SUMMARY
Beginning in 2004, chikungunya virus (CHIKV) went from an endemic pathogen limited to Africa and Asia that caused periodic outbreaks to a global pathogen. Given that outbreaks caused by CHIKV have continued and expanded, serious consideration must be given to identifying potential options for vaccines and therapeutics. Currently, there are no licensed products in this realm, and control relies completely on the use of personal protective measures and integrated vector control, which are only minimally effective. Therefore, it is prudent to urgently examine further possibilities for control. Vaccines have been shown to be highly effective against vector-borne diseases. However, as CHIKV is known to rapidly spread and generate high attack rates, therapeutics would also be highly valuable. Several candidates are currently being developed; this review describes the multiple options under consideration for future development and assesses their relative advantages and disadvantages.
KEYWORDS: chikungunya, vaccine, therapeutics
INTRODUCTION
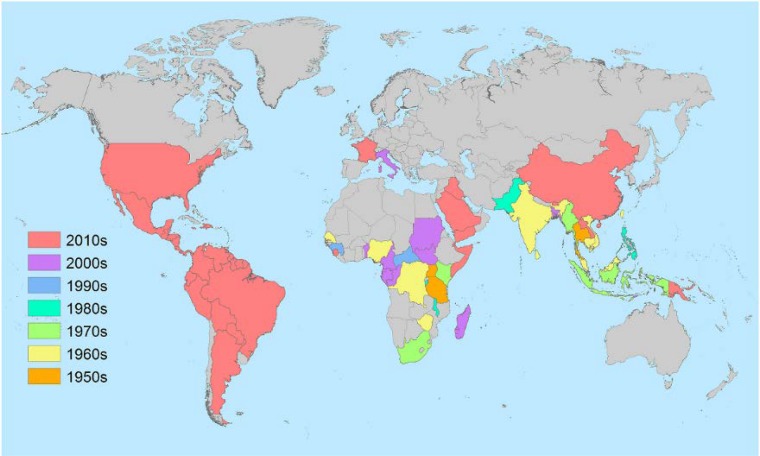
Chikungunya (CHIK) virus (CHIKV) was first identified during an outbreak of febrile polyarthralgia in modern-day Tanzania in 1952 to 1953 (1). Patients reported an illness that was nearly indistinguishable from dengue, consisting of high fever, joint pain, and a macropapular rash (now considered the classic triad of symptoms of CHIKV infection). The unusual severity of the joint pain was the factor prompting researchers to determine if the agent truly was due to dengue virus or another pathogen. The cause was indeed determined not to be dengue virus but a novel agent designated chikungunya virus, a local-dialect name given to indicate the severity of the pain. A number of subsequent large outbreaks due to this same agent, CHIKV, followed in the next 2 decades in urban centers of Thailand and India (Fig. 1). Urban outbreaks were first reported in Thailand in the late 1950s and early 1960s (2), where the scope of these outbreaks was unprecedented, involving attack rates of >30% (3). Urban outbreaks were also reported in Calcutta (modern-day Kolkata), India, in 1963 and in Madras (modern-day Chennai) State, India, from 1962 to 1964, where 40% of the population was infected (4, 5). Interestingly, while urban areas were the primary areas of reported activity, similarly high attack rates were found in rural Thailand (6). This suggested that while periodic outbreaks were occurring, there was endemic activity occurring in Southeast Asia. However, in spite of endemicity, from the 1980s to the turn of the century, no significant epidemic activity was reported. With the development of genetic methods to characterize the strains obtained in these outbreaks, it was revealed that strains were geographically linked, and thus, three genotypes were described for distinct clades representing strains from Central/East Africa (ECSA genotype), West Africa (West African genotype), and India/Southeast Asia (Asian genotype) (7). This geographic association with genotype remained unchanged until the outbreaks of the 21st century.
FIG 1.
Global distribution of CHIKV. Country colors correspond to the decade of the first reported identification of the local transmission of CHIKV by either serological, molecular, or virological detection methods. (Image created by Nicole Lindsey.)
Beginning around 2000, focal bursts of CHIKV activity were reported in Indonesia and the Republic of Congo, revealing that the virus was still circulating in its broad areas of endemicity (8, 9). However, in 2004, an outbreak that occurred in coastal Kenya (10) sparked the beginning of a decade-long, roving outbreak. The virus moved from Kenya to Comoros to La Reunion within approximately 1 year (11). In La Reunion, an amino acid mutation was detected (12), which led to a resurgence of the outbreak on this island, to hundreds of travel-associated cases being identified in Europe, and to the movement of the ECSA genotype out of Africa to India, where over 1 million cases were estimated within a single year (13). The movement of the virus of this ECSA genotype continued throughout Asia, Oceania, and the South Pacific (14–16), while public health officials prepared for the further movement of the virus to the Americas (17).
INTRODUCTION OF CHIKV INTO THE WESTERN HEMISPHERE
Given the rapid expansion of CHIKV throughout nearly all of the Eastern hemisphere, it was not unanticipated that the virus would arrive in the Western hemisphere as well. A number of elements necessary for an outbreak to occur were present in the Americas prior to the arrival of the virus. Primary among these factors was the presence of appropriate vectors, Aedes aegypti and Aedes albopictus, throughout virtually all of tropical America and some regions of the subtropics. These are the same species of mosquitoes that transmit dengue viruses, so anywhere that dengue can be found, a risk of CHIKV infection also exists. Second, because the virus had never circulated in the Americas, the population was completely naive, having no level of preexisting immunity. Similarly, because the virus had not previously been seen in this environment, physicians and public health officials had no training, reagents, or preparation for diagnosing infection by CHIKV. It was curious as to why there was no establishment of CHIKV in the Western hemisphere until the fall of 2013, as all of these conditions had existed for years, and there was evidence of repeated introductions via viremic travelers. Even more curious was that the establishment resulted from an introduction of the Asian genotype, which was not the major genotype that had been circulating throughout the Indian Ocean and Southeast Asia since 2004.
Detection of Asian genotype strains was reported in New Caledonia in 2011 during a period of intense surveillance for CHIKV activity throughout Asia. There were only approximately 2 dozen cases identified, but the finding that these isolates were of the Asian genotype gave conclusive evidence that this genotype was still actively circulating. In 2011 to 2012, Asian genotype CHIKV was also identified in Indonesia and the Philippines, with activity on multiple islands. The year 2013 saw further Asian genotype activity in a number of islands of the Pacific Ocean, including a large outbreak in the Federated States of Micronesia. The first report of the autochthonous transmission of CHIKV in the Americas was in December 2013 in the French Caribbean island of Martinique. Within just 1 year, the virus had moved to 43 countries of the Americas and affected over 1.1 million people (18).
Since the reemergence of CHIKV in 2004 in Kenya, the total number of cases globally has been estimated at over 3.4 million individuals. However, despite the high number of individuals affected over the past decade, there are still significant numbers of individuals at risk for infection. This risk includes not only those in the tropics but also those in areas in Asia, the United States, and Europe where A. albopictus is present in subtropical regions as well. Given the ongoing risk, the severity and chronicity of symptoms associated with CHIKV infection, and the need to protect travelers with the goal of containing CHIKV spread, vaccines and therapeutic products are urgently needed. Vaccines have been in development for over 50 years, with increased urgency in the past decade, and therapeutic products are now also being explored. Each of these products has advantages and limitations, which are described below.
BIOLOGY OF CHIKV
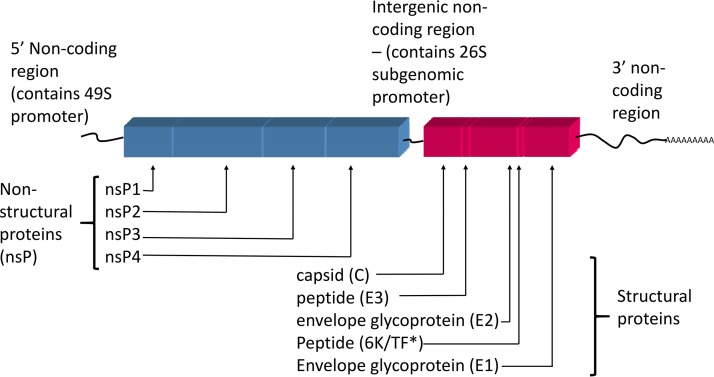
CHIKV has the characteristic genome structure and organization of all alphaviruses (family Togaviridae) (19). The genome consists of a single strand of positive-sense RNA that encodes 4 nonstructural proteins (nsP1 to -4) in the 5′ two-thirds of the genome and 3 structural proteins plus 3 peptides (capsid [C], envelope glycoprotein 1 [E1], envelope glycoprotein 2 [E2], 6K, transframe [TF], and E3) in the 3′ one-third of the genome (Fig. 2). The structural proteins are generated as a polyprotein (which is subsequently processed into mature, individual proteins) from a subgenomic mRNA, which results in large numbers of these proteins compared with the numbers of nonstructural proteins. The capsid protein associates with the genomic RNA to form nucleocapsids that are coated with the surface glycoproteins (E1 and E2) at the cell surface prior to budding. Like the structural proteins, the nonstructural proteins are translated as a polyprotein, which is cleaved by nsP2 and polyproteins containing nsP2. None of the nonstructural proteins are packaged in the final virions, so the humoral immune response primarily targets the predominant structural proteins present on the virion surface (E1 and E2). These surface proteins are also the target of most vaccine and antiviral strategies, as discussed below.
FIG 2.
Genome organization of CHIKV. Shown are both the order and presence of the structural and nonstructural proteins encoded in the CHIKV genome. *, for the 6K peptide, an alternate protein, designated the transframe protein (TF), can be generated due to a frameshift event that may occur during the translation of the 6K gene (19).
HISTORY OF CHIKV VACCINE DEVELOPMENT
CHIKV vaccine development began in response to one of the earliest documented urban outbreaks. The epidemic of Thai hemorrhagic fever that occurred in Bangkok, Thailand, in the early 1960s and that was in part caused by CHIKV (20, 21) led to research to develop a formalin-inactivated vaccine derived from suckling-mouse brains (SMB) infected with an African genotype strain of CHIKV. Neutralizing antibodies were generated at 15 days postinfection, demonstrating that this first product could be protective against CHIKV infection (22). A second early effort also utilized an African genotype strain that was inactivated by either formalin or UV irradiation (23) after 177 passages through SMB and 1 baby hamster kidney (BHK) cell passage. The vaccine was tested in a monkey model, where neutralizing (NT) antibodies were generated after a 3-dose immunization schedule. Interestingly, there were differences in the immune responses generated by the inactivation methods, with studies showing that UV inactivation was superior to formalin inactivation of this product. However, no additional work followed this early research on this formulation.
The most significant early CHIKV vaccine development was performed at the Walter Reed Army Institute of Research (WRAIR), which began with the systematic characterization of a distinct range of cell types, including chicken embryos (CE), SMB, and African green monkey kidney cells (GMKC) (24). Each product was formalin inactivated and subjected to potency testing in mice. Following 2 intraperitoneal (i.p.) injections at days 0 and 7, mice were challenged intracranially (i.c.) 14 days later. This initial assessment revealed that the CE preparation generated poor antibody and protective responses, while the GMKC product generated high levels of antibody and was protective against i.c. challenge. Curiously, a separate study indicated that the CE preparation generated up to 5 times more antibody than the GMKC preparation (25). It was subsequently determined that the titer of the virus prior to formalin inactivation was critical for a potent immune response, with a highly concentrated product being necessary. To avoid a concentration step with CE cells and to avoid potential allergic reactions that might be associated with a SMB product, the GMKC product was the formulation selected for further development. The GMKC product was next used in cross-strain challenges in multiple host systems, where both mice and monkeys were protected in challenge studies using 4 different CHIKV strains (24). An alternative method of inactivation of the GMKC product, using ether and a surfactant, was also evaluated (26). This method resulted in a strong antibody response that was protective against homologous virus challenge in mice. Interestingly, while hemagglutinating activity in addition to the NT antibody response was seen with this approach, this method was not pursued further.
To evaluate the safety of the formalin-inactivated product, a new strain (15561), obtained directly from human serum from a Thai patient, was chosen over the highly passaged initial strain. The first human testing with this purified product showed that most subjects developed NT antibody within just 2 weeks of a second dose (doses given on days 0 and 28), with no adverse events (27).
From this preliminary work at WRAIR, a live, rather than inactivated, vaccine was developed from the 15561 strain. A new attenuated strain, 181/clone 25 (181/25), was derived by 18 plaque-to-plaque passages of the parent product (28). Strain 181/25 had all the hallmarks of attenuation (29, 30), including a small-plaque phenotype, temperature sensitivity, decreased virulence (in mice), and low levels of viremia (in monkeys). When tested in mouse or monkey challenge models, this vaccine was protective and demonstrated reduced virulence compared to that of its nonattenuated parent strain. This was important since 181/25 was a live vaccine, demanding a more rigorous safety profile than inactivated options.
The favorable safety profile indicated that phase 1 clinical trials were appropriate for 181/25. After subcutaneous (s.c.) vaccination, all subjects seroconverted, with only low levels of viremia and symptoms comparable to those receiving a placebo (31). In a follow-up phase 2 double-blind, placebo-controlled trial, the safety profile was found to be acceptable; 98% of subjects developed NT antibodies, with 85% of these subjects maintaining antibody persistence at 1 year postvaccination. Vaccinees and placebo recipients demonstrated similar systemic reactions, with the exception of 5 vaccinees who developed transient arthralgia (32). However, despite some of these promising results, development efforts ended in 1998 due to limited funding and the unpredictable epidemiology of the virus (33).
Despite the termination of the U.S. military development program, the investigational new drug (IND) protocol for this vaccine candidate remained active until 2011 to allow the submission of reports from research studies. Additionally, stocks of vialed product were maintained in storage. The maintenance of these materials was fortuitous, as after the 2006 epidemic in La Reunion, the Ministry of Health of France requested access to these Department of Defense (DOD) materials for subsequent work to develop the vaccine (33). Other entities also performed follow-up studies with the vaccine candidate to further understand the mechanism of protection from both host and virus perspectives. One study used the vaccine virus in various strains of mice with deficiencies in their interferon (IFN) signaling to elucidate the role of distinct immune factors (34). Mice lacking all IFN receptors (IFN-α/β and -γ; AG129 strain) rapidly succumbed to the vaccine, while those maintaining IFN-γ receptors (A129) showed some signs of illness but recovered completely, suggesting a role for IFN in the development of protective immunity. Furthermore, when A129 mice were challenged with an ECSA genotype strain virus, all animals survived, confirming the at least partial role of IFN in the 181/25 vaccine response. Additional studies examined mutations associated with the 181/25 vaccine strain that are responsible for attenuation. A series of clones with individual or multiple mutations revealed that two E2 nonsynonymous mutations (at amino acid positions 12 and 82) generated the same attenuated phenotype as that of the parental virus (35). Subsequent work showed that the E2 residue at position 82 regulated tissue tropism and the host response via the differential utilization of glycosaminoglycans (GAGs) (36). Interestingly, some of those studies also revealed genetic reversion from the vaccine sequence to that of the parental sequence in both mice and humans (33, 35). Because so few mutations were found to be associated with attenuation in this vaccine, any reversion events would be important safety concerns for this product. While at least four additional entities requested the TSI-GSD-218 vaccine product for work toward licensure or comparative testing (including at least one company that was preparing for further clinical trials), as of 2012, a licensed product remains unavailable, and a range of other options to develop a licensed vaccine have been explored.
