Summary
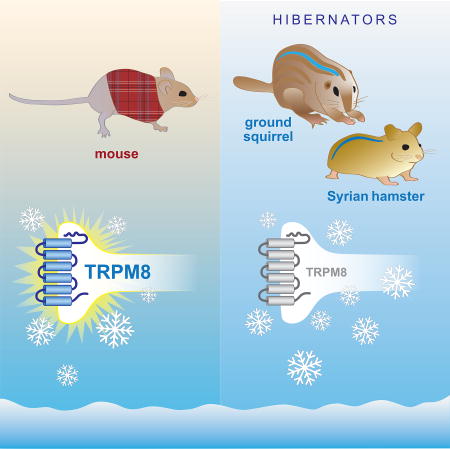
Thirteen-lined ground squirrels and Syrian hamsters are known for their ability to withstand cold during hibernation. We found that hibernators exhibit cold tolerance even in the active state. Imaging and electrophysiology of squirrel somatosensory neurons reveal a decrease in cold sensitivity of TRPM8-expressing cells. Characterization of squirrel and hamster TRPM8 showed that the channels are chemically-activated, but exhibit poor activation by cold. Cold sensitivity can be re-introduced into squirrel and hamster TRPM8 by transferring the transmembrane domain from the cold sensitive rat orthologue. The same can be achieved in squirrel TRPM8 by mutating only six amino acids. Reciprocal mutations suppress cold sensitivity of the rat orthologue, supporting functional significance of these residues. Our results suggest that ground squirrels and hamsters exhibit reduced cold sensitivity partially due to modifications in the transmembrane domain of TRPM8. Our study reveals molecular adaptations that accompany cold tolerance in two species of mammalian hibernators.
Graphical abstract
Introduction
Somatosensory system evolved to accommodate behavioral needs of various species and inhabit a wide spectrum of geographical ranges (Gracheva and Bagriantsev 2015). Cold sensitivity, a specific aspect of somatosensitivity, is a key physiological capacity pertinent to all vertebrates and invertebrates. In the somatosensory system, temperature changes are detected by primary afferent of somatosensory neurons localized within trigeminal and dorsal root ganglia (DRG). Cold receptors account for 15–20% of total neuronal population in DRG of mice and many other vertebrates (McKemy 2013). The molecular mechanism of cold sensitivity involves TRPM8, a cold-activated non-selective cation channel. TRPM8 mediates physiological responses to environmental cold below 26°C, and is activated by in the same temperature range in vitro (Bautista et al. 2007, Dhaka et al. 2007, McKemy et al. 2002, Peier et al. 2002). As a cold sensor, TRPM8 is an essential part of the thermosensory apparatus, which, along with other organs and systems, defines the range of temperature tolerance for a species and, ultimately, the breadth of its geographical habitat. An extreme example of temperature tolerance is demonstrated by mammalian hibernators that can withstand prolonged exposure to cold and extreme hypothermia (Carey et al. 2003). In order to survive harsh environmental conditions, hibernators must have developed adaptations at the molecular level, but most of them, including the suppressed ability to respond to cold, remain unknown. In this study, we explored the contribution of the somatosensory system to cold detection in two species of mammalian hibernators, the Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) and Syrian hamsters (Mesocricetus auratus).
Results
Squirrel TRPM8+ receptors are poorly sensitive to cold
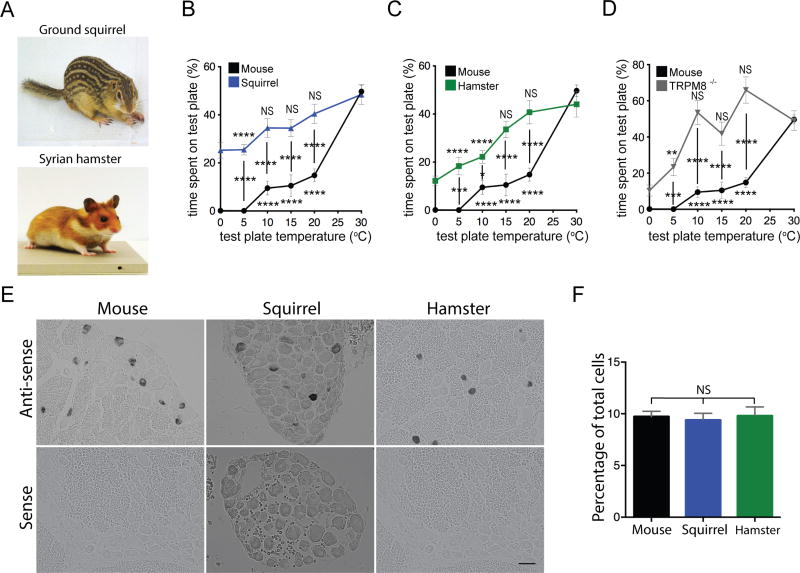
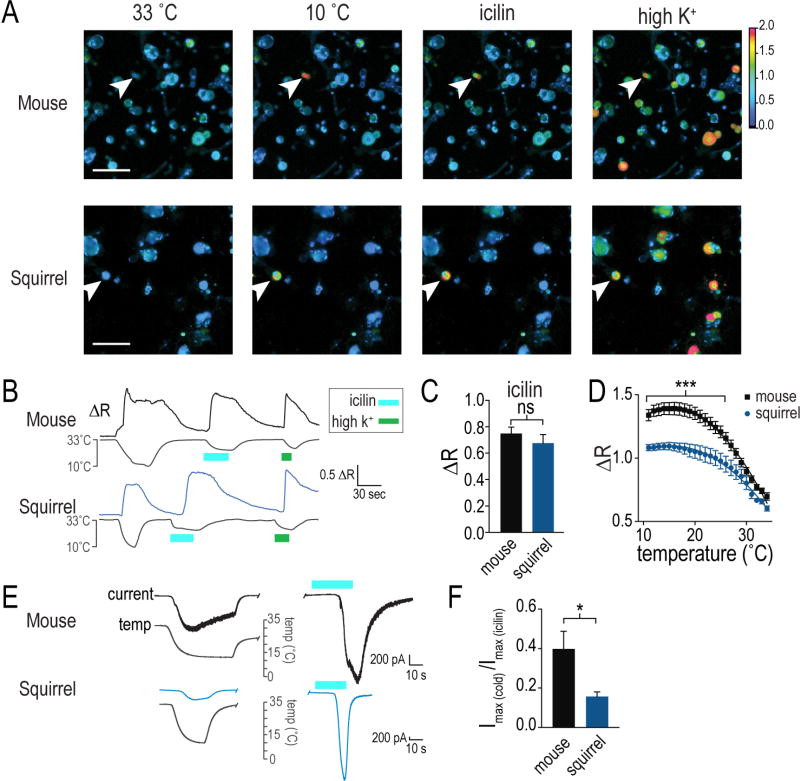
