Abstract
OBJECTIVE
To assess national differences in diabetes care and quality of life (QOL) between individuals with long-standing type 1 diabetes (≥50 years) in Canada and the U.S.
RESEARCH DESIGN AND METHODS
Cross-sectional data from identical surveys administered in the Canadian Study of Longevity in Diabetes and the Joslin Medalist Study, collected in 2013–2016 and 2005–2011, respectively, were compared. Laboratory values and ophthalmic examination were completed by clinical care physicians for Canadians and the Joslin Clinic for Americans. Univariate comparisons and multivariable regression for HbA1c, QOL, insulin pump use, and coronary artery disease (CAD) were performed. Nephropathy, CAD, and peripheral arterial disease (PAD) were self-reported; neuropathy was defined by a Michigan Neuropathy Screening Instrument (Questionnaire component) score ≥3, and proliferative retinopathy was documented from ophthalmic examination. QOL was self-reported on an ordinal scale.
RESULTS
Three hundred sixty-one Canadians and 668 Americans had similar ages (mean 65.78 years [SD 8.67] vs. 66.38 years [7.66], P = 0.27) and durations of diabetes (median 53.00 years [interquartile range 51.00, 58.00] vs. 53.00 years [51.00, 57.00], P = 0.51). Canadians had higher HbA1c (mean 7.53% [SD 1.03] [59 mmol/mol] vs. 7.22% [0.98] [55 mmol/mol], P < 0.0001), lower QOL (36.9% vs. 48.7% with “excellent” QOL, P = 0.0002), and less CAD (29.7% vs. 41.2%, P = 0.0003) and insulin pump use (43.3% vs. 55.6%, P = 0.0002). Other complication rates were similar. Residual differences for Canadians compared with Americans remained after adjustment for age, sex, CAD, PAD, education, and relevant a priori selected variables: 0.28% higher HbA1c (P = 0.0004); and odds ratios of 0.68 (95% CI 0.51, 0.90), 0.46 (0.31, 0.68), and 0.71 (0.52, 0.96) for higher QOL, CAD, and insulin pump use, respectively.
CONCLUSIONS
Although Canadians and Americans have similar rates of complications other than CAD, further research is required to understand why Canadians have higher HbA1c levels, lower QOL, and less insulin pump use.
Introduction
Type 1 diabetes is associated with microvascular and macrovascular complications, yet the identification of biomarkers predictive of complications has been limited (1,2). In subjects with long-standing type 1 diabetes (>50 years duration), ∼25% do not have complications, and this is not clearly attributed to superior glycemic control, although data are limited by a lack of longitudinal, historical glycemic control measurement (3–5).
We have previously described a Canadian cohort of extreme duration of type 1 diabetes (the Canadian Study of Longevity in Diabetes) in which common clinical variables, including contemporaneous hemoglobin A1c (HbA1c), were not associated with complications (6). The Joslin 50-Year Medalist Study showed limited association with classical risk factors for complications including HbA1c level, diabetes duration, and C-peptide level (7). The exception to this was the association of HbA1c and exercise with macrovascular disease (8). Alternatively, novel factors have been associated with protection from complications such as DNA damage checkpoint pathways and endothelial progenitor cells (5,9,10).
Canadians and Americans with type 1 diabetes are similar in clinical trial settings; however, national differences in diabetes characteristics have not been well studied in observational settings (11,12). Subjects in clinical trials may not be representative of the general population of individuals with type 1 diabetes, and the care of subjects while enrolled in clinical trials is unlikely to reflect “usual” care. Substantial differences between health care in Canada and the U.S. exist. In Canada, all medically necessary services are funded by the provincial health care plan in a single-payer system. Patients only pay for services not covered in the plan, such as medications and dental expenses. Each province has government-sponsored drug benefit programs for specific groups of individuals (e.g., seniors), and outside of these programs many Canadians purchase private insurance for medications through employee programs, though out-of-pocket costs are still common (13). In contrast, in the U.S., individuals <65 years of age may have employer-sponsored health insurance, and others have government coverage if elderly and/or disabled (Medicare) or of low socioeconomic status (SES) (Medicaid) (14).
An evaluation for similarities and differences between comparably ascertained cohorts of the Canadian Study of Longevity in Diabetes and the Joslin 50-Year Medalist Study is important as it is unknown whether differences in health care delivery, access to care, and compensation for health care and medications between Canada and the U.S. impact diabetes outcomes or measures of quality of care. These cohorts are ideal populations in which to study national differences because of the comparability resulting from very similar inclusion criteria and enrollment procedures.
Our objectives were to compare diabetes care indicators and quality of life (QOL) in a population of individuals with type 1 diabetes for >50 years between Canadians and Americans. We hypothesized that there would be similarities in Canadian and American metabolic control, diabetes complications, and key indicators of diabetes care, including QOL, insulin pump use, and use of renin-angiotensin-aldosterone system (RAAS) inhibitors (ACE inhibitors or aldosterone receptor blockers) and lipid-lowering medications.
Research Design and Methods
Study Design and Setting
The Joslin Medalist Study and the Canadian Study of Longevity in Diabetes have been described previously (4–7,15,16). The Joslin 50-Year Medalist Study and the Canadian Study of Longevity in Diabetes were initiated in 2005 and 2013 to study Joslin Medal awardees in the U.S. and Canada, respectively. The Joslin 50-Year Medal Program began awarding medals in 1970 to individuals throughout the world with type 1 diabetes for >50 years, based on original medical record documentation of diagnosis or three alternate forms of evidence of 50 years of insulin dependence since the time of diagnosis (5). Data for the Joslin Medalists included in this study were collected between 2005 and 2011 and encompass individuals from all of the 50 states in the U.S. (∼10% from Massachusetts, the location of the Joslin Diabetes Center), and data for the Canadian subjects were collected between 2013 and 2016. The programs in both countries were advertised in physician offices, on the Internet, at national diabetes foundations, on social media, and in print media.
