SUMMARY
This Practical Guidance for Clinical Microbiology document on the laboratory diagnosis of parasites from the gastrointestinal tract provides practical information for the recovery and identification of relevant human parasites. The document is based on a comprehensive literature review and expert consensus on relevant diagnostic methods. However, it does not include didactic information on human parasite life cycles, organism morphology, clinical disease, pathogenesis, treatment, or epidemiology and prevention. As greater emphasis is placed on neglected tropical diseases, it becomes highly probable that patients with gastrointestinal parasitic infections will become more widely recognized in areas where parasites are endemic and not endemic. Generally, these methods are nonautomated and require extensive bench experience for accurate performance and interpretation.
KEYWORDS: methods, parasites, gastrointestinal, diagnosis, parasitology, gastrointestinal infection
INTRODUCTION
This Practical Guidance for Clinical Microbiology document is intended to provide readers with practical information relevant to general hospital clinical microbiology laboratories for the recovery and identification of parasites from the gastrointestinal tract. Although the document is not designed for reference or research laboratories, it is important for general clinical laboratories to be aware of all relevant procedures, even those for which specimens are submitted to a reference laboratory.
The document is the result of a comprehensive literature review and expert consensus relevant to the topics under discussion; it also supports the education and training of microbiologists in clinical laboratories. However, it is not intended to provide didactic training related to human parasite life cycles, organism morphology, clinical disease, pathogenesis, treatment, or epidemiology and prevention.
As the world continues to “shrink” in terms of exposure to infectious diseases, it becomes much more likely that patients with gastrointestinal parasitic infections will be seen in areas where parasites are not endemic and will continue to increase in number in areas where they are endemic. Most procedures performed in diagnostic parasitology require a great deal of judgment and interpretation and are classified by the Clinical Laboratory Improvement Amendments of 1988 (1) as high-complexity procedures. The majority of these procedures are not automated and require considerable practice to produce accurate, clinically relevant results.
We have had extensive actual bench experience and bring to this project our accumulated knowledge and awareness of the many requirements necessary for excellence within a clinical laboratory. Although it is important to realize that not every laboratory will perform each procedure in exactly the same way, it is very important to understand the pros and cons of clinical procedure modifications. Very specific protocols containing detailed method directions are available; however, this document is designed to provide a complete understanding of the diagnostic methods rather than step-by-step method descriptions (2–10).
MEDICAL ASPECTS OF GASTROINTESTINAL PARASITIC DISEASES
Clinical Manifestations of Parasitic Diseases
There are many different clinical manifestations of parasitic infections of the gastrointestinal tract. The presentations of these parasitoses vary depending on the infecting parasite, as well as on a variety of host factors that are incompletely understood. However, it is clear that the patients with severely compromised immune responses usually have more-severe disease. These patients are also at risk for infections by parasites that do not commonly cause disease in immunocompetent individuals (11). The duration of parasitosis and the load of parasites also affect the clinical manifestations of disease.
Enteritis, diarrhea, and dysentery.
Infections of the gastrointestinal tract in the form of gastroenteritis, enteritis, or enterocolitis are common for certain intestinal parasites, such as Giardia lamblia (Giardia duodenalis, Giardia intestinalis), Cryptosporidium parvum or Cryptosporidium hominis, and Entamoeba histolytica, among others.
These infections usually manifest with some degree of abdominal pain, bloating, and diarrhea. The diarrhea ranges from stools with a watery consistency to dysentery. The types of diarrhea and consistency of the stool vary by pathogen. For example, Giardia lamblia (G. duodenalis, G. intestinalis) is classically associated with abundant, foul-smelling, watery diarrhea, whereas the presence of a dysenteric stool suggests a pathogen such as E. histolytica or Balantidium coli (12, 13). Symptoms can vary with certain pathogens, however. E. histolytica, for example, may be present in the stools of asymptomatic individuals (i.e., cyst shedders), may cause watery diarrhea, or may produce dysentery and bloody stool (13).
Invasive disease.
There are some gastrointestinal parasitic pathogens that may cause invasive disease, whereas there are others that, even in profoundly immunocompromised individuals, are usually not associated with tissue invasion. Ascarid parasites from other hosts, such as anisakids, burrow into the mucosa, which causes severe localized abdominal pain (14). This condition is essentially a more localized form of visceral larva migrans, since the nematode is in a biologically inappropriate host and wanders. Less commonly, the worm may penetrate through the muscularis propria and adventitia of the stomach or small intestine, causing a perforation. The worms in such instances may be found free in the abdominal cavity or embedded in the omentum.
Nematodes that have an indirect life cycle are by their nature invasive when the larvae penetrate the intestinal tract on their transpulmonary passage back to the intestines. Clinical pulmonary manifestations of primary infection and migration (i.e., Löffler's pneumonia) are not usually evident unless there is a large primary infection. Strongyloides stercoralis, however, establishes a chronic infection, which includes parthenogenic production of larvae that recapitulate the transpulmonary migration. This chronic infection is also usually subclinical, unless the infected individual becomes immunocompromised, and this subclinical condition includes the diminished immune response that occurs during normal aging. Transplant recipients are at risk for severe clinical disease. Strongyloides hyperinfection syndrome is a disease wherein there is uncontrolled replication of these helminths (15). Hyperinfection results in a substantial number of migrating larvae through the lungs and other organs, which, even with aggressive therapy, may result in death.
Some of the protozoal parasites, such as E. histolytica and B. coli, may produce locally invasive disease, with the penetration of the organism into the submucosa, forming the classic “flask-shaped ulcer” in histologic sections of the colon (16). Local invasive disease caused by E. histolytica may be contained by the inflammatory response, forming the so-called ameboma. The amoebae may also be transported to the liver via the portal vasculature to form an amebic liver abscess. Less commonly, a fistula may form between the hepatic abscess and the diaphragm and create a connection to the right pleural space, producing an amoebic empyema.
Nutritional depletion.
Nutritional depletion is another untoward consequence of gastrointestinal parasitic infections. The depletion of water and electrolytes is a danger in individuals with severe diarrhea. Immunocompromised individuals with infections caused by Cryptosporidium, Cystoisospora, and Giardia lamblia (G. duodenalis, G. intestinalis), among others, may have protracted voluminous diarrhea that results in severe dehydration and systemic electrolyte imbalance, which can cause death in these patients (17).
Other intestinal parasites compete with the human host for the absorption of nutrients. The classic example is that of pernicious anemia caused by vitamin B12 deficiency that results from the absorption of this nutrient by the large fish tapeworm, Diphyllobothrium latum (18). In other instances, it may be the competition for a variety of nutrients in food, which manifests as malnutrition (19). The effects include stunted growth, wasting, hunger, and more-specific signs of micronutrient deficiency. Malnutrition is more likely to occur in individuals with large burdens of worms that consume a substantial amount of the nutrients that are digested. Unfortunately, the individuals with the largest worm burdens are often people, commonly children, in resource-poor countries who already lack access to the recommended daily intake of food.
Hookworms pose a particular problem, as these helminths attach to the mucosa and access by means of teeth or cutting plates the highly vascular lamina propria of the intestine. These parasites ingest human blood, which results in anemia. The greater the hookworm load, the greater the anemia (20). As noted above, the individuals most likely to have a greater hookworm load are also those who live in resource-limited settings and who likely also do not receive the recommended daily allowance of protein and other iron-containing substances in their diets. Iron deficiency is thought to be the most common nutritional deficiency worldwide. Individuals with iron deficiency anemia may develop pica, which contributes to the acquisition of other geohelminths. There are also concerning associations between iron deficiency anemia, impaired cognition and learning, and delayed behavioral and psychomotor development (20).
Mechanical obstruction.
The presence of worms in the gastrointestinal tract can result in a number of problems. Ascaris has been reported to, on occasion, block the biliary and pancreatic ducts (21, 22). Obstruction of the common bile duct may result in abdominal pain, vomiting, and biliary colic. Other laboratory findings in these patients include elevated bilirubin and liver enzymes. The blockage may allow for overgrowth of bacterial microbiota and cause pyogenic cholangitis. Similarly, if the helminth enters and blocks the pancreatic duct, then pancreatitis ensues. The pancreatitis ranges from mild to severe. The diagnosis of these types of obstructions is often accomplished using endoscopic retrograde cholangiopancreatography (ERCP).
The appendix is a blind-pouch vestigial appendage of the colon. Occasionally, nematodes may enter the appendix. The mechanical movement of the worm may damage the mucosa, or overgrowth of the intestinal microbiota may result in appendicitis. Enterobius vermicularis has been shown to cause helminth-associated appendicitis, as has Ascaris lumbricoides (23). Less commonly, Taenia and other helminths have been associated with this disorder (24).
Intussusception and obstruction of the intestinal lumen may also occur with helminthic infections (25, 26). Intussusception occurs when one portion of the intestine overlaps an adjacent portion during peristaltic motion. There are many causes for intussusception, and parasitic infection is one of the rarer causes. Intussusception has been associated with Anisakis infection (25). Veterinarians are well acquainted with this disorder, as it is more common in animals. Overwhelming infections, particularly with large nematodes like Ascaris lumbricoides, may also result in complete obstruction of the intestinal lumen, which must be addressed surgically (26).
Parasitic Infections Acquired Abroad and Parasite Endemicity in the United States
The prevalences of the different types of gastrointestinal parasitic infection that are encountered vary by locale. This variation is impacted largely by the mechanism of transmission, the number of parasitized individuals in the area, the adequacy of public health measures to handle human and animal waste, and the ability of public health measures to provide clean drinking water for inhabitants of the area (27, 28). The prevalence of many gastrointestinal parasitic infections is therefore great in resource-poor countries that have a high burden of disease and inadequate public health facilities to handle waste and provide clean drinking water (28).
The mechanism of transmission is important for predicting which types of parasites are likely to be encountered. For example, one expects to encounter patients infected with Enterobius vermicularis in both resource-rich and resource-poor countries given that the eggs are infectious soon after passage and child-to-child transmission is possible either directly or through fomites (i.e., it does not matter if the children sharing toys are in Manhattan or in sub-Saharan Africa). In contrast, one is far less likely to encounter hookworm infections in locales where shoes are common than in areas where the citizens are often barefooted.
The number of parasitized individuals in the community affects the likelihood of infection or reinfection due to the increased number of opportunities for infection. For example, a child with pica in an area of low endemicity is less likely to acquire an infection by a geohelminth, such as Trichuris or Ascaris, than a similar child in an area where parasites are highly endemic because they are more likely to encounter parasite eggs in the dirt. Additionally, the areas that have large numbers of infected individuals are often resource poor and unable to appropriately handle human waste, so contamination of the environment and subsequent infections become the norm.
Resource-rich countries that adequately handle human waste significantly diminish the likelihood that parasitic cysts and eggs that originate from a human source will contaminate the environment, the food supply, or the drinking water. These countries forbid the use of human waste as fertilizer. There are nonhuman sources of some gastrointestinal pathogens (e.g., Cryptosporidium oocysts derived from animals) that may contaminate the water supply (29). Therefore, it is imperative that in addition to wastewater treatment being employed, modern drinking water treatment be employed. Failures in the system responsible for clean drinking water, which we often take for granted, demonstrate how important these systems are in public health. An often-cited example is that of the drinking water sources in Milwaukee, WI, in 1993 that were contaminated with Cryptosporidium hominis from human sewage effluent that impacted the drinking water facility intake, rather than water runoff from bovine feces (30). This contamination overwhelmed the clean drinking water facility's ability to inactivate the oocysts of Cryptosporidium and caused the largest waterborne outbreak by this parasite in U.S. history.
When the factors described above are considered, the list of gastrointestinal pathogens that one may expect to find in a citizen of the United States or another resource-rich country is very different from those encountered in an infected individual from a resource-poor region of the world where parasites are highly endemic. Trends in immigration may bring patients from countries where parasites are endemic who present with otherwise-rare or -infrequent pathogens. Additionally, travel is easier than ever, and adventurous excursions can place individuals at risk for infections that are uncommon in their home locale. Thus, taking a thorough clinical history is key (see below).
Medical Education and Consultation Related to Human Parasitic Infections
The expansion of medical knowledge in the past decade is incredible. The medical profession has responded through increased specialization and subspecialization. In the past, a surgeon might specialize as an orthopedic surgeon, whereas now, it is common to find practices with individuals who specialize in only knee or hip disease. Therefore, it is unreasonable to expect individuals who are not subspecialty trained in microbiology or infectious diseases to keep abreast of changes in clinical microbiology, one of the fastest-paced fields.
A clinical parasitologist or clinical microbiologist with expertise in parasitology is perfectly positioned to help educate physicians and provide guidance in test selection. These laboratorians, whenever possible, should participate in educating the next generation of physicians, not just to teach them at that point in their career but also to inform them that highly trained laboratorians remain available to assist them as needed throughout their professional careers. Additionally, this group should participate, whenever possible, in medical technology training programs. This training should go beyond the basic training of specimen processing, testing, and results reporting and should include preparing the technologist for his/her role as an integral member of the health care delivery team. Practicing medical technologists are the “front lines” and can notify and work with the laboratory director when unsuspected findings are discovered or untoward events occur. This type of engagement translates into improved patient care.
A clinical microbiologist should work with the medical staff to formulate the test requisition forms, which are largely becoming solely electronic. They should work to aid clinicians in finding and using the most appropriate test for the clinical scenario encountered (see below). They should play an active role in monitoring test utilization and use instances of inappropriate utilization as opportunities for education.
Importance of a Complete Patient History (Physician and Diagnostic Laboratory)
The importance of location in determining the type of parasite that the patient may have acquired has been noted above and should be disclosed as part of taking a thorough history. It remains remarkable after many years of practice and attendance in infectious diseases/microbiology teaching conferences how often the clues to the definitive diagnosis were present in the clinical history or, unfortunately, should have been present had a thorough clinical history been taken.
The clinical history is designed to discover epidemiologic risk factors that are important for guiding testing. In addition to general aspects of a history assessment, specific questions concerning past medical history, countries of previous residence, travel, outdoor activities, family, food, and drinking water should be addressed. Specific examples of the importance of each of these follow.
Where a patient lives or has lived is important for an assessment of the risk of having acquired parasites in the patient's native country that are not endemic in their current country (Cyclospora cases have been reported from the United States, Canada, and the United Kingdom). It is very common to find evidence of multiple gastrointestinal parasites in the stools of children who have been adopted from a resource-poor country where parasites are highly endemic into a low-prevalence, resource-rich country (31). If the history did not include the location of prior citizenship, then an ova and parasite examination (O&P) of the stool may not have been performed, as the individual may have been asymptomatic. Note that, technically, parasite eggs rather than ova are examined; however, the abbreviation O&P remains conventionally accepted. Although there are no accepted general guidelines in these cases, routine parasitology examinations (ova and parasites) may be an appropriate option. Travel history similarly discloses potential risks to the traveler (Cyclospora, Mexico), as does questioning about specific outdoor activities (Cryptosporidium, swimming in late summer, contact with calves). A history of backpacking and drinking stream water is classically associated with giardiasis, for example. Family history and past medical history are important to disclose inherited genetic diseases or other conditions that may put patients at increased risk for certain parasitic diseases. For example, individuals with common variable immunodeficiency are at increased risk for Giardia lamblia (G. duodenalis, G. intestinalis) infections, which tend to be severe (32). Food and drink histories are among the most important, since many parasitic infections are acquired through ingestion. For example, the discovery that a patient is a bear hunter would make one consider trichinellosis, when in the absence of a classical presentation it might otherwise not be considered (33).
Laboratory Test Menus and Trained Microbiologists
Laboratory type.
It should be recognized that not all laboratories offer the same types of services. Physician office-based laboratories may offer no parasitology but should have clear guidelines regarding the best test to perform for each clinical scenario and the materials for appropriate specimen collection and shipping. The smallest of these laboratories are usually able to perform moderately complex tests and, if the test volume is sufficient, may consider offering one of the easy-to-use, single-use, lateral-flow enzyme immunoassays (EIAs) for commonly encountered parasites, such as Giardia lamblia (G. duodenalis, G. intestinalis).
Small hospital laboratories may offer limited parasitology, the degree of which should be determined by test volume and technologist competency. It is important to critically assess competency for very low-volume tests, as it is difficult to remain proficient if testing is not commonly performed. In instances of low-volume testing, it may be better for the patient if the test is referred to a reference laboratory where expertise is maintained. Small hospital laboratories should be able to perform EIAs and pinworm prep examinations, although these are no longer common test requests. The performance of a full ova and parasite examination should be based on skill and competency. Many of the newer multiplex molecular diagnostic assays for gastrointestinal pathogens include some parasite pathogens, such as Giardia lamblia (G. duodenalis, G. intestinalis), Cryptosporidium, and E. histolytica (34). These are moderately or highly complex tests and represent options for expanded testing in small laboratories that may lack parasitology expertise.
Large hospital laboratories and reference laboratories should offer full parasitology services. These include enzyme immunoassays, full ova and parasite examination, modified acid-fast staining for Cryptosporidium, Cyclospora, and Cystoisospora, modified trichrome staining for microsporidian species, and the ability to identify adult helminths that may be passed in the stool. These laboratories will often also offer advanced molecular diagnostics for parasites. These tests may include FDA-approved multiplex assays for a variety of gastrointestinal pathogens or laboratory-developed tests for specific agents (e.g., PCR for Microsporidia). Thorough competency assessments are necessary, and participation in challenging parasite proficiency testing should be ongoing.
Test menu complexity.
The test menu should include the options available for each clinical scenario that are most commonly encountered. These options are many, and it is likely that a busy clinician may not always review these options thoroughly, which might lead to inappropriate test orders. Inappropriate orders have several adverse consequences. Foremost among these is the negative impact on patient care. If the clinician selects the wrong test, the diagnosis may be missed. If the wrong selection is discovered and the correct test is eventually performed, then the diagnosis is delayed and there is waste in performing the initial incorrect test. There have been instances where ordering personnel have checked all selection boxes based on the notion that “more is better,” and they will sort out the results later. Ordering many tests is a poor and wasteful practice that should be discouraged. Not only is this costly, but tests that are performed for which there is not a good pretest likelihood (i.e., the patient likely does not have the disease) are more likely to have a falsely positive result, which may result in additional and unnecessary work-up and further testing.
Therefore, it is important that test menus are designed to help guide clinicians to the most appropriate test, which can be done by highlighting salient aspects or best uses of a test after the test name listing and/or in the test directory. Examples of recommendations associated with particular tests are provided (Table 1).
TABLE 1.
Examples of use recommendations for select tests
| Test name | Use recommendation(s) |
|---|---|
| Giardia/Cryptosporidium enzyme immunoassay | Use when Giardia infection is most likely (e.g., to test for infectious diarrhea in a patient without a travel history). |
| Use to detect Cryptosporidium, which is a pathogen in immunocompetent and immunocompromised patients. | |
| Ova and parasite examination | Use predominantly when the patient has visited an area where parasites other than Giardia are endemic. |
| Modified acid-fast stain | Use when Cryptosporidium, Cyclospora, or Cystoisospora is suspected based on exposure and immunologic status. |
| Modified trichrome stain | Use for the detection of microsporidiosis, which is primarily a disease of immunocompromised hosts. |
| Pin worm prep | Use to collect eggs from the perianal skin. Do not order an O&P for the diagnosis of enterobiasis. |
| Baermann, agar plate culture, or Harada-Mori | Use when a negative O&P result is obtained from a symptomatic immunocompromised patient for whom there is a high suspicion of Strongyloides infection. |
| Multiplex molecular panels | Assays for gastrointestinal pathogens include some parasite pathogens, such as Giardia lamblia (G. duodenalis, G. intestinalis), Cryptosporidium, and E. histolytica (34). These are moderately or highly complex tests and represent options for expanded testing in smaller laboratories that may lack parasitology expertise. |
Test Ordering Options, Monitoring, and Intervention: Patient Clinical Relevance
Tests in the clinical parasitology section, with the exception of rapid immunoassays, are manual, are time-consuming, and require personnel expertise. Therefore, to preserve limited resources, these tests should be ordered judiciously. As noted above and in Table 2, designing a user-friendly test menu that guides the clinician to the appropriate test is important but has become challenging since in some electronic medical record systems tests may simply be listed alphabetically, requiring the providers to “hunt and peck” to find the right test. Additionally, some tests may sound alike, without differences clearly delineated. Therefore, it is not surprising that providers may select an inappropriate test. Test menu design is an area that is often not given due consideration and is therefore responsible for many unnecessary orders. Some laboratories may elect to use a case history form to guide appropriate testing (Fig. 1).
TABLE 2.
Approaches to test ordering for stool parasitology
| Patient and/or situation | Test(s) ordereda | Follow-up test(s) ordered |
|---|---|---|
| Patient with diarrhea and AIDS or another cause of immune deficiency; potential waterborne outbreak (municipal/city water supply) | Cryptosporidium or Giardia/Cryptosporidium immunoassay | If immunoassays are negative and symptoms continue, special tests for microsporidia (modified trichrome stain) and other coccidia (modified acid-fast stain) and an O&P should be performed. |
| Patient with diarrhea nursery school, day care center, camper backpacker; patient with diarrhea and potential waterborne outbreak (in a resort setting); patient with diarrhea from areas where Giardia is the most common parasite found | Giardia or Giardia/Cryptosporidium immunoassay (perform testing on two stools before reporting the patient as negative) (particularly relevant for areas of the United States where Giardia is the most common organism found) | If immunoassays are negative and symptoms continue, special tests for microsporidia and other coccidia (see above) and an O&P should be performed. |
| Patient with diarrhea and relevant travel history outside the United States; patient with diarrhea who is a past or present resident of a developing country; patient in an area of the United States where parasites other than Giardia are found (large metropolitan areas like Los Angeles, CA, New York, NY, Boston, MA, Miami, FL, etc.) | O&P, Entamoeba histolytica/E. dispar immunoassay, immunoassay for confirmation of E. histolytica (various tests for Strongyloides may be relevant [even in the absence of eosinophilia], particularly if there is any history of pneumonia [migrating larvae in the lungs], sepsis, or meningitis [fecal bacteria carried by migrating larvae], including an agar culture plate [the most sensitive diagnostic approach for Strongyloides]) | The O&P is designed to detect and identify a broad range of parasites (amoebae, flagellates, ciliates, Cystoisospora belli, helminths); if exams are negative and symptoms continue, special tests for coccidia (fecal immunoassays, modified acid-fast stains, autofluorescence) and microsporidia (modified trichrome stains, calcofluor white stains) should be performed. Fluorescent stains are also options. |
| Patient with unexplained eosinophilia and possible diarrhea; if chronic, the patient may also have a history of respiratory problems (larval migration) and/or sepsis or meningitis (hyperinfection) | O&P (recommended, although the agar plate culture for Strongyloides stercoralis [more sensitive than the O&P] is also recommended, particularly if there is any history of pneumonia [migrating larvae in lungs], sepsis, or meningitis [fecal bacteria carried by migrating larvae]) | If tests are negative and symptoms continue, additional O&Ps and special tests for microsporidia (modified trichrome stains, calcofluor white stains, fluorescent stains) and other coccidia (modified acid-fast stains, autofluorescence, fluorescent stains) should be performed. Serology for Strongyloides may also be recommended. |
| Patient with diarrhea (from suspected foodborne outbreak) | Test for Cyclospora cayetanensis (modified acid-fast stain, autofluorescence, fluorescent stains) | If tests are negative and symptoms continue, special procedures for microsporidia and other coccidia and an O&P should be performed. |
Depending on the particular immunoassay kit used, tests for various single or multiple organisms may be included. Selection of a particular kit depends on many variables: clinical relevance, cost, ease of performance, training, personnel availability, number of test orders, training of physician clients, sensitivity, specificity, equipment, and time to result, etc. Very few laboratories handle this type of testing in exactly the same way. Many options are clinically relevant and acceptable for good patient care. It is critical that the laboratory report indicate specifically which organisms can be identified using the kit; a negative report should list the organisms relevant to that particular kit. It is important to remember that sensitivity and specificity data for all of these fecal immunoassay kits (fluorescent-antibody assay, enzyme immunoassay, cartridge formats) are comparable.
FIG 1.
Example of a case history form that can be used to guide clinician ordering for stool ova and parasite testing.
It is also useful to periodically monitor who in the practice is ordering which tests. Most of the electronic order entry systems have the ability to create “order sets” for providers. Order sets decrease the time needed to search for individual tests. Unfortunately, these sets are often wasteful and filled with more tests than are needed. Order sets are not frequently curated and kept current. By way of example, if a primary care physician who sees predominantly individuals from areas where parasites are not endemic had a standard O&P in their “infectious diarrhea” order set, then the more-labor-intensive O&Ps would be regularly ordered when they would likely have been satisfied with the Giardia lamblia (G. duodenalis, G. intestinalis) immunoassay (IA) screening option.
There are a number of interventions that can be used to decrease unnecessary orders. Tailoring the order set for the pathogens most likely to be encountered is one approach, while educating clinicians is another option. For example, a key take-home message is that a patient from an area of endemicity or an immunocompromised patient may warrant additional tests.
A number of interventions can be useful in averting unnecessary testing, which includes testing for parasites. One of these is to electronically block same-day duplicate orders, should they occur (35). Another is to electronically block orders for O&Ps and stool cultures for patients who have been hospitalized longer than 3 days. In both instances, the clinician can override the electronic blockade by contacting the laboratory (2–6, 10). These interventions have diminished the number of orders that have been placed without thoughtful consideration, while still allowing clinicians to order the test if they really believe that it is clinically necessary.
Compromised Patients
The number of patients with compromised immune systems in our health care system continues to increase, due to the greater longevity of transplant recipients, better virologic control in human immunodeficiency virus (HIV)-infected individuals, and the use of newer “biologic” immunomodulating agents for diseases such as Crohn's disease, rheumatoid arthritis, and psoriasis. These patients are at risk for more-severe disease caused by commonly encountered agents, as well as disease caused by pathogens less commonly encountered in an immunocompetent host. Therefore, when investigating the cause of a likely infectious diarrheal syndrome in an immunocompromised host, both common and less common agents should be considered (5, 36). Although a patient is immunocompromised, an epidemiologic exposure history is still important to obtain, as this may disclose the most likely pathogen. Providers may choose to begin their investigation with the Giardia/Cryptosporidium immunoassay, given the excellent sensitivity of this assay and the inclusion of both a common pathogen (i.e., Giardia) and an organism known to infect immunocompromised hosts (i.e., Cryptosporidium). If negative, a clinical history will suggest the likelihood of a positive finding in the standard O&P. Both the modified acid-fast stain and a standard O&P will disclose the presence of Cystoisospora, an important pathogen in immunocompromised hosts, as well as Cyclospora, a foodborne pathogen associated with travel and the consumption of imported produce. Finally, if the cause remains undetermined, one should consider an assessment of Microsporidia. Although Microsporidia are taxonomically now classified as fungi, most testing remains in the parasitology sections of the laboratory (5). If the morphological assessment for Microsporidia is negative and clinical suspicion remains high, then a PCR analysis should be considered because of its superior sensitivity.
FACTORS WHICH INFLUENCE DIAGNOSTIC TEST PERFORMANCE
Use of Standard Precautions
Clinical laboratories should follow the requirements related to standard precautions, which state that all patients and all laboratory specimens are potentially infectious and should be handled accordingly (37–40). “Standard precautions” replaces earlier terms such as “blood and body fluid precautions,” “universal precautions,” and “body substance precautions” found in Occupational Safety and Health Administration (OSHA) documents. While the OSHA documents place the emphasis on blood-borne pathogens, such as HIV and hepatitis B and C viruses, standard precautions recognize that all infectious agents and all other potentially infectious material, except sweat, pose a risk to the health care worker (37).
Methods include those used to minimize exposure to infectious agents, to shield the laboratory worker from infectious material through a set of engineering and work practice controls, and to use personal protective equipment (PPE). In situations where differentiation between body fluid types is difficult or impossible, all body fluids shall be considered potentially infectious.
Also, the OSHA regulations require that employers provide hepatitis B vaccination and postexposure evaluation and follow-up, communicate the hazards to employees, and maintain appropriate records (40). Employees who decline immunization against hepatitis B virus are required to sign a hepatitis B vaccine declination form.
Equipment
Microscope for general use.
Good-quality microscopes and light sources are required for the examination of specimens for parasites. Organism detection and identification depend on morphological criteria, most of which must be seen under stereoscopic microscopes (low-magnification objectives) or regular microscopes equipped with low (10×), high dry (40×), and oil immersion (100×) objectives. Some laboratories recommend using a 50× or 60× oil immersion objective for scanning; this approach can be very beneficial, particularly if the 50× oil and 100× oil immersion objectives are placed side by side. The 40× high dry objective should be placed in the revolving nosepiece so that it is not next to any oil immersion objective; this helps prevent contaminating the 40× objective lens with oil, which can ruin the lens. Calibration of the microscope is essential; excellent references are available for training in this method (2–7). Although 5× oculars are acceptable, most laboratories select 10× oculars, preferably with a binocular adjustable tilting head. This flexibility allows the microscope to be used by numerous individuals. Thus, a selection of the following objectives will provide excellent options for the examination of specimens for parasites: 10×, 40×, 50/60× oil immersion, and 100× oil immersion. Some also equip the microscope with a 20× objective. All microscopes should be covered when not in use; this will help keep the instrument clean. Instrument calibration should be performed and documented yearly or more often if the instrument receives heavy use or is moved frequently. It is also mandatory that the lens of any oil immersion objective be cleaned with lens paper after each use. Use several layers and very little pressure to prevent removal of the coatings on external surfaces of the lens. Use new lens paper each use, and do not use laboratory wipes and/or tissues for this purpose, which may scratch the lens.
Centrifuge.
Overall, the centrifuge size and configuration depend on the method being used. Either a table or floor model centrifuge is acceptable. Generally, the centrifuge should hold 15-ml and/or 50-ml centrifuge tubes; this flexibility is recommended if commercial fecal concentration systems are used. A free-swinging or horizontal head is recommended. When routine centrifugation or a fecal concentration is performed, the sediment is deposited evenly on the bottom of the tube. Also, if the sediment surface is flat and the tube cannot be turned upside down (which will depend on the viscosity of the sediment), this configuration allows easy removal of the supernatant fluid from the sediment. Many laboratories currently use carrier cups with sealed closures; this feature, in addition to capped centrifuge tubes, will minimize any possible aerosol formation. It is generally recommended that the speed be checked and documented every 6 months or on a yearly basis.
Fume hood.
Chemical fume hoods are recommended when there is risk of exposure to hazardous fumes or splashes while chemical solutions are being prepared or dispensed. Airflow is generally controlled by a movable sash and should be in the range of 80 to 120 ft/min (1 ft = 30.48 cm). Chemical fume hoods are certified and documented annually. Although a fume hood is not required for diagnostic parasitology work, some laboratories keep the staining setup (trichrome and/or iron-hematoxylin) and formalin in a fume hood. Some laboratories also use fume hoods to reduce the odors found when fecal specimens are tested. Anything placed in the fume hood for storage (reagents, supplies, equipment) must not interfere with the proper airflow.
BSC.
A class II-A1 or II-A2 biological safety cabinet (BSC) is recommended for routine clinical laboratories. BSCs operate at a negative air pressure, air passes through a HEPA filter, and this vertical airflow acts as a protective barrier between the cabinet and the user. Although a BSC is not required for processing routine specimens in a diagnostic parasitology laboratory, some laboratories use class I (open-face) or class II (laminar-flow) BSCs for processing all unpreserved specimens (40). Use of a biological safety cabinet is recommended if the laboratory performs cultures for parasite isolation. However, remember that BSCs should not be used as fume hoods. Toxic, radioactive, or flammable vapors or gases are not removed by HEPA filters. When installed, have a class II BSC certified to meet standard 49 of the National Sanitation Foundation, Ann Arbor, MI (41). The cabinet must also be recertified at least annually and/or when it is moved, after filters are replaced, when the exhaust motor is repaired or replaced, and when any gaskets are removed or replaced. Record the date of recertification, the names of the individual and company performing the service, and any recommendations for future service.
Refrigerator-freezer.
Any general-purpose laboratory (non-explosion-proof) or household-type refrigerator-freezer (2 to 8°C) can be used in a parasitology laboratory. Even in a laboratory using explosion-proof refrigerators, solvents with flash points below refrigeration temperature should not be stored in these refrigerators. The temperature should be monitored and recorded on a daily basis; the same approach applies to the freezer. A proper seal on the gasket should be confirmed monthly, the condenser cleaned semiannually, and the refrigerator-freezer interior thoroughly cleaned annually.
Supplies.
Unless specific instrumentation and/or automated systems are in use, supplies for a diagnostic parasitology laboratory are often identical to those needed for routine testing throughout microbiology. Routine supplies include glassware (examples, graduated cylinders, flasks, bottles, funnels, centrifuge tubes, slides and coverslips, and pipettes). Depending on the relevant procedures, sterile glassware may be required as well. Other supplies might include gauze, culture tube racks, applicator sticks, storage boxes, filter paper, lens paper, forceps/scissors, micrometers (stage and disk), and appropriate biohazard containers. Although most clinical laboratories do not offer parasite cultures, if these are available, ATCC control organisms may be required.
Laboratory Technical Capabilities, Training, and Experience
Unfortunately, recruitment and retention of qualified individuals are major problems for most clinical laboratories throughout the United States and many other countries (42–45). The closure of training programs for medical laboratory scientists (MLS) and medical laboratory technicians (MLT) has contributed to this shortage; many of these closures were related to financial constraints. Between 1983 and 2009, approximately 64% of MLS programs were closed (42, 46). The number of MLS candidates passing the American Society of Clinical Pathology (ASCP) certification exam decreased from 6,000 in 1983 to a low of 1,892 in 2005 (http://www.ascp.org/certification). In spite of extensive recruitment from high school through college and the opening/reopening of training programs, there remains a large shortage of trained laboratory personnel. Most training programs are designed with two different segments, the first of which includes didactic training in various diagnostic disciplines and the second of which includes hands-on clinical training. Often, due to existing personnel shortages, the clinical laboratory training sites can no longer support the extensive teaching required for the clinical rotation portion of the training program.
Recognition of artifacts versus parasites.
In the developed world, where most parasites are not endemic, the detection and identification of helminths and protozoans can be challenging; however, the expertise in developing countries may be enhanced due to the common occurrence of parasite infections and a high positivity rate. Artifacts found in fecal specimens can closely resemble parasites, especially to an inexperienced microscopist. Several resources are essential in aiding in the identification. These include a calibrated ocular micrometer to determine the size range of the structure. Other necessary tools include publications that provide excellent images of parasites and artifacts, as well as tables with sizes and characteristics of various parasitic forms found in specimens (47–49).
Excellent websites where there are descriptions of parasites and their life cycles, galleries of parasite and artifact images, and a scientific question resource are available. These include www.CDC.gov/dpdx, which is operated by the Centers for Disease Control and Prevention in Atlanta, GA. Another excellent website is www.phsource.us, which was developed for the U.S. Air Force. The Atlas of Human Intestinal Protozoa (www.atlas-protozoa.com) provides excellent images. A virtual parasitology microscopy site, www.parasite-diagnosis.ch/home, is excellent and a good precursor to actual microscopy training. Another site is that of Medical Chemical Corporation (Para-Site), which contains diagnostic, morphological, and clinical tables, most frequently asked questions in diagnostic medical parasitology, and other educational information, including extensive case histories. There are also websites in Canada, including www.provlab.ab.ca in Alberta. All sites were accessed on 5 January 2016.
There are very specific details to study to determine whether a structure is a helminth egg or artifact. They include size, shape, color, presence of opercula, plugs, or spines, shell structure (thin or thick with mammillations, striations, pitting, other markings, shoulders, etc.) and internal features (hooklets, fibrils, yolk cells, miracidia, or other larvae) (5, 47, 48).
The same types of criteria are used for protozoans depending on their stage. Protozoan trophozoite characteristics include size, shape, nucleus (relative size, shape, position, karyosome, perikaryosomal space, peripheral chromatin, chromatin granules), the cytoplasm (amount of debris, pale/dark staining, vacuoles, food vacuoles), and other characteristics (axostyle, axonemes, cytostome, cilia, flagella) (5, 47, 48). Protozoan cyst characteristics include size, shape, cyst wall thickness, nucleus (relative size, shape, number of karyosomes, peripheral chromatin), and internal features (chromatoidal bars, vacuoles, axostyles, axonemes, median bodies, cilia, flagella, refractile bodies) (5, 47, 48).
One of the major difficulties is distinguishing parasites from artifacts and pseudoparasites found in fecal specimens and other specimens from the gastrointestinal tract. These elements or components include food residue and undigested products (including pollen), digested products, epithelial cells, mucus, and other secretions from the digestive tract, leukocytes, erythrocytes, and microorganisms such as bacteria and yeasts. It is essential that the microscopist be aware of these elements and differentiate them from the true parasitic forms. With difficult clinical organism differentiation, the laboratory may want to send the smears to a reference laboratory, where experts may be able to differentiate the artifacts from the parasites. Also, microscopic images and appropriate measurements can be sent to the reference laboratory electronically for consultation. Table 3 demonstrates some artifacts that may be present in stool specimens (47–49).
TABLE 3.
