Abstract
Voltage-gated KV7 channels (KV7.1 to KV7.5) are important regulators of the cell membrane potential in detrusor smooth muscle (DSM) of the urinary bladder. This study sought to further the current knowledge of KV7 channel function at the molecular, cellular, and tissue levels in combination with pharmacological tools. We used isometric DSM tension recordings, ratiometric fluorescence Ca2+ imaging, amphotericin-B perforated patch-clamp electrophysiology, and in situ proximity ligation assay (PLA) in combination with the novel compound N-(2,4,6-trimethylphenyl)-bicyclo[2.2.1]heptane-2-carboxamide (ML213), an activator of KV7.2, KV7.4, and KV7.5 channels, to examine their physiologic roles in guinea pig DSM function. ML213 caused a concentration-dependent (0.1–30 µM) inhibition of spontaneous phasic contractions in DSM isolated strips; effects blocked by the KV7 channel inhibitor XE991 (10 µM). ML213 (0.1–30 µM) also reduced pharmacologically induced and nerve-evoked contractions in DSM strips. Consistently, ML213 (10 µM) decreased global intracellular Ca2+ concentrations in Fura-2–loaded DSM isolated strips. Perforated patch-clamp electrophysiology revealed that ML213 (10 µM) caused an increase in the amplitude of whole-cell KV7 currents. Further, in current-clamp mode of the perforated patch clamp, ML213 hyperpolarized DSM cell membrane potential in a manner reversible by washout or XE991 (10 µM), consistent with ML213 activation of KV7 channel currents. Preapplication of XE991 (10 µM) not only depolarized the DSM cells, but also blocked ML213-induced hyperpolarization, confirming ML213 selectivity for KV7 channel subtypes. In situ PLA revealed colocalization and expression of heteromeric KV7.4/KV7.5 channels in DSM isolated cells. These combined results suggest that ML213-sensitive KV7.4- and KV7.5-containing channels are essential regulators of DSM excitability and contractility.
Introduction
Voltage-gated KV7 channels (KV7.1 to KV7.5), encoded by KCNQ genes (KCNQ1 to KCNQ5), are critically involved in establishing and maintaining the cell membrane potential of many excitable cells and tissue types (Jepps et al., 2009; Ng et al., 2011; Brueggemann et al., 2012a, 2014b; Evseev et al., 2013; Mani et al., 2013; Stott et al., 2014). In addition, emerging new evidence points to potential physiologic roles for the KV7 channels in detrusor smooth muscle (DSM), the main muscular component of the urinary bladder (Streng et al., 2004; Rode et al., 2010; Afeli et al., 2013; Anderson et al., 2013; Svalø et al., 2013, 2015; Stott et al., 2014; Provence et al., 2015; Mani et al., 2016).
Recent studies from our laboratory and others show that KV7 channels serve key functional roles in regulating DSM function in rats (Rode et al., 2010; Svalø et al., 2013), guinea pigs (Afeli et al., 2013; Anderson et al., 2013; Provence et al., 2015), pigs (Svalø et al., 2013), and humans (Svalø et al., 2015; Bientinesi et al., 2017). KV7 channel activation leads to hyperpolarization of the membrane potential, thereby decreasing Ca2+ influx through l-type voltage-gated Ca2+ channels, which precludes Ca2+-dependent activation of myosin light chain kinase necessary for the initiation of DSM contractility (Petkov, 2011; Stott et al., 2014). KV7 channels were initially termed “M channels” as pioneering studies in frog sympathetic neurons demonstrated the suppression of KV7 channel currents in response to Gq/11-coupled muscarinic acetylcholine receptor (mAChR) activation (Brown et al., 1980). However, whether mAChRs are involved in the regulation of KV7 channel activity in DSM is not known.
KV7 channel pore-forming α-subunits (KV7.1 to KV7.5) have been shown to form both homomeric and heteromeric channel conformations in experimental cell lines and native biologic systems, which significantly expands the molecular and functional diversity of the KV7 channel family (Abbott and Goldstein, 2001; Wrobel et al., 2012). Further, KV7 channel expression and function have also been shown to follow a certain level of subtype and tissue specificity. For example, KV7.1 channels have established roles in the repolarization of the cardiac action potential (Barhanin et al., 1996), heteromeric KV7.2/KV7.3 channels underlie the neuronal “M current” (Brown and Passmore, 2009), and homomeric or heteromeric combinations of KV7.4 and KV7.5 channel subtypes appear to be most critical in smooth muscle function (Haick and Byron, 2016). Understandably, the development of novel KV7 channel pharmacological modulators, with improved selectivity to distinguish among individual KV7 channel subtypes, is a highly sought endeavor. Indeed, several KV7 channel pharmacological modulators have emerged in recent years displaying improved selectivity for individual KV7 channel subtypes, including the KV7.2/KV7.3 channel activator ICA-069673 [N-(2-chloro-5-pyrimidinyl)-3,4-difluorobenzamide], a potent inhibitor of DSM excitability and contractility (Provence et al., 2015).
The compound N-(2,4,6-trimethylphenyl)-bicyclo[2.2.1]heptane-2-carboxamide (ML213) is a novel KV7 channel activator initially characterized as being selective for KV7.2 and KV7.4 channel subtypes displaying ∼80-fold selectivity over other KV7 channels (Yu et al., 2010, 2011), as well as greater potency than other Kv7 activators such as retigabine and BMS 204352 [(3S)-(+)-(5-chloro-2-methoxyphenyl)-1,3-dihydro-3-fluoro-6-(trifluoromethyl)-2H-indole-2-one] (Jepps et al., 2014). Subsequent in vitro pharmacological and electrophysiological studies in A7r5 vascular smooth muscle cells have reported ML213 as a potent activator of homomeric KV7.4 and KV7.5 channels and heteromeric KV7.4/KV7.5 channels (Brueggemann et al., 2014a). ML213 is currently the only known KV7 channel activator with this unique pharmacological selectivity profile. Nonetheless, studies seeking to identify whether heteromeric KV7 channels, specifically heteromeric KV7.4/KV7.5 channels, are expressed in DSM are currently absent. To identify the potential ML213-sensitive KV7 channel conformations expressed at the cellular level in DSM would greatly enhance our understanding of ML213 pharmacological effects on DSM function, while expanding our knowledge of the physiologic roles of KV7 channel subtypes in the urinary bladder.
Here, we used the novel KV7 channel activator ML213 to reveal insights into the subtype-specific KV7 channel functional roles in DSM excitability and contractility. This was achieved by a multidisciplinary experimental approach using isometric DSM tension recordings, ratiometric fluorescence Ca2+ imaging, and amphotericin-B perforated patch-clamp electrophysiology. Further, in situ proximity ligation assay (PLA) was conducted to elucidate whether KV7.4 and KV7.5 channels, key pharmacological targets of ML213, colocalize to form heteromeric channel complexes in DSM cells.
Materials and Methods
Ethical Approval for Animal Use.
Protocols for the use of experimental animals in the current study are consistent with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals and were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina, Columbia, SC (protocols #2186-100859-072114 and #2321-101157-092116).
Animal Housing, Euthanization, and DSM Tissue Acquisition.
Forty-one adult male Hartley-Albino guinea pigs (Charles River Laboratories, Raleigh, NC), 3–12 months of age and weighing 341−1191 g (average weight, 902 ± 10 g), were used for this study. Animals were housed in the Animal Resource Facilities at the University of South Carolina where they had free access to food and water and were exposed to 12-hour light/dark cycles at room temperature (22–23°C). Animals were euthanized via CO2 inhalation using a SMARTBOX Automated CO2 Delivery System (Euthanex Corp., Palmer, PA), followed by thoracotomy. The urinary bladder was then removed by an incision superior to the bladder neck. After dissection, the urinary bladder was cut open via a longitudinal incision originating from the urethral orifice. This allowed the bladder to be opened and pinned down flat to the base of a Sylgard-coated (Dow Corning, Corning, NY) Petri dish. Under a dissection microscope, microscissors and forceps were used to remove the entire mucosal layer and lamina propria, including the urothelium.
DSM Cell Isolation and Collection.
DSM single cells were freshly isolated using a combination of papain and collagenase, as previously described (Hristov et al., 2012b). DSM cells were used for electrophysiological experiments or in situ PLA within 12 hours after isolation. Intact DSM strips with urothelium removed were used for single-cell isolation, isometric DSM tension recordings, and ratiometric fluorescence Ca2+ imaging.
Isometric DSM Tension Recordings.
Isometric DSM tension recordings were conducted as previously described on isolated DSM strips void of urothelium (Hristov et al., 2012b). For experiments on spontaneous phasic, carbachol (CCh)-induced, and KCl-induced contractions, the DSM isolated strips were pretreated with the neuronal voltage-gated Na+ channel blocker tetrodotoxin (TTX; 1 µM) before the concentration-response effects of ML213 (0.1–30 µM) were assessed. Electrical field stimulation (EFS)–induced DSM contractions were stimulated with a PHM-152I programmable stimulator (Med Associates, Inc., Geogia, VT) in the absence of TTX. A 10- or 20-Hz EFS frequency, respectively, was delivered at 1-minute intervals, and ML213 (0.1–30 µM) was administered once a stable baseline and a contraction amplitude were achieved. In a separate protocol, EFS-induced DSM contractions were stimulated at 3-minute intervals over a wide range of frequencies (0.5, 2.0, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, and 50 Hz) in the absence (control) and then in the presence of ML213 (1 or 3 µM). The inhibitory effects of ML213 were normalized to the control (taken to be 100%) and expressed as percentages, with 100% indicating no effect and 0% indicating complete DSM relaxation.
Ratiometric Fluorescence Ca2+ Imaging.
Global intracellular Ca2+ concentrations of isolated DSM strips were measured using the ratiometric fluorescence Ca2+ probe Fura-2 AM, as previously described (Hristov et al., 2012a; Provence et al., 2015).
Perforated Patch-Clamp Electrophysiology.
KV7 currents were recorded using the amphotericin-B perforated patch-clamp technique in voltage-clamp mode. At a holding potential of −10 mV (corrected for junction potential), KV7 currents were recorded by applying 500-millisecond voltage steps at 10-mV intervals from voltages ranging from −80 to +40 mV. To effectively isolate KV7 currents, recordings were made in the presence of the selective large-conductance voltage-activated and Ca2+-activated K+ (BK) channel inhibitor paxilline (1 µM) and GdCl3 (50 µM), an inhibitor of nonselective cation channels and l-type voltage-gated Ca2+ channels (Mani et al., 2011; Brueggemann et al., 2012a). The amphotericin-B perforated patch-clamp technique in current-clamp mode (I = 0) was used to record the DSM cell membrane potential, as previously described (Afeli et al., 2013; Provence et al., 2015).
