Abstract
Benzodiazepines are commonly prescribed anxiolytics that pose abuse liability in susceptible individuals. Although it is well established that all drugs of abuse increase brain dopamine levels, and benzodiazepines are allosteric modulators of the GABAA receptor, it remains unclear how they alter dopamine release. Using in vivo fast-scan cyclic voltammetry, we measured diazepam-induced changes in the frequency and amplitude of transient dopamine release events. We found that diazepam concurrently increases the frequency and decreases the amplitude of transient dopamine release events in the awake and freely moving rat. The time course during which diazepam altered the frequency and amplitude of dopamine release events diverged, with the decreased amplitude effect being shorter lived than the increase in frequency, but both showing similar rates of onset. We conclude that diazepam increases the frequency of accumbal dopamine release events by disinhibiting dopamine neurons, but also decreases their amplitude. We speculate that the modest abuse liability of benzodiazepines is due to their ability to decrease the amplitude of dopamine release events in addition to increasing their frequency.
Introduction
Benzodiazepines (BZP) potentiate brain γ-aminobutyric acid (GABA) function by activating allosteric binding sites on the GABAA receptor (Sigel and Buhr, 1997). Potentiating GABAA function produces anxiolytic, sedative, and hypnotic effects in a receptor subtype–dependent manner (Rudolph and Möhler, 2006). Due to their anxiolytic properties, benzodiazepines are used clinically for the treatment of certain anxiety disorders (Dell’Osso and Lader, 2013). Many patients who are prescribed benzodiazepines take them responsibly and benefit from their therapeutic utility; however, administration of high doses of BZPs for extended durations can increase the odds of abuse in susceptible individuals (Griffiths and Weerts, 1997; Licata and Rowlett, 2008; Baldwin et al., 2013). Alarmingly, over 270,000 patients who visited emergency rooms in 2008 reported nonprescription use of benzodiazepines (Centers for Disease Control and Prevention, 2010). Such benzodiazepine-related incidents appear to be growing in prevalence, as the number of benzodiazepine-related emergency room visits increased by 82% over the preceding 5 years (Centers for Disease Control and Prevention, 2010). The current extent of BZP abuse remains unclear, as it can be difficult to disentangle pharmacotherapeutic use versus abuse; however, it is widely accepted that they are one of the most frequently prescribed anxiety medications in Western countries, where they are frequently misused (Umbricht and Velez, 2015). Despite the widespread use of BZPs, the way in which they influence the brain’s neurochemistry to promote abuse remains poorly characterized.
All drugs of abuse are thought to increase dopamine (DA) concentration within the nucleus accumbens (NAc) (Di Chiara and Imperato, 1988), a neural structure highly implicated in reward-seeking and motivation (Da Cunha et al., 2012; Floresco, 2015). The NAc primarily receives afferent dopaminergic input from the ventral tegmental area (VTA) (Swanson, 1982; Ikemoto, 2007). In the awake and behaving animal, VTA DA neurons exhibit two distinct firing patterns: tonic and phasic (Grace and Bunney, 1984; Grace et al., 2007). When at rest, these DA neurons exhibit a regular pacemaker pattern of low-frequency activity (1–5 Hz). This basal firing pattern provides a low concentration dopaminergic “tone” in the NAc. In contrast, when animals are exposed to drugs of abuse, VTA DA neurons fire in high-frequency bursts at rates greater than 20 Hz (Willuhn et al., 2010; Covey et al., 2014). This phasic pattern contributes to high-concentration, transient release events in the NAc that are thought to be integral in promoting abuse liability (Willuhn et al., 2010; Covey et al., 2014). Several recent reports have demonstrated that various drugs of abuse with diverging mechanisms of action, including cocaine (Stuber et al., 2005; Aragona et al., 2008), amphetamine (Covey et al., 2016), cannabinoids (Cheer et al., 2004; Oleson and Cheer, 2012), opiates (Fox et al., 2017), and nicotine (Cheer et al., 2007), increase both the frequency and amplitude of these transient release events (Covey et al., 2014).
BZPs are theorized to increase DA concentration via disinhibition of DA neurons in the VTA. BZP-induced activation of GABA receptors on GABAergic interneurons is thought to contribute to this disinhibition (Tan et al., 2010, 2011). Previous studies utilizing electrophysiological recordings and pharmacological manipulations of GABAA receptor demonstrate that BZPs do indeed increase the firing rate of putative VTA DA neurons in a GABAA receptor–dependent manner (O’Brien and White, 1987; Tan et al., 2010). A BZP-induced disinhibition of DA neural activity would also suggest that indices of NAc DA signaling should be enhanced. However, microdialysis studies generally report that BZPs decrease accumbal DA concentration (Zetterström and Fillenz, 1990; Invernizzi et al., 1991; Finlay et al., 1992, 1995; Takada et al., 1993; Murai et al., 1994; Dazzi et al., 1995; Hegarty and Vogel, 1995; Motzo et al., 1997; Yoshida et al., 1999; Bentue-Ferrer et al., 2001; Rada and Hoebel, 2005; Gomez-A et al., 2017), but see Bentue-Ferrer et al. (2001). Furthermore, a recent fast-scan cyclic voltammetry (FSCV) study reported that BZPs decrease the amplitude of electrically evoked accumbal DA concentrations (Gomez-A et al., 2017). In the current study, we predicted that a real-time analysis of BZP-induced accumbal DA release events would reveal an increase in their frequency, but also a decrease in their amplitude.