Human and nonhuman elements seen in fecal specimens
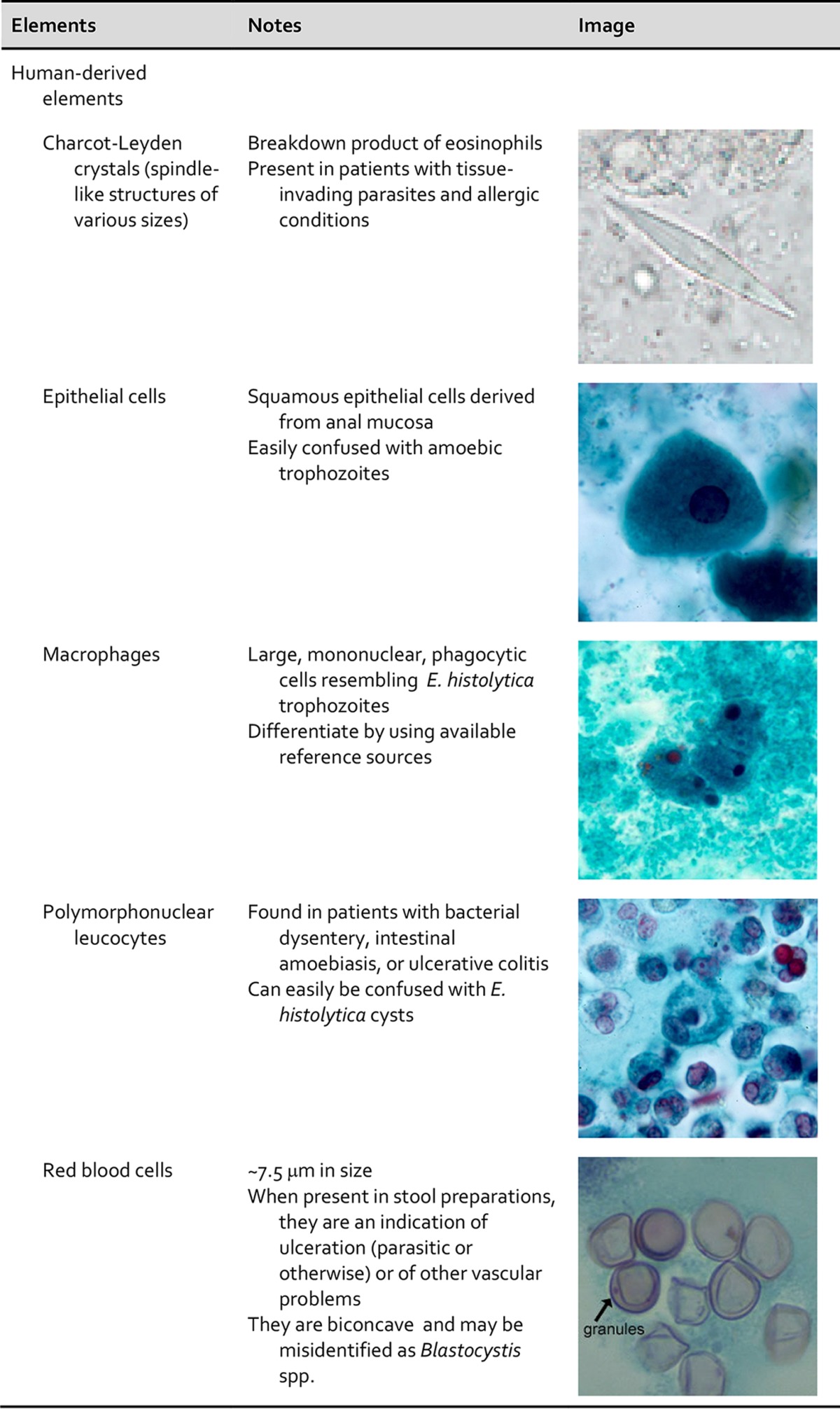
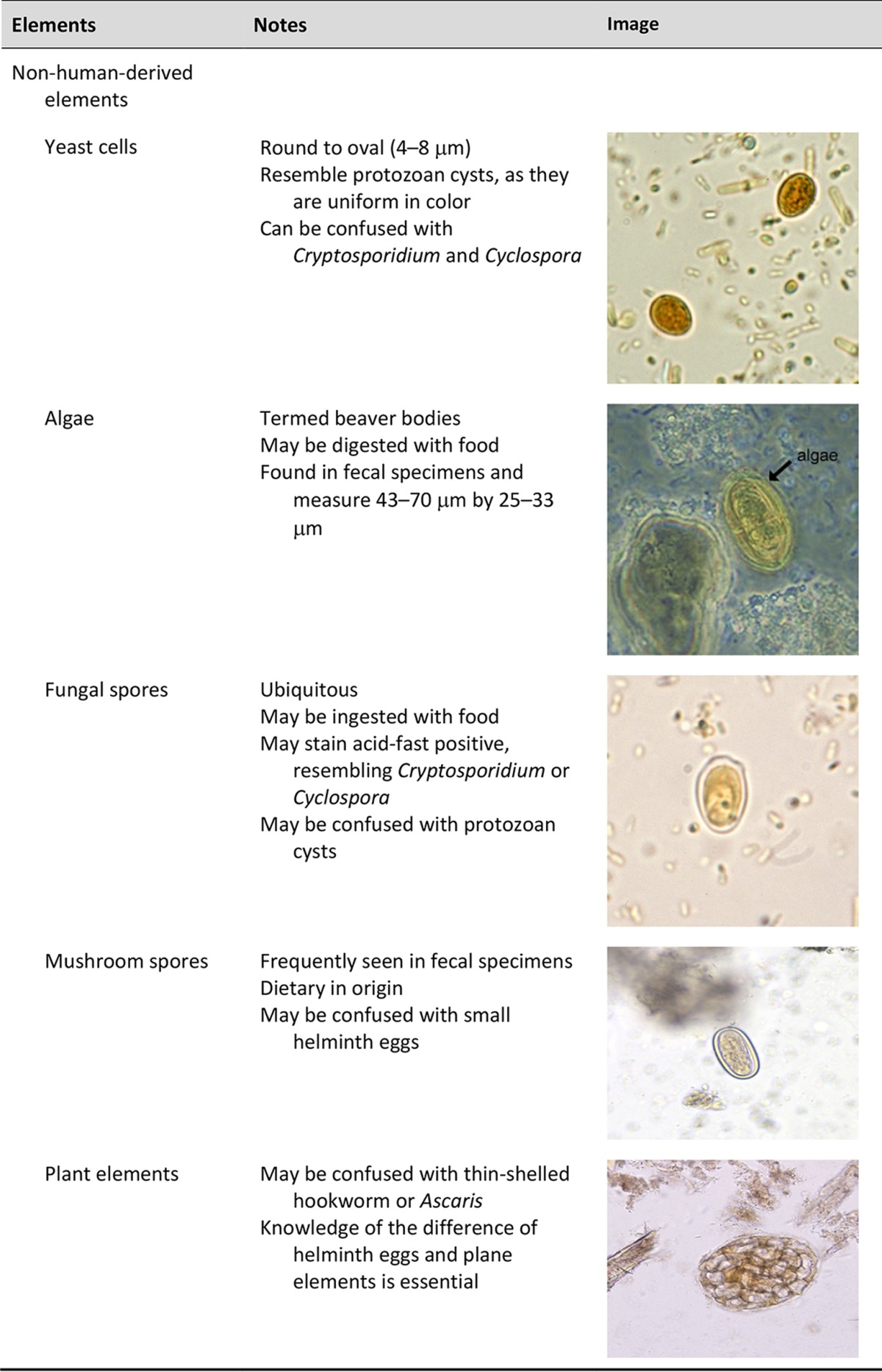
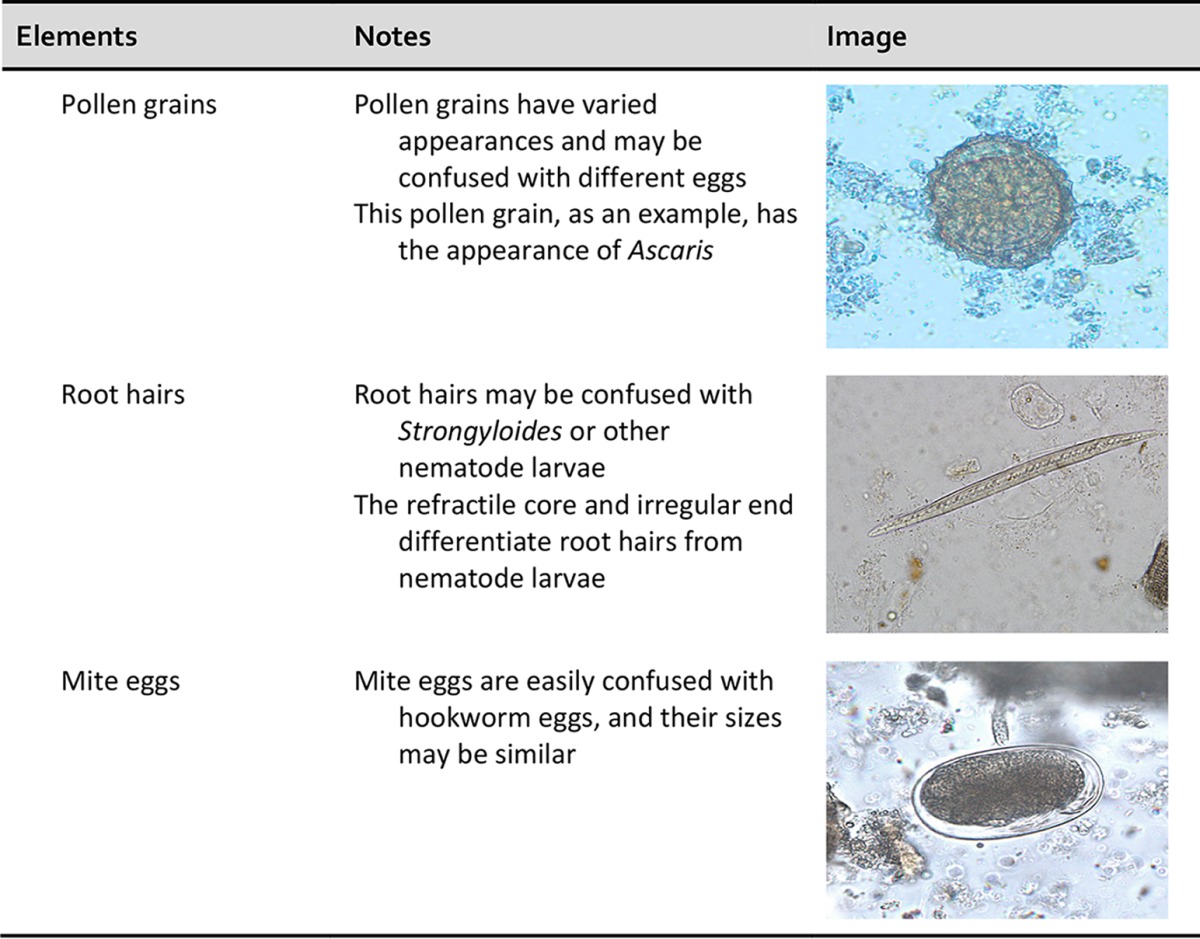
Importance of personnel knowledge of parasite life cycles.
In order to have a complete and thorough understanding of parasitology, it is imperative to have extensive knowledge of the life cycles of parasites infecting humans. The various parasite life cycles, including those of nematodes, cestodes, trematodes, and protozoa, may be simple or can be very complex. A complete patient travel and medical history, including dietary habits is necessary to provide clues for the investigation of parasites. Knowledge of parasitic life cycles should include the following.
- How parasitic infections are acquired. The routes of entry include the following.
- The ingestion of the infective stage.
- The ingestion of an intermediate host.
- The ingestion of a “transport vehicle” or host which contains the infective stage.
- The skin penetration of an infective stage.
The predilection site or the site where male and female parasites are found.
How a parasite reaches its final destination. The possible extension migration through the patient can cause other symptoms.
How the parasite leaves its definitive host to return to the environment.
The two life cycle stages: the infective stage entering the host and the diagnostic stage leaving the host.
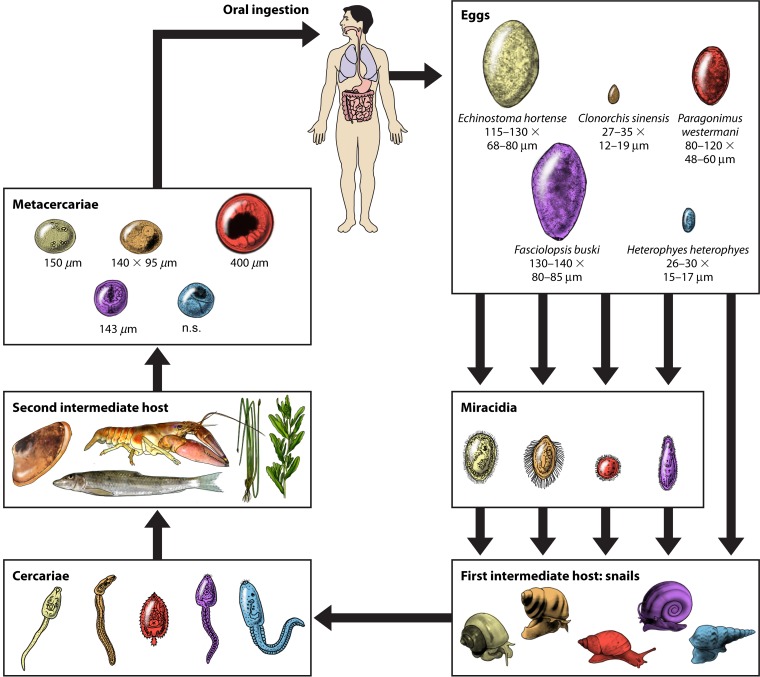
Protozoan life cycles have a sexual reproduction and/or an asexual reproduction phase. Organisms such as Cryptosporidium have a complex life cycle which corresponds to its pathogenicity. Helminthic life cycles may involve adult male and females with sexual reproduction, or the parasites may be hermaphroditic. Figure 2 illustrates the life cycle of trematodes to provide an example of the possible complexity. Life cycles of the various parasites are available on the www.CDC.gov/dpdx website complete with detailed descriptions to help understand the complexity of the individual life cycles.
FIG 2.
General life cycle of the trematodes. n.s., not shown. (Republished from reference 275 with permission.)
Parasitic forms in gastrointestinal tract specimens.
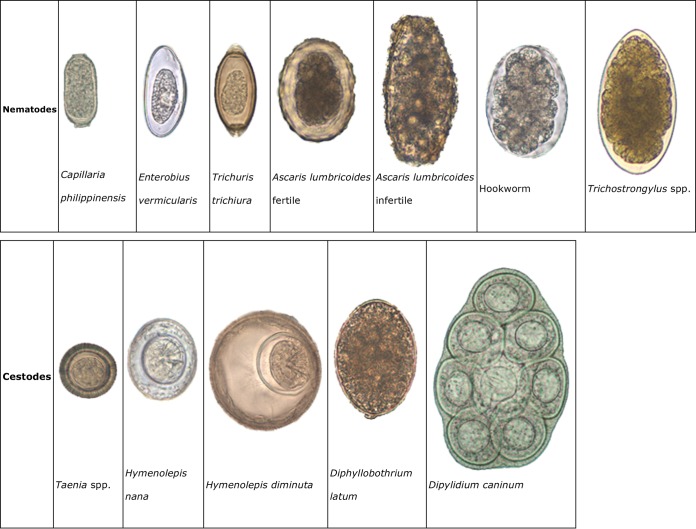
Human parasitic infections caused by intestinal helminths and protozoans are the most prevalent infections in developing countries. There are several different species of intestinal protozoans, pathogenic and nonpathogenic, that have similar characteristics, so accurate identification can be difficult because of the tiny differences. The protozoans are grouped according to their locomotor organelles. The largest group is the amoebae, and these organisms move with pseudopodia in the trophozoite form. There are specific criteria that are used to identify the trophozoite and cyst forms of amoebae. Because of the minute details in structure required to identify amoebae, flagellates, ciliates, apicomplexa, microsporidia, and helminths, numerous tables are available to assist users with the correct identifications of relevant parasites (see Appendix 1, Tables A1 to A4).
Communication between Clinicians and Laboratory Staff
Diagnostic decision making in clinical medicine is very dependent on clinical laboratory testing. The number, type, complexity, and cost of parasitology tests are growing, possibly creating more confusion regarding which tests are most appropriate at a time when emphasis is on reducing health care costs. This has led to scrutinizing the overordering of tests and ordering tests for the wrong purpose. Thus, it is important that physicians and laboratory staff communicate with each other to understand which laboratory tests are necessary and appropriate for diagnosis and treatment. In order for clinicians to maximize the expertise and resources of the laboratory staff, clinicians must provide accurate and relevant information, including the following. Additional information can be found in Fig. 1.
A complete recent and past travel history (not always available).
Relevant symptoms.
Medication which could suppress or alter parasite morphology.
The immune status, which may guide the investigation of opportunistic parasites.
Clinicians may not have the in-depth knowledge that is necessary to choose the appropriate parasitology test. There are several approaches that a laboratory can provide to facilitate selecting the appropriate laboratory test to establish a diagnosis. One very useful method includes medical algorithms. They are logical and sequential and can be automated and incorporated into software programs, as designed by medical microbiologists. Educating clinicians can be accomplished in a number of ways.
Providing laboratory test clarifications to clinicians (based on physician submitters in lab information systems) for relevant and timely testing information. This approach can reach the physicians requesting the parasitology testing most frequently.
Providing test comments with computerized reports regarding the appropriate use of tests or possible improvement to specimen collection, procedures, testing protocols to maximize diagnostic results.
The use of test menu descriptions to provide clinicians information for guidance in the investigation of particular parasites. The website www.cdc.gov/dpdx provides useful information regarding diagnostic assistance, parasite antigen detection, and molecular diagnosis.
Importance of Computer and Test Result Comments
Computer and test result comments can serve as excellent teaching aids for clinicians, many of whom may have little-to-no training in medical parasitology. When using these comments, it is recommended that clinicians use “canned” consistent comments (a template) rather than entering free-text comments. Specific examples can be seen in Appendix 2 (see Tables A5 and A6).
Training Clinicians Regarding the Diagnosis of Gastrointestinal Tract Parasitic Infections
As travel has become more accessible, the rate of tropical infections across the world is expected to increase; more health care professionals both at home and abroad are going to encounter these diseases more often. Disorders of parasitic etiology will play an important and expanding role in all aspects of medicine. Often, pathology personnel may be involved in training various groups of physicians, residents, and/or fellows regarding clinical aspects of medical parasitology diagnosis. In many cases, these students have had very limited exposure to these gastrointestinal protozoan and helminth infections. Tables for training can be found in Appendix 3, while a list of general medical parasitology references can be found in Appendix 4.
ROUTINE STOOL SPECIMEN PARASITE EXAMINATION METHODS
A routine ova and parasite examination (O&P) usually includes laboratory procedures that are designed to detect organisms in clinical specimens by using macroscopic and microscopic characteristics rather than culture, biochemical tests, and physical growth characteristics. Detection of stool parasites relies mainly on identification using bright-field microscopy and skilled laboratory personnel. More recently, commercial kits have been developed for the detection of fecal antigens (7, 50).
Test Selection and Patient Preparation
The test that is to be requested depends on which infectious agent is suspected. It is very important that the ordering physician be aware of what procedures are performed with the O&P and that this test may not recover all parasites. Additional stains for Microsporidia and Cyclospora/Cystoisospora/Cryptosporidium may need to be ordered separately, and immunoassays for selected organisms may also not be included in the O&P.
Collection of stool for parasite examination should always be performed before barium is administered to a patient for radiologic exams. Stool specimens containing the opaque, chalky suspension are unacceptable, and intestinal protozoa may be masked for 5 to 10 days after ingestion of barium. Other substances, such as castor oil or mineral oil, bismuth, antibiotics, including antimalarial medication, and nonabsorbable antidiarrheal preparations, may interfere with parasite recovery, and collection should be postponed for 5 to 10 days after administration to allow clearance of these substances.
Specimen Collection, Processing, and Shipping
There are many stool collection methods available for specimens suspected of containing parasites. When collection methods are selected, a thorough understanding of the advantages and disadvantages of each must be reviewed. Unless the stool specimens are properly collected and processed, these infections may not be detected. Therefore, specimen rejection criteria have become very important for the best results.
Fecal specimens should be collected in clean, wide-mouth containers with tight-fitting lids. The specimen should not be contaminated with water or urine, which may contain elements that can be mistaken for fecal parasites. Stool specimens should be placed in leak-proof bags when being transported to the laboratory for analysis. If postal delivery services are used, any diagnostic specimen must be packed according to national or international regulations (e.g., labeling with UN code 3373, the three-container approach) for packaging and shipping of biological specimens. Specimens need to be labeled with the proper patient identifiers, including patient's name and identification number along with the time and date of specimen collection. The specimen must also be accompanied by a request form indicating which laboratory procedures should be performed. Any additional relevant information should be included with the specimen submission.
It is recommended that multiple stool samples be examined prior to ruling out a parasitic infection. Historically, three specimens collected on alternate days within a 10-day period should be examined; however, some may argue that one or two stool exams are adequate. Many organisms, particularly intestinal protozoa, do not appear in the stool in consistent numbers; concentrations of trophozoites and cysts may vary on a daily basis. Physicians should be aware that the probability of detecting clinically relevant parasites in a single specimen may be as low as 50 to 60% but is >95% if three samples are examined by O&P (3, 51, 52).
Risk Management Issues
All fresh specimens should be handled carefully, since each specimen represents a potential source of infectious material. Standard safety precautions, including the use of personal protective equipment, the use of a biological safety cabinet when working with infectious materials, and proper handling of chemicals, should be followed (7, 53–56). Material safety data sheets (MSDS) should be reviewed for all reagents used in the laboratory. It is also mandatory that appropriate policies to prevent eating, drinking, or smoking within the laboratory are in place.
Formaldehyde vapor monitoring must be performed to ensure that the exposure does not pose a risk to laboratory personnel. Initial monitoring should be performed and repeated any time that there is a change in the use of formalin, which may result in an increase in exposure.
Quality control should be performed on a regular basis and documented. The frequency of quality control assessments will depend on the test and the expertise of personnel. Microscope calibration should be performed on all instruments used to examine specimens by the O&P.
Fresh or Preserved Specimens
Fresh stool specimens are mandatory for the recovery of motile protozoan trophozoites (amoebae, flagellates, ciliates). The trophozoite stage is normally found in cases of diarrhea. Once trophozoites have passed out of the body, they do not encyst but disintegrate if not examined promptly or put into preservative. Most helminth eggs and larvae, Cyclospora/Cystoisospora/Cryptosporidium oocysts, and microsporidial spores survive for extended periods of time. In general, liquid stools should be examined within 30 min of passage (not 30 min after arriving in the laboratory). If this is not possible, the specimen should be placed in a preservative and then transported to the laboratory. Once in preservative, motility will be lost. Semiformed or soft stools should be examined within 1 h of passage and usually contain both cysts and trophozoites. Formed stools should be examined within 24 h after passage and contain mainly cysts. If these specimens cannot be examined within the suggested time frames, again, the specimen should be placed in preservatives.
If there are delays from the time of passage until examination in the laboratory, the use of fecal preservatives should be considered. To preserve protozoan morphology and to prevent continued development of various helminth eggs and larvae, the stool can be placed in preservative immediately after passage (by the patient or hospital staff). Once placed in the preservative, adequate mixing of the specimen is mandatory. To ensure the proper ratio of preservative to stool, commercial vials are marked with a “fill-to” line on the collection container.
There are many preservative options provided by commercial vendors. When selecting an appropriate preservative, make sure that it is compatible with all stains and test kits used in your parasitology laboratory. Formalin, sodium acetate-acetic acid-formalin (SAF), mercuric chloride polyvinyl alcohol (PVA), modified (nonmercury) PVA, and nonformalin, nonmercury, non-PVA preservatives are commercially available (5, 57–59) (Table 4). Disposal regulations for compounds containing formalin and mercury are becoming stricter, and disposal is a factor.
TABLE 4.
Fecal fixatives used in diagnostic parasitology, i.e., with intestinal tract specimens, and testing compatibility with the fixativef
| Fixative | Concentrate | Permanent-stain smear (trichrome, iron-hematoxylin, special stains for coccidia and Microsporidia) | Immunoassays for Giardia lamblia and Cryptosporidium spp. | Comment(s) |
|---|---|---|---|---|
| 5%, 10% formalin | Yes | No | Yes | Concentrations and IAs (EIA, FA, Rapids) |
| 5%, 10% buffered formalin | Yes | No | Yes | Concentrations and IAs (EIA, FA, Rapids) |
| MIF | Yes | Polychrome IV stain | ND | No published data |
| SAF | Yes | Iron-hematoxylin (best) | Yes | Concentrations, permanent stains, and IAs (EIA, FA, Rapids) |
| Schaudinn's (Hg base), no PVAa | Rare | Yes | No | Permanent stains; Hg interferes with IAs; primarily used with fresh stool specimens (no fixative collection vials) |
| Schaudinn's (Hg base) + PVAa | Rare | Yes | No | Permanent stains; Hg and PVA interfere with IAs; considered the gold standard fixative for permanent stains |
| Schaudinn's (Cu base) + PVAb | Rare | Yes | No | Permanent stains; PVA interferes with IAs; stains not as good as with Schaudinn's fixative using Hg or Zn |
| Schaudinn's (Zn base) + PVAc | Rare | Yes | No | Permanent stains; PVA interferes with IAs; this is the same fixative as Total-Fix without PVA (see below) |
| EcoFix (PVA)d | Rare | Yes | No | Permanent stains; PVA interferes with IAs; works best with EcoStain, Wheatley's trichrome (2nd best) |
| Universal-fixativee Total-Fix | Yes | Yes | Yes | No formalin, no mercury, no PVA; concentrations, permanent stains, special stains, fecal IAs, PCR |
These two fixatives use the mercuric chloride base in the Schaudinn's fixative; this formulation is still considered to be the gold standard against which all other fixatives are evaluated (organism morphology after permanent staining).
This modification uses a copper sulfate base rather than mercuric chloride. The morphology of stained organisms is not as good as with Hg or Zn.
This modification (proprietary formula) uses a zinc base rather than mercuric chloride and works well with both trichrome and iron-hematoxylin.
This fixative uses a combination of ingredients but is prepared from a proprietary formula (contains PVA).
This modification uses a combination of ingredients (including zinc) but is prepared from a proprietary formula. The aim is to provide a universal fixative that can be used with fecal concentrations and with permanent-stain smears and available immunoassays for Giardia lamblia, Cryptosporidium spp., and Entamoeba histolytica (or the Entamoeba histolytica/E. dispar group). However, currently, fecal immunoassays for the Entamoeba histolytica/E. dispar group or Entamoeba histolytica (true pathogen) require fresh or frozen specimens; testing can also be performed from stool submitted in Cary-Blair transport medium. It is important to remember that immunoassays are performed on direct samples from the fixed stool, not on the sediment after concentration methods have been used; otherwise, antigens, especially soluble antigens, may be diluted and reduce assay sensitivity. However, centrifuged material can enhance the sensitivity of the direct fluorescent assay procedure and permanent stains, since the actual organisms (Cryptosporidium, Giardia) are seen microscopically.
IA, immunoassay; Cu, copper; EIA, enzyme immunoassay; FA, fluorescent antibody; Hg, mercury; MIF, merthiolate-iodine-formalin fixative; ND, no data; PVA, polyvinyl alcohol; Rapids, cartridge format membrane flow IAs; SAF, sodium acetate-acetic acid-formalin; Zn, zinc.
Commentary.
The most common collection option (original public health approach) within the United States is a two-vial system: one vial of 5% or 10% formalin or buffered formalin and one vial of fixative containing the plastic adhesive PVA. The formalin vial is used for the concentration and fecal immunoassays, while the PVA vial is used for the permanent-stain smear. Regulations for formalin (see below) were originally developed for industry, not clinical laboratories, where amounts of formalin tend to be quite low. However, a laboratory using any amount of formalin must be monitored (see below).
Semiuniversal fixatives.
Examples of a semiuniversal fixative are as follows: SAF (no mercury or PVA [contains formalin]) and EcoFix (Meridian Bioscience, Inc., Cincinnati, OH) (no mercury or formalin [contains PVA]).
Universal fixative.
Currently, Total-Fix (Medical Chemical Corporation, Torrance, CA) contains no formalin, no PVA, and no mercury. Total-Fix can be used without the addition of PVA to the fixative (an adequate drying time for smears prior to staining is the most important step [a minimum of 1 h in a 37°C incubator; more time is required for thick fecal smears]). The use of very hot slide warmers or a hot plate is not recommended. Total-Fix can be used for the concentration of, permanent-stain smears for, and special stains for Cyclospora/Cystoisospora/Cryptosporidium or Microsporidia. Fecal immunoassays can be used for Giardia, Cryptosporidium, and many of the molecular procedures, while formalin fixatives and those containing mercury and PVA cannot be used as preservatives to be analyzed by PCR.
Formalin fixative.
Formalin has been used for many years as an all-purpose fixative that is appropriate for helminth eggs and larvae and for protozoan cysts, oocysts, and spores. Two concentrations are commonly used: 5%, which is recommended for preservation of protozoan cysts, and 10%, which is recommended for helminth eggs and larvae. Although 5% is often recommended for all-purpose use, most commercial manufacturers provide 10%, which is more likely to kill all helminth eggs. To help maintain organism morphology, the formalin can be buffered with sodium phosphate buffers, i.e., neutral formalin. Selection of specific formalin formulations is at the user's discretion. Aqueous formalin will permit the examination of the specimen as a wet mount only, a technique much less accurate than a permanent-stain smear for the identification of intestinal protozoa. However, fecal immunoassays for Giardia lamblia (G. duodenalis, G. intestinalis) and Cryptosporidium spp. can be performed from the aqueous-formalin vial. Current fecal immunoassays for the Entamoeba histolytica/Entamoeba dispar group and Entamoeba histolytica are limited to fresh or frozen fecal specimens or Cary-Blair transport medium. After centrifugation, special stains for Cyclospora/Cystoisospora/Cryptosporidium (modified acid-fast stains) and the Microsporidia (modified trichrome stains) can be performed from the concentrate sediment obtained from formalin-preserved stool material. Use of the sediment provides a more sensitive test.
Ova and Parasite Examination
The most commonly performed test in the parasitology laboratory is the complete O&P. It consists of a direct wet mount, concentration, and permanent-stain smear (2–5).
Macroscopic examination.
A macroscopic examination should be performed on every unpreserved specimen and should provide information on the age of the specimen and physical characteristics. All specimens (preserved or unpreserved) should be macroscopically viewed because worms may be seen in the stool specimen and retrieved for identification.
Microscopic examination (direct wet-mount preparation).
The direct wet smear is prepared by mixing a small amount of fresh, unpreserved stool with a few drops of 0.85% saline and then examining the suspension under a 22- by 22-mm coverslip for motile protozoan trophozoites. The entire coverslip is examined with the low-power (10×) objective and low light intensity using Kohler illumination. Suspicious objects can be examined at 40×. The trophozoites are very pale and transparent and observed for motility. Helminth eggs, protozoan cysts, and Cyclospora/Cystoisospora/Cryptosporidium oocysts may also be observed on wet film. A drop of iodine can be added to the edge of the coverslip for color contrast; however, motility will be lost. According to the College of American Pathologists (CAP) checklist, it is not necessary to perform direct wet-mount exams on specimens received in preservative. Motility will not be observed from preserved specimens.
Microscopic examination (concentration procedures).
Fecal concentration is a routine part of a complete O&P. It allows detection of small numbers of organisms that may be missed on a direct wet mount. There are two types of concentration procedures, sedimentation and flotation. Both are used to concentrate helminth eggs and larvae, protozoan cysts, Cyclospora/Cystoisospora/Cryptosporidium oocysts, and microsporidial spores. Commercial concentration devices are available, and these devices may help to ensure user consistency during the performance of the procedure.
The formalin-ethyl acetate (FEA) sedimentation concentration procedure recovers all parasites present by centrifugation into a fecal pellet. Depending on the viscosity of the specimen, strain a small amount of specimen through two layers of gauze into a conical centrifuge tube. Add 0.85% saline or 10% formalin to the tube, mix, and centrifuge. Ethyl acetate is added to the fecal suspension prior to concentration as an extractor of debris and fat, and it leaves the parasites in the sediment at the bottom of the tube. It is mandatory that the fecal suspension be centrifuged at 500 × g for a minimum of 10 min. Decant and discard the supernatant, and resuspend the sediment with saline or 10% formalin. The sediment is examined as a wet preparation using 10× and 40× objectives, with or without iodine. Commercial concentration devices are available, and these devices help to ensure standardization when processing specimens and lead to improved parasite recovery. This procedure is the easiest to perform, allowing the broadest recovery of parasites, and is the least vulnerable to technical error (2–8).
The zinc sulfate flotation technique allows separation of most parasites from fecal debris. The high specific gravity of the solution floats the organisms, and examination of the top surface film allows the detection of parasites. The debris sinks to the bottom of the tube. This technique results in a cleaner wet-mount preparation than the sedimentation procedure. Some helminth eggs (heavy eggs, such as unfertilized Ascaris eggs and operculated eggs) will be found in the sediment layer; therefore, both the surface film and sediment must be examined. Also, the high specific gravity of the solution may distort the morphology of some parasites.
Microscopic examination (routine permanent-stain smears).
Detection and definitive identification of the protozoan trophozoites and cysts are best accomplished with the use of a permanent stained smear. Nuclear and cytoplasmic characteristics are enhanced with staining, allowing for organism recognition and identification. There are a number of staining techniques that can be used; however, the trichrome and iron-hematoxylin stains are most widely used. The permanent stain is examined using oil immersion objectives (100×), and a minimum of 300 fields should be examined before the result is determined to be negative. If organisms are seen after a shorter examination, a complete examination of 300 fields is recommended for the detection of other organisms that may be present in lower numbers. Permanent stains are not recommended for the identification of helminth eggs and larvae. These organisms often stain too darkly or are distorted, making identification difficult.
Wheatley's trichrome stain.
Wheatley's trichrome stain is a modification of the Gomori trichrome tissue stain and is used for routine fecal staining. Protozoan organisms will readily be seen on the trichrome stain. The fecal specimen is smeared onto a microscope slide. It is allowed to air dry prior to being stained. The slides are passed through a series of solutions, taking less than 1 h to stain. The stain is easy to perform and allows detection of protozoan trophozoites and cysts, white blood cells, red blood cells, Charcot-Leyden crystals, yeasts, and fecal debris. The color contrast (variations of red, blue, purple, and green) with the trichrome stain is more distinct than with the iron-hematoxylin stain, allowing for easier differentiation between organisms and artifacts. Although fecal specimens in preservatives can be stained with trichrome stain, PVA, modified PVA, and the newer nonformalin, non-PVA preservatives yield the best results.
Iron-hematoxylin.
There are many modifications of the iron-hematoxylin stain; however, the two most commonly used are the Spencer-Monroe and Tompkins-Miller procedures. Iron-hematoxylin was the stain used for most of the original descriptions of the intestinal protozoans. The stool smears are air dried and stained through a series of solutions. The contents of the specimen stain shades of grayish blue. Both methods can be used to stain fecal specimens in most preservatives, including SAF and merthiolate-iodine-formalin fixative (MIF). Other stains include Eco-Stain or the iron-hematoxylin–modified acid-fast combination stain.
METHODS FOR THE DETECTION OF CYCLOSPORA, CYSTOISOSPORA, CRYPTOSPORIDIUM, AND MICROSPORIDIA
Stains for Cyclospora, Cystoisospora, Cryptosporidium
The Cyclospora/Cystoisospora/Cryptosporidium parasites are apicomplexan protozoa, and they are intracellular, oocyst-forming parasites. The three genera recognized as causing human diarrhea and thought to be spread via contaminated food and water are Cyclospora, Cystoisospora, and Cryptosporidium. Cyclospora infection has been identified as common in tropical and subtropical regions, usually with a wet season peak. It is considered endemic in Haiti, Peru, Guatemala, Venezuela, Southeast Asia, Nepal, and India (60–62). There have been a series of large outbreaks of foodborne infection in the United States and Canada traced to imported berries and vegetables, including raspberries, mesclun, and basil. Symptoms include an acute watery diarrhea that may extend for several months if untreated (60–62). In AIDS patients, Cyclospora infection can also cause biliary tract involvement manifested by acalculous cholecystitis and cholangitis (62). Cystoisospora has a similar distribution: Latin America, the Middle East, Southeast Asia, and tropical areas in Africa. As with the other Cyclospora/Cystoisospora/Cryptosporidium infections, illness is more pronounced in the very young, travelers, and AIDS patients. In contrast to infection with Cyclospora and Cryptosporidium, Cystoisospora infection in AIDS patients may include systemic spread to lymph nodes, liver, and spleen. Despite appropriate treatment, infections may become chronic in these patients (63).
Why is it necessary to resort to staining or methods other than direct microscopy to identify these organisms? The spherical oocysts of Cryptosporidium are only 4 to 6 μm in diameter. At this size, they are too small to reliably identify in wet preparations by light microscopy. The unsporulated 8- to 10-μm oocysts of Cyclospora are perfectly round in outline and contain greenish inclusions, or morulas. However, despite these properties being quite identifiable, their comparatively small size means that they can be confused with smaller amoebae or pus cells. Although of larger size at 10 to 20 by 20 to 30 μm, the oocysts of Cystoisospora are unsporulated or contain a contracted sporont when passed. These oocysts are hyaline in appearance and can be poorly visualized by direct light microscopy. For all three species, the use of alternative methods other than direct microscopy is required to enhance detection.
Although these parasites are often considered together, there are features of Cryptosporidium that set it apart from other species. It is considered either as basal to all other Apicomplexa or more closely related to the gregarine parasites, with which Cryptosporidium shares many life cycle features. When describing the genus in 1907, Tyzzer (280) chose the name Cryptosporidium to emphasize the difference from other parasites, notably the absence of a sporocyst and the presence of naked sporozoa within the oocyst. These oocysts differ in having no need for sporocyst maturation, they are immediately infectious, and importantly, they are capable of autoinfection (64, 276). The immense significance of Tyzzer's discovery was not appreciated for many decades.
Further impetus for study of Cryptosporidium came from the veterinary world with the description of Cryptosporidium meleagridis in turkey poults in Scotland in 1955 (65). 1978 saw the recognition of Cryptosporidium associated with scouring in neonatal calves in Iowa (66). As the importance of cryptosporidial illness in calves was becoming recognized, Henriksen and Pohlenz in Denmark made the discovery that oocysts stain acid fast in a Ziehl-Neelsen (ZN) stain; this was a largely empirical finding based on testing a variety of stains from a neighboring bacteriology laboratory (67). The first report of human illness came in 1976 with the identification of Cryptosporidium in rectal biopsy specimens of a 3-year-old child (68). A subsequent review of more cases in humans suggested that infection seemed limited to immunosuppressed patients (69). In 1983, first reports describing Cryptosporidium in intestinal biopsy specimens of AIDS patients were seemingly in agreement with this theory. Initial efforts at staining oocysts from fecal specimens included the use of Giemsa and Kinyoun stains (69). Later that year, at a time when there were only an estimated 1,000 AIDS cases worldwide, a comparison study of specimens from eight patients using 15 different staining options confirmed that modified acid-fast staining performed best (70). Prior to the introduction of antiretroviral therapy, the diagnosis of Cryptosporidium was recognized as one of the AIDS-defining illnesses. The often-unrelenting symptoms included chronic diarrhea or severe cholera-like illness and could also have involved biliary tract colonization, cholecystitis, and cholangitis (71). The prediction that infection would be confined to the immunocompromised proved to be erroneous. Cryptosporidium was soon identified in immunocompetent patients as a cause of self-limiting, watery diarrhea of several weeks' duration; infection was also more common among young children than among adults (72). In subsequent years, Cryptosporidium has also been identified as a significant cause of illness in patients with malignancy and other types of immunodeficiency (73).
In the intervening years, many Cryptosporidium species and possible subspecies have been identified, with many exhibiting a very limited host range. The two species commonly detected in humans are C. parvum and C. hominis. The latter species does not typically infect animals, but C. parvum is commonly infects cattle. This species is recognized as an environmental problem in water catchments in rural communities. In total, there are approximately 20 species or genotypes of Cryptosporidium that have been identified as causing illness in humans, but most of these generally have a more restricted host range (74). The waterborne outbreak in Milwaukee, WI, that affected 400,000 people has been attributed to human feces (C. hominis from the sewage treatment plant) entering the drinking water system after a failure of the water treatment plant filtration system (30, 75).
With the advent of PCR assays, the dimension of illness caused by Cryptosporidium has been dramatically reestimated (76). In an analysis of the global burden of Cryptosporidium, estimates quoted show that 15 to 25% of diarrhea in childhood is attributable to this parasite (77). Furthermore, those most at risk are the immunocompromised, the malnourished, and infants of low birth weight. Review articles have stressed that any form of infection, even asymptomatic or postsymptomatic, can result in significantly reduced growth rates by the age of 6 to 9 years (78, 79). The importance of this infection has been confirmed in the Global Enteric Multicenter Study (GEMS) of the etiology of diarrhea in children (to 59 months) in sub-Saharan Africa and south Asia. Based on detection by immunoassay, results showed that Cryptosporidium infection was the second-most-common infection in infants and was associated with the highest risk of death in toddlers (80). PCR testing in Uganda has also shown concomitant respiratory tract involvement among children with diagnosed intestinal infection (80).
