male vs. female) | 0.564 (0.456–0.697) | 0.0001 | 0.708 | |
Bold print indicates statistical significance. CI, confidence interval; LVI, lymphovascular invasion; TIL, tumor-infiltrating lymphocytes.
Table III.
Univariate and multivariate analysis of variables in association with recurrence-free survival.
| Univariate analyses | Multivariate analyses | |||
|---|---|---|---|---|
| Variables | Risk ratio (95% CI) | P-value | Risk ratio (95% CI) | P-value |
| Age (<50 vs. ≥50 years) | 1.100 (0.893–1.354) | 0.371 | ||
| Site of lesion (trunk vs. extremities) | 0.935 (0.756–1.157) | 0.537 | ||
| Histology 1 (superficial spreading vs. others) | 1.618 (1.239–2.114) | 0.0001 | ||
| Histology 2 (nodular vs. others) | 0.497 (0.380–0.649) | 0.0001 | ||
| Clark's level (I–III vs. IV–V) | 2.408 (1.761–3.292) | 0.0001 | ||
| Breslow's thickness (<2 vs. ≥2 mm) | 3.059 (2.230–4.196) | 0.0001 | ||
| Neurotropism (present vs. absent) | 2.037 (0.993–4.178) | 0.052 | ||
| Mitotic rate (≤3 vs. >3/mm2) | 2.459 (1.859–3.253) | 0.0001 | 1.973 (1.090–3.574) | 0.025 |
| Ulceration (present vs. absent) | 2.833 (2.111–3.803) | 0.0001 | ||
| Vertical growth phase (present vs. absent) | 3.324 (1.352–8.170) | 0.009 | ||
| LVI (present vs. absent) | 2.037 (1.375–3.017) | 0.0001 | ||
| TIL (present vs. absent) | 0.811 (0.610–1.079) | 0.151 | ||
| Regression (present vs. absent) | 0.720 (0.494–1.050) | 0.088 | ||
| Precursor lesion (present vs. absent) | 0.798 (0.546–1.167) | 0.245 | ||
| BRAF (positive vs. negative) | 0.819 (0.454–1.480) | 0.508 | ||
| Node 1 (lymph node involvement, yes vs. no) | 3.035 (2.333–3.948) | 0.0001 | 3.521 (1.893–6.547) | 0.0001 |
| Node 2 (no. of involved lymph nodes, 1 vs. ≥2) | 1.356 (0.994–1.849) | 0.054 | ||
| Metastasis 1 (presence vs. absence) | 2.989 (2.113–4.227) | 0.0001 | ||
| Metastasis 2 (type M1a-b vs. M1c) | 0.667 (0.348–1.279) | 0.223 | ||
| Sex (male vs. female) | 0.594 (0.479–0.735) | 0.0001 | 0.749 | |
Bold print indicates statistical significance. CI, confidence interval; LVI, lymphovascular invasion; TIL, tumor-infiltrating lymphocytes.
Figure 1.
Overall survival curves in male (solid line) and female (dashed line) melanoma patients (P=0. 001). Cum, cumulative.
Figure 2.
Recurrence-free survival curves in male (solid line) and female (dashed line) melanoma patients (P=0. 001). Cum, cumulative.
Multivariate analysis
On multivariate analysis, higher mitotic rate (HR=1.973; 95% CI: 1.090–3.574; P=0.025) and lymph node involvement (HR=3.521; 95% CI: 1.893–6.547; P=0.0001) remained significantly associated with poorer RFS, whereas the presence of LVI (HR=3.001; 95% CI: 1.207–7.459; P=0.018) and disease recurrence (HR=10.90; 95% CI: 4.446–26.71; P=0.0001) exhibited a statistically significant association with poorer OS. However, sex was not found to be statistically significantly associated with either RFS (P=0.749) or OS (P=0.708) on multivariate analysis (Tables II and III).
Determination of confounding factors
With the purpose of determining the confounding factors for sex with respect to RFS and OS, forward step multivariate modifications were prepared, in which variables were added to the analyses one at a time, and whether sex had lost its significance was checked after each addition. Regarding OS, sex remained significant, with female patients exhibiting better survival with almost all the variables added to the analyses, except for neurotropism and vertical growth phase. Neurotropism and vertical growth phase confounded the significance of sex for OS (P=0.270 and P=0.357, respectively). When RFS was analysed using the forward step multivariate modification, the presence of lymph node involvement (P=0.071), vertical growth phase (P=0.077 and 0.368) and the presence of neurotropism (P=0.125) were found to be confounding factors for the significance of sex (Tables IV and V).