We characterized temperature sensitivity of active ground squirrels and hamsters (Figure 1A) using a two-plate temperature preference test (Laursen et al. 2016). We quantified the time spent by the animals on a reference plate set at 30°C or a test plate set to a temperature range from 0°C to 30°C. Consistent with earlier studies, mice strongly prefer 30°C over cooler temperatures and completely avoid temperatures below 10°C (Bautista et al. 2007, Dhaka et al. 2007). Squirrels and hamsters, on the other hand, exhibited significant preference to the 30°C plate only when the test plate reached 5°C and 10°C, respectively, and failed to show a complete avoidance even at 0°C (Figure 1B and C). The behavior exhibited by squirrels and hamsters is remarkably similar to that reported for mice with genomic ablation of TRPM8, a cold-activated ion channel (Figure 1D) (Bautista et al. 2007, Dhaka et al. 2007). We hypothesized that the apparent cold tolerance exhibited by squirrels and hamsters could be caused, at least partially, by either decreased abundance of TRPM8 neurons, or their diminished cold sensitivity. Using RNA in situ hybridization, we estimated that TRPM8 was expressed in 9.7% ± 0.5, 9.4% ± 0.7 and 9.8% ± 0.9 (mean ± SEM, n=1912–2870 cells) of neurons from, respectively, mouse, squirrel and hamster dorsal root ganglia (DRG), suggesting that the diminished cold sensitivity cannot be explained by a decrease in the number of cold-sensing cells (Figure 1E and F). To assess functional properties of neuronal cold receptors, we performed ratiometric calcium imaging of dissociated DRG neurons, focusing on cells activated by icilin, a specific agonist of TRPM8 (McKemy et al. 2002). As expected, all wild type mouse neurons sensitive to icilin (3.0% of 2352 neurons) were also sensitive to cold. We also detected robust icilin responses in a subset of squirrel DRG neurons (3.1% of 1177 neurons), demonstrating the presence of functional TRPM8 (Figure 2A and B). However, even though squirrel and mouse cells had identical icilin responses (Figure 2C) and all squirrel icilin-sensitive neurons were activated by cold, the amplitude of cold-evoked response was significantly diminished compared to mouse cells in the 10°C–25°C range, suggesting that squirrel neurons express TRPM8 with normal icilin, but impaired cold sensitivity (Figure 2D). In agreement with this, whole-cell electrophysiological recordings showed that the amplitude of cold-induced current normalized to icilin response is significantly lower in squirrel compared to mouse DRG neurons (Figure 2E and F). These data suggest that the apparent cold tolerance of squirrels can be explained, at least partially, by diminished cold sensitivity of TRPM8-expressing neuronal cold receptors.
Figure 1. Squirrel and hamster TRPM8 have diminished cold sensitivity.
(A) Image of a thirteen-lined ground squirrel and Syrian hamster (courtesy Gracheva lab). (B–D) Quantification of animal behavior in temperature preference tests shows the percentage of time spent by animals on the control (30°C) vs test (0°C–30°C) plate over 5 minutes. Data collected from 20 wild type mice, 8 TRPM8−/− mice, 19 squirrels and 19 hamsters for each temperature point. NS, not significant, P≥0.05, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, ordinary two-way ANOVA with Tukey post-hoc test. Statistical comparison is shown between species for each temperature (vertical lines) and within species vs reference 30°C plate (shown above or below symbols). (E) RNA in situ hybridization images showing expression of Trpm8 transcripts in tissue sections from mouse, squirrel and hamster DRG (scale bar, 50 µm). (F) Quantification of Trpm8 mRNA expression within mouse, squirrel, hamster DRG (mean ± SEM from 1912 neurons, 15 DRG sections for mouse; 2538 neurons, 20 DRG sections for squirrel; 2870 neurons, 20 DRG sections for hamster, from at least two animals for each species. NS, not significant, P≥0.05, one-way ANOVA with Dunnett’s post hoc test).