CURRENT VACCINE OPTIONS
After the reemergence of CHIKV in 2004 and its rapid expansion throughout the Indian Ocean and Southeast Asia and unexpected autochthonous transmission in Italy in 2007, there was renewed interest in developing a vaccine against CHIKV. A variety of research approaches were used to develop a product that would generate high levels of antibodies, provide lasting immunity, and require minimal and straightforward production needs. Options including virus-like particles (VLPs), subunit vaccines, vectored/chimeric vaccines, nucleic acid vaccines, and live attenuated vaccines have all been explored as possibilities (Table 1). The most developed of these products are reviewed below, and the advantages and disadvantages of each product are described.
TABLE 1.
Vaccine candidates under development and primary characteristics of each candidatea
| Vaccine type | CHIKV element(s) and source(s) (genotype) | Model system(s) | Immunization |
Challenge |
Humoral immune response(s) | Cell-mediated response(s) | Reference(s) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose(s) | Route(s) | Schedule(s) | Dose(s) | Route | Strain(s) (genotype) | ||||||
| VLP | Lentivirus-expressed C-E1, 37997 strain (WAf) | BALB/c mice, 6 to 8 wk of age | 19 μg VLP | i.m. | 2 and 6 wk | ND | ND | ND | IC50, 10,703 to 54,600 | ND | 37 |
| Rhesus macaques, 3 to 4 yr of age | 20 μg VLP | i.m. | 0, 4, and 24 wk | 1010 PFU, 15 wk p.i. | i.v. | LR 2006 OPY1 (ECSA) | IC50, 10,219 to 15,072 | ND | 131 | ||
| VLP | Lentivirus-expressed C, E2, and E1, 37997 strain (WAf) | Human clinical trial, phase 1 | 10, 20, or 40 μg | i.m. | 0, 4, and 20 wk | ND | ND | ND | IC50, 4,525 to 8,745 | ND | 40, 41 |
| VLP | Baculovirus-expressed C-E1, S27 strain (ECSA) | AG129 mice, 6 wk of age | 1 μg | s.c. | 0 and 21 days | 1,000 TCID50, 6 wk p.i. | i.p. | S27 (ECSA) | NT95, 40 to 80 | ND | 68 |
| C57BL/6 mice, 6 to 12 wk of age | 0.1 μg or 1 μg | s.c. | Single dose | 104 CCID50, 6 wk p.i. | s.c. | LR 2006 OPY1 (ECSA) | NT95, ∼1,100 | ND | 132 | ||
| VLP | Yeast-expressed C-E1, DRDE06 and DRDE07 strains (ECSA) | BALB/c mice, 4 wk or 2 days of age | 10, 20, or 40 μg | s.c. | 1, 14, and 28 days | ND | ND | ND | NT50, 128 to 2,048 | Th1/Th2 | 133 |
| Subunit | E1 and E2, S27 strain (ECSA) | AG129 mice, 6 wk of age | 2 μg | s.c. | 0 and 21 days | 1,000 TCID50, 6 wk p.i. | i.p. | S27 (ECSA) | NT95, <25 | ND | 67, 132 |
| Subunit | E2, IND-06-AP3 strain (ECSA) | BALB/c mice, 6 to 8 wk of age | 10, 20, or 50 μg | i.m. | 0 and 2 wk | 2.5 × 105 TCID50, 2 and 20 wk p.i. | i.n. | IND-06-AP3 (ECSA) | NT50, 56 to 2,560 | Th1 or Th2, depending on the adjuvant | 69 |
| Subunit | E1 and E2, DRDE-06 strain (ECSA) | BALB/c mice, age not specified | 40 μg | s.c. | 1, 21, and 35 days | ND | ND | ND | NT90, 32 to 512 | Th1/Th2 | 70 |
| Inactivated | Vero cell adapted, DRDE-06 strain (ECSA) | Swiss albino mice, 3 to 4 wk of age | 10, 25, or 50 μg | s.c. | 0, 14, and 28 days | ND | ND | ND | NT90, 6,400 | Th1/Th2 | 69, 134 |
| Chimeric virus (subunit) | Ad/CHIKV C-E1, LR2006 OPY1 strain (ECSA) | CD-1 or C57BL/6 mice, 6 to 8 wk of age | 108 IU | i.p. | Single dose | 104 CCID50 units, 6 wk p.i. | s.c. | LR2006 OPY1 (ECSA) and QIMR (Asian) | NT100, ∼2,000 | Th2/Th1 | 57 |
| Chimeric virus (VLP) | MV-CHIKV C-E1, 06-49 strain (ECSA) | CD46-IFNAR mice, 6 wk of age | 103 to 105 TCID50 | i.p. | Single dose or 0 and 30 days | 100 PFU | i.p. | 06-49 (ECSA) | NT90, 50 to 450; NT50, 450 to 4,050 | CMI (type not specified) | 45 |
| Chimeric virus (VLP) | MV-CHIKV C-E1, 06-49 strain (ECSA) | Human clinical trial, phase 1 | 1.5 × 104 to 3.0 × 105 TCID50 | NP (s.c. or i.m. per Priorix protocol) | 0 and 28 days or 0 and 90 days | NA | NA | NA | NT50, 5 to 433 | ND | 46 |
| Chimeric virus | MVA C-E1, LR2006 OPY1 strain (ECSA) | C57BL/6 mice, 5 to 8 wk of age | 1 × 107 to 2 × 107 PFU MVA-CHIKV | i.p. | 0 and 2 wk | 106 PFU, 7 wk p.i. | s.c. | LR 2006 OPY1 (ECSA) | NT50, 3 × 102 to 5 × 103 | CD8 response | 54 |
| Chimeric virus | MVA E3-E2, LR2006 OPY1 strain (ECSA) | BALB/c mice, 4 to 6 wk of age; A129 mice, 6 to 10 wk of age | 107 TCID50 | i.d. | 0 or 0 and 28 days | 104 PFU (BALB/c), 102 PFU (A129) 11 or 14 days p.i. | i.d. | LR 2006 OPY1 (ECSA) | NT50, ≤10 | CD4 response | 56 |
| Chimeric virus | MVA E3-E2, 6K-E1, and E3-E1, S27 strain (ECSA) | AG129 mice, 7 wk of age | 5 × 106 TCID50 | i.m. | 0 and 3 wk | 103 TCID50, 6 wk p.i. | i.p. | S27 (ECSA), IND/NL10 | NT100, 10 to 160 | ND | 135 |
| Chimeric virus | VSV E3-E1, S27 strain (ECSA) | C57BL/6 mice, 3 wk of age | 106 PFU | i.m. | Single dose | 104 PFU, 30 days p.i. | s.c. | LR 2006 OPY1 (ECSA) | NT80, 80 to 640 | CD8 response | 61 |
| Chimeric virus | EEEV-CHIKV, VEEV-CHIKV C-E1, CHIKV LR strain (ECSA) | NIH Swiss mice, C57BL/6 mice, >3 wk of age; CD-1 mice, 6 wk of age; A129 mice, 6 to 9 wk of age | 104 to 106 PFU | s.c. or i.p. | Single dose | 6.5 log10 PFU, 4 wk p.i.; 100 PFU, 5 wk p.i. (A129) | i.n. or s.c. | Ross (ECSA) or LR (ECSA) | NT80, 72 to 136, 20 to >640 | ND | 47, 50 |
| Chimeric virus | EILV-CHIKV C-E1, CHIKV-99659 strain (Asian) | C57BL/6 mice, 4 wk of age | 8.8 log10 PFU | s.c. | Single dose | 6.0 log10 PFU, 30 days p.i. | i.d. | 99659 strain (Asian) | NT80, ≥80 | CD4 and CD8 responses | 51 |
| IFN-α/βR−/− mice, 6 wk of age | 8.8 log10 PFU | s.c. | Single dose | 3.0 log10 PFU, 292 days p.i. | i.d. | 99659 strain (Asian) | NT80, 160 to 1,280 | ND | |||
| Cynomolgus macaques, 3 to 5 yr of age | 8.1 log10 PFU | i.m. | Single dose | 5.0 log10 PFU, 31 days p.i. | s.c. | LR (ECSA) | NT80, 80 to 640 | No CD4 response, no activated CD8 response | |||
| Live attenuated | CHIKV-IRES, LR strain (ECSA) | A129 mice, 3 or 10 wk of age | 104 PFU | i.d. | Single dose | 100 PFU, 94 days p.i. | i.d. | WT CHIKV (LR, ECSA) | NT80, >320 | ND | 64 |
| C57BL/6 mice, 3 wk of age | 105 PFU | s.c. | Single dose | 106 PFU, 3 wk p.i. | i.n. | Ross (ECSA) | Mean NT80, 62 | ND | |||
| A129 mice, 8 to 10 wk of age | 105 TCID50 | s.c. | Single dose | 100 PFU, 6 wk p.i., in CD4/CD8-depleted mice | i.d. | LR (ECSA) | Mean NT80, 1,152 | CD4 and CD8 responses | 136 | ||
| Cynomolgus macaques, >3 yr of age | 105 PFU | s.c. or i.d | Single dose | 105 PFU, 52 days p.i. | s.c. | WT CHIK-LR (ECSA) | NT50, 160 to 1,280; NT80, 80 to 640 | ND | 137 | ||
| Live attenuated | E2 TMD deletion mutants 37997 (WAf) | C57BL/6J mice, 28 days of age | ∼103 PFU | s.c. | Single dose | 103 PFU, 28 days p.i. | s.c. | SL15649 (ECSA) | NT50, 0 to ∼400 | ND | 138 |
| Live attenuated | 181/25, 15561 strain (Asian) | Human clinical trial | ∼105 PFU | s.c. | Single dose | NA | NA | NA | Geometric mean NT50, 582 | ND | 32, 33 |
| Live attenuated | 181/25, 15561 strain (Asian) | AG129 mice; A129 mice, 6 to 8 wk of age | 103 to 104 PFU | i.d. | Single dose | 100 PFU, day 38 or day 247 p.i.; A129 mice only | i.d. | CHIKV-LR (ECSA) | NT80, <10 to 160 | ND | 34 |
| Live attenuated | Δ6K and Δ5nsP3, LR 2006 OPY1 strain (ECSA) | C57BL/6 mice, 5 to 6 wk of age | 104 to 105 PFU | s.c. | Single dose or 0 and 3 wk | 106 PFU, 7 wk p.i. | s.c. | CHIKV-LR (ECSA) | NT50, 102 to 105 | Th1 response, CD8 T cell response | 76 |
| Live attenuated | Δ5nsP3, LR 2006 OPY1 strain (ECSA) | Cynomolgus macaques, 3 to 4 yr of age | 105 PFU | s.c. | Single dose | 7,000 to 10,000 PFU (100 AID50) | i.v. | CHIKV-LR 2006-OPY1 (ECSA) | NT50, >103 (persists for at least 300 days) | CD4 T cell response | 77 |
| Live attenuated | Heparin sulfate cell culture adapted, LR 2006 OPY1 strain (ECSA) | CD-1 mice, 21 days of age | 105 genome equivalents (∼103 PFU) | s.c. | Single dose | 103 PFU, 3 wk p.i. | NP | CHIKV-LR (ECSA) | Max of 104-fold NT change vs mock-infected cells | ND | 139 |
| Live attenuated | CHIKV-NoLS, LR 2006 OPY1 strain (ECSA) | C57BL/6 mice, 21 days of age | 104 PFU | s.c. | Single dose | 104 PFU, 30 days p.i. | s.c. | CHIKV-LR (ECSA) or Ross River virus | <10% cells infected at serum dilution of 10−1 | ND | 66 |
| DNA | Δ6K and Δ5nsP3, LR 2006 OPY1 strain (ECSA) | C57BL/6 mice, 5 to 6 wk of age | 20 μg DNA | i.d. with DermaVax electroporation | Single dose or 0 and 3 wk | 106 PFU, 7 wk p.i. | s.c. | CHIKV-LR (ECSA) | NT50, 102 to 104 | Th1 response, CD8 T cell response | 76 |
| DNA | C, E1, and E2, consensus sequence from multiple NCBI strains | C57BL/6 mice, 3 to 4 wk of age | 25 μg DNA | i.m. and electroporation | 0 and 2 wk or 0, 2, and 4 wk | ND | ND | ND | Ab generated (ELISA data only) | CMI, type not specified | 73 |
| C57BL/6 mice, 6 to 8 wk of age | 25 μg DNA | i.m. and electroporation | 0, 2, and 4 wk | 107 PFU, 35 days p.i. | i.n. | PC-08 (ECSA) | Ab generated | Th1 response | 75 | ||
| BALB/c mice, 8 wk of age | 25 μg DNA | i.m. and electroporation | 0, 2, and 4 wk | 107 PFU | i.n. | PC-08 (ECSA) | TCID50, 20 to 320 | CD8 T cell response | 74 | ||
| Rhesus monkeys, 4 to 8 yr of age | 1 mg | i.m. and electroporation | 0, 4, and 8 wk | ND | ND | ND | TCID50, 80 to 1,280 | CD8 T cell response | |||
| DNA | Complete coding sequence of the 181/25 strain (Asian) | BALB/c mice, 3 wk of age | 10 μg DNA | i.m. and electroporation | Single dose | 6 × 106 PFU | i.n. | Ross (ECSA) | NT80, 320 to 1,280; NT50, 640 to 10,240 | ND | 78 |
| DNA | DNA-launched MAb against CHIKV E1/E2, CHIKV LR (ECSA) | BALB/c mice, age not specified | 100 μg CVM1-IgG plasmid | Electroporation | Single dose | 107 PFU | s.c. or i.n. | Del-03 (ECSA) | IC50, 3 to 4.5 log10 units; 90 to 100% protection against mortality | No T cell response | 80 |
p.i., postimmunization; NT95, 95% neutralizing titer; IC50, 50% inhibitory concentration; ND, not done or not discussed; NA, not applicable; IU, infectious units; s.c., subcutaneous; i.p., intraperitoneal; i.v., intravenous; i.d., intradermal; i.n., intranasal; i.m., intramuscular; NP, data not provided; WAf, West African genotype; ECSA, East/Central/South African genotype; AID50, 50% animal infectious dose; Ad, adenovirus; VSV, vesicular stomatitis virus; CMI, cell-mediated immunity; EEEV, eastern equine encephalitis virus; EILV, Eilat virus; VEEV, Venezuelan equine encephalitis virus; MVA, modified vaccinia virus Ankara; MV, measles virus; WT, wild type; ELISA, enzyme-linked immunosorbent assay; Ab, antibody; MAb, monoclonal antibody; TMD, transmembrane domain. Shown is information on candidates reported through April 2017.
Virus-Like Particles
One of the first potential new CHIKV vaccines to reach advanced development was a VLP preparation. The product is generated from the CHIKV C-E3-E2-6K/TF-E1 polyprotein genes, using a cytomegalovirus CMV/R expression vector that was transfected into 293 human kidney cells (37). Because the entire structural gene cassette is present in the cells, a CHIKV VLP (structurally identical to the infectious virus but containing no nucleic acid) is generated. The resulting VLPs were purified by using buoyant density gradient sedimentation and quantitated to standardize doses. While VLPs representing both a West African genotype strain (37997) and an ECSA genotype strain (OPY-1) were initially developed, the 37997 variant yield was approximately 100-fold higher than that of the OPY-1 strain. Because the ongoing outbreak at the time of development of this product was due to the ECSA linage, there was some concern that a product of the West African genotype might not be protective against strains of other genotypes. However, the high percentage of amino acid similarity among all CHIKV strains (7) combined with serological cross-reactivity between CHIKV strains of different genotypes (38–40) suggested that while certain strains may lead to better vaccines, any CHIKV vaccine would be protective against viruses of all genotypes.
