Abstract
Microscopic colitis (MC) has rarely been described to be the cause of watery diarrhea in those with established inflammatory bowel disease (IBD), and instead has been presented as a herald syndrome to eventual IBD or incidentally found in asymptomatic IBD patients. We report a case series of 7 patients with IBD who presented with a watery diarrheal exacerbation due to new-onset MC. We propose that new-onset MC should be considered in the differential diagnosis of watery diarrhea occurring in patients with long-standing IBD and that evaluation should include colonoscopy with biopsies obtained throughout the colon.
Introduction
Microscopic colitis (MC) consists of the subtypes of collagenous colitis (CC) and lymphocytic colitis (LC). They often affect middle-aged and elderly women and present with chronic watery diarrhea and endoscopically normal mucosa.1,2 These colitides share the presence of mucosa with normal architecture, while exhibiting microscopic inflammation in the lamina propria and infiltration of the surface epithelium by lymphocytes to a varying degree. Additionally, they possess histologic identifiers unique to each subtype, such as a thickened basal collagen band in the case of CC, or the absence of a basal collagen band and more prominent surface epithelial lymphocytic infiltration in LC.2 Multiple cases of transformation from MC to various forms of inflammatory bowel disease (IBD) have been reported in the literature.3-6 Only a few case reports, however, have described the emergence of MC in patients with established IBD. While some of these articles characterize MC as being part of the IBD spectrum, others describe it as a rare association.7,8
Case Report
Patient 1
A 77-year-old woman with a 36-year history of ulcerative colitis (UC) that had been in clinical remission for 4 years on mesalamine presented with new-onset non-bloody watery diarrhea, weight loss, dehydration, and acute kidney injury. Stool studies for infection along with serological tests for celiac disease were negative. Initial flexible sigmoidoscopy indicated normal mucosa and negative biopsies. Follow-up colonoscopy 2 months later due to persistent diarrhea showed no active inflammation in the colon or terminal ileum, although biopsies indicated new-onset CC and showed thickened collagen plates throughout the colon (Figure 1). No drug-induced risk factors for MC were identified, though patient did experience a Clostridium difficile infection 4 months prior that had since resolved. The diarrhea resolved after treatment with oral budesonide; she was successfully weaned after 6 months of budesonide and has been asymptomatic for 1.5 years of follow-up.
Figure 1.
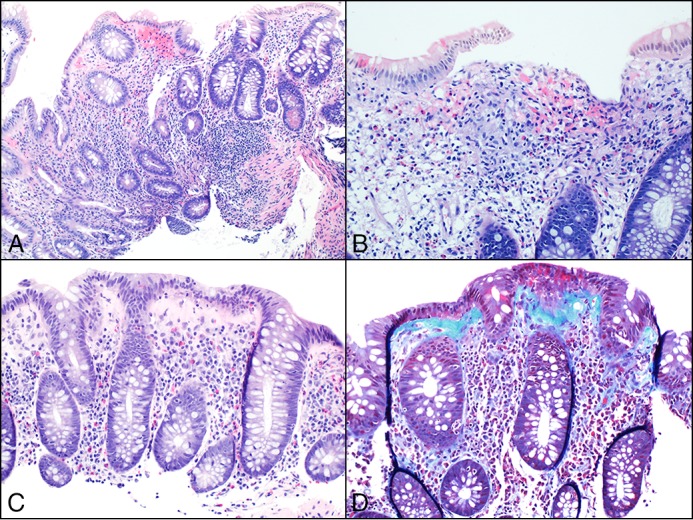
Histologic images from patient 1 showing disease progression. (A) Expansion of lamina propria, architectural glandular disarray, and focal cryptitis occurring during chronic colitis with mild activity. (B) Mucosal granulomas were also identified, supporting the diagnosis of Crohn’s colitis. (C) Loss of surface mucin and intraepithelial lymphocytes, a markedly thickened collagen plate, but no evidence of glandular disarray. (D) Trichrome stain highlighting the thickened collagen under the surface epithelium, confirming the diagnosis of collagenous colitis.
