SUMMARY
Brachyspira pilosicoli is a slow-growing anaerobic spirochete that colonizes the large intestine. Colonization occurs commonly in pigs and adult chickens, causing colitis/typhlitis, diarrhea, poor growth rates, and reduced production. Colonization of humans also is common in some populations (individuals living in village and peri-urban settings in developing countries, recent immigrants from developing countries, homosexual males, and HIV-positive patients), but the spirochete rarely is investigated as a potential human enteric pathogen. In part this is due to its slow growth and specialized growth requirements, meaning that it is not detectable in human fecal samples using routine diagnostic methods. Nevertheless, it has been identified histologically attached to the colon and rectum in patients with conditions such as chronic diarrhea, rectal bleeding, and/or nonspecific abdominal discomfort, and one survey of Australian Aboriginal children showed that colonization was significantly associated with failure to thrive. B. pilosicoli has been detected in the bloodstream of elderly patients or individuals with chronic conditions such as alcoholism and malignancies. This review describes the spirochete and associated diseases. It aims to encourage clinicians and clinical microbiologists to consider B. pilosicoli in their differential diagnoses and to develop and use appropriate diagnostic protocols to identify the spirochete in clinical specimens.
KEYWORDS: spirochete, Brachyspira, colitis, zoonosis, animals, epidemiology, diagnosis, control
INTRODUCTION
This review summarizes the characteristics of the anaerobic intestinal spirochete Brachyspira pilosicoli, the diseases that it causes in animals and in humans, its natural history, and methods for diagnosis and control. The review aims to increase understanding of this organism, particularly by medical scientists who may not have encountered it. Descriptions of the infections in animals provide comparative data that assist in understanding the spirochete's ecology and provide insights into its pathogenicity.
Although B. pilosicoli is recognized as being a pathogen in many mammalian and avian species, there is still uncertainty about the pathogenic importance of B. pilosicoli in humans, and its potential role as a human pathogen is rarely considered. This lack of attention occurs despite the high prevalence of colonization in certain human population groups, as revealed in targeted surveys. Infections with B. pilosicoli present numerous diagnostic difficulties, as the spirochete is anaerobic, grows slowly without forming colonies, and requires selective growth media. Although appropriate culture conditions and molecular-based methodology such as PCR assays are used to detect Brachyspira species in specialized veterinary diagnostic laboratories receiving samples from pigs, these methods are not routinely used in laboratories primarily handling samples from poultry or from other species of animals. Likewise, they are not available for use in clinical diagnostic laboratories that examine specimens from humans, except for a few laboratories that undertake special diagnostic investigations.
HISTORY AND NOMENCLATURE
Records from the earliest days of microscopy describe the presence of spirochetes in feces obtained from humans and animals. Some of these spirochetes undoubtedly were members of one or another of the nine officially recognized Brachyspira species. Key features of these species are presented in Table 1.
TABLE 1.
Names and characteristics of the nine officially named Brachyspira species
| Species | Hemolysis | Main host species | Diseasea | Previous names |
|---|---|---|---|---|
| B. pilosicoli | Weak | Pigs, chickens, and other species, including humans | Intestinal spirochetosis (AIS, PIS, HIS) | “Anquilina coli,” Serpulina pilosicoli |
| B. aalborgi | Weak | Humans and possibly other nonhuman primates | Intestinal spirochetosis (HIS) | NAb |
| B. hyodysenteriae | Strong | Pigs | Swine dysentery | Treponema hyodysenteriae, Serpulina hyodysenteriae |
| B. hampsonii | Strong | Pigs, waterbirds | Swine dysentery | NA |
| B. suanatina | Strong | Ducks, pigs | Swine dysentery | NA |
| B. intermedia | Weak | Chickens, pigs | AIS | Serpulina intermedia |
| B. innocens | Weak | Pigs, chickens, rats | Not recorded | Treponema innocens, Serpulina innocens |
| B. murdochii | Weak | Pigs, chickens, rats | Mild colitis in pigs | Serpulina murdochii |
| B. alvinipulli | Weak | Chickens | AIS | Serpulina alvinipulli |
AIS, avian intestinal spirochetosis; PIS, porcine intestinal spirochetosis; HIS, human intestinal spirochetosis.
NA, not applicable.
The first clear description of the spirochete that is now known as Brachyspira pilosicoli was made in the United Kingdom in 1980 (1). Weakly hemolytic isolate P43/6/78 (now the type strain of B. pilosicoli) was recovered from a fecal sample from a pig on a farm where weaned and growing pigs exhibited colitis and mucoid diarrhea. Pigs that subsequently were experimentally challenged orally with P43/6/78 developed colitis and diarrhea, and in one animal, large numbers of spirochete cells were found attached by one cell end to the luminal surface of colonocytes, giving the appearance of a “false brush border.” The condition was called intestinal spirochetosis (IS) to differentiate it from another, more severe colitis in pigs called swine dysentery (SD). The name “intestinal spirochetosis” previously had been used for a condition in humans with chronic diarrhea and other abdominal complaints, where spirochetes also were observed attached as a “false brush border” to the colorectal epithelium (2). The condition in humans was defined only on the basis of observing this characteristic end-on attachment of spirochetes in histological sections of the colon and/or rectum, as it had not proved possible to isolate and identify the associated microorganisms in the late 1960s.
In 1982 a small anaerobic spirochete named Brachyspira aalborgi was isolated from a colonic biopsy sample in a histologically identified human case of IS (human intestinal spirochetosis [HIS]) (3). This spirochete grew even more slowly than the porcine pathogen P43/6/78, taking more than 2 weeks to appear on the plates. To date, B. aalborgi has been detected in human beings and possibly in some species of nonhuman primates (4). For a number of years following this isolation, it was assumed that B. aalborgi was the (sole) etiological agent causing histologically identified IS in humans, even though B. pilosicoli had been found to cause a similar end-on attachment in colonized pigs (1).
Around the same time, in the early 1980s, and using methods similar to those used to cultivate intestinal spirochetes from pigs, unidentified spirochetes that were later recognized as B. pilosicoli were being isolated from the feces of humans with different nonspecific intestinal complaints (5–11), as well as from patients where HIS was histologically identified (12–15).
Also in the 1980s, and using the methods described for isolating porcine intestinal spirochetes, anaerobic spirochetes started to be identified and isolated from the feces and ceca of adult chickens with various clinical signs, including diarrhea and delays to the start of egg production (15–17). The condition was called avian intestinal spirochetosis (AIS). In some cases, spirochetes were observed forming a “false brush border” attached to the cecal epithelium (18), and this type of spirochete is now known to have been B. pilosicoli (19). The other two pathogenic species that may cause forms of AIS, Brachyspira intermedia and Brachyspira alvinipulli, do not appear to attach to the cecal epithelium in this characteristic way.
Work undertaken in the early to mid-1990s, using mainly multilocus enzyme electrophoresis (20–23) but also DNA-DNA hybridization, 16S rRNA gene sequencing, and biochemical testing (24–26), demonstrated that spirochetes resembling P43/6/78 that had been isolated from pigs, human beings, and poultry all belonged to a distinct new species of intestinal spirochete that is now known as B. pilosicoli (27). At that time this species was referred to as “Anguillina coli” (21, 22) or “group IV weakly hemolytic intestinal spirochetes” (25), before being officially named Serpulina pilosicoli (28). Subsequently, all species from the genus Serpulina were reassigned to the genus Brachyspira, and S. pilosicoli was renamed Brachyspira pilosicoli (27). Around the same time, B. pilosicoli was demonstrated colonizing wild ducks (29), domesticated turkeys (30), pheasants (31), rodents (32), dogs (33–35), young horses with chronic diarrhea (36), and a number of species of other animals and birds (37).
To date, IS in pigs (porcine intestinal spirochetosis [PIS]) has been associated only with B. pilosicoli. On the other hand, spirochete species other than B. pilosicoli sometimes may be involved in HIS and AIS. HIS generally is diagnosed on the appearance of attached spirochetes in histological sections from colorectal biopsy specimens, as most medical microbiology laboratories do not routinely use methods that are suitable for isolating and identifying these spirochetes. It is desirable that in the future appropriate diagnostic microbiological methods be used for identification of infections with B. pilosicoli in humans and poultry, as occurs with pigs. Furthermore, in cases where B. pilosicoli is identified as the etiological agent in HIS and AIS, it would aid clarity if the conditions were reported as “Brachyspira pilosicoli-associated HIS” and “Brachyspira pilosicoli-associated AIS,” respectively.
DESCRIPTION OF BRACHYSPIRA PILOSICOLI
The B. pilosicoli type strain is P43/6/78T (ATCC 51139T), and it was originally isolated from a pig with colitis and diarrhea (1). B. pilosicoli strains grow in an anaerobic environment at temperatures between 37 and 42°C. They are weakly beta-hemolytic on Trypticase soy blood agar, usually hydrolyze hippurate, and utilize d-cellobiose, d-fructose, l-fucose, d-galactose, N-acetyl-d-glucosamine, d-glucosamine, d-glucose, maltose, d-mannose, pyruvate, sucrose, d-trehalose, and d-ribose as growth substrates. Metabolic products generated include acetate, butyrate, ethanol, hydrogen, and carbon dioxide. The spirochete consumes substrate amounts of oxygen and has G+C contents in the range 27.4 to 27.9 mol%. Cells have tapered ends and are 4 to 10 μm long and 0.2 to 0.3 μm wide. Each spirochete cell has 8 or 10 periplasmic flagella, with 4 or 5 flagella attached through insertion discs at each cell end. In common with other spirochete species, these two sets of periplasmic flagella cross over near the midpoint of the cell (26).
Essential Cultural Characteristics
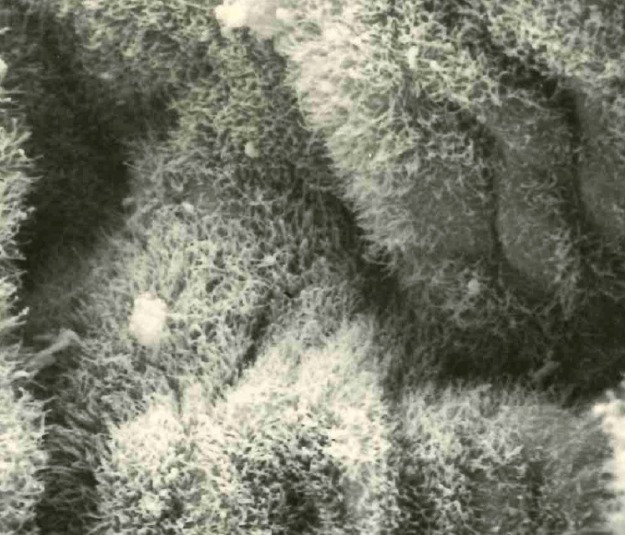
Brachyspira pilosicoli is a slow-growing anaerobe, typically taking 3 to 5 days before forming a thin flat film of growth surrounded by a zone of weak hemolysis on the surface of blood agar. The spirochete stains poorly with Gram's stain and is best observed with Warthin-Starry silver staining or by resuspending surface growth and observing the motile, flexuous, thin spiral- or comma-shaped cells under a phase-contrast or dark-field microscope (Fig. 1). The slow in vitro growth of B. pilosicoli means that it can be isolated only by using specialized selective media that inhibit overgrowth by other anaerobic members of the intestinal microbiota, and these plates are not routinely available commercially. Spirochetemia caused by B. pilosicoli can be detected using some manual blood culture systems, with an incubation period of up to 10 days, followed by subculture. Detection in the automated Bactec blood culture system with Anaerobic/F medium can take up to 15 days of incubation. The spirochete may not grow or signal in other blood culture systems (38).
FIG 1.
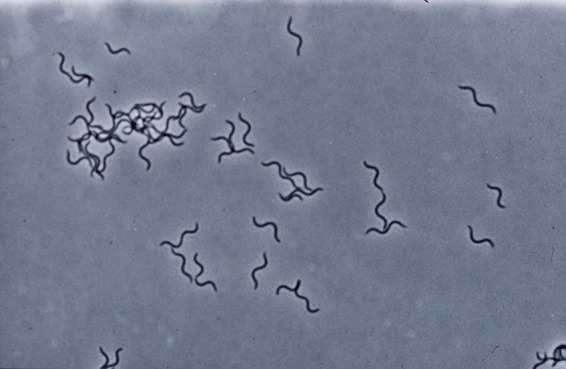
Appearance of motile, flexuous, thin, spiral-shaped cells of B. pilosicoli as observed under a phase-contrast microscope.
Strain Diversity and Population Structure
The strain diversity and population structure of B. pilosicoli have been analyzed using a variety of methods, including multilocus enzyme electrophoresis (20–23), pulsed-field gel electrophoresis (PFGE) (39), multilocus sequence typing (40), and variable-number tandem repeat analysis (41). Each of these methods has been applied to large collections of isolates recovered from different species and geographic origins. In each case the isolates in the collections were shown to be highly diverse, and the population structure was deduced to be recombinant (i.e., undergoing significant genetic recombination), as assessed by calculating the index of association (42). This is in contrast to the clonal population structure of Brachyspira hyodysenteriae, where recombination occurs less frequently.
Genomic Features
Analysis of the whole genome sequence of B. pilosicoli strain 95/1000, recovered from a pig, showed that it had a single circular chromosome of approximately 2.59 Mb (43). Unlike the case for the genome of B. hyodysenteriae strain WA1 (44), no extrachromosomal elements were found, but a previously unreported bacteriophage sequence was identified. To date, this is the second smallest genome of the different Brachyspira species to be reported, with only B. aalborgi 513T being marginally smaller. Genes for a bacteriophage-like gene transfer agent (GTA) were identified in strain 95/1000, confirming previous findings (45). Among other features, genes predicted to be involved with protection from oxidative stress were identified, including those encoding a glycine reductase complex that permits the use of glycine at the same time as protecting from oxidative stress, for aconitase and related enzymes in the incomplete citric acid cycle that allow synthesis of glutamate and allow the cycle to function during oxidative stress, and for NADH oxidase, an enzyme that consumes oxygen and is known to be present in all Brachyspira species (43). These enzyme activities are thought to help B. pilosicoli survive exposure to oxygen in the colon and in the external environment.
In a separate study, a comparative analysis of the genome of B. pilosicoli 95/1000 and the genome of B. hyodysenteriae WA1 identified a highly conserved 26-kb region shared by the two spirochete strains (46). Furthermore, the genomes of the enteric species Enterococcus faecalis and Escherichia coli contained substantial conservation of gene clusters across this same region, suggesting that it has been shared between these four species through horizontal gene transfer in the intestinal environment. This finding supports the observation that predicted proteins of B. hyodysenteriae WA1 had more similarities to proteins from E. coli and Clostridium species than they did to proteins from other spirochete species (46). A large number of the predicted proteins were associated with transport and metabolism, and again these genes may have been shared in the milieu of the large intestine through horizontal gene transfer.
More recently Mappley et al. (47) reported a comparative analysis of the genomes of B. pilosicoli strains 95/1000, B2904, and WesB, which were isolated from a pig, a chicken, and a human, respectively. The study included an analysis of phenotypic properties of the strains using a Biolog phenotype microarray. The sizes of the genomes of the three strains varied considerably (from approximately 2.59 to 2.89 Mb), and they showed major genome rearrangements that coincided mainly with the locations of mobile genetic elements. B. pilosicoli has been inferred to have a greater recombination rate than other Brachyspira species, apart from B. aalborgi (48), and of the three strains analyzed, WesB in particular appears to have undergone many recombination events. Hampson and Wang (48) speculated that the movement of large genome islands forms four potential pathways for genomic rearrangements in B. pilosicoli, while the activity of GTAs also may contribute to smaller-scale genome rearrangements (45, 49). Differences in the presence of some genes were identified between the strains, including those encoding glycine reductase complex components and transposases. Despite the small genome size range, B. pilosicoli strains encode more proteins than other Brachyspira species, possibly because of having a greater number of gene duplications and greater homogeneity than other Brachyspira species (48). The three strains of B. pilosicoli shared 2,132 genes, with 112 to 256 genes being unique to each strain (47). Different pairs of strains shared another 236, 38, and 19 genes, respectively. The three strains had similar distributions of features in the Cluster of Orthologous Genes database. Screening of the strains for utilization of 178 different carbon compounds demonstrated that their metabolic capacity was highly conserved. Differences were found only in seven of the carbon sources examined (d-mannose, d-glucuronic acid, d-mannitol, glucoronamide, β-d-allose, β-methyl-d-glucuronic acid, and l-sorbitol), and these correlated with observed genotypic differences between the strains.
Subsequently Lin et al. (50) described the genome of strain P43/6/78T, which was about 2.56 Mb and was most similar to the genome of strain 95/1000, also isolated from a pig. Most of the unique genes in P43/6/78T encoded hypothetical proteins, and some were considered to be potentially involved in O-antigen variation.
Cell Surface Properties
The B. pilosicoli outer envelope contains lipooligosaccharide (LOS) rather than lipopolysaccharide (LPS), and this material has been shown to be serologically heterogeneous among different strains (51). A number of specific outer membrane proteins and lipoproteins of B. pilosicoli have been described (52), but more work is needed to define their potential role in disease, including whether or not they may be involved in important functions such as attachment and/or in generating protective immunity. Recently, exposed proteins on the cell surface and peptides and proteins in extracellular growth medium were characterized using a high-resolution Orbitrap instrument with multiple liquid chromatography-tandem mass spectrometry (LC-MS/MS) runs (53). More than 29,000 peptides were identified, with 1,38 proteins being unique to B. pilosicoli. The levels of expression of these proteins varied considerably, and this emphasizes the need to consider gene expression when the biological significance of such proteins is investigated.
DISEASES CAUSED BY BRACHYSPIRA PILOSICOLI
Disease in Pigs
Background and occurrence.
The disease caused by infection with B. pilosicoli in pigs is called porcine intestinal spirochetosis (PIS) or porcine colonic spirochetosis (54). It is distinct from the more severe disease called swine dysentery (SD) resulting from infection with one or another related strongly hemolytic spirochetes, including Brachyspira hyodysenteriae, Brachyspira hampsonii, or Brachyspira suanatina (54). In SD, severe mucohemorrhagic colitis occurs, with bloody mucoid diarrhea and substantial loss of production. Although SD was recognized in the 1920s, the spirochetal etiology was not demonstrated for another 50 years (55, 56). Application of the methodology developed to isolate this anaerobic spirochete ultimately resulted in the discovery and description of the weakly hemolytic B. pilosicoli in 1980 (1).