The high-yield 37997 VLPs were injected intramuscularly into BALB/c mice in a two-dose series. A strong neutralizing antibody response was elicited against both homologous and heterologous strains (37). Follow-up studies in macaques showed that all animals injected with the VLPs developed neutralizing antibodies even after a single dose; the response was further increased after a booster dose. This product looked so promising that a phase 1 human trial was initiated to assess safety in adults, using a dose escalation format (41). There was no fever or arthralgia and no serious adverse events reported from this initial trial. All subjects developed neutralizing antibodies after the second vaccine dose, but at the final study time point, peak titers had dropped by approximately 4-fold. Not only did serum collected from the volunteers contain neutralizing antibodies, these antibodies were also found to be cross-protective against CHIKV strains of all genotypes (40), reinforcing the idea that a vaccine developed by using a CHIKV strain of any genotype would be broadly protective. The VLP approach is favored due to the safety profile of the product, since no live virus is present (there is no nucleic acid contained in the VLPs; therefore, it is replication incompetent). Production is also easy, and large stocks can be generated rapidly. However, because it is functionally a “killed” product, concerns of low immunogenicity have some merit.
Chimeric Vaccines
One of the most widely explored options for high levels of immunity is the use of a virus-vector system that incorporates genetic elements of CHIKV that are expressed, resulting in a strong antigen response. A number of these expression systems have been used for years for producing foreign gene products. These vectored vaccines typically have the advantage of continuous replication, generating more robust immune responses, and because they contain avirulent backbones, they frequently have increased safety. A number of different backbone platforms have been developed for CHIKV vaccines; these are described below, and their distinct traits are highlighted.
Measles virus-based chimeras.
The most advanced of these recombinant vaccines for CHIKV is based on the measles virus (MV) backbone. This system consists of a recombinant, live attenuated measles vaccine containing a CHIKV structural protein gene cassette. Because this system contains both the capsid protein and the envelope glycoprotein genes, upon expression, a CHIKV VLP is generated. As noted above, this results in a near-native presentation of the structural proteins with an immune response that mimics that against wild-type virus infection.
The measles virus backbone used for this CHIKV vaccine is based on the live attenuated Schwarz strain, which has a demonstrated record of safety and effectiveness (42). It was previously used as a vector for foreign antigen expression to develop protective immunity against West Nile virus (WNV) and dengue viruses in rodents and monkeys (43, 44), suggesting that it would likely be effective against alphavirus targets as well. For the CHIKV vaccine, the complete CHIKV structural coding region was inserted into the infectious MV cDNA. Western blotting, immunofluorescence analysis, and electron microscopy confirmed the expression of CHIKV E2 and capsid along with the detection of VLPs with a size and a morphology similar to those of wild-type CHIKV particles (45). Upon infection of CD45-IFNAR mice (lacking IFN-α/β receptors and expressing human MV receptors) with two doses given 4 weeks apart, all mice developed neutralizing CHIKV E2-specific antibodies. Fifty percent neutralization titers (NT50) ranged from 50 to 450 after the first dose and from 450 to 4,050 after a second dose, depending on the initial vaccine dose. Cell-mediated immunity was also detected even after the first immunization, as determined by an IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assay on CHIKV-stimulated splenocytes collected from vaccinated animals (45). When mice were challenged with 100 PFU of an ECSA genotype CHIKV strain 4 weeks after the second dose, 100% protection from death was seen with vaccine doses of 104 or higher.
While no nonhuman primate studies with this vaccine candidate were reported, phase 1 clinical trials have been completed. The trials included 3 different doses and 2 prime-boost schedules (46). The lowest dose (1.5 × 104 median tissue culture infection doses [TCID50]/0.05 ml) showed only low levels of seroconversion after the first dose, while the medium (7.5 × 104 TCID50/0.25 ml) and high (3.0 × 105 TCID50/1.0 ml) doses had ≥90% seroconversion after just one dose. All subjects seroconverted after 2 doses. Not unexpectedly, antibody titers were highest in the high-dose group and peaked after the booster dose. There were several severe adverse events following vaccination, including headache, injection site pain, influenza-like illness, and musculoskeletal pain, particularly in the high-dose group, making the medium dose the best compromise for immunogenicity and tolerability. In comparison with the only other CHIKV vaccine that had entered human clinical trials (live attenuated candidate 181/25), no arthralgic manifestations were noted with this candidate, likely due to the use of a MV backbone rather than the use of a live attenuated CHIKV strain.
One concern with this vaccine vector was the possibility of poor responses due to the presence of preexisting anti-MV immunity. However, anti-CHIKV antibody titers were generated in both MV-immune and naive mice, suggesting that previous vaccination of an individual with a MV vaccine would not prevent subsequent protection against the target of a chimeric MV-CHIKV vaccine product.
Alphavirus-based chimeras.
The next most developed set of chimeric vaccine options for CHIKV are those based on alternative alphaviruses. The earliest use of this approach involved the development of chimeras based on 3 other alphavirus backbones (47). One was an attenuated strain of Venezuelan equine encephalitis virus (VEEV) that is an IND product, the second was based on a Brazilian variant of Madariaga virus (formerly known as South American-type eastern equine encephalitis virus) that has been shown to be naturally attenuated in adult mice (48), and the third is a high-passage strain of Sindbis virus. In all chimeras, the nonstructural open reading frame (ORF) and the noncoding regions were from the alternative alphavirus, while the structural gene coding region was removed and replaced with the corresponding region of an ECSA genotype CHIKV strain. Previous work showed that chimeric alphaviruses containing the replication machinery of one alphavirus and the structural genes of another are highly attenuated (49). However, because they are also live vaccines, they continue to replicate and express the antigenic proteins of interest, typically eliciting a strong immune response. All three of these chimeras developed similar infection and immune response profiles when inoculated subcutaneously into 5-week-old outbred mice (47). No animals developed any detectable viremia in the first 3 days postimmunization, and all animals developed neutralizing antibodies (with a mean titer range of 40 to 136) by 3 weeks. When intranasally (i.n.) challenged with an ECSA strain of the virus at 21 days postimmunization, all mice remained healthy and had no detectable viremia. One of these chimeric viruses, VEEV-CHIKV, was later modified to increase the safety profile. The subgenomic promoter responsible for generating the structural polyprotein was inactivated/replaced by an internal ribosome entry site (IRES) derived from encephalomyocarditis virus (EMCV). By incorporating genetic elements from a third virus, the likelihood of reversion to virulence was greatly reduced (50). Subcutaneous inoculation of these mutated variants in 6-week-old CD-1 mice resulted in neutralizing antibodies by 28 days postimmunization. In a more sensitive mouse model, A129 mice (lacking IFN-α/β receptors) inoculated subcutaneously with 104 or 105 PFU of the VEEV–IRES-C–CHIKV variant showed no viremia and no mortality but developed neutralizing antibody responses. These animals also survived a lethal challenge dose. With any of the chimeric alphaviruses, consideration must be given to monitoring for compensatory, adaptive mutations that could modulate attenuation or lead to a reversion to virulence.
The most recent alphavirus chimeric variant utilizes the insect-only alphavirus Eilat virus (EILV) as the backbone containing the structural genes of CHIKV (51). The EILV-based backbone provides an additional layer of safety by lacking the capacity to replicate in vertebrate cells, thus functionally serving as a killed vaccine with enhanced immunogen expression. When tested in immunocompetent mice (C57BL/6), a high dose (8.8 log10 PFU) resulted in 80% seroconversion by just 4 days postimmunization. Challenge of these live EILV-CHIKV mice at 30 days postimmunization with CHIKV (99659 strain) prevented any viremia or footpad swelling. Curiously, formalin-inactivated EILV-CHIKV resulted in some delayed footpad swelling, suggesting that inactivation somewhat altered immunogenicity. In type I IFN-deficient mice (A129), neutralizing titers ranged from 40 to 1,280 at 289 days postimmunization, which were lower than the titers in comparator mice infected with the 181/25 candidate (titers of >1,280). However, challenge at day 292 resulted in complete protection from disease or viremia. Cynomolgus macaques similarly seroconverted by day 4 postvaccination and showed no signs of disease or viremia. Collectively, this work showed safety (without the need for inactivation) combined with strong immunogenicity and ease of production, making this a promising approach.
Vaccinia virus-based chimeras.
An alternative vector vaccine system that has been developed by multiple groups as a CHIKV vaccine is based on the highly attenuated modified vaccinia virus Ankara (MVA). One advantage of this system is the safety profile, as productive virus assembly does not occur in most mammalian cells (52), while the expression of foreign genes readily occurs. While the lack of generation of new particles might be thought to limit the amount of the antigen overall, the ability of MVA to upregulate early immune host responses typically results in strong immunogenicity of the foreign antigen (53). The first MVA-CHIKV system described included the complete CHIKV structural coding region of an ECSA genotype strain of the Indian Ocean lineage. Curiously, while no VLPs were detected with this expression vector, in vitro studies demonstrated that infected human monocytes and dendritic cells elicited innate IFN-β and proinflammatory cytokines (54). When used to infect C57BL/6 mice, a strong cell-mediated response was generated, as shown by the induction of CHIKV-specific memory T cells, where CD8+ T cell responses were directed primarily against E1 and E2. Unfortunately, the role of T cell responses in modulating CHIKV infection is not yet well defined (55). In addition to cell-mediated immunity, a strong humoral immune response was generated in all animals tested, with neutralizing antibody titers reaching >500, even after the administration of a single intraperitoneal vaccine dose of 107 PFU. Significantly, when mice were challenged with the wild-type virus, no CHIKV viremia was detected, and all animals were protected from disease (as measured by a lack of footpad swelling). A subsequent study using MVA as the vaccine vector incorporated only the CHIKV E3 and E2 proteins (56). This recombinant virus was injected into 2 mouse models: one immunocompetent mouse model (BALB/c) and one mouse model lacking type I IFN receptors (A129). The vaccine was found to be protective in both mouse models using a prime-boost system with doses of 107 50% tissue culture infective doses (TCID50) injected intradermally into the hind footpad. Curiously, virtually no neutralizing antibodies were detected, and the passive transfer of immune serum was not protective. Additionally, CD4+ cells appeared to be necessary for protection, confounding the understanding of the role of various immune effectors in disease modulation.
Adenovirus-based chimeras.
Another well-explored vector system that has been evaluated as a potential CHIKV vaccine vector was a nonreplicating, complex, adenovirus vector vaccine (CAdVax) with the structural polyprotein ORF of CHIKV incorporated (57). The use of an adenovirus vector system was attractive because these vectors have been shown to be stable, highly immunogenic, safe, and efficacious in a number of human clinical trials. Additionally, they are relatively easy to manufacture, making them a practical approach (58).
This adenovirus shuttle vector, encoding capsid, E3, E2, 6K/TF, and E1 of a CHIKV strain of the Indian Ocean lineage, was used to generate vector stocks in a packaging cell line. Outbred CD-1 mice given a single intraperitoneal dose of the vaccine (108 infectious units) developed CHIKV-specific IgG antibody by 2 weeks postvaccination. Similar, high levels of IgG antibodies were detected in inbred C57BL/6 mice postvaccination. Neutralizing antibodies were also generated after vaccination with the CAdVax-CHIK vaccine. When inbred mice were challenged in the footpad with 104 50% cell culture infectivity doses (CCID50) of either Asian or ECSA genotype strains of CHIKV, vaccinated animals developed no viremia or footpad swelling, in contrast to control animals, demonstrating protection against multiple genotypes of CHIKV with an ECSA genotype strain-based vaccine.
As with MV, concerns regarding the ability of the vaccine to provide protection if there was preexisting immunity to the adenovirus elements have been raised. While this specific point was not directly addressed with the CAdVax-CHIKV vaccine, studies of other CAdVax vaccine candidates have demonstrated that increasing the vaccine dose could overcome the effects of preexisting immunity (59). Because studies with the very sensitive New Zealand White rabbit model with the adenovirus vaccine showed no significant adverse events (57), it is anticipated that any higher doses of CAdVax-CHIKV would not be problematic.
Vesiculovirus-based chimeras.
The final chimeric virus system evaluated as a possible CHIKV vaccine utilizes a vesiculovirus backbone. The vesicular stomatitis virus (VSV) expression vector has been used in a number of vaccine studies by generating pseudotyped viruses with genes from several different pathogens (60). One VSV vaccine, designated VSVΔG-CHIKV, contains the CHIKV glycoprotein genes from an ECSA genotype CHIKV strain in place of the VSV G glycoprotein (61). This construct has the advantage of not inducing immunity against the vector itself (due to the lack of the G protein, which is the immunogenic element of VSV), making it possible to use this vector multiple times. A single dose of 106 PFU delivered intramuscularly to mice (C57BL/6) induced neutralizing antibodies but only if viral transcription and replication occurred; this indicates that any inactivated form of this VSV vaccine would likely be ineffective. When immunized mice were challenged s.c. in the footpad with 104 PFU of a CHIKV strain of the Indian Ocean lineage, the VSVΔG-CHIKV-vaccinated animals showed some footpad swelling, but it was significantly less than that in control animals. As might be expected for a VSV-based vaccine (62), VSVΔG-CHIKV-vaccinated animals developed cell-mediated immunity, particularly in response to the CHIKV E1 glycoprotein.
Live Attenuated Vaccines
While live attenuated vaccine options have long been considered, they were typically generated by repeated passage in cell culture. For the alphaviruses, this approach was used with CHIKV, as described above, as well as for VEEV (63). However, advances in alphavirus reverse genetic systems have allowed the development of rationally designed, attenuated options. These candidates contain very specific mutations or alterations of the parental virus genome that typically allow increased specificity, better safety profiles, and high levels of expression, allowing protection with only a single dose of the vaccine. Of course, reversion or compensatory mutations must be considered when using this approach.
One of the engineered live attenuated options for CHIKV that has been most developed is a full-length CHIKV clone where the natural subgenomic promoter was replaced with the IRES from encephalomyocarditis virus (64). The use of the IRES element functionally alters the host range, as viruses derived from the cDNA are incapable of replicating in mosquitoes. Thus, even if a vaccinee were to induce viremia, there would be no possible transmission to mosquitoes, thereby increasing the safety of the vaccine. This approach was also used previously with VEEV chimeric viruses (see above) to generate high levels of expression of the structural polyprotein with no risk of reversion to the wild type (65). The inclusion of the nonstructural CHIKV elements potentially adds to the CHIKV-specific immune response and reduces the degree of attenuation that often accompanies chimeric alphaviruses, particularly those based on an already attenuated parent strain.
The CHIKV-IRES vaccine candidate is based on a full-length clone of a wild-type strain from La Reunion (ECSA genotype). When the subgenomic promoter was removed and the IRES was inserted to drive the translation of the structural polyprotein, the resulting vaccine product was found to be highly attenuated in both infant outbred mice and mice lacking type I IFN receptors (64). In 6-day-old CD-1 mice inoculated subcutaneously with 105 PFU, no virus was detectable in any tissue examined, while mice inoculated with control virus strains (181/25 vaccine or an Indian Ocean lineage strain) had viremia through day 4 postvaccination. Ten-week-old A129 mice (which show signs of illness when infected with wild-type CHIKV) remained healthy when inoculated with 104 PFU intradermally (i.d.) in the footpad. Viremia developed in A129 mice given the CHIKV-IRES vaccine, but the level of viremia was statistically lower than that in 181/25-inoculated mice, and footpad swelling (greater than that in 181/25-inoculated mice) resulted. The attenuation of the CHIKV-IRES vaccine was more clear in 3-week-old A129 mice, where all mice survived vaccination (n = 4) without any signs of illness, while 181/25-inoculated mice all succumbed by day 8.