Materials and Methods
Subjects and Surgery.
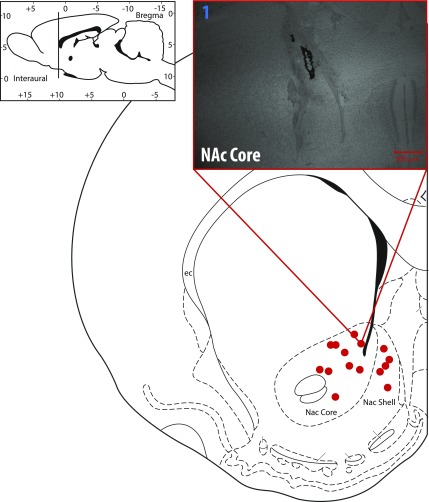
Catheterized male Long-Evans rats (Charles River Laboratories, Wilmington, MA) were singly housed under a 12:12 light:dark cycle with a 10 a.m. to 10 p.m. active period (dark phase). All experiments were conducted in the active phase. Rats (250–300 g at the time of surgery) were placed under isoflurane anesthesia (5% induction, 2% maintained) for surgery conducted in a Kopf stereotaxic apparatus. A guide cannula that mates with an electrode containing micromanipulator was implanted to be aimed at the nucleus accumbens core (+1.3 anterior-posterior, +1.4 medial-lateral relative to the bregma) or the nucleus accumbens shell (+1.7 anterior-posterior, +0.8 medial-lateral relative to the bregma) along with a contralateral Ag/AgCl reference electrode. Rats were given 3 days postsurgery to recover before experiments were conducted. The University of Colorado Denver Institutional Animal Care and Use Committee approved all experiments and procedures in advance.
FSCV.
Voltammetric recordings were conducted by lowering a glass-encased carbon-fiber microelectrode with a micromanipulator into the NAc and locking it into place. An initial wave form (−0.4 to 1.3 V, TarHeel CV filtered with cutoff frequency of 2 kHz for a scan rate of 400 V/s) was applied which allowed for the detection of DA via fast-scan cycle voltammograms taken every 100 ms. To increase electrode sensitivity, the wave form was first applied at 60 Hz for 30 minutes, but was reduced to 30 Hz before experimentation (Heien et al., 2003). To extract the DA component, principle component regression was applied to the raw voltammetric data as previously described (Heien et al., 2004). Specifically, DA and pH were resolved from the FSCV recordings using recording-specific training sets (n = 6/analyte) to produce pH background-subtracted (10 consecutive scans) DA concentration files for transient analysis. Prior to performing principle component regression, current-versus-time data were smoothed using the built-in TarHeel CV smoothing option (eight-point nearest-neighbor smoothing kernel). To increase the validity of calibration factors for DA assessment, we applied a recently developed computational model (Roberts et al., 2013) designed to calculate calibration factors for individual electrodes by applying known constants to background current values from each in vivo recording. By replicating Roberts et al. (2013), using 10 electrodes, we obtained a set of empirical values using multiple linear regression analysis. Our laboratory-specific coefficients are α = 4.71e−5, β = 17.185, γ = 8.324, and δ = −0.656. Using these coefficients, we can calculate calibration factors for individual electrodes used in vivo by simply entering the observed total background current and the switching potential corresponding to each individual file. We also found that total background current decreased over the course of the recording sessions; thus, each 1-minute dopamine concentration trace was further normalized to its total background current (Supplemental Fig. S1).
At the end of the experiment, animals were killed with CO2, and electrolytic lesions were performed to confirm electrode placement. Brains were extracted and frozen in −25°C 2-methylbutane, then stored at −80°C until sectioned coronally at 50 μm on a cryostat. Slices were dehydrated with baths of increasing ethanol concentration, stained with cresyl violet, preserved with histoclear (National Diagnostics, Atlanta, GA), and mounted for observation of lesion placement. Lesion placements are mapped and representative histology shown in Fig. 7.
Pharmacology.
Benzodiazepines were prepared in 1% Tween 20 and sonicated for 5 minutes to dissolve the BZPs into the solution. BZPs were administered intravenously in all in vivo experiments using a cumulative dosing approach (baseline, vehicle, 0.3, 0.56, and 1.0 mg/kg). The cumulative doses assume a half-life of >3 hours in the rat brain (Braestrup and Squires, 1977). Diazepam was purchased from Sigma-Aldrich (St. Louis, MO), and flumazenil was purchased from Tocris at leas (Bristol, UK).
Frequency Analysis.