Subclinical colonization of pigs with B. pilosicoli occurs commonly on some farms. On other farms, the spirochete may be isolated from diseased pigs alone or as part of a mixed infection with other enteric pathogens. Clinical cases of PIS usually occur in pigs soon after they are weaned, in recently mixed growing pigs that have been transferred to a new diet, or in finishing pigs at the last stage of production, after all antimicrobials are withdrawn from the diet. Changes of diet or other causes of disruption to the colonic microbiota, such as the use or cessation of use of oral antimicrobials, may predispose to spirochete proliferation and disease. PIS can affect groups of pigs of the same age or pigs of mixed ages.
Clinical signs.
Brachyspira pilosicoli is shed in the feces within 2 to 7 days of experimental inoculation, although the incubation period may extend up to 20 days. The first clinical signs usually are hollowing of the flanks and development of watery to mucoid diarrhea. The fecal consistency often changes to resemble wet cement or porridge, and it may glisten when mucus is present. Diarrhea may be green or brown and occasionally contains plugs of mucus and sometimes flecks of blood. Diarrhea usually lasts 2 to 14 days, although it may recur.
Affected pigs are unthrifty, have fecal staining of the perineal area and a hunched-up appearance, and sometimes are febrile, but they usually continue to eat. Pigs with recurrent diarrhea may show significant loss of body condition, poor feed conversion rates, and delays in reaching market weight (57).
Disease in Chickens
Background and occurrence.
Young meat-producing chickens (broilers) are susceptible to experimental infection with Brachyspira species, including B. pilosicoli (58–62), but under farm conditions they rarely suffer from AIS because they are slaughtered at around 6 weeks of age, before they are likely to have had significant exposure to Brachyspira species. On the other hand, AIS caused by infection with B. pilosicoli (and other Brachyspira species) commonly occurs in adult chickens laying table eggs and in breeding hens producing meat chickens (broiler breeders) (63–70). Infection is particularly common in chickens under free-range conditions (71, 72). Occasionally infection is reported in other poultry species, such as turkeys (30). Colonization and disease have been reported in many regions of the world (73–77). Infections can vary from being asymptomatic to severe, with increased mortality rates. Most commonly, infections are mild or moderate and generally are characterized by diarrhea with feces that are caramel in color and are frothy as a result of increased gas production.
Clinical signs.
The most economically significant outcomes of AIS are delayed onset and/or loss of egg production and an increase in fecal water content that results in “wet litter” (wet bedding material). In one early study, natural infection of two layer flocks with B. pilosicoli was associated with a 5% reduction in egg production, diarrhea in up to 25% of chickens, wet droppings, feces smeared on the feathers around the cloaca (“pasty vents”), lethargy, and depression (18). Subsequent observations of field cases have found that cases of AIS (not all of which necessarily were caused by or solely by B. pilosicoli) may result in increases in fecal water content, a lower growth rate, a delay in the onset of egg laying, and a decrease in numbers and quality of eggs (78–81). The shells of eggs from infected chickens may be contaminated with feces, and this limits opportunities for their sale as table eggs. The eggs also may be smaller, be lighter, have paler yolks, and have poorer shell quality than those from uninfected chickens. The progeny of chickens with AIS may be weak and show depressed weight gain, despite not being colonized (79, 82).
Experimental infection of adult breeder chickens with an avian B. pilosicoli strain resulted in a temporary increase in content of fecal water, fecal staining of eggshells, and a significant reduction in total production of eggs (79). The ceca of the infected birds were gassy, and the contents were frothy, fluid, and pale, but no gross or histological lesions were detected in the mucosa. Spirochetes were isolated but they were not found attached to the cecal epithelium. In another experiment, commercial-egg-laying chickens were experimentally challenged with one or the other of two strains of B. pilosicoli isolated from chickens. Approximately 80% of the chickens became colonized, and they had increased fecal water content and a reduction in weight gain, and the onset of egg laying was delayed (83).
Disease in Humans
Background and occurrence.
Relatively few studies have specifically investigated B. pilosicoli infections in humans. Most studies on “intestinal spirochetosis” have involved observation of colorectal biopsy specimens that show spirochetes attached to the epithelial surface, to form a “false brush border” (Fig. 2). Furthermore, in many such cases the species of spirochete present has not been identified. Publications have included case reports, analysis of archived histological samples, and reviews of the literature. Other sorts of studies have involved targeted examination for carriage of B. pilosicoli by analyzing fecal samples collected from individuals with different health statuses and from different regions. Frequently these studies have sought to determine risk factors for colonization and to look for associations with disease.
FIG 2.
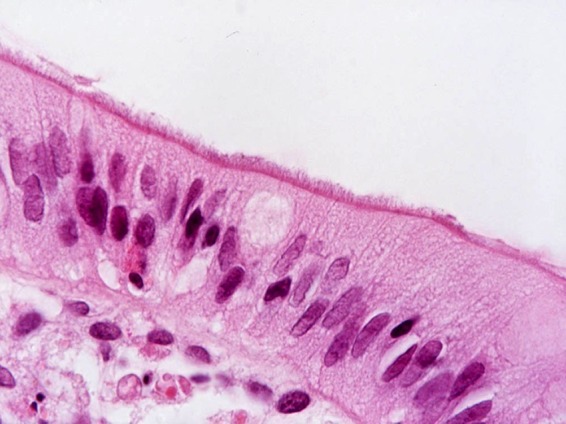
Appearance of a “false brush border” of Brachyspira pilosicoli cells attached by one cell end to the luminal surface of human colonic enterocytes in a patient diagnosed with HIS.
Clinical signs.
The majority of patients who have been described with histologically defined HIS have been from developed westernized countries, although rectal biopsy specimens showing false brush borders of spirochetes also have been recorded in rural patients from southern India (84) and from Brazil (85). Individuals with biopsy samples showing evidence of HIS may have one or another of a range of nonspecific symptoms attributable to an underlying chronic intestinal complaint for which other potential etiological factors have been excluded. Examples include abdominal pain, change in bowel habits, pseudoappendicitis, irritable bowel, diverticulitis, chronic nonwatery diarrhea, and rectal bleeding (86–91). On the other hand, HIS also has been observed as an incidental finding in biopsy specimens taken for other reasons; in such cases, and particularly where specific pathological changes are not found, the spirochetes appear to have a commensal relationship with the host (92). It has been difficult to reconcile these different findings, and particularly so as the identity of the spirochete species involved in different cases is rarely determined. Several reviews have concluded that clinical signs are more common where extensive spirochetal colonization occurs, with or without end-on attachment being observed, but particularly with spirochetal invasion occurring beyond the surface epithelium (93, 94). Clinical signs also appear to be more common and severe in colonized children, in patients who are infected with human immunodeficiency virus (HIV-positive [HIV+] patients), and in homosexual males than they are in other individuals (86, 87, 94).
Only one human experimental infection has been reported, where a volunteer (the author) drank cultures of B. pilosicoli strain WesB isolated from an Australian Aboriginal child with diarrhea (29). Examination of fecal samples demonstrated that the volunteer had become heavily colonized with the B. pilosicoli strain; he developed abdominal pain, bloating, and headaches, but diarrhea did not occur and B. pilosicoli was not isolated from the bloodstream. Treatment with oral metronidazole promptly removed the spirochetes from the feces, and the clinical symptoms ceased. Colonic biopsy specimens were not obtained. The results of this experiment are consistent with B. pilosicoli being able to cause nonspecific abdominal complaints in humans.
A number of patients with a B. pilosicoli spirochetemia have been described (95–101). In all cases these individuals have been chronically ill and/or immunocompromised, and the spirochetemia is likely to have been secondary to the immunosuppression. It remains unclear to what extent the spirochetemia may contribute to clinical signs in these patients, although some have shown multiorgan failure. Spirochetemia with B. pilosicoli is probably uncommon, since a study failed to identify any cases following examination of 1,063 blood samples from patients considered to be potentially at risk of being colonized (102).
PATHOGENESIS
The molecular mechanisms by which B. pilosicoli causes disease are not well understood, even though natural animal models are available. An early study looking for virulence-associated genes demonstrated that the spirochete lacks the attachment and invasion determinants found in the enterobacteria (103). Despite the recent availability of genome sequences, so far only a few B. pilosicoli attributes that might contribute to disease have been identified. Progress remains hampered by a lack of means for genetic manipulation of these bacteria, and without these tools, it is difficult to be able to determine the functional significance of individual genes or groups of genes whose products may be involved in the disease process.
Infection is thought to be predominantly by the direct fecal-oral route, with exposure to fecally contaminated water being postulated as a potentially important route of transmission (29, 104, 105). It is assumed that the spirochete passes through the stomach and small intestine protected within boluses of food or other organic matter, although it has been suggested that waterbirds also may acquire cecal infection from contaminated water in ponds by retroperistalsis of water up through the cloaca (70). B. pilosicoli cells show a chemoattraction to mucin solutions (106), and they produce enzymes, including the sialidase family-like proteins, that hydrolyze the mucus inner layer and may assist in penetration of the mucus overlying the colonic mucosa (43, 107, 108). B. pilosicoli is actively motile, with its periplasmic flagella generating a typical spirochetal “corkscrew-like” twisting motility that drives the spirochete forward, allowing it to penetrate the mucus. Swimming speeds increase as the viscosity of the medium increases, including at mucus concentrations that are equivalent to those found in the colon (106, 109). Different strains may vary both in their motility and in their chemotactic responses to mucin (106), and this could influence their relative colonization potential. Recently it has been demonstrated that expression profiles for different mucins may change in human patients with HIS who have different categories of lesions. It is not clear if these changes in mucin expression are driven by the spirochetes themselves or by associated alterations in other components of the intestinal microbiota (110). Given the important interactions between the spirochetes and mucus, these changes may be significant in the colonization process.
Once the spirochetes penetrate through the mucus overlying the enterocytes, one cell end attaches to the luminal surface of these cells (59, 111) (Fig. 3). Attachment occurs to mature apical enterocytes between crypt units but not to immature cells deeper in the crypts (112). B. pilosicoli surface lipoproteins may be involved in facilitating this attachment by undergoing protein-protein interactions with specific receptors on the cell surface (52). If a sufficient number of cells become attached, this gives the appearance of a “false brush border.” Where massive colonization occurs, it may cause a physical impedance to water and electrolyte absorption through the colonic enterocytes and hence contribute to diarrhea (88, 89) (Fig. 4).
FIG 3.
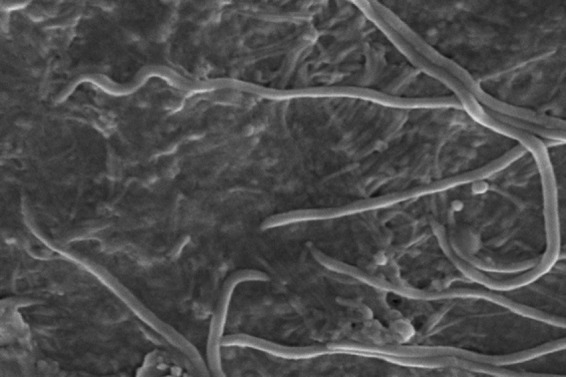
Scanning electron microscope image of B. pilosicoli cells attached by one cell end to a monolayer of cultured Caco-2 cells.
FIG 4.
Scanning electron microscope image of dense fields of B. pilosicoli cells attached by one cell end to the luminal surface of the colon in an experimentally infected pig.
In Vitro Models
Cellular attachment by B. pilosicoli strains has been studied in vitro using intestinal epithelial cell lines, but to date no specific adhesins or host cell receptors have been identified (111, 113). In infected Caco-2 cell monolayers, the cell junctions were the initial target sites for attachment (111). Subsequently the monolayers underwent a series of changes, consisting of an accumulation of actin at the cell junctions, a loss of integrity of tight junctions, and evidence for apoptosis occurring in the cells to which the spirochetes were attached. As assessed by quantitative reverse transcription-PCR, the colonized monolayers showed a significant upregulation of expression of interleukin-1β (IL-1β) and IL-8; in vivo these molecules may be responsible for attracting neutrophils to the infected colon and enhancing inflammation. Candidates that might cause cellular damage in vivo include the biological activities of LOS and/or of membrane proteases (107, 108, 111). Exposure of the monolayers to B. pilosicoli sonicates (containing LOS) caused a significant upregulation of IL-1β, tumor necrosis factor alpha (TNF-α), and IL-6, while culture supernatants and sonicates from the nonpathogenic Brachyspira innocens did not alter the expression of cytokines.
When B. pilosicoli cells were exposure to the stress hormone norepinephrine in vitro, they showed enhanced growth rates, increased attraction to mucin, and more cells attached to exposed Caco-2 cells (114). Norepinephrine may be present in the intestinal tract following periods of stress, so it is likely that this stimulation of the spirochete also can occur in vivo. Consequently, strains of B. pilosicoli may be more likely to cause disease in individuals who are experiencing stress; this may include the stress in pigs and chickens induced by intensive housing and similar stresses in people living under crowed and poor socioeconomic conditions or having concurrent disease.
Observations in Natural and Experimental Infections
Lesions seen in tissue samples obtained from individuals colonized by B. pilosicoli under natural and experimental conditions also may be used to provide clues to the pathogenesis of infection with the spirochete.
Gross appearance at necropsy or by endoscopy.
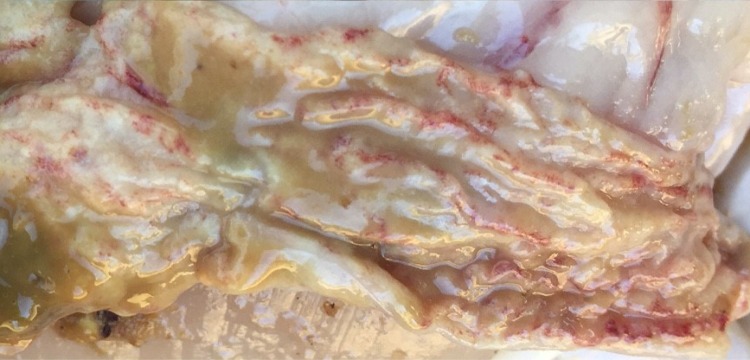
In experimentally infected pigs, gross alterations of the large intestines may be subtle, even in situations where the colonic mucosa shows microscopic evidence of severe spirochetal infection (115). In naturally infected pigs, the cecum and colon may be flaccid and fluid filled, with an edematous serosal surface and enlarged mesenteric and colonic lymph nodes. The large intestinal contents are usually watery, green to yellow, and frothy. Mild congestion and hyperemia of the mucosa may be observed, with some necrotic foci and erosions occurring (Fig. 5). Similar changes, including erosions in severe cases, may be seen in the ceca of adult chickens naturally infected with B. pilosicoli (Fig. 6). In chronic cases and in resolving lesions, hemorrhages may be covered by adherent fibrin, necrotic material, and digesta. In adult chickens, sometimes few if any gross lesions may be found in the large intestine, although the contents are often brown and frothy. In humans with histologically diagnosed HIS, the endoscopic appearance of the mucosa is commonly reported as normal, although there are other cases where the mucosa has been edematous, and erythematous spots or erosions have been present (reviewed in references 94, 116, and 117), including in a case where it was confirmed that B. pilosicoli was the spirochete involved (118). Biopsy specimens showing HIS have been found at sites throughout the colon and rectum, although a right-sided preference for positive biopsy specimens has been noted (119).
FIG 5.
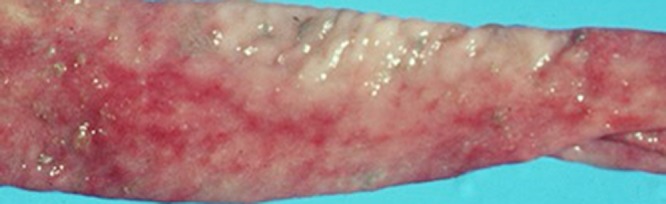
Appearance of the luminal surface of the opened colon of a pig experimentally infected with B. pilosicoli. The exposed mucosa shows congestion and hyperemia, with focal areas of superficial mucosal erosions and hemorrhages tracking along the length of the colon.
FIG 6.
Appearance of the luminal surface of an opened cecum of an adult laying chicken infected with B. pilosicoli. The exposed mucosa is covered by mucoid tan content. Areas of congestion, focal erosions, and hemorrhage can be seen in the mucosa underneath the contents.
A recent report from Japan found a significantly greater prevalence of HIS in samples from patients with sessile serrated adenomas/polyps (SSA/Ps) than in samples from control subjects, and a causal relationship was postulated (120). SSA/Ps are advanced premalignant lesions that serve as the precursors of many cases of colorectal cancer. A similar significant association was not found in Australian patients, although it was suggested that this might reflect the methodology used or population differences (121).
Microscopic appearance in different species.
Samples taken from experimentally and naturally infected untreated animals are more likely to show advanced lesions than are biopsy specimens taken from naturally infected humans, who would otherwise be treated if they showed clinical signs. Microscopic lesions in pigs with PIS are generally limited to the mucosa and submucosa but occasionally extend into the muscularis. The mucosa may be thickened, edematous, and occasionally hyperemic, with dilated, elongated crypts that are filled with mucus, cellular debris, and degenerate inflammatory cells (neutrophilic exocytosis), and there may be a mixed infiltrate consisting of neutrophils and lymphocytes in the lamina propria. B. pilosicoli cells occasionally may be seen invading through tight junctions between colonic enterocytes, within goblet cells, and in the lamina propria (37, 122). In the later stages of infection, inflammation may result in multifocal erosive, ulcerative, or mucoid colitis. The mucosa becomes thickened, and local ecchymotic or petechial hemorrhages occur on the surface, with the protozoan Balantidium coli occasionally being seen associated with the damaged porcine colonic mucosa (112). The crypt cell mitotic rate may be increased, resulting in immature, cuboidal, or squamous epithelium being present on the surface of the mucosa between crypts, leading to a reduction of absorptive surface area. As mentioned above, although sometimes focal and not always detected, the surface of the colonic enterocytes may be covered by a fringe of spirochetes attached end-on to form the characteristic “false brush border” (123, 124). Where this occurs, the spirochetes invaginate into the terminal web cytoplasm of individual colonocytes, with associated effacement of microvilli and disruption of microfilaments (54). An increase in eosinophil numbers in biopsy specimens has been reported in humans with HIS, although the species of spirochetes involved in the different cases was not identified (90). These patients also had an increased risk of having irritable bowel syndrome. In pigs, spirochetes also have been observed invading between epithelial cells in the extrusion zone that is located between adjacent crypt units (37). In chickens, the cytoplasm of enterocytes may appear damaged, with vacuolation, condensation, and fragmentation of the chromatin (59). In an immunocompetent heterosexual man with histological HIS, electron microscopy identified stunting of the microvilli together with spirochetes invading colonic epithelial cells, goblet cells, macrophages, and Schwann cells (125). In another patient, who had Crohn's disease and ankylosing spondylitis, spirochetes were found on the luminal surface of absorptive and goblet cells but also within the cytoplasm, in occasional macrophages within the lamina propria, and again within the occasional Schwann cell (126). Potentially, interference with Schwann cell function could influence neuronal myelation and function at the level of the gut wall or in other regions where the spirochete might invade. Interestingly, other spirochetes, including Treponema pallidum, Leptospira species, and some Borrelia species, also may show trophism toward neuronal tissue. In another study, spirochetal invasion was observed in two HIV+ patients where there was an extension of IS into the crypts of Lieberkühn, with spirochetal invasion of the colonic mucosa, and a marked inflammatory response that involved macrophages in the underlying lamina propria (127). Spirochetes that are presumed to have been B. pilosicoli also have been observed in the livers of immunocompromised human patients who had invasive colitis (128). There also has been a report of aborted fetuses from young women showing nontreponemal spirochetes in the lumen and mucosa of the intestine, as well as, less commonly, in other organs (129). There is a possibility that these spirochetes were invasive B. pilosicoli.
