Abstract
Rates of chronic conditions among pediatrics have been steadily increasing and medications used to treat these conditions have also shown a proportional increase. Most clinical trials focus on the safety of solitary medications in adult patients. However, data from these trials are often times extrapolated for use in pediatric patients who have different pharmacokinetic processes and physical profiles. As research increases and more drugs become available for pediatric use, the issue of polypharmacy becomes more of a concern. Polypharmacy is defined as the practice of administering or using multiple medications concurrently for the treatment of one to several medical disorders. With the increased rates of diagnosed complex disease states as prescribed mediations in pediatric patients, the prevalence and effect of polypharmacy in this patient population is largely a mystery. Polypharmacy falls within the realm of expertise of specialized pharmacists who can undertake medication therapy management services, medical chart reviews, and other services in pediatrics. Pharmacists have the time and knowledge to undertake pertinent interventions when managing polypharmacy and can play a major positive role in preventing adverse events. The aim of this paper is to review the literature on pediatric polypharmacy and provide insight into opportunities for pharmacists to help with management of polypharmacy. Information on adverse events, efficacy, and long-term outcomes with regard to growth and development of children subject to polypharmacy has yet to be published, leaving this realm of patient safety ripe for research.
Keywords: polypharmacy, pediatrics, pharmacists, involvement
Introduction
Rates of chronic conditions in pediatrics have been steadily increasing and medications used to treat these conditions have also shown a proportional increase.1,2 Most clinical trials for approving medications by the US Food and Drug Administration (FDA) focus on the safety and efficacy of solitary medications in adults. However, data from these trials are often times extrapolated for use in pediatric patients who have different pharmacokinetic processes and physical profiles. Clinical trials that focus on the safety, efficacy, and dosing parameters in pediatric patients are lacking, prompting use of “off-label” prescribing by physicians.3,4 With the limited availability of evidence-based protocols and practice guidelines, clinicians often rely on their best clinical judgment when managing pharmacotherapy for pediatric patients with multiple and/or complex disease states. The FDA has developed mandates for pediatric research and is providing incentives for researchers to improve the quality and quantity of available data.5–7 As research increases and more medications become available for use in pediatrics, the issue of polypharmacy is becoming more of a concern.
This article focuses on polypharmacy in pediatric patients and opportunities for pharmacists to help with its management. Polypharmacy is defined as the practice of administering or using multiple medications concurrently for the treatment of one to several medical disorders.8 With the increased rates of diagnosed complex disease states as prescribed mediations in pediatric patients, the prevalence of polypharmacy has increased. Some disease states warrant the use of multiple medications through guideline-driven recommendations, such as cystic fibrosis or human immunodeficiency virus. Yet, there are other disease states, such as autism spectrum disorder (ASD), where treatment recommendations are less defined, resulting in polypharmacy that may be harmful. Despite evidence highlighting rates of polypharmacy, its effect in the pediatric population is largely a mystery. A survey conducted between 1998 and 2007 reports that 56% of children used at least one medication product during that time, and of those products, over-the-counter medications were used most often.9 A patient is at risk for adverse events as the number of concomitant medications increases.10 Despite the detection of polypharmacy in pediatric patients, there are instances where polypharmacy is acceptable. Some providers may utilize polypharmacy to overlap treatment when switching to another agent, and others may use polypharmacy to stabilize patients who are experiencing exacerbations in the highly monitored inpatient setting.
Polypharmacy is different from medication overuse, which can be defined as the inappropriate overprescribing of a medication or therapy for treatment of a disease state. Examples of medication overuse include overprescribing asthma medication in children with persistent cough or overprescribing the use of short-acting beta-2 agonists for asthma exacerbations.11,12 Although medication overuse is an important concern for providers, polypharmacy may result from medication overuse with multiple therapies, and is a more subtle issue that should also be taken just as seriously. Certain medication combinations may result in drug–drug interactions in a patient population that may not be able to adequately describe or convey discomfort or pain. In addition, the complexity of care increases for pediatric patients since they rely on parents/guardians for continuity in maintaining medication lists, and at a single retail pharmacy, and also keeping physician appointments.
Polypharmacy falls within the realm of expertise of specialized pharmacists who are knowledgeable about medication therapy management services, are able to conduct chart reviews, and can participate in making pertinent pertinent medication-related interventions in pediatrics. Retail pharmacists can educate the parents on polypharmacy and make important interventions with regard to over-the-counter medications. We have demonstrated our usefulness in the clinical setting by improving the quality and reducing the cost of care through valuable interventions in adults.13–16 Pharmacists have the time and knowledge to make meaningful interventions when managing polypharmacy and can play a major positive role in preventing adverse events.
A systematic review of the literature was completed using Medline, EMBASE, and PubMed. Search terms included polypharmacy, pediatrics, concomitant pharmacotherapy, combined pharmacotherapy, children, adolescence, Medicaid, psychotropic medications, and side effects. Articles were selected based on their relevance regarding polypharmacy in pediatric patients. Considering the growth of chronic disease states in pediatric patients, there is a compelling lack of data showing the current rates of polypharmacy in many chronic conditions and its effects in our youth. The most common disease states for which off-label polypharmacy is documented include ASD and psychiatric disorders. To encourage hospital systems and specialists, our literature review highlights issues revolving around polypharmacy in hospital systems where the pharmacist may find areas to intervene. Insight into the prevalence of polypharmacy for many disease states has yet to be explored. Information on adverse events, efficacy, and long-term outcomes for the growth and development of children subject to polypharmacy are areas for future research concerning patient safety.
Polypharmacy in ASD and psychiatric disorders
Autism spectrum disorder
There is an abundance of research regarding polypharmacy and the use of psychotropic medications in patients with ASD (Table 1).17–25 Parent/guardian surveys and retrospective reviews of insurance databases have shown an increase in the use of psychotropic medications over the years. The only medications approved for the treatment of ASD by the FDA are risperidone and aripiprazole, both of which are indicated for treating irritability or aggression in patients.26,27 Yet, an overabundance of other medication classes, such as antidepressants, stimulants, tranquilizers/antipsychotics, anticonvulsants, antihypertensives, anxiolytics/sedatives/hypnotics, and benzodiazepines, are prescribed “off-label” without evidence from guidelines. Some providers may find these agents necessary to help stabilize symptoms in patients with ASD.22,25,28 If a patient has ASD and another psychiatric comorbidity, such as attention deficit disorder (ADD), the use of psychotropic agents also increases.22 Due to the lack of treatment protocols for ASD, physicians are forced to use a trial-and-error approach, which may result in polypharmacy and increased risk for pediatric patients.
Table 1.
