Abstract
We examined the association between plasma 25-hydroxyvitamin D [25(OH)D] concentration and islet autoimmunity (IA) and whether vitamin D gene polymorphisms modify the effect of 25(OH)D on IA risk. We followed 8,676 children at increased genetic risk of type 1 diabetes at six sites in the U.S. and Europe. We defined IA as positivity for at least one autoantibody (GADA, IAA, or IA-2A) on two or more visits. We conducted a risk set sampled nested case-control study of 376 IA case subjects and up to 3 control subjects per case subject. 25(OH)D concentration was measured on all samples prior to, and including, the first IA positive visit. Nine polymorphisms in VDR, CYP24A, CYP27B1, GC, and RXRA were analyzed as effect modifiers of 25(OH)D. Adjusting for HLA-DR-DQ and ancestry, higher childhood 25(OH)D was associated with lower IA risk (odds ratio = 0.93 for a 5 nmol/L difference; 95% CI 0.89, 0.97). Moreover, this association was modified by VDR rs7975232 (interaction P = 0.0072), where increased childhood 25(OH)D was associated with a decreasing IA risk based upon number of minor alleles: 0 (1.00; 0.93, 1.07), 1 (0.92; 0.89, 0.96), and 2 (0.86; 0.80, 0.92). Vitamin D and VDR may have a combined role in IA development in children at increased genetic risk for type 1 diabetes.
Introduction
Type 1 diabetes is a chronic autoimmune disease with increasing incidence worldwide and a geographical gradient in risk where incidence is higher at higher latitudes (1). Vitamin D represents a candidate protective factor for type 1 diabetes as it regulates the immune system and autoimmunity (2), and vitamin D status varies by latitude (3). Vitamin D supplementation in infancy may be associated with a lower risk of type 1 diabetes (4), yet there is substantial heterogeneity across studies (5). Moreover, dietary intake of vitamin D (from food and supplements) throughout childhood has not been found to be associated with either islet autoimmunity (IA) or the progression from IA to type 1 diabetes (6).
Previous studies are limited in that dietary intake is only one of a number of determinants of circulating 25-hydroxyvitamin D [25(OH)D], an established marker of vitamin D status that may more accurately reflect vitamin D exposure. Although 25(OH)D levels are lower in individuals with type 1 diabetes at diagnosis compared with control subjects (7,8), it is unclear whether levels are lower prior to diagnosis. Serum 25(OH)D levels in pregnancy (9,10) and at birth (11,12) are not consistently associated with development of IA or type 1 diabetes in the offspring. Although one cross-sectional analysis showed lower 25(OH)D concentrations in a young cohort of IA-positive subjects compared with IA-negative subjects (13), another showed no difference in 25(OH)D level by IA positivity (14). In prospective studies of children at increased risk for type 1 diabetes, 25(OH)D levels were not associated with IA development or progression to type 1 diabetes (6,15). Yet, subjects with adult-onset type 1 diabetes had lower 25(OH)D levels prior to diagnosis than control subjects (16,17), suggesting a protective effect in adult-onset disease.
These discrepant results may be due to features of study designs, population variation in 25(OH)D levels, and/or a failure to account for underlying genetic variation in the vitamin D pathway. A number of genes play a role in transporting 25(OH)D and 1,25(OH)2D (notably GC, which encodes the vitamin D binding protein [VDBP]), converting 25(OH)D to 1,25(OH)2D (CYP27B1), degrading 1,25(OH)2D (CYP24A1), and enabling the action of 1,25(OH)2D (VDR and RXRA). The VDR gene encodes the vitamin D receptor, which forms a heterodimer with the retinoid X receptor α, encoded by RXRA. In the presence of 1,25(OH)2D, this heterodimer binds to vitamin D response elements at many loci, altering gene expression in a number of biological pathways to influence disease risk. For example, there is a vitamin D response element influencing HLA-DRB1 gene expression. Variation in genes in the vitamin D pathway may modify the effectiveness of 25(OH)D concentration. In The Environmental Determinants of Diabetes in the Young (TEDDY) study, we examined whether 25(OH)D concentration in early infancy and throughout childhood is associated with development of IA. We hypothesized that the association between 25(OH)D concentration and IA is modified by genetic variants in the vitamin D metabolism pathway.
Research Design and Methods
TEDDY is a prospective study of children at increased risk of type 1 diabetes conducted in centers in the U.S. (Colorado, Georgia/Florida, Washington) and Europe (Finland, Germany, and Sweden). Detailed study design and methods have been previously published (18). Institutional review board approval and written informed consent was obtained for all children. The study is monitored by an External Evaluation Committee formed by the U.S. National Institutes of Health.
TEDDY Cohort
Between September 2004 and February 2010, a total of 424,788 newborn infants were screened for type 1 diabetes–associated HLA genotypes (19). The initial screening identified 21,589 eligible infants, of whom 8,676 were enrolled in the follow-up study before age 4 months.
Infants from the general population, with no first-degree relative with type 1 diabetes, were eligible if they had any one of the following HLA-DR-DQ genotypes: 1, DR3-DQ2/DR4-DQ8; 2, DR4-DQ8/DR4-DQ8; 3, DR4-DQ8/DR8-DQ4; or 4, DR3-DQ2/DR3-DQ2. Infants with a first-degree relative with type 1 diabetes were eligible with any above genotype or with 5, DR4-DQ8/DR4-DQ2.3; 6, DR4-DQ8/DR1-DQB 5.1; 7, DR4-DQ8/DR13-DQ6.4; 8, DR4-DQ8/DR9-DQ9.3; or 9, DR3-DQ2/DR9-DQ9.3. These HLA-DR-DQ genotypes are referred to in the text by abbreviated names listing only DR/DR genotypes (HLA-DR3/4 is defined as 1 above).
Clinic visits occurred every 3 months between 3 and 48 months of age, and every 6 months thereafter. The child’s weight and height (or length) were measured at each visit. Participants who lived far from the TEDDY clinic were placed on a long distance protocol, which permitted remote sampling with shipment overnight to the research clinic.
Serum autoantibodies to glutamate decarboxylase (GADA), insulinoma antigen-2 (IA-2A), and insulin (IAA) were measured in two harmonized core laboratories using radiobinding assays incorporating extensive quality control (20,21). Positive results due to maternal IgG transmission were removed. Persistent IA was defined as positive antibodies to the same antigen confirmed by both core laboratories in two consecutive samples, with the date of seroconversion defined as that of the first positive sample.