Spirochetemia.
Clear evidence for the invasive potential of strains of B. pilosicoli has come from reports of the spirochete being recovered from the bloodstream of elderly or critically ill humans with various immunosuppressive conditions (95–101). It is interesting that other spirochetal diseases, including those caused by Leptospira species and Borrelia species, have a spirochetemia as a central part of their pathogenesis.
B. pilosicoli has been demonstrated to attach to and agglutinate avian erythrocytes in vitro, and if this clumping of red cells occurs in the circulation, it might contribute to the severity of disease (130). Interestingly, relapsing fever Borrelia species also attach to and agglutinate erythrocytes, forming rosettes. B. pilosicoli spirochetemia has not yet been reported in naturally infected animals or birds, but this could reflect the fact that blood cultures are rarely taken from species other than humans, and the slow-growing anaerobic spirochete has specialized requirements for growth. In an experimental pig that received cyclophosphamide to suppress its immune system and then was injected intravenously with a B. pilosicoli culture, the spirochete was cultured from pericardial fluid after 5 days, thus confirming that it is able to survive for long periods at extraintestinal sites (131). Furthermore, in a group of 10 chickens experimentally inoculated orally with a B. pilosicoli strain isolated from a chicken, the spirochete was isolated from the livers of two chickens, and both had moderate to severe hepatic lipidosis. Lymphoid hyperplasia with proliferation of ellipsoid macrophages and germinal centers and the presence of pyknotic and karyorrhectic debris also was observed in the spleens of the birds (83). In a subsequent experiment, B. pilosicoli again was identified in the livers and spleens of chickens that were infected by the oral route (132).
Experimental Infection of Animals with Strains of B. pilosicoli Isolated from Humans
Evidence supporting the likely zoonotic potential of B. pilosicoli has come from studies in which strains of B. pilosicoli that were isolated from humans (and sometimes also from other species) were used to experimentally infect pigs (112), day-old chicks (58–61), laying chickens (133), or mice (134, 135). Typically, colonization and disease outcomes in these animals have been similar regardless of the species of origin of the isolates.
Pigs.
B. pilosicoli strain WesB, originally isolated from an Aboriginal child with diarrhea, was used to experimentally infect young newly weaned pigs (112). A control group was infected with B. pilosicoli strain 95/1000, originally isolated from a pig with PIS. Within 2 to 11 days, four pigs challenged with 95/1000 and two challenged with WesB became colonized and developed watery, mucoid diarrhea. The affected pigs had a moderate subacute mucosal colitis and had gross and histological changes similar to those reported in pigs with either natural or experimental PIS. Spirochetes were present within dilated intestinal crypts, and there was neutrophilic exocytosis and an increased secretion of mucus. In one pig, large numbers of spirochetes were observed attached to the colonic epithelium by one cell end. This study confirmed that human B. pilosicoli strain WesB had pathogenic potential in pigs.
Chickens.
In studies using newly hatched broiler chicks that were experimentally infected with isolates of B. pilosicoli recovered from humans, pigs, and/or dogs, either clinical signs were not observed (58) or the chicks developed watery diarrhea (59–62), sometimes with a depressed growth rate (59). Gross cecal lesions were not seen, but there were variable histological changes, including the characteristic occurrence of a dense mat of spirochetes attached to cecal enterocytes by one cell end (59–61), sometimes with a diffuse thickening of the cecal epithelial brush border (60). There was variable crypt elongation, with dilation of crypt lumina and a mild focal infiltration of heterophils (avian neutrophils) in the lamina propria. Sometimes spirochetes were found between enterocytes or producing gap-like lesions, and subepithelial accumulation of spirochetes and focal erosions without an inflammatory reaction were recorded (58). Vacuolation and protein deposition were found in the apical cytoplasm of some luminal enterocytes. In some cases, microvilli were obscured, damaged, or obliterated by large numbers of attached spirochetes, and there was disruption to the terminal web microfilaments. Individual spirochetes invaginated into the cellular membrane and indented into the terminal web cytoplasm but did not penetrate it (60).
The use of a human isolate of B. pilosicoli to experimental infect adult laying hens resulted in a persistent and significant increase in fecal water content (133). In this case, neither attachment of spirochetes nor gross pathological changes were found.
Mice.
Isolates of B. pilosicoli of human, avian, or porcine origin were used to experimentally infect lipopolysaccharide (LPS)-responsive C3H/HeOuJ [lps(n)/lps(n)] and LPS-hyporesponsive C3H/HeJ [lps(d)/lps(d)] mice via gastric intubation (135). Cecal tissue from mice infected with avian or porcine isolates revealed spirochetes attached end-on to the apical surface of enterocytes, but this was not found with the human isolate. There were no apparent differences in severity of cecal lesions between the two mouse strains infected with these isolates. Transmission electron microscopy showed spirochetes invaginated into the host cell membranes with a resultant effacement of microvilli and loss of the glycocalyx.
In another experiment, weanling C3H/HeJ mice fed on standard or experimental diets were inoculated with human strain WesB (134). The mice became colonized, but no end-on spirochetal attachment to the epithelial cells of the cecum was observed. Colonization was not associated with clinical signs.
Immunity
Host immune mechanisms directed against B. pilosicoli are poorly understood. Agglutinating serum antibodies have been recorded in pigs that have recovered from experimental infection (1), although no significant antibody levels against whole-cell preparations of B. pilosicoli were found in experimentally infected pigs with mild colitis 18 days after exposure (136). In other experiments, pigs developed low levels of serum IgG against B. pilosicoli whole-cell extracts after challenge (137). Mice experimentally infected with B. pilosicoli strains also developed serum antibody responses to the isolates (134, 135).
The existence of long-term colonization, involving intimate contact with the colonic epithelium, suggests that the spirochete may be able to subvert or evade immune mechanisms. If protection does occur, it may be strain specific, as B. pilosicoli strains show considerable antigenic variability in their surface LOS (51). Nothing is known about passive maternal immunity, although it may occur, as natural infection with B. pilosicoli has not been recorded in young chicks, piglets, or babies.
EPIDEMIOLOGY
Natural Habitat and Survival
Brachyspira pilosicoli colonizes the large intestines of many species of wild and domesticated birds and animals. It is regarded as a common pathogen of pigs and adult chickens, and on some farms infections with B. pilosicoli may be endemic. Cross-species transmission undoubtedly occurs, and zoonotic transmission is highly likely, although it has not been conclusively demonstrated (138). The spirochete is passed in feces, and it may survive for prolonged periods in natural water systems such as lakes and ponds, from which it can be isolated. These sorts of water bodies may contain nutrients and anaerobic sludge that would favor the spirochete's survival. For example, B. pilosicoli has been shown to remain viable in lake water held at 4°C for 66 days or at 25°C for 4 days (29). It can survive for 119 days in soil and for 210 days both in soil containing 10% pig feces and in pig feces alone (139). On the other hand, three different cell concentrations of B. pilosicoli survived in chicken cecal feces at 37°C for only between 2 and 17 h, with a maximum survival time of 72 to 84 h at 4°C (140). Chicken feces are acidic and often dry, and this may account for the relatively short survival time of B. pilosicoli in chicken sheds.
Dietary Influences on Colonization and Disease
As B. pilosicoli colonizes the large intestine, some aspects of the epidemiology of this species may be influenced by the diet consumed, as this influences the microbiota and physical and chemical conditions where colonization occurs. Analysis of risk factors on Danish pig farms showed that the use of home-mixed and/or nonpelleted meal diets was correlated with a reduced occurrence of PIS (141), while in experimentally infected pigs, feeding them a pelleted diet increased the risk of colonization compared with feeding meal (142). Under experimental conditions, the addition of carboxy methylcellulose to a pig diet both increased the viscosity of the intestinal contents and encouraged colonization with B. pilosicoli following experimental inoculation (143). Grains such as barley and rye have high levels of soluble nonstarch polysaccharide (“soluble fiber”), which also may increase the viscosity of the colonic contents, and therefore enhance the capacity of B. pilosicoli to colonize. Consistent with this, experimentally inoculated pigs that were fed diets where the main cereal source was cooked white rice, which is highly digestible and low in soluble fiber, showed less colonization with B. pilosicoli than control pigs fed on a standard wheat-based diet (136, 143). On the other hand, addition of insoluble fiber in the form of “distillers dried grains with solubles” to a standard North American pig diet (based mainly on corn and soybean meal) did not alter susceptibility to experimental infection with B. pilosicoli (144). There is some evidence that dietary influences on colonization may occur in species other than pigs. For example, in mice experimentally infected with a human strain of B. pilosicoli (WesB), supplementing a standard mouse diet with lactose and zinc bacitracin (ZnB) resulted in more animals becoming colonized, while supplementing just with zinc bacitracin did not support colonization (134). Differences between typical human diets consumed in developed and developing countries (and particularly in poorer villages and peri-urban areas) potentially could contribute to the much high rates of colonization in the latter settings.
Strain Typing Methods
As mentioned above, collections of B. pilosicoli isolates have been subjected to subspecific differentiation using a variety of methods. All studies have demonstrated the existence of extensive genetic heterogeneity in the species, and hence typing is only really useful when trying to trace individual strains in situations where local transmission is suspected. This approach can provide important epidemiological information to help devise control measures. Where different strains are present in individuals in a village or in animals on a farm, they either may have been independently introduced or may have arisen from “microevolution” of the original strains that were present at that location (39).
Most recently, the advent of next-generation sequencing (NGS) has allowed inexpensive whole-genomic sequencing so that techniques such as multilocus sequence typing now can be performed without the need to amplify and sequence individual genes. At the same time, the genome potentially can be screened for other genes, such as those predicted to be involved in virulence and/or antimicrobial susceptibility. To date NGS has not used with large numbers of B. pilosicoli isolates, although it has been applied to collections of B. hyodysenteriae isolates (145).
Pigs
PIS has been reported in most countries where pigs are reared. Investigations in different regions have found that a high proportion of farms often may be infected (146) and that infection is common in farms having pigs with persistent diarrhea problems (141, 147, 148). Increased recognition of the condition has resulted from improved diagnostic methods, the reduction in use of routine antimicrobial growth promoters that may have been inhibiting growth of the spirochete (149, 150), and the fact that other major intestinal diseases of pigs are now better controlled in many countries.
Transmission is believed to occur by the fecal/oral route, and infection may be introduced into naive herds by carrier pigs or by feral birds or animals that access the farm (32, 151, 152). B. pilosicoli can persist in the environment, and the disease can recur between batches of pigs if the premises are not adequately cleaned and disinfected. On one pig farm, B. pilosicoli was found in chickens, effluent pond water, and wild ducks visiting the effluent pond (151). An isolate recovered from the pond water had the same genetic type as one from a pig. These findings emphasize that water should not be recycled on piggeries and support previous suggestions that feral water birds may contaminate water supplies and so present a potential source of B. pilosicoli infection for pigs and for other animals (29).
The on-farm epidemiology of B. pilosicoli can vary considerably between farms (151). Sometimes the incidence is low and largely confined to pigs of one age group, while in other herds it may be widespread and associated with a number of different B. pilosicoli strains. The latter occurrence on certain farms might explain why PIS commonly recurs in convalescent animals or in those treated with antimicrobials. In such cases, reinfection may be with a different strain, possibly having different antigenic or other biological properties.
Chickens
AIS is rarely diagnosed, as the clinical signs usually are relatively mild and nonspecific, and most laboratories specializing in diseases of poultry do not regularly use diagnostic methods suitable for detecting intestinal spirochetes. On the other hand, epidemiological surveys have shown that infection is common in flocks of laying and breeding chickens in Europe, Scandinavia, North and South America, Malaysia, and Australia (63–70). Besides caged or housed flocks, outdoor free-range flocks and organic flocks with access to outside areas are commonly affected (71, 72, 76). In most regional studies, approximately 70% of laying flocks and 50% of breeding flocks contain chickens that are colonized by Brachyspira species. Within-flock prevalence varies from 10% to 95% of the tested samples. About 20% of these infected flocks contain chickens that are colonized with B. pilosicoli (63, 65, 67), and flocks with diarrhea or poor egg production are colonized more commonly than flocks with normal feces. Flocks with chickens that are over 40 weeks old are significantly more likely to be infected than flocks with younger chickens on the same farms (63, 65, 67, 76), and multi-age farms have the highest risk of infection (75). This pattern is likely to reflect relatively increased cumulated opportunities for transmission of the spirochetes to chickens as they age.
Humans
Prevalence based on colorectal biopsies.
There are a number of reasons why it is difficult to estimate the prevalence of B. pilosicoli infections in humans based on examination of colorectal biopsy specimens. First, biopsy specimens are regularly taken only from selected patients, and these include patients who may be suspected of having HIS (e.g., chronic diarrhea, rectal bleeding, abdominal pain, and/or bloating) and a much larger group of other patients with conditions such as suspected colonic adenomas, inflammatory polyps, or inflammatory bowel disease. Second, even where HIS may be present, not all cases are recognized by the histopathologist. Third, biopsy specimens are regularly taken only in facilities in developed countries, meaning that there is little information from much of the world. Fourth, even when HIS is observed, the majority of studies have not attempted to differentiate between B. aalborgi and B. pilosicoli as the etiological agent. Lastly, while there are numerous case reports where intestinal spirochetes have been seen attached by one of their cell ends to enterocytes in the colon and/or rectum, prompting a diagnosis of HIS, there are few control studies from the general population.
Although people undergoing colorectal biopsies are representative of only a small portion of the general population, in two studies where biopsy specimens from colorectal resections were examined for evidence of HIS, prevalences of 2.5% and 3.0% were found, in mid-Norway and South Norway, respectively (153, 154). This compares with detection rates of 20 to 50% in rectal biopsy specimens taken from homosexual males (155, 156) and 44% in biopsy specimens from HIV+ patients in other Western countries (13).
Anaerobic culture on selective medium of rectal biopsy specimens from 22 male homosexuals showing histological evidence of HIS resulted in the isolation of B. pilosicoli from 50% of the samples (14). In comparison, where molecular techniques have been used to identify the species of spirochetes present in HIS samples from the general human population, these have shown that B. aalborgi is the most common species involved (157). Examples from different studies include the following: 62.5% B. aalborgi and 0% B. pilosicoli (158); 86% B. aalborgi and 14.3% B. pilosicoli (159); 70% B. aalborgi and 6% B. pilosicoli (160); 100% B. pilosicoli and 0% B. aalborgi in 3 HIV+ homosexual males, with 78% B. aalborgi and 19% B. pilosicoli in 31 HIV− homosexual males (161); 80% B. aalborgi, 14% B. pilosicoli, and 6% both species (162); 100% B. aalborgi but 15% also B. pilosicoli (163); and 69% B. aalborgi, 30% B. pilosicoli, and 1% both species (164). In some studies, other, atypical strains also were identified. Although B. aalborgi is associated with many cases of HIS, the extent to which it can act as a potential human pathogen remains unclear.
Prevalence determined by fecal carriage.
A better understanding of B. pilosicoli prevalence has come from studies where the spirochete has been identified in feces specifically collected from different populations for this purpose. Identification has been by isolation from feces combined with PCR for species determination and/or by direct PCR detection on DNA extracted from the feces. A striking feature of the reported studies has been the high proportion of apparently “normal” individuals in developing countries who are colonized by B. pilosicoli. These have included native populations in Oman (26.7%, 11.4%) (10), India (25.6%) (165), Papua New Guinea (PNG) (22.8%) (104), Rwanda (22.6%, 14%) (166), and the island of Bali in Indonesia (11.8%) (105). Carriage also is common in rural Aboriginal Australians (32.6%) (11), among whom the standard of living is often much lower than that for other Australians. In Bangladesh, large numbers of intestinal spirochetes (presumed to be B. pilosicoli) were found in the feces of over a third of patients with cholera (167). The high prevalence of B. pilosicoli among individuals living in developing countries may be related to conditions such as increased exposure as a result of poor hygiene, environmental factors such as the poor quality of water supply and animal contact, host factors such as low immune status and coinfections, and nutritional deficiencies or differences in dietary ingredients compared to the case for more affluent Western populations (22, 104).
In contrast, infection with B. pilosicoli in the general population of developed countries appears to be uncommon. Of 1,679 Belgian patients with diarrhea, only 1.2% carried a spirochete called Treponema hyodysenteriae by the authors (166) but which was almost certainly B. pilosicoli. Similarly, spirochetes that later were identified as B. pilosicoli were present in fewer than 0.9% of non-Aboriginal Australians, including in 656 adults and children recorded as having gastrointestinal disturbances and in 39 healthy children (11, 22). In subsequent studies in Western Australia, B. pilosicoli was isolated from the feces of 10% of 151 rural Aboriginal patients with gastrointestinal complaints but from none of 142 non-Aboriginal rural patients with similar conditions (168). In a later study using PCR detection, B. pilosicoli was identified in the feces of 14.5% of rural Aborigines with gastrointestinal disorders but not in any non-Aboriginal patients or healthy individuals (169). Infection with B. pilosicoli in Aboriginal patients was significantly associated with having chronic diarrhea and with failure to thrive and being underweight in children. In another study, among 586 non-Aboriginal individuals who were long-term residents of Perth, Western Australia, including elderly patients in care or in hospital, children, and individuals with gastrointestinal disease, only one (elderly) person with a history of diverticulitis was culture positive for B. pilosicoli (170). Studies in Italy have successfully isolated B. pilosicoli from the feces of patients with gastrointestinal disease (7, 171), although their ethnicity, travel history, and background were not described.
Immigrants and visitors to developed countries.
