SUMMARY
Buruli ulcer is a noncontagious disabling cutaneous and subcutaneous mycobacteriosis reported by 33 countries in Africa, Asia, Oceania, and South America. The causative agent, Mycobacterium ulcerans, derives from Mycobacterium marinum by genomic reduction and acquisition of a plasmid-borne, nonribosomal cytotoxin mycolactone, the major virulence factor. M. ulcerans-specific sequences have been readily detected in aquatic environments in food chains involving small mammals. Skin contamination combined with any type of puncture, including insect bites, is the most plausible route of transmission, and skin temperature of <30°C significantly correlates with the topography of lesions. After 30 years of emergence and increasing prevalence between 1970 and 2010, mainly in Africa, factors related to ongoing decreasing prevalence in the same countries remain unexplained. Rapid diagnosis, including laboratory confirmation at the point of care, is mandatory in order to reduce delays in effective treatment. Parenteral and potentially toxic streptomycin-rifampin is to be replaced by oral clarithromycin or fluoroquinolone combined with rifampin. In the absence of proven effective primary prevention, avoiding skin contamination by means of clothing can be implemented in areas of endemicity. Buruli ulcer is a prototype of ecosystem pathology, illustrating the impact of human activities on the environment as a source for emerging tropical infectious diseases.
KEYWORDS: Mycobacterium ulcerans, Mycobacterium marinum, environmental mycobacteria, Buruli ulcer
INTRODUCTION
Large ulcers compatible with the diagnosis of Buruli ulcer were described by Sir Albert Cook in 1897 and by Kleinschmidt in northeastern Congo during the 1920s (1–4), but the causative agent, Mycobacterium ulcerans, was not isolated until 1948 in the Bairnsdale region of Victoria, Australia, by MacCallum et al. (5). The disease was finally named after Buruli (now called Nakasongola) County in Uganda, where the disease was described (6). The same infection has also been described under local names, according to the place where it occurred or was observed: Bairnsdale ulcer, Daintree ulcer, Mossman ulcer, and Searl ulcer in Australia, Tora and Mexican ulcer in Mexico (7), and mbasu, Kasongo ulcer, Kakerifu ulcer, La maladie mystérieuse de Daloa, and Mputa ya Luaka in African settings, where this infection has become more prevalent over the last few decades (8). Over the last decade, osteomyelitis has been an increasingly described form of the infection (9, 10). Still a query infection, Buruli ulcer is now known as a mycobacteriosis of the cutaneous and subcutaneous tissues caused by the nontuberculous bacterium Mycobacterium ulcerans (5, 11–18).
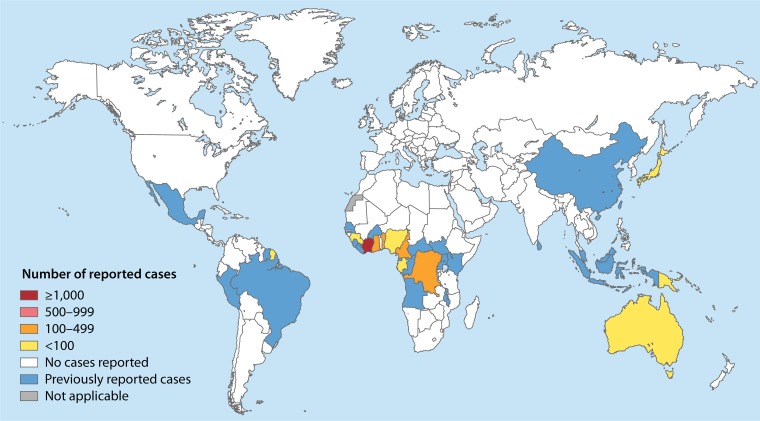
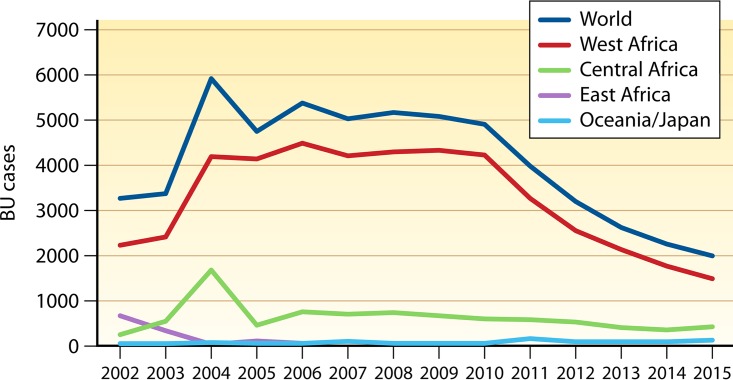
Buruli ulcer is a World Health Organization (WHO) reportable disease, reported in 33 countries in Southeast Asia, Australia, Africa, South America, and the Western Pacific, with impoverished rural communities of West and Central Africa being the most affected (Fig. 1) (14, 19, 20). Between 5,000 and 6,000 cases have been reported annually by 15 of the 33 reporting countries (21). Since only half of these countries regularly report data to the WHO, the full extent of the problem is unknown. Nevertheless, Buruli ulcer is regarded as the third-most-common mycobacterial infection in immunocompetent patients (15, 22) and is the second-most-common mycobacterial disease after tuberculosis in some countries with low endemicity for leprosy (23, 24). Buruli ulcer is one of the 17 tropical diseases classified as neglected diseases by the WHO, which recognized Buruli ulcer as an emerging public health problem in 1998 at the Yamoussoukro Conference (25). Starting in 2010, the number of registered cases regularly decreased in Africa, without a definitive explanation for that favorable trend (Fig. 2; Table 1). Causes for the decline in the overall incidence of Buruli ulcer remain purely speculative. Decline may reflect the positive effects of control programs or collateral effects of other health programs (26). In contrast, the incidence rose in Australia, from 32 cases in 2010 to 106 cases recorded in Victoria in 2015 (27). Understanding the epidemiological trends of Buruli ulcer has been obscured by the lack of definite knowledge regarding the reservoirs and modes of transmission of the causative agent, M. ulcerans, in every region of endemicity (19, 28, 29). Human-to-human transmission of Buruli ulcer has rarely been reported, suggesting environmental sources, as corroborated by several studies (30). Epidemiological studies have linked Buruli ulcer mainly to low-lying wetland areas and slow-moving rivers, especially in man-made environments (31–33). In West Africa and Central Africa, outbreaks of Buruli ulcer in the 1980s were linked to man-made changes in the natural environment (34, 35). More-recent studies have shown that in aquatic and swampy environments, M. ulcerans is detected in biofilms, soil, and aquatic insects (36–40).
FIG 1.
Global map representing countries that have reported cases of Buruli ulcer disease as of 2014 (344).
FIG 2.
Cases of Buruli ulcer (BU) reported in major areas of the world during the last decade. The correlation between Buruli ulcer cases in the world and in West Africa is 0.97.
TABLE 1.
New cases of Buruli ulcer reported from 2002 to 2015 by countries where Buruli ulcer is endemica
| Country | No. of new cases of Buruli ulcer in: |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | |
| Australia | 110 | 89 | 74 | 105 | 143 | 42 | 35 | 40 | 61 | 72 | 47 | 34 | 14 | 32 |
| Benin | 311 | 330 | 378 | 365 | 492 | 572 | 674 | 897 | 1,203 | 1,195 | 1,045 | 925 | 722 | 565 |
| Cote d'Ivoire | 549 | 827 | 1,039 | 1,386 | 1,659 | 2,533 | 2,679 | 2,242 | 2,191 | 1,872 | 1,564 | 1,153 | 768 | 750 |
| Cameroon | 133 | 126 | 133 | 160 | 256 | 287 | 323 | 312 | 230 | 271 | 265 | 914 | 223 | 132 |
| Congo | ND | ND | 6 | 38 | 56 | 107 | 147 | 126 | 99 | 370 | 53 | 235 | 180 | 102 |
| Democratic Republic of the Congo | 234 | 192 | 214 | 284 | 209 | 136 | 172 | 260 | 340 | 74 | 51 | 487 | 119 | 17 |
| Equatorial Guinea | ND | ND | ND | ND | 0 | ND | ND | ND | ND | ND | 3 | ND | ND | ND |
| Gabon | 40 | 47 | 59 | 45 | 59 | 65 | 41 | 53 | 32 | 54 | 91 | 43 | ND | ND |
| Ghana | 275 | 443 | 550 | 632 | 971 | 1,048 | 853 | 986 | 668 | 1,096 | 1,005 | 1,157 | 737 | 853 |
| Guinea | 72 | 46 | 96 | 82 | 59 | 24 | 61 | 80 | ND | 279 | 208 | 146 | 157 | ND |
| Japan | 3 | 7 | 10 | 4 | 10 | 9 | 5 | 2 | 3 | 1 | 1 | 1 | ND | ND |
| Liberia | 105 | ND | 8 | 21 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Nigeria | 113 | 65 | 23 | 40 | 4 | 7 | 24 | ND | ND | 9 | ND | ND | ND | ND |
| Papua New Guinea | 11 | 3 | ND | ND | 8 | 5 | 8 | 24 | 26 | ND | ND | 31 | 18 | 13 |
| Sierra Leone | ND | ND | ND | ND | 28 | ND | ND | 1 | ND | ND | ND | ND | ND | ND |
| South Sudan | ND | ND | ND | ND | ND | 4 | 5 | 3 | 8 | 38 | 24 | 4 | 360 | 568 |
| Togo | 81 | 67 | 37 | 51 | 52 | 67 | 52 | 95 | 141 | 40 | 317 | 800 | 38 | 96 |
| Uganda | ND | ND | ND | ND | ND | ND | 3 | 24 | 31 | 5 | 72 | 7 | 10 | 117 |
Data are from reference 344. ND, not determined.
The severe morbidity of Buruli ulcer and the high frequency of disabling sequelae contrast with the low mortality associated with the disease. As an example in Ghana, 2 patients of 102 died of sepsis and tetanus within 2 years (41). However, the disabling sequelae of Buruli ulcer have enormous physical and socioeconomic impacts on affected individuals (38).
Therefore, there is still a need for research concerning environmental reservoirs and sources, risk factors, and the contamination cycle in order to invent new protocols to fight Buruli ulcer. With this in mind, we herein review the current state of knowledge on Buruli ulcer in regions of endemicity and the management and environmental reservoirs of M. ulcerans around the world. We review the methods used for investigating M. ulcerans and suggest an intellectual framework for the potential sources or reservoirs of M. ulcerans.
METHODS
We performed a review of the literature through NCBI/PubMed, Google Scholar, published data from the WHO website, and the Web of Knowledge, using the following keywords: “Buruli ulcer,” “Mycobacterium ulcerans” AND “environment” AND “reservoir” AND “laboratory diagnosis” AND “clinic” and the related names of Buruli ulcer in countries of endemicity. We identified data up to March 2017. The titles and abstracts of the available articles were selected for their relevance to Buruli ulcer epidemiology, Buruli ulcer diagnosis, environmental factors (reservoirs, vehicle, source, M. ulcerans host), and the detection and isolation of M. ulcerans from environmental samples. The reference lists of the included papers were reviewed for additional references, including Web pages concerning the subject. We compared the geographical, ecological, and demographic characteristics of six West African countries with high rates of prevalence of Buruli ulcer (numbers of cases superior to 1/100,000 inhabitants) with those of six neighboring countries with low rates of prevalence of Buruli ulcer. Then, we downloaded from the Internet photos of farmers working in paddy fields in West Africa to analyze their degree of protective clothing when farming to correlate clothing with the main locations of Buruli ulcer lesions on the body. A comparison of the body temperatures at different points and the main locations of Buruli ulcer lesions was done.
M. ulcerans, the Agent of Buruli Ulcer
M. ulcerans has been shown to meet the four criteria (Koch's postulates) required to establish that an organism causes a disease: (i) it has been regularly isolated from Buruli ulcer-diseased tissues at various stages of the disease, (ii) it has been isolated in pure culture, (iii) its inoculation in appropriate laboratory animals reproduces the clinical and histopathological features of the disease, and (iv) the pathogen has been reisolated from the new host and shown to be the same as the originally inoculated pathogen. However, it must be noted that the absence of isolation from nondiseased skin has never been clearly reported (42–44).
M. ulcerans may date from the Jurassic Period, as its current repartition fits with the breakup of supercontinents 150 million years ago (45). Genome-based and gene-based phylogenetic reconstructions suggest that an ancestor common to M. ulcerans and its closest neighbor Mycobacterium marinum diverged by 470,000 to 1,200,000 years ago (46). M. ulcerans should therefore be regarded as a member of an M. marinum complex, also comprising Mycobacterium ulcerans subsp. shinshuense, Mycobacterium pseudoshottsii isolated from fish, and “Mycobacterium liflandii,” which has been isolated from Xenopus tropicalis and Xenopus laevis frogs (47). These species all produce the toxin mycolactone and form the so-called mycolactone-producing mycobacteria (MPM) but are not necessarily associated with Buruli ulcer (47). All MPM are thought to have evolved directly from M. marinum (48). In particular, M. ulcerans subsp. shinshuense has been described in China and Japan (49). It possesses a 174-kbp virulence plasmid coding for polyketide synthase, producing mycolactone (49). Within the M. marinum complex, the evolution of M. ulcerans has been marked by a reduction in the chromosome size, from 6.6 Mb in M. marinum to 5.8 Mb in M. ulcerans (50, 51). It is noteworthy that this region of difference between M. marinum and M. ulcerans comprises 28 to 22 PE-PPE genes, whose poorly characterized products have been shown to support the survival of M. marinum inside phagocytes (52). Proliferation of more than 200 copies of insertion sequence 2404 (IS2404) is another mark of genome decay. The genome of M. ulcerans Agy 99 (a strain isolated from a single individual in Ghana) contains two prophages, 18-kb phiMU01, encoding 18 coding DNA sequences (CDS) and 24-kb phiMU02, encoding 17 CDS. The two prophages look like other mycobacteriophages described for other Mycobacterium species with the same overall structure and contain CDS associated with replication functions. However, phiMU02 is probably nonfunctional due to the proliferation of the IS2606 insertion sequence, which has inactivated several genes (14). Accordingly, no phage has been reported to be associated with M. ulcerans in naturally or experimentally infected cells and tissues or in culture. Moreover, 14 mycobacteriophages have been tested for their ability to infect 18 different M. ulcerans strains, including the ATCC 35840 strain (which lacks mycolactone production), a rifampin-resistant strain, and 15 clinical isolates from various geographic origins, along with 2 M. marinum strains (53). A later study indicated that four mycobacteriophages, named Bxz2, D29, L5, and TM4, induced plaque formation of M. ulcerans but not M. marinum. However, plaque formation was not specific to M. ulcerans, as plaque formation was also observed in Mycobacterium tuberculosis and Mycobacterium bovis bacillus Calmette-Guérin (BCG). Furthermore, this study showed that M. ulcerans cell wall mycolactone was not involved in mycobacteriophage penetration into M. ulcerans (53). A second major genomic evolution event was the acquisition of a 174-kb plasmid called pMUM001, which is required for the synthesis of the major virulence factor mycolactone toxin (51). The replication site of this plasmid is more closely related to the one reported in the cryptic plasmid of Mycobacterium fortuitum (51).
M. ulcerans exhibits strong geographic diversity, as first suspected by partial 16S rRNA gene sequencing, which distinguished two subtypes of M. ulcerans linked to the Australian and African continents (11, 54). Further analysis of large sequence polymorphism in 12 regions of difference in 30 M. ulcerans isolates from diverse geographic origins indicated that M. ulcerans was involved in five insertion-deletion haplotypes that separated a so-called “classical lineage,” comprising most pathogenic genotypes from Africa, Australia, and Southeast Asia, and a so-called “ancestral lineage,” genetically closer to M. marinum, comprising isolates from Asia (China/Japan), South America, and Mexico (55).
It is estimated that these two M. ulcerans lineages diverged at the time of the emergence of Homo sapiens (250,000 to 400,000 years ago) (56), while the African isolates may have arisen in the past 18,000 years (46). Restriction fragment length polymorphism (RFLP) followed by IS2404 probe hybridization did not produce any band with M. marinum and yielded six M. ulcerans groups related to six geographic regions, including Africa, Australia, Mexico, Southeast Asia, Asia, and South America (57). All African isolates are genomically extremely closely related in the same cluster, and the classical-lineage M. ulcerans isolates from Australia also are all genomically extremely closely related and located in another cluster (58). A further genomic epidemiological study showed that isolates from West Africa (Côte d'Ivoire, Ghana, Togo, Benin) and Central Africa (Cameroon, Gabon, Congo-Brazzaville, Democratic Republic of the Congo in Bas-Congo, Angola) had identical mycobacterial interspersed repetitive unit-variable number of tandem repeat (MIRU-VNTR) profiles, their genomes differing in a limited number of single nucleotide polymorphisms (SNPs) (59, 60). This strong association between M. ulcerans genotype and the geographic origins of strains was interpreted as indicating that the reservoir of M. ulcerans was relatively fixed in space (50, 58, 60, 61). A further comparative whole-genome sequencing study of isolates from Africa showed that several distinct clonal complexes of M. ulcerans could be found in the same areas where Buruli ulcer is endemic (58, 60, 61). Likewise, two Cameroonian clonal complexes, differing by 828 SNPs, were shared by all members of the respective lineages (60).
These results suggested that some moving reservoir might be responsible for the introduction of M. ulcerans into a new area, where it further spread within human populations (58, 61). Recently, Vandelannoote et al. reconstructed the evolutionary history of M. ulcerans by comparing 165 M. ulcerans clinical isolates recovered between 1964 and 2012 in 11 African regions of endemicity (62). The authors identified two specific M. ulcerans lineages within the African continent: lineage Mu_A1, putatively dating from 68 BC, and lineage Mu_A2, which is more closely related to Papua New Guinea isolates (62). Bayesian analysis indicated that the Mu_A2 lineage was probably introduced in Africa as recently as 1800 AD, supporting the hypothesis of a human-mediated introduction in Africa (62). Genome-based analyses further indicated close relationships between the environment and patients' strains; this is true of M. ulcerans Agy 99 (51, 63). The DNA of this strain was recovered from a small mammal (Mastomys) in Côte d'Ivoire (64). In Ghana, genome types W, X, Y, and Z were found in both human and environmental samples (13, 63). Whole-genome sequencing of an M. ulcerans isolate from a ringtail possum isolated in Point Lonsdale, Australia, revealed extremely close genetic relationships with the genome sequence of a human isolate in the same township, suggesting a major role for mammals in the ecology of this mycobacterium (61, 65).
The genomic diversity of M. ulcerans is further reflected by the structural diversity of mycolactones, first identified in 1999 (66). Indeed, mycolactones are polyketides comprising a core lactone and a fatty acid side chain and belonging to the family of macrolides (67, 68), and six naturally occurring structural variants named A/B, C, D, E, F, and G have been characterized in the different MPM species (48, 69). M. liflandii produces mycolactone E (70, 71), while M. pseudoshottsii and M. marinum produce mycolactone F (72). Mycolactone F-producing mycobacteria do not culture at a temperature above 30°C, which likely limits their virulence for humans (72). Each M. ulcerans isolate produces one type of mycolactone, either A/B, C, or D, and different congeners of mycolactones are produced by the different geographical isolates; mycolactone A/B is produced by the African and Malaysian isolates, the Australian isolates produce toxic mycolactone C, while the Chinese isolates produce mycolactone D (67, 70–72). Indeed, clinical data indicate that M. ulcerans isolates collected in Australia, Asia, Central America, and Mexico are less pathogenic than African isolates (48, 71). Mycolactone synthesis is a complex process related to polyketide synthesis (PKS) (51). In brief, mycolactones are synthetized by polyketide synthases encoded by three large genes located in the 174-kb pMUM001 plasmid, mlsA1 and mlsA2, encoding the mycolactone core-producing PKS, and mlsB, encoding the side chain enzyme (51). After its synthesis, the toxin is secreted in bacterial-membrane-derived vesicles and concentrated in the extracellular matrix, which acts as a reservoir (68, 73). This synthesis is drastically downregulated by the presence of specific carbohydrates, such as glucose, maltose, and maltopentaose (74). Exposure to sunlight also causes its degradation and a loss of its biological activity. On the other hand, mycolactone preserves its structure and cytotoxic effects even after being heated at 100°C for 6 h. Outside the mycobacteria, mycolactones alter the Wiskott-Aldrich syndrome protein target and related scaffolding proteins (75), altering actin dynamics and cell adhesion with cell death (76). Mycolactone inhibits the function of the Sec61 translocation, which is responsible for protein translocation to the endoplasmic reticulum. This affects 30 to 50% of mammalian proteins, including circulating inflammatory mediators and proteins involved in lipid metabolism, coagulation, and tissue remodeling. Buruli ulcer patients have systemic and chronic defects in protein metabolism (77). Research has shown that the hypoalgesic effect observed in Buruli ulcer results from the activation of the angiotensin II type 2 receptor (AT2R), leading to neurite degeneration, cell death, and extensive coagulative necrosis (78). It was also shown that mycolactone decreased thrombomodulin expression on the surfaces of human dermal microvascular endothelial cells and that tissue necrosis might be caused by fibrin-driven ischemia (79). The identification of the Wiskott-Aldrich family proteins as molecular targets of the mycolactones would allow focusing the search for functional inhibitors of the toxins and probably provide the therapeutic tools of tomorrow (75, 76). All the A/B, C, and D mycolactones are toxins responsible for the damage observed in the skin and subcutaneous fat tissue, inducing apoptosis with minimal or no inflammation; unlike in other mycobacterioses, mycolactone does not induce lesions on healthy skin (66, 80).
However, a sole injection of mycolactone through the skin produces ulcers in guinea pigs (81), while a mutant deficient in mycolactone did not cause ulcers (66). Indeed, mycolactones have been shown to elicit a combination of ulcerative, analgesic, and anti-inflammatory effects in human skin by completely blocking the production of lipopolysaccharide (LPS)-dependent proinflammatory mediators posttranscriptionally (82–84). Mycolactone blunts the capacity of immune cells to produce inflammatory mediators by an independent mechanism of protein synthesis blockade (82). It has been demonstrated that mycolactone is sufficient to cause neurological damage (84, 85). Mycolactone can be detected in diseased skin samples from patients with Buruli ulcer by conventional thin-layer chromatography (86). The fact that the immunosuppression stops after removal of infected tissues supports the view that the systemic diffusion of mycolactone is responsible for its immunosuppressive effects (87). Indeed, mice injected by a radiolabeled form of the toxin (88) and clinical studies indicated that mycolactones diffuse from ulcerated lesions in clinically accessible samples. They also diffuse into the peripheral blood of Buruli ulcer patients (89), targeting mononuclear cells in peripheral blood and lymphoid organs, with a particular tropism for the spleen. The capacity of circulating lymphocytes to produce interleukin-2 upon stimulation is then hampered (88). The role of mycolactones during the environmental stages of M. ulcerans is unknown.
The study of M. ulcerans has been sharply limited by a lack of available isolates; none of the five environmental isolates advocated (39, 90, 91) have been deposited in public collections, and only 18 of 320 reported clinical isolates are available in public collections (see Table S1 in the supplemental material). Also, from 342 strains in the repertoire, only four complete M. ulcerans genomes have been reported: in Ghana (M. ulcerans Agy 99), the United States (M. ulcerans strain Harvey), Benin (M. ulcerans S4018), and Japan (M. ulcerans ATCC 33728) (Table S1). The lack of isolates may be due to intrinsic fastidiousness, rendering the isolation of M. ulcerans particularly susceptible to contaminant overgrowth (91, 92). Indeed, the M. ulcerans doubling time of 4.8 ± 0.3 days (93) correlates with the presence of only one chromosomic ribosomal operon, classifying M. ulcerans as a slow-growing mycobacterium (46). Optimal growth is obtained at 28 to 33°C under a 2.5 to 5% oxygen atmosphere and a final pH of 6.6 ± 0.2 at 25°C (94–98). The exposition of M. ulcerans to 41°C for 24 h kills more than 90% of the inoculum (22). This observation may have unanticipated practical implications for the culture of specimens that should not be exposed to high temperatures, such as the ones frequently encountered in tropical regions of endemicity. Moreover, M. ulcerans exhibits sunlight susceptibility, probably due to the lack of light-inducible carotenoids that protect M. marinum (46), linked to a stop codon in crtL, involved in pigment synthesis (14). This characteristic has been suggested to support the in vitro and in vivo susceptibility of M. ulcerans to purified methylene blue; all other tested mycobacteria, including M. marinum, are resistant to this dye (99). M. ulcerans was reported to grow in Middlebrook 7H9 broth, Middlebrook 7H10, and Middlebrook 7H11 agar media with oleic acid-albumin-dextrose-catalase (OADC) enrichment and Löwenstein-Jensen (LJ) medium. The addition of chitin to 7H9 Middlebrook broth was indirectly shown to increase the growth of one strain of M. ulcerans (100). Interestingly, the five available M. ulcerans genomes encoded a GH18 family member, compatible with a putative chitinase activity. Decontamination of environmental specimens is the key step for the isolation of M. ulcerans from environmental sources. F. Portaels and collaborators have tested several decontamination methods, including the Petroff method (101), incorporating sodium hydroxide (NaOH), the reversed Petroff method, and a mild decontamination method using HCl and oxalic acid treatment (102–104). All these methods proved to adversely affect the growth rate of M. ulcerans, but incorporation of egg yolk into the culture media limited the cytotoxic effects of these agents, especially the effect of oxalic acid. A recent study compared the effect of clinical sample decontamination with that of NaOH or oxalic acid, followed by inoculation in LJ medium slants with glycerol or inoculation in the same LJ medium slants supplemented with 2% PANTA (polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin). The decontamination methods did not differ in their effects on the recovery of M. ulcerans, but the use of inoculated media had a significant impact on the recovery of M. ulcerans. Indeed, the use of LJ medium slants with glycerol reduced the probability of M. ulcerans recovery by 65% (39). In the same study, the authors also compared the effects of the transport media on the growth of M. ulcerans and contamination cultures and found no significant difference between 7H9 medium containing PANTA and the antibiotic-free Amies medium Middlebrook (39). Using environmental specimens, the combination oxalic acid-NaOH gave more-effective results than the SDS-NaOH, NaOH-malachite green-cycloheximide, and N-acetyl–cysteine–NaOH combinations. Also, LJ medium supplemented with PANTA and mycobactin J best supported the growth of mycobacteria, including M. ulcerans, compared to isoniazid- or ethambutol-supplemented LJ medium (39, 40).
In contrast with the hundreds of clinical strains that have been isolated, only five isolates from the environment have been isolated (39, 91, 105) (Table S1). Many attempts to isolate M. ulcerans from flora and fauna failed (22, 105). The culture of diverse environmentally collected samples from areas where Buruli ulcer is endemic failed to yield M. ulcerans in the past (22), despite the parallel detection of M. ulcerans DNA sequences (19, 64, 106–113). Failure to culture M. ulcerans from environmental samples may possibly be attributable to inadequate sampling, conditions of transport, inadequate decontamination procedures, and the culture conditions of this fastidious heat-sensitive organism (22, 98). The initial isolation of an M. ulcerans strain was obtained from an aquatic Hemiptera from a Beninese sample collected by Portaels et al., who suggested that the disease resulted from exposure to a contaminated environment (91). This isolate was obtained after a 15-day incubation period in Bactec 12b broth and three successive passages in mouse footpads P1, P2, and P3 for 9 months, 6 months, and 12 months, followed by culture on LJ medium for 2 months (91). In contrast, recently, Aboagye et al. set up an efficient protocol and succeeded in obtaining a pure culture of two poorly characterized M. ulcerans strains in less than 6 months from soil and moss (39).
Cellular and Animal Models for M. ulcerans Infection
The fact that the pathogenesis of M. ulcerans is dependent on the temperature of the area where the bacteria were inoculated is the first notable characteristic of M. ulcerans (114). The second notable characteristic of M. ulcerans is its inability to penetrate intact skin and its inability to infect abraded skin, as demonstrated in an experimental infection of guinea pigs and mice (115). These results suggest that Buruli ulcer is dependent on the passive inoculation of M. ulcerans through intact skin as an alternative to ineffective passive passage through abraded skin, with the precise role of “biological needles,” such as mosquitoes and other insects, remaining to be studied in comparison with the effectiveness of mechanical needles. The third notable characteristic of M. ulcerans pathology is the presence of cell damage in the absence of an acute inflammatory response. Injection of mycolactone in guinea pig skin resulted in extensive tissue destruction and extensive apoptosis as the size of the lesion expanded (81). Knowing that apoptosis is associated with a lack of inflammatory response, these observations reproduced the observations made on Buruli ulcer lesions (81). In fact, these data indicate that Buruli ulcer is not an infectious disease depending on the multiplication of the pathogen but rather a toxemic disease caused mainly by mycolactone. It has been shown that the use of rifampin and streptomycin in the treatment of Buruli ulcer resulted in a rapid onset of local cellular immune responses associated with the phagocytosis of extracellular M. ulcerans. This may be related to declining levels of mycolactone in the tissue, thus leading to an enhanced chemotherapy-induced clearance of the infection (116). Mycolactone A/B causes apoptosis in keratinocyte stem cells (KSC) and transit-amplifying cells (TAC) extracted from human skin biopsy specimens even in small doses of 1 to 10 ng/ml. This apoptosis is dose dependent, as measured by morphological criteria, chromatin condensation, and nuclear fragmentation or as measured by the mitochondrial membrane potential. However, mycolactone A/B was less toxic in human keratinocyte cell lines (HaCaT). Only 25 to 30% of HaCaT cells were affected after treatment with 100 and 1,000 ng/ml of mycolactones A and B, respectively, compared to more than 60% TAC apoptosis at 1 ng/ml and 50% KSC apoptosis at 10 ng/ml. The apoptotic activity of mycolactone A/B was also tested on the human hepatoma cell line HuH7 and on the human epithelial embryonic kidney cell line HEK 293T, since mycolactone has renal and hepatic tropism when it diffuses into the blood (88). No apoptotic cells were detected after treatment with 1 to 1,000 ng/ml of mycolactone (117).
M. ulcerans probably escapes phagocytes during its first steps after intradermal inoculation, behaving as an extracellular pathogen, as observed mainly in cutaneous and subcutaneous lesions (118, 119); this is in opposition to what occurs in XTC2 cells and mice macrophage models, in which an intracellular growth phase for the pathogen has been reported (120, 121). It was shown that M. ulcerans bacilli were captured by phagocytes and were predominately intracellular organisms at 24 h postinfection, whereas examination of tissues of infected BALB/c mice harvested at the ulcerative stage (8 weeks postinfection) showed that M. ulcerans bacilli were exclusively in the extracellular compartment. This was also characterized by an extensive inflammatory infiltrate and the presence of neutrophils and major histocompatibility complex class II (MHC II) cells surrounding the bacterial foci (122). Accordingly, bone marrow-derived RAW264.7 macrophages, the dendritic cell line FSDC, and neutrophils, but not nonphagocytic L929 fibroblasts, were isolated from BALB/c mice phagocytizing M. ulcerans bacilli (123). In the same study, the authors showed that incubating bone marrow-derived macrophages with mycolactone significantly reduced their ability to phagocytize M. ulcerans bacilli. Furthermore, macrophages and dendritic cells infected with M. ulcerans exhibited alterations in their morphology similar to that after cytotoxicity from exogenously added mycolactone at 6 h postinfection (123). Apoptosis was observed as an important tissue destruction mechanism in human lesions associated with viable M. ulcerans cells (124). Nuclear fragmentation indicative of apoptosis was also observed before the death of cells at 24 h postinfection (123). Cells infected with M. ulcerans expressed less tumor necrosis factor alpha (TNF-α) and the transforming growth factor β (TGF-β) cytokine than cells infected with the M. ulcerans mutant, which does not produce mycolactone (123). In contrast, the macrophage inflammatory protein MIP-2, which is chemotactic and activating for neutrophils, was expressed more in cells infected with wild-type M. ulcerans than in cells infected with the M. ulcerans mutant. These data demonstrate an upregulation of inflammatory chemokines and a downregulation of inflammatory cytokines during infection with M. ulcerans (123). In a subsequent study, Torrado et al. reported that M. ulcerans induces the expression of gamma interferon (IFN-γ) at the infection sites of experimentally infected mice (125). Also, IFN-γ-deficient mice are more susceptible to M. ulcerans infection than wild-type mice when they are infected with intermediate or avirulent strains (118). In contrast, no difference in the susceptibilities to infection between IFN-γ-deficient and wild-type mice was noted when they were infected by the highly virulent strain, suggesting that the highly virulent strain of M. ulcerans has an adverse effect on the protective activity of IFN-γ on infected macrophages. Accordingly, by using bone marrow-derived macrophages, activated or not with IFN-γ, the authors showed that IFN-γ can activate macrophages to control the intracellular growth of avirulent and intermediate-virulence strains but not that of the highly virulent strain of M. ulcerans. The clinical observations of a recent study showed pronounced swelling of the infected footpads of IFN-γ-deficient mice; in contrast, nothing unusual was observed in wild-type mice after 5 weeks of infection (118). Histopathological analysis showed that IFN-γ-deficient mice exhibited more tissue necrosis, more edema, and a significantly greater bacterial load as measured by quantitative PCR (qPCR) than wild-type mice. These results suggest that IFN-γ activated the macrophages to eliminate intracellular bacteria at an early stage of infection (5 weeks) (118). Histological observations of adipose tissues from infected patients showed extensive necrosis of subcutaneous fatty tissues, which was directly correlated with mycobacterial invasion and toxin production (81, 126–128). This feature was also reproduced in infected pig skin with M. ulcerans (119). The histopathological analysis showed clusters of extracellular mycobacteria and fat cell ghosts after M. ulcerans infection and mycolactone injection (129). Furthermore, the interaction between M. ulcerans and adipose tissue was investigated using a human adipose cell model (128). After 24 h of incubation, electron microscopic observations showed an extracellular location of M. ulcerans and a cytotoxic effect on cells. Within 3 days, both apoptosis and necrosis were observed. Under the same conditions, cells were incubated with M. ulcerans culture filtrate and purified mycolactone. While M. ulcerans culture filtrate induced both necrosis and apoptosis, mycolactone induced only necrosis.
