SUMMARY
Human parechovirus (HPeV) is increasingly being recognized as a potentially severe viral infection in neonates and young infants. HPeV belongs to the family Picornaviridae and is currently divided into 19 genotypes. HPeV-1 is the most prevalent genotype and most commonly causes gastrointestinal and respiratory disease. HPeV-3 is clinically the most important genotype due to its association with severe disease in younger infants, which may partly be explained by its distinct virological properties. In young infants, the typical clinical presentation includes fever, severe irritability, and rash, often leading to descriptions of “hot, red, angry babies.” Infants with severe central nervous system (CNS) infections are at an increased risk of long-term sequelae. Considering the importance of HPeV as a cause of severe viral infections in young infants, we recommend that molecular diagnostic techniques for early detection be included in the standard practice for the investigation of sepsis-like illnesses and CNS infections in this age group.
KEYWORDS: HPeV, human parechovirus, infants, neonates, pediatrics, picornavirus, sepsis
INTRODUCTION
Human parechoviruses (HPeVs) were first isolated in 1956 and classified as enteroviruses (named echoviruses 22 and 23) but were not assigned to the separate genus Parechovirus until 1997 (1, 2). These viruses often cause gastrointestinal or respiratory illness in young children but infrequently cause disease in older children and adults (3, 4). In infants, clusters and outbreaks of HPeV infection are being recognized (5–9). These infants can present with a sepsis-like picture, often with central nervous system (CNS) involvement, which is difficult to differentiate clinically from bacterial sepsis (5, 10). They may present with seizures or significant neurological impairment while having only modestly increased levels of inflammatory markers and minimal cerebrospinal fluid (CSF) pleocytosis (11). Severe HPeV infections in infants are also associated with a risk of long-term complications (10–12).
The application of molecular diagnostic methods has enabled the early recognition of HPeV infections. Early recognition is important as it may reduce the use of antibiotics and shorten the duration of hospital admissions for patients with mild to moderate disease. It is also likely to lead to appropriate investigations and follow-up for potential complications in infants who are severely affected (5, 10, 13).
This review describes the virology, pathogenesis, immunology, epidemiology, clinical manifestations, diagnosis, and therapy of HPeV infections in infants and children.
VIROLOGY
Virus Structure and Genomic Organization
HPeV is classified in the family Picornaviridae, which includes viruses that infect both animals and humans (14). Picornaviruses are nonenveloped viruses with positive single-stranded RNA (ssRNA) enclosed in an icosahedral capsid (15). HPeV was first isolated in 1956 by Wigand and Sabin during studies on summer diarrhea. The first two viruses were classified as echoviruses 22 and 23 of the genus Enterovirus (1). The determination of the complete nucleotide sequence of echovirus 22 in 1992 suggested that both echoviruses 22 and 23 belong to an independent group of picornaviruses (16, 17). Currently, human parechoviruses are classified in the genus Parechovirus, which is divided into two species: Parechovirus A and Parechovirus B. Parechovirus A is currently subdivided into 19 genotypes, HPeV-1 to -19, based on phylogenetic analysis of VP1 sequences, while Parechovirus B comprises Ljungan viruses 1 to 4 (18, 19) (http://www.picornastudygroup.com/taxa/serotypes/serotypes.htm). This review focuses only on Parechovirus A.
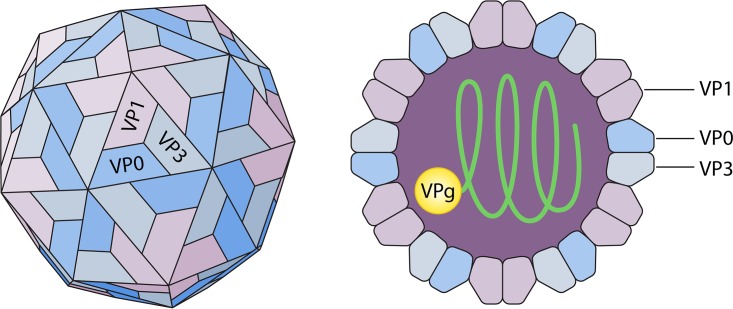
The HPeV virion has a diameter of 28 nm and contains a positive-sense, single-stranded RNA genome of approximately 7,300 nucleotides (16, 20). The HPeV capsid is composed of 60 protomers formed out of three nonidentical polypeptide chains (VP0, VP1, and VP3) (Fig. 1) (21). These three polypeptide chains are co- and posttranslationally formed out of one single polyprotein. Because the VP0 protein of HPeV remains intact and is not cleaved into VP2 and VP4 fragments as in other picornaviruses, the capsid contains only three instead of four different polypeptides (22, 23). The VP1 protein differs from the analogous capsid proteins in other picornaviruses by the blockage of the VP1 hydrophobic pocket.
FIG 1.
Structure of the human parechovirus virion. (Republished from reference 21 with permission of the publisher.)
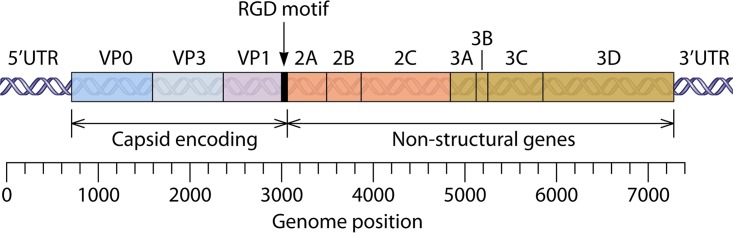
The HPeV genome is divided into four different areas: the 5′ untranslated region (UTR), one open reading frame (ORF), the 3′ UTR, and a poly(A) tract. The single ORF is translated into one polyprotein that is cleaved into several precursor molecules, which are eventually turned into both structural and nonstructural proteins (14, 24). The structural proteins of the capsid (VP0, VP1, and VP3) are located at the N-terminal portion of the polyprotein and followed by nonstructural proteins 2A, 2B, 2C, 3A, 3B, 3C, and 3D (Fig. 2) (15, 25).
FIG 2.
Genome organization of human parechovirus. (Republished from reference 25 with permission of the publisher.)
Cell Entry
The C terminus of VP1 of HPeV-1 contains an arginine-glycine-aspartic acid (RGD) motif, which plays a role in host cell recognition and attachment of several viruses through interactions with cell surface integrins (17, 26, 27). The RGD motif seems to be essential for the infectivity of HPeV-1, -2, -4, and -5. Interestingly, HPeV-3 differs from those genotypes as it lacks the RGD motif, which implies the use of a different receptor for cell entry (28). This might result in a change of tissue tropism and could partly explain why HPeV-3 infections have different epidemiology and clinical presentations compared to those of infections caused by other HPeV genotypes (29, 30). However, certain strains of HPeV-1, -5, -7, -8, -10, -11, -13, -14, and -15 were also found to lack an RGD motif (29, 31–33).
Biochemical and structural studies have shown that HPeV-1 binds to αVβ1, αVβ3, and αVβ6 integrin receptors. A recent study using an antibody blocking assay, immunofluorescence microscopy, and reverse transcription-quantitative PCR (RT-qPCR) suggested that HPeV-1 is internalized and replicates only in cell lines that express αVβ1 integrin and not in those with αVβ3 or αVβ6 integrins. After an interaction with cell surface integrins, HPeV-1 probably enters the host cell through the clathrin-dependent endocytic pathway (34–36).
Replication
During the replication cycle, HPeV-1 proteins can be located in the cellular endoplasmic reticulum (ER) at 30 min postinfection and in the cis-Golgi network after 60 min. At 4 h postinfection, capsid polypeptides had been synthesized and were detectable in the cytoplasm of infected cells. Within 6 to 8 h, HPeV-1 had completed an entire replication cycle (36).
Major changes in infected cells were found to be a dilated ER, which is stripped of its ribosomes, and a completely dispersed Golgi complex (37). HPeVs differ from most other picornaviruses because they do not shut off the protein synthesis of the infected cell during their own replication (38).
Evolution
Different genotypes are recognized based on phylogenetic differences in the VP1 region. Bayesian analysis indicated that the VP1 region evolves at a high rate of evolutionary change (∼10−3 substitutions per site per year). The Parechovirus A species probably diverged from its most recent common ancestor about 400 years ago and since then has evolved into different lineages. For example, it is estimated that HPeV-7 diverged from HPeV-3 around 150 years ago (39).
For most HPeV types, recombination seems to be an important factor in the evolution of the genus and may influence spread and pathogenicity. Recombination in HPeV occurs at a frequency similar to that of enteroviruses (40, 41). HPeV-3 differs from the other genotypes by undergoing little or no recombination and may have biological restrictions that prohibit recombination. One study showed no recombination in HPeV-3 strains, whereas 50% of the other HPeVs (types 1, 4, 5, and 6) isolated in the same year were recombinant. A different cell tropism, probably due to a lack of an RGD sequence, may contribute to this observation by reducing the chance of coinfection and, thus, recombination with other genotypes (40).
PATHOGENESIS AND HOST RESPONSE
Replication Sites
As HPeV predominantly affects the gastrointestinal and respiratory tracts, these locations might be considered to be the primary replication sites (25, 30). In a minority of cases, HPeV causes systemic illness by spreading hematogenously to other organs, including the brain or liver, that may act as secondary replication sites (25). In vitro studies showed that replication of HPeV types 1 to 6 is possible in many different cell lines (42). In a study that used HPeV-1 and HPeV-3 strains from patients with clinical symptoms, HPeV-3 strains showed better replication efficacy on a neural cell line (human neuroblastoma) than did HPeV-1. Furthermore, HPeV-3 isolates from patients with CNS disease showed better replication efficacy on neural cells than did isolates from patients without CNS disease (43).
Transmission
Transmission of HPeV is thought to take place easily between young children and occurs most frequently in those under 2 years of age (44). A Danish study recognized that the presence of a sibling <2 years old increased the risk of severe HPeV-3 infection 11-fold (45). Transmission can occur through the fecal-oral route from both asymptomatic and symptomatic infected individuals, in whom viral loads have been shown to be similar. The estimated median duration of shedding in stool is over 50 days (46, 47). Little data are available on transmission through the respiratory tract, but it has been suggested to be an acquisition route in children with CNS symptoms (48). Respiratory shedding is estimated to have a duration of 1 to 3 weeks (47).