Figure 2. Squirrel TRPM8 neurons have diminished cold sensitivity.
(A) Representative partial fields of view of Fura-2AM ratiometric calcium imaging in squirrel and mouse dissociated DRG neurons. White arrows indicate cold and icilin responding cells. Color coding denotes lowest and highest ratios from bottom to top. Scale bar = 100 µm. (B) Example traces from the images shown of squirrel and mouse neurons responding to cold, 10 µM icilin, 135 mM KCl (high K+). (C) Baseline-corrected peak calcium responses during icilin application (mean ± SEM, n=37 squirrel and 75 mouse icilin-sensitive neurons from a total of 1177 squirrel and 2532 mouse DRG neurons, obtained from 3 animals for each species; ns, not significant, P=0.4 Mann-Whitney U-test). (D) Population data of cold responses in icilin-sensitive neurons from squirrel and mouse binned by °C (mean ± SEM; 2-way ANOVA with Bonferroni correction: P<0.0001, main effect of species, temperature; ***0.001<P<0.05 for multiple comparisons at temperatures between 10–25°C, not significant (P≥0.05) between 26–34°C. (E) Example current traces evoked by cold and 10 µM icilin in dissociated mouse and squirrel DRG neurons held at −60mV in voltage-clamp mode. (F) Quantification of maximal inward current evoked in mouse and squirrel DRG neurons by temperature stimulation, normalized to maximal response to 10 µM icilin (*P<0.05, Mann-Whitney U-test, n=4 squirrel, 5 mouse DRG neurons from 3 animals for each species). See also Figure S1.
Squirrel and hamster TRPM8 have diminished cold sensitivity
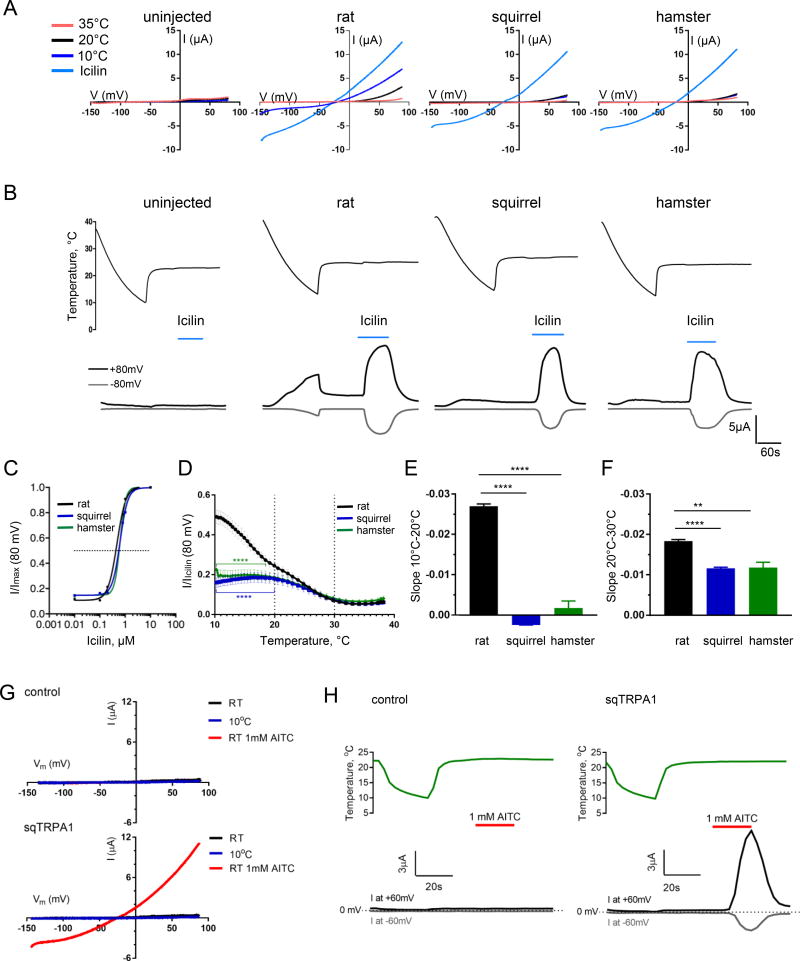
We cloned TRPM8 from squirrel and hamster DRG and analyzed their chemical and temperature sensitivity by two-electrode voltage clamp in Xenopus oocytes in comparison with rat TRPM8 (Figure S1A), a well characterized TRPM8 orthologue with properties similar to the mouse channel (McKemy et al. 2002, Peier et al. 2002). We found that squirrel and hamster TRPM8 are sensitive to icilin and menthol with EC50 indistinguishable from the rat orthologue (icilin EC50, mean ± SEM: 0.60±0.12 µM, 0.53±0.06 µM, 0.55±0.02 µM for rTRPM8, sqTRPM8 and hamTRPM8, respectively, n=7–8; menthol EC50: 38.42±4.80 µM, 32.08±3.45 µM, 35.04±4.59 µM for rTRPM8, sqTRPM8 and hamTRPM8, respectively, n=6, Figure 3A–C, S2A–D). These results agree with the presence of intact putative binding sites for these agonists in both squirrel and hamster TRPM8 (Figure S1A) (Bandell et al. 2006, Chuang et al. 2004). As expected, rat TRPM8 exhibited gradually increasing activity in response to cooling of the extracellular solution from 30°C to 20°C (linear activation slope k = −0.018 ± 0.000, mean ± SEM, n=10) and from 20°C to 10°C (k = −0.027 ± 0.000, n=10), with maximal cold-evoked current amplitude reaching ~51% of that evoked by 1µM icilin (Figure 3D–F). In contrast, squirrel and hamster TRPM8 activity only slightly increased in the 30°C to 20°C segment (k = −0.012 ± 0.000, n=10 and −0.012 ± 0.001, n=5 for sqTRPM8 and hamTRPM8, respectively) but remained virtually unchanged upon further cooling from 20°C to 10°C (k = 0.002 ± 0.000 and −0.002 ± 0.002 for sqTRPM8 and hamTRPM8, respectively Figure 3D–F). The maximal normalized cold-evoked amplitude for both orthologues was diminished to ~18% of that evoked by 1µM icilin (Figure 3D). Overall, cold responses of squirrel and hamster TRPM8 were significantly reduced compared to rat TRPM8 in, respectively, 10°C–20°C and 10°C–18.5°C range (Figure 3D). TRPM8 is known to undergo desensitization due to depletion of phosphatidylinositol 4,5-bisphosphate (PIP2) by calcium-activated phospholipase C (Liu and Qin 2005, Rohacs et al. 2005). This mechanism is unlikely the cause for the observed diminution of cold responses, since squirrel and hamster TRPM8 retain the putative PIP2-binding site in the C-terminus (Figure S1A) (Rohacs et al. 2005), and the removal of extracellular calcium failed to potentiate cold responses (Figure S2E and F). Thus, squirrel and hamster TRPM8 have diminished overall cold sensitivity and cannot track temperature changes in the 10°C–20°C range in vitro, consistent with the reduced cold responses of dissociated neurons and behavioral data.
Figure 3. Squirrel and hamster TRPM8 have diminished cold sensitivity.
(A, B) Exemplar current-voltage plots of responses to temperature ramps (35°C–10°C) and 1 µM icilin obtained by two-electrode voltage clamp in Xenopus oocytes expressing rat TRPM8, squirrel TRPM8, or hamster TRPM8. (C) Icilin dose-response curves for rat, squirrel and hamster TRPM8 orthologues (mean ± SEM, the error bars are smaller than symbols, n≥5 for each point). (D) Temperature-response profiles for TRPM8 orthologues normalized to the maximum icilin response. Data shown as mean ± SEM, n=5–10. ****0.0001<P<0.05 vs. rTRPM8 in the range indicated by brackets, not significant (P≥0.05) outside this range (two-way ANOVA with Dunnett’s post-hoc test). (E, F) Quantification of temperature response steepness (slope) obtained by fitting the data for the 10°C–20°C and 20°C–30°C segments in (D) to the linear equation (mean ± SEM, n=5–10, **P<0.01, ****P<0.0001, one-way ANOVA with Dunnett post-hoc test). (G, H) Exemplar current-voltage plots of responses to temperature ramps from room temperature (RT, 22°C) to 10°C, and 1 mM AITC obtained by two-electrode voltage clamp in water-injected Xenopus oocytes (control) or oocytes expressing squirrel TRPA1. The images are representative of >10 cells from 2 independent experiments. See also Figure S2.
We wondered whether the remaining cold sensitivity in squirrels is dictated by TRPA1, a polymodal ion channel which is expressed in a distinct neuronal population from TRPM8, and which was proposed to contribute to cold responses (del Camino et al. 2010, Memon et al. 2017). We therefore cloned TRPA1 from squirrel DRG and tested its temperature and chemical sensitivity. We found that squirrel TRPA1 is not activated by cooling to 10°C, even though the channel is activated by the specific agonist allyl isothiocyanate (Figure 3G and H). These data suggest that the residual responses to cold in squirrel neurons and in behavioral tests are mediated by other mechanisms.