In Situ PLA.
In situ PLA was performed on freshly isolated DSM cells using the Duolink In Situ Orange Starter Kit Goat/Rabbit (catalog #DUO92106; Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. DSM cells underwent fixation in 2% paraformaldehyde (10 minutes, 37°C) followed by two 15-minute washes in phosphate-buffered saline. DSM cells were then blocked in Duolink Blocking Solution (Sigma-Aldrich) (30 minutes, 37°C). Subsequently, DSM cells were incubated with the anti-rabbit KV7.4 (catalog #sc-50417; Santa Cruz Biotechnology, Dallas, TX) and anti-goat KV7.5 (catalog #sc-18048; Santa Cruz Biotechnology) antibodies or with the control anti-goat inositol triphosphate receptor (IP3R) antibody (catalog #sc-28614; Santa Cruz Biotechnology), respectively, in Duolink antibody diluent solution overnight at 4°C, followed by 2× 5-minute washes in Duolink 1× Wash Buffer A Solution (Sigma-Aldrich). DSM cells were then incubated with the Duolink PLA Probe Anti-Rabbit MINUS and Anti-Goat PLUS oligonucleotide-conjugated secondary antibodies (Sigma-Aldrich) (1 hour, 37°C), followed by 2× 5-minute washes in Duolink 1× Wash Buffer A Solution (Sigma-Aldrich). DSM cells were then incubated in ligase-ligation solution (30 minutes, 37°C), which hybridizes the two PLA MINUS and PLUS probes when in close proximity (<40 nm). After ligation, DSM cells were washed 2× for 2 minutes in Duolink 1× Wash Buffer A solution. Finally, DSM cells were incubated in amplification-polymerase solution (100 minutes, 37°C) containing nucleotides and fluorescently labeled oligonucleotides. The oligonucleotide arm of one PLA probe is a primer for rolling-circle amplification. The fluorescently labeled oligonucleotides hybridize to the rolling circle amplification product thus permitting detection. After amplification, DSM cells were washed 2× 10 minutes in 1× wash buffer B and then a 1-minute wash in 0.01× wash buffer B. Slides were then mounted using minimal volume of Duolink In Situ Mounting Medium with 4′,6-diamidino-2-phenylindole dihydrochloride (Sigma-Aldrich). PLA signals (Duolink In Situ Detection Reagents Orange; Sigma-Aldrich) (λexcitation/emission = 554/576 nm) were detected using a laser-scanning LSM 700 Confocal Microscope (Carl Zeiss, Oberkochen, Germany) with a 63× oil-immersion objective.
Solutions and Drugs.
Nominally Ca2+-free dissection solution contained the following (in mM): 80 monosodium glutamate; 55 NaCl; 6 KCl; 10 glucose; 10 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES); 2 MgCl2; NaOH was used to attain pH 7.3. Ca2+-containing physiologic saline solution contained the following (millimolar): 119 NaCl; 4.7 KCl; 24 NaHCO3; 1.2 KH2PO4; 2.5 CaCl2; 1.2 MgSO4; 11 glucose; aerated with 95% O2/5% CO2 to attain pH 7.4. Physiologic bath solution used for patch-clamp experiments was composed of the following (millimolar): 134 NaCl; 6 KCl; 1 MgCl2; 2 CaCl2; 10 glucose; and 10 HEPES; and NaOH was used to obtain pH 7.4. The patch-pipette solution contained the following (millimolar): 110 potassium aspartate; 30 KCl; 10 NaCl; 1 MgCl2; and 10 HEPES; 0.05 EGTA; NaOH was used to adjust the pH to 7.2. Amphotericin-B stock solution was prepared daily in dimethylsulfoxide and was included in the pipette solution (200–300 μg/ml) before the experiment and was replaced every 1–2 hours. ML213, TTX citrate, and XE991 were purchased from Tocris Bioscience (Minneapolis, MN). Fura-2 AM was purchased from Life Technologies (catalog #F1221; Carlsbad, CA). ML213 and Fura-2 AM were dissolved in dimethylsulfoxide as stock solutions, whereas XE991 and TTX citrate were dissolved in double-distilled water. All other chemicals were obtained from Fisher Scientific (Waltham, MA), Sigma-Aldrich, or Worthington Biochemical Corp. (Lakewood, NJ).
Data Analysis and Statistics.
Isometric DSM tension-recording experiments for spontaneous phasic, pharmacologically induced, and nerve-evoked contractions were conducted and analyzed as previously described (Hristov et al., 2012b; Afeli et al., 2013; Provence et al., 2015). Perforated whole-cell patch-clamp experiments were performed and analyzed using pCLAMP 10.3. Where specified in the summarized data, “n” represents the number of freshly isolated DSM strips or cells, and “N” represents the number of guinea pigs. Statistical analyses were based on n, the number of DSM cells or strips, respectively. For spontaneous contraction experiments, statistical analysis for the comparison of ML213 in the absence or presence of XE991, a two-way ANOVA test was performed followed by Bonferroni post hoc test. Paired Student’s t-tests or Wilcoxon matched-pairs signed rank tests were used for the remaining statistical analyses. Summarized data are reported as the mean ± S.E.M. or medians with 25th and 75th percentiles. P values <0.05 (two tailed) were considered to be statistically significant. CorelDRAW X3 Graphic Suite software (Corel Co., Mountain View, CA) and GraphPad Prism 4.03 or 7 software (GraphPad Software, La Jolla, CA) were used for data illustration and data analyses.
Results
ML213 Decreased Spontaneous Phasic Contractions in DSM Isolated Strips.
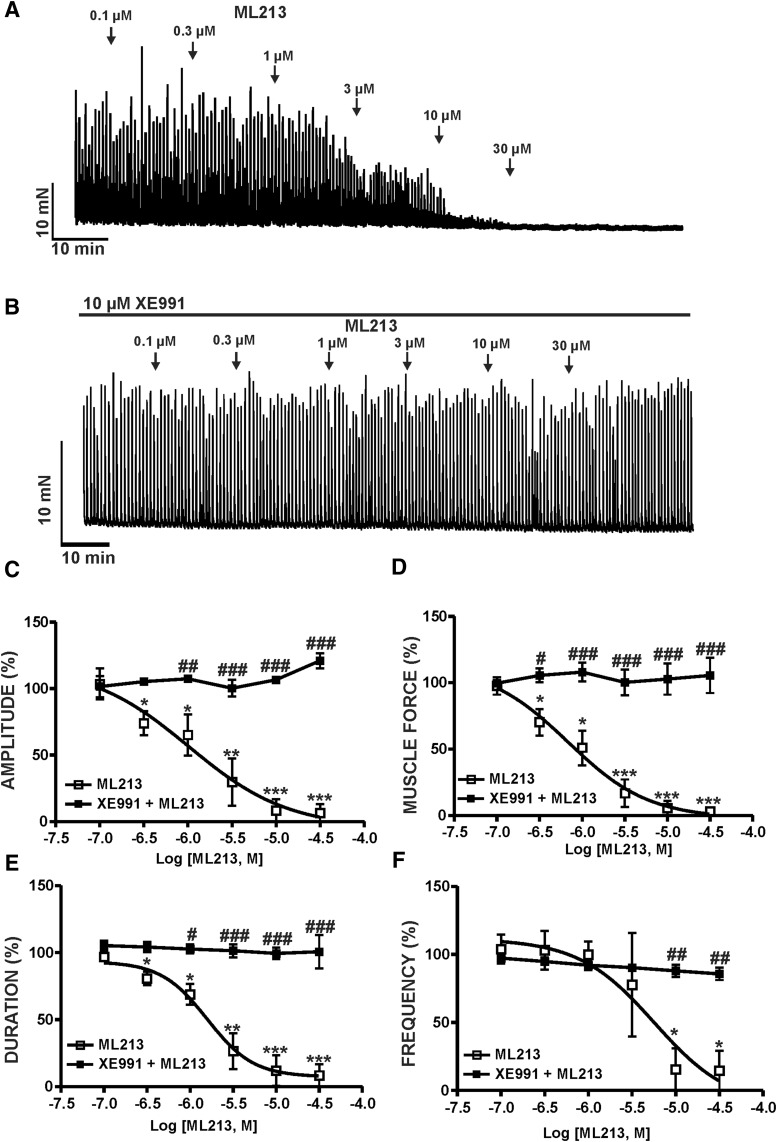
To explore the physiologic effects of KV7 channel pharmacological activation with ML213 on spontaneous phasic contractions, we conducted isometric DSM tension recordings on DSM isolated strips. ML213 (0.1–30 µM) caused a concentration-dependent inhibition of spontaneous phasic contractions in DSM isolated strips (n = 6, N = 6; P < 0.05) (Fig. 1; Supplemental Fig. 1). Pretreatment of DSM isolated strips with the KV7 channel inhibitor XE991 (10 µM) abolished ML213-induced (0.1–30 µM) inhibition of spontaneous phasic contractions, confirming selectivity for KV7 channels (n = 6, N = 6; P < 0.05) (Fig. 1; Supplemental Fig. 1). IC50 and maximal efficacy values for ML213 on spontaneous phasic contractions are reported in Table 1.
Fig. 1.
KV7 channel pharmacological activation with ML213 leads to an inhibition of spontaneous phasic contractions in DSM isolated strips. (A) A representative isometric DSM tension recording demonstrating that ML213 (0.1–30 µM) inhibited spontaneous phasic contractions in a DSM isolated strip. (B) A representative isometric DSM tension recording illustrating the KV7 channel inhibitor XE991 (10 µM) abolished the inhibitory effects of ML213 (0.1–30 µM) on DSM spontaneous phasic contractions. Cumulative concentration-response curves for ML213 on DSM phasic contraction: amplitude (C), muscle force (D), duration (E), and frequency (F) in the absence and presence of the KV7 channel inhibitor XE991 at 10 (micro)M (n = 6, N = 6; *P < 0.05; **P < 0.01; ***P < 0.001). #P < 0.05; ##P < 0.01; ###P < 0.001 indicate statistical significance in the effects of ML213 on spontaneous phasic contractions in the absence (control) versus the presence of XE991 (n = 6, N = 6; two-way ANOVA followed by Bonferroni post-test). See Table 1 for potency and maximum efficacy values and Supplemental Fig. 1 for the illustration of concentration responses, data for which are shown as the mean ± S.D.
