Abstract
Background
Vancomycin is the antibiotic of choice for the treatment of serious infections such as methicillin-resistant Staphylococcus aureus (MRSA). Inappropriate prescribing of vancomycin can lead to therapeutic failure, antibiotic resistance, and drug toxicity.
Objective
To examine the effectiveness of pharmacist-led implementation of a clinical practice guideline for vancomycin dosing and monitoring in a teaching hospital.
Methods
An observational pre–post study design was undertaken to evaluate the implementation of the vancomycin guideline. The implementation strategy principally involved education, clinical vignettes, and provision of pocket guidelines to accompany release of the guideline to the hospital Intranet. The target cohort for clinical behavioral change was junior medical officers, as they perform the majority of prescribing and monitoring of vancomycin in hospitals. Assessment measures were recorded for vancomycin prescribing, therapeutic drug monitoring, and patient outcomes.
Results
Ninety-nine patients, 53 pre- and 46 post-implementation, were included in the study. Prescribing of a loading dose increased from 9% to 28% (P=0.02), and guideline adherence to starting maintenance dosing increased from 53% to 63% (P=0.32). Dose adjustment by doctors when blood concentrations were outside target increased from 53% to 71% (P=0.12), and correct timing of initial concentration measurement increased from 43% to 57% (P=0.23). Appropriately timed trough concentrations improved from 73% to 81% (P=0.08). Pre-dose (trough) concentrations in target range rose from 33% to 44% (P=0.10), while potentially toxic concentrations decreased from 32% to 21% (P=0.05) post-implementation. Infection cure rates for patients increased from 85% to 96% (P=0.11) after the guideline was implemented.
Conclusion
The implementation strategy employed in this study demonstrated potential effectiveness, and should prompt additional larger studies to optimize strategies that will translate into improved clinical practice using vancomycin.
Keywords: antibiotics, Australia, behavioral medicine, clinical guidelines, implementation, intervention, pharmacists
Introduction
Vancomycin, after nearly 60 years of use, is still the intravenous antibiotic of choice for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infection.1–3 Inappropriate prescribing of vancomycin is associated with therapeutic failure, antibiotic resistance, and kidney toxicity.4 Therapeutic drug monitoring in patients receiving vancomycin has been shown to significantly increase clinical efficacy and to decrease the rate of kidney toxicity.5 A small number of newer antibiotics for the treatment of MRSA infection have been licensed by the US Food and Drug Administration (FDA) in recent years; however, it is critical to reserve these agents for when vancomycin fails.6 Development of clinical practice guidelines (CPGs) has been identified as a way to improve the utilization of vancomycin.7 Education and dissemination of CPGs on vancomycin prescribing and monitoring are measures to ensure best standard of care for patients receiving this antibiotic.8 A previous pilot study where a pharmacist implemented vancomycin dosing and monitoring guideline in a single surgical unit in our institution, Flinders Medical Centre, Bedford Park, South Australia, produced favorable and statistically significant results.9 It was unclear if a similar implementation strategy targeting physicians working in all medical and surgical units across our institution would produce similar results to the pilot. The aim of the current study was to examine the impact of implementation of a CPG for vancomycin dosing and monitoring across all medical and surgical units in our institution.