Review of polypharmacy in autism spectrum disorder for pediatric patients
| Reference | Study design | Length | Major objective | Patients, n (age range, years) | Results |
|---|---|---|---|---|---|
| Lake et al17 | Prospective survey | Unknown | Determine the rates of psychotropic medications and to identify child, parent, and service variables that are associated with psychotropic polypharmacy | 363 (2–30) | More than two psychotropic medications were prescribed for 26.4% of individuals, and 13.2% were prescribed more than two agents from the same therapeutic class Patient clinical variables significantly related to psychotropic polypharmacy: • History of hurting others (P<0.001) • History of self-injury (P=0.004) • History of psychiatric admission (P<0.001) • Psychiatric co-diagnosis (P<0.001) Significant parent variables include family counseling, having an additional child with ASD, parent crisis, and parent burden showed higher rates of polypharmacy |
| Schubart et al18 | Retrospective review of Medicaid Analytic eXtract data | 2000–2003 | Examine use of psychotropic over time | 2.2 million (3–17) | Approximately 65% of children with ASD received a psychotropic medication Most commonly prescribed medications include antipsychotics (39%), antidepressants (29%), stimulants (25%), mood stabilizers (16%), sedative hypnotics (14%), and anxiolytics (11%) Increased number of treatment days for psychotropic medications for children with ASD compared with those without ASD Within-class polypharmacy (60-day continuous use): • Antidepressants increased from 7.1% to 8.7% (P<0.001, chi-squared test) • Antipsychotics increased from 6.2% to 8.7% (P<0.001, chi-squared test) Between-class polypharmacy (60-day continuous use) increased from 26% to 30% (P<0.001, chi-squared test) |
| Spencer et al19 | Retrospective observational study | 2001–2009 | Examine use of psychotropic medications in pediatrics with private insurance | 33,656 (0–20) | Mean time of insurance enrollment: 75% for >3 years 35% (n=11,598) had multi-medication class polypharmacy Average polypharmacy episodes per person was 5.63 (median 4.00) Total days polypharmacy per subject averaged 525 days (median 345) |
| Coury et al20 | Prospective survey | 2007–2011 | Examine rates of psychotropic medications in adolescents with ASD receiving care through the Autism Speaks Autism Treatment Network | 2,853 (2–17) | More than one psychotropic medication was prescribed for 27% of patients; 15% of patients reported receiving one medication, 7.4% reported receiving two medications, and 4.5% reported receiving three or more medications Patients (n=442) with ASD and comorbid disorders such as ADHD, bipolar disorder, OCD, depression, or anxiety had increased rates of psychotropic therapy. Of these patients, 26% received two psychotropic agents, and 16% received three or more psychotropic agents. |
| Logan et al21 | Retrospective review | January 1, 2006 to December 31, 2007 |
Measures of prescription claims, any psychotropic prescriptions, multiple psychotropic prescriptions, number of prescriptions, and total over a 2-year period | 263 (8–15) | 20% (n=52) used multiple psychotropic classes; 40% (n=105) prescribed any psychotropic medication; of the 105 patients in this subgroup, 50% were prescribed multiple classes Patients who were older (>15 years) were prescribed more psychotropic medications Most common combinations were • ADHD medications and an antihypertensive (23%), antidepressant (14%), antipsychotic (13%), or sedative hypnotic (9%) • Antidepressants and an antihypertensive (12%) or antipsychotic (11%) |
| Frazier et al22 | Retrospective review of data from the prospective National Longitudinal Transition Study-2 | 2000–2009 | To examine the prevalence of medication use both overall and across specific medication classes | 890 (13–17) | Youth classified as having ASD + ADHD had higher rates of medication usage (58.2%) compared with youth classified as having only ASD (34.3%) or ADHD (49%) The majority of youth classified as having ASD were taking two or more medications (ASD only 52.1% and ASD + ADHD 58.2%). The highest number of youth taking three or more medications was in the ASD + ADHD groups (29.5%) |
| Rosenberg et al23 | Retrospective review of Interactive Autism Network parental survey data | 2007–2008 | To examine prescribing trends by medical specialty for children with ASD | 5,181 (0–17) | Nearly 10% reported concurrent use of medications in three more major classes to treat symptoms of ASD. Most common classes were stimulants, neuroleptics, and antidepressants For patients using at least two psychotropic medications (n=367), 25% had two types of prescribers, and 2% reported three types of prescribers |
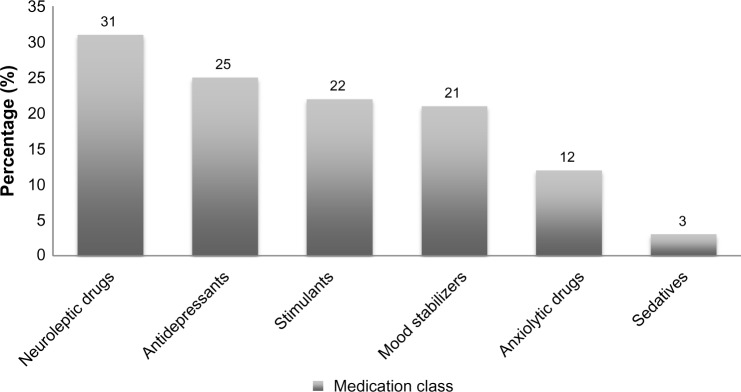
| Mandell et al24 | Retrospective review of Medicaid medical charts | 2001 | To estimate the prevalence of psychotropic medication use | 60,641 (0–21) | Concurrent use, a child having a prescription for three or more medications from different classes overlapping at least 30 days, occurred in 20% of patients Medication frequency: neuroleptic drugs (31%), antidepressants (25%), stimulants (22%), mood stabilizers (21%), anxiolytic drugs (12%), and sedatives (3%) |
| Oswald et al25 | Retrospective review | 2002 | To provide an overview of prescription practices for youth with autism, using prescription fill and refill histories | 2,390 (0–21) | Most prescribed medications include antidepressants (32.1%, 768 patients), stimulants (26.8%, 641 patients), tranquilizers/antipsychotics (23.4%, 561 patients), and anticonvulsants (14.4%, 343 patients) Number of different therapeutic class medications prescribed to an individual over 1 year varied (4.01±2.73; range 1–24) Antidepressants, antipsychotics, and anticonvulsants appeared to increase in usage as patients grew older |
Abbreviations: ASD, autism spectrum disorder; ADHD, attention deficit hyperactivity disorder; OCD, obsessive compulsive disorder.
Pediatric patients also have pharmacodynamic and pharmacokinetic profiles that are different from those in adults, and require close monitoring for efficacy and adverse events. Although these differences have not been studied extensively for many medications used to treat ASD, there still remains a heavy responsibility on providers to monitor patients for both signs of efficacy and side effects. Table 2 reviews the pharmacokinetic properties for atypical antipsychotics.29 The differences between pediatrics and pediatric and adult patients in the time to peak concentration, half life, and elimination processes for most patients do not show a common trend. When combined with other off-label medications, this form of polypharmacy may potentially cause more harm than benefit. Side effects of psychotropic medications include weight gain, sedation, headache, gastrointestinal problems, akathisia, and cognitive dysfunction.29 With one or more medication classes combined, the risk for drug–drug interactions and adverse effects increases. Further, there is little evidence examining the effects of these medications for the physiological and psychological development of pediatric patients. Because ASD is characterized by communication deficits, repetitive actions, and routines, or an intense focus on inappropriate items, confounded by the age of some pediatric patients, communicating adverse events and discomfort with medication may be problematic.30 Additionally, a study by Rosenberg et al found that some ASD patients may have more than one provider, which could contribute to further polypharmacy for some patients.23 There are limitations to the available research on this topic. Surveys of parents or guardians may provide incorrect or incomplete information, and refill histories do not ensure patients are taking all the medications listed. However, despite the limitations mentioned, the evidence available is compelling, and trends in pediatric polypharmacy for patients with ASD are still evident.
Table 2.