Transmembrane core domain plays an essential role in dictating cold sensitivity of TRPM8
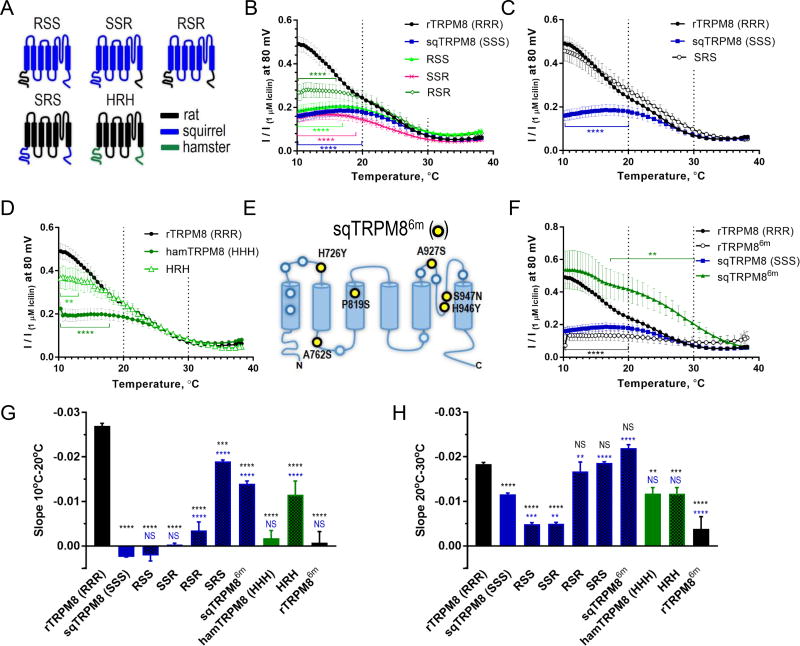
Even though the squirrel and hamster channels are highly homologous to the rat orthologue and display around 90% amino acid identity, they contain a number of amino acid substitutions in the putative intracellular, core transmembrane and extracellular regions (Figure S1A). To identify structural elements that underlie the diminished cold sensitivity in squirrel and hamster TRPM8, we generated chimeric channels with the robustly cold sensitive rat orthologue, and tested them by two-electrode voltage clamp in Xenopus oocytes. The substitution of both N- and C-terminal domains in squirrel TRPM8 with homologous domains from the rat channel (chimera RSR, Figure 4A) increased maximal normalized cold-evoked amplitude from 18% to 28%, while individual N- and C-terminal domains (chimeras RSS, SSR) had no effect (Figure 4B, G and H). Even though the potentiation of cold responses in the RSR chimera was substantial, it remained significantly lower than in the rat channel in the 10°–17°C range, prompting us to test the transmembrane core domain (Figure 4B). Strikingly, the substitution of the core transmembrane domain of squirrel TRPM8 with the rat homologue (chimera SRS, Figure 4A) conferred cold sensitivity to the extent indistinguishable from the rat channel in 10°C–30°C range (Figure 4C). Analogously, the transposition of the rat transmembrane core increased cold-evoked amplitude sensitivity of hamster TRPM8 (chimera HRH) in a broad range of temperatures except 10°C–13°C (Figure 4D). All the chimeras had unchanged sensitivity to icilin (Figure S2D and S3A). These data show that while N- and C-termini play a modulatory role (Tsuruda et al. 2006, Phelps and Gaudet 2007), the transmembrane domain alone can dictate cold responses of TRPM8.
Figure 4. Modulation of temperature sensitivity in squirrel and hamster TRPM8 orthologues.
(A) Topology diagram of TRPM8 chimeric channels. (B–D, F) Normalized temperature response profiles for chimeric channels between squirrel, hamster and rat TRPM8 (mean ± SEM, n=5–10; **0.01<P<0.05, ****0.0001<P<0.05 for data of the same color vs. rat TRPM8, in the range indicated by brackets, not significant (P≥0.05) outside this range (two-way ANOVA with Dunnett’s post-hoc test). (E) Topology diagram depicting the locations of the fifteen non-conserved amino acids in the transmembrane core of rat and squirrel TRPM8 (blue and yellow circles), and the six mutations that confer cold sensitivity to sqTRPM8 (yellow circles, sqTRPM86m). (G, H) Quantification of temperature response steepness (slope) obtained by fitting the data for the 10°C–20°C and 20°C–30°C segments in (B–D and F) to the linear equation (mean ± SEM, n=5–10). NS, not significant, P≥0.05, **P<0.01, ***P<0.001, ****P < 0.0001 vs. rTRPM8 (denoted by black symbols) or sqTRPM8 (denoted by blue symbols), one-way ANOVA with Dunnett post-hoc test. See also Figure S3.
The transmembrane domains of squirrel and rat TRPM8 differ by only fifteen amino acids (Figure 4E and S1A). To delineate molecular determinants of cold sensitivity, we subdivided the core transmembrane domain of TRPM8 into three blocks, each encompassing two transmembrane helices and containing, respectively, five, four and six amino acid differences between squirrel and rat TRPM8 (Figure S1A and S3B). Interestingly, transposition of any two of the three blocks (chimeras SR1–4S, SR3–6S, SR1–2,5–6S) or just the transmembrane domains 5 and 6 (chimera SR5–6S) from rat to squirrel TRPM8 was not sufficient to confer cold sensitivity to the same extent as transposition of the whole transmembrane domain (chimera SRS), suggesting that the functional amino acids are spread throughout the transmembrane core (Figure S3A–C, F and G).
By systematically replacing individual amino acids, we identified six residues in squirrel TRPM8 core, which when replaced by homologous residues from the rat channel (sqTRPM86m: H726Y, A762S, P819S, A927S, H946Y, S947N, Figure 4E and S1B) conferred robust cold sensitivity in the 10°C–20°C range (Figure 4F, S3A) without affecting icilin response (Figure S2D). Mutating any one of the six amino acids in sqTRPM86m back to the original squirrel residues (sqTRPM85m, Figure S3C) either abolished cold sensitivity (Figure S3D), or resulted in non-functional channels, as assessed by the absence of both icilin and cold responses. Conversely, reciprocal six point mutations in rat TRPM8 (rTRPM86m) significantly reduced cold responses (Figure 4F–H and S3A) without altering chemical sensitivity (Figure S2D). Thus, we conclude that these six amino acids in the transmembrane core are necessary for cold responses of squirrel and rat TRPM8.