Methods
Study design and procedure
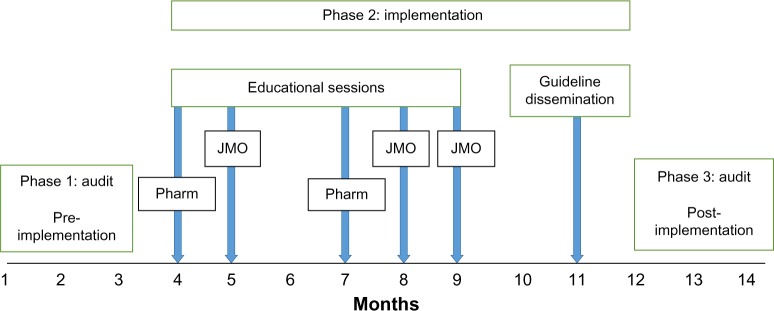
The present study was approved by the Southern Adelaide Clinical Human Research Ethics Committee (approval 12312/51711). The ethics application for this study contained a waiver of consent as participants were not going to be exposed to an increase risk of harm. The waiver of consent was consistent with the National Statement of Ethical Conduct in Human Research. The study was an observational pre–post design undertaken at Flinders Medical Centre, a teaching hospital with a wide variety of medical and surgical specialties, located in metropolitan South Australia. The study was comprised of three phases. Phase 1 was a retrospective audit of medical records of patients receiving vancomycin therapy over a 3-month consecutive period (pre-implementation). Phase 2 was an education program delivered to junior medical officers (JMOs) and registered pharmacists, dissemination of a pocket version of the guideline to these two groups, and release of the CPG to the hospital Intranet.
Pharmacists received education due to their supportive role to improve antibiotic use at organizational and practice level, which has been well documented.10 Release of the CPG was accompanied by a formal email sent from the hospital Trainee Medical Officer Unit to all JMOs advising them of the new guideline and requesting their adherence to it. Phase 3 was a subsequent audit of medical records of patients receiving vancomycin over a 3-month period of the following year (post-implementation) depicted in Figure 1. The same months were selected for audit pre- and post-implementation to avoid seasonal variance in the use of vancomycin. Resource allocation for implementation in this study was principally funded through partial salary support.
Figure 1.
Temporal schematic of audits and implementation of vancomycin clinical practice guideline.
Abbreviations: JMO, junior medical officers; Pharm, registered pharmacist.
Participants
JMOs
Medical officers (year 1 post-completion of medical school) registered with the Trainee Medical Officer Unit in our institution were included in the current study. JMOs were the chosen target cohort to measure behavioral change in their clinical practice, as they are the medical staff principally involved in prescribing, ordering of pathology tests, and interpreting test results to inform subsequent prescribing and monitoring of the intravenous antibiotic vancomycin.
Patients
Patients ≥18 years of age receiving vancomycin therapy were identified from vancomycin blood concentrations recorded in the daily hospital therapeutic drug monitoring report. Patients were eligible for inclusion if they had at least one measurable vancomycin concentration and had received more than one dose of vancomycin.
Intervention
CPG
The vancomycin dosing and monitoring guideline for adults that was implemented in this project was a modified version of the guideline used in a prior pilot study at our institution.9 Modifications to the guideline were: 1) an improved decision support table to assist prescribers to adjust the dose in response to results of vancomycin blood concentrations and kidney function; and 2) inclusion of an embedded hyperlink to a hospital Intranet-based creatinine clearance calculator (glomerular filtration rate [GFR] + calculator; Southern Adelaide Health Service, Adelaide, SA, Australia) to aid doctors to choose an appropriate individualized dose. Also included were general explanatory notes on how to use the guideline. The amended guideline underwent beta-testing by eight JMOs and two final-year medical students from Flinders University School of Medicine, co-located in our hospital, to ensure the guideline was “fit for purpose”. All JMOs and pharmacists were sent an email advising them of the release of the guideline and how to access it via the Intranet.
Key features of guideline
Prescribing was as follows: a loading dose of 25 mg/kg actual body weight (maximum 2 g vancomycin). Maintenance dosing was determined by creatinine clearance (CrCl): >90 mL/min, 1.5 g vancomycin 12 hourly; CrCl 60–90 mL/min, 1 g vancomycin 12 hourly; CrCl 20–59 mL/min, 1 g vancomycin 24 hourly; CrCl <20 mL/min, 1 g vancomycin every 2–7 days.
Therapeutic drug monitoring was as follows: The time initial blood concentration was to be measured was determined by CrCl; CrCl >60 mL/min required bleeding the patient before the fourth dose; CrCl 20–59 mL/min required bleeding the patient before the third dose; CrCl <20 mL/min required bleeding the patient at 48 hours post-dose. Subsequent monitoring was stipulated every 48 hours until stable blood concentration was achieved (target, 15–20 mg/L); thereafter, patients were to be bled twice weekly. Pre-dose (trough) blood concentrations were to be taken approximately 1 hour pre-dose.