Nested Case-Control Study
We conducted a nested case-control study using risk set sampling, as described previously (22), using data collected as of 31 May 2012. Briefly, 418 persistent IA case subjects were identified and 3 control subjects per case subject were selected. A control subject was a participant who had not developed persistent IA by the time the case subject to whom it was matched had developed IA, within ±45 days of the event time. Matching factors were clinical center, sex, and family history of type 1 diabetes to control for differences in geographic area, genetic background, and sample/data handling between clinical centers.
25(OH)D Measurement
Plasma from blood drawn into light-protected tubes at all visits up to the event time in case and control subjects was sent to the Genomics and Biomarkers Unit, National Institute for Health and Welfare, Helsinki, Finland. There, 25(OH)D concentrations were measured using the ARCHITECT 25-OH Vitamin D chemiluminescent microparticle immunoassay (CMIA). The assay was standardized against calibrators traceable to internal reference material (Sigma17938). Precision within the project, expressed as the coefficient of variation for four different external quality control samples included in each run, at concentration range 40.2–96.0 nmol/L, was 3.6–4.8%. The respective value for four internal control samples, at concentration range 37.9–104.4 nmol/L, was 2.7–4.8%. The laboratory was blinded to the external quality control samples and case-control status. The laboratory participates regularly in DEQAS (Vitamin D External Quality Assessment Scheme); during this project, mean bias ± SD from the ALTM (all-laboratory trimmed mean) for the participating laboratories was 3.8 ± 5.6% (n = 14), and mean bias ± SD from National Institute of Standards and Technology (NIST) target value was −5.0 ± 8.7% (n = 14) for samples ranging between 24.5 and 98.4 nmol/L.
The ARCHITECT CMIA method is a high throughput method with excellent reproducibility and is commonly used in clinical laboratories. The method requires about 10 µL of sample, making it an attractive choice in children’s studies, where limited sample volumes are available. Furthermore, it is relatively inexpensive. The reference method for 25(OH)D determination is based on liquid chromatography–tandem mass spectrometry, which is more specific and allows the separation of different isomers of vitamin D as well as measurement of low levels of them (including 1,25(OH)2D). Also, additional variability in results, potentially caused by differences in VDBP levels in pregnant women or in patients on dialysis, is avoided. These issues are, however, irrelevant in TEDDY. Therefore, because of excellent suitability for high throughput analysis, the low sample amount required, the excellent reproducibility of the method, and lack of any clinically interfering states, the ARCHITECT assay was chosen. Excellent agreement of the method with liquid chromatography–tandem mass spectrometry in the concentration range relevant to this study (slope 1.02, up to 80 nmol/L) was previously shown in population samples (23).
Vitamin D Gene Genotyping
Single nucleotide polymorphisms (SNPs) were genotyped using the Illumina Infinium ImmunoChip, a custom genotyping array of 195,806 SNPs selected for associations with autoimmune disease. SNPs with call rates <90% or allele distributions strongly deviating from Hardy-Weinberg equilibrium in control subjects (P < 10−6) (except for the HLA region) were discarded. Individuals with call rates <95% or those discordant with reported sex or prior genotyping were also discarded.
In order to estimate ancestry, we performed a principal components analysis with the ImmunoChip data on the entire TEDDY cohort, after selecting one subject per family, using EIGENSTRAT software.
For purposes of our targeted hypothesis regarding the vitamin D gene pathway and the effect of 25(OH)D, we identified SNPs in GC (rs7041), VDR [rs1544410 (BsmI), rs731236 (TaqI), rs11568820 (Cdx2), rs7975232 (ApaI)], CYP27B1 (rs10877012, rs4646536), CYP24A1 (rs4809959, rs2616277), and RXRA (rs3818740, rs10881582). rs731236 and rs10877012 were removed from analysis as they were in complete linkage disequilibrium with rs1544410 and rs4646536, respectively.
Statistical Analyses
Children who were missing either 25(OH)D levels or vitamin D gene data were excluded from analysis, resulting in 376 case and 1,041 control subjects (297 case subjects with 3 control subjects, 71 case subjects with 2 control subjects, and 8 case subjects with 1 control subject). The median age at seroconversion in case subjects was 21 months (minimum = 2, quartile [Q] 1 = 12, Q3 = 31, maximum = 72). The children had 25(OH)D measures on multiple time points prior to and including the seroconversion visit (minimum = 1, Q1 = 2, median = 3, Q3 = 4.5, maximum = 8). Conditional logistic regression was used to compare case and matched control subjects, adjusting for HLA genotype and the first two principal components (PC) from the principal components analysis described previously, in order to adjust for ancestry.
We identified factors influencing 25(OH)D concentration in our control subjects (Supplementary Table 1) to determine factors for adjustment to remove extraneous variation. We first explored taking the slope of 25(OH)D concentration over all time points in each child, as a measure of change in 25(OH)D over time. The subject-specific random slopes and random intercepts were generated from a linear mixed-effects model of 25(OH)D concentration at all time points, adjusting for season of blood draw, age of blood draw, year of blood draw, height, weight, long distance protocol, and sample set. Conditional logistic regression analysis, adjusting for HLA-DR3/4 status, showed that the adjusted slope of 25(OH)D concentration was not different between case and control subjects (odds ratio [OR] 0.87; 95% CI 0.36, 2.10), suggesting that change in 25(OH)D over time was not associated with IA. Hence, assuming a constant 25(OH)D in each child, we then calculated an average 25(OH)D concentration over all time points in each child (childhood 25(OH)D concentration) as a summary exposure variable. We created an adjusted 25(OH)D variable by regressing 25(OH)D concentration on the visit-level variables listed in Supplementary Table 1 using a general linear model. Residuals for each time point were obtained, and the averaged residual was compared between case and matched control subjects using conditional logistic regression adjusting for HLA-DR3/4 status and two PCs indicating ancestry. The adjusted childhood average 25(OH)D residual produced a similar association (OR 0.94; 95% CI 0.90, 0.98) to that obtained with measured 25(OH)D concentration (Table 2) (OR 0.93; 95% CI 0.89, 0.97), suggesting that accounting for visit-level variables such as season of blood collection was not necessary. We therefore continued our analysis with the measured 25(OH)D concentration for increased interpretability.