Pharmacokinetic properties of atypical antipsychotics in children versus adults
| Medication | FDA approval for ASD | Pharmacokinetics in adults | Pharmacokinetics in pediatrics | Monitoring information |
|---|---|---|---|---|
| Risperidone | Yes | Half-life: extensive metabolizers, 3 hours; poor metabolizers, 20 hours 9-hydroxyrisperidone – extensive metabolizers, 21 hours; poor metabolizers, 30 hours Intramuscular route 3–6 days Time to peak concentration: Oral route 1 hour 9-hydroxyrisperidone – extensive metabolizers, 3 hours; poor metabolizers, 17 hours Elimination: urine (70%), feces (14%) |
Similar to adults after appropriate adjustments of dose for weight | Blood pressure, heart rate (especially during dosing titration), mental status, AIMS, extrapyramidal symptoms, growth, body mass index, CBC with differential (monitor WBC and ANC especially for clozapine), liver enzymes (especially in obese children who are rapidly gaining weight), lipid profile, fasting blood glucose, and HbA1c |
| Aripiprazole | Yes | Half-life ~75 hours Time to peak concentration: Oral route 3–5 hours Intramuscular route 1–3 hours Elimination: feces (55%), urine (25%) |
Similar to adults for patients 10–17 years of age | |
| Olanzapine | No | Half-life: oral and intramuscular routes (short-acting) ~30 hours Time to peak concentration Oral route ~6 hours Elimination: urine Oral route 25 L/hour |
Half-life: oral and intramuscular routes (short-acting) ~37.2 hours Time to peak concentration Oral route ~4.7 hours Elimination: urine Oral route ~9.6 L/hour |
|
| Quetiapine | No | Half-life ~6 hours Time to peak concentration 1.5 hours Elimination: urine (73%) |
Half-life ~5.3 hours Time to peak concentration Oral route 0.5–3 hours |
|
| Ziprasidone | No | Half-life: Oral route 7 hours Time to peak concentration Oral route 6–8 hours Elimination: feces (66%) 7.5 mL/min/kg |
Half-life Oral route 3.3–4.1 hours Time to peak concentration Oral route 5–5.5 hours Elimination: feces (major) Oral route 11.5–13.1 mL/min/kg |
|
| Clozapine | No | Half-life ~12 hours Time to peak concentration Oral route 2.5 hours Elimination: urine (50%), feces (30%) |
Similar to adults. May see higher concentrations of desmethyl metabolite in comparison with clozapine (especially in females) when compared with adult data. | |
| Paliperidone | No | Half-life: Oral route 23 hours Intramuscular route 25–49 days Time to peak concentration Oral route ~24 hours Intramuscular route 13 days Elimination: urine (80%) |
Adolescents weighing >51 kg are similar to adults Adolescents weighing <51 kg had an observed increase of exposure by 23% (not considered clinically significant) |
Notes:
Adapted from Lexicomp Online. © 2015 Wolters Kluwer Clinical Drug Information, Inc. and its affiliates and/or licensors. All rights reserved.29
Abbreviations: AIMS, abnormal involuntary movement scale; ANC, absolute neutrophil count; ASD, autism spectrum disorder; CBC, complete blood count; FDA, US Food and Drug Administration; WBC, white blood cells.
Psychiatric disorders
An abundance of published information is available on the prevalence of polypharmacy in pediatric patients being treated for psychiatric disorders. Current research has made an effort to identify trends in prescribing and combinations of medications used to target areas for therapeutic intervention. The most common diagnoses were attention deficit hyperactivity disorder (ADHD), conduct disorder/oppositional defiant disorder, schizophrenia spectrum disorders, anxiety disorders, bipolar spectrum disorders, and ASDs.31,32
The increased use of second-generation antipsychotics (SGAs) along with other psychotropic medications has heightened concern about the metabolic effects in children and adolescents.31 Data are limited regarding the use of multiple medications for short-term control of acute behavioral problems or overlapping medications when changing a patient from one medication to another. Some of these practices may be supported by the American Academy of Child and Adolescent Psychiatry (AACAP) in special cases. AACAP states that “the use of multiple medications in refractory patients may, at times, be necessary but has not been studied rigorously and clinicians should proceed with caution”.33
A study by Kreider et al evaluated the frequency of polypharmacy when using a SGA combined with medications from the four psychotropic classes (stimulants, antidepressants, mood stabilizers, and alpha-agonists) in a sample Medicaid population from 2004 to 2008. The authors found that psychotropic polypharmacy was not being used for short-term behavioral problems.31 They also discovered that 85% of the SGA group had several prescriptions for concurrent SGA use during the year. There were also increased rates of use of SGA combined with other psychotropic classes, including mood stabilizers (52%), alpha-agonists (37%), antidepressants (32%), and stimulants (22%).
The AACAP recommends avoiding simultaneous use of multiple antipsychotics because of the possibility of significant risks and lack of evidence to support the use of polypharmacy.33 A review of 15 studies by Toteja et al found that the prevalence of antipsychotic polypharmacy was an average of one in ten to eleven youth who receive an antipsychotic medication.34 Like many review analyses, different study methods, populations, and clinical settings mean that it is difficult to make comparisons and draw inferences. Regardless of these limitations, the prevalence of antipsychotic polypharmacy is evident and requires research to identify if patients are receiving two or more antipsychotics after treatment failure, especially since this practice is not consistent with AACAP recommendations.
Jureidini et al reviewed trials that studied combination pharmacotherapy for psychiatric disorder in children and adolescents.35 The authors discovered a clinically significant difference between combination psychotherapy compared to monototherapy, when a central sympatholytic agent (eg, clonidine) was added to stimulant therapy for treatment of ADHD. Currently, this combination is approved by the FDA in the USA; however, further research is needed to support the use of polypharmacy with psychotropic medications.
Finally, another study examined the characteristics of pediatric patients who were prescribed psychotropic polypharmacy from 2002 to 2008 and were also enrolled in the Ohio Medicaid program.32 The rates of use of stimulants, antipsychotics, and alpha-agonists increased across all eligibility groups, with stimulants being the most prescribed. According to the authors, approximately 75% of low-income pediatric patients and 57.3% of those with disabilities received a stimulant medication. In addition, the patients who received the highest amount of polypharmacy, including medications from the same class, were those in foster care (27.3%), those who had disabilities (24.9%), and those who were from low-income families (11.5%). When examining polypharmacy between medication classes, the authors found that 22.3% of the foster care group, 19.5% of the disability group, and 9.0% of the low-income group received the most prescriptions. Psychotropic polypharmacy was highly evident in this trial; a significant increase for two or more antipsychotics across all groups was seen, with the foster group being the highest. These data correlate with raised concern over increasing rates of psychotropic polypharmacy in the foster care population.32,36 Pediatric patients in certain identifiable groups are at an increased risk for polypharmacy, and there should be more research and data investigating ways to augment therapy for these patients without increasing the risk of adverse events.
Other chronic pediatric conditions
Pediatric patients with chronic conditions such as asthma, epilepsy, or cystic fibrosis are treated with multiple medications; however, these patients are still at risk for adverse events due to the nature of polypharmacy. For example, pediatric patients with cystic fibrosis are treated with multiple medications supported by guidelines.37 These medications can include pancreatic enzyme supplements, multivitamins, antibiotics, anti-inflammatory agents, mucolytics, bronchodilators, and cystic fibrosis transmembrane conductance regulator potentiators. Although these patients will be on multiple medications for the duration of their lifetime, their regimens are documented as appropriate for optimal management of this disease state. Therefore, “rational” polypharmacy is founded on clinical evidence in this population, but should be heavily monitored for safety purposes. The same can be said for many chronic conditions that affect pediatric patients; however, there is a paucity of evidence to show polypharmacy between disease states, and the possible medication errors and adverse events that may occur because of it.