These recent figures illustrate that infection rates in developing countries have been significantly underestimated. In poorly resourced regions, where access to molecular techniques may not be available, it is important that attention is focused on improving existing technology, especially microscopy and staining techniques. The introduction of LED-sourced fluorescence has reduced the ongoing cost and maintenance of fluorescence microscopy, which should enhance the test options for the diagnosis of Cyclospora/Cystoisospora/Cryptosporidium and microsporidian parasites.
In considering alternative approaches for concentrating and staining oocysts of Cryptosporidium, it is essential to have a gold standard method for comparison. It is generally accepted that a fluorescent antibody test meets that requirement. First developed by Sterling and Arrowood (81), the assay was based on a monoclonal antibody to oocyst cell wall antigen. Similar versions of this assay are now marketed commercially by a number of manufacturers (71). Apart from cost, a limitation of this test is the requirement that the fecal smear must be processed or cleared in some way to ensure that fluorescence can easily be detected. This test will be described in more detail below.
Concentration of Cryptosporidium.
Unlike with other species, the oocyst of Cryptosporidium is very small; at 4 to 6 μm, it is too small to reliably identify by light microscopy when unstained. With profuse diarrhea, the number of oocysts can be very high; one estimate is 109/ml. At these numbers, diagnosis is relatively straightforward. However, detection is more difficult when oocyst numbers are low, as in asymptomatic infection or during the period of excretion after the cessation of symptoms. This period may extend to 2 months postinfection (82). Additionally, there is potential for inadvertent spread if convalescent cases are not recognized. Even subclinical infection is thought to have long-term health consequences for young children (77).
There have been two approaches to concentrating specimens. The flotation technique requires application of fecal specimen to a solution of higher density, such as zinc sulfate, Sheather's sucrose-phenol, or saturated sodium chloride solution. Sucrose-phenol has been favored for isolation of Cryptosporidium, with the oocysts recovered from the top meniscus after centrifugation. A disadvantage of this technique is that microscopy must be performed within 15 min of sedimentation. Otherwise, lysis and distortion of the oocysts can occur in the highly osmotic fluid. Additionally, the presence of sucrose may interfere with staining reactions (83, 84). This technique is unsuitable for recovery of some of the heavier helminth eggs and so is not generally regarded as applicable for use in screening for other parasites. Comparison studies of flotation versus the FEA concentration technique have provided varied results.
In one study, 703 stool specimens were processed by both methods during an outbreak of cryptosporidiosis. Despite results being comparable statistically, there was limited overlap of low-positivity results; approximately 20% of positive results were detected by a single method only (84). In contrast, in another study, dilutions of seeded fecal specimens were processed by both methods. In the FEA method, at the lowest positive cutoff level, there was nil recovery by flotation (85).
The FEA technique has more general application than flotation and has been favored for recovery of oocysts, principally because filtration followed by the extraction of fats by ethyl acetate gives a cleaner product, suitable for use in the direct fluorescent antibody (DFA) microscopy test. In 1991, Weber et al. (86) reviewed the efficiency of this process. They tested the threshold for detection using both a formed and a watery stool, supplemented with C. parvum oocysts at six different concentrations. Using 10% formalin and centrifugation at 500 × g for 2 min, the number of oocysts recovered by FEA represented a loss of 51.2% of expected numbers for liquid stool and 93.2% for the formed stool. The threshold for detection from liquid stool was 10,000 oocysts/g by acid-fast staining and 5,000 oocysts/g by DFA testing. There were high numbers of oocysts detected in the filter gauze and discarded supernatant. These rather-disconcerting findings were followed by a further report from the same research group, advocating a new FEA-flotation procedure. An increased centrifugation time of 10 min at 500 × g (now standard) did not improve yield from five seeded specimens but compacted the deposit, making DFA testing more difficult. In an additional flotation step, the deposit was suspended in water and layered on a solution of saturated sodium chloride. Following centrifugation, a clean, concentrated harvest of oocysts was recovered from the interface between the two solutions. Despite this modification, the limits of detection were still calculated to be 5 to 10,000 oocysts/g of stool. It was also noted that recovery was reduced from stools containing higher levels of fat.
Using a more typical cross-section of specimen types rather than seeded specimens, Clavel et al. (87) compared recoveries of Cryptosporidium oocysts from 73 stored positive specimens. By similarly extending the centrifugation time from 2 to 10 min, they found a 13% increase in the recovery of oocysts. Despite the increased fecal debris after extended centrifugation times, modified acid-fast stains could still be interpreted. A further examination of the FEA process was conducted in Brazil by Pacheco et al. (88), who again using stored positive specimens. In this study, 27 specimens were processed in two ways: by the FEA technique and by sedimentation by centrifugation (SC), without ethyl acetate extraction. Centrifugation was at 400 × g for 2 min. Unfortunately, this centrifugation speed is much lower than the 500 × g for 10 min now commonly used. Despite this discrepancy, the observations reflect the difficulties in recovery of oocysts of small size and low density. In the FEA process, the ring of ethyl acetate-extracted fats was also sampled and oocysts were detected in 93.3% of samples. The numbers in the fatty plug exceeded those in the deposit in 25.9% of these. This finding has bearing on the capacity to diagnose infection when oocyst numbers are low or when the patient has malabsorption and associated high fat levels in feces. Although the quality of the modified acid-fast smears prepared from the SC deposit was lower, with more stain residue, this method was the preferred option, as it avoided having to stain both the deposit and the ethyl acetate-fatty residue generated by the FEA method.
While there are doubts about the efficiency of recovery of oocysts and spores by the conventional formalin ethyl acetate method, at this stage, there are no practical alternatives. There is the added convenience of using only a single approach for concentrating eggs and cysts for microscopy. The effectiveness of this technique is that it produces a much cleaner product, due in large part to the ethyl acetate extraction of fats, which is of particular importance for immunofluorescence assay (IFA) staining of Cryptosporidium to reduce the level of background fluorescence. There may also be validity to the move to perform a modified concentration technique without using formalin (88, 89). Perhaps by removing formalin, subtle changes in the specific gravity of the suspending solution may be sufficient to ensure recovery of oocysts of very low density. In theory, flotation as a means of concentrating oocysts may offer more success in the recovery of structures of low density. However, as mentioned previously, the two methods have been compared directly, with no observed advantage in the use of flotation (84). Furthermore, the more complex nature of this procedure does not lend itself to large-scale processing of specimens.
Concentration of Cystoisospora and Cyclospora.
Commentary about the recovery of Cystoisospora and Cyclospora is more limited than for Cryptosporidium due largely to the lower frequency of detection. In comparing methods for concentrating Cystoisospora oocysts, Pacheco et al. (88) used FEA and sedimentation by centrifugation alone (SC). As they had observed for Cryptosporidium, they found that in the FEA concentration process, there were high losses of Cystoisospora oocysts in the ring of fatty debris and that best yield was obtained with centrifugation alone (89).
Similarly, Kimura et al. (89) screened fecal specimens in Nepal for Cyclospora by three methods: FEA, sucrose flotation, and direct smear fluorescence microscopy. The results using the last two techniques were not statistically different but were significantly higher than those obtained by FEA. Furthermore, the yield in FEA positives was much lower, with 55% containing only one oocyst in a set number of fields examined. They postulate that oocysts must get trapped in the fecal plug, again suggesting a low specific gravity.
Modified Acid-Fast Staining of Cyclospora/Cystoisospora/Cryptosporidium
The presence of acid-fast lipids in the cell wall and the property of autofluorescence are all common to Cryptosporidium, Cyclospora, and Cystoisospora (88, 90) (Fig. 3). This shared property means that the modified acid-fast stain still has relevance for the combined screening for all three parasites, especially in immunocompromised patients and for those with a history of overseas travel. At this stage, no other single test offers this flexibility. Henriksen and Pohlenz were the first (67) to recognize that lipids in the wall of Cryptosporidium oocysts could be stained with an acid-fast stain. Their method involved flooding a smear with strong carbol fuchsin, decolorizing with 5% sulfuric acid in alcohol, and counterstaining with methylene blue. This stain was referred to as a modified Ziehl-Neelsen (ZN) stain, as there was no heating step. An alternative approach adopted by Garcia et al. (70) was also referred to as a modified acid-fast stain. This method was more similar to that using the ZN stain in that heating of the carbol-fuchsin step was required. The atypical step was decolorizing with 5% H2SO4, without alcohol (acid-alcohol is used for acid-fast staining of mycobacteria). Garcia et al. also suggested the pretreatment of mucoid smears with 10% KOH to break up the mucus and improve adherence of the sample to the slide (70). In 1991, Casemore (91) described another method based on the standard solutions used for a ZN stain. Smears were stained for 20 min with either strong carbol-fuchsin or Kinyoun stain without heating and decolorized with 1% HCl in methanol, followed by counterstaining in malachite green or methylene blue.
FIG 3.
Permanent-stain options for various organisms, identified from left to right. (First row) Wheatley's trichrome stains of a Giardia lamblia trophozoite, an Entamoeba histolytica trophozoite with ingested RBCs, an Entamoeba coli cyst with red chromatoidal bars, and a Chilomastix mesnili cyst; (second row) iron-hematoxylin stains of a Dientamoeba fragilis trophozoite, Giardia lamblia cysts, an Entamoeba coli cyst, and an Iodamoeba bütschlii cyst; (third row) modified acid-fast stains of large Cyclospora cayetanensis organisms (both stained and unstained), a medium-sized Cryptosporidium sp. (with small artifacts), large Cyclospora cayetanensis organisms (with medium-sized Cryptosporidium sp. cells and a small artifact) (note that not all Cyclospora oocysts stain with modified acid-fast stain), and Cryptosporidium sp. organisms (note the presence of sporozoites in some oocysts [not seen in Cyclospora]); (fourth row) an iron-hematoxylin–modified acid-fast combination stain of Giardia lamblia and Cryptosporidium sp., a modified acid-fast–modified trichrome–Ryan blue combination stain of Cystoisospora belli microsporidial spores, a modified trichrome–Ryan blue stain of microsporidial spores, a modified trichrome–Weber green stain of microsporidial spores; (fifth row) DFA stain of Cryptosporidium oocysts (apple green color), autofluorescence of Cyclospora cayetanensis oocysts, Calcofluor white staining of microsporidial spores (in the circle), and a combination modified acid-fast–modified trichrome stain of Cryptosporidium oocysts (pink) and microsporidial spores (in circle).
There are many minor variations in performance of modified acid-fast stains, but the consensus now is to use 1 to 3% H2SO4 as a decolorizer and to avoid the use of alcohol (1% H2SO4 has been favored if the stain is for dual-purpose detection of Cyclospora). There is no heating step involved with the Kinyoun stain. It contains high concentrations of both phenol and basic fuchsin, and this is thought to give better penetration of the dye. It is postulated that phenol may change surface tension, allowing dye to enter hydrophobic surfaces. Once the dye is bound, it cannot be removed by acid treatment. Another variation in acid-fast staining is the addition of dimethyl sulfoxide (DMSO) to the phenol-basic fuchsin and the incorporation of acetic acid with malachite green to act as a combined decolorizer-counterstain. It was reported that by this method, staining of internal details of oocysts was more detailed than seen with conventional ZN stains (92). The action of DMSO is thought to allow better penetration of the fuchsin stain into the lipid layer.
Although Cryptosporidium, Cyclospora, and Cystoisospora are all identified as staining acid fast in modified acid-fast stains, shortcomings have been identified. One of the principal criticisms is that ghost cells or poorly stained oocysts occur, suggesting varied levels of uptake of the dye and ease of destaining (83). In particular, Cyclospora does not stain uniformly; there are usually many unstained ghost cells present (62). It has been suggested that to counteract this variability, the strength of decolorizer in the Kinyoun stain should be reduced to 1% H2SO4 (90). In contrast, the staining of Cystoisospora in the modified acid-fast stain is regarded as a suitable diagnostic test (88). Oocysts stain a mottled-pink color, but sporonts or sporocysts stain bright crimson (63).
To perform the modified acid-fast stain in our laboratory (NR), smears are prepared directly from fecal suspensions in SAF with no prior concentration step. Smears are briefly heat fixed on a hot plate at 60°C for 5 min and then fixed in methanol for 5 min. The prior, brief heat fixing is recommended to increase adherence and may improve staining of Cyclospora. The staining method used is that of Casemore (91), as described above. This method provides good contrast in staining and little obvious occurrence of ghost cells in control smears. The stained smear is first coated with a light layer of oil and examined at a ×100 magnification using strong lighting. Viewing at low magnification allows screening of a large area of the slide. Any pink objects are easily detected and are then inspected at ×1,000 magnification to identify the typical morphology of Cryptosporidium or other oocysts. Oocysts of Cryptosporidium are commonly found in thicker areas of the slide. Staining artifacts may be encountered; these generally take the form of variously sized, undifferentiated particles that are possibly coagulated protein. Bacterial and fungal spores may also stain acid fast and are seen quite commonly but can readily be distinguished by their morphology and intensity of staining. There is enormous variation in the choices of recipe used for modified acid-fast stains, ranging from ZN-strength carbol-fuchsin to the stronger Kinyoun reagent, applied with or without heating. For decolorizer, the options range from 1 to 10% H2SO4 with or without alcohol to the use of 1 to 3% HCl in alcohol. The choice of counterstain is usually either methylene blue or malachite green. The enormous variability underscores the necessity for adequate control material and for some estimation of the quantitative capability, perhaps in identifying standardized controls. In general, despite the variety of approaches used, the modified acid-fast stains can be used for the screening and identification of these species.
Combination acid-fast stains.
Some initiatives to improve efficiency in the processing of fecal specimens involve combining other tests with the acid-fast stain. Details of several methods designed to detect both Microsporidia and Cryptosporidium will be described below, in conjunction with methods for detection of Microsporidia (Fig. 3). Another innovative approach is to stain for both Cryptosporidium and amoebae and flagellates. This method, developed in Australia and marketed commercially, utilizes an initial acid-fast staining step followed by iron-hematoxylin staining (component stains from Thermo Fisher Scientific). The initial step uses ZN-strength or strong-carbol fuchsin, rather than the more concentrated Kinyoun stain. Cryptosporidium oocysts stain with a pink-gray hue with good contrast against the typical blue-gray color of iron-hematoxylin (93; A. Butcher, personal communication). Careful pH control is required to ensure consistent staining of protozoa.
Fluorescent acid-fast staining.
Another stain commonly used for detecting mycobacteria is the fluorescent auramine-phenol stain. This method has also proved useful for staining oocysts of Cryptosporidium (92). It is thought that auramine stains both lipids and DNA. The advantage of a fluorescent stain compared to other stains is that large areas of smear can be screened at low magnification. Criticisms of this method are that staining artifacts commonly occur and the staining of other species is of limited quality. Staining of Cystoisospora has been described as irregular (88); Cyclospora also stains very poorly, and the weak fluorescence renders this method unsuitable (90). Similarly, this approach has not been endorsed by the CDC in recommendations for screening for Cyclospora during food poisoning outbreaks (https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/investigation-2013-lab.html).
Safranin staining.
An additional method used for identifying Cryptosporidium is the Safranin stain, described by Baxby et al. (83) in 1984. This staining method is not straightforward; there are several steps which add to the complexity of the method. First, the heat-fixed slides must be fixed again in an acid-alcohol solution (3% HCl in methanol). For the next step, slides were stained in 1% aqueous safranin with heating to boiling. Slides were then washed and counterstained with 1% methylene blue. The authors noted that more oocysts stained than in comparable smears stained by a modified acid-fast stain. In 1997, Visvesvara et al. (93) adapted this method to stain Cyclospora, also finding that staining was superior to the modified acid-fast staining, with a higher number of oocysts detected. As in the Cryptosporidium stain, they also used heat-fixed slides and acid-methanol pretreatment. The heating step was replaced by microwave heating of smears immersed in safranin. Baxby et al. (83), had previously noted problems in batch-to-batch variation in the staining quality of safranin, but this could be remedied by adjusting the pH of the stain solution to 6.5 (93). Maratim et al. (94) in Kenya obtained equivalent results but found that the heating step is critical and that immersion in a water bath at 80°C for 15 min is an acceptable substitute for the microwave heating step. The major advantage of safranin staining is that a high percentage of oocysts of Cyclospora are stained compared to the percentage stained with modified acid-fast stains, which result in notoriously variable numbers stained.
The suitability of safranin staining to identify Cystoisospora oocysts has also been investigated. In a comparison of different staining methods for 15 Cystoisospora-positive, formalin-fixed stool samples, Pacheco et al. (88) found that modified acid-fast and auramine-rhodamine stains gave marginally better results than those achieved with safranin staining. Oocysts were counted in 20 microscopic fields in parallelly prepared smears of FEA deposits. Overall, the quality of modified acid-fast staining was observed to be more uniform. However, despite the median number of Cystoisospora oocysts in modified acid-fast stains being higher than in safranin stains, there was no significant difference detected between the two methods.
Fluorescent antibody staining.
In 1986 a direct fluorescent antibody (DFA) assay for detection of Cryptosporidium was developed (81) (Fig. 3). Although based on monoclonal antibodies directed against purified cell wall antigen of C. parvum, this format has proved over subsequent years to have activity against a wide range of Cryptosporidium species (95). In 1987 Garcia et al. (96) compared DFA with modified acid-fast staining and found that the fluorescent assay had a substantially higher sensitivity. At this time, the method included pretreatment of the stool sediment with 10% KOH, followed by washing in 10% formalin and centrifuging at 300 × g for 2 min. They estimated that DFA offered a 10-fold improvement in sensitivity compared with the reading of stained smears. By 1989, a commercially available assay was subjected to a trial and again proved superior to acid-fast staining (97). Subsequently, Garcia et al. (95) analyzed a commercially prepared combined Giardia/Cryptosporidium DFA test. Specimen preparation consisted of centrifugation at 500 × g for 10 min; there was no mention of KOH pretreatment. The ability to screen at a ×100 magnification was recognized as a major factor in improving sensitivity. DFA testing was easy to perform and could be used in less time than conventional assays.
Weber et al. (86) employed both modified acid-fast and DFA methods to investigate oocyst recovery from seeded specimens. The DFA test of FEA concentrates consistently showed a higher sensitivity, and the authors stressed that the FEA method was necessary to prepare a cleaner product, with less background fluorescence. However, they noted that a significant loss of oocysts occurred in the FEA technique. In another direct comparison of the two methods, specimens were screened. The inclusion of DFA testing improved the diagnosis of Cryptosporidium by 69.6%. This study used direct smears of fresh specimens, and it was noted that the staining was less intense when FEA concentrates were viewed, but oocysts were still easily identifiable (98). In evaluation studies of a variety of immunological techniques for the detection of 123 Cryptosporidium species, including EIA and lateral-flow devices, DFA testing was still considered to be the most accurate test (99–102).
Use of optical brighteners to detect Cryptosporidium.
Harrington (103) made a brief mention of the possible fluorescence of Cryptosporidium in two reports about application of fluorescent brighteners for detection of Microsporidia. He investigated whether these observations had validity by using a variety of pretreatments and variations in the method of staining. Slides were examined in violet light using a 395- to 400-nm excitation filter and 460- to 520-nm barrier filters. It was found that all four brighteners, including calcofluor white and Uvitex 2B, performed well, provided that the stain solution was prepared at 0.1% in 10% NaOH and was warmed to 60 to 65°C. However, as noted for the acid-fast staining, the degree of staining of oocysts within the same slide varied considerably, and it was suggested that the staining may be of the sporozoites rather than wall material. The degree of staining ranged from only a few speckles to solid bright fluorescence within the oocysts. The fluorescence associated with oocysts was much stronger than the dull, background staining of bacteria within these smears.
Autofluorescence of Cyclospora/Cystoisospora/Cryptosporidium.
By definition, autofluorescence is the capacity to absorb light in the UV range and reemit most of the absorbed energy as light of a longer wavelength. The oocysts Cyclospora, Cystoisospora, and Cryptosporidium are all recognized as having autofluorescent properties (90, 104). As mentioned previously, this characteristic can be attributed to the presence of high levels of tyrosine and the likely formation of dityrosine and 3,4-dihydroxyphenylalanine (DOPA) protein cross-linking in oocyst walls (105). Varea et al. (106) reported that at 365 nm (UV light), both Cryptosporidium and Cystoisospora appear violet and Cyclospora appears blue; under 405 nm (violet light) or 436 nm (blue light), they all appear green. No autofluorescence is seen at 546 nm (green light). The reported autofluorescence of Cryptosporidium is contrary to the view of others and is unexpected, as the oocyst wall of Cryptosporidium is not recognized as containing tyrosine but has high levels of histidine and cysteine (60, 107). The absence of any recorded attempts to diagnose Cryptosporidium on the basis of autofluorescence also suggests that the degree of reaction is not definitive. In contrast, Cyclospora is readily identified in UV light (62, 89, 108). The intensity of fluorescence is so strong that this technique is a CDC-recommended method for screening for this parasite in outbreak situations (https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/investigation-2013-lab.html). The fluorescence seems to be limited to the outer cell wall so that oocysts have the appearance of a fluorescent ring (90). In defining the expected colors of various species when viewed at different wavelengths, N. Ryan also emphasized that the choice of barrier filter can change the expected color.
Stains for the Microsporidia
There has been a long history of study of Microsporidia, with early focus being on insect Infections, but they have been recognized subsequently as parasites of all animal groups (109, 110). Microsporidia are eukaryotic species formerly considered protozoa but now thought to be more closely related to fungi. They exist by harnessing host cell metabolism to support a proliferative stage followed by spore formation. The identifying feature of microsporidian spores is that they contain a coiled tubule which is capable of evagination and penetration of the host cell membrane to transfer infective sporoplasm into the cell. Other features that are shared with fungi include the presence of chitin and trehalose, similarities in cell cycles, and certain gene organizations. Microsporidia are now considered to be highly derived fungi that underwent genetic and functional losses, thus resulting in one of the smallest eukaryotic genomes known. However, at this point, clinical and diagnostic issues and responsibilities may remain with the parasitologists.
Typical spore sizes range from 1.5 to 5 μm wide and 2 to 7 μm long; however, the organisms found in humans tend to be quite small, ranging from 1.5 to 2 μm long. Until recently, awareness and understanding of human infections have been limited; with increased understanding of AIDS and more recently other immunosuppressive illnesses, more attention has been focused on these organisms. Limited availability of electron microscopy (EM) capability has also played a role in the inability to recognize and diagnose these infections. However, with the introduction of newer diagnostic methods, the ability to identify these parasites has definitely improved, particularly in solid-organ and bone marrow transplant recipients.
Modified trichrome stains (chromotrope 2R).
There had been only rare reports of microsporidian infection in humans prior to 1985 when Enterocytozoon bieneusi was first identified as a cause of diarrhea in AIDS patients (111). Initial diagnosis of microsporidium infection had been based on EM study of small bowel biopsy specimens and identification of spores located in enterocytes. However, it was recognized that high numbers of spores could be present and that detection in feces should be possible. Detection was achieved by van Gool et al. (112), who detected Giemsa-stained spores of approximately 1.5 by 0.9 μm that had a characteristic central, lateral, or polar clear zone. However, spores were difficult to separate from bacteria of similar size and color.
A more practical approach was devised by Weber et al. (113), who found that by modifying Wheatley's trichrome stain, the spores could be stained a bright pink-red against a faint green background. Once again the spores were described as having clear zones or a belt-like stripe girding the spores diagonally or equatorially. Significantly the authors found that flotation or FEA methods of concentration resulted in loss of spores; methanol-fixed smears, prepared from formalin suspensions of feces, gave good results. Smears were made from only 10 μl of fecal suspension, spread over an area of 45 by 25 mm. There were two major modifications to Wheatley's trichrome recipe used for detection of protozoa (Fig. 3). The content of chromotrope 2R was increased 10-fold, and the staining time within the trichrome solution was extended to 90 min.
However, in our laboratory (NR), we found along with others that there was difficulty in recognizing the limits of smeared areas. In retrospect, extremely low volumes of specimen and very thin smears were being used, based on the theory that smears should not have overlapping layers of bacteria, which could prevent the discrimination of spores of comparable size. Practical advice on the theory of trichrome staining was sought, and modifications to this stain were based on the following points (114). The trichrome stains use acid stains, and the reproducibility of recipes is more certain if stains are maintained at optimum pH, hence the specification for an accurate pH 2.5. Background staining could be improved by use of aniline blue to replace fast green. Although both of these dyes compete with phosphotungstic acid, which is of similar size and can be considered a colorless dye, aniline blue is less inhibited. Furthermore, by reducing the phosphotungstic acid level from 0.7 to 0.25 g/100 ml, the degree of counterstaining was also increased. These changes were incorporated to produce the Ryan modification or trichrome blue stain (115). In addition to staining of fecal specimens, this stain proved useful for staining other specimen types, including sputum, urine, and nasopharyngeal aspirates. Contemporaneously within our clinical service, the histology laboratory tasked with staining intestinal biopsy specimens found this stain superior to modified Gram stains for the detection of Enterocytozoon bieneusi in enterocytes.
At this time, a second form of Microsporidia was described in AIDS patients (116). The spores of approximately 2 to 2.5 by 1.0 μm resembled Encephalitozoon cuniculi, but EM studies showed developing spores within a walled fibrillar network. In contrast to Enterocytozoon bieneusi, infection was not limited to enterocytes and could be detected within macrophages in the lamina propria. The species was named Septata intestinalis, but subsequent genetic and immunological characterization resulted in reclassification to Encephalitozoon intestinalis (117). Trichrome blue staining also detected this species; infection was not limited to intestinal sites and spores were detected in urine and nasal secretions (116). It was recognized that Enterocytozoon bieneusi was seen only in patients with CD4 counts of ≤50 but the threshold for Encephalitozoon intestinalis was ≤130 (118). Despite the considerable passage of time since these initial reports and the discovery of other species of Microsporidia infecting humans, these two species remain the only Microsporidia species found to cause heavy infection of the small bowel. Symptoms reported for those carrying either infection indicate an association with diarrheal illness (118).
In subsequent years, the incidence of Microsporidia has declined dramatically in response to better management of HIV infection with improvement in cellular immunity. However, awareness of the importance of microsporidian infections has again been raised through the recognition that transplant recipients and patients undergoing immunosuppressive therapy for illnesses such as rheumatoid arthritis may be vulnerable to infection (119–122). There is also evidence that decline in CD8 levels has been linked to infection (123). While these reports relate to microsporidian species causing myositis and systemic illness, it must be presumed that enteric infection can also occur in vulnerable patients.
Rapid trichrome stains.
After the initial reports of the validity of trichrome staining for Microsporidia, further modifications to these approaches have been developed. Kokoskin et al. (124) found that staining time for the Weber method could be reduced from 90 to 10 min at an optimum temperature of 50°C. These authors were also troubled by limited contrast in thin stained smears and recommended that in preparation of fecal smears, a thicker band be included at one end to aid inspection. Didier et al. (125) also exploited the enhanced efficiency of staining at higher temperature, selecting a staining time of 30 min at 37°C. The stain used was similar to the trichrome blue stain (115) adjusted to pH 2.5 but with the higher level of 0.7 g phosphotungstic acid, the same level as used in Wheatley's trichrome and Weber's modified trichrome. This method was noted to have a sensitivity rivalling that of the more nonspecific calcofluor stains (to be described below).
The “quick-hot Gram chromotrope” method represents a radical departure from other trichrome stains in that staining times are very rapid (126). It exploits the fact that most Microsporidia spores stain Gram-positive to various degrees. However, there are exceptions: Enterocytozoon bieneusi appears as Gram-variable, and cell-culture derived spores of Encephalitozoon intestinalis show incomplete staining with Gram-positive granules. This general Gram-positive character has been exploited in a stain that uses crystal violet, iodine fixation, then decolorizing but with no safranin counterstain. Following rinsing, slides are then stained for only 1 min at 55°C with a trichrome recipe consisting of 1.0% chromotrope 2R, 0.15% fast green, and 0.25% phosphotungstic acid. The decolorizing steps and transfer through increasing concentrations of alcohol are each of only 30-s duration. Spores stain as ovoid structures of a variable range of colors from red-pink, to red-violet, to dark violet. The advantage of this stain lies in the rapidity of the test and its applicability to examination of tissues where there is perhaps less likelihood of contaminating flora. In fecal smears the dark staining of Microsporidia is purported to distinguish them from more lightly stained bacteria and pink-red yeasts (126).
Although the Gram-positive property of spores can be exploited in this way, conversely the possibility of misinterpretation of Gram stains from tissue specimens should not be overlooked. One example is the report of an investigation of a case of endocarditis in a patient with negative HIV serology. Removal of his pacemaker revealed a fibrin vegetation with many foci of Gram-positive coccobacilli. These organisms were ultimately identified by EM studies as spores of Encephalitozoon cuniculi (127, 278). The Gram-positive nature of microsporidium spores suggests that the chitin wall structure is perhaps analogous to the dense cross-linked peptidoglycan layers of Gram-positive bacteria.
Optical brighteners.
As indicated previously, the study of microsporidian infection of insects extends as far back as the 19th century. Working in this field, Vavra reported in 1976 (127) that the spore wall is composed of an electron-dense outer layer, or exospore, which is protein-rich. The endospore, or inner, electron-transparent layer, is connected to the plasma membrane. The endospore structure is fibrillar in nature and recognized to contain chitin. In 1982, Vavra and Chalupsky (128) drew analogy with fluorescent staining of chitin in fungal tissues and showed that microsporidial spores also fluoresce in UV light following labeling with the optical brightener calcofluor white (Fig. 3).
In 1993, van Gool et al. (129) used fluorescent staining with a different optical brightener, Uvitex 2B, to detect Microsporidia spores in feces of AIDS patients. The method also used Evans blue as a nonfluorescent background stain. Fixation with formalin was noted to reduce the intensity of fluorescence. Shortly after this, Vavra et al. (130), exploiting their extensive experience with Microsporidia, used a large range of Microsporidia species, including three human isolates, to formulate some recommendations for a uniform approach to the use of optical brighteners. Excellent results were obtained using three different chemical forms of brightener, including the common agents, calcofluor white and Uvitex 2B. Consistent results were achieved with each when labeling was performed either in suspension or on dried smears. Optimum staining was obtained when the brightener was prepared at 0.001% in 0.1 M phosphate buffer at pH 7 to 8. Contrary to the finding of van Gool et al. (129), paraformaldehyde-treated spores gave strong fluorescence. However, it was noted that fluorescence of spores that had been stored for long periods was weak or nonexistent. Full restoration of fluorescence could be achieved either by washing in 1N NaOH prior to staining or by staining with a solution of whitener freshly dissolved in 1N NaOH.
These reports were followed by others that demonstrated the utility of this approach for the direct screening of fecal and biopsy specimens for Microsporidia. Other groups used the Uvitex 2B staining method of van Gool et al. (129) and reported good correlation with staining by a modified trichrome stain (131, 132). Similarly, Didier et al. (125) reported the successful application of calcofluor white, also with Evans blue counterstain, and noted that the method was easy to perform and that spores could even be detected in thicker areas of smears. Although regarded as a simple test to perform, there are some limitations. In screening 479 fecal specimens, Chioralia et al. (133) detected 119 with fluorescent oval structures present, but only six were confirmed as Microsporidia by modified trichrome stain, with yeast cells and bacterial spores accounting for other suspect objects. Didier et al. (125) also reported staining of yeasts but noted subtle differences in shape and color, with the cytoplasm in yeast cells staining an orange color. In a further distinction, it was emphasized that staining of Microsporidia spores should be restricted to the wall, not the internal content (130). Variation in the intensity of staining of spores has also been noted; spores were described as both bright-white and smaller reddish-brown structures for both Enterocytozoon bieneusi and Encephalitozoon intestinalis (129). Working with cell-culture-derived spores of the latter species, Didier et al. (125) also noted smaller, more intensely staining spores and slightly larger ones that stained less intensely. Despite these anomalies, staining with fluorescent brighteners has gained popularity and is commonly used as a quick screening method with confirmation based on the more complex, modified trichrome stains.
A review article about the use of fluorescence microscopy defines the correct combination of excitation and barrier filters to obtain fluorescence of good color and intensity. With the appropriate barrier filter, the expected color of Microsporidia oocysts should be bluish-white in ultraviolet or violet light (90). In addition to the simplicity of the staining with fluorescent whiteners, the other major advantage compared to trichrome stains is that slides can be screened at lower magnifications, allowing a wider coverage in a much shorter period of time.
The use of NaOH treatment of smears as suggested by Vavra et al. (130) as a means of restoring fluorescence to older stored specimens has not been fully recognized or employed. However, similar treatment is automatically provided in some commercially available stains. Fungi-Fluor, based on calcofluor white, was originally devised to detect fungal infections and contains 10 to 20% KOH as a clearing agent. This stain was used in a study to determine variation in excretion of microsporidial spores from stool to stool and between patients (134). In a further comparison of staining options, the commercially available stains Fungi-Fluor, calcofluor (Remel) and Fungiqual A (or Uvitex 2B) were used. Both calcofluor-based stains were combined with 10% KOH and used Evans blue as a quenching agent. Again excellent correlation with modified trichrome staining was achieved; formalin-preserved specimens stained well but fluorescence was less strong in the commonly used SAF fixative (135). The last observation may simply be due to the low pH and buffering capacity of SAF, which contains acetate buffer at pH 4.2, perhaps limiting the action of NaOH. The presumed effect of NaOH treatment may relate to the amphoteric nature of chitin. At high pH, the net negative charge may enhance binding of whitener.
Combined stains for Microsporidia and Cryptosporidium.
The use of trichrome blue stain to screen fecal specimens from HIV-positive patients has led occasionally to detection of previously undiagnosed Cryptosporidium infection. Unfortunately, staining lacked sufficient intensity to apply as a routine method. Sometimes oocysts were largely unstained but had clear rounded outlines or there were curved pale-pink structures inside, presumed to be sporozoites. However, one of the other characteristics of trichrome stains is that they are robust and can be used as a final procedure in combination with other stains. This feature was subsequently exploited in several combination assays for detection of both Cryptosporidium and Microsporidia.
To rationalize the approach to screening for opportunistic parasites in AIDS patients, Ignatius et al. (136) developed a combined acid-fast trichrome staining process for both of these parasites. The first process involved a Kinyoun stain for 10 min, followed by rinsing and a decolorization step of 0.5% HCl and then further rinsing, succeeded by staining with Didier's modified trichrome blue stain. Twelve Cryptosporidium specimens, one Cystoisospora specimen, and seven microsporidian specimens were correctly identified with this stain. Using a very similar approach, Reisner and Spring (137) also evaluated a combined, modified, acid-fast trichrome stain but used only commercially prepared reagents. In this instance, the trichrome blue stain was purchased from a commercial supplier who followed the original recipe for trichrome blue, prepared using only 0.25 g of phosphotungstic acid. In an analysis of their findings, Reisner and Spring (137) found good correlation between the trichrome and DFA stain for Cryptosporidium. All seven specimens positive for Microsporidia were also recognized by this method. Similarly, a combined trichrome safranin stain useful for detecting Cryptosporidium, Cyclospora, Cystoisospora, and Microsporidia has been described by a team working in Argentina (138).
Conclusions
Recent studies have highlighted the fact that Microsporidia are more widespread than first thought (281). Asymptomatic human and animal carriage illustrate that the environmental spread of spores is likely, especially through water (282). Those most at risk for severe infection include many categories of immunocompromised patients, not just HIV patients. Microsporidial infections have been detected in patients who have undergone bone marrow or solid-organ transplants and in patients with severe chronic illness requiring immunosuppressive therapies (119–123, 139, 140). The use of combination staining methods will reduce the laboratory requirement for single-focus assays. In this respect, combined stains that target Microsporidia and Cryptosporidium are of great practicality, as both constitute significant infections of immunocompromised and immunocompetent patients.
FECAL IMMUNOASSAYS
Antigen Detection
For most parasitic diseases, the antigen(s) is generally the assay component which has the most influence on test sensitivity and specificity. Parasites generally have more than one life cycle stage that may have both mutually shared antigens and stage-specific antigens. The matrix to which antigens are bound for use in a specific procedure also influences which antigen subset will be available for antibody binding.
The ova and parasite examination (O&P) is traditionally for detection of fecal parasites. Since the O&P is a labor-intensive, multistep test that requires a skilled microscopist, other test options have been developed. Immunoassays are available for a limited number of pathogenic protozoa, including Giardia lamblia (G. duodenalis, G. intestinalis), Cryptosporidium spp., the Entamoeba histolytica/Entamoeba dispar group, and Entamoeba histolytica. Commercially available antigen test formats include direct fluorescent antibody (DFA), enzyme immunoassay (EIA), and immunochromatographic lateral flow assays (LFAs) and are more sensitive and specific than the O&P (7, 95, 100, 101, 141–150). Immunoassays for antigen detection, however, are usually limited to one or two organisms only. When choosing which method is best for your laboratory, parameters such as test performance characteristics, skill level of technologists, availability of equipment, volume of requests, specimen collection requirements, and kit cost should be considered.
Fresh or preserved fecal samples are acceptable specimens for immunoassay testing. It is best to refer to the recommended collection procedures for each specific kit. All current EIA and LFA kits that include testing for the Entamoeba histolytica/E. dispar group and Entamoeba histolytica require fresh, unpreserved stool specimens only (149). Fresh, unpreserved specimens can be stored at 2 to 8°C and tested within 48 h or frozen. Most test kits for Giardia lamblia (G. duodenalis, G. intestinalis) and Cryptosporidium spp. accept stools preserved in a number of fecal fixatives. Ten percent formalin, merthiolate-iodine formalin (MIF), sodium acetate-acetic acid-formalin, non-polyvinyl alcohol (PVA) preservatives, and the single-vial universal fixatives are acceptable and should be tested within 2 months of collection. Stool specimens collected in Cary-Blair transport medium (or a similar medium) should be refrigerated or frozen and tested within 1 week. Specimens collected in mercury or PVA are not acceptable.
Immunodetection of antigens in stool specimens using the monoclonal-antibody-based DFA, EIA, and LFA formats are available through various manufacturers. Several kits combine tests to detect Cryptosporidium spp., Giardia lamblia (G. duodenalis, G. intestinalis), and the E. histolytica/E. dispar group (141–149). Only one EIA kit and one rapid test are specific for Entamoeba histolytica; additional kits are due to be released in the near future. All kits have high specificities (90 to 100%) and sensitivities (63 to 100%). DFA kits have sensitivities and specificities of >95%. Enhanced sensitivity for the DFA is attained by using concentrated fecal sediment. EIAs are also very sensitive; however, false-positive results have been known to occur (151).
Protozoa
(i) Entamoeba histolytica.