Implementation process
Education
Three 60-minute face-to-face education sessions on vancomycin prescribing and monitoring were provided to JMOs. Pharmacists received two educational sessions. Attendance at all education sessions was voluntary, with no incentives offered. The tutorial contained content on contemporary vancomycin treatment covering issues of antibiotic resistance – specifically how subtherapeutic dosing can promote bacterial resistance to vancomycin, the need for appropriate dosing, monitoring, issues of vancomycin kidney toxicity, and practical advice using clinical vignettes on how to determine an appropriate dosage regimen, how to monitor, interpret blood concentration results, and how to use this information to amend subsequent dosing. Education was delivered by the principal investigator, who has expertise in clinical education, antibiotics, and therapeutic drug monitoring. Fidelity of the education sessions was ensured by using the same content, and clinical vignettes were conducted over the same duration for all sessions.
Provision of printed material
A pocket laminated version of the guideline (10 cm ×6 cm), suitable for attachment to hospital identification badges, was provided to all JMOs and pharmacists.
Email alert
All JMOs and pharmacists were sent an email advising them of the existence of the guideline and how to access it via the Intranet.
Assessment measures
Patient characteristics
Medical records were used to extract details of patients; residence, comorbidities, and colonization with multi-resistant bacterial organisms. Indication for vancomycin, dosage, time of dose, and duration were recorded. Concomitant aminoglycoside antibiotic use was also recoded, as was the treating team (medical or surgical), length of stay in hospital, whether surgery was required to help resolve the infection, in addition to data on cure and readmission to hospital for the same infection. Laboratory data collected included serum creatinine (to determine kidney function), vancomycin blood concentrations, and microbiological data of organism and source of isolate. Vancomycin minimum inhibitory concentration (MIC) was determined using a Vitek® 2 compact (bioMerieux Inc., Durham, NC, USA) on MRSA isolates (when performed by a laboratory).
Clinical behavior of medical officers
Prescribing of vancomycin and ordering of pathology blood tests for vancomycin concentrations was assessed by audit of drug charts and medical records. Measurement was conducted on the proportion of patients prescribed: 1) a loading dose, 2) maintenance doses adherent to the guideline, 3) appropriate dose adjustment (ie, increase or decrease) by prescriber in response to blood concentrations of vancomycin outside target range, 4) measurement of initial vancomycin blood concentration adherent with the guideline (ie, after the appropriate number of doses), and 5) appropriately timed drawing of blood for vancomycin pre-dose (trough) blood concentration in relation to the time of last dose.
Patient outcomes
Electronic hospital pathology database and medical records were used to measure: 1) the number of days until patients attained a measured vancomycin blood concentration in target range, 2) the proportion of vancomycin concentrations patients attained within target range, 3) infection cure rates, and 4) the frequency of kidney toxicity.
Educational attendance and guideline measurement
The number of JMOs and pharmacists that attended educational sessions on vancomycin dosing and monitoring was recorded, as was the frequency of downloads of the guideline from the hospital Intranet.
Definitions
Guideline target range for vancomycin blood concentrations was 15–20 mg/L.11,12 Kidney toxicity was defined as a rise in serum CrCl of ≥50% or 0.5 mg/dL on 2 or more consecutive days of vancomycin therapy from baseline.13,14 Clinical cure was resolution of all clinical and laboratory signs and symptoms of infection.15
Statistical analysis
Data were stored in Microsoft Excel, and descriptive statistics were used to report results. The IBM Statistical Package for the Social Sciences (SPSS) version 22 was used to perform statistical testing. The Student’s t-test was performed to compare continuous variables, and the chi-square test was used to compare categorical variables to measure difference between the two groups. Observed difference were considered statistically significant when P<0.05.16
Results
Patients
There were 99 patients included in this study, with 53 pre- and 46 post-implementation. The median patient age was 75 years vs 63 years, respectively, and median weight was 78 kg vs 77 kg in the pre- and post-implementation groups, respectively. Patient characteristics are presented in Table 1, while indication for treatment and microbiological data are presented in Table 2.
