Abstract
Metronomic oral cyclophosphamide has gained increasing interest in recent years as a promising maintenance therapy in advanced, platinum-sensitive, high-grade serous ovarian cancer (HGSOC). Metronomic treatment with cyclophosphamide refers to the frequent, usually daily, administration of a low (oral) dose of cyclophosphamide with no prolonged drug-free breaks. Main advantages of this treatment are the effective reduction of tumour activity, oral administration in an outpatient setting, low cost and the low toxicity profile. Metronomic oral cyclophosphamide can benefit patients suffering from types of cancer known to be sensitive to alkylating agents, such as platinum-sensitive HGSOC. In recent years, several publications have underlined the advantage of this regimen and possible explanations were explored. We here present a patient with multiple recurrences of metastasized HGSOC, platinum-sensitive, with an on-going complete response to monotherapy with oral cyclophosphamide. This observation supports that patients with relapsing HGSOC who responded to platinum-based chemotherapy and cannot continue platinum-based chemotherapy because of toxicity, can be offered a course of metronomic cyclophosphamide. This case may serve as a reminder that old drugs can be used successfully even in the age of new upcoming therapy such as anti-angiogenic agents (VEGF inhibitors) and poly-ADP-ribose polymerase (PARP) inhibitors.
Keywords: cyclophosphamide, high-grade serous ovarian cancer, metronomic therapy
Introduction
The treatment of high-grade serous ovarian cancer (HGSOC) is complicated by the fact that it is usually in an advanced stage at the time of diagnosis. HGSOC accounts for 70–80% of ovarian cancer deaths, and overall survival has not changed significantly despite advances in therapy (www.seer.cancer.gov). HGSOC is distinctive in that it shows an initial favourable response to platinum-based chemotherapy (1). This response is probably related to a high rate of defects in the homologous recombination (HR) DNA repair pathway, mainly due to mutations in BRCA1 and BRCA2. Tumours with mutations in other components of the HR pathway (BRCAness phenotype) have the same likelihood of response to alkylating agents (2).
Regardless of extensive surgery and chemotherapy, over 80% of HGSOC patients relapse and require additional therapy. Metronomic oral cyclophosphamide has gained increasing interest in recent years as a promising maintenance therapy in advanced HGSOC after response to platinum based chemotherapy (3). It may cause long-term response in an outpatient setting with a low toxicity profile.
In the current study we present an exceptional responder: a patient with metastasized HGSOC who underwent different types of treatment modalities, including several episodes of platinum-based chemotherapy, and who after 13 years went into complete remission on low-dose and continuous oral cyclophosphamide alone. We discuss the possible underlying mechanism of this treatment with metronomic cyclophosphamide and the potential advantages compared to newer regimens.
Case report
In December 2003, a 47-year-old woman presented with obstipation, abdominal pain and a palpable mass in the lower right abdomen. The relevant previous medical history consisted of diabetes mellitus type I. She had no family history of breast or ovarian cancer. Ultrasound showed a solid and cystic mass of 14×14×8 cm and a CA-125 level of 5.659 kU/l. Exploratory laparotomy revealed a large mass with multiple peritoneal depositions. Subsequently, the patient underwent a bilateral ovariectomy, hysterectomy, omentectomy and resection of the rectosigmoid with closure of the rectal stump and formation of an end colostomy (Hartmann procedure). Macroscopic residual mass remained on the left iliacal external vein, and microscopic pathology demonstrated a high-grade papillary serous adenocarcinoma. A CT scan evaluation, performed before starting additional chemotherapy, revealed a mass between the liver and the right kidney, and presacral and para-aortal lymph node metastases. Six cycles of platinum-based chemotherapy, administered in accordance with the AGO-OVAR study (4), resulted in a partial response. This regimen was followed by a second debulking operation, reversal of the colostomy and another 3 cycles of chemotherapy. The patient had intra-abdominal recurrences in October 2005, in May 2007 and in February 2008, which were successfully treated with chemotherapy, consecutive paclitaxel and carboplatin, liposomal doxorubicin and further combinations of paclitaxel and carboplatin. In 2009, a recurrence at the lower part of the vagina was diagnosed. This was surgically removed and followed by radiotherapy. In 2011, a solitary liver metastasis was treated with radiofrequency ablation (RFA) and four cycles of cisplatin and etoposide. In August 2012, a tumour mass in the abdominal scar resulting from the RFA needle insertion was treated with radiotherapy, and at reoccurrence in May 2013, the tumour was resected. Tumour tissue at the next intra-abdominal reappearance in August 2014 was proven to be oestrogen receptor positive, and treatment was started with the aromatase inhibitor letrozole. Within several weeks, a slow biochemical progression could be observed. Several treatment options were considered.