Antigen-based fecal immunoassays include advantages over other methods currently used for diagnosis of amebiasis. Some of the assays differentiate E. histolytica from E. dispar, they have excellent sensitivity and specificity, they are readily usable by most laboratory personnel, and they have potential use as screening tools in situations such as waterborne outbreaks (144, 149). Because there are distinct genetic differences between E. dispar and E. histolytica, commercial kits have been developed to detect their presence and differentiate them in clinical samples. However, current antigen detection tests require the examination of fresh or frozen (not preserved) stool specimens, while many laboratories have switched to stool collection methods using various preservatives.
(ii) Cryptosporidium spp.
Detection of Cryptosporidium spp. in stool specimens relies on special staining techniques or fecal immunoassays. Permanent stains normally used in ova and parasite examinations do not adequately stain the organism. A number of immunoassay kits for antigen detection are commercially available and are more sensitive and specific than routine microscopic examination (101, 143–149). Stool specimens may be fresh, frozen, or fixed; however, polyvinyl alcohol-fixed specimens are usually unacceptable for use in fecal immunoassays. Based on published information, with rare exceptions, sensitivity and specificity data tend to be comparable for the EIA, DFA, and rapid cartridge formats for the detection of Cryptosporidium species antigen in fecal specimens (97, 98, 100–105). In general, PCR has proven to be more sensitive than microscopy and antigen detection immunoassays; however, cost and time remain issues for improvement.
(iii) Giardia lamblia (G. duodenalis, G. intestinalis).
Antigen detection in stool, duodenal fluids, and serum by DFA, IFA, enzyme-linked immunosorbent assay (ELISA), and immunoblotting methods has been reported (143–150). These tests are reliable and more sensitive than routine ova and parasite examinations. Commercial immunoassay kits are readily available. Users will have to evaluate which kit will be most useful for their own laboratories. Some of the methods may require fresh specimens, and stools fixed in preservatives may not be suitable. Also, some of the kits may not detect both trophozoites and cysts of G. lamblia (G. duodenalis, G. intestinalis) but may be selective for only one life cycle stage. In one case, the fecal antigen detection kit for fresh or frozen specimens detects but does not differentiate Giardia, Cryptosporidium, and Entamoeba histolytica (149).
Helminths.
Although many experimental studies have been undertaken for the detection of helminth antigens, commercial products are not readily available. Coproantigen detection has great potential for the diagnosis of strongyloidiasis, trichinosis, fascioliasis, and possibly Taenia species infections (5).
SPECIAL TECHNIQUES FOR ORGANISMS FOUND IN FECAL SPECIMENS
Larval-Nematode Detection
Special larval-nematode detection methods are used mainly for diagnosing Strongyloides infection when the direct fecal smear or the FEA concentration technique, both of which are relatively insensitive tests, yields negative results. Because Strongyloides are highly intermittent with their shedding patterns, which makes the routine ova and parasite examination unreliable, the consequences of a missed infection might have serious implications for patient management in certain population groups. It is therefore necessary to exclude this organism using a more sensitive testing process (152).
Various migration techniques can be used for the detection of Strongyloides (and other nematode larvae, such as hookworm and Trichostrongylus species) in human stools and are generally categorized into water emergence and agar plate culture methods, with the former becoming increasingly less popular. It is important to remember that all fecal specimens for any larval-detection method must be freshly passed and not refrigerated.
Water emergence methods.
(i) Baermann technique.
The Baermann method (6, 7, 47), originally designed in 1917, is a simple and time-honored but somewhat cumbersome way of recovering small numbers of larvae from stool. It appears to have lost favor to agar plate culture and other methods but is still commonly used, often in a modified form, for detecting larvae in soil samples and with veterinary diagnostics. The test is based on the premise that viable larvae in fresh nonrefrigerated stool will adapt to their free-living cycle by migrating through a bed of gauze and wire mesh into warm water after about an hour's incubation and concentrate at the bottom of the funnel apparatus, where they can then be disgorged for microscopic examination. An advantage of the Baermann method over other methods is the large amount of stool inoculum that can be used for detection of very light infections. It can also be offered as a “same-day service,” as the test takes only about 2 h to complete. A modification has been developed to obviate the space disadvantage of the original methods (153). Compared to the direct smear or FEA concentrate technique, the Baermann method increases the sensitivity of detection of S. stercoralis by 3.6 to 4 times (153).
(ii) Harada-Mori.
The Harada-Mori method (6, 7, 47) is a technique where fresh, unrefrigerated stool is inoculated onto the middle-to-upper portion of a strip of filter paper and partially immersed in a conical tube containing 3 to 5 ml of water. The capillary action of the filter paper ensures a moist transit route for larvae to migrate from the inoculum to the water, where they may be detected microscopically. This method takes up to 10 days to complete, with daily sampling of the deposit, which is a disadvantage if a quick turnaround time is needed to exclude the possibility of a Strongyloides infection. The Harada-Mori method is a more sensitive procedure than the direct smear and FEA concentration but inferior to the Baermann and agar plate migration methods (152).
(iii) Filter paper slant.
With the filter paper slant method, a strip of filter paper the same size as a microscope slide is placed on the slide and then into a petri dish with one end tilted on a piece of glass tubing or something similar and the other end immersed in water in the bottom of the dish. Feces are inoculated onto the filter paper and incubated for up to 10 days at 25 to 28°C. An advantage with this method is that daily examination for motile larvae can be quickly made using a stereomicroscope. Alternatively, a drop or two of water can be pipetted and examined by standard microscopy (5).
Agar plate culture method.
Agar plate culture procedures were introduced in the 1990s and found to be 1.6 to 6 times more sensitive than direct-smear, FEA concentration, or water emergence methods, as well as being inexpensive (nutrient agar plates are cheap and readily available). Agar plate culture is currently regarded as the method of choice (152–157), although two recent studies still report the Baermann method as being superior (154, 157). However, molecular methods for detecting Strongyloides have been shown in the main to be superior to parasitological methods (156, 157).
A nonselective nutrient agar medium that has been thoroughly dried is inoculated with approximately 2 g of fresh, nonrefrigerated feces of soft or loose consistency, directly onto the center of the medium. Although some manuals suggest specific recipes for the agar plate culture medium to be used, it appears that any routine nutrient bacteriological media without selective agents are satisfactory. Soften hard stool with isotonic saline. The lid rim should be taped to the base, sealed in an individual plastic bag, and incubated at warm room temperature for 2 days. Double-walled petri dishes with a glycerin moat are sometimes used to prevent larvae from migrating into the condensation water; however, these plates are not always readily available. As larvae migrate from the fecal inoculum and move over the agar surface, they leave characteristic tracks (furrows), appearing as radiating serpiginous lines which will subsequently develop coliform bacterial colonies. These lines should alert the technician to the presence of migrating larva, and a stereomicroscope with ×40 magnification can assist with their recognition on the plate surface. Rinsing the plate with 5 to 10% buffered formalin and then examining the washing sediment allows for larval identification. The 2-day incubation period used for Strongyloides does not appear to be sufficient for hookworm larvae.
In a study by Koga et al. (158), it was recommended that all plates be examined microscopically (enhanced with the use of a green filter) to detect tracking that would have been missed macroscopically. These authors suggested that all plates be washed with 10% buffered formalin, which is then pipetted into a conical centrifuge tube, and the sediment is examined under a ×40 magnification. This process reveals larvae where only tracks were initially seen and also where worms had become entrapped in bacterial colonies that had formed. Drying the plates was also considered an important factor, as free water on the agar surface diminished the viability of worms.
Safety alert.
With all techniques, it is imperative that safety precautions be adhered to because larvae can easily transform to the infective filariform stage and thus be potentially transmissible upon skin contact. Even condensation water under petri dish lids might harbor L3 larvae. Gloves must be worn at all times, and biological safety cabinet (BSC) and personal protective equipment (PPE), including splash prevention, should be used.
Estimation of Worm Burden
Although it is possible to correlate the egg production of Ascaris lumbricoides, Trichuris trichiura, the hookworms, and Schistosoma mansoni with worm burden, the quantitation of helminth eggs is rarely required in routine diagnostic laboratories. The number of eggs detected in a stool specimen does not significantly influence anthelmintic drug therapy, nor is it needed for posttreatment follow-up.
Most laboratories do not offer worm burden counts; however, when teaching students parasitology, a semiquantitative density chart (Table 5) can aid as a scoring guide of parasite recognition. This semiquantitation method is also used for proficiency testing reporting of helminths.
TABLE 5.
Semiquantitation scoring of helminth eggs and Blastocystis spp.a
| Density | Helminth eggs or larvae on a 10× wet prepn |
|---|---|
| Numerous/heavy/many | ≥10 eggs or larvae/field |
| Moderate | 3–9 eggs or larvae/field |
| Scanty/light/few | ≤2 eggs or larvae/5–10 fields |
| Rare/occasional | 2–5 organisms/entire 22- by 22-mm coverslip area |
Although Blastocystis spp. can be seen on a wet mount, the overall morphology, ability to find the organisms, and quantitation are enhanced from the permanent-stain smear (5).
Helminth egg counting is an inexact science, with many factors contributing to variations and errors in eggs per gram (EPG). These factors include the fecundity of worms and variability in daily egg production, stool consistency influenced by diet and fecal transit times, and the experience and accuracy of the testing technician. It should therefore be taken into consideration when applying the results of helminth egg counts that they do not necessarily correlate with or accurately predict the true worm burden of the host.
The most common egg counting techniques have traditionally been the direct smear (Beaver) (5–7, 47), the Stoll method (5–7, 47), and the Kato-Katz method (5–7, 47, 159–168); however, the FLOTAC (159–164) and McMaster (161, 166) methods are becoming increasingly more popular. Further studies are needed to validate the mini-FLOTAC with other quantitative techniques (McMaster and Kato-Katz) and in different settings where other soil-transmitted helminths are also endemic (169).
Hatching of Schistosome Eggs
Schistosome eggs are normally detected in stool or urine using either direct microscopy or concentration methods. Determining their viability is also a significant factor for the clinician to consider with patient management, as viable eggs represent an active infection. If they are nonviable, it may indicate a “burnt-out” infection or that treatment has been successful even though eggs can still be passed in stool or urine for months afterwards. If stool or urine has been preserved with a fixative or been refrigerated, the ability to determine the viability of eggs seen is nullified; therefore, the hatching of the eggs to release the miracidia in fresh, nonfixed stool is a procedure which not only confirms egg viability but may also increase the sensitivity of detection of a schistosomal infection (5). Certainly if schistosomiasis is suspected as a potential diagnosis, fresh, nonfixed specimens are recommended.
The standard method described for hatching eggs, particularly from those species whose eggs are found in stools, entails the use of an elbowed sidearm flask (few laboratories have these available), but similar vessels, such as Erlenmeyer flasks, are quite satisfactory (5–7).
Fresh, nonrefrigerated feces are homogenized in saline and coarse filtered through gauze. This process is twice repeated with the fecal sediment obtained. The saline suspension is then decanted, replaced with chlorine-free water, and added to a 500-ml sidearm flask so that the water rises 20 to 30 mm vertically in the side arm (the eggs will not hatch in saline). The flask is tightly covered in foil, leaving only the sidearm exposed. Place a bright light near the exposed sidearm and then stand the flask at room temperature, ensuring that the light does not heat the sidearm fluid. With a hand lens, observe for minute white, moving organisms in the sidearm and pipette a drop or two off for microscopic confirmation. An enhancement of this method applied to hatching the miracidia of S. mansoni was shown to be more sensitive than the Kato-Katz method (170). S. mansoni organisms are phototrophic and actively seek a light source; however, S. japonicum is reported to be not attracted to light (171), so this property should be taken into account when attempting to hatch eggs from this species.
The diagnosis of Schistosoma haematobium infection is confirmed by detecting the eggs in urine sediment or using a filtration technique, so there is seldom any point in using egg-hatching techniques for this species. Provided the specimen has not been preserved or refrigerated, viability can frequently be determined by observing either an active miracidium within the egg or flame cell movement (wafting) within the larva. There are four flame cells (sometimes termed solenocytes), one near each corner of the embryo (172). Trypan blue staining is another reliable way of determining viability (173). If viability is still undetermined, urine (preferably that collected between 10:00 a.m. and 2:00 p.m., when there is a daily peak excretion of eggs) is diluted approximately 1 in 10 with chlorine-free tap water in a large flask, in which miracidial hatching will occur in 10 to 15 min. Against a black background, they can be seen with the naked eye as actively motile minute white bodies. S. haematobium miracidia are relatively nonresponsive to light stimulation and will not rise to the source of an illuminated flask (174).
Note that since adult schistosomes may occur in unexpected sites (S. mansoni from urine), both urine and stool specimens must be collected without preservatives and should not be refrigerated before being processed. Regardless of the species suspected, both urine and stool should be examined for every patient with potential schistosomiasis.
Identification of Tapeworm Proglottids
India ink injection.
India ink injection is used for identification of the three tapeworms of the genus Taenia that infect humans (T. solium, T. saginata, T. asiatica), the most significant of which is the pork tapeworm T. solium, which can potentially cause serious neurological and eye disease, with subsequent public health consequences. Because superficially the proglottids of all Taenia spp. are very similar, it is important that an accurate distinction is made, as the sequelae of T. saginata and T. asiatica infection are of lesser significance.
The proglottids of tapeworms are the specimens required for identification purposes, as the eggs of all three species are indistinguishable without special procedures. It is important that species identification be made before the treatment of intestinal taeniasis, as drugs such as praziquantel result in the worm's disintegration in vivo. It is therefore highly unlikely that a complete or undamaged worm will be expelled. Likewise, the scolex, from which identification can often be made, is also unlikely to be recovered. However, from a public health perspective and because of the possibility of cysticercosis (Taenia solium), it is helpful for the clinician to know what species has been identified, if possible.
Proglottids are usually spontaneously passed but rapidly shrink and dry when exposed to the environment. For this testing, proglottids should ideally be submitted to the laboratory frozen or fresh and unfixed in saline or water. On arrival at the laboratory, they should be immediately floated in a petri dish of water. If fixation is required, 70% alcohol is preferable to formalin, which can render them hard and opaque and require prior clearing in lactophenol (50/50 liquid phenol crystals in lactic acid), which may take some hours. The passage of proglottids can be erratic, and if transportation delay is anticipated, the patient should be instructed to put the proglottids that they pass into a specimen container containing methylated spirits (or even drinking spirits) to prevent desiccation. Although India ink injection can be used to differentiate gravid proglottids, very effective empirical therapy and anticipated cure has subsequently reduced the use of this technique. Work in a BSC and use PPE to prevent exposure to potentially infective eggs.
Gravid proglottids, which morphologically are longer than they are wide, are essential for an accurate identification. Place the proglottid onto absorbent paper, and with a tuberculin-style syringe fitted with a 25- or 26-gauge needle, carefully inject India ink through the genital pore (which is a discrete protuberance situated about half way along one side of the segment) so that it ramifies throughout the uterine arms but not so forcefully that it ruptures into the body of the proglottid. It may take a number of attempts on various proglottids to get a “take,” i.e., to ensure that the ink has clearly and cleanly entered and filled each uterine arm. Using forceps, transfer the proglottid onto a glass slide, carefully place another slide on top, and press them together so that the proglottid is gently squashed centrally. Because the proglottid is quite slippery, this can be a frustrating procedure, but once in place, the slides can be temporarily held in a sandwich position with either rubber bands or paper clips.
Identification can then be made by counting the number of ink-stained lateral uterine branches on one side only that emerge from the median stem. A hand lens or a low-power stereomicroscope will probably be required to accurately determine the number. Taenia solium has between 7 and 13 lateral branches and T. saginata between 15 and 20. The gravid proglottids of T. asiatica are practically indistinguishable from those of T. saginata and as such are frequently confused. If specific identification is required, a molecular approach is necessary; however, this is a research option only and very rarely clinically significant (175).
To make a permanent preparation, put the slide through a series of alcohol baths (70%, 90%, and 100%) and two changes of xylene. Depending on the thickness of the proglottid, each alcohol and xylene bath will probably require at least overnight immersion. Once complete, the proglottid will appear parchment-like on one slide only. Unless it has floated off, do not attempt to reposition it, as it will be fragile and likely to tear. It can then be cover mounted with a permanent medium such as Euparal (176) or Eukitt.
If difficulty is experienced with proglottids that have contracted and thereby made India ink injection impractical, the uterine branches may be highlighted by staining with Semichon's acid carmine, although this procedure can take up to a week (5, 7). Longitudinal section cutting of formalin-fixed, paraffin-embedded gravid proglottids is also an alternative procedure; however, they can be somewhat difficult to identify from these sections (177).
The proglottids of Diphyllobothrium spp. (the fish tapeworm or broad tapeworm) are usually passed in chains of segments which can be up to half a meter long. Undamaged, they are quite characteristic; i.e., the proglottids are wider than long, with a rosette-shaped central uterus, the outline of which can be seen when gently crushed between two glass slides. India ink injection is difficult and need not be attempted. Characteristic eggs are usually found abundantly in the feces, or they likewise can be expelled from a gravid proglottid. Species identification of Diphyllobothrium by morphological means is very difficult; the use of DNA analysis and a specialist laboratory are required (178). Proglottids need to be preserved in alcohol rather than formalin for this testing.
Generally, other tapeworms can be identified by the eggs passed in stools. Refer to standard parasitology texts for identification criteria and morphological comparisons (5–7, 47, 48).
OTHER SPECIMENS FROM THE INTESTINAL TRACT
Sigmoidoscopy Specimens
Sigmoidoscopy is a clinical procedure that can be used to obtain specimens for the diagnosis of Entamoeba histolytica or schistosome infection, although the advent of sensitive immunoassays and DNA-based technologies for detecting E. histolytica (179) has probably reduced the need for this procedure. Sigmoidoscopy may still be indicated when permanent-stain films or immunoassays of a series of stool specimens are negative or equivocal for E. histolytica yet clinical suspicion remains. It is essential that there be a close liaison between the clinician and the laboratory scientist to ensure that specimens are correctly collected and processed.
Sigmoidoscopy may reveal the characteristic ulcers of intestinal amebiasis, especially in more severe disease, and allow the clinician to target lesions on the mucosa for specimen collection. Both direct wet preparations and slides for stained films should be made from material from the mucosal surface, which must be scraped or aspirated rather than wiped with absorbent tipped swabs. Specimens should also be taken from multiple sites, with each processed individually. If biopsy specimens for histological examination are taken, they should be preserved in 10% buffered formalin.
If a slide made for direct wet microscopy is to be of any value, a coverslip needs to be placed over the slide (suffused with a drop of saline) at the bedside, and the edges of the slide should be sealed with an agent such as nail polish to prevent drying before the slide is transported stat to the laboratory. At the laboratory, examine for trophozoite motility, initially under a 10× objective magnification. If trophic forms are detected, confirm any red blood cell (RBC) ingestion under a 40× objective magnification. An immediate diagnosis of E. histolytica can be made only if hematophagous trophozoites are seen in the wet preparation. Active directional motility is sometimes described as a diagnostic feature of E. histolytica, but this can be a subjective observation and is only suggestive at best. Nuclear detail cannot be seen in unstained preparations. The use of these direct mounts is currently uncommon and has been supplanted by the use of preserved material.
Fixatives such as those containing PVA or the newer single vials (universal fixatives) should be supplied at the bedside after sigmoidoscopy for making permanent slides for staining. Mucoid sigmoid material needs to be dabbed or scraped onto a glass slide and fixed immediately or mixed thoroughly with a drop or two of fixative containing PVA and then air dried. The making of permanent-stain slides should take precedence over the direct wet preparation, especially if the specimen amount is limited.
A permanent-stain film, such as one stained with trichrome, will confirm ingested RBCs for a definitive diagnosis of E. histolytica; however, the absence of such does not necessarily invalidate a diagnosis, and other features, such as nuclear details of trophozoites, need to be considered (5). The cyst stage of Entamoeba sp. is unlikely to be found from sigmoidoscopy specimens.
It should also be noted that although the WHO has recommended that intestinal E. histolytica infection be diagnosed with a specific test, such as E. histolytica-specific immunoassays (180), these methods have been validated only for fecal specimens and, as such, should not be used without additional validation for mucosal specimens collected after a sigmoidoscopy. DNA-based diagnostics have become increasingly more affordable and are about 100 times more sensitive than ELISA antigen tests (179).
The examination of sigmoid colon biopsy specimens is also considered a sensitive test for the rapid diagnosis of S. mansoni and S. japonicum infections, particularly if fecal testing is negative yet clinical suspicion remains high (181). A number of rectal biopsy specimens taken at sigmoidoscopy or proctoscopy from inflamed or granulomatous lesions or even random sites are gently crushed flat between two glass slides to near transparency and then examined microscopically (initially 40× objective). Some methods suggest soaking the tissue in isotonic saline for 30 to 60 min before examining the tissue (and, if need be, by hydrolysis of fresh tissue in 4% KOH for 18 h at 37°C or at 56°C for fixed tissue, although this procedure will invalidate observation of viable organisms). Schistosome eggs will appear as refractile bodies, often in clusters. Determining egg size and shape and the size and position of the spine definitively identifies the species, and observing flame cell movement within the miracidia (100× objective) will determine viability. Calcified eggs appear as black bodies. Occasionally, S. haematobium eggs are found in the rectal region and need to be differentiated from superficially similar Schistosoma intercalatum eggs. The latter are usually acid fast, so Ziehl-Neelsen staining can assist identification; other schistosome eggs, such as those of S. mansoni and Schistosoma mekongi, also exhibit an acid-fast shell outline (173). S. haematobium can also be diagnosed by demonstrating its characteristic eggs from biopsy specimens taken at cystoscopy using the same technique. Some biopsy specimens should also be preserved in 10% buffered formalin for histological sectioning and staining.
Duodenal Biopsy and Aspiration
At an endoscopy procedure, villus biopsies of the duodenal-jejunal region are usually taken as part of the investigation of upper intestinal disorders, including parasitic infections (5–7). Although biopsy specimens may be variously taken for histological, immunological, or biochemical studies, they are also valuable in detecting Giardia trophozoites in situ (and, more rarely, Strongyloides, Clonorchis [Opisthorchis], and microsporidial infection), especially if repeated stool examinations are negative but clinical suspicion remains. Aspirated duodenal fluid may also be collected for parasite examination.
Specimens collected by a physician at endoscopy for Giardia require immediate transportation at room temperature to the laboratory and need to be examined without delay. Biopsy specimens must be in isotonic saline to prevent desiccation.
Duodenal aspiration fluid, also collected at endoscopy, may sometimes be received for (primarily) Giardia examination (5–7). As with biopsy specimens, the fluid needs to be collected without any preservatives and sent immediately to the laboratory for stat examination. Depending upon the volume received, the specimen may need to be centrifuged (10 min at 500 × g). Examine the deposit for trophic forms, which, as for biopsy specimens, may exhibit only a barely noticeable fluttering movement. If there is mucus in the fluid, remove it with a Pasteur pipette for microscopic examination, as trophozoites tend to adhere to slough. There will be no movement if the specimen has become too cold, so identification will then need to be made by shape, binucleated appearance, and sucking disc on the organism's ventral side. Slides fixed in either Schaudinn's or PVA fixative should also be made for trichrome or iron-hematoxylin staining. If PVA fixative is to be used, mix 2 to 3 drops of the fixative onto a slide containing a drop or two of the aspiration deposit and mix well. Spread the specimen with an applicator stick to ensure that the preparation is thin and thoroughly dry before staining.
Strongyloides larvae in a wet preparation move and may demonstrate thrashing motility under a 10× objective magnification. Because the bile-stained eggs of Clonorchis (Opisthorchis), which have a size of around 30 μm, can easily be missed under ×10 magnification, scanning with a 40× objective is recommended. Examination for Microsporidia will require some specimens to be fixed in 10% buffered formalin and then centrifuged before slides are made for staining.
String Test or Gelatin Capsule Test
The string test, also known as the gelatin capsule method and marketed commercially as the Entero-Test, was devised in 1970 as a procedure to diagnose upper-small-bowel pathogens, especially Strongyloides (182). Although patient cooperation is required, it is a novel, simple, and rapid procedure used to obtain secretions from the duodenum-jejunum region and was also found to detect other parasites, including Giardia, Cryptosporidium, and Cystoisospora, without the need for endoscopy. More rarely, other parasites may also be found using this method, including Clonorchis (Opisthorchis), Fasciola, Trichostrongylus, and hookworm eggs. This testing procedure has also been applied to nonparasitological investigations, such as measuring mucosal inflammation in eosinophilic esophagitis and as a device to sample the esophageal microbiome. The use of the string test for diagnosing giardiasis has, however, probably declined over the years, being surpassed by sensitive fecal antigen detection tests that have since become available.
The string test is a gelatin capsule containing a coiled 90-cm line for children or a 140-cm line for adults and a 1-gram weight (5–7). The line consists of 20 cm of silicone rubber-covered thread and 70 cm of soft nylon yarn, one end of which is taped to the patient's cheek or ear. The capsule is swallowed and the line plays out, the gelatin dissolves in the stomach, and the weighted line passes through to the upper small intestine, where the weight becomes disengaged with the line and eventually is expelled in the stool. The line is left in position for around 4 h and then retrieved. Four to five drops of bile-stained mucus can be “milked” with thumb and forefinger off the terminal end of the string and expressed into a small tube, or the whole string can be placed into a petri dish, sealed with tape, and sent immediately to a laboratory. Disposable gloves must be worn when this procedure is performed.
A wet preparation is made from a drop of the fluid, and the complete coverslip area is examined for parasites. Giardia trophozoites adhere to any mucus portions and may exhibit fluttering motility (40× to 100× objectives), but a permanent-stain film should also be prepared. Mix a drop of the fluid on a slide and fix it in Schaudinn's fixative, or prepare a thin slide of the mucus mixed with a drop of PVA fixative and thoroughly dry the slide before staining. Other fecal fixatives appropriate for permanent staining are also acceptable. Strongyloides larvae are actively motile in the wet preparation (10× objective), but care must be taken to distinguish embryonating Strongyloides eggs from those of hookworm. Other helminth eggs are visible in the wet preparation (10× to 40× objectives). Although Cryptosporidium oocysts may appear as small refractile bodies and Cystoisospora oocysts are characteristically large and contain one or two sporonts, they need to be confirmed with a modified Ziehl-Neelsen stain or by demonstration of epifluorescence. Record also the color of the string, as a yellow-bile stain indicates that the line reached the duodenal-jejunal region.
STOOL CULTURE OF PROTOZOAN PARASITES
Introduction, Xenic, and Axenic Culture
Increasingly, culture of parasites has been deemphasized because of cutting-edge research in molecular biology that facilitates pathogen identification and diagnosis even when small numbers of organisms are present in a sample. Culture options are limited to large, more-complex laboratories and are not routinely available. It should be noted, however, that culturing parasites is essential for diverse areas of research where large numbers of parasites free of bacteria, fungi, and host materials are needed. An important advantage of in vitro culture, especially for axenic cultures, is its provision of a continuous supply of pure organisms without any interfering bacterial, fungal, or host tissue contamination. If practical, culture of the parasite should be attempted, even when identification of the organism has already been made, so that a bank of isolates can be established for further antigenic, molecular, and biochemical research, as well as epidemiologic investigations. In vitro culture is also invaluable for screening potential therapeutic agents that can interfere with the development of the parasite so that the infection can be terminated. Further, in vitro culture can be useful in elucidating isolate or strain differences that may provide helpful insights into the treatment of patients, as well as in developing and assessing the efficacies of vaccines. Additionally, cultured parasites are essential for developing animal models wherein experimental animals can be infected with the parasites to understand the pathological process and to develop effective candidate therapeutics. Although in vitro cultivation of the various parasites discussed here constitutes a continuing challenge, it nevertheless is a fruitful area of research (183).
Amoebae grown in association with unknown bacterial associates are called xenic cultures. If the amoebae are grown in association with a single known bacterium, the culture is called monoxenic; if amoebae are grown with several identified bacterial flora, then the culture is called polyxenic. If amoebae are grown as pure culture without any bacterial or other associated organisms, then the culture is called axenic.
Entamoeba histolytica
The history of cultivation of the intestinal parasites, especially Entamoeba histolytica, is long and dates back to 1925, when Boeck and Drbohlav (184) isolated and cultured E. histolytica in a diphasic egg slant medium that they had developed for culturing intestinal flagellates. Dobell and Laidlaw (185) modified the egg slant medium by replacing Locke's solution, which contains dextrose, with Ringer's solution and by replacing the liquid overlay with egg albumin or serum diluted with Ringer's solution and adding particulate rice starch at the interphase of the liquid overlay and solid slant. In 1946, Balamuth (186) reported on an improved egg yolk monophasic medium for the cultivation of intestinal amoebae. Robinson (187) developed a complex biphasic medium containing agar slants, Bacto peptone, erythromycin, a defined “R” medium with Escherichia coli, and phthalate solution. In 1982, Diamond (188) developed a new monophasic medium (TYSGM-9) for the cultivation of E. histolytica and several lumen-dwelling protists. This medium consisted of buffered salt solution, casein digest peptone, yeast extract, gastric mucin, heat-inactivated bovine serum, and rice starch in screw-cap tubes. The amoebae could easily be examined directly within the culture tube, and the density and movement of the amoebae could be monitored without removing an aliquot. Three media, modified Boeck and Drbohlav's egg slant medium, Robinson's medium, and Diamond's TYSGM-9, are used most often for the isolation and subsequent cultivation of E. histolytica (189). Culture of E. histolytica serves only as a supplemental procedure and never replaces the primary diagnosis by routine fecal examinations, PCR, or an E. histolytica-specific antigen assay. Even when the culture system is within quality control guidelines, a negative culture is still not definitive in ruling out the presence of E. histolytica.
Entamoeba dispar
Previously called nonpathogenic E. histolytica, E. dispar can be grown in several culture media (modified Boeck and Drbohlav's egg slant, Robinson's, and Diamond's TYSGM-9 media) that are normally used for cultivating E. histolytica. However, E. dispar resists growing in the axenic medium designed for E. histolytica. Clark reported growing E. dispar initially in the axenic medium supplemented with irradiated bacteria and later on with Escherichia coli fixed with either glutaraldehyde or formalin (190). After a few years of growing with the killed bacteria, the amoebae finally adapted to growth in the axenic medium without any bacterial supplementation (190). Kobayashi et al. (191) designed a medium (YIGADHA-S) containing yeast-iron-gluconic acid-dihydroxyacetone-serum and a vitamin mixture to cultivate E. dispar axenically. This medium is based on Diamond's casein-free yeast extract-iron-serum (YI-S) medium (192, 193). According to those authors, the main differences from YI-S medium are replacement of glucose by gluconic acid, addition of dihydroxyacetone and d-galacturonic acid monohydrate, and sterilization by filtration. This medium promoted the axenic growth of 5 strains of E. dispar (2 strains of nonhuman primate isolates and 3 strains of human isolates).
Blastocystis spp.
Blastocystis is an obligate anaerobic parasite with worldwide distribution. It is a common inhabitant of the gastrointestinal tracts of humans as well as a wide variety of vertebrates, including pigs, cattle, rats, mice, chickens, and reptiles. Although identified as a possible agent of gastrointestinal illness, its pathogenic status has not been clearly established (194). After being tossed around in various taxonomic groups, it has finally been classified as a stramenopile (along with golden brown algae, brown algae, chrysophytes, diatoms, water molds/oomycetes, slime nets, bicosoecids) based on the phylogenetic analysis of the 5 rRNAs (195). A commonly used culture medium for Blastocystis spp. is modified whole-egg slant medium with a Locke solution overlay used for the isolation of Entamoeba spp. (196); it contains 30% horse serum or 20% human serum (197). Blastocystis spp. can also be grown in Diamond's TP-S-1 or Balamuth's medium, although growth is not as good as that in the egg slant medium. If the tubes containing fecal material are positive for Blastocystis spp. after 48 h of incubation, then confirm the identification using a permanent-stain smear and/or a digital image. If negative, an additional 48 h of incubation can be performed after subculture.
Giardia lamblia (G. duodenalis, G. intestinalis)
Karapetyan (198) was the first to culture Giardia lamblia (G. duodenalis, G. intestinalis) in 1960 in a mixed-culture medium containing the yeast Candida guilliermondii and chick fibroblasts. In 1962 (199), he reported monoxenic cultivation of Giardia duodenalis (a rabbit isolate) with Saccharomyces cerevisiae. In 1970, axenic cultivation of Giardia from the rabbit, chinchilla, and cat (200) was achieved by Meyer after migrating the trophozoites in a U-shaped glass tube and separating the protozoa from the yeast. Giardia lamblia from the duodenal aspirate of a woman with an 8-year history of giardiasis was finally isolated and cultured with the accompanying yeast in two different liquid media (HSP-1 and HSP-2) (201). The HSP-1 medium consisted of Phytone Peptone, glucose, l-cysteine hydrochloride, Hanks' balance salt solution, and 15% human serum and antibiotics (penicillin and streptomycin). HSP-2 medium consisted of 100 ml HSP-1 medium plus 7.5 ml NCTC-135 (Gibco) and 2.5 ml glutathione-cysteine reducing solution. Since some of the human sera inhibited the growth of Giardia in the HSP-2 medium, Visvesvara (202) experimented with Diamond's TPS-1 medium used for the cultivation of E. histolytica. He found that G. lamblia (G. duodenalis, G. intestinalis) did not grow in autoclaved TP-S-1 medium but grew well in TPS-1 medium that was filter sterilized. Gordts et al. (203) cultured Giardia in TPS-1 medium by directly inoculating the parasites from the human intestines. Since Panmede (a liver digest and a component of the TPS-1 medium) was no longer available and liver digest from other sources did not support good growth of Giardia, efforts were made to come up with a medium that supported the growth of not only Giardia but other protists, including E. histolytica. Finally, Diamond's TYI-S-33 medium, devised to grow E. histolytica, was used by Keister (204), with some modification to support the growth of Giardia axenically. Because of the new manufacturing methods of some of the components (casein digest peptone), the growth of these parasites was somewhat erratic; a new medium (YI-S) consisting of yeast extract, iron, and serum, which supported the growth of both Giardia and E. histolytica, was designed (193). Bingham and Meyer (205) described a method of treating mature Giardia cysts with hydrochloric acid (pH 2), which allowed the excystation of the parasite and thus facilitated its axenic cultivation. In most cases, acid treatment kills off all bacteria, and the resulting culture is axenic. Therefore, this method is preferable because invasive procedures like collection of duodenal aspirate and biopsy specimen are negated.
Dientamoeba fragilis
Although Dientamoeba fragilis was considered nonpathogenic and thought to lack a cyst stage, recent studies indicate otherwise (206, 207). Dobell (208) was probably the first to establish thriving cultures of D. fragilis in a diphasic medium that consisted of a slant of inspissated horse serum overlaid with egg whites in Ringer's solution, with rice starch added at the confluence of the solid and liquid media. It has been cultured by others in Locke egg (LE) medium, TYSGM medium, Balamuth's egg yolk infusion medium, Robinson's medium, and other media with mixed bacterial flora.
Recently, Barratt et al. (209) reviewed the previous work on the culture media used and compared different culture media for their ability to support the growth of several isolates of D. fragilis, their optimum temperatures for growth, the optimum method for the cryopreservation of the isolates, and important bacterial associates in D. fragilis cultures. Based on their exhaustive research, Barratt et al. have shown that the best culture medium to grow D. fragilis is Loffler's medium, although the LE medium and Robinson's medium support fairly good growth of the organisms. Development of either a monoxenic or an axenic culture of D. fragilis has not yet been developed.
Balantidium (Neobalantidium) coli
Balantidium coli is a large, ciliated protozoon found in the gastrointestinal tracts of humans, nonhuman primates, pigs, and cattle, although its normal host is pigs. Humans can acquire the infection via the fecal-oral route, and human-to-human transmission may also occur. Humans may remain asymptomatic, as does the pig, or may develop dysentery similar to that caused by Entamoeba histolytica. B. coli is often referred to as an opportunistic pathogen (210, 211). Recently, the nomenclature of the mammal-infecting Balantidium genus has been changed to Neobalantidium based on the internal transcribed spacer (ITS) region of the small-subunit (SSU) ribosomal-DNA (rDNA) tree and morphological differences. Since Balantidium causes no pathology in poikilothermic hosts, but N. coli is pathogenic to warm-blooded animals, the term balantidiasis is retained to describe the infection in humans. Currently, the term Balantidium coli is still in use for human infections; however, the name change may occur in the future.
Although, Barret and Yarbrough (212) used a simple saline-serum medium for the cultivation of Balantidium, it can be isolated and cultivated in a wide variety of media, including LE, Jones', Robinson's, Balamuth's, Dobell's HSre with starch, and TYSGM-9 media, which have been used for the cultivation of E. histolytica (210). It can also be maintained monoxenically with Escherichia coli or other intestinal bacteria without much difficulty (210). It can be grown at a broad temperature range, from 25°C to 40°C (213).
Cryptosporidium spp.
The obligate, intracellular, protozoan parasites of the genus Cryptosporidium infect epithelial cells lining the digestive and respiratory tracts of a wide range of animal hosts. Typical of related parasites, Cryptosporidium features a complex life cycle, including asexual and sexual stages, culminating in the production of small, environmentally hardy oocysts. This monoxenous parasite can multiply to tremendous numbers in the host by “recycling” asexual stages and producing thin-walled oocysts in the gut that mature and release infectious sporozoites in the same host (autoinfection). Most human infections are attributed to two species: C. parvum (zoonotic transmission) and C. hominis (anthroponotic transmission) (214–216, 277), and the majority of in vitro studies have focused on isolates of these species.
Basic in vitro culture methods for Cryptosporidium species have been developed since the first report of success in 1983 (217). Indeed, cryptosporidial growth has been evaluated in a wide range of cell lines, but the majority of published in vitro studies use the following cell lines: human colonic tumor cells (HCT-8, CCl-224), Madin Darby canine kidney cells (MDCK, CCL-34), and human colonic adenocarcinoma cells (Caco-2, HTB-37). In vitro development of C. parvum through asexual and sexual stages is routinely accomplished in cell lines, and continuous culture has recently been reported (218, 219, 283).
Microsporidia
Although more than 1,200 species belonging to 143 genera of the Microsporidia have been described, only parasites belonging to 9 genera and 13 species are known to cause human disease. They are Anncaliia, Encephalitozoon, Enterocytozoon, Microsporidium, Nosema, Tubulinosema, Pleistophora, Trachipleistophora, and Vittaforma. However, Enterocytozoon bieneusi, the three species of Encephalitozoon (E. cuniculi, E. hellem, and E. intestinalis), and Anncaliia algerae are the most frequently found to infect the humans. Only short-term culture of E. bieneusi is possible so far. E. cuniculi, E. hellem, E. intestinalis, and Anncaliia algerae have been cultured from urine, sputum, and corneal smears and cryopreserved indefinitely (220). However, none of these Microsporidia have been cultured directly from the stool.
MOLECULAR METHODS
The first molecular assays have largely been replaced by rapid-cycle (i.e., real-time) assays and more recently by FDA-cleared products which are simple to use. The current trend in molecular microbiology is the introduction of syndrome-based tests. For example, rather than ordering tests for individual pathogens that may be responsible for gastroenteritis (e.g., rotavirus, stool culture, and O&P), there are options for ordering FDA-cleared multiplex panels that include a variety of pathogens that may be responsible for the disease.