Data Sources
Participants in both cohorts completed an identical survey consisting of a Diabetes and Health History Questionnaire, and Part 1 (questionnaire portion) of the Michigan Neuropathy Screening Instrument (MNSI). All variables for health history, medications, health care use, and education were thus determined by self-report in the same manner in the two cohorts. Coronary artery disease (CAD) was defined by a composite of self-reported history of CAD or specifically myocardial infarction, angina, coronary artery bypass grafting (CABG), or percutaneous coronary intervention (PCI), which is consistent with the definition of cardiovascular disease (CVD) used in previous Joslin Medal studies. Self-report of CAD is accurate when validated against the medical record (17). Neuropathy was determined by a score of ≥3 on the questionnaire component of the MNSI. QOL was rated by subject response to a single question to indicate QOL as poor, normal, good, or excellent.
Assessment of clinical and laboratory assessment parameters differed between the two cohorts. The Joslin Medalists had clinical and laboratory examinations performed at the Joslin Clinic and the Beetham Eye Institute. Blood samples were collected and processed in a single laboratory, and ophthalmic examinations were performed and retinopathy status was determined according to Early Treatment of Diabetic Retinopathy Study criteria. Blood pressure was measured in a systematic manner by a trained nurse, although because of logistical issues blood pressure was not measured in 13% of participants. In the Canadian cohort, clinical visits were not performed. Recent reports from optometrists or ophthalmologists performing routine clinical care were requested and used to determine retinopathy status, and primary care physicians were asked to complete an additional form with the date and values for most recent blood pressure, lipids, creatinine, HbA1c, and urine albumin-to-creatinine ratio (ACR). These laboratory tests were performed in the context of routine care and therefore were not completed in a central laboratory.
For this study, participants in both cohorts were classified as having either proliferative retinopathy or nonproliferative/no retinopathy.
Several objective definitions were used to determine the presence or absence of nephropathy, as follows: 1) estimated glomerular filtration rate (eGFR) of ≤45 mL/min, 2) eGFR of <60 mL/min in addition to urine ACR of >30 mg/g (3.4 mmol/mol), and 3) ACR of >18 mg/g (2 mg/mmol) in participants using a RAAS inhibitor or >30 mg/g (3.4 mg/mmol) in individuals not using a RAAS inhibitor.
Statistical Methods
Descriptive statistics were performed to assess completeness, distribution, biologic plausibility, and outliers. Missing data were assumed to be missing at random. Available-case analysis was used to report patient characteristics, and complete-case analysis was used for multivariable regression. Systolic and diastolic blood pressure values were not recorded in ∼25% of subjects (13% of Americans and 48% of Canadians) as a result of the methods of data collection described above. As a result, blood pressure was not included in multivariable models, though self-reported history of hypertension was included. Diabetes care indicators were compared between Canadians and American using χ2 tests for categorical variables, Student t tests for normally distributed continuous variables, and Wilcoxon rank sum tests for non-normally distributed continuous variables. Normally distributed data are presented as the mean (SD), and non-normally distributed data are presented as the median (interquartile range [IQR]). The following multivariable models were used: linear regression for HbA1c, ordinal logistic regression for QOL, and logistic regression for CAD and insulin pump use. All variables for multivariable models were selected a priori based on known or theoretical confounders. An α (type I error; two-tailed) threshold of <0.05 for all statistical tests was used. All statistical analysis was performed using SAS version 9.4 software.
Institutional Review Board Approval
All subjects provided informed consent, and approval to conduct the studies was received from institutional review boards at the University of Toronto and the Joslin Diabetes Center.
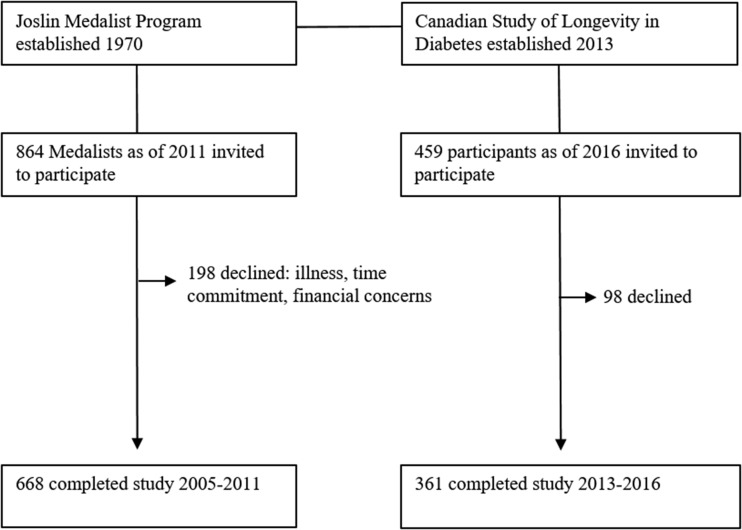
Results