Prescribing patterns for psychotropic and stimulant medications in pediatric patients
We have found very limited high-level evidence supporting the routine use of combination medications or polypharmacy in pediatric patients with psychiatric disorders. Some clinicians may utilize off-label supplemental medications in addition to first-line therapy in order to enhance or speed up a therapeutic response, or to treat behavioral issues such as aggression, anxiety, or insomnia.38 In the case of schizophrenia, guidelines acknowledge the use of additional therapy after clozapine has been fully exhausted for refractory psychotic illness and state that patients must be monitored for therapeutic efficacy and side effects.38 There are mixed reviews from case reports in adults concerning the combination of olanzapine with amisulpride or risperidone, and quetiapine with risperidone for occasions when clozapine cannot be used.39 Several reports suggest that aripiprazole combined with other non-clozapine atypical agents may worsen psychiatric illness.40–42 Although the prescribing of combination antipsychotic medication is of concern and appears to happen often in the treatment of schizophrenia, the frequency with which such prescribing occurs in pediatric patients is unknown.
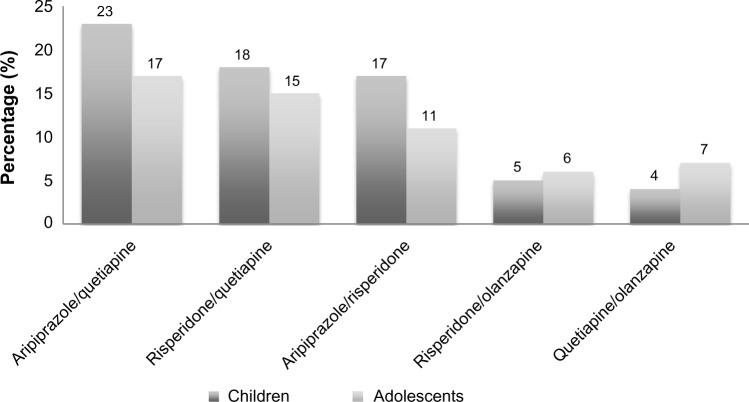
A study published in 2004 reviewed survey data from the National Center for Health Statistics from 1993 to 1999 with the aim of capturing US national prescribing trends for the use of combination therapy with stimulants and psychotropic medications. The prescribing of combination therapy involving stimulant medications increased from 3% in 1993–1994 to 15% in 1997–1998. Stimulant medications combined with psychotropic medications increased by 12.5% in 1993–1998.43 This study did not provide specifics on which stimulants and psychotropic medications were prescribed, however each was grouped by their medication classes. However, given the rate at which prescribing increased during that time period, it may be possible to extrapolate this information to the present. A retrospective study of the Florida Medicaid program found that 7% of children and adolescents were receiving multiple psychotropic medications, with rates increasing as the children grew older.44 There were more than 100 combinations of psychotropic medications prescribed, and the most common included aripiprazole/quetiapine, risperidone/quetiapine, aripiprazole/risperidone, risperidone/olanzapine, and quetiapine/olanzapine (Figure 1). When combining the Medicaid programs for California, Illinois, New York, or Texas in 2005, researchers found that psychotropic polypharmacy occurred in 28.8% of 282,910 pediatric patients for 14 days, 27.2% of patients for 30 days, and 20.9% of patients for 60 days.45 The most common combinations of medications for patients with long-term polypharmacy of longer than 60 days (n=55,611) included antipsychotics/stimulants (41%), antidepressants/antipsychotics (30%), and antipsychotics/anticonvulsants (23%). The specific medications in each class that were most commonly prescribed were not detailed.
Figure 1.
Percentage of commonly combined psychotropic medications using data from the Florida Medicaid Program from 2002 to 2007 for child and adolescent patients.
Note: Data from Constantine et al.44
The data regarding trends in polypharmacy for ASD are similar to those for psychiatric disorders. Mandell et al examined Medicaid claims for all 50 US states in 2001 and found that 20% (n=60,641) of pediatric patients used at least three medications concurrently.24 Neuroleptic medications followed by antidepressants and stimulants were prescribed most often (Figure 2). Yet again, there are no data describing the specific medications used or combined most often, and reports pertaining to adverse effects due to polypharmacy are not available. However, the data supplied are enough reason to investigate prescribing patterns in more detail in the future.
Figure 2.
Percentages of medication classes commonly used for autistic spectrum disorder from the 2001 US Medicaid Program for pediatric patients.
Note: Data from Mandell et al.24
The most current research available examining prescribing patterns and polypharmacy in pediatric patients was published in 2014.18 The authors reviewed Medicaid programs for 41 states from 2000 to 2003, and found that antipsychotic medications (39%, n=12,843), antidepressants (29%), and stimulants (25%) were the most commonly prescribed medications for patients diagnosed with ASD. Of note, there was an upward trend for use of antidepressant polypharmacy (7.1% to 8.7% in 3 years; P<0.001, chi-squared test) and antipsychotic polypharmacy (6.2% to 8.5%; P<0.001, chi-squared test) for ASD patients.
Most of the published studies highlight the use of polypharmacy in pediatric patients, especially for those suffering from psychiatric disorders. Despite the available data regarding the incidence of polypharmacy, changes in prescribing patterns and future guidelines cannot occur when there is a deficiency of evidence regarding specific medications prescribed erroneously in combinations that may be harmful to pediatric patients. There is a lack of reporting regarding the rates of adverse events due to polypharmacy between disease states (eg, medications for hypertension and diabetes). Evidence reflecting our current polypharmacy prescribing rates is scarce, and the most compelling and expansive research has been completed using data prior to 2003. Given the trial-and-error approach to treating most refractory psychiatric disorders and other chronic pediatric disease states, data regarding specific medications is important so that health care providers can make educated clinical choices. To our knowledge, there are no recently published data investigating polypharmacy trends in pediatrics, which creates a gap in health care by not providing insight into a very serious issue.
Polypharmacy in hospital systems
Inpatients
The Best Pharmaceuticals for Children Act, implemented in 2002, designed goals to decrease medication errors in hospitalized pediatric patients.46 Adverse events related to adult polypharmacy in the inpatient setting has been documented in the past, increasing patient safety concerns regarding administration of medication.47 These same safety concerns should be a priority for hospitalized pediatric patients, who may be at increased risk for adverse events due to differences in weight-based dosing protocols and “off-label” prescribing subsequent to lack of clinical evidence regarding efficacy and safety in this patient population.48 Lasky et al reviewed the medical records of 877,201 hospitalized pediatric patients in 2008.49 A total of 12,040,196 medications were charged to patients and 568 unique medications or combinations of medications were identified. The ten drugs prescribed most often were acetaminophen, lidocaine, ampicillin, gentamicin, fentanyl, ibuprofen, morphine, ondansetron, ceftriaxone, and albuterol. Acetaminophen was used in 14.7% of pediatric hospitalizations and lidocaine was used in 11.0%. The authors also reported that as pediatric patients aged, the use of certain medications became more or less prevalent. Pediatric patients under 2 years of age often received acetaminophen, lidocaine, ampicillin, gentamicin, ceftriaxone, albuterol, cefotaxime, fentanyl, lidocaine/prilocaine, and ibuprofen. Those more than 12 years of age frequently received ondansetron, fentanyl, acetaminophen, morphine, ibuprofen, oxytocin, lidocaine, midazolam, propofol, and ketorolac. The rates of simultaneous administration and adverse reactions for each patient were not investigated.