Innate Immune Response
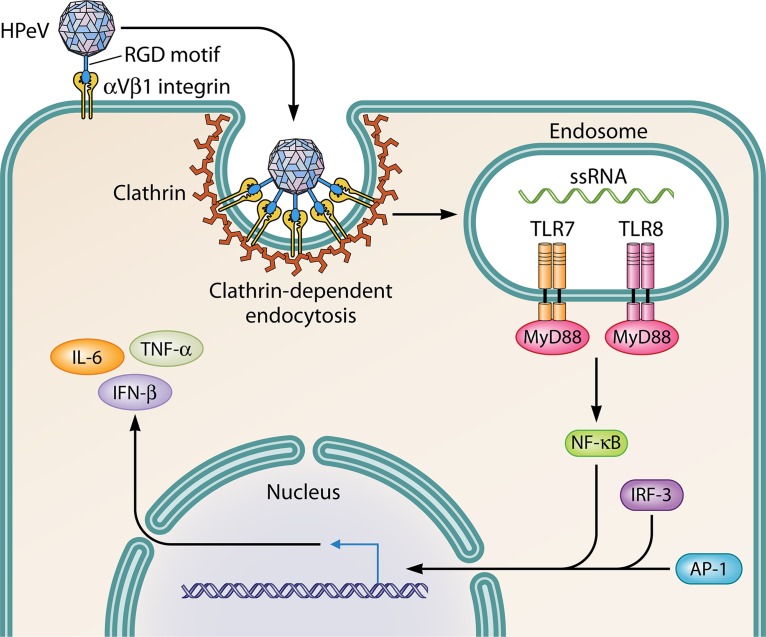
The innate immune system can recognize and respond quickly to specific components of viruses by inducing the production of cytokines by effector cells. Toll-like receptors (TLRs) are transmembrane proteins that have a key role in regulating innate immune responses against a variety of microbiological pathogens. Triantafilou et al. (49) studied in vitro immune responses to HPeV-1 and demonstrated that TLR8 and TLR7 act as host sensors for intracellular virus. These TLRs localize to endosomes, where they sense viral ssRNA and stimulate the secretion of inflammatory and regulatory cytokines (Fig. 3). Volpe (50) raised the possibility that this TLR activation may have a role in neuronal injury in infants with CNS infection by the inhibition of axonal growth and neuronal apoptosis. Interferon (IFN) expression is an important factor in the innate immune response, and both TLR-dependent and -independent pathways can be involved in the regulation of IFNs (49, 51).
FIG 3.
Cell entry and innate immune response. HPeV attaches to the cell surface by interaction of the RGD motif with αVβ1 integrin. The clathrin-dependent pathway facilitates the internalization of HPeV into endosomes, where a signaling cascade is mediated. Upon the activation of TLR7 and TLR8, the adaptor protein MyD88 stimulates NF-κB expression. The cooperative binding of the NF-κB p65 subunit, IFN regulatory factor 3 (IRF-3), and activator protein 1 (AP-1) to the type I IFN promoter region of the DNA induces IFN-β gene transcription (20), which results in the induction of the proinflammatory cytokines IFN-β, tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) (49).
Acquired Immune Response
The important antigenic site in HPeV-1 is thought to be the RGD motif of the VP1 protein, as described for other picornaviruses (52–54). Another antigenic site has been identified on the VP0 protein in a location that has not been reported to be antigenic in other picornaviruses (54). One study found evidence for immunogenic epitopes on all three capsid proteins (VP0, VP1, and VP3) (55).
Using antibody-producing B cells from human donors, Shakeel et al. (56) managed to produce two different monoclonal antibodies specific for HPeV-1: AM18 and AM28. The epitope of AM18 was located on the VP1 protein, including the RGD motif, and probably neutralizes the virus by aggregation and by blocking integrin binding to the capsid. AM28 recognizes a conformational epitope on the capsid composed of VP0 and VP3 loops from neighboring pentamers and probably inhibits RNA uncoating (56, 57).
EPIDEMIOLOGY AND CLINICAL SYNDROMES
Geographic Distribution
HPeV infections are common around the world and have been identified on every inhabited continent (48, 58–62). Reported prevalence rates vary depending on the age of the subjects included in the study population and the sampling sites chosen. HPeV-1 seems to be the most predominant genotype, although a study from Pakistan detected HPeV-15 in the majority of patients (31–33, 63).
Seasonality
The seasonality of HPeV infections shows considerable variability and appears to depend on the predominant genotype. HPeV-1 circulates throughout the year and does not show strong seasonality. National surveillance studies from the United States and Denmark described a small increase in the rate of HPeV-1 infections in the summer and autumn months, whereas Dutch data show a low rate in the summer months (4, 59, 64).
HPeV-3 shows a more variable temporal pattern of circulation. Years of high prevalence can alternate with years of a near absence of infections. A pattern of biannual cycles has been observed in northern Europe, where HPeV-3 infections have occurred much more frequently in even-numbered years between 2000 and 2010 (65, 66). The cause of this absence-and-reappearance pattern is unclear but may be related to the recombination dynamics of the viruses (67). A study from Japan did not show a biannual pattern of HPeV-3 infections but described annual differences with epidemic years in which there is a more pronounced peak of infections during the summer months (68). This preference for infections during summer and autumn has also been observed in several smaller studies from different continents (69–71). During the years of high HPeV-3 frequency, the annual distribution of HPeV in The Netherlands resembled that of enterovirus infections, which show a peak in the summer months. In odd years, this high frequency was not observed (65).
Outbreaks
The first report of nosocomial outbreaks of HPeV infections dates from 1968. HPeV-1 (echovirus 22) was detected during three different outbreaks of respiratory disease in 18 infants admitted to a premature nursery in New York (6). Another outbreak of HPeV-1 involved 19 neonatal intensive care unit (NICU) patients with gastrointestinal disease in Israel (7). A third nosocomial outbreak was reported in mostly premature neonates in Croatia, who developed gastrointestinal or respiratory symptoms of only mild to moderate severity (8).
HPeV-3 has also been associated with nosocomial outbreaks of severe disease. During an outbreak in an obstetric unit in Austria, HPeV-3 caused sepsis-like illness in 20.5% of neonates born within a 2-week time frame. However, the specific source of the infections could not be identified (9).
In a community outbreak in New South Wales, Australia, HPeV-3 was detected in 183 infants with an average age of 46 days during a 4-month period in spring and summer. Notification of medical staff of the clinical presentation of and management options for HPeV and syndromic surveillance during this outbreak resulted in a 30% decrease in hospital stays and possibly also a minimization of unnecessary antimicrobial drug use (62).
Studies in Yamagata, Japan, reported community HPeV-3 outbreaks associated mostly with myalgia every 2 to 3 years during the summer months (72). Another study from the same country but in a different region described an epidemic of HPeV-3 sepsis, sepsis-like syndrome, and encephalitis in 43 young infants under 4 months of age in the summer (73).
Seroprevalence and Asymptomatic Infections
Seropositivity for HPeV-1 is almost universal in adults, whereas only 10% of Dutch and about 73% of Japanese adults carry HPeV-3 antibodies (74–76). This difference in seroprevalence is likely to cause a lower level of maternal protection against HPeV-3 than against HPeV-1 in infants and might explain why symptomatic HPeV-3 infections tend to occur in much younger children and are associated with more severe disease than HPeV-1 infections. In Japan, seropositivity rates for HPeV-3 were significantly lower than those for HPeV-1 in cord blood samples of healthy full-term neonates at a cutoff titer of 1:4. Low antibody titers (<1:32) were found in about 40% of these infants (n = 175), suggesting that a high proportion of newborns lacked maternal protection against HPeV-3 infection. All of the infants with severe HPeV-3 infection (n = 45) had low antibody titers at disease onset. In this group, antibodies increased to a high level following infection, suggesting an important role for the development of neutralizing antibodies in fighting HPeV-3 infections (77).
A longitudinal study of 200 asymptomatic Finnish children 3 months of age and older, in which stool samples were tested, showed that infection rates increased rapidly during the first year of life and dropped again after 24 months of age. The cumulative incidence of infection was 48% by the age of 22 months (44). A comparable study from Norway detected HPeV at least once in 43% of children before 12 months of age and in 86% before 24 months of age. HPeV-1 was detected most frequently, but types 3 and 6 were also found (46).
Symptomatic Infections
Children under the age of 2 years are at the greatest risk of developing symptomatic HPeV infection. Generally, HPeV-1 is the predominant cause of disease, followed by types 3 and 6, respectively (3, 30, 65, 70). Children under 6 months of age present with the most severe course of the disease, related to higher rates of HPeV-3 infections (3, 11, 30, 68, 70). HPeV-3-infected children presenting with severe disease appear to be significantly younger than those with gastroenteritis (78). Some studies describe a high rate of premature birth in patients with severe HPeV-3 infections (11, 79–81). HPeV-6 tends to affect an older age group than the other two common types and is detected mainly in children >1 year of age (68, 82). Gastrointestinal symptoms are the most common presentation, whereas most severe infections are sepsis-like and CNS infections (68).
Gastroenteritis
Gastroenteritis is considered to be the most common manifestation of HPeV infections and can be caused by almost all HPeV types (30, 33, 83–85). HPeV-1 seems to be the most detected genotype, followed by HPeV-4 and -6 (31, 32, 59, 68, 83, 84, 86, 87). The majority of larger studies describing the epidemiology of gastroenteritis associated with HPeV were performed in Asian countries (60, 84, 88–90). In studies of more than 400 subjects with symptoms of gastroenteritis (seven studies), the estimated prevalence of HPeV infections in children <2 years of age with gastroenteritis ranged from 6.6% to 24.6% in Japan, The Netherlands, and Iran (29, 59, 60, 84, 88–90). Most patients were male (58.3% to 70.2%), and the median age of infected patients was between 10 and 17 months (29, 84, 88–90).
Of the hospitalized children with HPeV gastroenteritis in Sri Lanka, 90% were dehydrated, and fever and vomiting were present in 30 and 40% of the patients, respectively (31). A less severe course of disease has been described for HPeV-15 infections, in which fever and vomiting were uncommon (33). In contrast, HPeV-11 (n = 2) seems to be related to severe diarrhea (>10 episodes/day) with vomiting (31).
Some authors have questioned the clinical importance of HPeV detection in children with gastroenteritis. In a longitudinal observational study of healthy Norwegian infants, monthly stool samples were obtained, and symptoms of infections were recorded. No association between the presence of HPeV and reported symptoms of gastrointestinal or upper respiratory tract infections was found (46). Another study did not find a difference in HPeV prevalences or viral loads between hospitalized children with diarrhea and asymptomatic children (83). However, data from those studies are difficult to interpret due to low patient numbers, common coinfection with other viruses, and the fact that HPeV carriage in stool can be prolonged and occur long after symptoms have resolved.
Respiratory Tract Infections
HPeV-1, -3, and -6 are the most frequently identified genotypes in respiratory samples (48, 91, 92). HPeV is detected in both upper and lower respiratory tract infections and presents with nonspecific symptoms (6, 48). A study from 1968 of three outbreaks of respiratory disease among premature infants reported an initial presentation of coryza in 90% of HPeV-1 infections, which was often accompanied by cough or dyspnea. Forty percent of the patients had evidence of pneumonia upon X ray, and in 11% of patients, a morbilliform rash was seen. HPeV-infected patients could not be distinguished from HPeV-negative patients based on clinical symptoms, and the presentation was similar to that of respiratory syncytial virus in the same nursery (6). Respiratory tract symptoms have also been described for patients with HPeV-positive stool samples. Wheeze, cough, or coryza was reported for 30% to 58% of patients, and otitis media was reported for 30% of the patients (31, 93).