Studying the interactions with another phagocytic model, amoebae, brought additional data. It has been reported that M. ulcerans persisted inside Acanthamoeba polyphaga cells for 2 weeks, with an inoculum declining by 1 to 2 logs, as measured by culture (130). In a subsequent study of Acanthamoeba castellanii coculture, the authors showed that the number of M. ulcerans cells decreased by 90% over 28 days (29). These data suggest an improbable role of amoebae as sources or reservoirs of M. ulcerans. Temperature can partly explain divergent results obtained in animals, macrophages, and amoeba models. Indeed the optimal growth temperature for macrophages used in the experiments cited above is 37°C, while these experiments have been conducted at 32°C to mimic the optimal growth temperature of M. ulcerans (131). Using a suboptimal temperature can affect the antimicrobial activities of macrophages, such as cytokine production, antimicrobial peptide secretion, and activities and membrane dynamics required for phagolysosome biogenesis (132–134). Under these conditions, the survival and the multiplication of M. ulcerans cells in macrophages are facilitated.
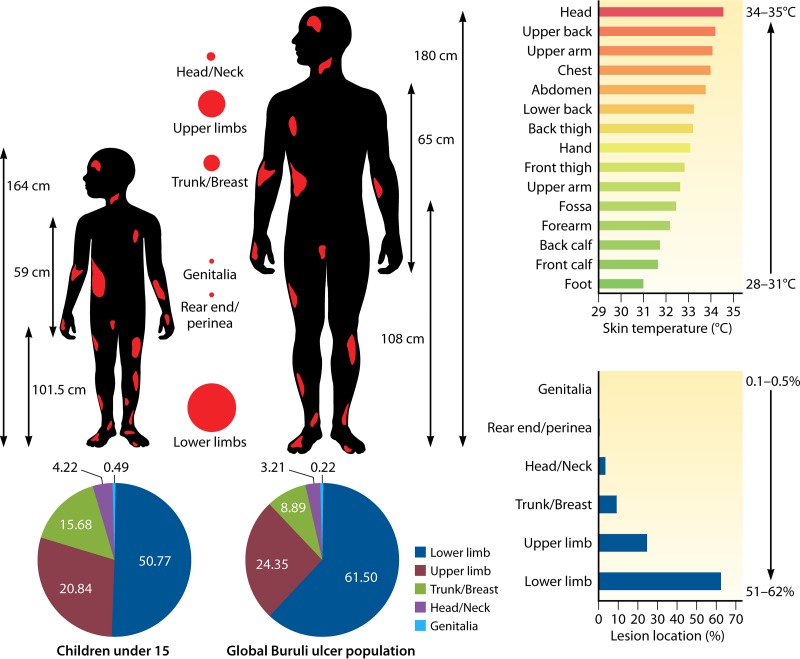
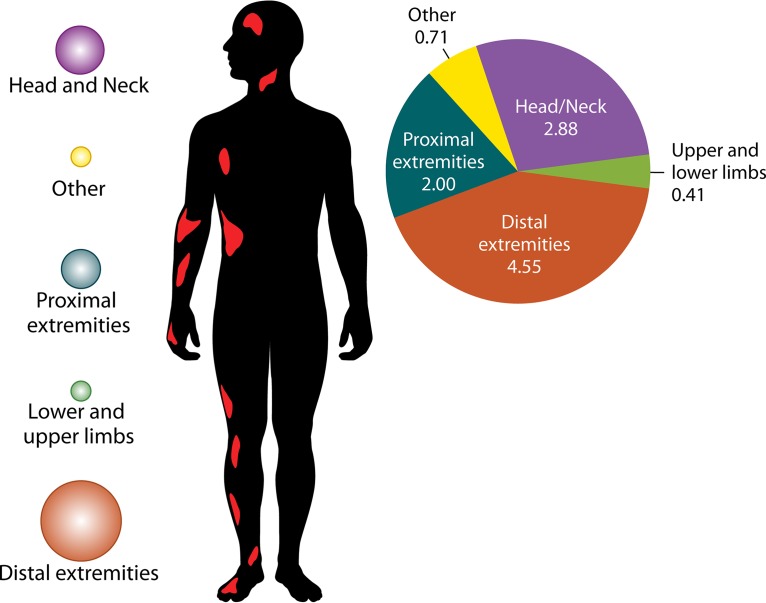
The unique microbiological features of M. ulcerans among the species of the genus Mycobacterium indicate that Buruli ulcer should be understood as a toxic effect of infection, with major features linked to the activities of the plasmid-encoded mycolactone, rather than to the replication of M. ulcerans. Indeed, M. ulcerans replication is strongly controlled by the local temperature, which is not the case with mycolactone (114). In the laboratory, the optimal temperature for replication is 30 to 33°C (20). This situation is indeed encountered in the same skin territories where Buruli ulcer lesions are more prevalent (Fig. 3). Accordingly, M. ulcerans does not disseminate in the bloodstream, and tissue lesions remain localized, despite the remote immunosuppressive neurotropic activities of mycolactone (88, 135). This is in agreement with animal studies, suggesting that the bacilli remain essentially localized within ulcerative lesions in subcutaneous tissues but not in the blood (123).
FIG 3.
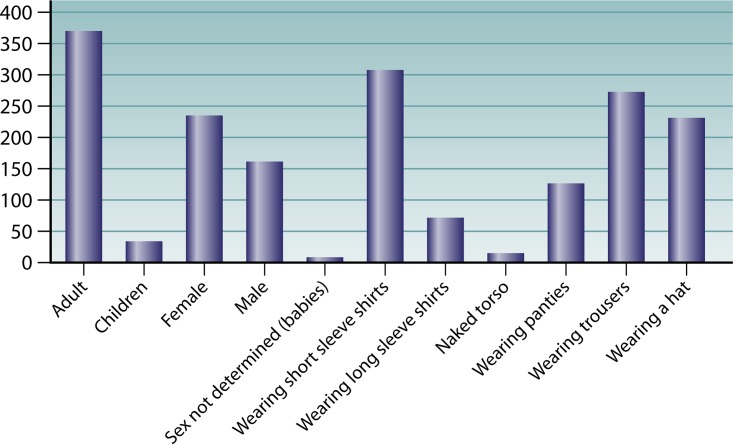
Pattern of distribution of Buruli ulcer lesions on the bodies of human patients in Africa. This figure is a composite of data from 10 independent studies (17, 23, 26, 155, 226, 235, 241, 242, 244, 253). The histograms show that there is an inverse correlation between the gradient of body temperature and the location of lesions.
M. ulcerans in the Environment
The fact that M. ulcerans exhibits a reduction in chromosome size compared to that of M. marinum suggests a reduction in the ecological niches, i.e., specialization (14, 136–138). Accordingly, genomic analysis has suggested that M. ulcerans may reside inside one or several hosts (14), in agreement with previous observations (22). However, it was demonstrated in an experimental study that it can live as a free-living organism in its environmental niches, where it can survive for a long time despite its fragility under certain climatic conditions, such as solar light, temperature elevation, and UV light (14, 29, 37). As discussed above, these aspects have been poorly investigated, as the vast majority of field studies have relied upon molecular biology methods, which gave no clues regarding the viability of the detected mycobacteria. M. ulcerans DNA has been detected in inanimate soil and aquatic environments, but most of the attempts to isolate it from these inanimate environments have failed (105, 139).
Molecular methods used to detect M. ulcerans DNA sequences in environmental specimens are summarized in Table 2. As for molecular targets, the insertion sequence IS2404 used in previous studies (98, 140, 141) was detected in other MPM (142, 143). The conventional IS2404 PCR assay alone cannot be relied upon for the specific detection of M. ulcerans. To increase the specificity of PCR assays, three independent repeated sequences in the M. ulcerans genome, i.e., two multicopy insertion sequences (IS2404, IS2606) and a multicopy sequence encoding the ketoreductase B domain (KR-B), need to be used (39, 113, 144, 145). Moreover, this multiplex PCR can control PCR inhibitors commonly present in environmental samples. Despite these limitations, molecular techniques have provided important clues in revealing the uncertain sources of M. ulcerans.
TABLE 2.
DNA targets for M. ulcerans and detection of related mycolactone-producing mycobacteria from environmental samples
| Mycobacterium | Presence of: |
Plasmid type | ||
|---|---|---|---|---|
| IS2404 sequence | IS2606 sequence | KR-B gene | ||
| Mycobacterium ulcerans | Yes | Yes | Yes | pMUM001 |
| Mycobacterium liflandii | Yes | Yes | Yes | pMUM002 |
| Mycobacterium pseudoshottsii | Yes | Yes | Yes | pMUM003 |
| Mycobacterium marinum | No/yes | Yes | Yes | pMM23 |
Detection of M. ulcerans DNA in Bodies of Water and Moss
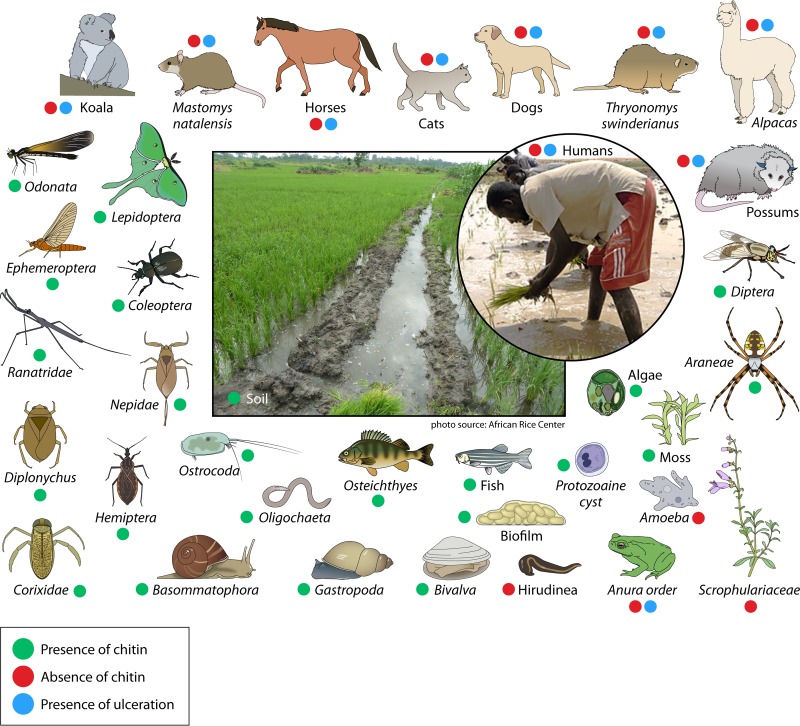
In Ghana, M. ulcerans DNA was detected in biofilms and water filtrate by amplifying the KR-B gene, which was then confirmed by VNTR-PCR (13), and in a body of water in an area of the Ashanti region where Buruli ulcer is endemic (145). Another study in Ghana detected M. ulcerans in biofilm, soil, filters, and detritus (63). Recently, using real-time PCR in samples to amplify the M. ulcerans IS2404 and KR-B genes, M. ulcerans was detected in stagnant water specimens, soil, water filtrate residues, and plants in Côte d'Ivoire, thereby confirming that water is a reservoir of M. ulcerans in areas of endemicity of Côte d'Ivoire (106, 146). Bodies of water act as vehicles for disseminating M. ulcerans strains (Fig. 4). Recently, Aboagye and collaborators detected M. ulcerans DNA in moss from Ghana, and then obtained a positive culture of M. ulcerans from this sample (39). In French Guiana (South America), M. ulcerans DNA was detected for the first time in water (112). In Louisiana (United States), an area where Buruli ulcer is not endemic, M. ulcerans DNA was detected in water and biofilms (147).
FIG 4.
Buruli ulcer risk factors and M. ulcerans reservoirs with chitin sources around paddy fields and swampy areas. The image of the Mali rice farmer is from https://commons.wikimedia.org/wiki/File:Mali_ricefarmers.jpg (from a United States Agency for International Development employee), and the central image is from the African Rice Center [https://commons.wikimedia.org/wiki/File:Sawah_rice_cultivation_in_inland_valleys_in_Ashanti_region,_Ghana_-_panoramio_(3).jpg].
Detection of M. ulcerans in Insects and Aquatic Animals
Aquatic insects have been implicated in the transmission of M. ulcerans and are considered potential vectors (16, 139, 148). Marsollier et al. subsequently carried out an experimental study demonstrating not only that Naucoridae concentrate M. ulcerans in their salivary glands but also that their bite transmits the infection to mice (16). Then M. ulcerans was detected in the salivary glands of water bugs belonging to the Naucoridae and Belostomatidae families and in snails. They are considered potential transient hosts of M. ulcerans, without offering favorable conditions for its growth and replication (16, 40, 141). In Côte d'Ivoire, M. ulcerans was detected by PCR in the Planorbidae family (planorbid and bulinini) (40). In Benin, it was demonstrated by the detection of the mycobacterium in the tissue of aquatic bugs captured during their migration toward water points that aquatic insects outside the aquatic context may be vectors of M. ulcerans (149). M. ulcerans DNA was detected in the tissues of water bugs (genera Micronecta and Diplonychus) (148), in aquatic insects (Belostomatidae, Hydrophilidae, and Naucoridae), and mollusks, supporting the hypothesis that the fauna in major foci where Buruli ulcer is endemic, especially in swampy areas of tropical and subtropical regions, may be a source of M. ulcerans infection (16, 22, 139, 141). M. ulcerans was isolated from an aquatic Hemiptera insect collected in Benin, and it was the first isolation of M. ulcerans after cultivation (91). It was detected in aquatic insects (Belostomatidae, Naucoridae, Corixidae, Ranatridae, and Nepidae) and in the saliva of Diplonychus sp. in Côte d'Ivoire (109) and in Benin (141). In Ghana, M. ulcerans was detected in Belostomatidae, Naucoridae, and Nepidae (150), and IS2404 PCR and VNTR analysis were used to detect M. ulcerans or M. liflandii in wild amphibians (frogs) and fish (Hemichromis bimaculatus) in Ghana (151). In Benin, collected samples of plants (cyperus, panicum, eichhornia) were used for the detection of M. ulcerans. The result was unsuccessful, but M. ulcerans strains were detected in insects (Naucoridae) dwelling in the plant roots (139). A study conducted in Ghana detected M. ulcerans in an invertebrate and vertebrate collection of specimens (13). Aquatic Heteroptera can bite humans and contaminate them with M. ulcerans, as well as contaminate water, which would ensure the dissemination of the germ from one pond to another. They can also infect humans outside aquatic environments because of their ability to fly many kilometers away from their source (61, 152). In Cameroon, M. ulcerans DNA was detected in communities of aquatic macroinvertebrates and vertebrates (153, 154).
In Benin, M. ulcerans was detected in about 8.7% of aquatic insects, but not in mosquitoes (Mansonia africana, Culex nebulosus, Culex quinquefasciatus, Anopheles pharoensis, Aedes vittatus, Culex decens, and Culex fatigans) or in other flying insects (107). Mosquitoes may not play a pivotal role in the ecology and transmission of M. ulcerans in the areas of endemicity studied (107), although a previous study in Ghana indicated the role of mosquitoes as vectors in the transmission of Buruli ulcer (155). In Australia, M. ulcerans DNA was detected in mosquitoes (Aedes camptorhynchus, Coquillettidia linealis, Anopheles annulipes, Culex australicus, Aedes notoscriptus) in several studies (19, 156). In Benin, several pathogenic free-living amoeba were isolated from water and biofilm specimens taken from protected and unprotected sources of water in villages known to have either high or low endemicity for Buruli ulcer, and no specimen was positive (157).
M. ulcerans strains were detected in aquatic plants in emergent zones from both lotic and lentic bodies of water in regions of endemicity of Ghana (158). These observations support the idea that aquatic plants are a reservoir of M. ulcerans and add a new potential link in the chain of transmission of M. ulcerans to humans (105). In Benin, M. ulcerans DNA was detected in stems and leaves of plants (107). Several plants were implicated as a growth factor for M. ulcerans in Côte d'Ivoire. This led to the use of Crinum calamistratum, Eriocephalus africanus, Vicia nana, and Vicia torta for the development of a new culture medium to cultivate M. ulcerans (159). We can conclude from this study that these aquatic plants contribute to the survival of M. ulcerans strains and might even play a central role in biofilm formation (Fig. 4; Table 3).
TABLE 3.
Detection and isolation of M. ulcerans strains from environmental samples around the worlda
| Reference | Country(ies) | Type(s) of samples collected | Reservoir(s) | Method(s) used |
|---|---|---|---|---|
| 207 | Ghana | Fecal specimens of domestic animals | None | qPCR (IS2404, KR-B) |
| 39 | Ghana | Soil, water, fungi, snails, moss, vegetation | Soil, moss | Ziehl-Neelsen, culture, heat shock protein 65, IS2404, IS2606, rpoB, ketoreductase gene |
| 150 | Ghana | Biting water bugs (Hemiptera: Naucoridae, Belostomatidae, Nepidae) | Belostomatidae, Naucoridae, Nepidae | Amplification of the ER domain in mlsA |
| 13 | Ghana | Macroinvertebrate/vertebrate, water filtrate, soil, biofilm | Anura order, Araneae, Coleoptera, Diptera, Ephemeroptera, Gastropoda, Hemiptera, Hirudinea, Lepidoptera, Odonata, Oligochaeta, Osteichthyes, Ostracoda, Basommatophora, Bivalva, Diptera, soil, water filtrate, biofilm, fish | ER PCR and IS2404 PCR, VNTR-PCR, DNA sequencing |
| 145 | Ghana | Environmental samples (water, detritus, trunk biofilm, plant biofilm) | Water | RT-PCR (IS2404, IS2606, KR-B) |
| 63 | Ghana | Soil, water filtrands, detritus, biofilm, small mammals | Biofilm, soil, filter, detritus, small mammal (Mastomys), mouse | IS2404, ER analysis, 16S rRNA and VNTR analysis, sequencing |
| 29 | Ghana | FLA from collected aerosols, biofilm plant, biofilm trunk, detritus, water | IS2404 detected in FLA from biofilm plant, biofilm trunk, water, detritus, aerosols | RT-PCR (IS2404, IS2606, KR-B) |
| 151 | Ghana | Water, fish, amphibians | Amphibian, fish | ER analysis, VNTR |
| 145 | Ghana | Environmental samples, organs of small mammals | Water | Real-time PCR |
| 428 | Ghana | Fish | Fish | Nested IS2404 PCR |
| 139 | Ghana, Benin | Plants from swamps areas, insects of plants roots | Insects (cyperus, panicum, eichhornia, Naucoridae) | Culture, nested IS2404 PCR |
| 141 | Benin | Belostomatidae (Appasus sp.), Dytiscidae, Hydrophilidae, Naucoridae (Naucoris sp., Macrocoris sp.), molluscs (Bulinus senegalensis), fish | Belostomatidae, Hydrophilidae, Naucoridae, molluscs, fish | Nested IS2404 PCR |
| 91 | Benin | Aquatic specimens | Hemiptera (Gerris sp.) | Culture positivity on LJ medium, nested IS2404 PCR |
| 149 | Benin | Aquatic insects | Diplonychus sp. | PCR (IS2404, KR-B) |
| 144 | Benin | Water filtrand, macrophytes, soil, excrement, biofilm, aquatic invertebrate taxa, fish, tadpoles | Water filtrand, well filtrand, pond/river filtrand, cistern filtrand, biofilm | PCR (IS2404, ER) |
| 107 | Benin | Mosquitoes (adults and larvae), vertebrates, aquatic insects and plants | Aquatic insects (Odonatan, Hemiptera, Coleoptera, Diptera), vertebrates (Anura, fish), plants | qPCR (IS2404, KR-B) |
| 16 | Côte d'Ivoire | Water bugs | Naucoridae | Nested IS2404 PCR |
| 105 | Côte d'Ivoire | Aquatic plants (Scrophulariaceae) | Scrophulariaceae | IS2404 qPCR, culture |
| 40 | Côte d'Ivoire | Snails (Planorbis sp., Bulinus sp.) | Planorbid, bulin | PCR |
| 148 | Côte d'Ivoire | |||
| 109 | Côte d'Ivoire | Aquatic Heteroptera | Diplonychus sp. (Belostomatidae), Naucoris sp. (Naucoridae), Micronecta sp. (Corixidae), Ranatra fusca (Ranatridae), Laccotrephes ater (Nepidae), Anisops sp. (Notonectidae) | qPCR (IS2404, KR-B) |
| 64 | Côte d'Ivoire | Small mammals | Mastomys natalensis | ER analysis, 16S rRNA, IS2404 PCR, sequencing |
| 106 | Côte d'Ivoire | Soil, stagnant water, plants, animal feces | Stagnant water, feces of Thryonomys swinderianus (agouti), soil | qPCR (IS2404, KR-B) |
| 146 | Côte d'Ivoire | Plant biofilms, water filtrate residues, plant detritus, soils | Plant biofilms, water filtrate residues, plant detritus, soils | ER analysis, 16S rRNA, IS2404-PCR, MIRU-VNTR |
| 108 | Japan | Environmental samples from a water channel in the patient's residence | Crayfish | Whole-genome amplification, touchdown PCR, DNA sequencing |
| 208 | Japan | Turtles | Turtles | PCR, nucleotide sequence analysis |
| 19 | Australia | Mosquitoes | Aedes camptorhynchus, Coquillettidia linealis, Anopheles annulipes, Culex australicus, Aedes notoscriptus | Real-time PCR (IS2404, IS2606, KR) |
| 156 | Australia | Mosquitoes | Anopheles sp. | Real-time PCR (IS2404, IS2606, KR) |
| 199 | Australia | Cats | Cats | Histological examination, Ziehl-Neelsen staining, PCR |
| 113 | Australia | Soil, sediment, mosquitos | Soil, sediment, mosquitos | PCR (IS2404, IS2606, KR) |
| 200 | Australia | Horses | Horses | Ziehl-Neelsen, IS2404 PCR |
| 201 | Australia | Dogs | Dogs | Real-time IS2404 PCR |
| 202 | Australia | Alpacas | Alpacas (Vicugna pacos) | IS2404 PCR |
| 204 | Australia | Koala | Koalas (Phascolarctos cinereus) | |
| 111 | Australia | Possums | Ringtail possums (Pseudocheirus peregrinus), brushtail possum (Trichosurus vulpecula), mountain brushtail possum (Trichosurus cunninghami) | IS2404 PCR |
| 112 | French Guiana (South America) | Water, filtered water | Water | qPCR (IS2404, KR-B) |
| 147 | United States (Louisiana) | Water, biofilms | Water, biofilms | IS2404 PCR |
| 153 | Cameroon | Aquatic communities (vertebrates and small invertebrates) | Vertebrates (Fish, Anura), Insecta (Odonata, Ephemeroptera, Hemiptera, Coleoptera, Diptera, Plecoptera, Lepidoptera), Mollusca, Crustacea (Decapoda, Cladocera), Annelida, Arachnida (Acari, Araneae) | qPCR (IS2404, KR-B) |
| 154 | Cameroon | Diptera, Hemiptera, Coleoptera, Odonata, Ephemeroptera | Diptera, Hemiptera, Coleoptera, Odonata, Ephemeroptera | qPCR (IS2404, KR-B) |
ER, enoyl reductase; FLA, free-living amoeba.
M. ulcerans in Environmental Biofilms
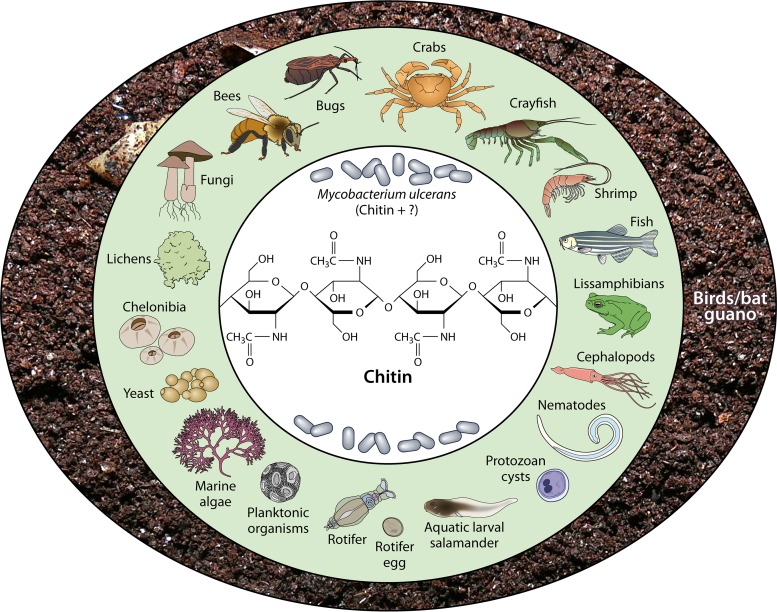
Biofilms are sessile microbial communities growing on surfaces, frequently embedded in a matrix of extracellular polymeric substances (160–162). The nature of the M. ulcerans biofilm is not fully elucidated. Chitin may be one component and an important nutrient source for M. ulcerans. Chitin is the (1→4)-β-linked homopolymer of N-acetyl-d-glucosamine (163). It is one of the most important carbohydrates of the fungal cell wall in the carapace of mud crabs (Scylla olivacea), the structural backbone of the exoskeletons of crustaceans (shrimp, crayfish, crabs), shells of Chelonibia patula, yeasts and lichens, marine algae (barnacle, Crustacea), rotifer eggshells (Brachionus plicatilis), adult females and egg shells of microfilariae (Onchocerca gibsoni, Onchocerca volvulus), Ascaris lumbricoides eggshells, the cuticle of microfilariae of Wuchereria bancrofti, the radulae of certain mollusks, insects, fish (zebrafish), lissamphibians, internal shells of cephalopods, some bird guano (penguin guano), and cysts of various protozoans (Fig. 5) (164–176). Although more-complex plants have no chitin, they do secrete chitin-degrading enzymes (chitinase), which is a common plant hydrolase that defends against pathogenic-fungus attacks (177). Chitin synthases (CHS) are widespread among eukaryotes and known to have a complex evolutionary history in some of the groups (178). The functional importance of each CHS in the growth and development of M. ulcerans should be investigated, because each CHS probably plays particular roles during the different developmental stages of bacteria in the environment (Fig. 4 and 5).
FIG 5.
Sources of chitin in the environment, West Africa. The middle circle includes primary sources, and the outer circle includes secondary sources.
Roles of Salts and Other Nutrients in the Maintenance of Environmental M. ulcerans
Salinity is one of the key environmental factors that limit crop growth and agricultural productivity. Hypersalinity is caused by an excessive concentration of soluble salts in the soil. The main ionic salt species are composed of sodium, calcium, and magnesium, appearing as chlorides and sulfates. Sodium chloride (NaCl) is the predominant salt. Salinity conditions occur in coastal, arid, and semiarid areas. In assuming that the salinity of the water and soil is a factor of M. ulcerans viability in the environment, we summarized soil and water salinity in Côte d'Ivoire as an example for West African countries, especially since M. ulcerans DNA was detected from a soil sample collected near rice paddy fields in Côte d'Ivoire (106, 146). The average salinity of lagoons in Côte d'Ivoire ranges between 4 and 19 mg/liter, whereas the salinity of rivers at their outlet in the south varied between 0 g/liter and 30 g/liter (179–185). The viability of M. ulcerans in salty areas has not been established, but in our laboratory, an experimental study proved that M. ulcerans strains could grow at a salinity above 20 g/liter (186). Soil salinity can be caused by the type of agriculture practiced in a given region. Therefore, it has been observed that intensive cultivation of rice for a short or long period is the basis of soil salinization and that the pH is below 8.5 in rice fields (187). It was shown that M. ulcerans followed seasonal dynamics and was present mainly in waters with a higher pH (188). In the United States, there were positive associations between pH levels and the concentrations of ammonia, dissolved oxygen, nitrate, nitrite, and sulfide in freshwater rivers where M. ulcerans DNA was detected in water and biofilms (147). Salinity is a major problem in tropical coastal regions having predominantly rice-based farming systems because of the intrusion of brackish water during the dry season through tidal movements and capillary rise from shallow saline groundwater. Salinization of rice paddies can cause a decrease in productivity if adequate irrigation methods are not used (187). Salinity continues to be high at the onset of the wet season, during and after rice transplantation, until sufficient rain washes it from the soil (189). Soil salinity also increases in proportion to sea proximity (Table 4). Recently, we proved that M. ulcerans strains could survive in soil for 4 months, suggesting that Buruli ulcer might be acquired through inoculation with watery soil as a transient source of infection (37). The increase in the incidence of Buruli ulcer in West Africa, especially in coastal areas, might be related to the construction of canals to irrigate rice fields. M. ulcerans is common in humid rural tropical areas where agriculture is the main activity of the population (190). In Ghana, a spatial relationship was demonstrated between the prevalence of Buruli ulcer and its proximity to drainage channels, farmlands, and the immunosuppressant arsenic found in soil (191). In Ghana, M. ulcerans was detected in soil by searching for the KR-B gene only (13) and recently by the use of several PCR systems detecting heat shock protein 65, IS2404, IS2606, rpoB, and the ketoreductase gene (39). Plants, aquatic invertebrates, amphibians, and specific water conditions might allow M. ulcerans to grow and persist in the environment (16, 37, 40, 40, 121, 146, 158, 192, 193). Rice fields include all the risk factors for transmission of Buruli ulcer. The environment of rice fields is always wet and muddy. Farmers with their families, including children less than 15 years old, work for several hours with limbs in permanent contact with muddy water and without adequate protection. Consequently, rice fields are the ideal breeding ground and source of M. ulcerans, with more potential reservoirs in the tropics (Fig. 4 and 6). Arsenic occurs naturally in the earth's crust, is widely distributed in the environment, and exists at an average concentration of approximately 5 mg/kg of soil (194, 195). There are many possible routes of human exposure to arsenic from both natural and anthropogenic sources (195). Natural mineralization and activities of microorganisms enhance arsenic mobilization in the environment, and human intervention has exacerbated arsenic contamination (194). A study conducted in Ghana to statistically quantify landscape characteristics and their relationship with the disease showed that arsenic levels in soil and gold mining areas were significant covariates and related to an increased risk of prevalence in the Amansie West District of Ghana (191). In the Amansie West District, which was one of the worst Buruli ulcer-affected districts, there are arsenic-enriched surface environments resulting from the oxidation of arsenic-bearing minerals occurring naturally in mineral deposits (191, 195). Proximity analyses, carried out to determine spatial relationships between Buruli ulcer in affected areas and arsenic-enriched farmlands and arsenic-enriched drainage channels in the Amansie West District, showed that the mean Buruli ulcer prevalence in settlements along arsenic-enriched drainage areas and within arsenic-enriched farmlands is greater than elsewhere (191, 195). Furthermore, the role of arsenic in the prevalence of Buruli ulcer has been questioned, and the results of a Ghanaian study suggest that arsenic in the environment may play a contributory role in M. ulcerans infection (191). The Amansie West District, which is drained by the Ofin River, had high caseloads of Buruli ulcer in 1998 (24), but in recent years, hardly any cases have been observed in the area (196).
TABLE 4.
River and lagoon salinity in Côte d'Ivoire, West Africa
| Site | Geographical position(s) | Avg salinity | pH | Reference |
|---|---|---|---|---|
| Lagune de Fresco | 2°50', 5°25W | 15.69 mg/liter | 7.52 | 179 |
| Lagune Aby | 2°51'–3°21E, 5°05'–5°22'N | 0.283–1.28 ppt | 6.96–7.8 | 180 |
| Baie des Milliardaires/Lagune Ebrié | 4°00'–4°10'W, 5°10'–5°20'N; 3°40'–4°50'W, 5°2'–5°10'N | 0.4–6.9‰ | 7.1–7.7 | 181 |
| Estuary zone/Grand-Lahou | 4°26'–5°20'N, 4°20'–5°20'W | 182 | ||
| Bac Sicor | 12.87‰ | 7.75 | ||
| Groguida | 18.95‰ | 7.74 | ||
| Kpanda | 18.95‰ | 7.74 | ||
| Braffedon | 18.95‰ | 7.74 | ||
| Fleuve Sassandra | Basse Côte d'Ivoire | 0–4% | 6.8–7.4 | 183 |
| Fleuve Bandama | Grand-Lahou (coast of Côte d'Ivoire) | 0–32‰ | 184 |
FIG 6.
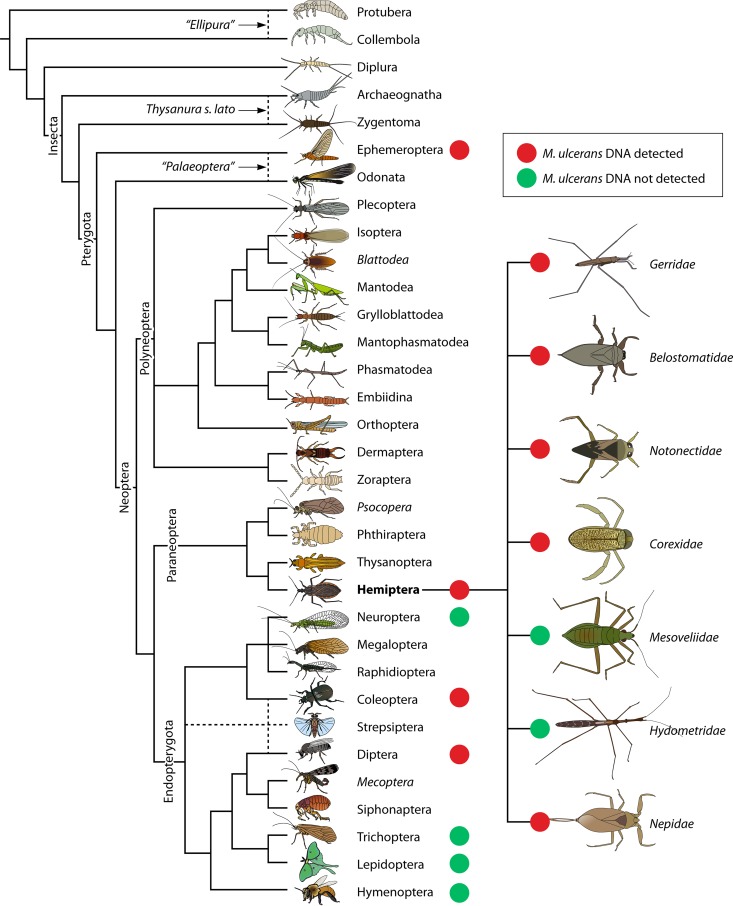
Cladogram of postulated relationships of extant hexapods, based on combined morphological and nucleotide sequence data showing M. ulcerans findings in insects (adapted from the work of Gullan and Cranston [427] with permission of the publisher [copyright Wiley-Blackwell]).