Table 2.
Association between 25(OH)D concentration and vitamin D sufficiency and IA in the TEDDY nested case-control study
| Vitamin D measure | All case subjects (n = 376) and control subjects (n = 1,041) | Case subjects with multiple autoantibodies (n = 230) and control subjects (n = 639) |
|---|---|---|
| Childhood 25(OH)D concentration† (per 5 nmol/L increase) |
0.93 (0.89–0.97) |
0.91 (0.87–0.96) |
| Early infancy 25(OH)D concentration‡ (per 5 nmol/L increase) |
0.94 (0.91–0.98) |
0.93 (0.89–0.97) |
| Childhood vitamin D sufficiency† (≥50 vs. <50 nmol/L) |
0.68 (0.52–0.89) |
0.61 (0.43–0.86) |
| Early infancy vitamin D sufficiency‡ (≥50 vs. <50 nmol/L) | 0.59 (0.44–0.79) | 0.57 (0.40–0.83) |
Data are OR (95% CI). Separate conditional logistic regression models were run for each vitamin D variable, adjusting for HLA-DR3/4 status and the first two PCs indicating ancestry. For the early infancy vitamin D measures, we also adjusted for age and season of sample collection at the first visit.
†Average of all visits prior to and including the seroconversion visit.
‡Defined by the first 25(OH)D measure occurring before 12 months of age in 360 case subjects and their 981 control subjects. There were 220 case subjects with multiple autoantibodies, with their 603 control subjects, with a measure before 12 months of age.
As previous studies have suggested the importance of early exposure (5), we examined 25(OH)D in early infancy by using the concentration at the first visit (early infancy 25(OH)D concentration). The first 25(OH)D measure was at 3 months of age in 68%, 6 months in 20%, 9 months in 7%, and ≥12 months in 5% of the children. Only those children whose first visit occurred before 12 months are included in the early infancy 25(OH)D analysis, resulting in 360 case and their control subjects (269 case subjects with 3 control subjects, 83 case subjects with 2 control subjects, and 8 case subjects with 1 control subject). In this analysis, we adjusted for season and age at the first sample, in addition to HLA and ancestry.
Log-linearity of the childhood 25(OH)D concentration and IA association was examined by plotting cumulative martingale residuals (24); the supremum test showed no indication of violating log-linearity, suggesting that it is appropriate to analyze 25(OH)D concentration as a continuous variable. However, given that the U.S. Institute of Medicine (25) and European guidelines (26) suggest that 25(OH)D concentrations ≥50 nmol/L are optimal, we also categorized 25(OH)D concentration based on this cut point, and defined childhood vitamin D sufficiency as an average 25(OH)D concentration over all time points of ≥50 nmol/L. Early infancy vitamin D sufficiency was defined as the first measured 25(OH)D concentration ≥50 nmol/L. Average childhood 25(OH)D concentration was within the sufficient range (25,26) in 58% of the children, whereas in early infancy, 49% of the children were vitamin D sufficient. As the Endocrine Society recommends attaining a 25(OH)D concentration above 75 nmol/L (27), in an exploratory analysis, we further divided the vitamin D sufficiency variable into 50–75 nmol/L and >75 nmol/L in order to examine whether the higher cutoff provides additional information regarding risk. Average childhood 25(OH)D was above 75 nmol/L in 10% of the children, whereas in early infancy, 12% of the children had 25(OH)D >75 nmol/L.
We tested for an interaction between 25(OH)D concentration and clinical center. The interaction term was not significant, suggesting that the association between 25(OH)D and IA did not differ by TEDDY clinical center. All analyses were performed using SAS, version 9.4. Two-sided P value < 0.05 was considered statistically significant.
When examining our a priori proposed 25(OH)D × vitamin D gene interactions, each SNP was analyzed additively by treating the number of minor alleles as a continuous variable. False discovery rate adjusted P values are also reported for multiple testing correction for nine SNPs (28).
Results
The childhood and early infancy mean 25(OH)D concentrations of the case and control subjects by the matching variables are shown in Table 1. Adjusting for HLA-DR3/4 and the first two PCs indicating ancestry, higher 25(OH)D concentrations throughout childhood and in early infancy were associated with lower IA risk (Table 2). Moreover, being vitamin D sufficient in childhood was associated with a 31% lower risk of IA compared with those that were insufficient. Similarly, early infancy vitamin D sufficiency was associated with a 40% lower risk of IA compared with those who were insufficient. When we further categorize the vitamin D sufficiency variable into 50–75 nmol/L and >75 nmol/L and compare these categories to vitamin D insufficiency (<50 nmol/L), we find a consistent inverse association between vitamin D status and IA at both the lower and higher categories of sufficiency (Supplementary Table 2), suggesting that further categorization of our vitamin D sufficiency variable is not informative. Therefore, our remaining analyses are conducted using 25(OH)D as a continuous variable and as a dichotomous vitamin D sufficiency variable, as previously defined.
Table 1.
Characteristics of subjects in the TEDDY nested case-control study
| Matching variable | Case subjects, n (%) | Plasma 25(OH)D concentration (nmol/L) |
|||
|---|---|---|---|---|---|
| Childhood* |
Early infancy† |
||||
| Case subjects | Control subjects | Case subjects | Control subjects | ||
| Clinical center |
|||||
| Colorado |
55 (14.6) |
50.6 ± 17.8 |
56.1 ± 15.4 |
42.6 ± 25.7 |
44.8 ± 22.6 |
| Georgia |
23 (6.1) |
58.3 ± 20.0 |
59.3 ± 17.0 |
51.2 ± 21.8 |
53.7 ± 21.1 |
| Washington |
35 (9.3) |
51.3 ± 14.6 |
56.1 ± 17.1 |
44.2 ± 18.7 |
46.7 ± 24.9 |
| Finland |
112 (29.8) |
43.1 ± 13.0 |
44.9 ± 13.7 |
41.1 ± 15.0 |
43.0 ± 15.8 |
| Germany |
29 (7.7) |
57.3 ± 23.1 |
65.0 ± 19.8 |
56.1 ± 29.9 |
64.1 ± 22.3 |
| Sweden |
122 (32.5) |
56.5 ± 14.6 |
59.5 ± 14.8 |
53.9 ± 20.3 |
60.4 ± 20.8 |
| Sex |
|||||
| Female |
167 (44.4) |
51.7 ± 17.0 |
54.2 ± 17.2 |
47.5 ± 21.5 |
49.8 ± 22.0 |
| Male |
209 (55.6) |
51.0 ± 16.7 |
55.0 ± 16.4 |
47.3 ± 21.4 |
52.2 ± 21.6 |
| FDR/GP status |
|||||
| FDR |
91 (24.2) |
47.4 ± 17.2 |
56.8 ± 17.3 |
42.5 ± 20.8 |
54.4 ± 23.3 |
| GP | 285 (75.8) | 52.6 ± 16.5 | 53.9 ± 16.5 | 48.9 ± 21.4 | 50.1 ± 21.2 |
Data are mean ± SD, unless otherwise stated. FDR, first-degree relative of an individual with type 1 diabetes;
GP, from the general population (no first-degree relative with type 1 diabetes).