Of 459 Canadians identified as having type 1 diabetes for >50 years, 361 (79%) participated in this study between 2013 and December 2016. Between April 2005 and August 2011, 668 (77% of eligible) Joslin Medalists participated and were assessed at the Joslin Diabetes Center. Nonparticipants cited illness, time commitment, or financial concerns as reasons for not participating (Fig. 1). Canadians and Americans were of similar age (mean 65.8 years [SD 8.7] vs. 66.4 years [7.7], P = 0.27) and had similar duration of diabetes (median 53.0 years [IQR 51.0, 58.0] vs. 53.0 years [51.0, 57.0], P = 0.51) (Table 1). Ninety-five percent of Canadians and Americans were Caucasian. The proportion of participants who were of male sex was similar (42.8% vs. 47.7%, P = 0.13). BMI did not differ between cohorts (median 25.1 kg/m2 [IQR 23.0, 28.3] vs. 25.7 kg/m2 [23.1, 28.4], P = 0.43). Canadians had lower systolic blood pressure (mean 128.2 mmHg [SD 15.3] vs. 132.8 mmHg [17.5], P = 0.0006) but higher diastolic blood pressure (67.0 mmHg [8.8] vs. 63.3 mmHg [8.8], P < 0.0001). Canadians had a lower frequency of annual physician visits, with 48.2% seeing a physician more than twice per year compared with 76.3% of Americans (P < 0.0001 for overall comparison of all categories). Canadians reported lower levels of education, with 53.5% completing postsecondary education, compared with 70% of Americans. Canadians also reported lower QOL, with a lower proportion of Canadians reporting excellent QOL (36.9% vs. 48.7%) and a higher proportion reporting poor QOL (3.1% vs. 1.25%, P = 0.0002 for overall comparison of all categories). Canadians had lower mean LDL compared with Americans (1.99 mmol/L [SD 0.71] vs. 2.11 mmol/L [0.63], P = 0.012). Interestingly, this occurred despite similar use of lipid-lowering medications (68.5% vs. 73.3%, P = 0.11). Canadians also had lower ACR (median 1.36 mmol/mol [IQR 0.80, 3.66] vs. 1.19 mmol/mol [0.52, 3.82], P = 0.018) despite similar use of RAAS inhibitors (71.9% vs. 61.9%, P = 0.52). Canadians had a mean HbA1c level ∼0.3% higher than Americans (7.53% [SD 1.03] [59 mmol/mol] vs. 7.22% [0.98] [55 mmol/mol], P < 0.0001). The proportions of subjects with HbA1c <7% (53 mmol/mol) were 30.1% and 39.6% for Canadians and Americans, respectively. Canadians also had a lower proportion of insulin pump use (43.3% vs. 55.6%, P = 0.0002).
Figure 1.
Study flow of participants.
Table 1.
Characteristics by nationality
| Characteristic | American (n = 668) | Canadian (n = 361) | P value |
|---|---|---|---|
| Age (years)** | 66.38 (7.66) | 65.78 (8.67) | 0.2729 |
| Age at diagnosis (years) | 11.00 [6.00, 15.00] | 11.00 [6.00, 16.00] | 0.6963* |
| Duration of diabetes (years) | 53.00 [51.00, 57.00] | 53.00 [51.00, 58.00] | 0.5154* |
| Male sex | 318 (47.7) | 154 (42.8)† | 0.13 |
| BMI (m/kg2) | 25.68 [23.14, 28.44] | 25.08 [22.96, 28.34] | 0.4328* |
| Missing | 78 (11.7) | 14 (3.9) | |
| SBP (mmHg)** | 132.82 (17.47) | 128.21 (15.33) | 0.0006 |
| Missing | 83 (12.5) | 174 (48.2) | |
| DBP (mmHg)** | 63.26 (8.77) | 66.95 (8.84) | <0.0001 |
| Missing | 83 (12.9) | 176 (48.8) | |
| Hypertension | 375 (58) | 214 (60.6) | 0.41 |
| Missing | 19 (2.9) | 8 (2.2) | |
| Insulin dose (units/kg) | 0.43 [0.34, 0.53] | 0.46 [0.37, 0.59] | 0.0009* |
| Missing | 82 (12.3) | 54 (15) | |
| Smoker (ever) | 295 (48.6) | 192 (53.8) | 0.12 |
| Missing | 59 (8.9) | 4 (1.1) | |
| Currently physically active | 509 (79) | 257 (72) | 0.012 |
| Missing | 22 (3.3) | 4 (1.1) | |
| No. annual physician visits | <0.0001 | ||
| <1 | 8 (1.2) | 15 (4.2) | |
| 1 | 29 (4.5) | 43 (12) | |
| 2 | 117 (18) | 128 (35.7) | |
| >2 | 496 (76.3) | 173 (48.2) | |
| Missing | 16 (2.4) | 2 (0.55) | |
| Highest education | <0.0001 | ||
| No high school | 2 (0.3) | 22 (6.2) | |
| High school | 51 (7.9) | 66 (18.5) | |
| Some college/university | 141 (21.8) | 78 (21.8) | |
| College/university degree | 202 (31.2) | 132 (37) | |
| Masters or doctoral degree | 191 (29.5) | 53 (14.8) | |
| Health professional | 60 (9.3) | 6 (1.7) | |
| Missing | 19 (2.9) | 4 (1.1) | |
| QOL | 0.0002 | ||
| Poor | 8 (1.25) | 11 (3.1) | |
| Normal | 55 (8.6) | 52 (14.5) | |
| Good | 264 (41.4) | 163 (45.5) | |
| Excellent | 311 (48.7) | 132 (36.9) | |
| Missing | 28 (4.2) | 3 (0.83) | |
| Laboratory values | |||
| Total cholesterol (mmol/L)‡** | 4.17 (0.88) | 4.13 (0.94) | 0.5046 |
| HDL (mmol/L)‡** | 1.65 (0.51) | 1.73 (0.53) | 0.0239 |
| Triglycerides (mmol/L)‡ | 0.76 [0.57, 1.05] | 0.80 [0.61, 1.07] | 0.073* |
| LDL (mmol/L)** | 2.11 (0.63) | 1.99 (0.71) | 0.0121 |
| Missing | 2 (0.3) | 58 (16.1) | |
| Renal outcomes | |||
| eGFR (mL/min/1.73 m2)** | 67.40 (19.97) | 68.04 (22.51) | 0.655 |
| Missing | 3 (0.45) | 21 (5.8) | |
| ACR (mg/mmol) | 1.36 [0.80, 3.66] | 1.19 [0.52, 3.82] | 0.0176* |
| Missing | 132 (19.8) | 85 (23.5) | |
| Most recent HbA1c, % (mmol/mol)** | 7.22 (0.98), 55 | 7.53 (1.03), 59 | <0.0001 |
| Missing | 0 | 12 (3.3) | |
| Medications | |||
| Insulin pump | 365 (55.6) | 155 (43.3) | 0.0002 |
| Missing | 9 (1.4) | 3 (0.83) | |
| RAAS inhibitor | 458 (69.9) | 258 (71.9) | 0.52 |
| Missing | 13 (1.9) | 2 (0.6) | |
| Lipid-lowering medication | 446 (68.5) | 263 (73.3) | 0.11 |
| Missing | 15 (2.3) | 2 (0.6) |
DBP, diastolic blood pressure; SBP, systolic blood pressure. Data are presented as mean (SD) (indicated by **), median [IQR], or frequency (% of available data).