In a large study in Great Britain where feces were cultured, a 1.5% prevalence of spirochetes having the growth and biochemical properties of B. pilosicoli was found. A striking feature was that the colonized individuals all either were of Indian descent and had recently visited the Indian subcontinent or were male homosexuals (8). The specific prevalences of carriage in these two subpopulations were 4.8% and 20.6%, respectively.
In a study of healthy immigrants to Western Australia, carriage was detected in 10.6% of the population, with the prevalence being significantly higher among individuals from the Middle East and from Africa than among individuals from other regions (170). In another study, a fecal carriage rate of 15% was found among 227 recent healthy immigrants to Australia (169). In the original study, feces from 17 of 180 (9.4%) Indonesians who either were short-term or medium-term visitors to Perth, Western Australia, were found to be culture positive for B. pilosicoli (170). The colonized individuals had lived in Perth for periods varying from 10 days to 4.5 years (with a median duration of 5 months).
Persistence of infection.
When the Indonesian visitors to Perth subsequently were resampled at different times, all the people who had been culture negative remained negative, and a few individuals who had been positive remained so after 5 months (170). Pairs of isolates that were recovered from two individuals after 4 and 5 months had the same pulsed-field gel electrophoresis (PFGE) types, while two isolates with different PFGE types were recovered 2 months apart from the same individual. It was suggested that culture-positive individuals were likely to have been either colonized while in Indonesia or infected while in Perth, perhaps as a result of contact with other colonized visiting Indonesians with whom they lived or socialized. There was no significant association between colonization with B. pilosicoli and clinical signs when the individuals were tested, but B. pilosicoli was 1.5 times (confidence interval, 0.55 to 4.6) more likely to be found in feces with a wet-clay consistency than in normal feces.
In a study in rural villagers in PNG where repeated samples were taken from individuals at different times, the results indicated that every individual was likely to be newly infected with a B. pilosicoli strain at least once a year, and on average, individuals were colonized for 4 months per year (104). Numerous different B. pilosicoli strains circulate in these environments, and mixed infections with different strains probably were common (28). The weight of infection was very high and may have impacted the vitality of the whole community.
Homosexuality and/or HIV status.
Infection with B. pilosicoli occurs at an increased frequency in males who have sex with males and in HIV+ patients. For example, B. pilosicoli was isolated from half of the rectal biopsy specimens obtained from homosexual males who were visiting a clinic for sexually transmitted diseases in Sydney, Australia (14), most of whom had nonspecific gastrointestinal symptoms and about half of whom were HIV+. Other studies have associated HIV+ status and an increased frequency of colonization with B. pilosicoli (155, 156). A number of case reports on fecal carriage of intestinal spirochetes have described isolation of spirochetes from homosexual males (5, 9, 172).
Age.
In a number of studies B. pilosicoli has been shown to be more prevalent in children than in adults. For example, Australian Aboriginal children who were aged between 2 and 18 years were significantly more likely to be colonized with B. pilosicoli than were children who were less than 2 years old or than were adults (11). In another study, carriage of B. pilosicoli again was significantly increased in 2- to 5-year-old Aboriginal children compared to older individuals (168). Similarly, colonized hospitalized Omani patients were more likely to be older than 2 years than younger (10). On the other hand, studies in both Bali and India found that carriage of B. pilosicoli was not associated with the age of the participants (105, 165). A review of the recent literature on HIS in children has been published (87).
Gender.
Most studies of B. pilosicoli carriage in heterosexual individuals have failed to record the gender of the individuals involved. In a study in PNG, no difference in carriage rate associated with gender was found (44), although studies of HIS in Norway found that the condition occurred more commonly in males than in females (153, 154).
Transmission between humans.
There are no reports of specific studies investigating transmission of human intestinal spirochetes, although the studies on fecal carriage have allowed analysis of environmental factors that may be important in transmission. It is presumed that infection occurs in humans via the oral route, following exposure to infected fecal material, in the same way as for animals (104). In PNG, colonization with B. pilosicoli was significantly more common in individuals living in villages than in those living in an urban area. Hygiene in the villages was poor, as modern sanitation and running water supplies were not available, and nearby streams were the only source of water (104). In Bali, the highest rate of colonization with B. pilosicoli occurred in a peri-urban area rather than in rural villages (105). This peri-urban area was crowded and contained pigs and other animal species, and the people obtained their water from shallow wells rather than having a supply of clean tap water from the city supplies. In India, B. pilosicoli was significantly more common in individuals who obtained their drinking water from a well or if their water was treated before use because of its poor quality (165). Taken together with the fact that B. pilosicoli may survive for long periods in water (29), these studies suggest that contaminated water sources may help to account for the high rates of intestinal carriage with B. pilosicoli found in developing communities.
Opportunities for cross-species transmission.
B. pilosicoli colonizes a wide range of hosts, and in households where animals and humans live in close proximity, there is potential for zoonotic transmission. Similarly, individuals working with intensively farmed pigs, chickens, or other farmed species may be at increased risk of exposure. The spirochete has been isolated from waterbird feces on the ground and from pond and lake water at picnic sites, including in a municipal zoological garden (29), as well as from the feces of puppies for sale in pet shops (34). These occurrences demonstrate the potential for transmission from animals to members of the general human community, and particularly to children. In one study, restriction enzyme analysis of B. pilosicoli strains from dogs and humans indicated that some of these strains were closely related (228). In another study, a B. pilosicoli isolate from an Australian dog and isolates recovered from Aboriginal children in the same community were found to be similar (22), again suggesting that dogs could be a reservoir for B. pilosicoli infections in humans. In PNG, among the village animals sampled, B. pilosicoli was detected only in four dogs and a duck and not in free-ranging village pigs (104). PFGE showed that a canine isolate from one PNG village was the same as an isolate from a human from another village, but no evidence was obtained to show that there could have been direct cross-species transmission. In a survey involving 101 dogs sampled in three tea estates in India, only one canine isolate of B. pilosicoli was obtained (166), and hence ownership of dogs did not represent a risk factor for carriage of B. pilosicoli by humans. In all three studies discussed above, the lower prevalence of B. pilosicoli in dogs than in humans indicates that it is unlikely that dogs serve as a major source of human infection in endemically infected populations, and indeed humans may act as a source of infection for village dogs in situations where they engage in coprophagy of human feces. In these environments, infection in humans seems more likely to come from other infected individuals or from contaminated water sources. B. pilosicoli also has been found on the carcasses of “spent” laying hens (hens discarded because they have stopped laying) in a supermarket in Belgium, and this may represent a potential source of transmission to humans who handle or consume this material but also to dogs that may be fed on raw chicken carcasses (173).
A summary of risk factors for human infection is presented in Table 2.
TABLE 2.
Factors associated with increased risk of human infection with B. pilosicoli
| Risk factor |
|---|
| Contamination of drinking-water supply with human or animal feces |
| Bathing in contaminated water (lakes, ponds) |
| Living in rural or peri-urban villages in developing countries |
| Living in rural Australian Aboriginal communities |
| Recent migration or travel from a developing country |
| Crowding and poor socioeconomic environment |
| Male homosexuality (men having sex with men) |
| HIV+ status |
| Coinfection with B. aalborgi and/or intestinal protozoa |
| Contact with animals? |
| For spirochetemia, debilitation and immunosuppression |
DIAGNOSIS
Clinical Signs
Clinical examination of individual pigs, chickens, and other animals, or groups of these animals, may reveal signs such as poor or uneven growth, wet feces, and species-specific effects such as delayed egg production that lead to a suspicion of infection. It is important to examine a cross-section of affected individuals, if these are available. In human beings, the occurrence of chronic diarrhea, failure to thrive in children, and/or other nonspecific intestinal symptoms, particularly in population groups that are known to be at increased risk, should prompt consideration of possible infection with B. pilosicoli. A definitive diagnosis requires the demonstration of B. pilosicoli, but the significance of this finding needs to be interpreted in the context of a complete diagnostic investigation.
Diagnostic Samples
In the case of production animal species, postmortem examination of several affected individuals should be undertaken if possible. The preferred diagnostic samples from all species include fresh feces and/or a series of samples of the colonic wall/biopsy specimens. In humans, endoscopy can be undertaken to look for suspicious areas that than can undergo biopsy and subsequent histopathological and microbiological investigation.
Laboratory Diagnosis
Smears.
Fecal or colonic contents or mucosal scrapings can be suspended and either wet or dried smears prepared and examined for the presence of microorganisms with the appearance of spirochetes. This simple screening approach is relatively nonspecific and has limited diagnostic value in itself.
Culture.
Brachyspira pilosicoli is anaerobic but will tolerate exposure to oxygen when plates are opened on the bench. Due to its slow growth rate and tendency to become overgrown by other, more rapidly growing species, selective solid medium systems are used for primary isolation from feces or colonic samples (174, 175). Typically, the medium consists of Trypticase soy agar supplemented with 5 to 10% defibrinated ovine or bovine blood, together with 1 to 5 antibiotics (including spectinomycin, rifampin, spiramycin, vancomycin, polymyxin, and/or colistin) to make the medium selective. A frequently used isolation plate contains 400 μg/ml spectinomycin and 25 μg/ml each of colistin and vancomycin (174). Alternatively, spectinomycin (400 μg/ml) can be used or can be used in combination with polymyxin B (5 μg/ml). Plates can be incubated in an anaerobic jar (with 94% H2 and 6% CO2) or anaerobic chamber (with 80% N2, 10% H2, and 10% CO2). Incubation can be at 37°C, but enhanced growth and selection occur at 41°C. Plates should be examined after 3 days and reexamined every 2 to 3 days for up to 2 weeks. The spirochetes form a low flat sheen of confluent growth with sharply defined edges. Growth sometimes penetrates the agar and is surrounded by weak hemolysis. Colonies generally are not formed, but some may occur on fastidious anaerobe agar after prolonged incubation. Growth can be confirmed by the characteristic morphology and motility of the spirochetes on wet mounts using dark-field or phase-contrast microscopy. Subcultures can be made on nonselective blood agar plates. The lack of colony formation and the potential presence of other Brachyspira species means that it is preferable to undertake repeated rounds of subculturing to obtain a pure culture before proceeding with other tests.
Secondary culture.
The spirochetes can be further propagated in anaerobic brain heart infusion broth supplemented with 10% fetal bovine serum incubated at 37 to 41°C. Growth has been shown to be enhanced by adding 1% oxygen to the N2 environment and by shaking the culture vigorously (176). The oxygen is consumed by NADH oxidase activity, while shaking presumably increases the gas/liquid interface and may replicate conditions occurring in the colon. The same physical conditions can be used to enhance growth in Kunkle's medium, a prereduced anaerobic Trypticase soy broth medium containing 2% fetal bovine serum and cholesterol (177). Under optimal conditions, growth of 108 to 109 cells per ml can be obtained in either of these broths within 2 to 3 days.
Blood cultures.
If spirochetemia is suspected, a series of blood samples should be taken and incubated in anaerobic blood culture bottles incorporating suitable detection methods. B. pilosicoli can be detected using some manual blood culture systems, with an incubation period of up to 10 days followed by subculture (38). The most commonly described media in which the spirochete have been detected are the Hémoline and BBL Septi-Chek formulations, although they have been detected in automated systems after prolonged incubation of up to 15 days (38, 95–101). Growth may not always be automatically signaled, and the medium should be visually checked for turbidity and aliquots examined for spirochete cells using phase-contrast microscopy.
Storage.
Isolated spirochetes can be stored at −80°C in 50% horse serum–1% brain heart infusion broth–10% glycerol, under which conditions they will survive for 5 years or more. B. pilosicoli also survives for extended periods at −80°C when cultures growing in Kunkle's broth are frozen directly.
Biochemical characteristics.
Biochemical characterization of intestinal spirochetes is no longer routinely undertaken in veterinary diagnostic laboratories. It was thought that the capacity to hydrolyze hippurate could be used to differentiate B. pilosicoli from other weakly hemolytic intestinal spirochete species, but that test is now considered less reliable as hippurate-negative isolates have been described (54).
MALDI-TOF MS.
In recent years, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has been adapted for rapid and inexpensive identification of isolates of Brachyspira species, including B. pilosicoli. Identification is based on the profile of spirochetal proteins, including ribosomal proteins and others with housekeeping functions (178).
Antigen detection methods.
Monoclonal antibodies (MAb) raised against cell envelope proteins of B. pilosicoli have been described (179, 180), and these potentially could be used in fluorescent or immunoperoxidase antibody tests on fecal smears or on histological specimens to identify this species (see “Immunohistochemistry” below). Unfortunately, these reagents are not commercially available. A MAb-based immunomagnetic separation of B. pilosicoli from feces prior to culture and PCR did not improve the sensitivity of detection in pig feces compared to direct culture and PCR (181).
PCR detection and identification.
Diagnosis by culture is routinely supported by the use of PCR tests for confirmation of the identity of isolates suspected to be B. pilosicoli. Growth from the primary isolation plate is picked off using a sterile wire or toothpick and added directly to the amplification mixture, or the spirochetes can be subcultured to purity before being subjected to PCR amplification for identification. In laboratories where appropriate culture methods are not available or where a rapid diagnosis is required, DNA can be directly extracted from feces or from colorectal biopsy specimens and used for PCR (157–159, 182, 183). PCRs also have been used on spirochetes recovered by laser capture from fixed cecal mucosa in infected turkeys (30).
The PCR targets that are most commonly used are the B. pilosicoli 16S rRNA gene, which contains a signature sequence for this species (26, 184), but other PCRs based on the 23S rRNA gene (185) and portions of the nox gene encoding NADH oxidase also have been described (186). Also, amplification of portions of the nox gene followed either by sequencing (186) or by restriction fragment length analysis of the product (187) has been used to determine the species Brachyspira isolates in specialized veterinary diagnostic laboratories.
Duplex and multiplex PCRs, detecting different Brachyspira species and sometimes also detecting other enteric pathogens, and quantitative PCR assays that allow spirochete quantitation also have been described for use in samples from pigs (188–192), chickens (189, 193), and humans (194).
Biopsy Specimens and Colonic Mucosa
Histology.
As indicated above, light and electron microscopic examination of colorectal samples may detect spirochetes attached to the mucosa. Although the visual detection of attached spirochetes is highly suggestive of an infection with B. pilosicoli, in cases of HIS, the spirochetes involved also could be B. aalborgi. The attached spirochetes are easier to visualize with silver-based stains than with hematoxylin and eosin staining.
Although end-on attachment of B. pilosicoli to colonic enterocytes is a common feature of the disease for all species, its occurrence may be localized, and it is not observed in all colonized individuals, including those with clinical signs (122). Consequently, failure to detect attached spirochetes in no way excludes the possibility that B. pilosicoli is colonizing the large intestine. To improve prospects for diagnosis, it is recommended that multiple biopsy specimens or samples be taken from throughout the colon to look for localized areas of attachment (91).
Imprint cytology.
A recent study has shown that imprint cytology of human colonic biopsy specimens can reveal spirochetes (subsequently identified as B. pilosicoli by PCR) that are not necessarily detected as a “false brush border” by histology (195).
Immunohistochemistry.
The application of antiserum to Treponema pallidum or Leptospira interrogans to histological sections can be used to assist with identifying the presence of attached spirochetes (85, 117, 163, 196). An alternative novel approach has been the use of in vivo confocal endomicroscopy on fresh tissue, with specific fluorescent staining used to identify areas of spirochete attachment (197).
In situ hybridization.
Another adjunct to diagnosis is the use of a fluorescent in situ hybridization technique which uses fluorescent oligonucleotide probes to visualize and localize spirochetes associated with the mucosa in formalin-fixed tissues. This approach has been used with colonic samples from animals (198, 199) and with colorectal biopsy specimens and fecal material from humans (164, 200). It has been combined with DNA extraction from samples, followed by PCR amplification of the 16S rRNA gene and DNA sequencing to identity the spirochetes involved (164). The technique also has been modified so that visualized spirochetes are isolated by laser capture microdissection before individual microcolonies are subjected to direct 16S rRNA gene PCR and DNA sequencing (201). An advantage of these techniques is that they provide simultaneous identification and localization of the spirochetes associated with the intestinal mucosa (Fig. 7).
FIG 7.
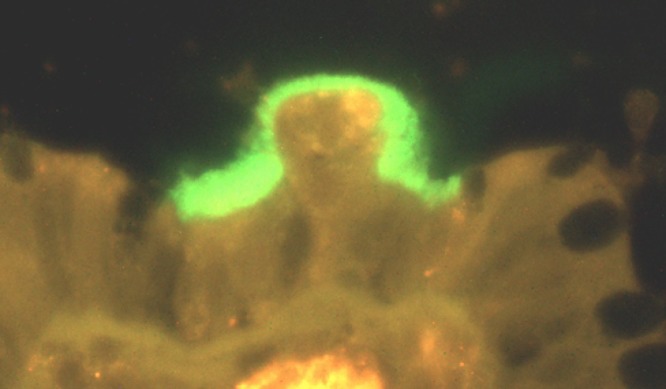
In situ hybridization with a labeled oligonucleotide probe specific to B. pilosicoli, showing localized attachment of a fringe of fluorescing spirochete cells to the surface of colonocytes in an infected pig.
DNA extraction and PCR.
As discussed above, fixed tissue (including archived tissue samples) also can be used for DNA extraction and PCR to identify attached spirochetes.
Serology
To date no reliable serological assays are available for detecting evidence of exposure to B. pilosicoli. A study using a recombinant B. pilosicoli ClpX protein as an enzyme-linked immunosorbent assay (ELISA) antigen gave cross-reactions with sera from humans who had no known exposure to the spirochete (202). Antigens with potential for use in serological assays have been identified but not have not been thoroughly tested for specificity (179, 180, 203).
PREVENTION
Vaccines
No commercial vaccines are available for prevention of B. pilosicoli infections. In pigs an autogenous bacterin induced systemic antibody titers, but the vaccinated animals still became colonized and developed diarrhea after experimental challenge (136). In mice, experimental vaccines based on two recombinant oligopeptide-binding proteins generated both systemic and colonic IgG antibody responses. After experimental challenge, the vaccinated mice were colonized for significantly fewer cumulative days, and in the case of one protein, fewer mice were colonized than in the control group (204). Oligopeptide-binding proteins may have potential for use as B. pilosicoli vaccine components; however, they require further testing in mice and other animal species.
Methods To Avoid Transmission of Infection
It can be difficult to avoid the introduction of B. pilosicoli into pig herds or flocks of chickens because of the presence of potential reservoir hosts such as wild birds and rodents that may access farms. This is a particular problem in uncontained outdoor production units. Similarly, humans living in rural villages in crowded conditions and lacking adequate hygienic facilities are at increased risk of exposure via the fecal-oral route. Provision of clean water that is not contaminated with the spirochete (and other pathogens) is a major consideration for the development of control measures.