TABLE 1.
IC50 and maximal efficacy values for the effects of ML213 on spontaneous phasic, KCl-induced (20 mM), CCh-induced (0.1 or 1 µM), and EFS-induced (10 or 20 Hz) phasic contractions in guinea pig DSM isolated strips
IC50 values are presented as the mean (95% confidence interval). Maximal efficacy is presented as the mean ± S.E.M. and is normalized to the control on a scale from 100% to 0% (full relaxation); n/a=not applicable (negligible effect on parameter).
| Condition | Amplitude | Muscle Force | Duration | Frequency | n/N |
|---|---|---|---|---|---|
| Spontaneous phasic contractions | 1.1 (0.5–2.4) μM; 95.6% ± 4.4% | 0.7 (0.2–2.8) μM; 96.8% ± 3.2% | 1.6 (0.8–2.9) μM; 91.6% ± 8.41% | 3.9 (0.8–20.3) μM; 85.4% ± 14.6% | 6/6 |
| KCl (20 mM)-induced contractions | 2.7 (1.4–5.1) μM; 99.2% ± 0.8% | 2.5 (1.2–5.3) μM; 99.1% ± 0.9% | 3.9 (1.9–8.2) μM; 90.3% ± 9.7% | n/a; 87.6% ± 8.2% | 7/7 |
| CCh (0.1 μM)-induced contractions | 3.5 (1.9–6.4) μM; 100% inhibition | 2.1 (1.1–3.9) μM; 100% inhibition | 3.0 (1.8–5.3) μM; 99.7% ± 0.29% | n/a; 100% inhibition | 6/6 |
| CCh (1 μM)-induced contractions | 6.2 (1.2–30.7) μM; 84% ± 9.1% | 4.4 (0.8–22.3) (micro)M; 92.1% ± 4.2% | 11.3 (2.1–61.2) (micro)M; 68% ± 9.5% | n/a; 47.1% ± 15.4% | 6/6 |
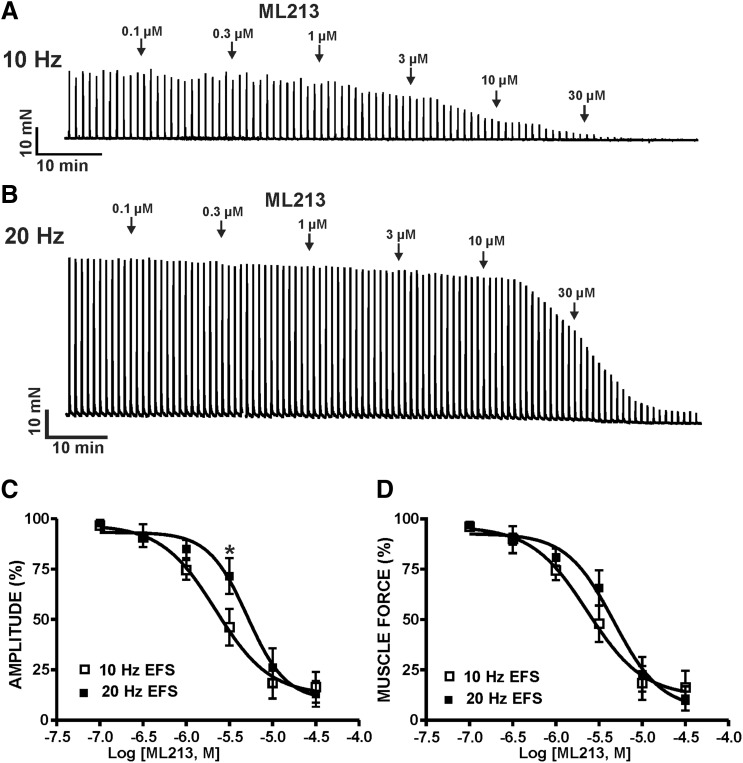
| 10-Hz EFS-induced contractions | 2.2 (1.3–3.7) μM; 83.6% ± 7.6% | 2.3 (1.3–4.1) μM; 83.9% ± 8.2% | n/a; 32.78% ± 13.5% | n/a | 7/6 |
| 20-Hz EFS-induced contractions | 5.1 (3.1–8.2) μM; 86.9% ± 6.4% | 4.7 (2.7–8.1) μM; 89.1% ± 6.0% | n/a; 37.46% ± 9.3% | n/a | 6/6 |
ML213 Decreased KCl-Induced Contractions in DSM Isolated Strips.
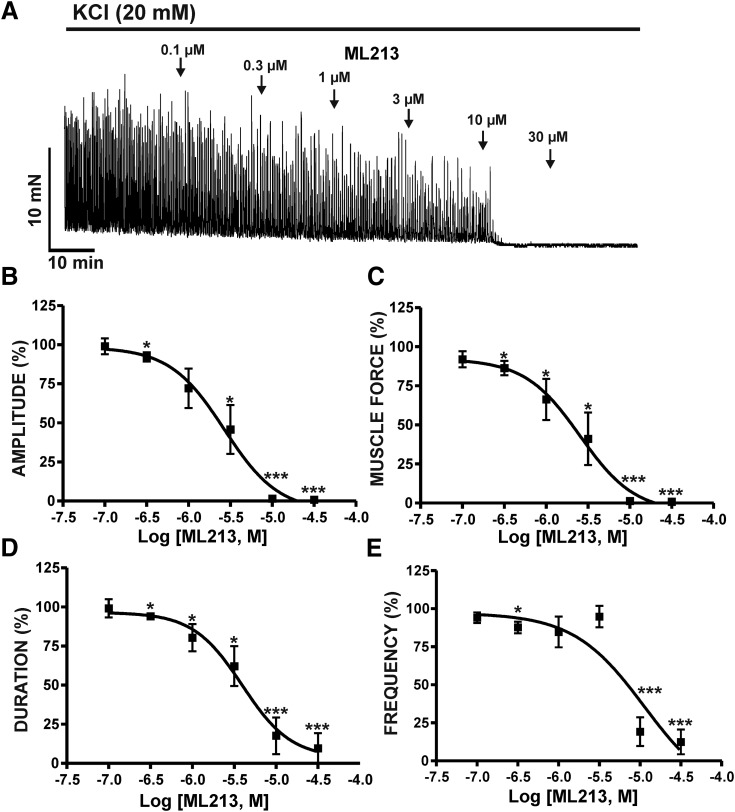
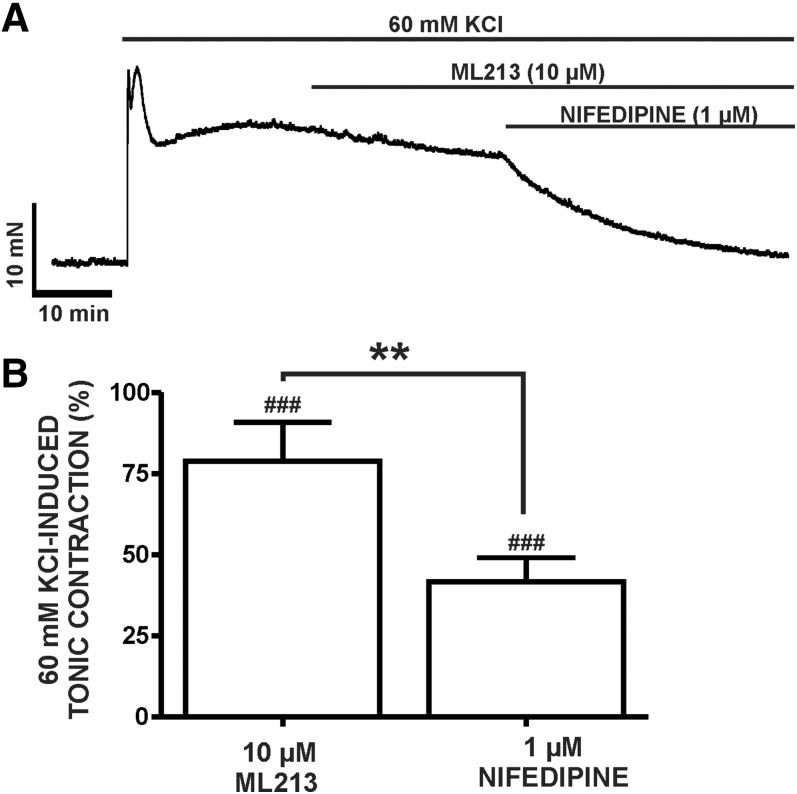
Next, we elucidated the effects of ML213 (0.1–30 µM) on DSM contractions induced by mild membrane depolarization using 20 mM KCl. In the presence of 20 mM KCl, ML213 (0.1–30 µM) caused a concentration-dependent inhibition of DSM contractions and exhibited a decrease in potency in comparison with ML213 inhibitory effects on spontaneous phasic contractions (Table 1) (n = 7, N = 7; P < 0.05) (Fig. 2; Supplemental Fig. 2). To further explore the role of K+ conductance in ML213-induced inhibition of DSM contractions and the potential direct interaction with l-type voltage-gated Ca2+ channels, we tested the effects of ML213 (10 μM) on tonic DSM contractions stimulated by high 60 mM KCl. Here, ML213 (10 μM) decreased 60 mM KCl-induced muscle tone to only 74% ± 3.5% of the control (n = 8, N = 4; P < 0.01) (Fig. 3; Supplemental Fig. 3). Subsequent application of the l-type voltage-gated Ca2+ channel inhibitor nifedipine, however, caused an inhibitory effect significantly greater in comparison with ML213 alone, decreasing DSM tone to 39.7% ± 6.4% of the control (n = 8, N = 4; P < 0.001) (Fig. 3; Supplemental Fig. 3). The difference between the inhibitory effects of ML213 (10 µM) and nifedipine in the presence of ML213 on tonic DSM contraction were statistically significant (n = 8, N = 4; P < 0.001) (Fig. 3; Supplemental Fig. 3). These data suggest that ML213 does not directly inhibit l-type voltage-gated Ca2+ channel activity in DSM.
Fig. 2.