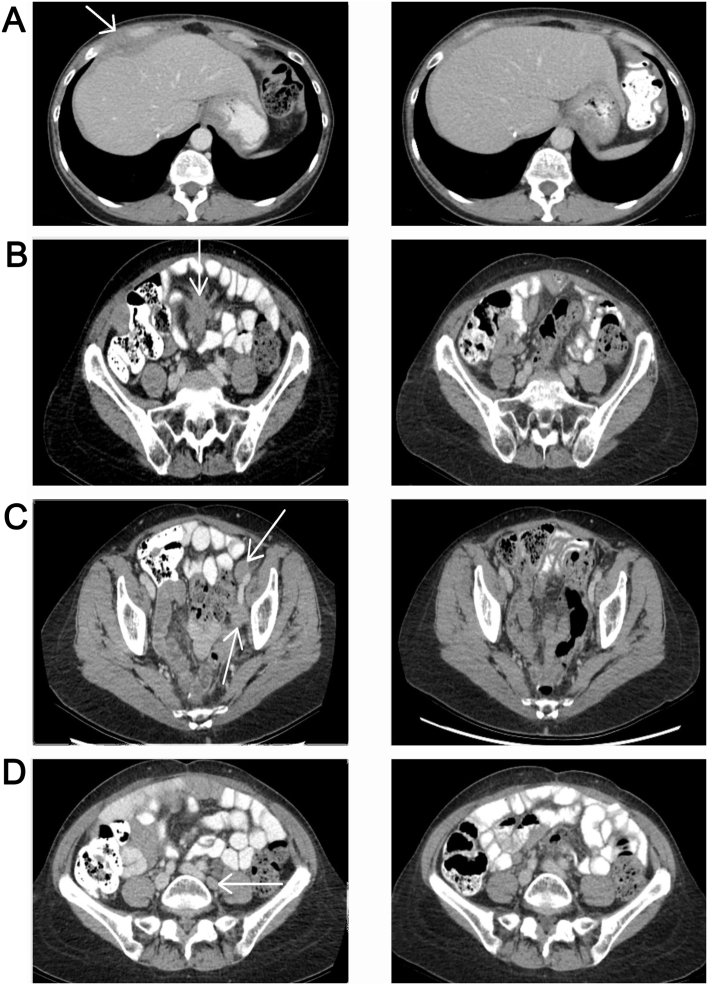
At that time, treatment with PARP inhibitors only occurred in clinical trials. Independent revision of microscopic pathology confirmed the high-grade papillary serous adenocarcinoma. The patient was invited to participate in a trial with olaparib but waived this option. Molecular mapping by then revealed no BRCA1 and BRCA2 gene mutations. Considering the solitary reccurrence, radiotherapy was started. In July 2015, a CT scan revealed new lymph node metastases and a 4-cm mass ventral of and connected to the right liver lobe (Fig. 1; left side). Although the patient had previously responded well to platinum-based (alkylating) chemotherapy, at this stage, several courses of neurotoxic chemotherapy and the pre-existing diabetes had caused significant neuropathy and retinopathy, which diminished her vision. Treatment with bevacizumab was therefore not an option. Otherwise, the clinical condition was excellent. In the Netherlands, bevacizumab is indicated only in first line treatment, at first recurrence of platinum sensitive ovarian cancer in combination with carboplatin and paclitaxel, or at recurrent platinum resistant ovarian cancer. Therefore, treatment with bevacizumab was no option. In addition, bevacizumab is contraindicated in this patient because of preexistent neuropathy. In July 2015, treatment with continuous low-dose oral cyclophosphamide (100 mg daily) was started and resulted in a complete remission of tumour load within 3 months. In May 2017, 22 months after the start of this treatment, the patient was still without any signs of cancer activity: CT-scan in January 2016 was negative (Fig. 1; right side), ultrasound in March 2017 was negative and tumour marker CA-125 had normalised (Fig. 2 clarifies the dose and cycles of chemotherapy and other interventions). Thus far, reports of cyclophosphamide-related toxicity have been limited to grade 1 fatigue, without signs of myelosuppression or physical discomfort. Treatment was interrupted once for several weeks, but this was not clinically relevant. Lately, several dose reductions were applied (50 mg p.o.) on the patient's request, partly due to her general aversion to drugs, as well as the drug-related toxicity symptoms (grade 1 fatigue). The patient is to proceed to treatment with cyclophosphamide until progressive disease or unacceptable toxicity.