For every 60 seconds of recording, a polynomic line was fitted to the DA concentration data using a custom MATLAB program (MathWorks, Natick, MA) that applies the following equation to each set of DA concentration data:
The coefficients (p1, p2, p3) with the largest R2 value were assigned for each 60 seconds of recording taken. To prevent the polynomic line from tracing up any transients (and artificially lowering the amplitude of any transients), only the values below the first fitted line were used to fit a second line with the following equation:
The degree of the polynomial was determined by finding the lowest AIC score with the following equation:
The coefficients (p1, p2, p3 ...) with the largest R2 value were assigned for each 60 seconds of recording taken for the second line. The second fitted line was subtracted from all values present in the original recording to adjust for drifting current and establish a true zero. Only values above 1 S.D. of the entire 60-second recording were considered nonbackground noise peaks. Peaks were programmatically determined by finding the local maximum. The peak transients were then analyzed with a set of criteria to determine a true transient. The criteria required that more than 0.5 seconds had elapsed between transients for them to be considered. If less than 0.5 seconds had elapsed, then the largest of the transients was recorded as the true transient. We assessed the effect of using three additional intertransient intervals (0.1, 0.3, and 1 second) in our analysis. Importantly, similar trends were observed across all conditions (Supplemental Fig. S2); however, we came to the conclusion that the 0.1 second criterion risks type-1 statistical error, whereas the 1 second criterion risks type-2 statistical error. Analysis of variance (ANOVA) and Bonferroni post-hoc tests were used to assess changes in DA concentration amplitude and frequency relative to vehicle.
Statistics.
All statistics were performed using SigmaPlot11 (Systat, San Jose, CA). First, Shapiro-Wilk was used to assess for normality, and Brown-Forsythe was used to assess for equal variance. Repeated-measures ANOVA and Bonferroni post-hoc tests were used for all analyses.
Results
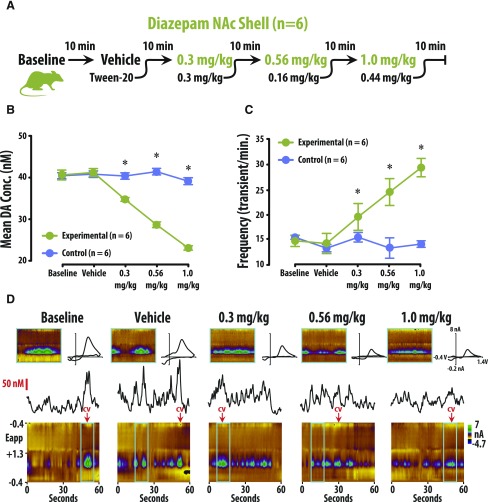
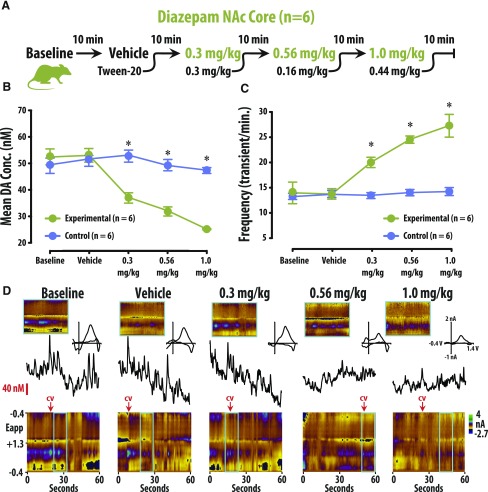
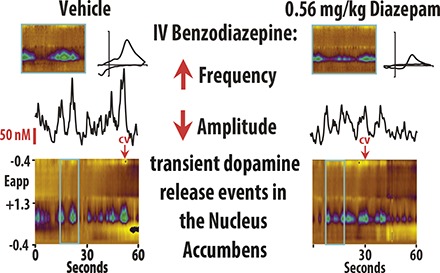
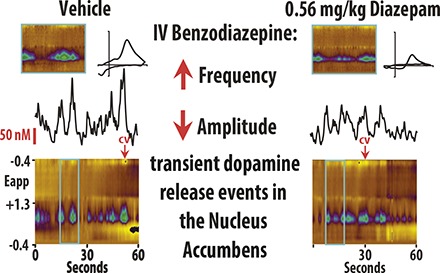
To assess the effects of benzodiazepines on DA release, we performed FSCV in both the NAc core and shell of awake and behaving rats. DA concentration and frequency were quantified after isolating transient release events from background current (Fig. 1; also see the Frequency Analysis section in Materials and Methods). Rats were treated with either vehicle or cumulative doses of diazepam (Figs. 2A and 3A). In our amplitude analysis, we observed a significant interaction between treatment group (vehicle vs. diazepam) and treatment condition (four consecutive injections) in both the shell [Fig. 2; F(4, 59) = 30.799, P < 0.01] and the core [Fig. 3; F(4, 59) = 18.009, P < 0.01] of the NAc. The effect of group depended upon the dosing condition, so that under baseline and vehicle conditions, there was no difference in amplitude between the diazepam and vehicle groups (n.s.). However, amplitude in both the shell and core was significantly lower in the diazepam group versus the vehicle group under the 0.3-, 0.56-, and 1-mg/kg cumulative dosing conditions (P < 0.05 for all conditions; Supplemental Tables S1 and S3). Similarly, the frequency analysis also revealed significant interaction between treatment group (vehicle vs. diazepam) and treatment condition (four consecutive injections) in both the shell [Fig. 2; F(4, 59) = 13.579, P < 0.01] and the core [Fig. 3; F(3, 63) = 18.305, P < 0.01] of the NAc. As in the amplitude analysis, the frequency of DA release events was not different between the diazepam and vehicle groups under baseline or vehicle conditions (n.s.). However, the frequency of DA release events in both the shell and core was significantly higher in the diazepam group versus the vehicle group under the 0.3-, 0.56-, and 1-mg/kg cumulative dosing conditions (P < 0.05 for all conditions; Supplemental Tables S2 and S4).