Buruli Ulcer in Animals
Buruli ulcer is by no means specific for humans, and studying M. ulcerans infection in animals may provide clues to the sources and transmission of the pathogen. In Ghana, small mammals within communities of endemicity may be susceptible to M. ulcerans infection and act as reservoirs; M. ulcerans Agy 99 was detected in lesions on Mastomys mouse tails caught in houses (63). In Côte d'Ivoire since the 1990s, the fish called tilapia (cichlid fish) has been suspected of being a reservoir of M. ulcerans (197), and M. ulcerans DNA was detected in fish collected in Benin (141) and in Ghana (13, 151). In Ghana, M. ulcerans DNA was also detected in amphibians (151). Recently, M. ulcerans DNA has been detected in the carcasses of small mammals, in Mastomys natalensis, in the mouse genus Mastomys, and in the stools of the small mammal Thryonomys swinderianus in Côte d'Ivoire and Ghana (63, 64, 106), suggesting that these animals may shelter and transport M. ulcerans. Later results corroborated an experimental study showing that T. swinderianus was susceptible to M. ulcerans infection (198) (Fig. 4). Small mammals living in close proximity to humans and commonly hunted animals, such as rabbits and rats, may therefore be potential sources of M. ulcerans (63). In Australia, M. ulcerans was detected in a cat (first known case in a cat) (199), horses (200), dogs (201), alpacas (Vicugna pacos) (202), possum species (111), koalas (Phascolarctos cinereus) (203–205), and frogs (206). These observations contrast with investigations conducted in Ghana, where M. ulcerans DNA was not detected in the feces of domestic animals in rural areas, showing that domestic animals are unlikely to be major reservoirs of M. ulcerans (207). In Japan, M. ulcerans DNA sequences were detected in turtles (Lissemys punctata punctata) and crayfish (108, 208, 209).
Buruli ulcer has also been encountered in aquatic invertebrates, mosquitoes (13, 16, 19, 91, 107, 113, 139, 141, 149, 150, 152–154, 156, 193), crayfish (108), amoeba, mollusks, crustaceans, annelida (29, 40, 141, 153), aerosols, water, biofilm, moss, detritus, feces, plants, and soil (13, 29, 39, 63, 93, 106, 107, 112, 113, 144–147, 210). The hypothesis most advanced to aggregate data issuing from the investigations on the environment is that M. ulcerans may be part of a food chain (211, 212).
Buruli Ulcer in Patients: Clinical Aspects
The usual clinical appearance of Buruli ulcer is a deep, rapidly developing chronic ulcer associated with necrosis of subcutaneous fat (34), often causing functional limitations which occur in as many as 25 to 50% of cases (22, 213). Prevention of disabilities and physiotherapy is now accepted as an integral part of therapy (214). The impact of the shift to pharmacological therapy on the occurrence of functional limitations has been studied by Barogui et al. (215). Most often, the diagnosis is made in the presence of a deep, rapidly developing chronic ulcer associated with necrosis of subcutaneous fat (34). Buruli ulcer evolves in three clinical stages, with a mean incubation period of 2 to 3 months but ranging between 3 weeks to almost a year. It includes (i) preulcerative lesions presenting as a nodule, papule, plaque, or edema; (ii) ulcerative lesions enlarging and contaminating underlying tissues, characterized by granulomatous healing and further fibrosis (216); and (iii) scars. A study conducted in the Democratic Republic of the Congo (former Zaire) showed that lesions appeared in body areas having undergone trauma, such as an accidental needlestick-like injury (scorpion stings). Nevertheless, 80% of cases detected early can be cured by an 8-week course of rifampin plus streptomycin, sometimes followed by a skin graft (15, 217, 218). Intact skin completely prevents Buruli ulcer, as M. ulcerans is unable to penetrate through intact skin by itself from an external route (115, 219). As for the mechanism of inoculation, two main hypotheses have been suggested. The first is that bacteria are injected into the skin through the bite of an insect or ectoparasite vector, and the second is that bacteria enter previous and open wounds from direct contact with the contaminated environment, aerosols from water surfaces, and water-dwelling fauna (22, 115, 220). An alternative hypothesis is that M. ulcerans is inhaled or ingested (220, 221) and reactivated in low-temperature areas of the body at the sites of trauma, but this hypothesis has not been challenged by any model or direct clinical observation (222, 223). An intriguing feature of Buruli ulcer is that 10.34% of patients have several localizations, the most parsimonious explanation being that M. ulcerans is inoculated several times (98, 224) (Fig. 7), perhaps by auto-inoculation from an index lesion. However, human-to-human transmission of M. ulcerans is extremely rare (22), with only one reported case after a human bite (225).
FIG 7.
Percentages of Buruli ulcer occurring in multiple locations on 1,702 patients.
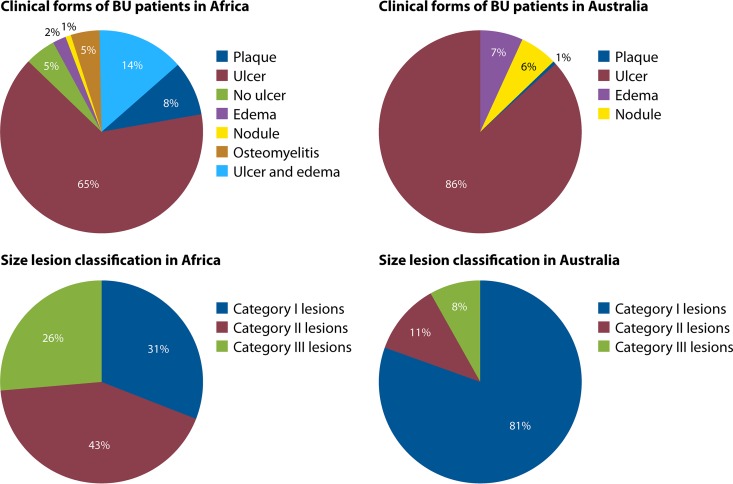
After the transcutaneous inoculation of M. ulcerans, the clinical presentation includes a papule, nodule, plaque, or edematous form, which eventually leads to extensive skin ulceration within 4 weeks with the classical, undermined borders (98, 226–231). The severe forms include osteomyelitis, reactive osteitis, and bone deformities (232, 233). One rare case of disseminated osteomyelitis has been reported following snake bite in an apparently nonimmunocompromised patient (234). Buruli ulcer is responsible for physical suffering, often leading to considerable disability if treatment has not been initiated quickly (18). The lesions are categorized according to the World Health Organization (WHO) classification as category I, which consist of lesions <5 cm at their widest diameter; category II, which consist of lesions between 5 and 15 cm at their widest diameter; and category III, which consist of lesions >15 cm at their widest diameter, lesions at critical sites, and multiple lesions (216). A comparison of Buruli ulcer clinical forms between African and Australian Buruli ulcer patients according to the WHO classification for lesion size is summarized in Fig. 8. In Africa, Buruli ulcer presents mainly as a disease of the skin and subcutaneous tissues, with rare extension to deeper tissues, including bone (97, 235, 236), and few extensions to muscle and bone, which are much more local (97, 235–237). In human immunodeficiency virus (HIV)-coinfected patients, even though systemic perturbations in the serum metabolome were reported (238) and severe Buruli ulcers were observed in some studies (239, 240), there was no disseminated infection.
FIG 8.
Comparison of the percentages of Buruli ulcer clinical forms and WHO-classified sizes of lesions between African and Australian Buruli ulcer patients.
Lesion Topography
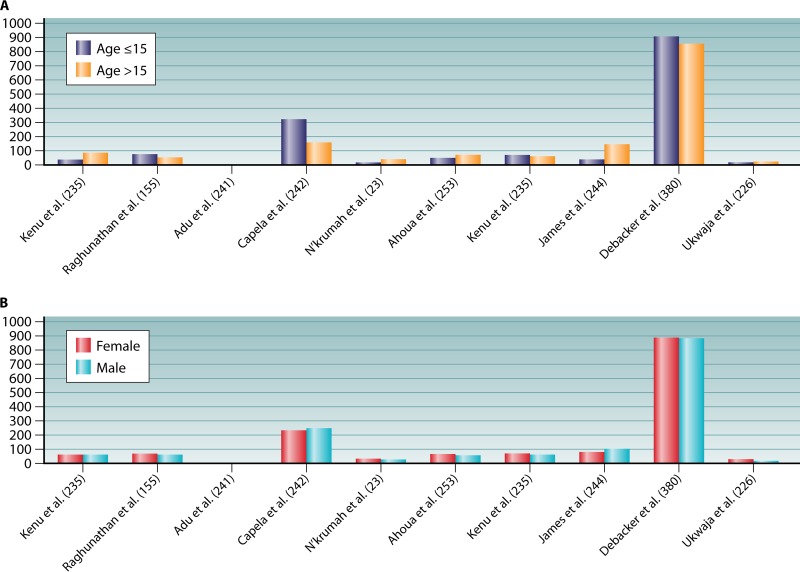
Lesion topography is not uniform on the body, and the pattern may not be random, as similar patterns have been reported in several countries, including Côte d'Ivoire, Ghana, Benin, Togo, and Nigeria. Neither the sex nor the age of the patient significantly alters the pattern of lesions. Approximately 80% of the lesions are located on the limbs, most commonly on the lower extremities, regardless of the age and sex of patients (Fig. 3) (20, 98). In Ghana, lesions were on the legs in 49% of patients and on the arms in 36% of patients, regardless of gender. Lesions on the distal extremities were observed in 61% of the patients, compared with lesions on the proximal extremities in 28% of patients (155). Males were significantly more likely than females to develop trunk lesions, but there was no gender difference for the extremities (155). A further study in Ghana found lesions distributed on the lower limbs (67.9%), upper limbs (21.4%), trunk/breast (0.9%), head/neck (6.2%), and both lower and upper limbs (3.6%) (235). Another study in Ghana found lesions distributed on the head/neck (6.8%), upper limbs (20.3%), trunk (1.7%), and lower limbs (71.2%) (241). In Benin, Capela et al. found that the different locations of Buruli ulcer lesions were the head/neck (1.3%), thorax/abdomen (9.0%), upper limbs (35.9%), and lower limbs (53.8%) (242). In Côte d'Ivoire, lesions were most frequently located on the lower limbs (76.5%) and upper limbs (17.5%) (23). In Togo, the main locations of lesions were upper limbs (39.5%), lower limbs (39.8%), and trunk/head (21.7%) (243). The first description of a large cohort in Nigeria found lesions on the lower limbs (56.7%), upper limbs (28.3%), other locations (5.5%), and disseminated locations (9.45%) (26). In Togo, a hospital study involving 180 patients found lesions on the upper limbs, trunk, head, and neck (244). Consequently, in the different countries where Buruli ulcer is endemic, the observations that lesions are predominantly distributed on the lower limbs (60%), upper limbs (30%), and other body parts (10%) (245, 246) are highly concordant (Fig. 3). These observations, which first indicated that clothed body parts are almost free of lesions, suggest that Buruli ulcer occurs on unclothed body parts and that clothes are sufficient to protect the skin against contamination by M. ulcerans or an injury inoculating M. ulcerans. By including factors which may moderate the pattern of lesions, we analyzed clothes and shoes worn in West Africa. The analysis of the clothing style used as protection by farmers and children in rice paddies from collected Web photos indicated that, while the majority of farmers wear pants for their farming activities, there remains the fact that the protection is inadequate, because they have to roll up their pants in the mud (Fig. 9). Thus, the extension of lesions is significantly correlated with an unclothed, unprotected skin surface. This observation suggests that Buruli ulcer may not result from the contamination of previous gross wounds by M. ulcerans but rather from passive or active transcutaneous inoculation by a plant, soil, water, insect, or small animal unable to penetrate clothing. In Australia, both using insect repellent and wearing long trousers were found to reduce the odds of contracting Buruli ulcer (18). Wearing clothing such as pants in areas with M. ulcerans in the environment seems to prevent the disease, and this explains the fact that, despite the presence of M. ulcerans in Louisiana wetlands, no cases of Buruli ulcer have been reported in health facilities (147). The most plausible mode of transmission is skin trauma at sites contaminated by M. ulcerans strains (31). Then, on unclothed body parts, we observed that the pattern of Buruli ulcer lesions was inversely correlated with the pattern of skin temperature. By comparing the gradient temperature of the body and the location of Buruli ulcer lesions, we found that there was an inverse correlation between the gradient of body temperature and the location of lesions (Fig. 3). Body temperature is maintained by thermoregulation, which depends on heat balance (247). Even if the core temperature of a healthy adult human is 36.8 ± 0.4°C in the normal physiological situation, it should be noted that the body temperature is not uniform and depends on the topography of the body portion (247, 248). The temperature of the skin over the entire body is not 37°C, as in the core, but varies between 28°C and 34.5°C, depending on the location (Fig. 3) (247). Skin temperature is compatible with the growth of M. ulcerans in the population living in tropical regions of West Africa, Central Africa, and East Africa (95, 249, 250). M. ulcerans can survive but does not grow at 37°C (97). The temperature sensitivity of M. ulcerans has long been recognized. It is sensitive to temperatures above 37°C (25, 251). These clinical observations correlate with observations of mice experimentally infected with M. ulcerans (114). In that study, Buruli ulcer lesions were observed regardless of the route of inoculation of M. ulcerans, demonstrating that the tail temperature was between 24.8 and 25.6°C and 11 to 12°C lower than the general body temperature (114).
FIG 9.
Clothing protection among farmers working in rice fields in West Africa. Analysis of 90 screenshots from the Internet of a total of 403 farmers.
As for the limited deep extension, we observed a significant inverse correlation between the prevalence of Buruli ulcer by skin region and the skin regional temperature (Fig. 3). This may be due to the facts that M. ulcerans itself lacks the protective pigments encoded by its close relative M. marinum and that the key virulence factor, mycolactone, is highly sensitive to solar radiation (68, 252). Reviewing the data indicates that both bare skin and skin temperature under 35°C significantly correlate with the pattern of distribution of Buruli ulcer skin lesions. Among 1,742 cases of Buruli ulcer from eight studies conducted in areas of endemicity of West Africa, multisite lesions were found. There were 49 (2.9%) disseminated lesions in the head and neck, 7 (0.41%) lesions located on the lower and upper limbs, 74 (4.34%) lesions disseminated to the distal extremities, 34 (1.99%) lesions disseminated to the proximal extremities, and 12 (0.7%) other disseminated lesions (23, 26, 155, 235, 241, 242, 244, 253). Because Buruli ulcer is not a systemic disease, the likely explanations for disseminated lesions may be multiple bites from contaminated insects, multiple contacts of wounds with sources or reservoirs of M. ulcerans, or body parts being scratched with hands that had been in contact with environmental M. ulcerans strains (99).
HIV Coinfection
Currently, the association between HIV infection and Buruli ulcer is not fully understood (254). In Africa, Buruli ulcer and HIV coinfection management is still a challenge for Buruli ulcer treatment. HIV positivity among Buruli ulcer patients was 8% in Ghana (254) and 2.6% in Benin (240), and in Cameroon the prevalence was approximately 4% in children, 17.0% in males, and 36.0% in females (255), which was higher than in the control population attending health facilities. HIV infection may affect the clinical presentation and severity of Buruli ulcer (254–256). A low CD4 cell count was significantly associated with a larger size of the main lesion (255). Studies have addressed the role of HIV as a risk factor for Buruli ulcer (155, 240, 255). Severe paradoxical reactions, including immune reconstitution inflammatory syndrome, can occur during the treatment of M. ulcerans-HIV-coinfected patients (86, 256, 257). As a consequence, the appropriate time to start antiretroviral therapy to minimize paradoxical reactions in relation to Buruli ulcer treatment with streptomycin and rifampin needs to be investigated (254). Mansonella perstans coinfection also needs to be considered in the diagnosis and treatment of Buruli ulcer. Nearly 23% of patients with Buruli ulcer in Ghana were coinfected with M. perstans, and this rate was higher than in the control population, in which 13% of patients were infected with M. perstans (258). Rarely, M. ulcerans and Leishmania braziliensis coinfection can be observed, and its corollary can be diagnostic confusion if the staff is not well trained and knowledgeable in the management of such diseases (259). At present, no specific underlying condition has been reported to support the development of Buruli ulcer. While hemoglobinopathies (hemoglobin sickle cell disease [HbSS]/sickle cell-hemoglobin C [SC]) were seven times more frequent in patients with Buruli ulcer osteomyelitis than in controls, these hemoglobinopathies were not associated with an increased prevalence of Buruli ulcer (260).
Differential Diagnosis of Buruli Ulcer
Buruli ulcer lesions can be confused with other cutaneous lesions, which is problematic, especially in tropical settings with limited access to laboratory facilities (261). Demographic and clinical criteria, including the age of the patient, the geographical area of residence, the location of lesions, and the presence of pain, help in the differential diagnosis. In Australia and other countries, the initial papular lesions are sometimes confused with insect bites (261). The differential diagnosis includes filariasis, leprosy, yaws, deep fungal infections (such as blastomycosis or coccidioidomycosis), mycetoma, ulcerative squamous cell carcinoma, abscesses, onchocerciasis, elephantiasis, scrofuloderma, mycosis, actinomycosis, herpes, cutaneous leishmaniasis, tropical phagedenic ulcer, venous ulcer, and noma (258, 261, 262).
Laboratory Diagnosis of Buruli Ulcer
In the past, Buruli ulcer was suspected on clinical evidence, but now the diagnosis can be confirmed by direct smear examination for acid-fast bacilli after Ziehl-Neelsen staining, and the test relies upon PCR targeting the genomic region IS2404, a test now widely available in regions of endemicity (26, 34, 95, 98, 233, 242, 261, 263). Microbiological diagnosis helps to reduce inappropriate administration of antibiotics also active against M. tuberculosis. Additional techniques, including culture of viable bacilli and histological staining, are used rarely. The current management of patients follows WHO recommendations and has been implemented for many years in countries of West and Central Africa (e.g., Côte d'Ivoire, Ghana, Togo, Benin, Cameroon, and the Democratic Republic of the Congo, as well as Nigeria recently and others) (95, 264–266). The quality of sample collection and the quality of the laboratory diagnosis of Buruli ulcer disease with microscopy, PCR, and histopathology have to be ensured by participation in external quality assurance systems (95). As for microscopy, it is possible to implement quality assurance for Ziehl-Neelsen staining, but it is difficult for auramine staining. The development of point-of-care (POC) tests is considered a research priority in order to make diagnosis more accessible to patients (267).
Useful Clinical Samples
Fine-needle aspiration and swab samples are usually used for the laboratory diagnosis of Buruli ulcer (265). The WHO recommends that a maximum of two swabs or two fine-needle aspirations be taken for each lesion, depending on the experience of the person performing the technique (265). There is no specific recommendation for the transport of specimens for PCR-based diagnosis. However, with regard to isolation and culture, which are no longer routinely practiced, temperatures should never exceed 32°C during specimen transportation (22). Tissue samples that had been placed for up to 21 days in a transport medium, namely, Middlebrook 7H9 broth supplemented with polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (Becton Dickinson, Sparks, MD), oleic acid, albumin, dextrose, catalase (Difco Laboratories, Detroit, MI), and 0.5% agar (also named semisolid transport medium) were still culture positive (97, 98). The application of harsh decontamination methods on specimens that contain few or rare organisms can be detrimental to the successful culture of M. ulcerans (22).
Microscopy
Optical microscopy is the diagnostic method most used in resource-limited settings. Several methods of staining are used; two are Ziehl-Neelsen staining and auramine staining (268). The Ziehl-Neelsen method is time-consuming and less sensitive than auramine staining, which improves sensitivity and turnaround time for the detection of acid-fast bacilli (269). The microscopic examination of skin exudate from an ulcer clinically suspected of being a Buruli ulcer is not the best tool for laboratory diagnosis, due to poor technical sensitivity (40 to 60%) (270). Nevertheless, it remains a good first means of investigation in an area of endemoepidemicity (271). Confirmation of clinically suspected cases of Buruli ulcer by microscopic examination occurs in 29% to 78% of cases (97, 102, 264, 272). Direct smear examination is easy to perform at a local level but has low sensitivity, below 60% (265). Nevertheless, it is the only test usually available in areas of endemicity (273). In general, the overall sensitivity of PCR is significantly higher than that of microscopic examination and culture (264).
Molecular Detection
PCR is considered the most sensitive method for the laboratory confirmation of Buruli ulcer. However, PCR remains expensive and involves reagents unsuitable for use in tropical countries with poor storage conditions, hindering the development of reliable qPCR diagnostic assays (274). It is highly sensitive and specific and is also reasonably rapid, but it requires trained personnel with specific equipment (274). Nevertheless, PCR is routinely performed in hospitals in countries such as Côte d'Ivoire, Ghana, Benin, Nigeria, Cameroon, and Togo, with the strengthening of laboratory capacity supported by national and international programs and nongovernmental organizations. IS2404 PCR has been used as reference method to confirm the presence of M. ulcerans in tissues (102, 272), and a dry-reagent-based PCR formulation has been proposed (261, 273). This procedure is based on the standard diagnostic IS2404 PCR developed by Stinear et al. (142) and has shown an excellent diagnostic sensitivity, >95% (261, 265, 274). The WHO recommends IS2404 qPCR amplification for the confirmation of Buruli ulcer diagnosis, because this technique is both the most rapid and the most sensitive (95). The dry-mix qPCR approach can be adapted for other sets of primers and probes, such as the ketoreductase-B (KR) domain of the M. ulcerans mycolactone polyketide synthase genes (95). Dry-reagent-based PCR was shown to be a reliable tool for the diagnosis of Buruli ulcer disease, and it is well adapted to tropical conditions (261, 273). The agreement rate between dry-reagent-based PCR and standard PCR was 91.7% for swab specimens and 95% for tissue specimens (273).
The loop-mediated isothermal amplification (LAMP) technique has also proven to be useful for the early diagnosis of Buruli ulcer (275, 276). Recently, LAMP was developed as a simple, robust, cost-effective technology and has been selected as a promising POC test candidate (267). The IS2404 detection-based LAMP assay employs lyophilized reagents (dry-reagent based, which provides significant advantages for application under tropical climate conditions) (267). The requirement of cold chains for transport and storage of reagents is avoided with the development of a dry-reagent-based LAMP assay employing lyophilized reagents (267). The sensitivities of IS2404 PCR, the conventional LAMP assay (83.22%), and IS2404 dry-reagent-based PCR (86.79%) were found to be comparable (267). LAMP was inferior in a study by Ablordey et al., but it can be used as a POC diagnostic test for Buruli ulcer (277).
Culture
Routine diagnosis of Buruli ulcer does not rely on culture, which offers the possibility of strain characterization and antibiotic susceptibility testing. M. ulcerans grows better at <35°C, which may explain the finding that bacilli do not disseminate in the blood of experimentally inoculated animals (123). Culture on LJ medium at 32°C is the most discriminatory method but is not very sensitive and takes more than 8 weeks, rendering it of little use to clinicians (274). The primary cultures of clinical specimens from swabs are usually positive within 9 to 12 weeks of incubation at 29 to 33°C, but a much longer incubation period of up to 9 months may be necessary for some isolates (98). Culture detects between 34% and 79% of positive cases but is not useful for immediate patient management (102, 265, 272), though culture is appropriate for the monitoring of antimycobacterial treatment (98, 264) as well as for performance of molecular epidemiology analyses, which are almost impossible to carry out directly from clinical specimens (94).
Histopathological Analysis
Histopathological examination is sensitive but expensive and requires a sophisticated laboratory, well-trained personnel, and invasive procedures (biopsy) (274). Histopathological analysis confirms >90% of clinically diagnosed cases and >70% of clinically suspected cases (102, 272). Its sensitivity is about 90% but requires a sophisticated laboratory and the use of invasive procedures (265), and histopathology is not available in most countries of endemicity for treatment decisions (270).
Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry
Initially, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) was used as a rapid and highly sensitive technique for the analysis of mycolic acids and hydrolysis products of mycolactone A/B by M. ulcerans (91, 278). It has recently been demonstrated that M. ulcerans and M. marinum can be separately identified directly from colonies by MALDI-TOF MS (279). Colonies of M. marinum are always identified as M. marinum by MALDI-TOF MS. However, colonies of M. ulcerans are identified as M. ulcerans but often as M. pseudoshottsii or M. shottsii (279).
Other Methods
Mycolactones A and B, which are specific to M. ulcerans and are present around infection sites, are therefore promising targets for the development of such a test (88). Because these toxins are specific to M. ulcerans, they represent a promising marker for developing a new diagnostic test (68). New methods based on detecting mycolactone have been proposed to replace the current WHO gold standard PCR method, which is expensive and not available in most areas of endemicity. Samples taken from the necrotic portion of ulcerative lesions provide mycolactone for analysis (265). One of these methods consists in the detection of mycolactone after its extraction from clinical samples by fluorescent thin-layer chromatography. The sensitivity of this technique is higher than that of microscopy or culture but lower than that of histology and PCR (270). More recently, a new molecular method based on detecting mycolactone by using RNA aptamers, which are an emerging novel class of detection molecules, has been proposed. However, this was a preliminary proof-of-concept report, and more tests must be done to approve this new method in the diagnosis of Buruli ulcer (280).
BURULI ULCER TREATMENT
Medical Treatment
Ciprofloxacin, sparfloxacin, ofloxacin, and amikacin are effective in vitro against M. ulcerans at a MIC between 0.5 mg/liter and 2 mg/liter (281). The MICs of rifampin, streptomycin, amikacin, moxifloxacin, R207910 (bedaquiline), linezolid, and PA-824 (pretomanid) were 2, 0.25, 1, 0.06, 0.06, 2, and 16 mg/liter, respectively, against the reference strain of M. ulcerans ATCC 19423. They were, respectively, 2, 0.5, 1, 0.25, 0.12, 1, and >16 mg/liter against isolate CU001 (282, 283). Rifamycins such as rifampin, with a MIC of 2 mg/liter, have exhibited the broadest range of activity against clinical and reference strains of M. ulcerans (281).
The MICs of clarithromycin ranged from 0.125 to 2 mg/liter at pH 6.6 and from <0.125 to 0.5 mg/liter at pH 7.4 (284). M. ulcerans was inhibited by dapsone (4-4′-diaminodiphenyl sulfone), with MICs varying between 0.3 and 0.1 mg/liter (285, 286). The bactericidal activity of rifampin combined with those of moxifloxacin or clarithromycin and of moxifloxacin with clarithromycin equaled that of rifampin combined with streptomycin, and such combinations are validated as orally administered treatments of Buruli ulcer (287). Accordingly, an animal study showed that oral daily administration of rifapentine plus clarithromycin was at least as effective as injected streptomycin plus oral rifampin (288). In vitro activity testing against clinical isolates of M. ulcerans showed MIC values ranging from 2 to 8 g/liter for milbemycin oxime and from 2 to 4 g/liter for selamectin (289) In the same experiment, ivermectin and moxidectin showed no significant activity, with a MIC of >32 g/liter (289). On the other hand, moxidectin was shown to inhibit the growth of M. ulcerans JKD8049 at 4 g/liter, and M. ulcerans strains were susceptible to ivermectin at 8 g/liter for M. ulcerans JKD8049 and at 4 g/liter for M. ulcerans 1117-13 (290). Further in vivo susceptibility tests with mice showed the superiority of the benzoxazinorifamycin KRM-1648 over rifampin (291). Likewise, the effectiveness of purified methylene blue against the initial stage of Buruli ulcer in mice was recently proven (99). Ciprofloxacin, sparfloxacin, ofloxacin, amikacin, and rifampin were shown to be effective in vitro against primary clinical and reference isolates of M. ulcerans in Ghana (281).
Using mouse models, rifampin, streptomycin, amikacin, moxifloxacin, R207910, and linezolid showed various bactericidal activities, while PA-824 failed to reduce the number of CFU in the footpads of infected mice (282). In this model, a few rifampin-resistant M. ulcerans mutants were isolated after the results of rifampin monotherapy, leading to the recommendation that rifampin should never be used as monotherapy in humans (90). In addition, Beissner et al. reported a rifampin-resistant clinical isolate from Ghana after monotherapy (292). These data indicate that rifampin should not be used as monotherapy. Accordingly, an 8-week treatment with rifampin-streptomycin sterilized an M. ulcerans infection in mice (287). Combined rifampin-amikacin, rifampin-clarithromycin-sparfloxacin, or rifampin-amikacin cured M. ulcerans-infected mice and prevented relapse up to 26 weeks after completion of treatment (293). The association of rifampin with moxifloxacin, R207910, or linezolid showed bactericidal effects equal to those of rifampin-streptomycin and rifampin-amikacin (282). Recently, a mouse model indicated that an oral intermittent 8-week regimen of rifapentine combined with clarithromycin was highly bactericidal and had better sterilizing activity than the conventional rifampin-streptomycin regimen (294). These in vitro and animal model data supported the proposal to shift from the once-standard streptomycin-based therapy to oral combinations. In Australia, fully oral combinations of rifampin with either clarithromycin or fluoroquinolones were shown to be effective and well tolerated (295). Moreover, a shorter 29-day therapy was shown to achieve an overall 95% success rate (296). All together, these data recently led the WHO to modify its recommendations for the treatment of Buruli ulcer in favor of oral combinations. Accordingly, the provisional guidelines of the WHO were changed and now state that streptomycin-based therapy is no longer the standard of care. Clofazimine has similar MICs against M. tuberculosis and M. ulcerans of 0.25 to 0.5 g/liter (297). Clofazimine alone blocks the multiplication of M. ulcerans in mouse footpads. In combination with rifampin, it eliminates the presence of M. ulcerans after 6 weeks of treatment, but its effectiveness is lower than that of the combination of rifampin-streptomycin and rifampin-clarithromycin in mouse footpads after 4 weeks of treatment (297). In this experiment, no relapses were observed in mice treated with rifampin-streptomycin and one relapse (5%) was observed in a mouse treated with rifampin-clofazimine, while relapses were observed in 50% of cases with the rifampin-clarithromycin combination (297) (Table 5).
TABLE 5.
Antibiotic and biocide susceptibility of M. ulceransa
| Drug (generic name) | Range | MIC50 | MIC90 |
|---|---|---|---|
| Rifampin | 0.12 to 4.0 | 0.5 | 2.0 |
| Streptomycin | 0.12 to 1.0 | 0.25 | 0.5 |
| Amikacin | 0.25 to 2.0 | 0.5 | 2.0 |
| Moxifloxacin | 0.015 to 0.5 | 0.12 | 0.5 |
| R207910 (bedaquiline) | 0.015 to 0.12 | 0.03 | 0.06 |
| Linezolid | 0.25 to 4.0 | 0.5 | 2.0 |
| PA-824 (pretomanid) | 4.0 to 16 | 16 | >16 |
| Saprofloxacin | 0.1 to 2 | ND | 0.5 |
| Ofloxacin | 0.1 to 2 | ND | 2 |
| Ciprofloxacin | 0.1 to 2 | ND | 1 |
| Clarithromycin | 0.125 to 4 | ND | 0.125 to 2 |
| Clofazimine | 0.06 to 2 | ND | 0.25 to 0.5 |
| Ivermectin | 0.125 to 64 | ND | >64 |
| Milbemycin oxime | 0.125 to 64 | ND | 1 to 8 |
| Moxidectin | 0.125 to 64 | ND | 16 to >64 |
| Selamectin | 0.125 to 64 | ND | 1 to 4 |
| Abamectin | 0.125 to 64 | ND | >64 |
| Doramectin | 0.125 to 64 | ND | >64 |
| Emamectin | 0.125 to 64 | ND | 16 to 32 |
| Eprinomectin | 0.125 to 64 | ND | >64 |
MICs are given in grams per liter. ND, not determined.
Early detection and management is very important in reducing morbidity and the disease's disfiguring nature. A key factor contributing to the steady increase of Buruli ulcer in resource-limited settings is improper practice of personal hygiene. Until the introduction of antibiotic therapy, the use of surgery to remove all infected tissue, with a wide safety margin to ensure the complete removal of infected tissues, was regarded as the most effective treatment (218, 298). Recurrence rates after surgical treatment without antibiotics vary from 16% to 28% (299). In addition, the cost of surgical treatment is far beyond the means of those most severely affected (299). Prevention of functional limitations and physiotherapy are now accepted as an integral part of therapy (214). The impact of the shift to pharmacological therapy on the occurrence of functional limitations has been studied by Barogui et al. (215). In this study, no differences in resulting functional limitations were observed between patients treated with surgery, antibiotics, or both. Since 2004, Buruli ulcer has been treated with 8 weeks of intramuscular injections of streptomycin (15 mg/kg) and oral rifampin (10 mg/kg) according to the previous WHO protocol of treatment with antibiotics, plus surgical excision and skin grafting (218, 299, 300). Without antibiotics, recurrence has been reported to be higher: as high as 48% (301). Since the introduction of antibiotic treatment, recurrence rates have receded remarkably (0 to 2%), and the requirement for surgical intervention has diminished (299). The combination of rifampin and streptomycin was effective for most patients with Buruli ulcer and proved to be a highly successful and practical treatment for all forms of M. ulcerans disease (217, 218). Streptomycin administration can cause both ototoxicity and nephrotoxicity (302). It was observed that cured patients were more likely to become reinfected rather than relapse (303). Compliance with the recommended 8-week treatment (218) is difficult to maintain, particularly in rural settings where health facilities are rare. The daily injection with streptomycin is problematic, as most patients live in remote areas with limited access to health care facilities. Proper hygiene with these injections is also a concern. Moreover, the antibiotic treatment may be accompanied by a clinical deterioration, known as a paradoxical reaction, which may be the result of restoration of local and systemic immune responses (304). For these reasons, an oral regimen avoiding intramuscular injections has been developed (294). In Japan, a combination of oral medication composed of rifampin, levofloxacin, and clarithromycin was successful in treating Buruli ulcer and showed better results than other chemotherapies. This treatment increases the probability of patient adherence and needs to be evaluated in a multisite study. It may also be the best way to decentralize patient care in rural areas with fewer resources (305). Combination oral therapy alone has been tested in Australia, and the results demonstrated that Buruli ulcer can be treated effectively using oral antibiotics alone, with an acceptable toxicity profile (295, 296). In Benin, an 8-week oral combination of clarithromycin and rifampin in Buruli ulcer patients was well tolerated, resulting in no treatment failures (306). Recent developments toward a fully oral therapy not including a quinolone but rather a combination of rifampin and clarithromycin were presented at the WHO Buruli ulcer meeting in March 2017. The provisional guideline was changed accordingly, and as of now, fully oral treatment has become standard therapy; streptomycin has been abandoned (306–308). The oral regimen with rifampin and clarithromycin is already recommended by the WHO and regularly administered in West African countries (e.g., Benin, Togo, and Ghana), though its effectiveness has not yet been proven by the ongoing randomized trial in West Africa.