Another study assessed the prevalence of exposure to medication in pediatric patients admitted to general or children’s hospitals.50 A total of 365,868 pediatric patients under 18 years of age had a total of 260,740 admissions to general hospitals and 491,451 admissions to children’s hospitals. Infant patients in children’s hospitals were exposed to an average of four medications and therapeutic agents on the 1st day of admission compared with three medications and therapeutic agents for general hospitals. Over a 30-day length of stay, pediatric patients were exposed to an average of four medications while in the care of children’s hospitals, compared to three medications for those admitted to general hospitals. In pediatric patients older than 1 year of age and hospitalized in children’s hospitals, the average level of exposure to medication and therapeutic agents was five medications on day 1, which increased to nine agents by day 30. For pediatric patients over 1 year of age in general hospitals, the average level of exposure to medications and therapeutic agents on the 1st day was five and increased to six by day 30. In pediatric patients >1 year of age in children’s hospitals, the largest amount of medication exposures was 13 agents on day 1 and rose to 20 exposures by day 30. Finally, for general hospitals, the largest amount of medication exposures for the same age group was 12 on the 1st day and rose to 15 by day 30. The authors also found that patients with a chronic disease had significantly increased exposure to medications and therapeutic agents. After adjusting for age, sex, length of hospital stay, and surgical encounters, this study found that children’s hospitals were 1.34 times more likely to expose their patients to medications and therapeutic agents. One can argue that children’s hospitals are more likely to encounter pediatric patients with chronic disease states requiring more medications. However, this study does not assess if the medications listed for each patient were actually administered. Medications were not matched to disease state, so an analysis of evidence-based regimens for each disease state was not available.
The above studies reveal areas of concern regarding the amount of medications administered to hospitalized pediatric patients. Despite the lack of a detailed analysis on use of medication, both studies shed light on the rates of medication used and call attention to the need for further research analyzing the effects of polypharmacy in hospital systems for this patient population. Pediatric patients are at risk for more adverse events (eg, acute renal failure) due to polypharmacy in the inpatient setting,51 yet there are some areas in the hospital where “rational” polypharmacy is permitted and practiced often. Hospital units dedicated to treating epilepsy, cystic fibrosis, and cancer are supported by guidelines where the benefits outweigh the risk of adverse events. Still, all medications should be monitored to reduce adverse events in the hospitalized child.
Emergency room
Children who are exposed to multiple medications, especially psychotropics, are at high risk of adverse events. Since there has been little research on rates of adverse events from psychotropic medications, Feinstein et al conducted a retrospective cohort study of emergency department visits to evaluate these rates. Of an estimated 144 million visits, 716,664 (0.5%) were associated with an adverse event.52 These authors found that children aged 0–18 years with a complex chronic condition (CCC), ie, those who “… have a chronic or life span shortening disease process, require lifelong medical care or rely on supportive technology (such as a tracheostomy ventilator, gastrostomy tube)”, were more likely to experience emergency department visits due to adverse events compared with other children.52 Medications that resulted in the most adverse drug events in children with CCC were psychotropic medications (18%) and in children without CCC were unclassified medications (39%). The authors concluded that children with CCC might have greater exposure to high-risk medications, a higher risk of drug–drug interactions, and more drug-disease interactions due to polypharmacy, which may be associated with a higher rate of adverse drug events. Since children with CCC seem to be at higher risk of adverse drug events, strategies to improve monitoring of drug interactions and to identify higher-risk drug regimens are necessary to reduce the occurrence of such events.52,53
Outpatients
Capturing rates of polypharmacy in pediatric patients in the outpatient setting has many challenges. Some pediatric patients may see more than one medical provider, go to more than one pharmacy, and may be under the care of different parents/guardians. Further, pediatric patients may be taking over-the-counter medications, which may interact with medications taken for chronic disease states. Chronic disease states like ADD, hypertension, diabetes, and asthma that require daily medications further increasing the risk of polypharmacy in the pediatric population.1 One study, which evaluated a medication reconciliation program in a pediatric health system, found that out of 2,745,523 clinic visits, 26% had 1–2 medications, 22% had 3–6 medications, and 11% had $7 medications.54 Each patient had an updated medication list at the end of each visit, and there was a 29% increase in the amount of medications prescribed. This study shows how the scale of polypharmacy may be underestimated by outpatient pediatricians and justifies the need to make medication reconciliation (a Joint Commission patient safety goal) a priority.
Cox et al monitored the use of chronic medications from 2002 to 2005 using the ambulatory administrative pharmacy claims of children who used the commercial insurance Express Scripts, Inc (St Louis, MO, USA).1 They examined the prevalence of use of antihypertensive, antihyperlipidemic, type 2 antidiabetic, antidepressant, ADD/ADHD, and asthma control medication classes. Medications used to treat asthma had the highest prevalence rate (29,500 children) followed by ADD/ADHD (25,400), and depression (15,700). Rates of medications used to treat diabetes more than doubled during the study years; however, the authors could not attribute this rise to childhood obesity. There was an increase in use of all medication classes examined, thus putting pediatric patients at increased risk of polypharmacy and drug–drug interactions. Another study published in 2012 examined trends in filling of outpatient pediatric prescriptions and found approximately 263.6 million prescriptions were dispensed between 2002 and 2010.55 The six therapeutic classes that had a statistically significant increase in sales included ADHD (46%), asthma (14%), oral corticosteroids (22%), contraceptives (93%), and antiepileptics (10%). The most frequently prescribed medication was methylphenidate followed by amphetamine/dextroamphetamine for the management of ADD/ADHD. Despite a decrease in sales over the 8-year period, systemic antibiotics accounted for nearly a quarter of all prescriptions dispensed.
The rates of medication use may be influenced by an increase in evidence-based recommendations from guidelines (for example, the updated pediatric asthma guidelines which recommend inhaled corticosteroids for all ages) as well as an increase in the level of provider comfort.56
Both studies had limitations which include, the lack of specifics on which medications were used together, if a patient had more than one chronic disease state, how many prescriptions a patient may be taking each month, and adherence to therapies. However, post-marketing data and prescription claims provide insight into the prescribing patterns for pediatric patients, and the data can be extrapolated to the general population. More research should be conducted capturing updated statistics on the use of popularly prescribed pediatric medications as commonly used guidelines are updated and the FDA approve new medications. With the rising rates of chronic medications in pediatrics, parents/guardians should be vigilant in maintaining stable medical networks and pharmacies while keeping informed of all medication regimens and changes.
Opportunities for pharmacists involvement
There is strong evidence to support the involvement of pharmacists as members of the health care team for pediatric patients.57–60 Due to the plethora of data that repeatedly show the worth and need for pharmacists as valued members of health systems, only a few articles are selected for review. Of particular note in the inpatient setting, a clinical pharmacy team consisting of pharmacy faculty, residents, and students performed a total of 4,605 interventions in 3,987 patients and prevented a total of 223 adverse drug events or medication errors. Physicians accepted the pharmacy team’s recommendations 91% of the time, and there was a cost saving of approximately $458,516. This dollar amount may not be fully represented because the dollar amounts for each intervention was defined for the adult population and the dollar amount for each intervention may have increased since the time of the study.61 Another study published in 2012 documented pharmacists’ interventions in an 87-bed pediatric hospital over approximately 2 months, and identified a total of 1,315 interventions (approximately 21 interventions daily). Of all the interventions documented, medication errors accounted for 24.5% and medication reconciliation errors accounted for 2.2%.62 More recently, pharmacists have also been able to demonstrate their value as members of the health care team by undertaking medication interventions, reducing length of stay, and/or improving adherence for pediatric patients hospitalized in several countries including Canada, the People’s Republic of China, Egypt, and Madrid.63–66
Computerized physician order entry (CPOE) has been utilized as a strategy to reduce medication errors and allows clinical pharmacists time to devote toward monitoring for potentially harmful medication errors. Although CPOE may be a useful tool to reduce medication errors, it may not be the most effective method. Wang et al evaluated the benefit of a CPOE system and the use of clinical pharmacists in a pediatric inpatient setting.67 The authors found that addition of CPOE reduced medication errors overall, but did not reduce potentially harmful administration errors. Many institutions have implemented CPOE and medication reconciliation as processes to improve patient safety, but computerized technology may not be the only solution.