Discussion
Here, we show that animals from two different Rodentia families, thirteen-lined ground squirrels (Sciuridae) and Syrian hamsters (Cricetidae), do not avoid cold as strongly as mice (Muridae), when given a choice between two plates with different temperatures. The apparent cold tolerance exhibited by squirrels and hamsters could be explained by a number of scenarios, including, but not limited to, the reduced ability to perceive cold by the somatosensory afferents, or the suppression of cold avoidance at the level of the CNS. Here, we specifically focused on the somatosensory component and analyzed the abundance and functional properties of TRPM8-expressing neuronal cold receptors. In mice, this population of neurons is responsible for the detection of a wide range of temperatures (0°C–26°C) via a mechanism that includes activation of the cold-gated ion channel TRPM8 (Bautista et al. 2007, Dhaka et al. 2007, McKemy et al. 2002, Peier et al. 2002, Pogorzala et al. 2013). Consistently, TRPM8-deficient mice show a significant reduction, but not a complete elimination, of cold-evoked responses at the behavioral, cellular and nerve fiber levels (Bautista et al. 2007, Dhaka et al. 2007, Milenkovic et al. 2014). The residual cold responses are attributed to the presence of additional cold-activated ion channels in TRPM8 neurons, as well as non-TRPM8 cold receptors (Knowlton et al. 2013, Memon et al. 2017, Pogorzala et al. 2013, Zimmermann et al. 2007, Lolignier et al. 2015). We focused on TRPM8-expressing neurons and show that these cells are present in squirrel and hamster DRG at proportions identical to mice, ruling out insufficient number of cold receptors as the cause of the observed behavioral phenotype. Functionally, however, squirrel neurons exhibit significantly reduced cold sensitivity compared to mouse cells, when assessed by ratiometric calcium imaging and electrophysiology. These data are consistent with the idea that the diminished cold sensitivity of peripheral cold receptors may contribute to species-specific cold tolerance we observed in the temperature preference test.
Since TRPM8 is a major cold-activated excitatory conduit in neuronal cold receptors, we cloned this channel from squirrel and hamster DRG and characterized its functional properties side-by-side with the rat orthologue in Xenopus oocytes. Consistent with earlier data, rat TRPM8 exhibited a progressive activation in response to gradual temperature decrease from 30°C to 10°C (McKemy et al. 2002). In striking contrast, we found that squirrel and hamster channels were significantly less sensitive to cold, exhibiting almost no change in activity below 20°C. At the same time, both orthologues retained sensitivity to icilin and menthol with an EC50 identical to rat TRPM8, demonstrating that the functional deficiencies in squirrel and hamster TRPM8 are modality-specific. Thus, the reduced cold sensitivity of TRPM8 explains, at least partially, the apparent cold tolerant phenotype exhibited by squirrels and hamsters in a two-plate preference test in the 10°C–30°C temperature range. Squirrels and hamsters remain sensitive to cooling below 10°C, suggesting the presence of a TRPM8-independent mechanism of cold detection. A number of molecules were suggested for this role, including TRPA1 and the voltage-gated sodium channels Nav1.8 and Nav1.9 (Lolignier et al. 2015, Memon et al. 2017, Zimmermann et al. 2007). Our data show that squirrel TRPA1 is not activated by temperature within the 10°C–22°C range, arguing against its role in cold detection in squirrels, although we were not able to test its activity at temperatures below 10°C.
In contrast to mice or rats, ground squirrels and hamsters can undergo prolonged periods of hibernation, during which their core body temperature drops to ambient and can be as low as 2°C–7°C (Bouma et al. 2011, Merriman et al. 2016, Carey et al. 2003, Tupone et al. 2017). While the mechanism of hibernation is complex and poorly understood, it seems clear that it involves significant modifications to the animal’s thermoregulatory responses, which normally rely on the integration and processing of inputs from both peripheral and internal thermosensory systems (Almeida et al. 2012, Weidler et al. 1974, Heller and Colliver 1974). Accordingly, pharmacological suppression of cold sensitivity in peripheral neurons via inhibition of TRPM8 triggers a complex systemic response, involving an increase in heat dissipation, decreased thermogenesis and, ultimately, decreased core body temperature (Feketa et al. 2013, Feketa and Marrelli 2015, Almeida et al. 2012). While not very many TRPM8 orthologues have been described, it is interesting to see that cold sensitivity of TRPM8 seems to follow the species’ core body temperature, being the lowest in cold-blooded frogs and highest in birds (Chuang et al. 2004, Gracheva and Bagriantsev 2015, Myers et al. 2009), whose body temperature is above that of rodents or humans. The squirrel and hamster TRPM8 orthologues described here are out of trend, prompting us to speculate that the apparent cold tolerance exhibited by squirrels and hamsters has evolved as a part of a complex physiological mechanism that supports hibernation. Conceivably, a suppressed sensitivity to environmental cold could be essential for both enduring the hibernation as well as entering it. To test this hypothesis would require the generation of transgenic animals expressing a robustly cold sensitive orthologue of TRPM8 from the native locus – an experiment that appears possible in a few years’ time.