*Average of measures from all visits prior to and including the seroconversion visit, which is the first of two consecutive visits at which the child tested positive for an autoantibody.
†Defined by the first 25(OH)D measure occurring before 12 months of age in 360 case subjects and their 981 control subjects.
In a secondary analysis in which we restricted the case definition to those who had multiple autoantibodies, which is strongly predictive of type 1 diabetes (29), we show similar statistically significant inverse associations between vitamin D and the outcome of multiple autoantibodies (Table 2).
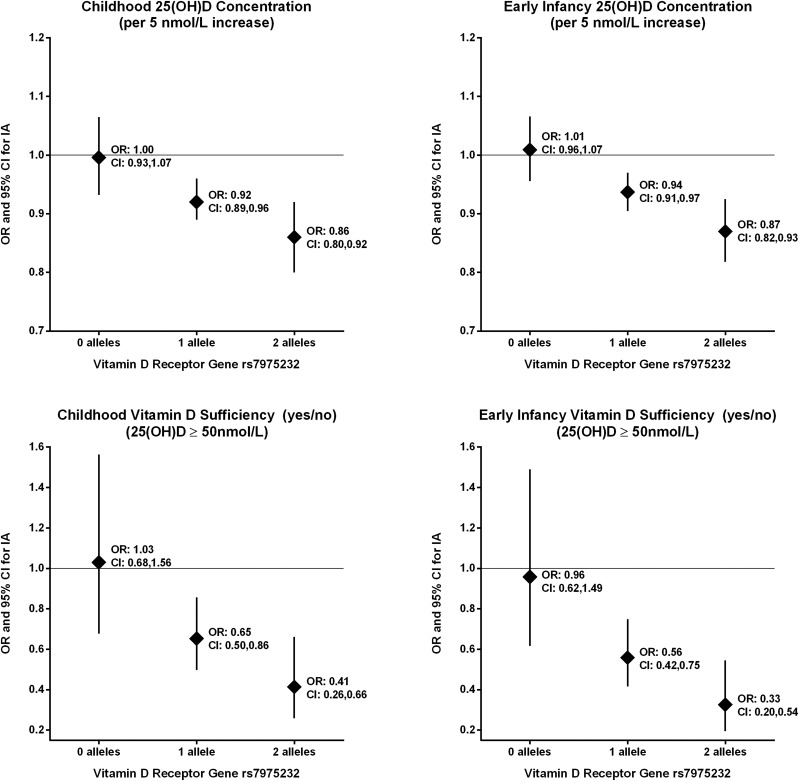
Our a priori hypothesis was that the association between 25(OH)D and IA would be modified by SNPs in the vitamin D metabolism genes. We detected significant interactions between the VDR SNP rs7975232 and childhood and early infancy 25(OH)D concentration (interaction P value = 0.0072 and 0.0019, respectively) and childhood and early infancy vitamin D sufficiency (interaction P value = 0.01 and 0.0051, respectively), adjusting for the HLA-DR3/4 and ancestry (Table 3). The association between childhood 25(OH)D and IA was modified by rs7975232, where for each additional minor allele, increased 25(OH)D concentration was associated with a greater decreased IA risk (Fig. 1). A similar relationship was seen for early infancy 25(OH)D concentration. The inverse association between vitamin D sufficiency and IA is absent in those with no minor alleles and becomes stronger with each additional allele; in those with two minor alleles, being vitamin D sufficient in childhood or in early infancy was associated with a 59% and 67%, respectively, lower risk of IA compared with those that were insufficient.
Table 3.
Test for interaction between vitamin D gene SNPs and the 25(OH)D variables on risk of IA
| Gene | SNP (minor allele) | Minor allele frequency | Nominal P value (P value adjusted by false discovery rate)* for SNP interaction with |
|||
|---|---|---|---|---|---|---|
| Childhood† 25(OH)D concentration | Early infancy‡ 25(OH)D concentration | Childhood vitamin D sufficiency | Early infancy vitamin D sufficiency | |||
|
GC |
rs7041 (T) |
0.42 |
0.67 (0.96) |
0.20 (0.60) |
0.19 (0.34) |
0.15 (0.45) |
|
CYP27B1 |
rs4646536 (C) |
0.33 |
0.29 (0.70) |
0.93 (0.96) |
0.46 (0.59) |
0.52 (0.71) |
|
CYP24A1 |
rs4809959 (A) |
0.50 |
0.51 (0.92) |
0.96 (0.96) |
0.04 (0.12) |
0.63 (0.71) |
|
CYP24A1 |
rs2616277 (A) |
0.17 |
0.85 (0.96) |
0.62 (0.80) |
0.61 (0.61) |
0.83 (0.83) |
|
VDR (BsmI) |
rs1544410 (A) |
0.39 |
0.23 (0.70) |
0.03 (0.14) |
0.18 (0.34) |
0.15 (0.45) |
|
VDR (ApaI) |
rs7975232 (C) |
0.46 |
0.0072 (0.06) |
0.0019 (0.017) |
0.01 (0.09) |
0.0051 (0.046) |
|
VDR (Cdx2) |
rs11568820 (A) |
0.19 |
0.31 (0.70) |
0.51 (0.77) |
0.04 (0.12) |
0.61 (0.71) |
|
RXRA |
rs3818740 (C) |
0.29 |
0.91 (0.96) |
0.47 (0.77) |
0.41 (0.59) |
0.31 (0.56) |
| RXRA | rs10881582 (A) | 0.25 | 0.96 (0.96) | 0.28 (0.63) | 0.56 (0.61) | 0.21 (0.47) |
All models include HLA-DR3/4 status and the first two PCs indicating ancestry. For the early infancy vitamin D exposures, we also adjusted for age and season of sample collection at the first visit.