In order to reduce transmission in endemically infected pig farms, in it is important to limit mixing of pigs from different age groups and sources. Avoiding rapid changes of diet at different stages of production, limiting stressors, and implementing management strategies that limit access to contaminated environments can reduce the impact of the infection. Replacing continuous-flow systems where pigs of all ages are present on a farm by “all-in/all-out” (batch) systems reduces the risk of perpetuating infection within a herd (146). Modification to the diet composition and/or physical form to reduce the viscosity of the intestinal contents or adding zinc oxide in the feed at 3 kg/ton may be helpful in limiting spirochete proliferation. In laying chickens, however, when zinc bacitracin (ZnB) was added to the diet, it enhanced colonization with B. pilosicoli following experimental infection (205). ZnB acts mainly on Gram-positive bacteria, and it is likely to have caused changes to the microbiota that directly or indirectly facilitated colonization by B. pilosicoli.
Disinfection.
Although B. pilosicoli is susceptible to many common disinfectants, the efficacy of some commercial products is reduced by the presence of organic matter (206). When alkaline salts, chlorine from a chlorine release agent, glutaraldehyde, hydrogen peroxide, iodine as an iodophor, and quaternary ammonium were tested, only alkaline salts failed to inactivate two concentrations of B. pilosicoli in the presence of organic matter in under a minute (139). Hence, cleaning and using disinfectants on fomites should assist in breaking cycles of infection between animals or humans living in close proximity.
TREATMENT
Antimicrobials
Antimicrobial susceptibility.
There are limited data available about the in vitro antimicrobial susceptibility of isolates of B. pilosicoli recovered from animals and humans, and much of the published work that is described below is quite old. The choice and availability of antimicrobials for use in animals are more limited than they are for human beings. For example, there are very few antimicrobials registered for treatment of chickens that lay eggs for human consumption, because of concerns about antimicrobial residues occurring in eggs. Antimicrobial use is less of a concern for breeder chickens where their eggs are not eaten by human beings. Furthermore, in recent years some drugs that were previously effective against B. pilosicoli, such as dimetridazole and carbadox, have been prohibited from use in production animal species in many countries.
The antimicrobial susceptibility of B. pilosicoli isolates recovered from animals and humans has been evaluated using an agar dilution method that is based on the Clinical and Laboratory Standards Institute guidelines developed for testing the susceptibility of anaerobic bacteria, as well as by using broth dilution testing as was originally developed for B. hyodysenteriae (207). The susceptibility patterns of different isolates vary, and hence it is recommended that isolates be tested for susceptibility prior to selection of the drugs to be used for treatment.
B. pilosicoli has penicillin-binding proteins in its cell envelope (208), and strains potentially are susceptible to penicillins, although resistance does occur. Alterations in penicillin-binding proteins that might lead to resistance through reduced binding have not been explored, and resistance to penicillins is attributed to the activity of a wide array of different and novel group D beta-lactamases that are produced by different strains of the species (209, 210). Resistances to macrolides, lincosamides, and/or pleuromutilins occur in some strains and are attributed to mutational changes on the binding sites on the 50S ribosomal subunit (211, 212). Interestingly, a recent metabonomics study has shown that B. pilosicoli cells exposed to the pleuromutilin tiamulin retained metabolic activity even at concentrations where they show significant growth inhibition (213).
Pigs.
Antimicrobials can be used either prophylactically or therapeutically to reduce B. pilosicoli infection in pig herds. Groups of affected pigs can be treated by water or feed medication, although parenteral treatment may be necessary for individual pigs that are severely affected. The main antimicrobials used to treat PIS in different countries are tiamulin, valnemulin, carbadox, dimetridazole, and lincomycin (214–216), although isolates that are resistant to one or more of these drugs occur.
Chickens.
Only a few drugs that are effective are available for use in egg-laying chickens, and the availability varies in different jurisdictions. Susceptibilities of isolates from chickens are similar to those found with pigs (217, 218). Tiamulin and lincomycin are the most commonly used antimicrobials for the control of AIS (64, 132, 219), although to avoid toxicity, tiamulin should not be used in combination with the commonly used ionophores such as monensin, salinomycin, or narasin. Effective long-term control of AIS in breeding flocks may require regular treatments with courses of antimicrobials, for example, given at 1- to 2-month intervals, together with thorough housecleaning and implementation of strict biosecurity measures designed to prevent spread of infection between houses.
Humans.
Analysis of the susceptibility of a collection of 123 isolates of B. pilosicoli from humans from a variety of different geographic origins found that they were susceptible to ceftriaxone, chloramphenicol, meropenem, metronidazole, moxifloxacin, and tetracycline but not to erythromycin or ciprofloxacin (220). Isolates exhibited various resistance to amoxicillin and clindamycin depending on their origin. Susceptibility to amoxicillin was restored by the presence of clavulanic acid, which suggests the involvement of β-lactamase in causing the resistance. Over half the isolates were β-lactamase positive as assessed using nitrocefin disks, and the positive samples had increased amoxicillin resistance.
Treatment with inidazole (a nitroimidazole similar to metronidazole) removed B. pilosicoli from 16 of 18 PNG villagers who were excreting the spirochete in their feces (42). Based on the in vitro susceptibility data and on many previous reports of successful treatment for HIS (although not all necessarily associated with B. pilosicoli), metronidazole has been recommended as the drug of choice for therapy (87, 94, 117, 221–223). Therapeutic doses of metronidazole used have varied from 500 to 1,000 mg/day for 10 to 14 days. The use of macrolide antibiotics with or without metronidazole also has been suggested as being the best therapeutic choice for children with HIS (87).
Other Potential Treatments
Lactobacillus spp. have been shown to have antagonistic activities against B. pilosicoli in vitro (224). Cell extracts of two Lactobacillus strains were shown to suppress motility and growth of B. pilosicoli in vitro and to significantly reduce adherence and invasion of the spirochete in a three-dimensional avian cecal organ culture model (225). Subsequently, colonization and signs of disease were reduced when L. reuteri LM1 was added to drinking water of chickens approaching the start of lay, prior to experimental infection with B. pilosicoli (226). Large-scale use of such probiotic cultures as prophylactics may be justified in some flocks with persistent AIS problems but also potentially could be used for prophylaxis or therapy in other species, including in humans.
Methods To Exclude B. pilosicoli
B. pilosicoli has been eradicated from a 60-sow pig herd by treatment of all animals with the antimicrobial tiamulin followed by relocation of the breeding herd, thorough cleaning and disinfection of the original premises, and then returning the adult animals to the farm (227). This protocol would be more difficult to follow in larger herds, and the existence of reservoir hosts such as wild birds and rodents presents an ongoing threat of reintroduction. Consequently, strict biosecurity measures would be required to maintain freedom from infection. The same considerations apply to poultry farms, where strict external biosecurity is required, together with biosecurity between sheds that house laying or breeding hens of different ages. In humans who have an increased risk of infection with B. pilosicoli (Table 1), improving personal hygiene and avoiding exposure to contaminated water or foodstuffs is recommended to reduce opportunities for infection and further transmission. Effective control also requires that health professionals have a better understanding of the existence of the spirochete and its natural history and that appropriate diagnostic techniques are available to detect it.
Biography

David J. Hampson qualified as a veterinarian at the Royal Veterinary College, University of London, in 1979 and received a Ph.D. in veterinary medicine from the University of Bristol in 1984. He was employed as a Lecturer in Veterinary Virology at Massey University in New Zealand, and in 1986 he moved to Murdoch University in Perth, Western Australia, as a Lecturer in Veterinary Microbiology. He was promoted to full Professor in 2000 and subsequently served as the Head of the Department of Veterinary Biology, Dean of the School of Veterinary and Biomedical Sciences, and Dean of the School of Veterinary and Life Sciences. He is currently Chair Professor of Pathobiology and Head of the Department of Infectious Diseases and Public Health at City University of Hong Kong. Professor Hampson has worked on Brachyspira species since 1986 and was involved in recognizing and characterizing many new species, including Brachyspira pilosicoli.
REFERENCES
- 1.Taylor DJ, Simmons JR, Laird HM. 1980. Production of diarrhoea and dysentery in pigs by feeding pure cultures of a spirochaete differing from Treponema hyodysenteriae. Vet Rec 106:326–332. doi: 10.1136/vr.106.15.324. [DOI] [PubMed] [Google Scholar]
- 2.Harland WA, Lee FD. 1967. Intestinal spirochaetosis. Br Med J 3:718–719. doi: 10.1136/bmj.3.5567.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hovind-Hougen K, Birch-Andersen A, Henrik-Nielsen R, Orholm M, Pedersen JO, Teglbjærg PS, Thaysen EH. 1982. Intestinal spirochaetosis: morphological characterization and cultivation of the spirochete Brachyspira aalborgi gen. nov., sp. nov. J Clin Microbiol 16:1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munshi MA, Taylor NM, Mikosza AS, Spencer PB, Hampson DJ. 2003. Detection by PCR and isolation assays of the anaerobic intestinal spirochete Brachyspira aalborgi from the feces of captive nonhuman primates. J Clin Microbiol 41:1187–1191. doi: 10.1128/JCM.41.3.1187-1191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tompkins DS, Waugh MA, Cooke EM. 1981. Isolation of intestinal spirochaetes from homosexuals. J Clin Pathol 34:1385–1387. doi: 10.1136/jcp.34.12.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanna A, Dettori G, Grillo R, Rossi A, Chiarenza D. 1982. Isolation and propagation of a strain of Treponema from the human digestive tract—preliminary report. L'igiene Moderna 77:287–297. [Google Scholar]
- 7.Sanna A, Dettori G, Aglianò AM, Branca G, Grillo R, Leone F, Rossi A, Parisi G. 1984. Studies of treponemes isolated from human gastrointestinal tract. L'igiene Moderna 81:959–973. [Google Scholar]
- 8.Tompkins DS, Foulkes SJ, Godwin PG, West AP. 1986. Isolation and characterisation of intestinal spirochaetes. J Clin Pathol 39:535–541. doi: 10.1136/jcp.39.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones MJ, Miller JN, George WL. 1986. Microbiological and biochemical characterization of spirochetes isolated from the feces of homosexual males. J Clin Microbiol 24:1071–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett SP. 1990. Intestinal spirochaetes in a Gulf Arab population. Epidemiol Infect 104:261–266. doi: 10.1017/S0950268800059434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JI, Hampson DJ. 1992. Intestinal spirochaetes colonizing Aborigines from communities in the remote north of Western Australia. Epidemiol Infect 109:133–141. [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper C, Cotton DWK, Hudson MJ, Kirkham N, Wilmott FEW. 1986. Rectal spirochaetosis in homosexual men: characterisation of the organism and pathophysiology. Genitourin Med 62:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Käsbohrer A, Gelderblom HR, Arasteh K, Heise W, Grosse G, L'age M, Schönberg A, Koch MA, Pauli G. 1990. Intestinale spirochätose bei HIV-infektion: verkommen, isolierung und morphologie der spirochäten. Deutsche Medizin Wochenschr 115:1499–1506. [DOI] [PubMed] [Google Scholar]
- 14.Trivett-Moore NL, Gilbert GL, Law CLH, Trott DJ, Hampson DJ. 1998. Isolation of Serpulina pilosicoli from rectal biopsy specimens showing evidence of intestinal spirochetosis. J Clin Microbiol 36:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davelaar FG, Smit HF, Hovind-Hougen K, Dwars RM, van der Valk PC. 1986. Infectious typhlitis in chickens caused by spirochetes. Avian Pathol 15:247–258. doi: 10.1080/03079458608436285. [DOI] [PubMed] [Google Scholar]
- 16.Dwars RM, Smit HF, Davelaar FG, van Veer TW. 1989. Incidence of spirochaetal infections in cases of intestinal disorder in chickens. Avian Pathol 18:591–595. doi: 10.1080/03079458908418634. [DOI] [PubMed] [Google Scholar]
- 17.Dwars RM, Smit HF, Davelaar FG. 1990. Observations on avian intestinal spirochaetosis. Vet Q 12:51–55. doi: 10.1080/01652176.1990.9694242. [DOI] [PubMed] [Google Scholar]
- 18.Trampel DW, Jensen NS, Hoffman LJ. 1994. Cecal spirochaetosis in commercial laying hens. Avian Dis 38:895–898. doi: 10.2307/1592131. [DOI] [PubMed] [Google Scholar]
- 19.McLaren AJ, Trott DJ, Swayne DE, Oxberry SL, Hampson DJ. 1997. Genetic and phenotypic characterization of intestinal spirochetes colonizing chickens, and allocation of known pathogenic isolates to three distinct genetic groups. J Clin Microbiol 35:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JI, McLaren AJ, Lymbery AJ, Hampson DJ. 1993. Human intestinal spirochetes are distinct from Serpulina hyodysenteriae. J Clin Microbiol 31:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JI, Hampson DJ, Lymbery AJ, Harders SJ. 1993. The porcine intestinal spirochaetes: identification of new genetic groups. Vet Microbiol 34:273–285. doi: 10.1016/0378-1135(93)90017-2. [DOI] [PubMed] [Google Scholar]
- 22.Lee JI, Hampson DJ. 1994. Genetic characterisation of intestinal spirochaetes, and their association with disease. J Med Microbiol 40:365–371. doi: 10.1099/00222615-40-5-365. [DOI] [PubMed] [Google Scholar]
- 23.Stanton TB, Trott DJ, Lee JI, McLaren AJ, Hampson DJ, Paster BJ, Jensen NS. 1996. Differentiation of intestinal spirochaetes by multilocus enzyme electrophoresis analysis and 16S rRNA sequence comparisons. FEMS Microbiol Lett 136:181–186. doi: 10.1111/j.1574-6968.1996.tb08046.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramanathan M, Duhamel GE, Mathiesen MR, Messier S. 1993. Identification and partial characterization of a group of weakly beta-hemolytic intestinal spirochetes of swine distinct from Serpulina innocens isolate B256. Vet Microbiol 37:53–64. doi: 10.1016/0378-1135(93)90182-7. [DOI] [PubMed] [Google Scholar]
- 25.Fellström C, Gunnarsson A. 1995. Phenotypical characterization of intestinal spirochaetes isolated from pigs. Res Vet Sci 59:1–4. doi: 10.1016/0034-5288(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 26.Fellström C, Pettersson B, Thomson JR, Gunnarsson A, Persson M, Johansson K-E. 1997. Identification of Serpulina species associated with porcine colitis by biochemical analysis and PCR. J Clin Microbiol 35:462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochiai S, Adachi Y, Mori K. 1997. Unification of the genera Serpulina and Brachyspira, and proposals of Brachyspira hyodysenteriae comb. nov., Brachyspira innocens comb. nov., and Brachyspira innocens comb. nov Microbiol Immunol 41:445–452. [DOI] [PubMed] [Google Scholar]
- 28.Trott DJ, Stanton TB, Jensen NS, Duhamel GE, Johnson JL, Hampson DJ. 1996. Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis. Int J Syst Bacteriol 46:206–215. doi: 10.1099/00207713-46-1-206. [DOI] [PubMed] [Google Scholar]
- 29.Oxberry SL, Trott DJ, Hampson DJ. 1998. Serpulina pilosicoli, water birds and water: potential sources of infection for humans and other animals. Epidemiol Infect 121:219–225. doi: 10.1017/S0950268898008863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shivaprasad HL, Duhamel GE. 2005. Cecal spirochetosis caused by Brachyspira pilosicoli in commercial turkeys. Avian Dis 49:609–613. doi: 10.1637/7383-052005.1. [DOI] [PubMed] [Google Scholar]
- 31.Webb DM, Duhamel GE, Mathiesen MR, Muniappa N, White AK. 1997. Cecal spirochetosis associated with Serpulina pilosicoli in captive juvenile ring-necked pheasants. Avian Dis 41:997–1002. doi: 10.2307/1592360. [DOI] [PubMed] [Google Scholar]
- 32.Backhans A, Johansson KE, Fellström C. 2010. Phenotypic and molecular characterization of Brachyspira spp. isolated from wild rodents. Environ Microbiol Rep 2:720–727. doi: 10.1111/j.1758-2229.2010.00165.x. [DOI] [PubMed] [Google Scholar]