A variety of molecular tools have been used to study parasites and parasite-host relationships. At the least one PCR assay can be found in the literature for essentially every human parasite. A combined Ovid Medline search of the entire database, which linked the keywords “molecular” and “parasite” yielded 16,893 results, whereas a search for “Giardia” and “PCR” yielded 647 results. Therefore, this section concentrates on more-recent applications, particularly those adapted to rapid-cycle PCR formats, multiplex assays, FDA-approved/cleared assays [the use of “cleared/510(k)” or “approved/premarket approval (PMA)” depends on the classification of the medical device], and other public health and clinically relevant studies.
Application to Parasitology
Molecular tools have been used for a variety of purposes in the study of parasitology. Many of these applications involve a further understanding of the biology of parasites and, although important, are not immediately relevant to this manuscript. Similarly, these tools have been used by veterinary parasitologists and environmental microbiologists. References to these studies will be made only when there is pertinence to human infections, such as when zoonotic transmission or food- or waterborne infections are studied. There have been no FDA-approved/cleared molecular diagnostic assays in the recent past. Numerous laboratory-developed assays that target a variety of parasites have been developed. Although numerous papers have disclosed excellent sensitivity and overall performance for many of these assays, there has been limited adoption in clinical microbiology laboratories for a number of reasons.
Methods of extraction and/or more-robust PCR assays needed to be developed to overcome the various inhibitors that may be found in stool (221). Additionally, and to this day, a single assay that affords detection of the full range of the parasites that may be found via an ova and parasite microscopic examination has not been developed. Furthermore, the medical community has been quite satisfied with the sensitivity of the Giardia and/or Cryptosporidium DFA microscopy and EIAs that are commercially available and moderately priced. The added cost of PCR for select pathogens, in conjunction with the need to retain the O&P, has been a deterrent to the incorporation of molecular diagnostics into clinical parasitology.
A variety of molecular-laboratory-developed tests (LDTs) that target parasites have been developed. This section will predominantly review LDTs that target common intestinal parasites, such as Giardia and Entamoeba histolytica, and parasites that infect immunocompromised hosts, such as Cryptosporidium and Microsporidia species. These results can be particularly helpful in outbreak investigations and epidemiological studies. It is also important to remember that some state public health laboratories require that stools positive for certain organisms (Cryptosporidium) be submitted for confirmatory testing and typing. The LDTs have been developed as both monoplex and multiplex assays, with each developer including targets of interest for particular reasons. Commercial vendors have responded to the needs of the clinical laboratory through the development of syndromic test panels. These syndromic panels target the most common causes of viral respiratory tract infections, the most frequent causes of meningitis and sepsis, and (pertinent to this review) the common causes of infectious enterocolitis. These panels may replace the stool culture bench, while the Giardia/Cryptosporidium EIAs will likely significantly decrease the number of O&Ps that are performed. A number of these assays have received FDA clearance at the time of this writing, and several contain parasite targets. These assays are prepackaged and easy to use and target the bacteria, viruses, and parasites most likely to cause infectious diarrhea. The compositions of panels vary slightly from vendor to vendor, but each represents a new diagnostic tool worthy of thorough consideration.
Laboratory-Developed Tests for Gastrointestinal Parasites, Monoplex
Whether a molecular assay for a particular parasite is clinically useful (i.e., it represents a practical assay for implementation in a clinical laboratory and may improve turnaround time) is a question that will ultimately be determined in laboratories across the country. Although an assay may be technically feasible to perform, it may not be practical to implement for a variety of reasons. Potential problems include (i) the inability to obtain sufficient material for an adequate validation, a process that is under increased scrutiny by the FDA; (ii) clinical volumes being insufficient to support implementing the assay; and (c) the fact that traditional methods are competitive and possibly detect other pathogens. For example, one may initially consider a PCR assay for Ascaris lumbricoides to represent an advance in testing due to improved sensitivity over the traditional O&P. Practically, however, Ascaris is not commonly encountered in resource-rich countries, which are likely able to afford such an assay. More importantly, this assay detects only Ascaris, whereas the traditional O&P, albeit less sensitive in this scenario, detects a variety of gastrointestinal parasites, as well as commensal protozoa. The introduction of molecular tests for gastrointestinal parasites is, therefore, complicated. The patient population served, the medical question(s) being asked of the laboratory, alternative approaches, and technical, economic, and practical feasibility must all be considered and largely aligned to successfully implement these assays.
Monoplex Giardia PCR.
A large number of PCR-based and other molecular tools to detect and characterize Giardia lamblia (G. duodenalis, G. intestinalis) have been described. These are often used to detect and characterize Giardia in animals, food handlers, water supplies, or other environmental sources (222–224). The genetic targets used for detection have included the genes for triose phosphate isomerase and the small ribosomal subunit (225). There are a number of genetic variants of Giardia lamblia (G. duodenalis, G. intestinalis), termed assemblages, which have been described. There are currently eight genetic assemblages, A to H. Certain assemblages, such as A and B, are more common in humans, whereas others are more common in cattle (e.g., A and E). Consideration of these variants is important for primer design; otherwise, primer-target nucleotide mismatch may occur, resulting in insufficient detection of certain assemblages (226).
Monoplex Entamoeba histolytica PCR.
Entamoeba histolytica is one of the more important gastrointestinal pathogens to detect because of the invasive nature of the disease that it causes. E. histolytica may cause hepatic amoebic abscesses and, rarely, pleuropulmonary disease, in addition to ulceration in the colon (227). Other sites of extraintestinal infections have been reported, but they are significantly less common (228). PCR has been used as a test complementary to serology for the diagnosis of amoebic hepatic liver abscess, since the stool is often free of amoebae during this phase of disease, and amoebae may be difficult to visualize and differentiate from histiocytes in the direct examination of aspirated abscess contents (229).
The morphological diagnosis of E. histolytica enterocolitis is challenging given the existence of morphologically identical or highly similar organisms, such as E. dispar. These organisms cannot be differentiated by morphological studies alone and are also characterized together by the commercially available enzyme immunoassays. Fortunately, these organisms can be differentiated by a variety of molecular methods.
Methods that have been used for differentiation of E. histolytica from other amoebae include PCR-restriction fragment length polymorphism (RFLP) analysis and real-time PCR with either SYBR green or specific probes (230–232). The use of highly sensitive tests that differentiate E. histolytica from E. dispar and other morphologically similar amoebae has allowed us to refine the determination of the prevalence and incidence of infections, to detect the presence of the organism in zoonotic hosts, to discover uncommon modes of transmission, and to study the epidemiology of the disease (233–237). Not surprisingly, the prevalence of infection by E. histolytica has been demonstrated to be lower than previously thought, since the methods used (i.e., microscopy and enzyme immunoassay) mischaracterized E. dispar as E. histolytica (238).
There have been a number of additional benefits from the expanded use of molecular methods for the detection and characterization of E. histolytica. Foremost, it has clarified the true sensitivity of the morphological methods that are still in use by most laboratories. For example, in a comparative evaluation of specimens from patients with dysentery in Egypt, Rashed et al. found that 40% were positive by microscopy but that 52% were positive by real-time PCR (239). Additionally, PCR has been used to evaluate the effectiveness of routine methods, such as formalin-ether sedimentation and trichrome staining, for the detection of E. histolytica (240). Additionally, there have been rigorous assessments of various types of nucleic acid amplification assays in an effort to determine the best approach for the detection of this pathogen (235, 240–244). In addition to a variety of PCR-based methods, other methods of nucleic acid amplification, such as loop-mediated isothermal amplification (LAMP), have been used to detect E. histolytica (245).
Molecular studies have disclosed a high degree of genetic polymorphism among strains of E. histolytica (246). Researchers have studied variation in genetic expression between virulent and avirulent strains to better understand the disease process (247). Perhaps most interesting, Jaiswal and colleagues studied the allelic variation in E. histolytica and correlated this with the clinical phenotypes of amebiasis (e.g., patients with dysentery versus asymptomatic cyst passers) and found correlations between certain alleles and disease states (248).
Monoplex Cryptosporidium species PCR.
As with Giardia, a variety of molecular assays have been described for a number of applications. In contrast to early thoughts, there are a variety of human and animal Cryptosporidium species that may infect humans. Assays predominantly for the study of Cryptosporidium in animals have been described. Zoonotic transmission of Cryptosporidium is well known from animals such as cattle, but even household pets may be a potential source of infection (249). The molecular characterization of Cryptosporidium from humans reveals clues to the sources of infection. Ebner et al., for example, performed a PCR-based survey of people with cryptosporidiosis from northern Australia and encountered C. hominis (subgenotype IdA18), the Cryptosporidium mink genotype (IIA16R1), and C. felis (225). When Iqbal et al. studied the prevalence of Cryptosporidium in the Qikiqtani region of Nunavut, Canada, and characterized its isolates, they discovered Cryptosporidium in 15.7% of patients with diarrhea; in contrast, Giardia was present in 4.6% (250). All the cryptosporidia in this study were C. parvum, subgenotype IIa, which suggested zoonotic transmission, although it was noted that human-to-human transmission could not be excluded. In rural Ethiopia, in contrast, the PCR-based prevalence disclosed 10.9% of the infections to be caused by Giardia, with only 1.1% caused by Cryptosporidium species (251). Although differences in the prevalences of particular pathogens vary by population and location, which is expected, molecular diagnostics remain an important means by which to detect and characterize these pathogens.
From a clinical point of view, however, it does not matter what species, strain, or genotype/subtype of Cryptosporidium is responsible for causing the infection; the patient management and treatment approach will be the same. There are no recognized drug susceptibility differences between cryptosporidial isolates or strains (so far), and general treatment (supportive therapy) is universal for all cryptosporidial diarrhea.
Cryptosporidium has been shown to be transmissible through contaminated water. Therefore, it is not surprising that a number of molecular assays have been described for testing water. These assays have been used for quantitation of parasites to study methods of water treatment and water-related outbreak tracking (230, 252, 253).
Molecular methods have been used to study and clarify the epidemiology of cryptosporidiosis. In addition to being used to study zoonotic and waterborne infection, these methods have been used to study the seasonality of disease, as well as differences in gender and age distributions (254). These tools have been used to study the burden of disease in resource-limited countries and further clarify the relatively high prevalence of disease in patients with HIV and in children in day care centers, in whom transmission readily occurs (255–257). Although these methods have been useful because of exquisite sensitivity and the ability to genotype the parasites, Frickmann et al. recommend caution when interpreting positive signals in high-prevalence settings, suggesting that these may be secondary to asymptomatic carriage or residual DNA from previous infections (258).
The introduction of PCR assays for Cryptosporidium species into clinical laboratories, particularly in the United States, has been somewhat limited due to the absence, until recently, of FDA-approved assays and because of the high quality of easy-to-use enzyme immunoassays. This will likely change in the future because of recently released FDA-cleared assays (see below). The excellent sensitivity of PCR-based assays for the detection of these pathogens has been demonstrated. Stensvold et al. used PCR to study Cryptosporidium infections in Denmark from 2010 to 2014 (259). They warn that outbreaks may not be detected if diagnostic tests of limited sensitivity continue to be used. An audit of diagnostics for cryptosporidiosis in 85 publicly funded clinical microbiology laboratories in England and Wales disclosed that only 1% (1/85) of the laboratories used PCR as the diagnostic method; the majority (80%; 68/85) used microscopy with either modified Ziehl-Neelsen or auramine phenol staining, and the remainder (19%; 16/85) used enzyme immunoassays.
Monoplex PCR for Microsporidia.
Although Microsporidia have been reclassified as fungi, the majority of testing is performed in a parasitology laboratory (279). Many laboratories still use traditional staining methods, such as the modified trichrome stain, for the detection of Microsporidia (113). These stains are challenging to interpret; therefore, diagnostic criteria have been developed and alternative staining methods (such as calcofluor white) used (133, 260, 261). Therefore, not surprisingly, a number of PCR-based assays have been developed.
Enhanced nucleic acid extraction methods have been studied as an important preanalytic parameter (262). One of the challenges in the molecular diagnosis of microsporidiosis is that a variety of taxonomically distinct Microsporidia species may cause disease. Early assays used traditional PCR and, in some instances, employed postamplification analysis, such as RFLP analysis to differentiate the most common species (263, 264). The developed assays commonly target the small-subunit rRNA, which contains both conserved regions for broad-range primer hybridization and taxonomically unique regions that may be used for species-level differentiation.
Rinder et al. performed an interesting blind study wherein 50 stool specimens were shared with six laboratories performing microscopy and six performing PCR. The sensitivity and specificity of the PCR were 67% and 98% and those for microscopy were 54% and 95%, respectively (265). Interestingly, these authors concluded that interlaboratory differences were likely more important than differences between methods.
Laboratory-Developed Tests for Gastrointestinal Parasites, Multiplex
There are a number of challenges in the construction and application of multiplex assays. In addition to all the challenges encountered with monoplex assays (e.g., inhibitors, primer and probe design, etc.), the opportunity for intermolecular interactions increases significantly with each additional primer and probe set added to the mixture. Therefore, sophisticated primer and probe design programs and molecular biologists who understand these interactions are needed to design functional, complex assays. Regardless of the expertise of the technician, these multiplex assays, by the sheer nature of the increased intermolecular interactions, have a lower analytical sensitivity than corresponding monoplex assays. The most important question for clinical applications, even if the analytical sensitivity is reduced, is “Is the analytical sensitivity sufficient to produce a clinical sensitivity to appropriately categorize all patients with disease?” When this is the case, then a multiplex assay that is clinically useful has been produced. Fortunately, advances in specimen preparation (i.e., extraction methods), more-robust PCR (i.e., with DNA polymerases that are less susceptible to inhibition), and advances in PCR design software have afforded the creation of clinically useful multiplex PCR assays for enteric pathogens.
A large number of laboratory-developed PCR assays that target a variety of pathogens, depending on the interest of the investigators and the population served, have been developed. A full review of these is beyond the scope of this text, but select assays are reviewed. These studies have been important to demonstrate the feasibility of this approach to the detection of parasites in a complex matrix.
Some researchers have developed multiplex assays that target only select protozoal enteric pathogens. Stark and colleagues undertook the development and assessment of a multiplex PCR for the detection of four enteric protozoal parasites, Giardia, Cryptosporidium, Dientamoeba fragilis, and Entamoeba histolytica (266). They evaluated the newly designed multiplex PCR both against monoplex PCRs directed against the same targets and against traditional microscopy using modified iron-hematoxylin staining. They evaluated 427 fecal specimens that were routinely submitted to a clinical microbiology laboratory. The multiplex PCR detected Giardia in 28 specimens, D. fragilis in 26 specimens, E. histolytica in 11 specimens, and Cryptosporidium in 9 specimens; these results were uniformly corroborated by the four monoplex assays that targeted these organisms. The sensitivities and specificities of the morphological assessment for the four pathogens were, respectively, as follows: 50% and 100% for Giardia, 38% and 99% for D. fragilis, 47% and 97% for E. histolytica, and 56% and 100% for Cryptosporidium.
Taniuchi et al. took a different approach to the screening of stool specimens for intestinal parasites, selecting to screen for both select protozoal and select helminthic pathogens (267). This assay combined the high sensitivity of PCR with the differentiating capability afforded by the Luminex bead technology. In short, Taniuchi and colleagues developed two multiplex PCR assays, one of which contains primer mixes that target protozoal parasites and another that targets helminthic parasites. The PCR products from these reactions were then hybridized with Luminex beads that contain species-specific probes for enteric parasites. They assessed 319 fecal specimens and compared this approach with another previously described multiplex assay. They reported an 83% sensitivity and 100% specificity for this approach and recommend it as a possible screen for enteric parasites. Interestingly, in another assay, Taniuchi and colleagues developed a multiplex assay that detected Cyclospora, Cryptosporidium, and microsporidial targets, which would be particularly useful for immunocompromised patients (268).
Basuni et al. also targeted both protozoa and helminths, but with a slightly different approach (269). They designed two multiplex PCR assays, one that targeted select protozoa and another that targeted select helminths. The intestinal protozoal assay targeted E. histolytica, Giardia, and Cryptosporidium, whereas the intestinal helminth assay targeted Ancylostoma duodenale, Ascaris lumbricoides, S. stercoralis, and Necator americanus. Both assays contained internal amplification controls. The results of these multiplex assays were compared with microscopic examinations performed on direct smears and following zinc-sulfate concentration and Kato-Katz thick-smear techniques performed on 225 fecal specimens from patients suspected of having infections. Microscopy detected the presence of eight specimens positive for helminths, whereas the multiplex assay detected 46 (P < 0.001). Similarly, only 4 specimens were found to contain protozoa by microscopy, whereas 18 were detected by PCR (P < 0.001). Although the enhanced sensitivity of the molecular approach is evident, the importance of a broadly inclusive panel was also evident, as three instances of T. trichiura infections were detected by microscopy, but this organism was not present in the helminth multiplex PCR, so it was missed by that method.
FDA-Cleared Multiplex Assays
There are several FDA-approved multiplex assays that include parasite targets, including the xTAG gastrointestinal pathogen panel (Luminex, Austin, TX) and the BioFire FilmArray gastrointestinal panel (bioMérieux, Durham, NC). The xTAG gastrointestinal pathogen panel was the first multiplex assay to receive FDA approval. This assay, which was originally formulated for the Luminex 100/200 system, has now been modified for the MAGPIX system. This modification retains the excellent performance of the assay on a new platform that is more user-friendly (i.e., it eliminates wash steps) and is a closed system. In addition to detecting 8 bacteria (9 in the international version) and 3 viruses, this assay detects Giardia, Cryptosporidium, and Entamoeba histolytica. An internal amplification control is also included in this assay.
Coste et al. compared this assay to conventional methods to study the etiologic agents of severe diarrhea in renal transplant recipients (270). Diarrheal stools from 49 patients were studied. Thirteen (23%) of the stool specimens were shown to contain an enteric pathogen by conventional methods, whereas 39 specimens (72%) were shown to contain an enteric pathogen using the xTAG gastrointestinal pathogen panel. Among these infected patients, one was found to have Giardia by conventional methods. When the specimens were studied with the xTAG gastrointestinal pathogen panel, the patient with Giardia was found to also have an infection with Campylobacter; additionally, another patient was found to be infected with Cryptosporidium, which was not discovered with conventional studies.
Perry et al. similarly disclosed advantages of a multiplex molecular platform when they compared two commercially available multiplex assays (i.e., the Luminex xTAG gastrointestinal pathogen panel and the Savyon Diagnostics gastrointestinal panel [available outside the United States]) with conventional diagnostics. They studied 1,000 clinical diarrheal stool specimens for the variety of pathogens included in these assays. Regarding intestinal parasites, Giardia was detected in 10 stools by the Luminex xTAG gastrointestinal pathogen panel, in 8 stools by the Savyon Diagnostics gastrointestinal panel, and in 6 stools by conventional diagnostics. The Savyon Diagnostics gastrointestinal panel contains D. fragilis, whereas the Luminex xTAG gastrointestinal pathogen panel does not. The Savyon assay detected D. fragilis in 45 stools, but no organisms of this species were detected by conventional diagnostics, and none, of course, were detected by the Luminex assay because D. fragilis was not included in the panel. The authors were able to corroborate the presence of D. fragilis in 44/45 of these stools by an alternative method (271). This study, again, confirms the need for expanded panels directed against a wider variety of parasites.
The FilmArray gastrointestinal panel is another commercially available product that has received FDA approval. In addition to detecting 12 enteric bacterial pathogens and five groups of viruses, this assay detects Cryptosporidium, Cyclospora, E. histolytica, and Giardia. The FilmArray reactions and analysis occur in a pouch that contains a number of chambers, wherein different reactions occur. Following a simple loading procedure, the pouch is placed into an instrument that controls the reactions and analysis. This simple-to-use approach brings complex molecular diagnostic capabilities to even small hospital laboratories. Buss and colleagues undertook a multicenter evaluation of the BioFire FilmArray gastrointestinal panel (272). This group prospectively collected and studied 1,556 clinical stool specimens and, in addition to the BioFire assay, tested these with conventional and other molecular assays. They determined the sensitivities of the FilmArray assay to be 100% for 12 of the 22 targets and >97.1% for 7 of the 22 targets, and the sensitivity could not be determined for the remaining analytes due to a low prevalence of the organism targets. The patients with parasitic infections detected in this study consisted of 27 patients with Giardia, 24 with Cryptosporidium, and a surprising 19 with Cyclospora, all of which were unsuspected and part of an outbreak. There were no infections with E. histolytica detected. A combination of traditional and molecular assays was used to determine the sensitivities and specificities of the individual parasites targeted within the BioFire FilmArray gastrointestinal panel. This study demonstrated the following sensitivities and specificities, respectively, for this assay: 100% and 99.5% for Giardia, 100% and 100% for Cyclospora, and 100% and 99.6% for Cryptosporidium.
Future Possibilities
There are always challenges that arise whenever new technologies are introduced and procedures are changed. One of the challenges with many organism-specific molecular diagnostic tests is that they detect only the organism for which they were designed. For example, a Giardia-specific PCR would be appropriately negative for a patient whose stool O&P disclosed Cystoisospora as the causative agent of infection. Broad-range and multiplex assays have addressed some of these issues, but gaps remain. The approach to building multiplex assays that address syndromes is generally good, since clinical findings alone are insufficient to differentiate the classes of the infectious agents causing disease (273). The current FDA-approved assays are excellent initial assays, but a second tier of assays is needed, particularly in parasitology. The helminths, including Strongyloides, and other parasites, such as D. fragilis, Blastocystis spp., Cystoisospora, and Microsporidia, among others, are not addressed. However, many of these inclusive panels are currently under development. Perhaps the most concerning challenge is the likely associated loss of microscopic morphological expertise when the majority of testing is converted from traditional to molecular assays. Next-generation sequencing, which consists of a variety of techniques to accomplish massive parallel sequencing, holds promise in several ways. Foremost, the usefulness of this type of technology to fully characterize the microbiomes of individuals with a particular disease, such as gastroenteritis, provides the opportunity to fully characterize all the microorganisms present in an attempt to determine the presence and type of pathogens, regardless of their taxonomic position. Furthermore, analysis of the transcriptome affords the ability to determine which microorganisms are likely involved in disease-producing processes and which are simply commensal or beneficial microbiota. Although this work and our understanding are nascent, this type of work will afford a more thorough understanding of the pathogens present, the pathophysiology of disease, and, finally, the construction of even more thorough syndrome-based assays.
CONCLUSIONS
In summary, we emphasize that this Practical Guidance for Clinical Microbiology document on the laboratory diagnosis of parasites from the gastrointestinal tract provides practical and clinically relevant guidelines for the recovery and identification of human parasites found in a particular body site. Generally, these methods are nonautomated and require extensive bench experience for accurate performance and interpretation. The information contained within this document is based on a comprehensive literature review and expert consensus on relevant diagnostic methods. The document content was not intended to include didactic information on human parasite life cycles, organism morphology, clinical disease, pathogenesis, treatment, or epidemiology and prevention. There are a number of excellent texts available that contain this type of information. As greater emphasis is placed on neglected tropical diseases, it is very likely that patients with gastrointestinal parasitic infections will become more widely recognized in areas of endemicity and nonendemicity. The capabilities of clinical microbiologists and other health care providers in diagnostic parasitology will remain highly important and in demand for the appropriate and comprehensive care of patients with these infections.
Biographies

Lynne S. Garcia, former Manager of the UCLA Clinical Microbiology Laboratory, is currently the Director of LSG & Associates, providing training, teaching, and consultation for diagnostic medical parasitology and health care administration. She has given over 400 presentations (international, national, and local) and published over 175 manuscripts, book chapters, and articles and is the author of Diagnostic Medical Parasitology (5) and Practical Guide to Diagnostic Parasitology (50), published by ASM Press, Washington, DC. She has been the Editor in Chief of the Clinical Microbiology Procedures Handbook (10) and is the Editor in Chief of Clinical Laboratory Management (45). She is a reviewer for 9 journals. She consults for the CAP Microbiology Resource Committee, was chair of the NCCLS Parasitology Subcommittee, and is a Fellow of the American Academy of Microbiology. Lynne is the recipient of the ASM bioMérieux Sonnenwirth Award for Leadership in Clinical Microbiology and the 2016 SCASM T. D. Beckwith Award for Accomplishments and Contributions to the Science of Microbiology and SCASM.

Michael Arrowood, Ph.D., is a research microbiologist in the Division of Foodborne, Waterborne, and Environmental Diseases at the Centers for Disease Control and Prevention. Dr. Arrowood completed a B.S. in biology at Southern Arkansas University, an M.S. in microbiology at Louisiana Tech University, and a Ph.D. in microbiology at the University of Arizona. Postdoctoral studies in parasitology continued at both the University of Arizona and Utah State University. Dr. Arrowood joined the CDC in Atlanta, GA, in 1991, where his primary focus is on Cryptosporidium and Cyclospora parasites. Since 1983, he has been involved in research on Cryptosporidium spp. and, more recently, on Cyclospora spp. He has developed standardized methods for the production and purification of the parasites from experimentally infected animals and naturally infected humans, developed monoclonal antibody-based diagnostic assays for cryptosporidial oocysts in stool and water samples, developed in vitro cultivation methods for C. parvum, and participated in the development of animal models of acute and chronic cryptosporidiosis in neonatal and adult mice. He has collaborated in the characterization of immunological responses to cryptosporidiosis, the evaluation of disinfection strategies for cryptosporidial oocysts, the characterization of potential anticryptosporidial drug therapies using in vitro culture and in vivo animal models, and the development of diagnostic methods for Cyclospora, as well as genome sequencing and analysis. He is author/coauthor of >100 refereed publications and 8 book chapters.

Evelyne Kokoskin, M.Sc., ART, received her master's degree from McGill University in microbiology and immunology. She also holds advanced certification as a registered technologist with the Canadian Society of Medical Laboratory Sciences in microbiology. In 2007, she was awarded the Fellowship of the Canadian Society of Medical Laboratory Sciences for her continued commitment to the profession. Evelyne spent over 20 years at the J. D. Maclean Centre for Tropical Diseases as a medical scientist in pursuit of intestinal, blood, and tissue parasitic diseases; her responsibilities included research and teaching and serving as head of the Quebec blood and tissue parasite reference and quality assurance program. She currently is the team leader for technical and educational development for the Public Health Ontario Laboratories. Her responsibilities include parasitology proficiency, exploring innovative technologies for in-house training and professional development, and developing and creating measureable competency assessments in different disciplines. She has extensive international teaching experience in medical laboratory sciences, particularly parasitology. She also is certified as a WHO malaria instructor and has authored an English and French malaria manual.

Graeme P. Paltridge was a senior medical laboratory scientist in microbiology with a specialist interest in diagnostic parasitology. He was the section manager of the Canterbury Health Laboratories (Christchurch, New Zealand) bacteriology and parasitology laboratory for 22 years. He has had extensive involvement in teaching parasitology, including teaching in developing countries, in particular Vietnam.

Dylan R. Pillai is a medical microbiologist and infectious diseases physician in Calgary, Canada. His main area of practice is in diagnostic parasitology, which includes using molecular methods with a research emphasis on malaria and drug resistance. He has field experience in several countries, including Laos, Peru, and Ethiopia.

Gary W. Procop, M.D., M.S., is Director of the Parasitology, Molecular Microbiology, Virology, and Mycology laboratories and Medical Director for Enterprise Test Utilization and Pathology Consultative Services at the Cleveland Clinic. He is past Chair of the Departments of Clinical and Molecular Pathology. He completed a Bachelor of Science at Eastern Michigan University, followed by an M.D. and M.S. at Marshall University School of Medicine. He did residency training in Anatomic and Clinical Pathology training at Duke University Medical Center and a Clinical Microbiology Fellowship at the Mayo Clinic. He is a diplomat of the American Board of Pathology in Anatomic and Clinical Pathology and in Medical Microbiology. He has given more than 550 scientific presentations and has 176 published manuscripts, 39 chapters, and two books to his credit. His primary interests are developing and promoting best practices in laboratory testing, the practical applications of molecular diagnostic methods for the diagnosis and treatment of infections, infectious disease pathology, mycology, and parasitology. He enjoys sailing, fencing, and genealogical research.

Norbert Ryan is a Senior Scientist in the Bacteriology Laboratory at the Victorian Infectious Diseases Reference Laboratory, Peter Doherty Institute for Infection and Immunity, Melbourne, Australia. Prior experience included working in microbiology at the former Fairfield Infectious Diseases Hospital, Melbourne, where there was a strong emphasis on diagnosis of travelers' diarrhea and other travel-related illnesses. Areas of interest other than parasitology include diagnosis of enteric infections, Legionnaires' disease, and sexually transmitted infections. Initial interest in parasitology stemmed from involvement in the (then) intensive health screening programs provided for refugees and migrants coming to Australia from countries such as Uganda, Eritrea, El Salvador, Vietnam, Laos, and Cambodia over the period 1985 to 1996. This extensive background in parasitology has led to provision of control material and participation in workshops and training programs. The attempted analysis of the principles and application of staining methods for diagnosis of protozoal infections and Microsporidia has been a specialty field.

Robyn Y. Shimizu has been a Clinical Laboratory Scientist in the Department of Pathology and Laboratory Medicine at the University of California, Los Angeles, CA, since 1979. She has assumed the role of a technical specialist and teaching coordinator in microbiology, concentrating in the areas of parasitology, mycology, and mycobacteriology. She has collaborated on numerous publications, including the Manual of Clinical Microbiology (56) and the Clinical Microbiology Procedures Handbook (2).

Govinda Visvesvara (Vish), Ph.D., officially retired from the Centers for Disease Control and Prevention, Atlanta, GA, on 30 September 2013 after a distinguished career in identifying, diagnosing, and improving methods for culturing free-living and pathogenic protists. During his tenure at the CDC, he isolated several organisms from clinical samples and correlated them with species found in the patients' environments, which is a critical step for better understanding how a disease is spread and, most importantly, how to prevent other people from becoming infected. Vish identified Acanthamoeba as the agent of keratitis in wearers of soft contact lenses and indicated that Brachiola algerae, a microsporidian, also can cause keratitis and other infections in humans. He discovered Balamuthia mandrillaris as the agent of meningoencephalitis in humans and other animals, discovered Naegleria fowleri as the agent of primary amoebic meningoencephalitis in animals, and identified Sappinia as the agent of a human brain infection. He established Giardia intestinalis cultures in cell-free medium and developed methods for immunofluorescence and other staining protocols for identifying Brachiola algerae, Giardia intestinalis, and Cyclospora. After the onset of HIV/AIDS and the recognition that several protists were the agents of opportunistic infections among immunodeficient patients, he added the Microsporidia, such as Encephalitozoon hellem, E. cuniculi, E. (Septata) intestinalis, and Enterocytozoon bieneusi, as well as Brachiola algerae and Vittaforma corneae, to his areas of expertise. His research has earned him numerous honors and awards, including election to fellowship in the American Academy of Microbiology and the CDC's McDade Award for Lifetime Scientific Achievement. He has published over 350 articles, book chapters, and reviews reporting work from morphological, immunologic, and molecular aspects of protists to proteomics and mass spectrometry. He enjoys traveling and photography.
APPENDIX 1
Tables A1 to A3 contain morphological and clinical information pertaining to some of the more common protozoa and helminths infecting humans. In addition to key diagnostic criteria, information related to body site is included. Table A4 contains relevant information for the development of learning tools for training physicians. Specific training and learning objectives are included, as well as laboratory information resources that may be helpful in making sure that important clinical information is included in physician training.
TABLE A1.
Characteristics of intestinal protozoa, in particular, trophozoites of common amoebaeb
| Organism(s) | Size (diam or length [μm])a | Motility | Visibility and no. of nuclei | Peripheral chromatin (stained) characteristic(s) | Karyosome (stained) | Cytoplasm appearance (stained) | Inclusions (stained) |
|---|---|---|---|---|---|---|---|
| Entamoeba histolytica | 12–60 (usual range, 15–20; invasive forms may be over 20) | Progressive and directional, with hyaline, finger-like pseudopodia; motility may be rapid | Difficult to see in unstained preparations; 1 nucleus | Fine granules that are uniform in size are present and usually evenly distributed; may have beaded appearance | Small and usually compact; centrally located but may also be eccentric | Finely granular, “ground-glass” appearance; clear differentiation of ectoplasm and endoplasm; if present, vacuoles are usually small | Noninvasive organism may contain bacteria; the presence of RBCs is diagnostic |
| Entamoeba dispar/Entamoeba moshkovskii | Same as E. histolytica | Same as E. histolytica | Same as E. histolytica | Same as E. histolytica | Same as E. histolytica | Same as E. histolytica | Organisms usually contain bacteria; RBCs are usually not present in the cytoplasm |
| Entamoeba hartmanni | 5–12 (usual range, 8–10) | Usually nonprogressive | Usually not seen in unstained preparations; 1 nucleus | Nucleus may stain more darkly than in E. histolytica/E. dispar and may be stain dependent, although morphologies are similar; chromatin may appear as a solid ring rather than beaded | Usually small and compact; may be centrally located or eccentric | Finely granular | May contain bacteria; no RBCs |
| Entamoeba coli | 15–50 (usual range, 20–25) | Sluggish nondirectional, with blunt, granular pseudopodia | Often visible in unstained preparations; 1 nucleus | May be clumped and unevenly arranged on the membrane; may also appear as a solid, dark ring with no beads or clumps | Large, not compact; may or may not be eccentric; may be diffuse and darkly stained | Granular, with little differentiation into ectoplasm and endoplasm; usually vacuolated | Bacteria, yeasts, other debris are found in inclusion bodies |
| Endolimax nana | 6–12 (usual range, 8–10) | Sluggish, usually nonprogressive | Occasionally visible in unstained preparations; 1 nucleus | Usually no peripheral chromatin; nuclear chromatin may be quite variable; perikaryosomal space is clear | Large, irregularly shaped; may appear “blot-like”; many nuclear variations are common; may mimic E. hartmanni or D. fragilis | Granular, vacuolated | Bacteria |
| Iodamoeba bütschlii | 8–20 (usual range, 12–15) | Sluggish, usually nonprogressive | Usually not visible in unstained preparations; 1 nucleus | Usually no peripheral chromatin | Large, may be surrounded by refractile granules that are difficult to see (“basket nucleus”) and create a darker perikaryosomal space | Granular, may be heavily vacuolated | Bacteria |
Wet preparation measurements (on permanent stains, organisms usually measure 1 to 2 μm less).
Adapted from reference 5.
TABLE A2.
Key characteristics of protozoa of the intestinal tract and urogenital systema
| Organism(s) (characteristic[s]) | Trophozoite or tissue stage | Cyst or other stage in specimen | Comments |
|---|---|---|---|
| Amoebae (shrinkage occurs in cyst forms in stained preparations, creating a halo which should be included in the measurement) | |||
| Entamoeba histolytica (pathogenic) | Cytoplasm is clean; the presence of RBCs is diagnostic, but the cytoplasm may also contain some ingested bacteria; peripheral nuclear chromatin is usually evenly distributed, with a central, compact karyosome | A mature cyst contains 4 nuclei; chromatoidal bars have smooth, rounded ends; the organism cannot be differentiated from E. dispar | Considered pathogenic; should be reported to public health authorities; trophozoites can be confused with macrophages and cysts can be confused with PMNs in stools |
| Entamoeba dispar (nonpathogenic) | Morphology identical to that of E. histolytica (confirmed by the presence of RBCs in the cytoplasm); if no RBCs are present, molecular testing or fecal immunoassays are necessary to confirm species designation | A mature cyst has a morphology identical to that of E. histolytica | Nonpathogenic; morphology resembles that of E. histolytica; these organisms should be reported as Entamoeba histolytica/E. dispar and reported to public health authorities; immunoassay reagents are now available to identify the Entamoeba histolytica/E. dispar group and to differentiate pathogenic E. histolytica and nonpathogenic E. dispar; some laboratories may decide to use these reagents on a routine basis, depending on the positivity rate and cost |
| Entamoeba histolytica/E. dispar (“group” or “complex” should be added to indicate that the two organisms are indistinguishable and require additional testing; some like to add the word “group” to indicate that the two organisms cannot be differentiated on the basis of morphology unless RBCs are seen within the cytoplasm or E. histolytica is confirmed using species-specific immunoassays) | Use the correct way to report, unless a species-specific immunoassay is used to identify E. histolytica or trophozoites are seen with ingested RBCs (E. histolytica) | ||
| Entamoeba hartmanni (nonpathogenic) | Looks identical to E. histolytica/E. dispar but is smaller (<12 μm); RBCs are not ingested | A mature cyst contains 4 nuclei but often has only 2; chromatoidal bars are often present and look like those of E. histolytica/E. dispar (size, <10 μm); very fine-looking organism | Shrinkage occurs on the permanent stain due to dehydration steps (especially in the cyst form); E. histolytica/E. dispar may actually be below the 12- and 10-μm cutoff limits and can be as much as 1.5 μm below the limits quoted for wet prepn measurements |
| Entamoeba coli (nonpathogenic) | Cytoplasm is dirty and may contain ingested bacteria or debris; peripheral nuclear chromatin is unevenly distributed, with a large, eccentric karyosome | A mature cyst contains 8 nuclei; more may be seen; chromatoidal bars (if present) tend to have sharp, pointed ends | If a smear is too thick or thin and if the stain is too dark or light, E. histolytica/E. dispar and E. coli can often be confused, since there is much overlap in morphology |
| Endolimax nana (nonpathogenic) | Cytoplasm is clean, not diagnostic, with a great deal of nuclear variation; there may even be some peripheral nuclear chromatin; perikaryosomal space is usually clean looking; normally only karyosomes are visible | The cyst is round to oval, with the 4 nuclear karyosomes being visible as miniature versions of the trophozoite karyosome | There is more nuclear variation in this amoeba than in any others; the organisms can be confused with Dientamoeba fragilis and/or E. hartmanni by inexperienced microscopists |
| Iodamoeba bütschlii (nonpathogenic) | Cytoplasm contains much debris; organisms are usually larger than E. nana but may look similar; large karyosome | The cyst contains a single nucleus (may be a basket nucleus) with bits of nuclear chromatin arranged on the nuclear membrane (the karyosome is the basket, the bits of chromatin are the handle); large glycogen vacuole; the perikaryosomal space is slightly darker due to the presence of chromatin fibrils | The glycogen vacuole stains brown with the addition of iodine in the wet prepn; a basket nucleus is more common in cysts but can be seen in trophozoites; the vacuole may be so large that the cyst collapses on itself |
| Blastocystis spp. (pathogenic; the organisms are undergoing review for possible reclassification; multiple strains or subtypes look the same [approx half are pathogenic, half are nonpathogenic]; numerous subtypes from different species are not all pathogenic for humans) | Trophozoites may/may not be seen; often in patients with diarrhea; difficult to identify | Central-body forms are the most common; there is tremendous size variation; the central area may or may not stain; the outer perimeter contains multiple nuclei (often seen as variously sized dots) | This is the most common gastrointestinal tract organism worldwide; it is much more common than Giardia or Dientamoeba (whose numbers tend to be equal, although Dientamoeba organisms are more common than Giardia organisms in many areas); symptomatic patients tend to be treated when >5 cysts/high-power field are reported |
| Flagellates | |||
| Giardia lamblia (pathogenic) | Trophozoites are teardrop shaped from the front and like a curved spoon from the side; they contain 2 nuclei, linear axonemes, and curved median bodies | Cysts are round to oval, containing 4 nuclei, axonemes, and median bodies | Organisms live in the duodenum, and multiple stool specimens may be negative; additional sampling techniques (aspiration, Entero-Test) may be needed; fecal immunoassays are helpful; assemblages A and B are pathogenic to humans; other assemblages have a narrow host specificity |
| Chilomastix mesnili (nonpathogenic) | Trophozoites are teardrop shaped; the cytostome is usually visible for identification; the nucleus is usually situated at the anterior end | The cyst is lemon shaped with 1 nucleus and a curved fibril, called the shepherd's crook (cytostome remnant) | The cyst can be identified much more easily than the trophozoite form; the trophozoite looks like some of the other small flagellates |
| Dientamoeba fragilis (pathogenic) | Cytoplasm contains debris; may contain 1 or 2 nuclei (chromatin is often fragmented into 4 packets) | The cyst form has now been identified; it appears to have a double wall; the percentage is quite low (∼1–2%); thus, it can be very difficult to find and identify | Tremendous size and shape range on a single smear; trophozoites with 1 nucleus can resemble E. nana; staining quality is important to produce packets, not a single “blob” |
| Trichomonas vaginalis (pathogenic) | Supporting rod (axostyle) is present; the undulating membrane comes halfway down the organism; small dots may be seen in the cytoplasm along the axostyle | No known cyst form | Recovered from the genitourinary system; often diagnosed at bedside with wet prepn (motility) |
| Pentatrichomonas hominis (nonpathogenic) | Supporting rod (axostyle) is present; the undulating membrane comes all the way down the organism; small dots may be seen in the cytoplasm along the axostyle; karyosome appears granular | No known cyst form | Recovered in stool; trophozoites may resemble other small flagellate trophozoites |
| Ciliates | |||
| Balantidium coli (pathogenic) | Very large trophozoites (50–100 μm long) covered with cilia; a large bean-shaped macronucleus is present; the very small micronucleus is difficult to see | Morphology is not significant, with the exception of a large, bean-shaped macronucleus; a small micronucleus is difficult to see | Rarely seen in the United States; causes severe diarrhea with large fluid loss; organisms are seen in proficiency test specimens or possibly people who work around pigs |
| Apicomplexa, coccidia | |||
| Cryptosporidium spp. (pathogenic) | Seen in the intestinal mucosa (edge of brush border), gallbladder, and lungs; present in biopsy specimens | Oocysts are seen in stool and/or sputum; organisms are acid fast and measure 4–6 μm; they are hard to find if only a few are present | Chronic infection occurs in a compromised host (internal autoinfective cycle), and self-cure occurs in an immunocompetent host; numbers of oocysts correlate with stool consistency; organisms can cause severe, watery diarrhea; oocysts are immediately infective when passed |
| Cyclospora cayetanensis (pathogenic) | Experience with this organism is not extensive; it may be difficult to identify in tissue; since patients are immunocompetent, biopsy specimens will rarely be required or requested | Oocysts are seen in stool (approx 8–10 μm in size); they are unsporulated and thus difficult to recognize as coccidia; they mimic Cryptosporidium on modified acid-fast-stained smears, they are larger, and they may appear almost colorless or darkly stained in acid-fast smears | Most infections are associated with immunocompetent individuals but may also be seen in immunosuppressed patients; may be associated with traveler's diarrhea; oocysts are not immediately infective when passed; within the United States, infections have been associated with contaminated food, including raspberries, basil, snow peas, and mesclun (baby lettuce leaves), which are considered “transmission vehicles; PCR can detect 40 or fewer oocysts per 100 g of raspberries or basil but has a detection limit of around 1,000 per 100 g in mesclun lettuce |
| Cystoisospora belli (pathogenic) | Seen in intestinal mucosal cells; seen in biopsy specimens; not as common as Cryptosporidium | Oocysts are seen in stool; organisms are acid fast; the best technique is concn, not a permanent-stain smear | Thought to be the only Cystoisospora sp. that infects humans; oocysts are not immediately infective when passed |
| Microsporidia Nosema spp. Encephalitozoon spp. Pleistophora spp. Trachipleistophora spp. Anncaliia sp. Enterocytozoon spp. Microsporidium spp. Vittaforma corneae Tubulinosema sp. | Developing stages are sometimes difficult to identify; spores can be identified by size, shape, and the presence of polar tubules | Depending on the genus involved, spores can be identified in stool or urine using the modified trichrome stain, calcofluor white, or immunoassay reagents (available outside the United States) | Spores are generally quite small (1–2.0 μm for Enterocytozoon spp.) and can easily be confused with other organisms or artifacts (particularly in stool); infections tend to be present in immunosuppressed patients; however, they are not limited to this patient group |
Modified from reference 10. RBC, red blood cells; PMN, polymorphonuclear neutrophils.