Evidence of pharmacists’ contributions in the outpatient or ambulatory care setting is slightly more limited. A study conducted in a Canadian hematology/oncology clinic found 165 drug-related problems in 58 patients, and pharmacists identified 99% of these problems. The authors also reported that 83.5% of the interventions completed had a positive impact on patient care.68 Condren and Boger evaluated the impact of a multidisciplinary asthma education program involving a pediatrician, pharmacist, and a nurse in a pediatric clinic.69 This retrospective analysis included 57 patients and compared outcomes in terms of decreased hospitalizations, emergency room visits, and systemic corticosteroid use for the year prior to enrollment in the clinic to 1 year after. The role of the pharmacist included asthma education, proper inhaler technique, performing spirometry, and follow-up telephone consultations. Hospitalizations were reduced by 82%, emergency department visits by 81%, and the amount of systemic corticosteroid exposure by 72% (P<0.001).69
The impact of a pharmacist’s participation in counseling sessions for epileptic patients and caregivers was evaluated in 2012.70 A total of 27 caregivers received a single counseling session in which the pharmacist provided information, including an explanation of medications, instructions on administration, management of side effects, and when to bring the child to the emergency department. The results of this study showed that post-counseling knowledge scores were significantly higher than pre-counseling knowledge scores (14.7±4.6 versus 10.4±3.4, P<0.005). The mean score for the caregivers’ level of confidence increased significantly after the counseling session (4.3 versus 3.6, P=0.002).
For the most part, interventions for reducing polypharmacy have been well documented in adults but evidence in children is limited.71 A retrospective study was conducted in outpatient pediatric clinics that assessed the implementation of an electronic medical record-based quality improvement intervention for documentation of a medication reconciliation process.54 The authors found improvement in documentation of medication reconciliation depending on the type of visit, the person placing the medication order, and quality-based incentives. Although the data are limited, medication reconciliation may be one of the main components for identifying polypharmacy in pediatric patients. Next, a single-center, prospective pilot study published in 2012 reviewed a pharmacist-managed medication reconciliation program for pediatric patients in outpatient clinics.72 The pharmacist on duty was responsible for speaking with the patient, family, caregiver, or retail pharmacy after a physician or nurse completed a medication history (MH) review. If there were any medication changes or interventions, the pharmacist updated the electronic medical record. A total of 100 MHs were included in the study and the mean number of medications documented prior to the pharmacist intervening was 4.4±3.3. After the pharmacist finalized the MH, the mean number of medications dropped to 4.3±3.9. It took an average of 15 minutes for the pharmacy to complete each MH. The authors reported a total of 309 discrepancies, which included missing medications (32.7%), “other” (25.2%), omitted information (22.3%), incorrect dose (8.4%), incorrect dose frequency (5.8%), incorrect medication (2.9%), and incorrect route of administration (2.6%). The “other” category mostly included discontinuation of medications no longer taken by the patient. Although the amount of medications reduced by the pharmacist was not statistically significant, the sample size was small, this research shows that further decreases in pediatric polypharmacy may have more value if reproduced on a larger scale.
Pharmacists have a unique opportunity to improve health care for pediatric patients by participating in the detection of polypharmacy through clinical pharmacy practice (making interventions, completing medication histories, discharge counseling) in the inpatient setting, and activities like medication reconciliation in the outpatient setting. A Joint Safety Commission goal for accreditation is for hospital systems to implement a process ensuring that there is an accurate medication list for the patient prior to starting a new medication.73 Many hospitals are using clinical pharmacists to implement the medication reconciliation process for their health systems and have evaluated the effectiveness of each process. Along with this goal for hospital accreditation, an integrated approach to monitoring drug therapy may help to decrease polypharmacy in pediatric patients. Physicians, parents/guardians, pharmacists, and other health care professionals should make a better effort to work cohesively by providing clear instructions, patient counseling, and appropriate follow-up. Clinical pharmacists who work with pediatric patients in the inpatient setting can participate in discharge counseling, which will decrease confusion for parents/guardians on drug administration, dose, and frequency, as well as the detection of side effects at home. In addition, pharmacists can assess drug–drug interactions and make valuable recommendations for alternative therapy should problems arise, prevent medication errors, and monitor patients for adverse events.
A good place for pharmacists to start documenting interventions regarding polypharmacy would be in pediatric patients who are prescribed psychotropic medications. The evidence is overwhelming with regard to use of multiple psychotropic medications in this patient population, and yet we were unable to find any evidence of an intervention or possible solution to the problem. If a single counseling session can improve caregiver understanding and possibly promote adherence and communication in the case of epilepsy, could the same strategy work for patients with psychiatric disorders? As the rates of chronic disease states continue to rise in pediatric patients, pharmacist should be on the front line monitoring multiple medication use within and between disease states by offering services such as medication therapy management and pharmacy-managed disease state clinics. The pediatric patient relies heavily on adults for health care, and the pharmacist should be part of the action plan to promote patient safety. Polypharmacy for pediatric patients is an area that is ripe for research, and pharmacists have a unique skill set with regard to solving this problem.
Conclusion
Prescribers and pharmacists should be aware of the potential drug interactions in pediatric patients taking multiple medications because of the lack of data on these effects. Ultimately, the goal is to implement the best evidence-based treatment for the patient and resolution of symptoms, and this should guide prescribing patterns. The rates of polypharmacy for many chronic conditions in pediatric patients have not been examined, making this area ripe for research. Here we have focused on general polypharmacy in pediatric patients in the hope that pharmacists can find opportunities to improve the care of pediatric patients and document their findings. A better understanding of which chronic illness are of concern, which medications are commonly prescribed in error, and present rates of polypharmacy will help to lay the foundation for interventions that will heighten patient safety. The uncertainty around adverse effects and the long-term effects of multiple medications makes pediatric polypharmacy an area that also needs development and research to promote safe and effective therapy for children and adolescents.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cox R, Halloran DR, Homan SM, Welliver S, Mager DE. Trends in the prevalence of chronic medication use in children: 2002–2005. Pediatrics. 2008;122:e1053–e1061. doi: 10.1542/peds.2008-0214. [DOI] [PubMed] [Google Scholar]
- 2.Perrin JM, Anderson E, Van Cleave J. The rise in chronic conditions among infants, children, and youth can be met with continuous health system innovations. Health Affairs. 2014;33:2009–2105. doi: 10.1377/hlthaff.2014.0832. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104:585–590. [PubMed] [Google Scholar]
- 4.Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290:905–911. doi: 10.1001/jama.290.7.905. [DOI] [PubMed] [Google Scholar]
- 5. FDA.gov Food and Drug Administration Modernization Act of 1997. [Accessed March 8, 2015]. Available from: http://www.fda.gov/regulatoryinformation/legislation/federalfooddrugandcosmeticactfdcact/significantamendmentstothefdcact/fdama/default.htm.
- 6. FDA.gov Best Pharmaceuticals for Children Act. Jan 4, 2002. [Accessed March 8, 2015]. Available from: http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/ucm049876.htm.
- 7. FDA.gov Food and Drug Administration Amendments Act of 2007. [Accessed March 8, 2015]. Available at: http://www.fda.gov/regulatoryinformation/legislation/federalfooddrugandcosmeticactfdcact/significantamendmentstothefdcact/foodanddrugadministrationamendmentsactof2007/default.htm.
- 8. Merriam-Webster.com [Accessed March 1, 2015]. Available from: http://www.merriam-webster.com/dictionary/polypharmacy.