*For continuous variables, indicates use of Wilcoxon rank sum test; Student’s t test used otherwise. For categorical variables, χ2 test used.
†One person missing.
‡None missing for Americans; missing for Canadians: 54 (15%) for total cholesterol, 55 (15.2%) for triglycerides, 53 (14.7%) for HDL.
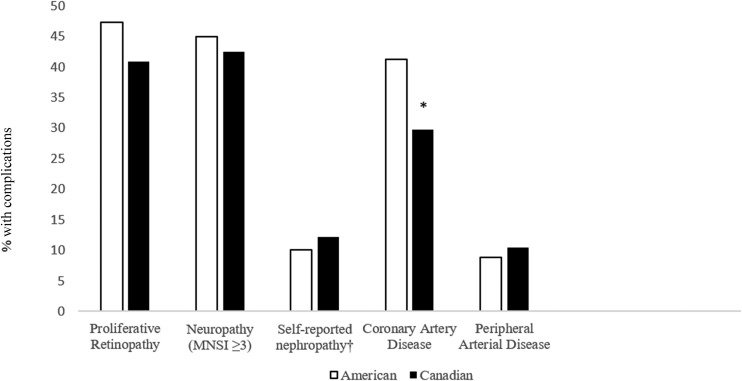
Complications did not differ by nationality with the exception of CAD, which was lower in the Canadian cohort (29.7% vs. 41.2%, P = 0.0003) (Fig. 2). Canadians had lower rates of revascularization (PCI or CABG): 86 (24%) compared with 221 (33.7%) for Americans (P = 0.0012). Both PCI and CABG were less common among Canadians compared with Americans (13.3% vs. 20.9% for PCI and 15.9% vs. 25.3% for CABG). Retinopathy and neuropathy were common in both cohorts (40.9% vs. 47.3% for retinopathy, 42.5% vs. 44.9% for neuropathy in Canadians and Americans, respectively). Nephropathy and peripheral arterial disease (PAD) were uncommon (14% vs. 13.6% for nephropathy based on an eGFR of ≤45 mL/min, 10.5% vs. 8.8% for PAD) for Canadians and Americans, respectively. Self-reported rates of nephropathy were higher in both countries than the objective determination based on ACR; however, there were no significant differences between countries for either self-reported or objective nephropathy.
Figure 2.
Prevalence of complications by nationality. *P < 0.05 for difference in prevalence by American vs. Canadian cohort. †For nephropathy differences in Americans vs. Canadians defined by the following: eGFR ≤45 mL/min, 13.6% vs. 14.4% (P = 0.72); ACR >2 mg/mmol while receiving RAAS inhibitor or >3.4 mg/mmol while not receiving RAAS inhibitor, 30.3% vs. 34.7% (P = 0.2); and eGFR <60 mL/min + ACR >3.4 mg/mmol, 13.6 vs. 17.4% (P = 0.15).
Multivariable models were used to assess residual differences according to nationality in key indicator variables, after adjustment for confounders selected a priori (Table 2). After adjustment, HbA1c level was 0.28% higher in Canadians compared with Americans. Variables that were significantly associated with higher HbA1c other than Canadian nationality were female sex, higher eGFR, higher BMI, and the presence of CAD and neuropathy. In contrast, insulin pump use was associated with lower HbA1c level.
Table 2.