TABLE A3.
Key characteristics of helminthsa
| Helminth(s) | Diagnostic stageb | Comments |
|---|---|---|
| Nematodes (roundworms) | ||
| Ascaris lumbricoides | Eggs are both fertilized (oval to round with a thick, mammillated/tuberculated shell) and unfertilized (tend to be more oval or elongate, with an exaggeratedly bumpy shell); eggs can be found in stool; adult worms are 10–12 in. and found in stool; rarely (in severe infections), migrating larvae can be found in sputum | Unfertilized eggs do not float by the flotation concn method; adult worms tend to migrate when irritated (by anesthesia or high fever); hence, patients from areas of endemic infection should be checked for infection prior to elective surgery |
| Trichuris trichiura (whipworm) | Eggs are barrel shaped with 2 clear, polar plugs; adult worms are rarely seen; eggs should be quantitated (rare, few, etc.), since light infections may not be treated | Dual infections with A. lumbricoides may be seen (both infections are acquired from ingestion of eggs from contaminated soil); in severe infections, rectal prolapse may occur in children, or bloody diarrhea can be mistaken for amoebiasis (these clinical manifestations are usually not seen in the United States) |
| Enterobius vermicularis (pinworm) | Eggs are football shaped with one flattened side; adult worms are about 3/8 in. long and white, with a pointed tail; females migrate from the anus and deposit eggs on the perianal skin | Causes itching in some patients; the test of choice is cellulose tape prepn; 4–6 consecutive daily tapes are necessary to rule out infection; symptomatic patients are often treated without actual confirmation of infection; eggs become infective within a few hours |
| Ancylostoma duodenale (Old World hookworm), Necator americanus (New World hookworm | Eggs of these two species are identical; they are oval with broadly rounded ends, a thin shell, and a clear space between the shell and developing embryo (8 to 16 cell stages); adult worms are rarely seen in clinical specimens | Causes blood loss anemia on differential smears in patients with heavy infections; if stool remains unpreserved for several hours or days, the eggs may continue to develop and hatch; rhabditiform larvae may resemble those of Strongyloides stercoralis |
| Strongyloides stercoralis | Rhabditiform larvae (noninfective) are usually found in the stool; they have a short buccal cavity or capsule with large genital primordial packet of cells (“short and sexy”); in very heavy infections, larvae are occasionally found in sputum; filariform (infective) larvae can be found in stool (with a slit in the tail) | May cause unexplained eosinophilia, abdominal pain, unexplained episodes of sepsis and/or meningitis, and pneumonia (migrating larvae) in compromised patients; the potential for internal autoinfection can maintain low-level infections for many years (a patient is asymptomatic or has eosinophilia); hyperinfection can occur in compromised patients (leading to disseminated strongyloidiasis and death); agar plate culture is the most sensitive diagnostic method; many infections are low level, and larvae are difficult to recover |
| Ancylostoma braziliensis (dog or cat hookworm | Humans are accidental hosts; larvae wander through the outer layer of the skin, creating tracks (causing severe itching and eosinophilia); no practical microbiological diagnostic tests exist | Cause of cutaneous larva migrans; the typical setup for infection is when dogs and cats defecate in sand boxes and hookworm eggs hatch and penetrate human skin when in contact with infected sand or soil (children playing in sand box) |
| Toxocara cati and T. canis (cat and dog ascarid | Humans are accidental hosts; infection is by ingestion of dog or cat ascarid eggs in contaminated soil; larvae wander through deep tissues (including the eye); can be mistaken for cancer of the eye; serologic tests are helpful for confirmation; infection causes eosinophilia | Cause of visceral larva migrans and ocular larva migrans; requests for laboratory services often originate in an ophthalmology clinic; serology may be helpful |
| Cestodes (tapeworms) | ||
| Taenia saginata (beef tapeworm) | A scolex (4 suckers, no hooklets) and gravid proglottid (with 12 branches on a single side) are diagnostic; eggs indicate Taenia spp. only (thick, striated shell containing a 6-hooked embryo or oncosphere); worm usually about 12–15 ft long | Adult worms cause symptoms in some individuals; infection occurs via ingestion of raw or poorly cooked beef; there is usually only a single worm per patient; individual proglottids may crawl from the anus; India ink can be injected into proglottids to visualize the uterine branches for identification |
| Taenia solium (pork tapeworm) | A scolex (4 suckers with hooklets) and gravid proglottid (with 12 branches on a single side) are diagnostic; eggs indicate Taenia spp. only (with a thick, striated shell containing a 6-hooked embryo or oncosphere); the worm is usually about 6–20 ft long | Adult worms cause gastrointestinal complaints in some individuals; cysticercosis (accidental ingestion of eggs) can cause severe central nervous system symptoms; infection is via ingestion of raw or poorly cooked pork; there is usually only a single worm per patient; occasionally 2 or 3 proglottids (hooked together) are passed; India ink can be injected into proglottids to visualize the uterine branches for identification; cysticerci are normally small and contained within an enclosing membrane; they occasionally develop as the “racemose” type, in which the worm tissue grows in the body like a metastatic cancer |
| Diphyllobothrium latum (broad fish tapeworm) | A scolex (lateral sucking groove) is present; the gravid proglottid is wider than long, with reproductive structures in the center “rosette”; eggs are operculated | Causes gastrointestinal complaints in some individuals; infection is via ingestion of raw or poorly cooked freshwater fish; the life cycle has 2 intermediate hosts (copepod and fish); worms may reach 30 ft long; the illness is associated with vitamin B12 deficiency in genetically susceptible groups (e.g., Scandinavians) |
| Hymenolepis nana (dwarf tapeworm) | Adult worms are not normally seen; eggs are round to oval and have a thin shell containing a 6-hooked embryo or oncosphere with polar filaments lying between the embryo and egg shell | Causes gastrointestinal complaints in some individuals; infection is via ingestion of eggs (the only life cycle in which the intermediate host [grain beetle] can be bypassed); the life cycle of the egg to the larval form to the adult can be completed in humans; this may be the most common tapeworm in the world |
| Hymenolepis diminuta (rat tapeworm) | Adult worms are not normally seen; eggs are round to oval and have a thin shell containing a 6-hooked embryo or oncosphere without polar filaments lying between the embryo and egg shell | Uncommon; eggs can be confused with H. nana eggs; eggs are submitted in proficiency testing specimens and must be differentiated from those of H. nana |
| Echinococcus granulosus | Adult worms are found only in carnivores (dog); hydatid cysts develop (primarily in the liver) when humans accidentally ingest eggs of the dog tapeworms; cysts contain daughter cysts and many scolices; a laboratory should examine fluid aspirated from a cyst at surgery | Humans are accidental intermediate hosts; the normal life cycle is from a dog to a sheep, with the hydatid cysts developing in the liver, lung, etc., of the sheep; human hosts may be unaware of their infection unless fluid leaks from the cyst (can trigger an anaphylactic reaction) or pain is felt at the cyst location |
| Echinococcus multilocularis | Adult worms are found only in carnivores (fox or wolf); hydatid cysts develop (primarily in the liver) when humans accidentally ingest eggs of the carnivore tapeworms; cysts grow like a metastatic cancer, with no limiting membrane | Humans are accidental intermediate hosts; prognosis is poor; surgical removal of the tapeworm tissue is very difficult; this organism is found in Canada, Alaska, and, less frequently, in the northern United States but is becoming more common in the United States, where the geographic range is moving further south |
| Trematodes (flukes) | ||
| Fasciolopsis buski (giant intestinal fluke) | Eggs are found in stool; they are very large and operculated (their morphology is like that of F. hepatica eggs) | Symptoms depend on worm burden; the organism is acquired from ingestion of plant material on which metacercariae have encysted (e.g., water chestnuts); worms are hermaphroditic |
| Fasciola hepatica (sheep liver fluke) | Eggs are found in stool; cannot be differentiated from those of F. buski | Symptoms depend on worm burden; the organism is acquired from ingestion of plant material on which metacercariae have encysted (e.g., watercress); worms are hermaphroditic |
| Clonorchis (Opisthorchis) sinensis (Chinese liver fluke) | Eggs are found in stool; very small (35 μm); they are operculated with shoulders, into which the operculum fits | Symptoms depend on worm burden; the organism is acquired from ingestion of raw fish; eggs can be missed unless a 40× objective is used for examination; eggs can resemble those of Metagonimus yokogawai and Heterophyes heterophyes (small intestinal flukes); worms are hermaphroditic |
| Paragonimus spp. (lung fluke) | Eggs are coughed up in sputum (brownish “iron filings” are egg packets); can be recovered in sputum or stool (if swallowed); are operculated with shoulders, into which the operculum fits | Symptoms depend on worm burden and egg deposition; infection is acquired from ingestion of raw crabs; eggs can be confused with those of D. latum; infections seen in the Orient (infections with Paragonimus mexicanus are found in Central and South America); Paragonimus kellicotti infections (rare) are seen in the United States; worms are hermaphroditic but often cross-fertilize with another worm if present |
| Schistosoma mansoni (blood fluke) | Eggs are recovered in stool (large lateral spine); specimens should be collected with no preservatives (to allow demonstration of egg viability); worms occur in veins of the large intestine | Acquired from skin penetration by a single cercaria from a freshwater snail; pathological findings are caused by the host immune response to the presence of eggs in tissues; adult worms in veins cause no problems; adult worms are separate sexes |
| Schistosoma haematobium (blood fluke) | Eggs are recovered in urine (large terminal spine); specimens should be collected with no preservatives (to allow demonstration of egg viability); worms occur in veins of the bladder | Acquired from skin penetration by a single cercaria from a freshwater snail; pathological findings are as with S. mansoni; 24-h and spot urine samples should be collected; chronic infection has an association with bladder cancer; adult worms are separate sexes |
| Schistosoma japonicum (blood fluke) | Eggs are recovered in stool (very small lateral spine); specimens should be collected with no preservatives (to allow demonstration of egg viability); worms occur in veins of the small intestine | Acquired from skin penetration by multiple cercariae from a freshwater snail; pathological findings are as with S. mansoni; infection is usually the most severe of the 3 Schistosoma species because of the original loading dose of infective cercariae from a freshwater snail (multiple cercariae stick together); symptoms are associated with egg production, which is greatest in S. japonicum infections |
Modified from reference 10.
1 in. = 2.54 cm; 1 ft = 30.48 cm.
TABLE A4.
Learning tools for training clinicians
| Objective | Type of activity | Learning toolsa | Method of assessment |
|---|---|---|---|
| Review the serological and microscopic diagnostic tests available for the detection and identification of parasites | Didactic | https://www.cdc.gov/dpdx/index.html; Approach to Parasitic Infections manual (Merck) | Accurately order tests based on the diagnosis and symptoms |
| Review the various parasites infecting humans and their morphology, life cycle, and epidemiology | Didactic | https://www.cdc.gov/dpdx/index.html; www.parasite-diagnosis.ch/home; WebMicroscope website; www.atlas-protozoa.com; other didactic prepared material | Pass the online quizzes for helminths and protozoa with 80% accuracy based on the didactic information |
| Review case histories for relevant information related to a correct diagnosis | Didactic | Reference 5; Medical Chemical Corporation website | Respond correctly to questions about the case and provide the correct parasite etiology |
| Microscopic examination of known concentrates and stained smears to review the morphological features of the various helminths and protozoans | Laboratory | Known concentrates of all protozoans; known Kinyoun-stained, modified-trichrome-stained, and other stained smears of all the protozoans; known concentrates of helminths; known macroscopic worms | Examine and identify unknown concentrates and stained smears with 80% accuracy |
| Review and observe the concn and staining procedures for fecal specimens | Laboratory and didactic | SOPs for specimen processing; relevant literature | Pass the online quiz |
| Review the routine operation and Kohler illumination of the microscope; review the calibration of the microscope | Laboratory | SOPs for microscope operation and maintenance; observation of Kohler illumination; use of websites for tutorials; http://www.microscopyu.com/; http://www.olympusmicro.com/ | Accurately set up the microscope for Kohler illumination; accurately measure various parasites for size accuracy |
| Report pathogenic and nonpathogenic parasites accurately with proper information | Didactic | SOPs; LIS of institution | Report examples of specimens with 100% accuracy |
SOPs, standard operating procedures; LIS, laboratory information system.
APPENDIX 2
Table A5 contains relevant computer comments used in the interpretation of result reports sent to physicians regarding the submission of stool specimens. In Table A6, additional report comments related to the reporting of the Entamoeba histolytica/E. dispar group, the reporting of nonpathogenic protozoa, and the reporting of Blastocystis spp. are provided. These report comments are extremely helpful in the clarification of reports for physicians in terms of pathogenicity and clinical relevance (5).
TABLE A5.
Computer comments
| Result or situation | Report comment(s) | Interpretation or discussion |
|---|---|---|
| Submission of stool specimens | ||
| Submission of a single stool specimen for ova and parasite examination | One stool specimen is not sufficient for the recovery of intestinal parasites (only a 50% recovery); 2 specimens are recommended, while 3 offer the best chance of organism recovery | While 3 specimens collected over a 10-day period are the best approach, receipt of 2 specimens is acceptable |
| Submission of 2 stool specimens for ova and parasite examination | Although submission of 2 stool specimens is acceptable, 3 specimens collected over a 10-day period provide the best approach for organism recovery | While 2 specimens are now considered acceptable, 3 specimens will allow the most complete percentage recovery of intestinal parasites present |
| Examination of fecal specimens | ||
| No parasites seen | Antibiotics such as metronidazole or tetracycline may interfere with the recovery of intestinal parasites, particularly the protozoa | If a patient is symptomatic and intestinal parasites are suspected, this comment may be helpful for the physician, particularly if the patient has received any of these antibiotics |
| Yeasts, budding yeast, and/or pseudohyphae | Reports of yeasts may or may not be clinically relevant due to possible specimen handling delays prior to fixation | Because yeasts can continue to grow within the stool prior to fixation, the results from the permanent-stain smear may or may not be clinically relevant; quantitate cells if the number is moderate or many or the cells are packed |
| Trophozoites containing ingested RBCs (Entamoeba histolytica) | Pathogenic; cause of amoebiasis | A positive result is based on the presence of ingested RBCs within the trophozoite's cytoplasm and/or a fecal immunoassay specific for the pathogen is positive (Entamoeba histolytica positive) |
| Trophozoites containing no ingested RBCs and/or cysts (Entamoeba histolytica/E. dispar group) | Differentiation between the pathogen Entamoeba histolytica and the nonpathogen Entamoeba dispar is not possible based on organism morphology; if ingested RBCs are not seen or cysts are present, you will be unable to differentiate the two organisms You will be unable to determine pathogenicity from the organism's morphology or from the patient's clinical condition, and treatment may be appropriate |
A fecal immunoassay specific for the pathogen, Entamoeba histolytica, can be performed on fresh stool to separate out E. histolytica and E. dispar
An immunoassay for the Entamoeba histolytica/E. dispar group complex will not differentiate the true pathogen, Entamoeba histolytica The fecal immunoassay specific for the pathogen Entamoeba histolytica requires fresh stool for testing (this can be added as another comment if you offer the differentiation test; see the entry below) |
| Differentiation of E. histolytica from E. dispar | To determine the presence or absence of pathogenic Entamoeba histolytica, submit a fresh stool specimen | The fecal immunoassay specific for the pathogen Entamoeba histolytica requires fresh stool for testing |
| Blastocystis spp. | Blastocystis spp. contain ~10 human subtypes, none of which can be differentiated on the basis of organism morphology; some are pathogenic and some are nonpathogenic; if no other pathogens are found, Blastocystis may be the cause of patient symptoms and other organisms capable of causing diarrhea should also be ruled out | Until there are testing options to differentiate between the pathogenic and nonpathogenic subtypes, it is important that physicians know that some strains of Blastocystis are pathogenic; quantitate these organisms (rare, few, moderate, many, packed) |
| Giardia lamblia (other names which refer to the same organism, Giardia lamblia, include Giardia intestinalis and Giardia duodenalis) | Pathogenic | If fecal immunoassays are performed, the testing of two separate stools (collected at least 1 day apart) is recommended before the patient is considered negative; the testing of two stools is not required for Cryptosporidium spp. |
| Entamoeba hartmanni, Entamoeba coli, Endolimax nana, Iodamoeba bütschlii, Chilomastix mesnili, Pentatrichomonas hominis, Enteromonas hominis, Retortamonas intestinalis, trophozoites and/or cysts | Nonpathogenic; treatment is not recommended; however, recovery of these organisms indicates that the patient has ingested something contaminated with fecal material (by the same infectivity route for pathogens) | It is important to report nonpathogens; a patient may be infected with one or more pathogen(s) not yet found |
| Microsporidia (fecal and urine specimens), Enterocytozoon bieneusi, Encephalitozoon intestinalis | The report indicates that microsporidial spores are present, probably Enterocytozoon bieneusi or Encephalitozoon intestinalis or both; these tend to disseminate from the gastrointestinal tract to the kidneys; identification to the genus/species level is not possible from stained smears | Enterocytozoon bieneusi and Encephalitozoon intestinalis are the two most likely organisms present; these comments are very helpful, especially in indicating that the two organisms cannot be identified to the genus or species level on the basis of calcofluor white or modified-trichrome-stained smears |
TABLE A6.
Optional comments for laboratory test reportsa
It is important to remember that educational information for your clients is critical to the success of your test reporting formats. The information in the table should be shared with your clients prior to changing your actual reporting formats. Your physician group may have a preference regarding additional comments. Information updates or newsletters are appropriate for this purpose. All of the comments in the table are optional, and wording can be changed to fit your circumstances. However, it is recommended that you select specific comments and try not to use “free text,” so that everyone reports test results in the same way each time. Adapted from reference 5.
It is important to remember that current fecal immunoassay kits for the detection of the Entamoeba histolytica/E. dispar group or for differentiation between the true pathogen (E. histolytica) and the nonpathogen (E. dispar) require fresh or frozen fecal specimens; although preserved specimens (generally preserved in a formalin-based fixative or some of the single-vial fixatives, universal fixative/no formalin/no mercury/no PVA/Total-Fix) can be used for the fecal immunoassays for Giardia lamblia or Cryptosporidium spp., they cannot be used for Entamoeba species testing.
APPENDIX 3
Parasitic Forms in Gastrointestinal Specimens
Human parasitic infections caused by intestinal helminths and protozoans are the most prevalent infections in developing countries (Tables A7 to A11 and Fig. A1 and A2). There are several different species of intestinal protozoans, pathogenic and nonpathogenic, which have similar characteristics, so accurate identification can be difficult because of the tiny differences. The protozoans are grouped according to their locomotor organelles. The largest group contains the amoebae, which move with pseudopodia in the trophozoite form. There are specific criteria that are used to identify the trophozoite and cyst forms of the amoebae. Because of the minute details in structure required to identify these organisms, a good-quality microscope and good staining procedures are essential.
Amoebae
Tables A7 and A8 illustrate the details of the morphology of the trophozoite and cyst forms of the different species. Although Dientamoeba fragilis is considered to be a flagellate, the flagella are internal and not visible by light microscopy. Since they look more like amoebae, D. fragilis is grouped with the amoebae. The cyst form has recently been confirmed; however, the number of cysts in a human clinical specimen is extremely limited. Therefore, they are not included in Table A8.
TABLE A7.
Morphology of trophozoite forms of amoebae
| Species | Size (length [μm]) | Motility | Nucleus characteristic(s) |
Cytoplasm characteristic(s) |
|||
|---|---|---|---|---|---|---|---|
| No. | Peripheral chromatin | Nuclear chromatin | Appearance | Inclusions | |||
| Entamoeba histolytica/E. dispar (E. histolytica is the true pathogen, and E. dispar is nonpathogenic) | 10–60; usual range, 15–20 (commensal form) and over 20 (invasive form) | Progressive, with hyaline, finger-like pseudopods | 1 (not visible in unstained preparations) | Fine granules; usually evenly distributed and uniform in size | Small, discrete; usually centrally located but occasionally eccentric | Finely granular | RBCs occasionally; noninvasive organisms may contain bacteria; no RBCs seen in E. dispar |
| Entamoeba hartmanni | 5–12; usual range, 8–10 | Usually nonprogressive but may occasionally be progressive | 1 (not visible in unstained preparations) | Similar to E. histolytica | Small, discrete, often eccentric | Finely granular | Bacteria |
| Entamoeba coli | 15–50; usual range, 20–25 | Sluggish, nonprogressive, with blunt pseudopods | 1 (often visible in unstained preparations) | Coarse granules, irregular in size and distribution | Large, discrete, usually eccentric | Coarse, often vacuolated | Bacteria, yeasts, or other materials |
| Entamoeba polecki | 10–25; usual range, 15–20 | Usually sluggish, similar to E. coli; occasionally, in diarrheic specimens, motility may be progressive | 1 (may be slightly visible in unstained preparations; occasionally may be irregularly distorted by pressure from vacuoles in cytoplasm) | Usually fine granules are evenly distributed; occasionally granules may be irregularly arranged; chromatin sometimes in plaques or crescents | Small, discrete, eccentric; occasionally large, diffuse, or irregular | Coarsely granular; may resemble E. coli; contains numerous vacuoles | Bacteria, yeasts |
| Endolimax nana | 6–12; usual range, 8–10 | Sluggish, usually nonprogressive with blunt pseudopods | 1 (visible occasionally in unstained preparations) | None | Large, irregularly shaped, blot-like | Granular, vacuolated | Bacteria |
| Iodamoeba bütschlii | 8–20; usual range, 12–15 | Sluggish, usually nonprogressive | 1 (not usually visible in unstained preparations) | None | Large, usually central; surrounded by refractile, achromatic granules; these granules are often not distinct even in stained slides | Coarsely granular, vacuolated | Bacteria, yeasts, or other materials |
| Dientamoeba fragilis | 5–15, usual range, 9–12 | Pseudopods are angular, serrated, or broad lobed and hyaline, almost transparent | 2 (in ∼20% of organisms, only 1 nucleus is present; nuclei are invisible in unstained preparations) | None | Large cluster of 4–8 granules (tetrad) | Finely granular | Bacteria |
TABLE A8.
Morphology of cyst forms of amoebae
| Species | Size (diam or length [μm]) | Shape | Nucleus characteristic(s) |
Cytoplasm characteristic(s) |
|||
|---|---|---|---|---|---|---|---|
| No. | Peripheral chromatin | Nuclear chromatin | Inclusions | Appearance | |||
| Entamoeba histolytica/E. dispar | 10–20; usual range, 12–15 | Usually spherical | 4 in mature cyst; occasionally 1 or 2 in immature cysts | Peripheral chromatin present; fine, uniform granules, evenly distributed | Small, discrete, usually centrally located | Present; elongated bars with bluntly rounded ends | Usually diffuse; concentrated mass often present in young cysts; stains reddish brown with iodine |
| Entamoeba hartmanni | 5–10; usual range, 6–8 | Usually spherical | 4 in mature cyst; 1 or 2 often seen in immature cysts | Similar to E. histolytica | Similar to E. histolytica | Present; elongated bars with bluntly rounded ends | Similar to E. histolytica |
| Entamoeba coli | 10–35; usual range, 15–25 | Usually spherical; occasionally oval, triangular, or other shapes | 8 in mature cysts; 16 or more are occasionally seen in supernucleated cysts; 2 or more are occasionally seen in immature cysts | Peripheral chromatin present; coarse granules are irregular in size and distribution but often appear more uniform than in trophozoites | Large, discrete, usually eccentric but occasionally centrally located | Present, but less frequently seen than in E. histolytica; usually splinter-like with pointed ends | Usually diffuse, but occasionally there is a well-defined mass in immature cysts; stain reddish brown with iodine |
| Entamoeba polecki | 9–18; usual range, 11–15 | Spherical or oval | 1, rarely 2; occasionally visible in unstained preparations | Usually fine granules that are evenly distributed | Usually small and eccentric | Present; many small bodies with angular or pointed ends or a few large ones; may be oval, rod-like, or irregular | Usually small, diffuse masses stain reddish brown with iodine; a dark area called an “inclusion mass” (possibly concentrated cytoplasm) is often also present; mass stains lightly with iodine |
| Endolimax nana | 5–10; usual range, 6–8 | Usually oval, may be round | 4 in mature cyst; immature cysts, 2, very rarely seen and may resemble cysts of Enteromonas hominis | None | Large (blot-like), usually central | Occasionally granules or small oval masses are seen, but bodies as seen in Entamoeba spp. are not present | Usually diffuse; a concentrated mass is seen occasionally in young cysts; stains reddish brown with iodine |
| Iodamoeba bütschlii | 5–20; usual range, 10–12 | Ovoidal, ellipsoidal, triangular, or other shapes | 1 in mature cysts | None | Large, usually eccentric; refractile, achromatic granules on one side of the karyosome; indistinct in iodine preparations | Occasionally granules are present, but chromatoid bodies as seen in Entamoeba spp. are not present | Compact, well-defined mass; stains dark brown with iodine |
Flagellates
Tables A9 and A10 illustrate the details of the trophozoite and cyst forms of the different species within flagellates. Some of the flagellates do not have a cyst form; therefore, no entry in Table A10 will be found for these organisms.
TABLE A9.
Morphology of trophozoite forms of flagellates
| Species | Size (length [μm]) | Shape | Motility | No. of nuclei | No. and positions of flagella | Other features |
|---|---|---|---|---|---|---|
| Pentatrichomonas hominis | 6–20; usual range, 11–12 | Pear shaped | Nervous, jerky | 1 (not visible in unstained mounts) | 3–5 anterior, 1 posterior | Undulating membrane extending the length of the body |
| Chilomastix mesnili | 6–24; usual range, 10–15 | Pear shaped | Stiff, rotary | 1 (not visible in unstained mounts) | 3 anterior, 1 in cytosome | Prominent cytostome extending 1/3–1/2 the length of the body; spiral groove across ventral surface |
| Giardia lamblia (G. duodenalis, G. intestinalis) | 10–20; usual range, 12–15 | Pear shaped | “Falling leaf” | 2 (not visible in unstained mounts) | 4 lateral, 2 ventral, 2 caudal | Sucking disk occupying 1/2–3/4 of the ventral surface; median bodies lying horizontally or obliquely in lower part of body |
| Enteromonas hominis | 4–10; usual range, 8–9 | Oval | Jerky | 1 (not visible in unstained mounts) | 3 anterior, 1 posterior | One side of body flattened; posterior flagellum extends free posteriorly or laterally |
| Retortamonas intestinalis | 4–9; usual range, 6–7 | Pear shaped or oval | Jerky | 1 (not visible in unstained mounts) | 1 anterior, 1 posterior | Prominent cytostome extending ∼1/2 the length of the body |
TABLE A10.
Morphology of cyst forms (flagellates)
| Species | Size (length [μm]) | Shape | No. of nuclei | Other features |
|---|---|---|---|---|
| Pentatrichomonas hominis | No cyst | |||
| Chilomastix mesnili | 6–10; usual range, 8–9 | Lemon shaped with anterior hyaline knob | 1 (not visible in unstained preparations) | Cytostome with supporting fibrils; usually visible in stained preparations |
| Giardia lamblia (G. duodenalis, G. intestinalis) | 8–19; usual range, 11–12 | Oval or ellipsoidal | Usually 4 (not distinct in unstained preparations; usually located at one end) | Fibrils or flagella appear longitudinally in unstained cysts; deeply staining fibers or fibrils may be seen lying laterally or obliquely across fibrils in the lower part of the cyst; cytoplasm often retracts from a portion of the cell wall |
| Enteromonas hominis | 4–10; usual range, 6–8 | Elongated or oval | 1–4 (2 usually lie at opposite ends of the cyst; not visible in unstained mounts) | Resembles E. nana cyst; fibrils or flagella are usually not seen |
| Retortamonas intestinalis | 4–9; usual range, 4–7 | Pear shaped or slightly lemon shaped | 1 (not visible in unstained mounts) | Resembles Chilomastix cyst; shadow outline of cytostome with supporting fibrils extends above nucleus |
Ciliates, Coccidia, Apicomplexa, and Blastocystis spp.
Table A11 illustrates the morphological details of the ciliates, the coccidians, the Apicomplexa, and Blastocystis spp. The most common pathogenic protozoan parasites are Giardia lamblia, Entamoeba histolytica, Dientamoeba fragilis, Cyclospora cayetanensis, and Cryptosporidium spp. If Blastocystis spp. are grouped with the pathogenic protozoa, they represent the most common parasites throughout the world and have been classified with the stramenopiles (brown algae) (5).
TABLE A11.
Morphology of the trophozoite and cyst forms of ciliates (Balantidium coli), coccidia, apicomplexa (Cryptosporidium), and Blastocystis spp.
| Species | Stage | Size (μm) | Shape | Motility | No. of nuclei | Other features |
|---|---|---|---|---|---|---|
| Balantidium coli | Trophozoite | 50–70 or more; usual range, 40–50 | Ovoid with tapering anterior end | Rotary, boring | 2 (1 large, kidney-shaped macronucleus and 1 small micronucleus immediately adjacent to the macronucleus; the macronucleus is occasionally visible in unstained preparations as a hyaline mass) | Body surface is covered by spiral, longitudinal rows of cilia; contractile vacuoles are present |
| Cyst | 45–65; usual range, 50–55 | Spherical or oval | 1 (large macronucleus visible in unstained preparations as a hyaline mass) | Macronucleus and contractile vacuole are visible in young cysts; in older cysts, internal structure appears granular | ||
| Cystoisospora belli | Oocyst | 25–30; usual range, 28–30 | Ellipsoidal | Nonmotile | Usual diagnostic stage is the immature oocyst with a single granular mass (zygote) within; the mature oocyst contains 2 sporocysts with 4 sporozoites each | |
| Sarcocystis hominis | Sporocyst | 13–17; usual range, 14–16 | Oval | Nonmotile | Mature oocysts with a thin wall collapsed around 2 sporocysts or free fully mature sporocysts with 4 sporozoites inside are usually seen in feces | |
| Sarcocystis suihominis | Sporocyst | 1–15; usual range, 12–13 | Oval | Nonmotile | Mature oocysts with a thin wall collapsed around 2 sporocysts or free fully mature sporocysts with 4 sporozoites inside are usually seen in feces | |
| Cryptosporidium spp. | Oocyst | 3–6; usual range, 4–5 | Spherical or oval | Nonmotile | Mature oocysts contain 4 “naked” sporozoites; no sporocysts are present; oocysts are immediately infective | |
| Cyclospora cayetanensis | Oocyst | 8–10 | Spherical | Nonmotile | May appear as “wrinkled” cellophane in stained preparations | Oocysts appear nonsporulated in clinical specimens; noninfectious |
| Blastocystis spp. | Vacuolated form | 5–30; usual range, 8–10 | Spherical, oval, or ellipsoidal | Nonmotile | 1, usually, but 2–4 may be present (located in “rim” of cytoplasm; in binucleated organisms, the 2 nuclei may be at opposite poles; in quadrinucleated forms, the 4 nuclei are evenly spaced around the periphery of the cell) | Cell contains large central body, or “vacuole” with a thin band, or “rim” of cytoplasm around the periphery; occasionally a ring of granules may be seen in the cytoplasm; there are at least 10 subtypes, about half of which are pathogenic (morphologically the same); quantitate (rare, few, moderate, many, packed) |
Microsporidia
“Microsporidia” is the general term for the obligate intracellular parasites belonging to the phylum Microsporidia. They produce resistant spores of various sizes. Because they are extremely small, measuring from 1 to 4 μm in size, and have unique features, special stains are necessary to detect and identify them. www.cdc.goc/dpdx is an excellent website for laboratory diagnosis, images, and links (5).
Helminths
Helminth infections are diagnosed by finding the characteristic eggs, which may vary in size from 25 to 180 μm, in the stool specimen, microscopic larvae, or macroscopic proglottids. The helminths are divided in two phyla: the Nematoda, or round worms, and the Platyhelminthes, which consist of the trematodes (flukes) and cestodes (tapeworms). Figure A1 contains images of nematode and cestode eggs, while Figure A2 contains images of trematode eggs.
FIG A1.
Morphological characteristics of nematode and cestode (helminth) eggs.
FIG A2.
Diagnostic images showing the morphological characteristics of trematode eggs. 1Paragonimus westermani is usually found in respiratory specimens; 2Schistosoma haematobium is usually passed in urine.
The eggs have some biological variation but are uniform in size, shape, and coloration within each species. Size charts, identification keys, etc. are available (5). Excellent references are available for assistance and can be found in Appendix 4.
APPENDIX 4
Below are a number of general references for medical parasitology, including publications on classification, biology, morphology, diagnosis, clinical symptoms, treatment, and epidemiology/prevention related to human parasitic infections. Many of the later publications contain extensive color illustrations, which may be valuable in training students, as well as for use as excellent bench resources for practicing microbiologists.
Ash LR, Orihel TC. 2007. Atlas of human parasitology, 5th ed. ASCP Press, Chicago, IL.
Ash LR, Orihel TC. 1991. Parasites: a guide to laboratory procedures and identification. ASCP Press, Chicago, IL.
Ash LR, Orihel TC, Savioli L. 1994. Bench aids for the diagnosis of intestinal parasites. World Health Organization, Geneva, Switzerland.
Beaty BJ, Marquardt WC (ed). 1996. The biology of disease vectors. University Press of Colorado, Niwot, CO.
Beaver PC, Jung RC, Cupp EW. 1984. Clinical parasitology, 9th ed. Lea & Febiger, Philadelphia, PA.
Binford CH, Connor DH. 1976. Pathology of tropical and extraordinary diseases. Armed Forces Institute of Pathology, Washington, DC.
Borchardt KA, Noble MA (ed). 1997. Sexually transmitted diseases. CRC Press, Inc., Boca Raton, FL.
Chernin E. 1977. A bicentennial sampler: milestones in the history of tropical medicine and hygiene. Am J Trop Med Hyg 26:1053–1104.
Cook GC, Zumla A (ed). 2008. Manson's tropical diseases, 22nd ed. The W. B. Saunders Co., Philadelphia, PA.
Cox FEG. 2005. History of human parasitology, chapter 1, part 1, p 1–3. In Cox FEG, Wakelin D, Gillespie SH, Despommier D (ed), Topley and Wilson’s microbiology and microbial infections, 10th ed (parasitology). Hodder Arnold, London, United Kingdom.
Del Brutto O, Garcia HH. 2014. Cysticercosis of the human nervous system. Springer-Verlag, Berlin, Germany.
Despommier DD, Gwadz RW, Hotez PJ (ed). 2005. Parasitic diseases, 5th ed. Springer-Verlag, New York, NY.