Results from studies on the relationship between HPeV detection in the respiratory tract and clinical disease are not uniform. High rates of coinfections (around two-thirds of infections) complicate this analysis (92, 94). A longitudinal study of HPeV-1 seroconversion in young children by Tauriainen et al. (95) showed a significant association of HPeV infections with otitis media, with an odds ratio of 6.14. A weaker association between HPeV infections and cough was found. Only 17% of the children in that study did not show any symptoms during HPeV infection. Kolehmainen et al. (96) reported contrasting results and found no relationship between HPeV-3 and HPeV-4 detection and clinical symptoms of respiratory tract infection and only a weak connection with acute otitis media.
Severe Infections
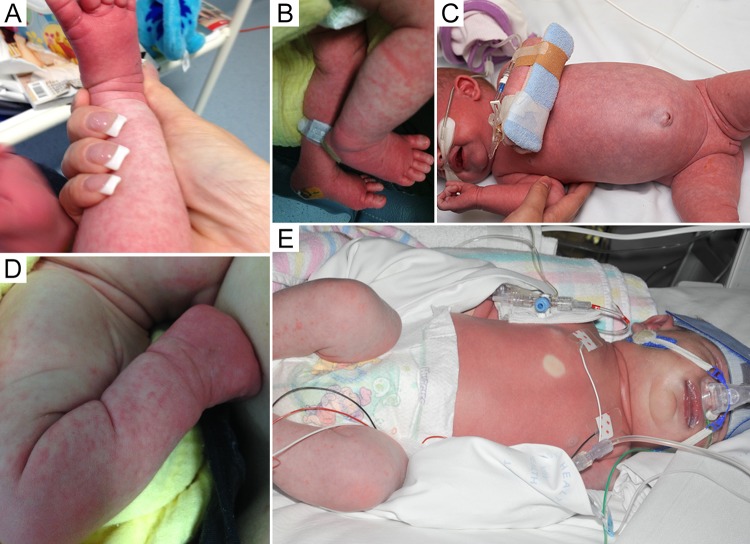
The majority of severe HPeV infections are caused by HPeV-3 and present in infants <3 months of age as asepsis, sepsis-like illness, or CNS infection (13, 30, 68, 97). Although most mild HPeV infections present with nonspecific symptoms, severe infections have some characteristic clinical features that may provide clues as to the etiology. In particular, when HPeV is known to be circulating in the community, presentations of “hot, red, angry babies” with features of sepsis should make clinicians consider HPeV infection (Fig. 4) (5, 62, 98, 99). During an HPeV-3 outbreak in Australia, the recognition of this triad of fever, rash, and severe irritability allowed the rapid and accurate identification of infected infants (5).
FIG 4.
Examples of clinical features of severe HPeV infection. (Republished from reference 5 with permission of Oxford University Press.)
Looking at studies of more than 20 patients with severe HPeV infection, fever was reported in 86% to up to 100% of infants (5, 13, 62, 73, 81, 98, 100). Most of those studies described irritability as the second most prevalent symptom, with proportions varying from 30% to 98%. Rashes appeared in 14 to 72% of patients and varied from a maculopapular or erythematous rash to a specific palmoplantar rash depending on the study and outbreak. In Japan in 2011, an outbreak of HPeV-3 caused a palmoplantar rash in 12 out of 15 patients, which was suggested to be related to a specific HPeV-3 strain (101). A much lower prevalence of rash (20%) was reported when another HPeV-3 lineage caused an outbreak of severe illness in Japanese infants in 2014 (73).
Poor suckling, tachycardia, and mottled skin are other common clinical characteristics, reported for 19 to 93%, 51 to 98%, and 18 to 76% of patients, respectively. Diarrhea, symptoms of upper respiratory tract infection, and abdominal distention (sometimes with an umbilical protrusion) have been described for up to 60, 39, and 75% of patients, respectively (5, 13, 62, 73, 81, 98, 100). Apnea has been described for 2 to 50% of patients, with the highest ratio being found in a study with a particularly high number of preterm infants (5, 73, 81, 98).
Enterovirus and HPeV infections often have similar clinical features, and distinguishing between the two infections based on clinical presentation alone may be difficult. Sharp et al. (102) compared clinical characteristics of infants with sepsis due to HPeV and those of infants with sepsis due to enterovirus and found a longer duration of fever and a higher maximum temperature in infants with HPeV infections. The mean duration of hospital admission for severe HPeV infections is mostly reported to be around 4 days, with an intensive care unit (ICU) admission rate of 9 to 50% (5, 10, 13, 62, 73, 81, 98, 100, 102, 103). Infants who were admitted to the ICU were significantly younger than those who were not (5).
Central Nervous System
HPeV-3 is the dominant genotype causing CNS infections, but other HPeV genotypes (e.g., HPeV-1, -5, and -6) are found occasionally (59, 79, 104, 105). Recent multicenter prospective research from Australia and the United States found that enteroviruses were the most common infectious causes of encephalitis in children, responsible for about 27% and 50% of infections, respectively (106, 107). Using a multiplex meningitis/encephalitis PCR panel for the rapid detection of 14 pathogens (viruses, bacteria, and yeast) in CSF samples, Leber et al. (107) found that HPeV was the second most common cause of infection in children <2 months of age in the United States (12/56 positive samples), followed by human herpesvirus 6, cytomegalovirus, and Streptococcus pneumoniae. Britton et al. (106) studied children <14 years of age in Australia and reported an infectious etiology in 59% of encephalitis cases. HPeV was the second most common infectious cause in this study, followed by herpes simplex virus, influenza virus, and Mycoplasma pneumoniae (106). The median age for children with CNS infection in various studies ranged between 9 days and 2.4 months (11, 79, 108, 109).
Seizures are a common presentation of HPeV infections of the CNS, with one study describing seizures in 90% of HPeV-infected infants with CNS involvement (79). In another study that compared HPeV-positive with HPeV-negative patients with suspected CNS infections, an association of HPeV with seizures and rash was reported (104).
More recent attention has been focused on the development of white matter damage in young infants (11, 110–112). HPeV white matter lesions are indistinguishable from those reported for enterovirus infections and hypoxic-ischemic encephalopathy and vary from diffuse signal intensity changes and punctate white matter lesions to cysts within the white matter (79, 110–112). In a study of 19 young infants with severe HPeV infections, intracranial hemorrhage was detected by ultrasound in three preterm neonates (81). The exact proportion of white matter damage in infants with HPeV CNS infections is unknown and may be underestimated, as in the absence of severe clinical findings, magnetic resonance imaging (MRI) is not always performed (79).
A nationwide study from Australia on HPeV encephalitis reported an ICU admission rate of 89% and invasive mechanical ventilation in 56% of the patients and showed that infants with HPeV encephalitis have a high risk of long-term complications related to the extent of white matter abnormalities. At discharge, three out of nine patients were described to have neurodevelopmental sequelae, but after 12 months of follow-up, this increased to five out of eight patients, two of whom were diagnosed with cerebral palsy and one of whom had central visual impairment (11).
Apart from meningoencephalitis, HPeV has also been reported to cause acute flaccid paralysis (85). The first cases were reported in Jamaica in 1986, when two out of six patients in an outbreak of acute flaccid paralysis tested positive for echovirus 22 (HPeV-1) (113). Only a small number of single cases have since been reported, in which types 1, 3, 6, and 12 were detected in children 1 year of age or younger (76, 85, 114, 115). HPeV also seems to be related to the development of acute disseminated encephalomyelitis (ADEM), which has been described in at least two separate cases (96, 116).
Miscellaneous
HPeV has been detected in patients with a large variety of other clinical presentations such as acute liver failure (HPeV-3) (117), hepatitis (HPeV-3) (5), hemolytic-uremic syndrome (HPeV-1) (118), myocarditis (HPeV-1 and -3) (119, 120), myalgia and myositis (HPeV-3) (72), herpangina (HPeV-6), hand-foot-and-mouth disease (HPeV-1 and -3) (88), apnea (HPeV-3) (121), sudden infant death syndrome (SIDS) (HPeV-1, -3, and -6) (122), hemophagocytic lymphohistiocytosis (HPeV-3) (123), extreme hyperferritinemia with a transient impairment of natural killer cell cytotoxicity (HPeV-3) (124), and Reye's syndrome (HPeV-6) (115).
LABORATORY DIAGNOSTICS
CSF and Blood Parameters
A lack of CSF pleocytosis and normal protein and glucose levels in CSF are common in patients with HPeV CNS infections (100, 125). A study describing young infants with cerebral HPeV infection and white matter changes upon MRI found an absence of cell reaction and normal glucose and protein levels in the CSF in eight out of nine patients (99). Similar results were recently described by another study: pleocytosis was absent, and the protein level was normal in all patients with HPeV encephalitis, including seven neonates with signs of diffusion restriction upon MRI (11). This phenomenon of uninflamed CSF was recognized previously in enteroviral CNS infections (110). In a study by Sharp et al. (102), CSF pleocytosis was less common in HPeV infections (2%) than in enterovirus infections (41%), and the average CSF white blood cell (WBC) counts and protein levels were also significantly lower in HPeV infections. The CSF glucose level was lower in enterovirus-infected patients than in HPeV-infected patients. Neopterin has also been suggested to be a potentially useful marker of CNS inflammation in HPeV infections (126). Elevated levels of neopterin have been described in two studies of three neonates with HPeV encephalitis and no pleocytosis, but further research should assess the possible role of neopterin in clinical decision-making (11, 126).
Infection parameters in blood are often within a normal range. Leukopenia was found in only 35% and an elevated C-reactive protein (CRP) level was found in 45% of hospitalized patients with severe HPeV infections. At admission, these rates were even lower, 15% and 20%, respectively (81). More severe leukopenia was associated with a more severe course of disease, and the mean white blood cell count was significantly lower in HPeV-infected than in enterovirus-infected patients.
Thrombocytopenia has been described for a minority of patients with severe HPeV infections, and reports of elevated aspartate aminotransferase (ASAT) and lactate dehydrogenase (LDH) levels vary from a low proportion to 100% of patients (81, 98, 99, 127). A deranged coagulation profile was found in 17 out of 31 patients presenting with HPeV sepsis (5).