Table 1.
Baseline characteristics of patients receiving vancomycin treatment
| Pre-implementation n=53 n, (%)* |
Post-implementation n=45 n, (%)* |
|
|---|---|---|
| Characteristic | ||
| Age years; median (IQR) | 75 (59–82) | 63 (46–75.5) |
| Male sex | 32 (60.32) | 31 (67.39) |
| Residence in RACF | 20 (37.73) | 10 (67.39) |
| Prior admission to hospital ≤12 months | 39 (73.58) | 31 (67.39) |
| Prior colonisation with MRO | ||
| In ≤12 months | ||
| MRSA | 20 (37.74) | 13 (28.26) |
| VRE | 11 (20.75) | 8 (17.39) |
| CrCL; median (IQR) (mL/min) | 77.28 (47.07–109.68) | 103.4 (70.63–129.8) |
| Comorbidities | ||
| Diabetes | 18 (33.96) | 10 (21.74) |
| Congestive heart failure | 6 (11.30) | 6 (13.04) |
| Ischemic heart disease | 10 (18.87) | 8 (17.39) |
| Valvular disease | 5 (9.43) | 5 (10.87) |
| Malignancy | 6 (11.32) | 12 (26.09) |
| Medication | ||
| Concomitant aminoglycoside | 8 (15.09) | 15 (32.61) |
| Penicillin/beta-lactam allergy | 20 (37.74) | 10 (21.74) |
| Treating team | ||
| Medical | 21 (39.62) | 25 (54.35) |
| Surgical | 32 (60.38) | 21 (45.65) |
Note:
Unless otherwise stated.
Abbreviations: IQR, interquartile range; RACF, residential aged care facility; MRO, multi-resistant organism; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; CrCl, creatine clearance; min, minute.
Table 2.
Infection site requiring vancomycin treatment and microbiological data
| Pre-implementation n=53 n, (%)* |
Post-implementation n=45 n, (%)* |
|
|---|---|---|
| Infection site | ||
| Bacteremia | 15 (11.32) | 16 (34.72) |
| Synovial/orthopedic | 3 (5.66) | 2 (4.34) |
| CNS/cranial | 5 (9.43) | 1 (2.17) |
| Skin and soft tissue infection | 17 (32.08) | 14 (30.43) |
| Osteomyelitis | 5 (9.43) | 6 (13.04) |
| Urinary | 1 (1.89) | 4 (8.70) |
| Respiratory | 6 (11.32) | 7 (15.21) |
| ENT | 1 (1.89) | 0 |
| GI/abdominal infection | 7 (13.2) | 2 (4.35) |
| Pyrexia of unknown origin | 6 (11.32) | 3 (6.52) |
| Bacterial organism# | ||
| MRSA | 18/56 (32.14) | 13/49 (26.53) |
| MIC (mg/L) performed | 11/18 (61) | 11/13 (85) |
| ≤0.5 | 4/11 (36) | 4/11 (36) |
| 1.0 | 5/11 (45) | 7/11 (63) |
| 2.0 | 2/11 (18) | 0 |
| Enterococcus spp | 6/56 (10.71) | 8/49 (16.33) |
| CoNS | 4/56 (7.14) | 5/49 (10.20) |
| Staphylococcus epidermis | 4/56 (7.14) | 5/49 (10.20) |
| MSSA | 4/56 (7.14) | 3/49 (6.12) |
| Streptococcus spp | 5/56 (8.92) | 0 |
| Other | 13/56 (23.2) | 8/49 (16.33) |
| No growth detected | 13/56 (23.2) | 8/49 (16.33) |
Notes:
Unless otherwise stated;
note, some patients had infection with more than one organism. Not all MRSA isolates had MIC performed.