This vaccine candidate was further tested in mouse challenge models. When 10-week-old vaccinated A129 mice were challenged with only 100 PFU, weight loss occurred, but all mice survived. Antibody 80% neutralization titers (NT80) prior to challenge were >640; postchallenge titers were not reported. An immunocompetent mouse challenge model was also examined with this vaccine. Three-week-old C57BL/6 mice were vaccinated with 105 PFU subcutaneously and later bled to determine antibody titers. All mice seroconverted with titers of ≥20. On the same day, mice were given an intranasal challenge of 106 PFU using an ECSA genotype CHIKV strain. No signs of illness resulted in any of the CHIKV-IRES-vaccinated mice, while 70% (7/10) of the sham-vaccinated mice died by day 10 postchallenge (64).
Another recent approach for attenuating CHIKV to serve as a vaccine has been to modify specific viral sequences associated with particular functions. One example was a modification of the nucleolar localization sequence (NoLS) located in the capsid protein (66). This sequence enables the capsid protein to be translocated to the host cell nucleolus. In encephalitic alphaviruses, this process results in the shutdown of host cell transcription, but the effect on arthralgic alphaviruses is unknown. Mutation of this site in the virus (CHIKV-NoLS) was shown to reduce replication in both mammalian and mosquito cells, and mice (C57BL/6) infected with this mutant showed no signs of disease compared with mice infected with the wild-type virus. Mice immunized with this strain were also protected from disease when challenged with the related Ross River virus (RRV), suggesting that broad alphavirus cross-protection could be achieved if the vaccine was appropriately designed and common viral functional elements were altered.
Subunit Vaccines
The use of individual CHIKV-specific proteins as subunit vaccines is attractive for a number of reasons, including safety and scalability. Because they contain no viral nucleic acid or infectious particles, they have a better safety profile than options that may result in infection, even if attenuated. The production of individual proteins is also an approach that can be easily developed in large-scale manufacturing facilities, leading to an increased ease of production. An understanding of the immunogenicity of the CHIKV glycoproteins E2 and E1 has led several groups to explore subunit vaccine products based on these envelope proteins.
One potential CHIKV subunit vaccine utilizes recombinant E2 and E1 (and the associated peptides E3 and 6K/TF) generated from a baculovirus expression system (67). The development of the proteins in an insect system leads to questions regarding appropriate glycosylation and cleavage; however, Western blot analyses have indicated that both proteins are indeed N-glycosylated (at least partially), are secreted, and are of the expected molecular weights but also are not fully processed from E3 (67). Antibodies generated against the E2 recombinant protein were capable of neutralizing CHIKV, indicating the potential of this subunit approach. Further studies examining immunogenicity indicate that these subunit proteins induce neutralizing antibodies in mice (AG129) but at much lower levels than intact VLPs (68).
Alternative subunit vaccine candidates, also based on the CHIKV E1 or E2 protein (without 6K/TF or E3), have been generated by using bacterial expression systems (69, 70). The E2 protein (or a truncated E2 variant [69]) was found to generate strong neutralizing antibody titers in mouse models, with neutralizing index values varying with the adjuvant used. BALB/c mice that were challenged with the truncated E2 protein also demonstrated complete protection against disease and sterilizing immunity when using most adjuvants. Cell-mediated immunity was demonstrated with these products, but the adjuvant played a role in the type and magnitude of responses generated.
DNA Vaccines
DNA vaccines constitute one of the newest categories of vaccine approaches for CHIKV. DNA vaccines have a number of advantages that make them attractive from both manufacturing and public health perspectives. The ease of production is a tremendous advantage, as is the safety associated with the DNA product itself. DNA is less sensitive to cold-chain storage conditions and therefore potentially has a much longer shelf life. Additionally, the ability of DNA-based vaccine products to develop both humoral and cell-mediated immunity is a significant improvement over traditional approaches and is particularly useful for those agents for which the pathway for protection is not characterized. However, DNA vaccines have shown only limited promise in most cases, with low immunogenicity in humans, the finding that large doses and repeated boosters are necessary, the lack of DNA uptake, and the need for adjuvants (71). Some work has been undertaken to overcome these limitations, such as the use of electroporation combined with immunization (72), but the overall platform still has limited support for development as advances in the methodologies are evaluated.
The development of DNA vaccines for CHIKV began with the construction of a series of plasmid vectors containing each of the primary structural proteins, capsid, E2, and E1 (73). Of particular interest is that these CHIKV genetic elements were not derived from any single strain but rather were derived from a consensus sequence of all strains available in the NCBI nucleotide database at the time. This strategy presumably eliminated any concerns associated with genotype differences that might impact broadly based efficacy. These vaccines were tested both in vitro and in vivo for expression, cellular responses, and the ability to generate an antibody response. This early work demonstrating efficient expression and the ability to generate both antibodies and cell-mediated immunity led to further evaluation in additional models, including nonhuman primates (74). The previous results were confirmed, with neutralizing antibodies and CD8 T cell responses being documented in both mice and monkeys. Additionally, outbred mice were protected against disease upon challenge after 3 immunization doses. Further development of the system included the addition of a plasmid with the nsP2 sequence, which was found to enhance the effect of the envelope protein vaccine (75).
A second generation of CHIKV DNA vaccines included the complete cDNA of attenuated forms of the CHIKV genome expressed from a plasmid vector. One such system is an infectious genome that was attenuated by deleting portions of the nsP3 gene or the complete 6K gene (76). A single immunization with this DNA generated high levels of neutralizing antibodies and strong T cell responses and protected mice against both viremia and foot swelling. Further challenge studies using a single dose of this vaccine candidate in a nonhuman primate model (cynomolgus macaques) resulted in protection against disease and generated antibodies that neutralized a heterologous Asian genotype strain to levels comparable to those of the homologous virus (77). While the attenuated virus demonstrated complete protection against challenge, there was still low levels of viremia upon immunization, perhaps suggesting only limited attenuation. However, these studies showed that the DNA-launched particles generated immunity and protection that were just as strong as those of wild-type viral particles, suggesting the significant potential of this approach. An additional example is that of infectious particles of the 181/25 live attenuated CHIKV strain launched from a cytomegalovirus promoter (78). This particular construct takes advantage of a DNA approach while using a known vaccine candidate that has been shown to be promising as a live product. As with the deletion DNA vaccines, this immunization DNA (iDNA) plasmid was efficacious and generated neutralizing antibodies in 100% of the mice tested, with no viremia being detected in any animal postchallenge. Perhaps even more significant for this particular candidate is that the reversion frequency for the mutations conferring attenuation is much lower than that in the 181/25 parental vaccine candidate (79). This suggests that in addition to the other advantages of DNA vaccines, the DNA-launched infectious-particle vaccines may also have a better safety profile than live attenuated options while maintaining the positive characteristics of a live product.
A recent and interesting DNA vaccine strategy is to launch sequence encoding anti-CHIKV monoclonal antibodies from a DNA plasmid (80). Because the plasmids generate a biologically active antibody, this approach can be used for both the generation of therapeutic antibodies as well as the rapid initiation of immunity without the laboratory phase associated with traditional antigen-generating vaccines. A plasmid containing heavy and light chain sequences specific for CHIKV envelope proteins constructed as full-length IgG (CVM1-IgG) generated CHIKV-specific antibodies upon electroporation into mice (BALB/c). This antibody neutralized multiple strains of both the ECSA and Asian genotypes. This approach benefits from both safety, as no virus elements are involved, as well as the capacity to be used as both a vaccine and a therapeutic agent, particularly in an emergency response situation.
HISTORY OF CHIKV THERAPEUTIC TREATMENTS
From the early known outbreaks of CHIK fever, treatment has been described as being supportive to reduce fever and pain using products such as analgesics, anti-inflammatory drugs, and antipyretics. An anecdotal comment from a patient with chronic CHIK arthralgia indicating that they felt less pain when taking antimalaria medication led to a pilot study in the early 1980s to evaluate the efficacy of chloroquine phosphate in treating CHIK symptoms (81). While that study was limited, results for 7 of 10 patients indicated that treatment with 250 mg/day of chloroquine phosphate for the 20-week study was effective. In spite of the promise of this treatment in this single study, the drug was not further evaluated until 2007, when a study on La Reunion Island suggested that there was no benefits for patients receiving this drug (82). However, a different study in India during the same outbreak found that chloroquine treatment provided improvements in patient symptoms (83). Curiously, this is the only drug that was studied for the treatment of CHIK prior to the large ongoing outbreaks of the 21st century, and given that these limited assessments provided apparently conflicting results, it is clear that larger and more systematic evaluations of this drug are needed to clearly determine its usefulness for treatment.
NEW OPTIONS FOR THERAPEUTIC AGENTS
While most emphasis on developments for CHIK control has been on vaccines, a growing number of studies, most of which were published within just the past 2 to 3 years, have addressed possible therapeutic options. A number of approaches have been examined for the development of therapeutics, including the use of traditional antiviral compounds; the synthesis of designer compounds; high-throughput screening in silico for existing products with possible efficacy against CHIKV; and the use of nucleic acid compounds, therapeutic monoclonal antibodies, and drugs that target host cell proteins (Table 2). There are advantages to each of these approaches, which are presented below, along with the likelihood of eventual application.
TABLE 2.
Therapeutic candidates under development and primary characteristics of each candidatea
| Type of therapeutic | Compound(s) | Model system(s) | CHIKV strain(s) (genotype) | Functional concentration |
Other measure(s) of inhibition | Selectivity index (CC50/EC50) |
In vivo trial or case study design |
Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | EC50 | CC50 | Dose(s) | Route | |||||||
| Known antiviral | Ribavirin | Vero cell culture | Ross C347 (ECSA) | 58 μM | 83 μM | NP | 1 to 24 | ND | ND | 84 | |
| Interferon alpha | >10,000 μM | 9.7 to 11.1 μM | >900 | ||||||||
| Ribavirin and interferon alpha | Vero cell culture | 181/25 (vaccine strain) (Asian) | NP | 833 μg/ml (ribavirin); 5,761 IU/ml (IFN-α) | NP | NP | ND | ND | 85 | ||
| Ribavirin and doxycycline | Vero cell culture, ICR mice | Clinical isolate (ECSA) | NP | 4.52 μM | NP | 92% inhibition of virus entry into cells | NP | 5 to 50 mg/kg of body wt | i.p. | 86 | |
| Picolinic acid | Vero cell culture | DRDE-06 (ECSA) | NP | NP | NP | CHIKV RNA concn decreased by 2 log10 units | NP | ND | ND | 140 | |
| Niclosamide | BHK-21 cell culture | S27 (ECSA), 0611aTw (ECSA), 0810bTw (ECSA) | NP | 0.85 to 0.95 μM | >20 μM | Decrease of viral titer up to 1.5 log10 units | 11.17 to 23.53 | ND | ND | 95 | |
| Nitazoxanide | BHK-21 cell culture | S27 (ECSA), 0611aTw (ECSA), 0810bTw (ECSA) | NP | 1.96 to 4.95 μM | 25 μM | Decrease of viral titer up to 2 log10 units | 5.05 to 16.34 | ND | ND | ||
| Curcumin | HeLa, BHK-21, Vero E6 | 06-049 (ECSA) | 3.89 μM | NP | 11.6 μM | NP | ND | ND | 101 | ||
| 293T | CHIKV E2/E1-pseudotyped lentivirus vector; CHIKV strain not specified | 3.90 to 10.79 μM | NP | <60 μM | NP | ND | ND | 102 | |||
| Berberine | BHK cell culture | LR2006 OPY1 (ECSA) | NP | 1.8 μM | >100 μM | Decrease of virus production by 5 log10 units | >55.6 | ND | ND | 99 | |
| Huh7.5 cell culture | NP | 1.9 μM | >100 μM | >52.6 | |||||||
| Berberine | Hek293 cell culture | LR 2006 OPY1 (ECSA), SGP11 (ECSA), CNR2023 (Asian) | NP | 4.5 μM | 202.6 μM | 45 | 100 | ||||
| HOS cell culture | NP | 12.2 μM | 429.5 μM | 35 | |||||||
| ATCC CRL-2522 cell culture | NP | 35.3 μM | NP | NP | |||||||
| C57BL/6 mice, 4 wk of age | ND | ND | ND | ND | 106 PFU virus | s.c. | |||||
| 10 mg/kg berberine | i.p. | ||||||||||
| Coumarin A | Vero, C6/36 cell culture | CHIKV ACol (Asian) | NP | 10.7 μg/ml | 3,150 μg/ml | 295.2 | ND | ND | 103 | ||
| Coumarin B | NP | 0.5 μg/ml | 549 μg/ml | 1,021.0 | ND | ND | |||||
| Voacangine | NP | 304.3 μg/ml | 1,136 μg/ml | 3.7 | ND | ND | |||||
| Lupeol acetate | NP | 538.5 μg/ml | 4,015 μg/ml | 7.5 | ND | ND | |||||
| Known antimicrobial | Flavaglines | HEK293T17 cell culture | Thai isolate (ECSA) | NP | 22.4 nM | 92 to 138.5 nM | NP | ND | ND | 87 | |
| Suramin | BHK-21, U2O8, MRC-5 cell culture | S27 (ECSA) | NP | 8.8 to 62.1 μM | ∼350 to 770 μM | 19.3 to >39.1 | ND | ND | 88 | ||
| Suramin | C57BL/6 mice, 4 wk of age | 0810bTw, 0611aTw, 0706aTw (ECSA) | ND | ND | ND | ND | 0.25 to 2 mg suramin | i.p. | 89 | ||
| 105 PFU CHIKV | s.c. | ||||||||||
| Flavanoid (silymarin) | Vero, BHK-21 cell culture | My/065/08/FN295485 (ECSA) | 16.9 μg/ml | 50 to 100 μg/ml | 305 to 425 μg/ml | 25.1 | ND | ND | 90 | ||
| Flavonoid (baicalin) | Virtual screening | NP | ND | ND | ND | Binding affinity of −9.8 kcal/mol | ND | ND | ND | 92 | |
| Flavonoid (naringenin) | Binding affinity of −8.4 kcal/mol | ||||||||||
| Flavonoid (quercetagetin) | Binding affinity of −8.6 kcal/mol | ||||||||||
| Flavonoid (baicalein) | Vero cell culture | FN295485 (ECSA) | 6.997 μM | ND | 356.3 μg/ml | 188.4 | ND | ND | 91 | ||
| Flavonoid (fisetin) | 29.5 μM | 194.4 μg/ml | 23.02 | ||||||||
| Flavonoid (quercetagetin) | 43.52 μM | 226.7 μg/ml | 16.3 | ||||||||
| Flavonoid (green tea catechin) | HEK293T cell culture | NP | 6.54 μg/ml | NP | NP | 40% decrease in infection rate at 10 μg/ml | NP | ND | ND | 96 | |
| Cardiac glycoside (lanatoside C) | BHK cell culture | D1225Y08 (ECSA) | NP | NP | NP | IC70, 1 μM; EC38, 1 μM | NP | ND | ND | 93 | |
| Cardiac glycoside (digoxin) | U-2 OS cell culture | SL15649 (ECSA), 181/25 (Asian) | NP | 48.8 to 108.9 nM | NP | NP | ND | ND | 94 | ||
| HSF cell culture | 43.9 nM | ||||||||||