Patient 2
A 57-year-old woman with a 1-year history of inflammatory colonic Crohn’s disease (CD) in previous remission on sulfasalazine presented with new-onset non-bloody watery diarrhea. Infectious stool studies and serologic markers for celiac disease were negative. Colonoscopy showed no active inflammation in the colon or terminal ileum, although biopsies indicated LC and showed characteristic intraepithelial lymphocytic infiltrates in the colon. No drug-induced risk factors for MC were identified. She was treated with oral budesonide, subsequently weaned after 6 months, and is asymptomatic 20 months after being weaned from budesonide.
Patient 3
A 38-year-old woman with a 10-year history of CD of the colon in previous remission on certrolizumab presented with new-onset non-bloody watery diarrhea. Her stool tests were negative for infection along with normal serologic markers for celiac disease. Capsule endoscopy and magnetic resonance enterography were both negative for small-bowel pathology. Colonoscopy showed pseudopolyps with no active inflammation in the colon or terminal ileum, although biopsies showed LC with characteristic focal basal lymphoplasmacytosis in the colon. No drug-induced risk factors for MC were identified. She was treated with oral budesonide, subsequently weaned, and has remained asymptomatic 1 year after treatment.
Patient 4
A 51-year-old woman with an 11-year history of UC in previous remission on mesalamine therapy presented with new-onset non-bloody watery diarrhea. Her stool studies were negative, and esophagogastroduodenoscopy, capsule endoscopy, and computed tomography enterography were normal. Colonoscopy showed no active inflammation in the colon or terminal ileum, although biopsies showed LC with increased intraepithelial lymphocytes and focal lamina propria lymphoplasmacytosis. Drug-induced risk factors for MC included nonsteroidal anti-inflammatory (NSAID) treatment, which was stopped at presentation to clinic. Her symptoms did not resolve by holding this medication. At the time of diagnosis, she reported that she smoked tobacco. She was treated with oral budesonide, subsequently weaned, and has remained asymptomatic at the last follow-up nearly 1 year later.
Patient 5
A 31-year-old woman with a 2-year history of UC in previous remission on mesalamine presented with new-onset non-bloody watery diarrhea. Stool evaluation for infection and serologic markers of celiac disease were both negative. Colonoscopy showed no active inflammation in the colon or terminal ileum, although biopsies indicated new-onset CC and showed thickened collagen plates throughout the colon. No drug-induced risk factors for MC were identified. She was treated with oral budesonide and loperamide as needed; she was subsequently weaned from budesonide and is asymptomatic after 5 years of follow-up.
Patient 6
A 29-year-old woman with a 1-year history of UC in previous remission on balsalazide presented with new-onset non-bloody watery diarrhea. Stool studies and serum celiac panel were both negative. Magnetic resonance enterography was also negative for small-bowel pathology. Colonoscopy showed patchy pseudopolyps in the cecum without active inflammation in the colon or terminal ileum, although biopsies showed increased intraepithelial lymphocytes and focal lamina propria lymphoplasmacytosis, indicative of LC. No drug-induced risk factors for MC were identified. The patient did recently experience a refractory case of C. difficile colitis, which resolved after a fecal microbiota transplant. She was treated with oral budesonide and subsequently weaned, but she had a recurrence of watery diarrhea, abdominal pain, and urgency. Repeat colonoscopy with biopsies showed LC. Reescalation of budesonide therapy resolved symptoms, and the patient is currently maintained asymptomatically on 3 mg oral budesonide.