*The nominal P value of the interaction term is from the Wald test. In parentheses, we provide the P value corrected by the false discovery rate (28).
†Average of measures from all visits prior to and including the seroconversion visit.
‡The first 25(OH)D measure occurring before 12 months of age in 360 case subjects and their 981 control subjects.
Figure 1.
ORs and 95% CIs for decreased risk of IA associated with 25(OH)D concentration (5 nmol/L increase) and being vitamin D sufficient (≥50 nmol/L) versus insufficient by number of minor alleles (the C allele) at rs7975232 in the vitamin D receptor gene. Analyses are adjusted for HLA-DR3/4 and the first two PCs indicating ancestry; and ORs are calculated from the SNP × vitamin D measure interaction terms. For childhood vitamin D measures, we analyzed 376 case subjects and their control subjects (297 case subjects with 3 control subjects, 71 case subjects with 2 control subjects, and 8 case subjects with 1 control subject) with complete plasma 25(OH)D and vitamin D genetic data. For the early infancy vitamin D measures, we analyzed 360 case subjects and their control subjects (269 case subjects with 3 control subjects, 83 case subjects with 2 control subjects, and 8 case subjects with 1 control subject).
In our secondary analysis using only those with multiple autoantibodies for our case subjects, we detected similar interactions between rs7975232 and childhood 25(OH)D concentration (interaction P = 0.0199), early infancy 25(OH)D concentration (interaction P = 0.0055), childhood vitamin D sufficiency (interaction P = 0.0065), and early infancy vitamin D sufficiency (interaction P = 0.0111) on IA risk.
Discussion
In this large prospective study of children at increased type 1 diabetes risk, we found that higher plasma 25(OH)D concentration is associated with a decreased risk of IA. The inverse association was seen when considering the entire cohort, but upon further analysis was primarily seen in those carrying a specific variant in the VDR gene. These relationships remained after limiting our case subjects to those with multiple autoantibodies, a definition that is strongly predictive of type 1 diabetes (29). Our data suggest that vitamin D level and VDR may have a combined role in conferring risk of developing IA, suggesting that the underlying mechanism involves vitamin D action.
Genetic modification of the effect of 25(OH)D may offer an explanation for the inconsistencies in the evidence regarding the putative protective role of vitamin D in type 1 diabetes. Our observation that increasing 25(OH)D concentration was associated with decreased IA risk only in those with minor alleles at VDR rs7975232 is novel and suggests that this underlying susceptibility may be related to the ability to adequately use vitamin D. The previous studies of at-risk children that failed to find an association between 25(OH)D and either IA or type 1 diabetes (6,15) had not explored interactions between 25(OH)D and VDR variants. Previous studies also had fewer IA cases than TEDDY, perhaps resulting in reduced power to detect the relatively small effect size that is observed when not accounting for the interaction.
Vitamin D acts through the vitamin D receptor encoded by the VDR gene. Functional VDR variants act within a complex gene network conferring greater gene activity in part via enhanced gene transcription or mRNA stability, ultimately modulating expression of VDR target genes (30) through vitamin D response elements. The genetic information in our study was derived from SNPs measured in an array platform, which incompletely characterize the target genes and cannot precisely identify potential causal variants. In addition, we lack functional data to explore why and how the identified VDR variants may modify 25(OH)D associations.
Nevertheless, rs7975232 represents the ApaI RFLP, one of three nonfunctional variants (BsmI, ApaI, and TaqI) that are in linkage disequilibrium with each other and with the polyA VNTR in the 3′UTR of VDR, which helps regulate gene expression. PolyA variants (falling into a bimodal distribution of short and long polyA regions) are associated with differences in VDR activity (31). The BsmI-ApaI-TaqI haplotype containing the ApaI(rs7975232) minor allele (linked to the short polyA region) appears to display better vitamin D response, as measured by in vivo response to calcitriol treatment and serum osteocalcin levels (30). This is consistent with our finding that the effect of 25(OH)D concentration is more pronounced in those with minor alleles at rs7975232.
Although we do not have a measure of the more biologically active 1,25(OH)2D3 in the TEDDY children, circulating 25(OH)D concentration has been shown to predict levels of 1,25(OH)2D3 (32,33). The VDBP, which transports 25(OH)D to the kidney for conversion to 1,25(OH)2D, likely plays an important role in the body’s ability to use 25(OH)D effectively. Although we did not measure VDBP levels in TEDDY, we found that 25(OH)D concentration did not interact with rs7041 in GC (the gene that encodes VDBP) on the risk of IA (Table 3).
The epidemiological data suggest a possible scenario where greater VDR activity for a given amount of 1,25(OH)2D could provide protection in situations of low 25(OH)D. For example, the BsmI BB genotype was associated with type 1 diabetes risk only in environments of high 25(OH)D levels (34). In another autoimmune disease, systemic lupus erythematosus, CYP24A1 rs4809959 modified the effect of 25(OH)D, where for each additional minor allele, increased 25(OH)D was associated with further decreased systemic lupus erythematosus risk (35). Low 25(OH)D concentration was associated with a composite outcome of incident hip fracture, myocardial infarction, cancer, and mortality only in those who had one or more minor allele at VDR loci (36). Other studies have found a combined effect of VDR variants and 25(OH)D on risk of breast cancer (BsmI) (37), prostate cancer (Cdx2) (38), and asthma (TaqI) (39).