Multivariable models for the outcomes HbA1c (linear regression), QOL (ordinal logistic regression), CAD, and pump use (logistic regressions)
| Predictors | Model outcome |
|||||||
|---|---|---|---|---|---|---|---|---|
| HbA1c (n = 788) |
QOL (low to high) (n = 866) |
CAD (n = 761) |
Pump (n = 878) |
|||||
| Adjusted β | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Nationality (reference = American) | 0.28 | 0.0003 | 0.71 (0.53, 0.95) | 0.02 | 0.46 (0.31, 0.68) | <0.0001 | 0.71 (0.52, 0.97) | 0.030 |
| Age | 0.0028 | 0.56 | 1.00 (0.99, 1.02) | 0.65 | 1.05 (1.02, 1.08) | 0.0001 | 0.95 (0.93, 0.96) | <0.0001 |
| Sex (reference = male) | 0.47 | <0.0001 | 0.94 (0.71, 1.25) | 0.67 | 0.64 (0.44, 0.93) | 0.02 | 1.57 (1.16, 2.13) | 0.0033 |
| Insulin pump | −0.15 | 0.030 | 1.21 (0.92, 1.6) | 0.17 | ||||
| Exercise | 1.8 (1.30, 2.49) | 0.0004 | 0.79 (0.52, 1.20) | 0.28 | 1.34 (0.94, 1.90) | 0.11 | ||
| Annual physician visits, n (reference <1) | ||||||||
| 1 | −0.08 | 0.77 | ||||||
| 2 | −0.27 | 0.27 | ||||||
| >2 | −0.22 | 0.36 | ||||||
| HbA1c | 0.76 (0.65, 0.87) | 0.0001 | 1.23 (1.03, 1.47) | 0.024 | 0.79 (0.68, 0.93) | 0.0041 | ||
| eGFR | 0.0042 | 0.029 | 0.98 (0.97, 0.99) | 0.0004 | ||||
| BMI | 0.014 | 0.068 | 0.99 (0.95, 1.03) | 0.53 | ||||
| CAD | 0.20 | 0.0088 | 0.89 (0.66, 1.20) | 0.44 | 0.90 (0.66, 1.24) | 0.52 | ||
| PAD | 0.15 | 0.24 | 0.59 (0.36, 0.95) | 0.029 | 5.22 (2.71, 10.07) | <0.0001 | 0.52 (0.30, 0.89) | 0.018 |
| Nephropathy (self-report) | 0.23 | 0.055 | 0.63 (0.41, 0.98) | 0.050 | 1.32 (0.74, 2.36) | 0.35 | 1.65 (1.01, 2.69) | 0.045 |
| Neuropathy (MNSI score ≥3) | 0.14 | 0.044 | 0.69 (0.53, 0.91) | 0.0086 | 1.61 (1.20, 2.15) | 0.0015 | ||
| Proliferative retinopathy (by examination) | 0.066 | 0.34 | 0.75 (0.57, 0.98) | 0.037 | 0.99 (0.73, 1.32) | 0.92 | ||
| Lipid-lowering medication | 3.69 (2.34, 5.81) | <0.0001 | ||||||
| LDL | 0.86 (0.64, 1.15) | 0.30 | ||||||
| RAAS inhibitor | 0.77 (0.50, 1.18) | 0.24 | ||||||
| Smoking | 1.10 (0.78, 1.55) | 0.59 | ||||||
| Hypertension | 1.41 (0.96, 2.06) | 0.08 | ||||||
| Education (reference = no high school) | ||||||||
| High school | 0.28 | 0.22 | 0.63 (0.24, 1.64) | 0.35 | 2.94 (0.83, 10.40) | 0.095 | 1.07 (0.37, 3.10) | 0.91 |
| Some university/college | 0.13 | 0.56 | 0.66 (0.26, 1.64) | 0.37 | 2.97 (0.88, 10.05) | 0.08 | 1.26 (0.45, 3.50) | 0.66 |
| University/college | 0.15 | 0.48 | 0.89 (0.36, 2.2) | 0.80 | 1.87 (0.56, 6.23) | 0.31 | 1.14 (0.41, 3.11) | 0.80 |
| Masters or doctoral | 0.11 | 0.62 | 1.27 (0.50, 3.20) | 0.61 | 1.85 (0.54, 6.33) | 0.33 | 1.54 (0.55, 4.30) | 0.41 |
| Health professional | 0.17 | 0.50 | 0.87 (0.31, 2.44) | 0.78 | 1.39 (0.36, 5.44) | 0.64 | 1.38 (0.44, 4.30) | 0.59 |
Canadians had lower QOL than Americans after adjustment for possible confounders, with an odds ratio (OR) of being in a higher QOL category of 0.71 (95% CI 0.53, 0.95) for Canadians compared with Americans. Higher HbA1c and the presence of neuropathy, nephropathy, retinopathy, and PAD were also independently associated with reduced odds of being in a higher QOL category. A lower prevalence of CAD in Canadians also persisted after adjustment, with an OR of 0.46 (95% CI 0.31, 0.68) for Canadians. Other variables significantly associated with the presence of CAD were older age, male sex, higher HbA1c level, lower eGFR, lipid-lowering medication use, and nephropathy. LDL level was not independently associated with CAD.
To explore whether insulin pump use was related to higher HbA1c level in Canadians, an analysis stratified by insulin pump use was performed. A higher HbA1c level of ∼0.3% in Canadians was found in both pump users (7.42% [58 mmol/mol] vs. 7.13% [54 mmol/mol], P = 0.001) and non–pump users (7.51% [59 mmol/mol] vs. 7.34% [57 mmol/mol], P = 0.0083). After adjustment, Canadians had reduced odds (OR 0.71 [95% CI 0.52, 0.97]) of pump use compared with Americans. Factors significantly associated with reduced pump use in the combined cohort were older age, male sex, higher HbA1c level, the presence of PAD, and the absence of nephropathy and neuropathy.
Conclusions
Canadians and Americans with long-standing type 1 diabetes in the Canadian Study of Longevity in Diabetes and the Joslin Medalist Study have similar age, duration of diabetes, ethnicity, sex distribution, and BMI. Even after adjustment for potential confounders, there were significant differences between countries, with Canadians having a higher HbA1c level by 0.3%, worse QOL, and lower rates of insulin pump use, which was contrary to our hypothesis. Interestingly, despite higher HbA1c levels, Canadians had superior lipid parameters. Although the rates of proliferative retinopathy, nephropathy, neuropathy, and PAD were similar, Canadians had significantly lower rates of CAD, even after adjustment.