Cell Culture
Traditionally, viral culture has been used for the diagnosis of HPeV infections, followed by virus neutralization testing for typing (1, 88). However, the commercial availability of reagents remains limited to HPeV-1 and HPeV-2. In cell culture, HPeV can produce an enterovirus-like cytopathic effect (128). Nonetheless, cell culture lacks sensitivity, with HPeV-3 being especially fastidious and other HPeV types having various growth efficiencies, even in the same cell culture (13, 42, 129, 130).
Molecular Methods
The emergence of HPeV-3 as a cause of severe pediatric disease has promoted the development and application of HPeV-specific RT-PCR diagnostic assays. Assays targeting the enterovirus 5′ UTR will not identify HPeV infection because of sequence heterogeneity within the 5′ UTRs of these two genera (16, 131, 132). HPeV real-time RT-PCRs are currently the preferred diagnostic tests for HPeV infections, as these assays are specific and have a sensitivity that is 100- to 1,000-fold higher than that of cell culture (130, 133). HPeV PCR was implemented in Dutch laboratories in 2004 and facilitated an increase in the rate of detection of parechovirus infections by a factor of 8 (65), while the addition of HPeV-specific PCR to the analysis of CSF samples in children <5 years of age with neonatal sepsis or central nervous system symptoms led to a 31% increase in rates of detection of viral causes (13). Diagnostic RT-PCRs are not type specific, and the identification of individual genotypes requires genetic sequencing of the VP1 regions (134). In October 2015, the first multiplex meningitis/encephalitis-specific PCR panel for the identification of 14 different pathogens (including HPeV) from one CSF sample was approved by the U.S. Food and Drug Administration (FDA) (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm466360.htm). In a multicenter prospective study of CSF specimens of pediatric and adult patients, the FilmArray panel was shown to be sensitive and specific for HPeV and most other pathogens (107). Enterovirus-specific PCRs such as Cepheid Xpert EV are commonly used in the United States but cannot detect HPeV (135).
HPeVs can be detected in stool samples, respiratory secretions, blood, and CSF samples (124). In patients with HPeV-3 sepsis-like illness, the virus can be detected in serum from the day of symptom onset. Serum viral loads were highest upon admission to the hospital and decreased rapidly in the following days (73). The rate of detection of HPeV has been shown to be highest with stool samples. In a comparison of HPeV detection rates in different pediatric specimens from symptomatic children <16 years of age, sensitivities of RT-PCR of 95% for stool samples, 84% for CSF, 79% for blood, 64% for nasopharyngeal specimens, and 57% for urine were shown (130). Even in patients with meningitis, the sensitivities of PCR for the detection of HPeV are comparable between stool and CSF samples (130).
In patients with potential sepsis-like illness or CNS infection due to HPeV, PCR testing of both serum and CSF samples may be useful. A recent study from Scotland compared the viral loads in CSF and plasma in children being evaluated for CNS infection, and in children with confirmed HPeV infection, there was a 1,000- to 10,000-fold-higher viral load in serum than in CSF (125).
Although HPeV PCR has become part of the standard workup for viral encephalitis in the United Kingdom, testing for the virus is not yet routine in many countries (136). In Australia and New Zealand, it is not in the consensus guidelines for encephalitis, and in the United States, only a few laboratories have the capacity to even test for HPeV (137, 138). However, HPeV assays are now becoming commercially available, along with quality assurance panels for assay validation (Quality Control for Molecular Diagnostics). PCR is essential for the diagnosis of HPeV infections and may reduce the use of antibiotics and shorten the duration of ICU stays by providing an early diagnosis (5).
THERAPY
The current management of severe HPeV infections involves early recognition and supportive care. To date, no antiviral drug has been shown to be effective against HPeV, and no vaccines are currently available to protect against infection.
Intravenous immunoglobulins (IVIGs) have been used for the treatment of severe disease due to HPeV. An infant with severe, dilated cardiomyopathy caused by HPeV-1 showed full recovery after treatment with IVIG (139). This infant had no detectable HPeV-specific antibodies prior to treatment, and the high titer of specific anti-HPeV-1 antibody after treatment suggests that IVIG may have had a role in the child's recovery.
However, IVIG may not be effective against all HPeV genotypes. Westerhuis et al. (43) showed that IVIG and specific antibodies efficiently neutralized HPeV-1 in vitro, while most HPeV-3 strains could not be neutralized. In addition, considerable variability in the levels of HPeV-neutralizing antibodies present in different IVIG preparations may explain this finding. A study from The Netherlands found very low neutralizing antibody titers in IVIG preparations and in the serum of HPeV-3-infected donors, but a study from Japan reported high HPeV-3-neutralizing antibody titers in six different IVIG preparations (43, 77). This is probably related to the much higher HPeV-3 seroprevalence in Japanese adults (140). Further clinical trials would be needed before IVIG could be recommended for the routine treatment of HPeV infections.
Little research has been performed on specific therapy for HPeV infections. Pleconaril and itraconazole, both of which possess in vitro activity against certain picornaviruses, do not seem to inhibit HPeV replication (141, 142). Pleconaril acts on picornaviruses by binding to a hydrophobic pocket within the VP1 protein, and its lack of an effect on HPeV might be related to the blockage of this pocket in HPeV (23). HPeV-1 also seems to be partly resistant to brefeldin A, which inhibits viral protein transportation (143). A greater knowledge of HPeV virology and the functions of specific proteins in the viral replication cycle might be useful in providing novel targets for antiviral therapy (144).
CONCLUSION
HPeV infections are increasingly being recognized as an important cause of sepsis-like disease and CNS infections in infants <3 months of age. Severe disease with a presentation of a “hot, red, angry baby” is commonly associated with HPeV-3 infection. CSF and blood infection parameters are often within normal ranges, which may not reflect the severity of the illness. Patients with CNS involvement frequently present with seizures, and the risk of long-term sequelae in infants with CNS involvement has only recently been recognized. Molecular diagnostic methods are essential for early diagnosis and should be implemented in the standard workup for sepsis and meningitis in neonates and young infants. Until now, no effective treatment for HPeV infections has been identified. Further research is needed to establish the value of IVIG as a treatment for severe HPeV infections. A greater understanding of HPeV virology and the functions of specific proteins in the viral replication cycle is urgently needed as a pathway to providing novel targets for antiviral therapy (144).
SEARCH STRATEGY AND SELECTION CRITERIA
References for this review were identified through searches of PubMed by use of the term “parechovirus,” “HPeV,” “echovirus 22,” or “echovirus 23.” Relevant articles resulting from these searches and relevant references cited in those articles were reviewed. Articles published in English, French, German, and Dutch were included. The most recent search was conducted on 23 February 2017, and no other date limits were used.
ACKNOWLEDGMENTS
We thank Anja Werno for her critical feedback. This study was made possible by the support of the Department of Pediatrics, University of Otago, Christchurch School of Medicine, and the Microbiology Department, Canterbury Health Laboratories, New Zealand.
We declare no competing interests.
The article was written by all authors.
Biographies

Laudi Olijve, M.Sc., completed her medical training at the Radboud University Nijmegen in The Netherlands (2016). During her final year of medical training, she performed research on pediatric Kingella kingae infections at the University of Otago, Christchurch, New Zealand. She is currently coordinating a study on osteoarticular infections in preschoolers in New Zealand and Australia through the University of Otago while gaining clinical experience at the ETZ Hospital in Tilburg, The Netherlands.

Lance Jennings is semiretired, retaining roles as Scientific Adviser/Virology, Canterbury District Health Board, and Clinical Associate Professor in the Pathology Department, University of Otago, New Zealand. He is a cofounder and a Director of the Asia Pacific Alliance for the Control of Influenza (APACI) and is chairperson of the International Society for Influenza and Other Respiratory Virus Diseases (ISIRV). His research interests focus on the epidemiology, diagnosis, treatment, and prevention of influenza and other respiratory virus infections. He has been instrumental in the development of influenza control strategies for New Zealand, including the introduction of free influenza vaccines, the establishment of influenza awareness education (NISG), and pandemic planning.

Tony Walls is a Pediatric Infectious Diseases Specialist who works at the University of Otago, Christchurch, New Zealand. He did his clinical training in Pediatrics in New Zealand and specialist training at Great Ormond St Hospital for Children in London, United Kingdom. Following the completion of his specialist training, he worked for several years as an Infectious Diseases Specialist at Sydney Children's Hospital. He has a longstanding interest in viral infections in children and completed an M.D. through the University of London on adenovirus infections in children post-stem cell transplantation. His current research interests include vaccinations and their safety as well as bone and joint infections in preschoolers.
REFERENCES
- 1.Wigand R, Sabin AB. 1961. Properties of ECHO types 22, 23 and 24 viruses. Arch Gesamte Virusforsch 11:224–247. doi: 10.1007/BF01241688. [DOI] [PubMed] [Google Scholar]
- 2.International Committee on Taxonomy of Viruses. 1997. Virus taxonomy: release history. http://ictvonline.org/taxonomyReleases.asp.