- 33.Duhamel GE, Trott DJ, Muniappa N, Mathiesen MR, Tarasiuk K, Lee JI, Hampson DJ. 1998. Canine intestinal spirochetes consist of Serpulina pilosicoli and a newly identified group provisionally designated “Serpulina canis” sp. nov. J Clin Microbiol 36:2264–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxberry SL, Hampson DJ. 2003. Colonization of pet shop puppies with Brachyspira pilosicoli. Vet Microbiol 93:167–174. doi: 10.1016/S0378-1135(03)00017-8. [DOI] [PubMed] [Google Scholar]
- 35.Hidalgo A, Rubio P, Osorio J, Carvajal A. 2010. Prevalence of Brachyspira pilosicoli and “Brachyspira canis” in dogs and their association with diarrhoea. Vet Microbiol 6:356–360. doi: 10.1016/j.vetmic.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Hampson DJ, Lester GD, Phillips ND, La T. 2006. Isolation of Brachyspira pilosicoli from weanling horses with chronic diarrhoea. Vet Rec 158:661–662. doi: 10.1136/vr.158.19.661. [DOI] [PubMed] [Google Scholar]
- 37.Duhamel GE. 2001. Comparative pathology and pathogenesis of naturally acquired and experimentally induced colonic spirochetosis. Anim Health Res Rev 2:3–17. [PubMed] [Google Scholar]
- 38.Brooke CJ, Margawani KR, Pearson AK, Riley TV, Robertson ID, Hampson DJ. 2000. Evaluation of blood culture systems for detection of the intestinal spirochaete Brachyspira (Serpulina) pilosicoli in human blood. J Med Microbiol 49:1031–1036. doi: 10.1099/0022-1317-49-11-1031. [DOI] [PubMed] [Google Scholar]
- 39.Atyeo RF, Oxberry SL, Hampson DJ. 1996. Pulsed-field gel electrophoresis for sub-specific differentiation of Serpulina pilosicoli (formerly “Anguillina coli”). FEMS Microbiol Lett 141:77–81. doi: 10.1111/j.1574-6968.1996.tb08366.x. [DOI] [PubMed] [Google Scholar]
- 40.Neo E, La T, Phillips ND, Alikhani Y, Hampson DJ. 2013. The pathogenic intestinal spirochaete Brachyspira pilosicoli forms a diverse recombinant species demonstrating some local clustering of related strains and potential for zoonotic spread. Gut Pathogens 5:24. doi: 10.1186/1757-4749-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neo E, La T, Phillips ND, Hampson DJ. 2013. Multiple locus variable number tandem repeat analysis (MLVA) of the pathogenic intestinal spirochaete Brachyspira pilosicoli. Vet Microbiol 163:299–304. doi: 10.1016/j.vetmic.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Trott DJ, Mikosza ASJ, Combs BG, Oxberry SL, Hampson DJ. 1998. Population genetic analysis of Serpulina pilosicoli and its molecular epidemiology in villages in the Eastern Highlands of Papua New Guinea. Int J Syst Bacteriol 48:659–668. doi: 10.1099/00207713-48-3-659. [DOI] [PubMed] [Google Scholar]
- 43.Wanchanthuek P, Bellgard MI, La T, Ryan K, Moolhuijzen P, Chapman B, Black M, Schibeci D, Hunter A, Barrero R, Phillips ND, Hampson DJ. 2010. The complete genome sequence of the pathogenic intestinal spirochete Brachyspira pilosicoli and comparison with other Brachyspira genomes. PLoS One 57:e11455. doi: 10.1371/journal.pone.0011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellgard MI, Wanchanthuek P, La T, Ryan K, Moolhuijzen P, Albertyn Z, Shaban B, Motro Y, Dunn DS, Schibeci D, Hunter A, Barrero R, Phillips ND, Hampson DJ. 2009. Genome sequence of the pathogenic intestinal spirochete Brachyspira hyodysenteriae reveals adaptations to its lifestyle in the porcine large intestine. PLoS One 4:e4641. doi: 10.1371/journal.pone.0004641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motro Y, La T, Bellgard MI, Dunn DS, Phillips ND, Hampson DJ. 2009. Identification of genes associated with prophage-like gene transfer agents in the pathogenic intestinal spirochaetes Brachyspira hyodysenteriae, Brachyspira pilosicoli and Brachyspira intermedia. Vet Microbiol 134:340–345. doi: 10.1016/j.vetmic.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 46.Motro Y, Dunn DS, La T, Phillips ND, Hampson DJ, Bellgard MI. 2008. Intestinal spirochaetes of the genus Brachyspira share a partially conserved 26 kilobase genomic region with Enterococcus faecalis and Escherichia coli. Microbiol Insights 1:1–9. [Google Scholar]
- 47.Mappley LJ, Black ML, Abuoun M, Darby AC, Woodward MJ, Parkhill J, Bellgard MI, La T, Phillips ND, La Ragione RM, Hampson DJ. 2012. Comparative genomics of Brachyspira pilosicoli strains: genome rearrangements, reductions and correlation of genetic complement with phenotypic diversity. BMC Genomics 13:454. doi: 10.1186/1471-2164-13-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampson DJ, Wang P. 7 September 2017. Colonic spirochetes: what has genomics taught us? Curr Top Microbiol Immunol doi: 10.1007/82_2017_48. [DOI] [PubMed] [Google Scholar]
- 49.Zuerner RL, Stanton TB, Minion FC, Li C, Charon NW, Trott DJ, Hampson DJ. 2004. Genetic variation in Brachyspira: chromosomal rearrangements and sequence drift distinguish B. pilosicoli from B. hyodysenteriae. Anaerobe 10:229–237. doi: 10.1016/j.anaerobe.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Lin C, den Bakker HC, Suzuki H, Lefébure T, Ponnala L, Sun Q, Stanhope MJ, Wiedmann M, Duhamel GE. 2013. Complete genome sequence of the porcine strain Brachyspira pilosicoli P43/6/78(T.). Genome Announc 1:e00215-12. doi: 10.1128/genomeA.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee BJ, Hampson DJ. 1999. Lipooligosaccharide profiles of Serpulina pilosicoli strains, and their serological cross-reactivities. J Med Microbiol 48:411–415. doi: 10.1099/00222615-48-4-411. [DOI] [PubMed] [Google Scholar]
- 52.Trott DJ, Alt DP, Zuerner RL, Wannemuehler MJ, Stanton TB. 2001. The search for Brachyspira outer membrane proteins that interact with the host. Anim Health Res Rev 2:19–30. [PubMed] [Google Scholar]
- 53.Casas V, Vadillo S, San Juan C, Carrascal M, Abian J. 2016. The exposed proteomes of Brachyspira hyodysenteriae and B. pilosicoli. Front Microbiol 7:1103. doi: 10.3389/fmicb.2016.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hampson DJ, Burrough ER. Swine dysentery and Brachyspiral colitis. In Zimmerman JJ, Karriker L, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J (ed), Diseases of swine, 11th ed, in press Wiley-Blackwell, Ames, IA, USA. [Google Scholar]
- 55.Taylor DJ, Alexander TJL. 1971. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J 127:58–61. [DOI] [PubMed] [Google Scholar]
- 56.Harris DL, Glock RD, Christensen CR, Kinyon JM. 1972. Swine dysentery. I. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction of the disease. Vet Med Small Anim Clin 67:61–64. [PubMed] [Google Scholar]
- 57.Thomson JR, Smith WJ, Murray BP. 1998. Investigations into field cases of porcine colitis with particular reference to infection with Serpulina pilosicoli. Vet Rec 142:235–239. doi: 10.1136/vr.142.10.235. [DOI] [PubMed] [Google Scholar]
- 58.Dwars RM, Davelaar FG, Smit HF. 1992. Infection of broiler chicks (Gallus domesticus) with human intestinal spirochaetes. Avian Pathol 21:559–568. doi: 10.1080/03079459208418877. [DOI] [PubMed] [Google Scholar]
- 59.Trott DJ, McLaren AJ, Hampson DJ. 1995. Pathogenicity of human and porcine intestinal spirochetes in one-day-old specific pathogen-free chicks: an animal model of intestinal spirochetosis. Infect Immun 63:3705–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muniappa N, Duhamel GE, Mathiesen MR, Bargar TW. 1996. Light microscopic and ultrastructural changes in the ceca of chicks inoculated with human and canine Serpulina pilosicoli. Vet Pathol 33:542–550. doi: 10.1177/030098589603300509. [DOI] [PubMed] [Google Scholar]
- 61.Trott DJ, Hampson DJ. 1998. Evaluation of day-old specific-pathogen-free chicks as an experimental model for pathogenicity testing of intestinal spirochaete species. J Comp Pathol 118:365–381. doi: 10.1016/S0021-9975(07)80012-0. [DOI] [PubMed] [Google Scholar]
- 62.Prapasarakul N, Lugsomya K, Disatian S, Lekdumrongsak T, Banlunara W, Chetanachan P, Hampson DJ. 2011. Faecal excretion of intestinal spirochaetes by urban dogs, and their pathogenicity in a chick model of intestinal spirochaetosis. Res Vet Sci 91:38–43. doi: 10.1016/j.rvsc.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 63.Stephens CP, Hampson DJ. 1999. Prevalence and disease association of intestinal spirochaetes in chickens in eastern Australia. Avian Pathol 28:447–454. doi: 10.1080/03079459994461. [DOI] [PubMed] [Google Scholar]
- 64.Burch DGS, Harding C, Alvarez R, Valks M. 2006. Treatment of a field case of avian intestinal spirochaetosis caused by Brachyspira pilosicoli with tiamulin. Avian Pathol 35:211–216. doi: 10.1080/03079450600711011. [DOI] [PubMed] [Google Scholar]
- 65.Bano L, Merialdi G, Bonilauri P, Dall'Anese G, Capello K, Comin D, Cattoli G, Sanguinetti V, Hampson DJ, Agnoletti F. 2008. Prevalence, disease associations and risk factors for colonization with intestinal spirochaetes (Brachyspira spp.) in flocks of laying hens in north-eastern Italy. Avian Pathol 37:281–286. doi: 10.1080/03079450802043726. [DOI] [PubMed] [Google Scholar]
- 66.Feberwee A, Hampson DJ, Phillips ND, La T, van der Heijen HMJF, Wellenberg GJ, Dwars RM, Landman WJM. 2008. Identification of Brachyspira hyodysenteriae and other pathogenic Brachyspira species in chickens from laying flocks with diarrhea or reduced production or both. J Clin Microbiol 46:593–600. doi: 10.1128/JCM.01829-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myers SE, Dunn PA, Phillips ND, La T, Hampson DJ. 2009. Brachyspira intermedia and Brachyspira pilosicoli are commonly found in older laying flocks in Pennsylvania. Avian Dis 53:533–537. doi: 10.1637/8900-042709-Reg.1. [DOI] [PubMed] [Google Scholar]
- 68.Mappley LJ, La Ragione RM, Woodward MJ. 2014. Brachyspira and its role in avian intestinal spirochaetosis. Vet Microbiol 168:245–260. doi: 10.1016/j.vetmic.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 69.Le Roy CI, Mappley LJ, La Ragione RM, Woodward MJ, Claus SP. 2015. Brachyspira pilosicoli-induced avian intestinal spirochaetosis. Microb Ecol Health Dis 26:28853. doi: 10.3402/mehd.v26.28853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hampson DJ. 2018. Avian intestinal spirochetosis. In Swayne DE, Boulianne M, McDougald L, Nair V, Logue C, Suarez D (ed), Diseases of poultry, 14th ed, in press Wiley-Blackwell, Ames, IA, USA. [Google Scholar]
- 71.Wagenaar J, Bergen MA, Graaf L, Landman WJ. 2003. Free-range chickens show a higher incidence of Brachyspira infections in the Netherlands, p 16 In Thomson JR. (ed), Proceedings of the 2nd International Conference on Colonic Spirochaetal Infections of Animals and Humans, Eddleston, Scotland, UK. [Google Scholar]
- 72.Hess C, Zloch A, Bilic I, Hacksteiner K, Kuchling S, Hess M. 2017. High prevalence of Brachyspira spp. in layers kept in alternative husbandry systems associated with frequent species variations from end of rearing to slaughter. Avian Pathol 46:481–487. doi: 10.1080/03079457.2017.1315049. [DOI] [PubMed] [Google Scholar]
- 73.McLaren AJ, Hampson DJ, Wylie SL. 1996. The prevalence of intestinal spirochaetes in poultry flocks in Western Australia. Aust Vet J 74:319–321. [DOI] [PubMed] [Google Scholar]
- 74.Phillips ND, La T, Hampson DJ. 2005. A cross-sectional study to investigate the occurrence and distribution of intestinal spirochaetes (Brachyspira spp.) in three flocks of laying hens. Vet Microbiol 105:189–198. doi: 10.1016/j.vetmic.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 75.Medhanie GA, McEwen SA, Weber L, Sanei B, Cooley L, Houghton S, Slavic D, Guerin MT. 2013. Risk factors associated with the colonization of Ontario layer chicken flocks with Brachyspira species. Prev Vet Med 109:304–311. doi: 10.1016/j.prevetmed.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 76.Amin MM, Phillips ND, La T, Robertson ID, Hampson DJ. 2014. Intestinal spirochaetes (Brachyspira spp.) colonizing flocks of layer and breeder chickens in Malaysia. Avian Pathol 43:501–505. doi: 10.1080/03079457.2014.966056. [DOI] [PubMed] [Google Scholar]
- 77.Illanes NV, Tamiozzo PJ, Cabral A, Bertone J, Romanini S, Yaciuk R, Vázquez M, Pelliza BR. 2016. Detection of Brachyspira pilosicoli and other Brachyspira species in Argentine poultry farms. Rev Argent Microbiol 48:67–70. doi: 10.1016/j.ram.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Dwars RM, Davelaar FG, Smit HF. 1992. Influence of infection with avian intestinal spirochaetes on the faeces of laying hens. Avian Pathol 21:427–429. [DOI] [PubMed] [Google Scholar]
- 79.Dwars RM, Davelaar FG, Smit HR. 1993. Infection of broiler parent hens with avian intestinal spirochaetes: effects on egg production and chick quality. Avian Pathol 22:37–41. doi: 10.1080/03079459308418957. [DOI] [PubMed] [Google Scholar]
- 80.Stephens CP, Hampson DJ. 2001. Intestinal spirochete infections of chickens: a review of disease associations, epidemiology and control. Anim Health Res Rev 2:83–91. [PubMed] [Google Scholar]
- 81.Stephens CP, Hampson DJ. 2002. Experimental infection of broiler breeder hens with the intestinal spirochaete Brachyspira (Serpulina) pilosicoli causes reduced egg production. Avian Pathol 31:169–175. doi: 10.1080/03079450120118667. [DOI] [PubMed] [Google Scholar]
- 82.Smit HR, Dwars RM, Davelaar FG, Wijtten GAW. 1998. Observations on the influence of intestinal spirochaetosis in broiler breeders on the performance of their progeny and egg production. Avian Pathol 27:133–141. doi: 10.1080/03079459808419314. [DOI] [PubMed] [Google Scholar]
- 83.Mappley LJ, Tchórzewska MA, Nunez A, Woodward MJ, La Ragione RM. 2013. Evidence for systemic spread of the potentially zoonotic intestinal spirochaete Brachyspira pilosicoli in experimentally challenged laying chickens. J Med Microbiol 62:297–302. doi: 10.1099/jmm.0.052126-0. [DOI] [PubMed] [Google Scholar]
- 84.Mathan M, Mathan V. 1985. Rectal mucosal morphological abnormalities in normal subjects in a southern India; a tropical colonopathy? Gut 26:710–717. doi: 10.1136/gut.26.7.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Brito T, Sandoval MP, Silva AG, Saad RC, Colaiacova W. 1996. Intestinal spirochaetosis: first cases reported in Brazil and the use of immunohistochemistry as an aid in histopathological diagnosis. Rev Inst Med Trop Sao Paulo 38:45–52. doi: 10.1590/S0036-46651996000100009. [DOI] [PubMed] [Google Scholar]
- 86.Marthinsen L, Willen R, Carlen B, Lindberg E, Varendh G. 2002. Intestinal spirochetosis in eight pediatric patients from Southern Sweden. APMIS 110:571–579. doi: 10.1034/j.1600-0463.2002.11007809.x. [DOI] [PubMed] [Google Scholar]
- 87.Carpentieri DF, Souza-Morones S, Gardetto JS, Ross HM, Downey K, Ingebo K, Siaw E. 2010. Intestinal spirochetosis in children: five new cases and a 20-year review of the literature. Pediatr Dev Pathol 13:471–475. doi: 10.2350/09-10-0725-CR.1. [DOI] [PubMed] [Google Scholar]
- 88.Rodgers FG, Rogers C, Shelton AP, Hawkey CJ. 1986. Proposed pathogenic mechanism for the diarrhea associated with human intestinal spirochetes. Am J Clin Pathol 86:679–682. doi: 10.1093/ajcp/86.5.679. [DOI] [PubMed] [Google Scholar]
- 89.Gad A, Willén R, Furugård K, Fors B, Hradsky M. 1977. Intestinal spirochaetosis as a cause of longstanding diarrhoea. Ups J Med Sci 82:49–54. doi: 10.3109/03009737709179059. [DOI] [PubMed] [Google Scholar]
- 90.Walker MM, Talley NJ, Inganäs L, Engstrand L, Jones MP, Nyhlin H, Agréus L, Kjellstrom L, Öst Å, Andreasson A. 2015. Colonic spirochetosis is associated with colonic eosinophilia and irritable bowel syndrome in a general population in Sweden. Hum Pathol 46:277–283. doi: 10.1016/j.humpath.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 91.Helbing R, Osterheld M-C, Vaudaux B, Jaton K, Nydegger A. 2012. Intestinal spirochetosis mimicking inflammatory bowel disease in children. BMC Pediatr 2012 12:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Mook WN, Koek GH, van der Ven AJ, Ceelen TL, Bos RP. 2004. Human intestinal spirochaetosis: any clinical significance? Eur J Gastroenterol Hepatol 16:83–87. doi: 10.1097/00042737-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 93.Körner M, Gebbers JO. 2003. Clinical significance of human intestinal spirochetosis—a morphologic approach. Infection 31:341–349. [DOI] [PubMed] [Google Scholar]
- 94.Tsinganou E, Gebbers JO. 2010. Human intestinal spirochetosis—a review. Ger Med Sci 8:Doc01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lambert T, Goursot G. 1982. Diarhe aigue avec hemocultures et coprocultures positives a Treponema. Med Malad Infect 12:276–278. doi: 10.1016/S0399-077X(82)80028-0. [DOI] [Google Scholar]
- 96.Fournié-Amazouz E, Baranton G, Carlier JP, Chambreuil G, Jolivet AG, Hermes I, Lemarie C, St Girons I. 1995. Isolations of intestinal spirochaetes from the blood of human patients. J Hosp Infect 30:160–162. doi: 10.1016/0195-6701(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 97.Trott DJ, Jensen NS, Saint Girons I, Oxberry SL, Stanton TB, Lindquist D, Hampson DJ. 1997. Identification and characterization of Serpulina pilosicoli isolates from the blood of critically ill patients. J Clin Microbiol 35:482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanavaki S, Mantadakis E, Thomakos N, Pefanis A, Matsiota-Bernard P, Karabela S, Samonis G. 2002. Brachyspira (Serpulina) pilosicoli spirochetemia in an immunocompromised patient. Infection 30:175–177. doi: 10.1007/s15010-002-2175-1. [DOI] [PubMed] [Google Scholar]