Functional analysis of chimeras between TRPM8 orthologues showed that cold sensitivity of the squirrel and hamster channels can be restored if their transmembrane cores are replaced with the homologous domain from rat TRPM8, strongly supporting the idea that the transmembrane core is a key determinant of cold sensitivity. Whether the core domain senses temperature directly, or allosterically responds to a discrete temperature sensor located elsewhere in the channel (Arrigoni et al. 2016), remains to be determined. The squirrel’s transmembrane core differs from rat’s by fifteen amino acids, and transposition of only six of them is sufficient to restore cold sensitivity. Conversely, changing the six amino acids in the rat channel to their squirrel analogs significantly diminishes cold sensitivity, demonstrating that these sites are crucial for cold responses of both channels. None of these residues, which are scattered throughout the core without forming an obvious cluster, have been implicated in chemical- or voltage sensitivity of TRPM8 (Bandell et al. 2006, Chuang et al. 2004, Voets et al. 2007). This indicates that the changes that occurred in squirrel TRPM8 structure have been selected to specifically suppress cold responses. Interestingly, the six residues are not conserved between squirrel and hamster TRPM8 (Figure S1B). Moreover, of the six residues four are identical between hamster and rat TRPM8. Together with the observation that the transposition of the rat transmembrane core onto hamster TRPM8 confers cold sensitivity, strongly support the idea that squirrel and rat channels have lost sensitivity to cold via non-identical changes in the transmembrane domain.
Recently, we reported that ground squirrels are tolerant to noxious heat, partially due to diminished heat sensitivity of the TRPV1 channel in peripheral nociceptors (Laursen et al. 2016). Similar to TRPM8, the suppression of temperature sensitivity in squirrel TRPV1 is specific, as the channel remains sensitive to chemical agonists, such as protons and capsaicin, preserving its role in inflammation. The modality-specific diminution of temperature responses, rather than a complete obliteration of the functional gene, suggest that squirrel and hamster TRPM8 may retain other important physiological functions, which currently remain obscure.
Experimental procedures
Further details and outlines of resources used in this work can be found in Supplemental Experimental Procedures.
Animals
Animals were housed in a pathogen-free facility at Yale University. All animal procedures were performed in compliance with the Office of Animal Research Support of Yale University (protocol 2015-11497). Summer active squirrels, hamsters and mice were housed on a 12-h light/dark cycle under standard laboratory conditions with ad libitum access to food and water. Thirteen-lined ground squirrels were maintained on a diet of dog food (Iams) supplemented with sunflower seeds, superworms, and fresh vegetables.
Temperature preference assay
Behavioral experiments on mice (8–14 weeks old male C57BL/6 mice and TRPM8−/− in C57BL/6 background, approximate weight 25g), active squirrels (1–1.5 year old males, approximate weight 200g; of note, squirrels become sexually mature at 8–12 month, and their lifespan is 8–10 years) and hamsters (8–14 weeks old males, approximate weight 120g) were performed in May-July. For the two-plate temperature preference/aversion assay, animals were placed into a chamber containing one floor plate set to a control temperature of 30°C and the other set to a test temperature between 30°C-0°C (T2CT, Bioseb, France). Animals were placed onto the control plate and recorded as they freely explored both sides of the chamber for a total of 5 minutes. Plate order was reversed between groups and test days. Animal was considered to cross from one plate to the other plate when the animal’s 4 paws crossed into new plate, even though all animals used for analysis touched the experimental plate at least one time with their front paws.
Tissue collection
Whole DRG were fixed in 4% paraformaldehyde for RNA in situ hybridization or homogenized in the Trizol reagent for RNA extraction. For functional analyses of dissociated neurons, DRG were treated with collagenase P followed by 0.25% trypsin, and suspended in DMEM media supplemented with 10% FBS and penicillin/streptomycin.
Statistical Analysis
Data were obtained from at least two independent experiments and analyzed with GraphPad Prism 6.0 (GraphPad Software, Inc). Sample size and statistical tests are reported in figure legends. Mann-Whitney U-test was used for pair-wise comparisons, ordinary one- or two-way ANOVA with post-hoc correction were used for multiple comparisons. Statistical tests were chosen based on normality of distributions and variance equality, or lack thereof, and the number of samples. Unless indicated otherwise, data were reported as mean ± SEM, significance displayed as not significant (NS), P>0.05, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Supplementary Material
Acknowledgments
We thank members of the Gracheva and Bagriantsev laboratories for their contributions throughout the project. This study was partly funded by fellowships from the Beckman Foundation, Rita Allen Foundation and NIH grants 1R01NS091300-01A1 and 3R01NS091300-02S1 to E.O.G; by American Heart Association grant 14SDG17880015 and NSF grant 1453167 to S.N.B.; and by the Axle Tech International Endowed Professorship to D.K.M. V.MC. was partially supported by NSF Postdoctoral Fellowship. E.R.S. was supported by a postdoctoral fellowship from the Arnold and Mabel Beckman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
The authors declare no competing financial interests. V.M-C., E.R.S., M.M., E.O.G. and S.N.B. designed and performed experiments. V.M-C., E.R.S., M.M., E.O.G. and S.N.B. collected and analyzed data. D.K.M. supplied squirrels and advised on behavioral experiments. V.M-C., E.R.S., S.N.B. and E.O.G. wrote the manuscript with contributions from M.M., and D.K.M. E.O.G. and S.N.B. conceived the study and provided guidance and supervision throughout the project.