Patient 7
A 60-year-old woman with a 30-year history of UC in previous remission on mesalamine presented with new-onset non-bloody watery diarrhea. Stool studies for infection along with serological tests for celiac disease were negative. Colonoscopy showed no active inflammation in colon or terminal ileum, although biopsies showed LC with increased intraepithelial lymphocytes and focal lamina propria lymphoplasmacytosis. In terms of drug-induced risk factors for MC, the patient was currently prescribed an NSAID, a proton pump inhibitor, a selective seritonin reuptake inhibitor, and a statin drug. Her NSAID and proton pump inhibitor were discontinued without resolution of symptoms. She was then treated with 9 mg oral budesonide, and the symptoms resolved. The patient is asymptomatic at her most recent follow-up 16 months later.
Discussion
Patients with long-standing IBD on maintenance therapy often experience diarrhea suggestive of a flare of their disease.9,10 This may be due to causes other than exacerbation of their underlying IBD, such as gastrointestinal (GI) infections (C. difficile colitis, cytomegalovirus colitis), bile salt diarrhea, bacterial overgrowth, NSAID use, or irritable bowel syndrome. Clinical workup of diarrheal exacerbations in patients with IBD starts with excluding GI infections and, if negative, evaluating for worsening of the underlying disease.
In contrast to a prior series in which almost entirely asymptomatic MC cases were identified, we describe 7 IBD patients with new-onset watery diarrhea heralding their MC diagnosis.7 Our series is also the first to describe patients with colonic CD developing MC, as all prior published cases occurred in patients with UC. We also identified potential risk factors associated with the development of MC, namely infections (C. difficile) and certain drugs. Associated risk factors for the development of MC in the non-IBD population include female sex, increasing age, smoking (both former and current), concomitant autoimmune disease, certain medications, and C. difficile colitis.1,11,12 Six of our 7 patients exhibited risk factors for development of MC. Interestingly, 2 patients had refractory C. difficile infections prior to the emergence of their MC, one of whom required a fecal microbiota transplant. It has been proposed that chronic C. difficile infection in patients with IBD, especially in those requiring fecal microbiota transplant, may drive the development of MC.13 This may be due to perturbation of the gut microbiota, which then may give rise to MC.
Diagnosing new-onset MC is challenging when it exists in a patient with quiescent colitis, which can look very similar histologically to MC.14 At low magnification, there is normal crypt architecture and diffuse infiltration of the lamina propria by lymphocytes and plasma cells, including basal lymphoplasmacytosis.14-16 The one distinguishing feature is that MC invariably has an increase in intraepithelial lymphocytes in the surface epithelium, which is typically not a feature of IBD.17 Therefore, MC can easily be misdiagnosed as quiescent colitis.
All of our cases were evaluated by board-certified GI pathologists who utilized current pathologic standards, such as intraepithelial lymphocytosis and thickened collagen bands to diagnose microscopic colitides. The diagnostic histologic criteria used for patients with established IBD included basal lymphoplasmacytosis, cryptitis, crypt abscesses, ulceration, Paneth cell metaplasia, and glandular architectural distortion.
When a patient with UC experiences diarrheal exacerbation, a flexible sigmoidoscopy with biopsy is often performed. However, several studies have shown that the highest diagnostic yield for MC come from biopsies of the right (70%) and transverse colons (83%), and that up to 70% of rectal biopsies in patients with MC are false negatives.18,19 This is because changes are more marked in the proximal colon and the distal colon may be spared in CC. The disease may also be patchy in its distribution. The changes of LC are more uniformly and diffusely distributed throughout the large intestine. Random rectal biopsies may give rise to false negatives, whereas additional left colon (sigmoid or descending colon) biopsies increase the chances of arriving at the correct diagnosis. In our series, one diagnosis of CC was missed after flexible sigmoidoscopic exam with left colon biopsies alone. We suggest that endoscopic investigation of non-bloody diarrheal exacerbations in IBD patients in which MC is suspected should be accompanied by biopsies of all anatomical colon segments to increase diagnostic yield. The recognition of the coexistence of MC in IBD patients may one day improve clinical outcomes in the IBD population.