KV7 channel pharmacological activation with ML213 leads to an inhibition of 20 mM KCl-induced phasic contractions in DSM isolated strips. (A) A representative isometric DSM tension recording demonstrating the concentration-dependent inhibition of 20 mM KCl-induced DSM phasic contractions by ML213 (0.1–30 µM). Cumulative concentration-response curves for the inhibitory effects of ML213 on DSM phasic contraction: amplitude (B), muscle force (C), duration (D), and frequency (E) (n = 7, N = 7; *P < 0.05; ***P < 0.001; two-tailed paired Student’s t-test). See Table 1 for potency and maximum efficacy values and Supplemental Fig. 2 for illustration of summary concentration-responses, data for which are shown as the mean ± S.D.
Fig. 3.
Differential inhibitory effects of ML213 and nifedipine on 60 mM KCl-induced tonic contractions in DSM isolated strips. (A) A representative isometric DSM tension recording demonstrating the differential effects of 10 µM ML213 and 1 µM nifedipine upon a depolarization-induced DSM tonic contraction caused by 60 mM KCl in a DSM isolated strips. (B) Summarized data demonstrating the differential inhibitory effects of ML213 (10 µM) and nifedipine (1 µM) + ML213 on DSM 60 mM KCl-induced tonic contraction (n = 8, N = 4; ###P < 0.001 versus control; and **P < 0.001 ML213 versus nifedipine + ML213, respectively; two-tailed paired Student’s t-test). See Supplemental Fig. 3 for the display of summary responses as scatterplots with means.
CCh Attenuated ML213-Induced Inhibition of DSM Contractions in a Concentration-Dependent Manner.
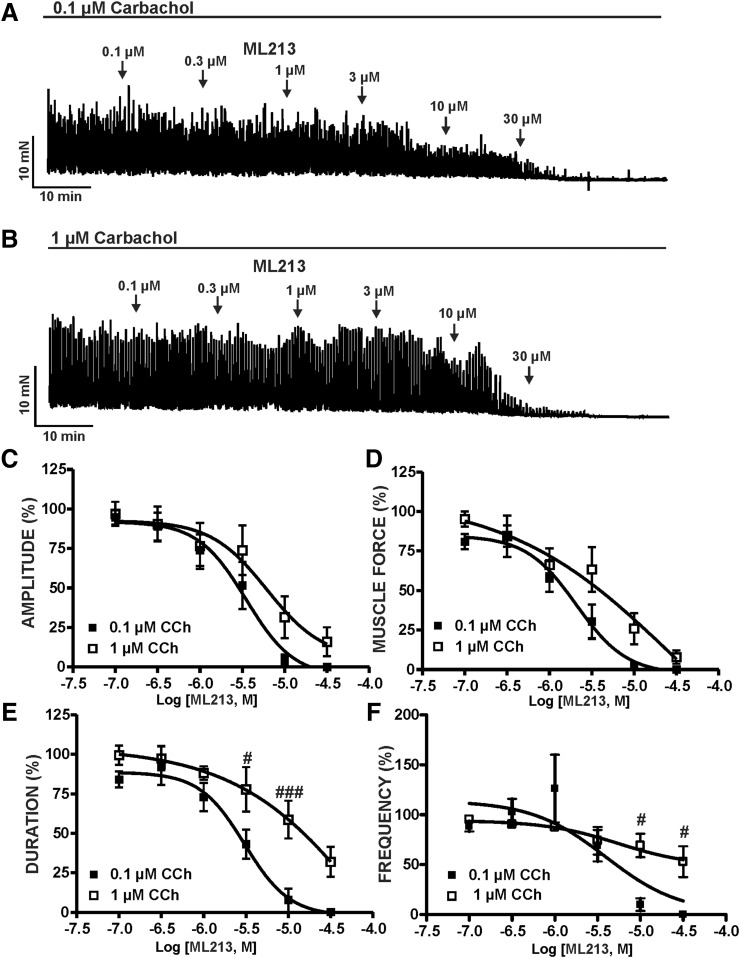
We next sought to determine the potential involvement of mAChR signaling in ML213-induced inhibition of DSM contractility. This was achieved by examining the inhibitory effects of ML213 (0.1–30 µM) on DSM contractions stimulated by the mAChR agonist CCh (0.1 or 1 µM). ML213 (0.1–30 µM) caused a concentration-dependent inhibition of both 0.1 and 1 µM CCh (n = 6, N = 6 for both; P < 0.05) (Fig. 4; Supplemental Fig. 4). However, in comparison with 0.1 µM, mAChR stimulation with 1 µM CCh markedly reduced the potency of ML213 on DSM contraction duration and muscle force (Table 1). IC50 and maximal efficacy values for ML213 on 0.1 and 1 µM CCh-induced DSM contractions are reported in Table 1 (see also Fig. 4).
Fig. 4.
KV7 channel pharmacological activation with ML213 leads to an inhibition of CCh-induced DSM phasic contractions. (A) A representative isometric DSM tension recording demonstrating the concentration-dependent inhibition of 0.1 µM CCh-induced phasic contractions in a DSM isolated strip. (B) A representative isometric DSM tension recording demonstrating the concentration-dependent inhibition of 1 µM CCh-induced phasic contractions in a DSM isolated strip. Cumulative concentration-response curves for ML213 on CCh-induced (0.1 or 1 µM) DSM phasic contraction amplitude (C), muscle force (D), duration (E), and frequency (F) (n = 6, N = 6 for both data sets; #P < 0.05; ###P < 0.001; two-tailed paired Student’s t-test). See Table 1 for potency and maximum efficacy values and Supplemental Fig. 4 for the illustration of summary concentration-responses, data for which are shown as the mean ± S.D. ANOVA analyses revealed significant differences for ML213-induced relaxation in the presence of 0.1 (micro)M CCh versus 1 (micro)M CCh for muscle force (P = 0.006) and duration (P < 0.001); for amplitude the difference just failed to reach significance (P = 0.055).
ML213 Decreased Nerve-Evoked Contractions in DSM Isolated Strips.
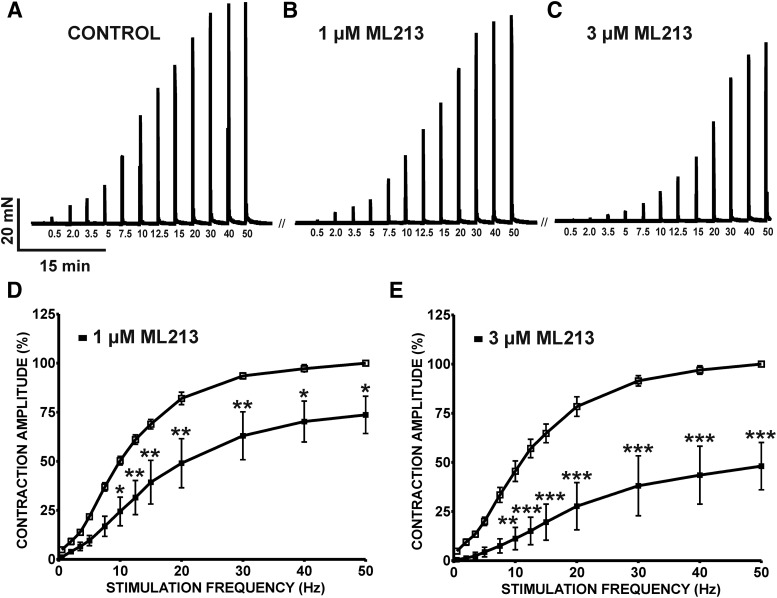
To further explore potential roles for mAChRs in ML213-induced inhibition of DSM contractions, ML213 (0.1–30 µM) inhibitory effects were examined on nerve-evoked contractions stimulated by either 10- or 20-Hz EFS. ML213 (0.1–30 µM) caused a concentration-dependent inhibition of EFS-induced contractions stimulated continuously at both 10- and 20-Hz EFS frequencies (n = 7, N = 6 for 10 Hz; n = 6, N = 6 for 20 Hz) (Fig. 5; Supplemental Fig. 5). IC50 and maximal efficacy values for ML213 on 10- and 20-Hz EFS-induced contractions are reported in Table 1. In comparison with 10-Hz EFS, the potency of ML213 on 20-Hz EFS-induced contractions was reduced by more than 2-fold (see Table 1). Next, ML213 (1 or 3 µM) inhibitory effects were examined on contractions induced over a range of EFS frequencies (0.5–50 Hz). ML213 (1 or 3 µM) decreased the amplitude of 10- to 50-Hz EFS-induced contractions (n = 6, N = 6; P < 0.05) (Fig. 6; Supplemental Fig. 6).
Fig. 5.
KV7 channel pharmacological activation with ML213 leads to an inhibition of nerve-evoked contractions induced by 10- and 20-Hz EFS in DSM isolated strips. (A) A representative isometric DSM tension recording demonstrating the concentration-dependent inhibition of 10 Hz EFS-induced contractions by ML213 (0.1–30 µM) in DSM isolated strips. (B) A representative isometric DSM tension recording illustrating the concentration-dependent inhibition of 20-Hz EFS-induced contractions by ML213 (0.1–30 µM) in DSM isolated strips. Cumulative concentration-response curves for ML213 on DSM EFS-induced contraction amplitude (C) and muscle force (D) (n = 7, N = 6 for 10 Hz; n = 6, N = 6 for 20 Hz; *P < 0.05 for 10 versus 20 Hz; two-tailed paired Student’s t-tests). See Table 1 for potency and maximum efficacy values and Supplemental Fig. 5 for illustration of summary concentration-responses, data for which are shown as the mean ± S.D.
Fig. 6.
ML213 decreases 0.5- to 50-Hz EFS-induced contractions in DSM isolated strips. The original isometric DSM tension recordings of 0.5- to 50-Hz EFS-induced contractions in DSM isolated strips before (control) (A) and after (B) the addition of 1 µM ML213 and 3 µM ML213 (C). Cumulative frequency-response curves demonstrating the inhibitory effects of 1 µM ML213 (D) and 3 µM ML213 (E) on the amplitude of 0.5- to 50-Hz EFS-induced contractions in DSM isolated strips (n = 6, N = 6 for both data sets; *P < 0.05; **P < 0.01; ***P < 0.001; two-tailed paired Student’s t-test). See Supplemental Fig. 6 for the display of summary frequency-responses, data for which are shown as the mean ± S.D.