Figure 1.
CT abdomen before (left) and after (right) starting treatment with metronomic cyclophosphamide. Left, CT scan of the abdomen (July, 2015) revealing (A) a mass ventral of and connected to the right liver-lobe and (B) central in the mesenterium, (C) new lymph node metastases left of the inguinal arteria and (D) left para-illiacal. Right, CT scan of the abdomen (in January 2016) with disappearance of the masses.
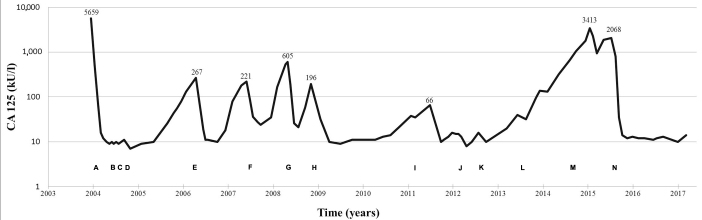
Figure 2.
Treatment strategies in time and levels of CA125 as a marker of response. (A) Debulking procedure (bilateral ovarectomy, hysterectomy, omentectomy and resection of rectosigmoid). (B) AGO-OVAR study: Paclitaxel (135 mg/m2 d1), carboplatin (AUC 5 d1), gemcitabine (800 mg/m2 d1&8): q 3 weeks ×6. (C) Second look debulking of residual masses. (D) AGO-OVAR study: paclitaxel (135 mg/m2 d1), carboplatin (AUC 5 d1), gemcitabine (800 mg/m2 d1&8): q 3 weeks ×3. (E) Paclitaxel (80 mg/m2), carboplatin (AUC 2): q weeks ×4. (F) Liposomal doxorubicin (50 mg/m2 d1): q 4 weeks ×6. (G) Paclitaxel (80 mg/m2 d1-8-15) and carboplatin (AUC 2): q 4 weeks ×2. (H) Resection vaginal recurrence followed by radiotherapy (20 Gy). (I) Radiofrequency ablation solitary liver metastasis. (J) Cisplatine (70 mg/m2): q 2 weeks ×4, followed by etoposide (50 mg p.o daily, 4 months). (k) Radiotherapy abdominal scar (20 Gy). (L) Resection recurrence in abdominal scar. (M) Letrozol (2.5 mg daily). (N) Cyclophosphamide (100 mg p.o. daily).
Discussion
This case study presents a patient with metastasized HGSOC who underwent different types of treatment modalities, including several episodes of platinum-based chemotherapy, and who after 13 years went in a complete remission on low-dose and continuous oral cyclophosphamide alone. Cyclophosphamide, a mustard-alkylating agent, is one of the most important, the eldest and the most effective alkylating cytostatic (5). Metronomic treatment with cyclophosphamide consists of frequent, usually daily, administration of a low (oral) dose of cyclophosphamide without prolonged drug-free breaks. The efficacy of metronomic cyclophosphamide can be explained by an anti-angiogenic effect that continuously inhibits the endothelial cells that attempts to restore the vascular status of the tumour. Furthermore, previous studies described a direct effect on the induction of tumour stem cell differentiation, which can cause a selective reduction in circulating regulatory T cells. This reduction also leads to a suppression of conventional T cells and natural killer cells, with restoration of peripheral T-cell proliferation and innate killing activities (6–8). As the dose in metronomic treatment is far below the maximum tolerated dose (MTD), it causes fewer side effects. This strategy can benefit patients suffering from types of cancer known to be sensitive to alkylating agents, such as lymphomas, breast cancer and ovarian cancer (9).