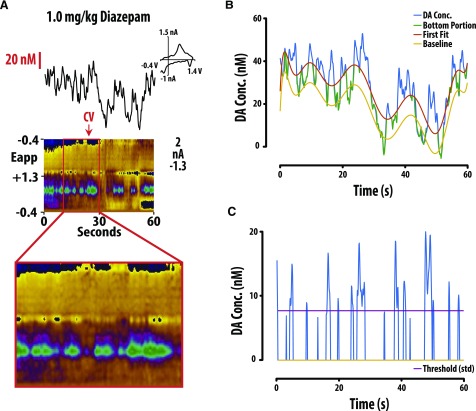
Fig. 1.
DA frequency was determined by establishing threshold concentration values to isolate transient release events from background current. (A) Representative color plot and associated DA concentration (Conc.) when 1.0 mg/kg diazepam is administered i.v. Representative color plots topographically depict the voltammetric data with time on the x-axis, applied scan potential (Eapp) on the y-axis, and background-subtracted faradaic current shown on the z-axis in pseudocolor. DA release events can be identified by an oxidation peak (green) at +0.6 V. (B) Extracted DA concentration was then fitted with a polynomic line shifted by +3 times the standard deviation of a region with minimal transient activity. (C) Only release events above the threshold (3× S.D.) that were more than 0.5 seconds apart were counted as transients. Conc., concentration; CV, cyclic voltammogram.
Fig. 2.
Diazepam decreased amplitude of DA concentration (Conc.) and increased the frequency of transient release events in the NAc shell. (A) Tenminute baseline recording preceded the i.v. of vehicle, 0.3, 0.56, and 1.0 mg/kg diazepam (cumulative). (B) Diazepam decreased DA concentration versus vehicle-treated rats (mean ± S.E.M.). (C) Diazepam increased the frequency of DA release events versus vehicle-treated rats (mean ± S.E.M.). (D) Representative color plots and associated DA concentration traces under baseline, vehicle, 0.3, 0.56, and 1.0 mg/kg conditions.
Fig. 3.
Diazepam decreased DA concentration (Conc.) amplitude but increased the frequency of release events in the NAc Core. (A) Ten-minute recordings in the NAc core were conducted to establish a baseline, and then after vehicle, 0.3, 0.56, and 1.0 mg/kg cumulative doses (i.v.) of diazepam. (B) DA concentration (mean ± S.E.M.) decreased in the NAc core after diazepam administration (vs. vehicle-treated rats). (C) The frequency of transient DA release events (mean ± S.E.M.) increased in the NAc core after diazepam administration (vs. vehicle-treated rats). (D) Representative color plots and associated DA concentration traces for baseline, vehicle, 0.3, 0.56, and 1.0 mg/kg cumulative doses of diazepam.
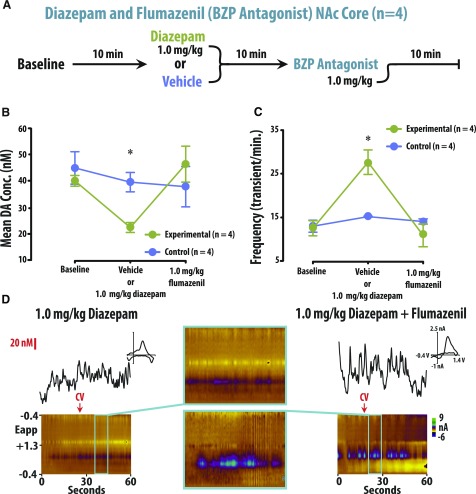
The effects of diazepam on dopamine release were reversed by the selective BZP antagonist flumazenil (Fig. 4). Two groups of rats were used: an experimental group that received diazepam and then flumazenil, and a control group that received diazepam and then flumazenil. These two groups were assessed under three different conditions: baseline, diazepam or vehicle, then flumazenil (Fig. 4A). A two-way repeated-measures ANOVA revealed a significant interaction between group and treatment condition [F(2, 23) = 7.554, P < 0.01] with the group effect being dependent upon the treatment. No significant difference in either frequency or amplitude was observed between groups under baseline or flumazenil conditions. However, amplitude was significantly lower (P < 0.05 vs. vehicle control group; Supplemental Table S5) and frequency was significantly higher (P < 0.05 vs. vehicle control group; Supplemental Table S6) in the experimental group following diazepam treatment.
Fig. 4.
The BZP receptor antagonist flumazenil reversed the decreased amplitude and increased frequency induced by the administration of diazepam. (A) Ten-minute recordings of the NAc core were conducted following administration of either 1.0 mg/kg diazepam and 1.0 mg/kg flumazenil (experimental group) or vehicle and 1.0 mg/kg flumazenil (control group). (B and C) In the experimental group (vs. control), diazepam decreased amplitude and increased frequency, effects that were reversed by flumazenil. (D) Representative color plots and associated DA concentration (Conc.) traces for 1.0 mg/kg diazepam and 1.0 mg/kg diazepam with 1.0 mg/kg flumazenil.