Warming the affected skin at 38 to 39°C may improve the outcome of extensive or relapsing lesions, but observations are anecdotal (309). The theoretical frame for such practice includes the optimal growth of M. ulcerans at 32°C and better cellular microbicidy at 39°C (309). Accordingly, in the search for innovative treatments, the efficacy of phase change material (PCM) thermotherapy as local thermotherapy was proven in a phase 2 clinical trial in Cameroon to be a highly effective, simple, inexpensive, and safe treatment for M. ulcerans disease. PMC involves applying temperature from 39°C to 42°C to the skin surface. It has potential as a home-based remedy for lesions suspected of being Buruli ulcers at the community level, where laboratory confirmation is not available (251, 298, 310). Phototherapy and UV therapy are sometimes used to treat human skin diseases, such as psoriasis or eczema, but rarely in infectious disease and may be a therapeutic solution for the treatment of Buruli ulcer (68).
It has been established that the standard first-line treatment for tropical ulcers is a combination of penicillin and metronidazole (311). Antibiotics such as beta-lactams (penicillin, ampicillin, cefuroxime, cefixime, flucloxacillin), macrolides (erythromycin, clarithromycin), aminoglycosides (amikacin, gentamicin), quinolones (ciprofloxacin), cyclines (tetracycline), phenicol (chloramphenicol), and sulfamethoxazole-trimethoprim (co-trimoxazole) have been used for the treatment of Buruli ulcer secondary infections, which are often thought to be responsible for the severe complications in Buruli ulcer (283, 284). The role of Staphylococcus aureus has recently been investigated (312) with the alternative hypothesis that paradoxical inflammation is causing severe complications.
Traditional Medicine
Traditional treatments remain the first option for poor populations in Africa, who may have restricted access to synthetic products due to their cost and accessibility (313, 314). However, the use of traditional treatment as first-line therapy, lay perception, and self-medication contribute to longer delays in diagnosis and treatment (315, 316). Such treatment is considered devastating, expensive, and ineffective in some cases (317). According to a socio-anthropological study conducted in Benin, the main steps in traditional treatment were diagnosis, removal of necrotic tissue, wound care, and exorcism (314). In the history of the development of new therapeutic molecules, plants have always occupied a preponderant place as sources for new pharmacological molecules (318). In Africa, much effort is spent in the pharmacological study of medicinal plants used in traditional medicine for the treatment of Buruli ulcer. Ricinus communis, Cyperus cyperoides, Nicotiana tabacum, Mangifera indica, Solanum rugosum, Carica papaya, and Moringa oleifera have demonstrated clinical efficacy (319). Another study in West Africa showed that active extracts from 10 plant species (Alstonia boonei, Annona reticulata, Annona senegalensis, Bridelia ferruginea, Carica papaya, Eucalyptus globulus, Polyalthia suaveolens, Sorindeia juglandifolia, Spathodea campanulata, and Zanthoxylum zanthoxyloides) and one extract from Cleistopholis showed activity against M. ulcerans (320). These plants were from different families, namely, Annonaceae, Apocynaceae, Bignoniaceae, Caricaceae, Compositae, Euphorbiaceae, Myrtaceae, Phyllanthaceae, and Rutaceae (320). These medications are used as decoctions, infusions, powders, pomade, and macerations and taken orally or applied to wounds (319). Further studies are required to isolate and characterize the active ingredients in the extracts of these plants. In a study conducted in Benin, it was proven that the extract from aerial parts of Holarrhena floribunda had significant antimycobacterial activity against M. ulcerans (318). Natural products represent potential alternatives to standard therapies for use as curative medications for M. ulcerans disease (319). Plants with medicinal potential should be scrutinized for biologically active compounds by the bioassay-guided fractionation approach to provide new insights for finding novel therapeutics for Buruli ulcer control (319). Given that traditional healers represent a parallel point of entry into the health system to support people suffering from Buruli ulcer with products that have often proven their effectiveness, there is a need for health authorities to better supervise this area. However, the involvement of plants and the possible role of local herbal therapies are not evidence based; it is rather opinion based and speculative and requires special attention by authorities and scientists.
Medical Prevention
There is no proven effective primary prevention of M. ulcerans infection. Nevertheless, our partial knowledge of the sources and transmission of environmental M. ulcerans does suggest some measures of prevention, the efficacy of which remains to be measured, that are, to date, the most effective methods to reduce disease transmission. Indeed, mandatory early detection through active case finding, early laboratory-confirmed diagnosis, and early initiation of treatment to prevent long-term sequelae do not prevent additional cases of noncontagious Buruli ulcer (321, 322).
Due to the significant reduction in the quality of life of patients presenting with extensive tissue scarring, a Buruli ulcer vaccine would be greatly beneficial to the worldwide community (323). Despite the efforts for the development of vaccines against Buruli ulcer disease, there is still no effective preventive vaccine for Buruli ulcer (324–329). Antibodies to surface antigens of M. ulcerans do not seem to have a protective effect (330). BCG vaccination status provides relatively short-term immune protection from M. ulcerans infection and prevents osteomyelitis (31, 331). Preliminary data suggest that BCG effectively serves as a vehicle to M. ulcerans antigens, warranting further studies to improve efficacy (323). Since prevention is not possible in the absence of either an effective vaccine or a clear understanding of the mode of transmission, a major control strategy for Buruli ulcer consists in early detection and treatment, depending on effective laboratory confirmation of suspected cases (270). Currently, preventive measures include clothing in the course of pastoral work, the quick disinfection of wounds after an injury with running water and soap (30), and a swimming prohibition in the presence of an open wound, as well as the use of insecticides and impregnated mosquito nets in homes.
Buruli Ulcer Prevalence in the Population
Before 2010, the prevalence of Buruli ulcer was increasing in West Africa and Central Africa (34, 35, 332–335). The resurgence of Buruli ulcer in the world has led the scientific community to a better understanding of the disease, including its reservoirs and modes of transmission, as well as risk factors. The most affected countries are in West Africa, with Côte d'Ivoire being among the most affected countries in the world (19, 336, 429) (Fig. 10; Table 1). In 2014, 1,736 of 2,151 (80.7%) cases of Buruli ulcer reported to the WHO by African countries were from West Africa (Table 1). At the beginning of 2014, 12 of the 15 countries regularly reporting data to the WHO reported nearly 2,200 new cases, which represents a decrease of about 50% compared to the number in 2009, when 5,000 cases were reported. Except in a couple of countries (Japan, Australia), the number of cases has declined since 2010 in most areas of endemicity. The exact cause of this decline is unknown, but it may be a positive side effect of the fight against coreservoirs and covectors of other targeted infections in the tropics (232).
FIG 10.
Countries reporting Buruli ulcer to the WHO and prevalence of Buruli ulcer, 2002 to 2015.
Epidemiology of Buruli Ulcer
More than half of the new cases of Buruli ulcer reported annually around the world are from West Africa (Fig. 2). Among 15 West African countries, countries along the Gulf of Guinea, including Benin (17, 337), Côte d'Ivoire (197, 334), Nigeria (338), Ghana (24, 339, 340), Sierra Leone (341), Togo (342), and Guinea (343), are reporting new cases to the WHO (Fig. 10; Table 1). Eight West African countries declared 83.6% (range, 80.89% to 86.30%) of the total number of cases over the past 10 years (Fig. 2) (34, 35, 332–335, 344), with Côte d'Ivoire being among the most affected countries in the world (19, 336) (Fig. 10; Table 1). Côte d'Ivoire, Ghana, Benin, Togo, Guinea, and Nigeria (343) (Fig. 1 and 10; Table 1) have regularly reported new cases to the WHO during the last 2 decades, and these countries have the highest prevalence of the disease (20 to 158 cases per 100,000) (Fig. 10). Mali is a new potential African country where the disease is endemic, with a recent report of cases (345). Notably, Buruli ulcer has never been reported in Niger, Cabo Verde, Sao Tome, Principe, Chad, and Guinea-Bissau.
In Côte d'Ivoire and Ghana, Buruli ulcer is the second leading cause of mycobacterial infection after tuberculosis (23, 24). Affected populations live in rural areas, and children less than 15 years of age account for about 70% of cases (25, 63). The first probable case of Buruli ulcer in Ghana was reported in the Greater Accra Region in 1971, and more than 2,000 cases were reported between 1991 and 1997 (24). In Côte d'Ivoire, the first detection of Buruli ulcer occurred in 1981, but the number of cases clearly increased in 1987 and then became a national public health problem (334). In Nigeria, Buruli ulcer cases were first reported from Benue in 1967 (346).
In central Africa, Buruli ulcer foci have been reported in Gabon, Cameroon, Congo, the Democratic Republic of the Congo, South Sudan, Angola, the Central African Republic, and Equatorial Guinea (347–350) but never in Sao Tome, Principe, or Chad. In Cameroon, the first case of Buruli ulcer was reported in 1969 (351), in 1950 in the Democratic Republic of the Congo (352), and in 1998 in Angola (353).
In East Africa during the 1960s, many cases of Buruli ulcer were reported in Uganda, especially in Buruli County, which eventually provided the name for this disease (2). Cases of Buruli ulcer were reported in other countries of East Africa, such as Kenya (354) and Sudan (131). In South Africa, the first cases of Buruli ulcer were reported in 2001 in Malawi (355).
The true-incidence data of the disease in each of the West, Central, East, and South African countries are difficult to collect because not all patients attend health facilities (because of the lack of information about the disease, because of the lack of financial means, or because of the social stigma associated with chronic wounds), surveillance measures are poor, and there is a lack of case confirmation in health facilities (13, 356). All the African countries where Buruli ulcer is endemic do not necessarily have a systematically organized health system for monitoring and reporting Buruli ulcer cases. Programs are often put in place, but they do not work efficiently in some countries.
In Oceania, Bairnsdale ulcer (Buruli ulcer) was first reported in 1935 as a series of unusual painless ulcers in a patient from Southeast Australia (3). Since 1991, its incidence has progressively increased in Australia (18). However, elderly patients comprise a significant proportion of Buruli ulcer patients in Australian populations (18, 230, 231, 357). In continental Asia, the first reported case of M. ulcerans infection in China was described in 2000 (358). In eastern Asia, the first reported culture-documented case occurred in Japan in 1980 (359). An M. ulcerans isolate was recovered from a 19-year-old girl (360). These isolates were distinguished (on the basis of mycolic acid patterns) from previous M. ulcerans isolates and were reported to form a subcluster named M. ulcerans subsp. shinshuense (M. ulcerans ATCC 33728) (360). M. ulcerans subsp. shinshuense was confirmed to be the etiologic agent of Buruli ulcer in Japan (361). The first cases of Buruli ulcer in Malaysia in Southeast Asia were described in 1958 (362) and in 1983 in Kiribati, which is located in the Central Pacific region (363). Subsequently, Buruli ulcer was considered an emerging disease in Papua New Guinea (364).
In the Americas, Buruli ulcer has been diagnosed in South America, in Mexico since 1953 (352), and in French Guiana (112, 365) and Peru (366) since 1969. The first Brazilian case was reported in 2007 (367). French Guiana was qualified as the only area in the Americas where Buruli ulcer is endemic, with an average incidence of 2.09/100,000 (368). From 1969 until 2007, only 11 cases of Buruli ulcer were reported in Peru, but no countrywide survey has been conducted to evaluate its true prevalence there (366). Indeed, in Peru, Buruli ulcer is probably both infrequent and underreported and may often be misdiagnosed as leishmaniasis, which is more prevalent and better known (366).
Geography of Buruli Ulcer
Buruli ulcer is not a ubiquitous infection but is rather located in some large geographic areas scattered in 1 of the 33 countries which report cases to the WHO in Australia, Asia, Africa, and the Americas (Fig. 1). In each country where Buruli ulcer is endemic, there is a distinct geographical distribution, depending on environmental factors. In all these countries, Buruli ulcer occurs in specific discrete foci, suggesting a space-confined distribution pattern (369). To create an overview of the common characteristics of countries where Buruli ulcer is endemic, we observed that most of these countries are located within a belt limited by latitudes 10°N and 10°S (Fig. 10) and in moderate, nontropical climate areas, including Australia and Japan (233). In Australia, where the disease and the agent were first described, the incidence of Buruli ulcer has progressively risen since 1991 (18). Although Buruli ulcer is usually regarded as a disease of tropical and subtropical climates, an increasing number of cases have been recorded in temperate southeastern Australia (18, 370). Areas of endemicity include mainly coastal Victoria, particularly the Mornington Peninsula and Bellarine Peninsula, northern Queensland near Mossman, the Capricorn Coast of Queensland near Yeppoon, and the tropical northern coast near Darwin (370). Buruli ulcer has moved as far in as Melbourne's southeast suburbs, including Bentleigh, Hampton, and Cheltenham (27), but no case has been linked to Tasmania, South Australia, or southern Western Australia. There have been cases in southern New South Wales near the border with Victoria (27). In Australia, environmental factors associated with Buruli ulcer prevalence included a low elevation with forested land cover (371). Likewise, in Côte d'Ivoire (372) and Benin (32), areas of endemicity are characterized by a high density of forest cover and low density of urban cover.
In continental Asia, the reported case of M. ulcerans infection in China had occurred at the highest latitudes in the Northern Hemisphere and was caused by M. ulcerans subspecies shinshuense (358). In Japan, the majority of cases are distributed in typically temperate, mountainous regions located between latitudes 34°N and 38°N, mountainous terrains at an altitude of 2,000 m in the case of the mountain ranges of Hida, Kiso, and Akaishi on Honshu, and 1,400 m in the cases of Hidaka on Hokkaido (358, 361, 373).
We reviewed the characteristics of these foci in West Africa, which occupies approximately one-fifth of the continent. The vast majority of this region is composed of plains rising to 300 m above sea level, but the northern section is composed of a semiarid terrain known as the Sahel, a transitional zone between the Sahara and the savannahs and forests of western Sudan (374) (Fig. 10). In West Africa, regions with reported cases of Buruli ulcer are all characterized by their proximity to a river, which connects the coast to mountains of >1,500 m that are less than 500 km away from the coast. In Benin, it was observed that the mean prevalences of Buruli ulcer significantly correlated inversely with elevation, from 60.7 cases/10,000 inhabitants in villages with an elevation below 50 m to 10.2/10,000 inhabitants in villages with an elevation between 50 and 100 m to 5.4/10,000 inhabitants in villages with an elevation above 100 m (144, 375). However, cases were reported at a minimum distance of 15 km from the coast of the Atlantic Ocean and a maximum distance of 18 km in a study conducted in Benin (144). In a study conducted in Benin by Portaels et al., an inverse relationship between the prevalence of the disease and the distance that a patient lived from a river was found. The prevalence gradually increased from 0.6 to 32.6/1,000 inhabitants when the distance from a river was less than 10 km (131). This observation correlates with our recent report that M. ulcerans tolerates a degree of salinity above 20 g/liter (186). In West Africa, where the disease is most prevalent, a dramatic increase in the incidence of Buruli ulcer has been reported by countries mostly along the Gulf of Guinea (17, 197, 334–342, 376). In all these countries, Buruli ulcer occurs in specific discrete foci, suggesting a space-confined distribution pattern (369). We reviewed the characteristics of these foci in West Africa, which occupies approximately one-fifth of the continent. The vast majority of this region is composed of plains lying at 300 m above sea level (374) (Fig. 10).
Seasonal factors may affect the epidemiology of M. ulcerans. In Cameroon, M. ulcerans dynamics are largely driven by seasonal climatic factors (188). In Ghana, the incidence of Buruli ulcer peaked at the end of the rainy season in September and October (333). It was recently shown in Ghana that the proportion of positive M. ulcerans samples recorded was higher during the months with higher rainfall levels (11%) than during the dry season months (3%) (210). This demonstrates that there is a seasonal pattern to the presence of M. ulcerans in the environment, which may be related to recent rainfall or water in the soil (210). In Cameroon, M. ulcerans dynamics are largely driven by seasonal climatic factors (188). In the United States (Louisiana), the environmental investigation of M. ulcerans DNA by IS2404 qPCR revealed seasonal variations in the prevalences of M. ulcerans, with a notable decrease in prevalence in the samples collected during autumn every year in the areas between latitude 30.003537 and longitude 92.021235 (147). No cases of human Buruli ulcer have been reported in Louisiana, suggesting that the environmental distribution of M. ulcerans is not limited to areas where Buruli ulcer is endemic and that infections caused by M. ulcerans are not limited to humans (147).
In Central Africa, it was shown that cases of Buruli ulcer peaked in March, suggesting that the risk is highest during the rainy season (377). In Cameroon, in the Nyong River distance model, the risk of Buruli ulcer decreased when the distance to the river increased, with a dose-response relationship (378). In Japan, there is a dynamic seasonal appearance of M. ulcerans in the environment, which may contribute to the seasonal variation of Buruli ulcer occurrence (108).
Outbreaks of Buruli ulcer have been attributed in many cases to environmental disturbances, such as flooding, agricultural deforestation, increases in the sizes of irrigated areas for cultivation, and construction of dams or damming of rivers (31, 33, 34, 347, 370). It also was shown that the areas where Buruli ulcer is highly endemic are located most often in lowland areas (375). Environmental factors, such as climate, soil, geology, and geochemistry, may indirectly influence or contribute to M. ulcerans infection (379). Several screenings of M. ulcerans in environmental samples have been done (Table 3). In countries with a constantly high incidence of Buruli ulcer, temperature and humidity generally follow the same trends, with average temperatures ranging between 22°C and 33°C, which is the optimum temperature required for the growth and survival of M. ulcerans (23, 94–97). The average relative humidity is 85% in the southern areas of these countries and 71% in the north. The annual sunshine duration varies with the seasons, and the average has been estimated at 1,762 h (249, 250).
In Japan, it was proven that there is a dynamic seasonal appearance of M. ulcerans in the environment, which may contribute to the seasonal variation of Buruli ulcer occurrence (108). In Central Africa, it was shown that the cases of Buruli ulcer peaked in March, suggesting that the risk is at its highest during the rainy season (377). In each country of endemicity, there is a distinct geographical distribution, depending on environmental factors.
Buruli ulcer, which is rampant in foci of endemicity and scattered, but limited, in general in marshes, floodplains, and close to lakes or rivers, is an ancient disease and widespread in the world and seems to have currently reached a new level through the extension of its usual foci and impact. Man-made changes in the environment may provide new opportunities for ecological niches for M. ulcerans and new opportunities for contact between populations and these niches.
Descriptive Epidemiology of Buruli Ulcer
Age.
M. ulcerans infection affects primarily children between 5 and 15 years of age (233).
Children less than 15 years of age represent approximately 42% of the overall population in West Africa. This proportion is approximately 41% in countries with a high prevalence of Buruli ulcer (Côte d'Ivoire, Ghana, Benin, Guinea, Togo, Nigeria) and 43% in countries with a lower prevalence or in which it is not endemic (Burkina Faso, Sierra Leone, Senegal, Guinea Bissau, Liberia, Mali) (P < 0.05). In 10 independent studies conducted in Ghana, Côte d'Ivoire, Benin, and Nigeria (23, 26, 155, 226, 235, 241, 242, 244, 253, 380), 50.7% of patients were less than 15 years old, and the median age was 18 years (Fig. 11). The peak age group in West Africa studies was 5 to 15 years, although Buruli ulcer can affect any age group (334, 340, 380). The highest detection rates were found sometimes in 75- to 79-year-old patients in West Africa, probably due to the reactivation of disease from a latent infection of M. ulcerans (380). It was proven in Africa that children less than 5 years old rarely develop antibody responses to the 18-kDa small heat shock protein (shsp) of M. ulcerans and thus seem to be considerably less exposed to the pathogen than older children (381, 382). As Buruli ulcer is not known to be an immunizing infection, this may reflect a greater exposition to sources and vectors (381, 382).
FIG 11.
Numbers of patients by age class (A) and sex (B) in studies conducted in West Africa (A) and the sex ratio of patients (B).
Sex ratio.
In West Africa, the female population represents about 49.52% of the overall population, with a sex ratio of 1.02. This proportion is approximately 49.87% in six countries with a high prevalence of Buruli ulcer (Côte d'Ivoire, Ghana, Benin, Guinea, Togo, Nigeria), while it is 50.99% in countries with a lower prevalence or in which it is not endemic (Burkina Faso, Sierra Leone, Senegal, Guinea Bissau, Liberia, Mali) (P < 0.05). The sex ratio is 1.01 in countries where Buruli ulcer is endemic and 0.96 in the other countries. In 10 studies conducted in the countries of endemicity of West Africa (23, 26, 155, 226, 235, 241, 242, 244, 253, 380), the global calculated sex ratio for Buruli ulcer patients (male to female) was 0.97 (0.90 to 1.04) (Fig. 11).
Farming Activities in Swampy Areas as a Risk Factor for Buruli Ulcer
In West Africa, the emergence and distribution of Buruli ulcer cases are clearly linked to aquatic ecosystems, and recent data suggest that different modes of transmission occur in specific areas and epidemiological settings (110, 150, 235, 347). Since the 1970s, some authors have formulated the hypothesis that patients may be infected through minor wounds or skin abrasions via contact with water containing M. ulcerans or by insect bites (99, 150, 383). Prior to recent studies, it was difficult to establish the epidemiological and ecological evidence linking the source of M. ulcerans to swamps and slow-flowing water (379). Past epidemiological studies have associated Buruli ulcer with human activity near, or within, slow-flowing or still bodies of water that have been created or disturbed by humans. This postulate was considered because there is strong epidemiological evidence linking the source of M. ulcerans to swamps and slow-flowing water or stagnant water (379). Residence near an aquatic environment has been identified as a consistent risk factor for M. ulcerans infection in Africa (155, 192, 334, 384). The proximity to rivers and water reservoirs has long been implicated in the emergence of Buruli ulcer in West African countries and, particularly in rural areas, especially in children less than 15 years old (63). In Côte d'Ivoire and Ghana, infections were reported to occur near rivers (198, 385, 386). The increased incidence of Buruli ulcer in Côte d'Ivoire was very much related to areas around dammed rivers and corroborates the first reported case of M. ulcerans infection in Côte d'Ivoire, a young patient living near the artificial Kossou Lake in the center of the country (386, 387). Irrigated rice and banana fields and deforested irrigation and aquaculture installations are zones for high-risk Buruli ulcer in Côte d'Ivoire (372); Buruli ulcer has also emerged in some communities (20, 153). Cases described in Nigeria were associated with the Benue River Valley in 1967 or a small artificial lake (338, 346). Similarly, in Liberia, cases were reported after dam construction following the introduction of swamp rice to replace upland rice (rice grown on dry soil) (341, 388). In Ghana, cases have clustered along the Densu River (385). The proximity of villages to rivers was a risk factor for contracting Buruli ulcer in Benin and Ghana (155, 389), and the link between a watery ecosystem and the emergence of Buruli ulcer was proven. In West Africa, Buruli ulcer afflicts primarily rural farmers in swampy environments. Also, it is thought that the use of river water for domestic purposes may contribute to the high prevalence of Buruli ulcer in settings of endemicity (389, 390). Another epidemiological study in Benin showed that foci of endemicity are organized primarily around the valley of the Ouémé and Kouffo Rivers. The communes of Lalo, Ouinhi, Bonu, Adjohoun, and Ze are the most affected (246). The first two reported patients with Buruli ulcer in Togo established a geographical continuum of the disease in all countries bordering the Gulf of Guinea (342). Cases reported in Burkina Faso (335) and Sierra Leone (391) were also related to an aquatic environment. A study conducted in Ghana suggested that swimming or activities on riverbanks were risk factors for contracting Buruli ulcer (192). Three at-risk areas for M. ulcerans disease were identified in Togo: the Laguna coastal area, marshy inland areas where market crops and rice are cultivated, and river valley areas (244). The foci of the disease are associated with environmental changes due to logging and mining and the creation or the extension of swampy areas, such as the construction of dams or lakes for the development of agriculture by irrigation, and are associated with exposure to river areas and sometimes with flooding (25, 155, 338).
The exposed skin of farmers and their activities in rural areas may facilitate the transmission of the pathogen (155). It has been demonstrated that there is a link between a watery ecosystem and the emergence of Buruli ulcer; preventive public health programs based on strategies that provide protected water supply systems to villages must be developed to reduce the frequency of the disease (389) (Fig. 4).
Raghunathan et al. identified wading in a river and streams in tropical climates as a risk factor for Buruli ulcer (155). A recent case-control study in Ghana showed that the risk factors for Buruli ulcer are contacts with wetland, insect bites in water, use of adhesive when injured, and bathing in the river (235). Other risk factors in Ghana were exposure to river areas, the presence of arsenic in the environment, exposed skin, use of water from rivers and ponds for drinking, and being between 2 and 14 years old (155, 191, 192). In Côte d'Ivoire, farming near the river was a risk factor (334). Another study in Côte d'Ivoire showed that regular contacts with unprotected surface water and the absence of protective equipment during agricultural activities were identified as the main factors associated with the risk of contracting Buruli ulcer (23). The contact with water was due mainly to agricultural activities (e.g., rice farming, market gardening, and fishing) and washing/bathing/swimming activities (23). The same conclusions about risk factors in Côte d'Ivoire were obtained previously by Ahoua et al. and Marston et al., and they concluded that young children and women having daily water-related activities were most at risk (253, 334). In Nigeria, the area of endemicity in Ogun state is divided into two drainage basins, the Yewa and Ogun Rivers, which are considered to be risk areas for Buruli ulcer (26). In Togo, three risk areas in swampy areas were identified: the Laguna coastal area, marshy inlands where market crops and rice are cultivated, and river valleys (244). Risk factors identified in Benin were the use of water from swamps, agricultural activities, being <15 years old or >49 years old, BCG vaccination status, and improper wound care (30, 33, 380). The greatest risk factors for acquiring Buruli ulcer included residing in an area of endemicity, close proximity to specific bodies of water, and being less than 15 years old (20) (Fig. 4). A fundamental research study conducted in Ghana with Buruli ulcer patients and control patients showed that a genetic polymorphism in the SLC11A1 gene played a role in the susceptibility to Buruli ulcer, with an estimated 13% population-attributable risk (392).
Protective Factors
Raghunathan et al. found that wearing a shirt while farming, sharing indoor living space with livestock, and bathing with toilet soap appeared to be protective (155). Covering limbs during farming and the use of alcohol after insect bites were also found to be protective factors against Buruli ulcer in Ghana (235). Wearing long pants was protective against M. ulcerans infection in Côte d'Ivoire and Australia (18, 334). N′krumah et al. found that wearing protective equipment before being in contact with surface water was a protective factor against Buruli ulcer (23) (Fig. 4). In Benin, the use of mosquito bed nets was considered to be a protective factor (30). In Australia, Quek et al. showed that immediately washing a wound received outdoors was found to decrease the odds of disease (18). In Ghana, it was proven that patients with Buruli ulcer who had received BCG vaccination had a shorter duration of the ulcer than those who were not vaccinated (333). A further study in Uganda showed that any protective effect was of short duration (393). Minimizing contact with water or soil around regions where Buruli ulcer is endemic, particularly in the presence of cuts or abrasions, had a protective effect.
Coepidemiology of Buruli Ulcer with Prevalent Infections
In an attempt to narrow the spectrum of potential reservoirs and vectors for M. ulcerans, we created a map of 10 infectious diseases that are prevalent in the same geographical belt as Buruli ulcer, with a focus on their vectors and reservoirs. We then focused on four infections in the tropics with significant overlap of Buruli ulcers.
Malaria
Malaria is the most important insect-transmitted human disease, and progress in its control has been slow, especially in Africa, where approximately 90% of cases occur (394, 395). Sub-Saharan Africa is home to localities with the highest global malaria transmission levels and, hence, high malarial morbidity and mortality. Human malarial protozoa are transmitted by mosquitoes of the genus Anopheles, including A. arabiensis, A. gambiae, A. melas, and A. merus. Anopheles arabiensis is considered mostly zoophilic compared to the highly anthropophilic A. gambiae but still plays a very important role in malaria transmission (394). The transmission of malaria in the coastal areas of West Africa is almost constant throughout the year. Further north, transmission varies from 1 month to 11 months of the year. While the role of mosquitoes in the transmission of M. ulcerans has not been demonstrated in West Africa, M. ulcerans DNA has been detected in mosquitoes (Aedes camptorhynchus, Coquillettidia linealis, Anopheles annulipes, Culex australicus, Aedes notoscriptus) trapped in Australia (19). Experimentally, mosquito larvae (Aedes aegypti, A. albopictus, Ochlerotatus triseriatus, Culex restuans larvae) can ingest wild-type M. ulcerans and M. marinum and remain infected throughout larval development (396). Evidence that implicates mosquitoes in the transmission of M. ulcerans in southeastern Australia has been established (18). The role of mosquitoes in transmission in Africa remains controversial. In particular, mosquito bites do not explain the unequal left-right distributions of lesions reported in some studies (308, 340). However, the past 15 years have seen unprecedented progress in malaria prevention and control by scaling up vector control interventions, particularly in sub-Saharan Africa (397, 398). Faced with the heavy burden of malaria, African countries decided in 2000 at the Abuja Summit to pay special attention to the fight against this disease (399). In 2005, they decided that at least 60% of the people who were most vulnerable to this disease, especially children under 5 years of age and pregnant women, should benefit from the best possible combination of personal and community protective measures, such as mosquito nets impregnated with insecticides, long-lasting insecticidal nets (LLINs), and other existing and available interventions to prevent infection and disease. This target was set at 80% for 2010 by the Organization of African Unity (OAU), currently replaced by the African Union (AU) (397–399). The insecticide-treated net kills or keeps away mosquitoes and other insects, such as head lice, bed bugs, and fleas. Numerous types of insecticide are used to treat the net: deltamethrin, lambda-cyhalothrin, alpha-cypermethrin, cyfluthrin, etofenprox, and permethrin (397). The WHO Global Malaria Program (WHO GMP) recommends three primary interventions for effective malaria control: the diagnosis and treatment of patients, the use of insecticide-treated nets (ITNs), and indoor residual spraying (IRS) (398). To strengthen the fight against malaria, African countries have benefited from Global Fund grants and technical support from other partners. The main aim was to contribute to the reduction of morbidity and mortality due to malaria between 2008 and 2014. Among the objectives of the application to the Global Fund is increasing the ITN utilization rate to at least 80% for people exposed to malaria, particularly pregnant women and children under 5 years of age. This mass distribution campaign has complemented LLIN distribution between 2008 and 2009 in most sub-Saharan countries. IRS is a major intervention for malaria control. There are currently 12 insecticides recommended for IRS, including dichlorodiphenyltrichloroethane, pyrethroids, and carbamates, which were used efficiently against vectors of malaria by national malaria programs to scale up global malaria control and elimination (400). Given the incertitude as the role of mosquitoes in the transmission of M. ulcerans in Africa, the fact that these preventive measures caused a decrease in Buruli ulcer cases in Africa remains controversial.
Filariasis
In Africa, lymphatic filariasis or elephantiasis is a neglected tropical disease (401). The environmental conditions for lymphatic filariasis transmission occur around the forest and savannah regions of West Africa (401). Lymphatic filariasis is caused by the filarial worms Wuchereria bancrofti, Brugia malayi, and Brugia timori, which are endemic in 55 countries (401–403). The transmission of lymphatic filariasis in Africa is predicted to appear across much of the coastal and savannah areas of West Africa (401), thus, in the same areas as Buruli ulcer. In Ghana, lymphatic filariasis caused by W. bancrofti nematodes is found in several regions where Buruli ulcer is endemic (258). Approximately 80% of the people living in areas that require preventive chemotherapy to stop the spread of infection live in the following 10 countries: Angola, Cameroon, Côte d'Ivoire, the Democratic Republic of the Congo, India, Indonesia, Mozambique, Myanmar, Nigeria, and the United Republic of Tanzania (402). Culex species mosquitoes are the major vectors of W. bancrofti (404). The major Anopheles vectors in West Africa are A. gambiae sensu lato and the Anopheles funestus group (405). The World Health Assembly resolution WHA50.29 (406) encourages eliminating lymphatic filariasis. In response, the WHO launched its Global Programme to Eliminate Lymphatic Filariasis (GPELF) in 2000 (401). In 2012, the WHO's neglected tropical diseases roadmap reconfirmed the target date for achieving elimination by 2020 (402). Between 2000 and 2014, 5.63 billion treatments were delivered to more than 1 billion people at least once in 63 countries, considerably reducing transmission in many places. Recent research data showed that the transmission of lymphatic filariasis in at-risk populations has dropped by 43% since the beginning of the GPELF (402). Depending on the parasite vector species, measures such as insecticide-treated nets, indoor residual spraying, and personal protection measures may help protect people from infection. Vector control has, in specific settings, contributed to the elimination of lymphatic filariasis in the absence of large-scale preventive chemotherapy (402). Between 2000 and 2009, nine of the West African countries achieved full coverage of their entire at-risk populations after the launch of the GPELF with the mass drug administration (MDA) of a single dose of diethylcarbamazine or ivermectin plus albendazole (403, 407). In West African countries, due to the fact that onchocerciasis is coendemic with lymphatic filariasis, ivermectin plus albendazole in a single dose per year was used for MDA (407). In addition to interrupting transmission, MDA provides significant collateral health benefits, such as reduced morbidity from intestinal worms and ectoparasites (291, 402, 407). Vector control to reduce mosquito populations was one of the WHO GPELF priorities for the interruption of transmission by the recommended use of techniques such as insecticide-treated bed nets and curtains as well as residual spraying as effective vector control tools (403). Insecticide resistance among the vectors of lymphatic filariasis in Africa has been reported. The kdr mutation responsible for resistance to pyrethroids has been found in the M and S forms of A. gambiae sensu stricto (408). The GPELF is based on the MDA, but the vector control activities of the Roll Back Malaria campaign have a significant capacity to eliminate the risk of transmission of W. bancrofti in areas of coendemicity (408). We have formulated the following postulate, which states that the annual decrease in the number of cases of Buruli ulcer since 2010s in the world, and particularly in West Africa, is due to the associated benefit of the WHO GPELF program by the MDA (including ivermectin) to all populations at risk. This assumption is reinforced by two recent studies in which experiments demonstrated that two avermectins could inhibit the growth and kill M. ulcerans strains from both Africa and Australia (289, 290) (Table 5).