ML213 Decreased Global Intracellular Ca2+ Concentrations in DSM Isolated Strips.
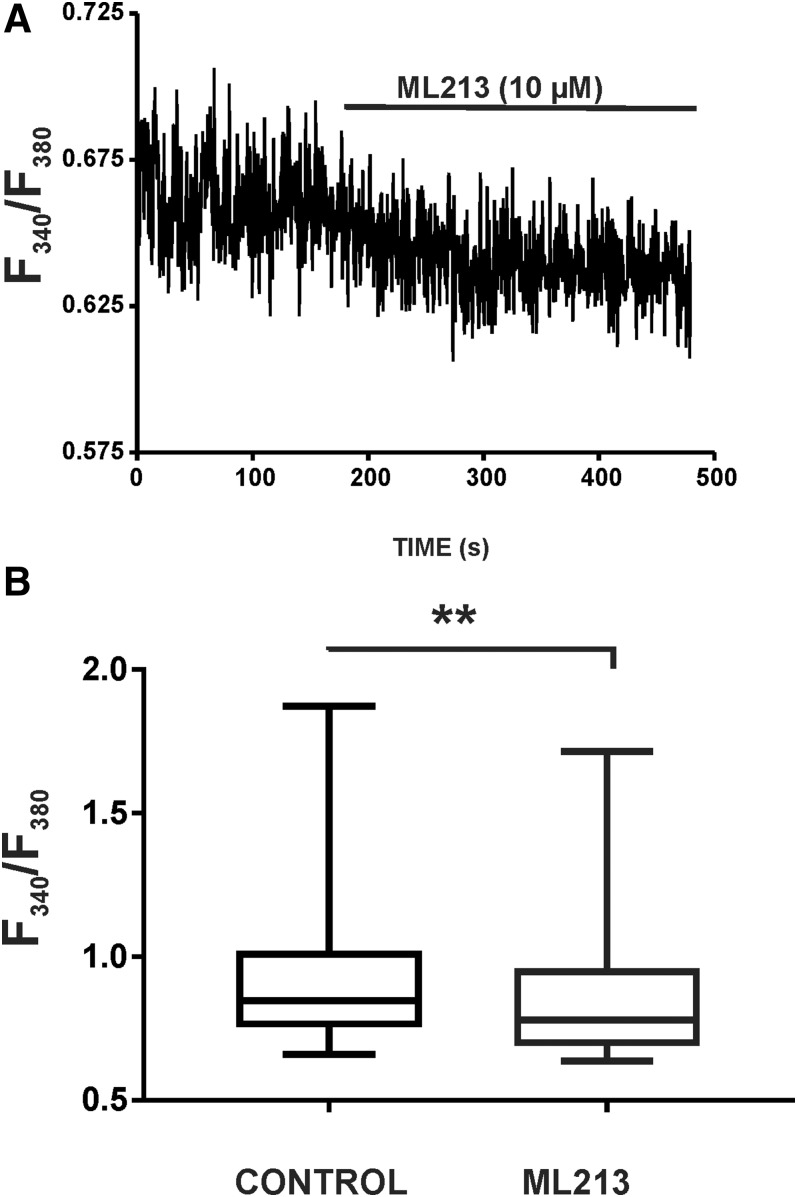
We next aimed to assess the effects of ML213 on global intracellular Ca2+ concentrations of isolated DSM strips loaded with Fura-2. This was achieved using the ratiometric fluorescence Ca2+ indicator Fura-2 AM and measuring the emission at 510 nm with excitation at 340 and 380 nm. Under control conditions in the absence of ML213, the median intensity (F340/F380) was 0.85 (0.75–1.02, 25th to 75th percentiles) (n = 8, N = 6), and in the presence of ML213 (10 µM) the median F340/F380 ratio decreased to a median of 0.78 (0.69–0.96) (n = 8, N = 6; P < 0.01) (Fig. 7; Supplemental Fig. 7). These results suggest that KV7 channel pharmacological activation with ML213 critically regulates global intracellular Ca2+ concentrations in DSM isolated strips and thus DSM contractility.
Fig. 7.
ML213 decreases global intracellular Ca2+ concentrations in DSM isolated strips. (A) Original recordings illustrating that ML213 (10 µM) significantly decreased global intracellular Ca2+ levels in a Fura-2–loaded DSM strip. (B) Summarized data (as box-whisker plots) representing the reduction in global Ca2+ levels by 10 µM ML213 in DSM isolated strips (n = 8, N = 6; **P < 0.01; two-tailed Wilcoxon matched-pairs signed rank test). See Supplemental Fig. 7 for the display of summary data as scatterplots with medians.
ML213 Enhanced the Amplitude of Whole-Cell KV7 Currents in Freshly Isolated DSM Cells.
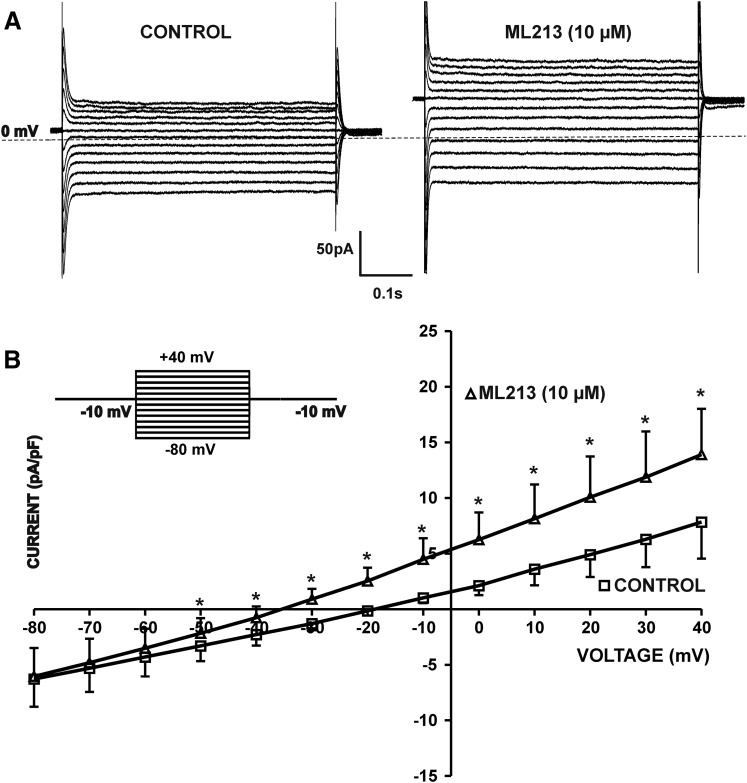
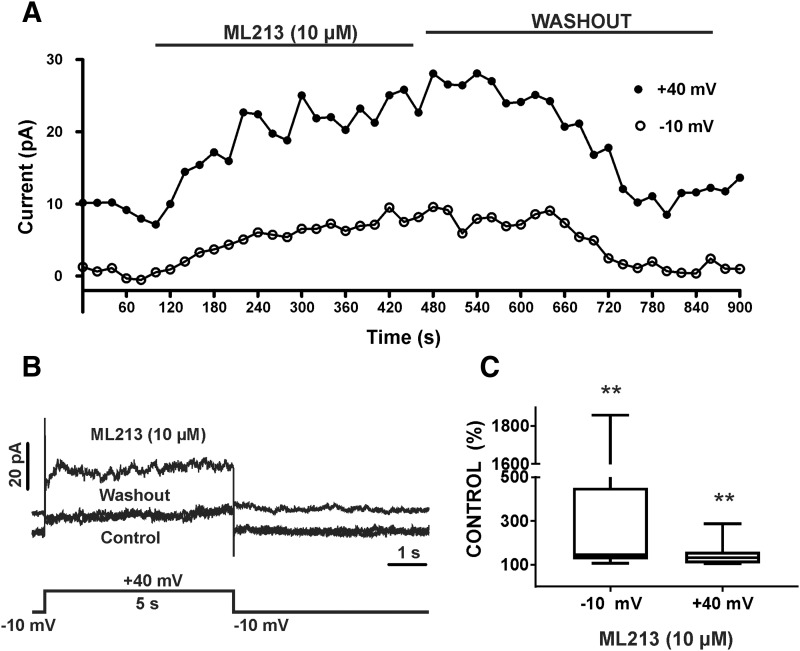
We next sought to examine the pharmacological effects of ML213 on whole-cell KV7 currents using the perforated patch-clamp technique in voltage-clamp mode. In the first series of experiments, KV7 currents were recorded at a holding potential of −10 mV wherein 500-millisecond voltage steps were applied at 10 mV intervals from −80 to +40 mV. To effectively isolate KV7 currents, recordings were made in the presence of the selective BK channel inhibitor paxilline (1 µM) and GdCl3 (50 µM), an inhibitor of nonselective cation and l-type voltage-gated Ca2+ channels. Further, the more depolarized holding potential of −10 mV that we used ensures inactivation of other non-KV7 K+ channels, such as inward-rectifying K+ channels (Brueggemann et al., 2012b). ML213 (10 µM) caused a significant increase in the amplitude of whole-cell KV7 currents at all voltages from −50 to +40 mV (n = 7, N = 5; P < 0.05) (Fig. 8). We further sought to examine the time course for the pharmacological effects of ML213 on KV7 currents in DSM cells. This was achieved using a one-step voltage-clamp protocol with a 5-second duration stimulus applied every 20 seconds from a −10 mV holding potential to +40 mV. ML213 (10 µM) caused a significant increase in the whole-cell KV7 current amplitude median values to 145.7% (125.3%–451.5%, 25th and 75th percentiles) and 132.1% (107.0%–159.2%, 25th and 75th percentiles), respectively, in comparison with the control (n = 8, N = 5; P < 0.01) (Fig. 9; Supplemental Fig. 9).
Fig. 8.
ML213 (10 µM) enhanced whole-cell KV7 currents in freshly isolated DSM cells. (A) Representative whole-cell patch-clamp recordings illustrating the potentiation of KV7 currents induced by the introduction of ML213 in the bath external solution. Paxilline (1 µM) and GdCl3 (50 µM) were present in the solution. (B) Current-voltage relationships obtained in the absence (control) or presence of 10 µM ML213 in a whole-cell voltage-clamp recording. The two curves represent the current-voltage relationship expressed as normalized current in the absence or presence of 10 µM ML213 (n = 7, N = 5, *P < 0.05; two-tailed paired Student’s t-test). (B, top left insert) The 500-millisecond voltage-step protocol from −80 to +40 mV with a holding potential at −10 mV. See Supplemental Fig. 8 for current-voltage plots, data for which are shown as the mean ± S.D.