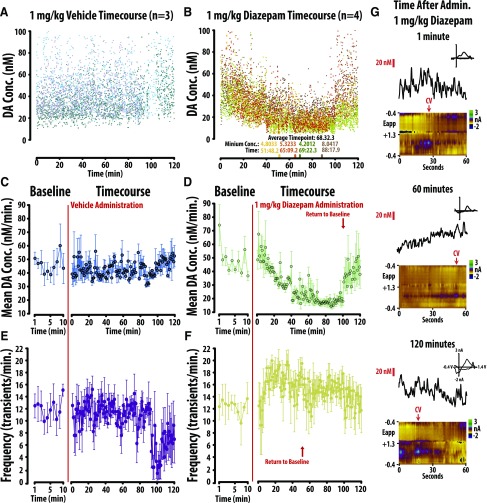
To further explore the mechanism of action of BZPs, we performed a time course-study to assess if the diazepam-induced changes in frequency and amplitude occur in parallel (Fig. 5). Diazepam (1.0 mg/kg) or vehicle was administered to separate groups, and changes in accumbal core DA concentration were recorded over 120 minutes. To illustrate subject-specific trends and overall variability, individual DA concentration points are depicted in Fig. 5, A and B. Vehicle failed to produce a significant change in either amplitude or frequency (Fig. 5, C and E; n.s.). Separate one-way repeated-measures ANOVA revealed diazepam significantly changed dopamine amplitude [Fig. 5D; F(120, 483) = 1.519, P < 0.01] and frequency [Fig. 5E; F(120, 483) = 1.306, P = 0.032]. We defined a return to baseline as the first point where a significant change from baseline was directly followed by an insignificant change from baseline. Bonferroni post-hoc analysis revealed that diazepam-induced decreases in the amplitude of DA transients recovered at minute 101, whereas diazepam-induced increases in the frequency of events recovered at minute 52 (P < 0.05 vs. baseline). The divergent time course may suggest that distinct mechanisms alter BZP-induced changes in DA frequency and amplitude.
Fig. 5.
The changes in frequency versus amplitude of DA induced by 1.0 mg/kg diazepam occur across divergent time-courses. All DA concentration data are plotted across the 120 minute recording session following vehicle (A) and diazepam (B) treatment. (C, D) In comparison to vehicle (left), diazepam decreases mean (+/- SEM) dopamine concentration, an effect that recovered after 100min. (E, F). In comparison to vehicle (left), diazepam increased the mean frequency (+/- SEM) of dopamine release events, an effect that recovered after 50min. (G) Representative color plots and associated DA concentration traces for 1.0 mg/kg diazepam at 1 minute, 60 minutes, and 120 minutes.
To further analyze the effects of diazepam on the frequency and amplitude of DA transients, we created histograms depicting total values (frequency and amplitude) across all conditions (core, shell, and all doses of diazepam; Fig. 6). The histograms illustrate that there is both a diazepam-induced loss of higher amplitude DA events and an increase in lower amplitude DA events.
Fig. 6.
Histograms showing total transients in the shell (top) and core (bottom) across baseline, vehicle, and all diazepam conditions. These figures illustrate both a diazepam-induced loss of higher-amplitude DA events and an increase in lower amplitude DA events.
Discussion
BZPs are a commonly used pharmacotherapy for anxiety disorders that can exhibit abuse liability in susceptible individuals. Although it is well established that BZPs are allosteric modulators of the GABAA receptor (Tan et al., 2010, 2011), it remains unclear how they alter DA release. All drugs of abuse are thought to increase brain DA levels (Di Chiara and Imperato, 1988), and the electrophysiology literature indicates that BZPs disinhibit VTA DA neurons (Tan et al., 2010, 2011); however, the microdialysis literature generally reports that BZPs decrease accumbal DA concentration (Zetterström and Fillenz, 1990; Invernizzi et al., 1991; Finlay et al., 1992, 1995; Takada et al., 1993; Murai et al., 1994; Dazzi et al., 1995; Hegarty and Vogel, 1995; Motzo et al., 1997; Yoshida et al., 1999; Bentue-Ferrer et al., 2001; Rada and Hoebel, 2005; Gomez-A et al., 2017), but see Bentue-Ferrer et al. (2001). Although microdialysis studies show a net reduction in extracellular DA concentration, the temporal resolution provided by this technique makes it difficult to discern whether a reduction in the amplitude of transient release events contributes to this effect. Additionally, a recent FSCV study demonstrated that BZPs decrease electrically evoked DA release in the NAc of anesthetized rats (Gomez-A et al., 2017). Our study builds upon these data by demonstrating the effects of diazepam on naturally occurring accumbal transient release events in the awake and freely moving animal.