Schistosomiasis
Schistosomiasis, also known as bilharziosis, is caused by several species of parasitic platyhelminthes of the genus Schistosoma, which can infect the urinary tract or the intestines of hosts. Of the 207 million estimated cases of schistosomiasis worldwide, 93% occur in sub-Saharan Africa. Schistosoma haematobium and Schistosoma mansoni are endemic throughout the continent. Transmission is usually associated with poor socio-economic conditions. Compared to the other schistosomes, S. haematobium is responsible for approximately two-thirds of the schistosomiasis cases in sub-Saharan Africa. S. haematobium infection is highly endemic in many Buruli ulcer foci in West Africa, with a striking increase in transmission after river dams were constructed (409). Approximately 76% of the population lives near rivers, lakes, and other bodies of water contaminated with snail intermediate hosts (410), which are also incriminated as potential reservoirs of M. ulcerans. The infection has been associated with water resource development projects, such as dams and irrigation projects, and slow-flowing or stagnant water, where the snail, an intermediate host of the parasite, breeds (410, 411). The disease is essentially an infection of rural and agricultural communities, where the way of life promotes contamination of inland water with human excreta (412). Schistosomiasis and Buruli ulcer have increased rapidly in the tropical wetlands of West and Central Africa since the 1980s, particularly after irrigation and dam construction (413, 414). Whether schistosomiasis was a risk factor for Buruli ulcer by driving the host immune response toward a predominantly Th2 pattern (409) has been disputed (413, 414). The highest prevalence and intensities of human schistosomiasis occur in school-aged children, adolescents, and young adults (410), as with Buruli ulcer. The control strategies include control of the intermediate snail host, use of molluscicides, chemotherapy, and improved sanitation and health education (412). The WHO strategy for schistosomiasis control focuses on reducing the disease using periodic, targeted treatments with praziquantel through large-scale treatment of affected populations (407).
Cutaneous Leishmaniasis
Leishmaniasis in HIV-coinfected patients is a significant yet neglected public health problem in West Africa (415). It is a vector-borne parasitic disease of humans and mammals caused by cell-infecting flagellate protozoa of the genus Leishmania, transmitted by female phlebotomine sand flies (415, 416). In most African countries, the disease is typically caused by one of two species, Leishmania major or Leishmania tropica (410). The areas of endemicity of leishmaniasis are governed by the presence of the sand fly vector, their dietary preferences, and their ability to promote the internal development of specific Leishmania species (417). Sand fly species of the genera Phlebotomus and Sergentomyia are two putative vectors in the transmission of Leishmania in West Africa (418). In South America, Leishmania braziliensis, Leishmania guyanensis, and Leishmania panamensis are responsible for cutaneous ulcers (259, 419). One case of M. ulcerans and L. braziliensis coinfection in a European traveler in South America raised the question of possible cotransmission of the two pathogens (259).
Areas of Uncertainties and Perspectives
Sources of infection.
M. ulcerans has been detected in soil, biofilms, aquatic insects, fish, amphibia, and wildlife, confirming the epidemiologic evidence linking Buruli ulcer to aquatic and marshy environments. This is illustrated by the clear colocalization between rice fields and regions in Côte d'Ivoire where Buruli ulcer is endemic (372). However, the exact biotopes where M. ulcerans resides and which constitute sources of infection remain unknown. Amoebae are natural hosts of several microbial pathogens, such as certain mycobacteria (Mycobacterium smegmatis, M. marinum, Mycobacterium simiae, Mycobacterium avium) (420). Other studies showed that M. shottsii, M. pseudoshottsii, and M. marinum bacilli were internalized by A. polyphaga trophozoites (421–424). Therefore, amoebae can be a serious niche for the investigation of environmental strains of M. ulcerans in settings where Buruli ulcer is endemic. In a recent high-throughput carbon substrate profile of M. ulcerans in our laboratory, we found a significant association between the M. ulcerans core biologome and bacteria, fungi, algae, and mollusks. We concluded that environmental M. ulcerans research should increase its focus on fungi, algae, and mollusks, because they contain the nutrients necessary for the survival of M. ulcerans (211).
In addition, the route of transmission remains enigmatic. Current hypotheses regarding the role of mosquito and water bug bites are not supported by the current distribution of the disease in human populations. However, a mosquito bite might be one form of skin lesion among others giving the opportunity to M. ulcerans to penetrate the skin. Consequently, further laboratory studies may clarify the role of mosquitoes in the transmission of M. ulcerans to people from the local environment or wildlife.
Variations in the incidence of Buruli ulcer.
Buruli ulcer is an infectious pathology related to ecosystems in areas of endemicity, and the incidence of Buruli ulcer is driven mainly by variations in the ecosystems, but significant variations are unpredictable. For example, it could not be anticipated that in Ghana, soil arsenic is significantly associated with the persistence of the disease in specific areas contaminated by this mineral (191).
Targeted interventions against Buruli ulcer.
Early detection and treatment of the disease has been implemented by national Buruli ulcer control programs to reduce the morbidity and disability associated with the disease. Multifaceted activities at the community level are organized for the early detection of cases, with information, education, and communication campaigns in communities and schools, training of village health workers, and strengthening of community-based surveillance systems. Since the creation of national programs with WHO support in the fight against Buruli ulcer in the 2000s by health authorities of the countries concerned, valuable efforts have been made to control and fight this disease (425, 426).
CONCLUSIONS
M. ulcerans is a prototype of an opportunistic inoculated pathogen, and Buruli ulcer is a prototype for ecosystem pathology. However, the exact ecosystems in which M. ulcerans resides are still unknown, as are the sources of infection for the populations in areas of endemicity and the exact circumstances of transmission. Efforts must be made to unravel exact sources of infection by substituting isolation and culture of environmental specimens with an exclusive PCR-only-based approach. Active and continuous surveillance in countries at risk of Buruli ulcer is needed for mapping the areas of endemicity in order to implement targeted control actions. An effective strategy to reduce the incidence of Buruli ulcer should involve compliance with protective equipment during agricultural activities, avoidance of contact with surface water, and community capacity building through training and sensitization. It is necessary to improve the means of prevention through ongoing identification of the most at-risk M. ulcerans infection factors in areas of high endemicity. Preventive public health policies for protecting water supply systems in villages must be implemented to reduce the frequency of this infectious disease. Educational programs should especially target the population groups at risk. A better understanding of the ecology of M. ulcerans and its route of transmission is very important for enhanced knowledge of disease epidemiology in order to establish control and prevention strategies. Given the current decline in the incidence of Buruli ulcer since 2010, it is necessary to conduct thorough investigations to better understand the factors involved in the decreased incidence to improve Buruli ulcer control strategies for each setting where Buruli ulcer is endemic. The search for efficient, natural, and active products against M. ulcerans should be encouraged in resource-limited settings, because they are part of the natural heritage of these populations. They are financially affordable and can be used at the earliest stage.
In conclusion, elucidating the sources of contamination and the modes of transmission by tentative isolation of M. ulcerans from environmental samples is a priority for efficient guiding of the fight against this neglected “tropical” disease.
Supplementary Material
ACKNOWLEDGMENTS
D.Z., A.B., and R.B.D.T. benefit from a Ph.D. grant from the IHU Méditerranée Infection.
D.Z., A.B., and R.B.D.T. retrieved references and drafted the manuscript. D.Z. drew all the original figures. M.D. proposed the thematic review and drafted the manuscript.
Biographies

Dezemon Zingue is from the Centre Muraz research institute and holds a doctorate in pharmacy (2005) from the University of Ouagadougou (Burkina Faso). He received his Master of Science in health systems engineering from the Université de Nice Sophia Antipolis in 2007 and then his Master of Science in human pathology from Aix-Marseille Université in 2014. Since 2014, he has continued his work as a Ph.D. student at Aix-Marseille Université in the Research Unit in Infectious and Tropical Emergent Diseases (URMITE) on a scholarship from Méditerranée Infection, exploring the Mycobacterium ulcerans reservoirs and the development of new laboratory techniques.

Amar Bouam holds a doctorate in veterinary medicine (D.V.M.). He obtained his degree in veterinary medicine in 2014 at the Institute of Veterinary Science of Blida, Blida, Algeria. Then he completed a Master's degree in zoonoses at the University of Limoges, Limoges, France. Since 2015, he has been a Ph.D. student and is working on Buruli ulcer under the supervision of Professor Michel Drancourt in the Research Unit in Infectious and Tropical Emergent Diseases (URMITE), directed by Professor Didier Raoult.

Roger B. D. Tian obtained his Ph.D. degree at the URMITE, directed by Professor Didier Raoult. He has particular interest in tropical infections, including Buruli ulcer.

Michel Drancourt is a professor of medical microbiology in IHU Méditerranée Infection and head of the Microbiology Laboratory at Marseille's public hospital. His research topics include mycobacteria, neglected organisms, and paleomicrobiology, and he participated in the promotion of the point of care in clinical microbiology.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CMR.00045-17.
REFERENCES
- 1.UBG. 1969. The Uganda Buruli group. BCG vaccination against Mycobacterium ulcerans infection (Buruli ulcer). First results of a trial in Uganda. Lancet i:111–115. [PubMed] [Google Scholar]
- 2.Clancey JK, Dodge OG, Lunn HF, Oduori ML. 1961. Mycobacterial skin ulcers in Uganda. Lancet ii:951–954. [DOI] [PubMed] [Google Scholar]
- 3.Alsop DG. 1972. The Bairnsdale ulcer. Aust N Z J Surg 41:317–319. [PubMed] [Google Scholar]
- 4.Meyers WM. 1995. Mycobacterial infections of the skin. Trop Pathol (Spez Pathol Anat) 8:291–377. doi: 10.1007/978-3-642-57863-2_9. [DOI] [Google Scholar]
- 5.MacCallum P, Tolhurst JC, Buckle G, Sissons HA. 1948. A new mycobacterial infection in man. J Pathol Bacteriol 60:93–102. doi: 10.1002/path.1700600111. [DOI] [PubMed] [Google Scholar]
- 6.Lunn HF. 1965. Effects of climate on the localization and geographic distribution of mycobacterial skin ulcers. Dermatol Int 4:111–114. doi: 10.1111/j.1365-4362.1965.tb05134.x. [DOI] [PubMed] [Google Scholar]
- 7.Coloma JN, Navarrete-Franco G, Iribe P, López-Cepeda LD. 2005. Ulcerative cutaneous mycobacteriosis. Int J Leprosy 73:5–12. [DOI] [PubMed] [Google Scholar]
- 8.Kibadi K, Ajoulat I, Meyers WM, Mokassa L, Muyembe T, Portaels F. 2007. Étude des appellations et des représentations attachées à l'infection à Mycobacterium ulcerans dans différents pays endémiques d'Afrique. Med Trop 67:241–248. [PubMed] [Google Scholar]
- 9.Phanzu DM, Suykerbuyk P, Imposo DB, Lukanu PN, Minuku JB, Lehman LF, Saunderson P, de Jong BC, Lutumba PT, Portaels F, Boelaert M. 2011. Effect of a control project on clinical profiles and outcomes in Buruli ulcer: a before/after study in Bas-Congo, Democratic Republic of Congo. PLoS Negl Trop Dis 5:e1402. doi: 10.1371/journal.pntd.0001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent QB, Ardant M-F, Adeye A, Goundote A, Saint-André J-P, Cottin J, Kempf M, Agossadou D, Johnson C, Abel L, Marsollier L, Chauty A, Alcaïs A. 2014. Clinical epidemiology of laboratory-confirmed Buruli ulcer in Benin: a cohort study. Lancet Global Health 2:422–430. doi: 10.1016/S2214-109X(14)70223-2. [DOI] [PubMed] [Google Scholar]
- 11.Portaels F, Fonteyene PA, de Beenhouwer H, de Rijk P, Guedenon A, Hayman J, Meyers MW. 1996. Variability in 3′ end of 16S rRNA sequence of Mycobacterium ulcerans is related to geographic origin of isolates. J Clin Microbiol 34:962–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. 2004. Buruli ulcer disease. Wkly Epidemiol Rec 79:194–199. [PubMed] [Google Scholar]
- 13.Williamson HR, Benbow ME, Nguyen KD, Beachboard DC, Kimbirauskas RK, McIntosh MD, Quaye C, Ampadu EO, Boakye D, Merritt RW, Small PL. 2008. Distribution of Mycobacterium ulcerans in buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl Trop Dis 2:e205. doi: 10.1371/journal.pntd.0000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, Meurice G, Simon D, Bouchier C, Ma L, Tichit M, Porter JL, Ryan J, Johnson PD, Davies JK, Jenkin GA, Small PL, Jones LM, Tekaia F, Laval F, Daffe M, Parkhill J, Cole ST. 2007. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res 17:192–200. doi: 10.1101/gr.5942807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh DS, Portaels F, Meyers WM. 2011. Buruli ulcer: advances in understanding Mycobacterium ulcerans infection. Dermatol Clin 29:1–8. doi: 10.1016/j.det.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Marsollier L, Robert R, Aubry J, Saint Andre JP, Kouakou H, Legras P, Manceau AL, Mahaza C, Carbonnelle B. 2002. Aquatic insects as a vector for Mycobacterium ulcerans. Appl Environ Microbiol 68:4623–4628. doi: 10.1128/AEM.68.9.4623-4628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Guédénon A, Scott JT, Dramaix M, Portaels F. 2004. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, southern Benin, 1997–2001. Emerg Infect Dis 10:1391–1398. doi: 10.3201/eid1008.030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quek TY, Athan E, Henry MJ, Pasco JA, Redden-Hoare J, Hughes A, Johnson PD. 2007. Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg Infect Dis 13:1661–1666. doi: 10.3201/eid1311.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson PD, Azuolas J, Lavender CJ, Wishart E, Stinear TP, Hayman JA. 2007. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis 13:1653–1660. doi: 10.3201/eid1311.061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merritt RW, Walker ED, Small PLC, Wallace JR, Johnson PDR, Benbow ME, Boakye DA. 2010. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis 4:e911. doi: 10.1371/journal.pntd.0000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. 2014. Buruli ulcer (Mycobacterium ulcerans infection). Fact Sheet no. 199. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 22.Portaels F, Chemlal K, Elsen P, Johnson PD, Hayman JA, Hibble J, Kirkwood R, Meyers WM. 2001. Mycobacterium ulcerans in wild animals. Rev Sci Tech 20:252–264. doi: 10.20506/rst.20.1.1270. [DOI] [PubMed] [Google Scholar]
- 23.N′krumah RTAS, Koné B, Tiembre I, Cissé G, Pluschke G, Tanner M, Utzinger J. 2016. Socio-environmental factors associated with the risk of contracting Buruli ulcer in Tiassalé, South Côte d'Ivoire: a case-control study. PLoS Negl Trop Dis 10:e0004327. doi: 10.1371/journal.pntd.0004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amofah G, Bonsu F, Tetteh C, Okrah J, Asamoa K, Asiedu K, Addy J. 2002. Buruli ulcer in Ghana: results of a national case search. Emerg Infect Dis 8:167–170. doi: 10.3201/eid0802.010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asiedu K, Sherpbier R, Raviglione M (ed). 2000. Buruli ulcer. Mycobacterium ulcerans infection. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/66164/1/WHO_CDS_CPE_GBUI_2000.1.pdf. [Google Scholar]
- 26.Marion E, Carolan K, Adeye A, Kempf M, Chauty A, Marsollier L. 2015. Buruli ulcer in south western Nigeria: a retrospective cohort study of patients treated in Benin. PLoS Negl Trop Dis 9:e3443. doi: 10.1371/journal.pntd.0003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booker C. 4 August 2016. Flesh-eating Buruli ulcer cases soar as disease spreads to Melbourne. The Age, Melbourne, Victoria, Australia: http://www.theage.com.au/victoria/flesheating-buruli-ulcer-cases-soar-as-disease-spreads-to-melbourne-20160804-gqksnw.html. [Google Scholar]
- 28.Aboagye SY, Asare P, Otchere ID, Koka E, Mensah GE, Yirenya-Tawiah D, Yeboah-Manu D. 2017. Environmental and behavioral drivers of Buruli ulcer disease in selected communities along the Densu River Basin of Ghana: a case-control study. Am J Trop Med Hyg 96:1076–1083. doi: 10.4269/ajtmh.16-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amissah NA, Gryseels S, Tobias NJ, Ravadgar B, Suzuki M, Vandelannoote K, Durnez L, Leirs H, Stinear TP, Portaels F, Ablordey A, Eddyani M. 2014. Investigating the role of free-living amoebae as a reservoir for Mycobacterium ulcerans. PLoS Negl Trop Dis 8:e3148. doi: 10.1371/journal.pntd.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nackers F, Johnson RC, Glynn JR, Zinsou C, Tonglet R, Portaels F. 2007. Environmental and health-related risk factors for Mycobacterium ulcerans disease (Buruli ulcer) in Benin. Am J Trop Med Hyg 77:834–836. [PubMed] [Google Scholar]
- 31.Walsh DS, Portaels F, Meyers WM. 2008. Buruli ulcer (Mycobacterium ulcerans infection). Trans R Soc Trop Med Hyg 102:969–978. doi: 10.1016/j.trstmh.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Wagner T, Benbow ME, Brenden TO, Qi J, Johnson RC. 2008. Buruli ulcer disease prevalence in Benin, West Africa: associations with land use/cover and the identification of disease clusters. Int J Health Geogr 7:25. doi: 10.1186/1476-072X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debacker M, Portaels F, Aguiar J, Steunou C, Zinsou C, Meyers W, Dramaix M. 2006. Risk factors for Buruli ulcer, Benin. Emerg Infect Dis 12:1325–1331. doi: 10.3201/eid1209.050598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Josse R, Guedenon A, Darie H, Anagonou S, Portaels F, Meyers WM. 1995. Mycobacterium ulcerans cutaneous infections: Buruli ulcers. Med Trop (Mars) 55:363–373. (In French.) [PubMed] [Google Scholar]
- 35.Giles-Vernick T, Owona-Ntsama J, Landier J, Eyangoh S. 2015. The puzzle of Buruli ulcer transmission, ethno-ecological history and the end of “love” in the Akonolinga district, Cameroon. Soc Sci Med 129:20–27. doi: 10.1016/j.socscimed.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Nyabadza F, Bonyah E. 2015. On the transmission dynamics of Buruli ulcer in Ghana: insights through a mathematical model. BMC Res Notes 8:656. doi: 10.1186/s13104-015-1619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian RD, Lepidi H, Nappez C, Drancourt M. 2016. Experimental survival of Mycobacterium ulcerans in watery soil, a potential source of Buruli ulcer. Am J Trop Med Hyg 94:89–92. doi: 10.4269/ajtmh.15-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Werf TS, van der Graaf WT, Tappero JW, Asiedu K. 1999. Mycobacterium ulcerans infection. Lancet 354:1013–1018. doi: 10.1016/S0140-6736(99)01156-3. [DOI] [PubMed] [Google Scholar]
- 39.Aboagye SY, Danso E, Ampah KA, Nakobu Z, Asare P, Otchere ID, Röltgen K, Yirenya-Tawiah D, Yeboah-Manu D. 2016. Isolation of nontuberculous mycobacteria from the environment of Ghanian communities where Buruli ulcer is endemic. Appl Environ Microbiol 82:4320–4329. doi: 10.1128/AEM.01002-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsollier L, Sévérin T, Aubry J, Merritt RW, Saint André J-P, Legras P, Manceau A-L, Chauty A, Carbonnelle B, Cole ST. 2004. Aquatic snails, passive hosts of Mycobacterium ulcerans. Appl Environ Microbiol 70:6296–6298. doi: 10.1128/AEM.70.10.6296-6298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asiedu K, Etuaful S. 1998. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am J Trop Med Hyg 59:1015–1022. doi: 10.4269/ajtmh.1998.59.1015. [DOI] [PubMed] [Google Scholar]
- 42.Henle FGJ. 1840. Von den Miasmen und Contagien, p 1–82. In Pathologische Untersuchungen. Friedrich Gustav Jacob Henle, Berlin, Germany. [Google Scholar]
- 43.Koch R. 1882. Die Aetiologie der Tuberkulose. Berl Klin Wochenschr 19:221–230. [Google Scholar]
- 44.Cule J. 1988. Sir William Osler and his Welsh connections. Postgrad Med J 64:568–574. doi: 10.1136/pgmj.64.753.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayman J. 1984. Mycobacterium ulcerans: an infection from Jurassic time. Lancet ii:1015–1016. [DOI] [PubMed] [Google Scholar]
- 46.Stinear TP, Jenkin GA, Johnson PDR, Davies JK. 2000. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J Bacteriol 182:6322–6330. doi: 10.1128/JB.182.22.6322-6330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pidot SJ, Asiedu K, Käser M, Fyfe JAM, Stinear TP. 2010. Mycobacterium ulcerans and other mycolactone-producing mycobacteria should be considered a single species. PLoS Negl Trop Dis 4:e663. doi: 10.1371/journal.pntd.0000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pidot SJ, Hong H, Seemann T, Porter JL, Yip MJ, Men A, Johnson M, Wilson P, Davies JK, Leadlay PF, Stinear TP. 2008. Deciphering the genetic basis for polyketide variation among mycobacteria producing mycolactones. BMC Genomics 9:462–462. doi: 10.1186/1471-2164-9-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakanaga K, Yotsu RR, Hoshino Y, Suzuki K, Makino M, Ishii N. 2013. Buruli ulcer and mycolactone-producing mycobacteria. Jpn J Infect Dis 66:83–88. doi: 10.7883/yoken.66.83. [DOI] [PubMed] [Google Scholar]
- 50.Doig KD, Holt KE, Fyfe JAM, Lavender CJ, Eddyani M, Portaels F, Yeboah-Manu D, Pluschke G, Seemann T, Stinear TP. 2012. On the origin of Mycobacterium ulcerans, the causative agent of Buruli ulcer. BMC Genomics 13:258–258. doi: 10.1186/1471-2164-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stinear TP, Mve-Obiang A, Small PL, Frigui W, Pryor MJ, Brosch R, Jenkin GA, Johnson PD, Davies JK, Lee RE, Adusumilli S, Garnier T, Haydock SF, Leadlay PF, Cole ST. 2004. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc Natl Acad Sci U S A 101:1345–1349. doi: 10.1073/pnas.0305877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh VK, Berry L, Bernut A, Singh S, Carrere-Kremer S, Viljoen A, Alibaud L, Majlessi L, Brosch R, Chaturvedi V, Geurtsen J, Drancourt M, Kremer L. 2016. A unique PE_PGRS protein inhibiting host cell cytosolic defenses and sustaining full virulence of Mycobacterium marinum in multiple hosts. Cell Microbiol 18:1489–1507. doi: 10.1111/cmi.12606. [DOI] [PubMed] [Google Scholar]
- 53.Rybniker J, Kramme S, Small PL. 2006. Host range of 14 mycobacteriophages in Mycobacterium ulcerans and seven other mycobacteria including Mycobacterium tuberculosis—application for identification and susceptibility testing. J Med Microbiol 55:37–42. doi: 10.1099/jmm.0.46238-0. [DOI] [PubMed] [Google Scholar]
- 54.Huys G, Rigouts L, Chemlal K, Portaels F, Swings J. 2000. Evaluation of amplified fragment length polymorphism analysis for inter- and intraspecific differentiation of Mycobacterium bovis, M. tuberculosis, and M. ulcerans. J Clin Microbiol 38:3675–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Käser M, Rondini S, Naegeli M, Stinear T, Portaels F, Certa U. 2007. Evolution of two distinct phylogenetic lineages of the emerging human pathogen Mycobacterium ulcerans. BMC Evol Biol 7:177. doi: 10.1186/1471-2148-7-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi W, Käser M, Röltgen K, Yeboah-Manu D, Pluschke G. 2009. Genomic diversity and evolution of Mycobacterium ulcerans revealed by next-generation sequencing. PLoS Pathog 5:e1000580. doi: 10.1371/journal.ppat.1000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chemlal K, Huys G, Fonteyne PA, Vincent V, Lopez AG, Rigouts L, Swings J, Meyers WM, Portaels F. 2001. Evaluation of PCR-restriction profile analysis and IS2404 restriction fragment length polymorphism and amplified fragment length polymorphism fingerprinting for identification and typing of Mycobacterium ulcerans and M. marinum. J Clin Microbiol 39:3272–3278. doi: 10.1128/JCM.39.9.3272-3278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamelas A, Ampah KA, Aboagye S, Kerber S, Danso E, Asante-Poku A, Asare P, Parkhill J, Harris SR, Pluschke G, Yeboah-Manu D, Röltgen K. 2016. Spatiotemporal co-existence of two Mycobacterium ulcerans clonal complexes in the Offin River Valley of Ghana. PLoS Negl Trop Dis 10:e0004856. doi: 10.1371/journal.pntd.0004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stragier P, Ablordey A, Bayonne LM, Lugor YL, Sindani IS, Suykerbuyk P, Wabinga H, Meyers WM, Portaels F. 2006. Heterogeneity among Mycobacterium ulcerans isolates from Africa. Emerg Infect Dis 12:844–847. doi: 10.3201/eid1205.051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolz M, Bratschi MW, Kerber S, Minyem JC, Um Boock A, Vogel M, Bayi PF, Junghanss T, Brites D, Harris SR, Parkhill J, Pluschke G, Lamelas Cabello A. 2015. Locally confined clonal complexes of Mycobacterium ulcerans in two Buruli ulcer endemic regions of Cameroon. PLoS Negl Trop Dis 9:e0003802. doi: 10.1371/journal.pntd.0003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ablordey AS, Vandelannoote K, Frimpong IA, Ahortor EK, Amissah NA, Eddyani M, Durnez L, Portaels F, de Jong BC, Leirs H, Porter JL, Mangas KM, Lam MMC, Buultjens A, Seemann T, Tobias NJ, Stinear TP. 2015. Whole genome comparisons suggest random distribution of Mycobacterium ulcerans genotypes in a Buruli ulcer endemic region of Ghana. PLoS Negl Trop Dis 9:e0003681. doi: 10.1371/journal.pntd.0003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandelannoote K, Meehan CJ, Eddyani M, Affolabi D, Phanzu DM, Eyangoh S, Jordaens K, Portaels F, Mangas K, Seemann T, Marsollier L, Marion E, Chauty A, Landier J, Fontanet A, Leirs H, Stinear TP, de Jong BC. 2017. Multiple introductions and recent spread of the emerging human pathogen Mycobacterium ulcerans across Africa. Genome Biol Evol 9:414–426. doi: 10.1093/gbe/evx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narh CA, Mosi L, Quaye C, Dassi C, Konan DO, Tay SCK, de Souza DK, Boakye DA, Bonfoh B. 2015. Source tracking Mycobacterium ulcerans infections in the Ashanti Region, Ghana. PLoS Negl Trop Dis 9:e0003437. doi: 10.1371/journal.pntd.0003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dassi C, Mosi L, Akpatou B, Narh C, Quaye C, Konan D, Djaman J, Bonfoh B. 2015. Detection of Mycobacterium ulcerans in Mastomys natalensis and potential transmission in Buruli ulcer endemic areas in Côte d'Ivoire. Mycobact Dis 5:184. [Google Scholar]
- 65.Fyfe JA, Lavender CJ, Handasyde KA, Legione AR, O'Brien CR, Stinear TP, Pidot SJ, Seemann T, Benbow ME, Wallace JR, McCowan C, Johnson PD. 2010. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis 4:0000791. doi: 10.1371/journal.pntd.0000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small PL. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 67.Gunawardana G, Chatterjee D, George KM, Brennan P, Whittern D, Small PLC. 1999. Characterization of novel macrolide toxins, mycolactones A and B, from a human pathogen, Mycobacterium ulcerans. J Am Chem Soc 121:6092–6093. doi: 10.1021/ja990017l. [DOI] [Google Scholar]
- 68.Marion E, Prado S, Cano C, Babonneau J, Ghamrawi S, Marsollier L. 2012. Photodegradation of the Mycobacterium ulcerans toxin, mycolactones: considerations for handling and storage. PLoS One 7:e33600. doi: 10.1371/journal.pone.0033600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong H, Stinear T, Porter J, Demangel C, Leadlay PF. 2007. A novel mycolactone toxin obtained by biosynthetic engineering. Chembiochem 8:2043–2047. doi: 10.1002/cbic.200700411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhodes MW, Kator H, Kotob S, van Berkum P, Kaattari I, Vogelbein W, Floyd MM, Butler WR, Quinn FD, Ottinger C, Shotts E. 2001. A unique Mycobacterium species isolated from an epizootic of striped bass (Morone saxatilis). Emerg Infect Dis 7:896–899. doi: 10.3201/eid0705.017523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mve-Obiang A, Lee RE, Portaels F, Small PL. 2003. Heterogeneity of mycolactones produced by clinical isolates of Mycobacterium ulcerans: implications for virulence. Infect Immun 71:774–783. doi: 10.1128/IAI.71.2.774-783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranger BS, Mahrous EA, Mosi L, Adusumilli S, Lee RE, Colorni A, Rhodes M, Small PLC. 2006. Globally distributed mycobacterial fish pathogens produce a novel plasmid-encoded toxic macrolide, mycolactone F. Infect Immun 74:6037–6045. doi: 10.1128/IAI.00970-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tafelmeyer P, Laurent C, Lenormand P, Rousselle JC, Marsollier L, Reysset G, Zhang R, Sickmann A, Stinear TP, Namane A, Cole ST. 2008. Comprehensive proteome analysis of Mycobacterium ulcerans and quantitative comparison of mycolactone biosynthesis. Proteomics 8:3124–3138. doi: 10.1002/pmic.200701018. [DOI] [PubMed] [Google Scholar]
- 74.Deshayes C, Angala SK, Marion E, Brandli I, Babonneau J, Preisser L, Eyangoh S, Delneste Y, Legras P, De Chastellier C, Stinear TP, Jackson M, Marsollier L. 2013. Regulation of mycolactone, the Mycobacterium ulcerans toxin, depends on nutrient source. PLoS Negl Trop Dis 7:e2502. doi: 10.1371/journal.pntd.0002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guenin-Mace L, Veyron-Churlet R, Thoulouze MI, Romet-Lemonne G, Hong H, Leadlay PF, Danckaert A, Ruf MT, Mostowy S, Zurzolo C, Bousso P, Chretien F, Carlier MF, Demangel C. 2013. Mycolactone activation of Wiskott-Aldrich syndrome proteins underpins Buruli ulcer formation. J Clin Invest 123:1501–1512. doi: 10.1172/JCI66576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarfo FS, Phillips R, Wansbrough-Jones M, Simmonds RE. 2016. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol 18:17–29. doi: 10.1111/cmi.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phillips RO, Sarfo FS, Landier J, Oldenburg R, Frimpong M, Wansbrough-Jones M, Abass K, Thompson W, Forson M, Fontanet A, Niang F, Demangel C. 2014. Combined inflammatory and metabolic defects reflected by reduced serum protein levels in patients with Buruli ulcer disease. PLoS Negl Trop Dis 8:e2786. doi: 10.1371/journal.pntd.0002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anand U, Sinisi M, Fox M, MacQuillan A, Quick T, Korchev Y, Bountra C, McCarthy T, Anand P. 2016. Mycolactone-mediated neurite degeneration and functional effects in cultured human and rat DRG neurons: mechanisms underlying hypoalgesia in Buruli ulcer. Mol Pain 20:1–11. doi: 10.1177/1744806916654144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogbechi J, Ruf MT, Hall BS, Bodman-Smith K, Vogel M, Wu HL, Stainer A, Esmon CT, Ahnstrom J, Pluschke G, Simmonds RE. 2015. Mycolactone-dependent depletion of endothelial cell thrombomodulin is strongly associated with fibrin deposition in Buruli ulcer lesions. PLoS Pathog 11:e1005011. doi: 10.1371/journal.ppat.1005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oliveira MS, Fraga AG, Torrado E, Castro AG, Pereira JP, Filho AL, Milanezi F, Schmitt FC, Meyers WM, Portaels F, Silva MT, Pedrosa J. 2005. Infection with Mycobacterium ulcerans induces persistent inflammatory responses in mice. Infect Immun 73:6299–6310. doi: 10.1128/IAI.73.10.6299-6310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.George KM, Pascopella L, Welty DM, Small PLC. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect Immun 68:877–883. doi: 10.1128/IAI.68.2.877-883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guenin-Mace L, Baron L, Chany AC, Tresse C, Saint-Auret S, Jonsson F, Le Chevalier F, Bruhns P, Bismuth G, Hidalgo-Lucas S, Bisson JF, Blanchard N, Demangel C. 2015. Shaping mycolactone for therapeutic use against inflammatory disorders. Sci Transl Med 7:289ra85. doi: 10.1126/scitranslmed.aab0458. [DOI] [PubMed] [Google Scholar]
- 83.Hall BS, Hill K, McKenna M, Ogbechi J, High S, Willis AE, Simmonds RE. 2014. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog 10:e1004061. doi: 10.1371/journal.ppat.1004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song OR, Marion E, Comoglio Y, Babonneau J, Guerineau N, Sandoz G, Yeramian E, Brodin P, Marsollier L. 2016. Mycolactone: the amazing analgesic mycobacterial toxin. Med Sci (Paris) 32:156–158. (In French.) doi: 10.1051/medsci/20163202007. [DOI] [PubMed] [Google Scholar]
- 85.En J, Goto M, Nakanaga K, Higashi M, Ishii N, Saito H, Yonezawa S, Hamada H, Small PLC. 2008. Mycolactone is responsible for the painlessness of Mycobacterium ulcerans infection (Buruli ulcer) in a murine study. Infect Immun 76:2002–2007. doi: 10.1128/IAI.01588-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarfo FS, Phillips RO, Zhang J, Abass MK, Abotsi J, Amoako YA, Adu-Sarkodie Y, Robinson C, Wansbrough-Jones MH. 2014. Kinetics of mycolactone in human subcutaneous tissue during antibiotic therapy for Mycobacterium ulcerans disease. BMC Infect Dis 14:1471–2334. doi: 10.1186/1471-2334-14-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diaz D, Dobeli H, Yeboah-Manu D, Mensah-Quainoo E, Friedlein A, Soder N, Rondini S, Bodmer T, Pluschke G. 2006. Use of the immunodominant 18-kilodalton small heat shock protein as a serological marker for exposure to Mycobacterium ulcerans. Clin Vaccine Immunol 13:1314–1321. doi: 10.1128/CVI.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong H, Coutanceau E, Leclerc M, Caleechurn L, Leadlay PF, Demangel C. 2008. Mycolactone diffuses from Mycobacterium ulcerans-infected tissues and targets mononuclear cells in peripheral blood and lymphoid organs. PLoS Negl Trop Dis 2:e325. doi: 10.1371/journal.pntd.0000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarfo FS, Le Chevalier F, Aka N, Phillips RO, Amoako Y, Boneca IG, Lenormand P, Dosso M, Wansbrough-Jones M, Veyron-Churlet R, Guenin-Mace L, Demangel C. 2011. Mycolactone diffuses into the peripheral blood of Buruli ulcer patients—implications for diagnosis and disease monitoring. PLoS Negl Trop Dis 5:19. doi: 10.1371/journal.pntd.0001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marsollier L, Honore N, Legras P, Manceau AL, Kouakou H, Carbonnelle B, Cole ST. 2003. Isolation of three Mycobacterium ulcerans strains resistant to rifampin after experimental chemotherapy of mice. Antimicrob Agents Chemother 47:1228–1232. doi: 10.1128/AAC.47.4.1228-1232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Portaels F, Meyers WM, Ablordey A, Castro AG, Chemlal K, de Rijk P, Elsen P, Fissette K, Fraga AG, Lee R, Mahrous E, Small PLC, Stragier P, Torrado E, Van Aerde A, Silva MT, Pedrosa J. 2008. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl Trop Dis 2:e178. doi: 10.1371/journal.pntd.0000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bratschi MW, Ruf M-T, Andreoli A, Minyem JC, Kerber S, Wantong FG, Pritchard J, Chakwera V, Beuret C, Wittwer M, Noumen D, Schürch N, Um Book A, Pluschke G. 2014. Mycobacterium ulcerans persistence at a village water source of Buruli ulcer patients. PLoS Negl Trop Dis 8:e2756. doi: 10.1371/journal.pntd.0002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marsollier L, Stinear T, Aubry J, Saint Andre JP, Robert R, Legras P. 2004. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl Environ Microbiol 70:1097–103. doi: 10.1128/AEM.70.2.1097-1103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eddyani M, Debacker M, Martin A, Aguiar J, Johnson CR, Uwizeye C, Fissette K, Portaels F. 2008. Primary culture of Mycobacterium ulcerans from human tissue specimens after storage in semisolid transport medium. J Clin Microbiol 46:69–72. doi: 10.1128/JCM.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Portaels F. (ed). 2014. Laboratory diagnosis of Buruli ulcer: a manual for health-care providers, p 117. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 96.Palomino JC, Obiang AM, Realini L, Meyers WM, Portaels F. 1998. Effect of oxygen on growth of Mycobacterium ulcerans in the BACTEC system. J Clin Microbiol 36:3420–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eddyani M, Portaels F. 2007. Survival of Mycobacterium ulcerans at 37 degrees C. Clin Microbiol Infect 13:1033–1035. doi: 10.1111/j.1469-0691.2007.01791.x. [DOI] [PubMed] [Google Scholar]
- 98.Portaels F, Johnson P, Meyers WM (ed). 2001. Buruli ulcer. Diagnosis of Mycobacterium ulcerans disease. A manual for health care providers. World Health Organization, Geneva, Switzerland. http://whqlibdoc.who.int/hq/2001/WHO_CDS_CPE_GBUI_2001.4.pdf.