- 3.Janes VA, Minnaar R, Koen G, van Eijk H, Dijkman-de Haan K, Pajkrt D, Wolthers KC, Benschop KS. 2014. Presence of human non-polio enterovirus and parechovirus genotypes in an Amsterdam hospital in 2007 to 2011 compared to national and international published surveillance data: a comprehensive review. Euro Surveill 19(46):pii=20964. doi: 10.2807/1560-7917.ES2014.19.46.20964. [DOI] [PubMed] [Google Scholar]
- 4.van der Sanden S, de Bruin E, Vennema H, Swanink C, Koopmans M, van der Avoort H. 2008. Prevalence of human parechovirus in The Netherlands in 2000 to 2007. J Clin Microbiol 46:2884–2889. doi: 10.1128/JCM.00168-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatami A, McMullan BJ, Webber M, Stewart P, Francis S, Timmers KJ, Rodas E, Druce J, Mehta B, Sloggett NA, Cumming G, Papadakis G, Kesson AM. 2015. Sepsis-like disease in infants due to human parechovirus type 3 during an outbreak in Australia. Clin Infect Dis 60:228–236. doi: 10.1093/cid/ciu784. [DOI] [PubMed] [Google Scholar]
- 6.Berkovich S, Pangan J. 1968. Recoveries of virus from premature infants during outbreaks of respiratory disease: the relation of ECHO virus type 22 to disease of the upper and lower respiratory tract in the premature infant. Bull N Y Acad Med 44:377–387. [PMC free article] [PubMed] [Google Scholar]
- 7.Birenbaum E, Handsher R, Kuint J, Dagan R, Raichman B, Mendelson E, Linder N. 1997. Echovirus type 22 outbreak associated with gastro-intestinal disease in a neonatal intensive care unit. Am J Perinatol 14:469–473. doi: 10.1055/s-2007-994182. [DOI] [PubMed] [Google Scholar]
- 8.Ljubin-Sternak S, Juretic E, Santak M, Plesa M, Forcic D, Vilibic-Cavlek T, Aleraj B, Mlinaric-Galinovic G. 2011. Clinical and molecular characterization of a parechovirus type 1 outbreak in neonates in Croatia. J Med Virol 83:137–141. doi: 10.1002/jmv.21848. [DOI] [PubMed] [Google Scholar]
- 9.Strenger V, Diedrich S, Boettcher S, Richter S, Maritschnegg P, Gangl D, Fuchs S, Grangl G, Resch B, Urlesberger B. 2016. Nosocomial outbreak of parechovirus 3 infection among newborns, Austria, 2014. Emerg Infect Dis 22:1631–1634. doi: 10.3201/eid2209.151497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergnano S, Kadambari S, Whalley K, Menson EN, Martinez-Alier N, Cooper M, Sanchez E, Heath PT, Lyall H. 2015. Characteristics and outcomes of human parechovirus infection in infants (2008-2012). Eur J Pediatr 174:919–924. doi: 10.1007/s00431-014-2483-3. [DOI] [PubMed] [Google Scholar]
- 11.Britton PN, Dale RC, Nissen MD, Crawford N, Elliott E, Macartney K, Khandaker G, Booy R, Jones CA, PAEDS-ACE Investigators. 2016. Parechovirus encephalitis and neurodevelopmental outcomes. Pediatrics 137:e20152848. doi: 10.1542/peds.2015-2848. [DOI] [PubMed] [Google Scholar]
- 12.Verboon-Maciolek MA, Utrecht FG, Cowan F, Govaert P, van Loon AM, de Vries LS. 2008. White matter damage in neonatal enterovirus meningoencephalitis. Neurology 71:536. doi: 10.1212/01.wnl.0000324706.94229.88. [DOI] [PubMed] [Google Scholar]
- 13.Wolthers KC, Benschop KS, Schinkel J, Molenkamp R, Bergevoet RM, Spijkerman IJ, Kraakman HC, Pajkrt D. 2008. Human parechoviruses as an important viral cause of sepsislike illness and meningitis in young children. Clin Infect Dis 47:358–363. doi: 10.1086/589752. [DOI] [PubMed] [Google Scholar]
- 14.Knowles NJ, Hovi T, Hyypiä T, King AMQ, Lindberg AM, Pallansch MA, Palmenberg AC, Simmonds P, Skern T, Stanway G, Yamashita T, Zell R. 2012. Picornaviridae, p 855–880. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy. Classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 15.Tapparel C, Siegrist F, Petty TJ, Kaiser L. 2013. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol 14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Hyypia T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, Auvinen P, Kinnunen L, Stanway G. 1992. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci U S A 89:8847–8851. doi: 10.1073/pnas.89.18.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanway G, Kalkkinen N, Roivainen M, Ghazi F, Khan M, Smyth M, Meurman O, Hyypia T. 1994. Molecular and biological characteristics of echovirus 22, a representative of a new picornavirus group. J Virol 68:8232–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Shi Y, Xia Y. 2016. Genome analysis revealed novel genotypes and recombination of the human parechoviruses prevalent in children in Eastern China. Gut Pathog 8:52. doi: 10.1186/s13099-016-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuchaona W, Khamrin P, Yodmeeklin A, Saikruang W, Kongsricharoern T, Ukarapol N, Okitsu S, Hayakawa S, Ushijima H, Maneekarn N. 2015. Detection and characterization of a novel human parechovirus genotype in Thailand. Infect Genet Evol 31:300–304. doi: 10.1016/j.meegid.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Chang JT, Yang CS, Chen YS, Chen BC, Chiang AJ, Chang YH, Tsai WL, Lin YS, Chao D, Chang TH. 2015. Genome and infection characteristics of human parechovirus type 1: the interplay between viral infection and type I interferon antiviral system. PLoS One 10:e0116158. doi: 10.1371/journal.pone.0116158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiss Institute of Bioinformatics. 2011. ViralZone. Swiss Institute of Bioinformatics, Lausanne, Switzerland: http://www.expasy.org/viralzone. [Google Scholar]
- 22.Kalynych S, Palkova L, Plevka P. 2015. The structure of human parechovirus 1 reveals an association of the RNA genome with the capsid. J Virol 90:1377–1386. doi: 10.1128/JVI.02346-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shakeel S, Westerhuis BM, Domanska A, Koning RI, Matadeen R, Koster AJ, Bakker AQ, Beaumont T, Wolthers KC, Butcher SJ. 2016. Multiple capsid-stabilizing interactions revealed in a high-resolution structure of an emerging picornavirus causing neonatal sepsis. Nat Commun 7:11387. doi: 10.1038/ncomms11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghazi F, Hughes PJ, Hyypia T, Stanway G. 1998. Molecular analysis of human parechovirus type 2 (formerly echovirus 23). J Gen Virol 79(Part 11):2641–2650. [DOI] [PubMed] [Google Scholar]
- 25.Harvala H, Wolthers KC, Simmonds P. 2010. Parechoviruses in children: understanding a new infection. Curr Opin Infect Dis 23:224–230. doi: 10.1097/QCO.0b013e32833890ca. [DOI] [PubMed] [Google Scholar]
- 26.Ruoslahti E. 1996. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 27.Boonyakiat Y, Hughes PJ, Ghazi F, Stanway G. 2001. Arginine-glycine-aspartic acid motif is critical for human parechovirus 1 entry. J Virol 75:10000–10004. doi: 10.1128/JVI.75.20.10000-10004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Sunaidi M, Williams CH, Hughes PJ, Schnurr DP, Stanway G. 2007. Analysis of a new human parechovirus allows the definition of parechovirus types and the identification of RNA structural domains. J Virol 81:1013–1021. doi: 10.1128/JVI.00584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benschop K, Thomas X, Serpenti C, Molenkamp R, Wolthers K. 2008. High prevalence of human parechovirus (HPeV) genotypes in the Amsterdam region and identification of specific HPeV variants by direct genotyping of stool samples. J Clin Microbiol 46:3965–3970. doi: 10.1128/JCM.01379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benschop KS, Schinkel J, Minnaar RP, Pajkrt D, Spanjerberg L, Kraakman HC, Berkhout B, Zaaijer HL, Beld MG, Wolthers KC. 2006. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin Infect Dis 42:204–210. doi: 10.1086/498905. [DOI] [PubMed] [Google Scholar]
- 31.Pham NT, Takanashi S, Tran DN, Trinh QD, Abeysekera C, Abeygunawardene A, Khamrin P, Okitsu S, Shimizu H, Mizuguchi M, Ushijima H. 2011. Human parechovirus infection in children hospitalized with acute gastroenteritis in Sri Lanka. J Clin Microbiol 49:364–366. doi: 10.1128/JCM.02151-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pham NT, Chan-It W, Khamrin P, Nishimura S, Kikuta H, Sugita K, Baba T, Yamamoto A, Shimizu H, Okitsu S, Mizuguchi M, Ushijima H. 2011. Detection of human parechovirus in stool samples collected from children with acute gastroenteritis in Japan during 2007-2008. J Med Virol 83:331–336. doi: 10.1002/jmv.21740. [DOI] [PubMed] [Google Scholar]
- 33.Alam MM, Khurshid A, Shaukat S, Rana MS, Sharif S, Angez M, Nisar N, Naeem M, Zahoor Zaidi SS. 2013. Human parechovirus genotypes -10, -13 and -15 in Pakistani children with acute dehydrating gastroenteritis. PLoS One 8:e78377. doi: 10.1371/journal.pone.0078377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seitsonen J, Susi P, Heikkila O, Sinkovits RS, Laurinmaki P, Hyypia T, Butcher SJ. 2010. Interaction of alphaVbeta3 and alphaVbeta6 integrins with human parechovirus 1. J Virol 84:8509–8519. doi: 10.1128/JVI.02176-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merilahti P, Tauriainen S, Susi P. 2016. Human parechovirus 1 infection occurs via alphaVbeta1 integrin. PLoS One 11:e0154769. doi: 10.1371/journal.pone.0154769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joki-Korpela P, Marjomaki V, Krogerus C, Heino J, Hyypia T. 2001. Entry of human parechovirus 1. J Virol 75:1958–1967. doi: 10.1128/JVI.75.4.1958-1967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krogerus C, Egger D, Samuilova O, Hyypia T, Bienz K. 2003. Replication complex of human parechovirus 1. J Virol 77:8512–8523. doi: 10.1128/JVI.77.15.8512-8523.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coller BA, Chapman NM, Beck MA, Pallansch MA, Gauntt CJ, Tracy SM. 1990. Echovirus 22 is an atypical enterovirus. J Virol 64:2692–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faria NR, de Vries M, van Hemert FJ, Benschop K, van der Hoek L. 2009. Rooting human parechovirus evolution in time. BMC Evol Biol 9:164. doi: 10.1186/1471-2148-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benschop KS, Williams CH, Wolthers KC, Stanway G, Simmonds P. 2008. Widespread recombination within human parechoviruses: analysis of temporal dynamics and constraints. J Gen Virol 89:1030–1035. doi: 10.1099/vir.0.83498-0. [DOI] [PubMed] [Google Scholar]