Table IV.
Forward step multivariate modifications of sex in association with overall survival.
| Model | Modifications | Sex HR | (95% CI) | P-value |
|---|---|---|---|---|
| A | Crude (sex only) | 0.564 | (0.456–0.697) | 0.0001 |
| B | Model A+histology 1 | 0.600 | (0.455–0.790) | 0.0001 |
| C | Model B+age | 0.600 | (0.455–0.790) | 0.0001 |
| D | Model C+Breslow's thickness | 0.580 | (0.431–0.795) | 0.001 |
| E | Model D+Clark's level | 0.579 | (0.422–0.796) | 0.001 |
| F | Model E+mitotic rate | 0.588 | (0.418–0.827) | 0.002 |
| G | Model F+ulceration | 0.593 | (0.418–0.839) | 0.003 |
| H | Model G+node 1 | 0.580 | (0.389–0.863) | 0.007 |
| I | Model H+metastasis 1 | 0.587 | (0.395–0.874) | 0.009 |
| J | Model I+recurrence | 0.600 | (0.399–0.901) | 0.014 |
| K | Model J+site of lesion | 0.600 | (0.399–0.901) | 0.014 |
| L | Model K+regression | 0.563 | (0.359–0.885) | 0.013 |
| M | Model L+TIL | 0.623 | (0.395–0.982) | 0.042 |
| N | Model M+neurotropism | 0.270 | ||
| O | Model M+vertical growth phase | 0.357 |
Age, <50 vs. ≥50 years; site of lesion, trunk vs. extremities; histology 1, superficial spreading vs. others; Clark's level, I–III vs. IV–V; Breslow's thickness, <2 vs. ≥2 mm; neurotropism, present vs. absent; mitotic rate, ≤3 vs. >3/mm2; ulceration, present vs. absent; vertical growth phase, present vs. absent; TIL, present vs. absent; regression, present vs. absent; precursor lesion, present vs. absent; BRAF, positive vs. negative; node 1, presence of lymph node involvement (yes vs. no); metastasis 1, presence vs. absence; recurrence, yes vs. no; sex, male vs. female. HR, hazard ratio; CI, confidence interval; TIL, tumor-infiltrating lymphocytes.
Table V.
Forward step multivariate modifications of sex in association with recurrence-free survival.
| Model | Modifications | Sex HR | (95% CI) | P-value |
|---|---|---|---|---|
| A | Crude (sex only) | 0.594 | (0.479–0.735) | 0.0001 |
| B | Model A+histology 1 | 0.575 | (0.439–0.755) | 0.0001 |
| C | Model B+age | 0.575 | (0.439–0.755) | 0.0001 |
| D | Model C+Breslow's thickness | 0.632 | (0.471–0.848) | 0.002 |
| E | Model D+Clark's level | 0.648 | (0.479–0.878) | 0.005 |
| F | Model E+mitotic rate | 0.657 | (0.475–0.908) | 0.011 |
| G | Model F+ulceration | 0.676 | (0.484–0.943) | 0.021 |
| H | Model G+site of lesion | 0.676 | (0.484–0.943) | 0.021 |
| I | Model H+metastasis 1 | 0.673 | (0.482–0.939) | 0.020 |
| J | Model I+node 1 | 0.071 | ||
| K | Model I+vertical growth phase | 0.077 | ||
| L | Model J+vertical growth phase | 0.368 | ||
| M | Model I+neurotropism | 0.125 |
Age, <50 vs. ≥50 years; site of lesion, trunk vs. extremities; histology 1, superficial spreading vs. others; Clark's level, I–III vs. IV–V; Breslow's thickness, <2 vs. ≥2 mm; neurotropism, present vs. absent; mitotic rate, ≤3 vs. >3/mm2; ulceration, present vs. absent; vertical growth phase, present vs. absent; TIL, present vs. absent; regression, present vs. absent; precursor lesion, present vs. absent; BRAF, positive vs. negative; node 1, presence of lymph node involvement (yes vs. no); metastasis 1, presence vs. absence; recurrence, yes vs. no; sex, male vs. female. HR, hazard ratio; CI, confidence interval; TIL, tumor-infiltrating lymphocytes.