There are several possible explanations for the differences observed between Canadians and Americans. First, it is unknown whether the differences we observed are specific to populations of individuals with long-standing diabetes or whether these differences are also apparent when comparing the general Canadian and American populations. For example, Americans had higher rates of revascularization procedures, and it is unknown whether these procedures were performed in response to ischemic events (reflecting a higher rate of CAD) or whether a greater number of diagnostic procedures leading to intervention are performed in the U.S. (reflecting a surveillance bias). In a comparison of health care services use after myocardial infarction in individuals with CVD (and not specifically diabetes), the rates of revascularization were substantially lower in Canadians than in Americans. This suggests that the differences we observed in national revascularization rates are not specific to the longevity cohorts in this analysis (18).
Temporal differences in diabetes management may explain some findings because the American data for this study were collected between 2005 and 2011, whereas Canadian data were collected between 2013 and 2016. American Diabetes Association (ADA) guidelines are updated annually and Canadian Diabetes Association (CDA) guidelines were published in 2008 and 2013. In recent years, there has been increasing emphasis on less stringent HbA1c targets in certain populations. In the 2011 ADA guidelines (19), a less stringent HbA1c target was recommended for high-risk populations (Grade C); however, no threshold was specified. Although the 2008 CDA guidelines (20) were similar, the 2013 guidelines had a greater emphasis on individualizing HbA1c targets with a figure devoted to this concept and a specific HbA1c target of up to 8.5% (69 mmol/mol) recommended (Grade D). Even compared with the most recent 2017 ADA guidelines (21), the HbA1c threshold recommended for high-risk populations is more conservative in the CDA guidelines (8.5% [69 mmol/mol]) than the ADA guidelines (8% [64 mmol/mol]). Though the HbA1c level was higher in Canadians, it is reassuring that complication rates (particularly for CAD) were similar between Canadians and Americans. This corroborates previous studies, which have found that current HbA1c level does not correlate with complications in observational studies of long-standing type 1 diabetes (3,4,22).
The 2013 CDA guidelines also have more liberal indications for statin and RAAS inhibitors, with recommendations for the use of both medications in all individuals over 55 years of age, or at any age if a microvascular complication is present. Statin use is recommended for primary prevention in all individuals over 40 years of age or any individual above 30 years of age with >15 years duration of diabetes. The 2013 CDA guidelines also have an online, user-friendly, quick-reference tool for vascular protection. In contrast, the 2011 ADA guidelines recommended statin for primary prevention only in individuals above 40 years of age with an additional risk factor. Even in comparison with the most recent 2017 ADA guidelines, statin is recommended in individuals under 40 years of age for primary prevention only if an additional cardiovascular risk factor is present, and, additionally, RAAS inhibitors are not universally recommended based on age or the presence of microvascular complication. Even though rates of use of statins and RAAS inhibitors were similar in our study and these guidelines were published in the final years of recruitment for these studies, the differences may reflect a general culture that facilitates the treatment of vascular risk factors for primary prevention of CVD to a greater extent in Canada. Americans may benefit from a more aggressive strategy toward vascular protection and consideration of a simple tool for practitioners to consult for vascular protection. LDL was not independently associated with CAD, which is likely a result of the predominance of lipid-lowering medication use in these cohorts and a greater impact of medication use, rather than to LDL level itself, on the risk of CAD.
Disparities in key diabetes indicators (HbA1c, insulin pump use, and CAD) between Canadians and Americans in this analysis remained despite adjustment for confounders. This signifies residual, unexplained differences, which we hypothesize may be the result of a combination of practice patterns reflecting local training and resources, as well as differences in health care systems, that were not captured by our data. Although we assessed the frequency of annual physician visits, we did not collect detailed information regarding health care access and usage, such as specialist involvement, proximity to health services, insurance status, and medication coverage.
Contrary to our hypothesis, Canadians had worse self-reported QOL than Americans, which has been observed previously in several settings. In a multinational clinical trial of type 1 and type 2 diabetes assessing health-related QOL, baseline treatment satisfaction was higher in Americans than in Canadians (23). In a population of patients 1 year after a myocardial infarction, QOL was higher in Americans than in Canadians (18). These studies suggest that Americans may report higher QOL than Canadians, though this is not a consistent finding. For example, a meta-analysis (24) of American and Canadian patients with retinal disease, in which one-third of subjects had diabetic retinopathy, found no differences between countries for four different measures of health-related QOL. It is possible that Americans either truly have higher QOL than Canadians or there is a systematic inflation in their reporting of QOL. In support of this, a study using a time tradeoff methodology to assess utility values for the impact of hypoglycemia demonstrated that Canadians with diabetes had higher values of disutility per hypoglycemic event than Americans, indicating a perception of more severe impact on QOL (25). Another possible explanation for our finding is the influence of SES and education. In individuals over 65 years of age, health-related QOL is associated with household income in the U.S. but not in Canada (26). Though we did not assess SES, education may the best marker of SES for health outcomes, and Canadians in our study had lower levels of education than Americans (27).
We hypothesized that differences between Canadians and Americans would be the result of differences in access to care and funding for health services and medications. In other populations of individuals with diabetes, Canadians have been observed to have more frequent visits with general practitioners and eye specialists than Americans but less frequent care by other specialists such as endocrinologists (11,28). However, the disparities in health care access associated with SES are more pronounced in the U.S. than in Canada. In an examination of national differences in subjects enrolled in the Diabetes Control and Complications Trial (DCCT), higher education was associated with more frequent physician visits in the U.S., but this association was not seen in Canada (11). Uninsured Americans and particularly ethnic minority groups have less access to care than Canadians and report worse QOL, whereas the opposite is true for insured Americans (29). The subjects in our samples likely reflect selection bias for higher education and Caucasian ethnicity, which may have minimized differences due to health care systems that would be more apparent in samples reflecting a broader spectrum of SES.