Abbreviations: GI, gastrointestinal; Spp, bacterial species; MRSA, methicillin-resistant Staphylococcus aureus; CoNS, coagulate negative S. aureus; MSSA, methicillin-sensitive S. aureus; MIC, minimum inhibitory concentration; CNS, central nervous system; ENT, ear nose and throat.
Clinical behavior of medical officers
Prescribing of vancomycin loading doses increased significantly from five (9.43%) to 13 (28.27%) doses (P=0.02). The proportion of maintenance doses prescribed that were adherent to the guideline increased without significance from 52.83% to 63.04% (P=0.32). The frequency of prescribers amending (increasing or decreasing) a dose when a vancomycin blood concentration was either low or high, increased non-significantly from 53.85% pre-implementation to 70.59% post-implementation (P=0.12). The appropriate timing when the initial vancomycin blood concentration was measured (after the correct number of doses based on kidney function) increased from 43.40% to 56.52% post-implementation (P=0.23). Appropriately measured pre-dose (trough) vancomycin blood concentrations in relation to the time the previous dose was administered improved non-significantly from 72.57% to 80.57% (P=0.08) (Table 3).
Table 3.
Clinical behavior of medical officers and patient outcomes
| Pre-implementation n=53 n, (%)* |
Post-implementation n=45 n, (%)* |
P-values | |
|---|---|---|---|
| Clinical behaviour | |||
| Prescribing a loading dose | 5 (9.43) | 13 (28.27) | 0.02 |
| Adherent maintenance dose | 28 (52.83) | 29 (63.04) | 0.32 |
| Dosage adjusted correctly | 21/39 (53.85) | 24/34 (70.59) | 0.12 |
| Adherent timing of initial conc | 23 (43.40) | 26 (56.52) | 0.23 |
| Adherent pre-dose conc | 164 (72.57) | 26 (80.57) | 0.08 |
| Patient outcomes | |||
| Days of admission; median (IQR) | 20 (10.5–32.5) | 16 (9–29.5) | 0.13 |
| Days of vanco treatment; median (IQR) | 10 (4.25–13.75) | 6 (4–16.5) | 0.31 |
| Days until first conc in target; median (IQR) | 5 (4.25–13.75) | 4 (3.5–5.5) | 0.12 |
| Conc in target range | 54 (32.93) | 60 (42.55) | 0.10 |
| Potentially kidney toxic conc | 52 (31.37) | 30 (21.28) | 0.05 |
| Sterile site cure | 27/30 (90) | 19/19 (100) | 0.27 |
| Non-sterile site cure | 22/28 (78.57) | 27/29 (93.10) | 0.14 |
| All cure of infection | 49/58 (84.48) | 46/48 (95.83) | 0.11 |
| Kidney toxicity | 6 (11.32) | 5 (10.87) | 1.0 |
Note:
Unless otherwise stated.
Abbreviations: Conc, blood vancomycin concentration; Vanco, vancomycin; IQR, interquartile range.
Patient outcomes
The median time for patients to attain an in-target vancomycin trough concentration in their blood decreased from 5 (interquartile range [IQR] 4.25–13.75) to 4 (IQR, 3–5.5) days (P=0.12) post-implementation. The proportion of vancomycin blood concentrations in our CPG target range (15–20 mg/L) increased non-significantly from 32.93% to 42.55% (P=0.10), while the proportion of concentrations in the lower shoulder range (10–14.9 mg/L) remained unchanged at 20.73% pre-implementation and 20.57% post-implementation. Potentially kidney toxic concentrations (>20 mg/L) decreased from 52 (31.37%) to 30 (21.28%) post-implementation (P=0.05). The incidence of nephrotoxicity observed did not change from 11.32% pre- and 10.87% post-implementation.