Discussion
In the present study, a significant non-adjusted (crude) favorable OS was found for female patients (P=0.0001) in the univariate analysis. This significance in OS favoring women disappeared as the multivariate analysis was performed adjusting for the variables that exhibited statistical significance in the univariate analysis. Thus, a forward step multivariate modification was developed and the significance of sex difference remained with all the models, except for neurotropism and vertical growth phase. Neurotropism and vertical growth phase, the only two confounders, contradicted the results of previous similarly designed studies that identified Breslow's thickness and site of the primary disease as the only confounders for sex difference (6,9,12). Our finding may be the result of the smaller number of patients in ‘neurotropism-present’ and ‘vertical growth phase-absent’ groups compared with their counterparts (23 vs. 499 and 50 vs. 422, respectively). This vast difference in patient numbers between groups may compromise the result of the analysis. Provided these two covariates with exclusively small patient numbers were omitted, the sex survival advantage remained independently significant and unaffected by any other factor.
Women are generally more inclined to conform to the screening protocols and, if necessary, are more likely to seek access to medical care compared with men (13); thus, their lesions may be diagnosed and treated at an earlier stage, when the lesion is thinner and before it becomes ulcerated. Ulceration and increased thickness have already been identified as the most powerful tumor-related poor prognostic predictors (10,12). Similarly, the present study also observed that men had thicker and ulcerated lesions and that they had tumors located more often on the trunk, compared with women, in whom the tumors were more often located in the extremities. As truncal melanoma spreads more often to distant regions compared with lower extremity lesions, and melanoma on the lower extremities metastasizes more frequently to adjoining regions compared with the upper extremity lesions, it may be concluded that truncal melanomas have a significantly worse prognosis compared with their extremity counterparts, and that, if the lesion is located in the lower extremity, there is an outcome advantage over upper extremity lesions (14). One of the limitations in our study was that, in our data, the primary site was classified as truncal vs. extremity; thus, we were unable to determine the prognostic difference between the lesions located in the upper and lower extremities.
However, in the present study, as well as in numerous other studies, sex remained an independent significant survival predictor after adjusting for factors that are affected by behavioral differences. One of these studies revealed that men were 1.9 times more likely to succumb to melanoma after adjusting for factors such as Breslow's thickness, primary lesion site, nodal involvement, and presence or absence of metastasis (11). Liu et al found that there was no correlation between melanoma thickness and time to diagnosis, which also refuted the possible role of behavioral differences between men and women (15). A prior study stated that the delay in diagnosis did not account for thicker melanoma and poorer prognosis; instead, it was attributed to the rapidly growing aggressive tumors that were more likely to occur in older men, thus explaining the better survival rate in women (16).
Whether hormones exerted an effect on the sex-related survival difference was also investigated. Tamoxifen was studied in a randomized trial and was not found to improve the survival outcome in metastatic melanoma (17). Contrary to this finding, another in vitro study demonstrated that tamoxifen significantly hindered cell invasion in melanoma; in addition, it was found that, apart from melanoma, breast, esophageal, pancreatic, lung, gastric and colorectal cancers and soft tissue sarcomas exhibited the same female survival advantage (18). Neither melanoma diagnosed during pregnancy was found to affect survival rate, nor the pregnancy that occurred before or after the diagnosis of melanoma was shown to affect the survival outcome of melanoma; furthermore, oral contraceptive use and hormone replacement therapies were not found to be associated with increased risk of melanoma development and/or prognosis (19). In the present study, we were unable to assess the definitive role of sex hormones in melanoma, due to relevant information (such as menopausal status or oral contraceptive use of the study group) missing from our data, which was defined as another limitation of this study.
Numerous other factors were hypothesized to be possible explanations for the survival advantage of female melanoma patients, such as oxidative stress, drugs and ethanol consumption (5,20). Men are known to express lower amounts of antioxidant enzymes; therefore, they may produce a higher level of radical oxygen species, thus enhancing metastasis via multiple pathways.