Disclosures
Author contributions: B. Megna and F. Caldera wrote the manuscript and searched the literature. S. Saha, A. Wald, R. Agni, and KA Matkowskyj wrote the manuscript. F. Caldera is the article guarantor.
Financial disclosure: S. Saha is a consultant for UCB Biosciences, Inc.
Informed consent was obtained for this case report.
References
- 1.Gentile NM, Khanna S, Loftus EV Jr, et al. The epidemiology of microscopic colitis in Olmsted County from 2002 to 2010: A population-based study. Clin Gastroenterol Hepatol. 2014; 12:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Münch A, Langner C. Microscopic colitis: Clinical and pathologic perspectives. Clin Gastroenterol Hepatol. 2015; 13:228–36. [DOI] [PubMed] [Google Scholar]
- 3.Pokorny CS, Kneale KL, Henderson CJ. Progression of collagenous colitis to ulcerative colitis. J Clin Gastroenterol. 2001; 32:435–38. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese C, Fabbri A, di Febo G. Colitis evolving into ulcerative colitis. Gut. 2005; 54:1347–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aqel B, Bishop M, Krishna M, et al. Collagenous colitis evolving into ulcerative colitis: A case report and review of the literature. Dig Dis Sci. 2003; 48:2323–7. [DOI] [PubMed] [Google Scholar]
- 6.Janczewska I, Stål P, Sandstedt B. [Transformation of microscopic colitis to inflammatory bowel disease]. Lakartidningen. 2007; 104:1597–8. [PubMed] [Google Scholar]
- 7.Jegadeesan R, Liu X, Pagadala MR, et al. Microscopic colitis: Is it a spectrum of inflammatory bowel disease? World J Gastroenterol. 2013; 19:4252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque M, Florin T. Progression of ulcerative colitis to collagenous colitis: Chance, evolution or association? Inflamm Bowel Dis. 2007; 13:1321.. [DOI] [PubMed] [Google Scholar]
- 9.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105:501–23. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Hanauer SB, Sandborn WJ, et al. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104:465–83. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Banares F, de Sousa MR, Salas A, et al. Epidemiological risk factors in microscopic colitis: A prospective case-control study. Inflamm Bowel Dis. 2013; 19:411–7. [DOI] [PubMed] [Google Scholar]
- 12.Erim T, Alazmi WM, O'Loughlin CJ, et al. Collagenous colitis associated with Clostridium difficile: A cause effect? Dig Dis Sci. 2003; 48:1374–5. [DOI] [PubMed] [Google Scholar]
- 13.Tariq R, Smyrk T, Pardi DS, et al. New-onset microscopic colitis in an ulcerative colitis patient after fecal microbiota transplantation. Am J Gastroenterol. 2016; 111:751–2. [DOI] [PubMed] [Google Scholar]
- 14.Ayata G, Ithamukkala S, Sapp H, et al. Prevalence and significance of inflammatory bowel disease-like morphologic features in collagenous and lymphocytic colitis. Am J Surg Pathol. 2002; 26:1414–23. [DOI] [PubMed] [Google Scholar]
- 15.Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology. 2015; 66:613–26. [DOI] [PubMed] [Google Scholar]
- 16.Robert D, Odze M, and Goldblum J. Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 3rd ed. Philadelphia: Saunders; 2015. [Google Scholar]
- 17.Goldstein NS, Gyorfi T. Focal lymphocytic colitis and collagenous colitis: Patterns of Crohn's colitis? Am J Surg Pathol. 1999; 23:1075–81. [DOI] [PubMed] [Google Scholar]
- 18.Offner FA, Jao RV, Lewin KJ, et al. Collagenous colitis: A study of the distribution of morphological abnormalities and their histological detection. Hum Pathol. 1999; 30:451–7. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, Mazzoleni G, Riddell RH. Distribution of collagenous colitis: Utility of flexible sigmoidoscopy. Gut. 1992; 33:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