Fig. 9.
Time course of ML213-induced activation of KV7 channel currents in freshly isolated DSM cells. (A) Effects of ML213 on KV7 currents recorded using a one-step voltage-clamp protocol with a 5-second duration stimulus applied every 20 seconds from a −10-mV holding potential to +40 mV. (B) Representative traces (and voltage step used) of the experiment plotted in (A) for the control, ML213 (10 µM), and washout of ML213. (C) Summary data for the percentage of KV7 currents in the presence of ML213 compared with control at −10 and +40 mV, respectively (n = 8, N = 5, **P < 0.01; two-tailed Wilcoxon matched-pairs signed rank test). Summarized data illustrate the average of four to six points of each steady-state current. See Supplemental Fig. 9 for display of summary data as scatterplots with medians.
ML213 Hyperpolarizes the Resting Membrane Potential in Freshly Isolated DSM Cells.
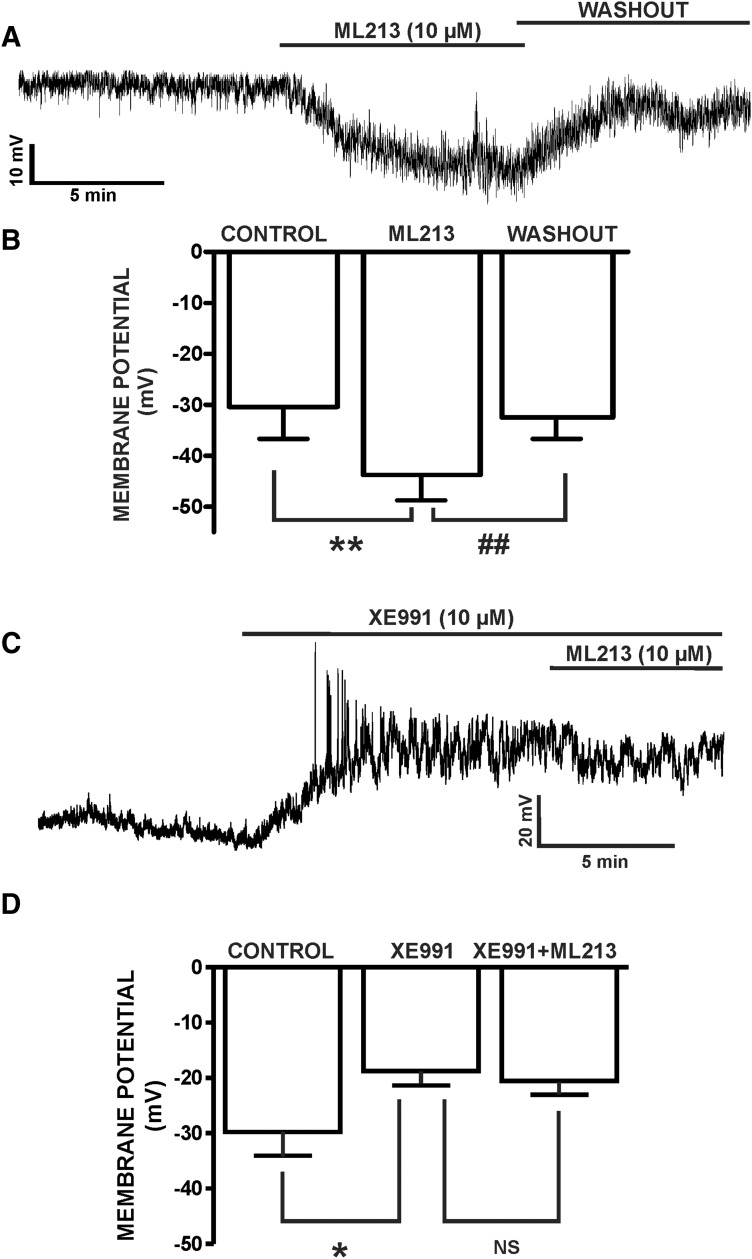
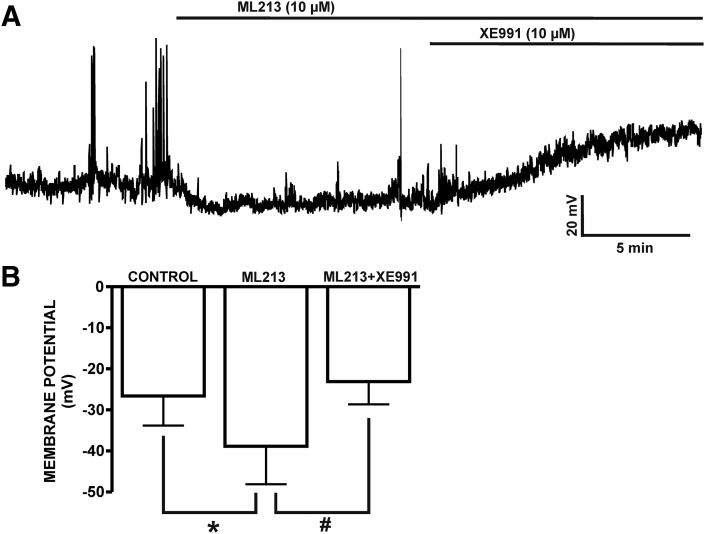
Using the perforated whole-cell patch-clamp technique in current-clamp mode, we next sought to determine the physiologic role of ML213-sensitive KV7 channels in regulating the DSM cell membrane potential. ML213-induced inhibitory effects were examined in five DSM isolated cells from five different animals, which had an average resting membrane potential of −30.4 ± 6.2 mV. Subsequently, ML213 hyperpolarized the DSM cell membrane potential to −43.8 ± 5.0 mV (n = 5, N = 5; P < 0.01) (Fig. 10; Supplemental Fig. 10). ML213-induced DSM cell membrane hyperpolarization was fully reversible by washout of ML213 (Fig. 10, A and B; Supplemental Fig. 10A). On the contrary, XE991 (10 µM) depolarized the DSM cell membrane potential from −29.8 ± 4.3 to −18.7 ± 2.7 mV and blocked ML213 (10 µM) hyperpolarization at −20.6 ± 2.8 mV (n = 8, N = 4; P < 0.05) (Fig. 10, C and D; Supplemental Fig. 10B), confirming ML213 selectivity for the KV7 channels. XE991 (10 µM) was also effective in reversing the hyperpolarizing effects of ML213 from −39.2 ± 9.2 to −23.2 ± 5.5 mV (control was −26.6 ± 7.2 mV) (n = 7, N = 6; P < 0.05) (Fig. 11; Supplemental Fig. 11). These combined results suggest key involvement for ML213-sensitive KV7.2, KV7.4, and KV7.5 channels in regulating the DSM cell membrane potential.
Fig. 10.
ML213 hyperpolarizes the resting membrane potential in freshly isolated DSM cells. (A) A representative membrane potential recording in current-clamp mode illustrating that ML213 (10 µM) hyperpolarized the DSM cell membrane potential in a freshly isolated DSM cell and that this effect was reversible by washout. (B) Summarized data for the hyperpolarizing effects of ML213 (10 µM) on the DSM cell membrane potential (n = 5, N = 5; **P < 0.01) and for the washout (n = 5, N = 5, ##P < 0.01). (C) A representative membrane potential recording in current-clamp mode demonstrating that XE991 (10 µM) depolarized the DSM cell membrane potential and blocked ML213 (10 µM) hyperpolarization in a freshly isolated DSM cell. (D) Summarized data for XE991-induced (10 µM) depolarization in freshly isolated DSM cells and the lack of ML213 (10 µM) hyperpolarizing effects in the presence of 10 µM XE991 (n = 8, N = 4; *P < 0.05; two-tailed paired Student’s t-test), NS, nonsignificant. See Supplemental Fig. 10 for display of summary data as scatterplots with means.
Fig. 11.
ML213-induced hyperpolarization is reversed by KV7 channel inhibition with XE991. (A) A representative current-clamp recording from a freshly isolated DSM cell illustrating hyperpolarization of the DSM cell membrane potential by ML213 (10 µM) and that the ML213-induced hyperpolarization is reversed by the KV7 channel inhibitor XE991 (10 µM). (B) Summary data for the hyperpolarizing effects of ML213 (10 µM) on the DSM cell membrane potential (n = 7, N = 5, *P < 0.05) effects reversible by 10 µM XE991 (n = 7, N = 5; #P < 0.05; two-tailed paired Student’s t-test). See Supplemental Fig. 11 for display of summary data as scatterplots with means.
KV7.4 and KV7.5 Channel α-Subunits Assemble to Form Heteromeric KV7.4/KV7.5 Channel Subtypes in Freshly Isolated DSM Cells.
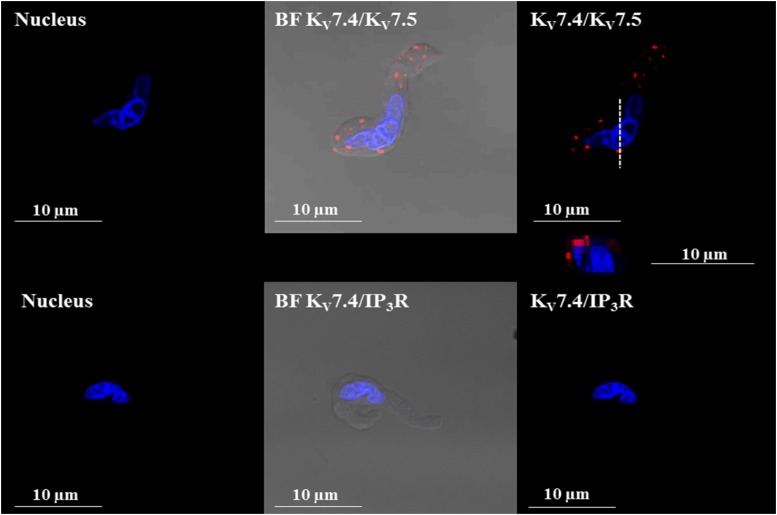
Using in situ PLA, we sought to determine whether KV7.4 and KV7.5 channels, among the key targets of ML213, form heteromeric channels in freshly isolated DSM cells. In situ PLA demonstrated that KV7.4 and KV7.5 channel subunits associate to form heteromeric KV7.4/KV7.5 channels in isolated DSM cells. As shown in Fig. 12, in situ PLA experiments demonstrated the colocalization of KV7.4 and KV7.5 channel α-subunits within 40 nm and within the vicinity of the DSM cell membrane. Primary antibodies against the IP3R, which would not be expected to colocalize with members of the KV7 channel family, were used as a negative control. Coincubation of the KV7.4 channel primary antibody with the primary antibody of the IP3Rs yielded no PLA fluorescent signal (Fig. 12). These combined results suggest that KV7.4 and KV7.5 channel subunits assemble to form heteromeric KV7.4/KV7.5 channel subtypes in DSM cells.