- 9.Vernacchio L, Kely J, Kaufman DW, Mitchell AA. Medication use among children <12 years of age in the United States: results from the Slone Survey. Pediatrics. 2008;124:446–454. doi: 10.1542/peds.2008-2869. [DOI] [PubMed] [Google Scholar]
- 10.Kunac DL, Kennedy J, Austin N, Reith D. Incidence, preventability, and impact of adverse drug events (ADEs) and potential ADEs in hospitalized children in New Zealand: a prospective observational cohort study. Paediatr Drugs. 2009;11:153–160. doi: 10.2165/00148581-200911020-00005. [DOI] [PubMed] [Google Scholar]
- 11.Thomson F, Masters IB, Change AB. Persistent cough in children and overuse of medications. J Paediatr Child Health. 2002;38:578–581. doi: 10.1046/j.1440-1754.2002.00045.x. [DOI] [PubMed] [Google Scholar]
- 12.Butz AM, Tsoukleris M, Donithan M, et al. Patterns of inhaled anti-inflammatory medication use in young underserved children with asthma. Pediatrics. 2006;118:2504–2513. doi: 10.1542/peds.2006-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumock GT, Meed PD, Ploetz PA, Vermeulen LC. Economic evaluations of clinical pharmacy services: 1988–1995. Pharmacotherapy. 1996;16:1188–1208. [PubMed] [Google Scholar]
- 14.Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59:2070–2077. doi: 10.1093/ajhp/59.21.2070. [DOI] [PubMed] [Google Scholar]
- 15.Kopp BJ, Mrsan M, Erstad BL, Duby JJ. Cost implications of and potential adverse events prevented by interventions of critical care pharmacists. Am J Health Syst Pharm. 2007;64:2483–2487. doi: 10.2146/ajhp060674. [DOI] [PubMed] [Google Scholar]
- 16.Rijdt T, Willems L, Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm. 2008;65:1161–1172. doi: 10.2146/ajhp070506. [DOI] [PubMed] [Google Scholar]
- 17.Lake JK, Weiss JA, Dergal J, Lunsky Y. Child, parent, and service predictors of psychotropic polypharmacy among adolescents and young adults with an autism spectrum disorder. J Child Adolesc Psychopharmacol. 2014;24:486–493. doi: 10.1089/cap.2014.0011. [DOI] [PubMed] [Google Scholar]
- 18.Schubart JR, Camacho F, Leslie D. Psychotropic medication trends among children and adolescents with autism spectrum disorder in the Medicaid program. Autism. 2013;18:631–637. doi: 10.1177/1362361313497537. [DOI] [PubMed] [Google Scholar]
- 19.Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132:833–840. doi: 10.1542/peds.2012-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coury DL, Anagnostou E, Manning-Courtney P, et al. Use of psychotropic medication in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130:s69–s78. doi: 10.1542/peds.2012-0900D. [DOI] [PubMed] [Google Scholar]
- 21.Logan SL, Nicholas JS, Carpenter LA, et al. High prescription drug utilization and associated costs among Medicaid-eligible children with autism spectrum disorders identified by a population-based surveillance network. Ann Epidemiol. 2012;22:1–8. doi: 10.1016/j.annepidem.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frazier TW, Shattuck PT, Narendorf SC, Cooper BP, Wagner M, Spitznagel EL. Prevalence and correlates of psychotropic medication use in adolescents with autism spectrum disorder with and without caregiver-reported attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21:571–579. doi: 10.1089/cap.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg RE, Mandell DS, Farmer JE, Law JK, Marvin AR, Law PA. Psychotropic medication use among children with autism spectrum disorders enrolled in a national registry, 2007–2008. J Child Adolesc Psychopharmacol. 2010;40:342–351. doi: 10.1007/s10803-009-0878-1. [DOI] [PubMed] [Google Scholar]
- 24.Mandell DS, Morales KH, Macrus SC, Stahmer AC, Doshi J, Polsky DE. Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics. 2008;121:e441–e488. doi: 10.1542/peds.2007-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oswald DP, Soneklar NA. Medication use among children with autism-spectrum disorders. J Child Adolesc Psychopharmacol. 2007;17:348–355. doi: 10.1089/cap.2006.17303. [DOI] [PubMed] [Google Scholar]
- 26. FDA.gov Risperdal (prescribing information] [Accessed March 1, 2015]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020272s056,020588s044,021346s033,021444s03lbl.pdf.
- 27. FDA.gov New pediatric labeling information database. [Accessed March 1, 2015]. Available from: http://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase.
- 28.Zito JM, Derivan AT, Kratochvil CJ, Safer DJ, Fegert JM, Greenhill LL. Off-label psychopharmacologic prescribing for children: history supports close clinical monitoring. Child Adolesc Psychiatry Ment Health. 2008;2:1–11. doi: 10.1186/1753-2000-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lexicomp Online, Pediatric and Neonatal Lexi-Drugs, Hudson, OH. USA: Lexi-Comp Inc.; [Accessed February 15, 2015]. Available from http://online.lexi.com/crlsql/servlet/crlonline. [Google Scholar]
- 30.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition. Arlington, VA, USA: American Psychiatric Publishing; 2013. [Google Scholar]
- 31.Kreider AR, Matone M, Bellonci C, et al. Growth in the concurrent use of antipsychotics with other psychotropic medications in Medicaid-enrolled children. J Am Acad Child Adolesc Psychiatry. 2014;53:960–970. doi: 10.1016/j.jaac.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Fontanella CA, Warner LA, Phillips GS, et al. Trends in psychotropic polypharmacy among youth enrolled in Ohio Medicaid, 2002–2008. Psychiatr Serv. 2014;65:1332–1340. doi: 10.1176/appi.ps.201300410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. AACAP.org American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medications in children and adolescents. [Accessed March 2, 2015]. Available from: http://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf.
- 34.Toteja N, Gallego JA, Saito E, et al. Prevalence and correlates of antipsychotic polypharmacy in children and adolescents receiving antipsychotic treatment. Int J Neuropsychopharmacol. 2014;17:1095–1105. doi: 10.1017/S1461145712001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jureidini J, Tonkin A, Jureidini E. Combination pharmacotherapy for psychiatric disorders in children and adolescents: prevalence, efficacy, risks and research needs. Pediatr Drugs. 2013;15:377–391. doi: 10.1007/s40272-013-0032-6. [DOI] [PubMed] [Google Scholar]
- 36.Brenner SL, Southerland DG, Burns BJ, et al. Use of psychiatric medications among youth in treatment foster care. J Child Fam Stud. 2014;23:666–674. doi: 10.1007/s10826-013-9882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 38.Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25:383–399. doi: 10.2165/11587810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Chan J, Sweeting M. Combination therapy with nonclozapine atypical antipsychotic medications: a review of current evidence. J Psychopharmacol. 2007;21:657–664. doi: 10.1177/0269881106071334. [DOI] [PubMed] [Google Scholar]
- 40.DeQuardo JR. Worsened agitation with aripiprazole: adverse effect of dopamine partial agonism? J Clin Psychiatry. 2004;61:132–133. doi: 10.4088/jcp.v65n0122b. [DOI] [PubMed] [Google Scholar]
- 41.Ramaswamy S, Vijay D, William M, Sattar SP, Praveen F, Petty F. Aripiprazole possibly worsens psychosis. Int Clin Psychopharmacol. 2004;19:45–48. doi: 10.1097/00004850-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Reeves RR, Mack JE. Worsening schizoaffective disorder with aripiprazole. Am J Psychiatry. 2004;161:1308. doi: 10.1176/appi.ajp.161.7.1308. [DOI] [PubMed] [Google Scholar]
- 43.Bhatara V, Feil M, Hoagwood K, et al. National trends in concomitant psychotropic medication with stimulants in pediatric visits: practice vs knowledge. J Atten Disord. 2004;7:217–226. doi: 10.1177/108705470400700404. [DOI] [PubMed] [Google Scholar]
- 44.Constantine RJ, Boaz T, Tandon R. Antipsychotic polypharmacy in treatment of children and adolescents in a fee-for-service component of a state Medicaid program. Clin Ther. 2010;32:949–959. doi: 10.1016/j.clinthera.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Patel A, Sherer J, et al. The definition and prevalence of pediatric psychotropic polypharmacy. Psychiatr Serv. 2011;62:1450–1455. doi: 10.1176/appi.ps.000642011. [DOI] [PubMed] [Google Scholar]
- 46. FDA.gov Food and Drug Administration Amendments Act of 2007. [Accessed March 8, 2015]. Available at: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/ucm148011.htm.