- 99.Tian RBD, Asmar S, Napez C, Lépidi H, Drancourt M. 2017. Effectiveness of purified methylene blue in an experimental model of Mycobacterium ulcerans infection. Int J Antimicrob Agents 49:290–295. doi: 10.1016/j.ijantimicag.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 100.Sanhueza D, Chevillon C, Colwell R, Babonneau J, Marion E, Marsollier L, Guegan JF. 2016. Chitin promotes Mycobacterium ulcerans growth. FEMS Microbiol Ecol 92:27. doi: 10.1093/femsec/fiw067. [DOI] [PubMed] [Google Scholar]
- 101.Petroff SA. 1915. A new and rapid method for the isolation and cultivation of the tubercle bacillus directly from sputum and feces. J Exp Med 21:38–42. doi: 10.1084/jem.21.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Portaels F, Agular J, Fissette K, Fonteyne PA, De Beenhouwer H, de Rijk P, Guedenon A, Lemans R, Steunou C, Zinsou C, Dumonceau JM, Meyers WM. 1997. Direct detection and identification of Mycobacterium ulcerans in clinical specimens by PCR and oligonucleotide-specific capture plate hybridization. J Clin Microbiol 35:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collins CH. 1952. Oxalic acid and acid-iron peroxide in the routine culture of tubercle bacilli from sputum. Tubercle 33:149–151. doi: 10.1016/S0041-3879(52)80084-4. [DOI] [PubMed] [Google Scholar]
- 104.Yajko DM, Nassos PS, Sanders CA, Gonzalez PC, Reingold AL, Horsburgh CR Jr, Hopewell PC, Chin DP, Hadley WK. 1993. Comparison of four decontamination methods for recovery of Mycobacterium avium complex from stools. J Clin Microbiol 31:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marsollier L, Stinear T, Aubry J, Saint André JP, Robert R, Legras P, Manceau A-L, Audrain C, Bourdon S, Kouakou H, Carbonnelle B. 2004. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl Environ Microbiol 70:1097–1103. doi: 10.1128/AEM.70.2.1097-1103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tian RB, Niamke S, Tissot-Dupont H, Drancourt M. 2016. Detection of Mycobacterium ulcerans DNA in the environment, Ivory Coast. PLoS One 11:e0151567. doi: 10.1371/journal.pone.0151567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zogo B, Djenontin A, Carolan K, Babonneau J, Guegan J-F, Eyangoh S, Marion E. 2015. A field study in Benin to investigate the role of mosquitoes and other flying insects in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis 9:e0003941. doi: 10.1371/journal.pntd.0003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo Y, Degang Y, Ohtsuka M, Ishido Y, Ishii N, Suzuki K. 2015. Detection of Mycobacterium ulcerans subsp. shinshuense DNA from a water channel in familial Buruli ulcer cases in Japan. Future Microbiol 10:461–469. doi: 10.2217/fmb.14.152. [DOI] [PubMed] [Google Scholar]
- 109.Konan KL, Doannio JM, Coulibaly NG, Ekaza E, Marion E, Asse H, Kouassi D, N′Goran KE, Dosso M, Marsollier L, Aubry J. 2015. Detection of the IS2404 insertion sequence and ketoreductase produced by Mycobacterium ulcerans in the aquatic Heteroptera in the health districts of Dabou and Tiassale in Cote d'Ivoire. Med Sante Trop 25:44–51. (In French.). doi: 10.1684/mst.2014.0363. [DOI] [PubMed] [Google Scholar]
- 110.Garchitorena A, Ngonghala CN, Texier G, Landier J, Eyangoh S, Bonds MH, Guégan J-F, Roche B. 2015. Environmental transmission of Mycobacterium ulcerans drives dynamics of Buruli ulcer in endemic regions of Cameroon. Sci Rep 5:18055. doi: 10.1038/srep18055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O'Brien CR, Handasyde KA, Hibble J, Lavender CJ, Legione AR, McCowan C, Globan M, Mitchell AT, McCracken HE, Johnson PD, Fyfe JA. 2014. Clinical, microbiological and pathological findings of Mycobacterium ulcerans infection in three Australian possum species. PLoS Negl Trop Dis 8:e2666. doi: 10.1371/journal.pntd.0002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris A, Gozlan R, Marion E, Marsollier L, Andreou D, Sanhueza D, Ruffine R, Couppie P, Guegan JF. 2014. First detection of Mycobacterium ulcerans DNA in environmental samples from South America. PLoS Negl Trop Dis 8:e2660. doi: 10.1371/journal.pntd.0002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fyfe JAM, Lavender CJ, Johnson PDR, Globan M, Sievers A, Azuolas J, Stinear TP. 2007. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl Environ Microbiol 73:4733–4740. doi: 10.1128/AEM.02971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Feldman WH, Karlson AG, Herrick JF. 1957. Mycobacterium ulcerans: pathogenesis of infection in mice, including determinations of dermal temperatures. Am J Pathol 33:1163–1179. [PMC free article] [PubMed] [Google Scholar]
- 115.Williamson HR, Mosi L, Donnell R, Aqqad M, Merritt RW, Small PL. 2014. Mycobacterium ulcerans fails to infect through skin abrasions in a guinea pig infection model: implications for transmission. PLoS Negl Trop Dis 8:e2770. doi: 10.1371/journal.pntd.0002770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schutte D, Umboock A, Pluschke G. 2009. Phagocytosis of Mycobacterium ulcerans in the course of rifampicin and streptomycin chemotherapy in Buruli ulcer lesions. Br J Dermatol 160:273–283. doi: 10.1111/j.1365-2133.2008.08879.x. [DOI] [PubMed] [Google Scholar]
- 117.Bozzo C, Tiberio R, Graziola F, Pertusi G, Valente G, Colombo E, Small PL, Leigheb G. 2010. A Mycobacterium ulcerans toxin, mycolactone, induces apoptosis in primary human keratinocytes and in HaCaT cells. Microbes Infect 12:1258–1263. doi: 10.1016/j.micinf.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 118.Bieri R, Bolz M, Ruf M-T, Pluschke G. 2016. Interferon-γ is a crucial activator of early host immune defense against Mycobacterium ulcerans infection in mice. PLoS Negl Trop Dis 10:e0004450. doi: 10.1371/journal.pntd.0004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bolz M, Ruggli N, Borel N, Pluschke G, Ruf MT. 2016. Local cellular immune responses and pathogenesis of Buruli ulcer lesions in the experimental Mycobacterium ulcerans pig infection model. PLoS Negl Trop Dis 10:e0004678. doi: 10.1371/journal.pntd.0004678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torrado E, Fraga AG, Castro AG, Stragier P, Meyers WM, Portaels F, Silva MT, Pedrosa J. 2007. Evidence for an intramacrophage growth phase of Mycobacterium ulcerans. Infect Immun 75:977–987. doi: 10.1128/IAI.00889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Drancourt M, Jarlier V, Raoult D. 2002. The environmental pathogen Mycobacterium ulcerans grows in amphibian cells at low temperatures. Appl Environ Microbiol 68:6403–6404. doi: 10.1128/AEM.68.12.6403-6404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marsollier L, Aubry J, Coutanceau E, Andre JP, Small PL, Milon G, Legras P, Guadagnini S, Carbonnelle B, Cole ST. 2005. Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell Microbiol 7:935–943. doi: 10.1111/j.1462-5822.2005.00521.x. [DOI] [PubMed] [Google Scholar]
- 123.Coutanceau E, Marsollier L, Brosch R, Perret E, Goossens P, Tanguy M, Cole ST, Small PL, Demangel C. 2005. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell Microbiol 7:1187–1196. doi: 10.1111/j.1462-5822.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 124.Walsh DS, Meyers WM, Portaels F, Lane JE, Mongkolsirichaikul D, Hussem K, Gosi P, Myint KSA. 2005. High rates of apoptosis in human Mycobacterium ulcerans culture-positive Buruli ulcer skin lesions. Am J Trop Med Hyg 73:410–415. [PubMed] [Google Scholar]
- 125.Torrado E, Fraga AG, Logarinho E, Martins TG, Carmona JA, Gama JB, Carvalho MA, Proença F, Castro AG, Pedrosa J. 2010. IFN-γ-dependent activation of macrophages during experimental infections by Mycobacterium ulcerans is impaired by the toxin mycolactone. J Immunol 184:947–955. doi: 10.4049/jimmunol.0902717. [DOI] [PubMed] [Google Scholar]
- 126.Driskell R, Jahoda CAB, Chuong C-M, Watt F, Horsley V. 2014. Defining dermal adipose tissue. Exp Dermatol 23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hayman J. 1993. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J Clin Pathol 46:5–9. doi: 10.1136/jcp.46.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dobos KM, Small PL, Deslauriers M, Quinn FD, King CH. 2001. Mycobacterium ulcerans cytotoxicity in an adipose cell model. Infect Immun 69:7182–7186. doi: 10.1128/IAI.69.11.7182-7186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bolz M, Ruggli N, Ruf M-T, Ricklin ME, Zimmer G, Pluschke G. 2014. Experimental infection of the pig with Mycobacterium ulcerans: a novel model for studying the pathogenesis of Buruli ulcer disease. PLoS Negl Trop Dis 8:e2968. doi: 10.1371/journal.pntd.0002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gryseels S, Amissah D, Durnez L, Vandelannoote K, Leirs H, De Jonckheere J, Silva MT, Portaels F, Ablordey A, Eddyani M. 2012. Amoebae as potential environmental hosts for Mycobacterium ulcerans and other mycobacteria, but doubtful actors in Buruli ulcer epidemiology. PLoS Negl Trop Dis 6:e1764. doi: 10.1371/journal.pntd.0001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Portaels F, Silva MT, Meyers WM. 2009. Buruli ulcer. Clin Dermatol 27:291–305. doi: 10.1016/j.clindermatol.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 132.Lind TK, Darre L, Domene C, Urbanczyk-Lipkowska Z, Cardenas M, Wacklin HP. 2015. Antimicrobial peptide dendrimer interacts with phosphocholine membranes in a fluidity dependent manner: a neutron reflection study combined with molecular dynamics simulations. Biochim Biophys Acta 1848:2075–2084. doi: 10.1016/j.bbamem.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 133.Bag N, Yap DH, Wohland T. 2014. Temperature dependence of diffusion in model and live cell membranes characterized by imaging fluorescence correlation spectroscopy. Biochim Biophys Acta 1838:802–813. doi: 10.1016/j.bbamem.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 134.Capo X, Martorell M, Sureda A, Batle JM, Tur JA, Pons A. 2016. Docosahexaenoic diet supplementation, exercise and temperature affect cytokine production by lipopolysaccharide-stimulated mononuclear cells. J Physiol Biochem 72:421–434. doi: 10.1007/s13105-016-0490-8. [DOI] [PubMed] [Google Scholar]
- 135.Phillips R, Sarfo FS, Guenin-Mace L, Decalf J, Wansbrough-Jones M, Albert ML, Demangel C. 2009. Immunosuppressive signature of cutaneous Mycobacterium ulcerans infection in the peripheral blood of patients with Buruli ulcer disease. J Infect Dis 200:1675–1684. doi: 10.1086/646615. [DOI] [PubMed] [Google Scholar]
- 136.Rolain JM, Vayssier-Taussat M, Saisongkorh W, Merhej V, Gimenez G, Robert C, Le Rhun D, Dehio C, Raoult D. 2013. Partial disruption of translational and posttranslational machinery reshapes growth rates of Bartonella birtlesii. mBio 4:e00115-. doi: 10.1128/mBio.00115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Georgiades K, Raoult D. 2011. The rhizome of Reclinomonas americana, Homo sapiens, Pediculus humanus and Saccharomyces cerevisiae mitochondria. Biol Direct 6:55. doi: 10.1186/1745-6150-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gil R, Sabater-Munoz B, Latorre A, Silva FJ, Moya A. 2002. Extreme genome reduction in Buchnera spp.: toward the minimal genome needed for symbiotic life. Proc Natl Acad Sci U S A 99:4454–4458. doi: 10.1073/pnas.062067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Portaels F, Elsen P, Guimaraes-Peres A, Fonteyne P-A, Meyers WM. 1999. Insects in the transmission of Mycobacterium ulcerans infection. Lancet 353:986. doi: 10.1016/S0140-6736(98)05177-0. [DOI] [PubMed] [Google Scholar]
- 140.Ross BC, Marino L, Oppedisano F, Edwards R, Robins-Browne RM, Johnson PD. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol 35:1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kotlowski R, Martin A, Ablordey A, Chemlal K, Fonteyne P-A, Portaels F. 2004. One-tube cell lysis and DNA extraction procedure for PCR-based detection of Mycobacterium ulcerans in aquatic insects, molluscs and fish. J Med Microbiol 53:927–933. doi: 10.1099/jmm.0.45593-0. [DOI] [PubMed] [Google Scholar]
- 142.Stinear T, Ross BC, Davies JK, Marino L, Robins-Browne RM, Oppedisano F. 1999. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J Clin Microbiol 37:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stragier P, Ablordey A, Durnez L, Portaels F. 2007. VNTR analysis differentiates Mycobacterium ulcerans and IS2404 positive mycobacteria. Syst Appl Microbiol 30:525–530. doi: 10.1016/j.syapm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 144.Williamson HR, Benbow ME, Campbell LP, Johnson CR, Sopoh G, Barogui Y, Merritt RW, Small PLC. 2012. Detection of Mycobacterium ulcerans in the environment predicts prevalence of Buruli ulcer in Benin. PLoS Negl Trop Dis 6:e1506. doi: 10.1371/journal.pntd.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vandelannoote K, Durnez L, Amissah D, Gryseels S, Dodoo A, Yeboah S, Addo P, Eddyani M, Leirs H, Ablordey A, Portaels F. 2010. Application of real-time PCR in Ghana, a Buruli ulcer-endemic country, confirms the presence of Mycobacterium ulcerans in the environment. FEMS Microbiol Lett 304:191–194. doi: 10.1111/j.1574-6968.2010.01902.x. [DOI] [PubMed] [Google Scholar]
- 146.Tano MB, Dassi C, Mosi L, Koussémon M, Bonfoh B. 2017. Molecular characterization of mycolactone producing mycobacteria from aquatic environments in Buruli ulcer non-endemic areas in Côte d'Ivoire. Int J Environ Res Public Health 14:178. doi: 10.3390/ijerph14020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hennigan CE, Myers L, Ferris MJ. 2013. Environmental distribution and seasonal prevalence of Mycobacterium ulcerans in southern Louisiana. Appl Environ Microbiol 79:2648–2656. doi: 10.1128/AEM.03543-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Doannio JM, Konan KL, Dosso FN, Kone AB, Konan YL, Sankare Y, Ekaza E, Coulibaly ND, Odehouri KP, Dosso M, Sess ED, Marsollier L, Aubry J. 2011. Micronecta sp (Corixidae) and Diplonychus sp (Belostomatidae), two aquatic Hemiptera hosts and/or potential vectors of Mycobacterium ulcerans (pathogenic agent of Buruli ulcer) in Cote d'Ivoire. Med Trop (Mars) 71:53–57. (In French.) [PubMed] [Google Scholar]
- 149.Marion E, Deshayes C, Chauty A, Cassisa V, Tchibozo S, Cottin J, Doannio J, Marot A, Marsollier L. 2011. Detection of Mycobacterium ulcerans DNA in water bugs collected outside the aquatic environment in Benin. Med Trop (Mars) 71:169–172. (In French.) [PubMed] [Google Scholar]
- 150.Benbow ME, Williamson H, Kimbirauskas R, McIntosh MD, Kolar R, Quaye C, Akpabey F, Boakye D, Small P, Merritt RW. 2008. Aquatic invertebrates as unlikely vectors of Buruli ulcer disease. Emerg Infect Dis 14:1247–1254. doi: 10.3201/eid1408.071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Willson SJ, Kaufman MG, Merritt RW, Williamson HR, Malakauskas DM, Benbow ME. 2013. Fish and amphibians as potential reservoirs of Mycobacterium ulcerans, the causative agent of Buruli ulcer disease. Infect Ecol Epidemiol 3:10.3402/iee.v3i0.19946. doi: 10.3402/iee.v3i0.19946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Konan KL, Fofana D, Koné AB, Konan YL, Assé H, Kouassi D, Dosso M, Marsollier L, Aubry J, N′Goran K, Doannio JMC. 2015. Inventory of aquatic heteroptera in ponds near the villages of six health districts, endemic to Buruli ulcer in Côte d'Ivoire (West Africa). Experiment 31:2012–2021. http://www.experimentjournal.com/expadmin/pdf_files/Konan%20K.L%20et%20al,%20The%20Experiment,%202015.,Vol.%2031(1),%202012-2021.pdf. [Google Scholar]
- 153.Garchitorena A, Roche B, Kamgang R, Ossomba J, Babonneau J, Landier J, Fontanet A, Flahault A, Eyangoh S, Guegan JF, Marsollier L. 2014. Mycobacterium ulcerans ecological dynamics and its association with freshwater ecosystems and aquatic communities: results from a 12-month environmental survey in Cameroon. PLoS Negl Trop Dis 8:e2879. doi: 10.1371/journal.pntd.0002879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carolan K, Garchitorena A, García-Peña GE, Morris A, Landier J, Fontanet A, Le Gall P, Texier G, Marsollier L, Gozlan RE, Eyangoh S, Lo Seen D, Guégan J-F. 2014. Topography and land cover of watersheds predicts the distribution of the environmental pathogen Mycobacterium ulcerans in aquatic insects. PLoS Negl Trop Dis 8:e3298. doi: 10.1371/journal.pntd.0003298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Raghunathan PL, Whitney EAS, Asamoa K, Stienstra Y, Taylor TH, Amofah GK, Ofori-Adjei D, Dobos K, Guarner J, Martin S, Pathak S, Klutse E, Etuaful S, van der Graaf WTA, van der Werf TS, King CH, Tappero JW, Ashford DA. 2005. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis 40:1445–1453. doi: 10.1086/429623. [DOI] [PubMed] [Google Scholar]
- 156.Lavender CJ, Fyfe JAM, Azuolas J, Brown K, Evans RN, Ray LR, Johnson PDR. 2011. Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in southeastern Australia. PLoS Negl Trop Dis 5:e1305. doi: 10.1371/journal.pntd.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eddyani M, De Jonckheere JF, Durnez L, Suykerbuyk P, Leirs H, Portaels F. 2008. Occurrence of free-living amoebae in communities of low and high endemicity for Buruli ulcer in southern Benin. Appl Environ Microbiol 74:6547–6553. doi: 10.1128/AEM.01066-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McIntosh M, Williamson H, Benbow ME, Kimbirauskas R, Quaye C, Boakye D, Small P, Merritt R. 2014. Associations between Mycobacterium ulcerans and aquatic plant communities of West Africa: implications for Buruli ulcer disease. Ecohealth 11:184–196. doi: 10.1007/s10393-013-0898-3. [DOI] [PubMed] [Google Scholar]
- 159.Mougin B, Tian RBD, Drancourt M. 2015. Tropical plant extracts modulating the growth of Mycobacterium ulcerans. PLoS One 10:e0124626. doi: 10.1371/journal.pone.0124626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Heydorn A, Ersbøll BK, Hentzer M, Parsek MR, Givskov M, Molin S. 2000. Experimental reproducibility in flow-chamber biofilms. Microbiology 146:2409–2415. doi: 10.1099/00221287-146-10-2409. [DOI] [PubMed] [Google Scholar]
- 161.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. 1987. Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 162.Garrett TR, BM, Zhang Z. 2008. Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18:1049–1056. doi: 10.1016/j.pnsc.2008.04.001. [DOI] [Google Scholar]
- 163.Gooday GW. 1990. The ecology of chitin degradation. Adv Microb Ecol 11:387–430. doi: 10.1007/978-1-4684-7612-5_10. [DOI] [Google Scholar]
- 164.Fajardo-Somera RA, Johnk B, Bayram O, Valerius O, Braus GH, Riquelme M. 2015. Dissecting the function of the different chitin synthases in vegetative growth and sexual development in Neurospora crassa. Fungal Genet Biol 75:30–45. doi: 10.1016/j.fgb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 165.Sarbon NM, Sandanamsamy S, Kamaruzaman SF, Ahmad F. 2015. Chitosan extracted from mud crab (Scylla olivicea) shells: physicochemical and antioxidant properties. J Food Sci Technol 52:4266–4275. doi: 10.1007/s13197-014-1522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kaya M, Karaarslan M, Baran T, Can E, Ekemen G, Bitim B, Duman F. 2014. The quick extraction of chitin from an epizoic crustacean species (Chelonibia patula). Nat Prod Res 28:2186–2190. doi: 10.1080/14786419.2014.927469. [DOI] [PubMed] [Google Scholar]
- 167.Kappel L, Gaderer R, Flipphi M, Seidl-Seiboth V. 2016. The N-acetylglucosamine catabolic gene cluster in Trichoderma reesei is controlled by the Ndt80-like transcription factor RON1. Mol Microbiol 99:640–657. doi: 10.1111/mmi.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ruocco N, Costantini S, Guariniello S, Costantini M. 2016. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 21:551. doi: 10.3390/molecules21050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fesel PH, Zuccaro A. 2016. β-Glucan: crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet Biol 90:53–60. doi: 10.1016/j.fgb.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 170.Klusemann J, Kleinow W, Peters W. 1990. The hard parts (trophi) of the rotifer mastax do contain chitin: evidence from studies on Brachionus plicatilis. Histochemistry 94:277–283. doi: 10.1007/BF00266628. [DOI] [PubMed] [Google Scholar]
- 171.Brydon LJ, Gooday GW, Chappell LH, King TP. 1987. Chitin in egg shells of Onchocerca gibsoni and Onchocerca volvulus. Mol Biochem Parasitol 25:267–272. doi: 10.1016/0166-6851(87)90090-9. [DOI] [PubMed] [Google Scholar]
- 172.Sromova D, Lysek H. 1990. Visualization of chitin-protein layer formation in Ascaris lumbricoides egg-shells. Folia Parasitol 37:77–80. [PubMed] [Google Scholar]
- 173.Zhu KY, Merzendorfer H, Zhang W, Zhang J, Muthukrishnan S. 2016. Biosynthesis, turnover, and functions of chitin in insects. Annu Rev Entomol 61:177–196. doi: 10.1146/annurev-ento-010715-023933. [DOI] [PubMed] [Google Scholar]
- 174.Tang WJ, Fernandez JG, Sohn JJ, Amemiya CT. 2015. Chitin is endogenously produced in vertebrates. Curr Biol 25:897–900. doi: 10.1016/j.cub.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Frederiksen RF, Paspaliari DK, Larsen T, Storgaard BG, Larsen MH, Ingmer H, Palcic MM, Leisner JJ. 2013. Bacterial chitinases and chitin-binding proteins as virulence factors. Microbiology 159:833–847. doi: 10.1099/mic.0.051839-0. [DOI] [PubMed] [Google Scholar]
- 176.Zhang YZ, Chen Q, Liu CH, Liu YB, Yi P, Niu KX, Wang YQ, Wang AQ, Yu HY, Pu ZE, Jiang QT, Wei YM, Qi PF, Zheng YL. 2016. Chitin synthase gene FgCHS8 affects virulence and fungal cell wall sensitivity to environmental stress in Fusarium graminearum. Fungal Biol 120:764–774. doi: 10.1016/j.funbio.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 177.Wan J, Zhang X-C, Stacey G. 2008. Chitin signaling and plant disease resistance. Plant Signal Behav 3:831–833. doi: 10.4161/psb.3.10.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Morozov AA, Likhoshway YV. 2016. Evolutionary history of the chitin synthases of eukaryotes. Glycobiology 26:635–639. doi: 10.1093/glycob/cww018. [DOI] [PubMed] [Google Scholar]
- 179.Issola Y, Kouassi A, Dongui B, Biemi J. 2008. Caractéristiques physico-chimiques d'une lagune côtière tropicale: lagune de Fresco (Côte d'Ivoire). Afr Sci 4:368–393. [Google Scholar]
- 180.Kambiré O, Adingra AA, Eblin SG, Aka N, Kakou AC, Koffi-Nevry R. 2014. Characterization of waters of an estuarine lagoon of the Ivory Coast: the Aby lagoon. Larhyss J 20:95–110. (In French.) [Google Scholar]
- 181.Inza B, Soro M, Etchian A, Trokourey A, Bokra Y. 2009. Caractérisation physico-chimique des eaux et des sédiments de surface de la baie des milliardaires, lagune Ébrié, Cote d'Ivoire. Rev Ivoir Sci Technol 13:139–154. [Google Scholar]
- 182.Konan K, Kouassi K, Kouamé K, Kouassi A, Gnakri D. 2013. Hydrologie et hydrochimie des eaux dans la zone de construction du chenal du port de pêche de Grand-Lahou, Côte d'Ivoire. Int J Biol Chem Sci 7:819–831. [Google Scholar]
- 183.N′Guessan YA, Wango T-E, Konan KE, Adingra A, Amani EM, Monde S, Affian K, Aka K. 2015. Hydrologie et morphologie de l'estuaire du fleuve Sassandra, Basse Côte d'Ivoire. Afr Sci 11:161–172. [Google Scholar]
- 184.Wognin V, Monde S, Affian K, Coulibaly A, Aka K. 2007. Waters model circulation in the estuary of Bandama (Ivory Coast). Rivers flows and tide condition's incidence. Sud Sci Technol 5:12. [Google Scholar]
- 185.Abe J, Bakayoko S, Bamba SB, Koffi KP. 1993. Morphology and hydrodynamic in the Bandama inlet. J Ivoir Océanol Limnol 2:9–24. [Google Scholar]
- 186.Asmar S, Sassi M, Phelippeau M, Drancourt M. 2016. Inverse correlation between salt tolerance and host-adaptation in mycobacteria. BMC Res Notes 9:249. doi: 10.1186/s13104-016-2054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lacharme M. 2001. Le contrôle de la salinité dans les rizières. Fascicule 9, Mémento Technique de Riziculture. Coopération Française, Paris, France. [Google Scholar]
- 188.Garchitorena A, Guégan J-F, Léger L, Eyangoh S, Marsollier L, Roche B. 2015. Mycobacterium ulcerans dynamics in aquatic ecosystems are driven by a complex interplay of abiotic and biotic factors. Elife 4:e07616. doi: 10.7554/eLife.07616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dramé KN, Manneh B, Ismail AM. 2013. Rice genetic improvement for abiotic stress tolerance in Africa, p 144–160. In Wopereis MCS, Johnson DE, Ahmadi N, Tollens E, Jalloh A (ed), Realizing Africa's rice promise. CABI. [Google Scholar]
- 190.Wansbrough-Jones M, Phillips R. 2006. Buruli ulcer: emerging from obscurity. Lancet 367:1849–1858. doi: 10.1016/S0140-6736(06)68807-7. [DOI] [PubMed] [Google Scholar]
- 191.Duker AA, Carranza EJM, Hale M. 2004. Spatial dependency of Buruli ulcer prevalence on arsenic-enriched domains in Amansie West District, Ghana: implications for arsenic mediation in Mycobacterium ulcerans infection. Int J Health Geogr 3:19–19. doi: 10.1186/1476-072X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Aiga H, Amano T, Cairncross S, Domako JA, Nanas OK, Coleman S. 2004. Assessing water-related risk factors for Buruli ulcer: a case-control study in Ghana. Am J Trop Med Hyg 71:387–392. [PubMed] [Google Scholar]
- 193.Marsollier L, Aubry J, Saint-Andre JP, Robert R, Legras P, Manceau AL, Bourdon S, Audrain C, Carbonnelle B. 2003. Ecology and transmission of Mycobacterium ulcerans. Pathol Biol 51:490–495. doi: 10.1016/S0369-8114(03)00151-2. [DOI] [PubMed] [Google Scholar]
- 194.Duker AA, Carranza EJ, Hale M. 2005. Arsenic geochemistry and health. Environ Int 31:631–641. doi: 10.1016/j.envint.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 195.Garelick H, Jones H, Dybowska A, Valsami-Jones E. 2008. Arsenic pollution sources. Rev Environ Contam Toxicol 197:17–60. [DOI] [PubMed] [Google Scholar]
- 196.Ampah KA, Asare P, Binnah DD-G, Maccaulley S, Opare W, Röltgen K, Pluschke G, Yeboah-Manu D. 2016. Burden and historical trend of Buruli ulcer prevalence in selected communities along the Offin River of Ghana. PLoS Negl Trop Dis 10:e0004603. doi: 10.1371/journal.pntd.0004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Darie H, Le Guyadec T, Touze JE. 1993. Epidemiological and clinical aspects of Buruli ulcer in Ivory Coast. 124 recent cases. Bull Soc Pathol Exot 86:272–276. [PubMed] [Google Scholar]
- 198.Addo P, Adu-Addai B, Quartey M, Abbas M, Okang I, Owusu E, Ofori-Adjei D, Awumbila B. 2006. Clinical and histopathological presentation of Buruli ulcer in experimentally infected grasscutters (Thryonomys swinderianus). Internet J Trop Med 3:1–15. [Google Scholar]
- 199.Elsner L, Wayne J, O'Brien CR, McCowan C, Malik R, Hayman JA, Globan M, Lavender CJ, Fyfe JA. 2008. Localised Mycobacterium ulcerans infection in a cat in Australia. J Feline Med Surg 10:407–412. doi: 10.1016/j.jfms.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.van Zyl A, Daniel J, Wayne J, McCowan C, Malik R, Jelfs P, Lavender CJ, Fyfe JA. 2010. Mycobacterium ulcerans infections in two horses in south-eastern Australia. Aust Vet J 88:101–106. doi: 10.1111/j.1751-0813.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 201.O'Brien CR, McMillan E, Harris O, O'Brien DP, Lavender CJ, Globan M, Legione AR, Fyfe JA. 2011. Localised Mycobacterium ulcerans infection in four dogs. Aust Vet J 89:506–510. doi: 10.1111/j.1751-0813.2011.00850.x. [DOI] [PubMed] [Google Scholar]
- 202.O'Brien C, Kuseff G, McMillan E, McCowan C, Lavender C, Globan M, Jerrett I, Oppedisano F, Johnson P, Fyfe J. 2013. Mycobacterium ulcerans infection in two alpacas. Aust Vet J 91:296–300. doi: 10.1111/avj.12071. [DOI] [PubMed] [Google Scholar]
- 203.Mitchell PJ, Jerrett IV, Slee KJ. 1984. Skin ulcers caused by Mycobacterium ulcerans in koalas near Bairnsdale, Australia. Pathology 16:256–260. doi: 10.3109/00313028409068533. [DOI] [PubMed] [Google Scholar]
- 204.Mitchell PJ, McOrist S, Bilney R. 1987. Epidemiology of Mycobacterium ulcerans infection in koalas (Phascolarctos cinereus) on Raymond Island, southeastern Australia. J Wildl Dis 23:386–390. doi: 10.7589/0090-3558-23.3.386. [DOI] [PubMed] [Google Scholar]
- 205.McOrist S, Jerrett IV, Anderson M, Hayman J. 1985. Cutaneous and respiratory tract infection with Mycobacterium ulcerans in two koalas (Phascolarctos cinereus). J Wildl Dis 21:171–173. doi: 10.7589/0090-3558-21.2.171. [DOI] [PubMed] [Google Scholar]
- 206.Tobias NJ, Doig KD, Medema MH, Chen H, Haring V, Moore R, Seemann T, Stinear TP. 2013. Complete genome sequence of the frog pathogen Mycobacterium ulcerans ecovar liflandii. J Bacteriol 195:556-564. doi: 10.1128/JB.02132-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tobias NJ, Ammisah NA, Ahortor EK, Wallace JR, Ablordey A, Stinear TP. 2016. Snapshot fecal survey of domestic animals in rural Ghana for Mycobacterium ulcerans. PeerJ 4:e2065. doi: 10.7717/peerj.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sakaguchi K, Iima H, Hirayama K, Okamoto M, Matsuda K, Miyasho T, Kasamatsu M, Hasegawa K, Taniyama H. 2011. Mycobacterium ulcerans infection in an Indian flap-shelled turtle (Lissemys punctata punctata). J Vet Med Sci 73:1217–1220. doi: 10.1292/jvms.10-0386. [DOI] [PubMed] [Google Scholar]