| Vero cell culture | 67.3 nM | ||||||||||
| ST2 cell culture | 16.2 μM | ||||||||||
| C2C12 cell culture | 23.2 μM | ||||||||||
| Vitamin C | Human case | Unknown | ND | ND | ND | Symptoms resolved | ND | 100 g/day | i.v. | 97 | |
| Chloroquine | Human clinical trial | Infected patients (ECSA) | ND | ND | ND | ND | 250 mg/day | Oral | 81 | ||
| Designer molecules | nsP2 protease inhibitors (dimethylbenzaldehyde derivative) | BHK-21 cell culture | LR2006 OPY1 (ECSA) | ∼50 to 100 μM | 1.5 to >100 μM | >200 μM | >2.1 to >133.3 | ND | ND | 104 | |
| Benzimidazole derivative (compound A) | Vero cell culture | SL10571, S27, (ECSA) BaH306 (Asian) | NP | 0.54 to 0.98 μM | 3.70 μM | NP | ND | ND | 105 | ||
| Benzo-coumarin-arene conjugates | Vero cell culture | 899 (ECSA) | NP | 10.2 to >331 μM | 13.8 to >284 μM | 1.6 to 11.5 | ND | ND | 107 | ||
| LATA-PAP1-THAN peptide fusion protein | Vero cell culture, ICR mice, 5–6 wk of age | SGEHICHS277108 (ECSA) | NP | 11.2 μg/ml | NP | 89% plaque reduction in vitro, 100% survival in mice at 0.75 mg/kg | ND | 0.5 to 1 mg/kg | i.p. | 108 | |
| [1,2,3]Triazolo[4,5-d]pyrimidin-7(6H)-ones | Vero cell culture | 899, LR2006 OPY1 (ECSA) | NP | 0.75 to >490 μM | 82 to >872 μM | >200 | ND | ND | 106 | ||
| Benzimidazole/thiosemicarbazone hybrid (MBZM-N-IBT) | Vero cell culture | S27, DRCE06 (ECSA) | NP | 38.68 to 58.93 μM | >800 μM | Reduction in infectious particle release | >21 | ND | ND | 109 | |
| Nucleic acids | Oligonucleotide (PMO) targeting AUG of ORFs | HeLa cell culture | SGEHICHD122508 (ECSA) | NP | NP | NP | >96% cell viability at 10 μM, 1- to 3-log10 reduction in CHIKV titers | NP | 116 | ||
| BALB/c mice | LK(EH)CH 6708 (ECSA) | 5, 10, and 15 μg/g | i.p. | ||||||||
| Ribonucleoside analog (β-d-N4-hydroxycytidine) | Huh-7, BHK-21, Vero cell culture | CNR20235 (Asian), LR2006 OPY1 (ECSA) | NP | 0.2 to 1.8 μM | 7.7 μM | NP | ND | ND | 141 | ||
| siRNA (against nsP3, E1) | Vero E6 cell culture | DRCE06 (ECSA) | NP | NP | NP | 96.3 to 99.6% reduction of virus titer at 25 nmol siRNA | NP | ND | ND | 112 | |
| siRNA (against nsP1, E2) | Vero E6 cell culture | 061573 (ECSA) | NP | NP | NP | >90% inhibition in cell culture | NP | 113 | |||
| Swiss albino, C57BL/6 mice, 3 to 4 wk of age | 100% inhibition of viremia | 20 to 25 μg | i.v. | ||||||||
| miRNA (against nsP1, nsP2, C) | Vero cell culture | DRDE-07 (ECSA) | ND | ND | ND | Decreases in RNA load and infectious virus titers | ND | ND | ND | 114 | |
| shRNAs (against E1, nsP1, C) | HeLa, BHK-21, RD cell culture | Various strains (ECSA and Asian) | NP | NP | NP | 0.5- to 3.2-log10 reduction in CHIKV titers | NP | 115 | |||
| C57BL/6 suckling mice | 60 to 100% survival | 10, 30, and 60 μg | i.p. | ||||||||
| Monoclonal antibodies | Human MAb C9 | HEK293T cell culture | S27 (ECSA) | 0.1 to 0.4 μg/ml | NP | NP | 100% survival; NT80, 0.3 μg/ml | NP | 0.5 mg MAb/mouse | i.p. | 118 |
| C57BL/6 mice, 6 wk of age | 121 | ||||||||||
| Mouse MAb CK47 (anti-E1) | 293, Vero, B7, PAI cell culture | SL11131, clinical isolate (ECSA) | 0.8 to 250 μg/ml | NP | NP | CHIKV titer reduction of ∼2.5 log10 units | NP | NP | NP | 121 | |
| Human MAbs, various | Vero 81 cell culture, ifnar−/− mice, 6 wk of age | SL15649 (ECSA) | NP | 0.6 to 5,200 ng/ml | NP | 50 to 100% survival in mice | NP | 50 μg | i.p. | 119 | |
| Disease-modifying antirheumatic drug (CTLA4-Ig) with human MAb (4N12) | C57BL/6 mice, 4 wk of age | LR2006 (ECSA) | ND | ND | ND | Eliminated foot swelling, 100- to 10,000-fold decrease in viral load | ND | 300 μg CTLA4-Ig and 300 μg anti-CHIKV MAb | i.p. | 122 | |
| Host cell targets | Prostratin | BGM, Vero, HEL, ATCC CRL-2522 cell culture | CHIKV-899 (ECSA) | NP | 0.2 to 8 μM | >100 μM | Decreased viral RNA levels | NP | ND | ND | 123 |
| Multiple small-molecule inhibitors (i.e., TOFA, pimozide) | HEK293 cell culture | C21 (not specified) | <0.01 to 3.36 μM | NP | NP | Decreased levels of viral replication | NP | 124 | |||
| C57BL/6J mice | Footpad swelling | 20 mg/kg (pimozide), 25 mg/kg (TOFA) | Oral (pimozide), i.p. (TOFA) | ||||||||
| SAT1 (spermidine-spermine acetyltransferase) | Huh7, BHK-21 cell culture | La Reunion 06-049 (ECSA) | ND | ND | ND | Enhanced transcription but limited virion production | ND | 125 | |||
IC50, 50% inhibitory concentration; CC50, 50% cytotoxicity concentration; EC50, 50% effective concentration; ND, not done; NP, not provided; HSF, human synovial fibroblasts; TOFA, 5-tetradecyloxy-2-furoic acid. Shown is information on candidates reported through April 2017.
Known Antimicrobial Compounds
One of the first routes for identifying a suitable antiviral compound is to test those agents that have been found to have activity against other viruses. One such study of CHIKV examined the abilities of several well-known and characterized antiviral agents, including ribavirin, 6-azauridine, glycyrrhizin, and interferon, to control CHIKV replication in Vero cell culture (84). All of these compounds were found to inhibit the replication of CHIKV and to reduce virus titers by as much as 5 log10 units. Unfortunately, for two of the compounds tested, the concentrations required were high (glycyrrhizin) or the chemical was not yet approved for use in humans as an antiviral (6-azauridine). However, the other two compounds, ribavirin and interferon alpha, were even more effective when used in combination (85) but need to be further tested and evaluated in animal models of CHIKV infection. Another study further demonstrated the inhibitory effects of ribavirin when used in combination with doxycycline in an ICR mouse model of infection (86). This work demonstrated that multiple points in the viral life cycle could be interrupted when a combination of drugs was used. These compounds not only have been shown to have broad antiviral activity but also are already approved for this purpose for humans. In addition to evaluating compounds that are known to have antiviral activity, classes of compounds shown to inhibit other pathogens or that have cytoprotective properties have also been evaluated. These compounds include chemicals such as flavaglines (87), suramin (88, 89), flavonoids (such as silymarin) (90–92), cardiac glycosides (93, 94), and other compounds from existing FDA-approved drug libraries (95). While some of these compounds were effective only if given prior to CHIKV infection, all of these compounds show some efficacy against CHIKV in cell culture systems. “Natural” products that may have antiviral properties are a subset of chemicals of particular interest. Two such widely known chemicals, green tea catechin and vitamin C, were among the first chemicals to be preliminarily evaluated for activity against CHIKV (96, 97). Epigallocatechin gallate interfered with CHIKV attachment to and entry into HEK293T cells, while high doses of vitamin C delivered intravenously resolved symptoms in a single CHIKV-infected patient with shoulder and knee pain. A number of additional recent studies have further examined the role of natural products, particularly those derived from plants, seeds, and spices, in generating anti-CHIKV activity (98–103). Even though some of these products have been used as traditional medicines, their modes of action are not always known. However, recent work with natural products has moved to understanding the mechanism(s) of inhibition, which could lead to further modification of the compounds to increase functionality and/or reduce toxicity. For example, curcumin has been shown to block the entry of enveloped viruses in several mammalian cell lines (101, 102), while treatment with berberine altered cellular protein kinase activation and resulted in reductions of viral RNA and protein expression levels (99, 100). Natural products have the appeal of avoiding the introduction of synthetic products, which can be perceived to be unhealthy, but they can also have the drawback of limited bioavailability. It is clear that further and systematic evaluations in additional systems are needed for a range of these possible anti-CHIKV agents.
Synthesis of Designer Compounds
The novel approach of developing designer chimeric compounds has also been undertaken to identify agents effective against CHIKV. Most of these studies focused on particular chemical structures that may be present in drugs with activity against other viruses or pathogens. In one example, computer-aided design of molecules that would “dock” onto the nsP2 protein and serve as protease inhibitors was performed. These designer molecules were synthesized and evaluated for their ability to inhibit protein activity (104). Several of these unnamed compounds, all having common core structures, were shown to inhibit nsP2 functionality in vitro, demonstrating the potential utility of targeted designer molecules based on in silico characteristics. Other studies have screened existing compound libraries for their ability to inhibit the development of cytopathic effects (CPE) after virus infection of cell culture (105, 106). Examples of this approach include variants of compounds possessing a benzimidazole structure (105) and the [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones (106). In cell culture, these agents are effective at low micromolar concentrations against CHIKV and sometimes against other alphaviruses. Another chimeric group of compounds, the benzouracil-coumarin-arene conjugates, involves the combination of 3 distinct moieties that have antiviral activity and distinct activities in an effort to increase effectiveness (107). A variation of the combined-moiety approach is to use shortened peptides rather than whole proteins. These individual peptides, each having some antiviral activity, are fused, as in the case of a latarcin-PAP1-thanatin (LATA-PAP1-THAN) peptide fusion protein, which is more effective than the individual peptide elements (108). While the concept of mixing multiple domains that have complementary functionalities is a clever one, the resulting products do not always demonstrate the anticipated effectiveness. For example, 22 distinct benzouracil-coumarin-arene compounds were synthesized, but only 5 were active against CHIKV in Vero cell culture (107). In contrast, distinct elements that had not been shown to have antiviral activity but were projected to be effective in combination by in silico analysis were effective in the case of a thiosemicarbazone with a benzimidazole group attached (109). Unfortunately, while these combination compounds, particularly peptides, are designed to show high specificity and selectivity, they frequently require extensive large-scale production steps, which may limit their manufacturability. However, this targeted approach to the development of agents with specific activities provides an intelligent design platform for the generation of therapeutics that may have multiple modes of action, which could prevent the development of resistance.
High-Throughput Screening
Assessing the potential of millions of different chemicals to interfere with viral processes is another strategy for identifying compounds that may have particular activity against CHIKV. As this is not a practical approach without high-throughput capacity, methods to screen millions of structures have been described. A natural place to start when considering this massive number of compounds is the utilization of in silico screening. One such study involved a virtual screening simulation of 5 million compounds using the nsP2 protein of CHIKV (viral protease) as the target (110). A series of 26 compounds was identified after performing an analysis of “structure-activity relationships,” but only a few of these compounds tested in cell culture systems demonstrated efficacy in preventing cell death when used at micromolar concentrations. The approach of using in silico screening is theoretically sound but may depend on models of the various proteins being targeted if the actual structures have not been resolved. It is also clearly just a first step in the process, as any computer-identified molecules must ultimately demonstrate biological efficacy.
A more direct approach to evaluate large libraries of compounds is to screen all of them in a biological assay. This obviously requires methodologies that can rapidly handle large numbers of individual tests. The use of a cell-based high-throughput ATP/luminescence screening assay is one approach that has been described to identify inhibitors of CHIKV (111). Using a 384-well-plate format, a liquid-dispensing system mixes cells with suspensions of compounds and the virus. Cells are incubated for 2 days, and the ATP levels are measured, providing an indication of cell viability. Thousands of compounds could be screened by using such an approach in just a matter of weeks, providing researchers with targets for follow-up studies irrespective of whether or not there is any previous information on the antiviral properties of a particular molecule. As high-throughput screening methods have become more feasible and common, multiple studies have used this style of approach to screen natural compound libraries, known antimicrobial libraries, bioactive small-molecule compound libraries, and approved drug libraries (described in other sections and in Table 2). This has the advantage of potentially identifying drugs that already have approval for usage in humans, thus reducing the time needed for regulatory processes.
Nucleic Acid-Based Antivirals
The use of nucleic acids to directly target the viral genome is a strategy that has been used for years against viral pathogens from a number of families. For CHIKV, oligomers, nucleoside analogs, small interfering RNAs (siRNAs), microRNAs (miRNAs), and short hairpin RNAs (shRNAs) have all been evaluated to see if they can inhibit viral replication or translation. Some of the first inhibitors to be tested were siRNAs targeting the nsP3 and E1 genes (112). When transfected into Vero cells, these siRNAs inhibited CHIKV replication by over 99% at 24 h postinfection. However, the protective ability did not last for 72 h when control and test cultures showed similar replication values, presumably due to the instability of the siRNAs. Subsequent studies showed that siRNAs against E1 and nsP1 were able to protect outbred mice when administered at 72 h postinfection (113). miRNA approaches may be preferable over siRNA approaches due to their greater stability. One study using miRNA targeting 3 different proteins (nsP1, nsP2, capsid) demonstrated up to 99.8% reductions in plaques in Vero cell culture at 24 h posttreatment (114). The use of shRNAs is another approach that directly targets the viral genome. One study included shRNAs designed to target capsid, E1, and nsP1 of CHIKV (115). These molecules were used in both cell culture and mice (C57BL/6), and the E1 shRNA inhibited multiple strains of CHIKV and was effective in both cells and suckling mice. Other approaches use nucleoside analogs to interfere with replication or specific phosphorodiamidate morpholino oligomers (PMOs) to sterically block ribosome assembly on the RNA molecule, effectively inhibiting translation. One study using PMOs in suckling BALB/c mice showed that PMOs afforded 100% protection against CHIKV when administered prophylactically (116). While all of these nucleic acid-based approaches demonstrate potential therapeutic value, they all suffer from the same limitation in being extremely specific for the sequences that they target. This could be problematic for RNA viruses like CHIKV due to their high mutational frequencies. However, these antivirals can be easily and rapidly generated in the case of public health emergencies and can be more effective when used in combination.