Sub-analysis found eight of the eleven (72.73%) participants (four of six pre-and four of five post-implementation) with nephrotoxicity had one or more potentially toxic vancomycin concentrations >20 mg/L. In contrast, in the 87 participants without nephrotoxicity, 37 of 87 (42.53%) (14 pre- and 23 post-implementation) had one or more vancomycin concentrations >20 mg/L (P=0.31).
All cures of infection for which vancomycin was prescribed increased from 84.48% pre-implementation to 95.83% post-implementation (P=0.11) (Table 3). Sub-analysis of the three of eleven (27.27%) infections that failed to respond to therapy (two pre- and one post-implementation) involved patients that had one or more sub-therapeutic vancomycin concentrations <10 mg/L. No association was observed between clinical failure and van-comycin concentrations <10 mg/L (P=0.33).
Educational attendance and guideline process measures
Fifty-one of 75 (68%) JMOs registered with the hospital Trainee Medical Officer Unit had documented attendance at voluntary educational sessions provided on vancomycin dosing and monitoring. Thirty-five of 47 (74%) pharmacists from the study site attended an education session. From uploading the guideline to the hospital Intranet until the close of the study, the guideline had a monthly download mean of 86.5 (standard deviation [SD] 21.06).
Discussion
The implementation of a vancomycin CPG across medical and surgical units was associated with a statistically significant increase in the number of patients being prescribed loading doses. This result is meaningful, as a recent systematic review found that doctors prescribing loading doses of vancomycin enabled their patients to more rapidly attain blood target levels of vancomycin known to kill bacteria.17 There was a non-significant trend to an increase in adherent measurement of pre-dose (trough) blood concentrations. This result is also meaningful, as it has been previously reported that vancomycin concentrations collected at inappropriate times produce spurious results, leading doctors to make incorrect treatment decisions.18 There was a substantial reduction of borderline significance in the proportion of potentially toxic concentrations (>20 mg/mL), which have been associated with kidney toxicity.19–21 This finding warrants further investigation to confirm this observation. Doctors’ prescribing of appropriate vancomycin maintenance doses increased from approximately half to nearly two-thirds (63%) that were guideline-adherent. This compares closely with 64% appropriate maintenance dosing achieved in another study post-implementation of vancomycin guidelines,22 and is considerably better than (50%) the result that was reported in a study conducted in a Hong Kong teaching hospital.23 Post-implementation in the current study, there was a much larger improvement in dosage adjustments, from 54% pre-implementation to 71% post-implementation, made by JMOs when trough concentrations were outside target range, suggesting patients were more closely monitored in the current study.
Adherence to the CPG for measurement timing when pre-dose (trough) blood concentrations were taken (relative to time of preceding dose) increased in excess of 80% post-implementation in the present study and compared similarly (78%) to a study conducted in California that implemented vancomycin guidelines.24 While the effect size of provision of pocket guidelines is unknown, a Cochrane review on the effect of printed educational material on professional practice and health care outcomes found providing written material to health care staff did have a beneficial effect.25 The effect of implementation on the proportion of vancomycin concentrations in the target range (43%) did reproduce the result observed in the pilot study (44%),9 but was non-significant in the current study. Forty-three percent of all concentrations within our CPG target range highlights the fact that there is still considerable work to be done to improve this result; however, some authorities use a wider target range (10–20 mg/L),26 and when our results were measured against this range, some 63% of our concentrations were within range.
The rates of nephrotoxicity observed in the present study remained encouragingly unchanged despite the post-implementation group receiving many more loading doses, and having double the percentage of patients concomitantly receiving aminoglycoside antibiotics, which are also known to cause kidney toxicity.27,28 Since the introduction of the CPG, the median duration of vancomycin therapy decreased from 10 days pre-implementation to 6 days post-implementation. It is not possible to determine if this reduction was due to reasons such as the prescribing of more loading doses, thus enabling the antibiotic to act more rapidly, or because doctors were requesting blood tests more promptly and were adherent to the guideline. The cure rate for both sterile and non-sterile infections also improved somewhat post-implementation.