1,25(OH)2D3 autoregulates the expression of the VDR gene in target cells (40,41), and nearly all immune cells express the VDR, suggesting a plausible mechanism by which 1,25(OH)2D3 is immunomodulatory. 1,25(OH)2D3 has both pro- and anti-inflammatory actions, which may be particularly important for autoimmune disease. As monocytes differentiate into dendritic cells, 1,25(OH)2D3 pushes them toward a tolerogenic state characterized by anti-inflammatory IL-10 dominance over proinflammatory cytokines such as IL-12 and TNFa. Conversely, 1,25(OH)2D3 stimulates the initial differentiation of monocytes into proinflammatory macrophages producing IL-1B and cathelicidin for effective pathogen clearance. However, 1,25(OH)2D3 subsequently decreases macrophage production of a variety of proinflammatory factors while increasing anti-inflammatory IL-10 to restore macrophage balance (reviewed in Dankers et al. [42]). Taken in total, these actions are consistent with decreased autoimmunity without compromising immune defense against pathogens.
1,25(OH)2D promotes immune tolerance by not only shifting the balance of the body’s T-cell response from Th1 to a Th2 response but also by maintaining B-cell homeostasis (reviewed in Altieri et al. [43]) and thus may decrease the risk of autoimmune diseases such as type 1 diabetes. Vitamin D deficiency in early life led to an earlier onset and higher risk of diabetes in the genetically predisposed NOD mouse (44). Likewise, treatment with 1,25(OH)2D3 or its structural analogs may result in a decrease in insulitis and diabetes development in NOD mice (reviewed in Mathieu [45]). However, in humans, low-dose 1,25(OH)2D offered little improvement on insulin requirements in subjects with new-onset diabetes (46,47), although this may have been because treatment occurred too late in the disease process.
Although we had adequate power to detect the described interaction between 25(OH)D and VDR, other genetic interactions of smaller magnitude might be missed here and would require even larger studies for detection. We were not able to examine the association between IA and vitamin D deficiency, as only 6% of the children in our nested case-control study were vitamin D deficient, as defined by average childhood 25(OH)D concentration <30 nmol/L. Moreover, we cannot exclude the possibility that 25(OH)D concentration may be marking confounding variables such as a different lifestyle, social class, or eating habits.
Vitamin D insufficiency (i.e., 25(OH)D concentration <50 nmol/L) is relatively common, detected in 42% of the at-risk children in TEDDY, and in 22–67% of patients with new-onset type 1 diabetes (48–50). Although ours is an observational study, our results suggest that attaining vitamin D sufficiency in children at risk for developing type 1 diabetes may have a protective role. Interventions in infants and children via vitamin D supplementation (51) or dietary modification (52) have been successful in attaining vitamin D sufficiency. Whether or not these efforts should target children who have specific variants in the VDR gene requires further investigation.
In conclusion, this study indicates that higher concentration of circulating 25(OH)D in combination with VDR genotype may decrease risk of IA in children who are at increased risk for type 1 diabetes, as defined by HLA genotype or family history of type 1 diabetes. Further studies are needed to confirm these associations and to enhance knowledge of how variation in vitamin D metabolism genes may alter individual responsiveness to vitamin D. Measurement of an individual’s vitamin D status, along with genetic variation within vitamin D metabolism genes, may help identify individuals most responsive to intervention.
Supplementary Material
Article Information
Funding. This work is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, and contract no. HHSN267200700014C), National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, JDRF, and Centers for Disease Control and Prevention. This work was supported in part by the National Institutes of Health National Center for Advancing Translational Sciences Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
The funders of this study were represented on the Steering Committee and played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.N. drafted the manuscript. J.M.N., B.F., I.E., Å.L., O.S., J.T., M.R., A.-G.Z., J.-X.S., B.A., J.K., S.M.V., and W.H. contributed to the study concept and design. J.M.N., H.-S.L., B.F., I.E., U.U., J.Y., Å.L., O.S., J.T., M.R., A.-G.Z., J.-X.S., S.O.-G., W.-M.C., S.S.R., J.S., J.K., S.M.V., and W.H. contributed to the acquisition, analysis, or interpretation of data. J.M.N., Å.L., J.T., M.R., A.-G.Z., J.-X.S., J.K., S.M.V., and W.H. supervised the study. H.-S.L., B.F., I.E., U.U., J.Y., Å.L., O.S., J.T., M.R., A.-G.Z., J.-X.S., S.O.-G., W.-M.C., S.S.R., J.S., B.A., J.K., S.M.V., and W.H. contributed to the critical revision of the manuscript for important intellectual content. H.-S.L., W.-M.C., S.S.R., and J.K. contributed to the statistical analysis. I.E., O.S., J.T., M.R., A.-G.Z., S.O.-G., W.-M.C., S.S.R., J.S., J.K., S.M.V., and W.H. contributed to the administrative, technical, or material support. Å.L., O.S., J.T., M.R., A.-G.Z., J.-X.S., J.K., and W.H. obtained funding. J.M.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 14th International Congress of the Immunology of Diabetes Society, Munich, Germany, 12–16 April 2015, and the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0802/-/DC1.
A complete list of the members of the TEDDY Study Group can be found in Supplementary Data online.