Differences in access and funding may also explain less pump use in the Canadian participants. For Canadian adults, costs toward pump supplies are covered in some provinces universally, and in other provinces coverage is based on household income or drug plan. Government funding is available for the full cost of insulin pumps only in Ontario and Alberta, in which 67% of the Canadian participants in this study reside. Individuals without government funding for pumps either pay out of pocket or rely on additional insurance (30). In the U.S., individuals over 65 years of age who choose to purchase Medicare Part B (which is a nominal cost) receive funding for insulin pump and supplies (31). Eighteen states have regulations where state insurance law mandates coverage for insulin pumps; however, it is difficult to determine whether this is enforced (32). Unfortunately, we did not have documentation of insurance or Medicare/Medicaid status in the Joslin Medalist participants or private medication coverage for the Canadian participants.
The strengths of this analysis include being the first national comparison of older individuals with type 1 diabetes. Surveys were designed to harmonize data collection with the Joslin Medalist Study allowing comparison. There are, however, several limitations to this study. Survivorship and volunteer bias were present and may affect generalizability but would not affect comparisons since they were similar in both countries because of identical inclusion criteria and accrual methods. In addition, both cohorts may have had a selection bias toward higher SES and Caucasian participants because the proportion of other ethnicities was lower than in other large epidemiologic studies of type 1 diabetes (1,33,34). There is a possibility of measurement error since all complications other than retinopathy and neuropathy were determined by self-report, and QOL was not measured using a standardized scale with demonstrated validity. Blood pressure was not systematically measured. Finally, the study was cross-sectional, and therefore we can only make inferences of association, not causation.
In summary, in a comparison between Canadians and Americans with long-standing type 1 diabetes, complication rates were similar other than lower CAD among Canadians. Canadians had worse glycemic control, lower QOL, and less insulin pump use, yet superior achievement of nonglycemic targets. Our findings differ from national comparisons of clinical trial participants with type 1 diabetes, which may not reflect usual, local practice. The disparities discovered in this study require future research and strategies to optimize management for individuals with type 1 diabetes in both Canada and the U.S.
Article Information
Funding. This work was supported by Diabetes Canada and JDRF Canada (grant 17-2013-312) and its Canadian Clinical Trial Network, as well as Randy and Jenny Frisch and The Harvey and Annice Frisch Family Fund. A.W. has received a postdoctoral fellowship award from the JDRF Canadian Clinical Trial Network, the Dr. Fernand Labrie Fellowship Research Award from the Canadian Society of Endocrinology and Metabolism, and a Canadian Institutes of Health Research Canada Graduate Scholarship Award. Y.L. is the recipient of a Canadian Diabetes Association fellowship award, and D.Z.C. receives operating funding from the Banting & Best Diabetes Centre, University of Toronto, and the Canadian Institutes of Health Research. The Joslin Medalist Study is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (P30-DK-036836, UL1-RR-025758-03, R24-DK-083957-01, and DP3-DK-094333-01), JDRF (17-2013-310), the Beatson Cancer Charity, and contributions by Tom Beatson and many Joslin Medalists.
The funding organizations had no role in the design of the study; the collection, analysis, and interpretation of data; or the decision to approve publication of the completed manuscript.
Duality of Interest. H.A.K. has received support from Sanofi. J.A.L. has received speaker honoraria or consulting fees from Novo Nordisk, Merck Sharp & Dohme, AstraZenca, and Eli Lilly. M.H.B. has been on advisory boards for and has had research support from Novartis Canada, Bayer Canada, and Allergan Canada and has been a consultant to Bayer Canada and Alcon Canada. D.Z.C. has received speaker honoraria from Janssen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Merck and has received research grant support from AstraZeneca, Merck, and Boehringer Ingelheim. B.A.P. has received speaker honoraria from Medtronic, Johnson & Johnson, Roche, GlaxoSmithKline Canada, Novo Nordisk, and Sanofi; has received research grant support from Medtronic and Boehringer Ingelheim; and serves as a consultant for NeuroMetrix. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.W. and B.A.P. performed the primary analysis and wrote the manuscript as primary authors. L.E.L., H.A.K., L.J.T., S.D., G.B., M.A.F., J.A.L., A.O., Y.L., M.H.B., N.P., V.B., and D.Z.C. contributed to discussion and reviewed and edited this work. A.W. and B.A.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016, and the 2016 Canadian Diabetes Association Professional Conference and Annual Meetings, Ottawa, ON, Canada, 26–29 October 2016.