In recent years, several publications have underlined the advantage of this regimen for patients with recurrent HGSOC (10–12). Moreover, Kummar et al demonstrated in a small randomized trial of oral cyclophosphamide with or without velaparib (a PARP inhibitor) that low-dose cyclophosphamide is associated with responses and prolonged disease stabilization in pre-treated HGSOCs (11). There was a 19,4% (7/36 patients) overall response in the cyclophosphamide-only arm. In this arm, four of the patients who responded had BRCA-mutant ovarian cancer (including the patient who had a CR) and two had HGSOC. Notably, the addition of velaparib to cyclophosphamide did not increase the response rate (overall response of 11,8%, 4/34 patients). Major restrictions of this study are the relatively small group of patients and a low dose of velaparib. The efficacy of olaparib, another PARP inhibitor, in ovarium cancer is undisputed (13). It would be interesting to compare the efficacy of metronomic cyclophosphamide and PARP inhibitors in this group of patients. Unfortunately, large randomized studies of metronomic cyclophosphamide versus PARP inhibitors are lacking.
Alternative treatment options for patients with HGSOC include liposomal doxorubicin, gemcitabine and topotecan. However, those do not contribute to a long-term remission and are associated with significant toxicities (14,15). More recently, additional compounds have become available. Firstly, the combination of bevacizumab (a monoclonal antibody against VEGF) with chemotherapy has been approved in the Netherlands (16). Secondly, PARP inhibitors are indicated for patients with a defect in the homologue recombinant (HR) DNA repair pathway, which is present in 51% of HGSOCs (17). These HR defects make HGSOC sensitive to alkylating chemotherapy, e.g., platinum compounds and cyclophosphamide, and other DNA-damaging agents, such as PARP inhibitors (18,19). Notably, a benefit of PARP inhibitors has also been reported in non-BRCA-mutant tumours, suggesting that the indication for these agents may be expanded (19). Nevertheless, PARP inhibitors have three main disadvantages. Firstly, recent studies demonstrated that HGSOCs become resistant to PARP inhibitors (11). Secondly, PARP inhibitors as well as bevacizumab combined with chemotherapy may cause significant side effects (15,16,19). By contrast, low-dose cyclophosphamide is well tolerated (20,21). The main known toxicities, such as cytopenia, haemorrhagic cystitis or cardiac toxicity, occur when using high-dose cyclophosphamide. Low-dose cyclophosphamide, however, may cause some degree of leukopenia (7). Lastly, the costs of oral metronomic cyclophosphamide treatment are significantly lower than the costs of administering intravenous cytotoxic drugs or novel treatments such as PARP inhibitors. In this context, it should be noted that in most countries treatment strategies for HGSOCs do no longer routinely include cyclophosphamide (intravenous or oral).
The main limitation of a case report is that its findings cannot be generalized. However, exceptional responders may provide insights into the contribution of factors that are important for therapy response and long-term survival. We here presented an exceptional responder and described the advantages of metronomic cyclophosphamide. Further research should be undertaken to compare the efficacy, toxicity and cost- effectiveness of metronomic cyclophosphamide and newer agents such as PARP inhibitors in HGSOC ovarian cancer. Although the ovarian cancer in this case does not have BRCA1 or BRCA2 mutation, the genomic profile of this tumour shows BRCAness (on the basis of classifiers developed and validated in mamma carcinoma, as there are no classifiers developed and validated in ovarian cancer), class 4 pathogenic variation in TP53 and class 5 pathogenic variation in GNAS. There was no mutation in ATM. We are aware that NBS1, RAD51, BARD1, GAPDH and other damage response genes can also be tested. In retrospect, it would be interesting to examine these other damage response genes, as has been pointed out by Lee et al (22), particular as exposition of some of these genes is associated with aggressive parameters in epithelial ovarian cancers. However, these were not available during molecular tumor analysis at that time. Genomic profiling of tumours also is an interesting and possible important part of future possibilities to predict therapy response.
We here presented an exceptional responder: a patient with metastasized HGSOC, who underwent different types of treatment modalities, including several episodes of platinum-based chemotherapy. She eventually, after 13 years of disease, went in complete remission on low-dose and continuous oral cyclophosphamide alone. In our opinion, this case may serve as a reminder that old drugs can be used successfully even in the age of targeted therapy and molecularly designed drugs. Metronomic cyclophosphamide can be considered first instead of PARP inhibitors in patients with HGSOC who responded to platinum-based chemotherapy and cannot continue platinum-based chemotherapy because of toxicity, independent of BRCA mutation or BRCA-ness tumour profile. Metronomic cyclophosphamide has several advantages, which include effective reduction of tumour activity, oral administration, good tolerability and low cost. Further research should be undertaken to compare the efficacy, toxicity and cost- effectiveness of metronomic cyclophosphamide and newer agents, such as PARP inhibitors, in HGSOC ovarian cancer.