A strength of the guideline implemented in this study is that it was based on contemporary international and national vancomycin consensus guidelines.11,29 The guideline was developed and endorsed by local opinion leaders in the fields of pharmacy, infectious diseases, and clinical pharmacology from the hospital it was implemented in. This is meaningful, as a systematic review of the influence of local opinion leaders showed that their influence was successful in promoting evidence-based practice.30 Importantly, the CPG was beta-tested on JMOs and final-year medical students, who were the target audience, and the outcomes used to measure the impact of guideline implementation were highly objective. Finally, details of the implementation were provided. This is important, as it has been reported that studies involving guideline implementation often do not provide enough information about the implementation process to be informative to others seeking to change practice.31 Further, it has been reported that there is an imperfect evidence base in guideline dissemination and implementation studies, with little consideration given to resource allocation.32 The current study adds to the evidence base on this topic.
Limitations
The present study has a number of limitations. Review of “usual clinical care” paper-based medical records and medication charts is problematic, as data extraction can be difficult; however, the accuracy of research assistants performing data collection was audited and was found to be of high accuracy. While the educational component of implementation included JMOs assigned to various different medical and surgical units, the study was conducted in a single hospital, and thus, the findings may not necessarily be generalizable. The small sample size is a clear limitation. While there was improvement in some outcome measures, there is still substantial room for improvement. From the attendance records, we were unable to confirm if some JMOs or pharmacists went to more than one education session, thus potentially reducing the total count of professionals receiving education. In addition, there may be selection bias in effect, as those professionals that were interested to improve their knowledge and skills were the ones that attended the educational sessions.
Conclusion
The pharmacist implementation of a CPG across medical and surgical units significantly increased the proportion of loading doses prescribed by doctors for patients receiving vancomycin. A larger-powered study may help determine if proportional improvements observed in other measures of prescribing and monitoring vancomycin translate into statistically significant changes in clinical behavior and meaningful outcomes for patients and their doctors.
Acknowledgments
This study was part funded by an Auditmaker Grant (AUD1104) administered through the Society of Hospital Pharmacists of Australia, Research and Development Grants Advisory Committee. None of the funding bodies played a role in the study design.
Footnotes
Disclosure
CJP was funded by a National Health and Medical Research Council of Australia – Translating Research into Practice Fellowship (1035960) during the period of this work. The authors report no other conflicts of interest in this work.
References
- 1.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 2.Rybak MJ, Rotschafer JC, Rodvold KA. Vancomycin: over 50 years later and still a work in progress. Pharmacotherapy. 2013;33:1253–1255. doi: 10.1002/phar.1382. [DOI] [PubMed] [Google Scholar]
- 3.Moellering RC., Jr Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(Suppl 1):S3–S4. doi: 10.1086/491708. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano C, Haase KK, Hall R. Use of vancomycin pharmacokinetic-pharmacodynamic properties in the treatment of MRSA infections. Expert Rev Anti Infect Ther. 2010;8:95–106. doi: 10.1586/eri.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye ZK, Tan HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS One. 2013;8(10):e77169. doi: 10.1371/journal.pone.0077169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu T, Stockmann C, Balch AH, Spigarelli MG, Sherwin CM. Evolution of interventional vancomycin trials in light of new antibiotic development in the USA, 1999–2012. Int J Antimicrob Agents. 2014;43:215–222. doi: 10.1016/j.ijantimicag.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Ye ZK, Li C, Shai SD. Guidelines for therapeutic drug monitoring of vancomycin: a systematic review. PLoS One. 2014;9(6):e99044. doi: 10.1371/journal.pone.0099044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duguid M, Cruickshank M, editors. Antimicrobial stewardship in Australian hospitals. Sydney, NSW: Australian Commission on Safety and Quality in Health Care; 2011. [Accessed May 24, 2015]. Available from: http://www.safetyandquality.gov.au/our-work/healthcare-associated-infection/antimicrobial-stewardship/book/ [Google Scholar]