Antibody Therapies
The use of human immune sera to treat human infections by passive transfer, particularly those that have no other known treatment, has occurred for decades for infection with exotic viruses. One study using this approach utilized immunoglobulins purified from plasma of CHIKV-infected donors to protect both adult and neonatal mice challenged with a CHIKV strain of the Indian Ocean lineage (117). With the advent of monoclonal antibodies, this concept has been further tailored for the targeted treatment of infections, and a number of studies have investigated the therapeutic potential of monoclonal antibodies against CHIKV. One way to obtain CHIKV-specific monoclonal antibodies is to transform B cells from individuals who have recovered from CHIKV infection followed by screening for anti-CHIKV activity and limiting-dilution cloning to obtain pure monoclonal populations. By using this approach, a neutralizing monoclonal antibody was generated, which mapped to the acid-sensitive region of the E2 glycoprotein that is important for virus entry into cells (118). The use of this antibody at up to 18 h postinfection in adult C57BL/6 mice completely protected them from disease. Another study of human-derived monoclonal antibodies targeting the E2 protein examined a panel of 30 candidates derived from a single donor (119). Thirteen of these candidates, all mapping to the A domain of E2, were found to have broad and effective neutralizing activity, and this subset was protective in a lethal mouse model (interferon receptor-deficient mice), even up to 60 h postinfection. This is not unexpected given that antibody binding to the A domain can block fusion, likely by inhibiting needed conformational changes. While antibodies targeting E2 are clear choices for monoclonal antibody treatment to prevent virus binding and entry into cells (120), a mouse monoclonal antibody targeting the E1 glycoprotein has also been shown to be able to prevent the release of progeny CHIKV from infected cells (121). While not tested in vivo, understanding the mechanism of this interference with the release of the virus provides a possible additional approach for therapy.
More recently, combined treatment using monoclonal antibodies and disease-modifying antirheumatic drugs (DMARDs) is a novel approach that has been shown to control CHIKV arthritis disease in a C57BL/6 mouse model by nearly completely eliminating foot swelling combined with eliminating infectious virus (122). This concept of immunomodulatory drug treatment (to block immune responses, as with other inflammatory arthritis-form therapies) combined with specific anti-CHIKV monoclonal antibodies may be applied to a range of infectious diseases.
Targeting Host Cell Pathways
While nearly all of the classical approaches for the therapeutic treatment of an infectious arbovirus are directed at the pathogen itself, the relatively new idea of targeting host cell pathways or host cell molecules necessary for viral replication is a creative and promising option for disease control (123–125). One such example is the use of the molecule prostratin, an activator of protein kinase C (PKC) enzymes, to inhibit CHIKV replication (123). This molecule was previously shown to inhibit the entry of HIV into cells by decreasing necessary receptors for the virus and was therefore tested for effectiveness against CHIKV. Treatment of kidney cells or skin fibroblasts with prostratin reduced the CHIKV RNA load, the production of infectious progeny, and the accumulation of viral nsP1 and C proteins. Interestingly, the stage of inhibition occurred after virus entry, suggesting a mechanism of action distinct from that of HIV inhibition. Another example involved the targeting of spermidine and spermine to reduce the intracellular level of polyamines, which are found in some DNA virions and are required for multiple steps in the CHIKV cell cycle (125). Treatment of mammalian cells with SAT1 to deplete spermidine/spermine led to decreased RNA virus replication, extending the known function of this inhibitor to include affecting RNA viruses. While both of these examples used host cell targets that had been shown to have effects on other viruses, this approach could still be used even if little is known about the biology of the virus. Using a genomewide loss-of-function screening approach to identify host factors affecting virus production, a number of cellular targets that could be therapeutic agents were found (124). These target proteins or pathways were as diverse as fatty acid synthesis, calmodulin signaling, and ATPases. When small-molecule inhibitors of these various targets were applied in vivo, reductions in CHIKV replication and foot swelling occurred. The effects were enhanced when these inhibitors were used in combination, suggesting that the use of multiple drugs targeting distinct pathways could be highly effective, with little likelihood of resistance developing.
SUMMARY AND LIKELY PROSPECTS FOR THE FUTURE
Because CHIKV outbreaks were of such a large magnitude and the virus has essentially caused a pandemic in the past decade, a significant amount of effort has been invested in developing products for both prophylaxis and treatment. Unfortunately, like most arboviral outbreaks, as the epidemics wane or there is simply “information fatigue,” the motivation for further antiviral development tends to decrease over time. However, the sheer number of individuals still at risk for CHIKV infection, the incredibly debilitating nature of the disease, and the potential for chronic musculoskeletal conditions suggest that all the vaccine and therapeutic efforts are warranted and should be continued. In evaluating the various products that have been produced, however, it is clear that there are some limitations in direct comparisons of the products and, ultimately, for selecting a product for further development and clinical trials. One significant challenge is that there are numerous different virus strains used, different animal models with different routes of both vaccination and challenge, and different methods for evaluating efficacy. Having the CHIKV scientific community agree upon certain parameters for product (both vaccine and therapeutic) evaluation would greatly aid in direct comparisons. The use of virus-specific networks and conferences dedicated to individual priority viruses (126) could be venues for discussing and perhaps establishing standard parameters such as animal models, challenge strains of the virus to use, the timing of immunizations/challenges, and evaluation criteria that would allow for more direct comparisons of the various approaches. Another challenge is the need to develop products for a range of users (i.e., for those living in areas of endemicity, travelers, and immunocompromised individuals, etc.). Because different approaches may be more desirable for different target groups, a single approach or platform may be challenging to select.
Of significant concern from a public health perspective is that when large outbreaks of arboviral disease occur and then burn out, there is a lack of drive from partners to bring vaccines to market. Because a license is issued not only for the finished product but also for the processes to produce, test, and release that product, the amounts of funding and effort needed to bring a new vaccine to market are staggering. This includes having both raw materials and component supplies that are consistently available in the same format for years, having a facility that is designed and built for commercial manufacturing, and having knowledgeable and skilled technical staff to ensure the integrity of the processes. The entire process to license a new product, including the research phase, typically ranges from 5 to 18 years and can cost as much as $500 million (127). Justifiably, commercial entities need to balance these costs with eventual returns. When predicting the next outbreak is nearly impossible and another high-priority agent emerges for which a vaccine is warranted (such as the global spread of Zika virus) (128), it is challenging to advocate for the rapid development and deployment of protective vaccines and drugs for any particular individual pathogen. However, this is exactly what is needed for these zoonotic agents that have entered new ecological niches and will be with us from this point forward. One recent suggestion for overcoming some of these challenges would be to create a global vaccine development fund that could “provide the resources and the momentum to carry vaccines from their conception in academic and government laboratories and small biotechnology firms to development and licensure by industry” (129). In particular, this fund would be utilized to develop vaccines against disease targets that have a low prioritization or incentive because the market is too small to justify the investment risks. Eleven pathogens, including CHIKV, were recently noted to belong to this category according to a World Health Organization research and development blueprint for action (130). A resource such as this would be invaluable for financing, but a parallel step is to continue to develop and refine broad-spectrum antiviral platforms and strategies that can be used when they are desperately needed but that will also serve as a springboard for the next emerging need. Finally, while the challenges in developing a CHIKV vaccine are numerous, the science behind virtually all of the approaches described here is sound, and given the biology and great transmissibility of CHIKV, the development of both vaccines and therapeutics for distribution is a goal worth achieving.
ACKNOWLEDGMENTS
I thank Nicole Lindsey for assistance in preparing Fig. 1.
The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Biography

Ann M. Powers, Ph.D., F.A.S.T.M.H., received her Ph.D. in Molecular Arbovirology from Colorado State University. She performed postdoctoral research training at the University of Texas Medical Branch (UTMB) focusing on molecular determinants of mosquito infectivity and alphaviral evolution. She became an assistant professor at UTMB prior to joining the CDC in 2000. Currently, she is a Research Microbiologist and Lead of the Virology Team in the Arboviral Diseases Branch. In her current position, work focuses on investigations of virus-mosquito interactions, identification of alphavirus elements of virulence, and field-based ecology and surveillance projects, and she oversees laboratories involved in arboviral vaccine development, animal model development, and immunobiology. She has held numerous additional international and national positions, including serving as Chair of the Togaviridae Study Group for the International Committee on Taxonomy of Viruses, Chairman of the American Committee on Arthropod-Borne Viruses, a Deputy Editor for the journal PLoS Neglected Tropical Diseases, and Councilor for the American Society for Tropical Medicine and Hygiene. She has authored over 140 scientific publications, book chapters, and public health documents on alphaviruses and other mosquito-borne viruses.
REFERENCES
- 1.Robinson MC. 1955. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. I. Clinical features. Trans R Soc Trop Med Hyg 49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- 2.Jupp PG, McIntosh BM. 1988. Chikungunya virus disease, p 137–157. In Monath TP. (ed), The arbovirus: epidemiology and ecology, vol II CRC Press, Boca Raton, FL. [Google Scholar]
- 3.Halstead SB, Scanlon JE, Umpaivit P, Udomsakdi S. 1969. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. IV. Epidemiologic studies in the Bangkok metropolitan area. Am J Trop Med Hyg 18:997–1021. [DOI] [PubMed] [Google Scholar]
- 4.Myers RM, Carey DE. 1967. Concurrent isolation from patient of two arboviruses, Chikungunya and dengue type 2. Science 157:1307–1308. doi: 10.1126/science.157.3794.1307. [DOI] [PubMed] [Google Scholar]
- 5.Rao TR. 1966. Recent epidemics caused by chikungunya virus in India, 1963-1965. Sci Cult 32:215. [Google Scholar]
- 6.Halstead SB, Udomsakdi S, Scanlon JE, Rohitayodhin S. 1969. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. V. Epidemiologic observations outside Bangkok. Am J Trop Med Hyg 18:1022–1033. doi: 10.4269/ajtmh.1969.18.1022. [DOI] [PubMed] [Google Scholar]
- 7.Powers AM, Brault AC, Tesh RB, Weaver SC. 2000. Re-emergence of Chikungunya and O'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol 81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 8.Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim R, Djauzi, Wandra T, Master J, Kosasih H, Hartati S, Beckett C, Sedyaningsih ER, Beecham HJ III, Corwin AL. 2005. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg 99:128–141. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Pastorino B, Muyembe-Tamfum JJ, Bessaud M, Tock F, Tolou H, Durand JP, Peyrefitte CN. 2004. Epidemic resurgence of Chikungunya virus in democratic Republic of the Congo: identification of a new central African strain. J Med Virol 74:277–282. doi: 10.1002/jmv.20168. [DOI] [PubMed] [Google Scholar]
- 10.Sergon K, Njuguna C, Kalani R, Ofula V, Onyango C, Konongoi LS, Bedno S, Burke H, Dumilla AM, Konde J, Njenga MK, Sang R, Breiman RF. 2008. Seroprevalence of chikungunya virus (CHIKV) infection on Lamu Island, Kenya, October 2004. Am J Trop Med Hyg 78:333–337. [PubMed] [Google Scholar]
- 11.Renault P, Solet JL, Sissoko D, Balleydier E, Larrieu S, Filleul L, Lassalle C, Thiria J, Rachou E, de Valk H, Ilef D, Ledrans M, Quatresous I, Quenel P, Pierre V. 2007. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005-2006. Am J Trop Med Hyg 77:727–731. [PubMed] [Google Scholar]
- 12.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Brehin AC, Cubito N, Despres P, Kunst F, Rey FA, Zeller H, Brisse S. 2006. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med 3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dash PK, Parida MM, Santhosh SR, Verma SK, Tripathi NK, Ambuj S, Saxena P, Gupta N, Chaudhary M, Babu JP, Lakshmi V, Mamidi N, Subhalaxmi MV, Rao PV, Sekhar K. 2007. East Central South African genotype as the causative agent in reemergence of chikungunya outbreak in India. Vector Borne Zoonotic Dis 7:519–527. doi: 10.1089/vbz.2006.0648. [DOI] [PubMed] [Google Scholar]
- 14.Cao-Lormeau VM, Musso D. 2014. Emerging arboviruses in the Pacific. Lancet 384:1571–1572. doi: 10.1016/S0140-6736(14)61977-2. [DOI] [PubMed] [Google Scholar]
- 15.Roth A, Hoy D, Horwood PF, Ropa B, Hancock T, Guillaumot L, Rickart K, Frison P, Pavlin B, Souares Y. 1 July 2014. Preparedness for threat of chikungunya in the Pacific. Emerg Infect Dis doi: 10.3201/eid2008.130696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, Guillaumot L, Souares Y. 2014. Concurrent outbreaks of dengue, chikungunya and Zika virus infections—an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro Surveill 19(41):pii=20929. doi: 10.2807/1560-7917.ES2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 17.PAHO. 2011. Preparedness and response for chikungunya virus: introduction in the Americas. Pan American Health Organization, Washington, DC: http://new.paho.org/hq/index.php?option=com_content&view=article&id=6464%3Apaho%2C-cdc-publish-guide-on-preparing-for-chikungunya-virus-introduction-in-the-americas&catid=740%3Anews-press-releases&Itemid=1926&lang=en. [Google Scholar]
- 18.Petersen LR, Powers AM. 19 January 2016. Chikungunya: epidemiology. F1000Res doi: 10.12688/f1000research.7171.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder JE, Kulcsar KA, Schultz KL, Riley CP, Neary JT, Marr S, Jose J, Griffin DE, Kuhn RJ. 2013. Functional characterization of the alphavirus TF protein. J Virol 87:8511–8523. doi: 10.1128/JVI.00449-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halstead SB, Yammerat C, Scanlon JE. 1963. The Thai hemorrhagic fever epidemic of 1962, a preliminary report. J Med Assoc Thai 46:449–462. [Google Scholar]