References
- Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32(6):2086–99. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni C, Rohaim A, Shaya D, Findeisen F, Stein RA, Nurva SR, Mishra S, McHaourab HS, Minor DL., Jr Unfolding of a Temperature-Sensitive Domain Controls Voltage-Gated Channel Activation. Cell. 2016;164(5):922–36. doi: 10.1016/j.cell.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9(4):493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–8. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bouma HR, Kroese FG, Kok JW, Talaei F, Boerema AS, Herwig A, Draghiciu O, van Buiten A, Epema AH, van Dam A, Strijkstra AM, Henning RH. Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2011;108(5):2052–7. doi: 10.1073/pnas.1008823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83(4):1153–81. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43(6):859–69. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30(45):15165–74. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54(3):371–8. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Feketa VV, Balasubramanian A, Flores CM, Player MR, Marrelli SP. Shivering and tachycardic responses to external cooling in mice are substantially suppressed by TRPV1 activation but not by TRPM8 inhibition. Am J Physiol Regul Integr Comp Physiol. 2013;305(9):R1040–50. doi: 10.1152/ajpregu.00296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feketa VV, Marrelli SP. Induction of therapeutic hypothermia by pharmacological modulation of temperature-sensitive TRP channels: theoretical framework and practical considerations. Temperature (Austin) 2015;2(2):244–57. doi: 10.1080/23328940.2015.1024383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Bagriantsev SN. Evolutionary adaptation to thermosensation. Curr Opin Neurobiol. 2015;34C:67–73. doi: 10.1016/j.conb.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Heller HC, Colliver GW. CNS regulation of body temperature during hibernation. Am J Physiol. 1974;227(3):583–9. doi: 10.1152/ajplegacy.1974.227.3.583. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33(7):2837–48. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen WJ, Schneider ER, Merriman DK, Bagriantsev SN, Gracheva EO. Low-cost functional plasticity of TRPV1 supports heat tolerance in squirrels and camels. Proc Natl Acad Sci U S A. 2016;113(40):11342–11347. doi: 10.1073/pnas.1604269113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25(7):1674–81. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolignier S, Bonnet C, Gaudioso C, Noel J, Ruel J, Amsalem M, Ferrier J, Rodat-Despoix L, Bouvier V, Aissouni Y, Prival L, Chapuy E, Padilla F, Eschalier A, Delmas P, Busserolles J. The Nav1.9 channel is a key determinant of cold pain sensation and cold allodynia. Cell Rep. 2015;11(7):1067–78. doi: 10.1016/j.celrep.2015.04.027. [DOI] [PubMed] [Google Scholar]
- McKemy DD. The molecular and cellular basis of cold sensation. ACS Chem Neurosci. 2013;4(2):238–47. doi: 10.1021/cn300193h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–8. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Memon T, Chase K, Leavitt LS, Olivera BM, Teichert RW. TRPA1 expression levels and excitability brake by KV channels influence cold sensitivity of TRPA1-expressing neurons. Neuroscience. 2017;353:76–86. doi: 10.1016/j.neuroscience.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman DK, Sajdak BS, Li W, Jones BW. Seasonal and post-trauma remodeling in cone-dominant ground squirrel retina. Exp Eye Res. 2016;150:90–105. doi: 10.1016/j.exer.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic N, Zhao WJ, Walcher J, Albert T, Siemens J, Lewin GR, Poulet JF. A somatosensory circuit for cooling perception in mice. Nat Neurosci. 2014;17(11):1560–6. doi: 10.1038/nn.3828. [DOI] [PubMed] [Google Scholar]
- Myers BR, Sigal YM, Julius D. Evolution of thermal response properties in a cold-activated TRP channel. PLoS One. 2009;4(5):e5741. doi: 10.1371/journal.pone.0005741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–15. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Phelps CB, Gaudet R. The role of the N terminus and transmembrane domain of TRPM8 in channel localization and tetramerization. J Biol Chem. 2007;282(50):36474–80. doi: 10.1074/jbc.M707205200. [DOI] [PubMed] [Google Scholar]
- Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci. 2013;33(13):5533–41. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8(5):626–34. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Tsuruda PR, Julius D, Minor DL., Jr Coiled coils direct assembly of a cold-activated TRP channel. Neuron. 2006;51(2):201–12. doi: 10.1016/j.neuron.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Cano G, Morrison SF. Thermoregulatory inversion: a novel thermoregulatory paradigm. Am J Physiol Regul Integr Comp Physiol. 2017;312(5):R779–R786. doi: 10.1152/ajpregu.00022.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol. 2007;3(3):174–82. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- Weidler DJ, Earle AM, Myers GG, Sieck GC. Effect of hypothalamic lesions on temperature regulation in hibernating ground squirrels. Brain Res. 1974;65(1):175–9. doi: 10.1016/0006-8993(74)90345-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447(7146):855–8. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.