In a study that analyzed a total of 11,774 cases, women were found to exhibit significantly higher survival rates, and the risk for progression was significantly lower for women across all stages of melanoma. Accordingly, melanomas in female patients were less likely to metastasize to lymph nodes or visceral organs; furthermore, even if they did metastasize, the significant survival advantage was sustained after the first progression and lymph node metastasis (6). In a subsequent study on stage I/II melanoma, it was observed that men had worse prognostic characteristics at diagnosis, and they retained a progression disadvantage of ~30% after diagnosis (7). In 2013, the same group analyzed survival differences across sexes in stage III and IV melanoma patients (8). They again demonstrated the continuity of independent female survival advantage following adjustment for known prognostic factors, despite lymph node and distant metastasis. Based on this result, an intrinsic biological mechanism was considered to affect the progression and outcome of melanoma at each stage of the disease. A number of previous studies had already demonstrated that there was no difference between men and women in the mutation rates of melanoma-associated genes (e.g. BRAF, NRAS and KIT), indicating that primary melanoma genetics did not differ across sexes (21–23). Thus, instead of melanoma-related biological causes, intrinsic host-related factors were suggested to be implicated in the female survival advantage.
Mitotic rate is the number of mitoses/mm2, indicating the degree of proliferating cells within the tumor; therefore, a high mitotic rate suggests a rapidly growing cancer and portends a poor prognosis (24). It was found that women maintained the survival advantage, even if they had melanomas with high mitotic rates.
A forward step Cox proportional hazards model was used, which was similar to the model used by Joosse et al (9). In this modification, mitotic rate was not shown to affect the survival advantage of women. As mentioned earlier, apart from neurotropism and vertical growth phase, none of the remaining variables confounded the significance of sex, and the small patient number in the groups of these two covariates was considered to cause this confounding effect. Provided these two factors were omitted, the female survival advantage persisted and was independently significant. Therefore, we concurred with the researchers who suggested the presence of a female biological trait as the explanation for the independent survival advantage. In light of the above mentioned findings, it is strongly believed that the elucidation of this yet unknown female biological trait will alter the approach to melanoma. In conclusion, sex is of utmost importance among the prognostic factors for melanoma and, therefore, it is recommended that the focus of research be directed to the role of sex in the pathogenesis of melanoma.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Clark WH, Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–727. [PubMed] [Google Scholar]
- 3.Spatz A, Batist G, Eggermont AM. The biology behind prognostic factors of cutaneous melanoma. Curr Opin Oncol. 2010;22:163–168. doi: 10.1097/CCO.0b013e328337fe8f. [DOI] [PubMed] [Google Scholar]
- 4.Lasithiotakis K, Leiter U, Meier F, Eigentler T, Metzler G, Moehrle M, Breuninger H, Garbe C. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer. 2008;112:1795–1804. doi: 10.1002/cncr.23359. [DOI] [PubMed] [Google Scholar]
- 5.Joosse A, De Vries E, van Eijck CH, Eggermont AM, Nijsten T, Coebergh JW. Reactive oxygen species and melanoma: An explanation for gender differences in survival? Pigment Cell Melanoma Res. 2010;23:352–364. doi: 10.1111/j.1755-148X.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- 6.Joosse A, de Vries E, Eckel R, Nijsten T, Eggermont AM, Hölzel D, Coebergh JW, Engel J. Munich Melanoma Group: Gender differences in melanoma survival: Female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131:719–726. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
- 7.Joosse A, Collette S, Suciu S, Nijsten T, Lejeune F, Kleeberg UR, Coebergh JW, Eggermont AM, de Vries E. Superior outcome of women with stage I/II cutaneous melanoma: Pooled analysis of four European organisation for research and treatment of cancer phase III trials. J Clin Oncol. 2012;30:2240–2247. doi: 10.1200/JCO.2011.38.0584. [DOI] [PubMed] [Google Scholar]