The paradoxical electrophysiological and microdialysis findings might suggest that BZPs disinhibit DA neurons, but also decrease the concentration of DA released into the NAc through independent mechanisms. It is possible that the electrophysiological results represent a disinhibition of DA neural activity in the VTA (O’Brien and White, 1987; Tan et al., 2010), which increases both the frequency and amplitude of accumbal transient release events. By contrast, the microdialysis results (Zetterström and Fillenz, 1990; Invernizzi et al., 1991; Finlay et al., 1992, 1995; Takada et al., 1993; Murai et al., 1994; Dazzi et al., 1995; Hegarty and Vogel, 1995; Motzo et al., 1997; Yoshida et al., 1999; Bentue-Ferrer et al., 2001; Rada and Hoebel, 2005; Gomez-A et al., 2017) and the recent anesthetized FSCV study (Gomez-A et al., 2017) might represent a localized suppression of accumbal DA release events. However, it should also be noted that the aforementioned electrophysiological studies identified dopamine neurons solely using electrophysiological criteria—which is now considered insufficient to determine whether a recorded unit is DAergic or GABAergic (Ungless et al., 2004; Ungless and Grace, 2012).
In the current study, we predicted that a real-time analysis of BZP-induced accumbal DA release events would reveal an increase in their frequency, but also a decrease in their amplitude. To address this hypothesis, we performed FSCV in the awake and freely moving rat and observed that the BZP diazepam increased the frequency of DA release events while also decreasing their amplitude. The time course over which BZPs altered the frequency and amplitude of DA release events diverged, with the decreased amplitude effect being shorter lived than the increase in frequency, but both showing similar rates of onset. The divergent time course may suggest that these two effects are governed by distinct neural mechanisms. We observed similar effects in both the core and shell regions of the NAc, and they were reversed by treatment with the selective BZP-site antagonist flumazenil.
Emerging evidence suggests that a unifying action of all abused substances is their ability to increase DA transient release events (Covey et al., 2014). Although many mechanisms of action have been identified through which abused substances increase brain DA concentrations, a unifying feature is that all drugs of abuse increase high-concentration transient release events (Covey et al., 2014). To date, most drugs of abuse, including cocaine, amphetamine, opiates, cannabis, and nicotine, have been shown to increase both the frequency and amplitude of transient release events (Covey et al., 2014). However, in the current study, we found that BZPs increase the frequency, but decrease the amplitude of transient release events. Similar results have been reported with ethanol (Cheer et al., 2007; Robinson et al., 2009), which is similar to BZPs in that it is known to activate GABA receptors (Valenzuela and Jotty, 2015; Ostroumov et al., 2016). It is possible that NAc-localized GABA modulation of VTA dopaminergic terminals may contribute to reductions in amplitude of DA release.
BZPs are known to produce moderate abuse liability (Griffiths and Weerts, 1997; Licata and Rowlett, 2008; Baldwin et al., 2013) and potentiate brain reward function (Straub et al., 2010), but also alter the rewarding and reinforcing properties of other abused drugs. Sedative-hypnotics, including BZPs, are frequently coabused with psychostimulants to self-medicate insomnia, agitation, and irritability (Wesson and Smith, 1985). Similarly, sedative-hypnotics can be used to treat anxiety and psychosis in cocaine abusers (Wesson and Smith, 1985). Cocaine was first shown to produce anxiogenic-like stimulus effects in the preclinical literature by Wood and Lal (1987). Using drug discrimination, these authors demonstrated that the anxiogenic drug pentylenetetrazol generalizes to cocaine withdrawal (Wood and Lal, 1987), an effect that was blocked by diazepam (Wood et al., 1989). The benzodiazepine alprazolam was also shown to reduce the discriminative stimulus effects of amphetamine (Rush et al., 2004). A variety of benzodiazepines have also been shown to reduce distress ultrasonic vocalization in rodents (Rowlett et al., 2001; Hodgson et al., 2008). Goeders et al. (1993) and Goeders (1997, 2002) expanded upon these findings by demonstrating that benzodiazepines concurrently reduce stress-associated adrenocorticosteroids and cocaine self-administration. More recently, their group demonstrated that a combination of the BZPs metyrapone and oxazepam reduces cocaine self-administration under an FR4 schedule in rats (Goeders and Guerin, 2008) and cocaine craving and cocaine taking in a double-blind, randomized, placebo-controlled pilot study (Kablinger et al., 2012). Benzodiazepines are also commonly used to treat symptoms of withdrawal during detoxification from alcohol (Kosten and O’Connor, 2003; Zindel and Kranzler, 2014). Given the well established yet complex interactions between the hypothalamic–pituitary–adrenal axis and the mesocorticolimbic system (Ungless et al., 2003; Beckstead et al., 2009), it is likely that benzodiazepines alter the abuse potential of other drugs through both direct and indirect actions on both systems. Further studies are needed to determine how BZP-induced changes in DA release are altered during polydrug administration and in animal models of drug addiction.
Fig. 7.
Electrolytic lesions confirm electrode placement within the NAc core and shell. Representative image (upper right) of NAc core lesion.
Acknowledgments
We thank Dr. Dan P. Covey and Dr. Joseph F. Cheer for helpful comments in the preparation of this manuscript.
Abbreviations
- ANOVA
analysis of variance
- BZP
benzodiazepine
- DA
dopamine
- FSCV
fast-scan cyclic voltammetry
- NAc
nucleus accumbens
- VTA
ventral tegmental area
Authorship Contributions
Participated in research design: Brodnik, España, Oleson.