Eldridge BF, Edman JD (ed). 2004. Medical entomology, 2nd ed. Kluwer Academic Publishers, Norwell, MA.
Farrar, J, Hotez PJ, Junghanss T, Kang G, Laloo D, White NJ. 2013. Manson's tropical diseases, 23rd ed. Elsevier Health Sciences, London, United Kingdom.
Faust EC. 1949. Human helminthology, 3rd ed. Lea & Febiger, Philadelphia, PA.
Fayer R (ed). 2007. Cryptosporidium and cryptosporidiosis, 2nd ed. CRC Press, Inc, Boca Raton, FL.
Foster WD. 1965. A history of parasitology. E. & S. Livingstone, London, United Kingdom.
Garcia HH, Tanowitz HB, Del Brutto OH. 2013. Neuroparasitology and tropical neurology, 3rd ed. Elsevier, Oxford, United Kingdom.
Garcia LS. 2009. Practical guide to diagnostic parasitology, 2nd ed. ASM Press, Washington, DC.
Garcia LS. 2016. Diagnostic medical parasitology, 6th ed. ASM Press, Washington, DC.
Goddard J. 2012. Physician's guide to arthropods of medical importance, 6th ed. CRC Press, Inc, Boca Raton, FL.
Goddard J. 2012. Public health entomology. CRC Press Inc, Boca Raton, FL.
Harwood RF, James MT. 1979. Entomology in human and animal health. Macmillan, New York, NY.
Hoeppli R. 1962. Parasites and parasitic infections in early medicine and science. University of Malaya Press, Singapore.
Horsburgh CR, Jr, Nelson AM (ed). 1997. Pathology of emerging infections. ASM Press, Washington, DC.
Horsfall WR. 1962. Medical entomology: arthropods and human disease. The Ronald Press Co, New York, NY.
John DT, Petri WA. 2006. Medical parasitology, 9th ed. The W B Saunders Co, Philadelphia, PA.
Jong EC, McMullen R. 2003. The travel and tropical medicine manual, 3rd ed. The W B Saunders Co, Philadelphia, PA.
Kean BH, Mott KE, Russell AJ. 1978. Tropical medicine and parasitology classic investigations. Cornell University Press, Ithaca, NY.
Kettle DS. 1995. Medical and veterinary entomology, 2nd ed. CAB International, Wallingford, United Kingdom.
Klassen-Fischer MK, Meyers WM, Neafie RC. 2011. Topics on the pathology of protozoan and invasive arthropod diseases, African trypanosomiasis. Uniformed Services University of the Health Sciences, Bethesda, MD.
Kokoskin E. 2001. The malaria manual. McGill University Centre for Tropical Diseases, Montreal, Canada.
Lambert HP, Farrar WE. 1982. Infectious diseases illustrated. The W B Saunders Co, Philadelphia, PA.
Lujan HD, Svard SG (ed). 2011. Giardia, a model organism. Springer-Verlag, Vienna, Austria.
Magill, AJ, Meyers WM, Neafie RC, Klassen-Fischer MK. 2011. Topics on the pathology of protozoan and invasive arthropod diseases, cutaneous leishmaniasis. Uniformed Services University of the Health Sciences, Bethesda, MD.
Magill AJ, Meyers WM, Klassen-Fischer MK, Neafie RC. 2011. Topics on the pathology of protozoan and invasive arthropod diseases, visceral leishmaniasis. Uniformed Services University of the Health Sciences, Bethesda, MD.
Magill AJ, Ryan ET, Sullivan T, Hill DR. 2012. Hunter's tropical medicine, 9th ed. The W B Saunders Co, Philadelphia, PA.
Mansour TE, Mansour JM. 2011. Chemotherapeutic targets in parasites. Cambridge University Press, Cambridge, United Kingdom.
Mehlhorn H, Tan KSW, Yoshikawa H. 2013. Blastocystis: pathogen or passenger? Springer Verlag, Berlin, Germany.
Meyers WM, Neafie RC, Marty AM, Wear DJ (ed). 2000. Pathology of infectious diseases, vol 1. Helminthiases. Armed Forces Institute of Pathology, Washington, DC. Note that the latest volume of Topics on the Pathology of Protozoa and Invasive Arthropod Diseases (274) is now available. See the introduction by M. K. Klassen-Fischer and R. C. Neafie.
Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (ed). 2007. Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
Murrell KD, Fried B (ed). 2007. Food-borne parasitic zoonoses. Springer-Verlag, New York, NY.
Nelson AM, Horsburgh CR, Jr. 1998. Pathology of emerging infections 2. ASM Press, Washington, DC.
Orihel TC, Ash LR. 1995. Parasites in human tissues. ASCP Press, Chicago, IL.
Orihel TC, Ash LR, Ramachandran CP. 1996. Bench aids for the diagnosis of filarial infections. World Health Organization, Geneva, Switzerland.
Ortega YR. 2006. Foodborne parasites. Springer-Verlag, New York, NY.
Peters W, Gilles HM. 1995. Color atlas of tropical medicine and parasitology. Mosby-Wolfe, New York, NY.
Reeder MM, Palmer PES. 1981. The radiology of tropical diseases with epidemiological, pathological and clinical correlation. The Williams & Wilkins Co, Baltimore, MD.
Roberts LS, Janovy J, Nadler S. 2012. Foundations of parasitology, 9th ed. The C V Mosby Co, St Louis, MO.
Rondanelli EG, Scaglia M. 1993. Atlas of human protozoa. Masson, Milan, Italy.
Scarpignato C, Rampal P (ed). 1995. Travelers' diarrhea: recent advances. Karger Publishers, Farmington, CT.
Scott HH. 1939. A history of tropical medicine. Edward Arnold & Co, London, United Kingdom.
Secor WE, Colley DG (ed). 2010. Schistosomiasis. Springer-Verlag, New York, NY.
Sherman IW (ed). 1998. Malaria. ASM Press, Washington, DC.
Sherman IW. 2008. Reflections on a century of malaria biochemistry. Academic Press, Inc, San Diego, CA.
Smith KCV. 1973. Insects and other arthropods of medical importance. John Wiley & Sons, Inc, New York, NY.
Spencer FM, Monroe LS. 1976. The color atlas of intestinal parasites, 2nd ed. Charles C Thomas, Publisher, Springfield, IL.
Staines HM, Krishna S (ed). 2012. Treatment and prevention of malaria. Birkhauser Verlag AG, Basel, Germany.
Steele JH. 1982. CRC handbook series in zoonoses. CRC Press, Inc, Boca Raton, FL.
Sullivan D, Krishna S (ed). 2010. Malaria: drugs, disease and post-genomic biology. Springer-Verlag, Berlin, Germany.
Sun T. 1999. Parasitic disorders, 2nd ed. The Williams & Wilkins Co, Baltimore, MD.
Taylor AER, Baker JR. 1978. Methods of cultivating parasites in vitro. Academic Press Inc, New York, NY.
Taylor MA, Coop RL, Wall RL. 2007. Veterinary parasitology, 3rd ed. Blackwell Publishing, Chicester, United Kingdom.
Von Brand T. 1979. Biochemistry and physiology of endoparasites. Elsevier/North Holland Publishing Co, New York, NY.
Warren KS, Mahmoud AAF. 1990. Tropical and geographical medicine, 2nd ed. McGraw Hill Book Co, New York, NY.
Wittner M, Weiss LM (ed). 1999. The microsporidia and microsporidiosis. ASM Press, Washington, DC.
Yamaguchi T. 1981. Color atlas of clinical parasites. Lea & Febiger, Philadelphia, PA.
Zaman V. 1994. Atlas of medical parasitology. 3rd ed. Lea & Febiger, Philadelphia, PA.
Footnotes
[This article was published on 15 November 2017 with the title “Laboratory Diagnosis of Parasites from the Gastrointestinal Tract.” The title was updated in the current version, posted on 20 July 2020.]
REFERENCES
- 1.US Food and Drug Administration, Center for Medical Services, Centers for Disease Control and Prevention. 1988. Clinical laboratory improvement amendments (CLIA). FDA, Washington, DC: https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/IVDRegulatoryAssistance/ucm124105.htm. [Google Scholar]
- 2.Leber AL. (ed). 2016. Clinical microbiology procedures handbook, 4th ed ASM Press, Washington, DC. [Google Scholar]
- 3.Garcia LS. 2004. Parasitology, p 9.0.1–9.10.8.3. In Isenberg HD. (ed), Clinical microbiology procedures handbook, 2nd ed, vol 2 ASM Press, Washington, DC. [Google Scholar]
- 4.Isenberg HD. (ed). 1995. Essential procedures for clinical microbiology. ASM Press, Washington, DC. [Google Scholar]
- 5.Garcia LS. 2016. Diagnostic medical parasitology, 6th ed ASM Press, Washington, DC. [Google Scholar]
- 6.Garcia LS, Smith JW, Fritsche TR. 2003. Selection and use of laboratory procedures for the diagnosis of parasitic infections of the gastrointestinal tract. Cumitech 30A. ASM Press, Washington, DC. [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2005. Procedures for the recovery and identification of parasites from the intestinal tract, 2nd ed Approved guideline M28 A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Parasitology Subcommittee, Microbiology Section of Scientific Assembly, American Society for Medical Technology. 1978. Recommended procedures for the examination of clinical specimens submitted for the diagnosis of parasitic-infections. Am J Med Technol 44:1101–1106. [PubMed] [Google Scholar]
- 9.College of American Pathologists. 2012. Commission on Laboratory Accreditation inspection checklist. College of American Pathologists, Chicago, IL. [Google Scholar]
- 10.Garcia LS. (ed). 2010. Clinical microbiology procedures handbook, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 11.Hunter PR, Nichols G. 2002. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev 15:145–154. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellanger AP, Scherer E, Cazorla A, Grenouillet F. 2013. Dysenteric syndrome due to Balantidium coli: a case report. New Microbiol 36:203–205. [PubMed] [Google Scholar]
- 13.Pritt BS, Clark CG. 2008. Amebiasis Mayo. Clin Proc 83:1154–1159. doi: 10.4065/83.10.1154. [DOI] [PubMed] [Google Scholar]
- 14.Sohn WM, Na BK, Kim TH, Park TJ. 2015. Anisakiasis: report of 15 gastric cases caused by anisakis type I larvae and a brief review of Korean anisakiasis. Korean J Parasitol 53:465–470. doi: 10.3347/kjp.2015.53.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geri G, Rabbat A, Mayaux J, Zafrani L, Chalumeau-Lemoine L, Guidet B, Azoulay E, Pène F. 2015. Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature. Infection 43:691–698. doi: 10.1007/s15010-015-0799-1. [DOI] [PubMed] [Google Scholar]
- 16.Procop G, Pritt BS. 2014. Pathology of infectious diseases, 1st ed, p 610–643. Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 17.Levine GI. 1991. Parasitic diseases. Diseases associated with acquired immunodeficiency syndrome. Prim Care 18:129–152. [PubMed] [Google Scholar]
- 18.Von Bonsdorff B. 1956. Diphyllobothrium latum as a cause of pernicious anemia. Exp Parasitol 5:207–230. doi: 10.1016/0014-4894(56)90015-7. [DOI] [PubMed] [Google Scholar]
- 19.de Gier B, Campos Ponce M, van de Bor M, Doak CM, Polman K. 2014. Helminth infections and micronutrients in school-age children: a systematic review and meta-analysis. Am J Clin Nutr 99:1499–1509. doi: 10.3945/ajcn.113.069955. [DOI] [PubMed] [Google Scholar]
- 20.Brooker S, Bethony J, Hotez PJ. 2004. Human hookworm infection in the 21st century. Adv Parasitol 58:197–288. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel A, Lakshimi CP, Pottakkat B. 2012. Biliary ascariasis: mimicker of retained bile duct stone. Dig Endosc 24:480. doi: 10.1111/j.1443-1661.2012.01338.x. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal R, Mittal S, Jain M, Lamba GS. 2012. Severe acute pancreatitis caused by Ascaris lumbricoides. Trop Gastroenterol 33:235–236. doi: 10.7869/tg.2012.61. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz M, Akbulut S, Kutluturk K, Sahin N, Arabaci E, Ara C, Yilmaz S. 2013. Unusual histopathological findings in appendectomy specimens from patients with suspected acute appendicitis. World J Gastroenterol 19:4015–4022. doi: 10.3748/wjg.v19.i25.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarti I, Gangopadhyay M, Bandopadhyay A, Das NK. 2014. A rare case of gangrenous appendicitis by eggs of Taenia species. J Parasit Dis 38:135–137. doi: 10.1007/s12639-012-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yorimitsu N, Hiraoka A, Utsunomiya H, Imai Y, Tatsukawa H, Tazuya N, Yamago H, Shimizu Y, Hidaka S, Tanihira T, Hasebe A, Miyamoto Y, Ninomiya T, Abe M, Hiasa Y, Matsuura B, Onji M, Michitaka K. 2013. Colonic intussusception caused by anisakiasis: a case report and review of the literature. Intern Med 52:223–226. doi: 10.2169/internalmedicine.52.8629. [DOI] [PubMed] [Google Scholar]
- 26.Andrade AM, Perez Y, Lopez C, Collazos SS, Andrade AM, Ramirez GO, Andrade LM. 2015. Intestinal obstruction in a 3-year-old girl by Ascaris lumbricoides infestation: case report and review of literature. Medicine 94:e655. doi: 10.1097/MD.0000000000000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. 2013. Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. sites, 1996–2012. MMWR Morb Mortal Wkly Rep 62:283–287. [PMC free article] [PubMed] [Google Scholar]
- 28.Jejaw A, Zeynudin A, Zemene E, Belay T. 2014. Status of intestinal parasitic infections among residents of Jimma Town, Ethiopia. BMC Res Notes 7:502. doi: 10.1186/1756-0500-7-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels ME, Shrivastava A, Smith WA, Sahu P, Odagiri M, Misra PR, Panigrahi P, Suar M, Clasen T, Jenkins MW. 2015. Cryptosporidium and Giardia in humans, domestic animals and village water sources in rural India. Am J Trop Med Hyg 93:596–600. doi: 10.4269/ajtmh.15-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mac Kenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med 331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 31.Staat DD, Klepser ME. 2006. International adoption: issues in infectious diseases. Pharmacotherapy 26:1207–1220. doi: 10.1592/phco.26.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oksenhendler E, Gérard L, Fieschi C, Malphettes M, Mouillot G, Jaussaud R, Viallard JF, Gardembas M, Galicier L, Schleinitz N, Suarez F, Soulas-Sprauel P, Hachulla E, Jaccard A, Gardeur A, Théodorou I, Rabian C, Debré P, DEFI Study Group. 2008. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis 46:1547–1554. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 33.Wilson NO, Hall RL, Montgomery SP, Jones JL. 2015. Trichinellosis surveillance—United States, 2008–2012. MMWR Surveill Summ 64(Suppl 1):1–8. [PubMed] [Google Scholar]
- 34.Khare R, Espy MJ, Cebelinski E, Boxrud D, Sloan LM, Cunningham SA, Pritt BS, Patel R, Binnicker MJ. 2014. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J Clin Microbiol 52:3667–3673. doi: 10.1128/JCM.01637-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Procop GW, Yerian LM, Wyllie R, Harrison AM, Kottke-Marchant K. 2014. Duplicate laboratory test reduction using a clinical decision support tool. Am J Clin Pathol 141:718–723. doi: 10.1309/AJCPOWHOIZBZ3FRW. [DOI] [PubMed] [Google Scholar]
- 36.Stark D, Barratt JLN, van Hal S, Marriott D, Harkness J, Ellis JT. 2009. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin Microbiol Rev 22:634–650. doi: 10.1128/CMR.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. 2014. Protection of laboratory workers from occupationally acquired infections; approved guideline—4th ed CLSI document M29-A4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 38.National Committee for Clinical Laboratory Standards. 2002. Implementing a needlestick and sharps injury prevention program in the clinical laboratory; a report. Standard X3-R. NCCLS, Wayne, PA. [Google Scholar]
- 39.Sewell DL. 1995. Laboratory-associated infections and biosafety. Clin Microbiol Rev 8:389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller JM, Astles R, Baszler T, Chapin K, Carey R, Garcia L, Gray L, Larone D, Pentella M, Pollock A, Shapiro DS, Weirich E, Wiedbrauk D. 2011. Guidelines for safe work practices in human and animal medical diagnostic laboratories. MMWR Suppl 60:1–100. [PubMed] [Google Scholar]
- 41.National Sanitation Foundation. 1987. NSF standard no. 49 for class II (laminar flow) biohazard cabinetry. National Sanitation Foundation, Ann Arbor, MI. [Google Scholar]
- 42.Cearlock D. 2009. NAACLS programs, graduates, pass rates and placements: findings from the 2009 annual survey of programs. NAACLA News 103:3. [Google Scholar]
- 43.Carvalho J. 2011. Importance of clinical microbiologists for U.S. healthcare infrastructure. Clin Lab Sci 24:136–141. [PubMed] [Google Scholar]
- 44.Cortelyou-Ward K, Ramirez B, Rotarius T. 2011. The laboratory workforce shortage: a managerial perspective. Health Care Manag (Frederick) 30:148–155. [DOI] [PubMed] [Google Scholar]
- 45.Garcia LS. 2014. Clinical laboratory management, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 46.Kibak P. 2008. The worsening shortage of lab staff. What's being done to turn it around? Clin Lab News 34:1–4. [Google Scholar]
- 47.Garcia LS. 2007. Diagnostic medical parasitology, 5th ed ASM Press, Washington, DC. [Google Scholar]
- 48.Ash LR, Orihel TC. 2007. Atlas of human parasitology, 5th ed American Society of Clinical Parasitologists, Lawrence, KS. [Google Scholar]
- 49.Colmer-Hamood JA. 2001. Artifacts mimicking ova and parasites. Lab Med 32:80–84. doi: 10.1309/0VTA-YUXK-F20G-T20E. [DOI] [Google Scholar]
- 50.Garcia LS. 2009. Practical guide to diagnostic parasitology, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 51.Hiatt RA, Markell EK, Ng E. 1995. How many stool examinations are necessary to detect pathogenic intestinal protozoa? Am J Trop Med Hyg 53:36–39. doi: 10.4269/ajtmh.1995.53.36. [DOI] [PubMed] [Google Scholar]
- 52.Marti H, Koella JC. 1993. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol 31:3044–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.US Department of Health and Human Services. 2009. Biosafety and biomedical laboratories, 5th ed US Government Printing Office, Washington, DC. [Google Scholar]
- 54.Melvin DM, Brooke MM. 1982. Laboratory procedures for the diagnosis of intestinal parasites, 3rd ed US Department of Health, Education, and Welfare publication (CDC) 82-8282. US Printing Office, Washington, DC. [Google Scholar]
- 55.National Committee for Clinical Laboratory Standards. 2002. Clinical laboratory waste management. Approved standard GP5-2A. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 56.Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry MA, Richter SS, Warnock DW (ed). 2015. Manual of clinical microbiology, 11th ed ASM Press, Washington, DC. [Google Scholar]
- 57.Scholten TH, Yang J. 1974. Evaluation of unpreserved and preserved stools for the detection and identification of intestinal parasites. Am J Clin Pathol 62:563–567. doi: 10.1093/ajcp/62.4.563. [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Scholten T. 1977. A fixative for intestinal parasites permitting the use of concentration and permanent staining procedures. Am J Clin Pathol 67:300–304. doi: 10.1093/ajcp/67.3.300. [DOI] [PubMed] [Google Scholar]
- 59.Garcia LS, Shimizu RY, Shum A, Bruckner DA. 1993. Evaluation of intestinal protozoan morphology in polyvinyl alcohol preservative comparison of zinc sulfate- and mercuric chloride-based compounds for use in Schaudinn's fixative. J Clin Microbiol 31:307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herwaldt BL. 2000. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis 31:1040–1057. doi: 10.1086/314051. [DOI] [PubMed] [Google Scholar]
- 61.Abanyie F, Harvey RR, Harris JR, Wiegand RE, Gaul L, Desvignes-Kendrick M, Irvin K, Williams I, Hall RL, Herwaldt B, Gray EB, Qvarnstrom Y, Wise ME, Cantu V, Cantey PT, Bosch S, Silva DAAJ, Fields A, Bishop H, Wellman A, Beal J, Wilson N, Fiore AE, Tauxe R, Lance S, Slutsker L, Parise M. 2015. 2013 multistate outbreaks of Cyclospora cayetanensis infections associated with fresh produce focus on the Texas investigations. Epidemiol Infect 143:3451–3458. doi: 10.1017/S0950268815000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortega YR, Sanchez R. 2010. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin Microbiol Rev 23:218–234. doi: 10.1128/CMR.00026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindsay DS, Dubey JP, Blagburn BL. 1997. Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin Microbiol Rev 10:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzipori S, Widmer G. 2008. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol 24:184–189. doi: 10.1016/j.pt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slavin D. 1955. Cryptosporidium meleagridis (sp. nov.). J Comp Pathol Ther 65:262–266. doi: 10.1016/S0368-1742(55)80025-2. [DOI] [PubMed] [Google Scholar]
- 66.Pohlenz J, Bemrick W, Moon H, Cheville N. 1978. Bovine cryptosporidiosis: a transmission and scanning electron microscopic study of some stages in the life cycle and of the host-parasite relationship. Vet Pathol 15:417–427. doi: 10.1177/030098587801500318. [DOI] [PubMed] [Google Scholar]
- 67.Henriksen SA, Pohlenz JF. 1981. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand 22:594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nime FA, Burek JD, Page DL, Holscher MA, Yardley JH. 1976. Acute enterocolitis in a human being infected with the protozoan Cryptosporidium. Gastroenterology 70:592–598. [PubMed] [Google Scholar]
- 69.Ma P, Soave R. 1983. Three-step stool examination for cryptosporidiosis in 10 homosexual men with protracted watery diarrhea. J Infect Dis 147:824–828. doi: 10.1093/infdis/147.5.824. [DOI] [PubMed] [Google Scholar]
- 70.Garcia LS, Bruckner DA, Brewer TC, Shimizu RY. 1983. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J Clin Microbiol 18:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao L, Cama V. 2011. Cryptosporidium, p 2180–2189. In Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (ed), Manual of clinical microbiology, 10th ed ASM Press, Washington, DC. [Google Scholar]
- 72.Casemore DP, Sands RL, Curry A. 1985. Cryptosporidium species a “new” human pathogen. J Clin Pathol 38:1321–1336. doi: 10.1136/jcp.38.12.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouzid M, Hunter PR, Chalmers RM, Tyler KM. 2013. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev 26:115–134. doi: 10.1128/CMR.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ryan U, Hijjawi N. 2015. New developments in Cryptosporidium research. Int J Parasitol 45:367–373. doi: 10.1016/j.ijpara.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 75.Fayer R, Morgan U, Upton SJ. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int J Parasitol 30:1305–1322. doi: 10.1016/S0020-7519(00)00135-1. [DOI] [PubMed] [Google Scholar]
- 76.Ajjampur S, Rajendran P, Ramani S, Banerjee I, Monica B, Sankaran P, Rosario V, Arumugam R, Sarkar R, Ward H. 2008. Closing the diarrhoea diagnostic gap in Indian children by the application of molecular techniques. J Med Microbiol 57:1364–1368. doi: 10.1099/jmm.0.2008/003319-0. [DOI] [PubMed] [Google Scholar]
- 77.Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. 1999. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four–seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg 61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 79.Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA Jr. 2012. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis 54:185–192. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mor SM, Tumwine JK, Ndeezi G, Srinivasan MG, Kaddu-Mulindwa DH, Tzipori S, Griffiths JK. 2010. Respiratory cryptosporidiosis in HIV-seronegative children in Uganda: potential for respiratory transmission. Clin Infect Dis 50:1366–1372. doi: 10.1086/652140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sterling CR, Arrowood MJ. 1986. Detection of Cryptosporidium sp. infections using a direct immunofluorescent assay. Pediatr Infect Dis 5:S139–S142. doi: 10.1097/00006454-198601001-00022. [DOI] [PubMed] [Google Scholar]
- 82.Shirley DA, Moonah SN, Kotloff KL. 2012. Burden of disease from cryptosporidiosis. Curr Opin Infect Dis 25:555–563. doi: 10.1097/QCO.0b013e328357e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baxby D, Blundell N, Hart CA. 1984. The development and performance of a simple, sensitive method for the detection of Cryptosporidium oocysts in faeces. J Hyg (Lond) 93:317–323. doi: 10.1017/S0022172400064858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McNabb SJ, Hensel DM, Welch DF, Heijbel H, McKee GL, Istre GR. 1985. Comparison of sedimentation and flotation techniques for identification of Cryptosporidium sp. oocysts in a large outbreak of human diarrhea. J Clin Microbiol 22:587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weber R, Bryan RT, Juranek DD. 1992. Improved stool concentration procedure for detection of Cryptosporidium oocysts in fecal specimens. J Clin Microbiol 30:2869–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weber R, Bryan RT, Bishop HS, Wahlquist SP, Sullivan JJ, Juranek DD. 1991. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J Clin Microbiol 29:1323–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clavel A, Arnal A, Sanchez E, Varea M, Quilez J, Ramirez I, Castillo FJ. 1996. Comparison of 2 centrifugation procedures in the formalin-ethyl acetate stool concentration technique for the detection of Cryptosporidium oocysts. Int J Parasitol 26:671–672. doi: 10.1016/0020-7519(96)00057-4. [DOI] [PubMed] [Google Scholar]
- 88.Pacheco FTF, Martins SRAS, Oliveira RR, Alcantara-Neves NM, Silva MP, Soares NM, Teixeira MCA. 2013. Differences in detection of Cryptosporidium and Isospora (Cystoisospora) oocysts according to the fecal concentration or staining method used in a clinical laboratory. J Parasitol 99:1002–1008. doi: 10.1645/12-33.1. [DOI] [PubMed] [Google Scholar]
- 89.Kimura K, Kumar Rai S, Takemasa K, Ishibashi Y, Kawabata M, Belosevic M, Uga S. 2004. Comparison of three microscopic techniques for diagnosis of Cyclospora cayetanensis. FEMS Microbiol Lett 238:263–266. doi: 10.1016/j.femsle.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 90.Harrington BJ. 2008. Microscopy of 4 pathogenic enteric protozoan parasites: a review. Lab Med 39:231–238. doi: 10.1309/83G3PE2H1V2NY9FK. [DOI] [Google Scholar]
- 91.Casemore DP. 1991. ACP Broadsheet 128: June 1991. Laboratory methods for diagnosing cryptosporidiosis. J Clin Pathol 44:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bronsdon MA. 1984. Rapid dimethyl sulfoxide-modified acid-fast stain of Cryptosporidium oocysts in stool specimens. J Clin Microbiol 19:952–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Visvesvara GS, Moura H, Kovacs-Nace E, Wallace S, Eberhard ML. 1997. Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. J Clin Microbiol 35:730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maratim AC, Kamar KK, Ngindu A, Akoru CN, Diero L, Sidle J. 2002. Safranin staining of Cyclospora cayetanensis oocysts not requiring microwave heating. Br J Biomed Sci 59:114–115. doi: 10.1080/09674845.2002.11783646. [DOI] [PubMed] [Google Scholar]
- 95.Garcia LS, Shum AC, Bruckner DA. 1992. Evaluation of a new monoclonal antibody combination reagent for direct fluorescence detection of Giardia cysts and Cryptosporidium oocysts in human fecal specimens. J Clin Microbiol 30:3255–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garcia LS, Brewer TC, Bruckner DA. 1987. Fluorescence detection of Cryptosporidium oocysts in human fecal specimens by using monoclonal antibodies. J Clin Microbiol 25:119–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rusnak J, Hadfield TL, Rhodes MM, Gaines JK. 1989. Detection of Cryptosporidium oocysts in human fecal specimens by an indirect immunofluorescence assay with monoclonal antibodies. J Clin Microbiol 27:1135–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alles AJ, Waldron MA, Sierra LS, Mattia AR. 1995. Prospective comparison of direct immunofluorescence and conventional staining methods for detection of Giardia and Cryptosporidium spp. in human fecal specimens. J Clin Microbiol 33:1632–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chalmers RM, Campbell BM, Crouch N, Charlett A, Davies AP. 2011. Comparison of diagnostic sensitivity and specificity of seven Cryptosporidium assays used in the UK. J Med Microbiol 60:1598–1604. doi: 10.1099/jmm.0.034181-0. [DOI] [PubMed] [Google Scholar]
- 100.Garcia LS, Shimizu RY. 1997. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol 35:1526–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kehl KS, Cicirello H, Havens PL. 1995. Comparison of four different methods for detection of Cryptosporidium species. J Clin Microbiol 33:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP. 2003. Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J Clin Microbiol 41:623–626. doi: 10.1128/JCM.41.2.623-626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrington BJ. 2009. The staining of oocysts of Cryptosporidium with the fluorescent brighteners Uvitex 2B and calcofluor white. Lab Med 40:219–223. doi: 10.1309/LM8PT49NZVVYONCB. [DOI] [Google Scholar]
- 104.Bialek R, Binder N, Dietz K, Knobloch J, Zelck UE. 2002. Comparison of autofluorescence and iodine staining for detection of Isospora belli in feces. Am J Trop Med Hyg 67:304–305. doi: 10.4269/ajtmh.2002.67.304. [DOI] [PubMed] [Google Scholar]
- 105.Belli SI, Wallach MG, Luxford C, Davies MJ, Smith NC. 2003. Roles of tyrosine-rich precursor glycoproteins and dityrosine- and 3,4-dihydroxyphenylalanine-mediated protein cross-linking in development of the oocyst wall in the coccidian parasite Eimeria maxima. Eukaryot Cell 2:456–464. doi: 10.1128/EC.2.3.456-464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Varea M, Clavel A, Doiz O, Castillo FJ, Rubio MC, Gomez-Lus R. 1998. Fuchsin fluorescence and autofluorescence in Cryptosporidium, Isospora and Cyclospora oocysts. Int J Parasitol 28:1881–1883. doi: 10.1016/S0020-7519(98)00146-5. [DOI] [PubMed] [Google Scholar]
- 107.Mai K, Sharman PA, Walker RA, Katrib M, Souza DD, McConville MJ, Wallach MG, Belli SI, Ferguson DJ, Smith NC. 2009. Oocyst www formation and composition in coccidian parasites. Mem Inst Oswaldo Cruz 104:281–289. doi: 10.1590/S0074-02762009000200022. [DOI] [PubMed] [Google Scholar]
- 108.Eberhard ML, Pieniazek NJ, Arrowood MJ. 1997. Laboratory diagnosis of Cyclospora infections. Arch Pathol Lab Med 121:792–797. [PubMed] [Google Scholar]
- 109.Cali A, Neafie RC, Takvorian PM. 2011. Microsporidiosis. Defense Technical Information Center, Fort Belvoir, VA: http://www.dtic.mil/get-tr-doc/pdf?AD=ADA547526. [Google Scholar]
- 110.Mathis A, Weber R, Deplazes P. 2005. Zoonotic potential of the microsporidia. Clin Microbiol Rev 18:423–445. doi: 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desportes I, Charpentier YL, Galian A, Bernard F, Cochand-Priollet B, Lavergne A, Ravisse P, Modigliani R. 1985. Occurrence of a new microsporidian: Enterocytozoon bieneusi ng, n. sp., in the enterocytes of a human patient with AIDS. J Protozool 32:250–254. doi: 10.1111/j.1550-7408.1985.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 112.van Gool T, Hollister WS, Schattenkerk WE, Van den Bergh Weerman MA, Terpstra WJ, van Ketel RJ, Reiss P, Canning EU. 1990. Diagnosis of Enterocytozoon bieneusi microsporidiosis in AIDS patients by recovery of spores from faeces. Lancet 336:697–698. doi: 10.1016/0140-6736(90)92198-Q. [DOI] [PubMed] [Google Scholar]
- 113.Weber R, Bryan RT, Owen RL, Wilcox RCM, Goprelkin L, Visvesvara GS. 1992. Improved light-microscopical detection of microsporidial spores in stool and duodenal aspirates. N Engl J Med 326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 114.Shoobridge MP. 1983. A new principle in polychrome staining: a system of automated staining, complementary to hematoxylin and eosin, and usable as a research tool. Stain Technol 58:245–258. doi: 10.3109/10520298309066797. [DOI] [PubMed] [Google Scholar]
- 115.Ryan NJ, Sutherland G, Coughlan K, Globan M, Doultree J, Marshall J, Baird RW, Pedersen J, Dwyer B. 1993. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol 31:3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cali A, Kotler DP, Orenstein JM. 1993. Septata intestinalis n. g., n. sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Eukaryot Microbiol 40:101–112. doi: 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 117.Hartskeerl RA, Van Gool T, Schuitema AR, Didier ES, Terpstra WJ. 1995. Genetic and immunological characterization of the microsporidian Septata intestinalis Cali, Kotler and Orenstein, 1993: reclassification to Encephalitozoon intestinalis. Parasitology 110:277–285. doi: 10.1017/S0031182000080860. [DOI] [PubMed] [Google Scholar]
- 118.Leder K, Ryan N, Spelman D, Crowe SM. 1998. Microsporidial disease in HIV-infected patients: a report of 42 patients and review of the literature. Scand J Infect Dis 30:331–338. doi: 10.1080/00365549850160594. [DOI] [PubMed] [Google Scholar]
- 119.Field AS, Paik JY, Stark D, Qiu MR, Morey A, Plit ML, Canning EU, Glanville AR. 2012. Myositis due to the microsporidian Anncaliia (Brachiola) algerae in a lung transplant recipient. Transpl Infect Dis 14:169–176. doi: 10.1111/j.1399-3062.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- 120.Levine DJ, Riley DJ, Jorgensen JH, McClain WD, Tio F, Visvesvara GS, Abboud-Werner SL. 2013. Key diagnostic features of granulomatous interstitial nephritis due to Encephalitozoon cuniculi in a lung transplant recipient. Am J Surg Pathol 37:447–452. doi: 10.1097/PAS.0b013e31827e1968. [DOI] [PubMed] [Google Scholar]
- 121.Hocevar SN, Paddock CD, Spak CW, Rosenblatt R, Diaz-Luna H, Castillo I, Luna S, Friedman GC, Antony S, Stoddard RA, Tiller RV, Peterson T, Blau DM, Sriram RR, da Silva A, de Almeida M, Benedict T, Goldsmith CS, Zaki SR, Visvesvara GS, Kuehnert MJ, Microsporidia Transplant Transmission Investigation Team. 2014. Microsporidiosis acquired through solid organ transplantation: a public health investigation. Ann Int Med 160:213–220. doi: 10.7326/M13-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Watts MR, Chan RC, Cheong EY, Brammah S, Clezy KR, Tong C, Marriott D, Webb CE, Chacko B, Tobias V, Outhred AC, Field AS, Prowse MV, Bertouch JV, Stark D, Reddel SW. 2014. Anncaliia algerae microsporidial myositis. Emerg Infect Dis 20:185–191. doi: 10.3201/eid2002.131126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghosh K, Weiss LM. 2012. T cell response and persistence of the microsporidia. FEMS Microbiol Rev 36:748–760. doi: 10.1111/j.1574-6976.2011.00318.x. [DOI] [PubMed] [Google Scholar]
- 124.Kokoskin E, Gyorkos TW, Camus A, Cedilotte L, Purtill T, Ward B. 1994. Modified technique for efficient detection of microsporidia. J Clin Microbiol 32:1074–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA. 1995. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol 33:3138–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moura H, Schwartz DA, Bornay-Llinares F, Sodre FC, Wallace S, Visvesvara GS. 1997. A new and improved “quick-hot Gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch Pathol Lab Med 121:888–893. [PubMed] [Google Scholar]
- 127.Vávra J. 1976. Development of the Microsporidia, p 87–109. In Bulla LA Jr, Cheng TC (ed), Comparative pathobiology, vol 1 Biology of the Microsporidia. Springer Science, New York, NY. [Google Scholar]
- 128.Vavra J, Chalupsky J. 1982. Fluorescence staining of microsporidian spores with the brightener calcofluor white-M2R. J Protozool 29:3. [Google Scholar]
- 129.van Gool T, Snijders F, Reiss P, Eeftinck Schattenkerk JK, van den Bergh Weerman MA, Bartelsman JF, Bruins JJ, Canning EU, Dankert J. 1993. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J Clin Pathol 46:694–699. doi: 10.1136/jcp.46.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vavra J, Dahbiova R, Hollister WS, Canning EU. 1993. Staining of microsporidian spores by optical brighteners with remarks on the use of brighteners for the diagnosis of AIDS associated human microsporidioses. Folia Parasitol 40:267–272. [PubMed] [Google Scholar]
- 131.DeGirolami PC, Ezratty CR, Desai G, McCullough A, Asmuth D, Wanke C, Federman M. 1995. Diagnosis of intestinal microsporidiosis by examination of stool and duodenal aspirate with Weber's modified trichrome and Uvitex 2B strains. J Clin Microbiol 33:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ignatius R, Henschel S, Liesenfeld O, Mansmann U, Schmidt W, Köppe S, Schneider T, Heise W, Futh U, Riecken EO, Hahn H, Ullrich R. 1997. Comparative evaluation of modified trichrome and Uvitex 2B stains for detection of low numbers of microsporidial spores in stool specimens. J Clin Microbiol 35:2266–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chioralia G, Trammer T, Kampen H, Seitz HM. 1998. Relevant criteria for detecting Microsporidia in stool specimens. J Clin Microbiol 36:2279–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goodgame R, Stager C, Marcantel B, Alcocer E, Segura AM. 1999. Intensity of infection in AIDS-related intestinal microsporidiosis. J Infect Dis 180:929–932. doi: 10.1086/314914. [DOI] [PubMed] [Google Scholar]
- 135.Conteas CN, Sowerby T, Berlin GW, Dahlan F, Nguyen A, Porschen R, Donovan J, LaRiviere M, Orenstein JM. 1996. Fluorescence techniques for diagnosing intestinal microsporidiosis in stool, enteric fluid, and biopsy specimens from acquired immunodeficiency syndrome patients with chronic diarrhea. Arch Pathol Lab Med 120:847–853. [PubMed] [Google Scholar]
- 136.Ignatius R, Lehmann M, Miksits K, Regnath T, Arvand M, Engelmann E, Futh U, Hahn H, Wagner J. 1997. A new acid-fast trichrome stain for simultaneous detection of Cryptosporidium parvum and microsporidial species in stool specimens. J Clin Microbiol 35:446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reisner BS, Spring J. 2000. Evaluation of a combined acid-fast–trichrome stain for detection of Microsporidia and Cryptosporidium parvum. Arch Pathol Lab Med 124:777–779. doi:. [DOI] [PubMed] [Google Scholar]
