Studies including ours indicate that systemic inhibition of dipeptidyl peptidase 4 (DPP4), an ubiquitously expressed peptidase, by pharmaceutical inhibitors improved atherosclerosis (1,2). However, the role of immune cell–derived DPP4 is not well defined in atherosclerosis. Our previous work demonstrated that DPP4 expression on monocytes/macrophages was increased in obesity and associated with the degree of insulin resistance (3). To test if the obesity-related increase of monocyte DPP4 plays a role in vascular disease, we investigated the relationship of monocyte DPP4 expression with human aortic atherosclerosis, obesity, and lipid metabolism. A total of 14 control volunteers and 27 atherosclerotic patients without diagnosed heart, lung, or liver diseases and without prescription drugs (except ACE inhibitors and angiotensin receptor blockers) were selected from Aliskiren Effect on Aortic Plaque Progression (ALPINE), a phase 4 clinical trial (clinical trial reg. no. NCT01417104, clinicaltrials.gov). Presence of aortic atherosclerotic plaque (Fig. 1A) was confirmed by high-resolution three-dimensional MRI.
Figure 1.
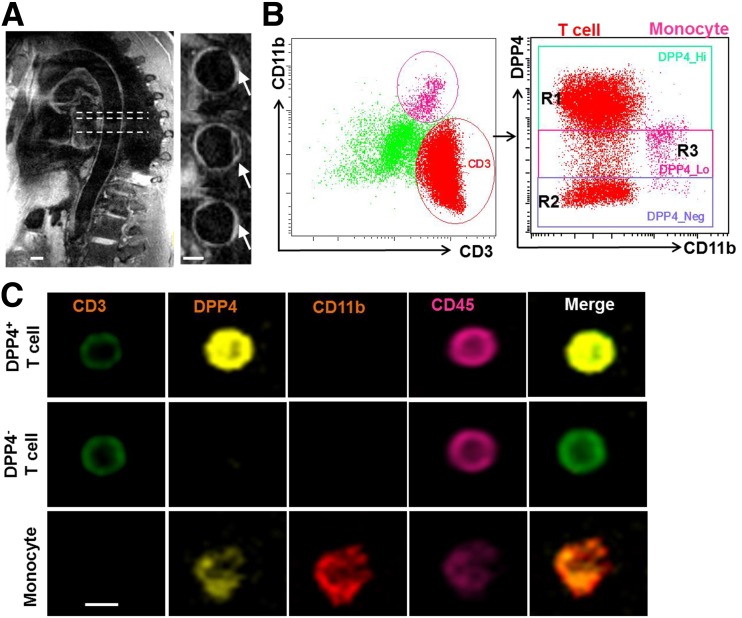
A: High-resolution three-dimensional dark-blood MRI was used to confirm the aortic atherosclerotic plaque in patients with atherosclerotic disease. Arrows indicate the thickening of the aortic wall. Scale bars, 10 mm. B and C: DPP4 expression on circulating immune cells. Peripheral blood mononuclear cells were isolated from healthy volunteers, and CD11b+ monocyte and CD3+ T cells were gated for the detection of DPP4 using an imaging flow cytometer. Representative scatter plots (B) and cell images (C) are shown. Scale bar, 5 μm. R1, region 1 (region with high DPP4 expression); R2, region 2 (region without DPP4 expression); R3, region 3 (region with low DPP4 expression).
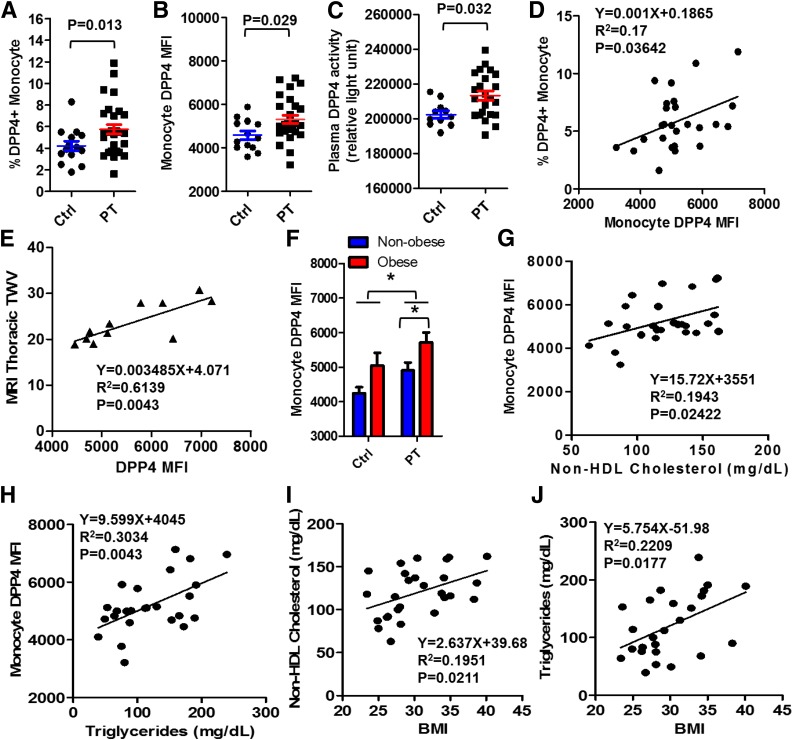
Flowsight imaging flow cytometry confirmed a moderate expression of DPP4 on circulating CD11b+ monocytes (Fig. 1B and C). The frequency of high-expression DPP4 (DPP4+) monocytes in the peripheral blood (Fig. 2A) and DPP4 expression level as indicated by DPP4 mean fluorescence intensity (MFI) on circulating monocytes (Fig. 2B) were increased in patients with atherosclerosis, even after adjusting for obesity and current prescription drug therapy (ACE inhibitors and angiotensin receptor blockers). In accordance with cellular expression, a higher level of plasma DPP4 enzymatic activity was noted in atherosclerosis (Fig. 2C). There was also a positive correlation between the frequency of DPP4+ monocytes and DPP4 MFI (Fig. 2D). In addition, atherosclerotic burden was positively correlated with monocyte DPP4 expression (Fig. 2E) but not with plasma DPP4 activity (data not shown). Since obesity may upregulate DPP4 (3), we examined whether the increase of DPP4 in atherosclerosis was associated with obesity. Monocyte DPP4 expression was further increased in obese (BMI ≥30) atherosclerotic patients compared with nonobese (BMI <30) patients (Fig. 2F).