Conducted experiments: Schelp, Rakowski, Pultorak, Sambells.
Performed data analysis: Schelp, Oleson.
Wrote or contributed to the writing of the manuscript: Schelp, Oleson.
Footnotes
Funding for the project was provided by the National Institutes of Health [Grants R01DA031900 and R03DA038734], National Science Foundation [Grant IOS-1557755], a Boettcher Young Investigator Award, and a NARSAD Young Investigator Award.
The authors have no conflicts to disclose.
The work was previously presented as a poster presentation: Benzodiazpines and their dual administration with ethanol increase accumbal transient dopamine release events (2016) RAKOWSKI D R, SCHELP SA, BRODNIK Z, ESPAÑA RA, PULTORAK KJ, OLESON EB. The 2016 Society for Neuroscience Annual Meeting, San Diego, CA.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. (2008) Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci 28:8821–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Aitchison K, Bateson A, Curran HV, Davies S, Leonard B, Nutt DJ, Stephens DN, Wilson S. (2013) Benzodiazepines: risks and benefits. A reconsideration. J Psychopharmacol 27:967–971. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT. (2009) CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology 34:1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentué-Ferrer D, Reymann JM, Tribut O, Allain H, Vasar E, Bourin M. (2001) Role of dopaminergic and serotonergic systems on behavioral stimulatory effects of low-dose alprazolam and lorazepam. Eur Neuropsychopharmacol 11:41–50. [DOI] [PubMed] [Google Scholar]
- Braestrup C, Squires RF. (1977) Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci USA 74:3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2010) Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep 59:705–709. [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci 24:4393–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. (2007) Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci 27:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA. (2016) Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur J Neurosci 43:1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Roitman MF, Garris PA. (2014) Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci 37:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha C, Gomez-A A, Blaha CD. (2012) The role of the basal ganglia in motivated behavior. Rev Neurosci. 23:747–767. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Motzo C, Imperato A, Serra M, Gessa GL, Biggio G. (1995) Modulation of basal and stress-induced release of acetylcholine and dopamine in rat brain by abecarnil and imidazenil, two anxioselective gamma-aminobutyric acidA receptor modulators. J Pharmacol Exp Ther 273:241–247. [PubMed] [Google Scholar]
- Dell’osso B, Lader M. (2013) Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry 28:7–20. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, Damsma G, Fibiger HC. (1992) Benzodiazepine-induced decreases in extracellular concentrations of dopamine in the nucleus accumbens after acute and repeated administration. Psychopharmacology (Berl) 106:202–208. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. (1995) Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience 64:619–628. [DOI] [PubMed] [Google Scholar]
- Floresco SB. (2015) The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66:25–52. [DOI] [PubMed] [Google Scholar]
- Fox ME, Rodeberg NT, Wightman RM. (2017) Reciprocal catecholamine changes during opiate exposure and withdrawal. Neuropsychopharmacology. 42: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. (1997) A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology 22:237–259. [DOI] [PubMed] [Google Scholar]
- Goeders NE. (2002) The HPA axis and cocaine reinforcement. Psychoneuroendocrinology 27:13–33. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. (2008) Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacol Biochem Behav 91:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, McNulty MA, Guerin GF. (1993) Effects of alprazolam on intravenous cocaine self-administration in rats. Pharmacol Biochem Behav 44:471–474. [DOI] [PubMed] [Google Scholar]
- Gomez-A A, Fiorenza AM, Boschen SL, Sugi AH, Beckman D, Ferreira ST, Lee K, Blaha CD, Da Cunha C. (2017) Diazepam inhibits electrically evoked and tonic dopamine release in the nucleus accumbens and reverses the effect of amphetamine. ACS Chem Neurosci 8:300–309. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. (1984) The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4:2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30:220–227. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM. (1997) Benzodiazepine self-administration in humans and laboratory animals--implications for problems of long-term use and abuse. Psychopharmacology (Berl) 134:1–37. [DOI] [PubMed] [Google Scholar]
- Hegarty AA, Vogel WH. (1995) The effect of acute and chronic diazepam treatment on stress-induced changes in cortical dopamine in the rat. Pharmacol Biochem Behav 52:771–778. [DOI] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. (2004) Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem 76:5697–5704. [DOI] [PubMed] [Google Scholar]
- Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM. (2003) Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst (Lond) 128:1413–1419. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Guthrie DH, Varty GB. (2008) Duration of ultrasonic vocalizations in the isolated rat pup as a behavioral measure: sensitivity to anxiolytic and antidepressant drugs. Pharmacol Biochem Behav 88:341–348. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Brain Res Rev 56:27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi R, Pozzi L, Samanin R. (1991) Release of dopamine is reduced by diazepam more in the nucleus accumbens than in the caudate nucleus of conscious rats. Neuropharmacology 30:575–578. [DOI] [PubMed] [Google Scholar]