- 21.Halstead SB, Buescher EL. 1961. Hemorrhagic disease in rodents infected with virus associated with Thai hemorrhagic fever. Science 134:475–476. doi: 10.1126/science.134.3477.475. [DOI] [PubMed] [Google Scholar]
- 22.Kitaoka M. 1967. Japanese encephalitis vaccine including a preliminary report on dengue fever and chikungunya vaccines. Jpn J Med Sci Biol 20:41–56. [PubMed] [Google Scholar]
- 23.Nakao E, Hotta S. 1973. Immunogenicity of purified, inactivated chikungunya virus in monkeys. Bull World Health Organ 48:559–562. [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison VR, Binn LN, Randall R. 1967. Comparative immunogenicities of chikungunya vaccines prepared in avian and mammalian tissues. Am J Trop Med Hyg 16:786–791. doi: 10.4269/ajtmh.1967.16.786. [DOI] [PubMed] [Google Scholar]
- 25.White A, Berman S, Lowenthal JP. 1972. Comparative immunogenicities of chikungunya vaccines propagated in monkey kidney monolayers and chick embryo suspension cultures. Appl Microbiol 23:951–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckels KH, Harrison VR, Hetrick FM. 1970. Chikungunya virus vaccine prepared by Tween-ether extraction. Appl Microbiol 19:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. 1971. Production and evaluation of a formalin-killed chikungunya vaccine. J Immunol 107:643–647. [PubMed] [Google Scholar]
- 28.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE Jr, Lupton HW. 1986. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine 4:157–162. doi: 10.1016/0264-410X(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 29.Eckels KH, Harrison VR, Summers PL, Russell PK. 1980. Dengue-2 vaccine: preparation from a small-plaque virus clone. Infect Immun 27:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halstead SB, Marchette NJ, Diwan AR, Palumbo NE, Putvatana R. 1984. Selection of attenuated dengue 4 viruses by serial passage in primary kidney cells. II. Attributes of virus cloned at different dog kidney passage levels. Am J Trop Med Hyg 33:666–671. doi: 10.4269/ajtmh.1984.33.666. [DOI] [PubMed] [Google Scholar]
- 31.McClain DJ, Pittman PR, Ramsburg HH, Nelson GO, Rossi CA, Mangiafico JA, Schmaljohn AL, Malinoski FJ. 1998. Immunologic interference from sequential administration of live attenuated alphavirus vaccines. J Infect Dis 177:634–641. doi: 10.1086/514240. [DOI] [PubMed] [Google Scholar]
- 32.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. 2000. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg 62:681–685. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- 33.Hoke CH Jr, Pace-Templeton J, Pittman P, Malinoski FJ, Gibbs P, Ulderich T, Mathers M, Fogtman B, Glass P, Vaughn DW. 2012. US Military contributions to the global response to pandemic chikungunya. Vaccine 30:6713–6720. doi: 10.1016/j.vaccine.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Partidos CD, Weger J, Brewoo J, Seymour R, Borland EM, Ledermann JP, Powers AM, Weaver SC, Stinchcomb DT, Osorio JE. 2011. Probing the attenuation and protective efficacy of a candidate chikungunya virus vaccine in mice with compromised interferon (IFN) signaling. Vaccine 29:3067–3073. doi: 10.1016/j.vaccine.2011.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorchakov R, Wang E, Leal G, Forrester NL, Plante K, Rossi SL, Partidos CD, Adams AP, Seymour RL, Weger J, Borland EM, Sherman MB, Powers AM, Osorio JE, Weaver SC. 2012. Attenuation of Chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the E2 envelope glycoprotein. J Virol 86:6084–6096. doi: 10.1128/JVI.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashbrook AW, Burrack KS, Silva LA, Montgomery SA, Heise MT, Morrison TE, Dermody TS. 2014. Residue 82 of the chikungunya virus E2 attachment protein modulates viral dissemination and arthritis in mice. J Virol 88:12180–12192. doi: 10.1128/JVI.01672-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. 2010. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat Med 16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powers AM. 2011. Genomic evolution and phenotypic distinctions of chikungunya viruses causing the Indian Ocean outbreak. Exp Biol Med (Maywood) 236:909–914. doi: 10.1258/ebm.2011.011078. [DOI] [PubMed] [Google Scholar]
- 39.Chua CL, Sam IC, Merits A, Chan YF. 2016. Antigenic variation of East/Central/South African and Asian chikungunya virus genotypes in neutralization by immune sera. PLoS Negl Trop Dis 10:e0004960. doi: 10.1371/journal.pntd.0004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goo L, Dowd KA, Lin TY, Mascola JR, Graham BS, Ledgerwood JE, Pierson TC. 2016. A virus-like particle vaccine elicits broad neutralizing antibody responses in humans to all chikungunya virus genotypes. J Infect Dis 214:1487–1491. doi: 10.1093/infdis/jiw431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang LJ, Dowd KA, Mendoza FH, Saunders JG, Sitar S, Plummer SH, Yamshchikov G, Sarwar UN, Hu Z, Enama ME, Bailer RT, Koup RA, Schwartz RM, Akahata W, Nabel GJ, Mascola JR, Pierson TC, Graham BS, Ledgerwood JE, VRC 311 Study Team . 2014. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet 384:2046–2052. doi: 10.1016/S0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- 42.Brandler S, Tangy F. 2008. Recombinant vector derived from live attenuated measles virus: potential for flavivirus vaccines. Comp Immunol Microbiol Infect Dis 31:271–291. doi: 10.1016/j.cimid.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Despres P, Combredet C, Frenkiel MP, Lorin C, Brahic M, Tangy F. 2005. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis 191:207–214. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 44.Brandler S, Ruffie C, Najburg V, Frenkiel MP, Bedouelle H, Despres P, Tangy F. 2010. Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine 28:6730–6739. doi: 10.1016/j.vaccine.2010.07.073. [DOI] [PubMed] [Google Scholar]
- 45.Brandler S, Ruffie C, Combredet C, Brault JB, Najburg V, Prevost MC, Habel A, Tauber E, Despres P, Tangy F. 2013. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine 31:3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 46.Ramsauer K, Schwameis M, Firbas C, Mullner M, Putnak RJ, Thomas SJ, Despres P, Tauber E, Jilma B, Tangy F. 2015. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis 15:519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- 47.Wang E, Volkova E, Adams AP, Forrester N, Xiao SY, Frolov I, Weaver SC. 2008. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine 26:5030–5039. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguilar PV, Adams AP, Wang E, Kang W, Carrara AS, Anishchenko M, Frolov I, Weaver SC. 2008. Structural and nonstructural protein genome regions of eastern equine encephalitis virus are determinants of interferon sensitivity and murine virulence. J Virol 82:4920–4930. doi: 10.1128/JVI.02514-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paessler S, Fayzulin RZ, Anishchenko M, Greene IP, Weaver SC, Frolov I. 2003. Recombinant Sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J Virol 77:9278–9286. doi: 10.1128/JVI.77.17.9278-9286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang E, Kim DY, Weaver SC, Frolov I. 2011. Chimeric chikungunya viruses are nonpathogenic in highly sensitive mouse models but efficiently induce a protective immune response. J Virol 85:9249–9252. doi: 10.1128/JVI.00844-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erasmus JH, Auguste AJ, Kaelber JT, Luo H, Rossi SL, Fenton K, Leal G, Kim DY, Chiu W, Wang T, Frolov I, Nasar F, Weaver SC. 2017. A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat Med 23:192–199. doi: 10.1038/nm.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volz A, Sutter G. 2013. Protective efficacy of modified vaccinia virus Ankara in preclinical studies. Vaccine 31:4235–4240. doi: 10.1016/j.vaccine.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog 5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Garcia-Arriaza J, Cepeda V, Hallengard D, Sorzano CO, Kummerer BM, Liljestrom P, Esteban M. 2014. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J Virol 88:3527–3547. doi: 10.1128/JVI.03418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teo TH, Lum FM, Lee WW, Ng LF. 2012. Mouse models for chikungunya virus: deciphering immune mechanisms responsible for disease and pathology. Immunol Res 53:136–147. doi: 10.1007/s12026-012-8266-x. [DOI] [PubMed] [Google Scholar]
- 56.Weger-Lucarelli J, Chu H, Aliota MT, Partidos CD, Osorio JE. 2014. A novel MVA vectored Chikungunya virus vaccine elicits protective immunity in mice. PLoS Negl Trop Dis 8:e2970. doi: 10.1371/journal.pntd.0002970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D, Suhrbier A, Penn-Nicholson A, Woraratanadharm J, Gardner J, Luo M, Le TT, Anraku I, Sakalian M, Einfeld D, Dong JY. 2011. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine 29:2803–2809. doi: 10.1016/j.vaccine.2011.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seregin SS, Amalfitano A. 2009. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin Biol Ther 9:1521–1531. doi: 10.1517/14712590903307388. [DOI] [PubMed] [Google Scholar]
- 59.Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. 2003. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol 77:10780–10789. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts A, Buonocore L, Price R, Forman J, Rose JK. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol 73:3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chattopadhyay A, Wang E, Seymour R, Weaver SC, Rose JK. 2013. A chimeric vesiculo/alphavirus is an effective alphavirus vaccine. J Virol 87:395–402. doi: 10.1128/JVI.01860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zinkernagel RM, Adler B, Holland JJ. 1978. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp Cell Biol 46:53–70. [DOI] [PubMed] [Google Scholar]
- 63.Berge TO, Banks IS, Tigertt WD. 1961. Attenuation of Venezuelan equine encephalomyelitis virus by in vitro cultivation in guinea pig heart cells. Am J Hyg (Lond) 73:209–218. [Google Scholar]
- 64.Plante K, Wang E, Partidos CD, Weger J, Gorchakov R, Tsetsarkin K, Borland EM, Powers AM, Seymour R, Stinchcomb DT, Osorio JE, Frolov I, Weaver SC. 2011. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog 7:e1002142. doi: 10.1371/journal.ppat.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerbois M, Volkova E, Forrester NL, Rossi SL, Frolov I, Weaver SC. 2013. IRES-driven expression of the capsid protein of the Venezuelan equine encephalitis virus TC-83 vaccine strain increases its attenuation and safety. PLoS Negl Trop Dis 7:e2197. doi: 10.1371/journal.pntd.0002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor A, Liu X, Zaid A, Goh LY, Hobson-Peters J, Hall RA, Merits A, Mahalingam S. 2017. Mutation of the N-terminal region of chikungunya virus capsid protein: implications for vaccine design. mBio 8:e01970-16. doi: 10.1128/mBio.01970-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Metz SW, Geertsema C, Martina BE, Andrade P, Heldens JG, van Oers MM, Goldbach RW, Vlak JM, Pijlman GP. 2011. Functional processing and secretion of chikungunya virus E1 and E2 glycoproteins in insect cells. Virol J 8:353. doi: 10.1186/1743-422X-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Metz SW, Martina BE, van den Doel P, Geertsema C, Osterhaus AD, Vlak JM, Pijlman GP. 2013. Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits. Vaccine 31:6092–6096. doi: 10.1016/j.vaccine.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 69.Kumar M, Sudeep AB, Arankalle VA. 2012. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine 30:6142–6149. doi: 10.1016/j.vaccine.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 70.Khan M, Dhanwani R, Rao PV, Parida M. 2012. Subunit vaccine formulations based on recombinant envelope proteins of chikungunya virus elicit balanced Th1/Th2 response and virus-neutralizing antibodies in mice. Virus Res 167:236–246. doi: 10.1016/j.virusres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. 2011. Clinical applications of DNA vaccines: current progress. Clin Infect Dis 53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sallberg M, Frelin L, Ahlen G, Sallberg-Chen M. 2015. Electroporation for therapeutic DNA vaccination in patients. Med Microbiol Immunol 204:131–135. doi: 10.1007/s00430-014-0384-8. [DOI] [PubMed] [Google Scholar]
- 73.Muthumani K, Lankaraman KM, Laddy DJ, Sundaram SG, Chung CW, Sako E, Wu L, Khan A, Sardesai N, Kim JJ, Vijayachari P, Weiner DB. 2008. Immunogenicity of novel consensus-based DNA vaccines against chikungunya virus. Vaccine 26:5128–5134. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mallilankaraman K, Shedlock DJ, Bao H, Kawalekar OU, Fagone P, Ramanathan AA, Ferraro B, Stabenow J, Vijayachari P, Sundaram SG, Muruganandam N, Sarangan G, Srikanth P, Khan AS, Lewis MG, Kim JJ, Sardesai NY, Muthumani K, Weiner DB. 2011. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis 5:e928. doi: 10.1371/journal.pntd.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bao H, Ramanathan AA, Kawalakar O, Sundaram SG, Tingey C, Bian CB, Muruganandam N, Vijayachari P, Sardesai NY, Weiner DB, Ugen KE, Muthumani K. 2013. Nonstructural protein 2 (nsP2) of chikungunya virus (CHIKV) enhances protective immunity mediated by a CHIKV envelope protein expressing DNA vaccine. Viral Immunol 26:75–83. doi: 10.1089/vim.2012.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hallengard D, Kakoulidou M, Lulla A, Kummerer BM, Johansson DX, Mutso M, Lulla V, Fazakerley JK, Roques P, Le Grand R, Merits A, Liljestrom P. 2014. Novel attenuated chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J Virol 88:2858–2866. doi: 10.1128/JVI.03453-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roques P, Ljungberg K, Kummerer BM, Gosse L, Dereuddre-Bosquet N, Tchitchek N, Hallengard D, Garcia-Arriaza J, Meinke A, Esteban M, Merits A, Le Grand R, Liljestrom P. 2017. Attenuated and vectored vaccines protect nonhuman primates against chikungunya virus. JCI Insight 2:e83527. doi: 10.1172/jci.insight.83527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tretyakova I, Hearn J, Wang E, Weaver S, Pushko P. 2014. DNA vaccine initiates replication of live attenuated chikungunya virus in vitro and elicits protective immune response in mice. J Infect Dis 209:1882–1890. doi: 10.1093/infdis/jiu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hidajat R, Nickols B, Forrester N, Tretyakova I, Weaver S, Pushko P. 2016. Next generation sequencing of DNA-launched chikungunya vaccine virus. Virology 490:83–90. doi: 10.1016/j.virol.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muthumani K, Block P, Flingai S, Muruganantham N, Chaaithanya IK, Tingey C, Wise M, Reuschel EL, Chung C, Muthumani A, Sarangan G, Srikanth P, Khan AS, Vijayachari P, Sardesai NY, Kim JJ, Ugen KE, Weiner DB. 2016. Rapid and long-term immunity elicited by DNA-encoded antibody prophylaxis and DNA vaccination against chikungunya virus. J Infect Dis 214:369–378. doi: 10.1093/infdis/jiw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brighton SW. 1984. Chloroquine phosphate treatment of chronic chikungunya arthritis. An open pilot study. South Afr Med J 66:217–218. [PubMed] [Google Scholar]
- 82.De Lamballerie X, Boisson V, Reynier JC, Enault S, Charrel RN, Flahault A, Roques P, Le Grand R. 2008. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis 8:837–839. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- 83.Chopra A, Saluja M, Venugopalan A. 2014. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol 66:319–326. doi: 10.1002/art.38221. [DOI] [PubMed] [Google Scholar]