- 99.Bait-Merabet L, Thille AA, Legrand P, Brun-Buisson C, Cattoir V. 2008. Brachyspira pilosicoli bloodstream infections: case report and review of the literature. Ann Clin Microbiol Antimicrob 7:19. doi: 10.1186/1476-0711-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zeeshan M, Irfan S, Ahmed I. 2009. Brachyspira species blood stream infection. J Pak Med Assoc 59:723–724. [PubMed] [Google Scholar]
- 101.Prim N, Pericas R, Español M, Rivera A, Mirelis B, Coll P. 2011. Bloodstream infection due to Brachyspira pilosicoli in a patient with multiorgan failure. J Clin Microbiol 49:3697–3699. doi: 10.1128/JCM.00680-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brooke CJ, Hampson DJ, Riley TV, Lum G. 2001. Failure to detect Brachyspira pilosicoli in the bloodstream of Australian patients. J Clin Microbiol 39:4219. doi: 10.1128/JCM.39.11.4219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hartland EL, Mikosza ASJ, Robins-Browne RM, Hampson DJ. 1998. Examination of Serpulina pilosicoli for attachment and invasion determinants of Enterobacteria. FEMS Microbiol Lett 165:59–63. doi: 10.1111/j.1574-6968.1998.tb13127.x. [DOI] [PubMed] [Google Scholar]
- 104.Trott DJ, Combs BG, Mikosza AS, Oxberry SL, Robertson ID, Passey M, Taime J, Sehuko R, Hampson DJ. 1997. The prevalence of Serpulina pilosicoli in humans and domestic animals in the Eastern Highlands of Papua New Guinea. Epidemiol Infect 119:369–379. doi: 10.1017/S0950268897008194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Margawani KR, Robertson ID, Brooke JC, Hampson DJ. 2004. Prevalence, risk factors and molecular epidemiology of Brachyspira pilosicoli in humans on the island of Bali, Indonesia. J Med Microbiol 53:325–332. doi: 10.1099/jmm.0.05415-0. [DOI] [PubMed] [Google Scholar]
- 106.Naresh R, Hampson DJ. 2010. Attraction of Brachyspira pilosicoli to mucin. Microbiology 156:191–197. doi: 10.1099/mic.0.030262-0. [DOI] [PubMed] [Google Scholar]
- 107.Muniappa N, Duhamel GE. 1997. Outer membrane-associated serine protease of intestinal spirochetes. FEMS Microbiol Lett 154:159–164. doi: 10.1111/j.1574-6968.1997.tb12638.x. [DOI] [PubMed] [Google Scholar]
- 108.Dassanayake RP, Caceres NE, Sarath G, Duhamel GE. 2004. Biochemical properties of membrane-associated proteases of Brachyspira pilosicoli isolated from humans with intestinal disorders. J Med Microbiol 53:319–323. doi: 10.1099/jmm.0.45549-0. [DOI] [PubMed] [Google Scholar]
- 109.Nakamura S, Adachi Y, Goto T, Magariyama Y. 2006. Improvement in motion efficiency of the spirochete Brachyspira pilosicoli in viscous environments. Biophys J 90:3019–3026. doi: 10.1529/biophysj.105.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ogata S, Shimizu K, Tominaga S, Nakanishi K. 2017. Immunohistochemical study of mucins in human intestinal spirochetosis. Hum Pathol 62:126–133. doi: 10.1016/j.humpath.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 111.Naresh R, Song Y, Hampson DJ. 2009. The intestinal spirochete Brachyspira pilosicoli attaches to cultured Caco-2 cells and induces pathological changes. PLoS One 4:e8352. doi: 10.1371/journal.pone.0008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trott DJ, Huxtable CR, Hampson DJ. 1996. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infect Immun 64:4648–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muniappa N, Ramanathan MR, Tarara RP, Westerman RB, Mathiesen MR, Duhamel GE. 1998. Attachment of human and rhesus Serpulina pilosicoli to cultured cells and comparison with a chick infection model. J Spiro Tick-borne Dis 5:44–53. [Google Scholar]
- 114.Naresh R, Hampson DJ. 2011. Exposure to norepinephrine enhances Brachyspira pilosicoli growth, attraction to mucin and attachment to Caco-2 cells. Microbiology 157:543–547. doi: 10.1099/mic.0.044594-0. [DOI] [PubMed] [Google Scholar]
- 115.Jensen TK, Boye M, Møller K. 2004. Extensive intestinal spirochaetosis in pigs challenged with Brachyspira pilosicoli. J Med Microbiol 53:309–312. doi: 10.1099/jmm.0.05403-0. [DOI] [PubMed] [Google Scholar]
- 116.Ngwa T, Peng JL, Choi E, Tayarachakul S, Liangpunsakul S. 2016. Colonic spirochetosis in a 60-year-old immunocompetent patient: case report and review. J Investig Med High Impact Case Rep 4:2324709616662671. doi: 10.1177/2324709616662671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Umeda S, Serizawa H, Kobayashi T, Toyonaga T, Saito E, Nakano M, Higuchi H, Tsunematsu S, Watanabe N, Hibi T, Morinaga S. 2017. Clinical significance of human intestinal spirochetosis: a retrospective study. Nihon Shokakibyo Gakkai Zasshi 114:230–237. doi: 10.11405/nisshoshi.114.230. [DOI] [PubMed] [Google Scholar]
- 118.Umeno J, Matsumoto T, Nakamura S, Yoshino S, Hirahashi M, Yao T, Iida M. 2007. Intestinal spirochetosis due to Brachyspira pilosicoli: endoscopic and radiographic features. J Gastroenterol 42:253–256. doi: 10.1007/s00535-006-1988-6. [DOI] [PubMed] [Google Scholar]
- 119.Ogata S, Shimizu K, Nakanishi K. 2015. Human intestinal spirochetosis: right-side preference in the large intestine. Ann Diagn Pathol 19:414–417. doi: 10.1016/j.anndiagpath.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 120.Omori S, Mabe K, Hatanaka K, Ono M, Matsumoto M, Takahashi M, Yoshida T, Ono S, Shimizu Y, Sugai N, Suzuki A, Katsuki S, Fujii T, Kato M, Asaka M, Sakamoto N. 2014. Human intestinal spirochetosis is significantly associated with sessile serrated adenomas/polyps. Pathol Res Pract 210:440–443. doi: 10.1016/j.prp.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 121.Young JP, Price TJ, Moore J, Ruszkiewicz AR. 2016. Human intestinal spirochetosis and its relationship to sessile serrated adenomas in an Australian population. Pathol Res Pract 212:751–753. doi: 10.1016/j.prp.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 122.Thomson JR, Smith WJ, Murray BP, McOrist S. 1997. Pathogenicity of three strains of Serpulina pilosicoli in pigs with a naturally acquired intestinal flora. Infect Immun 65:3693–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jacques M, Girard C, Higgins R, Goyette G. 1989. Extensive colonization of the porcine colonic epithelium by a spirochete similar to Treponema innocens. J Clin Microbiol 27:1139–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Girard C, Lemarchand T, Higgins R. 1995. Porcine colonic spirochetosis: a retrospective study of eleven cases. Can Vet J 36:291–294. [PMC free article] [PubMed] [Google Scholar]
- 125.Padmanabhan V, Dahlstrom J, Maxwell G, Kaye A, Clarke A, Barrett PJ. 1996. Invasive intestinal spirochaetosis: a report of three cases. Pathology 28:283–296. doi: 10.1080/00313029600169174. [DOI] [PubMed] [Google Scholar]
- 126.Antonakopoulos G, Newman J, Wilkinson M. 1982. Intestinal spirochaetosis: an electron microscopic study of an unusual case. Histopathology 6:477–488. doi: 10.1111/j.1365-2559.1982.tb02744.x. [DOI] [PubMed] [Google Scholar]
- 127.Guccion JG, Benator DA, Zeller J, Termanini B, Saini N. 1995. Intestinal spirochetosis and acquired immunodeficiency syndrome: ultrastructural studies of two cases. Ultrastruct Pathol 19:15–22. [DOI] [PubMed] [Google Scholar]
- 128.Kostman JR, Patel M, Catalano E, Camacho J, Hoffpauir J, DiNubile MJ. 1995. Invasive colitis and hepatitis due to previously uncharacterized spirochetes in patients with advanced human immunodeficiency virus infection. Clin Infect Dis 21:1159–1165. doi: 10.1093/clinids/21.5.1159. [DOI] [PubMed] [Google Scholar]
- 129.Abramowsky C, Beyer-Patterson P, Cortinas E. 1991. Nonsyphilitic spirochetosis in second-trimester fetuses. Pediatr Pathol 11:827–838. doi: 10.3109/15513819109065480. [DOI] [PubMed] [Google Scholar]
- 130.Naresh R, Hampson DJ. 2014. Strains of the intestinal spirochaete Brachyspira pilosicoli attach to and aggregate erythrocytes. Lett Appl Microbiol 58:65–69. doi: 10.1111/lam.12158. [DOI] [PubMed] [Google Scholar]
- 131.Hampson DJ, Robertson ID, Oxberry SL. 1998. Extraintestinal colonisation by Serpulina pilosicoli in an experimentally inoculated pig, p 54. In Done S, Thomson J, Varley M (ed), Proceedings of the 15th International Pig Veterinary Society Congress, Birmingham, England. [Google Scholar]
- 132.Woodward MJ, Mappley L, Le Roy C, Claus SP, Davies P, Thompson G, La Ragione RM. 2015. Drinking water application of Denagard® Tiamulin for control of Brachyspira pilosicoli infection of laying poultry. Res Vet Sci 103:87–95. doi: 10.1016/j.rvsc.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 133.Jamshidi A, Hampson DJ. 2003. Experimental infection of layer hens with a human isolate of Brachyspira pilosicoli. J Med Microbiol 52:361–364. doi: 10.1099/jmm.0.05040-0. [DOI] [PubMed] [Google Scholar]
- 134.Jamshidian M, La T, Phillips ND, Hampson DJ. 2004. Brachyspira pilosicoli colonization in experimentally infected mice can be facilitated by dietary manipulation. J Med Microbiol 53:313–318. doi: 10.1099/jmm.0.05393-0. [DOI] [PubMed] [Google Scholar]
- 135.Sacco RE, Trampel DW, Wannemuehler MJ. 1997. Experimental infection of C3H mice with avian, porcine, or human isolates of Serpulina pilosicoli. Infect Immun 65:5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hampson DJ, Robertson ID, La T, Oxberry SL, Pethick DW. 2000. Influences of diet and vaccination on colonisation of pigs with the intestinal spirochaete Brachyspira (Serpulina) pilosicoli. Vet Microbiol 73:75–84. doi: 10.1016/S0378-1135(99)00200-X. [DOI] [PubMed] [Google Scholar]
- 137.Zhang P, Duhamel GE. 2002. Serum antibody responses of pigs following challenge with Brachyspira pilosicoli, p 188 Proc 17th Congr Int Pig Vet Soc, Ames, IA. [Google Scholar]
- 138.Hampson DJ, Oxberry SL, La T. 2006. Potential for zoonotic transmission of Brachyspira pilosicoli. Emerg Infect Dis 12:869–870. doi: 10.3201/eid1205.051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boye M, Baloda SB, Leser TD, Moller K. 2001. Survival of Brachyspira hyodysenteriae and B. pilosicoli in terrestrial microcosms. Vet Microbiol 81:33–40. doi: 10.1016/S0378-1135(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 140.Phillips ND, La T, Hampson DJ. 2003. Survival of intestinal spirochaete strains from chickens in the presence of disinfectants and in faeces held at different temperatures. Avian Pathol 32:639–643. doi: 10.1080/03079450310001610677. [DOI] [PubMed] [Google Scholar]
- 141.Stege H, Jensen TK, Møller K, Baekbo P, Jorsal SE. 2001. Risk factors for intestinal pathogens in Danish finishing pig herds. Prev Vet Med 50:153–164. doi: 10.1016/S0167-5877(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 142.Lindecrona RH, Jensen TK, Møller K. 2004. Influence of diet on the experimental infection of pigs with Brachyspira pilosicoli. Vet Rec 154:264–267. doi: 10.1136/vr.154.9.264. [DOI] [PubMed] [Google Scholar]
- 143.Hopwood D, Pethick D, Hampson DJ. 2002. Increasing the viscosity of the intestinal contents stimulates proliferation of enterotoxigenic Escherichia coli and Brachyspira pilosicoli in weaner pigs. Br J Nutr 88:523–532. doi: 10.1079/BJN2002694. [DOI] [PubMed] [Google Scholar]
- 144.Wilberts BL, Arruda PH, Kinyon JM, Frana TS, Wang C, Magstadt DR, Madson DM, Patience JF, Burrough ER. 2014. Investigation of the impact of increased dietary insoluble fiber through the feeding of distillers dried grains with solubles (DDGS) on the incidence and severity of Brachyspira-associated colitis in pigs. PLoS One 2014 9:e114741. doi: 10.1371/journal.pone.0114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.La T, Rohde J, Phillips ND, Hampson DJ. 2016. Comparison of Brachyspira hyodysenteriae isolates recovered from pigs in apparently healthy multiplier herds with isolates from herds with swine dysentery. PLoS One 11:e0160362. doi: 10.1371/journal.pone.0160362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stege H, Jensen TK, Møller K, Baekbo P, Jorsal SE. 2000. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev Vet Med 46:279–292. doi: 10.1016/S0167-5877(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 147.Fellström C, Pettersson B, Johansson K-E, Lundeheim N, Gunnarsson A. 1996. Prevalence of Serpulina species in relation to diarrhea and feed medication in pig-rearing herds in Sweden. Am J Vet Res 57:807–811. [PubMed] [Google Scholar]
- 148.Møller K, Jensen TK, Jorsal SE, Leser TD, Carstensen B. 1998. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly beta-haemolytic intestinal spirochaetes, Salmonella enterica, and haemolytic Escherichia coli from swine herds with and without diarrhoea among growing pigs. Vet Microbiol 62:59–72. [DOI] [PubMed] [Google Scholar]
- 149.Heinonen M, Fossi M, Jalli J-P, Saloniemi H, Tuovinen V. 2000. Detectability and prevalence of Brachyspira species in herds rearing health class feeder pigs in Finland. Vet Rec 146:343–347. doi: 10.1136/vr.146.12.343. [DOI] [PubMed] [Google Scholar]
- 150.Jensen VF, Jorsal SL, Toft N. 2017. A cross-sectional study of oral antibacterial treatment patterns in relation to specific diarrhoeal pathogens in weaner pigs. Vet Microbiol 203:18–27. doi: 10.1016/j.vetmic.2017.01.038. [DOI] [PubMed] [Google Scholar]
- 151.Oxberry SL, Hampson DJ. 2003. Epidemiological studies of Brachyspira pilosicoli in two Australian piggeries. Vet Microbiol 93:109–120. doi: 10.1016/S0378-1135(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 152.Phillips ND, La T, Adams PJ, Harland BL, Fenwick SG, Hampson DJ. 2009. Detection of Brachyspira hyodysenteriae, Lawsonia intracellularis and Brachyspira pilosicoli in feral pigs. Vet Microbiol 134:294–299. doi: 10.1016/j.vetmic.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 153.Lindboe CF, Tostrup NE, Nersund R, Rekkavik G. 1993. Human intestinal spirochaetosis in mid-Norway. A retrospective histopathological study with clinical correlations. APMIS 101:858–864. [PubMed] [Google Scholar]
- 154.Lindboe CF. 2001. The prevalence of human intestinal spirochetosis in Norway. Anim Health Res Rev 2:117–119. [PubMed] [Google Scholar]
- 155.McMillan A, Lee FD. 1981. Sigmoidoscopic and microscopic appearance of the rectal mucosa in homosexual men. Gut 22:1035–1041. doi: 10.1136/gut.22.12.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Law CL, Grierson JM, Stevens SM. 1994. Rectal spirochaetosis in homosexual men: the association with sexual practices, HIV infection and enteric flora. Genitourin Med 70:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mikosza ASJ, Hampson DJ. 2001. Human intestinal spirochetosis: Brachyspira aalborgi and/or Brachyspira pilosicoli? Anim Health Res Rev 2:101–110. [PubMed] [Google Scholar]
- 158.Mikosza ASJ, La T, Brooke CJ, Lindboe CF, Ward PB, Heine RG, Guccion JG, de Boer WB, Hampson DJ. 1999. Polymerase chain reaction amplification from fixed tissue indicates the frequent involvement of Brachyspira aalborgi in human intestinal spirochetosis. J Clin Microbiol 37:2093–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mikosza ASJ, La T, de Boer WB, Hampson DJ. 2001. The comparative prevalence of Brachyspira (Serpulina) pilosicoli and Brachyspira aalborgi as the etiologic agents of histologically identified intestinal spirochetosis in Australia. J Clin Microbiol 39:347–350. doi: 10.1128/JCM.39.1.347-350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kraatz W, Thunberg U, Pettersson B, Fellström C. 2001. Human intestinal spirochetosis diagnosed with colonoscopy and analysis of partial 16S rDNA sequences of involved spirochetes. Anim Health Res Rev 2:111–116. [PubMed] [Google Scholar]
- 161.Tateishi Y, Takahashi M, Horiguchi S, Funata N, Koizumi K, Okudela K, Hishima T, Ohashi K. 2015. Clinicopathologic study of intestinal spirochetosis in Japan with special reference to human immunodeficiency virus infection status and species types: analysis of 5265 consecutive colorectal biopsies. BMC Infect Dis 15:13. doi: 10.1186/s12879-014-0736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sato H, Nakamura S, Habano W, Wakabayashi G, Adachi Y. 2010. Human intestinal spirochaetosis in northern Japan. J Med Microbiol 59:791–796. doi: 10.1099/jmm.0.017376-0. [DOI] [PubMed] [Google Scholar]
- 163.Tanahashi J, Daa T, Gamachi A, Kashima K, Kondoh Y, Yada N, Yokoyama S. 2008. Human intestinal spirochetosis in Japan; its incidence, clinicopathologic features, and genotypic identification. Mod Pathol 21:76–84. [DOI] [PubMed] [Google Scholar]
- 164.Rojas P, Petrich A, Schulze J, Wiessner A, Loddenkemper C, Epple HJ, Sterlacci W, Vieth M, Kikhney J, Moter A. 2017. Distribution and phylogeny of Brachyspira spp. in human intestinal spirochetosis revealed by FISH and 16S rRNA-gene analysis. Anaerobe 47:25–32. doi: 10.1016/j.anaerobe.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 165.Munshi MA, Traub RJ, Robertson ID, Mikosza AS, Hampson DJ. 2004. Colonization and risk factors for Brachyspira aalborgi and Brachyspira pilosicoli in humans and dogs on tea estates in Assam, India. Epidemiol Infect 132:137–144. doi: 10.1017/S095026880300116X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goossens H, Lejour M, De Mol P, De Boeck M, Verhaeghen G, Dekegel D, Butzler JP. 1983. Isolation of Treponema hyodysenteriae from human faecal specimens: a curiosity or a new enteropathogen?, abstr A267. Abstr Eur Congr Clin Microbiol, Bologna, Italy. [Google Scholar]