- 8.Joosse A, Collette S, Suciu S, Nijsten T, Patel PM, Keilholz U, Eggermont AM, Coebergh JW, de Vries E. Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: A pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials. J Clin Oncol. 2013;31:2337–2346. doi: 10.1200/JCO.2012.44.5031. [DOI] [PubMed] [Google Scholar]
- 9.Joosse A, van der Ploeg AP, Haydu LE, Nijsten TE, de Vries E, Scolyer RA, Eggermont AM, Coebergh JW, Thompson JF. Sex differences in melanoma survival are not related to mitotic rate of the primary tumor. Ann Surg Oncol. 2015;22:1598–1603. doi: 10.1245/s10434-014-4166-8. [DOI] [PubMed] [Google Scholar]
- 10.Mervic L, Leiter U, Meier F, Eigentler T, Forschner A, Metzler G, Bartenjev I, Büttner P, Garbe C. Sex differences in survival of cutaneous melanoma are age dependent: An analysis of 7338 patients. Melanoma Res. 2011;21:244–252. doi: 10.1097/CMR.0b013e32834577c8. [DOI] [PubMed] [Google Scholar]
- 11.de Vries E, Nijsten TE, Visser O, Bastiaannet E, van Hattem S, Janssen-Heijnen ML, Coebergh JW. Superior survival of females among 10,538 Dutch melanoma patients is independent of Breslow thickness, histologic type and tumor site. Ann Oncol. 2008;19:583–589. doi: 10.1093/annonc/mdm498. [DOI] [PubMed] [Google Scholar]
- 12.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American joint committee on cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 13.Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Goydos JS, Beitsch PD, Urist MM, Ariyan S, Sussman JJ, Edwards MJ, et al. Gender-related differences in outcome for melanoma patients. Ann Surg. 2006;243:693–698. doi: 10.1097/01.sla.0000216771.81362.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leiter U, Meier F, Schittek B, Garbe C. The natural course of cutaneous melanoma. J Surg Oncol. 2004;86:172–178. doi: 10.1002/jso.20079. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Dowling JP, Murray WK, McArthur GA, Thompson JF, Wolfe R, Kelly JW. Rate of growth in melanomas: Characteristics and associations of rapidly growing melanomas. Arch Dermatol. 2006;142:1551–1558. doi: 10.1001/archderm.142.12.1551. [DOI] [PubMed] [Google Scholar]
- 16.Richard MA, Grob JJ, Avril MF, Delaunay M, Thirion X, Wolkenstein P, Souteyrand P, Dreno B, Bonerandi JJ, Dalac S, et al. Melanoma and tumor thickness: Challenges of early diagnosis. Arch Dermatol. 1999;135:269–274. doi: 10.1001/archderm.135.3.269. [DOI] [PubMed] [Google Scholar]
- 17.Lens MB, Reiman T, Husain AF. Use of tamoxifen in the treatment of malignant melanoma. Cancer. 2003;98:1355–1361. doi: 10.1002/cncr.11644. [DOI] [PubMed] [Google Scholar]
- 18.Molife R, Lorigan P, MacNeil S. Gender and survival in malignant tumours. Cancer Treat Rev. 2001;27:201–209. doi: 10.1053/ctrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 19.Jhaveri MB, Driscoll MS, Grant-Kels JM. Melanoma in pregnancy. Clin Obstet Gynecol. 2011;54:537–545. doi: 10.1097/GRF.0b013e318236e18b. [DOI] [PubMed] [Google Scholar]
- 20.Le Marchand L, Saltzman BS, Hankin JH, Wilkens LR, Franke AA, Morris SJ, Kolonel LN. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232–245. doi: 10.1093/aje/kwj115. [DOI] [PubMed] [Google Scholar]
- 21.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scolyer RA, Kefford RF. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 22.Devitt B, Liu W, Salemi R, Wolfe R, Kelly J, Tzen CY, Dobrovic A, McArthur G. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res. 2011;24:666–672. doi: 10.1111/j.1755-148X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 23.Kong Y, Si L, Zhu Y, Xu X, Corless CL, Flaherty KT, Li L, Li H, Sheng X, Cui C, et al. Large-scale analysis of KIT aberrations in Chinese patients with melanoma. Clin Cancer Res. 2011;17:1684–1691. doi: 10.1158/1078-0432.CCR-10-2346. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JF, Soong SJ, Balch CM, Gershenwald JE, Ding S, Coit DG, Flaherty KT, Gimotty PA, Johnson T, Johnson MM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: An analysis of patients in the multi-institutional American joint committee on cancer melanoma staging database. J Clin Oncol. 2011;29:2199–2205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]