- 209.Ohtsuka M, Kikuchi N, Yamamoto T, Suzutani T, Nakanaga K, Suzuki K, Ishii N. 2014. Buruli ulcer caused by Mycobacterium ulcerans subsp shinshuense: a rare case of familial concurrent occurrence and detection of insertion sequence 2404 in Japan. JAMA Dermatol 150:64–67. doi: 10.1001/jamadermatol.2013.6816. [DOI] [PubMed] [Google Scholar]
- 210.Aboagye SY, Ampah KA, Ross A, Asare P, Otchere ID, Fyfe J, Yeboah-Manu D. 2017. Seasonal pattern of Mycobacterium ulcerans, the causative agent of Buruli ulcer, in the environment in Ghana. Microb Ecol 74:350–361. doi: 10.1007/s00248-017-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zingue D, Bouam A, Militello M, Drancourt M. 2017. High-throughput carbon substrate profiling of Mycobacterium ulcerans suggests potential environmental reservoirs. PLoS Negl Trop Dis 11:e0005303. doi: 10.1371/journal.pntd.0005303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Morris AL, Guegan JF, Andreou D, Marsollier L, Carolan K, Le Croller M, Sanhueza D, Gozlan RE. 2016. Deforestation-driven food-web collapse linked to emerging tropical infectious disease, Mycobacterium ulcerans. Sci Adv 2:e1600387. doi: 10.1126/sciadv.1600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stienstra Y, van Roest MH, van Wezel MJ, Wiersma IC, Hospers IC, Dijkstra PU, Johnson RC, Ampadu EO, Gbovi J, Zinsou C, Etuaful S, Klutse EY, van der Graaf WT, van der Werf TS. 2005. Factors associated with functional limitations and subsequent employment or schooling in Buruli ulcer patients. Trop Med Int Health 10:1251–1257. doi: 10.1111/j.1365-3156.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 214.Lehman L, Simonet V, Saunderson P, Agbenorku P. 2006. Buruli ulcer: prevention of disability (POD). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 215.Barogui Y, Johnson RC, van der Werf TS, Sopoh G, Dossou A, Dijkstra PU, Stienstra Y. 2009. Functional limitations after surgical or antibiotic treatment for Buruli ulcer in Benin. Am J Trop Med Hyg 81:82–87. [PubMed] [Google Scholar]
- 216.Sizaire V, Nackers F, Comte E, Portaels F. 2006. Mycobacterium ulcerans infection: control, diagnosis, and treatment. Lancet Infect Dis 6:288–296. doi: 10.1016/S1473-3099(06)70464-9. [DOI] [PubMed] [Google Scholar]
- 217.WHO. 2012. Treatment of Mycobacterium ulcerans disease (Buruli ulcer): guidance for health workers. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 218.WHO. 2004. Global Buruli ulcer initiative. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 219.Wallace JR, Mangas KM, Porter JL, Marcsisin R, Pidot SJ, Howden BO, Omansen TF, Zeng W, Axford JK, Johnson PDR, Stinear TP. 2017. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis 11:e0005553. doi: 10.1371/journal.pntd.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Johnson PD, Stinear TP, Hayman JA. 1999. Mycobacterium ulcerans—a mini-review. J Med Microbiol 48:511–513. doi: 10.1099/00222615-48-6-511. [DOI] [PubMed] [Google Scholar]
- 221.Hayman J. 1991. Postulated epidemiology of Mycobacterium ulcerans infection. Int J Epidemiol 20:1093–1098. doi: 10.1093/ije/20.4.1093. [DOI] [PubMed] [Google Scholar]
- 222.Connor DH, Lunn HF. 1965. Mycobacterium ulcerans infection (with comments on pathogenesis). Int J Lepr 33:698–709. [PubMed] [Google Scholar]
- 223.Duker AA, Portaels F, Hale M. 2006. Pathways of Mycobacterium ulcerans infection: a review. Environ Int 32:567–573. doi: 10.1016/j.envint.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 224.Pszolla N, Sarkar MR, Strecker W, Kern P, Kinzl L, Meyers WM, Portaels F. 2003. Buruli ulcer: a systemic disease. Clin Infect Dis 37:e78–. doi: 10.1086/377170. [DOI] [PubMed] [Google Scholar]
- 225.Debacker M, Zinsou C, Aguiar J, Meyers WM, Portaels F. 2003. First case of Mycobacterium ulcerans disease (Buruli ulcer) following a human bite. Clin Infect Dis 36:67–68. doi: 10.1086/367660. [DOI] [PubMed] [Google Scholar]
- 226.Ukwaja KN, Meka AO, Chukwuka A, Asiedu KB, Huber KL, Eddyani M, Chukwu JN, Anyim MC, Nwafor CC, Oshi DC, Madichie NO, Ekeke N, Njoku M, Ntana K. 2016. Buruli ulcer in Nigeria: results of a pilot case study in three rural districts. Infect Dis Poverty 5:39. doi: 10.1186/s40249-016-0119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mavinga Phanzu D, Suykerbuyk P, Saunderson P, Ngwala Lukanu P, Masamba Minuku JB, Imposo DB, Mbadu Diengidi B, Kayinua M, Tamfum Muyembe JJ, Tshindele Lutumba P, de Jong BC, Portaels F, Boelaert M. 2013. Burden of Mycobacterium ulcerans disease (Buruli ulcer) and the underreporting ratio in the territory of Songololo, Democratic Republic of Congo. PLoS Negl Trop Dis 7:e2563. doi: 10.1371/journal.pntd.0002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bayonne Manou LS, Portaels F, Eddyani M, Book AU, Vandelannoote K, de Jong BC. 2013. Mycobacterium ulcerans disease (Buruli ulcer) in Gabon: 2005–2011. Med Sante Trop 23:450–457. (In French.) [DOI] [PubMed] [Google Scholar]
- 229.Porten K, Sailor K, Comte E, Njikap A, Sobry A, Sihom F, Meva'a A, Eyangoh S, Myatt M, Nackers F, Grais RF. 2009. Prevalence of Buruli ulcer in Akonolinga health district, Cameroon: results of a cross sectional survey. PLoS Negl Trop Dis 3:466. doi: 10.1371/journal.pntd.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.O'Brien DP, Friedman ND, Cowan R, Pollard J, McDonald A, Callan P, Hughes A, Athan E. 2015. Mycobacterium ulcerans in the elderly: more severe disease and suboptimal outcomes. PLoS Negl Trop Dis 9:e0004253. doi: 10.1371/journal.pntd.0004253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Friedman ND, Athan E, Hughes AJ, Khajehnoori M, McDonald A, Callan P, Rahdon R, O'Brien DP. 2013. Mycobacterium ulcerans disease: experience with primary oral medical therapy in an Australian cohort. PLoS Negl Trop Dis 7:e2315. doi: 10.1371/journal.pntd.0002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.WHO. 2016. Buruli ulcer (Mycobacterium ulcerans infection). WHO, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs199/en/ Accessed October 2017. [Google Scholar]
- 233.Yotsu RR, Murase C, Sugawara M, Suzuki K, Nakanaga K, Ishii N, Asiedu K. 2015. Revisiting Buruli ulcer. J Dermatol 42:1033–1041. doi: 10.1111/1346-8138.13049. [DOI] [PubMed] [Google Scholar]
- 234.Hofer M, Hirschel B, Kirschner P, Beghetti M, Kaelin A, Siegrist CA, Suter S, Teske A, Bottger EC. 1993. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N Engl J Med 328:1007–1009. doi: 10.1056/NEJM199304083281405. [DOI] [PubMed] [Google Scholar]
- 235.Kenu E, Nyarko KM, Seefeld L, Ganu V, Käser M, Lartey M, Calys-Tagoe BNL, Koram K, Adanu R, Razum O, Afari E, Binka FN. 2014. Risk factors for Buruli ulcer in Ghana—a case control study in the Suhum-Kraboa-Coaltar and Akuapem South Districts of the eastern region. PLoS Negl Trop Dis 8:e3279. doi: 10.1371/journal.pntd.0003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lagarrigue V, Portaels F, Meyers WM, Aguiar J. 2000. Buruli ulcer: risk of bone involvement! Apropos of 33 cases observed in Benin. Med Trop 60:262–266. (In French.) [PubMed] [Google Scholar]
- 237.Walsh DS, Dela Cruz EC, Abalos RM, Tan EV, Walsh GP, Portaels F, Meyers WM. 2007. Clinical and histologic features of skin lesions in a cynomolgus monkey experimentally infected with Mycobacterium ulcerans (Buruli ulcer) by intradermal inoculation. Am J Trop Med Hyg 76:132–134. [PubMed] [Google Scholar]
- 238.Niang F, Sarfo FS, Frimpong M, Guenin-Mace L, Wansbrough-Jones M, Stinear T, Phillips RO, Demangel C. 2015. Metabolomic profiles delineate mycolactone signature in Buruli ulcer disease. Sci Rep 5:17693. doi: 10.1038/srep17693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vincent QB, Ardant M-F, Marsollier L, Chauty A, Alcaïs A. 2014. HIV infection and Buruli ulcer in Africa. Lancet Infect Dis 14:796–797. doi: 10.1016/S1473-3099(14)70882-5. [DOI] [PubMed] [Google Scholar]
- 240.Johnson RC, Nackers F, Glynn JR, de Biurrun Bakedano E, Zinsou C, Aguiar J, Tonglet R, Portaels F. 2008. Association of HIV infection and Mycobacterium ulcerans disease in Benin. AIDS 22:901–903. doi: 10.1097/QAD.0b013e3282f7690a. [DOI] [PubMed] [Google Scholar]
- 241.Adu EJK, Ampadu E. 2015. Mycobacterium ulcerans disease in the middle belt of Ghana: an eight-year review from six endemic districts. Int J Mycobacteriol 4:138–142. doi: 10.1016/j.ijmyco.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 242.Capela C, Sopoh GE, Houezo JG, Fiodessihoué R, Dossou AD, Costa P, Fraga AG, Menino JF, Silva-Gomes R, Ouendo EM, Rodrigues F, Pedrosa J. 2015. Clinical epidemiology of Buruli ulcer from Benin (2005–2013): effect of time-delay to diagnosis on clinical forms and severe phenotypes. PLoS Negl Trop Dis 9:e0004005. doi: 10.1371/journal.pntd.0004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Beissner M, Arens N, Wiedemann F, Piten E, Kobara B, Bauer M, Herbinger K-H, Badziklou K, Banla Kere A, Löscher T, Nitschke J, Bretzel G. 2015. Treatment outcome of patients with Buruli ulcer disease in Togo. PLoS Negl Trop Dis 9:e0004170. doi: 10.1371/journal.pntd.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.James K, Attipou KK, James YE, Blakime M, Tignokpa N, Abete B. 2003. Buruli ulcer in Togo: a hospital study. Sante 13:43–47. [PubMed] [Google Scholar]
- 245.Berger S. 2016. Tropical skin ulcers: global status. Gideon Informatics, Los Angeles, CA. [Google Scholar]
- 246.Johnson RC, Sopoh GE, Barogui Y, Dossou A, Fourn L, Zohoun T. 2008. Surveillance system for Buruli ulcer in Benin: results after four years. Sante 18:9–13. (In French.) [DOI] [PubMed] [Google Scholar]
- 247.Jiao J, Yao L, Li Y, Wong S, Sau-Fan Ng F, Teng Y, Guo Y. 2012. Thermal physiology and local responses of human body during exercise in hot conditions. J Fiber Bioeng Inform 5:115–124. doi: 10.3993/jfbi06201201. [DOI] [Google Scholar]
- 248.Aschoff J, Wever R. 1958. Kern und Schale im Wfumehaushalt des Menschen. Naturwissenschaften 45:477–485. doi: 10.1007/BF00635546. [DOI] [Google Scholar]
- 249.Brou G, Houndonougbo F, Aboh A, Mensah G, Fantodji A. 2012. Effet de la variation temporelle de la température ambiante journalière sur le poids des œufs de poules pondeuses ISA Brown en Côte-d'Ivoire. Int J Biol Chem Sci 6:2158–2169. [Google Scholar]
- 250.Miller CS, Gosling WD. 2014. Quaternary forest associations in lowland tropical West Africa. Quat Sci Rev 84:7–25. doi: 10.1016/j.quascirev.2013.10.027. [DOI] [Google Scholar]
- 251.Vogel M, Bayi PF, Ruf M-T, Bratschi MW, Bolz M, Um Boock A, Zwahlen M, Pluschke G, Junghanss T. 2016. Local heat application for the treatment of Buruli ulcer: results of a phase II open label single center non comparative clinical trial. Clin Infect Dis 62:342–350. doi: 10.1093/cid/civ883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PD, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, Clarke K, Cronin A, Davis P, Goodhead I, Holroyd N, Jagels K, Lord A, Moule S, Mungall K, Norbertczak H, Quail MA, Rabbinowitsch E, Walker D, White B, Whitehead S, Small PL, Brosch R, Ramakrishnan L, Fischbach MA, Parkhill J, Cole ST. 2008. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res 18:729–741. doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ahoua L, Guetta A, Ekanza E, Bouzid S, N′Guessan R, Dosso M. 2009. Risk factors for Buruli ulcer in Côte d'Ivoire: results of a case-control study, August 2001. Afr J Biotechnol 8:536–546. [Google Scholar]
- 254.Tuffour J, Owusu-Mireku E, Ruf M-T, Aboagye S, Kpeli G, Akuoku V, Pereko J, Paintsil A, Bonney K, Ampofo W, Pluschke G, Yeboah-Manu D. 2015. Challenges associated with management of Buruli ulcer/human immunodeficiency virus coinfection in a treatment center in Ghana: a case series study. Am J Trop Med Hyg 93:216–223. doi: 10.4269/ajtmh.14-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Christinet V, Comte E, Ciaffi L, Odermatt P, Serafini M, Antierens A, Rossel L, Nomo A-B, Nkemenang P, Tsoungui A, Delhumeau C, Calmy A. 2014. Impact of human immunodeficiency virus on the severity of Buruli ulcer disease: results of a retrospective study in Cameroon. Open Forum Infect Dis 1:ofu021. doi: 10.1093/ofid/ofu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Toll A, Gallardo F, Ferran M, Gilaberte M, Iglesias M, Gimeno JL, Rondini S, Pujol RM. 2005. Aggressive multifocal Buruli ulcer with associated osteomyelitis in an HIV-positive patient. Clin Exp Dermatol 30:649–651. doi: 10.1111/j.1365-2230.2005.01892.x. [DOI] [PubMed] [Google Scholar]
- 257.Komenan K, Elidjé EJ, Ildevert GP, Yao KI, Kanga K, Kouamé KA, Abdoulaye S, Hamdam KS, Yao YP, Jean-Marie K. 2013. Multifocal Buruli ulcer associated with secondary infection in HIV positive patient. Case Rep Med 2013:348628. doi: 10.1155/2013/348628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Phillips RO, Frimpong M, Sarfo FS, Kretschmer B, Beissner M, Debrah A, Ampem-Amoako Y, Abass KM, Thompson W, Duah MS, Abotsi J, Adjei O, Fleischer B, Bretzel G, Wansbrough-Jones M, Jacobsen M. 2014. Infection with Mansonella perstans nematodes in Buruli ulcer patients, Ghana. Emerg Infect Dis 20:1000–1003. doi: 10.3201/eid2006.131501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mougin B, Avenel-Audran M, Hasseine L, Martin L, Cottin J, Pomares C, Delaunay P, Marty P, Ravel C, Chabasse D, Abgueguen P. 2011. A cutaneous ulcer resulting from Mycobacterium ulcerans—Leishmania braziliensis coinfection in South America. Am J Trop Med Hyg 85:897–899. doi: 10.4269/ajtmh.2011.11-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nackers F, Tonglet R, Slachmuylder V, Johnson RC, Robert A, Zinsou C, Glynn JR, Portaels F, Gala JL. 2007. Association between haemoglobin variants S and C and Mycobacterium ulcerans disease (Buruli ulcer): a case-control study in Benin. Trop Med Int Health 12:511–518. doi: 10.1111/j.1365-3156.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- 261.Siegmund V, Adjei O, Racz P, Berberich C, Klutse E, van Vloten F, Kruppa T, Fleischer B, Bretzel G. 2005. Dry-reagent-based PCR as a novel tool for laboratory confirmation of clinically diagnosed Mycobacterium ulcerans-associated disease in areas in the tropics where M. ulcerans is endemic. J Clin Microbiol 43:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Guarner J, Bartlett J, Whitney EA, Raghunathan PL, Stienstra Y, Asamoa K, Etuaful S, Klutse E, Quarshie E, van der Werf TS, van der Graaf WT, King CH, Ashford DA. 2003. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis 9:651–656. doi: 10.3201/eid0906.020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tabah EN, Nsagha DS, Bissek AC, Njamnshi AK, Bratschi MW, Pluschke G, Um Boock A. 2016. Buruli ulcer in Cameroon: the development and impact of the National Control Programme. PLoS Negl Trop Dis 10:e0004224. doi: 10.1371/journal.pntd.0004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Herbinger K-H, Adjei O, Awua-Boateng N-Y, Nienhuis WA, Kunaa L, Siegmund V, Nitschke J, Thompson W, Klutse E, Agbenorku P, Schipf A, Reu S, Racz P, Fleischer B, Beissner M, Fleischmann E, Helfrich K, van der Werf TS, Lüscher T, Bretzel G. 2009. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease. Clin Infect Dis 48:1055–1064. doi: 10.1086/597398. [DOI] [PubMed] [Google Scholar]
- 265.WHO. 2008. Guidance on sampling techniques for laboratory-confirmation of Mycobacterium ulcerans infection (Buruli ulcer disease). WHO, Geneva, Switzerland: http://www.who.int/buruli/Guidance_sampling_techniques_MU_infection.pdf. [Google Scholar]
- 266.Beissner M, Herbinger KH, Bretzel G. 2010. Laboratory diagnosis of Buruli ulcer disease. Future Microbiol 5:363–370. doi: 10.2217/fmb.10.3. [DOI] [PubMed] [Google Scholar]
- 267.Beissner M, Phillips RO, Battke F, Bauer M, Badziklou K, Sarfo FS, Maman I, Rhomberg A, Piten E, Frimpong M, Huber KL, Symank D, Jansson M, Wiedemann FX, Banla Kere A, Herbinger KH, Loscher T, Bretzel G. 2015. Loop-mediated isothermal amplification for laboratory confirmation of Buruli ulcer disease—towards a point-of-care test. PLoS Negl Trop Dis 9:e0004219. doi: 10.1371/journal.pntd.0004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Affolabi D, Bankole H, Ablordey A, Hounnouga J, Koutchakpo P, Sopoh G, Aguiar J, Dossou A, Johnson RC, Anagonou S, Portaels F. 2008. Effects of grinding surgical tissue specimens and smear staining methods on Buruli ulcer microscopic diagnosis. Trop Med Int Health 13:187–190. doi: 10.1111/j.1365-3156.2007.01989.x. [DOI] [PubMed] [Google Scholar]
- 269.Shinnick TM, Iademarco MF, Ridderhof JC. 2005. National plan for reliable tuberculosis laboratory services using a systems approach. Recommendations from CDC and the Association of Public Health Laboratories Task Force on Tuberculosis Laboratory Services. MMWR Recomm Rep 54:1–12. [PubMed] [Google Scholar]
- 270.Wadagni A, Frimpong M, Phanzu DM, Ablordey A, Kacou E, Gbedevi M, Marion E, Xing Y, Babu VS, Phillips RO, Wansbrough-Jones M, Kishi Y, Asiedu K. 2015. Simple, rapid Mycobacterium ulcerans disease diagnosis from clinical samples by fluorescence of mycolactone on thin layer chromatography. PLoS Negl Trop Dis 9:e0004247. doi: 10.1371/journal.pntd.0004247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.N′Guessan K, Kouassi Y, Bouzid S, Ehuie P, Koffi K, Oniangue C, Aka N, Dosso M. 2001. Value and limits of microscopy of exudates in Mycobacterium ulcerans cutaneous infection in Cote d'Ivoire. Bull Soc Pathol Exot 94:9–10. (In French.) [PubMed] [Google Scholar]
- 272.Asiedu K, Wansbrough-Jones M. 2007. Mycobacterium ulcerans infection (Buruli or Bairnsdale ulcer): challenges in developing management strategies. Med J Aust 186:55–56. [DOI] [PubMed] [Google Scholar]
- 273.Siegmund V, Adjei O, Nitschke J, Thompson W, Klutse E, Herbinger KH, Thompson R, van Vloten F, Racz P, Fleischer B, Loescher T, Bretzel G. 2007. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clin Infect Dis 45:68–75. doi: 10.1086/518604. [DOI] [PubMed] [Google Scholar]
- 274.Babonneau J, Bernard C, Marion E, Chauty A, Kempf M, Robert R, Marsollier L. 2015. Development of a dry-reagent-based qPCR to facilitate the diagnosis of Mycobacterium ulcerans infection in endemic countries. PLoS Negl Trop Dis 9:e0003606. doi: 10.1371/journal.pntd.0003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.de Souza DK, Quaye C, Mosi L, Addo P, Boakye DA. 2012. A quick and cost effective method for the diagnosis of Mycobacterium ulcerans infection. BMC Infect Dis 12:1471–2334. doi: 10.1186/1471-2334-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Njiru ZK, Yeboah-Manu D, Stinear TP, Fyfe JA. 2012. Rapid and sensitive detection of Mycobacterium ulcerans by use of a loop-mediated isothermal amplification test. J Clin Microbiol 50:1737–1741. doi: 10.1128/JCM.06460-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ablordey A, Amissah DA, Aboagye IF, Hatano B, Yamazaki T, Sata T, Ishikawa K, Katano H. 2012. Detection of Mycobacterium ulcerans by the loop mediated isothermal amplification method. PLoS Negl Trop Dis 6:e1590. doi: 10.1371/journal.pntd.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Laval F, Laneelle MA, Deon C, Monsarrat B, Daffe M. 2001. Accurate molecular mass determination of mycolic acids by MALDI-TOF mass spectrometry. Anal Chem 73:4537–4544. doi: 10.1021/ac0105181. [DOI] [PubMed] [Google Scholar]
- 279.Zingue D, Flaudrops C, Drancourt M. 2016. Direct matrix-assisted laser desorption ionisation time-of-flight mass spectrometry identification of mycobacteria from colonies. Eur J Clin Microbiol Infect Dis 35:1983–1987. doi: 10.1007/s10096-016-2750-5. [DOI] [PubMed] [Google Scholar]
- 280.Sakyi SA, Aboagye SY, Otchere ID, Liao AM, Caltagirone TG, Yeboah-Manu D. 2016. RNA aptamer that specifically binds to mycolactone and serves as a diagnostic tool for diagnosis of Buruli ulcer. PLoS Negl Trop Dis 10:e0004950. doi: 10.1371/journal.pntd.0004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Thangaraj HS, Adjei O, Allen BW, Portaels F, Evans MR, Banerjee DK, Wansbrough-Jones MH. 2000. In vitro activity of ciprofloxacin, sparfloxacin, ofloxacin, amikacin and rifampicin against Ghanaian isolates of Mycobacterium ulcerans. J Antimicrob Chemother 45:231–233. doi: 10.1093/jac/45.2.231. [DOI] [PubMed] [Google Scholar]
- 282.Ji B, Lefrançois S, Robert J, Chauffour A, Truffot C, Jarlier V. 2006. In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob Agents Chemother 50:1921–1926. doi: 10.1128/AAC.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yeboah-Manu D, Kpeli GS, Ruf MT, Asan-Ampah K, Quenin-Fosu K, Owusu-Mireku E, Paintsil A, Lamptey I, Anku B, Kwakye-Maclean C, Newman M, Pluschke G. 2013. Secondary bacterial infections of buruli ulcer lesions before and after chemotherapy with streptomycin and rifampicin. PLoS Negl Trop Dis 7:e2191. doi: 10.1371/journal.pntd.0002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Portaels F, Traore H, De Ridder K, Meyers WM. 1998. In vitro susceptibility of Mycobacterium ulcerans to clarithromycin. Antimicrob Agents Chemother 42:2070–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pattyn SR, van Ermengem J. 1968. DDS sensitivity of mycobacteria. Antagonistic effect of PABA for M. ulcerans and M. kansasii. Int J Lepr Other Mycobact Dis 36:427–431. [PubMed] [Google Scholar]
- 286.Portaels F, Van den Breen L, Pattyn SR. 1982. Sensitivity of mycobacteria to dapsone. Arzneimittelforschung 32:1124–1125. [PubMed] [Google Scholar]
- 287.Ji B, Chauffour A, Robert J, Lefrancois S, Jarlier V. 2007. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother 51:3737–3739. doi: 10.1128/AAC.00730-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Almeida D, Converse PJ, Ahmad Z, Dooley KE, Nuermberger EL, Grosset JH. 2011. Activities of rifampin, rifapentine and clarithromycin alone and in combination against Mycobacterium ulcerans disease in mice. PLoS Negl Trop Dis 5:e933. doi: 10.1371/journal.pntd.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Scherr N, Pluschke G, Thompson CJ, Ramón-García S. 2015. Selamectin is the avermectin with the best potential for Buruli ulcer treatment. PLoS Negl Trop Dis 9:e0003996. doi: 10.1371/journal.pntd.0003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Omansen TF, Porter JL, Johnson PD, van der Werf TS, Stienstra Y, Stinear TP. 2015. In-vitro activity of avermectins against Mycobacterium ulcerans. PLoS Negl Trop Dis 9:e0003549. doi: 10.1371/journal.pntd.0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hopla CE, Durden LA, Keirans JE. 1994. Ectoparasites and classification. Rev Sci Tech 13:985–1017. doi: 10.20506/rst.13.4.815. [DOI] [PubMed] [Google Scholar]
- 292.Beissner M, Awua-Boateng NY, Thompson W, Nienhuis WA, Klutse E, Agbenorku P, Nitschke J, Herbinger KH, Siegmund V, Fleischmann E, Adjei O, Fleischer B, van der Werf TS, Loscher T, Bretzel G. 2010. A genotypic approach for detection, identification, and characterization of drug resistance in Mycobacterium ulcerans in clinical samples and isolates from Ghana. Am J Trop Med Hyg 83:1059–1065. doi: 10.4269/ajtmh.2010.10-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Dega H, Bentoucha A, Robert J, Jarlier V, Grosset J. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob Agents Chemother 46:3193–3196. doi: 10.1128/AAC.46.10.3193-3196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chauffour A, Robert J, Veziris N, Aubry A, Jarlier V. 2016. Sterilizing activity of fully oral intermittent regimens against Mycobacterium ulcerans infection in mice. PLoS Negl Trop Dis 10:e0005066. doi: 10.1371/journal.pntd.0005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Friedman ND, Athan E, Walton AL, O'Brien DP. 2016. Increasing experience with primary oral medical therapy for Mycobacterium ulcerans disease in an Australian cohort. Antimicrob Agents Chemother 60:2692–2695. doi: 10.1128/AAC.02853-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cowan R, Athan E, Friedman ND, Hughes AJ, McDonald A, Callan P, Fyfe J, O'Brien DP. 2015. Mycobacterium ulcerans treatment—can antibiotic duration be reduced in selected patients? PLoS Negl Trop Dis 9:e0003503. doi: 10.1371/journal.pntd.0003503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Converse PJ, Tyagi S, Xing Y, Li SY, Kishi Y, Adamson J, Nuermberger EL, Grosset JH. 2015. Efficacy of rifampin plus clofazimine in a murine model of Mycobacterium ulcerans disease. PLoS Negl Trop Dis 9:e0003823. doi: 10.1371/journal.pntd.0003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Junghanss T, Um Boock A, Vogel M, Schuette D, Weinlaeder H, Pluschke G. 2009. Phase change material for thermotherapy of Buruli ulcer: a prospective observational single centre proof-of-principle trial. PLoS Negl Trop Dis 3:17. doi: 10.1371/journal.pntd.0000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.WHO. 2012. Guidance for health workers. Treatment of Mycobacterium ulcerans disease (Buruli ulcer). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 300.Chauty A, Ardant M-F, Adeye A, Euverte H, Guédénon A, Johnson C, Aubry J, Nuermberger E, Grosset J. 2007. Promising clinical efficacy of streptomycin-rifampin combination for treatment of Buruli ulcer (Mycobacterium ulcerans Disease). Antimicrob Agents Chemother 51:4029–4035. doi: 10.1128/AAC.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Teelken MA, Stienstra Y, Ellen DE, Quarshie E, Klutse E, van der Graaf WT, van der Werf TS. 2003. Buruli ulcer: differences in treatment outcome between two centres in Ghana. Acta Trop 88:51–56. doi: 10.1016/S0001-706X(03)00170-0. [DOI] [PubMed] [Google Scholar]
- 302.Klis S, Stienstra Y, Phillips RO, Abass KM, Tuah W, van der Werf TS. 2014. Long term streptomycin toxicity in the treatment of Buruli ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis 8:e2739. doi: 10.1371/journal.pntd.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Eddyani M, Vandelannoote K, Meehan CJ, Bhuju S, Porter JL, Aguiar J, Seemann T, Jarek M, Singh M, Portaels F, Stinear TP, de Jong BC. 2015. A genomic approach to resolving relapse versus reinfection among four cases of Buruli ulcer. PLoS Negl Trop Dis 9:e0004158. doi: 10.1371/journal.pntd.0004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nienhuis WA, Stienstra Y, Abass KM, Tuah W, Thompson WA, Awuah PC, Awuah-Boateng NY, Adjei O, Bretzel G, Schouten JP, van der Werf TS. 2012. Paradoxical responses after start of antimicrobial treatment in Mycobacterium ulcerans infection. Clin Infect Dis 54:519–526. doi: 10.1093/cid/cir856. [DOI] [PubMed] [Google Scholar]
- 305.Sugawara M, Ishii N, Nakanaga K, Suzuki K, Umebayashi Y, Makigami K, Aihara M. 2015. Exploration of a standard treatment for Buruli ulcer through a comprehensive analysis of all cases diagnosed in Japan. J Dermatol 42:588–595. doi: 10.1111/1346-8138.12851. [DOI] [PubMed] [Google Scholar]
- 306.Chauty A, Ardant M-F, Marsollier L, Pluschke G, Landier J, Adeye A, Goundoté A, Cottin J, Ladikpo T, Ruf T, Ji B. 2011. Oral treatment for Mycobacterium ulcerans infection: results from a pilot study in Benin. Clin Infect Dis 52:94–96. doi: 10.1093/cid/ciq072. [DOI] [PubMed] [Google Scholar]
- 307.WHO. 2017. Neglected tropical diseases. Biennial meeting of the Global Buruli ulcer Initiative, Geneva, 20 to 22 March 2017. WHO, Geneva, Switzerland. [Google Scholar]
- 308.Nienhuis WA, Stienstra Y, Thompson WA, Awuah PC, Abass KM, Tuah W, Awua-Boateng NY, Ampadu EO, Siegmund V, Schouten JP, Adjei O, Bretzel G, van der Werf TS. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664–672. doi: 10.1016/S0140-6736(09)61962-0. [DOI] [PubMed] [Google Scholar]
- 309.Johnson PDR, Hayman JA, Quek TY, Fyfe JAM, Jenkin GA, Buntine JA, Athan E, Birrell M, Graham J, Lavender CJ. on behalf of the Mycobacterium ulcerans Study Team. 2007. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Med J Aust 186:64–68. [DOI] [PubMed] [Google Scholar]
- 310.Braxmeier S, Hellmann M, Beck A, Umboock A, Pluschke G, Junghanss T, Weinlaeder H. 2009. Phase change material for thermotherapy of Buruli ulcer: modelling as an aid to implementation. J Med Eng. Technol 33:559–566. doi: 10.1080/03091900903067457. [DOI] [PubMed] [Google Scholar]
- 311.Rathnayake D, Sinclair R. 2014. Tropical and exotic dermatoses and ulcers. Aust Fam Physician 43:604–609. [PubMed] [Google Scholar]