- 41.Williams CH, Panayiotou M, Girling GD, Peard CI, Oikarinen S, Hyoty H, Stanway G. 2009. Evolution and conservation in human parechovirus genomes. J Gen Virol 90:1702–1712. doi: 10.1099/vir.0.008813-0. [DOI] [PubMed] [Google Scholar]
- 42.Westerhuis BM, Jonker SC, Mattao S, Benschop KS, Wolthers KC. 2013. Growth characteristics of human parechovirus 1 to 6 on different cell lines and cross-neutralization of human parechovirus antibodies: a comparison of the cytopathic effect and real time PCR. Virol J 10:146. doi: 10.1186/1743-422X-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westerhuis BM, Koen G, Wildenbeest JG, Pajkrt D, de Jong MD, Benschop KS, Wolthers KC. 2012. Specific cell tropism and neutralization of human parechovirus types 1 and 3: implications for pathogenesis and therapy development. J Gen Virol 93:2363–2370. doi: 10.1099/vir.0.043323-0. [DOI] [PubMed] [Google Scholar]
- 44.Kolehmainen P, Oikarinen S, Koskiniemi M, Simell O, Ilonen J, Knip M, Hyoty H, Tauriainen S. 2012. Human parechoviruses are frequently detected in stool of healthy Finnish children. J Clin Virol 54:156–161. doi: 10.1016/j.jcv.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen NM, Midgley SE, Nielsen AC, Christiansen CB, Fischer TK. 2016. Severe human parechovirus infections in infants and the role of older siblings. Am J Epidemiol 183:664–670. doi: 10.1093/aje/kwv206. [DOI] [PubMed] [Google Scholar]
- 46.Tapia G, Cinek O, Witso E, Kulich M, Rasmussen T, Grinde B, Ronningen KS. 2008. Longitudinal observation of parechovirus in stool samples from Norwegian infants. J Med Virol 80:1835–1842. doi: 10.1002/jmv.21283. [DOI] [PubMed] [Google Scholar]
- 47.Wildenbeest JG, Benschop KS, Bouma-de Jongh S, Wolthers KC, Pajkrt D. 2016. Prolonged shedding of human parechovirus in feces of young children after symptomatic infection. Pediatr Infect Dis J 35:580–583. doi: 10.1097/INF.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 48.Sharp J, Bell J, Harrison CJ, Nix WA, Oberste MS, Selvarangan R. 2012. Human parechovirus in respiratory specimens from children in Kansas City, Missouri. J Clin Microbiol 50:4111–4113. doi: 10.1128/JCM.01680-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Triantafilou K, Vakakis E, Orthopoulos G, Ahmed MA, Schumann C, Lepper PM, Triantafilou M. 2005. TLR8 and TLR7 are involved in the host's immune response to human parechovirus 1. Eur J Immunol 35:2416–2423. doi: 10.1002/eji.200526149. [DOI] [PubMed] [Google Scholar]
- 50.Volpe JJ. 2008. Neonatal encephalitis and white matter injury: more than just inflammation? Ann Neurol 64:232–236. doi: 10.1002/ana.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hiscott J. 2007. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem 282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 52.Pulli T, Lankinen H, Roivainen M, Hyypia T. 1998. Antigenic sites of coxsackievirus A9. Virology 240:202–212. doi: 10.1006/viro.1997.8908. [DOI] [PubMed] [Google Scholar]
- 53.Verdaguer N, Mateu MG, Andreu D, Giralt E, Domingo E, Fita I. 1995. Structure of the major antigenic loop of foot-and-mouth disease virus complexed with a neutralizing antibody: direct involvement of the Arg-Gly-Asp motif in the interaction. EMBO J 14:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joki-Korpela P, Roivainen M, Lankinen H, Poyry T, Hyypia T. 2000. Antigenic properties of human parechovirus 1. J Gen Virol 81:1709–1718. doi: 10.1099/0022-1317-81-7-1709. [DOI] [PubMed] [Google Scholar]
- 55.Alho A, Marttila J, Ilonen J, Hyypia T. 2003. Diagnostic potential of parechovirus capsid proteins. J Clin Microbiol 41:2294–2299. doi: 10.1128/JCM.41.6.2294-2299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shakeel S, Westerhuis BM, Ora A, Koen G, Bakker AQ, Claassen Y, Wagner K, Beaumont T, Wolthers KC, Butcher SJ. 2015. Structural basis of human parechovirus neutralization by human monoclonal antibodies. J Virol 89:9571–9580. doi: 10.1128/JVI.01429-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westerhuis BM, Benschop KS, Koen G, Claassen YB, Wagner K, Bakker AQ, Wolthers KC, Beaumont T. 2015. Human memory B cells producing potent cross-neutralizing antibodies against human parechovirus: implications for prevalence, treatment, and diagnosis. J Virol 89:7457–7464. doi: 10.1128/JVI.01079-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lekana-Douki SE, Nkoghe D, Drosten C, Ngoungou EB, Drexler JF, Leroy EM. 2014. Viral etiology and seasonality of influenza-like illness in Gabon, March 2010 to June 2011. BMC Infect Dis 14:373. doi: 10.1186/1471-2334-14-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer TK, Midgley S, Dalgaard C, Nielsen AY. 2014. Human parechovirus infection, Denmark. Emerg Infect Dis 20:83–87. doi: 10.3201/eid2001.130569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thongprachum A, Takanashi S, Kalesaran AF, Okitsu S, Mizuguchi M, Hayakawa S, Ushijima H. 2015. Four-year study of viruses that cause diarrhea in Japanese pediatric outpatients. J Med Virol 87:1141–1148. doi: 10.1002/jmv.24155. [DOI] [PubMed] [Google Scholar]
- 61.Drexler JF, Grywna K, Stocker A, Almeida PS, Medrado-Ribeiro TC, Eschbach-Bludau M, Petersen N, da Costa-Ribeiro H Jr, Drosten C. 2009. Novel human parechovirus from Brazil. Emerg Infect Dis 15:310–313. doi: 10.3201/eid1502.081028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cumming G, Khatami A, McMullan BJ, Musto J, Leung K, Nguyen O, Ferson MJ, Papadakis G, Sheppeard V. 2015. Parechovirus genotype 3 outbreak among infants, New South Wales, Australia, 2013-2014. Emerg Infect Dis 21:1144–1152. doi: 10.3201/eid2107.141149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen HF, Zheng XY, Chen XM, Shi TL, Yao YX, Yuan Q, Chen Q, Yu SY. 2015. Diversity and recombination of human parechovirus in children with acute gastroenteritis in Guangzhou, China. J Med Virol 87:296–302. doi: 10.1002/jmv.24030. [DOI] [PubMed] [Google Scholar]
- 64.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA, Centers for Disease Control and Prevention. 2006. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ 55:1–20. [PubMed] [Google Scholar]
- 65.van der Sanden SM, Koopmans MP, van der Avoort HG. 2013. Detection of human enteroviruses and parechoviruses as part of the national enterovirus surveillance in The Netherlands, 1996-2011. Eur J Clin Microbiol Infect Dis 32:1525–1531. doi: 10.1007/s10096-013-1906-9. [DOI] [PubMed] [Google Scholar]
- 66.Harvala H, McLeish N, Kondracka J, McIntyre CL, McWilliam Leitch EC, Templeton K, Simmonds P. 2011. Comparison of human parechovirus and enterovirus detection frequencies in cerebrospinal fluid samples collected over a 5-year period in Edinburgh: HPeV type 3 identified as the most common picornavirus type. J Med Virol 83:889–896. doi: 10.1002/jmv.22023. [DOI] [PubMed] [Google Scholar]
- 67.Calvert J, Chieochansin T, Benschop KS, McWilliam Leitch EC, Drexler JF, Grywna K, da Costa Ribeiro H Jr, Drosten C, Harvala H, Poovorawan Y, Wolthers KC, Simmonds P. 2010. Recombination dynamics of human parechoviruses: investigation of type-specific differences in frequency and epidemiological correlates. J Gen Virol 91:1229–1238. doi: 10.1099/vir.0.018747-0. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe K, Hirokawa C, Tazawa T. 30 August 2016. Seropositivity and epidemiology of human parechovirus types 1, 3, and 6 in Japan. Epidemiol Infect doi: 10.1017/S0950268816001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walters B, Penaranda S, Nix WA, Oberste MS, Todd KM, Katz BZ, Zheng X. 2011. Detection of human parechovirus (HPeV)-3 in spinal fluid specimens from pediatric patients in the Chicago area. J Clin Virol 52:187–191. doi: 10.1016/j.jcv.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Piralla A, Furione M, Rovida F, Marchi A, Stronati M, Gerna G, Baldanti F. 2012. Human parechovirus infections in patients admitted to hospital in Northern Italy, 2008-2010. J Med Virol 84:686–690. doi: 10.1002/jmv.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han TH, Chung JY, You SJ, Youn JL, Shim GH. 2013. Human parechovirus-3 infection in children, South Korea. J Clin Virol 58:194–199. doi: 10.1016/j.jcv.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 72.Mizuta K, Yamakawa T, Kurokawa K, Chikaoka S, Shimizu Y, Itagaki T, Katsushima F, Katsushima Y, Ito S, Aoki Y, Matoba Y, Tanaka S, Yahagi K. 2016. Epidemic myalgia and myositis associated with human parechovirus type 3 infections occur not only in adults but also in children: findings in Yamagata, Japan, 2014. Epidemiol Infect 144:1286–1290. doi: 10.1017/S0950268815002873. [DOI] [PubMed] [Google Scholar]
- 73.Aizawa Y, Suzuki Y, Watanabe K, Oishi T, Saitoh A. 2016. Clinical utility of serum samples for human parechovirus type 3 infection in neonates and young infants: the 2014 epidemic in Japan. J Infect 72:223–232. doi: 10.1016/j.jinf.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Tauriainen S, Martiskainen M, Oikarinen S, Lonnrot M, Viskari H, Ilonen J, Simell O, Knip M, Hyoty H. 2007. Human parechovirus 1 infections in young children—no association with type 1 diabetes. J Med Virol 79:457–462. doi: 10.1002/jmv.20831. [DOI] [PubMed] [Google Scholar]
- 75.Westerhuis B, Kolehmainen P, Benschop K, Nurminen N, Koen G, Koskiniemi M, Simell O, Knip M, Hyoty H, Wolthers K, Tauriainen S. 2013. Human parechovirus seroprevalence in Finland and The Netherlands. J Clin Virol 58:211–215. doi: 10.1016/j.jcv.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 76.Ito M, Yamashita T, Tsuzuki H, Takeda N, Sakae K. 2004. Isolation and identification of a novel human parechovirus. J Gen Virol 85:391–398. doi: 10.1099/vir.0.19456-0. [DOI] [PubMed] [Google Scholar]