- 9.Phillips CJ, Doan H, Quinn S, Kirkpatrick CM, Gordon DL, Doogue MP. An educational intervention to improve vancomycin prescribing and monitoring. Int J Antimicrob Agents. 2013;41:393–394. doi: 10.1016/j.ijantimicag.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Hulscher ME, Grol RT, van der Meer JW. Antibiotic prescribing in hospitals: a social and behavioural approach. Lancet Infect Dis. 2010;10:167–175. doi: 10.1016/S1473-3099(10)70027-X. [DOI] [PubMed] [Google Scholar]
- 11.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;43:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 12.Gould IM, Cauda R, Esposito S, Gudiol F, Mazzei T, Garau J. Management of serious methicillin-resistant Staphylococcus aureus infections: what are the limits? Int J Antimicrob Agents. 2011;37:202–209. doi: 10.1016/j.ijantimicag.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78:743–750. [PubMed] [Google Scholar]
- 14.Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34:653–661. doi: 10.1002/phar.1423. [DOI] [PubMed] [Google Scholar]
- 15.Mandell GL, Bennett JE, Dolin RD. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014. [Google Scholar]
- 16.Peacock JL, Peacock PJ. Oxford Handbook of Medical Statistics. Oxford: Oxford University Press; 2012. [Google Scholar]
- 17.Reardon J, Lau TT, Ensom MH. Vancomycin loading doses: a systematic review. Ann Pharmacother. 2015;49:557–565. doi: 10.1177/1060028015571163. [DOI] [PubMed] [Google Scholar]
- 18.Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early? Frequency and impact on clinical actions. Am J Clin Pathol. 2012;137:472–478. doi: 10.1309/AJCPDSYS0DVLKFOH. [DOI] [PubMed] [Google Scholar]
- 19.Wong-Beringer A, Joo J, Tse E, Beringer P. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents. 2011;37:95–101. doi: 10.1016/j.ijantimicag.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration–time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 21.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dib JG, Al-Tawfiq JA, Al Abdulmohsin S, Mohammed K, Jenden PD. Improvement in vancomycin utilization in adults in a Saudi Arabian Medical Center using the Hospital Infection Control Practices Advisory Committee guidelines and simple educational activity. J Infect Public Health. 2009;2:141–146. doi: 10.1016/j.jiph.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee VW, Lyon DJ, Fung KS, et al. Appropriateness of vancomycin use before and after guideline implementation. Am J Health Syst Pharm. 2003;60:949–950. doi: 10.1093/ajhp/60.9.949. [DOI] [PubMed] [Google Scholar]
- 24.Swartling M, Gupta R, Dudas V, Guglielmo BJ. Short term impact of guidelines on vancomycin dosing and therapeutic monitoring. Int J Clin Pharm. 2012;34:282–285. doi: 10.1007/s11096-012-9614-6. [DOI] [PubMed] [Google Scholar]
- 25.Giguère A, Légaré F, Grimshaw J, et al. Printed educational materials: effects on professional practice and healthcare outcomes [review] Cochrane Database Syst Rev. 2012;10:CD004398. doi: 10.1002/14651858.CD004398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto K, Takesue Y, Ohmagari N, et al. Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2013;19:365–380. doi: 10.1007/s10156-013-0599-4. [DOI] [PubMed] [Google Scholar]
- 27.Turnidge J. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am. 2013;17:503–528. doi: 10.1016/s0891-5520(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 28.Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007;45:753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 29.Antibiotic Expert Group . Therapeutic Guidelines: Antibiotic (Version 14) Melbourne, VIC: Therapeutic Guidelines Limited; 2010. [Google Scholar]
- 30.Flodgren G, Parmelli E, Doumit G, et al. Local opinion leaders: effects on professional practice and health care outcomes [review] Cochrane Database Syst Rev. 2011;8:CD000125. doi: 10.1002/14651858.CD000125.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients [review] Cochrane Database Syst Rev. 2013;4:CD003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 32.Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8:iii–iv. 1–72. doi: 10.3310/hta8060. [DOI] [PubMed] [Google Scholar]