References
- 1.Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ, Bast RC, Jr, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15:668–679. doi: 10.1038/nrc4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu G, Yang D, Sun Y, Shmulevich I, Xue F, Sood AK, Zhang W. Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer. Pharmacogenomics. 2012;13:1523–1535. doi: 10.2217/pgs.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrandina G, Corrado G, Mscilini F, Malaguti P, Samaritani R, Distefano M, Masciullo V, Di Legge A, Saveresse A, Scambia G. Metronomic oral cyclophosphamide (MOC) in the salvage therapy of heavily treated recurrent ovarian cancer patients: A retrospective, multicenter study. BMC Cancer. 2014;14:947. doi: 10.1186/1471-2407-14-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Bois A, Belau A, Wagner U, Pfisterer J, Schmalfeldt B, Richter B, Staehle A, Jackisch C, Lueck HJ, Schroeder W, et al. A phase II study of paclitaxel, carboplatin, and gemcitabine in previously untreated patients with epithelial ovarian cancer FIGO stage IC-IV (AGO-OVAR protocol OVAR-8) Gynecol Oncol. 2005;96:444–451. doi: 10.1016/j.ygyno.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 7.Madondo MT, Quinn M, Plebanski M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer Treat Rev. 2016;42:3–9. doi: 10.1016/j.ctrv.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 8.André N, Carré M, Pasquier E. Metronomics: Towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11:413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 9.Scharovsky OG, Mainetti LE, Rozados VR. Metronomic chemotherapy: Changing the paradigm that more is better. Curr Oncol. 2009;16:7–15. doi: 10.3747/co.v16i2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samaritani R, Corrado G, Vizza E, Sbiroli C. Cyclophosphamide ‘metronomic’ chemotherapy for palliative treatment of a young patient with advanced epithelial ovarian cancer. BMC Cancer. 2007;7:65. doi: 10.1186/1471-2407-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kummar S, Oza AM, Fleming GF, Sullivan DM, Gandara DR, Naughton MJ, Villalona-Calero MA, Morgan RJ, Jr, Szabo PM, Youn A, et al. Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res. 2015;21:1574–1582. doi: 10.1158/1078-0432.CCR-14-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perroud HA, Scharovsky OG, Rozados VR, Alasino CM. Clinical response in patients with ovarian cancer treated with metronomic chemotherapy. Ecancermedicalscience. 2017;11:723. doi: 10.3332/ecancer.2017.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustign G, Scott C, Meier W, Shapira-Frommer R, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 14.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 15.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, Wagner U, Stähle A, Stuart G, Kimmig R, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 16.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MY, Yi J, Nycum LR. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network: Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bast RC, Jr, Mills GB. Personalizing therapy for ovarian cancer: BRCAness and beyond. J Clin Oncol. 2010;28:3545–3548. doi: 10.1200/JCO.2010.28.5791. [DOI] [PubMed] [Google Scholar]
- 19.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 20.El-Husseiny K, Motawei H, Ali MS. Continuous low-dose oral cyclophosphamide and methotrexate as maintenance therapy in patients with advanced ovarian carcinoma after complete clinical response to platinum and paclitaxel chemotherapy. Int J Gynecol Cancer. 2016;26:437–442. doi: 10.1097/IGC.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 21.Ferrandina G, Corrado G, Mascilini F, Malaguti P, Samaritani R, Distefano M, Masciullo V, Di Legge A, Saverese A, et al. Metronomic oral cyclophosphamide (MOC) in the salvage therapy of heavily treated recurrent ovarian cancer patients: a retrospective, multicenter study. BMC Cancer. 2014;14:947. doi: 10.1186/1471-2407-14-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YK, Park NH, Lee H. Clinicopathological values of NBS1 and DNA damage response genes in epithelial ovarian cancers. Exp Mol Med. 2015;47:e195. doi: 10.1038/emm.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]