- 138.Ponce de Leon P, Flaherty P, Zdero M. 1999. A new trichromic safranin stain for the detection of Cryptosporidium parvum, Cyclospora cayetanensis, species of Microsporidia and Isospora belli in fecal material. Rev Latinoam Microbiol 41:211–214. (In Spanish.) [PubMed] [Google Scholar]
- 139.Orenstein JM, Russo P, Didier ES, Bowers C, Bunin N, Teachey DT. 2005. Fatal pulmonary microsporidiosis due to encephalitozoon cuniculi following allogeneic bone marrow transplantation for acute myelogenous leukemia. Ultrastruct Pathol 29:269–276. doi: 10.1080/01913120590951257. [DOI] [PubMed] [Google Scholar]
- 140.Choudhary MM, Metcalfe MG, Arrambide K, Bern C, Visvesvara GS, Pieniazek NJ, Bandea RD, Deleon-Carnes M, Adem P, Choudhary MM, Zaki SR, Saeed MU. 2011. Tubulinosema sp. microsporidian myositis in immunosuppressed patient. Emerg Infect Dis 17:1727–1730. doi: 10.3201/eid1709.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Priest JW, Kwon JP, Moss DM, Roberts JM, Arrowood MJ, Dworkin MS, Juranek DD, Lammie PJ. 1999. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol 37:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Priest JW, Moss DM, Visvesvara GS, Jones CC, Li A, Isaac-Renton JL. 2010. Multiplex assay detection of immunoglobulin G antibodies that recognize Giardia intestinalis and Cryptosporidium parvum antigens. Clin Vaccine Immunol 17:1695–1707. doi: 10.1128/CVI.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Garcia LS, Shimizu RY. 2000. Detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens using the ColorPAC combination rapid solid-phase qualitative immunochromatographic assay. J Clin Microbiol 38:1267–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garcia LS, Shimizu RY, Bernard CN. 2000. Detection of Giardia lamblia, Entamoeba histolytica/E. dispar, and Cryptosporidium parvum antigens in human fecal specimens using the EIA Triage parasite panel. J Clin Microbiol 38:3337–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garcia LS, Shimizu RY, Novak S, Carroll M, Chan F. 2003. Commercial assay for detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens by rapid solid-phase qualitative immunochromatography. J Clin Microbiol 41:209–212. doi: 10.1128/JCM.41.1.209-212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shimelis T, Tadesse E. 2014. Performance evaluation of point-of-care test for detection of Cryptosporidium stool antigen in children and HIV infected adults. Parasit Vectors 7:227. doi: 10.1186/1756-3305-7-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alexander LC, Niebel M, Jones B. 2013. The rapid detection of Cryptosporidium and Giardia species in clinical stools using the Quik Chek immunoassay. Parasitol Int 62:552–553. doi: 10.1016/j.parint.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 148.Minak J, Kabir M, Mahmud I, Liu Y, Liu L, Haque R, Petri WA Jr. 2012. Evaluation of rapid antigen point-of-care tests for detection of Giardia and Cryptosporidium species in human fecal specimens. J Clin Microbiol 50:154–156. doi: 10.1128/JCM.01194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Christy NCV, Hencke JD, Escueta-De Cadiz A, Nazib F, von Thien H, Yagita K, Ligaba S, Haque R, Nozaki T, Tannish E, Herein JF, Petri WA Jr. 2012. Multisite performance evaluation of an enzyme-linked immunosorbent assay for detection of Giardia, Cryptosporidium, and Entamoeba histolytica antigens in human stool. J Clin Microbiol 50:1762–1763. doi: 10.1128/JCM.06483-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Scheffler EH, Van Etta LL. 1994. Evaluation of rapid commercial enzyme immunoassay for detection of Giardia lamblia in formalin-preserved stool specimens. J Clin Microbiol 32:1807–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Centers for Disease Control and Prevention (CDC). 2002. Manufacturer's recall of rapid assay kits based on false positive Cryptosporidium antigen tests—Wisconsin, 2001–2002. MMWR Morb Mortal Wkly Rep 51:189. [PubMed] [Google Scholar]
- 152.Requena-Mendez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Munoz J. 2013. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7:e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hernández-Chavarría F, Avendaño L. 2001. A simple modification of the Baermann method for diagnosis of strongyloidiasis. Mem Inst Oswaldo Cruz 96:805–807. doi: 10.1590/S0074-02762001000600011. [DOI] [PubMed] [Google Scholar]
- 154.Steinmann P, Zhou X-N, Du Z-W, Jiang J-Y, Wang L-B, Wang X-Z, Li L-H, Marti H, Utzinger J. 2007. Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis 1:e75. doi: 10.1371/journal.pntd.0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khanna V, Tilak K, Prakash PY, Mulkopadhyay D. 2015. Modified agar plate culture method for culture of Strongyloides stercoralis. Trop Parasitol 5:136–138. doi: 10.4103/2229-5070.162535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Buonfrate D, Formenti F, Perandin F, Bisoffi Z. 2015. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect 21:543–552. doi: 10.1016/j.cmi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 157.Sharifdini M, Mirhendi H, Ashrafi K, Hosseini M, Mohebali M, Khodadadi H, Kia EB. 2015. Comparison of nested polymerase chain reaction and real-time polymerase chain reaction with parasitological methods for the detection of Strongyloides stercoralis in human fecal samples. Am J Trop Med Hyg 93:1285–1291. doi: 10.4269/ajtmh.15-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, Takatsuka N, Kita K, Ohtomo H. 1991. A modified agar plate method for detection of. Strongyloides stercoralis. Am J Trop Med Hyg 45:518–521. doi: 10.4269/ajtmh.1991.45.518. [DOI] [PubMed] [Google Scholar]
- 159.Barda B, Zepherine H, Rinaldi L, Cringoli G, Burion R, Clementi M, Albonico M. 2013. Mini-FLOTAC and Kato-Katz: helminth eggs watching on the shore of Lake Victoria. Parasit Vectors 6:220. doi: 10.1186/1756-3305-6-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barda BD, Rinaldi L, Ianniello D, Zepherine H, Salvo F, Sadutshang T, Cringoli G, Clementi M, Albonico M. 2013. Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: experience from the field. PLoS Negl Trop Dis 7:e2344. doi: 10.1371/journal.pntd.0002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barda B, Cajal P, Villagran E, Cimino R, Juarez M, Krolewiecki A, Rinaldi L, Cringoli G, Burioni R, Albonico M. 2014. Mini-FLOTAC, Kato-Katz and McMaster: three methods, one goal; highlights from north Argentina. Parasit Vectors 7:271. doi: 10.1186/1756-3305-7-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Glinz D, Kigbafori KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, Rinaldi L, Cringoli G, Goran EKN, Utzinger J. 2010. Comparing diagnostic accuracy of Kato-Katz, Koga plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis 4:e754. doi: 10.1371/journal.pntd.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Habtamu K, Degarege A, Ye-Ebiyo Y, Erko B. 2011. Comparison of the Kato-Katz and FLOTAC techniques for the diagnosis of soil-transmitted helminth infections. Parasitol Int 60:398–402. doi: 10.1016/j.parint.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 164.Knopp S, Speich B, Hattendorf J, Rinaldi L, Mohammed K, Khamis S, Mohammed A, Albonico M, Rollinson D, Marti D, Cringoli G, Utzinger J. 2011. Diagnostic accuracy of Kato-Katz and FLOTAC for assessing anthelmintic drug efficacy. PLoS Negl Trop Dis 5:e1036. doi: 10.1371/journal.pntd.0001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kongs A, Marks G, Verle P, Van der Stuyft P. 2001. The unreliability of the Kato-Katz technique limits its usefulness for evaluating S. mansoni infections. Trop Med Int Health 6:163–169. doi: 10.1046/j.1365-3156.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- 166.Levecke B, Behnke JM, Ajjampur SRS, Albonico M, Ame SM, Charlier J, Geiger SM, Hoa NTV, Ngassam RIK, Kotze AC, McCarthy JS, Montresor A, Periago MV, Vercruysse J. 2011. A comparison of the sensitivity and fecal egg counts of the McMaster egg counting and Kato-Katz thick smear methods for soil-transmitted helminths. PLoS Negl Trop Dis 5:e1201. doi: 10.1371/journal.pntd.0001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Odongo-Aginya EI, Taylor MG, Sturrock RF, Ackers JP, Doehring E. 1995. Field evaluation of an improved Kato-Katz thick smear technique for quantitative determination of helminth eggs in faeces. Trop Med Parasitol 46:275–277. [PubMed] [Google Scholar]
- 168.Ramsan M, Montresor A, Foum A, Ameri H, Di Matteo L, Albonico M, Savioli L. 1999. Independent evaluation of the Nigrosin-Eosin modification of the Kato-Katz technique. Trop Med Int Health 4:46–49. doi: 10.1046/j.1365-3156.1999.00356.x. [DOI] [PubMed] [Google Scholar]
- 169.World Health Organization. 1991. Basic laboratory methods in medical parasitology, p 25–28. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 170.Jurberg AD, de Oliveira AA, Lenz HL, Coelho PMZ. 2008. A new miracidia hatching device for diagnosing schistosomiasis. Mem Inst Oswaldo Cruz 103:112–114. doi: 10.1590/S0074-02762008005000005. [DOI] [PubMed] [Google Scholar]
- 171.Beaver PC, Jung RC, Cupp EW. 1984. Clinical parasitology, 9th ed, p 743 Lea & Febiger, Philadelphia, PA. [Google Scholar]
- 172.Matsunaga K, Nojima H, Koech DK. 1987. Dependence of hatching of Schistosoma haematobium miracidia on physical and biological factors. Parasitol Res 74:55–60. doi: 10.1007/BF00534933. [DOI] [PubMed] [Google Scholar]
- 173.Cheesbrough M. 1998. District laboratory practice in tropical countries, part 1, p 238–239. The Bath Press, Bath, Great Britain. [Google Scholar]
- 174.Bradley DJ. 1967. The measurement of schistosome populations, p 301–327. Mostofi FK. (ed), Bilharziasis. International Academy of Pathology, Special Monograph, 1st ed Springer-Verlag, Berlin, Germany. [Google Scholar]
- 175.Galán-Puchades MT, Fuentes MV. 2013. Taenia asiatica: the most neglected human Taenia and the possibility of cysticercosis. Korean J Parasitol 51:51–54. doi: 10.3347/kjp.2013.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Berlin OGW, Miller M. 1980. Euparal as a permanent mounting medium for helminth eggs and proglottids. J Clin Microbiol 12:700–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mayta H, Talley A, Gilman RH, Jimenez J, Verastegui M, Ruiz M, Garcia HH, Gonzalez AE. 2000. Differentiating Taenia solium and Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis. J Clin Microbiol 38:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wicht B, Yanagida T, Scholz T, Ito A, Juan A, Jiménez JA, Brabec J. 2010. Multiplex PCR for differential identification of broad tapeworm (Cestoda: Diphyllobothrium) infecting humans. J Clin Microbiol 48:3111–3116. doi: 10.1128/JCM.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2007. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev 20:511–532. doi: 10.1128/CMR.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.WHO/PAHO/UNESCO. 1997. Report of a consultation of experts on amoebiasis. Wkly Epidemiol Rep W H O 72:97–99. [Google Scholar]
- 181.Gryseels B, Strickland GT. 2013. Schistosomiasis, p 867–883. In Magill AJ, Ryan ET, Hill D, Solomon T (ed), Hunter's tropical medicine and emerging infectious diseases, 9th ed Saunders-Elsevier, London, United Kingdom. [Google Scholar]
- 182.Beal CB, Viens P, Grant RGL, Hughes JM. 1970. A new technique for sampling duodenal content. Demonstration of upper small-bowel pathogens. Am J Trop Med Hyg 19:349–352. doi: 10.4269/ajtmh.1970.19.349. [DOI] [PubMed] [Google Scholar]
- 183.Visvesvara GS, Garcia LS. 2002. Culture of protozoan parasites. Clin Microbiol Rev 15:327–328. doi: 10.1128/CMR.15.3.327-328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boeck WC, Drbohlav J. 1925. The cultivation of Endamoeba (sic) histolytica. Am J Hyg 5:271–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dobell C, Laidlaw PP. 1926. On the cultivation of Entamoeba histolytica and some other entozoic amoebae. Parasitology 18:238–318. doi: 10.1017/S0031182000005205. [DOI] [Google Scholar]
- 186.Balamuth W. 1946. Improved egg yolk infusion for cultivation of Entamoeba histolytica and other intestinal protozoa. Am J Clin Pathol 16:380–384. doi: 10.1093/ajcp/16.6.380. [DOI] [PubMed] [Google Scholar]
- 187.Robinson GL. 1968. The laboratory diagnosis of human parasitic amoebae. Trans R Soc Trop Med Hyg 62:285–294. doi: 10.1016/0035-9203(68)90170-3. [DOI] [PubMed] [Google Scholar]
- 188.Diamond LS. 1982. A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen dwelling protozoa. J Parasitol 68:958–959. doi: 10.2307/3281016. [DOI] [PubMed] [Google Scholar]
- 189.Diamond LS. 1987. Entamoeba, Giardia and Trichomonas, p 1–28. In Taylor AER, Baker JR (ed), In vitro methods for parasite cultivation. Academic Press, New York, NY. [Google Scholar]
- 190.Clark CG. 1995. Axenic cultivation of Entamoeba dispar Brumpt 1925, Entamoeba insolita Geiman and Wichterman 1937 and Entamoeba ranarum Grassi 1879. J Eukaryot Microbiol 42:590–593. doi: 10.1111/j.1550-7408.1995.tb05912.x. [DOI] [PubMed] [Google Scholar]
- 191.Kobayashi S, Imal E, Haghighi A, Khalifa SA, Tachibana H, Tsutomu T. 2005. Axenic cultivation of Entamoeba dispar in newly designed yeast extract-iron-gluconic acid-dihydroacetone-serum medium. J Parasitol 91:1–4. doi: 10.1645/GE-3386. [DOI] [PubMed] [Google Scholar]
- 192.Visvesvara GS. 1992. Parasite culture: Entamoeba histolytica, p 7.9.1.1–7.9.1.8. Isenberg HD. (ed), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, DC. [Google Scholar]
- 193.Diamond LS, Clark CG, Cunnick CC. 1995. YI-S, a casein-free medium for axenic cultivation of Entamoeba histolytica, related Entamoeba, Giardia intestinalis and Trichomonas vaginalis. J Eukaryot Microbiol 42:277–278. doi: 10.1111/j.1550-7408.1995.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 194.Scanlan PD. 2012. Blastocystis: past pitfalls and present and future perspectives. Trends Parasitol 28:327–334. doi: 10.1016/j.pt.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 195.Silberman JD, Sogin ML, Leipe DD, Clark GC. 1996. Human parasite finds taxonomic home. Nature 380:398. doi: 10.1038/380398a0. [DOI] [PubMed] [Google Scholar]
- 196.Diamond LS. 1983. Lumen dwelling protozoa: Entamoeba, trichomonads and Giardia, p 65–109. In Jensen JB. (ed), In vitro cultivation of protozoan parasites. CRC, Boca Raton, FL. [Google Scholar]
- 197.Zierdt CH, Swan JC. 1981. Generation time and growth rate of the human intestinal parasite Blastocystis hominis. J Protozool 28:483–485. doi: 10.1111/j.1550-7408.1981.tb05324.x. [DOI] [PubMed] [Google Scholar]
- 198.Karapetyan AE. 1960. Methods of Lamblia cultivation. Tsitologia 2:379–384. [Google Scholar]
- 199.Karapetyan A. 1962. In vitro cultivation of Giardia duodenalis. J Parasitol 48:337–340. doi: 10.2307/3275191. [DOI] [PubMed] [Google Scholar]
- 200.Meyer AE. 1970. Isolation and axenic cultivation of Giardia trophozoites from the rabbit, chinchilla, and cat. Exp Parasitol 27:179–183. doi: 10.1016/0014-4894(70)90023-8. [DOI] [PubMed] [Google Scholar]
- 201.Meyer AE. 1976. Giardia lamblia: isolation and axenic cultivation. Exp Parasitol 39:101–105. doi: 10.1016/0014-4894(76)90016-3. [DOI] [PubMed] [Google Scholar]
- 202.Visvesvara GS. 1980. Axenic growth of Giardia lamblia in Diamond's TP-S-1 medium. Trans R Soc Trop Med Hyg 74:213–215. doi: 10.1016/0035-9203(80)90249-7. [DOI] [PubMed] [Google Scholar]
- 203.Gordts B, Retorė P, Cadranel S, Hemelhof W, Rahman M, Butzler JP. 1984. Routine culture of Giardia lamblia trophozoites from human duodenal aspirates. Lancet ii:1347–1348. [DOI] [PubMed] [Google Scholar]
- 204.Keister DB. 1983. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg 77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 205.Bingham AK, Meyer EA. 1979. Giardia excystation can be induced in vitro in acidic solutions. Nature 277:301–302. doi: 10.1038/277301a0. [DOI] [PubMed] [Google Scholar]
- 206.Johnson EH, Windsor JJ, Clark CG. 2004. Emerging from obscurity: biological, clinical, and diagnostic aspects of Dientamoeba fragilis. Clin Microbiol Rev 17:533–570. doi: 10.1128/CMR.17.3.553-570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stark D, Garcia LS, Barratt JLN, Phillips O, Roberts T, Marriott D, Harkness J, Ellis JT. 2014. Description of Dientamoeba fragilis cyst and precystic forms from human samples. J Clin Microbiol 52:2680–2683. doi: 10.1128/JCM.00813-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dobell C. 1940. Researches on the intestinal protozoa of monkeys and man. X. The life history of Dientamoeba fragilis: observations, experiments, and speculations. Parasitology 32:417–461. [Google Scholar]
- 209.Barratt JLN, Banik GR, Harkness J, Mariott D, Ellis JT, Stark D. 2010. Newly defined conditions for the in vitro cultivation and cryopreservation of Dientamoeba fragilis: new techniques set to fast track molecular studies on this organism. Parasitology 137:1867–1878. doi: 10.1017/S0031182010000764. [DOI] [PubMed] [Google Scholar]
- 210.Schuster FL, Ramirez-Avila L. 2008. Current world status of Balantidium coli. Clin Microbiol Rev 21:626–638. doi: 10.1128/CMR.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Garcia LS. 2008. Balantidium coli, p 353–366. In Khan NA. (ed), Emerging protozoan pathogens. Taylor & Francis, New York, NY. [Google Scholar]
- 212.Barret HP, Yarbrough N. 1921. A method for the cultivation of Balantidium coli. Am J Trop Med 1:161. doi: 10.4269/ajtmh.1921.s1-1.161. [DOI] [Google Scholar]
- 213.Cox FEG. 1961. The cultivation of Balantidium coli throughout its viable temperature range. Ann Trop Med Parasitol 55:305–308. doi: 10.1080/00034983.1961.11686051. [DOI] [PubMed] [Google Scholar]
- 214.Pieniazek NJ, Bornay-Llinares FJ, Slemenda SB, da Silva AJ, Moura IN, Arrowood MJ, Ditrich O, Addiss DG. 1999. New Cryptosporidium genotypes in HIV-infected persons. Emerg Infect Dis 5:444–449. doi: 10.3201/eid0503.990318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Morgan U, Weber R, Xiao L, Sulaiman I, Thompson RC, Ndiritu W, Lal A, Moore A, Deplazes P. 2000. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J Clin Microbiol 38:1180–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xiao L, Bern C, Limor J, Sulaiman I, Roberts J, Checkley W, Cabrera L, Gilman RH, Lal AA. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis 183:492–497. doi: 10.1086/318090. [DOI] [PubMed] [Google Scholar]
- 217.Woodmansee DB, Pohlenz JFL. 1983. Development of Cryptosporidium sp. in a human rectal tumor cell line, p 306–319. In Proceedings of the Fourth International Symposium on Neonatal Diarrhea Veterinary Infectious Diseases Organization (VIDO), University of Saskatchewan, Saskatoon, Canada. [Google Scholar]
- 218.Arrowood MJ. 2007. In vitro cultivation, p 499–525. In Fayer R, Xiao L (ed), Cryptosporidium and cryptosporidiosis, 2nd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 219.Karanis P, Aldeyarbi HM. 2011. Evolution of Cryptosporidium in vitro culture. Int J Parasitol 41:1231–1242. doi: 10.1016/j.ijpara.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 220.Visvesvara GS. 2002. In vitro culture of Microsporidia of clinical importance. Clin Microbiol Rev 15:401–413. doi: 10.1128/CMR.15.3.401-413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hawash Y. 2014. DNA extraction from protozoan oocysts/cysts in feces for diagnostic PCR. Korean J Parasitol 52:263–271. doi: 10.3347/kjp.2014.52.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shin JC, Reyes AW, Kim SH, Kim S, Park HJ, Seo KW, Song KH. 2015. Molecular detection of Giardia intestinalis from stray dogs in animal shelters of Gyeongsangbuk-do (province) and Daejeon, Korea. Korean J Parasitol 53:477–481. doi: 10.3347/kjp.2015.53.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Colli CM, Bezagio RC, Nishi L, Ferreira EC, Falavigna-Guilherme AL, Gomes ML. 2015. Food handlers as a link in the chain of transmission of Giardia duodenalis and other protozoa in public schools in southern Brazil. Trans R Soc Trop Med Hyg 109:601–603. doi: 10.1093/trstmh/trv062. [DOI] [PubMed] [Google Scholar]
- 224.Taran-Benshoshan M, Ofer N, Dalit VO, Aharoni A, Revhun M, Nitzan Y, Nasser AM. 2015. Cryptosporidium and Giardia removal by secondary and tertiary wastewater treatment. J Environ Sci Health A Tox Hazard Subst Environ Eng 50:1265–1273. doi: 10.1080/10934529.2015.1055152. [DOI] [PubMed] [Google Scholar]
- 225.Ebner J, Koehler AV, Robertson G, Bradbury RS, Jex AR, Haydon SR, Stevens MA, Norton R, Joachim A, Gasser RB. 2015. Genetic analysis of Giardia and Cryptosporidium from people in Northern Australia using PCR-based tools. Infect Genet Evol 36:389–395. doi: 10.1016/j.meegid.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 226.Van Lith L, Soba B, Vizcaino VV, Svard S, Sprong H, Tosini F, Pozio E, Caccio SM. 2015. A real-time assemblage-specific PCR assay for the detection of Giardia duodenalis assemblages A, B and E in fecal samples. Vet Parasitol 211:28–34. doi: 10.1016/j.vetpar.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 227.Patricio C, Amaral P, Lourenco J. 2014. An uncommon case of hepatopulmonary amoebiasis. BMJ Case Rep 2014:bcr2014204129. doi: 10.1136/bcr-2014-204129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Asano H, Kaneuchi M, Furuta I, Yamaya Y, Hatanaka KC, Takeda M, Matsuno Y, Sakuragi N. 2014. Genital infection caused by Entamoeba histolytica confirmed by polymerase chain reaction analyses. J Obstet Gynaecol Res 40:1441–1444. doi: 10.1111/jog.12351. [DOI] [PubMed] [Google Scholar]
- 229.Hartmeyer GN, Hogh SV, Chen M, Holt H, Skov MN, Kemp M. 2013. Need for species-specific detection for the diagnosis of amoebiasis in a non-endemic setting. Scand J Infect Dis 45:868–871. doi: 10.3109/00365548.2013.816440. [DOI] [PubMed] [Google Scholar]
- 230.Fontecha GA, Garcia K, Rueda MM, Sosa-Ochoa W, Sanchez AL, Leiva B. 2015. A PCR-RFLP method for the simultaneous differentiation of three Entamoeba species. Exp Parasitol 151-152:80–83. doi: 10.1016/j.exppara.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 231.Gomes Tdos S, Garcia MC, de Souza Cunha F, Werneck de Macedo H, Peralta JM, Peralta RH. 2014. Differential diagnosis of Entamoeba spp. in clinical stool samples using SYBR green real-time polymerase chain reaction. Sci World J 2014:645084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Calderaro A, Gorrini C, Bommezzadri S, Piccolo G, Dettori G, Chezzi C. 2006. Entamoeba histolytica and Entamoeba dispar: comparison of two PCR assays for diagnosis in a non-endemic setting. Trans R Soc Trop Med Hyg 100:450–457. doi: 10.1016/j.trstmh.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 233.Yakoob J, Abbas Z, Beg MA, Naz S, Khan R, Jafri W. 2012. Entamoeba species associated with chronic diarrhoea in Pakistan. Epidemiol Infect 140:323–328. doi: 10.1017/S0950268811000215. [DOI] [PubMed] [Google Scholar]
- 234.Velasco JM, Yoon IK, Mason CJ, Jarman RG, Bodhidatta L, Klungthong C, Silapong S, Valderama MT, Wongstitwilairoong T, Torres AG, De Cecchis DP, Pavlin JA. 2011. Applications of PCR (real-time and MassTag) and enzyme-linked immunosorbent assay in diagnosis of respiratory infections and diarrheal illness among deployed U.S. military personnel during exercise Balikatan 2009, Philippines. Mil Med 176:1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lau YL, Jamaiah I, Rohela M, Fong MY, Siti CO, Siti FA. 2014. Molecular detection of Entamoeba histolytica and Entamoeba dispar infection among wild rats in Kuala Lumpur, Malaysia. Trop Biomed 31:721–727. [PubMed] [Google Scholar]
- 236.Hung CC, Chang SY, Ji DD. 2012. Entamoeba histolytica infection in men who have sex with men. Lancet Infect Dis 12:729–736. doi: 10.1016/S1473-3099(12)70147-0. [DOI] [PubMed] [Google Scholar]
- 237.Herbinger KH, Fleischmann E, Weber C, Perona P, Loscher T, Bretzel G. 2011. Epidemiological, clinical, and diagnostic data on intestinal infections with Entamoeba histolytica and Entamoeba dispar among returning travelers. Infection 39:527–535. doi: 10.1007/s15010-011-0155-z. [DOI] [PubMed] [Google Scholar]
- 238.Rodulfo H, Ahmar B, Rodriguez ME, Mora L, De Donato M. 2012. Nested PCR reveals elevated over-diagnosis of E. histolytica in Barcelona, Venezuela. Invest Clin 53:365–377. [PubMed] [Google Scholar]
- 239.Rashed SM, Nasr Mel S, Abd-Allah KF, Eraky MA, Nagieb MM. 2011. Comparative study between PCR and microscopic examination in diagnosing. Entamoeba histolytica. J Egypt Soc Parasitol 41:89–98. [PubMed] [Google Scholar]
- 240.Anuar TS, Al-Mekhlafi HM, Abdul Ghani MK, Abu Bakar E, Azreen SN, Salleh FM, Moktar N. 2013. Evaluation of formalin-ether sedimentation and trichrome staining techniques: its effectiveness in detecting Entamoeba histolytica/dispar/moshkovskii in stool samples. J Microbiol Methods 92:344–348. doi: 10.1016/j.mimet.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 241.ElBakri A, Samie A, Ezzedine S, Odeh RA. 2013. Differential detection of Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii in fecal samples by nested PCR in the United Arab Emirates (UAE). Acta Parasitol 58:185–190. doi: 10.2478/s11686-013-0128-8. [DOI] [PubMed] [Google Scholar]
- 242.Lau YL, Anthony C, Fakhrurrazi SA, Ibrahim J, Ithoi I, Mahmud R. 2013. Real-time PCR assay in differentiating Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii infections in Orang Asli settlements in Malaysia. Parasit Vectors 6:250. doi: 10.1186/1756-3305-6-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Moon JH, Cho SH, Yu JR, Lee WJ, Cheun HI. 2011. PCR diagnosis of Entamoeba histolytica cysts in stool samples. Korean J Parasitol 49:281–284. doi: 10.3347/kjp.2011.49.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Foo PC, Chan YY, See Too WC, Tan ZN, Wong WK, Lalitha P, Lim BH. 2012. Development of a thermostabilized, one-step, nested, tetraplex PCR assay for simultaneous identification and differentiation of Entamoeba species, Entamoeba histolytica and Entamoeba dispar from stool samples. J Med Microbiol 61:1219–1225. doi: 10.1099/jmm.0.044552-0. [DOI] [PubMed] [Google Scholar]
- 245.Singh P, Mirdha BR, Ahuja V, Singh S. 2013. Loop-mediated isothermal amplification (LAMP) assay for rapid detection of Entamoeba histolytica in amoebic liver abscess. World J Microbiol Biotechnol 29:27–32. doi: 10.1007/s11274-012-1154-7. [DOI] [PubMed] [Google Scholar]
- 246.ElBakri A, Samie A, Ezzedine S, Odeh R. 2014. Genetic variability of the serine-rich Entamoeba histolytica protein gene in clinical isolates from the United Arab Emirates. Trop Biomed 31:370–377. [PubMed] [Google Scholar]
- 247.Freitas MA, Alvarenga AC, Fernandes HC, Gil FF, Melo MN, Pesquero JL, Gomes MA. 2014. Differentially expressed genes of virulent and nonvirulent Entamoeba histolytica strains identified by suppression subtractive hybridization. Biomed Res Int 2014:285607. doi: 10.1155/2014/285607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jaiswal V, Ghoshal U, Mittal B, Dhole TN, Ghoshal UC. 2014. Association between allelic variation due to short tandem repeats in tRNA gene of Entamoeba histolytica and clinical phenotypes of amoebiasis. Acta Trop 133:1–7. doi: 10.1016/j.actatropica.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 249.Osman M, Bories J, El Safadi D, Poirel MT, Gantois N, Benamrouz-Vanneste S, Delhaes L, Hugonnard M, Certad G, Zenner L, Viscogliosi E. 2015. Prevalence and genetic diversity of the intestinal parasites Blastocystis sp. and Cryptosporidium spp. in household dogs in France and evaluation of zoonotic transmission risk. Vet Parasitol 214:167–170. doi: 10.1016/j.vetpar.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 250.Iqbal A, Goldfarb DM, Slinger R, Dixon BR. 2015. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in diarrhoeic patients in the Qikiqtani Region, Nunavut, Canada. Int J Circumpolar Health 74:27713. doi: 10.3402/ijch.v74.27713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Flecha MJ, Benavides CM, Tissiano G, Tesfamariam A, Cuadros J, de Lucio A, Bailo B, Cano L, Fuentes I, Carmena D. 2015. Detection and molecular characterisation of Giardia duodenalis, Cryptosporidium spp. and Entamoeba spp. among patients with gastrointestinal symptoms in Gambo Hospital, Oromia Region, southern Ethiopia. Trop Med Int Health 20:1213–1222. doi: 10.1111/tmi.12535. [DOI] [PubMed] [Google Scholar]
- 252.Bonilla JA, Bonilla TD, Abdelzaher AM, Scott TM, Lukasik J, Solo-Gabriele HM, Palmer CJ. 2015. Quantification of protozoa and viruses from small water volumes. Int J Environ Res Public Health 12:7118–7132. doi: 10.3390/ijerph120707118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Li N, Neumann NF, Ruecker N, Alderisio KA, Sturbaum GD, Villegas EN, Chalmers R, Monis P, Feng Y, Xiao L. 2015. Development and evaluation of three real-time PCR assays for genotyping and source tracking Cryptosporidium spp. in water. Appl Environ Microbiol 81:5845–5854. doi: 10.1128/AEM.01699-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.El-Badry AA, Al-Antably AS, Hassan MA, Hanafy NA, Abu-Sarea EY. 2015. Molecular seasonal, age and gender distributions of Cryptosporidium in diarrhoeic Egyptians: distinct endemicity. Eur J Clin Microbiol Infect Dis 34:2447–2453. doi: 10.1007/s10096-015-2502-y. [DOI] [PubMed] [Google Scholar]
- 255.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators. 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wumba RD, Zanga J, Mbanzulu KM, Mandina MN, Kahindo AK, Aloni MN, Ekila MB. 2015. Cryptosporidium identification in HIV-infected humans. Experience from Kinshasa, the Democratic Republic of Congo. Acta Parasitol 60:638–644. [DOI] [PubMed] [Google Scholar]
- 257.Goni P, Almagro-Nievas D, Cieloszyk J, Lobez S, Navarro-Mari JM, Gutierrez-Fernandez J. 2015. Cryptosporidiosis outbreak in a child day-care center caused by an unusual Cryptosporidium hominis subtype. Enferm Infecc Microbiol Clin 33:651–655. doi: 10.1016/j.eimc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 258.Frickmann H, Schwarz NG, Rakotozandrindrainy R, May J, Hagen RM. 2015. PCR for enteric pathogens in high-prevalence settings. What does a positive signal tell us? Infect Dis 47:491–498. [DOI] [PubMed] [Google Scholar]
- 259.Stensvold CR, Ethelberg S, Hansen L, Sahar S, Voldstedlund M, Kemp M, Hartmeyer GN, Otte E, Engsbro AL, Nielsen HV, Molbak K. 2015. Cryptosporidium infections in Denmark, 2010–2014. Dan Med J 62:A5086. [PubMed] [Google Scholar]
- 260.Luna VA, Stewart BK, Bergeron DL, Clausen CR, Plorde JJ, Fritsche TR. 1995. Use of the fluorochrome calcofluor white in the screening of stool specimens for spores of Microsporidia. Am J Clin Pathol 103:656–659. doi: 10.1093/ajcp/103.5.656. [DOI] [PubMed] [Google Scholar]
- 261.Beauvais B, Sarfati C, Molina JM, Lesourd A, Lariviere M, Derouin F. 1993. Comparative evaluation of five diagnostic methods for demonstrating Microsporidia in stool and intestinal biopsy specimens. Ann Trop Med Parasitol 87:99–102. doi: 10.1080/00034983.1993.11812742. [DOI] [PubMed] [Google Scholar]
- 262.Muller A, Stellermann K, Hartmann P, Schrappe M, Fatkenheuer G, Salzberger B, Diehl V, Franzen C. 1999. A powerful DNA extraction method and PCR for detection of Microsporidia in clinical stool specimens. Clin Diagn Lab Immunol 6:243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Fedorko DP, Nelson NA, Cartwright CP. 1995. Identification of Microsporidia in stool specimens by using PCR and restriction endonucleases. J Clin Microbiol 33:1739–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kock NP, Petersen H, Fenner T, Sobottka I, Schmetz C, Deplazes P, Pieniazek NJ, Albrecht H, Schottelius J. 1997. Species-specific identification of Microsporidia in stool and intestinal biopsy specimens by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis 16:369–376. doi: 10.1007/BF01726365. [DOI] [PubMed] [Google Scholar]
- 265.Rinder H, Janitschke K, Aspock H, Da Silva AJ, Deplazes P, Fedorko DP, Franzen C, Futh U, Hunger F, Lehmacher A, Meyer CG, Molina JM, Sandfort J, Weber R, Loscher T. 1998. Blinded, externally controlled multicenter evaluation of light microscopy and PCR for detection of Microsporidia in stool specimens. The Diagnostic Multicenter Study Group on Microsporidia. J Clin Microbiol 36:1814–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Stark D, Al-Qassab SE, Barratt JL, Stanley K, Roberts T, Marriott D, Harkness J, Ellis JT. 2011. Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. J Clin Microbiol 49:257–262. doi: 10.1128/JCM.01796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Taniuchi M, Verweij JJ, Noor Z, Sobuz SU, Lieshout L, Petri WA Jr, Haque R, Houpt ER. 2011. High throughput multiplex PCR and probe-based detection with Luminex beads for seven intestinal parasites. Am J Trop Med Hyg 84:332–337. doi: 10.4269/ajtmh.2011.10-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Taniuchi M, Verweij JJ, Sethabutr O, Bodhidatta L, Garcia L, Maro A, Kumburu H, Gratz J, Kibiki G, Houpt ER. 2011. Multiplex polymerase chain reaction method to detect Cyclospora, Cystoisospora, and Microsporidia in stool samples. Diagn Microbiol Infect Dis 71:386–390. doi: 10.1016/j.diagmicrobio.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Basuni M, Mohamed Z, Ahmad M, Zakaria NZ, Noordin R. 2012. Detection of selected intestinal helminths and protozoa at Hospital Universiti Sains Malaysia using multiplex real-time PCR. Trop Biomed 29:434–442. [PubMed] [Google Scholar]
- 270.Coste JF, Vuiblet V, Moustapha B, Bouin A, Lavaud S, Toupance O, de Rougemont A, Benejat L, Megraud F, Wolak-Thierry A, Villena I, Chemla C, Le Magrex E, de Champs C, Andreoletti L, Rieu P, Leveque N. 2013. Microbiological diagnosis of severe diarrhea in kidney transplant recipients by use of multiplex PCR assays. J Clin Microbiol 51:1841–1849. doi: 10.1128/JCM.03366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Perry MD, Corden SA, Howe RA. 2014. Evaluation of the Luminex xTAG gastrointestinal pathogen panel and the Savyon Diagnostics gastrointestinal infection panel for the detection of enteric pathogens in clinical samples. J Med Microbiol 63:1419–1426. doi: 10.1099/jmm.0.074773-0. [DOI] [PubMed] [Google Scholar]
- 272.Buss SN, Leber A, Chapin K, Fey PD, Bankowski MJ, Jones MK, Rogatcheva M, Kanack KJ, Bourzac KM. 2015. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol 53:915–925. doi: 10.1128/JCM.02674-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chemaly RF, Yen-Lieberman B, Schindler SA, Goldfarb J, Hall GS, Procop GW. 2003. Rotaviral and bacterial gastroenteritis in children during winter: an evaluation of physician ordering patterns. J Clin Virol 28:44–50. doi: 10.1016/S1386-6532(02)00237-8. [DOI] [PubMed] [Google Scholar]
- 274.Meyers WM, Firpo A, Wear DJ. 2011. Topics on the pathology of protozoan and invasive arthropod diseases. Armed Forces Institute of Pathology, Washington, DC: http://www.dtic.mil/dtic/tr/fulltext/u2/a547773.pdf, accessed 9 January 2016. [Google Scholar]
- 275.Keiser J, Utzinger J. 2009. Food-borne trematodiasis. Clin Microbiol Rev 22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bird RG, Smith MD. 1980. Cryptosporidiosis in man: parasite life cycle and fine structural pathology. J Pathol 132:217–233. doi: 10.1002/path.1711320304. [DOI] [PubMed] [Google Scholar]
- 277.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 278.Martinelli Filho M, Ribeiro HB, Paula LJ, Nishioka SA, Tamaki WT, Costa R, Siqueira SF, Kawakami JT, Higuchi ML. 2009. Endocarditis secondary to Microsporidia giant vegetation in a pacemaker user. Circulation 119:e386–e388. doi: 10.1161/CIRCULATIONAHA.108.817312. [DOI] [PubMed] [Google Scholar]
- 279.Keeling PJ. 2003. Congruent evidence from alpha-tubulin and beta-tubulin gene phylogenies for a zygomycete origin of Microsporidia. Fungal Genet Biol 38:298–309. doi: 10.1016/S1087-1845(02)00537-6. [DOI] [PubMed] [Google Scholar]
- 280.Tyzzer EE. 1907. A sporozoan found in the peptic glands of the common mouse. Proc Soc Exp Biol Med 5:12–13. [Google Scholar]
- 281.Sood AB, Debeic MR, Yeh S, Grossniklaus HE, Randleman JB. 2016. Microsporidial stromal keratitis and endophthalmitis in an immunocompetent patient. J Ophthalmic Inflamm Infect 6:30. doi: 10.1186/s12348-016-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chan KS, Koh TH. 2015. Microsporidian eye infection from recreational activities. J Clin Microbiol 53:753. doi: 10.1128/JCM.03192-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Morada M, Lee S, Bunther-Cummins L, Weiss LM, Widmer G, Tzipori S, Yarlett N. 2016. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int J Parasitol 46:21–29. doi: 10.1016/j.ijpara.2015.07.006. [DOI] [PubMed] [Google Scholar]