- 84.Briolant S, Garin D, Scaramozzino N, Jouan A, Crance JM. 2004. In vitro inhibition of chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antiviral Res 61:111–117. doi: 10.1016/j.antiviral.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Gallegos KM, Drusano GL, D'Argenio DZ, Brown AN. 2016. Chikungunya virus: in vitro response to combination therapy with ribavirin and interferon alfa 2a. J Infect Dis 214:1192–1197. doi: 10.1093/infdis/jiw358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rothan HA, Bahrani H, Mohamed Z, Teoh TC, Shankar EM, Rahman NA, Yusof R. 2015. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS One 10:e0126360. doi: 10.1371/journal.pone.0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wintachai P, Thuaud F, Basmadjian C, Roytrakul S, Ubol S, Desaubry L, Smith DR. 2015. Assessment of flavaglines as potential chikungunya virus entry inhibitors. Microbiol Immunol 59:129–141. doi: 10.1111/1348-0421.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ho YJ, Wang YM, Lu JW, Wu TY, Lin LI, Kuo SC, Lin CC. 2015. Suramin inhibits chikungunya virus entry and transmission. PLoS One 10:e0133511. doi: 10.1371/journal.pone.0133511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuo SC, Wang YM, Ho YJ, Chang TY, Lai ZZ, Tsui PY, Wu TY, Lin CC. 2016. Suramin treatment reduces chikungunya pathogenesis in mice. Antiviral Res 134:89–96. doi: 10.1016/j.antiviral.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 90.Lani R, Hassandarvish P, Chiam CW, Moghaddam E, Chu JJ, Rausalu K, Merits A, Higgs S, Vanlandingham D, Abu Bakar S, Zandi K. 2015. Antiviral activity of silymarin against chikungunya virus. Sci Rep 5:11421. doi: 10.1038/srep11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lani R, Hassandarvish P, Shu MH, Phoon WH, Chu JJ, Higgs S, Vanlandingham D, Abu Bakar S, Zandi K. 2016. Antiviral activity of selected flavonoids against chikungunya virus. Antiviral Res 133:50–61. doi: 10.1016/j.antiviral.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 92.Seyedi SS, Shukri M, Hassandarvish P, Oo A, Shankar EM, Abubakar S, Zandi K. 2016. Computational approach towards exploring potential anti-chikungunya activity of selected flavonoids. Sci Rep 6:24027. doi: 10.1038/srep24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheung YY, Chen KC, Chen H, Seng EK, Chu JJ. 2014. Antiviral activity of lanatoside C against dengue virus infection. Antiviral Res 111:93–99. doi: 10.1016/j.antiviral.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Ashbrook AW, Lentscher AJ, Zamora PF, Silva LA, May NA, Bauer JA, Morrison TE, Dermody TS. 2016. Antagonism of the sodium-potassium ATPase impairs chikungunya virus infection. mBio 7:e00693-16. doi: 10.1128/mBio.00693-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang YM, Lu JW, Lin CC, Chin YF, Wu TY, Lin LI, Lai ZZ, Kuo SC, Ho YJ. 2016. Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission. Antiviral Res 135:81–90. doi: 10.1016/j.antiviral.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weber C, Sliva K, von Rhein C, Kummerer BM, Schnierle BS. 2015. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral Res 113:1–3. doi: 10.1016/j.antiviral.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez MJ, Miranda-Massari JR, Berdiel MJ, Duconge J, Rodriguez-Lopez JL, Hunninghake R, Cobas-Rosario VJ. 2014. High dose intraveneous vitamin C and chikungunya fever: a case report. J Orthomol Med 29:154–156. [PMC free article] [PubMed] [Google Scholar]
- 98.Chan YS, Khoo KS, Sit NWW. 2016. Investigation of twenty selected medicinal plants from Malaysia for anti-chikungunya virus activity. Int Microbiol 19:175–182. doi: 10.2436/20.1501.01.275. [DOI] [PubMed] [Google Scholar]
- 99.Varghese FS, Kaukinen P, Glasker S, Bespalov M, Hanski L, Wennerberg K, Kummerer BM, Ahola T. 2016. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antiviral Res 126:117–124. doi: 10.1016/j.antiviral.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 100.Varghese FS, Thaa B, Amrun SN, Simarmata D, Rausalu K, Nyman TA, Merits A, McInerney GM, Ng LF, Ahola T. 2016. The antiviral alkaloid berberine reduces chikungunya virus-induced mitogen-activated protein kinase signaling. J Virol 90:9743–9757. doi: 10.1128/JVI.01382-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M. 2017. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res 142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 102.von Rhein C, Weidner T, Henss L, Martin J, Weber C, Sliva K, Schnierle BS. 2016. Curcumin and Boswellia serrata gum resin extract inhibit chikungunya and vesicular stomatitis virus infections in vitro. Antiviral Res 125:51–57. doi: 10.1016/j.antiviral.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 103.Gomez-Calderon C, Mesa-Castro C, Robledo S, Gomez S, Bolivar-Avila S, Diaz-Castillo F, Martinez-Gutierrez M. 2017. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymosa on dengue and chikungunya virus infections. BMC Complement Altern Med 17:57. doi: 10.1186/s12906-017-1562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Das PK, Puusepp L, Varghese FS, Utt A, Ahola T, Kananovich DG, Lopp M, Merits A, Karelson M. 2016. Design and validation of novel chikungunya virus protease inhibitors. Antimicrob Agents Chemother 60:7382–7395. doi: 10.1128/AAC.02586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wada Y, Orba Y, Sasaki M, Kobayashi S, Carr MJ, Nobori H, Sato A, Hall WW, Sawa H. 2017. Discovery of a novel antiviral agent targeting the nonstructural protein 4 (nsP4) of chikungunya virus. Virology 505:102–112. doi: 10.1016/j.virol.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 106.Gigante A, Canela MD, Delang L, Priego EM, Camarasa MJ, Querat G, Neyts J, Leyssen P, Perez-Perez MJ. 2014. Identification of [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones as novel inhibitors of chikungunya virus replication. J Med Chem 57:4000–4008. doi: 10.1021/jm401844c. [DOI] [PubMed] [Google Scholar]
- 107.Hwu JR, Kapoor M, Tsay SC, Lin CC, Hwang KC, Horng JC, Chen IC, Shieh FK, Leyssen P, Neyts J. 2015. Benzouracil-coumarin-arene conjugates as inhibiting agents for chikungunya virus. Antiviral Res 118:103–109. doi: 10.1016/j.antiviral.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 108.Rothan HA, Bahrani H, Shankar EM, Rahman NA, Yusof R. 2014. Inhibitory effects of a peptide-fusion protein (latarcin-PAP1-thanatin) against chikungunya virus. Antiviral Res 108:173–180. doi: 10.1016/j.antiviral.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 109.Mishra P, Kumar A, Mamidi P, Kumar S, Basantray I, Saswat T, Das I, Nayak TK, Chattopadhyay S, Subudhi BB, Chattopadhyay S. 2016. Inhibition of chikungunya virus replication by 1-[(2-methylbenzimidazol-1-yl)methyl]-2-oxo-indolin-3-ylidene]amino]thiourea (MBZM-N-IBT). Sci Rep 6:20122. doi: 10.1038/srep20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bassetto M, De Burghgraeve T, Delang L, Massarotti A, Coluccia A, Zonta N, Gatti V, Colombano G, Sorba G, Silvestri R, Tron GC, Neyts J, Leyssen P, Brancale A. 2013. Computer-aided identification, design and synthesis of a novel series of compounds with selective antiviral activity against chikungunya virus. Antiviral Res 98:12–18. doi: 10.1016/j.antiviral.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Gong EY, Bonfanti JF, Ivens T, Van der Auwera M, Van Kerckhove B, Kraus G. 2013. Development of a high-throughput antiviral assay for screening inhibitors of chikungunya virus and generation of drug-resistant mutations in cultured cells. Methods Mol Biol 1030:429–438. doi: 10.1007/978-1-62703-484-5_32. [DOI] [PubMed] [Google Scholar]
- 112.Dash PK, Tiwari M, Santhosh SR, Parida M, Lakshmana Rao PV. 2008. RNA interference mediated inhibition of Chikungunya virus replication in mammalian cells. Biochem Biophys Res Commun 376:718–722. doi: 10.1016/j.bbrc.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 113.Parashar D, Paingankar MS, Kumar S, Gokhale MD, Sudeep AB, Shinde SB, Arankalle VA. 2013. Administration of E2 and NS1 siRNAs inhibit chikungunya virus replication in vitro and protects mice infected with the virus. PLoS Negl Trop Dis 7:e2405. doi: 10.1371/journal.pntd.0002405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saha A, Bhagyawant SS, Parida M, Dash PK. 2016. Vector-delivered artificial miRNA effectively inhibited replication of chikungunya virus. Antiviral Res 134:42–49. doi: 10.1016/j.antiviral.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lam S, Chen KC, Ng MM, Chu JJ. 2012. Expression of plasmid-based shRNA against the E1 and nsP1 genes effectively silenced chikungunya virus replication. PLoS One 7:e46396. doi: 10.1371/journal.pone.0046396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lam S, Chen H, Chen CK, Min N, Chu JJ. 2015. Antiviral phosphorodiamidate morpholino oligomers are protective against chikungunya virus infection on cell-based and murine models. Sci Rep 5:12727. doi: 10.1038/srep12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Couderc T, Khandoudi N, Grandadam M, Visse C, Gangneux N, Bagot S, Prost JF, Lecuit M. 2009. Prophylaxis and therapy for chikungunya virus infection. J Infect Dis 200:516–523. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Selvarajah S, Sexton NR, Kahle KM, Fong RH, Mattia KA, Gardner J, Lu K, Liss NM, Salvador B, Tucker DF, Barnes T, Mabila M, Zhou X, Rossini G, Rucker JB, Sanders DA, Suhrbier A, Sambri V, Michault A, Muench MO, Doranz BJ, Simmons G. 2013. A neutralizing monoclonal antibody targeting the acid-sensitive region in chikungunya virus E2 protects from disease. PLoS Negl Trop Dis 7:e2423. doi: 10.1371/journal.pntd.0002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith SA, Silva LA, Fox JM, Flyak AI, Kose N, Sapparapu G, Khomandiak S, Ashbrook AW, Kahle KM, Fong RH, Swayne S, Doranz BJ, McGee CE, Heise MT, Pal P, Brien JD, Austin SK, Diamond MS, Dermody TS, Crowe JE Jr. 2015. Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against chikungunya virus. Cell Host Microbe 18:86–95. doi: 10.1016/j.chom.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clayton AM. 2016. Monoclonal antibodies as prophylactic and therapeutic agents against chikungunya virus. J Infect Dis 214:S506–S509. doi: 10.1093/infdis/jiw324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Masrinoul P, Puiprom O, Tanaka A, Kuwahara M, Chaichana P, Ikuta K, Ramasoota P, Okabayashi T. 2014. Monoclonal antibody targeting chikungunya virus envelope 1 protein inhibits virus release. Virology 464–465:111–117. doi: 10.1016/j.virol.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 122.Miner JJ, Cook LE, Hong JP, Smith AM, Richner JM, Shimak RM, Young AR, Monte K, Poddar S, Crowe JE Jr, Lenschow DJ, Diamond MS. 2017. Therapy with CTLA4-Ig and an antiviral monoclonal antibody controls chikungunya virus arthritis. Sci Transl Med 9:eaah3438. doi: 10.1126/scitranslmed.aah3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abdelnabi R, Amrun SN, Ng LF, Leyssen P, Neyts J, Delang L. 2017. Protein kinases C as potential host targets for the inhibition of chikungunya virus replication. Antiviral Res 139:79–87. doi: 10.1016/j.antiviral.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 124.Karlas A, Berre S, Couderc T, Varjak M, Braun P, Meyer M, Gangneux N, Karo-Astover L, Weege F, Raftery M, Schonrich G, Klemm U, Wurzlbauer A, Bracher F, Merits A, Meyer TF, Lecuit M. 2016. A human genome-wide loss-of-function screen identifies effective chikungunya antiviral drugs. Nat Commun 7:11320. doi: 10.1038/ncomms11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mounce BC, Poirier EZ, Passoni G, Simon-Loriere E, Cesaro T, Prot M, Stapleford KA, Moratorio G, Sakuntabhai A, Levraud JP, Vignuzzi M. 2016. Interferon-induced spermidine-spermine acetyltransferase and polyamine depletion restrict Zika and chikungunya viruses. Cell Host Microbe 20:167–177. doi: 10.1016/j.chom.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 126.Akkina R, Ellerbrok H, Hall W, Hasegawa H, Kawaguchi Y, Kleanthous H, McSweegan E, Mercer N, Romanowski V, Sawa H, Vahlne A. 2017. 2016 International meeting of the Global Virus Network. Antiviral Res 142:21–29. doi: 10.1016/j.antiviral.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Plotkin S, Robinson JM, Cunningham G, Iqbal R, Larsen S. 2017. The complexity and cost of vaccine manufacturing—an overview. Vaccine 35:4064–4071. doi: 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Plotkin SA. 2016. Zika as still another argument for a new path to vaccine development. Clin Microbiol Infect 22:294–295. doi: 10.1016/j.cmi.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Plotkin SA, Mahmoud AA, Farrar J. 2015. Establishing a global vaccine-development fund. N Engl J Med 373:297–300. doi: 10.1056/NEJMp1506820. [DOI] [PubMed] [Google Scholar]
- 130.Rottingen JA, Gouglas D, Feinberg M, Plotkin S, Raghavan KV, Witty A, Draghia-Akli R, Stoffels P, Piot P. 2017. New vaccines against epidemic infectious diseases. N Engl J Med 376:610–613. doi: 10.1056/NEJMp1613577. [DOI] [PubMed] [Google Scholar]
- 131.Kramer RM, Zeng Y, Sahni N, Kueltzo LA, Schwartz RM, Srivastava IK, Crane L, Joshi SB, Volkin DB, Middaugh CR. 2013. Development of a stable virus-like particle vaccine formulation against chikungunya virus and investigation of the effects of polyanions. J Pharm Sci 102:4305–4314. doi: 10.1002/jps.23749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Metz SW, Gardner J, Geertsema C, Le TT, Goh L, Vlak JM, Suhrbier A, Pijlman GP. 2013. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS Negl Trop Dis 7:e2124. doi: 10.1371/journal.pntd.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saraswat S, Athmaram TN, Parida M, Agarwal A, Saha A, Dash PK. 2016. Expression and characterization of yeast derived chikungunya virus like particles (CHIK-VLPs) and its evaluation as a potential vaccine candidate. PLoS Negl Trop Dis 10:e0004782. doi: 10.1371/journal.pntd.0004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tiwari M, Parida M, Santhosh SR, Khan M, Dash PK, Rao PV. 2009. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of chikungunya virus. Vaccine 27:2513–2522. doi: 10.1016/j.vaccine.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 135.van den Doel P, Volz A, Roose JM, Sewbalaksing VD, Pijlman GP, van Middelkoop I, Duiverman V, van de Wetering E, Sutter G, Osterhaus AD, Martina BE. 2014. Recombinant modified vaccinia virus Ankara expressing glycoprotein E2 of chikungunya virus protects AG129 mice against lethal challenge. PLoS Negl Trop Dis 8:e3101. doi: 10.1371/journal.pntd.0003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chu H, Das SC, Fuchs JF, Suresh M, Weaver SC, Stinchcomb DT, Partidos CD, Osorio JE. 2013. Deciphering the protective role of adaptive immunity to CHIKV/IRES a novel candidate vaccine against chikungunya in the A129 mouse model. Vaccine 31:3353–3360. doi: 10.1016/j.vaccine.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roy CJ, Adams AP, Wang E, Plante K, Gorchakov R, Seymour RL, Vinet-Oliphant H, Weaver SC. 2014. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis 209:1891–1899. doi: 10.1093/infdis/jiu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Piper A, Ribeiro M, Smith KM, Briggs CM, Huitt E, Nanda K, Spears CJ, Quiles M, Cullen J, Thomas ME, Brown DT, Hernandez R. 2013. Chikungunya virus host range E2 transmembrane deletion mutants induce protective immunity against challenge in C57BL/6J mice. J Virol 87:6748–6757. doi: 10.1128/JVI.03357-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gardner CL, Hritz J, Sun C, Vanlandingham DL, Song TY, Ghedin E, Higgs S, Klimstra WB, Ryman KD. 2014. Deliberate attenuation of chikungunya virus by adaptation to heparan sulfate-dependent infectivity: a model for rational arboviral vaccine design. PLoS Negl Trop Dis 8:e2719. doi: 10.1371/journal.pntd.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sharma R, Fatma B, Saha A, Bajpai S, Sistla S, Dash PK, Parida M, Kumar P, Tomar S. 2016. Inhibition of chikungunya virus by picolinate that targets viral capsid protein. Virology 498:265–276. doi: 10.1016/j.virol.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 141.Ehteshami M, Tao S, Zandi K, Hsiao HM, Jiang Y, Hammond E, Amblard F, Russell OO, Merits A, Schinazi RF. 2017. Characterization of β-d-N4-hydroxycytidine as a novel inhibitor of chikungunya virus. Antimicrob Agents Chemother 61:e02395-16. doi: 10.1128/AAC.02395-16. [DOI] [PMC free article] [PubMed] [Google Scholar]