Fig. 12.
PLA shows that KV7.4/KV7.5 heteromeric channels are expressed in isolated guinea pig DSM cells. Images of a freshly isolated DSM cell stained with the combination of anti-KV7.4 and anti-KV7.5 channel antibodies (top panels). PLA fluorescent signals are shown in red. No PLA fluorescent signals were observed when the anti-KV7.4 antibody was coincubated with the anti-IP3R antibody (bottom panels). An orthogonal cross-sectional image of the DSM cell displaying PLA signals was obtained after z-stack scans collected along the viewing plane indicated by the dashed (- - -) lines and is represented directly below the top right panel. BF, bright field; DAPI, 4′,6-diamidine-2′-phenylindole dihydrochloride (nuclear staining shown in blue).
Discussion
The current study used the novel compound ML213, a potent and selective activator of KV7.2-, KV7.4-, and KV7.5-containing channels, to elucidate their physiologic roles in DSM function. ML213 inhibited spontaneous, pharmacologically induced, and nerve-evoked DSM contractions and decreased global intracellular Ca2+ concentrations in DSM strips. ML213 enhanced whole-cell KV7 currents and hyperpolarized the resting membrane potential of freshly isolated DSM cells. In situ PLA studies further revealed the expression of heteromeric KV7.4/KV7.5 channels within the vicinity of the DSM cell membrane.
Previous studies on guinea pig, including those from our group, demonstrated a role for KV7 channels in DSM function (Afeli et al., 2013; Anderson et al., 2013; Provence et al., 2015). Here, ML213 promoted concentration-dependent inhibition of spontaneous phasic contractions with IC50 values of 1.1 and 0.7 µM, respectively (Fig. 1; Table 1). The observed inhibitory effects of ML213 on DSM contractile activity are consistent with previous reports in human and pig DSM (Svalø et al., 2013, 2015; Bientinesi et al., 2017). Based on our data, ML213 displays greater potency in comparison with retigabine, the KV7.1 channel activator l-364373, and the KV7.2/KV7.3 channel activator ICA-069673 for inhibiting DSM spontaneous phasic contraction amplitude and muscle force (Afeli et al., 2013; Anderson et al., 2013; Provence et al., 2015).
ICA-069673 and ML213 are known to potently activate homomeric KV7.4 channels; however, the key distinction between these two compounds is the ability for ML213 to potently activate both homomeric KV7.5 and heteromeric KV7.4/KV7.5 channels (Brueggemann et al., 2014a). The greater potency for ML213 to inhibit spontaneous phasic, pharmacologically induced, and EFS-induced DSM contractions (Figs. 1–6), in comparison with ICA-069673 (Provence et al., 2015), supports the involvement of KV7.4- or KV7.5-containing channels in DSM function. Similar observations were reported using ML213 in rodent mesenteric arteries, where the KV7.4 channels subtypes are predominately expressed (Jepps et al., 2014).
ML213 inhibited DSM contractions induced by the mAChR agonist CCh in a concentration-dependent manner (Fig. 4). Consistently, ML213 exhibited a reduced effect on amplitude for 20-Hz EFS-induced DSM contractions in comparison with 10-Hz contractions (Fig. 5). ML213 inhibitory effects on CCh- and EFS-induced DSM contractions are in line with previous reports in pigs and humans (Svalø et al., 2013, 2015). These results suggest that cellular pathways leading to the activation KV7 channels may function in opposition to mAChR signaling. However, confirming the existence of a functional link between KV7 channels and mAChRs in DSM requires future studies. Parasympathetic nerves release ACh and ATP, which act on mAChRs and purinergic receptors, respectively, to trigger urinary bladder smooth muscle contraction during micturition (Andersson and Arner, 2004; Werner et al., 2007; Heppner et al., 2009), with ACh-induced activation of mAChRs being the primary mechanism (Andersson and Arner, 2004). EFS evokes ACh release from parasympathetic nerve terminals, which leads to depolarization of the DSM cell membrane potential and associated DSM contractions (Nausch et al., 2010). Although mAChR regulation of KV7 channel activity is consistent with early studies in neurons (Brown et al., 1980), whether a similar regulatory mechanism exists in DSM remains unknown. A recent study in airway smooth muscle reported that, unlike neuronal KV7 channels (Delmas and Brown, 2005), mAChR activation by CCh did not exhibit significant inhibitory effects on KV7 currents (Evseev et al., 2013). Thus, KV7 channel activation in DSM may act in opposition to mAChRs through an indirect mechanism, for example, the modulation of l-type voltage-gated Ca2+ channels. Consistent with ML213 inhibitory effects on DSM contractions, we further observed attenuation of global intracellular Ca2+ concentrations by ML213 in DSM strips (Fig. 7).
For the first time, we report the activation of whole-cell KV7 currents by ML213 in freshly isolated DSM cells (Figs. 8 and 9). The use of a more depolarized holding potential (−10 mV) in our study ensured inactivation of other non-KV7 K+ channels, such as inward-rectifying K+ channels (Brueggemann et al., 2012b). Also, at a −10-mV holding potential, some of the KV7 channels are open and generate quantifiable currents, which are not contaminated by the presence of nonselective cation currents due to the inclusion of GdCl3. Thus, the stimulatory effects of ML213 on KV7 currents are evident (Fig. 8; Supplemental Fig. 8). Our data support the concept that the hyperpolarizing currents after the addition of ML213 are a direct result of KV7 channel pharmacological activation. In a series of experiments, marginal kinetics of voltage dependence of activation of KV7 channels were revealed using a short–voltage step (500-millisecond) protocol. Longer voltage step protocols (5 seconds) with multiple voltage steps may result in time-dependent rundown after several minutes of recording. Hence, we sought to examine the time course effects of ML213 on KV7 current activity by performing a one-step 5-second voltage-clamp protocol from a holding potential of −10 mV to one of +40 mV at 20-second intervals (Fig. 9). This protocol allowed for the recording of KV7 channel currents without encountering substantial current rundown. The stimulatory effects of ML213 on KV7 currents were modest but statistically significant (Figs. 8 and 9), although it is well established that small changes in DSM cell excitability can have pronounced effects on DSM contractility (Petkov et al., 2001; Petkov, 2014). Indeed, we observed very robust ML213 inhibitory effects on DSM contractility (Figs. 1–4). Overall, the experimental design for our electrophysiological experiments aimed not only to examine KV7 current activity and its response to pharmacological activation with ML213, but also to mimic a physiologic environment characterized by a more depolarized resting membrane potential, which normally occurs during the action potential. Activation of ML213-sensitive KV7 channels was further shown to hyperpolarize the DSM cell membrane potential (Fig. 10), consistent with ML213 inhibitory effects on global intracellular Ca2+ concentrations and DSM contractility.
KV7 channel α-subunits are known to form functional homomeric and heteromeric channel conformations in vitro and in vivo (Soldovieri et al., 2011; Stott et al., 2014), with KV7.4 and KV7.5 being prominent in smooth muscle tissues (Gamper and Shapiro, 2015). In vascular myocytes, ML213 has been shown to activate heteromeric KV7.4/KV7.5 channels in addition to the homomeric channels composed of either KV7.4 or KV7.5 channel α-subunits (Yu et al., 2010; Brueggemann et al., 2014a). In addition to KV7.2 channel expression in guinea pig DSM (Afeli et al., 2013; Anderson et al., 2013; Provence et al., 2015), KV7.4 (Anderson et al., 2013) and KV7.5 (Afeli et al., 2013; Anderson et al., 2013) channel α-subunits have been identified. However, whether these KV7 channel subunits, in particular KV7.4 or KV7.5, associate to form functional heteromers in DSM remained unknown until now. We confirmed the expression of heteromeric KV7.4/KV7.5 channels in isolated DSM cells using in situ PLA. PLA fluorescent signals for KV7.4/KV7.5 α-subunit colocalization were uniformly detected through the DSM cell periphery, suggesting their plasmalemmal expression (Fig. 12). These findings, which are consistent with studies on rodent vasculature (Chadha et al., 2014), provide strong evidence confirming heteromeric KV7.4/KV7.5 channel expression in DSM cells. Although heteromeric KV7.4/KV7.5 channels are potently activated by ML213 pharmacological activation, it has not yet been reported whether heteromeric KV7.2/KV7.3 channels or other KV7.2-containing channels, are ML213 sensitive. However, the potential activation of KV7.2/KV7.3 channels, for example, by ML213, would presumably be mediated by the KV7.2 channel subunit because KV7.3 channel subtypes are insensitive to ML213.
In addition, further studies to elucidate the precise stoichiometry of KV7 channels and their associations with β-regulatory subunits (KCNE1–5, (beta) accessory subunits of Kv7 channels) will be required to further understand the potential ML213 targets in DSM. Indeed, a recent study (Jepps et al., 2015) in rodent arterial myocytes demonstrated KCNE4 colocalization with KV7.4 and KV7.5 channels, respectively. Also, the coexpression of KCNE4 with KV7.4 channels was found to increase KV7.4 channel expression and current amplitudes (Jepps et al., 2015). Moreover, a report from bovine DSM suggests that KV7.4 channel subtypes are the most abundantly expressed; findings consistent with emerging studies in humans demonstrating KV7.4 mRNA expression as the primary KV7 channel expressed in DSM (Svalø et al., 2015; Bientinesi et al., 2017). A separate group reported KV7.5 channel mRNA expression as the most highly expressed KV7 channel subtype in human DSM (Argentieri et al., 2002). Although ML213 and ICA-069673 activate KV7.2 channels, their differential selectivity for KV7.4- and KV7.5-containing channels demonstrates the capacity for subtype-specific pharmacological targeting.