Figure 2.
A–C: DPP4 expression on circulating monocytes from obese patients with cardiovascular disease and healthy control subjects. Twenty-seven atherosclerotic patients and 14 healthy control subjects were recruited, and peripheral blood mononuclear cells were isolated for the detection of DPP4. The frequency of DPP4+ monocytes in the peripheral blood (A), DPP4 MFI on circulating monocytes (B), and plasma DPP4 enzymatic activity (C) in atherosclerotic patients and healthy control subjects are shown. P values adjusted for BMI and current prescription drug therapy are shown in the figures. D: Correlation analysis of the frequency of DPP4+ monocytes and DPP4 MFI. E: Regression analysis of atherosclerotic burden as measured by thoracic total wall volume (TWV) and monocyte DPP4 expression. F: Monocyte DPP4 MFI in obese and nonobese atherosclerotic patients or healthy control subjects. *P < 0.05. G: Correlation between monocyte DPP4 expression and non-HDL cholesterol level. H: Correlation between monocyte DPP4 expression and triglyceride level. I: Correlation between BMI and non-HDL cholesterol level. J: Correlation between BMI and triglyceride level. Ctrl, control subjects; PT, atherosclerotic patients.
Regression analyses of monocyte DPP4 expression with metabolic parameters showed monocyte DPP4 expression was positively correlated with plasma levels of triglycerides and non-HDL cholesterol (Fig. 2G and H) but not with fasting blood glucose or insulin levels. Given that obesity is an important correlate of abnormal cholesterol/triglyceride metabolism (Fig. 2I and J), lipid mediators of insulin resistance/obesity may drive DPP4 expression. Toll-like receptors (TLRs) have been suggested to mediate LDL-induced inflammatory signaling in cardiometabolic disease (4), and TLR activation by lipopolysaccharide upregulates DPP4 on monocytes and macrophages (3). Therefore, TLR pathways might be involved in the monocyte DPP4 upregulation in obesity and atherosclerosis. However, further studies are required to explore the precise mechanisms underlying obesity-induced DPP4 upregulation. Atherosclerosis is characterized by imbalanced lipid metabolism and maladaptive inflammation caused by cholesterol-laden macrophage accumulation in the arterial wall. Given the importance of DPP4 in mediating both inflammation (3) and dyslipidemia (5), increased expression of DPP4 may play a pathogenic role in obesity-associated atherosclerosis progression by promoting dyslipidemia and vascular inflammation. In summary, our results suggest an important role for monocyte DPP4, linking obesity and dyslipidemia with atherosclerosis.
Article Information
Acknowledgments. The authors thank clinical staff (Lisa Hafer at The Ohio State University, Kylene Broadwater and Steven Sawicki at the University of Maryland School of Medicine) for their help with subject recruitment and sample collection.
Funding. This work was supported by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (K01DK105108), American Heart Association (17GRNT33670485), American Association of Immunologists (CIIF-8745), and National Natural Science Foundation of China (81670431). X.R. was supported by grants from the National Institute of Environmental Health Sciences (K99ES026241) and the National Institute of Diabetes and Digestive and Kidney Diseases (T32DK098107).
Duality of Interest. S.I.T. was previously employed by Bristol-Myers Squibb and currently owns stock in that company. S.R. has served as a consultant for Takeda Pharmaceuticals and Boehringer Ingelheim. J.Z. has received funding support from Boehringer Ingelheim (IIS2015-10485). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. X.R., C.X., and J.Z. researched data. G.M. and J.V. were responsible for MRI measurement of aortic plaque. X.R. and J.Z. wrote the manuscript. S.R. and J.Z. reviewed and edited the manuscript. J.A.D. and C.S. contributed to clinical tissue collection. M.B.F., X.J.S., M.J.Q., and S.I.T. contributed to discussion of experimental design. J.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Shah Z, Kampfrath T, Deiuliis JA, et al. . Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011;124:2338–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mita T, Katakami N, Shiraiwa T, et al.; Collaborators on the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE) Trial . Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE): a randomized controlled trial [published correction appears in Diabetes Care 2017;40:808]. Diabetes Care 2016;39:455–464 [DOI] [PubMed] [Google Scholar]
- 3.Zhong J, Rao X, Deiuliis J, et al. . A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 2013;62:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem 2003;278:1561–1568 [DOI] [PubMed] [Google Scholar]
- 5.Xiao C, Dash S, Morgantini C, Adeli K, Lewis GF. Gut peptides are novel regulators of intestinal lipoprotein secretion: experimental and pharmacological manipulation of lipoprotein metabolism. Diabetes 2015;64:2310–2318 [DOI] [PubMed] [Google Scholar]