- 312.Amissah NA, Chlebowicz MA, Ablordey A, Tetteh CS, Prah I, van der Werf TS, Friedrich AW, van Dijl JM, Stienstra Y, Rossen JW. 2017. Virulence potential of Staphylococcus aureus isolates from Buruli ulcer patients. Int J Med Microbiol 307:223–232. doi: 10.1016/j.ijmm.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 313.Yemoa A, Gbenou J, Affolabi D, Moudachirou M, Bigot A, Anagonou S, Portaels F, Quetin-Leclercq J, Martin A. 2011. Buruli ulcer: a review of in vitro tests to screen natural products for activity against Mycobacterium ulcerans. Planta Med 77:641–646. doi: 10.1055/s-0030-1250642. [DOI] [PubMed] [Google Scholar]
- 314.Johnson RC, Makoutode M, Hougnihin R, Guedenon A, Ifebe D, Boko M, Portaels F. 2004. Traditional treatment for Buruli ulcer in Benin. Med Trop 64:145–150. (In French.) [PubMed] [Google Scholar]
- 315.Webb BJ, Hauck FR, Houp E, Portaels F. 2009. Buruli ulcer in West Africa: strategies for early detection and treatment in the antibiotic era. East Afr J Public Health 6:144–147. [DOI] [PubMed] [Google Scholar]
- 316.Kibadi K, Boelaert M, Kayinua M, Minuku JB, Muyembe-Tamfum JJ, Portaels F, Lefevre P. 2009. Therapeutic itineraries of patients with ulcerated forms of Mycobacterium ulcerans (Buruli ulcer) disease in a rural health zone in the Democratic Republic of Congo. Trop Med Int Health 14:1110–1116. doi: 10.1111/j.1365-3156.2009.02324.x. [DOI] [PubMed] [Google Scholar]
- 317.Aujoulat I, Johnson C, Zinsou C, Guedenon A, Portaels F. 2003. Psychosocial aspects of health seeking behaviours of patients with Buruli ulcer in southern Benin. Trop Med Int Health 8:750–759. doi: 10.1046/j.1365-3156.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 318.Yemoa A, Gbenou J, Affolabi D, Moudachirou M, Bigot A, Anagonou S, Portaels F, Martin A, Quetin-Leclercq J. 2015. Beninese medicinal plants as a source of antimycobacterial agents: bioguided fractionation and in vitro activity of alkaloids isolated from Holarrhena floribunda used in traditional treatment of Buruli ulcer. Biomed Res Int 2015:835767. doi: 10.1155/2015/835767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tsouh Fokou PV, Nyarko AK, Appiah-Opong R, Tchokouaha Yamthe LR, Addo P, Asante IK, Boyom FF. 2015. Ethnopharmacological reports on anti-Buruli ulcer medicinal plants in three West African countries. J Ethnopharmacol 172:297–311. doi: 10.1016/j.jep.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 320.Tsouh Fokou PV, Kissi-Twum AA, Yeboah-Manu D, Appiah-Opong R, Addo P, Tchokouaha Yamthe LR, Ngoutane Mfopa A, Fekam Boyom F, Nyarko AK. 2016. In vitro activity of selected West African medicinal plants against Mycobacterium ulcerans disease. Molecules 21:445. doi: 10.3390/molecules21040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.O'Brien DP, Jenkin G, Buntine J, Steffen CM, McDonald A, Horne S, Friedman ND, Athan E, Hughes A, Callan PP, Johnson PD. 2014. Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: guideline update. Med J Aust 200:267–270. doi: 10.5694/mja13.11331. [DOI] [PubMed] [Google Scholar]
- 322.WHO. 2012. Treatment of Mycobacterium ulcerans disease (Buruli ulcer): guidance for health workers, p 73 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 323.Hart BE, Hale LP, Lee S. 2015. Recombinant BCG expressing Mycobacterium ulcerans Ag85A imparts enhanced protection against experimental Buruli ulcer. PLoS Negl Trop Dis 9:e0004046. doi: 10.1371/journal.pntd.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ahorlu CK, Koka E, Yeboah-Manu D, Lamptey I, Ampadu E. 2013. Enhancing Buruli ulcer control in Ghana through social interventions: a case study from the Obom sub-district. BMC Public Health 13:59. doi: 10.1186/1471-2458-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Roupie V, Pidot SJ, Einarsdottir T, Van Den Poel C, Jurion F, Stinear TP, Huygen K. 2014. Analysis of the vaccine potential of plasmid DNA encoding nine mycolactone polyketide synthase domains in Mycobacterium ulcerans infected mice. PLoS Negl Trop Dis 8:e2604. doi: 10.1371/journal.pntd.0002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Tanghe A, Content J, Van Vooren JP, Portaels F, Huygen K. 2001. Protective efficacy of a DNA vaccine encoding antigen 85A from Mycobacterium bovis BCG against Buruli ulcer. Infect Immun 69:5403–5411. doi: 10.1128/IAI.69.9.5403-5411.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tanghe A, Dangy JP, Pluschke G, Huygen K. 2008. Improved protective efficacy of a species-specific DNA vaccine encoding mycolyl-transferase Ag85A from Mycobacterium ulcerans by homologous protein boosting. PLoS Negl Trop Dis 2:199. doi: 10.1371/journal.pntd.0000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Tanghe A, Adnet PY, Gartner T, Huygen K. 2007. A booster vaccination with Mycobacterium bovis BCG does not increase the protective effect of the vaccine against experimental Mycobacterium ulcerans infection in mice. Infect Immun 75:2642–2644. doi: 10.1128/IAI.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Watanabe M, Nakamura H, Nabekura R, Shinoda N, Suzuki E, Saito H. 2015. Protective effect of a dewaxed whole-cell vaccine against Mycobacterium ulcerans infection in mice. Vaccine 33:2232–2239. doi: 10.1016/j.vaccine.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 330.Bolz M, Kerber S, Zimmer G, Pluschke G. 2015. Use of recombinant virus replicon particles for vaccination against Mycobacterium ulcerans disease. PLoS Negl Trop Dis 9:e0004011. doi: 10.1371/journal.pntd.0004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.van der Werf TS, Stienstra Y, Johnson RC, Phillips R, Adjei O, Fleischer B, Wansbrough-Jones MH, Johnson PD, Portaels F, van der Graaf WT, Asiedu K. 2005. Mycobacterium ulcerans disease. Bull World Health Organ 83:785–791. [PMC free article] [PubMed] [Google Scholar]
- 332.Aguiar J, Domingo M, Guédénon A, Meyers W, Steunou C, Portaels F. 1997. L'ulcère de Buruli, une maladie mycobactérienne importante et en recrudescence au Bénin. Bull Seances Acad R Sci Outre Mer 3:325–356. [Google Scholar]
- 333.Amofah GK, Sagoe-Moses C, Adjei-Acquah C, Frimpong EH. 1993. Epidemiology of Buruli ulcer in Amansie West district, Ghana. Trans R Soc Trop Med Hyg 87:644–645. doi: 10.1016/0035-9203(93)90272-R. [DOI] [PubMed] [Google Scholar]
- 334.Marston BJ, Diallo MO, Horsburgh CR, Diomande I, Saki MZ, Kanga J-M, Patrice GB, Lipman HB, Ostroff SM, Good RC. 1995. Emergence of Buruli ulcer disease in the Daloa region of Cote D'Ivoire. Am J Trop Med Hyg 52:219–224. doi: 10.4269/ajtmh.1995.52.219. [DOI] [PubMed] [Google Scholar]
- 335.Ouoba K, Sano D, Traore A, Ouedraogo R, Sakande B, Sanou A. 1998. Buruli ulcers in Burkina Faso: a propos of 6 cases. Tunis Med 76:46–50. [PubMed] [Google Scholar]
- 336.Coulibaly-N′Golo GM, Ekaza E, Coulibaly B, Aka N, N′Guessan RK, Thiberge JM, Caro V, Brisse S, Bretin-Dosso M. 2011. Multilocus VNTR analysis of Mycobacterium ulcerans strains isolated in Cote d'Ivoire. J Infect Dev Ctries 5:59–63. [DOI] [PubMed] [Google Scholar]
- 337.Muelder K. 1988. Buruli ulcer in Benin. Trop Doct 18:53. doi: 10.1177/004947558801800204. [DOI] [PubMed] [Google Scholar]
- 338.Oluwasanmi JO, Solanke TF, Olurin EO, Itayemi SO, Alabi GO, Lucas AO. 1976. Mycobacterium ulcerans (Buruli) skin ulceration in Nigeria. Am J Trop Med Hyg 25:122–128. doi: 10.4269/ajtmh.1976.25.122. [DOI] [PubMed] [Google Scholar]
- 339.Bayley AC. 1971. Buruli ulcer in Ghana. Br Med J 2:401–402. doi: 10.1136/bmj.2.5758.401-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.van der Werf TS, van der Graaf WT, Groothuis DG, Knell AJ. 1989. Mycobacterium ulcerans infection in Ashanti region, Ghana. Trans R Soc Trop Med Hyg 83:410–413. doi: 10.1016/0035-9203(89)90521-X. [DOI] [PubMed] [Google Scholar]
- 341.Monson MH, Gibson DW, Connor DH, Kappes R, Hienz HA. 1984. Mycobacterium ulcerans in Liberia: a clinicopathologic study of 6 patients with Buruli ulcer. Acta Trop 41:165–172. [PubMed] [Google Scholar]
- 342.Meyers WM, Tignokpa N, Priuli GB, Portaels F. 1996. Mycobacterium ulcerans infection (Buruli ulcer): first reported patients in Togo. Brit J Dermatol 134:1116–1121. doi: 10.1046/j.1365-2133.1996.d01-914.x. [DOI] [PubMed] [Google Scholar]
- 343.Ratmanov P, Mediannikov O, Raoult D. 2013. Vectorborne diseases in West Africa: geographic distribution and geospatial characteristics. Trans R Soc Trop Med Hyg 107:273–284. doi: 10.1093/trstmh/trt020. [DOI] [PubMed] [Google Scholar]
- 344.WHO. 2015. Distribution of Buruli ulcer, worldwide, 2015. Abstr WHO Annu Meet Buruli Ulcer http://gamapserver.who.int/mapLibrary/Files/Maps/Buruli_2015.png?ua:1. [Google Scholar]
- 345.Bessis D, Kempf M, Marsollier L. 2015. Mycobacterium ulcerans disease (Buruli ulcer) in Mali: a new potential African endemic country. Acta Dermatol Venereol 95:489–490. doi: 10.2340/00015555-1942. [DOI] [PubMed] [Google Scholar]
- 346.Gray HH, Kingma S, Kok SH. 1967. Mycobacterial skin ulcers in Nigeria. Trans R Soc Trop Med Hyg 61:712–714. doi: 10.1016/0035-9203(67)90139-3. [DOI] [PubMed] [Google Scholar]
- 347.Johnson PDR, Stinear T, Small PLC, Pluschke G, Merritt RW, Portaels F, Huygen K, Hayman JA, Asiedu K. 2005. Buruli ulcer (M. ulcerans infection): new insights, new hope for disease control. PLoS Med 2:e108. doi: 10.1371/journal.pmed.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Minime-Lingoupou F, Beyam N, Zandanga G, Manirakiza A, N′Domackrah A, Njuimo S, Eyangoh S, Cottin J, Marsollier L, Marion E, Portaels F, Le Faou A, Bercion R. 2010. Buruli ulcer, Central African Republic. Emerg Infect Dis 16:746–748. doi: 10.3201/eid1604.090195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kibadi K, Panda M, Tamfum J-JM, Fraga AG, Filho AL, Anyo G, Pedrosa J, Nakazawa Y, Suykerbuyk P, Meyers WM, Portaels F. 2008. New foci of Buruli ulcer, Angola and Democratic Republic of Congo. Emerg Infect Dis 14:1790–1792. doi: 10.3201/eid1411.071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Marion E, Obvala D, Babonneau J, Kempf M, Asiedu KB, Marsollier L. 2014. Buruli ulcer disease in Republic of the Congo. Emerg Infect Dis 20:1070–1072. doi: 10.3201/eid2006.131498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ravisse P. 1977. L'ulcere cutane a Mycobacterium ulcerans au Cameroun. I. Etude clinique, epidemiologique et histologique. Bull Soc Pathol Exot 70:109–124. [PubMed] [Google Scholar]
- 352.Janssens PG PS, Meyers WM, Portaels F. 2005. Buruli ulcer: an historical overview with updating to 2005. Bull Seances Acad R Sci Outre Mer 51:165–199. [Google Scholar]
- 353.Bar W, Rusch-Gerdes S, Richter E, Marquez de Bar G, Dittmer C, Papsdorf H, Stosiek P, de Rijk PB, Meyers WM, Portaels F. 1998. Mycobacterium ulcerans infection in a child from Angola: diagnosis by direct detection and culture. Trop Med Int Health 3:189–196. doi: 10.1046/j.1365-3156.1998.00225.x. [DOI] [PubMed] [Google Scholar]
- 354.Walsh DS, Eyase F, Onyango D, Odindo A, Otieno W, Waitumbi JN, Bulimo WD, Schnabel DC, Meyers WM, Portaels F. 2009. Clinical and molecular evidence for a case of Buruli ulcer (Mycobacterium ulcerans infection) in Kenya. Am J Trop Med Hyg 81:1110–1113. doi: 10.4269/ajtmh.2009.09-0313. [DOI] [PubMed] [Google Scholar]
- 355.Komolafe OO. 2001. Buruli ulcer in Malawi—a first report. Malawi Med J 13:37–38. doi: 10.4314/mmj.v13i2.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Stienstra Y, van der Graaf WT, Asamoa K, van der Werf TS. 2002. Beliefs and attitudes toward Buruli ulcer in Ghana. Am J Trop Med Hyg 67:207–213. doi: 10.4269/ajtmh.2002.67.207. [DOI] [PubMed] [Google Scholar]
- 357.O'Brien DP, Robson M, Friedman ND, Walton A, McDonald A, Callan P, Hughes A, Rahdon R, Athan E. 2013. Incidence, clinical spectrum, diagnostic features, treatment and predictors of paradoxical reactions during antibiotic treatment of Mycobacterium ulcerans infections. BMC Infect Dis 13:416–416. doi: 10.1186/1471-2334-13-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Faber WR, Arias-Bouda LM, Zeegelaar JE, Kolk AH, Fonteyne PA, Toonstra J, Portaels F. 2000. First reported case of Mycobacterium ulcerans infection in a patient from China. Trans R Soc Trop Med Hyg 94:277–279. doi: 10.1016/S0035-9203(00)90320-1. [DOI] [PubMed] [Google Scholar]
- 359.Mikoshiba H, Shindo Y, Matsumoto H, Mochizuki M, Tsukamura M. 1982. A case of typical mycobacteriosis due to Mycobacterium ulcerans-like organism. Nihon Hifuka Gakkai Zasshi 92:557–565. (In Japanese.) [PubMed] [Google Scholar]
- 360.Tsukamura M, Kaneda K, Imaeda T, Mikoshiba H. 1989. A taxonomic study on a mycobacterium which caused a skin ulcer in a Japanese girl and resembled Mycobacterium ulcerans. Kekkaku 64:691–697. (In Japanese.) [PubMed] [Google Scholar]
- 361.Nakanaga K, Hoshino Y, Yotsu RR, Makino M, Ishii N. 2011. Nineteen cases of Buruli ulcer diagnosed in Japan from 1980 to 2010. J Clin Microbiol 49:3829–3836. doi: 10.1128/JCM.00783-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Pettit JH, Marchette NJ, Rees RJ. 1966. Mycobacterium ulcerans infection. Clinical and bacteriological study of the first cases recognized in South East Asia. Br J Dermatol 78:187–197. [DOI] [PubMed] [Google Scholar]
- 363.Christie M. 1987. Suspected Mycobacterium ulcerans disease in Kiribati. Med J Aust 146:600–604. [DOI] [PubMed] [Google Scholar]
- 364.Jacquemart Y, Josse R. 2002. Papua New Guinea. Med Trop 62:583–588. [PubMed] [Google Scholar]
- 365.Sambourg E, Dufour J, Edouard S, Morris A, Mosnier E, Reynaud Y, Sainte-Marie D, Nacher M, Guegan JF, Couppie P. 2014. Paradoxical reactions and responses during antibiotic treatment for Mycobacterium ulcerans infection (Buruli ulcer). Four cases from French Guiana. Ann Dermatol Venereol 141:413–418. (In French.) doi: 10.1016/j.annder.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 366.Guerra H, Palomino JC, Falconí E, Bravo F, Donaires N, Van Marck E, Portaels F. 2008. Mycobacterium ulcerans disease, Peru. Emerg Infect Dis 14:373–377. doi: 10.3201/eid1403.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.dos Santos VM, Noronha FL, Vicentina EC, Lima CC. 2007. Mycobacterium ulcerans infection in Brazil. Med J Aust 187:63–64. [DOI] [PubMed] [Google Scholar]
- 368.Reynaud Y, Millet J, Couvin D, Rastogi N, Brown C, Couppié P, Legrand E. 2015. Heterogeneity among Mycobacterium ulcerans from French Guiana revealed by multilocus variable number tandem repeat analysis (MLVA). PLoS One 10:e0118597. doi: 10.1371/journal.pone.0118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Osei FB, Duker AA. 2015. Analysis of Buruli ulcer prevalence in Amansie West District: a geostatistical approach. Austin Biom Biostat 2:1011. [Google Scholar]
- 370.Quek TYJ, Henry MJ, Pasco JA, O'Brien DP, Johnson PDR, Hughes A, Cheng AC, Redden-Hoare J, Athan E. 2007. Mycobacterium ulcerans infection: factors influencing diagnostic delay. Med J Aust 187:561–563. [DOI] [PubMed] [Google Scholar]
- 371.van Ravensway J, Benbow ME, Tsonis AA, Pierce SJ, Campbell LP, Fyfe JA, Hayman JA, Johnson PD, Wallace JR, Qi J. 2012. Climate and landscape factors associated with Buruli ulcer incidence in Victoria, Australia. PLoS One 7:e51074. doi: 10.1371/journal.pone.0051074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Brou T, Broutin H, Elguero E, Asse H, Guegan J-F. 2008. Landscape diversity related to Buruli ulcer disease in Côte d'Ivoire. PLoS Negl Trop Dis 2:e271. doi: 10.1371/journal.pntd.0000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yoshikawa T, Kaizuka S, Ōta Y. 1981. Landforms of Japan. University of Tokyo Press, Tokyo, Japan. [Google Scholar]
- 374.Herman JS, Chiodini PL. 2011. West Africa, p 128–138. In Petersen E, Chen LH, Schlagenhauf P (ed), West Africa, infectious diseases: a geographic guide. Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 375.Sopoh GE, Johnson RC, Anagonou SY, Barogui YT, Dossou AD, Houézo JG, Phanzu DM, Tente BH, Meyers WM, Portaels F. 2011. Buruli ulcer prevalence and altitude, Benin. Emerg Infect Dis 17:153–154. doi: 10.3201/eid1701.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Muelder K, Nourou A. 1990. Buruli ulcer in Benin. Lancet 336:1109–1111. doi: 10.1016/0140-6736(90)92581-2. [DOI] [PubMed] [Google Scholar]
- 377.Landier J, Constantin de Magny G, Garchitorena A, Guegan JF, Gaudart J, Marsollier L, Le Gall P, Giles-Vernick T, Eyangoh S, Fontanet A, Texier G. 2015. Seasonal patterns of Buruli ulcer incidence, Central Africa, 2002–2012. Emerg Infect Dis 21:1414–1417. doi: 10.3201/eid2108.141336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Landier J, Gaudart J, Carolan K, Lo Seen D, Guégan J-F, Eyangoh S, Fontanet A, Texier G. 2014. Spatio-temporal patterns and landscape-associated risk of Buruli ulcer in Akonolinga, Cameroon. PLoS Negl Trop Dis 8:e3123. doi: 10.1371/journal.pntd.0003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Portaels F. 1995. Epidemiology of mycobacterial diseases. Clin Dermatol 13:207–222. doi: 10.1016/0738-081X(95)00004-Y. [DOI] [PubMed] [Google Scholar]
- 380.Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Scott JT, Dramaix M, Portaels F. 2004. Mycobacterium ulcerans disease: role of age and gender in incidence and morbidity. Trop Med Int Health 9:1297–1304. doi: 10.1111/j.1365-3156.2004.01339.x. [DOI] [PubMed] [Google Scholar]
- 381.Roltgen K, Bratschi MW, Ross A, Aboagye SY, Ampah KA, Bolz M, Andreoli A, Pritchard J, Minyem JC, Noumen D, Koka E, Um Boock A, Yeboah-Manu D, Pluschke G. 2014. Late onset of the serological response against the 18 kDa small heat shock protein of Mycobacterium ulcerans in children. PLoS Negl Trop Dis 8:e2904. doi: 10.1371/journal.pntd.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ampah KA, Nickel B, Asare P, Ross A, De-Graft D, Kerber S, Spallek R, Singh M, Pluschke G, Yeboah-Manu D, Roltgen K. 2016. A sero-epidemiological approach to explore transmission of Mycobacterium ulcerans. PLoS Negl Trop Dis 10:e0004387. doi: 10.1371/journal.pntd.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.UBG. 1971. The Uganda Buruli Group. Epidemiology of Mycobacterium ulcerans infection (Buruli ulcer) at Kinyara, Uganda. Trans R Soc Trop Med Hyg 65:763–775. [DOI] [PubMed] [Google Scholar]
- 384.Sopoh GE, Barogui YT, Johnson RC, Dossou AD, Makoutodé M, Anagonou SY, Kestens L, Portaels F. 2010. Family relationship, water contact and occurrence of Buruli ulcer in Benin. PLoS Negl Trop Dis 4:e746. doi: 10.1371/journal.pntd.0000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Mensah-Quainoo EK. 1998. A study of the magnitude and determinants of Buruli ulcer disease in the Ga District of Ghana, p 33 Abstr Int Conf Buruli Ulcer Contr Res, Yamoussoukro, Cote d'Ivoire, 6 to 8 July 1998 http://apps.who.int/iris/bitstream/10665/64317/1/WHO_TB_98.252.pdf. [Google Scholar]
- 386.Aujoulat I, Huguet-Ribas M-P, Koita Y. 1996. L'ulcère de Buruli: un problème de santé publique méconnu appellant une mobilization internationale. Développement et santé. Rev Int Perfect Méd Sanit 125:22–30. [Google Scholar]
- 387.Peraudin ML, Herrault A, Desbois JC. 1980. Ulcère cutanée à Mycobacterium ulcerans (ulcère de Buruli). Ann Pediatr (Paris) 27:687–692. [PubMed] [Google Scholar]
- 388.Ziefer AM, Connor DH, Gibson DW. 1981. Mycobacterium ulcerans. Infection of two patients in Liberia. Int J Dermatol 20:362–367. doi: 10.1111/j.1365-4362.1981.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 389.Johnson RC, Makoutodé M, Sopoh GE, Elsen P, Gbovi J, Pouteau LH, Meyers WM, Boko M, Portaels F. 2005. Buruli ulcer distribution in Benin. Emerg Infect Dis 11:500–501. doi: 10.3201/eid1103.040597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Stoffel V, Barthelme B, Chague F. 2005. Tropical ecopathology: up hill and down dale Buruli ulcer. Sante Publique 17:191–197. doi: 10.3917/spub.052.0191. [DOI] [PubMed] [Google Scholar]
- 391.Gibson J. 1975. Buruli ulcers in Bo. Bull Sierra Leone Med Dental Assoc 2:64–66. [Google Scholar]
- 392.Stienstra Y, van der Werf TS, Oosterom E, Nolte IM, van der Graaf WT, Etuaful S, Raghunathan PL, Whitney EA, Ampadu EO, Asamoa K, Klutse EY, te Meerman GJ, Tappero JW, Ashford DA, van der Steege G. 2006. Susceptibility to Buruli ulcer is associated with the SLC11A1 (NRAMP1) D543N polymorphism. Genes Immun 7:185–189. doi: 10.1038/sj.gene.6364281. [DOI] [PubMed] [Google Scholar]
- 393.Smith PG, Revill WD, Lukwago E, Rykushin YP. 1976. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans R Soc Trop Med Hyg 70:449–457. doi: 10.1016/0035-9203(76)90128-0. [DOI] [PubMed] [Google Scholar]
- 394.Collins FH, Paskewitz SM. 1995. Malaria: current and future prospects for control. Annu Rev Entomol 40:195–219. doi: 10.1146/annurev.en.40.010195.001211. [DOI] [PubMed] [Google Scholar]
- 395.WHO. 2003. Climate change and human health: risks and responses. WHO, Geneva, Switzerland. [Google Scholar]
- 396.Wallace JR, Gordon MC, Hartsell L, Mosi L, Benbow ME, Merritt RW, Small PLC. 2010. Interaction of Mycobacterium ulcerans with mosquito species: implications for transmission and trophic relationships. Appl Environ Microbiol 76:6215–6222. doi: 10.1128/AEM.00340-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.WHO. 2002. Instructions for treatment and use of insecticide-treated mosquito nets. Use insecticide-treated mosquito nets to sleep in peace—and protect your health. WHO, Geneva, Switzerland. [Google Scholar]
- 398.WHO/GMP. 2009. Global Malaria Programme. Insecticide-treated mosquito nets: a WHO position statement. WHO, Geneva, Switzerland: http://files.givewell.org/files/DWDA%202009/Interventions/Nets/itnspospaperfinal.pdf. [Google Scholar]
- 399.OAU. 4 June 2000. African Summit on Roll Back Malaria. The Abuja Declaration on Roll Back Malaria in Africa by the African Heads of State and Government. USAID, Washington, DC: https://www.usaid.gov/sites/default/files/documents/1864/abuja.pdf. [Google Scholar]
- 400.WHO. 2011. The use of DDT in malaria vector control: World Health Organization position statement. WHO, Geneva, Switzerland. [Google Scholar]
- 401.Cano J, Rebollo MP, Golding N, Pullan RL, Crellen T, Soler A, Hope LAK, Lindsay SW, Hay SI, Bockarie MJ, Brooker SJ. 2014. The global distribution and transmission limits of lymphatic filariasis: past and present. Parasit Vectors 7:466. doi: 10.1186/s13071-014-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.WHO. 2016. Lymphatic filariasis. Fact Sheet 102. WHO, Geneva, Switzerland. [Google Scholar]
- 403.WHO. 2010. Global programme to eliminate lymphatic filariasis. WHO GPELF Progress Report 2000–2009 and strategic plan 2010–2020. WHO, Geneva, Switzerland. [Google Scholar]
- 404.Abdullah S, Adazu K, Masanja H, Diallo D, Hodgson A, Ilboudo-Sanogo E, Nhacolo A, Owusu-Agyei S, Thompson R, Smith T, Binka FN. 2007. Patterns of age-specific mortality in children in endemic areas of Sub-Saharan Africa. Am J Trop Med Hyg 77:99–105. doi: 10.1016/0035-9203(83)90028-7. [DOI] [PubMed] [Google Scholar]
- 405.Boakye DA, Wilson MD, Appawu MA, Gyapong J. 2004. Vector competence, for Wuchereria bancrofti, of the Anopheles populations in the Bongo district of Ghana. Ann Trop Med Parasitol 98:501–508. doi: 10.1179/000349804225003514. [DOI] [PubMed] [Google Scholar]
- 406.World Health Assembly. 1997. Elimination of lymphatic filariasis as a public health problem. World Health Assembly Resolution and Decision WHA50.29. WHO, Geneva, Switzerland: http://www.who.int/neglected_diseases/mediacentre/WHA_50.29_Eng.pdf. [Google Scholar]
- 407.WHO. 2006. Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. WHO, Geneva, Switzerland. [Google Scholar]
- 408.de Souza DK, Koudou B, Kelly-Hope LA, Wilson MD, Bockarie MJ, Boakye DA. 2012. Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit Vectors 5:259. doi: 10.1186/1756-3305-5-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Stienstra Y, van der Graaf WT, te Meerman GJ, The TH, de Leij LF, van der Werf TS. 2001. Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors. Trop Med Int Health 6:554–562. doi: 10.1046/j.1365-3156.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 410.Hotez PJ, Kamath A. 2009. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Imevbore A, Ofoezie I, Obot E. 1988. Vector-borne disease problems of small scale water resources development projects in Kano State: snail vectors of schistosomiasis. Afrancet 1:17–23. [Google Scholar]
- 412.Akinwale OP, Ajayi MB, Akande DO, Gyang PV, Adeleke MA, Adeneye AK, Adebayo MO, Dike AA. 2010. Urinary schistosomiasis around Oyan Reservoir, Nigeria: twenty years after the first outbreak. Iranian J Public Health 39:92–95. [PMC free article] [PubMed] [Google Scholar]
- 413.Scott JT, Johnson RC, Aguiar J, Debacker M, Kestens L, Guedenon A, Gryseels B, Portaels F. 2004. Schistosoma haematobium Infection and Buruli ulcer. Emerg Infect Dis 10:551–552. doi: 10.3201/eid1003.020514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Stienstra Y, van der Werf TS, van der Graaf WT, Secor WE, Kihlstrom SL, Dobos KM, Asamoa K, Quarshi E, Etuaful SN, Klutse EY, King CH. 2004. Buruli ulcer and schistosomiasis: no association found. Am J Trop Med Hyg 71:318–321. [PubMed] [Google Scholar]
- 415.Kimutai A, Ngure P, Tonui W, Gicheru M, Nyamwamu L. 2009. Leishmaniasis in Northern and Western Africa: a review. Afr J Infect Dis 3:14–25. [Google Scholar]
- 416.Nzelu CO, Kato H, Puplampu N, Desewu K, Odoom S, Wilson MD, Sakurai T, Katakura K, Boakye DA. 2014. First detection of Leishmania tropica DNA and Trypanosoma species in Sergentomyia sand flies (Diptera: Psychodidae) from an outbreak area of cutaneous leishmaniasis in Ghana. PLoS Negl Trop Dis 8:e2630. doi: 10.1371/journal.pntd.0002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Kweku MA, Odoom S, Puplampu N, Desewu K, Nuako GK, Gyan B, Raczniak G, Kronmann KC, Koram K, Botero S, Boakye D, Akuffo H. 2011. An outbreak of suspected cutaneous leishmaniasis in Ghana: lessons learnt and preparation for future outbreaks. Global Health Action 2011:4. doi: 10.3402/gha.v4i0.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Romero GAS, Boelaert M. 2010. Control of visceral leishmaniasis in Latin America—a systematic review. PLoS Negl Trop Dis 4:e584. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Veland N, Valencia BM, Alba M, Adaui V, Llanos-Cuentas A, Arevalo J, Boggild AK. 2013. Simultaneous infection with Leishmania (Viannia) braziliensis and L. (V.) lainsoni in a Peruvian patient with cutaneous leishmaniasis. Am J Trop Med Hyg 88:774–777. doi: 10.4269/ajtmh.12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Adekambi T, Ben Salah S, Khlif M, Raoult D, Drancourt M. 2006. Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl Environ Microbiol 72:5974–5981. doi: 10.1128/AEM.03075-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Gupta T, Fine-Coulson K, Karls R, Gauthier D, Quinn F. 2013. Internalization of Mycobacterium shottsii and Mycobacterium pseudoshottsii by Acanthamoeba polyphaga. Can J Microbiol 59:570–576. doi: 10.1139/cjm-2013-0079. [DOI] [PubMed] [Google Scholar]
- 422.Steinert M, Birkness K, White E, Fields B, Quinn F. 1998. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl Environ Microbiol 64:2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Lamrabet O, Mba Medie F, Drancourt M. 2012. Acanthamoeba polyphaga-enhanced growth of Mycobacterium smegmatis. PLoS One 7:11. doi: 10.1371/journal.pone.0029833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wilson MD, Boakye DA, Mosi L, Asiedu K. 2011. In the case of transmission of Mycobacterium ulcerans in Buruli ulcer disease, Acanthamoeba species stand accused. Ghana Med J 45:31–34. doi: 10.4314/gmj.v45i1.68920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Aka P. 2002. Buruli ulcer, an emerging disease in public health in Cote D'Ivoire (Ivory Coast). Forum Nord Derm Ven 7:22–26. [Google Scholar]
- 426.Affolabi D, Tanimomo-Kledjo B, Anyo G, Johnson RC, Anagonou SY, Portaels F. 2008. Setting up a national reference laboratory for Buruli ulcer: the case of Benin. Trop Med Int Health 13:365–368. doi: 10.1111/j.1365-3156.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- 427.Gullan PJ, Cranston PS. 2014. The insects: an outline of entomology, 5th ed, p 190–226. Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 428.Eddyani M, Ofori-Adjei D, Teugels G, De Weirdt D, Boakye D, Meyers WM, Portaels F. 2004. Potential role for fish in transmission of Mycobacterium ulcerans disease (Buruli ulcer): an environmental study. Appl Environ Microbiol 70:5679–5681. doi: 10.1128/AEM.70.9.5679-5681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Kanga JM, Kacou ED. 2001. Epidemiological aspects of Buruli ulcer in Côte d'Ivoire: results of a national survey. Bull Soc Pathol Exot 94:46–51. (In French.) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.