Because our in situ PLA experiments provided decisive evidence confirming the expression of heteromeric KV7.4/KV7.5 channel subtypes in DSM (Fig. 12), these channels may be among the key pharmacological targets for ML213. However, homomeric KV7.4 and KV7.5 channel subtypes may also be critically involved as a previous report revealed ML213 to be equally potent for KV7.4- and KV7.5-containing channels (Brueggemann et al., 2014a). The ability to fine-tune KV7 channel pharmacological modulators to exhibit improved selectivity among KV7.4, KV7.5, and KV7.4/KV7.5 channels may represent a substantial breakthrough in the development of novel and efficacious therapeutic modalities for lower urinary tract dysfunction.
In conclusion, our study demonstrates that ML213-sensitive KV7.4- and KV7.5-containing channels are essential regulators of DSM excitability and contractility. KV7 channel activation by the novel activator ML213 leads to the activation of KV7 currents, hyperpolarization of the membrane potential, a decrease in global intracellular Ca2+ concentration, and the attenuation of DSM contractility.
Acknowledgments
We thank Dr. James W. Hardin for the critical evaluation of our data and statistical methods. We also thank Dr. Kiril L. Hristov for help with some of the experiments; and Dr. Whitney Kellett, Dr. John Malysz, Dr. Viktor Yarotskyy, and Ms. Sarah Maxwell for critical evaluation of the manuscript.
Abbreviations
- BK
large-conductance voltage-activated and Ca2+-activated K+ channel
- CCh
carbachol
- DSM
detrusor smooth muscle
- EFS
electrical field stimulation
- ICA-069673
N-(2-chloro-5-pyrimidinyl)-3,4-difluorobenzamide
- IP3R
inositol triphosphate receptor
- KV
voltage-gated K+ channel
- mAChR
muscarinic acetylcholine receptor
- ML213
N-(2,4,6-Trimethylphenyl)-bicyclo[2.2.1]heptane-2-carboxamide
- PLA
proximity ligation assay
- TTX
tetrodotoxin
- XE991
10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride
Authorship Contributions
Participated in research design: Provence, Angoli, and Petkov.
Conducted experiments: Provence, Angoli, and Petkov.
Performed data analysis: Provence, Angoli, and Petkov.
Wrote or contributed to the writing of the manuscript: Provence, Angoli, and Petkov.
Footnotes
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
This study was supported by a grant from the National Institutes of Health R01 DK106964 to Georgi V. Petkov. Aaron Provence was supported by an NIH pre-doctoral fellowship F31 DK104528 under Dr. Petkov's mentorship.
References
- Abbott GW, Goldstein SA. (2001) Potassium channel subunits encoded by the KCNE gene family: physiology and pathophysiology of the MinK-related peptides (MiRPs). Mol Interv 1:95–107. [PubMed] [Google Scholar]
- Afeli SA, Malysz J, Petkov GV. (2013) Molecular expression and pharmacological evidence for a functional role of kv7 channel subtypes in Guinea pig urinary bladder smooth muscle. PLoS One 8:e75875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson UA, Carson C, Johnston L, Joshi S, Gurney AM, McCloskey KD. (2013) Functional expression of KCNQ (Kv7) channels in guinea pig bladder smooth muscle and their contribution to spontaneous activity. Br J Pharmacol 169:1290–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE, Arner A. (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986. [DOI] [PubMed] [Google Scholar]
- Argentieri T, Sheldon J, and Bowlby M (2002) inventors, American Home Products Corporation, assignee. Methods for modulating bladder function. U.S. patent 6,348,486. 2002 Feb 19.
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. (1996) K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384:78–80. [DOI] [PubMed] [Google Scholar]
- Bientinesi R, Mancuso C, Martire M, Bassi PF, Sacco E, Currò D. (2017) KV7 channels in the human detrusor: channel modulator effects and gene and protein expression. Naunyn Schmiedebergs Arch Pharmacol 390:127–137. [DOI] [PubMed] [Google Scholar]
- Brown AM, Akaike N, Tsuda Y, Morimoto K. (1980) Ion migration and inactivation in the calcium channel. J Physiol (Paris) 76:395–402. [PubMed] [Google Scholar]
- Brown DA, Passmore GM. (2009) Neural KCNQ (Kv7) channels. Br J Pharmacol 156:1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Haick JM, Cribbs LL, Byron KL. (2014a) Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA-069673. Mol Pharmacol 86:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Haick JM, Neuburg S, Tate S, Randhawa D, Cribbs LL, Byron KL. (2014b) KCNQ (Kv7) potassium channel activators as bronchodilators: combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am J Physiol Lung Cell Mol Physiol 306:L476–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Kakad PP, Love RB, Solway J, Dowell ML, Cribbs LL, Byron KL. (2012a) Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am J Physiol Lung Cell Mol Physiol 302:L120–L132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Mani BK, Haick J, Byron KL. (2012b) Exploring arterial smooth muscle Kv7 potassium channel function using patch clamp electrophysiology and pressure myography. J Vis Exp (67):e4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA. (2014) Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol 34:887–893. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. (2005) Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6:850–862. [DOI] [PubMed] [Google Scholar]
- Evseev AI, Semenov I, Archer CR, Medina JL, Dube PH, Shapiro MS, Brenner R. (2013) Functional effects of KCNQ K(+) channels in airway smooth muscle. Front Physiol 4:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. (2015) KCNQ channels, in Handbook of Ion Channels (Zheng J, Trudeau M. eds) pp 275–306, CRC Press, Boca Raton, FL. [Google Scholar]
- Haick JM, Byron KL. (2016) Novel treatment strategies for smooth muscle disorders: targeting Kv7 potassium channels. Pharmacol Ther 165:14–25. [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Werner ME, Nausch B, Vial C, Evans RJ, Nelson MT. (2009) Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J Physiol 587:5275–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Chen M, Afeli SA, Cheng Q, Rovner ES, Petkov GV. (2012a) Expression and function of K(V)2-containing channels in human urinary bladder smooth muscle. Am J Physiol Cell Physiol 302:C1599–C1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Chen M, Soder RP, Parajuli SP, Cheng Q, Kellett WF, Petkov GV. (2012b) KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 302:C360–C372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Bentzen BH, Stott JB, Povstyan OV, Sivaloganathan K, Dalby-Brown W, Greenwood IA. (2014) Vasorelaxant effects of novel Kv 7.4 channel enhancers ML213 and NS15370. Br J Pharmacol 171:4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Carr G, Lundegaard PR, Olesen SP, Greenwood IA. (2015) Fundamental role for the KCNE4 ancillary subunit in Kv7.4 regulation of arterial tone. J Physiol 593:5325–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Greenwood IA, Moffatt JD, Sanders KM, Ohya S. (2009) Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol 297:G107–G115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Brueggemann LI, Cribbs LL, Byron KL. (2011) Activation of vascular KCNQ (Kv7) potassium channels reverses spasmogen-induced constrictor responses in rat basilar artery. Br J Pharmacol 164:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, O’Dowd J, Kumar L, Brueggemann LI, Ross M, Byron KL. (2013) Vascular KCNQ (Kv7) potassium channels as common signaling intermediates and therapeutic targets in cerebral vasospasm. J Cardiovasc Pharmacol 61:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Robakowski C, Brueggemann LI, Cribbs LL, Tripathi A, Majetschak M, Byron KL. (2016) Kv7.5 potassium channel subunits are the primary targets for PKA-dependent enhancement of vascular smooth muscle Kv7 currents. Mol Pharmacol 89:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausch B, Heppner TJ, Nelson MT. (2010) Nerve-released acetylcholine contracts urinary bladder smooth muscle by inducing action potentials independently of IP3-mediated calcium release. Am J Physiol Regul Integr Comp Physiol 299:R878–R888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, et al. (2011) Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol 162:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV. (2011) Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV. (2014) Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am J Physiol Regul Integr Comp Physiol 307:R571–R584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. (2001) Low levels of K(ATP) channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280:R1427–R1433. [DOI] [PubMed] [Google Scholar]
- Provence A, Malysz J, Petkov GV. (2015) The novel KV7.2/KV7.3 channel opener ICA-069673 reveals subtype-specific functional roles in guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 354:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode F, Svalø J, Sheykhzade M, Rønn LC. (2010) Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur J Pharmacol 638:121–127. [DOI] [PubMed] [Google Scholar]
- Soldovieri MV, Miceli F, Taglialatela M. (2011) Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda) 26:365–376. [DOI] [PubMed] [Google Scholar]
- Stott JB, Jepps TA, Greenwood IA. (2014) K(V)7 potassium channels: a new therapeutic target in smooth muscle disorders. Drug Discov Today 19:413–424. [DOI] [PubMed] [Google Scholar]
- Streng T, Christoph T, Andersson KE. (2004) Urodynamic effects of the K+ channel (KCNQ) opener retigabine in freely moving, conscious rats. J Urol 172:2054–2058. [DOI] [PubMed] [Google Scholar]
- Svalø J, Bille M, Parameswaran Theepakaran N, Sheykhzade M, Nordling J, Bouchelouche P. (2013) Bladder contractility is modulated by Kv7 channels in pig detrusor. Eur J Pharmacol 715:312–320. [DOI] [PubMed] [Google Scholar]
- Svalø J, Sheykhzade M, Nordling J, Matras C, Bouchelouche P. (2015) Functional and molecular evidence for Kv7 channel subtypes in human detrusor from patients with and without bladder outflow obstruction. PLoS One 10:e0117350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. (2007) Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292:R616–R624. [DOI] [PubMed] [Google Scholar]
- Wrobel E, Tapken D, Seebohm G. (2012) The KCNE tango–how KCNE1 interacts with Kv7.1. Front Pharmacol 3:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wu M, Hopkins C, Engers J, Townsend S, Lindsley C, McManus OB, Li M. (2011) A small molecule activator of KCNQ2 and KCNQ4 channels, in Probe Reports from the NIH Molecular Libraries Program, National Center for Biotechnology Information 2010-, Bethesda, MD. [PubMed] [Google Scholar]
- Yu H, Wu M, Townsend SD, Zou B, Long S, Daniels JS, McManus OB, Li M, Lindsley CW, Hopkins CR. (2011) Discovery, synthesis, and structure activity relationship of a series of N-aryl-bicyclo[2.2.1]heptane-2-carboxamides: characterization of ML213 as a novel KCNQ2 and KCNQ4 potassium channel opener. ACS Chem Neurosci 2:572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]