- 77.Aizawa Y, Watanabe K, Oishi T, Hirano H, Hasegawa I, Saitoh A. 2015. Role of maternal antibodies in infants with severe diseases related to human parechovirus type 3. Emerg Infect Dis 21:1966–1972. doi: 10.3201/eid2111.150267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wildenbeest JG, Benschop KS, Minnaar RP, Bouma-de Jongh S, Wolthers KC, Pajkrt D. 2014. Clinical relevance of positive human parechovirus type 1 and 3 PCR in stool samples. Clin Microbiol Infect 20:O640–O647. doi: 10.1111/1469-0691.12542. [DOI] [PubMed] [Google Scholar]
- 79.Verboon-Maciolek MA, Groenendaal F, Hahn CD, Hellmann J, van Loon AM, Boivin G, de Vries LS. 2008. Human parechovirus causes encephalitis with white matter injury in neonates. Ann Neurol 64:266–273. doi: 10.1002/ana.21445. [DOI] [PubMed] [Google Scholar]
- 80.Bissel SJ, Auer RN, Chiang CH, Kofler J, Murdoch GH, Nix WA, Painter M, Richer M, Sartelet H, Wang G, Wiley CA. 2015. Human parechovirus 3 meningitis and fatal leukoencephalopathy. J Neuropathol Exp Neurol 74:767–777. doi: 10.1097/NEN.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 81.Kurz H, Prammer R, Bock W, Ollerieth R, Bernert G, Zwiauer K, Aberle JH, Aberle SW, Fazekas T, Holter W. 2015. Intracranial hemorrhage and other symptoms in infants associated with human parechovirus in Vienna, Austria. Eur J Pediatr 174:1639–1647. doi: 10.1007/s00431-015-2583-8. [DOI] [PubMed] [Google Scholar]
- 82.Bubba L, Martinelli M, Pellegrinelli L, Primache V, Tanzi E, Pariani E, Binda S. 2017. A 4-year study on epidemiologic and molecular characteristics of human parechoviruses and enteroviruses circulating in children younger than 5 years in Northern Italy. Pediatr Infect Dis J 36:13–19. doi: 10.1097/INF.0000000000001344. [DOI] [PubMed] [Google Scholar]
- 83.Zhang DL, Jin Y, Li DD, Cheng WX, Xu ZQ, Yu JM, Jin M, Yang SH, Zhang Q, Cui SX, Liu N, Duan ZJ. 2011. Prevalence of human parechovirus in Chinese children hospitalized for acute gastroenteritis. Clin Microbiol Infect 17:1563–1569. doi: 10.1111/j.1469-0691.2010.03390.x. [DOI] [PubMed] [Google Scholar]
- 84.Yip CC, Lo KL, Que TL, Lee RA, Chan KH, Yuen KY, Woo PC, Lau SK. 2014. Epidemiology of human parechovirus, Aichi virus and salivirus in fecal samples from hospitalized children with gastroenteritis in Hong Kong. Virol J 11:182. doi: 10.1186/1743-422X-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alam MM, Khurshid A, Shaukat S, Sharif S, Rana MS, Angez M, Naeem M, Zaidi SS. 2012. Identification of human parechovirus genotype, HPeV-12, in a paralytic child with diarrhea. J Clin Virol 55:339–342. doi: 10.1016/j.jcv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Zhong H, Lin Y, Sun J, Su L, Cao L, Yang Y, Xu J. 2011. Prevalence and genotypes of human parechovirus in stool samples from hospitalized children in Shanghai, China, 2008 and 2009. J Med Virol 83:1428–1434. doi: 10.1002/jmv.22114. [DOI] [PubMed] [Google Scholar]
- 87.Chen H, Yao Y, Liu X, Xiao N, Xiao Y, Huang Y, Chen Q, Yu S. 2014. Molecular detection of human parechovirus in children with acute gastroenteritis in Guangzhou, China. Arch Virol 159:971–977. doi: 10.1007/s00705-013-1915-0. [DOI] [PubMed] [Google Scholar]
- 88.Ito M, Yamashita T, Tsuzuki H, Kabashima Y, Hasegawa A, Nagaya S, Kawaguchi M, Kobayashi S, Fujiura A, Sakae K, Minagawa H. 2010. Detection of human parechoviruses from clinical stool samples in Aichi, Japan. J Clin Microbiol 48:2683–2688. doi: 10.1128/JCM.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chieochansin T, Vichiwattana P, Korkong S, Theamboonlers A, Poovorawan Y. 2011. Molecular epidemiology, genome characterization, and recombination event of human parechovirus. Virology 421:159–166. doi: 10.1016/j.virol.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 90.Ghazi F, Ataei Z, Dabirmanesh B. 2012. Molecular detection of human parechovirus type 1 in stool samples from children with diarrhea. Int J Infect Dis 16:e673–e676. doi: 10.1016/j.ijid.2012.05.1020. [DOI] [PubMed] [Google Scholar]
- 91.Cabrerizo M, Calvo C, Trallero G, Luz Garcia-Garcia M, Arroyas M, Sanchez V, Pozo F, Casas I. 2013. Molecular epidemiology of human parechoviruses in children with acute respiratory infection in Spain. Pediatr Infect Dis J 32:802–803. doi: 10.1097/INF.0b013e31828bbe46. [DOI] [PubMed] [Google Scholar]
- 92.Harvala H, Robertson I, McWilliam Leitch EC, Benschop K, Wolthers KC, Templeton K, Simmonds P. 2008. Epidemiology and clinical associations of human parechovirus respiratory infections. J Clin Microbiol 46:3446–3453. doi: 10.1128/JCM.01207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pajkrt D, Benschop KS, Westerhuis B, Molenkamp R, Spanjerberg L, Wolthers KC. 2009. Clinical characteristics of human parechoviruses 4-6 infections in young children. Pediatr Infect Dis J 28:1008–1010. doi: 10.1097/INF.0b013e3181a7ab5f. [DOI] [PubMed] [Google Scholar]
- 94.Moe N, Pedersen B, Nordbo SA, Skanke LH, Krokstad S, Smyrnaios A, Dollner H. 2016. Respiratory virus detection and clinical diagnosis in children attending day care. PLoS One 11:e0159196. doi: 10.1371/journal.pone.0159196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tauriainen S, Oikarinen S, Taimen K, Laranne J, Sipila M, Lonnrot M, Ilonen J, Simell O, Knip M, Hyoty H. 2008. Temporal relationship between human parechovirus 1 infection and otitis media in young children. J Infect Dis 198:35–40. doi: 10.1086/588677. [DOI] [PubMed] [Google Scholar]
- 96.Kolehmainen P, Jaaskelainen A, Blomqvist S, Kallio-Kokko H, Nuolivirta K, Helminen M, Roivainen M, Lappalainen M, Tauriainen S. 2014. Human parechovirus type 3 and 4 associated with severe infections in young children. Pediatr Infect Dis J 33:1109–1113. doi: 10.1097/INF.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 97.Harvala H, Robertson I, Chieochansin T, McWilliam Leitch EC, Templeton K, Simmonds P. 2009. Specific association of human parechovirus type 3 with sepsis and fever in young infants, as identified by direct typing of cerebrospinal fluid samples. J Infect Dis 199:1753–1760. doi: 10.1086/599094. [DOI] [PubMed] [Google Scholar]
- 98.Selvarangan R, Nzabi M, Selvaraju SB, Ketter P, Carpenter C, Harrison CJ. 2011. Human parechovirus 3 causing sepsis-like illness in children from midwestern United States. Pediatr Infect Dis J 30:238–242. doi: 10.1097/INF.0b013e3181fbefc8. [DOI] [PubMed] [Google Scholar]
- 99.Verboon-Maciolek MA, Krediet TG, Gerards LJ, de Vries LS, Groenendaal F, van Loon AM. 2008. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J 27:241–245. doi: 10.1097/INF.0b013e31815c1b07. [DOI] [PubMed] [Google Scholar]
- 100.Schuffenecker I, Javouhey E, Gillet Y, Kugener B, Billaud G, Floret D, Lina B, Morfin F. 2012. Human parechovirus infections, Lyon, France, 2008-10: evidence for severe cases. J Clin Virol 54:337–341. doi: 10.1016/j.jcv.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 101.Shoji K, Komuro H, Miyata I, Miyairi I, Saitoh A. 2013. Dermatologic manifestations of human parechovirus type 3 infection in neonates and infants. Pediatr Infect Dis J 32:233–236. doi: 10.1097/INF.0b013e31827b1fd0. [DOI] [PubMed] [Google Scholar]
- 102.Sharp J, Harrison CJ, Puckett K, Selvaraju SB, Penaranda S, Nix WA, Oberste MS, Selvarangan R. 2013. Characteristics of young infants in whom human parechovirus, enterovirus or neither were detected in cerebrospinal fluid during sepsis evaluations. Pediatr Infect Dis J 32:213–216. doi: 10.1097/INF.0b013e318276b328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cabrerizo M, Trallero G, Pena MJ, Cilla A, Megias G, Munoz-Almagro C, Del Amo E, Roda D, Mensalvas AI, Moreno-Docon A, Garcia-Costa J, Rabella N, Omenaca M, Romero MP, Sanbonmatsu-Gamez S, Perez-Ruiz M, Santos-Munoz MJ, Calvo C, Study Group of Enterovirus and Parechovirus Infections in Children under 3 Years-Old, Spain, PI12-00904. 2015. Comparison of epidemiology and clinical characteristics of infections by human parechovirus vs. those by enterovirus during the first month of life. Eur J Pediatr 174:1511–1516. doi: 10.1007/s00431-015-2566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karsch K, Obermeier P, Seeber L, Chen X, Tief F, Muhlhans S, Hoppe C, Conrad T, Bottcher S, Diedrich S, Rath B. 2015. Human parechovirus infections associated with seizures and rash in infants and toddlers. Pediatr Infect Dis J 34:1049–1055. doi: 10.1097/INF.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 105.Harvala H, Calvert J, Van Nguyen D, Clasper L, Gadsby N, Molyneaux P, Templeton K, McWilliams Leitch C, Simmonds P. 2014. Comparison of diagnostic clinical samples and environmental sampling for enterovirus and parechovirus surveillance in Scotland, 2010 to 2012. Euro Surveill 19(15):pii=20772 http://www.eurosurveillance.org/content/10.2807/1560-7917.ES2014.19.15.20772. [DOI] [PubMed] [Google Scholar]
- 106.Britton P, Dale R, Blyth C, Clark J, Crawford N, Marshall HS, Elliott E, Macartney K, Booy R, Jones C. 2016. The causes and clinical features of childhood encephalitis in Australia: a multicentre, prospective, cohort study. Open Forum Infect Dis 3:1169–1169. doi: 10.1093/ofid/ofw172.872. [DOI] [PubMed] [Google Scholar]
- 107.Leber AL, Everhart K, Balada-Llasat JM, Cullison J, Daly J, Holt S, Lephart P, Salimnia H, Schreckenberger PC, DesJarlais S, Reed SL, Chapin KC, LeBlanc L, Johnson JK, Soliven NL, Carroll KC, Miller JA, Dien Bard J, Mestas J, Bankowski M, Enomoto T, Hemmert AC, Bourzac KM. 2016. Multicenter evaluation of BioFire FilmArray Meningitis/Encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol 54:2251–2261. doi: 10.1128/JCM.00730-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Felsenstein S, Yang S, Eubanks N, Sobrera E, Grimm JP, Aldrovandi G. 2014. Human parechovirus central nervous system infections in southern California children. Pediatr Infect Dis J 33:e87–e91. doi: 10.1097/INF.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 109.Escuret A, Mirand A, Dommergues MA, Couzon B, Foucaud P, Peigue-Lafeuille H, Marque-Juillet S. 2013. Epidemiology of parechovirus infections of the central nervous system in a French pediatric unit. Arch Pediatr 20:470–475. (In French.) doi: 10.1016/j.arcped.2013.02.066. [DOI] [PubMed] [Google Scholar]