Contributor Information
Collaborators: The TEDDY Study Group, Marian Rewers, Kimberly Bautista, Judith Baxter, Ruth Bedoy, Daniel Felipe-Morales, Kimberly Driscoll, Brigitte I. Frohnert, Patricia Gesualdo, Michelle Hoffman, Rachel Karban, Edwin Liu, Jill Norris, Adela Samper-Imaz, Andrea Steck, Kathleen Waugh, Hali Wright, Jorma Toppari, Olli G. Simell, Annika Adamsson, Suvi Ahonen, Heikki Hyöty, Jorma Ilonen, Sanna Jokipuu, Tiina Kallio, Leena Karlsson, Miia Kähönen, Mikael Knip, Lea Kovanen, Mirva Koreasalo, Kalle Kurppa, Tiina Latva-aho, Maria Lönnrot, Elina Mäntymäki, Katja Multasuo, Juha Mykkänen, Tiina Niininen, Sari Niinistö, Mia Nyblom, Petra Rajala, Jenna Rautanen, Anne Riikonen, Mika Riikonen, Jenni Rouhiainen, Minna Romo, Tuula Simell, Ville Simell, Maija Sjöberg, Aino Stenius, Maria Leppänen, Sini Vainionpää, Eeva Varjonen, Riitta Veijola, Suvi M. Virtanen, Mari Vähä-Mäkilä, Mari Åkerlund, Katri Lindfors, Jin-Xiong She, Desmond Schatz, Diane Hopkins, Leigh Steed, Jamie Thomas, Janey Adams, Katherine Silvis, Michael Haller, Melissa Gardiner, Richard McIndoe, Ashok Sharma, Joshua Williams, Gabriela Young, Stephen W. Anderson, Laura Jacobsen, Anette G. Ziegler, Andreas Beyerlein, Ezio Bonifacio, Michael Hummel, Sandra Hummel, Kristina Foterek, Nicole Janz, Mathilde Kersting, Annette Knopff, Sibylle Koletzko, Claudia Peplow, Roswith Roth, Marlon Scholz, Joanna Stock, Katharina Warncke, Lorena Wendel, Christiane Winkler, Åke Lernmark, Daniel Agardh, Carin Andrén Aronsson, Maria Ask, Jenny Bremer, Ulla-Marie Carlsson, Corrado Cilio, Emelie Ericson-Hallström, Lina Fransson, Thomas Gard, Joanna Gerardsson, Rasmus Bennet, Monica Hansen, Gertie Hansson, Susanne Hyberg, Fredrik Johansen, Berglind Jonsdottir, Helena Elding Larsson, Marielle Lindström, Markus Lundgren, Maria Månsson-Martinez, Maria Markan, Jessica Melin, Zeliha Mestan, Karin Ottosson, Kobra Rahmati, Anita Ramelius, Falastin Salami, Sara Sibthorpe, Birgitta Sjöberg, Ulrica Swartling, Evelyn Tekum Amboh, Carina Törn, Anne Wallin, Åsa Wimar, Sofie Åberg, William A. Hagopian, Michael Killian, Claire Cowen Crouch, Jennifer Skidmore, Josephine Carson, Maria Dalzell, Kayleen Dunson, Rachel Hervey, Corbin Johnson, Rachel Lyons, Arlene Meyer, Denise Mulenga, Alexander Tarr, Morgan Uland, John Willis, Dorothy Becker, Margaret Franciscus, MaryEllen Dalmagro-Elias Smith, Ashi Daftary, Mary Beth Klein, Chrystal Yates, Jeffrey P. Krischer, Michael Abbondondolo, Sarah Austin-Gonzalez, Maryouri Avendano, Sandra Baethke, Rasheedah Brown, Brant Burkhardt, Martha Butterworth, Joanna Clasen, David Cuthbertson, Christopher Eberhard, Steven Fiske, Dena Garcia, Jennifer Garmeson, Veena Gowda, Kathleen Heyman, Francisco Perez Laras, Hye-Seung Lee, Shu Liu, Xiang Liu, Kristian Lynch, Jamie Malloy, Cristina McCarthy, Steven Meulemans, Hemang Parikh, Chris Shaffer, Laura Smith, Susan Smith, Noah Sulman, Roy Tamura, Ulla Uusitalo, Kendra Vehik, Ponni Vijayakandipan, Keith Wood, Jimin Yang, Beena Akolkar, Kasia Bourcier, Thomas Briese, Suzanne Bennett Johnson, Eric Triplett, Liping Yu, Dongmei Miao, Polly Bingley, Alistair Williams, Kyla Chandler, Saba Rokni, Claire Williams, Rebecca Wyatt, Gifty George, Sian Grace, Iris Erlund, Irma Salminen, Jouko Sundvall, Jaana Leiviskä, Nina Kangas, Petra Arohonka, Henry Erlich, Steven J. Mack, Anna Lisa Fear, Sandra Ke, Niveen Mulholland, Stephen S. Rich, Wei-Min Chen, Suna Onengut-Gumuscu, Emily Farber, Rebecca Roche Pickin, Jordan Davis, Dan Gallo, Jessica Bonnie, and Paul Campolieto
References
- 1.Tuomilehto J. The emerging global epidemic of type 1 diabetes. Curr Diab Rep 2013;13:795–804 [DOI] [PubMed] [Google Scholar]
- 2.Rosen Y, Daich J, Soliman I, Brathwaite E, Shoenfeld Y. Vitamin D and autoimmunity. Scand J Rheumatol 2016;45:439–447 [DOI] [PubMed] [Google Scholar]
- 3.Kimlin MG. Geographic location and vitamin D synthesis. Mol Aspects Med 2008;29:453–461 [DOI] [PubMed] [Google Scholar]
- 4.Hyppönen E, Läärä E, Reunanen A, Järvelin M-R, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001;358:1500–1503 [DOI] [PubMed] [Google Scholar]
- 5.Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients 2013;5:3551–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson M, Brady H, Yin X, et al. . No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 2011;54:2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pozzilli P, Manfrini S, Crinò A, et al.; IMDIAB group . Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res 2005;37:680–683 [DOI] [PubMed] [Google Scholar]
- 8.Littorin B, Blom P, Schölin A, et al. . Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006;49:2847–2852 [DOI] [PubMed] [Google Scholar]
- 9.Miettinen ME, Reinert L, Kinnunen L, et al. . Serum 25-hydroxyvitamin D level during early pregnancy and type 1 diabetes risk in the offspring. Diabetologia 2012;55:1291–1294 [DOI] [PubMed] [Google Scholar]
- 10.Sørensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes 2012;61:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadario F, Savastio S, Pagliardini V, et al. . Vitamin D levels at birth and risk of type 1 diabetes in childhood: a case-control study. Acta Diabetol 2015;52:1077–1081 [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen R, Thorsen SU, Cohen AS, et al. . Neonatal vitamin D status is not associated with later risk of type 1 diabetes: results from two large Danish population-based studies. Diabetologia 2016;59:1871–1881 [DOI] [PubMed] [Google Scholar]
- 13.Raab J, Giannopoulou EZ, Schneider S, et al. . Prevalence of vitamin D deficiency in pre-type 1 diabetes and its association with disease progression. Diabetologia 2014;57:902–908 [DOI] [PubMed] [Google Scholar]
- 14.Reinert-Hartwall L, Honkanen J, Härkönen T, et al.; DIABIMMUNE Study Group . No association between vitamin D and β-cell autoimmunity in Finnish and Estonian children. Diabetes Metab Res Rev 2014;30:749–760 [DOI] [PubMed] [Google Scholar]
- 15.Mäkinen M, Mykkänen J, Koskinen M, et al. . Serum 25-hydroxyvitamin D concentrations in children progressing to autoimmunity and clinical type 1 diabetes. J Clin Endocrinol Metab 2016;101:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munger KL, Levin LI, Massa J, Horst R, Orban T, Ascherio A. Preclinical serum 25-hydroxyvitamin D levels and risk of type 1 diabetes in a cohort of US military personnel. Am J Epidemiol 2013;177:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorham ED, Garland CF, Burgi AA, et al. . Lower prediagnostic serum 25-hydroxyvitamin D concentration is associated with higher risk of insulin-requiring diabetes: a nested case-control study. Diabetologia 2012;55:3224–3227 [DOI] [PubMed] [Google Scholar]