- Kablinger AS, Lindner MA, Casso S, Hefti F, DeMuth G, Fox BS, McNair LA, McCarthy BG, Goeders NE. (2012) Effects of the combination of metyrapone and oxazepam on cocaine craving and cocaine taking: a double-blind, randomized, placebo-controlled pilot study. J Psychopharmacol 26:973–981. [DOI] [PubMed] [Google Scholar]
- Kosten TR, O’Connor PG. (2003) Management of drug and alcohol withdrawal. N Engl J Med 348:1786–1795. [DOI] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. (2008) Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav 90:74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzo C, Porceddu ML, Dazzi L, Sanna A, Serra M, Biggio G. (1997) Enhancement by flumazenil of dopamine release in the nucleus accumbens of rats repeatedly exposed to diazepam or imidazenil. Psychopharmacology (Berl) 131:34–39. [DOI] [PubMed] [Google Scholar]
- Murai T, Koshikawa N, Kanayama T, Takada K, Tomiyama K, Kobayashi M. (1994) Opposite effects of midazolam and β-carboline-3-carboxylate ethyl ester on the release of dopamine from rat nucleus accumbens measured by in vivo microdialysis. Eur J Pharmacol 261:65–71. [DOI] [PubMed] [Google Scholar]
- O’Brien DP, White FJ. (1987) Inhibition of non-dopamine cells in the ventral tegmental area by benzodiazepines: relationship to A10 dopamine cell activity. Eur J Pharmacol 142:343–354. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF. (2012) A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med 2:a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA. (2016) Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron 92:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Hoebel BG. (2005) Acetylcholine in the accumbens is decreased by diazepam and increased by benzodiazepine withdrawal: a possible mechanism for dependency. Eur J Pharmacol 508:131–138. [DOI] [PubMed] [Google Scholar]
- Roberts JG, Toups JV, Eyualem E, McCarty GS, Sombers LA. (2013) In situ electrode calibration strategy for voltammetric measurements in vivo. Anal Chem 85:11568–11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. (2009) Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res 33:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Tornatzky W, Cook JM, Ma C, Miczek KA. (2001) Zolpidem, triazolam, and diazepam decrease distress vocalizations in mouse pups: differential antagonism by flumazenil and β-Carboline-3-carboxylate-t-butyl ester (β-CCt). J Pharmacol Exp Ther 297:247–253. [PubMed] [Google Scholar]
- Rudolph U, Möhler H. (2006) GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol 6:18–23. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Wagner FP, Hays LR, Glaser PE. (2004) Alprazolam attenuates the behavioral effects of d-amphetamine in humans. J Clin Psychopharmacol 24:410–420. [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr A. (1997) The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 18:425–429. [DOI] [PubMed] [Google Scholar]
- Straub CJ, Carlezon WA, Jr, Rudolph U. (2010) Diazepam and cocaine potentiate brain stimulation reward in C57BL/6J mice. Behav Brain Res 206:17–20. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. (2005) Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology 30:853–863. [DOI] [PubMed] [Google Scholar]
- Swanson LW. (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9:321–353. [DOI] [PubMed] [Google Scholar]
- Takada K, Murai T, Kanayama T, Koshikawa N. (1993) Effects of midazolam and flunitrazepam on the release of dopamine from rat striatum measured by in vivo microdialysis. Br J Anaesth 70:181–185. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy J-M, Rudolph U, Lüscher C. (2010) Neural bases for addictive properties of benzodiazepines. Nature 463:769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Rudolph U, Lüscher C. (2011) Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci 34:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht A, Velez ML. (2015) Benzodiazepine abuse and addiction, in Textbook of Addiction Treatment: International Perspectives. pp 343–365. Springer, Milan.
- Ungless MA, Grace AA. (2012) Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303:2040–2042. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. (2003) Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron 39:401–407. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Jotty K. (2015) Mini-review: effects of ethanol on GABAA receptor-mediated neurotransmission in the cerebellar cortex--recent advances. Cerebellum 14:438–446. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Smith DE. (1985) Cocaine: treatment perspectives, in Cocaine use in America. Epidemiologic and Clinical Perspectives National Institute on Drug Abuse Research Monograph Series 61, pp 193–202, National Institute on Drug Abuse, Rockville, MD. [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. (2010) Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse, in Behavioral Neuroscience of Drug Addiction (Self DW, Staley JK. eds), pp 29–71, Springer-Verlag, Berlin, Heidelberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Lal H. (1987) Anxiogenic properties of cocaine withdrawal. Life Sciences 41:1431–36. [DOI] [PubMed] [Google Scholar]
- Wood DM, Laraby PR, Lal H. (1989) A pentylenetetrazol‐like stimulus during cocaine withdrawal: blockade by diazepam but not haloperidol. Drug Dev Res 16:269–276. [Google Scholar]
- Yoshida Y, Koide S, Hirose N, Takada K, Saigusa T, Koshikawa N. (1999) In vivo microdialysis evidence that midazolam facilitates propofol‐induced reduction in rat accumbal dopamine release. Neurosci Res Commun 25:121–127. [Google Scholar]
- Zetterström T, Fillenz M. (1990) Local administration of flurazepam has different effects on dopamine release in striatum and nucleus accumbens: a microdialysis study. Neuropharmacology 29:129–134. [DOI] [PubMed] [Google Scholar]
- Zindel LR, Kranzler HR. (2014) Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J Stud Alcohol Drugs Suppl 75 (Suppl 17):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]