- 110.Verboon-Maciolek MA, Groenendaal F, Cowan F, Govaert P, van Loon AM, de Vries LS. 2006. White matter damage in neonatal enterovirus meningoencephalitis. Neurology 66:1267–1269. doi: 10.1212/01.wnl.0000208429.69676.23. [DOI] [PubMed] [Google Scholar]
- 111.Amarnath C, Helen Mary T, Periakarupan A, Gopinathan K, Philson J. 2016. Neonatal parechovirus leucoencephalitis—radiological pattern mimicking hypoxic-ischemic encephalopathy. Eur J Radiol 85:428–434. doi: 10.1016/j.ejrad.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 112.Gupta S, Fernandez D, Siddiqui A, Tong WC, Pohl K, Jungbluth H. 2010. Extensive white matter abnormalities associated with neonatal parechovirus (HPeV) infection. Eur J Paediatr Neurol 14:531–534. doi: 10.1016/j.ejpn.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 113.Figueroa JP, Ashley D, King D, Hull B. 1989. An outbreak of acute flaccid paralysis in Jamaica associated with echovirus type 22. J Med Virol 29:315–319. doi: 10.1002/jmv.1890290418. [DOI] [PubMed] [Google Scholar]
- 114.Legay V, Chomel JJ, Fernandez E, Lina B, Aymard M, Khalfan S. 2002. Encephalomyelitis due to human parechovirus type 1. J Clin Virol 25:193–195. doi: 10.1016/S1386-6532(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 115.Watanabe K, Oie M, Higuchi M, Nishikawa M, Fujii M. 2007. Isolation and characterization of novel human parechovirus from clinical samples. Emerg Infect Dis 13:889–895. doi: 10.3201/eid1306.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Obermeier PE, Karsch K, Hoppe C, Seeber L, Schneider J, Muhlhans S, Chen X, Tief F, Kaindl AM, Weschke B, Bottcher S, Diedrich S, Rath B. 2016. Acute disseminated encephalomyelitis after human parechovirus infection. Pediatr Infect Dis J 35:35–38. doi: 10.1097/INF.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 117.Bigelow AM, Scott JP, Hong JC, Cronin DC, Vitola BE, Fons RA, Petersen TL. 2016. Human parechovirus as a cause of isolated pediatric acute liver failure. Pediatrics 138:e20160233. doi: 10.1542/peds.2016-0233. [DOI] [PubMed] [Google Scholar]
- 118.O'Regan S, Robitaille P, Mongeau JG, McLaughlin B. 1980. The hemolytic uremic syndrome associated with ECHO 22 infection. Clin Pediatr (Phila) 19:125–127. doi: 10.1177/000992288001900207. [DOI] [PubMed] [Google Scholar]
- 119.Mardekian SK, Fortuna D, Nix A, Bhatti T, Wiley CA, Flanders A, Urtecho J, Sloane J, Ahmad J, Curtis MT. 2015. Severe human parechovirus type 3 myocarditis and encephalitis in an adolescent with hypogammaglobulinemia. Int J Infect Dis 36:6–8. doi: 10.1016/j.ijid.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 120.Russell SJ, Bell EJ. 1970. Echoviruses and carditis. Lancet i:784–785. doi: 10.1016/S0140-6736(70)91021-4. [DOI] [PubMed] [Google Scholar]
- 121.Nirei J, Aizawa Y, Okazaki M, Kobayashi A, Onozuka J, Numata O, Oishi T, Saitoh A. 2016. Human parechovirus type 3 infection: cause of apnea in infants born prematurely. Pediatr Int 58:400–402. doi: 10.1111/ped.12869. [DOI] [PubMed] [Google Scholar]
- 122.Sedmak G, Nix WA, Jentzen J, Haupt TE, Davis JP, Bhattacharyya S, Pallansch MA, Oberste MS. 2010. Infant deaths associated with human parechovirus infection in Wisconsin. Clin Infect Dis 50:357–361. doi: 10.1086/649863. [DOI] [PubMed] [Google Scholar]
- 123.Aviner S, Sofer D, Shulman LM, Bibi H, Weitzman S. 2014. Hemophagocytic lymphohistiocytosis associated with parechovirus 3 infection. J Pediatr Hematol Oncol 36:e251–e253. doi: 10.1097/MPH.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 124.Casas-Alba D, Martinez-Monseny A, Monfort L, Munoz-Almagro C, Cabrerizo M, Deya A, Launes C. 2016. Extreme hyperferritinemia in dizygotic twins with human parechovirus-3 infection. Pediatr Infect Dis J 35:1366–1368. doi: 10.1097/INF.0000000000001333. [DOI] [PubMed] [Google Scholar]
- 125.Harvala H, Griffiths M, Solomon T, Simmonds P. 2014. Distinct systemic and central nervous system disease patterns in enterovirus and parechovirus infected children. J Infect 69:69–74. doi: 10.1016/j.jinf.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 126.Brownell AD, Reynolds TQ, Livingston B, McCarthy CA. 2015. Human parechovirus-3 encephalitis in two neonates: acute and follow-up magnetic resonance imaging and evaluation of central nervous system markers of inflammation. Pediatr Neurol 52:245–249. doi: 10.1016/j.pediatrneurol.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 127.Yuzurihara SS, Ao K, Hara T, Tanaka F, Mori M, Kikuchi N, Kai S, Yokota S. 2013. Human parechovirus-3 infection in nine neonates and infants presenting symptoms of hemophagocytic lymphohistiocytosis. J Infect Chemother 19:144–148. doi: 10.1007/s10156-012-0420-9. [DOI] [PubMed] [Google Scholar]
- 128.Benschop K, Minnaar R, Koen G, van Eijk H, Dijkman K, Westerhuis B, Molenkamp R, Wolthers K. 2010. Detection of human enterovirus and human parechovirus (HPeV) genotypes from clinical stool samples: polymerase chain reaction and direct molecular typing, culture characteristics, and serotyping. Diagn Microbiol Infect Dis 68:166–173. doi: 10.1016/j.diagmicrobio.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 129.Joki-Korpela P, Hyypia T. 1998. Diagnosis and epidemiology of echovirus 22 infections. Clin Infect Dis 27:129–136. doi: 10.1086/514615. [DOI] [PubMed] [Google Scholar]
- 130.de Crom SC, Obihara CC, de Moor RA, Veldkamp EJ, van Furth AM, Rossen JW. 2013. Prospective comparison of the detection rates of human enterovirus and parechovirus RT-qPCR and viral culture in different pediatric specimens. J Clin Virol 58:449–454. doi: 10.1016/j.jcv.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 131.Benschop K, Molenkamp R, van der Ham A, Wolthers K, Beld M. 2008. Rapid detection of human parechoviruses in clinical samples by real-time PCR. J Clin Virol 41:69–74. doi: 10.1016/j.jcv.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 132.Legay V, Chomel JJ, Lina B. 2002. Specific RT-PCR procedure for the detection of human parechovirus type 1 genome in clinical samples. J Virol Methods 102:157–160. doi: 10.1016/S0166-0934(01)00435-9. [DOI] [PubMed] [Google Scholar]
- 133.Nix WA, Maher K, Johansson ES, Niklasson B, Lindberg AM, Pallansch MA, Oberste MS. 2008. Detection of all known parechoviruses by real-time PCR. J Clin Microbiol 46:2519–2524. doi: 10.1128/JCM.00277-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nix WA, Maher K, Pallansch MA, Oberste MS. 2010. Parechovirus typing in clinical specimens by nested or semi-nested PCR coupled with sequencing. J Clin Virol 48:202–207. doi: 10.1016/j.jcv.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 135.Pillet S, Billaud G, Omar S, Lina B, Pozzetto B, Schuffenecker I. 2010. Multicenter evaluation of the ENTEROVIRUS R-gene real-time RT-PCR assay for the detection of enteroviruses in clinical specimens. J Clin Virol 47:54–59. doi: 10.1016/j.jcv.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 136.Kneen R, Michael BD, Menson E, Mehta B, Easton A, Hemingway C, Klapper PE, Vincent A, Lim M, Carrol E, Solomon T, National Encephalitis Guidelines Development and Stakeholder Groups. 2012. Management of suspected viral encephalitis in children—Association of British Neurologists and British Paediatric Allergy, Immunology and Infection Group national guidelines. J Infect 64:449–477. doi: 10.1016/j.jinf.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 137.Britton PN, Eastwood K, Paterson B, Durrheim DN, Dale RC, Cheng AC, Kenedi C, Brew BJ, Burrow J, Nagree Y, Leman P, Smith DW, Read K, Booy R, Jones CA, Australasian Society of Infectious Diseases, Australasian College of Emergency Medicine, Australian and New Zealand Association of Neurologists, Public Health Association of Australia. 2015. Consensus guidelines for the investigation and management of encephalitis in adults and children in Australia and New Zealand. Intern Med J 45:563–576. doi: 10.1111/imj.12749. [DOI] [PubMed] [Google Scholar]
- 138.Abedi GR, Watson JT, Pham H, Nix WA, Oberste MS, Gerber SI. 2015. Enterovirus and human parechovirus surveillance—United States, 2009-2013. MMWR Morb Mortal Wkly Rep 64:940–943. doi: 10.15585/mmwr.mm6434a3. [DOI] [PubMed] [Google Scholar]
- 139.Wildenbeest JG, Wolthers KC, Straver B, Pajkrt D. 2013. Successful IVIG treatment of human parechovirus-associated dilated cardiomyopathy in an infant. Pediatrics 132:e243–e247. doi: 10.1542/peds.2012-1136. [DOI] [PubMed] [Google Scholar]
- 140.Tanaka S, Aoki Y, Matoba Y, Yahagi K, Itagaki T, Matsuzaki Y, Mizuta K. 2016. Seroepidemiology of human parechovirus types 1, 3, and 6 in Yamagata, Japan, in 2014. Microbiol Immunol 60:854–858. doi: 10.1111/1348-0421.12456. [DOI] [PubMed] [Google Scholar]
- 141.Strating JR, van der Linden L, Albulescu L, Bigay J, Arita M, Delang L, Leyssen P, van der Schaar HM, Lanke KH, Thibaut HJ, Ulferts R, Drin G, Schlinck N, Wubbolts RW, Sever N, Head SA, Liu JO, Beachy PA, De Matteis MA, Shair MD, Olkkonen VM, Neyts J, van Kuppeveld FJ. 2015. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep 10:600–615. doi: 10.1016/j.celrep.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van de Ven AA, Douma JW, Rademaker C, van Loon AM, Wensing AM, Boelens JJ, Sanders EA, van Montfrans JM. 2011. Pleconaril-resistant chronic parechovirus-associated enteropathy in agammaglobulinaemia. Antivir Ther 16:611–614. doi: 10.3851/IMP1792. [DOI] [PubMed] [Google Scholar]
- 143.Gazina EV, Mackenzie JM, Gorrell RJ, Anderson DA. 2002. Differential requirements for COPI coats in formation of replication complexes among three genera of Picornaviridae. J Virol 76:11113–11122. doi: 10.1128/JVI.76.21.11113-11122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van der Linden L, Wolthers KC, van Kuppeveld FJ. 2015. Replication and inhibitors of enteroviruses and parechoviruses. Viruses 7:4529–4562. doi: 10.3390/v7082832. [DOI] [PMC free article] [PubMed] [Google Scholar]