- 18.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 19.Hagopian WA, Erlich H, Lernmark A, et al.; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011;12:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonifacio E, Yu L, Williams AK, et al. . Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babaya N, Yu L, Miao D, et al. . Comparison of insulin autoantibody: polyethylene glycol and micro-IAA 1-day and 7-day assays. Diabetes Metab Res Rev 2009;25:665–670 [DOI] [PubMed] [Google Scholar]
- 22.Lee H-S, Burkhardt BR, McLeod W, et al.; TEDDY study group . Biomarker discovery study design for type 1 diabetes in The Environmental Determinants of Diabetes in the Young (TEDDY) study. Diabetes Metab Res Rev 2014;30:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowling KG, Hull G, Sundvall J, Lamberg-Allardt C, Cashman KD. Improved accuracy of an tandem liquid chromatography-mass spectrometry method measuring 24R,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D metabolites in serum using unspiked controls and its application to determining cross-reactivity of a chemiluminescent microparticle immunoassay. J Chromatogr A 2017;1497:102–109 [DOI] [PubMed] [Google Scholar]
- 24.Borgan Ø, Zhang Y. Using cumulative sums of martingale residuals for model checking in nested case-control studies. Biometrics 2015;71:696–703 [DOI] [PubMed] [Google Scholar]
- 25.Ross AC, Manson JE, Abrams SA, et al. . The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiro A, Buttriss JL. Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull 2014;39:322–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al.; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300 [Google Scholar]
- 29.Ziegler AG, Rewers M, Simell O, et al. . Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uitterlinden AG, Fang Y, Van Meurs JBJ, Pols HAP, Van Leeuwen JPTM. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004;338:143–156 [DOI] [PubMed] [Google Scholar]
- 31.Whitfield GK, Remus LS, Jurutka PW, et al. . Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol 2001;177:145–159 [DOI] [PubMed] [Google Scholar]
- 32.Lagunova Z, Porojnicu AC, Vieth R, Lindberg FA, Hexeberg S, Moan J. Serum 25-hydroxyvitamin D is a predictor of serum 1,25-dihydroxyvitamin D in overweight and obese patients. J Nutr 2011;141:112–117 [DOI] [PubMed] [Google Scholar]
- 33.Engelman CD, Fingerlin TE, Langefeld CD, et al. . Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab 2008;93:3381–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tizaoui K, Kaabachi W, Hamzaoui A, Hamzaoui K. Contribution of VDR polymorphisms to type 1 diabetes susceptibility: systematic review of case-control studies and meta-analysis. J Steroid Biochem Mol Biol 2014;143:240–249 [DOI] [PubMed] [Google Scholar]
- 35.Young KA, Munroe ME, Guthridge JM, et al. . Combined role of vitamin D status and CYP24A1 in the transition to systemic lupus erythematosus. Ann Rheum Dis 2017;76:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin GP, Robinson-Cohen C, de Boer IH, et al. . Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 2012;308:1898–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowe LC, Guy M, Mansi JL, et al. . Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer 2005;41:1164–1169 [DOI] [PubMed] [Google Scholar]
- 38.Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Hollis BW, Giovannucci E. Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer risk. Prostate 2007;67:911–923 [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulou A, Kouis P, Middleton N, et al. . Association of vitamin D receptor gene polymorphisms and vitamin D levels with asthma and atopy in Cypriot adolescents: a case-control study. Multidiscip Respir Med 2015;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zella LA, Kim S, Shevde NK, Pike JW. Enhancers located in the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 2007;103:435–439 [DOI] [PubMed] [Google Scholar]
- 41.Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol Endocrinol 2010;24:128–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol 2017;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altieri B, Muscogiuri G, Barrea L, et al. . Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev Endocr Metab Disord 2017;18:335–346 [DOI] [PubMed] [Google Scholar]
- 44.Giulietti A, Gysemans C, Stoffels K, et al. . Vitamin D deficiency in early life accelerates type 1 diabetes in non-obese diabetic mice. Diabetologia 2004;47:451–462 [DOI] [PubMed] [Google Scholar]
- 45.Mathieu C. Vitamin D and diabetes: Where do we stand? Diabetes Res Clin Pract 2015;108:201–209 [DOI] [PubMed] [Google Scholar]
- 46.Walter M, Kaupper T, Adler K, Foersch J, Bonifacio E, Ziegler AG. No effect of the 1alpha,25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care 2010;33:1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bizzarri C, Pitocco D, Napoli N, et al.; IMDIAB Group . No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care 2010;33:1962–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huynh T, Greer RM, Nyunt O, et al. . The association between ketoacidosis and 25(OH)-vitamin D levels at presentation in children with type 1 diabetes mellitus. Pediatr Diabetes 2009;10:38–43 [DOI] [PubMed] [Google Scholar]
- 49.Franchi B, Piazza M, Sandri M, Mazzei F, Maffeis C, Boner AL. Vitamin D at the onset of type 1 diabetes in Italian children. Eur J Pediatr 2014;173:477–482 [DOI] [PubMed] [Google Scholar]
- 50.Al-Zubeidi H, Leon-Chi L, Newfield RS. Low vitamin D level in pediatric patients with new onset type 1 diabetes is common, especially if in ketoacidosis. Pediatr Diabetes 2016;17:592–598 [DOI] [PubMed] [Google Scholar]
- 51.Gallo S, Comeau K, Vanstone C, et al. . Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 2013;309:1785–1792 [DOI] [PubMed] [Google Scholar]
- 52.Brett NR, Lavery P, Agellon S, et al. . Dietary vitamin D dose-response in healthy children 2 to 8 y of age: a 12-wk randomized controlled trial using fortified foods. Am J Clin Nutr 2016;103:144–152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.