References
- 1.Miller RG, Secrest AM, Ellis D, Becker DJ, Orchard TJ. Changing impact of modifiable risk factors on the incidence of major outcomes of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2013;36:3999–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 3.Bain SC, Gill GV, Dyer PH, et al. Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 2003;20:808–811 [DOI] [PubMed] [Google Scholar]
- 4.Keenan HA, Costacou T, Sun JK, et al. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year Medalist Study. Diabetes Care 2007;30:1995–1997 [DOI] [PubMed] [Google Scholar]
- 5.Sun JK, Keenan HA, Cavallerano JD, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50-year Medalist Study. Diabetes Care 2011;34:968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisman A, Rovinski R, Farooqi MA, et al. Commonly measured clinical variables are not associated with burden of complications in long-standing type 1 diabetes: results from the Canadian Study of Longevity in Diabetes. Diabetes Care 2016;39:e67–e68 [DOI] [PubMed] [Google Scholar]
- 7.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He ZH, D’Eon SA, Tinsley LJ, et al. Cardiovascular disease protection in long-duration type 1 diabetes and sex differences. Diabetes Care 2015;38:e73–e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt S, Gupta MK, Khamaisi M, et al. Preserved DNA damage checkpoint pathway protects against complications in long-standing type 1 diabetes. Cell Metab 2015;22:239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez SL, Gong JH, Chen L, et al. Characterization of circulating and endothelial progenitor cells in patients with extreme-duration type 1 diabetes. Diabetes Care 2014;37:2193–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth GL, Zinman B, Redelmeier DA. Diabetes care in the U.S. and Canada. Diabetes Care 2002;25:1149–1153 [DOI] [PubMed] [Google Scholar]
- 12.Perkins BA, Halpern EM, Orszag A, et al. Sensor-augmented pump and multiple daily injection therapy in the United States and Canada: post-hoc analysis of a randomized controlled trial. Can J Diabetes 2015;39:50–54 [DOI] [PubMed] [Google Scholar]
- 13.Canadian Institute for Health Information Prescribed Drug Spending in Canada, 2016: A Focus on Public Drug Programs. Ottawa, ON, Canada, Canadian Institute for Health Information, 2016 [Google Scholar]
- 14.Ridic G, Gleason S, Ridic O. Comparisons of health care systems in the United States, Germany and Canada. Mater Sociomed 2012;24:112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai JW, Boulet G, Halpern EM, et al. Cardiovascular disease guideline adherence and self-reported statin use in longstanding type 1 diabetes: results from the Canadian study of longevity in diabetes cohort. Cardiovasc Diabetol 2016;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulet G, Halpern EM, Lovblom LE, et al. Prevalence of insulin pump therapy and its association with measures of glycemic control: results from the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Technol Ther 2016;18:298–307 [DOI] [PubMed] [Google Scholar]
- 17.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–1103 [DOI] [PubMed] [Google Scholar]
- 18.Mark DB, Naylor CD, Hlatky MA, et al. Use of medical resources and quality of life after acute myocardial infarction in Canada and the United States. N Engl J Med 1994;331:1130–1135 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng AY; Canadian Diabetes Association Clinical Practice Guidelines Expert Committee . Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes 2013;37(Suppl. 1):S1–S3 [DOI] [PubMed] [Google Scholar]
- 21.Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes 2017;9:320–324 [DOI] [PubMed] [Google Scholar]
- 22.Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003;26:1374–1379 [DOI] [PubMed] [Google Scholar]
- 23.Kotsanos JG, Vignati L, Huster W, et al. Health-related quality-of-life results from multinational clinical trials of insulin lispro. Assessing benefits of a new diabetes therapy. Diabetes Care 1997;20:948–958 [DOI] [PubMed] [Google Scholar]
- 24.Johnson D, Hollands S, Hollands H, Schweitzer K, Almeida D, Sharma S. QOL amongst American vs. Canadian patients with retinal diseases. Curr Opin Ophthalmol 2010;21:227–232 [DOI] [PubMed] [Google Scholar]
- 25.McDonough C, Dunkley AJ, Aujla N, Morris D, Davies MJ, Khunti K. The association between body mass index and health-related QOL: influence of ethnicity on this relationship. Diabetes Obes Metab 2013;15:342–348 [DOI] [PubMed] [Google Scholar]
- 26.Huguet N, Kaplan MS, Feeny D. Socioeconomic status and health-related QOL among elderly people: results from the Joint Canada/United States Survey of Health. Soc Sci Med 2008;66:803–810 [DOI] [PubMed] [Google Scholar]
- 27.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health 1992;82:816–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klarenbach SW, Jacobs P. International comparison of health resource utilization in subjects with diabetes: an analysis of Canadian and American national health surveys. Diabetes Care 2003;26:1116–1122 [DOI] [PubMed] [Google Scholar]
- 29.Lasser KE, Himmelstein DU, Woolhandler S. Access to care, health status, and health disparities in the United States and Canada: results of a cross-national population-based survey. Am J Public Health 2006;96:1300–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diabetes Canada. Access to medications, devices and supplies & your rights [article online], 2017. Available from http://www.diabetes.ca/diabetes-and-you/know-your-rights/access-to-medications-devices-supplies-your-rights. Accessed 16 March 2017
- 31.American Diabetes Association. Medicare [article online], 2017. Available from http://www.diabetes.org/living-with-diabetes/health-insurance/medicare.html?referrer=https://www.google.ca/. Accessed 16 March 2017
- 32.Cauchi R, Mason K, Chung Y, Thangasamy A. Diabetes health coverage: state laws and programs [article online], 2016. Available from http://www.ncsl.org/research/health/diabetes-health-coverage-state-laws-and-programs.aspx. Accessed 16 March 2017 [Google Scholar]
- 33.Walsh MG, Zgibor J, Borch-Johnsen K, Orchard TJ; DiaMond Investigators . A multinational comparison of complications assessment in type 1 diabetes: the DiaMond Substudy of Complications (DiaComp) level 2. Diabetes Care 2004;27:1610–1617 [DOI] [PubMed] [Google Scholar]
- 34.Daniels M, DuBose SN, Maahs DM, et al.; T1D Exchange Clinic Network . Factors associated with microalbuminuria in 7,549 children and adolescents with type 1 diabetes in the T1D Exchange clinic registry. Diabetes Care 2013;36:2639–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]