- 47.Joshua L, Devi P, Guido S. Adverse drug reactions in medical intensive care unit of a tertiary care hospital. Pharmacoepidemiol Drug Saf. 2009;18:639–645. doi: 10.1002/pds.1761. [DOI] [PubMed] [Google Scholar]
- 48.Committee on Drugs, American Academy of Pediatrics Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pedatrics. 1995;95:286–294. [PubMed] [Google Scholar]
- 49.Lasky T, Ernst F, Greenspan J, Wang S, Gonzalez L. Estimating pediatric inpatient medication use in the United States. Pharmacoepidemiol Drug Saf. 2011;20:76–82. doi: 10.1002/pds.2063. [DOI] [PubMed] [Google Scholar]
- 50.Feudtner C, Dai D, Hexem K, Luan X, Metjian TA. Prevalence of polypharmacy exposure among hospitalized children in the United States. Arch Pediatr Adolesc Med. 2012;166:9–16. doi: 10.1001/archpediatrics.2011.161. [DOI] [PubMed] [Google Scholar]
- 51.Rizkalla N, Feudtner C, Dai D, et al. Patterns of medication exposures in hospitalized pediatric patients with acute kidney injury requiring renal replacement therapy. Crit Care Med. 2012;40:1–328. doi: 10.1097/PCC.0b013e31829f5bc8. [DOI] [PubMed] [Google Scholar]
- 52.Feinstein JA, Feudtner C, Kempe A. Adverse drug event-related emergency department visits associated with complex chronic conditions. Pediatrics. 2014;133:1575–1585. doi: 10.1542/peds.2013-3060. [DOI] [PubMed] [Google Scholar]
- 53.Stone BL, Boehme S, Mundorff MB, et al. Hospital admission medication reconciliation in medically complex children: an observation study. Arch Dis Child. 2010;95:250–255. doi: 10.1136/adc.2009.167528. [DOI] [PubMed] [Google Scholar]
- 54.Rappaport D, Collins B, Koster A, et al. Implementing medication reconciliation in outpatient pediatrics. Pediatrics. 2011;128:e1600–e1607. doi: 10.1542/peds.2011-0993. [DOI] [PubMed] [Google Scholar]
- 55.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US Children, 2002–2010. Pediatrics. 2012;130:23–31. doi: 10.1542/peds.2011-2879. [DOI] [PubMed] [Google Scholar]
- 56.National Heart, Lung, and Blood Institute. Guidelines for the diagnosis and management of Asthma (EPR-3) US Department of Health and Human Services National Institutes of Health. Oct, 2007. [Accessed June 21, 2015]. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/summary-report-2007.
- 57.Munzenberger P, Emmanuel S, Heins M. The role of a pharmacist on the pediatric unit of a general hospital. Am J Hosp Pharm. 1972;29:755–760. [PubMed] [Google Scholar]
- 58.Koren G, Reich A, Hales B. Use of clinical pharmacists to prevent medication errors in children. J Pharm Technol. 1991;7:219–221. doi: 10.1177/875512259100700607. [DOI] [PubMed] [Google Scholar]
- 59.Falck KA, Darsey EH, Naughton MJ. Pharmacy interventions in multidisciplinary pediatric intensive care unit. Crit Care Med. 1997;2:162–167. [Google Scholar]
- 60.Krupicka MI, Bratton SL, Sonnenthal K, et al. Impact of a pediatric clinical pharmacist in the pediatric intensive care unit. Crit Care Med. 2002;30:919–921. doi: 10.1097/00003246-200204000-00035. [DOI] [PubMed] [Google Scholar]
- 61.Condren ME, Haase MR, Luedtke SA, Gaylor AS. Clinical activities of an academic pediatric pharmacy team. Ann Pharmacother. 2004;38:574–578. doi: 10.1345/aph.1D384. [DOI] [PubMed] [Google Scholar]
- 62.Cunningham KJ. Analysis of clinical interventions and the impact of pediatric pharmacists on medication error prevention in a teaching hospital. J Pediatr Pharmacol Ther. 2012;17:365–373. doi: 10.5863/1551-6776-17.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Virani A, Crown N. The impact of a clinical pharmacist on patient and economic outcomes in a child and adolescent health unit. Can J Hosp Pharm. 2003;56:158–162. [Google Scholar]
- 64.Zhang C, Zhang L, Haung L, et al. Clinical pharmacist on medical care of pediatric inpatients: a single-center randomized control trial. PLoS One. 2012;7:e30856. doi: 10.1371/journal.pone.0030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alagha H, Badary O, Ibrahim H, Sabri NA. Reducing prescribing errors in the pediatric intensive care unit: an experience from Egypt. Acta Paediatr. 2011;100:e169–e174. doi: 10.1111/j.1651-2227.2011.02270.x. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Llamazares C, Calleja-Hernandez MA, Manrique-Rodriguez S, Pérez-Sanz C, Duran-García E, Sanjurjo-Saez M. Impact of clinical pharmacist interventions in reducing pediatric prescribing errors. Arch Dis Child. 2012;97:564–568. doi: 10.1136/archdischild-2011-301239. [DOI] [PubMed] [Google Scholar]
- 67.Wang JK, Herzog NS, Kaulshal R, Park C, Mochizuki C, Weingarten SR. Prevention of pediatric medication errors by hospital pharmacists and the potential benefit of computerized physician order entry. Pediatrics. 2007;1:e77–e85. doi: 10.1542/peds.2006-0034. [DOI] [PubMed] [Google Scholar]
- 68.Taylor TL, Dupuis LL, Nicksy D, et al. Clinical pharmacy services in a pediatric hematology/oncology clinic: a description and assessment. Can J Hosp Pharm. 1999;52:18–23. [Google Scholar]
- 69.Condren M, Boger J. Impact of a pediatric clinic-based multidisciplinary asthma education and management program. J Pediatr Pharmacol Ther. 2005;10:254–258. doi: 10.5863/1551-6776-10.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen C, Lee D, Hie S. The impact of pharmacist’s counseling on pediatric patients’ caregiver’s knowledge on epilepsy and its treatment in a tertiary hospital. Int J Clin Pharm. 2013;35:829–834. doi: 10.1007/s11096-013-9817-5. [DOI] [PubMed] [Google Scholar]
- 71.Costello I, Wong I, Nunn A. A literature review to identify interventions to improve the use of medicines in children. Child Care Health Dev. 2004;30:647–665. doi: 10.1111/j.1365-2214.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 72.Provine AD, Simmons EM, Bhagat PH. Establishment and evaluation of pharmacist-managed admission medication history and reconciliation process for pediatric patients. J Pediatr Pharmacol Ther. 2014;19:98–102. doi: 10.5863/1551-6776-19.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. JointCommission.org Hospital: 2015 National Patient Safety Goals. [Accessed March 1, 2015]. Available from: http://www.jointcommission.org/hap_2015_npsgs/