- 167.Nelson EJ, Tanudra A, Chowdhury A, Kane AV, Qadri F, Calderwood SB, Coburn J, Camilli A. 2009. High prevalence of spirochetosis in cholera patients, Bangladesh. Emerg Infect Dis 15:571–573. doi: 10.3201/eid1504.081214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brooke CJ, Clair AN, Mikosza ASJ, Riley TV, Hampson DJ. 2001. Carriage of intestinal spirochaetes by humans: epidemiological data from Western Australia. Epidemiol Infect 127:369–374. doi: 10.1017/S095026880100588X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brooke CJ, Riley TV, Hampson DJ. 2006. Comparison of prevalence and risk factors for faecal carriage of the intestinal spirochaetes Brachyspira aalborgi and Brachyspira pilosicoli in four Australian populations. Epidemiol Infect 134:627–634. doi: 10.1017/S0950268805005170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Margawani KR, Robertson ID, Hampson DJ. 2009. Isolation of the anaerobic intestinal spirochaete Brachyspira pilosicoli from long-term residents and Indonesian visitors to Perth, Western Australia. J Med Microbiol 58:248–252. doi: 10.1099/jmm.0.004770-0. [DOI] [PubMed] [Google Scholar]
- 171.Peruzzi S, Gorrini C, Piccolo G, Calderaro A, Dettori G, Chezzi C. 2007. Human intestinal spirochaetosis in Parma: a focus on a selected population during 2002-2005. Acta Biomed 78:128–132. [PubMed] [Google Scholar]
- 172.Ruane PJ, Nakata MM, Reinhardt JF, George WL. 1989. Spirochete-like organisms in the human gastrointestinal tract. Rev Infect Dis 11:184–196. doi: 10.1093/clinids/11.2.184. [DOI] [PubMed] [Google Scholar]
- 173.Verlinden M, Pasmans F, Garmyn A, De Zutter L, Haesebrouck F, Martel A. 2012. Occurrence of viable Brachyspira spp. on carcasses of spent laying hens from supermarkets. Food Microbiol 32:321–324. doi: 10.1016/j.fm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 174.Jenkinson SR, Wingar CR. 1981. Selective medium for the isolation of Treponema hyodysenteriae. Vet Rec 109:384–385. doi: 10.1136/vr.109.17.384. [DOI] [PubMed] [Google Scholar]
- 175.Kunkle RA, Kinyon JM. 1988. Improved selective medium for the isolation of Treponema hyodysenteriae. J Clin Microbiol 26:2357–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stanton TB, Lebo DF. 1988. Treponema hyodysenteriae growth under various culture conditions. Vet Microbiol 18:177–190. doi: 10.1016/0378-1135(88)90063-6. [DOI] [PubMed] [Google Scholar]
- 177.Kunkle RA, Harris DL, Kinyon JM. 1986. Autoclaved liquid medium for propagation of Treponema hyodysenteriae. J Clin Microbiol 24:669–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Calderaro A, Piccolo G, Montecchini S, Buttrini M, Gorrini C, Rossi S, Arcangeletti MC, De Conto F, Medici MC, Chezzi C. 2012. MALDI-TOF MS analysis of human and animal Brachyspira species and benefits of database extension. J Proteom 8:273–280. [DOI] [PubMed] [Google Scholar]
- 179.Lee BJ, Hampson DJ. 1995. A monoclonal antibody reacting with the cell envelope of spiochaetes isolated from cases of intestinal spirochaetosis in pigs and humans. FEMS Microbiol Lett 131:179–184. doi: 10.1111/j.1574-6968.1995.tb07774.x. [DOI] [PubMed] [Google Scholar]
- 180.Tenaya IWM, Penhale JP, Hampson DJ. 1998. Preparation of diagnostic polyclonal and mnoclonal antibodies against outer envelope proteins of Serpulina pilosicoli. J Med Microbiol 47:317–324. doi: 10.1099/00222615-47-4-317. [DOI] [PubMed] [Google Scholar]
- 181.Corona-Barrera E, Smith DG, La T, Hampson DJ, Thomson JR. 2004. Immunomagnetic separation of the intestinal spirochaetes Brachyspira pilosicoli and Brachyspira hyodysenteriae from porcine faeces. J Med Microbiol 53:301–307. doi: 10.1099/jmm.0.05500-0. [DOI] [PubMed] [Google Scholar]
- 182.Mikosza ASJ, La T, Margawani KR, Brooke CJ, Hampson DJ. 2001. PCR detection of Brachyspira aalborgi and Brachyspira pilosicoli in human faeces. FEMS Microbiol Lett 197:167–170. doi: 10.1111/j.1574-6968.2001.tb10599.x. [DOI] [PubMed] [Google Scholar]
- 183.Mikosza ASJ, Hampson DJ, Koopmans MPG, van Duynhoven YTHP. 2003. Presence of Brachyspira aalborgi and B. pilosicoli in feces of patients with diarrhea. J Clin Microbiol 41:4492–4493. doi: 10.1128/JCM.41.9.4492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Park NY, Chung CY, McLaren AJ, Atyeo RF, Hampson DJ. 1995. Polymerase chain reaction for identification of human and porcine spirochaetes recovered from cases of intestinal spirochaetosis. FEMS Microbiol Lett 125:225–259. doi: 10.1111/j.1574-6968.1995.tb07362.x. [DOI] [PubMed] [Google Scholar]
- 185.Leser TD, Møller K, Jensen TK, Jorsal SE. 1997. Specific detection of Serpulina hyodysenteriae and potentially pathogenic weakly beta-haemolytic porcine intestinal spirochetes by polymerase chain reaction targeting 23S rDNA. Mol Cell Probes 11:363–372. doi: 10.1006/mcpr.1997.0129. [DOI] [PubMed] [Google Scholar]
- 186.Atyeo RF, Stanton TB, Jensen NS, Suriyaarachichi DS, Hampson DJ. 1999. Differentiation of Serpulina species by NADH oxidase gene (nox) sequence comparisons and nox-based polymerase chain reaction tests. Vet Microbiol 67:47–60. doi: 10.1016/S0378-1135(99)00030-9. [DOI] [PubMed] [Google Scholar]
- 187.Rohde J, Rothkamp A, Gerlach GF. 2002. Differentiation of porcine Brachyspira species by a novel nox PCR-based restriction fragment length polymorphism analysis. J Clin Microbiol 40:2598–2600. doi: 10.1128/JCM.40.7.2598-2600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.La T, Phillips ND, Hampson DJ. 2003. Development of a duplex PCR assay for the detection of Brachyspira hyodysenteriae and Brachyspira pilosicoli in pig feces. J Clin Microbiol 41:3372–3375. doi: 10.1128/JCM.41.7.3372-3375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Song Y, Hampson DJ. 2009. Development of a multiplex qPCR for detection and quantitation of pathogenic intestinal spirochaetes in pigs and chickens. Vet Microbiol 137:129–136. doi: 10.1016/j.vetmic.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 190.Willems H, Reiner G. 2010. A multiplex real-time PCR for the simultaneous detection and quantitation of Brachyspira hyodysenteriae, Brachyspira pilosicoli and Lawsonia intracellularis in pig faeces. Berl Munch Tierarztl Wochenschr 123:205–209. [PubMed] [Google Scholar]
- 191.Ståhl M, Kokotovic B, Hjulsager CK, Breum SØ, Angen Ø. 2011. The use of quantitative PCR for identification and quantification of Brachyspira pilosicoli, Lawsonia intracellularis and Escherichia coli fimbrial types F4 and F18 in pig feces. Vet Microbiol 151:307–314. doi: 10.1016/j.vetmic.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 192.Borgström A, Scherrer S, Kirchgässner C, Schmitt S, Frei D, Wittenbrink MM. 2017. A novel multiplex qPCR targeting 23S rDNA for diagnosis of swine dysentery and porcine intestinal spirochaetosis. BMC Vet Res 13:42. doi: 10.1186/s12917-016-0939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Phillips ND, La T, Hampson DJ. 2006. Development of a two-step nested duplex PCR assay for the rapid detection of Brachyspira pilosicoli and Brachyspira intermedia in chicken faeces. Vet Microbiol 116:239–245. doi: 10.1016/j.vetmic.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 194.Westerman LJ, Stel HV, Schipper ME, Bakker LJ, Neefjes-Borst EA, van den Brande JH, Boel EC, Seldenrijk KA, Siersema PD, Bonten MJ, Kusters JG. 2012. Development of a real-time PCR for identification of Brachyspira species in human colonic biopsies. PLoS One 7:e52281. doi: 10.1371/journal.pone.0052281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ogata S, Higashiyama M, Adachi Y, Ohara I, Nishiyama J, Okusa Y, Takeo H, Sato K, Nakanishi K, Kawai T. 2010. Imprint cytology detects floating Brachyspira in human intestinal spirochetosis. Hum Pathol 41:249–254. doi: 10.1016/j.humpath.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 196.Ogata S, Shimizu K, Oda T, Tominaga S, Nakanishi K. 2016. Immunohistochemical detection of human intestinal spirochetosis. Hum Pathol 58:128–133. doi: 10.1016/j.humpath.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 197.Günther U, Epple HJ, Heller F, Loddenkemper C, Grünbaum M, Schneider T, Zeitz M, Bojarski C. 2008. In vivo diagnosis of intestinal spirochaetosis by confocal endomicroscopy. Gut 57:1331–1333. [PubMed] [Google Scholar]
- 198.Boye M, Jensen TK, Møller K, Leser TD, Jorsal SE. 1998. Specific detection of the genus Serpulina, S. hyodysenteriae and S pilosicoli in porcine intestines by fluorescent rRNA in situ hybridization Mol Cell Probes 12:323–330. [DOI] [PubMed] [Google Scholar]
- 199.Jensen TK, Boye M, Ahrens P, Korsager B, Teglbjaerg PS, Lindboe CF, Moller K. 2001. Diagnostic examination of human intestinal spirochetosis by fluorescent in situ hybridization for Brachyspira aalborgi, Brachyspira pilosicoli, and other species of the genus Brachyspira (Serpulina). J Clin Microbiol 39:4111–4118. doi: 10.1128/JCM.39.11.4111-4118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schmiedel D, Epple HJ, Loddenkemper C, Ignatius R, Wagner J, Hammer B, Petrich A, Stein H, Göbel UB, Schneider T, Moter A. 2009. Rapid and accurate diagnosis of human intestinal spirochetosis by fluorescence in situ hybridization. J Clin Microbiol 47:1393–1401. doi: 10.1128/JCM.02469-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Klitgaard K, Mølbak L, Jensen TK, Lindboe CF, Boye M. 2005. Laser capture microdissection of bacterial cells targeted by fluorescence in situ hybridization. Biotechniques 39:864–868. [DOI] [PubMed] [Google Scholar]
- 202.Movahedi A, Hampson DJ. 2007. Distribution of the clpX gene in Brachyspira species and reactivity of recombinant Brachyspira pilosicoli ClpX with sera from mice and humans. J Med Microbiol 56:930–936. doi: 10.1099/jmm.0.47004-0. [DOI] [PubMed] [Google Scholar]
- 203.Casas V, Rodríguez-Asiain A, Pinto-Llorente R, Vadillo S, Carrascal M, Abian J. 2017. Brachyspira hyodysenteriae and B. pilosicoli proteins recognized by sera of challenged pigs. Front Microbiol 8:723. doi: 10.3389/fmicb.2017.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Movahedi A, Hampson DJ. 2010. Evaluation of recombinant Brachyspira pilosicoli oligopeptide-binding proteins as vaccine candidates in a mouse model of intestinal spirochaetosis. J Med Microbiol 59:353–359. doi: 10.1099/jmm.0.015842-0. [DOI] [PubMed] [Google Scholar]
- 205.Jamshidi A, Hampson DJ. 2002. Zinc bacitracin enhances colonisation by the intestinal spirochaete Brachyspira pilosicoli in experimentally infected layer hens. Avian Pathol 31:293–298. doi: 10.1080/03079450220136493. [DOI] [PubMed] [Google Scholar]
- 206.Corona-Barrera E, Smith DGE, Murray B, Thomson JR. 2004. Efficacy of seven disinfectant sanitisers on field isolates of Brachyspira pilosicoli. Vet Rec 154:473–474. doi: 10.1136/vr.154.15.473. [DOI] [PubMed] [Google Scholar]
- 207.Karlsson M, Oxberry SL, Hampson DJ. 2002. Antimicrobial susceptibility testing of Australian isolates of Brachyspira hyodysenteriae using a new broth dilution method. Vet Microbiol 84:123–133. doi: 10.1016/S0378-1135(01)00444-8. [DOI] [PubMed] [Google Scholar]
- 208.Dassanayake RP, Sarath G, Duhamel GE. 2005. Penicillin-binding proteins in the pathogenic intestinal spirochete Brachyspira pilosicoli. Antimicrob Agents Chemother 49:1561–1563. doi: 10.1128/AAC.49.4.1561-1563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mortimer-Jones SM, Phillips ND, La T, Naresh R, Hampson DJ. 2008. Penicillin resistance in the intestinal spirochaete Brachyspira pilosicoli associated with OXA-136 and OXA-137, two new variants of the class D beta-lactamase OXA-63. J Med Microbiol 57:1122–1128. doi: 10.1099/jmm.0.2008/001552-0. [DOI] [PubMed] [Google Scholar]
- 210.La T, Neo E, Phillips ND, Hampson DJ. 2015. Genes encoding ten newly designated OXA-63 group class D β-lactamases identified in strains of the pathogenic intestinal spirochaete Brachyspira pilosicoli. J Med Microbiol 64:1425–1435. doi: 10.1099/jmm.0.000162. [DOI] [PubMed] [Google Scholar]
- 211.Karlsson M, Fellström C, Johansson KE, Franklin A. 2004. Antimicrobial resistance in Brachyspira pilosicoli with special reference to point mutations in the 23S rRNA gene associated with macrolide and lincosamide resistance. Microb Drug Resist 10:204–208. [DOI] [PubMed] [Google Scholar]
- 212.Pringle M, Poehlsgaard J, Vester B, Long KS. 2004. Mutations in ribosomal protein L3 and 23S ribosomal RNA at the peptidyl transferase centre are associated with reduced susceptibility to tiamulin in Brachyspira spp. isolates. Mol Microbiol 54:1295–1306. doi: 10.1111/j.1365-2958.2004.04373.x. [DOI] [PubMed] [Google Scholar]
- 213.Le Roy CI, Passey JL, Woodward MJ, La Ragione RM, Claus SP. 2017. Metabonomics-based analysis of Brachyspira pilosicoli's response to tiamulin reveals metabolic activity despite significant growth inhibition. Anaerobe 45:71–77. doi: 10.1016/j.anaerobe.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 214.Hommez J, Devriese LA, Castryck F, Miry C, Lein A, Haesebrouck F. 1998. Susceptibility of different Serpulina species in pigs to antimicrobial agents. Vlaams Diergen Tijdschrift 67:32–35. [Google Scholar]
- 215.Pringle M, Landén A, Franklin A. 2006. Tiamulin resistance in porcine Brachyspira pilosicoli isolates. Res Vet Sci 80:1–4. doi: 10.1016/j.rvsc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 216.Pringle M, Landén A, Unnerstad HE, Molander B, Bengtsson B. 2012. Antimicrobial susceptibility of porcine Brachyspira hyodysenteriae and Brachyspira pilosicoli isolated in Sweden between 1990 and 2010. Acta Vet Scand 54:54. doi: 10.1186/1751-0147-54-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Trampel DW, Kinyon JM, Jensen NS. 1999. Minimum inhibitory concentration of selected antimicrobial agents for Serpulina isolated from chickens and rheas. J Vet Diagn Invest 11:379–382. doi: 10.1177/104063879901100418. [DOI] [PubMed] [Google Scholar]
- 218.Hampson DJ, Stephens CP, Oxberry SL. 2006. Antimicrobial susceptibility testing of Brachyspira intermedia and Brachyspira pilosicoli isolates from Australian chickens. Avian Pathol 35:12–16. doi: 10.1080/03079450500465643. [DOI] [PubMed] [Google Scholar]
- 219.Stephens CP, Hampson DJ. 2002. Evaluation of tiamulin and lincomycin for the treatment of broiler breeders experimentally infected with the intestinal spirochaete Brachyspira pilosicoli. Avian Pathol 31:299–304. doi: 10.1080/03079450220136501. [DOI] [PubMed] [Google Scholar]
- 220.Brooke CJ, Hampson DJ, Riley TV. 2003. In vitro antimicrobial susceptibility of Brachyspira pilosicoli isolates from humans. Antimicrob Agents Chemother 47:2354–2357. doi: 10.1128/AAC.47.7.2354-2357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Peghini PL, Guccion JG, Sharma A. 2000. Improvement of chronic diarrhea after treatment for intestinal spirochetosis. Digest Dis Sci 45:1006–1010. doi: 10.1023/A:1005597729899. [DOI] [PubMed] [Google Scholar]
- 222.Esteve M, Salas A, Fernández-Bañares F, Lloreta J, Mariné M, Gonzalez CI, Forné M, Casalots J, Santaolalla R, Espinós JC, Munshi MA, Hampson DJ, Viver JM. 2006. Intestinal spirochetosis and chronic watery diarrhea: clinical and histological response to treatment and long-term follow up. J Gastroent Hepatol 21:1326–1333. doi: 10.1111/j.1440-1746.2006.04150.x. [DOI] [PubMed] [Google Scholar]
- 223.Takezawa T, Hayashi S, Adachi Y, Sunada K, Hayashi Y, Nishimura N, Yano T, Miyata T, Yamamoto H, Hirai Y, Sugano K. 2012. Human intestinal spirochetosis in an immunocompromised host: evaluation of eradication therapy by endoscopy, histopathology and bacteriology. Clin J Gastroenterol 5:69–73. doi: 10.1007/s12328-011-0265-2. [DOI] [PubMed] [Google Scholar]
- 224.Bernardeau M, Gueguen M, Smith DGE, Corona-Barrera E, Vernoux JP. 2009. In vitro antagonistic activities of Lactobacillus spp. against Brachyspira hyodysenteriae and Brachyspira pilosicoli. Vet Microbiol 138:184–190. doi: 10.1016/j.vetmic.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 225.Mappley LJ, Tchórzewska MA, Cooley WA, Woodward MJ, La Ragione RM. 2011. Lactobacilli antagonize the growth, motility, and adherence of Brachyspira pilosicoli: a potential intervention against avian intestinal spirochetosis. Appl Environ Microbiol 77:5402–5411. doi: 10.1128/AEM.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mappley LJ, Tchórzewska MA, Nunez A, Woodward MJ, Bramley PM, La Ragione RM. 2013. Oral treatment of chickens with Lactobacillus reuteri LM1 reduces Brachyspira pilosicoli-induced pathology. J Med Microbiol 62:287–296. doi: 10.1099/jmm.0.051862-0. [DOI] [PubMed] [Google Scholar]
- 227.Fossi M, Heinonen M, Pohjanvirta T, Pelkonen S, Peltoniemi AT. 2001. Eradication of endemic Brachyspira pilosicoli infection from a farrowing herd: a case report. Anim Health Res Rev 2:53–57. [PubMed] [Google Scholar]
- 228.Koopman MBH, Kasboher A, Beckamn G, van der Zeijst BAM, Kusters JG. 1993. Genetic similarity of intestinal spirochetes from humans and various animal species. J Clin Microbiol 31:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]